Introduction
S100 calcium binding protein A4 (S100A4) is
controlled directly by the Wnt/β-catenin signal pathway and is
involved in a number of biological mechanisms, i.e. cell movement,
surviving, differentiation, and organization of the cytoskeleton
(1–5). In addition, S100A4 plays an important
role in the biology of stem cells (2,4,5). In
S100A4 deficient mice tumor development and metastasis are
suppressed (6). S100A4 is
overexpressed in tumor cells with metastatic phenotype, while
inhibition of S100A4 expression reduces the metastatic potential of
tumor cells (7,8).
Cysteine-rich angiogenic inducer 61 (CYR61, CCN1) is
part of the CCN intercellular signaling protein family and has
various physiological functions, which are strongly dependent on
the respective tissue (9,10). In some tissues integrin-dependent
proliferation, migration and adhesion is induced by CYR61 (11). In addition, CYR61 can cause
directed migration as chemoattractant (12,13).
Cyr61 has an important role during vascularization in embryonic
development, angiogenesis, and wound healing (10,14,15).
In addition, CYR61 plays an important role in skeleton formation
and development of the neural system (14,15).
Furthermore, CYR61 is involved in transformation processes of the
extracellular membrane (16).
CYR61-caused effects in tumor tissues depend also strongly on the
respective kind of tissue and stage of the disease (17–23).
In breast cancers CYR61 leads to increased vascularization of
tumors, increased cell proliferation, increased migration and a
general increase of tumorigenicity (18,24).
A significant connection between CYR61 overexpression and stage of
cancer, tumor size, lymph node infestation, and poor survival
prognosis can be determined for cancers such as breast cancer,
glioma and squamous cell carcinoma (18,20,24,25).
Between 50 and 60% of human breast cancers and in
addition most breast cancer cell lines express receptors for
gonadotropin-releasing hormone (GnRH) (26–29).
Approximately 74% of triple-negative breast cancers express GnRH
receptors (30). Previous work
showed that invasion of breast cancer cells through an artificial
basement membrane was increased when they were cocultured with
human primary osteoblasts (hOB) (31). We demonstrated that invasion in
vitro (31) and metastasis
in vivo (32) of GnRH
receptor-positive breast cancer cells was time- and
dose-dependently reduced by GnRH analogs. We have now analyzed
whether GnRH treatment affects S100A4 and CYR61 in mesenchymal
transformed breast cancer cells.
Materials and methods
Cell lines and culture conditions
Human breast cancer cell lines MDA-MB-231 and MCF-7
(wild-type) and osteoblast-like osteosarcoma cell line MG63 were
obtained from American Type Culture Collection (ATCC, Manassas, VA,
USA). In order to guarantee the identity of the cell lines over the
years, cells were expanded after purchase and aliquots were stored
in liquid nitrogen. Every half year a new frozen stock was opened
and expanded to carry out the experiments. Cells were cultured at
37°C in a humidified atmosphere of 5% CO2 in air in
phenol red-free Dulbecco's minimal essential medium (DMEM, PAA
Laboratories GmbH, Pasching, Austria) supplemented with 10%
charcoal-stripped fetal calf serum (cs-FCS) from Allgaeu BioTech
Service (Görisried, Germany).
Human tissues
To analyze expression of GnRH receptor, S100A4, and
CYR61 in specimens of human breast cancers we used human tissue
arrays (US Biomax, Inc., Rockville, MD, USA) containing
paraffin-embedded human normal and malignant breast tissue
specimens, whose characteristics are outlined in Table I. Informed consent for the use of
human tissues was obtained in accordance with the ethics guidelines
that were effective at the time of collection and processing.
 | Table IImmunohistochemical detection of
S100A4, CYR61 and GnRH receptor in human breast cancers, normal and
other non-malignant breast tissues. |
Table I
Immunohistochemical detection of
S100A4, CYR61 and GnRH receptor in human breast cancers, normal and
other non-malignant breast tissues.
| No. | Age | Path.
diagnosis | Type | Grade | TNM | AR | ERα | PR | HER2 | S100A4 | CYR61 | GnRH-R |
|---|
| 1 | 50 | Normal | Normal | | | + – ++, 30% | + – ++, 15% | − | − | − | − | + |
| 2 | 43 | Normal | Normal | | | + – ++, 20% | +, 5% | ++, 15% | ++ | − | − | + |
| 3 | 50 | Normal | Normal | | | + – ++, 20% | + – ++, 15% | +, 5% | − | − | − | + |
| 4 | 30 | Periductual
mastitis | Hyperplasia | | | + – ++, 20% | + – ++, 5% | ++, 5% | − | − | − | − |
| 5 | 52 | Hyperplasia | Hyperplasia | | | − | + – ++, 10% | ++, 10% | − | + | − | + |
| 6 | 38 | Hyperplasia | Hyperplasia | | | − | + – ++, 5% | ++, 5% | − | + | + | + |
| 7 | 41 | Hyperplasia | Hyperplasia | | | +, 20% | +, 15% | ++, 20% | − | + | + | − |
| 8 | 40 | Fibrocystic
changes | Hyperplasia | | | + – ++, 20% | + – ++, 15% | + – ++, 20% | + | + | + | + |
| 9 | 41 | Fibrocystic
changes | Hyperplasia | | | +, 20% | + – ++, 15% | ++ – +++, 30% | + | ++ | + | + |
| 10 | 39 | Fibroadenoma | Benign | | | +, 5% | + – ++, 5% | + – ++, 10% | + | − | − | + |
| 11 | 16 | Fibroadenoma | Benign | | | +, 50% | ++ – +++, 80% | ++ – +++, 80% | +/− | − | − | + |
| 12 | 34 | Fibroadenoma | Benign | | | + – ++, 30% | ++ – +++, 20% | ++ – +++, 20% | + | − | − | + |
| 13 | 38 | Phyllodes
sarcoma | Sarcoma | | TisN0M0 | + – ++, 10% | + – ++, 10% | ++ – +++, 50% | + – ++ | + | − | − |
| 14 | 52 | Intraductal
carcinoma | In Situ | I | TisN0M0 | +/− | +++, 80% | − | ++ – +++ | + | + | + |
| 15 | 33 | Intraductal
carcinoma | In Situ | I | TisN0M0 | +/− | ++, 20% | ++ – +++, 50% | ++ – +++ | + | + | + |
| 16 | 37 | Invasive ductal
carcinoma (part. intraductal) | In Situ | I–II | T3N1M1 | + – ++, 20% | ++ – +++, 60% | ++ – +++, 50% | + | + | ++ | + |
| 17 | 53 | Ductal carcinoma
in situ | In Situ | I | TisN0M0 | +, 15% | − | − | +++ | + | + | ++ |
| 18 | 61 | Ductal papillary
adenocarcinoma | Malignant | I–II | T2N0M0 | +, 15% | + – ++, 20% | + – ++, 10% | + | +++ | ++ | ++ |
| 19 | 41 | Invasive ductal
carcinoma | Malignant | II | T2N0M0 | ++, 5% | ++, 5% | ++ – +++, 10% | + | ++ | ++ | + |
| 20 | 55 | Invasive ductal
carcinoma | Malignant | III | T3N2M0 | + – ++, 5% | +, 50% | + – ++, 5% | + | +++ | ++ | + |
| 21 | 44 | Invasive ductal
carcinoma | Malignant | II–III | T3N0M0 | − | − | − | − | +++ | + | ++ |
| 22 | 72 | Invasive ductal
carcinoma | Malignant | II | T2N0M0 | ++, 20% | − | − | ++ – +++ | ++ | + | ++ |
| 23 | 41 | Invasive ductal
carcinoma | Malignant | II | T3N2M0 | − | +, 5% | − | +++ | ++ | + | ++ |
| 24 | 18 | Invasive ductal
carcinoma | Malignant | II–III | T2N0M0 | +/− | + – ++, 5% | + – ++, 5% | + | + | + | − |
| 25 | 31 | Invasive ductal
carcinoma | Malignant | II–III | T2N0M0 | +/− | − | − | +++ | +++ | + | ++ |
| 26 | 54 | Invasive ductal
carcinoma | Malignant | II | T2N0M0 | − | − | − | +++ | ++ | + | − |
| 27 | 75 | Invasive ductal
carcinoma | Malignant | III | T3N0M0 | ++, 30% | +, 10% | + – ++, 10% | − | ++ | + | + |
| 28 | 30 | Invasive ductal
carcinoma | Malignant | I–II | T3N0M0 | − | − | + – ++, 5% | − | +++ | + | + |
| 29 | 43 | Invasive ductal
carcinoma | Malignant | III | T3N0M0 | − | − | − | − | ++ | + | ++ |
| 30 | 37 | Invasive ductal
carcinoma | Malignant | III | T2N0M0 | − | − | + – ++, 5% | +++ | ++ | − | + |
| 31 | 42 | Invasive ductal
carcinoma | Malignant | II–III | T4N2MX | − | +, 50% | ++ – +++, 60% | + | ++ | ++ | − |
| 32 | 30 | Invasive ductal
carcinoma | Malignant | II | T2N0M0 | − | − | − | − | +++ | +/− | ++ |
| 33 | 39 | Invasive ductal
carcinoma | Malignant | I–II | T3N0M0 | − | ++ – +++, 50% | + – ++, 50% | +/− | ++ | +++ | + |
| 34 | 46 | Invasive ductal
carcinoma | Malignant | III | T2N0M0 | +/− | +, 5% | − | + | ++ | + | − |
| 35 | 40 | Invasive ductal
carcinoma | Malignant | II | T3N1M0 | − | +, 10% | + – ++, 10% | − | ++ | + | + |
| 36 | 58 | Invasive ductal
carcinoma | Malignant | II | T3N0M0 | − | − | − | ++ – +++ | ++ | + | − |
| 37 | 35 | Invasive ductal
carcinoma | Malignant | III | T2N0M0 | ++ – +++, 60% | ++ – +++, 30% | + – ++, 10% | +++ | +++ | +++ | ++ |
| 38 | 53 | Invasive ductal
carcinoma | Malignant | II–III | T2N0M0 | ++ – +++, 10% | + – ++, 10% | − | +++ | ++ | ++ | − |
| 39 | 50 | Invasive ductal
carcinoma | Malignant | II–III | T4N3M1 | − | +, 5% | +, 5% | + | +++ | + | + |
| 40 | 48 | Invasive ductal
carcinoma | Malignant | II–III | T2N0M0 | +, 5% | ++ – +++, 30% | ++ – +++, 50% | +/− | ++ | +++ | + |
| 41 | 51 | Invasive ductal
carcinoma | Malignant | II | T3N1M0 | +/− | ++, 50% | + – ++, 15% | − | ++ | ++ | − |
| 42 | 43 | Invasive ductal
carcinoma | Malignant | II–III | T4N2MX | − | − | − | +++ | +++ | + | − |
| 43 | 43 | Invasive ductal
carcinoma | Malignant | II–III | T3N1M0 | +/− | − | − | + – ++ | +++ | +/− | ++ |
| 44 | 40 | Invasive ductal
carcinoma | Malignant | III | T3N0M0 | − | − | − | +++ | +++ | + | + |
| 45 | 47 | Invasive ductal
carcinoma | Malignant | III | T3N1M0 | − | − | − | ++ – +++ | ++ | + | ++ |
| 46 | 49 | Invasive ductal
carcinoma | Malignant | II–III | T3N1M0 | + – ++, 5% | ++ – +++, 20% | ++ – +++, 60% | + | +++ | +++ | − |
| 47 | 58 | Invasive mucinous
adenocarcinoma | Malignant | | T3N2M0 | − | − | − | + – ++ | ++ | +/− | ++ |
| 48 | 40 | Invasive lobular
carcinoma | Malignant | III | T2N0M0 | +/− | ++ – +++, 50% | + – ++, 15% | − | + | +++ | + |
Immunohistochemistry
The tissue array slides were deparaffinized and
rehydrated. Antigens were retrieved by incubation with 0.01 M
citrate buffer (pH 6.0) in a microwave (700 W) for 5 min.
Endogenous peroxidase activity was quenched by treatment with 3%
hydrogen peroxide solution for 6 min. After washing in PBS, the
slides were treated with polyclonal rabbit anti-human GnRH receptor
antiserum (33), polyclonal rabbit
anti-human S100A4 antibody (Abcam, Cambridge, UK), or polyclonal
rabbit anti-human CYR61 antibody (Abcam) in a 1:10,000, 1:1,000, or
1:1,000 dilution, respectively in 1% BSA in 10 mM Tris, pH 8.0, 500
mM NaCl and 0.1% Tween-20 (TBST) for 1 h and, after being washed,
were detected with the ready-to-use secondary horseradish
peroxidase-conjugated anti-rabbit IgG antibody detection system
according to the instructions of the supplier (Zymed Laboratories,
San Francisco, CA, USA). Controls were performed by substitution of
the primary antiserum with pre-immune serum of the same rabbit
(anti-human GnRH receptor) or by omission of the primary antibody.
Counterstaining was performed using Meyer's hematoxylin for 10 sec.
The slides were then dehydrated, cleared, mounted with Permount,
and studied by light microscopy.
Co-culture and microinvasion assay
Invasion was measured by assessment of the breast
cancer cell migration rate through an artificial basement membrane
in a modified Boyden chamber, where the breast cancer cells and the
MG63 cells were grown without direct cell-to-cell contact. The
membrane of the cell culture insert (upper well) consisted of
polycarbonate (8-μm pore diameter, Millipore, Schwalbach,
Germany) and was coated on ice with Matrigel®
[extracellular matrix (ECM) gel; Becton Dickinson Biosciences,
Heidelberg, Germany] diluted 1:2 in serum-free DMEM. Breast cancer
cells were seeded into the upper wells (inserts) of the chamber,
while the MG63 cells were seeded into the lower wells. The cells
were cultured in DMEM supplemented with 10% charcoal-stripped fetal
calf serum (cs-FCS) without phenol red for 12 h to allow the cells
to attach. Thereafter the upper wells were placed on top of the
lower wells (time point t0) and the breast cancer cells
were treated right away with increasing concentrations of GnRH
agonist Triptorelin (10−11-10−5 M) every 24 h
for 96 h, with polyclonal rabbit anti-human S100A4 antibody (Abcam;
15 μg/ml) or with polyclonal rabbit anti-human CYR61
antibody (Abcam; 15 μg/ml) for 96 h. A non-specific
polyclonal rabbit isotype control antibody was used as negative
control (Abcam). After 96 h the invaded breast cancer cells under
the membrane were counted. Controls were performed by omission of
the MG63 cells.
Generation of aggressive MCF-7 cells
Mesenchymal transformed MCF-7 cells (MCF-7-EMT) were
generated as described by Ziegler et al (34). Briefly, single-cell suspensions of
wild-type MCF-7 cells (MCF-7 WT) were suspended at a density of
40,000 cells/ml in DMEM/F-12 containing 5 μg/ml insulin
(Sanofi-Aventis, Frankfurt, Germany), 0.5 mg/ml hydrocortisone, 2%
B27 supplement (Invitrogen, Darmstadt, Germany), 20 ng/ml epidermal
growth factor (EGF; Sigma, Deisenhofen, Germany), 20 ng/ml
fibroblast growth factor-2 (FGF-2; Sigma), and 1%
penicillin/streptomycin (PAA Laboratories GmbH) before incubation
on ultralow adherence six-well plates (2.5 ml per plate; Corning,
Lowell, MA, USA). For prolonged mammosphere culture (5–6 weeks) the
cells were passaged weekly. Mammospheres were harvested, incubated
with trypsin for 3 min at 37°C, and dissociated with a 21-gauge
needle. After checking for single cells, the cells were pelleted
and suspended in mammosphere culture medium to 40,000 cells/ml
before culturing on ultralow adherence plates. To show changes in
morphology, bright field images of living MCF-7 WT cells and
MCF-7-EMT cells were taken.
Isolation of mRNA and cDNA synthesis
The cells were detached immediately with 0.5 g
trypsin (Biochrom, Berlin, Germany) and 5 mmol EDTA in 1 liter
PBS/bovine serum albumin. Total RNA was prepared by the RNeasy
protocol (Qiagen, Hilden, Germany). The concentration of RNA in
each sample was determined by photospectroscopy. First-strand cDNA
was generated by reverse transcription of 1 mg total RNA, using
p(dT)15 primers (Roche Diagnostics, Mannheim, Germany) with
MMLV-reverse transcriptase, according to the instructions of the
suppliers (Life Technologies, Karlsruhe, Germany). After
determination of the concentrations of the cDNAs, the samples were
used for PCR analysis. The integrity of the samples was tested by
RT-PCR of the ribosomal housekeeping gene L7.
Semiquantitative PCR amplification
The cDNAs (2 ng) were amplified in a 50 μl
reaction volume containing 10 mM Tris/HCl (pH 8.3), 50 mM potassium
chloride, 1.5 mM magnesium chloride, 200 mM of each of the dNTPs, 1
mM of the appropriate primers (S100A4 (239 bp): forward primer,
5′-TCT CTC CTC AGC GCT TCT TC-3′; backward primer, 5′-GCT GTC CAA
GTT GCT CAT CA-3′; CYR61 (241 bp): forward primer, 5′-CTC CCT GTT
TTT GGA ATG GA-3′; backward primer, 5′-TGG TCT TGC TGC ATT TCT
TG-3′; L7 (357 bp), forward primer, 5′-AGA TGT ACA GAA CTG AAA
TTC-3′; backward primer, 5′-ATT TAC CAA GAG ATC GAG CAA-3′) and
1.25 U AmpliTaq Gold polymerase (Applied Biosystems, Weiterstadt,
Germany) in an Applied Biosystems DNA thermal cycler 9600.
Twenty-five (S100A4), 27 (CYR61) or 18 (L7) cycles of amplification
representing the exponential phase of the PCR were carried out:
Denaturation at 94°C for 60 sec; annealing at 58°C (S100A4), 55°C
(CYR61) or 54°C (L7) for 60 sec; followed by extension at 72°C for
60 sec. The PCR products were separated by gel electrophoresis in
1.5% agarose and visualized by ethidium bromide staining on a UV
transilluminator. For correct densitometric analysis, the S100A4 or
the CYR61 fragment and the L7 fragment were run on the same gel.
For quantification the bands were analyzed using a Biometra BioDoc
Analysis system (Biometra, Göttingen, Germany). Expression levels
were calculated in comparison with the expression levels of the
control (=100%). Expression levels of the housekeeping gene L7 were
used for standardization.
Statistical analysis
All experiments were repeated at least three times
with different passages of the respective cell lines. The data were
tested for significant differences by un-paired two-tailed t-test
or by one-way analysis of variance followed by Tukey's multiple
comparisons test for comparison of individual groups, after a
Bartlett test had shown that variances were homogeneous using
GraphPad Prism 6.01 software (GraphPad Software Inc., La Jolla, CA,
USA).
Results
Correlation of invasiveness and
expression of S100A4 and CYR61
Invasion of MDA-MB-231 breast cancer cells measured
by assessment of breast cancer cell migration rate through an
artificial basement membrane in a modified Boyden chamber was
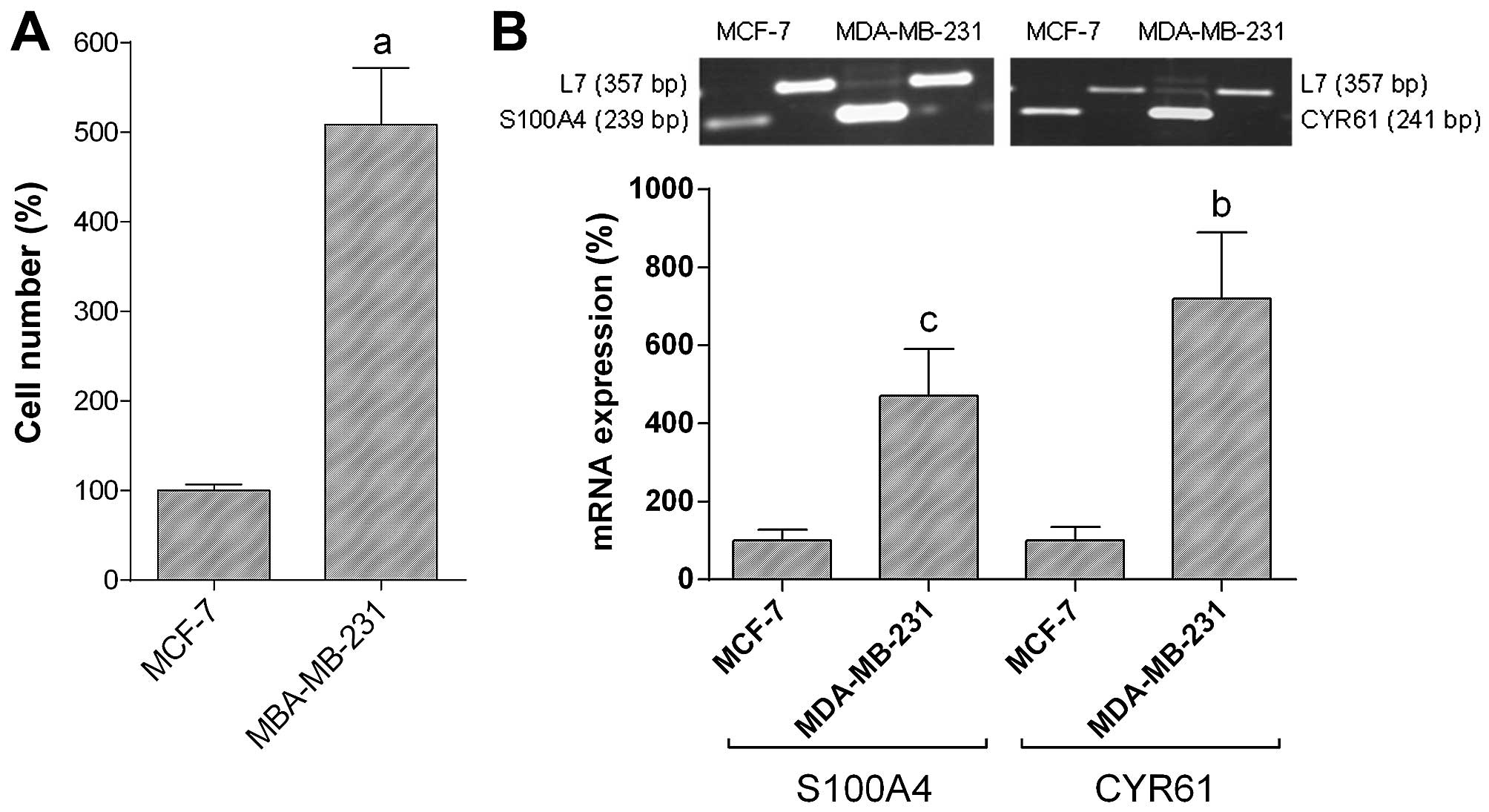
5-fold higher (588.8±63.2% vs. MCF-7; =100%; P<0.001) as
compared with MCF-7 breast cancer cells (Fig. 1A). Expression of S100A4 mRNA in
MDA-MB-231 breast cancer cells was 4.7-fold higher (470.0±120.9 vs.
MCF-7; =100%; P<0.05) as compared with MCF-7 breast cancer cells
(Fig. 1B). CYR61 mRNA expression
in MDA-MB-231 breast cancer cells was 7.2-fold higher (719.5±169.7
vs. MCF-7; =100%; P<0.01) as compared with MCF-7 breast cancer
cells (Fig. 1B).
Expression of S100A4 and effects of
anti-S100A4 antibody treatment on invasion of MCF-7-EMT and
MDA-MB-231 cells
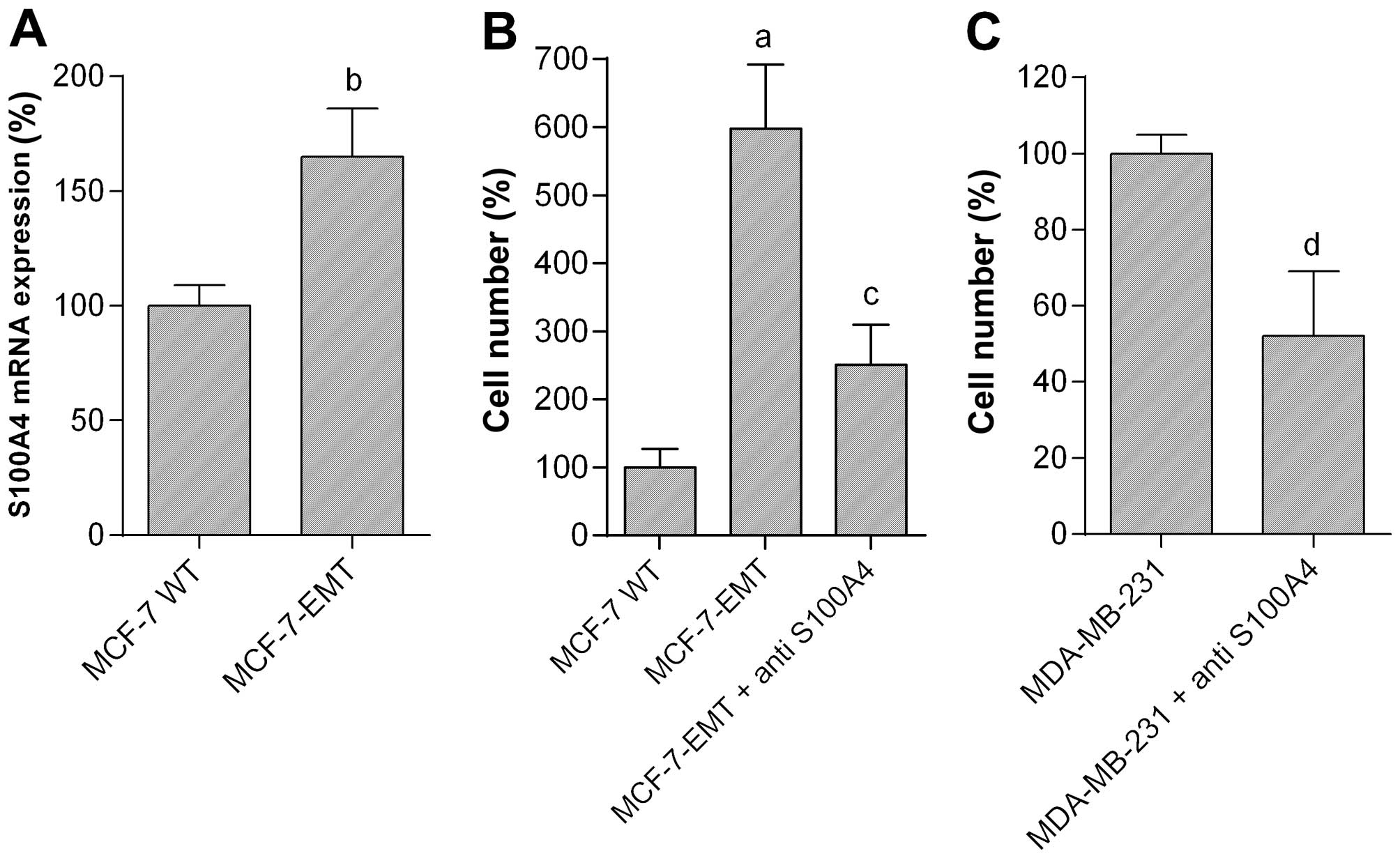
Mesenchymal transformed MCF-7 cells (MCF-7-EMT)
showing significantly increased invasion in contrast to wild-type
MCF-7 (MCF-7 WT) cells were generated using prolonged mammosphere
culture (34,35). Expression of S100A4 mRNA in
MCF-7-EMT cells was significantly increased to 165.1±21.0% as
compared with MCF-7 WT cells (=100%; P<0.05) (Fig. 2A).
To emphasize the importance of S100A4 we observed
the invasiveness of MCF-7-EMT and MDA-MB-231 breast cancer cells
after treatment with anti-human S100A4 antibody (Fig. 2B and C). MCF-7-EMT cells cultured
in a modified Boyden chamber showed significant increased invasion
in contrast to the wild-type cells (Fig. 2B). Invasion of MCF-7-EMT cells was
6-fold higher (598.3±94.5% vs. MCF-7 WT; P<0.001) in comparison
to MCF-7 WT cells. Treatment with S100A4 antibody led to a
significant decrease of invaded MCF-7-EMT cells to 251.1±59.2% of
control (P<0.01 vs. MCF-7-EMT) (Fig. 2B). The naturally high invasiveness
of MDA-MB-231 cells was reduced to 52.4±17.2% of control (=100%;
P<0.001) after treatment with S100A4 antibody (Fig. 2C). Controls using a non-specific
isotype control antibody showed no effects (not shown).
Expression of CYR61 and effects of
anti-CYR61 antibody treatment on invasion of MCF-7-EMT and
MDA-MB-231 cells
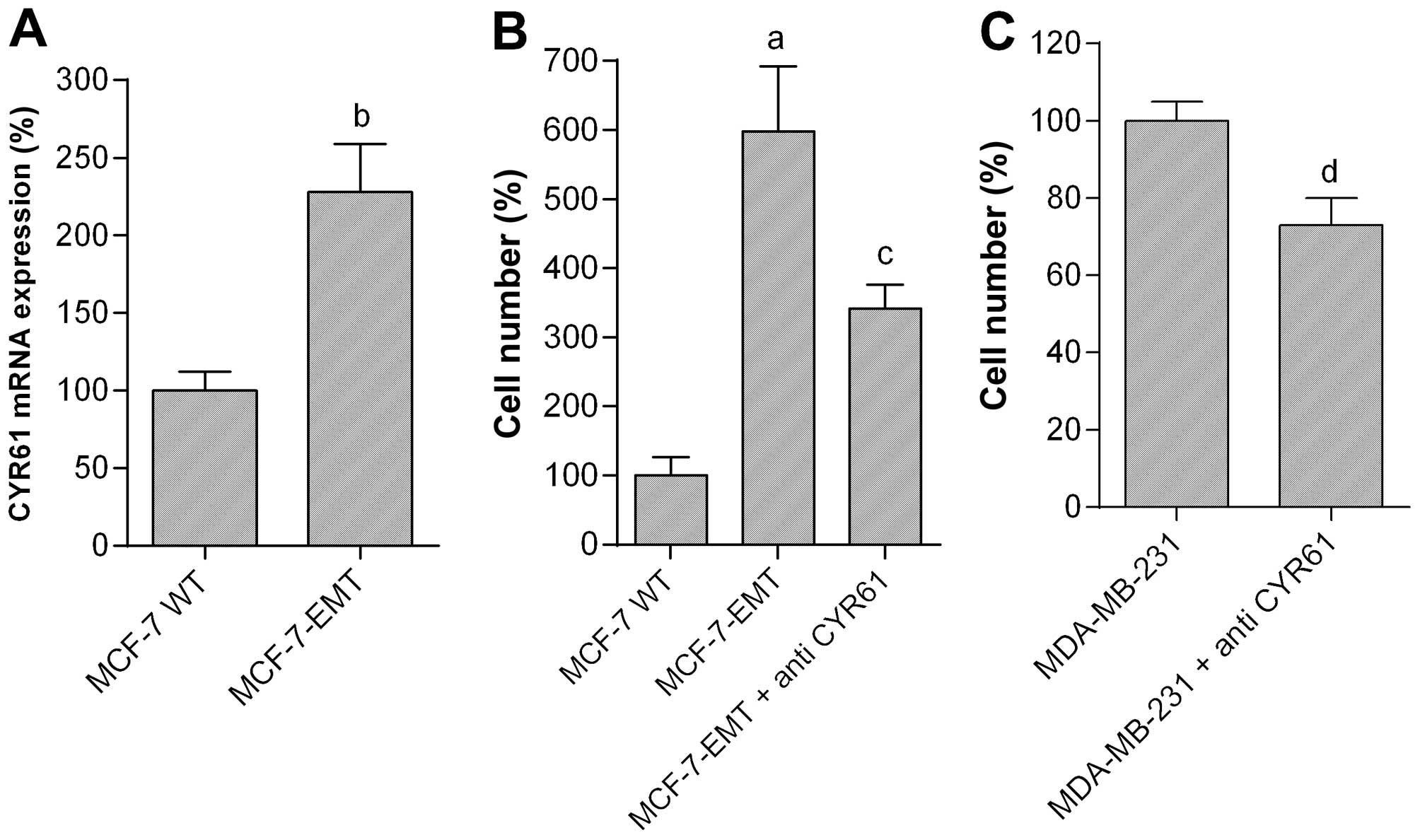
Expression of CYR61 mRNA in MCF-7-EMT cells was
significantly increased to 228.2±30.9% as compared with MCF-7 WT
cells (=100%; P<0.01) (Fig.
3A). To analyze the role of CYR61 we observed the invasiveness
of MCF-7-EMT and MDA-MB-231 breast cancer cells after treatment
with anti-human CYR61 antibody (Fig.
3B and C). The 6-fold increased invasion of MCF-7-EMT cells in
comparison to MCF-7 WT cells (598.3±94.5% vs. MCF-7 WT; P<0.001)
was significantly decreased to 341.4±35.3% of control (P<0.05
vs. MCF-7-EMT) after treatment with CYR61 antibody (Fig. 3B). The naturally high invasiveness
of MDA-MB-231 cells was reduced to 73.1±7.0% of control (=100%;
P<0.05) after treatment with CYR61 antibody (Fig. 3C). Controls using a non-specific
isotype control antibody showed no effects (not shown).
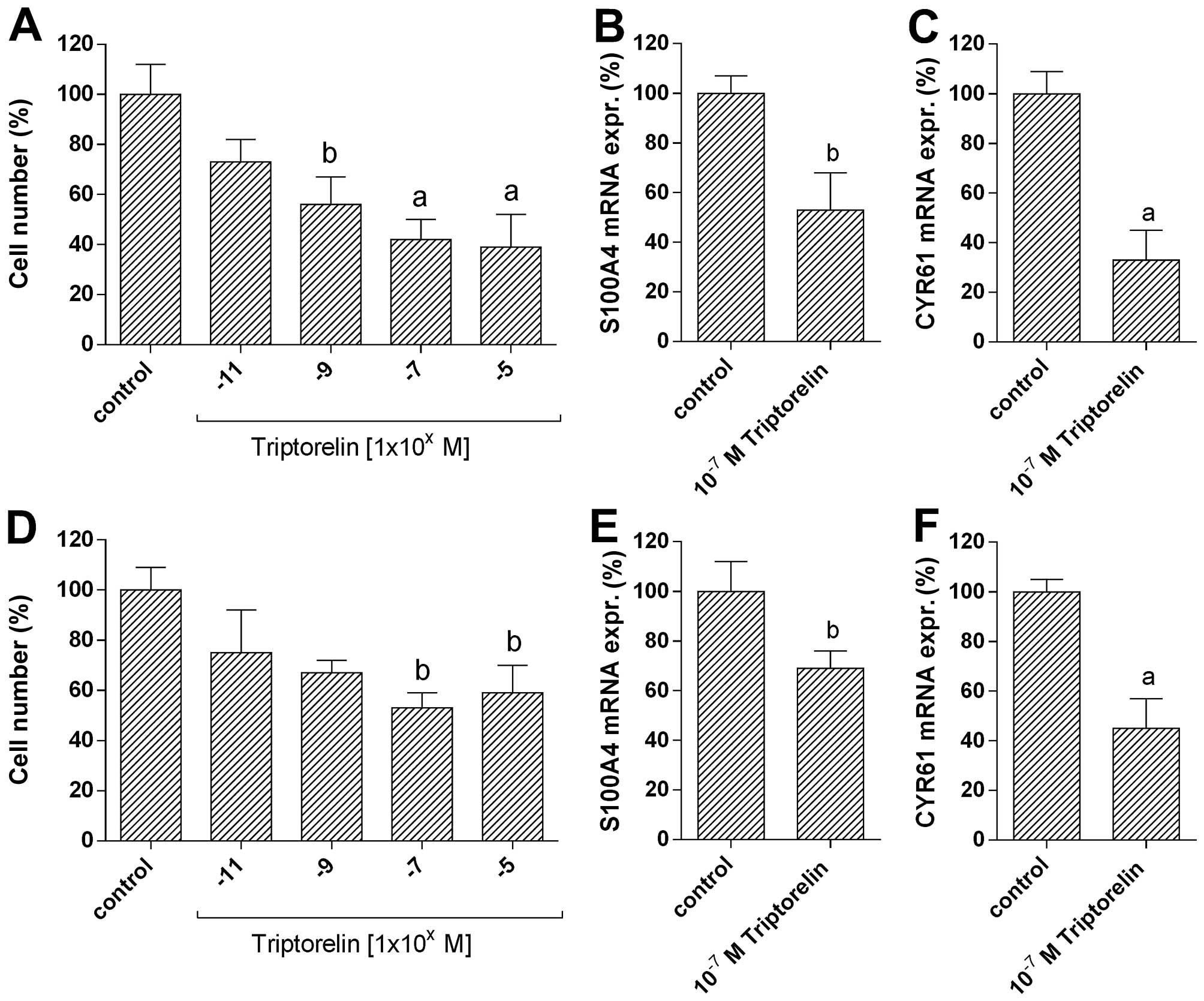
Effects of GnRH agonist treatment on
invasion and expression of S100A4 and CYR61
In the GnRH receptor-positive breast cancer cell
lines MCF-7-EMT (Fig. 4A) and
MDA-MB-231 (Fig. 4D), the number
of invaded cells was dose-dependently reduced by 5 days of
treatment with increasing concentrations
(10−11-10−5 M) of GnRH agonist
Triptorelin.
At 10−11 M Triptorelin concentration a
slight decrease in number of invaded MCF-7-EMT cells to 73.2±9.1%
of control (=100%; not significant) was observed (Fig. 4A). At 10−9 M
concentration of Triptorelin, the reduction in cell number was
significant (55.8±10.9% of control; 100%; P<0.05). At
10−7 M Triptorelin concentration a significant decrease
in number of invaded MCF-7-EMT cells to 42.0±7.9% of control (100%;
P<0.01) was observed. Triptorelin at 10−5 M had
almost the same inhibitory effects on cell invasion (38.7±12.5% of
control (100%; P<0.01).
At 10−11 M Triptorelin concentration the
number of invaded MDA-MB-231 cells was slightly decreased to
75.1±16.8% of control (100%; not significant) (Fig. 4D). At 10−9 M
concentration of Triptorelin the number of invaded MDA-MB-231 cells
was reduced to 67.2±5.4% of control (100%; not significant). The
inhibitory effects were maximal at 10−7 M concentration
of Triptorelin and corresponded to 53.1±5.8% of control (100%;
P<0.05). Triptorelin at 10−5 M showed comparable
inhibitory effects on cell invasion (58.9±10.7% of control (100%;
P<0.05).
To analyze whether GnRH plays a role in S100A4 and
CYR61 function, MCF-7-EMT (Fig. 4B and
C) and MDA-MB-231 (Fig. 4E and
F) cells were treated with GnRH agonist Triptorelin and mRNA
expression of S100A4 and CYR61 was measured. After 48 h of
treatment with Triptorelin (10−7 M) S100A4 mRNA
expression in MCF-7-EMT cells was significantly reduced to
53.2±14.7% of control (100%; P<0.05) (Fig. 4B). Expression of CYR61 mRNA in
MCF-7-EMT cells was significantly decreased to 33.1±10.8% of
control (100%; P<0.01) (Fig.
4C). Treatment of MDA-MB-231 cells with 10−7 M
Triptorelin for 48 h resulted in a significant decrease of S100A4
mRNA expression to 68.7±7.1% of control (100%; p<0.05) (Fig. 4E), whereas CYR61 mRNA was
significantly reduced to 45.0±11.9% of control (100%; P<0.01)
(Fig. 4F).
Immunohistochemical detection of S100A4,
CYR61 and GnRH receptor in human breast cancers, normal and other
non-malignant breast tissues
Following data were given by the supplier of the
tissue arrays (materials and methods section): Age of the patients,
pathology diagnosis, tissue type, tumor grading, and TNM
classification (Table I).
Expression levels of androgen receptor (AR), estrogen receptor α
(ERα), progesterone receptor (PR) and human epidermal growth factor
receptor 2 (HER2) were known (Table
I).
In biopsy specimens of breast hyperplasia and
malignant breast cancers S100A4 and CYR61 were found highly
expressed (Table I). Carcinoma
in situ showed lower expression of S100A4 and CYR61, whereas
normal breast tissues and benign fibroadenoma were S100A4 and CYR61
negative. In most cases, high CYR61 expression, but not high S100A4
expression was correlated with high ERα expression. Benign
fibroadenoma with high ERα expression remained CYR61 negative.
Correlations to expression of AR, PR and HER2 were not found. GnRH
receptor expression was detectable in 4 of 6 cases of benign
fibroadenoma (67%), in 3 of 3 cases of benign fibroadenoma (100%),
in 4 of 4 cases of carcinoma in situ (100%), and in 22 of 31
cases of malignant breast cancers (71%).
Discussion
We have established a coculture model to mimic the
in vivo invasion process and to analyze EMT (31,36,37).
Using this model we can induce non invading MCF-7 breast cancer
cells to substantial invasion and thus to markedly increase the
number of cells undergoing EMT (31,34).
By prolonged mammosphere culture we have generated a mesenchymal
transformed MCF-7 cell line (MCF-7-EMT), which in contrast to
wild-type MCF-7 cells (MCF-7 WT) exhibit significantly increased
invasive behavior in vitro and in vivo as well as
increased expression of EMT-related genes (34).
S100A4 and CYR61 play important roles in EMT,
invasion and metastasis by promoting cancer cell motility (6,11,38,39).
We found both genes highly expressed in high invasive MDA-MB-231
breast cancer cells. Jiang et al demonstrated a significant
increase of CYR61 expression in breast cancers in comparison to
normal breast tissue (40). This
increase is correlated with poor prognosis, lymph node status as
well as metastatic propagation (40,41).
Jenkinson et al showed a clear influence of S100A4 on
invasiveness of breast cancer cells (42). Invasion of breast cancer cells with
S100A4 overexpression was strongly increased as compared with the
non-transfected controls.
MCF-7 cells have no invasive behavior. In addition,
MCF-7 cells show very low expression of S100A4 and CYR61. After
mesenchymal transition, invasion and expression of S100A4 and CYR61
of MCF-7 cells were found to be markedly increased. The increased
invasion of MCF-7-EMT cells was reduced by anti-S100A4 and
anti-CYR61 antibodies. In addition, invasion of naturally high
invasive MDA-MB-231 cells was decreased by anti-S100A4 and
anti-CYR61 antibodies showing the important role of these factors.
Nguyen et al have attributed the migration-promoting effect
of CYR61 to MMP-1 expression (43). In a fibroblast-directed migration
assay, loss of CYR61 in breast cancer cells led to inhibition of
MMP-1. In the same assay, absence of MMP-1 activity in the
fibroblasts inhibited CYR61-permitted migration of the breast
cancer cells (43).
Previously we showed that in vitro invasion
and in vivo metastasis of GnRH receptor-positive breast
cancer cells is time- and dose-dependently reduced by GnRH analogs
(31,32). Now we have analyzed whether GnRH
treatment affects increased invasion and expression of S100A4 and
CYR61 in mesenchymal transformed breast cancer cells. Treatment of
mesenchymal transformed MCF-7-EMT and naturally high invasive
MDA-MB-231 cells with GnRH agonist Triptorelin resulted in a
significant decrease of invasion and expression of S100A4 and
CYR61. Lin et al reported that neutralizing of CYR61 using
an anti-CYR61 antibody resulted in inhibition of breast cancer
growth and metastasis in vivo (44). We used a non-toxic and easy to
apply GnRH agonist to reduce CYR61 and S100A4 expression. In
addition, our earlier studies showed anti-metastatic activities of
GnRH agonists without undesirable side effects (31,32).
However, overexpression of CYR61 in endometriosis could not be
reduced after therapy with GnRH agonist Leuprorelin (45).
To further show the clinical significance of S100A4
and CYR6, we have analyzed their expression in biopsy specimens.
Both, S100A4 and CYR61 were found highly expressed in biopsy
specimens of malignant breast cancers, whereas their expression in
carcinoma in situ was much lower. Normal breast tissues and
benign fibroadenoma were S100A4 and CYR61 negative. Noteworthy, we
found in breast hyperplasia a high expression as well, although its
biological background and relevance remains uncertain. High CYR61
expression but not high S100A4 expression seems to be associated
with high estrogen receptor α (ERα) expression. It is known that
expression of CYR61 is induced by estrogen (18). Correlation of high CYR61 expression
with high ERα expression was found in many studies (18,46–49).
However, benign fibroadenoma with high ERα expression remained
CYR61 negative, indicating that additional mechanisms regulate
CYR61 expression.
To use GnRH agonists for treatment of invasive
breast cancer it is essential that these tumors express GnRH
receptors. We found GnRH receptor expression in approximately 71%
of malignant breast cancers (n=31). In a recent study we were able
to demonstrate GnRH receptor expression in approximately 74% of
triple-negative breast cancer specimens (n=42) (30). Another study found GnRH receptor
expression in all analyzed triple-negative breast cancers (n=16)
(50).
In conclusion, our findings suggest that S100A4 and
CYR61 play major roles in breast cancer invasion. Treatment with
GnRH agonist Triptorelin decreases invasion and expression of
S100A4 and CYR61. The precise mechanisms remain unclear and are
part of our current research. The use of GnRH agonists or similar
S100A4 and CYR61 blocking treatments might represent novel
anti-metastatic therapeutic approaches and should be further
explored.
Acknowledgements
We would like to thank Sonja Blume for her excellent
technical assistance.
References
|
1
|
Stein U, Arlt F, Walther W, Smith J,
Waldman T, Harris ED, Mertins SD, Heizmann CW, Allard D, Birchmeier
W, et al: The metastasis-associated gene S100A4 is a novel target
of beta-catenin/T-cell factor signaling in colon cancer.
Gastroenterology. 131:1486–1500. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ito M and Kizawa K: Expression of
calcium-binding S100 proteins A4 and A6 in regions of the
epithelial sac associated with the onset of hair follicle
regeneration. J Invest Dermatol. 116:956–963. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mazzucchelli L: Protein S100A4: Too long
overlooked by pathologists? Am J Pathol. 160:7–13. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Morris RJ, Liu Y, Marles L, Yang Z,
Trempus C, Li S, Lin JS, Sawicki JA and Cotsarelis G: Capturing and
profiling adult hair follicle stem cells. Nat Biotechnol.
22:411–417. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tumbar T, Guasch G, Greco V, Blanpain C,
Lowry WE, Rendl M and Fuchs E: Defining the epithelial stem cell
niche in skin. Science. 303:359–363. 2004. View Article : Google Scholar
|
|
6
|
Grum-Schwensen B, Klingelhofer J, Berg CH,
El-Naaman C, Grigorian M, Lukanidin E and Ambartsumian N:
Suppression of tumor development and metastasis formation in mice
lacking the S100A4(mts1) gene. Cancer Res. 65:3772–3780. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Saleem M, Kweon MH, Johnson JJ, Adhami VM,
Elcheva I, Khan N, Bin Hafeez B, Bhat KM, Sarfaraz S, Reagan-Shaw
S, et al: S100A4 accelerates tumorigenesis and invasion of human
prostate cancer through the transcriptional regulation of matrix
metalloproteinase 9. Proc Natl Acad Sci USA. 103:14825–14830. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mahon PC, Baril P, Bhakta V, Chelala C,
Caulee K, Harada T and Lemoine NR: S100A4 contributes to the
suppression of BNIP3 expression, chemoresistance, and inhibition of
apoptosis in pancreatic cancer. Cancer Res. 67:6786–6795. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lau LF and Lam SC: The CCN family of
angiogenic regulators: The integrin connection. Exp Cell Res.
248:44–57. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Holbourn KP, Acharya KR and Perbal B: The
CCN family of proteins: Structure-function relationships. Trends
Biochem Sci. 33:461–473. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kireeva ML, Mo FE, Yang GP and Lau LF:
Cyr61, a product of a growth factor-inducible immediate-early gene,
promotes cell proliferation, migration, and adhesion. Mol Cell
Biol. 16:1326–1334. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schütze N, Schenk R, Fiedler J, Mattes T,
Jakob F and Brenner RE: CYR61/CCN1 and WISP3/CCN6 are
chemoattractive ligands for human multipotent mesenchymal stroma
cells. BMC Cell Biol. 8:452007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin BR, Chang CC, Chen LR, Wu MH, Wang MY,
Kuo IH, Chu CY, Chang KJ, Lee PH, Chen WJ, et al: Cysteine-rich 61
(CCN1) enhances chemotactic migration, transendothelial cell
migration, and intravasation by concomitantly up-regulating
chemokine receptor 1 and 2. Mol Cancer Res. 5:1111–1123. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
O'Brien TP and Lau LF: Expression of the
growth factor-inducible immediate early gene cyr61 correlates with
chondrogenesis during mouse embryonic development. Cell Growth
Differ. 3:645–654. 1992.PubMed/NCBI
|
|
15
|
Dornbach LM and Lyons KM: Genetic analysis
of CCN gene function in mammalian development. CCN Proteins. A New
Damily of Cell Growth and Differentiation Regulators. Perbal BV and
Takigawa M: Imperial College Press; London; Hackensack, NJ: pp.
135–152. 2005, View Article : Google Scholar
|
|
16
|
Brigstock DR: Regulation of angiogenesis
and endothelial cell function by connective tissue growth factor
(CTGF) and cysteine-rich 61 (CYR61). Angiogenesis. 5:153–165. 2002.
View Article : Google Scholar
|
|
17
|
Sun ZJ, Wang Y, Cai Z, Chen PP, Tong XJ
and Xie D: Involvement of Cyr61 in growth, migration, and
metastasis of prostate cancer cells. Br J Cancer. 99:1656–1667.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xie D, Miller CW, O'Kelly J, Nakachi K,
Sakashita A, Said JW, Gornbein J and Koeffler HP: Breast cancer.
Cyr61 is over-expressed, estrogen-inducible, and associated with
more advanced disease. J Biol Chem. 276:14187–14194.
2001.PubMed/NCBI
|
|
19
|
Xie D, Yin D, Tong X, O'Kelly J, Mori A,
Miller C, Black K, Gui D, Said JW and Koeffler HP: Cyr61 is
overexpressed in gliomas and involved in integrin-linked
kinase-mediated Akt and beta-catenin-TCF/Lef signaling pathways.
Cancer Res. 64:1987–1996. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kok SH, Chang HH, Tsai JY, Hung HC, Lin
CY, Chiang CP, Liu CM and Kuo MY: Expression of Cyr61 (CCN1) in
human oral squamous cell carcinoma: An independent marker for poor
prognosis. Head Neck. 32:1665–1673. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Holloway SE, Beck AW, Girard L, Jaber MR,
Barnett CC Jr, Brekken RA and Fleming JB: Increased expression of
Cyr61 (CCN1) identified in peritoneal metastases from human
pancreatic cancer. J Am Coll Surg. 200:371–377. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen PP, Li WJ, Wang Y, Zhao S, Li DY,
Feng LY, Shi XL, Koeffler HP, Tong XJ and Xie D: Expression of
Cyr61, CTGF, and WISP-1 correlates with clinical features of lung
cancer. PLoS One. 2:e5342007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chien W, Kumagai T, Miller CW, Desmond JC,
Frank JM, Said JW and Koeffler HP: Cyr61 suppresses growth of human
endometrial cancer cells. J Biol Chem. 279:53087–53096. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Evtimova V, Zeillinger R and Weidle UH:
Identification of genes associated with the invasive status of
human mammary carcinoma cell lines by transcriptional profiling.
Tumour Biol. 24:189–198. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Goodwin CR, Lal B, Zhou X, Ho S, Xia S,
Taeger A, Murray J and Laterra J: Cyr61 mediates hepatocyte growth
factor-dependent tumor cell growth, migration, and Akt activation.
Cancer Res. 70:2932–2941. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fekete M, Wittliff JL and Schally AV:
Characteristics and distribution of receptors for
[D-TRP6]-luteinizing hormone-releasing hormone, somatostatin,
epidermal growth factor, and sex steroids in 500 biopsy samples of
human breast cancer. J Clin Lab Anal. 3:137–147. 1989. View Article : Google Scholar
|
|
27
|
Baumann KH, Kiesel L, Kaufmann M, Bastert
G and Runnebaum B: Characterization of binding sites for a
GnRH-agonist (buserelin) in human breast cancer biopsies and their
distribution in relation to tumor parameters. Breast Cancer Res
Treat. 25:37–46. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Moriya T, Suzuki T, Pilichowska M, Ariga
N, Kimura N, Ouchi N, Nagura H and Sasano H: Immunohistochemical
expression of gonadotropin releasing hormone receptor in human
breast carcinoma. Pathol Int. 51:333–337. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mangia A, Tommasi S, Reshkin SJ, Simone G,
Stea B, Schittulli F and Paradiso A: Gonadotropin releasing hormone
receptor expression in primary breast cancer: Comparison of
immunohistochemical, radioligand and western blot analyses. Oncol
Rep. 9:1127–1132. 2002.PubMed/NCBI
|
|
30
|
Fost C, Duwe F, Hellriegel M, Schweyer S,
Emons G and Grundker C: Targeted chemotherapy for triple-negative
breast cancers via LHRH receptor. Oncol Rep. 25:1481–1487.
2011.PubMed/NCBI
|
|
31
|
von Alten J, Fister S, Schulz H, Viereck
V, Frosch KH, Emons G and Gründker C: GnRH analogs reduce
invasiveness of human breast cancer cells. Breast Cancer Res Treat.
100:13–21. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schubert A, Hawighorst T, Emons G and
Gründker C: Agonists and antagonists of GnRH-I and −II reduce
metastasis formation by triple-negative human breast cancer cells
in vivo. Breast Cancer Res Treat. 130:783–790. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gründker C, Schlotawa L, Viereck V, Eicke
N, Horst A, Kairies B and Emons G: Antiproliferative effects of the
GnRH antagonist cetrorelix and of GnRH-II on human endometrial and
ovarian cancer cells are not mediated through the GnRH type I
receptor. Eur J Endocrinol. 151:141–149. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ziegler E, Hansen MT, Haase M, Emons G and
Gründker C: Generation of MCF-7 cells with aggressive metastatic
potential in vitro and in vivo. Breast Cancer Res Treat.
148:269–277. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gründker C, Bauerschmitz G, Knapp J,
Schmidt E, Olbrich T and Emons G: Inhibition of SDF-1/CXCR4-induced
epithelial-mesenchymal transition by kisspeptin-10. Breast Cancer
Res Treat. 152:41–50. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Olbrich T, Ziegler E, Türk G, Schubert A,
Emons G and Gründker C: Kisspeptin-10 inhibits bone-directed
migration of GPR54-positive breast cancer cells: Evidence for a
dose-window effect. Gynecol Oncol. 119:571–578. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Magliocco A and Egan C: Breast cancer
metastasis: Advances trough the use of in vitro co-culture model
systems. Breast Cancer - Focusing Tumor Microenvironment, Stem
Cells and Metastasis. Gunduz M and Gunduz E: InTech; Rijeka,
Croatia: 2011, View
Article : Google Scholar
|
|
38
|
Kim EJ and Helfman DM: Characterization of
the metastasis-associated protein, S100A4. Roles of calcium binding
and dimerization in cellular localization and interaction with
myosin. J Biol Chem. 278:30063–30073. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Leask A and Abraham DJ: All in the CCN
family: Essential matricellular signaling modulators emerge from
the bunker. J Cell Sci. 119:4803–4810. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jiang WG, Watkins G, Fodstad O,
Douglas-Jones A, Mokbel K and Mansel RE: Differential expression of
the CCN family members Cyr61, CTGF and Nov in human breast cancer.
Endocr Relat Cancer. 11:781–791. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xie D, Nakachi K, Wang H, Elashoff R and
Koeffler HP: Elevated levels of connective tissue growth factor,
WISP-1, and CYR61 in primary breast cancers associated with more
advanced features. Cancer Res. 61:8917–8923. 2001.PubMed/NCBI
|
|
42
|
Jenkinson SR, Barraclough R, West CR and
Rudland PS: S100A4 regulates cell motility and invasion in an in
vitro model for breast cancer metastasis. Br J Cancer. 90:253–262.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Nguyen N, Kuliopulos A, Graham RA and
Covic L: Tumor-derived Cyr61(CCN1) promotes stromal matrix
metalloproteinase-1 production and protease-activated receptor
1-dependent migration of breast cancer cells. Cancer Res.
66:2658–2665. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lin J, Huo R, Wang L, Zhou Z, Sun Y, Shen
B, Wang R and Li N: A novel anti-Cyr61 antibody inhibits breast
cancer growth and metastasis in vivo. Cancer Immunol Immunother.
61:677–687. 2012. View Article : Google Scholar
|
|
45
|
Absenger Y, Hess-Stumpp H, Kreft B,
Krätzschmar J, Haendler B, Schütze N, Regidor PA and Winterhager E:
Cyr61, a deregulated gene in endometriosis. Mol Hum Reprod.
10:399–407. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Dhar A and Ray A: The CCN family proteins
in carcinogenesis. Exp Oncol. 32:2–9. 2010.PubMed/NCBI
|
|
47
|
Tsai MS, Bogart DF, Castañeda JM, Li P and
Lupu R: Cyr61 promotes breast tumorigenesis and cancer progression.
Oncogene. 21:8178–8185. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tsai MS, Bogart DF, Li P, Mehmi I and Lupu
R: Expression and regulation of Cyr61 in human breast cancer cell
lines. Oncogene. 21:964–973. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sampath D, Winneker RC and Zhang Z: Cyr61,
a member of the CCN family, is required for MCF-7 cell
proliferation: Regulation by 17beta-estradiol and overexpression in
human breast cancer. Endocrinology. 142:2540–2548. 2001.PubMed/NCBI
|
|
50
|
Buchholz S, Seitz S, Schally AV, Engel JB,
Rick FG, Szalontay L, Hohla F, Krishan A, Papadia A, Gaiser T, et
al: Triple-negative breast cancers express receptors for
luteinizing hormone-releasing hormone (LHRH) and respond to LHRH
antagonist cetrorelix with growth inhibition. Int J Oncol.
35:789–796. 2009.PubMed/NCBI
|