Introduction
Clear cell adenocarcinoma (CCC) of the ovary
accounts for 10% of epithelial ovarian cancers and is an entity
distinct from other ovarian carcinomas. For example, CCC exhibits a
more chemoresistant phenotype than other histological types, which
leads to a poorer prognosis. Although the combination of
carboplatin and paclitaxel has been established as the standard
therapy for ovarian cancer (1),
with serous and endometrioid adenocarcinomas responding well to
this regimen, CCC shows lower response rates (2,3).
Moreover, the incidence of CCC has been increasing; it is now
estimated to account for 20% of ovarian cancers in Japan and ~5–6%
in Europe (3). New target-based
therapies for the treatment of CCC remains an unmet need in these
patients.
Apoptotic cell death is critical for the maintenance
of tissue homeostasis in healthy organisms, providing an efficient
and safe mechanism to remove unwanted cells. Impairment of
apoptosis is a crucial step in tumor development (4) and renders tumor cells more resistant
to conventional cytotoxic therapy (5). The key regulators of apoptosis are
interacting proteins of the Bcl-2 family, which has been subdivided
into three subfamilies: a pro-survival subfamily (Bcl2 and the
closely related proteins Bcl-XL, Bcl-w and
Mcl-1) and two pro-apoptotic subfamilies that include the initiator
BH3-only proteins (Bim, PUMA, BAD and NOXA) and the cell death
mediators (BAX and BAK) (6,7). The
interactions among these intracellular proteins determine whether a
cell lives or dies, and alterations in their expression and
function are associated with cancer development (8,9).
ABT737 is a small-molecule inhibitor of the anti-apoptotic proteins
Bcl-2, Bcl-XL and Bcl-w, which shows
single-agent activity and increases sensitivity to chemotherapeutic
agents (10). However, cells
expressing high levels of Mcl-1 show resistance to ABT737 (11). Mcl-1 is also thought to be a
crucial pro-survival factor responsible for resistance to
antitublin agents such as paclitaxel (12). Therefore, inhibiting Mcl-1, as well
as Bcl-2, Bcl-XL and Bcl-w, may be required
to overcome ovarian cancer chemo-resistance. Recently, Mcl-1
inhibitor molecule 1 (MIM1), a selective small-molecule inhibitor
of Mcl-1 (IC50, 4.8 μM) that overcomes Mcl-1-dependent
leukemia cell survival, was reported (13). MIM1 selectively affects Mcl-1, with
no effect on Bcl-2 or Bcl-XL. In this report we
investigated the effect of combining paclitaxel with ABT-737 or/and
MIM1 on ovarian cancer cell viability.
Bcl2-associated athanogene 3 (BAG3) is one of six
BAG family proteins. Via its BAG domain, BAG3 interacts with and
regulates the folding of Hsc70/Hsp70 (14,15).
In addition to the BAG domain, BAG3 contains a WW domain near its
N-terminus and a proline-rich region (multiple PXXP motifs)
(16,17), and our earlier study showed that
BAG3 also regulates cell motility and tumor growth, invasion and
metastasis (18). Moreover,
several lines of evidence indicate that downregulation of BAG3
enhances chemotherapy-mediated apoptosis among cancer cells
(19–21), which is consistent with the recent
finding that BAG3 stabilizes Mcl-1, Bcl-2 and Bcl-XL,
thereby promoting cancer cell survival (22,23).
The precise mechanism by which BAG3 exerts these effects remains
unclear. It is known, however, that the deubiquitinase USP9X
(ubiquitin-specific peptidase 9, X-linked) is among the proteins
that co-immunoprecipitate with Mcl-1 (24), and that removing the poly-ubiquitin
chains from Mcl-1 stabilizes it and confers resistance to
apoptosis. In this manner, USP9X reportedly promotes tumor cell
survival (25).
In this study, we investigated the mechanism by
which BAG3 confers chemoresistance to ovarian cancer cells. We
found that BAG3 stabilizes Mcl-1 by interacting with USP9X. We
previously showed that BAG3 expression relates to clinical severity
and prognosis in ovarian cancer patients (26). Here we assessed Mcl-1 expression in
ovarian cancer tissues and its correlation with the clinical state,
and evaluated the effect of ABT-737 and MIM1 on ovarian cancer cell
sensitivity to paclitaxel.
Materials and methods
Cells and cell culture
Two established ovarian cancer cell lines were used
in this study. The AMOC2 line was established from a serous
adenocarcinoma, while the ES2 line was established from a clear
cell carcinoma. AMOC2 cells were cultured in RPMI-1640 (Gibco,
Grand Island, NY, USA) supplemented with 10% fetal bovine serum
(Hyclone, Logan, UT, USA) and 1% penicillin/streptomycin (Gibco).
ES2 cells were cultured in Dulbecco's modified Eagle's medium
(DMEM) (Gibco) supplemented with 10% FBS and 1%
penicillin/streptomycin. Both cell lines were maintained in a
CO2 incubator (5% CO2) at 37°C.
BAG3 overexpression
AMOC2 cells were transfected with the expression
vector pcDNA-BAG3, encoding full-length BAG3, or with empty pcDNA
vector (control) using Lipofectamine 3000 regent (Invitrogen,
Carlsbad, CA, USA) according to the manufacturer's protocol. After
24 h, the cells were split and allowed to adhere overnight.
Real-time quantitative reverse
transcription PCR (qRT-PCR) for microRNA
Total RNA was extracted from cells using TRIzol
regent (Invitrogen), after which reverse transcription was
performed with 10 μg of total RNA using a TaqMan®
MicroRNA Reverse Transcription kit (Applied Biosystems, Foster, CA,
USA) and sequence-specific RT primers from the TaqMan MicroRNA
assays (Applied Biosystems) according to the manufacturer's
instructions. Separate reverse transcription reactions were run for
each TaqMan MicroRNA assay on every RNA sample. qRT-PCR was
performed with cDNA using inventoried TaqMan MicroRNA assays and
TaqMan Universal Master Mix II (Applied Biosystems). The assay was
performed in triplicate, and the PCR amplification was performed
using a StepOnePlus™ Real-Time PCR system (Applied Biosystems).
Gene expression was calculated using the 2−ΔCt method.
Mature miRNA transfection
Using Lipofectamine RNAiMAX (Invitrogen), cells
grown in 6-cm dishes were transfected with miRNA-29b mirVana miRNA
mimic (Ambion, Austin, TX, USA) to augment miR-29b activity, or
with an inactive negative control for miRNA-29b mirVana miRNA mimic
(Ambion). Alternatively, they were transfected with miRNA-29b
mirVana miRNA inhibitor (Ambion) to diminish miR-29b activity or
with a negative control for miRNA-29b mirVana miRNA inhibitor
(Ambion).
Gene silencing using a short hairpin RNA
(shRNA) vector
A gene silencing vector (pLTRH1) containing RNA
polymerase promoter producing shRNA specific for BAG3 was used to
transfect ES2 and AMOC2 cells. Oligonucleotides
[5′-GATCCCGTACCTGATGATCGAAGAGTTTCAAGAGAACTCTTCGATCATCAGGTATTTTTGGAG-3′
(sense) and
5′-TCGACTTCCAAAAAATACCTGATGATCGAAGAGTTCTCTTGAAACTCTTCGATCATCAGGTACGG-3′
(antisense)] specific for mouse bag3 were synthesized and subcloned
into the Bg1II and Sa1I sites, downstream of the H1
promoter (27). G3T-hi
amphotrophic packaging cells (Takara Bio, Shiga, Japan) were
transfected with pLTRH1bag3 puro or empty pLTRH1 puro vector
according to the manufacturer's instructions to obtain a retroviral
supernatant, which was added at a 1:5 ratio to DMEM or RPMI-1640
supplemented with 10% FBS and then used to infect ES2 or AMOC2
cells. Infected cells were then selected by incubation in medium
containing 1.0 μg/ml puromycin (Gibco) for 48 h after infection.
High-responder clones to BAG3 knockdown were selected for
subsequent experiments.
siRNA transfection
Cells were transfected with Mcl-1 small interfering
RNA (siRNA) (#6315; Cell Signaling, MA, USA), control siRNA (#6568;
Cell Signaling), USP9X siRNA (SR305407; OriGene, MD, USA) or
control siRNA (SR300004; OriGene) by Lipofectamine RNAiMAX
(Invitrogen), and lysates were prepared 48 h after
transfection.
Lysate production
Cell lysates were produced from subcon-fluent cell
cultures. After scraping the cells from dishes, they were placed in
RIPA buffer [50 mM Tris-HCl (pH 8.0), 150 mM NaCl, 0.1% SDS, 1%
NP40 and 0.5% sodium deoxycholate] containing a protease inhibitor
cocktail (1:100 dilution; Thermo Scientific, Rockford, IL, USA).
The cells were then lysed by sonication, after which the lysates
were centrifuged at 15,000 rpm for 15 min at 4°C to pellet the
nuclei. The supernatant was then collected as the cell lysate.
Western blotting
After measuring the protein content, lysates was
diluted in 2X sample buffer [0.5 M Tris-HCl (pH 6.8), 10% SDS,
β-mercaptoethanol and 1% BPB] and boiled for 5 min at 100°C.
Samples containing 20 μg of protein were then electrophoresed (200
V for 35 min) on SDS polyacryl-amide gel, and the separated
proteins were transferred onto PVDF membranes. After blocking with
5% non-fat dry milk in TBS [10 mM sodium phosphate (pH 7.8), 150 mM
NaCl and 0.05% Tween-20], the membrane was probed with the
following primary antibodies: anti-BAG3 (1:1,000 dilution; gift of
Dr S. Takayama), anti-Mcl-1 (1:500 dilution; S-19; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), anti-Bcl-XL
(1:1,000 dilution; #2762; Cell Signaling), anti-PARP (1:1,000
dilution; #9542; Cell Signaling), anti-USP9X (1:5,000 dilution;
ab99343, Abcam, Cambridge, UK) and anti-β-actin (1:5,000 dilution;
A5441; Sigma-Aldrich, St. Louis, MO, USA). The protein was
visualized using ECL Prime Western Blotting Detection Reagent and
ImageQuant LAS 500 (GE Healthcare, Buckinghamshire, UK).
Cell viability assay
To test the responsiveness of the cells to
paclitaxel under various culture conditions, cells were plated in
5% serum-containing medium in 96-well plates (5,000 cells/well) and
incubated at 37°C under a 5% CO2 atmosphere. After 24 h,
the medium was replaced with medium containing the indicated
concentration of paclitaxel (Nippon Kayaku Co., Ltd., Tokyo,
Japan), MIM1 (Merck Millipore, Darmstadt, Germany), ABT737 (Selleck
Chemicals, Houston, TX, USA) or a combination of the three. Cell
viability assays were then performed after 48 h using a Cell
Proliferation Kit II (XTT; Roche Diagnostics, Mannheim, Germany).
After the desired incubation period, 50 μl of XTT labeling mixture
was added to each well, and the cells were incubated for an
additional 4 h, after which absorbance at 492 nm was recorded using
an ELISA plate reader.
Co-immunoprecipitation
Cell lysates were prepared in immunoprecipitation
(IP) lysis buffer (25 mM Tris, 150 mM NaCl, 1 mM EDTA, 1% Nonidet
P-40, 5% glycerol and protease inhibitor cocktail (1:100 dilution,
pH 7.4; Thermo Scientific) and clarified by centrifugation (14,000
x g, 20 min). Anti-USP9X (Abcam) and anti-Myc-tag mAb (1:100
dilution; M192-3; MBL, Co., Ltd., Nagoya, Japan) were used for
immunoprecipitation. Dynabeads Protein G (Life Technologies,
Carlsbad, CA, USA) were incubated with antibody for 30 min at 4°C.
After washing beads with IP buffer, the beads with the immobilized
antibody were incubated with lysates for 30 min at 4°C. The beads
were then washed three times with IP buffer, and the complexes were
eluted in 2X SDS sample buffer (5 min at 100°C), resolved by
SDS-PAGE and analyzed by western blotting.
Patients for clinical and pathological
analysis
Primary cancer tissue specimens were obtained from
51 patients operated on for ovarian cancer at Sapporo Medical
University Hospital. All samples were preserved at −80°C until
used. In addition, the patient's clinical and pathological data
were obtained from their medical records. None of the participating
patients received preoperative treatment (e.g., neoadjuvant
chemotherapy). All patients signed a consent form to
participate.
Statistical analysis
Student's t-tests were used for statistical
evaluation of the data. The Kaplan-Meier product limit method was
used to compare progression-free survival among the patients, and
the data obtained were evaluated using the log-rank test. Values of
P<0.05 were considered significant. SPSS 22.0 (IBM, Armonk, NY,
USA) was used in analysis.
Results
Mcl-1 expression in primary ovarian
cancer tissue
We previously found that BAG3 is associated with
significantly higher risks of cancer progression and relapse
(26), and that BAG3 upregulates
Mcl-1 and mediates chemoresistance in ovarian cancer cells
(28). In this study, we first
investigated the expression of Mcl-1 in ovarian cancer patients.
Lysates were prepared from tissue samples collected from 51 ovarian
cancer patients and analyzed by immunoblotting. The patient
characteristics are shown in Table
I. Mcl-1 expression was detected in 11 (21.6%) of the 51
samples. Upon application of FIGO stage classification, we found
that the rate of Mcl-1 positivity was higher at stages III (9/28,
32.1%) and IV (1/4, 25%) than at stages I (1/17, 5.8%) and II (0/2,
0%), but there was no significant difference in Mcl-1 expression
among FIGO stages, and no significant correlation between Mcl-1
expression and histologic type. Nonetheless, Kaplan-Meier analysis
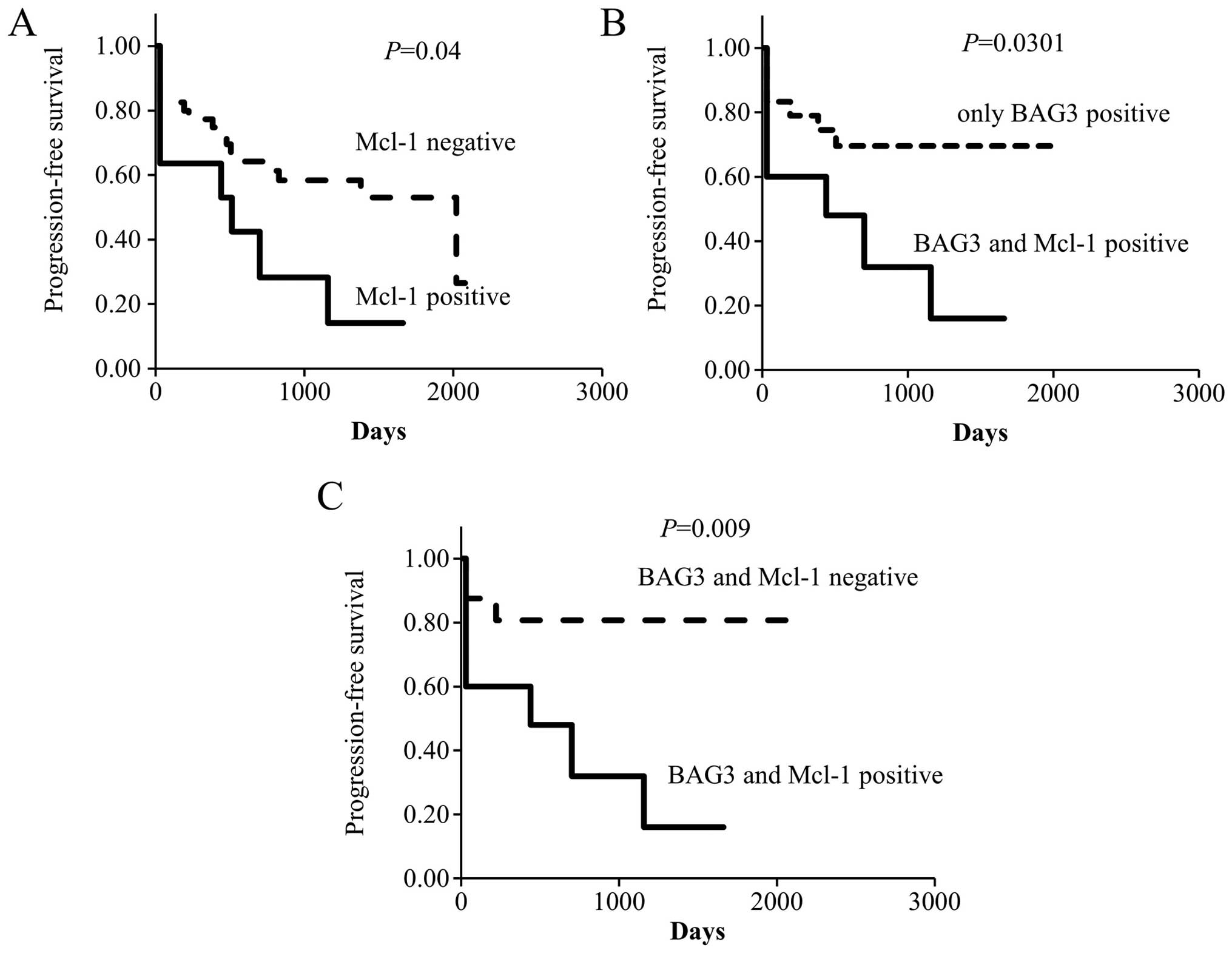
indicated that the progression-free survival (PFS) was poorer among
Mcl-1-positive patients than Mcl-1-negative patients (median
duration of PFS: 512 vs. 2,020 days, P=0.04; Fig. 1A).
 | Table IMcl-1 expression and
clinicopathological features in ovarian cancer. |
Table I
Mcl-1 expression and
clinicopathological features in ovarian cancer.
|
Characteristics | Value | Mcl-1 positive N
(%) |
|---|
| Number | 51 | 11 (21.6) |
| Age (mean,
range) | 52, 19–82 | |
| Histology |
| Serous | 12 | 4 (33.3) |
| Endometrioid | 17 | 5 (29.4) |
| Clear | 10 | 1 (10) |
| Mucinous | 6 | 1 (16.6) |
| Others | 6 | 0 (0) |
| Stage |
| I | 17 | 1 (5.8) |
| II | 2 | 0 (0) |
| III | 28 | 9 (32.1) |
| IV | 4 | 1 (25) |
To investigate the correlation between BAG3 and
Mcl-1 expression in ovarian cancer patients, we classified
BAG3-positive patients as being only BAG3-positive (Mcl-1 negative)
or both BAG3- and Mcl-1-positive. Of the 34 BAG3-positive patients,
Mcl-1 expression was detected in 10. Kaplan-Meier analysis showed
that the PFS of patients in the BAG3- and Mcl-1-positive group was
significantly poorer than in the only BAG3-positive group
(P=0.0301; Fig. 1B). The median
PFS in the BAG3- and Mcl-1-positive group was 440 days. By
contrast, the only BAG3-positive group has not yet reached a median
PFS time in our analysis. Moreover, Kaplan-Meier analysis showed
that the PFS of patients in the BAG3- and Mcl-1-positive group was
significantly poorer than in the BAG3- and Mcl-1-negative group
(P=0.009; Fig. 1C). Because only
Mcl-1-positive patients (BAG3 negative) in total were 2 cases, we
could not compare only Mcl-1-positive patients to other patients.
These results suggest that positivity for BAG3 and Mcl-1 may be a
key determinant of disease progression in ovarian cancer.
Sensitivity to paclitaxel and expression
of BAG3 and Mcl-1 in ovarian cancer cell lines
Earlier study from our group showed that BAG3
knockdown increases the chemosensitivity of ES2 clear ovarian
cancer cells, but BAG3 knockdown had no significant effect on the
chemosensitivity of AMOC2 serous ovarian cancer cells (28). To clarify the mechanism for this
difference, we initially compared the sensitivity to paclitaxel of
AMOC2 and ES2 cells using XTT viability assays. When AMOC2 and ES2
cells were treated for 48 h with increasing concentrations of
paclitaxel, we found AMOC2 cells to be significantly more sensitive
(P<0.0001) to the drug than ES2 cells (Fig. 2A). In addition, western blotting
showed that BAG3, Mcl-1 and Bcl-XL proteins were all
more strongly expressed in ES2 cells than AMOC2 cells (Fig. 2B). Furthermore, we confirmed that
silencing Mcl-1increased the chemosensitivity of ES2 cells
(Fig. 2C and D). These results
suggest that BAG3 may mediate chemoresistance by interacting with
anti-apoptotic Bcl2 family proteins.
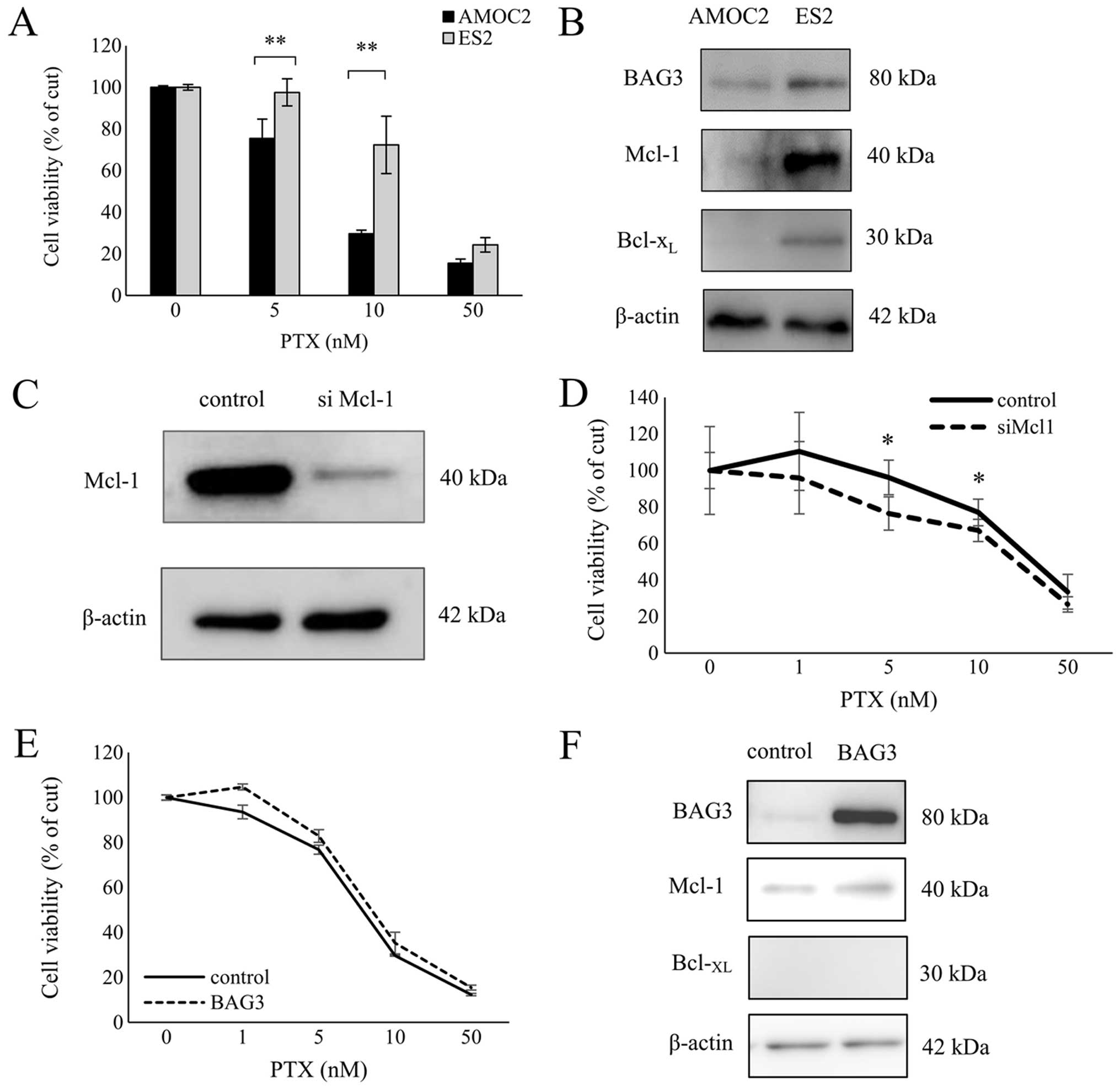 | Figure 2BAG3 and Mcl-1 expression correlates
with paclitaxel resistance in ovarian cancer cells. (A) AMOC2 cells
and ES2 cells were treated for 48 h with the indicated
concentrations of paclitaxel and then subjected to XTT assays.
Symbols depict means ± SD. **P<0.01. (B) Western
blotting showing levels of BAG3, Mcl-1 and Bcl-XL
protein in AMOC2 and ES2 cells. As a loading control, the blots
were reprobed with anti-β-actin antibody. (C) Western blot analysis
showing Mcl-1 silencing in ES2 cells. As a loading control, the
blots were reprobed using mouse monoclonal anti-β-actin antibody.
(D) ES2 cells were transfected with Mcl-1 siRNA (siMcl-1) or
non-targeting siRNA (control). Twenty-four hours later paclitaxel
was added to a concentration of 0, 1, 5, 10 or 50 nM, and the cells
were incubated for an additional 48 h, after which viability was
assessed in XTT assays. Shown are means ± SD;
*P<0.05. (E) AMOC2 ovarian cancer cells were
transfected with BAG3 expression vector (AMOC2-BAG3) or a control
vector (AMOC2-pcDNA). Twenty-four hours later paclitaxel was added
to a concentration of 0, 1, 5, 10 or 50 nM, and the cells were
incubated for an additional 48 h, after which viability was
assessed in XTT assays. (F) Western blot analysis of BAG3, Mcl-1
and Bcl-XL expression in AMOC2-BAG3 and AMOC2-pcDNA
cells. As a loading control, the blots were reprobed using mouse
monoclonal anti-β-actin antibody. |
Overexpression of BAG3 has only a small
effect on sensitivity to paclitaxel in AMOC2 serous ovarian cancer
cells
We previously showed that in AMOC2 serous ovarian
cancer cells, which express Mcl-1 only weakly and are more
sensitive to paclitaxel than ES2 cells, BAG3 knockdown has little
effect on sensitivity to paclitaxel (28). To further investigate the
association between chemoresistance and BAG3 overexpression in
vitro, AMOC2 cells were transfected with full-length BAG3. The
resultant overexpression of BAG3 led to only slight increases in
Mcl-1 expression and paclitaxel resistance, which were not
statistically significant (Fig. 2E and
F).
Varying miR-29b expression has only a
slight effect on sensitivity to paclitaxel in AMOC2 serous ovarian
cancer cells
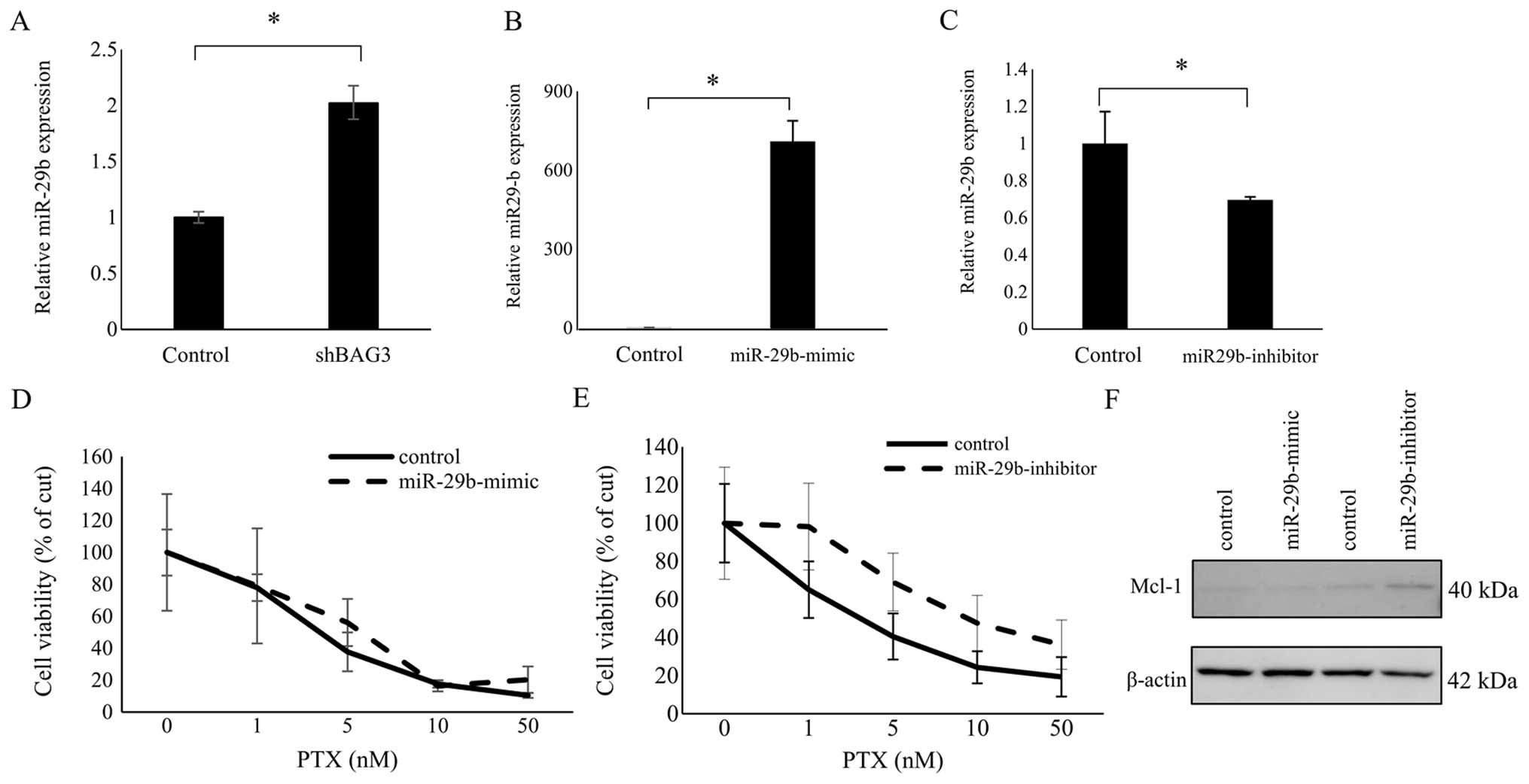
We have also shown that BAG3 knockdown downregulates
Mcl-1 expression in ES2 cells through upregulation of microRNA-29b
(28). We therefore next
investigated the association between BAG3 and miR-29b in AMOC2
cells. As in ES2 cells, knocking down BAG3 expression using shBAG3
enhanced miR-29b expression in AMOC2 cells (Fig. 3A). In addition, when we transfected
AMOC2 cells with miRNA-29b mirVana miRNA mimic to augment miR-29b
activity or with miRNA-29b mirVana miRNA inhibitor to diminish
miR-29b activity, qRT-PCR confirmed that miR-29b expression was
markedly increased in AMOC2 cells transfected with miR29b mimic
(Fig. 3B), but decreased in AMOC2
cells transfected with the miR29b inhibitor (Fig. 3C). Unlike in ES2 cells (28), however, transfection of AMOC2 cells
with miR-29b-mimic had no effect on Mcl-1 expression or paclitaxel
sensitivity, as compared to the miR-control (Fig. 3D and F). On the other hand, Mcl-1
expression was increased somewhat in AMOC2 cells transfected with
the miR-29b-inhibitor, and those cells were more refractory to
paclitaxel than cells transfected with the miR-29b-inhibitor
negative control, though the difference was not statistically
significant (Fig. 3E and F). These
findings imply that although BAG3 may enhance Mcl-1 expression and
chemoresistance in some degree in AMOC2 cells, the effect is mild,
inducing little or no endogenous Mcl-1 expression. Nonetheless,
these results are consistent with the idea that positivity for both
BAG3 and Mcl-1 correlates with a poor prognosis in ovarian cancer
patients.
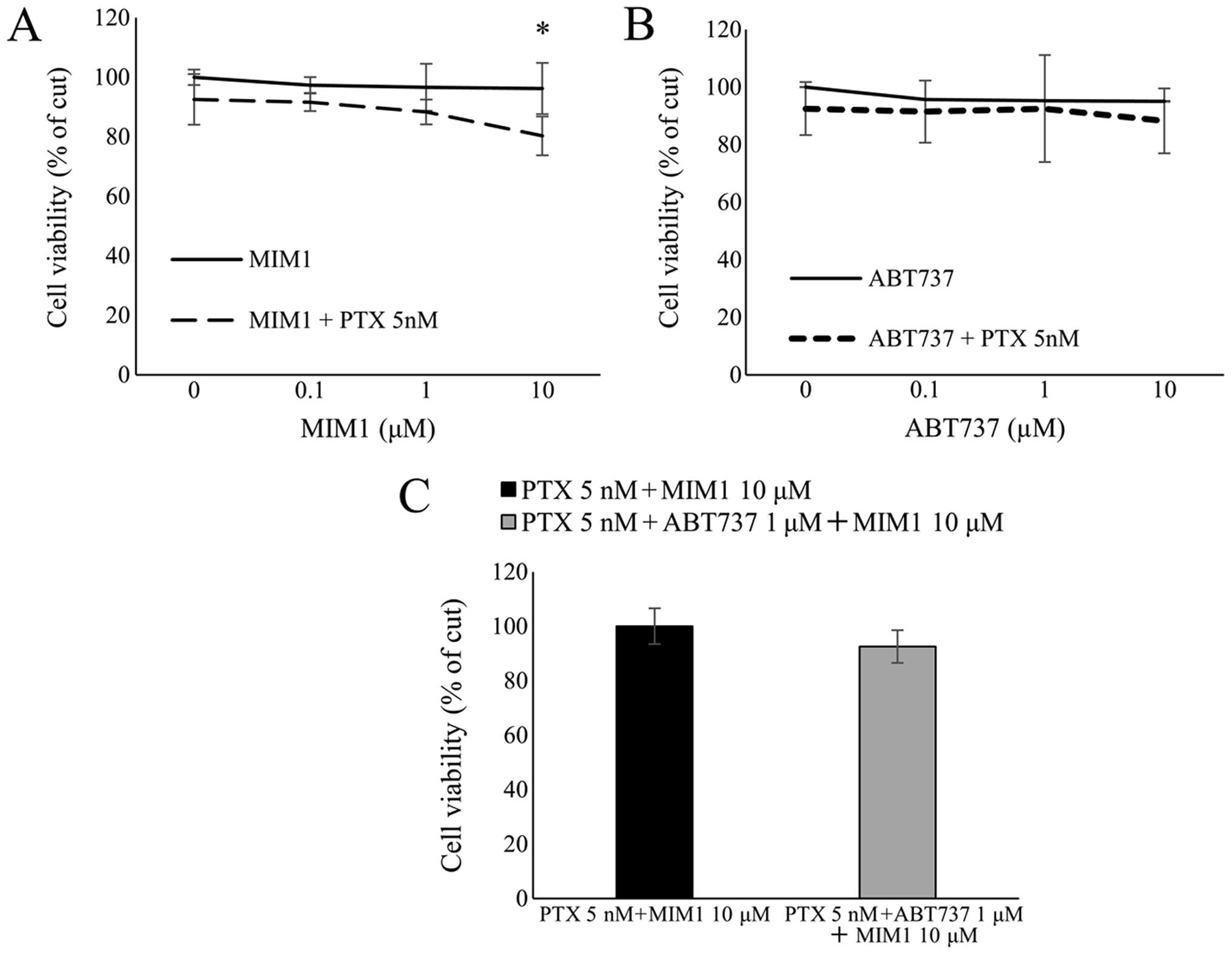
Mcl-1-specific high-affinity antagonist
MIM1 increases sensitivity of ES2 cells to paclitaxel
We next used XTT viability assays to assess the
effect on cell proliferation of the Mcl-1 antagonist MIM1, alone
and in combination with paclitaxel. ES2 cells were treated for 48 h
with increasing concentrations of MIM1 alone or in combination with
a low concentration of paclitaxel (5 nM). Treatment with the
paclitaxel/MIM1 combination significantly reduced cell viability,
whereas MIM1 had no effect on cell viability by itself (Fig. 4A). Thus MIM1 appears to increase
the sensitivity of Mcl-1-expressing cells to paclitaxel. We also
examined the effect of combining paclitaxel with ABT737, a Bcl-2,
Bcl-XL and Bcl-w antagonist, in a similar
manner. We found that ABT737 alone or in combination with
paclitaxel had little effect on ES2 cell viability (Fig. 4B), which is consistent with earlier
reports showing that Mcl-1 mediates ABT737 resistance (11,22,29,30).
BAG3 knockdown enhances the effect of the
triple-drug combination of paclitaxel, MIM1 and ABT737
We also evaluated the effect of combining paclitaxel
(5 nM), MIM1 (10 μM) and ABT737 (1 μM) and found that the effect of
the triple-drug combination on cell viability was not different
from that of paclitaxel and MIM1 (Fig.
4C). We speculated that, as suggested previously, BAG3
stabilizes and upregulates Mcl-1 (22,28)
and that as a consequence the inhibitory effect of MIM1 is not
sufficient to increase the efficacy of ABT737. To test this idea,
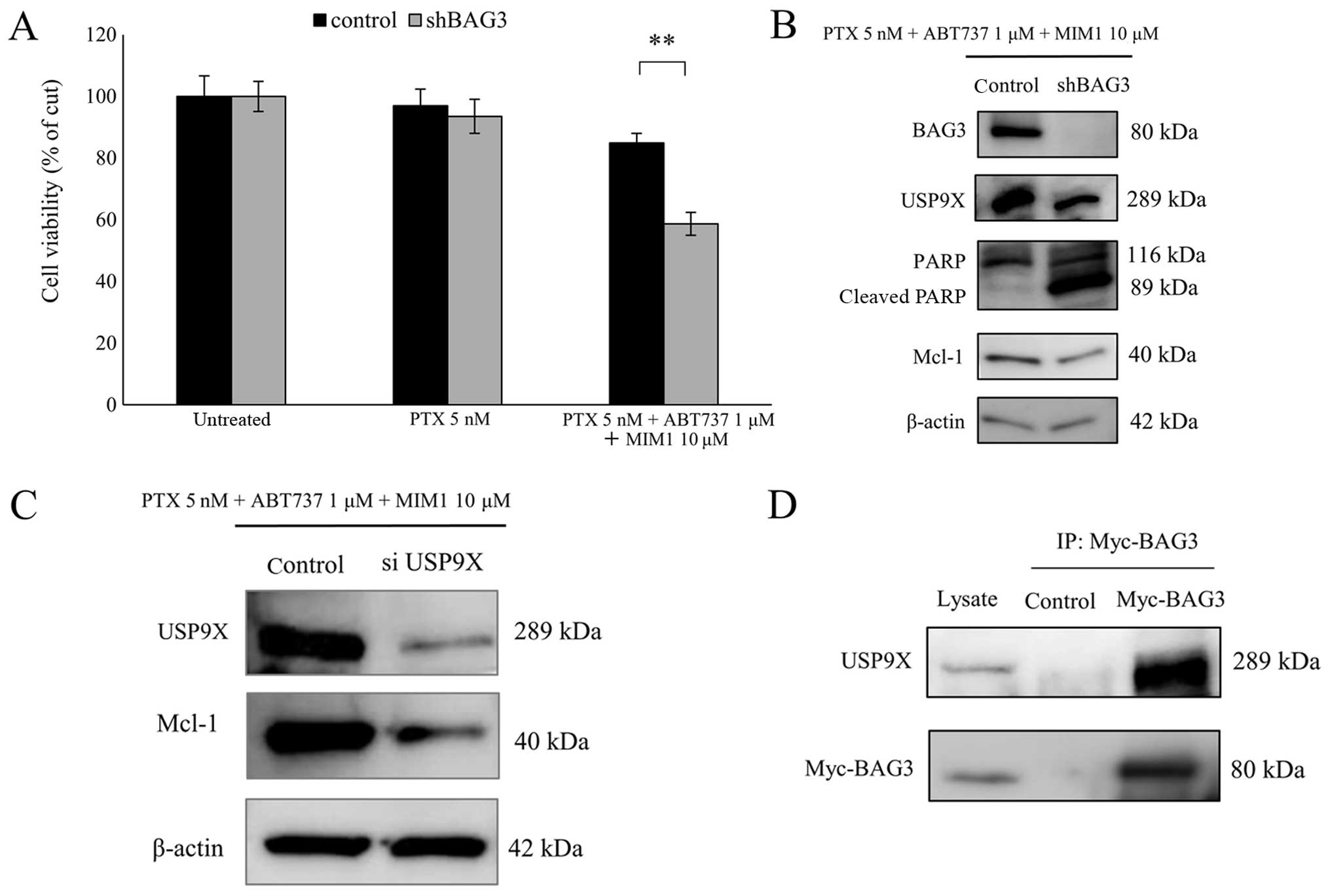
ES2 cells were infected with a retroviral vector encoding shRNA
targeting BAG3 (ES2-shBAG3). As expected, BAG3 knockdown
significantly enhanced the effect of the triple-drug combination on
cell viability (Fig. 5A). In
ES2-shBAG3 cells treated with the triple-drug combination, Mcl-1
expression was reduced, as compared to control, and PARP cleavage,
an indicator of caspase activation, were observed (Fig. 5B). These results suggest that
stabilization of Mcl-1 by BAG3 both confers resistance to
chemotherapy and contributes to the anti-apoptotic activity within
ovarian cancer cells.
Interaction between BAG3 and USP9X
Finally, we tested whether levels of the
deubiquitinase USP9X, which stabilizes Mcl-1 by negatively
regulating its ubiquitination, are affected by BAG3. We initially
found that USP9X levels are reduced by BAG3 knockdown (Fig. 5B) and Mcl-1 levels are decreased by
silencing USP9X (Fig. 5C). Then to
determine whether BAG3 directly interacts with USP9X, we
transfected a Myc-tagged BAG3 expression construct into ES2 cells.
Thereafter, immunoprecipitation of the Myc epitope from lysates of
the Myc-BAG3 transfectants also pulled down endogenous USP9X,
whereas neither protein was precipitated from control lysates
(Fig. 5D). Apparently, BAG3 and
USP9X directly interact within ES2 ovarian cancer cells. Taken
together, these results suggest that BAG3 binds to and stabilizes
USP9X, which in turn stabilizes Mcl-1 and promotes resistance to
apoptosis.
Discussion
In this study, we investigated the relationship
between the combined expression of BAG3 and Mcl-1 in ovarian cancer
tissue and patient prognosis and chemoresistance. We previously
showed that tumoral BAG3 expression was associated with a
significantly increased risk of disease progression and recurrence
(26). Our present analysis
indicates that Mcl-1 expression in primary ovarian cancer tissues
is associated with a poor prognosis and that expression of both
BAG3 and Mcl-1 is associated with a significantly poorer prognosis
than expression of BAG3 alone. These findings suggest that tumoral
BAG3 does not act alone in contributing to a poor prognosis in
ovarian cancer patients, but acts in combination with Mcl-1. In
vitro, ES2 clear ovarian cancer cells endogenously express high
levels of Mcl-1 and BAG3, and show less sensitivity to paclitaxel
than AMOC2 serous ovarian cancer cells, which endogenously express
only low levels of Mcl-1 and BAG3. BAG3 knockdown in ES2 cells
suppresses Mcl-1 expression and increases sensitivity to paclitaxel
(28), which is consistent with
the idea that combined expression of both BAG3 and Mcl-1 correlates
with a poor prognosis in ovarian cancer patients, and that BAG3 and
Mcl-1 act in concert to mediate anti-apoptotic activity in ovarian
cancer cells.
In many cancers, Mcl-1 is a key mediator that
enables cancer cells to overcome oncogenic stress-induced
apoptosis. For instance, Mcl-1 is critical for the development and
maintenance of acute myeloid leukemia (31,32),
and high levels of Mcl-1 are often associated with chemotherapeutic
resistance and relapse (33,34).
Moreover, Mcl-1 is not inhibited by the Bcl-2 antagonist ABT737 and
is considered responsible for cancer cell resistance to ABT737
(11,22,29,30).
Consistent with those earlier findings, our results show that
ABT737 does not increase paclitaxel sensitivity in Mcl-1-expressing
ES2 cells. On the other hand, MIM1, a selectively Mcl-1 inhibitor
(13), increased somewhat the
sensitivity of ES2 cells to paclitaxel. However, adding ABT737 to
the paclitaxel/MIM1 combination provided no additional benefit. A
large body of evidence, including our earlier research, indicates
that BAG3 induces chemoresistance through upregulation of Mcl-1
expression (22,23,28).
We therefore hypothesized that in the presence of BAG3, the
inhibitory effect of MIM1 would not be sufficient to suppress Mcl-1
activity, and thus ABT737 would also be without effect. Indeed, we
found that BAG3 knockdown (ES2-shBAG3) using a retroviral shRNA
vector increased the sensitivity of ES2 cells to the combination of
paclitaxel, MIM1 and ABT737. Several studies have investigated the
mechanism by which BAG3 positively regulates Mcl-1 expression.
Using a colon cancer cell line, Jacobs and Marnett demonstrated
that heat shock factor 1 (HSF1) upregulates Mcl-1 expression and
that the effect is mediated by BAG3 (23). In addition, it is now known that
BAG3 binds to and stabilizes Mcl-1 (22), and that BAG3 also upregulates Mcl-1
expression by inhibiting miR-29b expression (28). Here we provide the first evidence
of yet another molecular mechanism by which BAG3 upregulates Mcl-1
expression.
It is now known that four different E3
ubiquitin-ligases (Mule, SCFβ-TrCP, SCFFbw7 and Trim17) (35–38)
mediate Mcl-1 ubiquitination, while the deubiquitinase USP9X acts
to suppress Mcl-1 ubiquitination (24). We found that BAG3 binds to and
stabilizes USP9X and that USP9X expression is downregulated during
apoptosis in ES2 cells after BAG3 knockdown. In addition, our
results show that downregulation of Mcl-1 expression correlates
with attenuated USP9X expression, suggesting BAG3 upregulates Mcl-1
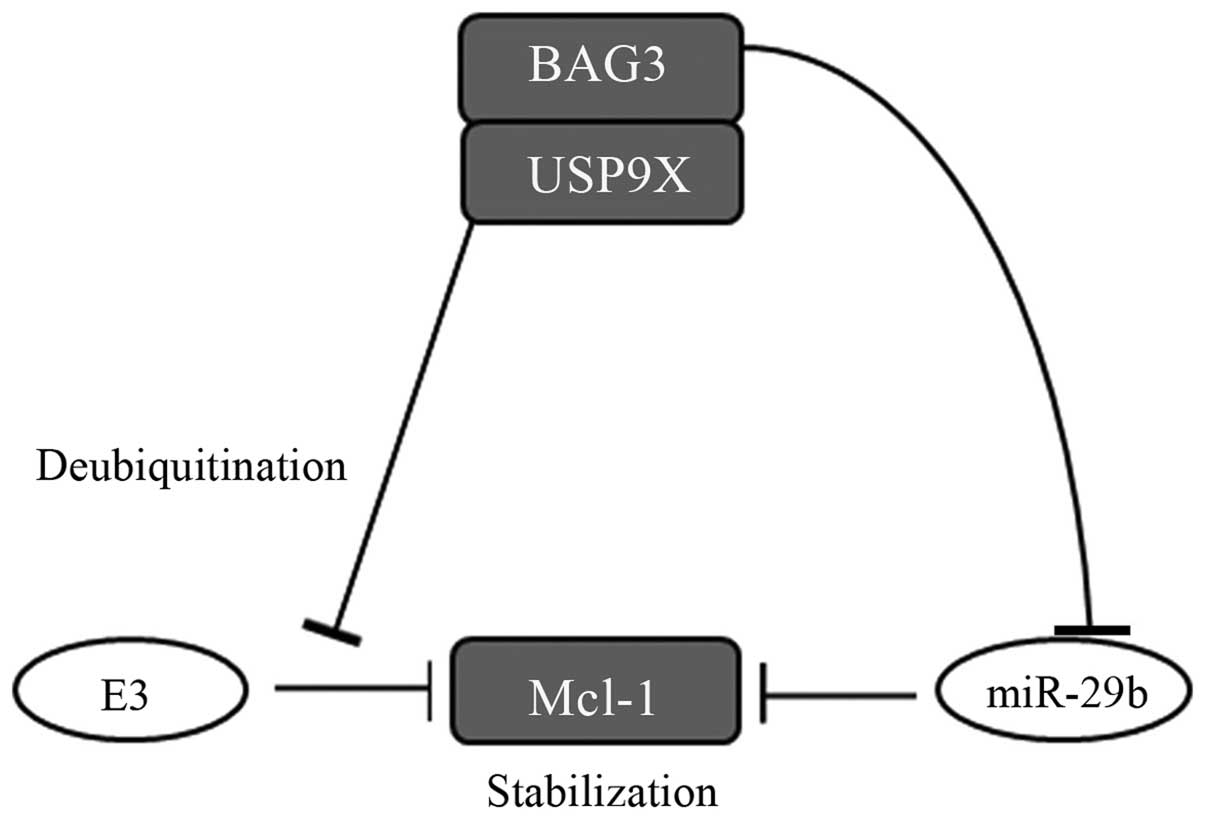
levels via USP9X. A schematic diagram showing a possible mechanism
to explain the BAG3 activity is shown in Fig. 6. Notably, BAG1, another BAG family
protein, also reportedly binds to USP9X and regulates the stability
of Mcl-1 (39). Both BAG1 and BAG3
contain conserved BAG domains via which they bind Hsp70 (14,15),
interact with the proteasome and modulate Hsp70 client protein
degradation (40,41). We therefore suggest that BAG3 also
interacts with USP9X and that the stability of USP9X is determined
to both BAG3 and BAG1.
In conclusion, we have shown that the combined
expression of BAG3 and Mcl-1 correlates with a poor prognosis in
ovarian cancer patients and with resistance to paclitaxel. Mcl-1
inhibition by MIM1 effectively increased paclitaxel sensitivity,
overcoming the chemoresistance of ovarian cancer cells. Addition of
the Bcl-2 antagonist ABT737 increased paclitaxel sensitivity
further, but only after BAG3 knockdown. We suggest BAG3 binds to
USP9X to stabilize Mcl-1 levels, and that BAG3 and Mcl-1 are
potentially useful biomarkers of the responsiveness of ovarian
cancer to paclitaxel. Moreover, BAG3 would likely be a useful
therapeutic target, particularly if targeted in combination with
Bcl-2 family proteins, for the treatment of chemoresistant ovarian
cancers.
Acknowledgements
The authors would like to thank Dr S. Takayama for
providing a gene silencing vector (pLTRH1) specific for BAG3 and
rabbit anti-BAG3 antibody.
Abbreviations:
|
BAG3
|
Bcl-2-associated athanogene 3
|
|
CCC
|
clear cell adenocarcinoma
|
|
XTT
|
2,
3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide
|
|
PTX
|
paclitaxel
|
|
PFS
|
progression-free survival
|
|
shRNA
|
short hairpin RNA
|
|
IP
|
immunoprecipitation
|
References
|
1
|
McGuire WP, Hosk ins WJ, Brady MF, Kucera
PR, Partridge EE, Look KY, Clarke-Pearson DL and Davidson M:
Cyclophosphamide and cisplatin compared with paclitaxel and
cisplatin in patients with stage III and stage IV ovarian cancer. N
Engl J Med. 334:1–6. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Itamochi H, Kigawa J and Terakawa N:
Mechanisms of chemoresistance and poor prognosis in ovarian clear
cell carcinoma. Cancer Sci. 99:653–658. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Anglesio MS, Carey MS, Köbel M, Mackay H
and Huntsman DG; Vancouver Ovarian Clear Cell Symposium Speakers.
Clear cell carcinoma of the ovary: A report from the first Ovarian
Clear Cell Symposium, June 24th, 2010. Gynecol Oncol. 121:407–415.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Johnstone RW, Ruefli AA and Lowe SW:
Apoptosis: A link between cancer genetics and chemotherapy. Cell.
108:153–164. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cory S, Huang DCS and Adams JM: The Bcl-2
family: Roles in cell survival and oncogenesis. Oncogene.
22:8590–8607. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yip KW and Reed JC: Bcl-2 family proteins
and cancer. Oncogene. 27:6398–6406. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Czabotar PE, Lessene G, Strasser A and
Adams JM: Control of apoptosis by the BCL-2 protein family:
Implications for physiology and therapy. Nat Rev Mol Cell Biol.
15:49–63. 2014. View
Article : Google Scholar
|
|
9
|
Llambi F, Moldoveanu T, Tait SW,
Bouchier-Hayes L, Temirov J, McCormick LL, Dillon CP and Green DR:
A unified model of mammalian BCL-2 protein family interactions at
the mitochondria. Mol Cell. 44:517–531. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Oltersdorf T, Elmore SW, Shoemaker AR,
Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges
J, Hajduk PJ, et al: An inhibitor of Bcl-2 family proteins induces
regression of solid tumours. Nature. 435:677–681. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
van Delft MF, Wei AH, Mason KD, Vandenberg
CJ, Chen L, Czabotar PE, Willis SN, Scott CL, Day CL, Cory S, et
al: The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and
efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized.
Cancer Cell. 10:389–399. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wertz IE, Kusam S, Lam C, Okamoto T,
Sandoval W, Anderson DJ, Helgason E, Ernst JA, Eby M, Liu J, et al:
Sensitivity to antitubulin chemotherapeutics is regulated by MCL1
and FBW7. Nature. 471:110–114. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cohen NA, Stewart ML, Gavathiotis E,
Tepper JL, Bruekner SR, Koss B, Opferman JT and Walensky LD: A
competitive stapled peptide screen identifies a selective small
molecule that overcomes MCL-1-dependent leukemia cell survival.
Chem Biol. 19:1175–1186. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Takayama S, Xie Z and Reed JC: An
evolutionarily conserved family of Hsp70/Hsc70 molecular chaperone
regulators. J Biol Chem. 274:781–786. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rosati A, Ammirante M, Gentilella A,
Basile A, Festa M, Pascale M, Marzullo L, Belisario MA, Tosco A,
Franceschelli S, et al: Apoptosis inhibition in cancer cells: A
novel molecular pathway that involves BAG3 protein. Int J Biochem
Cell Biol. 39:1337–1342. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Einbond A and Sudol M: Towards prediction
of cognate complexes between the WW domain and proline-rich
ligands. FEBS Lett. 384:1–8. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sudol M, Chen HI, Bougeret C, Einbond A
and Bork P: Characterization of a novel protein-binding module -
the WW domain. FEBS Lett. 369:67–71. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Iwasaki M, Homma S, Hishiya A, Dolezal SJ,
Reed JC and Takayama S: BAG3 regulates motility and adhesion of
epithelial cancer cells. Cancer Res. 67:10252–10259. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rosati A, Bersani S, Tavano F, Dalla Pozza
E, De Marco M, Palmieri M, De Laurenzi V, Franco R, Scognamiglio G,
Palaia R, et al: Expression of the antiapoptotic protein BAG3 is a
feature of pancreatic adenocarcinoma and its overexpression is
associated with poorer survival. Am J Pathol. 181:1524–1529. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Festa M, Del Valle L, Khalili K, Franco R,
Scognamiglio G, Graziano V, De Laurenzi V, Turco MC and Rosati A:
BAG3 protein is overexpressed in human glioblastoma and is a
potential target for therapy. Am J Pathol. 178:2504–2512. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chiappetta G, Basile A, Barbieri A, Falco
A, Rosati A, Festa M, Pasquinelli R, Califano D, Palma G, Costanzo
R, et al: The anti-apoptotic BAG3 protein is expressed in lung
carcinomas and regulates small cell lung carcinoma (SCLC) tumor
growth. Oncotarget. 5:6846–6853. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Boiani M, Daniel C, Liu X, Hogarty MD and
Marnett LJ: The stress protein BAG3 stabilizes Mcl-1 protein and
promotes survival of cancer cells and resistance to antagonist
ABT-737. J Biol Chem. 288:6980–6990. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jacobs AT and Marnett LJ: HSF1-mediated
BAG3 expression attenuates apoptosis in 4-hydroxynonenal-treated
colon cancer cells via stabilization of anti-apoptotic Bcl-2
proteins. J Biol Chem. 284:9176–9183. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schwickart M, Huang X, Lill JR, Liu J,
Ferrando R, French DM, Maecker H, O'Rourke K, Bazan F,
Eastham-Anderson J, et al: Deubiquitinase USP9X stabilizes MCL1 and
promotes tumour cell survival. Nature. 463:103–107. 2010.
View Article : Google Scholar
|
|
25
|
Yan J, Zhong N, Liu G, Chen K, Liu X, Su L
and Singhal S: Usp9x- and Noxa-mediated Mcl-1 downregulation
contributes to pemetrexed-induced apoptosis in human non-small-cell
lung cancer cells. Cell Death Dis. 5:e13162014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Suzuki M, Iwasaki M, Sugio A, Hishiya A,
Tanaka R, Endo T, Takayama S and Saito T: BAG3 (BCL2-associated
athanogene 3) interacts with MMP-2 to positively regulate invasion
by ovarian carcinoma cells. Cancer Lett. 303:65–71. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Homma S, Iwasaki M, Shelton GD, Engvall E,
Reed JC and Takayama S: BAG3 deficiency results in fulminant
myopathy and early lethality. Am J Pathol. 169:761–773. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sugio A, Iwasaki M, Habata S, Mariya T,
Suzuki M, Osogami H, Tamate M, Tanaka R and Saito T: BAG3
upregulates Mcl-1 through downregulation of miR-29b to induce
anticancer drug resistance in ovarian cancer. Gynecol Oncol.
134:615–623. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Konopleva M, Contractor R, Tsao T, Samudio
I, Ruvolo PP, Kitada S, Deng X, Zhai D, Shi YX, Sneed T, et al:
Mechanisms of apoptosis sensitivity and resistance to the BH3
mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 10:375–388.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tahir SK, Yang X, Anderson MG,
Morgan-Lappe SE, Sarthy AV, Chen J, Warner RB, Ng SC, Fesik SW,
Elmore SW, et al: Influence of Bcl-2 family members on the cellular
response of small-cell lung cancer cell lines to ABT-737. Cancer
Res. 67:1176–1183. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Glaser SP, Lee EF, Trounson E, Bouillet P,
Wei A, Fairlie WD, Izon DJ, Zuber J, Rappaport AR, Herold MJ, et
al: Mechanisms of chemoresistance and poor prognosis in ovarian
clear cell carcinoma. Genes Dev. 26:120–125. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xiang Z, Luo H, Payton JE, Cain J, Ley TJ,
Opferman JT and Tomasson MH: Mcl1 haploinsufficiency protects mice
from Myc-induced acute myeloid leukemia. J Clin Invest.
120:2109–2118. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wei G, Twomey D, Lamb J, Schlis K, Agarwal
J, Stam RW, Opferman JT, Sallan SE, den Boer ML, Pieters R, et al:
Gene expression-based chemical genomics identifies rapamycin as a
modulator of MCL1 and glucocorticoid resistance. Cancer Cell.
10:331–342. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wuillème-Toumi S, Robillard N, Gomez P,
Moreau P, Le Gouill S, Avet-Loiseau H, Harousseau JL, Amiot M and
Bataille R: Mcl-1 is overexpressed in multiple myeloma and
associated with relapse and shorter survival. Leukemia.
19:1248–1252. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhong Q, Gao W, Du F and Wang X:
Mule/ARF-BP1, a BH3-only E3 ubiquitin ligase, catalyzes the
polyubiquitination of Mcl-1 and regulates apoptosis. Cell.
121:1085–1095. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ding Q, He X, Hsu JM, Xia W, Chen CT, Li
LY, Lee DF, Liu JC, Zhong Q, Wang X, et al: Degradation of Mcl-1 by
beta-TrCP mediates glycogen synthase kinase 3-induced tumor
suppression and chemosensitization. Mol Cell Biol. 27:4006–4017.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Inuzuka H, Shaik S, Onoyama I, Gao D,
Tseng A, Maser RS, Zhai B, Wan L, Gutierrez A, Lau AW, et al:
SCF(FBW7) regulates cellular apoptosis by targeting MCL1 for
ubiquitylation and destruction. Nature. 471:104–109. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Magiera MM, Mora S, Mojsa B, Robbins I,
Lassot I and Desagher S: Trim17-mediated ubiquitination and
degradation of Mcl-1 initiate apoptosis in neurons. Cell Death
Differ. 20:281–292. 2013. View Article : Google Scholar :
|
|
39
|
Aveic S, Pigazzi M and Basso G: BAG1: The
guardian of anti-apoptotic proteins in acute myeloid leukemia. PLoS
One. 6:e260972011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lüders J, Demand J and Höhfeld J: The
ubiquitin-related BAG-1 provides a link between the molecular
chaperones Hsc70/Hsp70 and the proteasome. J Biol Chem.
275:4613–4617. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Doong H, Rizzo K, Fang S, Kulpa V,
Weissman AM and Kohn EC: CAIR-1/BAG-3 abrogates heat shock
protein-70 chaperone complex-mediated protein degradation:
Accumulation of poly-ubiquitinated Hsp90 client proteins. J Biol
Chem. 278:28490–28500. 2003. View Article : Google Scholar : PubMed/NCBI
|