Introduction
Cancer is the second most common cause of death
among children aged 1–14 years. Leukemia accounts for one-third of
all cancers diagnosed in children, 78% of which is acute
lymphoblastic leukemia (ALL) (1)
and 10% acute myeloid leukemia (2). ALL is characterized by the
uncontrolled production within the bone marrow of hematopoietic
precursor cells of the lymphoid or myeloid series. Nearly 85% of
ALL cases have a B-cell immunophenotype precursor and approximately
15% show a T-cell phenotype (3).
The development of cancer has been associated with
malignant-like cells that express low levels of immunogenic surface
molecules and are poor T-cell stimulators, which facilitates their
escape from cellular antineoplastic immune responses (4). The immune system protects against
invading pathogens and transformed cells, including cancer cells;
the activation of the innate immune system via pattern recognition
receptors such as Toll-like receptors (TLRs) is well established.
TLRs are type I membrane glycoproteins with an extracellular domain
containing leucine-rich repeats, which is required for the
recognition of pathogen-associated molecular patterns and
damage-associated molecular patterns. TLRs also have a cytoplasmic
Toll/interleukin-1 receptor (TIR) domain, required for downstream
signaling (5). The TLR family is
composed of 12 and 10 functional receptors in mice and humans,
respectively. Human TLRs are mainly expressed in immune-related
cells, such as monocytes, neutrophils, macrophages, dendritic cells
(DC), T, B and natural killer (NK) cells (6). The expression or upregulation of TLRs
has been demonstrated in solid tumors and tumor cell lines, but
their expression or role in the pathogenesis and development of
acute leukemia in children remains unclear (7).
The aim of the present study was to evaluate the
expression of TLR1, TLR3, TLR4, TLR7 and TLR9 in peripheral blood
mononuclear cells (PBMCs) from children with acute lymphoblastic
leukemia.
Materials and methods
PBMCs were obtained from 50 pediatric patients
diagnosed with acute lymphoblastic leukemia (ALL) prior to any
treatment. As controls, PBMC samples were obtained from 20 children
attending the ophthalmology and orthopedics services. All
procedures were approved by the Research, Ethics and Biosafety
Committee of the Hospital Infantil de México Federico Gómez
(Registry HIM/2011/022), following the International Guidelines for
Biomedical Research Involving Human Beings (CIOMS-WHO 1993), and
the Ethical Principles for Medical Research Involving Human
Subjects of the World Medical Association and by the National
Committee of Scientific Research. Samples of peripheral blood were
collected after informed consent from the parents. The demographic
and clinical data of the included patients are summarized in
Table I.
 | Table IDemographic and clinical data of
patients with acute lymphoblastic leukemia (ALL) and controls. |
Table I
Demographic and clinical data of
patients with acute lymphoblastic leukemia (ALL) and controls.
| Characteristics | ALL (n=50) | Control (n=20) |
|---|
| Median age | 7 (range, 3 months to
15 years) | 8 (range, 4–15
years) |
| Gender | 23 F/27 M | 9 F/11 M |
| ALL
immunophenotype | Pro-B 6 | |
| Pre-B 23 | |
| B 13 | |
| T 8 | |
| BM blast infiltration
(%) | 70–99 | |
| Risk
stratification | SR 7 | |
| HR 43 | |
Isolation of PBMCs
PBMCs were obtained from peripheral blood collected
according to the international and institutional guidelines from
pediatric patients diagnosed with ALL prior to any treatment or
transfusion; cells were isolated by density gradient with
Lymphoprep™ (Axis-Shield, Oslo, Norway) according to the
manufacturer's instructions.
Immunofluorescence analysis
PBMCs (1×105) from patients with ALL were
dropped onto clean glass coverslips, incubated for 15 min at room
temperature in PBS containing 4% paraformaldehyde, and then washed
in 0.1% PBS/Tween-20. The cells were blocked with Power Block
Universal Blocking reagent (BioGenex, San Ramón, CA, USA) in a
humidified chamber for 10 min. The fixed cells were incubated with
primary antibodies (goat polyclonal IgG anti-TLR1, anti-TLR3,
anti-TLR4, anti-TLR7 or anti-TLR9; Santa Cruz Biotechnology,
Heidelberg, Germany) at room temperature for 1 h. After two washes
with 0.1% PBS/Tween-20, cells were incubated with secondary
antibodies (FITC-coupled goat anti-rabbit IgG (H+L); Jackson
ImmunoResearch, West Grove, PA, USA) for 40 min at room temperature
and then washed twice with 0.1% PBS/Tween-20. Some glass coverslips
with PBMCs were also incubated with 50 nM of
LysoTracker® (Molecular Probes Corp., CA, USA) for 30
min at room temperature and then washed twice with 0.1%
PBS/Tween-20. Nuclei were stained for 20 min at room temperature
with DRAQ7 (Abcam, Cambridge, MA, USA). Finally, cells were washed
twice in PBS/Tween-20. The coverslips were mounted on glass slides
using Vectashield mounting medium (Vector Laboratories, Burlingame,
CA, USA) and dried overnight in the dark.
Confocal microscopy analysis
Cells were visualized using an Axiovert 100M LSM5
confocal microscope (Carl Zeiss, Jena, Germany); with a ×63 oil
objective. Briefly, 10 micrographs were captured at ×40 using a
zoom of 1.5, 488 and 543 nm lasers, and BP 505–550 nm and LP 650-nm
filters. In each experiment, images from different samples were
taken consecutively using identical settings. Images were analyzed
using ImageJ software. The results were expressed as medians of the
media of the fluorescence intensity (MFI).
Statistical analysis
Prism software (version 5.01; Graphpad Software
Inc., La Jolla, CA, USA) was used for statistical analysis. The
comparison between groups was performed by comparing medians with
the Mann-Whitney U test. The differences between phenotypes were
analyzed using the Kruskal-Wallis test. P-values <0.05 were
considered significant for both tests.
Results
Expression of TLR1 and TLR4
The patients with ALL comprised 23 girls and 27
boys; the control children were 9 girls and 11 boys. The average
age of the patients with ALL was 7±4 years (range, 3 months to 15
years), and 8±4 years for the controls (range, 4–15 years). The
expression of TLRs in PBMCs from all patients and controls was
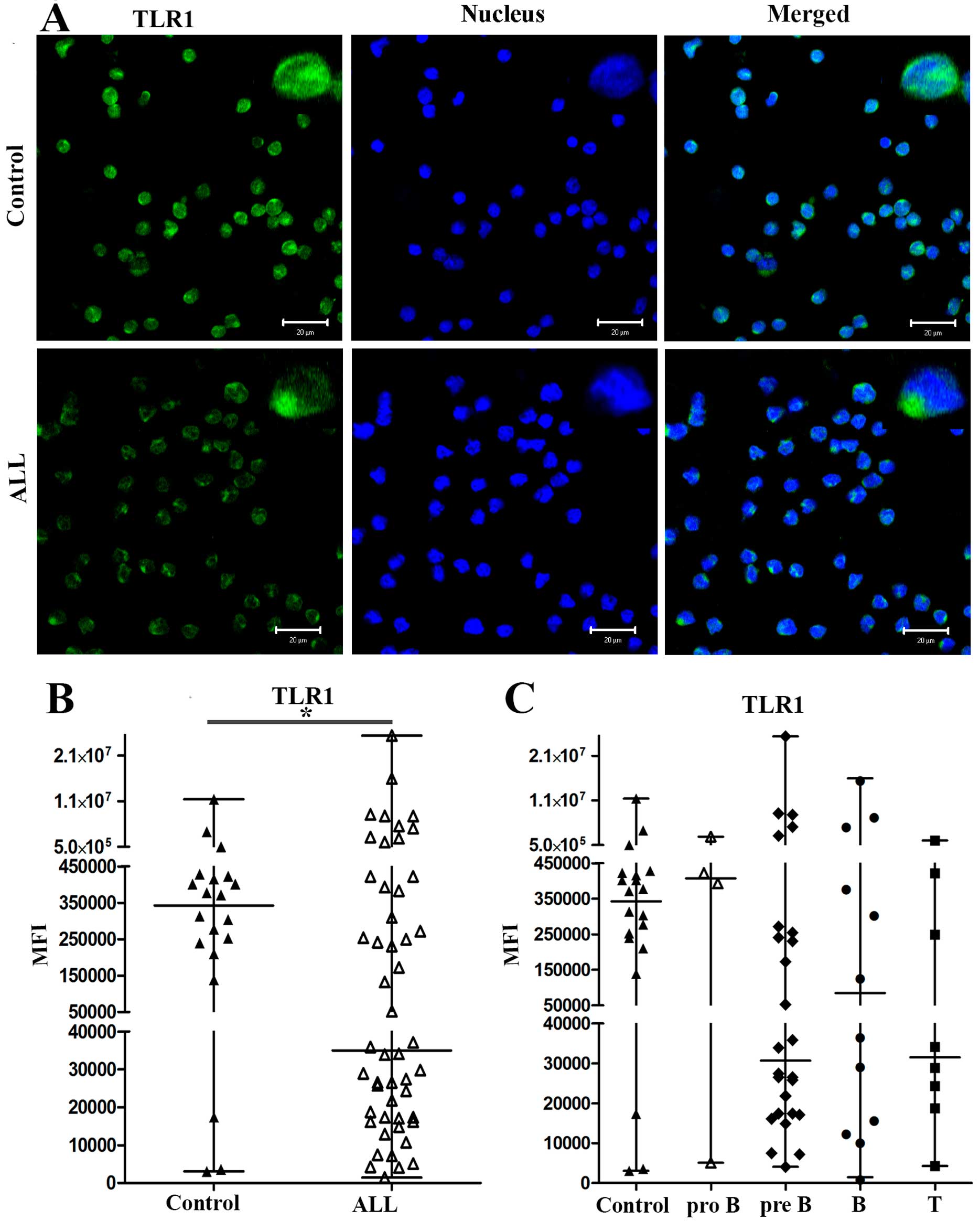
observed by immunofluorescence. Expression of TLR1 for one
representative control and for one representative patient with ALL
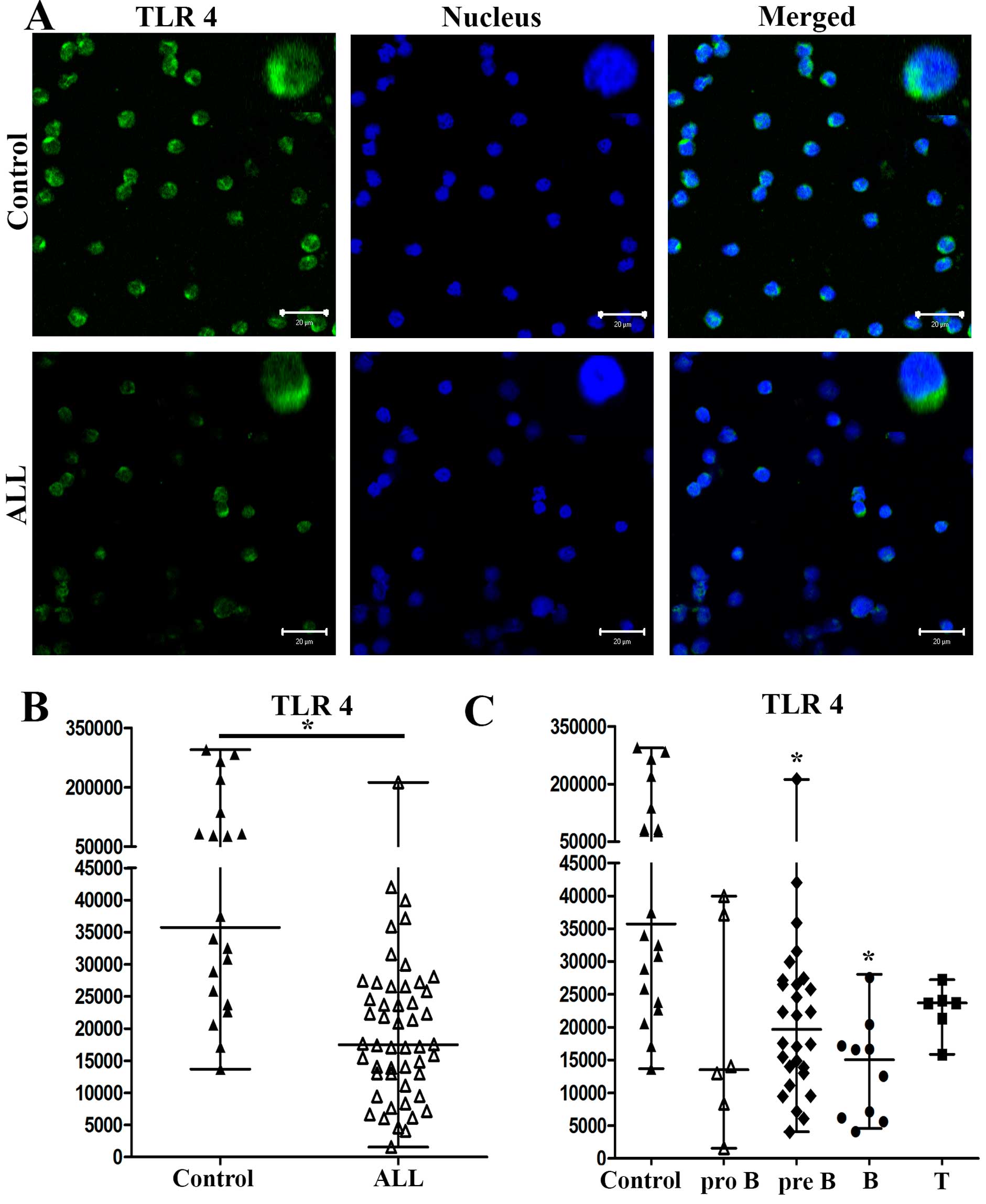
is shown in Fig. 1A; TLR4
expression of one representative control and for one representative
patient with ALL is shown in Fig.
2A. In patients with ALL, TLR1 and TLR4 expression was lower
than in control patients. The median values of the mean
fluorescence intensity (MFI) for TLR1 in cells from 50 patients
with ALL and 20 controls were 35,017 and 342,460, respectively
(P<0.0349) (Fig. 1B). The
medians of the MFI for TLR4 in cells from patients with ALL and
controls were 17,478 and 35,745, respectively (P<0.0001)
(Fig. 2B).
When we analyzed the expression levels of TLR1 and
TLR4 in PBMCs from patients with different subtypes of ALL (pro-B,
pre-B, B and T), we found that the median values of the MFI for
TLR1 in pro-B, pre-B, B and T ALL were 407,631, 30,676, 84,650 and
31,531, respectively, and 342,460 for controls (Fig. 1C). The medians of the MFI for TLR4
from patients with pro-B, pre-B, B and T ALL were 13,520, 19,696,
15,067 and 23,680, respectively and 35,745 for controls (Fig. 2C). The differences in TLR4
expression were only significant between ALL pre-B and B subtypes
compared with controls (P<0.0002).
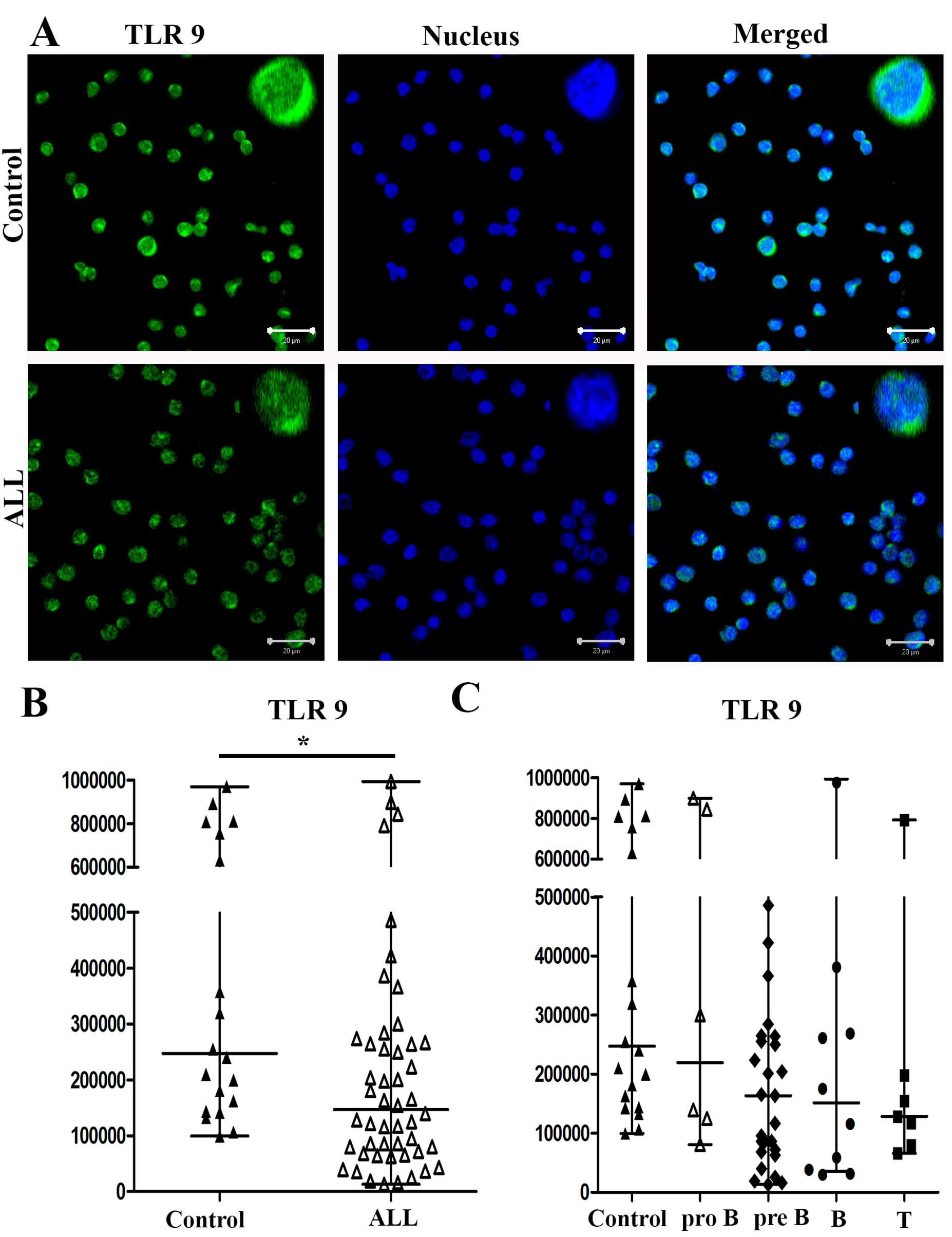
Expression of TLR3, TLR7 and TLR9
By immunofluorescence, we observed different
expression levels of TLR3, TLR7 and TLR9 in PBMCs from patients
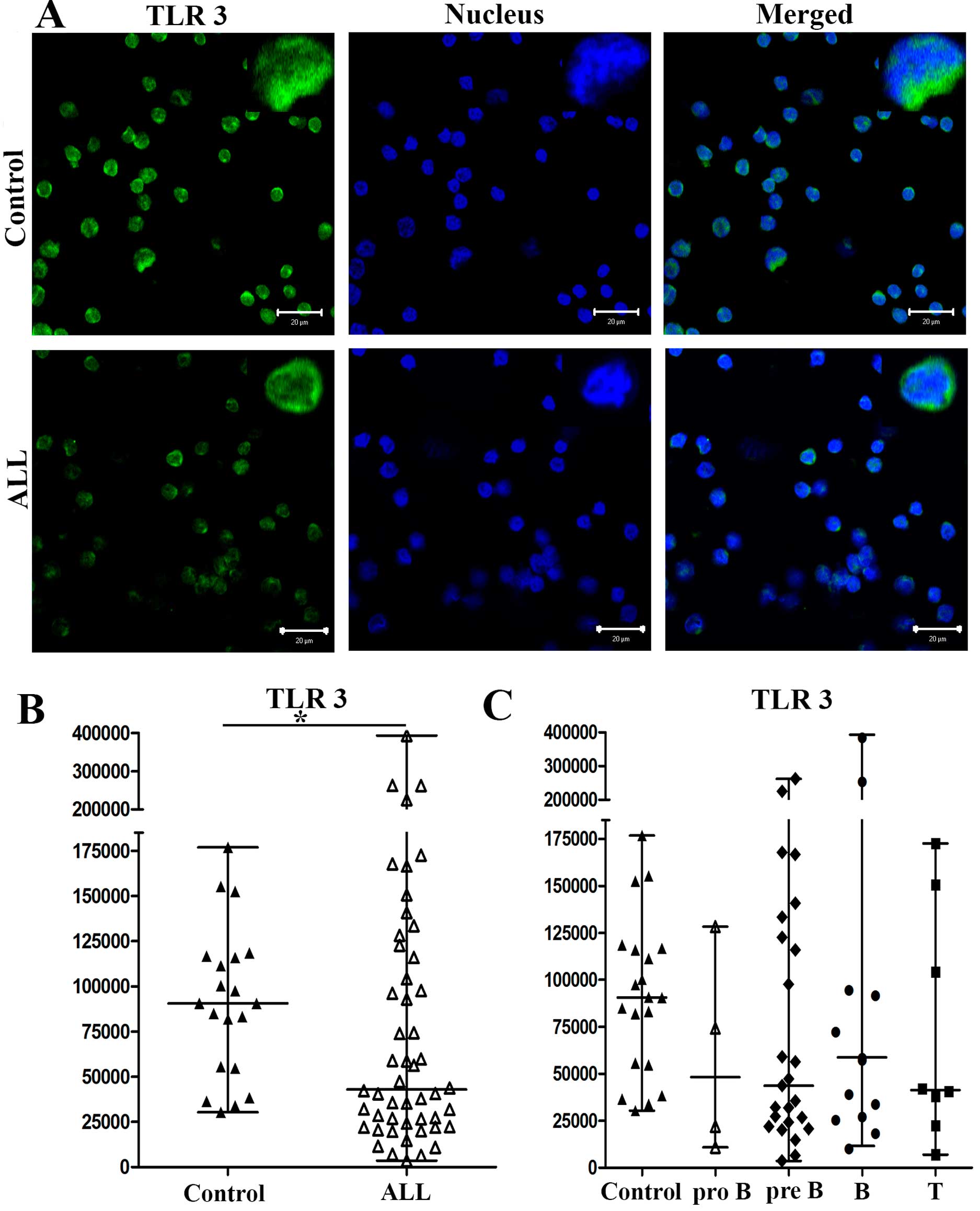
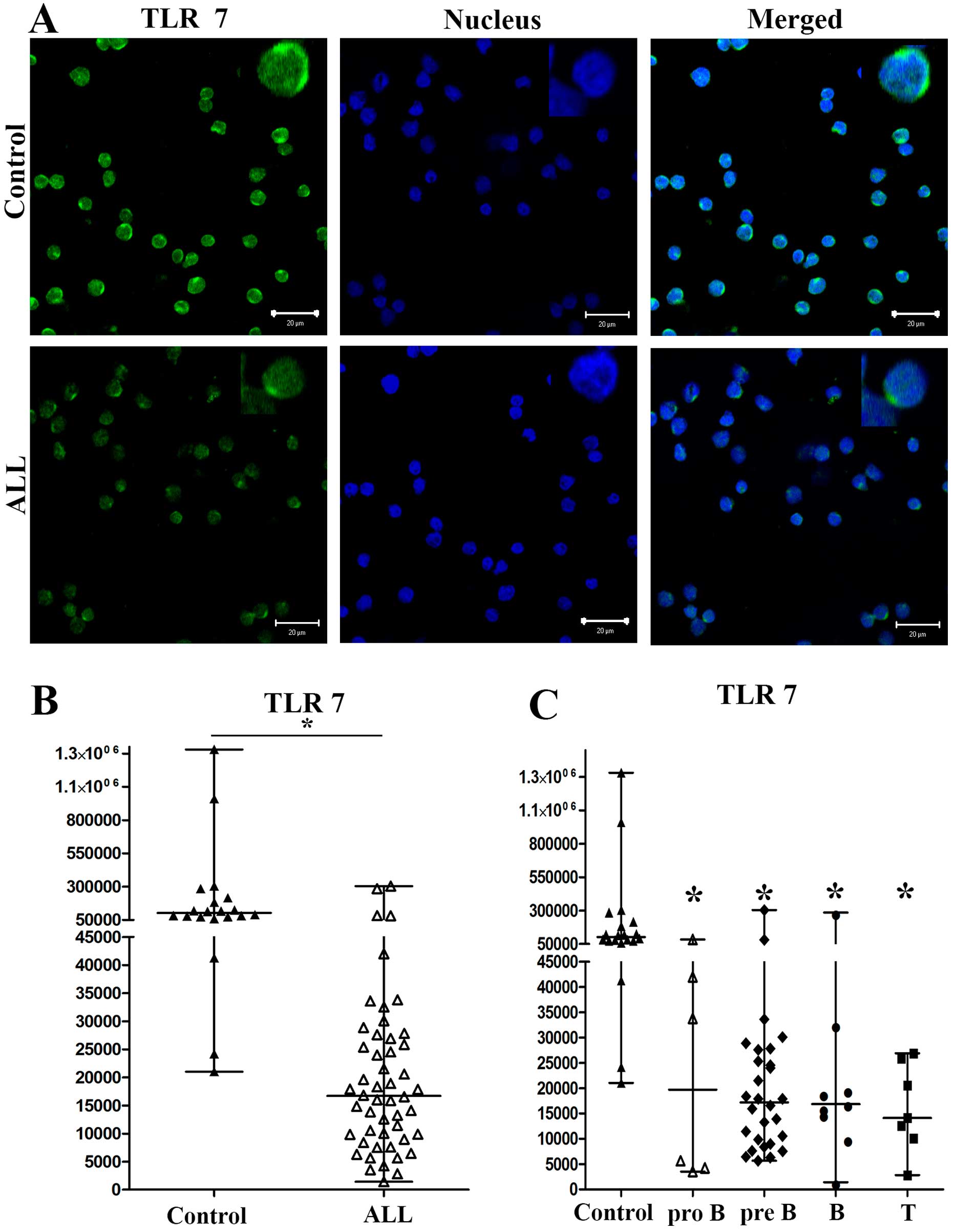
with ALL and controls. In Figs.
3Figure 4–5, we show the results for a
representative control and of a representative patient with ALL
(Figs. 3A, 4A and 5A, respectively). The medians of the MFI
for TLR3 on cells from patients with ALL and controls were 42,900
and 90,551, respectively (P<0.0252) (Fig. 3B). The medians of the MFI for TLR7
on cells from patients with ALL and controls were 16,696 and
102,557, respectively (P<0.0001) (Fig. 4B). Finally, the medians of the MFI
for TLR9 on cells from patients with ALL and controls were 147,157
and 247,630, respectively (P<0.0038) (Fig. 5B).
When we analyzed the expression levels of TLR3, TLR7
and TLR9 in PBMCs from patients with pro-B, pre-B, B and T ALL, the
medians of the MFI for TLR3 from patients with pro-B, pre-B, B and
T ALL were 48,160, 43,674, 58,708 and 41,323, respectively, and
90,551 for controls (Fig. 3C). The
medians of the MFI for TLR7 from pro-B, pre-B, B and T ALL were
19,732, 17,213, 16,841 and 14,127, respectively, and 102,557 for
controls (Fig. 4C). The medians of
the MFI for TLR9 from patients with pro-B, pre-B, B and T ALL were
219,857, 163,306, 151,555 and 128,391, respectively, and 247,630
for controls (Fig. 5C). The
differences in TLR7 expression between ALL subtypes pro-B, pre-B, B
and T compared with controls were all significant
(P<0.0001).
When we used LysoTracker, a fluorescent probe for
lysosomes, we did not find TLR1 and TLR4 in lysosomes of PBMCs from
patients with ALL or controls. However, TLR3, TLR7 and TLR9 were
present in lysosomes of PBMCs from both patients with ALL and
controls (data no shown).
Discussion
In the present study, we report reduced expression
of TLR1, TLR3, TLR4, TLR7 and TLR9 in PBMCs from patients with ALL
compared with controls. We observed substantial heterogeneity in
the level and type of TLR expression in the patients with ALL. Our
results also showed that Pro-B, Pre-B, B and T ALL phenotypes
presented the lowest levels of TLR7 expression while Pre-B and B
ALL phenotypes presented the lowest levels of TLR4 expression.
There were no significant differences for the other TLRs and
leukemia phenotypes, possibly because there was a broad
distribution of expression and only a small number of patients with
each ALL phenotype. We observed TLR3, TLR7 and TLR9 but not TLR1
and TLR4 in lysosomes of PBMCs from ALL patients and controls.
Studies have analyzed the expression of TLRs in
tonsil tissues (8), autoimmune
diseases (9–11), in solid cancers (12), hematological cancers such as
multiple myeloma and chronic myeloid leukemia (CML) (13,14),
and in murine models (15,16), cell lines (17) and PBMCs from CML patients (18). However, this is the first study to
analyze the expression level of TLRs in PBMCs from pediatric
patients with ALL.
One study demonstrated TLR1 and TLR6 mRNA expression
in all cell types of PBMCs in adult humans by quantitative RT-PCR;
this group also detected high expression of TLR7 mRNA and TLR9 mRNA
in DCs and marked levels of TLR2 mRNA in monocytes (19). Naïve B cells expressed low to
undetectable levels of TLR1, TLR2 and TLR4, while memory B cells
expressed constitutively high levels of TLR6, TLR7, TLR10 and
especially TLR9 (18). Another
study found high expression levels of TLR1, TLR2, TLR7, TLR9 and
TLR10 in germinal center and memory B cells in tonsils, while TLR9
levels were lower in circulating blood B cells (8). Other studies have also noted that
specific subsets of human peripheral T cells might selectively
express different TLRs (TLR1–5, 7 and 9 mRNA), although at greatly
varying levels (19).
Specifically, regulatory T cells (Treg), but not naïve T cells,
expressed TLR8 (20); Treg also
expressed TLR4 and TLR5 mRNA (21,22).
In the present study, we report lower expression
levels of all TLRs studied in PBMCs from ALL patients compared with
that in the controls; the lowest expression of TLR4 and TLR7 was
observed in children with Pro-B and B ALL subtypes. TLR expression
may differ depending on stimulus, environment, subset, and cell
type and probably age group. The TLR expression profile appears to
be influenced by the location of the cells; in this case it is of
interest because it occurs in a pathological condition and because
all studied TLRs were expressed at lower levels than in the
controls.
As a way to study TLR expression in hematological
cancer, Bourke et al (17)
used a panel of cell lines derived from different leukemia and
lymphoma cell types to demonstrate expression of TLR9 and/or TLR10,
although pre-B-cell lines were negative. Other studies reported
highly variable expression of mRNA for TLRs1–7 in B-cell precursor
ALL cell lines (23). Expression
of TLRs in PBMCs from patients with B-cell chronic lymphocytic
leukemia (B-CLL) was restricted to TLR7, 8 and 9 (18). TLR7 is expressed by plasmacytoid
DCs and memory B cells (24).
Studies in patients with CLL showed TLR1, TLR2, TLR6 and TLR10
expression (25). Patients with
acute myeloid leukemia (AML) expressed TLR2, 4 and 9 in
monocyte-derived DCs; healthy individuals strongly expressed TLR2
and TLR4, and TLR9 was expressed at a lower level in both groups
(26–28). Nevertheless, pre-B ALL cells
expressed TLR1, 7 and 9, and low levels of TLR3, 4 and 5. This may
be a consequence of malignant differentiation or just a reflection
of the normal B-cell precursor phenotype (5).
We report low expression of TLR1, 3, 4, 7 and 9 in
PBMCs from pediatric patients with ALL compared with those from
control children. These results show that healthy children and
those with neoplasia express different levels of TLRs. It is
probable that the age of these patients adds to these differences,
because age plays an important role in linking innate and adaptive
immune responses in normal and pathologic conditions. A high level
of heterogeneity exists between different reports studying patients
and cell lines; in some cases, this may be associated with disease
activity.
Most studies of TLR4 expression levels, including
our own, have reported it as decreased in patients with leukemia,
which might be related to the development or presence of a leukemic
clone of cells in lymphocytic leukemia and myeloid leukemia
(29,30). Thus, further studies are required
to determine whether this decreased TLR4 expression contributes to
the pathogenesis of leukemic clone development through an
associated depressed immune surveillance (31), or whether patients have an
increased risk of disease progression and poor prognosis with the
development of autoimmune complications (29).
The decreased TLR4 expression in pre-B and B ALL
subtypes observed in this study might therefore be a reflection of
an impaired host response toward the malignant clonal populations.
In the present study, levels of TLR4 expression in both subsets of
ALL were lower than in controls; therefore, depressed resistance to
the challenge of leukemic transformation might be associated with a
lack of sufficient host TLR4 (30). However, normal B cells also
responded to TLR7 activation by increasing costimulatory molecule
expression, cytokine production and by becoming more sensitive to
killing by cytotoxic effectors (25). These findings suggest a potential
role for TLR4 and TLR7 in disease progression and in inhibition of
effective immunotherapy.
Physiologically, various diseases may alter the
immune regulatory function of TLRs; stimulation with different
ligands could be crucial for the antitumor immune response.
Bekeredjian-Ding et al (31) showed that in peripheral blood B
cells of healthy donors, type I interferon (IFN), induced during
infections, triggered TLR7 expression and polyclonal B-cell
expansion and also B-cell differentiation toward plasma cells. In
addition, Reid et al (32)
showed that the impact of CpG stimulation on precursor B ALL cell
lines and B ALL bone marrow biopsy samples from pediatric patients
was characterized by increased CD40 expression but only small
changes in CD86 levels and no induction of CD80 expression. CpG
stimulation of ALL blasts produced increased levels of
interleukin-6 (IL-6), IL-8, IL-10 and IFN-γ, but no detectable
IL-12p70, and reduced secretion of IL-5. Agonists of TLR2, TLR4,
TLR7 and TLR9 led to detectable changes in costimulatory molecule
expression in ALL; only TLR2 and TLR9 stimulation influenced T-cell
responses (24,30). Unlike the findings in other B-cell
leukemia types, no change in proliferation was observed for pre-B
ALL cells in the presence of ligands for TLR2, TLR7 and TLR9
(32).
TLR expression is present in both normal immune
cells and in malignant cells, but with distinctive patterns
(6), so the expression of TLRs in
pre-B leukemic blasts compared with normal B cells may be the
result of malignant transformation or simply a reflection of the
underlying phenotype of the precursor cells. TLR agonists might
serve to strengthen effectively the response of the immune system
in leukemia.
The expression of TLRs has been demonstrated in some
tumors and tumor cell lines, but the role of TLRs in pathogenesis
and/or development of acute leukemia remains unclear.
The expression of TLRs in hematological cancer has
been demonstrated in murine models, cell lines, bone marrow, B and
T cells of peripheral blood samples from patients with non-Hodgkin
lymphoma, multiple myeloma, AML, CML and in healthy volunteers.
However, the expression of TLRs in PBMCs from pediatric patients
with ALL has not been reported previously; this is the first study
reporting the expression of TLRs 1, 3, 4, 7 and 9 in PBMCs from
children with ALL.
The present study provides information on the
expression of TLRs in cells of the immune system that are crucial
for the antitumor immune response. Although the possible role of
TLR in the pathogenesis of these diseases is unclear, the low level
of expression of TLRs in patients with ALL may partially explain
the deficiency that exists in the antitumor immune response of
these patients. The observed variations could also explain the
differences in the responses of the patients to autoimmune
diseases, infection, and neoplastic responses, including active
disease. Although our results show low expression of some TLRs,
further studies are necessary to address the expression of TLRs in
different mononuclear cell types. Variation between cell
populations must be analyzed to determine whether the differences
found between patients with ALL and controls are related to
different levels of expression in different types of mononuclear
cells and/or subtypes of ALL.
Acknowledgements
The present study was supported by the Hospital
Infantil de México Federico Gómez, grant HIM/2011/022,
HIM/2012/024, 06720 Mexico City, Mexico. María A. Sánchez-Cuaxospa
acknowledges the scholarship provided by CONACyT. This study
constitutes a partial fulfillment of the Graduate Program of
Doctorado en Ciencias Biomédicas of the Universidad Nacional
Autonóma de México (UNAM).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stock W and Pui CH: Chapter 17. Acute
lymphoblastic leukemia and lymphoblastic lymphoma. ASH-SAP. pp.
489–510. 2010
|
|
3
|
Hossain MJ, Xie L and Caywood EH:
Prognostic factors of childhood and adolescent acute myeloid
leukemia (AML) survival: Evidence from four decades of US
population data. Cancer Epidemiol. 39:720–726. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rakoff-Nahoum S and Medzhitov R: Toll-like
receptors and cancer. Nat Rev Cancer. 9:57–63. 2009. View Article : Google Scholar
|
|
5
|
Chiron D, Bekeredjian-Ding I,
Pellat-Deceunynck C, Bataille R and Jego G: Toll-like receptors:
Lessons to learn from normal and malignant human B cells. Blood.
112:2205–2213. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Harsini S, Beigy M, Akhavan-Sabbagh M and
Rezaei N: Toll-like receptors in lymphoid malignancies:
Double-edged sword. Crit Rev Oncol Hematol. 89:262–283. 2014.
View Article : Google Scholar
|
|
7
|
Fabricius D, Breckerbohm L, Vollmer A,
Queudeville M, Eckhoff SM, Fulda S, Strauss G, Debatin KM,
Jahrsdörfer B and Meyer LH: Acute lymphoblastic leukemia cells
treated with CpG oligodeoxynucleotides, IL-4 and CD40 ligand
facilitate enhanced anti-leukemic CTL responses. Leukemia.
25:1111–1121. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Månsson A, Adner M, Höckerfelt U and
Cardell LO: A distinct Toll-like receptor repertoire in human
tonsillar B cells, directly activated by PamCSK, R-837 and CpG-2006
stimulation. Immunology. 118:539–548. 2006.PubMed/NCBI
|
|
9
|
Marshak-Rothstein A: Toll-like receptors
in systemic auto-immune disease. Nat Rev Immunol. 6:823–835. 2006.
View Article : Google Scholar
|
|
10
|
Krieg AM and Vollmer J: Toll-like
receptors 7, 8, and 9: Linking innate immunity to autoimmunity.
Immunol Rev. 220:251–269. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Klonowska-Szymczyk A, Wolska A, Robak T,
Cebula-Obrzut B, Smolewski P and Robak E: Expression of toll-like
receptors 3, 7, and 9 in peripheral blood mononuclear cells from
patients with systemic lupus erythematosus. Mediators Inflamm.
2014:3814182014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Green TL, Santos MF, Ejaeidi AA, Craft BS,
Lewis RE and Cruse JM: Toll-like receptor (TLR) expression of
immune system cells from metastatic breast cancer patients with
circulating tumor cells. Exp Mol Pathol. 97:44–48. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu Y, Zhao Y, Huang H, Chen G, Wu X, Wang
Y, Chang W, Zhu Z, Feng Y and Wu D: Expression and function of
toll-like receptors in multiple myeloma patients: Toll-like
receptor ligands promote multiple myeloma cell growth and survival
via activation of nuclear factor-kappaB. Br J Haematol.
150:543–553. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bernasconi NL, Onai N and Lanzavecchia A:
A role for Toll-like receptors in acquired immunity: Up-regulation
of TLR9 by BCR triggering in naive B cells and constitutive
expression in memory B cells. Blood. 101:4500–4504. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Genestier L, Taillardet M, Mondiere P,
Gheit H, Bella C and Defrance T: TLR agonists selectively promote
terminal plasma cell differentiation of B cell subsets specialized
in thymus-independent responses. J Immunol. 178:7779–7786. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gururajan M, Jacob J and Pulendran B:
Toll-like receptor expression and responsiveness of distinct murine
splenic and mucosal B-cell subsets. PLoS One. 2:e8632007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bourke E, Bosisio D, Golay J, Polentarutti
N and Mantovani A: The toll-like receptor repertoire of human B
lymphocytes: Inducible and selective expression of TLR9 and TLR10
in normal and transformed cells. Blood. 102:956–963. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Spaner DE, Miller RL, Mena J, Grossman L,
Sorrenti V and Shi Y: Regression of lymphomatous skin deposits in a
chronic lymphocytic leukemia patient treated with the Toll-like
receptor-7/8 agonist, imiquimod. Leuk Lymphoma. 46:935–939. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hornung V, Rothenfusser S, Britsch S, Krug
A, Jahrsdörfer B, Giese T, Endres S and Hartmann G: Quantitative
expression of toll-like receptor 1–10 mRNA in cellular subsets of
human peripheral blood mononuclear cells and sensitivity to CpG
oligodeoxynucleotides. J Immunol. 168:4531–4537. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Peng G, Guo Z, Kiniwa Y, Voo KS, Peng W,
Fu T, Wang DY, Li Y, Wang HY and Wang RF: Toll-like receptor
8-mediated reversal of CD4+ regulatory T cell function.
Science. 309:1380–1384. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Crellin NK, Garcia RV, Hadisfar O, Allan
SE, Steiner TS and Levings MK: Human CD4+ T cells
express TLR5 and its ligand flagellin enhances the suppressive
capacity and expression of FOXP3 in CD4+CD25+
T regulatory cells. J Immunol. 175:8051–8059. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Caramalho I, Lopes-Carvalho T, Ostler D,
Zelenay S, Haury M and Demengeot J: Regulatory T cells selectively
express toll-like receptors and are activated by
lipopolysaccharide. J Exp Med. 197:403–411. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Corthals SL, Wynne K, She K, Shimizu H,
Curman D, Garbutt K and Reid GS: Differential immune effects
mediated by Toll-like receptors stimulation in precursor B-cell
acute lymphoblastic leukaemia. Br J Haematol. 132:452–458.
2006.PubMed/NCBI
|
|
24
|
Spaner DE, Shi Y, White D, Mena J, Hammond
C, Tomic J, He L, Tomai MA, Miller RL, Booth J, et al:
Immunomodulatory effects of Toll-like receptor-7 activation on
chronic lymphocytic leukemia cells. Leukemia. 20:286–295. 2006.
View Article : Google Scholar
|
|
25
|
Muzio M, Scielzo C, Bertilaccio MT,
Frenquelli M, Ghia P and Caligaris-Cappio F: Expression and
function of toll like receptors in chronic lymphocytic leukaemia
cells. Br J Haematol. 144:507–516. 2009. View Article : Google Scholar
|
|
26
|
Schmitt A, Li L, Giannopoulos K, Greiner
J, Reinhardt P, Wiesneth M and Schmitt M: Quantitative expression
of Toll-like receptor-2, -4, and -9 in dendritic cells generated
from blasts of patients with acute myeloid leukemia. Transfusion.
48:861–870. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rybka J, Butrym A, Wróbel T, Jaźwiec B,
Stefanko E, Dobrzyńska O, Poręba R and Kuliczkowski K: The
expression of Toll-like receptors in patients with acute myeloid
leukemia treated with induction chemotherapy. Leuk Res. 39:318–322.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rozková D, Novotná L, Pytlík R, Hochová I,
Kozák T, Bartůnková J and Spísek R: Toll-like receptors on B-CLL
cells: Expression and functional consequences of their stimulation.
Int J Cancer. 126:1132–1143. 2010. View Article : Google Scholar
|
|
29
|
Barcellini W, Imperiali FG, Zaninoni A,
Reda G, Consonni D, Fattizzo B, Lonati S, Nobili L, Zanella A and
Cortelezzi A: Toll-like receptor 4 and 9 expression in B-chronic
lymphocytic leukemia: Relationship with infections, autoimmunity
and disease progression. Leuk Lymphoma. 55:1768–1773. 2014.
View Article : Google Scholar
|
|
30
|
Webb RN, Cruse JM and Lewis RE: Decreased
TLR4 gene expression in leukemic leukocyte populations. Exp Mol
Pathol. 87:117–126. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bekeredjian-Ding IB, Wagner M, Hornung V,
Giese T, Schnurr M, Endres S and Hartmann G: Plasmacytoid dendritic
cells control TLR7 sensitivity of naive B cells via type I IFN. J
Immunol. 174:4043–4050. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Reid GS, She K, Terrett L, Food MR,
Trudeau JD and Schultz KR: CpG stimulation of precursor B-lineage
acute lymphoblastic leukemia induces a distinct change in
costimulatory molecule expression and shifts allogeneic T cells
toward a Th1 response. Blood. 105:3641–3647. 2005. View Article : Google Scholar : PubMed/NCBI
|