Introduction
Diffuse large B-cell lymphoma (DLBCL) is an
aggressive and the most common subtype of non-Hodgkin lymphoma,
accounting for 30–40% of all newly diagnosed cases (1). DLBCLs are highly heterogeneous at the
molecular level (2); gene
expression profiling studies have identified ≥2 major molecular
subtypes of DLBCL, germinal center B-cell-like (GCB) and activated
B-cell-like (ABC) DLBCL, which differ with respect to the
expression of hundreds of genes and have distinct prognoses
(3). The biggest difference
between ABC-DLBCL and GCB-DLBCL is that the ABC subtype has a lower
cure rate. Significantly, after R-CHOP treatment, the ABC subtype
shows an inferior outcome compared with that of the GCB subtype
(3-year progression-free survival, ~40% versus 75%; p<0.001)
(4). Therefore, it is very
important to find potential therapeutic targets for ABC-DLBCL.
Heme oxygenase-1 (HO-1), also known as heat shock
protein 32, is a stress-related cytoprotective molecule that is
expressed constitutively in various neoplastic cells (5). HO-1 can be strongly induced in
response to cellular stress and oxidative stimuli, such as nitric
oxide (6), heat shock (7), and inflammatory cytokines (8). Moreover, under pathological or stress
conditions, increased HO-1 expression can lead to resistance to
apoptosis, promotion of cell proliferation, and alleviation of
inflammation (9).
Recently, the relationships between high HO-1
expression, drug resistance, and promotion of cell proliferation
have been extensively studied (10–14).
Our previous studies have shown that HO-1 is highly expressed in
acute myelogenous leukemia (AML), myelodysplastic syndromes, and
chronic myelogenous leukemia (CML) (15–19),
confirming that HO-1 plays an important role as an anti-apoptotic
molecule and could be a potential target for treatment. However,
the role of HO-1 in DLBCL remains to be elucidated. In this study,
based on analyses of its protein and mRNA levels in 31 DLBCL
patients, we found that HO-1 was characteristically overexpressed
in ABC-DLBCLs.
ABC-DLBCL is characterized by constitutive
activation of the nuclear factor-κB (NF-κB) pathway (20,21).
NF-κB is a key transcription factor that promotes cell survival and
proliferation, and inhibition of apoptosis (22,23).
Targeting of NF-κB and its downstream genes can trigger apoptosis
in ABC-DLBCL cells (20,24,25).
However, NF-κB regulates hundreds of genes, not all of which have
been thoroughly investigated.
Many studies have been performed to clarify the
relationship between NF-κB and HO-1 in various diseases. Recently,
several studies have indicated that NF-κB can induce the expression
of HO-1 (26–28). Li et al demonstrated that
NF-κB activation is important for the upregulation of HO-1
(29). Rushworth et al
demonstrated that nuclear factor erythroid 2-related factor 2
(Nrf2) expression is regulated by high levels of nuclear NF-κB in
AML cells (30) and that Nrf2 is
found directly upstream of HO-1 (31,32).
To the best of our knowledge, the relevance of NF-κB and HO-1 in
DLBCLs has not yet been clarified. Considering the relationship
between HO-1 and NF-κB, we hypothesized that the high level of HO-1
expression in ABC-DLBCL is due to the constitutive activation of
NF-κB. Furthermore, the NF-κB-regulated HO-1 may play an
anti-apoptotic role, which leads to the dismal therapeutic outcomes
in ABC-DLBCL.
Therefore, we analyzed the influence of HO-1 on
vincristine (VCR)- and/or dexamethasone (DXM)-induced proliferation
inhibition and apoptosis in OCI-ly10 cells. An approach involving
knockdown of HO-1 gene expression via lentivirus-mediated siRNA
delivery and another involving HO-1 overexpression were used.
Furthermore, we focused on whether HO-1 overexpression plays an
anti-apoptotic role in ABC-DLBCL and explored the possible
mechanism involved.
Materials and methods
Study cohort
From 2008 to 2013, 32 newly diagnosed patients with
DLBCL who had not received therapy were included in the study.
Their paraffin-embedded tissues and fresh-frozen tumor tissues were
obtained from the files of the Affiliated Hospital of Guiyang
Medical University after necessary informed consent and/or
exemption had been acquired. All DLBCL diagnoses were made
according to the World Health Organization classification system
(33). After determining the
cell-of-origin subtypes of DLBCL using formalin-fixed
paraffin-embedded (FFPE) tissue as previously described (34), 20 patients were grouped into the
ABC subtype and 11 patients into the GCB subtype; only 1 patient
was considered to have an unclassified subtype and was excluded
from the study. The 31 patients in the study included 19 males and
12 females, aged 20–90 years (median age, 58 years). All patients
underwent surgical resection of tumor tissue. Eleven normal lymph
nodes (confirmed as normal tissues by pathology) from non-tumor
adjacent tissues of gastric cancer patients were also selected as
negative controls and 5 spleen samples from healthy individuals
were selected as the positive control. The study was approved by
the institutional review board (Affiliated Hospital of Guiyang
Medical University), and informed consent was obtained in
accordance with the Declaration of Helsinki, prior to obtaining
fresh tumor tissue in each case. Patient characteristics are
summarized in Table I.
 | Table IClinical characteristics of diffuse
large B-cell lymphoma patients. |
Table I
Clinical characteristics of diffuse
large B-cell lymphoma patients.
| Characteristic | n (%) |
|---|
| Median age,
years | 58 |
| Clinical stage III,
IV | 16 (51.6) |
| Entranodal sites
>1 | 13 (41.9) |
| ECOG PS ≥2 | 12 (38.7) |
| Lactate
dehydrogenase > 1N | 17 (54.8) |
| B symptoms
present | 19 (61.3) |
| Ki-67 ≥50% | 21 (67.7) |
| IPI score |
| Low (0–1) | 11 (35.4) |
| Low intermediate
(2) | 6 (19.4) |
| High intermediate
(3) | 8 (25.8) |
| High (4–5) | 6 (19.4) |
| Subtype |
| Activated
B-cell-like | 20 (64.5) |
| Germinal center
B-cell-like | 11 (35.5) |
Cells
The human DLBCL cell lines OCI-ly10, OCI-ly19 were
purchased from Deutsche Sammlung von Mikroorganismen und
Zellkulturen. The cell lines were cultured at 37°C in a 5%
humidified atmosphere in RPMI-1640 medium supplemented with 20%
fetal bovine serum (Gibco BRL; Life Technologies, Carlsbad, CA,
USA), penicillin (100 U/ml), and streptomycin (100 μg/ml). Normal
human B lymphocytes were purified from blood donor buffy coats
using the human B-cell enrichment cocktail from StemCell
Technologies.
Reagents
The following reagents were used: fetal bovine serum
(Gibco BRL); RPMI-1640 medium (Gibco BRL); dimethyl sulfoxide
(DMSO; Sigma-Aldrich, St. Louis, MO, USA); Annexin V-fluorescein
isothiocyanate (FITC)/propidium iodide (PI) apoptosis detection kit
(BD Biosciences, San Jose, CA, USA); primary antibodies such as
p-p65S536 and p-IκB-αS32/S36 for western blot
analysis (Cell Signaling Technology, Beverly, MA, USA); secondary
antibodies (Li-Cor Corp., Lincoln, NE, USA); TRIzol reagent (Life
Technologies); and VCR and DXM (Sigma-Aldrich).
Immunohistochemistry
Immunohistochemistry was performed on the study
cohort samples obtained from the archives of Affiliated Hospital of
Guiyang Medical University, using 5-μm-thick FFPE tissue sections.
Slides were deparaffinized and pretreated with 10 mM citrate (pH
6.0; Zymed, South San Francisco, CA, USA) in a steam pressure
cooker (Decloaking Chamber; BioCare Medical, Walnut Creek, CA, USA)
and subsequently washed in distilled water. All further steps were
performed at room temperature in a hydrated chamber. Slides were
pretreated with Peroxidase Block (Dako USA, Carpinteria, CA, USA)
for 5 min to quench endogenous peroxidase activity. Primary mouse
anti-HO-1 antibody (1:120 dilution; Beyotime, Shanghai, China) was
incubated in the Dako diluent (Dako USA) for 1 h. The specificity
of the HO-1 antibody was previously confirmed by immunoblotting an
HO-1-positive control cell line, demonstrating reactivity with a
single band of appropriate molecular weight. Slides were washed in
50 mM Tris (Tris(hydroxymethyl) aminomethane)-Cl (pH 7.4) and
incubated with anti-mouse horseradish peroxidase (HRP)-conjugated
antibody solution (Envision+ Detection kit; Dako USA) for 30 min.
After further washing, immunoperoxidase staining was developed with
the chromogen diaminobenzidine (Dako USA), and slides were
counterstained with Harris hematoxylin (Polyscientific, Bay Shore,
NY, USA).
Quantitative analysis and staining
interpretation
In order to evaluate HO-1 expression, immunostained
sections were scored semiquantitatively according to the proportion
of tumor cells stained and the staining intensity, as previously
described (35,36). Briefly, all specimens were assessed
using the immunoreactive score (IRS), which was calculated by
multiplying the staining intensity (grade 0, none; 1, weak; 2,
moderate; 3, strong) by the percentage of positively stained cells
(0, <5.0%; 1, 5–25%; 2, 26–50%; 3, >51%). For HO-1 staining,
samples with staining intensity greater than or equal to the median
value were categorized as high, whereas those with staining
intensity lesser than the median value were categorized as low. We
defined IRS ≥4 as positive and IRS <4 as negative.
For each tumor section, 5 randomly chosen fields and
≥500 cancer cells were analyzed. Slides were examined using an
E-400 microscope (Nikon, Tokyo, Japan) to produce digital images
that were visualized using a computer-aided image analysis system
(Win ROOF version 5.0; Mitani, Fukui, Japan). Slides were evaluated
twice at different times by 2 investigators who were blinded to the
clinicopathological features and survival data.
Viral transduction
Sequences containing human HO-1 (HO-1,
5′-GCGTTTACCCGCCATCCGCACCCTAGGAGATCTCA GCCACAG-3′) and small
interfering RNA targeting human HO-1 (siRNA-HO-1,
5′-TGGTAGGGCTTTATGCCATGT TTCAAGAGAACATGGCATAAAGCCCTACTTTTTTC-3′)
were selected with Invitrogen designer software. Retroviruses were
generated by transfecting empty plasmid vectors containing enhanced
green fluoresence protein (EGFP) or vectors containing human
HO-1-EGFP/siRNA-HO-1-EGFP into 293FT packaging cells, using FuGENE
HD6. Lentiviral stocks were concentrated using the Lenti-X
concentrator, and titers were determined with the Lenti-X qRT-PCR
Titration kit (Shanghai Innovation Biotechnology Co., Ltd., China).
Finally, 4 recombinant lentiviral vectors were constructed:
lentivirus-V5-D-TOPO-HO-1-EGFP (L-HO-1), lentivirus-V5-D-TOPO-EGFP
(TOPO-EGFP), lentivirus-pRNAi-U6.2-EGFP-siHO-1 (siHO-1), and
lentivirus-pRNAi-U6.2-EGFP (RNAi-EGFP). For transduction, cells
were plated onto 12-well plates at 2.5×105 cells/well
and infected with the lentiviral stocks at a multiplicity of
infection of 10 in the presence of polybrene (10 μg/ml) and then
analyzed by fluorescence microscopy (Olympus, Tokyo, Japan) and
western blotting at 48 h post-transduction. OCI-ly10 cells and
OCI-ly19 cells were transduced with L-HO-1, siHO-1, RNAi-EGFP and
TOPO-EGFP, respectively.
MTT assay
The effects of HO-1 on the proliferation of OCI-ly10
cells, as well as the responses to VCR and DXM, were determined by
using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) assay. Cells were seeded in 96-well plates at a
density of 5,000 cells/well. The cell line was exposed to different
concentrations of DXM (0.5, 1, 2, 4, 6, 8, 10 and 12 mM) and VCR
(0.025, 0.05, 0.1, 0.2, 0.4, 0.8, 1.6 and 3.2 μM) for 24 h. After
the treatment, 20 μl of MTT dye (5 mg/ml; Sigma-Aldrich) was added
to each well. After 4 h of incubation at 37°C, the culture medium
was removed. Subsequently, 150 μl of DMSO was added and thoroughly
mixed for 10 min. Spectrometric absorbance at 570 nm was measured
with a microplate reader. The experiments were performed 5 times
for each group. The survival rate (SR) was measured using the
following equation: SR (%) =
(ATreatment/AControl) × 100%. The
concentration that produced 50% cytotoxicity (IC50) was
determined using GraphPad Prism 5.0 software (GraphPad Software
Inc., San Diego, CA, USA).
Flow cytometry
Cells were harvested, washed with phosphate-buffered
saline (PBS), and stained with an Annexin V-FITC/PI apoptosis kit
(BD Biosciences) according to the manufacturer's instructions.
Apoptotic cells were detected using a FACScan flow cytometer
(Becton-Dickinson, Franklin Lakes, NJ, USA), and the data were
analyzed using CellFIT software.
RNA extraction and qPCR
Total RNA was extracted from FFPE tissue sections of
samples obtained from DLBCL patients by using the RNAprep Pure FFPE
kit (TianGen Biotech, Beijing, China) according to the
manufacturer's instructions. Total RNA from OCI-ly10 cells was
extracted using TRIzol reagent according to the manufacturer's
instructions. qPCR was performed using the SYBR Green PCR Master
Mix (TianGen Biotech) and PRISM 7500 Real-Time PCR detection system
(ABI PRISM, USA). The HO-1 expression level was analyzed relative
to that of the β-actin gene. Primers for qPCR were as follows:
HO-1-F, 5′-ACCCATGACA CCAAGGACCAGA-3′;HO-1-R,5′-GTGTAAGGACCCATCG
GAGAAGC-3′; β-actin-F, 5′-GAGACCTTCAACACCC CAGC-3′; and β-actin-R,
5′-ATGTCACGCACGATTTCCC-3′. The reaction mixture contained cDNA,
primers, and SYBR Master Mix and had a total volume of 20 μl. The
thermal cycling conditions used were 1 min at 94°C, followed by 40
cycles at 94°C for 10 sec and 60°C for 15 sec.
Immunofluorescence staining
The correlationship of p65 and HO-1 was examined by
immunofluorescence. OCI-ly10 cells were plated on 6-well culture
plates, Bay11-7082 pretreated or left untreated for 1 h, and
incubated with TNF-α (15 ng/ml). Then the cells were rinsed in PBS
and fixed by incubation with 4% formaldehyde in PBS for 15 min at
room temperature. After washing with PBS, cells were permeabilized
with PBS containing 0.25% Triton X-100. The cells were blocked in
PBS containing 5% BSA for 30 min. Next, with further washing, cells
were incubated with a rabbit anti-HO-1 monoclonal antibody and a
mouse anti-p65 monoclonal antibody, diluted at 1:200 with confining
liquid, for 2 h at room temperature. After washing with PBS, cells
were incubated with CY3-conjugated goat anti-mouse antibody (1:200)
and FITC-conjugated goat anti-rabbit antibody (1:200) for 30 min,
respectively. Then, the nucleus was stained with DAPI
(4,6-diamidino-2-phenylindole) and cells were photographed by
fluorescent microscopy.
Western blot analysis
To analyze fresh tumor tissues, proteins were
extracted from a total of 5×106 cells. To analyze the
suspended cells, proteins were extracted from a total of
2×107 cells. Total proteins were extracted by lysing
cells in RIPA buffer containing 1 mM phenylmethanesulfonyl fluoride
(Solarbio Science & Technology, Beijing, China). Cytoplasmic
and nuclear proteins were extracted using a nuclear and cytoplasmic
protein extraction kit (Beyotime) according to the manufacturer's
instructions. Equal amounts of protein from the lysates were
resolved using 10% SDS-PAGE gels and transferred to polyvinylidene
difluoride membranes (Millipore Corp., Milford, MA, USA) in order
to analyze protein expression by western blot analysis. The
membranes were blocked with 5% non-fat milk in Tris-buffer at room
temperature for 2 h and then incubated overnight at 4°C with
primary antibody against the protein of interest. After the
membrane was washed with PBS with 0.1% Tween-20, it was incubated
with the appropriate HRP-conjugated secondary antibody, and protein
levels were detected with enhanced chemiluminescence (7Sea
Biotech). The optical densities were analyzed using Quantity One
software.
Statistical analysis
Statistical analysis of the data was conducted using
SPSS version 19 software package (SPSS, Chicago, IL, USA). All data
are presented as the mean ± standard error of the mean (SEM).
Statistical analyses were performed using analysis of variance
(ANOVA) and Student's t-test. The Fisher's exact test was used to
assess the relationship between HO-1 expression and
clinicopathological features. The optical density for the western
blot assay was quantified with Quantity One software. A p-value of
<0.05 was considered statistically significant.
Results
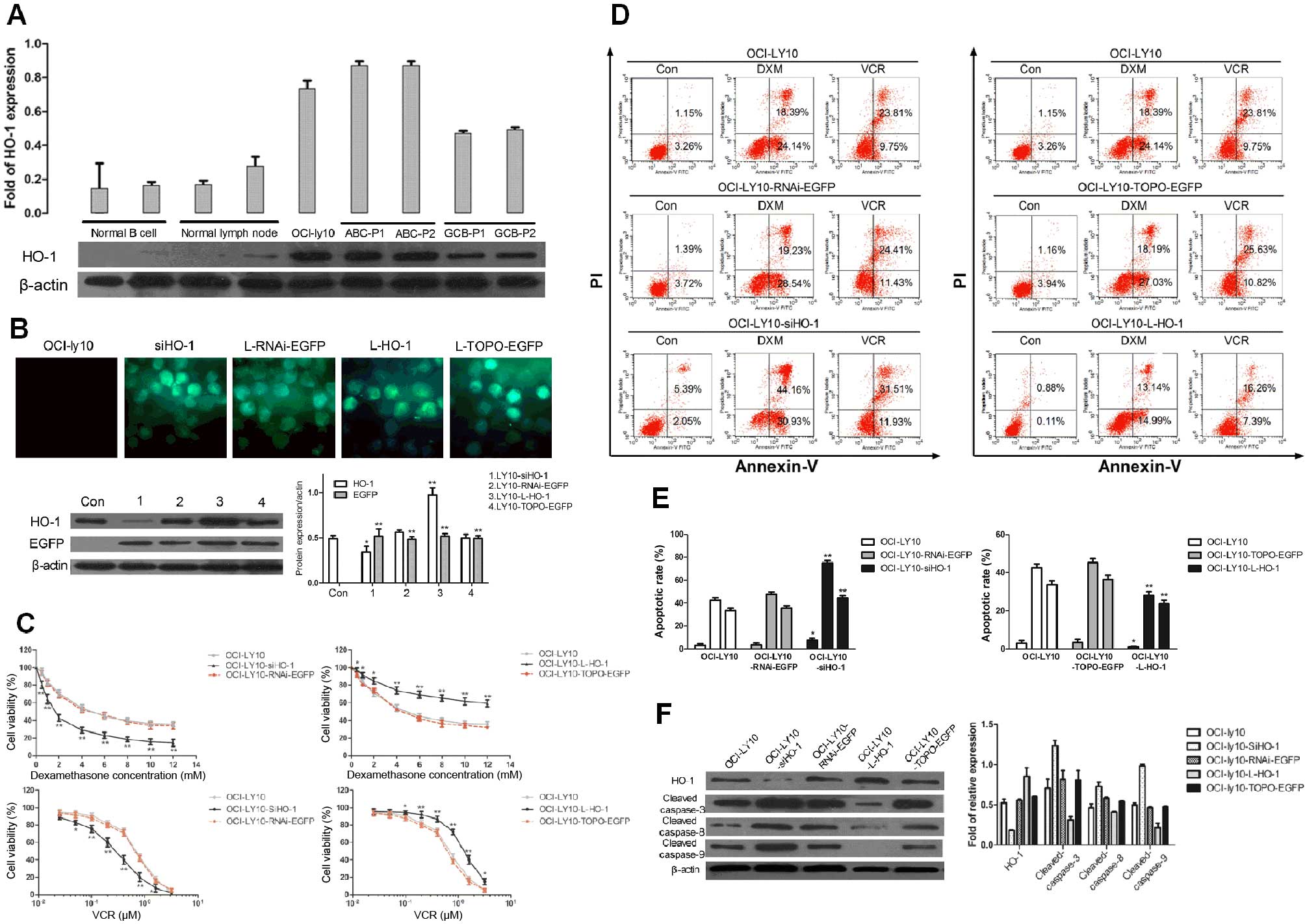
HO-1 is characteristically overexpressed
in ABC-DLBCL patients
Upon immunohistochemical analysis of surgical
resections of DLBCL tissues and normal lymph nodes, HO-1 expression
was mainly detected in the cytoplasm and membrane of tumor cells,
whereas nuclear staining was almost undetectable (Fig. 1A). The mean IRS was 4, and samples
were considered positive when IRS was ≥4. Finally, 18 of 31 (58.1%)
patients were found to have tumors positive for HO-1 expression,
whereas normal lymph nodes were negative for HO-1 expression. Among
these positive samples, we found that there was a significantly
higher percentage of HO-1 positivity in ABC-DLBCL (75%) than in
GCB-DLBCL (27.3%) (p=0.021). We also detected HO-1 mRNA expression
in FFPE tissue sections, and the data showed that the expression of
HO-1 was significantly higher in ABC-DLBCL than in GCB-DLBCL
(p<0.01) and the negative control group (p<0.01) (Fig. 1B). These results indicated that
high HO-1 expression is characteristic of ABC-DLBCL.
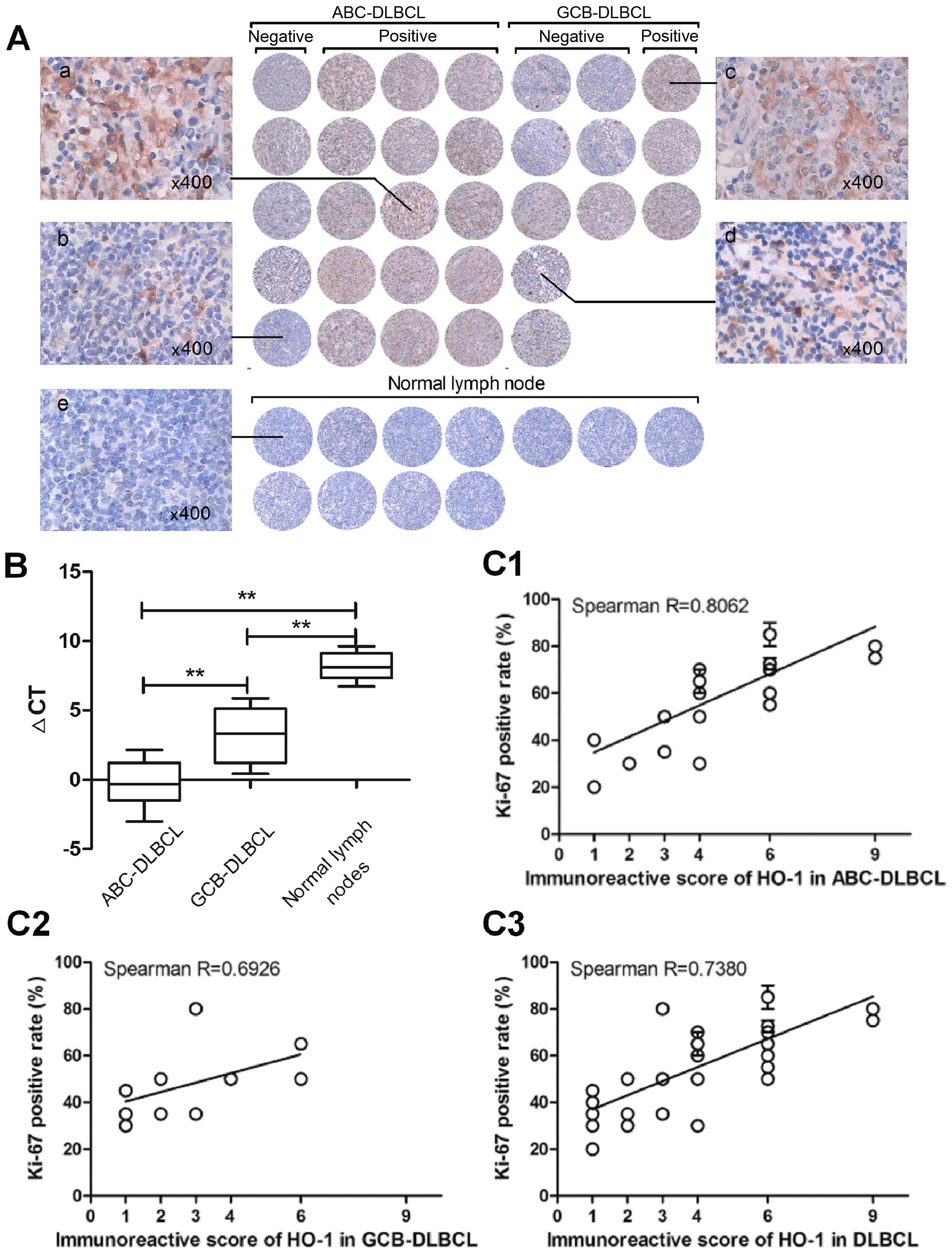 | Figure 1HO-1 is overexpressed in most
ABC-DLBCL patients. (A) Overview of the immunohistochemical results
for the 31 diffuse large B-cell lymphoma (DLBCL) tissue samples.
Each field of view was ≥60% of the original image (x200). Labels a
and c are representative cases of DLBCL-positive for HO-1
expression, b and d are representative cases of DLBCL-negative for
HO-1 expression, and e is representative case of normal lymph
nodes. (B) Differential expression of HO-1 mRNA in 31 DLBCL
patients and 11 normal lymph nodes, as determined by qPCR. Data are
presented as the ΔCT of HO-1 expression in the patient samples,
with higher ΔCTs indicating lower expression.
**p<0.01. (C) Scatterplot representing the
correlation of HO-1 expression with the Ki-67 positive rate in
DLBCL, GCB-DLBCL and in ABC-DLBCL patient samples. Spearman
correlation coefficient R for HO-1 positivity degree versus Ki-67
positive rate: in DLBCL, R=0.7380, p<0.01; in ABC-DLBCL,
R=0.8062, p<0.01; in GCB-DLBCL, R=0.6926, p<0.01. |
HO-1 expression correlates strongly with
Ki-67 expression in DLBCL patients
HO-1 protein expression in DLBCL patients was
further evaluated according to the clinicopathological
characteristics of DLBCL; the results are summarized in Table II. Age and gender were not
determinant factors of HO-1 expression. The rate of HO-1 positivity
was significantly higher in patients with >1 site of extranodal
involvement (11/13, 84.6%) than in patients with only 1 site of
extranodal involvement (7/18, 38.9%) (p=0.025). However, no
significant correlation was observed between the T stage and
International Prognostic Index (IPI) score, performance status,
lactate dehydrogenase levels, and B symptoms. The HO-1 positivity
rate was strongly related with Ki-67 expression in DLBCL patients
(R=0.7380; p<0.01). We then further analyzed the relationship
between the expression of HO-1 and Ki-67 in ABC-DLBCLs and observed
a more obvious correlation (R=0.8062; p<0.01) (Fig. 1C).
 | Table IICorrelation between HO-1 expression
and clinicopathological features of DLBCL patients. |
Table II
Correlation between HO-1 expression
and clinicopathological features of DLBCL patients.
| | HO-1
expression | |
|---|
| |
| |
|---|
|
Characteristics | No.
n=31 | Negative
n=13 | Positive
n=18 | p-value |
|---|
| Age, years; n
(%) | | | | 0.717 |
| ≤60 | 17 | 8 (47.1%) | 9 (52.9%) | |
| >60 | 14 | 5 (35.7%) | 9 (64.3%) | |
| Gender | | | | 0.689 |
| Female | 8 | 4 (50.0%) | 4 (50.0%) | |
| Male | 23 | 9 (39.1%) | 14 (60.9%) | |
| Performance
status | | | | 0.262 |
| <2 | 19 | 6 (31.6%) | 13 (68.4%) | |
| ≥2 | 12 | 7 (58.3%) | 5 (41.7%) | |
| T stage | | | | 0.285 |
| I or II | 15 | 8 (53.3%) | 7 (46.7%) | |
| III or IV | 16 | 5 (31.3%) | 11 (68.8%) | |
| Extranodal sites
involved | | | | 0.025 |
| 1 site | 18 | 11 (61.1%) | 7 (38.9%) | |
| >1 site | 13 | 2 (15.4%) | 11 (84.6%) | |
| LDH levela | | | | 0.703 |
| Normal | 12 | 4 (33.3%) | 8 (66.7%) | |
| High | 17 | 8 (47.1%) | 9 (52.9%) | |
| IPI | | | | 0.275 |
| Low to
low-intermediate | 17 | 9 (52.9%) | 8 (47.1%) | |
| High-intermediate
to high | 14 | 4 (28.6%) | 10 (71.4%) | |
| B symptoms | | | | 0.710 |
| Absent | 12 | 6 (50.0%) | 6 (50.0%) | |
| Present | 19 | 7 (36.8%) | 12 (63.2%) | |
| Ki-67
expressionb | | | | <0.01 |
| Positive | 21 | 4 (19.0%) | 17 (81.0%) | |
| Negative | 10 | 9 (90.0%) | 1 (10.0%) | |
| Subtype | | | | 0.021 |
| ABC | 20 | 5 (25.0%) | 15 (75.0%) | |
| GCB | 11 | 8 (72.7%) | 3 (27.3%) | |
High expression of HO-1 can inhibit
apoptosis in the ABC-DLBCL cell line OCI-ly10
Given a previous study showing that HO-1 expression
and the Ki-67 positivity rate were closely related, and since Ki-67
is a proliferation-associated nuclear antigen (37), we concluded that HO-1 plays a role
in promoting proliferation and preventing apoptosis, as previously
described (5,9). HO-1 expression was compared among the
ABC-derived cell line OCI-ly10, other different groups of cell
lines, and cancer cells from randomly selected DLBCL patients.
Western blot analysis showed that HO-1 was expressed at low levels
in normal B cells, normal lymph nodes, and GCB-DLBCL cells, whereas
ABC-DLBCL cells and OCI-ly10 cells had high levels of HO-1
expression (Fig. 2A).
In order to further investigate the effects of HO-1
on apoptosis, we used a lentiviral system to regulate HO-1
expression. Expression of HO-1 in OCI-ly10 cells was downregulated
upon transduction with siHO-1 and upregulated upon transduction
with L-HO-1. Western blotting results showed that HO-1 was highly
expressed in OCI-ly10-Lentivirus-V5-D-TOPO-HO-1-EGFP
(OCI-ly10-L-HO-1) cells but poorly expressed in
OCI-ly10-lentivirus-pRNAi-U6.2-EGFP-siHO-1 (OCI-ly10-siHO-1) cells
after 48 h of transduction, which indicates successful
transductions of the vectors (Fig.
2B).
Subsequently, we examined the viability of OCI-ly10
cells in response to varying concentrations of DXM and VCR, which
are commonly used chemotherapeutic drugs in DLBCLs. OCI-ly10,
OCI-ly10-L-HO-1, OCI-ly10-TOPO-EGFP, OCI-ly10-siHO-1, and
OCI-ly10-RNAi-EGFP cells were cultured with DXM and VCR at
different concentrations. With increasing concentration of DXM or
VCR, the viability of cells decreased gradually. At the same
concentration of DXM or VCR, cells with a high HO-1 expression
level had significantly higher viability rates than their
counterparts with low expression (Fig.
2C). The apoptosis rate of high HO-1-expressing groups was the
lowest, whereas that of low HO-1-expressing groups was the highest.
These results were validated by the Annexin V-FITC/PI assay after
induction of cells with 1 mM DXM or 0.5 μM VCR for 24 h (Fig. 2D). Interestingly, HO-1 expression
was negatively correlated with the expression of apoptotic
proteins, including cleaved caspase-3, cleaved caspase-8, and
cleaved caspase-9, when cells were treated with 0.5 μM VCR
(Fig. 2F). Hence, these data
demonstrate that upregulation of the HO-1 expression can lead to
the inhibition of OCI-ly10 cell apoptosis, indicating that HO-1 has
an anti-apoptotic effect.
Characteristic HO-1 overexpression in
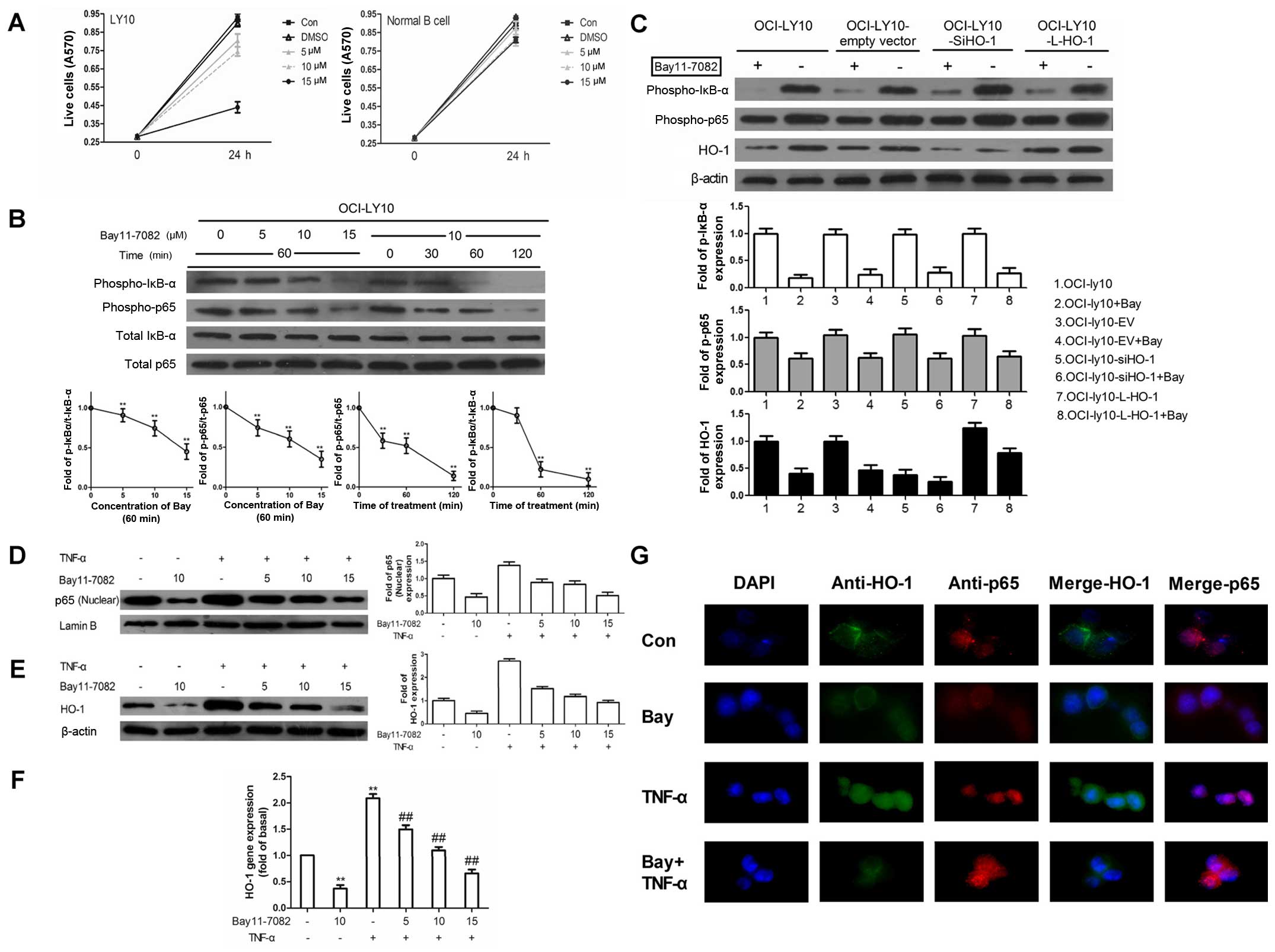
ABC-DLBCL is driven by constitutively activated NF-κB
Having confirmed that HO-1 is associated with
apoptosis, the next step was to investigate the mechanism of the
HO-1 expression increase in ABC-DLBCL. Previous studies indicated
that HO-1 expression is regulated by NF-κB (26–29).
Because NF-κB is known to be constitutively activated in ABC-DLBCL,
we hypothesized that the characteristic HO-1 overexpression in
ABC-DLBCL could be regulated by NF-κB.
To test this hypothesis, we treated OCI-ly10 cells
with Bay11-7082, an inhibitor of IκB-α phosphorylation. Bay11-7082
inhibited the phosphorylation of IκB-α and p65 in a time- and
dose-dependent manner, which represented the activation of NF-κB
(Fig. 3B). Considering the high
cytotoxicity of Bay11-7082 at a concentration of 15 μM (Fig. 3A), we finally chose the
concentration of 10 μM to treat OCI-ly10 cells for 60 min. Then,
western blot analysis of the protein levels of p-p65 and p-IκB-α
readout of phosphorylation of IκB-α and p65) and HO-1, in different
transduction groups untreated or treated with Bay11-7082, was
performed. As shown in Fig. 3C,
HO-1 expression could be inhibited by Bay11-7082 and siHO-1.
Moreover, the combination of siHO-1 and Bay11-7082 significantly
reduced HO-1 expression compared with the empty vector group.
However, the expression of p-p65 and p-IκB-α was only slightly
changed after silencing of HO-1 expression. Similarly, transduction
of HO-1 had no effect on p-p65 and p-IκB-α. These results indicated
that HO-1 was downstream of NF-κB.
The translocation of p65 is known to be an important
indicator of NF-κB activation. Therefore, tumor necrosus factor-α
(TNF-α) was used to activate the nuclear translocation of p65 in
the absence and presence of Bay11-7082, which is also an NF-κB
pharmacological inhibitor. In the absence of inhibitor, a
significant response of p65 in the nucleus was obtained within 15
min. As shown in Fig. 3D and E,
pretreatment with Bay11-7082 caused attenuation of the
TNF-α-induced p65 translocation and HO-1 protein level in a
concentration-dependent manner. In addition, Bay11-7082 decreased
the HO-1 mRNA expression (Fig.
3F). Similarly, immunofluorescence staining showed that the
TNF-α-induced translocation of p65 to the nucleus was blocked by
pretreatment with Bay11-7082 (Fig.
3G). Double staining showed that the majority of HO-1 was
localized in the cytoplasm of OCI-ly10 cells, where it was
decreased while p65 was blocked. These results suggest that
regulation of NF-κB can affect the expression of HO-1.
To further validate the results, 10 fresh tumor
tissues from ABC-DLBCL patients were randomly selected and the
NF-κB relative protein expression was analyzed (Fig. 4A). Cytosolic HO-1 expression was
positively correlated with nuclear p65 expression (R=0.9146) and
with the phosphorylated forms of p65 and IκB-α, which were obtained
from the whole-cell lysate (R=0.9369 and R=0.8169, respectively)
(Fig. 4B). These results suggest
that HO-1 expression is positively correlated with the degree of
NF-κB activation in ABC-DLBCL. Moreover, it can be concluded that
NF-κB is found upstream of HO-1 in ABC-DLBCL, regulating HO-1
expression and thereby affecting cell apoptosis.
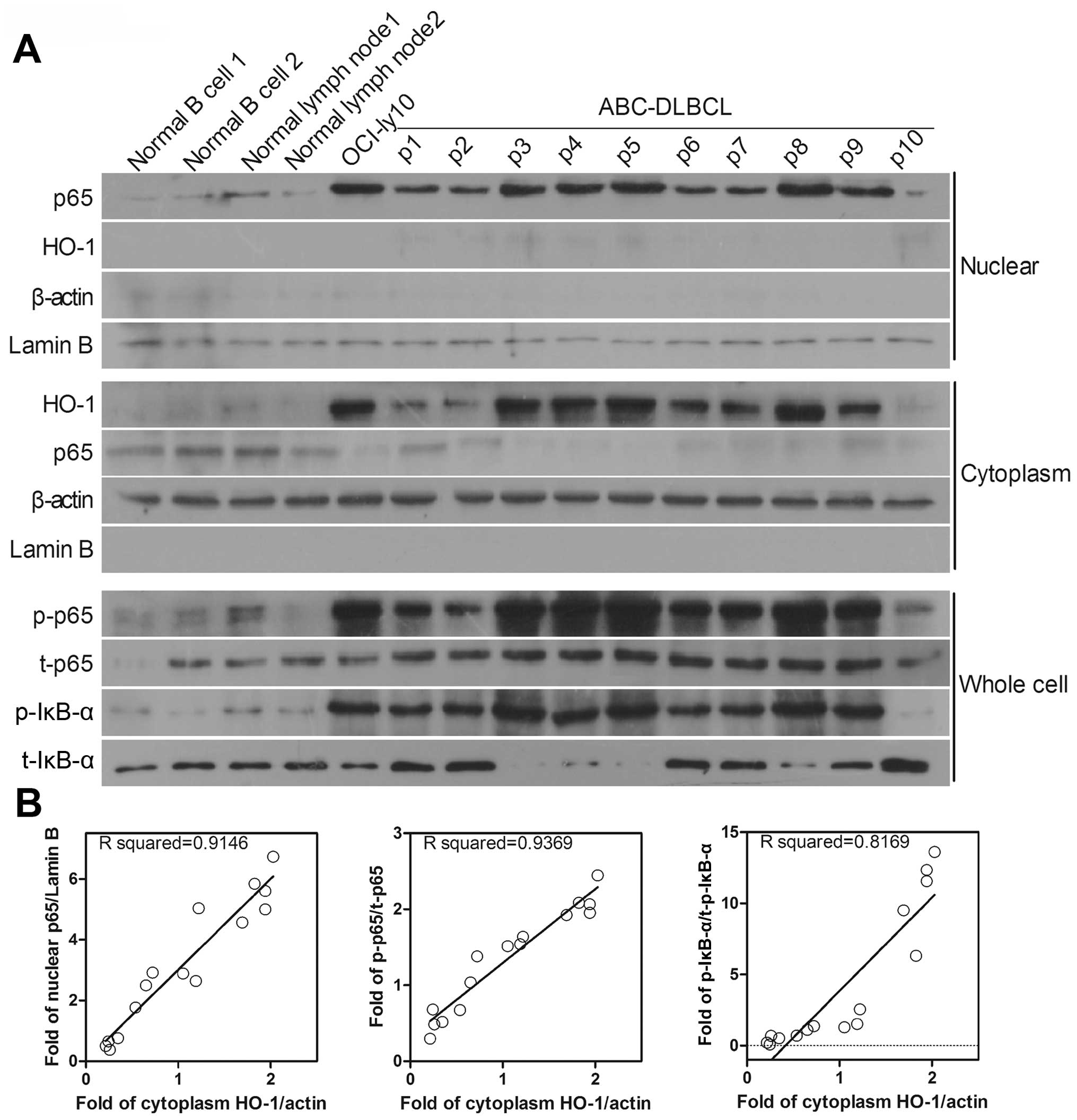 | Figure 4Protein levels of HO-1 and NF-κB are
increased in ABC-DLBCL patients. (A) Nuclear and cytosolic western
blots were reprobed for p65 and HO-1 expression. Whole-cell western
blots were reprobed for p-IκB-α, p-p65, t-p65, and t-IκB-α
expression. Lamin B was used as the nuclear protein reference
whereas β-actin was used as the cytosolic and whole-cell protein
references. (B) Scatterplot representing the correlation of HO-1
expression with the degree of NF-κB activation in ABC-DLBCL patient
samples. For protein levels of nuclear p65 versus cytosolic HO-1
(B, left), the Spearman correlation coefficient R=0.9146,
p<0.01; for protein levels of phosphorylated p65 versus
cytosolic HO-1 (B, middle), R=0.9369, p<0.01; for protein levels
of phosphorylated p-IκB-α versus cytosolic HO-1 (B, right),
R=0.8169, p<0.01. |
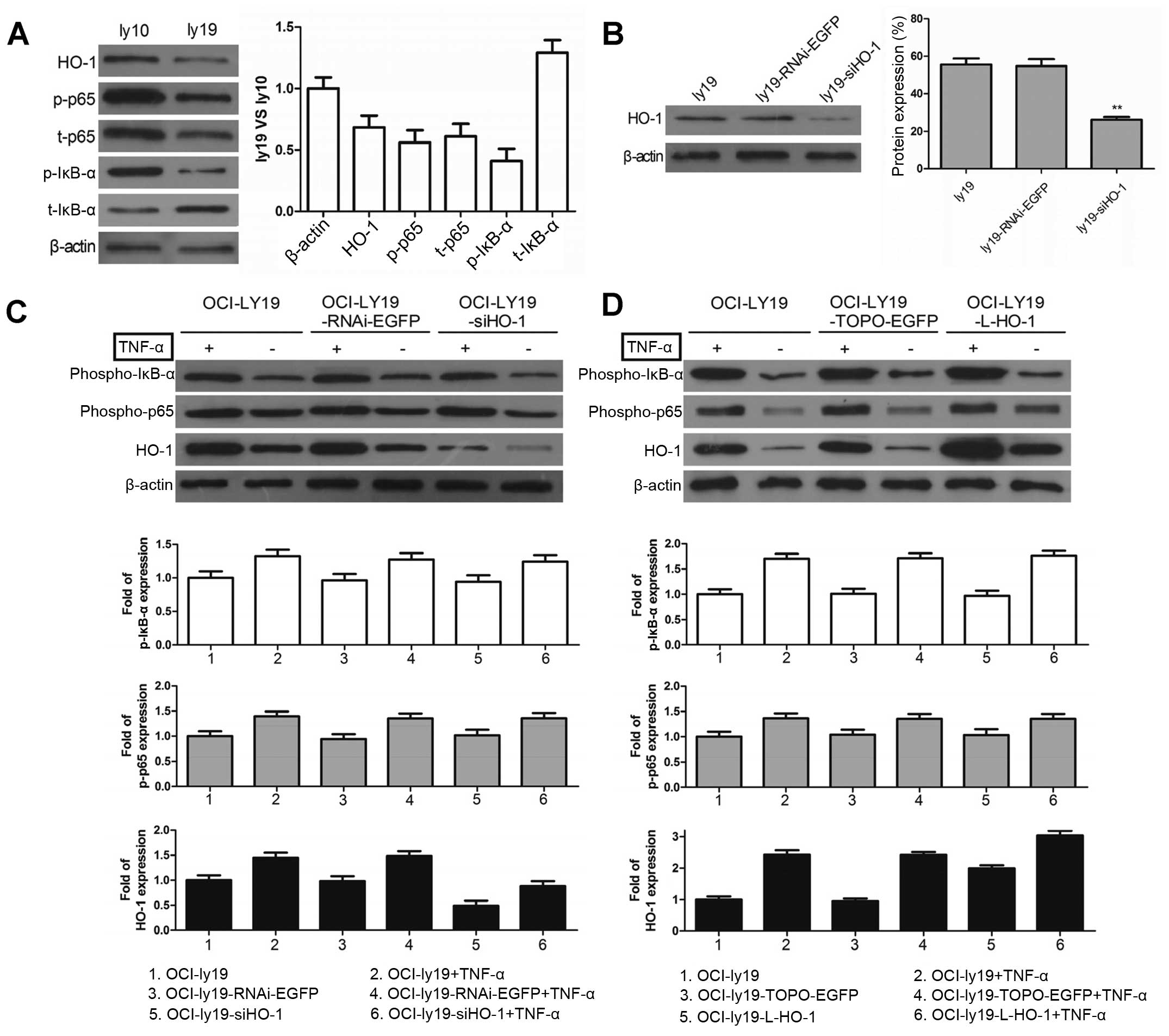
Activated NF-κB-mediated upregulation of
HO-1 expression inhibits apoptosis in GCB-DLBCL cells
Having shown that HO-1 overexpression is
attributable to the constitutively activated NF-κB in ABC-DLBCL, we
next wanted to determine whether activated NF-κB will cause changes
in HO-1 expression in GCB-DLBCL cells that have low NF-κB activity
(21). We compared the HO-1
expression and NF-κB activity levels in the ABC-DLBCL cell line
OCI-ly10 with those in the GCB-DLBCL cell line OCI-ly19. The
results showed that the aforementioned levels were lower in
OCI-ly19 cells than in OCI-ly10 cells (Fig. 5A). We then used TNF-α to activate
NF-κB and observed the HO-1 expression. After successfully knocking
down HO-1 expression in OCI-ly19 cells with si-HO-1 (Fig. 5B), we incubated ly19,
ly19-RNAi-EGFP, and ly19-siHO-1 with or without TNF-α. HO-1
expression was upregulated after the TNF-α-mediated increased
phosphorylation of p65 and IκB-α (Fig.
5C). Furthermore, HO-1 expression was superinduced in the
presence of both TNF-α and L-HO-1 (Fig. 5D). However, there was no effect on
p-p65 and p-IκB-α when regulating HO-1 first. The results indicated
that HO-1 expression can be upregulated by NF-κB in GCB-DLBCL
cells.
Poly(ADP-ribose) polymerase-1 (PARP-1), which can be
proteolytically cleaved by caspase-3 at the DEVD peptide sequence
site to generate an 85- and a 24-kDa fragment, is a known caspase-3
substrate. In OCI-ly10 cells, consistent with the decreased HO-1
protein levels, Bay11-7082 treatment or Lenti-siHO-1 expression
increased the PARP cleavage activity, and their combination
resulted in maximum cleavage of PARP (Fig. 5E). Furthermore, knockdown of HO-1
was associated with increased cleaved PARP in OCI-ly19 cells.
However, such HO-1 decrease was reversed upon treatment with the
NF-κB activator TNF-α.
Subsequently, apoptosis was detected in OCI-ly10
cells by flow cytometry, and the combination of Bay11-7082 and
siHO-1 significantly increased the apoptotic event (Fig. 5F). However, there were no
significant differences in apoptosis after either siHO-1 or
Bay11-7082 treatment alone. In OCI-ly19 cells, the combination of
TNF-α and siHO-1 could rescue the apoptotic phenotype caused by
HO-1 silencing in OCI-ly19 cells (Fig.
5G). These results demonstrated that the NF-κB-mediated
overexpression of HO-1 not only plays an anti-apoptotic role in
ABC-DLBCL cells but also inhibits apoptosis in GCB-DLBCL cells.
Thus, since HO-1 overexpression may be responsible for the dismal
outcomes in ABC-DLBCL, a combined target comprising NF-κB and HO-1
may provide a new therapeutic option for this DLBCL subtype.
Discussion
HO-1 is well known as a stress-related
cytoprotective molecule and is overexpressed in various tumors as
well as hematological malignancies such as BCR-ABL-positive CML and
most AMLs. Moreover, HO-1 overexpression is related to increased
tumor proliferation and resistance to apoptosis. Currently, HO-1
has been confirmed as a novel target for AML and CML (14,19).
However, to the best of our knowledge, the relationship between
HO-1 and DLBCL has not yet been elucidated. Thus, we focused on the
expression of HO-1 and its role in DLBCL.
In this study, we showed that a high level of HO-1
expression is characteristic of the ABC lymphoma subtype. At the
same time, HO-1 had a low level of expression in the GCB lymphoma
subtype, but the expression was still significantly higher than
that in normal lymph nodes due to the fact that HO-1 can be induced
by various pathological stimuli. Analysis of the
clinicopathological features possibly related to HO-1 showed that
its high-level of expression was correlated with high levels of
Ki-67 in DLBCL; this correlation was more obvious in ABC-DLBCL.
Ki-67 is a nuclear antigen associated with tumor invasion and
proliferation, and can be used as an index to judge the
proliferation and malignancy degree of non-Hodgkin lymphoma
(38). Miller et al
demonstrated that a high proliferative index due to increased Ki-67
is inversely related with overall survival (39). Katzenberger et al
demonstrated that the Ki-67 proliferation index is a quantitative
indicator of clinical risk in mantle cell lymphoma (40). In the promotion of proliferation,
Ki-67 and HO-1 have common points. Moreover, the rate of HO-1
positivity was significantly higher in patients with >1 site of
extranodal involvement. These results suggest that, in addition to
its widely known roles in tumor cells as a protective molecule
against various stresses and in supporting rapid tumor growth, HO-1
may be associated with tumor invasion and proliferation.
Moreover, no significant association of HO-1
expression with other clinicopathological features of DLBCL was
observed, including age, gender, performance status, IPI score,
lactate dehydrogenase level, B symptoms, and T stage of tumors.
However, it was observed that tissues of III/IV stage and with
high-intermediate to high IPI scores showed higher levels of HO-1
expression. These results may be attributed to the use of a small
number of patient samples.
Since HO-1 is overexpressed in ABC-DLBCL and may be
associated with proliferation, we investigated the effect of high
HO-1 expression on the ABC-DLBCL cell line OCI-ly10 after HO-1
overexpression or knockdown by lentiviral vector delivery. Despite
incubation with DXM or VCR, HO-1 overexpression resulted in a
higher viability rate and lower apoptotic rate. Moreover, HO-1 was
negatively correlated with the cleaved forms of the caspase family
of proteins. Numerous studies had reported that HO-1 overexpression
plays an anti-apoptotic role and leads to drug resistance in
hematological malignancies such as AML and CML (14,19).
Our study demonstrated that HO-1 can also suppress apoptosis in
ABC-DLBCL cells in vitro. Our next step would be to study
the anti-apoptotic effect of HO-1 in vivo.
To date, several studies have indicated that NF-κB
can regulate the induction of HO-1 expression (26–28).
Li et al demonstrated that NF-κB activation is necessary for
basal levels of cardiac HO-1 protein expression (29). We hypothesized that the high basal
expression of HO-1 is due to NF-κB which is constitutively
activated in ABC-DLBCL. NF-κB, a pathogenetic hallmark of
ABC-DLBCL, promotes cell survival and proliferation and inhibits
apoptosis (2). In this study,
activated NF-κB increased the expression of HO-1, whereas NF-κB
inhibition had the opposite effect. Expression of p-p65 and p-IκB-α
was not affected by the changes in HO-1, and this verified that
HO-1 is directly downstream of NF-κB. On comparing the protein
levels of NF-κB and HO-1 in 10 randomly selected patients of
ABC-DLBCL and control groups, we found that HO-1 was constitutively
activated in ABC-DLBCLs. Furthermore, phosphorylation of the NF-κB
subunit p65 and of IκB-α may be responsible for the expression of
HO-1.
Further studies were performed on the
GCB-DLBCL-derived cell line OCI-ly19 that has a low level of NF-κB
activity. After the TNF-α-mediated activation of NF-κB, increased
HO-1 expression was observed. Taken together, these results show
that basal HO-1 expression is under the control of NF-κB. Moreover,
if the basal NF-κB activity is stimulated in OCI-ly19 cells, HO-1
will be subsequently induced and therefore provides a second line
of defense against anticancer drugs. However, combining the
inhibition of NF-κB and HO-1 in OCI-ly10 cells significantly
increased apoptosis and thus may suggest a new target for therapy
of ABC-DLBCL.
A bigger sample pool of DLBCL patients is required
to perform further investigations to verify HO-1 expression in
different DLBCL subtypes. The underlying signaling pathway remains
to be further elucidated since the mechanism of how HO-1 overcomes
apoptosis is unclear.
In conclusion, the characteristic overexpression of
HO-1 is mediated by constitutively activated NF-κB in ABC-DLBCL.
HO-1 expression inhibits apoptosis in ABC-DLBCL, whereas HO-1
silencing promotes apoptosis. Increasing the expression of HO-1 in
GCB-DLBCL-derived OCI-ly19 cells can lead to drug resistance.
Furthermore, the combination of NF-κB and HO-1 may provide a new
target for the therapy of ABC-DLBCL. The findings herein provide
valuable experimental evidence for the targeted therapy of
ABC-DLBCL.
Acknowledgements
This study was supported, in part, by the National
Natural Science Foundation of China (Nos. 81070444, 81270636,
81360501 and 81470006), International Cooperation Project of
Guizhou Province (No. 2011-7010), Social Project of Guizhou
Province (No. 2011-3012), Provincial Government Special Fund of
Guizhou Province (No. 2010-84), Science and Technology Foundation
of Guizhou Province (J word[2010]-2164), Union Project of Guizhou
Province Science and Technology (LH word, [2015]-7386) and Project
of Science and Technology Bureau of Guiyang City (No.
2012103-36).
References
|
1
|
World Health Organization. Classification
of Tumors of Haematopoietic and Lymphoid Tissues. IARC Press; Lyon:
2008
|
|
2
|
Nogai H, Dörken B and Lenz G: Pathogenesis
of non-Hodgkin's lymphoma. J Clin Oncol. 29:1803–1811. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Alizadeh AA, Eisen MB, Davis RE, Ma C,
Lossos IS, Rosenwald A, Boldrick JC, Sabet H, Tran T, Yu X, et al:
Distinct types of diffuse large B-cell lymphoma identified by gene
expression profiling. Nature. 403:503–511. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lenz G, Wright G, Dave SS, Xiao W, Powell
J, Zhao H, Xu W, Tan B, Goldschmidt N, Iqbal J, et al;
Lymphoma/Leukemia Molecular Profiling Project. Stromal gene
signatures in large-B-cell lymphomas. N Engl J Med. 359:2313–2323.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jozkowicz A, Was H and Dulak J: Heme
oxygenase-1 in tumors: Is it a false friend? Antioxid Redox Signal.
9:2099–2117. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Foresti R, Clark JE, Green CJ and
Motterlini R: Thiol compounds interact with nitric oxide in
regulating heme oxygenase-1 induction in endothelial cells.
Involvement of superoxide and peroxynitrite anions. J Biol Chem.
272:18411–18417. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stuhlmeier KM: Activation and regulation
of Hsp32 and Hsp70. Eur J Biochem. 267:1161–1167. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Terry CM, Clikeman JA, Hoidal JR and
Callahan KS: Effect of tumor necrosis factor-alpha and
interleukin-1 alpha on heme oxygenase-1 expression in human
endothelial cells. Am J Physiol. 274:H883–H891. 1998.PubMed/NCBI
|
|
9
|
Miyazaki T, Kirino Y, Takeno M, Samukawa
S, Hama M, Tanaka M, Yamaji S, Ueda A, Tomita N, Fujita H, et al:
Expression of heme oxygenase-1 in human leukemic cells and its
regulation by transcriptional repressor Bach1. Cancer Sci.
101:1409–1416. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Furfaro AL, Piras S, Passalacqua M,
Domenicotti C, Parodi A, Fenoglio D, Pronzato MA, Marinari UM,
Moretta L, Traverso N, et al: HO-1 up-regulation: A key point in
high-risk neuroblastoma resistance to bortezomib. Biochim Biophys
Acta. 1842:613–622. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee SE, Yang H, Jeong SI, Jin YH, Park CS
and Park YS: Induction of heme oxygenase-1 inhibits cell death in
crotonaldehyde-stimulated HepG2 cells via the PKC-δ-p38-Nrf2
pathway. PLoS One. 7:e416762012. View Article : Google Scholar
|
|
12
|
Zhang L, Liu YL, Chen GX, Cui B, Wang JS,
Shi YL, Li LP and Guo XB: Heme oxygenase-1 promotes Caco-2 cell
proliferation and migration by targeting CTNND1. Chin Med J (Engl).
126:3057–3063. 2013.
|
|
13
|
Kongpetch S, Kukongviriyapan V, Prawan A,
Senggunprai L, Kukongviriyapan U and Buranrat B: Crucial role of
heme oxygenase-1 on the sensitivity of cholangiocarcinoma cells to
chemotherapeutic agents. PLoS One. 7:e349942012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mayerhofer M, Gleixner KV, Mayerhofer J,
Hoermann G, Jaeger E, Aichberger KJ, Ott RG, Greish K, Nakamura H,
Derdak S, et al: Targeting of heat shock protein 32 (Hsp32)/heme
oxygenase-1 (HO-1) in leukemic cells in chronic myeloid leukemia: A
novel approach to overcome resistance against imatinib. Blood.
111:2200–2210. 2008. View Article : Google Scholar
|
|
15
|
Ma D, Fang Q, Wang P, Gao R, Sun J, Li Y,
Hu XY and Wang JS: Downregulation of HO-1 promoted apoptosis
induced by decitabine via increasing p15INK4B promoter
demethylation in myelodysplastic syndrome. Gene Ther. 22:287–296.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wei S, Wang Y, Chai Q, Fang Q, Zhang Y and
Wang J: Potential crosstalk of Ca2+-ROS-dependent
mechanism involved in apoptosis of Kasumi-1 cells mediated by heme
oxygenase-1 small interfering RNA. Int J Oncol. 45:2373–2384.
2014.PubMed/NCBI
|
|
17
|
Ma D, Fang Q, Wang P, Gao R, Wu W, Lu T,
Cao L, Hu X and Wang J: Induction of heme oxygenase-1 by
Na+-H+ exchanger 1 protein plays a crucial
role in imatinib-resistant chronic myeloid leukemia cells. J Biol
Chem. 290:12558–12571. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang P, Ma D, Wang J, Fang Q, Gao R, Wu W,
Lu T and Cao L: Silencing HO-1 sensitizes SKM-1 cells to apoptosis
induced by low concentration 5-azacytidine through enhancing p16
demethylation. Int J Oncol. 46:1317–1327. 2015.PubMed/NCBI
|
|
19
|
Lin X, Fang Q, Chen S, Zhe N, Chai Q, Yu
M, Zhang Y, Wang Z and Wang J: Heme oxygenase-1 suppresses the
apoptosis of acute myeloid leukemia cells via the JNK/c-JUN
signaling pathway. Leuk Res. 39:544–552. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Davis RE, Brown KD, Siebenlist U and
Staudt LM: Constitutive nuclear factor kappaB activity is required
for survival of activated B cell-like diffuse large B cell lymphoma
cells. J Exp Med. 194:1861–1874. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Compagno M, Lim WK, Grunn A, Nandula SV,
Brahmachary M, Shen Q, Bertoni F, Ponzoni M, Scandurra M, Califano
A, et al: Mutations of multiple genes cause deregulation of
NF-kappaB in diffuse large B-cell lymphoma. Nature. 459:717–721.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Davis RE, Ngo VN, Lenz G, Tolar P, Young
RM, Romesser PB, Kohlhammer H, Lamy L, Zhao H, Yang Y, et al:
Chronic active B-cell-receptor signalling in diffuse large B-cell
lymphoma. Nature. 463:88–92. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lenz G and Staudt LM: Aggressive
lymphomas. N Engl J Med. 362:1417–1429. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ngo VN, Davis RE, Lamy L, Yu X, Zhao H,
Lenz G, Lam LT, Dave S, Yang L, Powell J, et al: A loss-of-function
RNA interference screen for molecular targets in cancer. Nature.
441:106–110. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lam LT, Davis RE, Pierce J, Hepperle M, Xu
Y, Hottelet M, Nong Y, Wen D, Adams J, Dang L, et al: Small
molecule inhibitors of IkappaB kinase are selectively toxic for
subgroups of diffuse large B-cell lymphoma defined by gene
expression profiling. Clin Cancer Res. 11:28–40. 2005.PubMed/NCBI
|
|
26
|
Lavrovsky Y, Schwartzman ML, Levere RD,
Kappas A and Abraham NG: Identification of binding sites for
transcription factors NF-kappa B and AP-2 in the promoter region of
the human heme oxygenase 1 gene. Proc Natl Acad Sci USA.
91:5987–5991. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kurata S, Matsumoto M, Tsuji Y and
Nakajima H: Lipopolysaccharide activates transcription of the heme
oxygenase gene in mouse M1 cells through oxidative activation of
nuclear factor kappa B. Eur J Biochem. 239:566–571. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lin CC, Chiang LL, Lin CH, Shih CH, Liao
YT, Hsu MJ and Chen BC: Transforming growth factor-beta1 stimulates
heme oxygenase-1 expression via the PI3K/Akt and NF-kappaB pathways
in human lung epithelial cells. Eur J Pharmacol. 560:101–109. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li Q, Guo Y, Ou Q, Cui C, Wu WJ, Tan W,
Zhu X, Lanceta LB, Sanganalmath SK, Dawn B, et al: Gene transfer of
inducible nitric oxide synthase affords cardioprotection by
upregulating heme oxygenase-1 via a nuclear
factor-{kappa}B-dependent pathway. Circulation. 120:1222–1230.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rushworth SA, Zaitseva L, Murray MY, Shah
NM, Bowles KM and MacEwan DJ: The high Nrf2 expression in human
acute myeloid leukemia is driven by NF-κB and underlies its
chemoresistance. Blood. 120:5188–5198. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ogborne RM, Rushworth SA and O'Connell MA:
Alpha-lipoic acid-induced heme oxygenase-1 expression is mediated
by nuclear factor erythroid 2-related factor 2 and p38
mitogen-activated protein kinase in human monocytic cells.
Arterioscler Thromb Vasc Biol. 25:2100–2105. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rushworth SA, Chen XL, Mackman N, Ogborne
RM and O'Connell MA: Lipopolysaccharide-induced heme oxygenase-1
expression in human monocytic cells is mediated via Nrf2 and
protein kinase C. J Immunol. 175:4408–4415. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ai ZL, Zhu CH, Min M, Wang J, Lan CH, Fan
LL, Sun WJ and Chen DF: The role of hepatic liver X receptor α- and
sterol regulatory element binding protein-1c-mediated lipid
disorder in the pathogenesis of non-alcoholic steatohepatitis in
rats. J Int Med Res. 39:1219–1229. 2011. View Article : Google Scholar
|
|
34
|
Scott DW, Wright GW, Williams PM, Lih CJ,
Walsh W, Jaffe ES, Rosenwald A, Campo E, Chan WC, Connors JM, et
al: Determining cell-of-origin subtypes of diffuse large B-cell
lymphoma using gene expression in formalin-fixed paraffin-embedded
tissue. Blood. 123:1214–1217. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gandini NA, Fermento ME, Salomón DG,
Blasco J, Patel V, Gutkind JS, Molinolo AA, Facchinetti MM and
Curino AC: Nuclear localization of heme oxygenase-1 is associated
with tumor progression of head and neck squamous cell carcinomas.
Exp Mol Pathol. 93:237–245. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Miyata Y, Kanda S, Mitsunari K, Asai A and
Sakai H: Heme oxygenase-1 expression is associated with tumor
aggressiveness and outcomes in patients with bladder cancer: A
correlation with smoking intensity. Transl Res. 164:468–476. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gerdes J, Schwab U, Lemke H and Stein H:
Production of a mouse monoclonal antibody reactive with a human
nuclear antigen associated with cell proliferation. Int J Cancer.
31:13–20. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Szczuraszek K, Mazur G, Jeleń M, Dziegiel
P, Surowiak P and Zabel M: Prognostic significance of Ki-67 antigen
expression in non-Hodgkin's lymphomas. Anticancer Res.
28A:1113–1118. 2008.
|
|
39
|
Miller TP, Grogan TM, Dahlberg S, Spier
CM, Braziel RM, Banks PM, Foucar K, Kjeldsberg CR, Levy N, Nathwani
BN, et al: Prognostic significance of the Ki-67-associated
proliferative antigen in aggressive non-Hodgkin's lymphomas: A
prospective Southwest Oncology Group trial. Blood. 83:1460–1466.
1994.PubMed/NCBI
|
|
40
|
Katzenberger T, Petzoldt C, Höller S,
Mäder U, Kalla J, Adam P, Ott MM, Müller-Hermelink HK, Rosenwald A
and Ott G: The Ki67 proliferation index is a quantitative indicator
of clinical risk in mantle cell lymphoma. Blood. 107:34072006.
View Article : Google Scholar : PubMed/NCBI
|