Introduction
Salivary gland carcinoma (SGC) is a rare malignancy
with an incidence rate of approximately 1 per million per year in
the United States (1) and Europe
(2). SGC may arise in all major or
minor salivary glands and may have a variety of histologic and
biologic characteristics that have been summarized in a recent
WHO-classification schema (3).
With regard to all locations, mucoepidermoid carcinomas,
polymorphous low-grade adenocarcinoma and the adenoid cystic
carcinoma (SACC) are the most frequently occurring histologic types
(4,5). Studies with patient-collectives from
Western Europe found that SACC are the most frequent type (2,6).
Although the etiology of SGC remains unclear, prior exposure to
radiation, a history of benign salivary gland tumor and a
deficiency of nutrients, particularly vitamins A and C have been
identified as SGC risk factors (7–9).
Epidemiologic studies indicate that the increased rates of SGC
after exposure to carcinogens like nickel, chrome, asbestos, cement
are possible, but there is no proof yet. Increasingly genetic
aberrations are identified in SGC, none of them specific for a
certain histological type. DNA of a variety of viruses with
oncogenic potential such as EBV, CMV and HHV-6 to 8 was isolated
from SGC, but this finding could not be linked to the development
of malignancy yet (10).
Today, human papillomavirus (HPV) has been widely
accepted as a cause for a subgroup of squamous cell carcinoma of
the head and neck area (HNSCC) (11,12).
Infections with these viruses are linked to the highest risk for
transformation to invasive carcinoma (13). There is very rare and sometimes
controversial information about HPV infections in SGC and SACC
specifically (14–19) suggesting an association between HPV
and certain salivary gland neoplasms. However, the clinical
significance of these findings remains elusive. One intention of
the present study was to systematically investigate the HPV status
in SACC tumor tissues. In HPV-related HNSCC and cervical carcinoma,
p16 expression correlates highly with the presence of oncogenic
HPV-DNA (20). Therefore, p16 is
considered a specific marker equivalent to DNA-sequencing for
high-risk HPV (HR-HPV) and is widely used in routine
histopathological screening (20).
Clinically important is that the presence of high-risk HPV and p16
expression in HNSCC is correlated with a better survival as
compared to tumors with no evidence of HPV infection (21–23).
Both HPV-DNA and the expression of p16 were investigated in the
present study cohort of SACC.
In HNSCC, HPV-positive tumors are characterized by
certain clinical and pathological characteristics, the expression
of certain cell cycle proteins and genetic changes that are
different from HPV-negative HNSCC (24,25).
TP53 mutations are by far the most common and present in ~70% of
HPV-negative HNSCC patients, but rare in HPV-related HNSCC as p53
is targeted by E6 protein (26).
The prognosis also changes with the status of epithelial growth
factor receptor (EGFR) expression. In one study, HNSCC positive for
p16 expression showed a better 5-year-survival (FYS) than
p16-negative tumors (84 vs. 49%). If EGFR and p16 were co-expressed
the FYS increased to 93%. If tumors were only positive for EGFR,
but negative for p16, FYR was decreased (21). In analogy to investigate the
significant differences in survival from the subgroups classified
by HPV infection in the HNSCC, we also performed p53 and EGFR
immunostaining in SACC specimens with the aim to evaluate the
association between p53 and EGFR expression with HPV status in
patients with SACC. Until now no relevant universal prognostic
indicators could be derived from clinical data, histological or
immunohistochemical markers. Ki-67 has been reported as a
proliferation marker related to a poor survival in SGC (27).
Since the advances in treatment modalities have not
achieved a significant impact on the survival of patients with
SACC, the discovery of biomarkers and potential targets are of high
interest. In the present study, we propose to identify HPV-subtypes
in SACC and to correlate them with other characteristics of the
patients with the aim to uncover potentially novel prognostic and
predictive molecular signatures and therapeutic targets.
Materials and methods
Patients and tissue specimens
The present study was approved by the Institutional
Review Board of Charité-Universitätsmedizin Berlin, Germany
(EA4/035/08). Sixty-seven consecutive patients with sufficient
amounts of banked biological material, data on clinical follow-up
and the confirmed pathologic diagnosis of SACC, and with no prior
history of malignancies and treatments were included. Archival
formalin-fixed, paraffin-embedded (FFPE) SACC of the head and neck
area were obtained from the Department of Pathology, Charité
University Medicine, Campus Benjamin Franklin, Germany and were
collected from 1982 to 2007. Data on the TNM classification were
retrieved from the pathology database and patient charts. Overall
survival (OS) and recurrence-free survival (RFS) was calculated
from the date of diagnosis.
DNA extraction and HPV genotyping
FFPE tissues were sliced at 10 μm thickness and
collected in a sterile, nucleic acid-free microtube. For each tube,
3 sections of a total of 30 μm thickness were carefully collected.
The proceeding and the following section of each series was stained
by hematoxylin and evaluated for the presence of tumor tissue. As a
control for cross contamination, HPV free tissue specimens of
murine origin, which were always negative for HPV and β-globin,
were cut in between SACC patients specimens. After
deparaffinization of FFPE tissues, the genomic DNA was extracted by
QIAamp DNA FFPE Tissue kit (Qiagen, Hilden, Germany) following the
manufacturer's instructions. DNA-concentrations were measured by
NanoDrop (NanoDrop™ 2000/2000c spectrophotometers; Thermo Fisher
Scientific GmbH, Dreieich, Germany). Then
GP5+/bio-6+ PCR followed by Multiplexed
Genotyping readout was performed exactly as described by Schmitt
et al (28,29). Each PCR experiment included
specimens from cervical cancers, which were proved infected by
high-risk HPV as positive control, and several specimens lacking
template DNA as contamination control.
Following PCR amplification, 10 μl of each reaction
mixture was subjected to hybridization to Luminex beads coupled to
HPV type-specific probes and analysed on a Bio-Plex 200 (Bio-Rad
Laboratories, Hercules, CA, USA). The results were concluded as the
mean fluorescence intensity (MFI) of at least 50 beads per set. For
each sample, the assay was performed twice in independent
experiments.
The following HPV types were discriminated and
genotyped: high-risk HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56,
58, 59, 68, 73 and 82; putative HR (pHR) types 26, 53 and 66; and
low-risk (LR) types 6, 11, 40, 42, 43, 44 and 70 (29,30).
Importantly, within this assay, it is possible to
detect multiple simultaneous infections (28). A sample was regarded positive when
the MFI value was 3-fold higher than the background MFI value and
borderline at 2-fold. The assay detects 10–1000 HPV copies
depending on the HPV type. Net MFI values above 5 MFI were defined
as positive reactions. A β-globin specific PCR was included to
verify sufficient DNA content.
To assess DNA-fragmentation a specific
PCR was performed
Some FFPE sections dated back to 1982. Due to
non-buffered formalin use until 1995 this may have led to DNA
fragmentation and therefore false negative HPV-PCR. Therefore, on
each sample with a negative β-globin result a PCR was performed to
assess the grade of DNA-fragmentation as previously described.
Finally, the presence of 100-, 200-, 300- and 400-bp bands was
evaluated on a 2% agarose gel (31).
Immunohistochemistry
Immunostaining of SACC and surrounding normal
specimens was done using 5-μm sections of FFPE from the same series
of sections that were used for DNA extraction. The sections were
processed by the avidin-biotin-peroxidase method (ABC) using the
Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA, USA)
and stained with a mouse anti-p16 antibody (clone DCS-50;
Neomarkers, Inc., Fremont, CA, USA) or mouse anti-human EGFR
monoclonal antibody (Dako, Glostrup, Denmark). A biotinylated
secondary goat anti-mouse antibody was added for 20 min at room
temperature after a washing step. Then the color reaction was
developed using the APAAP-Red-kit (Dako), and counterstaining was
done with hemalaun solution. Antibody titrations and isotype
control antibodies were used to determine optimal staining
conditions.
p16 expression was examined by three independent
experienced investigators in a blinded manner, and considered
positive if nuclear and cytoplasmic staining was positive.
A semi-quantitative analysis was done, assessing
percentage and pattern of staining in the tissue (normal and tumor)
using a grading of 0 (negative staining) to 3 (strong staining).
The p16 antibody reactivity was scored (32) as follows: negative (<1% of the
cells were positive), sporadic (isolated cells were positive, but
<5%), focal (small cell clusters, but <25% of the cells were
positive) and diffuse (>25% of the cells were stained).
Depending on these criteria, the results were further classified as
being negative, weak (+), moderate (++) and strong (+++),
respectively.
Statistical analysis
Statistical analysis was performed using SPSS 16.0
software (SPSS, Inc., Chicago, IL, USA). Categorical variables were
expressed as percentages and frequencies, and numerical variables
were represented as mean ± SD. Qualitative data were compared using
the Chi-square or Fisher's exact test as appropriate. Overall
survival (OS) and locoregional recurrence-free survival (LRFS) were
determined by using the Kaplan-Meier (KM) method. The Cox
multivariate regression model was applied to evaluate hazard ratio
(HR) and P-value with the aim to compare the factors with
prognostic potential. A P-value of <0.05 was regarded as
statistically significant.
Results
Patients
Study participants were between 16 and 90 years of
age (median 61.3 years; female to male ratio 2.05:1) and only
patients with no prior history of malignancies were included.
Details of the demographic and clinical data for the 67
investigated patients are summarized in Table I. Twenty-three patients (34%)
underwent surgery alone as their sole treatment, 41 (62%) patients
underwent surgery and received postoperative radiation, 3 (4%)
patients underwent surgery and received postoperative
radiochemotherapy. The median follow-up in this population was 102
months (interquartile range, 3–265) among surviving patients.
Twenty-six patients (39%) developed recurrence after initial
treatments. Thirty-seven patients (55%) had died at the time of
last follow-up.
 | Table ICorrelation between
clinicopathological characteristics and examined variables in 67
patients with adenoid cystic carcinoma. |
Table I
Correlation between
clinicopathological characteristics and examined variables in 67
patients with adenoid cystic carcinoma.
| | HPV-DNA
detection | | p16 expression | | Ki-67
expression | | p53 expression | | EGFR
expression | |
|---|
| |
| |
| |
| |
| |
| |
|---|
| Subgroup | n (%) | Pos (%) | Neg (%) | P-value | Pos (%) | Neg (%) | P-value | ++/+++ (%) | + (%) | Undefined (%) | P-value | Pos (%) | Neg (%) | P-value | Pos (%) | Neg (%) | P-value |
|---|
| Gender | | | | | | | | | | | | | | | | | |
| Female | 44 (66) | 19 (68) | 25 (64) | 0.80 | 27 (69) | 17 (61) | 0.60 | 16 (64) | 27 (68) | 1 (50) | 0.79 | 19 (56) | 25 (76) | 0.12 | 25 (66) | 19 (66) | 1.00 |
| Male | 23 (34) | 9 (32) | 14 (36) | | 12 (31) | 11 (39) | | 9 (36) | 13 (32) | 1 (50) | | 15 (44) | 8 (24) | | 13 (34) | 10 (34) | |
| Age (years) | | | | | | | | | | | | | | | | | |
| ≤60 | 32 (48) | 10 (36) | 22 (56) | 0.14 | 16 (41) | 16 (57) | 0.22 | 10 (40) | 21 (53) | 1 (50) | 0.44 | 13 (38) | 19 (58) | 0.14 | 17 (45) | 15 (52) | 0.63 |
| >60 | 35 (52) | 18 (64) | 17 (44) | | 23 (59) | 12 (43) | | 15 (60) | 19 (47) | 1 (50) | | 21 (62) | 14 (42) | | 21 (55) | 14 (48) | |
| Primary site | | | | | | | | | | | | | | | | | |
| Parotid | 17 (25) | 10 (36) | 7 (18) | 0.13 | 11 (28) | 6 (21.5) | 0.43 | 5 (20) | 12 (30) | 0 (0) | 0.56 | 5 (15) | 12 (36) | 0.12 | 8 (21) | 9 (31) | 0.65 |
| Submandibular | 10 (15) | 2 (7) | 8 (21) | | 4 (10) | 6 (21.5) | | 5 (20) | 5 (13) | 0 (0) | | 6 (18) | 4 (12) | | 6 (16) | 4 (14) | |
| Minor salivary
gland | 40 (60) | 16 (57) | 24 (62) | | 24 (62) | 16 (57) | | 15 (60) | 23 (57) | 2 (100) | | 23 (67) | 17 (52) | | 24 (63) | 16 (55) | |
| Tumor stage | | | | | | | | | | | | | | | | | |
| T1–2 | 21 (31) | 9 (32) | 12 (31) | 1.0 | 12 (31) | 9 (32) | 1.00 | 7 (28) | 13 (33) | 1 (50) | 0.78 | 8 (24) | 13 (39) | 0.18 | 14 (37) | 7 (24) | 0.30 |
| T3–4 | 44 (66) | 18 (64) | 26 (67) | | 25 (64) | 19 (68) | | 18 (82) | 25 (63) | 1 (50) | | 26 (76) | 18 (55) | | 23 (61) | 21 (72) | |
| Tx | 2 (3) | 1 (4) | 1 (2) | | 2 (5) | 0 (0) | | 0 (0) | 2 (4) | 0 (0) | | 0 (0) | 2 (6) | | 1 (2) | 1 (3) | |
| Lymph node
metastasis | | | | | | | | | | | | | | | | | |
| N0 | 57 (85) | 25 (89) | 32 (82) | 0.45 | 32 (82) | 25 (89) | 1.00 | 20 (80) | 35 (87) | 2 (100) | 0.25 | 30 (88) | 27 (82) | 1.00 | 35 (92) | 22 (76) | 0.07 |
| N1–2 | 8 (12) | 2 (7) | 6 (15) | | 5 (13) | 3 (11) | | 5 (20) | 3 (8) | 0 (0) | | 4 (12) | 4 (12) | | 2 (5) | 6 (20) | |
| Nx | 2 (3) | 1 (4) | 1 (3) | | 2 (5) | 0 (0) | | 0 (0) | 2 (5) | 0 (0) | | 0 (0) | 2 (6) | | 1 (3) | 1 (3) | |
| M-stage | | | | | | | | | | | | | | | | | |
| M0 | 59 (88) | 23 (82) | 36 (92) | 0.22 | 34 (87) | 25 (89) | 1.00 | 19 (76) | 38 (95) | 2 (100) | 0.03 | 30 (88) | 29 (88) | 0.67 | 32 (84) | 27 (94) | 0.22 |
| M1 | 6 (9) | 4 (6) | 2 (5) | | 3 (8) | 3 (11) | | 5 (20) | 1 (3) | 0 (0) | | 4 (12) | 2 (6) | | 5 (13) | 1 (3) | |
| Mx | 2 (3) | 1 (10) | 1 (3) | | 2 (5) | 0 (0) | | 1 (4) | 1 (2) | 0 (0) | | 0 (0) | 2 (6) | | 1 (3) | 1 (3) | |
| Resection margins
(primary) | | | | | | | | | | | | | | | | | |
| R0 | 31 (46) | 12 (43) | 19 (49) | 0.80 | 18 (46) | 13 (46) | 1.00 | 14 (56) | 16 (40) | 1 (50) | 0.31 | 17 (50) | 14 (42) | 0.63 | 16 (42) | 15 (52) | 0.47 |
| R1–2 | 36 (54) | 16 (57) | 20 (51) | | 21 (54) | 15 (54) | | 11 (44) | 24 (60) | 1 (50) | | 17 (50) | 19 (58) | | 22 (58) | 14 (48) | |
| Treatment
modality | | | | | | | | | | | | | | | | | |
| Surgery only | 23 (34) | 8 (29) | 15 (38) | 0.64 | 13 (33) | 10 (36) | 0.94 | 7 (28) | 15 (38) | 1 (50) | 0.48 | 9 (26) | 14 (42) | 0.37 | 12 (32) | 11(38) | 0.83 |
| Surgery + RT | 41 (62) | 19 (67) | 22 (56) | | 24 (62) | 17 (68) | | 16 (64) | 24 (60) | 1 (50) | | 23 (68) | 18 (55) | | 24 (63) | 17 (59) | |
| Surgery + RT +
chemotherapy | 3 (4) | 1 (4) | 2 (6) | | 2 (5) | 1 (4) | | 2 (8) | 1 (2) | 0 (0) | | 2 (6) | 1 (3) | | 2 (5) | 1 (3) | |
| Recurrence | | | | 0.62 | | | 0.45 | | | | 1.0 | | | 0.14 | | | 0.04 |
| No | 41 (61) | 16 (57) | 25 (37) | | 22 (56) | 19 (68) | | 15 (60) | 25 (63) | 1 (50) | | 24 (71) | 17 (52) | | 19 (50) | 22 (76) | |
| Yes | 26 (39) | 12 (43) | 14 (36) | | 17 (41) | 9 (32) | | 10 (40) | 15 (37) | 1 (50) | | 10 (29) | 16 (48) | | 19 (50) | 7 (24) | |
HPV status
HPV-DNA positivity was identified in 28 (42%) of 67
patients in this study cohort of SACC patients (Table II). Subtypes identified were HPV
16, 18, 11, 33, 45, 56 and 59. Frequency was as follows: 17 cases
were HPV-16 positive, 4 cases were HPV 18-positive, 3 cases were
HPV 11-positive, 2 cases were co-positive for HPV 33 and 59, 1 case
was positive for HPV 45, 1 case was co-positive for HPV 45 and 56
(Table II).
 | Table IIThe presence of human papillomavirus
subtypes detected in samples from patients with adenoid cystic
carcinoma. |
Table II
The presence of human papillomavirus
subtypes detected in samples from patients with adenoid cystic
carcinoma.
| n | HPV-neg n (%) | HPV 16 n (%) | HPV 18 n (%) | HPV 11a n (%) | HPV 33, 59b n (%) | HPV 45, 56 n
(%) | HPV 45 n (%) |
|---|
| Primary site |
| Parotid | 17 | 7 (17.9) | 6 (37.5) | 3 (75) | 0 (0) | 0 (0) | 0 (0) | 1 (50) |
| Submandibular | 10 | 8 (20.5) | 1 (6.2) | 1 (25) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Minor salivary
gland | 40 | 24 (61.5) | 9 (56.3) | 0 (0) | 3 (100) | 2 (100) | 1 (100) | 1 (50) |
No significant associations were observed between
HPV-DNA status and clinicopathological parameters (Table I). Ten of 17 (59%) patients were
HPV-positive in parotid, 2 of 8 (25%) patients were HPV-positive in
submandibular and 16 of 40 (40%) patients were HPV-positive in the
minor salivary glands. There was no significant relation of HPV
infections with the tumor sites.
IHC was performed to detect p16INK4a
protein and its cellular localization. Overall positivity of
p16INK4a was 58%. The correlation between positive
HPV-DNA status and p16 expression in total specimens was highly
significant (P<0.001) (Table
III). A total of 89% of HPV-DNA-positive primary tumors
co-expressed p16 and 64% of HPV-DNA negative tumors were
p16-negative. However, 11% did not co-express HR-HPV and p16.
Positive p16-status has a specificity of 89% for HPV-DNA positivity
in this study. Remarkably, in tumors of three patients low-risk HPV
11 was present as the only type detected.
 | Table IIICorrelation between HPV-DNA detection
with p16, Ki-67, p53 and EGFR expression. |
Table III
Correlation between HPV-DNA detection
with p16, Ki-67, p53 and EGFR expression.
| Variables | Total
(n=67)
n (%) | HPV-positive
(n=28)
n (%) | HPV-negative
(n=39)
n (%) | P-value |
|---|
| p16 expression | | | | <0.001 |
| Negative | 28 (42) | 3 (11) | 25 (64) | |
| Positive | 39 (58) | 25 (89) | 14 (36) | |
| Ki-67
expression | | | | 0.539 |
| 1 | 40 (60) | 18 (64) | 22 (56) | |
| 2 | 17 (25) | 8 (29) | 9 (23) | |
| 3 | 8 (12) | 2 (7) | 6 (15) | |
| Undefined | 2 (3) | 0 (0) | 2 (5) | |
| p53 expression | | | | 0.258 |
| 0 | 33 (49) | 11 (39) | 22 (56) | |
| 1 | 6 (9) | 2 (7) | 4 (10) | |
| 2 | 6 (9) | 2 (7) | 4 (10) | |
| 3 | 22 (33) | 13 (47) | 9 (24) | |
| EGFR
expression |
| 0 | 29 (43) | 12 (43) | 17 (44) | 0.99 |
| 1 | 13 (20) | 5 (18) | 8 (21) | |
| 2 | 7 (10) | 3 (11) | 4 (10) | |
| 3 | 18 (27) | 8 (29) | 10 (25) | |
Expression of Ki-67, p53 and EGFR in
SACC
Forty (60%) of 67 specimens weakly expressed Ki-67,
while 27 (40%) cases showed moderate or strong expression. Higher
expression of Ki-67 was correlated with M1 stage (P=0.03).
Thirty-four out of 67 (51%) specimens stained p53-positive.
However, p53 expression did not show any correlation with
clinicopathological parameters (Table
I). Thirty-eight (57%) of 67 specimens were EGFR positive.
EGFR-negative patients with SACC have shown a lower rate of
recurrence (Table I).
The correlation of HR-HPV-DNA positivity and Ki-67,
p53 and EGFR expression were further analyzed (Table III). No significant relation was
found.
Factors affecting survival
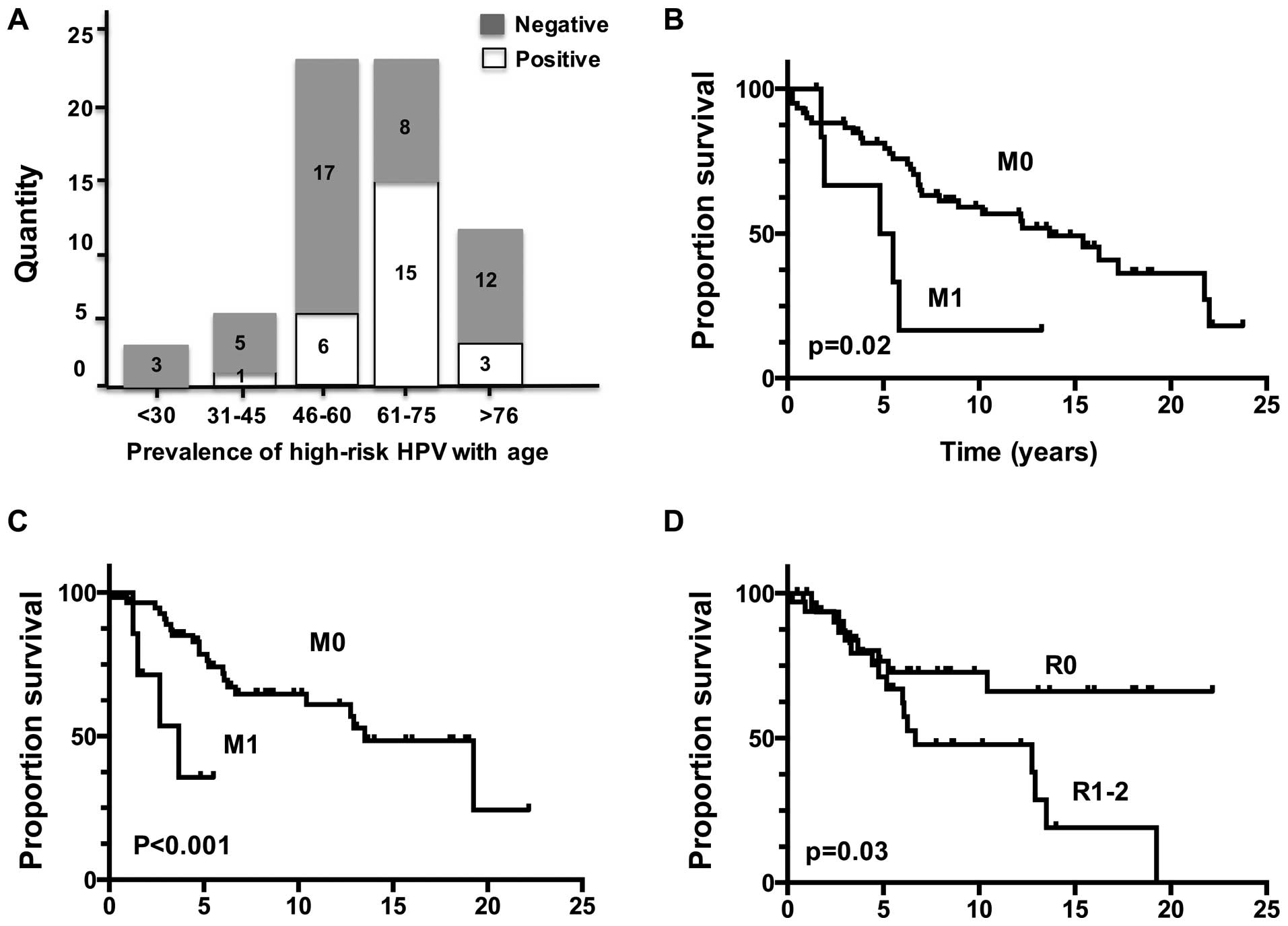
Kaplan-Meier survival estimations indicated that
SACC patients with M1 stage (P=0.02) had a reduced OS (Fig. 1). From the Kaplan-Meier analysis of
the recurrence-free survival, we found that SACC patients with M1
stage (P<0.001) and positive resection margins (P=0.03) had an
increased recurrence rate.
Univariate and multivariate analyses of the
association of clinical and demographic variables with overall
survival of SACC patients are presented in Table IV. Univariate analyses for SACC
showed that the status of metastases was significantly associated
with poor survival of SACC patients (P=0.028). Multivariate
analyses for SACC showed that the status of metastases (P=0.033)
and p16 expression (P=0.005) was an independent prognostic
factor.
 | Table IVUnivariate and multivariate analysis
for factors with potential influence on overall survival in 67
patients with adenoid cystic carcinoma. |
Table IV
Univariate and multivariate analysis
for factors with potential influence on overall survival in 67
patients with adenoid cystic carcinoma.
| Univariate | Multivariate |
|---|
|
|
|
|---|
| Hazard ratio | P-value | Hazard ratio | 95% CI | P-value |
|---|
| Tumor stage (pT 3–4
vs. pT 1–2) | 1.122 | 0.477 | 0.8 | 0.377–1.899 | 0.865 |
| Lymph node
metastasis (pN 2–3 vs. pN 0–1) | 1.088 | 0.769 | 1.196 | 0.406–3.520 | 0.746 |
| Metastases
(positive vs. negative) | 2.977 | 0.028 | 3.973 | 1.121–14.083 | 0.033 |
| HPV (positive vs.
negative) | 1.164 | 0.652 | 1.606 | 0.694–3.715 | 0.268 |
| p16 (positive vs.
negative) | 0.657 | 0.207 | 0.286 | 0.119–0.685 | 0.005 |
| Ki-67 (+ vs.
++/+++) | 1.366 | 0.354 | 1.318 | 0.591–2.937 | 0.5 |
| p53 (positive vs.
negative) | 1.495 | 0.233 | 1.869 | 0.841–4.155 | 0.125 |
| EGFR (positive vs.
negative) | 0.691 | 0.221 | 0.535 | 0.245–1.171 | 0.118 |
| Resection (R1–2 vs.
R0) | 1.345 | 0.378 | 1.928 | 0.828–4.487 | 0.128 |
Treatment
modality
Surgery only
Surgery + RT
Surgery + RT + chemotherapy | 1.025 | 0.933 | 0.763 | 0.370–1.574 | 0.465 |
Univariate analysis showed that both M1 stage
(P=0.001) and positive resection margins (R1–2) (P=0.03) were
significantly associated with a local recurrence risk of SACC
patients (Table V). However,
multivariate analyses for SACC showed that M1 stage (P=0.002),
negative HPV status (P=0.041) and the presence of a positive
resection margin (R1–2) (P=0.012) were independent factors for the
risk of local recurrence. High grade of Ki-67 expression has shown
a trend for occurrence of local recurrence (P=0.067).
 | Table VUnivariate and multivariate analysis
for factors with potential impact on recurrence-free survival in 67
patients with adenoid cystic carcinoma. |
Table V
Univariate and multivariate analysis
for factors with potential impact on recurrence-free survival in 67
patients with adenoid cystic carcinoma.
| Univariate | Multivariate |
|---|
|
|
|
|---|
| Hazard ratio | P-value | Hazard ratio | 95% CI | P-value |
|---|
| Tumor stage (pT 3–4
vs. pT 1–2) | 2.420 | 0.062 | 1.781 | 0.585–5.423 | 0.310 |
| Lymph node
metastasis (pN 2–3 vs. pN 0–1) | 1.848 | 0.221 | 1.912 | 0.537–6.810 | 0.317 |
| Metastases
(positive vs. negative) | 1.351 | 0.001 | 1.468 | 1.154–1.867 | 0.002 |
| HPV (positive vs.
negative) | 1.788 | 0.154 | 3.313 | 1.051–10.445 | 0.041 |
| p16 (positive vs.
negative) | 1.314 | 0.513 | 0.955 | 0.282–3.232 | 0.941 |
| Ki-67 (+ vs.
++/+++) | 1.321 | 0.497 | 2.628 | 0.935–7.385 | 0.067 |
| p53 (positive vs.
negative) | 0.593 | 0.211 | 0.515 | 0.190–1.394 | 0.191 |
| EGFR (positive vs.
negative) | 0.931 | 0.670 | 1.291 | 0.869–1.916 | 0.206 |
| Resection (R1–2 vs.
R0) | 2.468 | 0.030 | 4.945 | 1.423–17.182 | 0.010 |
Treatment
modality
Surgery only
Surgery + RT
Surgery + RT + chemotherapy | 1.046 | 0.903 | 0.617 | 0.187–2.030 | 0.426 |
Discussion
The first objective of the present study was to
investigate a potential presence of persisting HPV infections in a
larger number of SACC patients as one subgroup of the most
frequently occurring carcinomas of the salivary glands. The second
aim was to evaluate its potential impact on clinicopathological
parameters, disease recurrence and survival. Twenty-eight of 67
SACC patients with HPV positivity were identified demonstrating a
more diverse HPV type spectrum than seen in HNSCC. Subtypes 16, 18,
11, 33, 45, 46 and 59 were detected. HPV 16 was the most prevalent
subtype seen in 16 patients in accordance to HNSCC. The presence of
HPV 11 and 56 were limited to minor salivary glands. Multiple
infections of HPV 33 and 59 or 45 and 56 were also found in minor
salivary glands. The prevalence of HPV infections was not related
to the tumor sites. The subtype variability as well as its relation
with tumor sites will be of interest for further investigations in
a larger cohort. To date, HPV-related carcinomas of the head and
neck tend to be squamous cell carcinomas. There is very sparse
information on HPV infection in tumors of the major and minor
salivary glands as compared to tumors from other head and neck
regions. Hafed et al (33)
conducted an analysis of HPV types 16 and 18 in 34 salivary gland
neoplasms including 7 SACC. No evidence of HPV infection was found
in the 7 SACC specimen. They used, however, in situ
hybridization which is prone to lower sensitivity, and
subjectivity. Moreover, HR-HPV was not detectable in a study of
primary sinonasal tract and nasopharyngeal adenoid cystic
carcinomas (34). Bishop et
al (17) found that all
specimens were HR-HPV-negative in the 184 salivary gland tumors
that arose outside of the sinonasal tract which included 98 ACCs,
while 8 HR-HPV-related carcinomas with adenoid cystic-like features
were identified that arose in the sinonasal tract. A recent study
reported a negative HPV status in a study cohort of salivary gland
neoplasms by a sensitive and specific PCR assay (16). To the best of our knowledge, this
study is the first to report the evidence of positivity for HPV
infection with both high-risk (HPV 16, 18, 33, 45, 56 and 59) and
low-risk types (HPV 11) in patients with SACC from using a highly
sensitive PCR and a well controlled study material for DNA
integrity.
p16 positivity has been introduced to be a surrogate
marker of HPV infection in cervical and head and neck cancer
(35). In the present study, the
expression of p16-positive IHC and HPV-DNA positivity was
significantly correlated in patients with SACC. However, and in
contrast to HNSCC (23), both
HPV-DNA positivity and p16 expression did not show any correlation
with clinicopathological parameters. As we have seen from our
previous studies (35,36) and others (23,37)
in HNSCC, a certain discrepancy rate of HPV status and p16
expression was observed. In the present study cohort of SACC
patients, the subgroup categories of
HPV+/p16+ patients included 37.3%, of
HPV−/p16− 37.3%, of
HPV−/p16+ 21% and of
HPV+/p16− 4.4% patients. In our recent
meta-analysis, we found a significantly improved 5-year overall
survival for HPV+/p16+ HNSCC, intermediate
for the HPV−/p16+ subgroup and the shortest
survival in HPV+/p16− and
HPV−/p16− HNSCC (23). Therefore, a p16 IHC followed by HPV
detection is suggested for subgroup classification referring to
prognosis and therapy strategies. In the present study, no
significant differences were found when we performed further
analysis on these subgroups in relation to clinicopathological
parameters and outcome in SACC patients (data not shown).
Multivariate analysis has shown that p16 positivity was an
independent marker (Table IV)
indicating a better survival in patients with SACC, while a
presence of HPV-DNA was related to a better recurrence-free
survival. This overlap, however, may be due to the limited size of
the study cohort.
The two viral oncogenes promoting tumor progression
are the HR-HPV derived E6 and E7 genes. E6 protein inactivates p53
thereby inhibiting apoptosis, while E7 protein activates the cell
cycle by inhibiting the tumor-suppressor-protein pRb complex with
E2F. This results in liberation of the transcription factor E2F and
by a positive feedback loop in upregulation of p16 (38). Hence, p16 can be used as a
surrogate marker for HPV-related cancer. However, HPV-independent
pathways of oncogenesis can also lead to an overexpression of p16.
The cellular function of p16 has been recently identified. Knock
out of p16 in HPV E7 expressing cells can lead to induction of
apoptosis showing a physiological role in transformation despite
its original role in the process of senescence (39–42).
In another study inactivation of the p16 promotor by methylation
has been shown (43). These
findings suggested the multiple roles of p16 in carcinogenesis of
malignancies in addition to HPV-related pathogenesis, explaining
the discrepancy of HPV-DNA and p16 expression (16,23).
Further laboratory and clinical studies should be designed with the
aim to elucidate the underlying biological cause of the distinct
HPV/p16 pattern.
To date, there is no obvious improvement of survival
over time which has been reviewed in a large European study on SACC
of the head and neck with a 65% 10-year survival rate (44). In the present study, the overall
survival rate of SACC patients was 68%. The potential importance of
measurements of certain biomarkers and clinicopathological
parameters with impact on disease recurrence and survival are
underscored in this study. We found a significantly higher
expression of EGFR but not p53 in SACC patients developing
recurrence. Despite this finding EGFR expression did not show a
significant correlation to survival in SACC patients. In HNSCC,
anti-EGFR antibodies such as cetuximab are approved for treatment.
However, the addition of cetuximab to radiation or chemo-radiation
has resulted in limited benefits to date for the majority of
patients with head and neck cancer. Furthermore, EGFR expression
levels have not consistently predicted clinical responses to
cetuximab. The exact role of EGFR in HNSCC is still debated. As a
measure for a high proliferative index, a higher Ki-67
immunoreactivity has been reported to be related to a poor survival
in SGC (27). Our data confirmed
its potential prognostic value in the investigated patient cohort
with SACC. In addition, there was an association between higher
Ki-67 expression and metastases in SACC patients. In HNSCC,
HPV-negative tumors appear to be enriched for EGFR and TP53
mutations (25). Herein, there
were no correlations of HPV status with p53, EGFR and Ki-67
expression in SACC. Nevertheless, investigations beyond this
initial study that incorporate larger numbers of patients with SACC
are still necessary to further validate these data.
The standard combination treatment for SACC is
radical surgical resection and postoperative radiotherapy. In this
study cohort, the treatment modality did not achieve significant
impact on survival. Patients with tumor-free resection margins, as
expected, had a better recurrence-free survival. SACC will easily
infiltrate into adjacent tissues and local recurrences occur
despite the above treatment. Therefore, a complete resection with
free margin should be achieved wherever possible to reduce the rate
of recurrence.
Taken together, our results underscore
stratification factors of potential clinical value. Positive
resection margins were observed to be related to a disease
recurrence despite radiotherapy. The development of distant
metastases continues to determine the treatment outcome and
predicts a poor survival. In accordance to other studies, higher
grade of Ki-67 positivity, representative for a more proliferative
tumor, indicates a poorer survival. Most importantly, our results
presented for the first time that HPV infection is prevalent in
ACCs. Although there were no significant correlations between HPV
status and other clinicopathological parameters for survival, the
importance of measuring the presence of HPV, and of expression of
p53 and EGFR for patient stratification is still of interest to
reveal associations of molecularly defined subgroups of SACC. Our
findings suggest that p16 positivity could be used as an
independent biomarker in predicting prognosis of SACC. Hopefully,
the knowledge derived from this study and others will provide new
insights into the multiple questions still open in relation to the
etiology and carcinogenesis of SACC and treatment strategies.
Acknowledgements
We are grateful to Berliner Krebsgesellschaft e.V.
for financial support. The expert technical assistance by Ursula
Schiller and Heidrun Wolter is gratefully acknowledged.
References
|
1
|
Carvalho AL, Nishimoto IN, Califano JA and
Kowalski LP: Trends in incidence and prognosis for head and neck
cancer in the United States: a site-specific analysis of the SEER
database. International journal of cancer Int J Cancer.
114:806–816. 2005. View Article : Google Scholar
|
|
2
|
Bjørndal K, Krogdahl A, Therkildsen MH,
Overgaard J, Johansen J, Kristensen CA, Homøe P, Sørensen CH,
Andersen E, Bundgaard T, et al: Salivary gland carcinoma in Denmark
1990–2005: A national study of incidence, site and histology.
Results of the Danish Head and Neck Cancer Group (DAHANCA). Oral
Oncol. 47:677–682. 2011. View Article : Google Scholar
|
|
3
|
Barnes LEJ, Reichart P and Sidransky D:
World Health Organization Classification of Tumours. Pathology and
Genetics, Head and Neck Tumours. IARC Press; Lyon: 2005
|
|
4
|
Bell RB, Dierks EJ, Homer L and Potter BE:
Management and outcome of patients with malignant salivary gland
tumors. J Oral Maxillofac Surg. 63:917–928. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Buchner A, Merrell PW and Carpenter WM:
Relative frequency of intra-oral minor salivary gland tumors: A
study of 380 cases from northern California and comparison to
reports from other parts of the world. J Oral Pathol Med.
36:207–214. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Terhaard C1, Lubsen H, Van der Tweel I,
Hilgers FJ, Eijkenboom WM, Marres HA, Tjho-Heslinga RE, de Jong JM
and Roodenburg JL; Dutch Head and Neck Oncology Cooperative Group.
Salivary gland carcinoma: independent prognostic factors for
locoregional control, distant metastases, and overall survival:
results of the Dutch head and neck oncology cooperative group. Head
Neck. 26:681–692; discussion 692–683. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Whatley WS, Thompson JW and Rao B:
Salivary gland tumors in survivors of childhood cancer. Otolaryngol
Head Neck Surg. 134:385–388. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zheng R, Wang LE, Bondy ML, Wei Q and
Sturgis EM: Gamma radiation sensitivity and risk of malignant and
benign salivary gland tumors: A pilot case-control analysis.
Cancer. 100:561–567. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Licitra L, Grandi C, Prott FJ, Schornagel
JH, Bruzzi P and Molinari R: Major and minor salivary glands
tumours. Crit Rev Oncol Hematol. 45:215–225. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Klussmann JP, Müller A, Wagner M,
Guntinas-Lichius O, Jungehuelsing M, Sloots T, Ablashi DV and
Krueger GR: Human herpesvirus type 8 in salivary gland tumors. J
Clin Virol. 16:239–246. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Herrero R, Castellsagué X, Pawlita M,
Lissowska J, Kee F, Balaram P, Rajkumar T, Sridhar H, Rose B,
Pintos J, et al; IARC Multicenter Oral Cancer Study Group. Human
papillomavirus and oral cancer: The International Agency for
Research on Cancer multicenter study. J Natl Cancer Inst.
95:1772–1783. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Leemans CR, Braakhuis BJ and Brakenhoff
RH: The molecular biology of head and neck cancer. Nat Rev Cancer.
11:9–22. 2011. View
Article : Google Scholar
|
|
13
|
Khan MJ, Castle PE, Lorincz AT, Wacholder
S, Sherman M, Scott DR, Rush BB, Glass AG and Schiffman M: The
elevated 10-year risk of cervical precancer and cancer in women
with human papillomavirus (HPV) type 16 or 18 and the possible
utility of type-specific HPV testing in clinical practice. J Natl
Cancer Inst. 97:1072–1079. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vageli D, Sourvinos G, Ioannou M,
Koukoulis GK and Spandidos DA: High-risk human papillomavirus (HPV)
in parotid lesions. Int J Biol Markers. 22:239–244. 2007.PubMed/NCBI
|
|
15
|
Lin FC, Chen PL, Tsao TY, Li CR, Jeng KC
and Tsai SC: Prevalence of human papillomavirus and Epstein-Barr
virus in salivary gland diseases. J Int Med Res. 42:1093–1101.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Senft E, Lemound J, Stucki-Koch A,
Gellrich NC, Kreipe H and Hussein K: Expression of cyclin-dependent
kinase inhibitor 2A 16, tumour protein 53 and epidermal growth
factor receptor in salivary gland carcinomas is not associated with
oncogenic virus infection. Int J Oral Sci. 7:18–22. 2015.
View Article : Google Scholar
|
|
17
|
Bishop JA, Yonescu R, Batista D,
Yemelyanova A, Ha PK and Westra WH: Mucoepidermoid carcinoma does
not harbor transcriptionally active high risk human papillomavirus
even in the absence of the MAML2 translocation. Head Neck Pathol.
8:298–302. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Isayeva T, Said-Al-Naief N, Ren Z, Li R,
Gnepp D and Brandwein-Gensler M: Salivary mucoepidermoid carcinoma:
Demonstration of transcriptionally active human papillomavirus
16/18. Head Neck Pathol. 7:135–148. 2013. View Article : Google Scholar :
|
|
19
|
Isayeva T, Li Y, Maswahu D and
Brandwein-Gensler M: Human papillomavirus in non-oropharyngeal head
and neck cancers: A systematic literature review. Head Neck Pathol.
6(Suppl 1): S104–S120. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Klussmann JP, Gültekin E, Weissenborn SJ,
Wieland U, Dries V, Dienes HP, Eckel HE, Pfister HJ and Fuchs PG:
Expression of p16 protein identifies a distinct entity of tonsillar
carcinomas associated with human papillomavirus. Am J Pathol.
162:747–753. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Reimers N, Kasper HU, Weissenborn SJ,
Stützer H, Preuss SF, Hoffmann TK, Speel EJ, Dienes HP, Pfister HJ,
Guntinas-Lichius O and Klussmann JP: Combined analysis of HPV-DNA,
p16 and EGFR expression to predict prognosis in oropharyngeal
cancer. International journal of cancer Int J Cancer.
120:1731–1738. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fakhry C and Gillison ML: Clinical
implications of human papillomavirus in head and neck cancers. J
Clin Oncol. 24:2606–2611. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Coordes A, Lenz K, Qian X, Lenarz M,
Kaufmann AM and Albers AE: Meta-analysis of survival in patients
with HNSCC discriminates risk depending on combined HPV and p16
status. Eur Arch Otorhinolaryngol. Jul 31–2015.(Epub ahead of
print). View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sepiashvili L, Bruce JP, Huang SH,
O'Sullivan B, Liu FF and Kislinger T: Novel insights into head and
neck cancer using next-generation ‘omic’ technologies. Cancer Res.
75:480–486. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wichmann G1, Rosolowski M, Krohn K, Kreuz
M, Boehm A, Reiche A, Scharrer U, Halama D, Bertolini J, Bauer U,
et al: The role of HPV RNA transcription, immune response-related
gene expression and disruptive TP53 mutations in diagnostic and
prognostic profiling of head and neck cancer. Int J Cancer.
137:2846–2857. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Klussmann JP, Mooren JJ, Lehnen M,
Claessen SM, Stenner M, Huebbers CU, Weissenborn SJ, Wedemeyer I,
Preuss SF, Straetmans JM, et al: Genetic signatures of HPV-related
and unrelated oropharyngeal carcinoma and their prognostic
implications. Clin Cancer Res. 15:1779–1786. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Luukkaa H, Klemi P, Leivo I, Vahlberg T
and Grénman R: Prognostic significance of Ki-67 and p53 as tumor
markers in salivary gland malignancies in Finland: An evaluation of
212 cases. Acta Oncol. 45:669–675. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schmitt M, Bravo IG, Snijders PJ, Gissmann
L, Pawlita M and Waterboer T: Bead-based multiplex genotyping of
human papillomaviruses. J Clin Microbiol. 44:504–512. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schmitt M, Dondog B, Waterboer T and
Pawlita M: Homogeneous amplification of genital human alpha
papillomaviruses by PCR using novel broad-spectrum GP5+
and GP6+ primers. J Clin Microbiol. 46:1050–1059. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Muñoz N, Bosch FX, de Sanjosé S, Herrero
R, Castellsagué X, Shah KV, Snijders PJ and Meijer CJ;
International Agency for Research on Cancer Multicenter Cervical
Cancer Study Group. Epidemiologic classification of human
papillomavirus types associated with cervical cancer. N Engl J Med.
348:518–527. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Van Dongen JJ, Langerak AW, Brüggemann M,
Evans PA, Hummel M, Lavender FL, Delabesse E, Davi F, Schuuring E,
García-Sanz R, et al: Design and standardization of PCR primers and
protocols for detection of clonal immunoglobulin and T-cell
receptor gene recombinations in suspect lymphoproliferations:
Report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia.
17:2257–2317. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Klaes R, Friedrich T, Spitkovsky D, Ridder
R, Rudy W, Petry U, Dallenbach-Hellweg G, Schmidt D and von Knebel
Doeberitz M: Overexpression of p16(INK4A) as a specific marker for
dysplastic and neoplastic epithelial cells of the cervix uteri. Int
J Cancer. 92:276–284. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hafed L, Farag H, Shaker O and El-Rouby D:
Is human papilloma virus associated with salivary gland neoplasms?
An in situ-hybridization study. Arch Oral Biol. 57:1194–1199. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Thompson LD, Penner C, Ho NJ, Foss RD,
Miettinen M, Wieneke JA, Moskaluk CA and Stelow EB: Sinonasal tract
and nasopharyngeal adenoid cystic carcinoma: A clinicopathologic
and immunophenotypic study of 86 cases. Head Neck Pathol. 8:88–109.
2014. View Article : Google Scholar :
|
|
35
|
Qian X, Wagner S, Ma C, Coordes A, Gekeler
J, Klussmann JP, Hummel M, Kaufmann AM and Albers AE: Prognostic
significance of ALDH1A1-positive cancer stem cells in patients with
locally advanced, metastasized head and neck squamous cell
carcinoma. J Cancer Res Clin Oncol. 140:1151–1158. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Qian X, Wagner S, Ma C, Klussmann JP,
Hummel M, Kaufmann AM and Albers AE: ALDH1-positive cancer
stem-like cells are enriched in nodal metastases of oropharyngeal
squamous cell carcinoma independent of HPV status. Oncol Rep.
29:1777–1784. 2013.PubMed/NCBI
|
|
37
|
Robinson M, Sloan P and Shaw R: Refining
the diagnosis of oropharyngeal squamous cell carcinoma using human
papillomavirus testing. Oral Oncol. 46:492–496. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hoffmann TK, Arsov C, Schirlau K, Bas M,
Friebe-Hoffmann U, Klussmann JP, Scheckenbach K, Balz V, Bier H and
Whiteside TL: T cells specific for HPV16 E7 epitopes in patients
with squamous cell carcinoma of the oropharynx. Int J Cancer.
118:1984–1991. 2006. View Article : Google Scholar
|
|
39
|
Ressler S, Bartkova J, Niederegger H,
Bartek J, Scharffetter-Kochanek K, Jansen-Dürr P and Wlaschek M:
p16INK4A is a robust in vivo biomarker of cellular aging in human
skin. Aging Cell. 5:379–389. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
McLaughlin-Drubin ME, Park D and Munger K:
Tumor suppressor p16INK4A is necessary for survival of cervical
carcinoma cell lines. Proc Natl Acad Sci USA. 110:16175–16180.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pauck A, Lener B, Hoell M, Kaiser A,
Kaufmann AM, Zwerschke W and Jansen-Dürr P: Depletion of the cdk
inhibitor p16INK4a differentially affects proliferation of
established cervical carcinoma cells. J Virol. 88:5256–5262. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Vékony H, Röser K, Löning T, Raaphorst FM,
Leemans CR, Van der Waal I and Bloemena E: Deregulated expression
of p16INK4a and p53 pathway members in benign and malignant
myoepithelial tumours of the salivary glands. Histopathology.
53:658–666. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li J, El-Naggar A and Mao L: Promoter
methylation of p16INK4a, RASSF1A, and DAPK is frequent in salivary
adenoid cystic carcinoma. Cancer. 104:771–776. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ciccolallo L, Licitra L, Cantú G, Gatta G
and Group EW; EUROCARE Working Group. Survival from salivary glands
adenoid cystic carcinoma in European populations. Oral Oncol.
45:669–674. 2009. View Article : Google Scholar
|