Introduction
Apoptosis is the process of programmed cell death
that includes many biochemical changes, such as nuclear
fragmentation, cell shrinkage and chromatin condensation. It plays
a crucial role in the growth, development, metabolism and
maintaining homeostasis in multicellular organisms (1). If some internal or external factors
effect the production of apoptosis, a disruption of the balance
between cell proliferation and apoptosis would lead to the
development of cancer (2).
Inhibitor of apoptosis protein (IAP) is one of the best known
factors which can promote cancer development by inhibiting cell
apoptosis (3).
As a member of IAP family, X-linked inhibitor of
apoptosis protein (XIAP) contains three baculoviral IAP repeat
(BIR) domains and one really interesting new gene (RING)-finger
domain (4). The BIR1, BIR2 and
BIR3 domains of XIAP are the main structure for inhibiting
apoptosis, due to their capability of inhibiting caspases. The BIR2
domain plays an important role in blocking caspase-3 and -7, and
the BIR3 domain is required for inhibition of caspase-9 (5). Like other IAP members, XIAP contains
RING domain in its C terminal, which is relative to its
self-ubquitination. Besides, its RING domain also promotes
ubquitination of other proteins and degrades them through
ubiquitin-proteasome pathway (6).
Second mitochondrial-derived activator of caspase
(Smac) is located in mitochondria. It releases into cytosol under
some apoptotic induction signals, such as chemotherapy or
radiotherapy (7). Its main
mechanism of apoptotic induction is the interaction between Smac
and IAPs. Smac binds to IAPs and reverses the inhibition of
caspases by IAPs, and then induces the caspase cascade and cell
apoptosis (8). For example, Smac
binds to the BIR domains of XIAP through its N-terminus and
prevents XIAP from binding caspase-3, -7 and -9, then, the caspases
were activated and amplified to trigger cell apoptosis (9).
XIAP and Smac are two important prognostic
biomarkers for malignant tumors because they determine the
sensitivity of cancer cells to apoptosis (10–14).
Usually, the expression of XIAP is much higher in cancer tissues
than that in normal tissues, and the increased XIAP expression is
correlated with poor prognostic and decreased survival in patients
(15–17). While Smac expression was reduced in
cancer tissues compared to normal tissues, and this reduction is
more significant during cancer progression with advanced malignant
phenotypes (18,19). It has been reported that the
expression of XIAP and Smac are negatively correlated in many types
of cancer, such as testicular germ cell tumors, renal cell
carcinomas and gastric adenocarcinomas (19–21).
However, its mechanisms are still unknown.
In the present study, we found that there was a
negative correlation between Smac and XIAP at the level of protein
but not mRNA in NSCLC patients. Overexpression of mature Smac
induced lung cancer cells apoptosis, and this apoptotic induction
was inhibited by co-transfection with XIAP plasmid. It is because
XIAP promoted the ubiquitination and degradation of Smac. Besides,
nude mouse xenograft experiment further proved the relation and the
function of Smac and XIAP in vivo.
Materials and methods
Clinical samples
Primary tumor specimens were obtained from 26
patients with NSCLC who underwent pulmonary surgery at the First
Affiliated Hospital of Xi'an Jiaotong University. Normal lung
tissues were collected at >5 cm from the edge of the tumor. All
patients had a single tumor, without distant metastasis and none of
them had been previously treated with chemo- or radiotherapy. The
study design and procedure were approved by the Ethics Committee of
the hospital and each participant signed an informed consent
document prior to enrollment.
RT-PCR
Total RNA was extracted from tissues using RNeasy
Mini kits (Qiagen, Inc., Valencia, CA, USA) and reverse
transcription was performed with SuperScript III First-Strand kits
(Invitrogen Life Technologies, Carlsbad, CA, USA) according to the
manufacturer's instructions. The DNA was amplified with PfuUltra
DNA Polymerases & PCR Master Mixes (Agilent Technologies, Palo
Alto, CA, USA) on an S1000™ Thermal Cycler (Bio-Rad Laboratores,
Richmond, CA, USA), with the following protocol: initial
denaturation at 95°C for 3 min, then 30 cycles of 95°C for 30 sec,
60°C for 30 sec, and 72°C for 1 min, and then final extension at
72°C for 5 min. GAPDH gene was used as a control. The following PCR
primer sequences were used for Smac forward,
5′-ATCAGAGCCTCATTCCCTTAGT-3′ and reverse,
5′-TGGTGTTTTGAAGTCATCTCAGC-3′; XIAP forward,
5′-TGCTCACCTAACCCCAAGAG-3′ and reverse, 5′-ATCTGCCATGGATGGATTTC-3′;
GAPDH forward, 5′-CACCAACTGCTTAGCACCCC-3′ and reverse,
5′-TGCTCAGTGTAGCCCAGGATG-3′. The RT-PCR assay was analyzed by grey
value analysis using Quantity One software; the target/internal
relative grey-scale value indicates the corresponding mRNA
expression level.
Western blot analysis and
co-immunoprecipitation assay
Lung tissues or treated cells were lysed in protein
extraction buffer [50 mM Tris-Cl (pH 7.5), 150 mM NaCl, 1 mM EDTA
(pH 8.0), 1% Triton X-100, and protease inhibitor cocktail (Roche
Molecular Biochemicals, Indianapolis, IN, USA)] for 30 min on ice.
Western blot analysis and co-immunoprecipitation assay were
performed as previously described (22) using the following antibodies:
anti-Smac rabbit monoclonal antibody (1:1,000; ab32023), anti-XIAP
rabbit polyclonal antibody (1:2,000; ab21278), anti-β-actin rabbit
polyclonal antibody (1:5,000; ab75186) (both from Abcam, Cambridge,
UK); anti-GAPDH rabbit polyclonal IgG antibody (1:1,000, sc-25778;
Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA); anti-Flag tag
mouse monoclonal IgG antibody (1:1,000, F1804; Sigma-Aldrich, St.
Louis, MO, USA); anti-myc tag mouse monoclonal antibody (1:1000;
2276S; Cell Signaling Technology, Inc., Danvers, MA, USA);
anti-ubiquitin rabbit monoclonal antibody (1:2000; ab140601;
Abcam).
Construction of plasmids and
transfection
Full-length XIAP and Smac were generated by PCR from
A549 cell cDNA using the following primers: XIAP: forward
5′-AGATCTGGATCCACTTTTAACAGTTTTGAAGG-3′ and reverse
5′-TCTAGAGCGGCCGCTTAAGACATAAAAATTTTTTGCTTG-3′. Smac: sense
5′-AAGCTTGGATCCGCGGCTCTGAAGAGTTGGCTGTCG-3′ and reverse
5′-TCTAGAGCGGCCGCTCAATCCTCACGCAGGTAGGCCTCC-3′; Ub-mSmac was
generated by overlapping PCR using primiers: forward
5′-AGATCTGGATCCATGCAGATCTTCGTGAAAACCCTTACCGGC-3′ and reverse
5′-TCTAGAGCGGCCGCTCAATCCTCACGCAGGTAGGCCTCC-3′. The resulting
fragments were digested by BamHI and NotI (Thermo
Fisher Scientific, Rockford, IL, USA), and cloned into
pcDNA3.0-Flag. The plasmids were transfected into A549 or H460
cells with Lipofectamine 2000 (Invitrogen-Life Technologies).
Western blotting was used to identify protein overexpression in
cells.
MTT assay
For cell viability assays, 5×103 A549 and
H460 cells, with or without overexpression of Smac or mSmac, were
plated into each well of 96-well plates. After 48 h, 20 μl of MTT
(Sigma-Aldrich) was added to each well for 4 h. Then, the
supernatant was discarded and 100 μl DMSO was added to each well,
and the absorbance at 490 nm (A490) was measured with a microplate
reader (Bio-Rad Laboratories). Each cell viability assay was
performed with five replicate wells. Cell viability (%) was
calculated as follows: Cell viability = the average A490 in an
experimental chemotherapy group/the average A490 in the blank
control group x 100%.
Flow cytometry
Cells were plated into 24-well plates with a
concentration of 1×105/well. The following day, cells
were transfected with mSmac and/or XIAP. After 48 h, cells were
trypsinized and incubated with 5 μl propidium iodide and 5 μl
Annexin V-FITC (Invitrogen) for 15 min. Samples were then analyzed
for apoptosis by a FACScan flow cytometer (BD Biosciences, Franklin
Lakes, NJ, USA).
Immunofluorescence
Lung cancer cells were fixed with 3%
paraformaldehyde and permeabilized using 0.2% Triton X-100. Then
the fixed cells were incubated with anti-Flag tag mouse monoclonal
IgG antibody (1:1,000, F1804; Sigma-Aldrich). The secondary
antibody is a labeled goat anti-mouse IgG antibody (A11001;
Invitrogen, Carlsbad, CA, USA). Fluorescence confocal images were
captured using a LSM 5 Pascal Laser Scanning Microscope (Carl Zeiss
AG, Oberkochen, Germany) and Laser Scanning Microscope LSM PASCAL
software (version 4.2 SP1).
Caspase-3 activity assay
Cells were washed with cold PBS twice, resuspended
in lysis buffer and kept on ice for 15 min. The lysate was
centrifuged at 16,000 x g at 4°C for 15 min. Supernatants were
collected and caspase-3 activity was measured using caspase-3
activity assay kit (Beyotime Institute of Biotechnology, Shanghai,
China) according to the manufacturer's instructions. The working
principles of this kit are based on the cleavage of the caspase-3
substrate, Ac-DEVD-pNA. The release of p-nitroanilide (pNA) was
qualified by determining the absorbance with the microplate reader
at 405 nm. Caspase-3 activity was expressed as the ratio of control
cells.
Nude mouse xenograft studies
Four-week-old BALB/c athymic nude mice (4–5-weeks
old and weighing 16–20 g) were purchased from the Center of
Laboratory Animals of Xi'an Jiaotong University and were bred at
pathogen-free conditions. All animal experiments were approved by
the Ethics Committee of the First Affiliated Hospital of Xi'an
Jiaotong University. H460 cells were transfected with empty vector,
Ub-mSmac or Ub-mSmac+XIAP, and stably-transfected cells were
selected in medium supplemented with 1 mg/ml G418 (Sigma-Aldrich)
for one month. Western blotting was used to identify the cells with
significant overexpression. Cells (5×106) were harvested
from tissue culture dishes and subcutaneously injected into the
right flank of each nude mice. Tumor volumes were measured every
three days by calipers and calculated using the formula: Tumor
volume = length × width2 × 1/2. After one month, the
mice were sacrificed and the tumors were removed for IHC
staining.
IHC staining
The expression of XIAP, Smac and Ki-67 was evaluated
by immunohistochemical (IHC) analyses in 4-μm sections of the 10%
formaldehyde-fixed and paraffin-embedded blocks. The tissue slides
were deparaffinized in xylene and rehydrated through a series of
graded alcohols. To exhaust endogenous peroxidase activity, the
slides were treated with 3% H2O2 for 10 min.
Then, the slides were immersed in 0.01 M citrate buffer, pH 6.0,
for 3 min in a pressure cooker at 125°C, then placed at room
temperature for 30 min to cool down. After washing with
phosphate-buffered saline (PBS), the slides were pre-incubated for
30 min in 10% normal goat serum and then incubated with anti-Smac
rabbit monoclonal antibody (1:400; ab32023), anti-XIAP rabbit
polyclonal antibody (1:400; ab21278; both from Abcam); anti-Ki-67
rabbit monoclonal antibody (1:400, 9027; Cell Signaling
Technology). After washing with PBS, the slides were incubated in a
donkey anti-rabbit IgG/HRP secondary antibody (1:250; bs-0295D-HRP;
Beijing Bioss, Inc., Beijing, China) at room temperature for 30
min. All slides were generated using a diaminobenzidine chromogen
solution. The counterstaining was performed with hematoxylin. The
slides then were dehydrated, cleared and mounted.
Both the intensity and extent of staining were taken
into consideration when analyzing the data. The extent of staining
was scored from 0 to 100% (1, 1–25%, 2, 26–50%, 3, 51–75%, 4,
76–100%) and the intensity of staining was scored from 0 to 2 (0,
none; 1, weak to moderate; 2, strong). The IHC score was determined
as high expression (+): score ≥3; low expression (−): score ≤2.
Statistical analysis
Data reported are the mean ± SD deviation of three
independent experiments and evaluated by the Student t-tests or
one-way ANOVA. The normal distribution and equality of variances of
the data in clinical samples were detected by Kolmogorov-Smirnov
test and Levene's test separately. The difference of XIAP or Smac
expression between cancer tissues and their paired normal tissues
was evaluated by paired-samples t-test. The correlation between the
expression of XIAP and Smac was evaluated by Pearson's correlation
analysis. Statistical analyses were performed using the SPSS
version 13.0 (SPSS, Inc., Chicago, IL, USA). Significant
differences were established at P<0.05.
Results
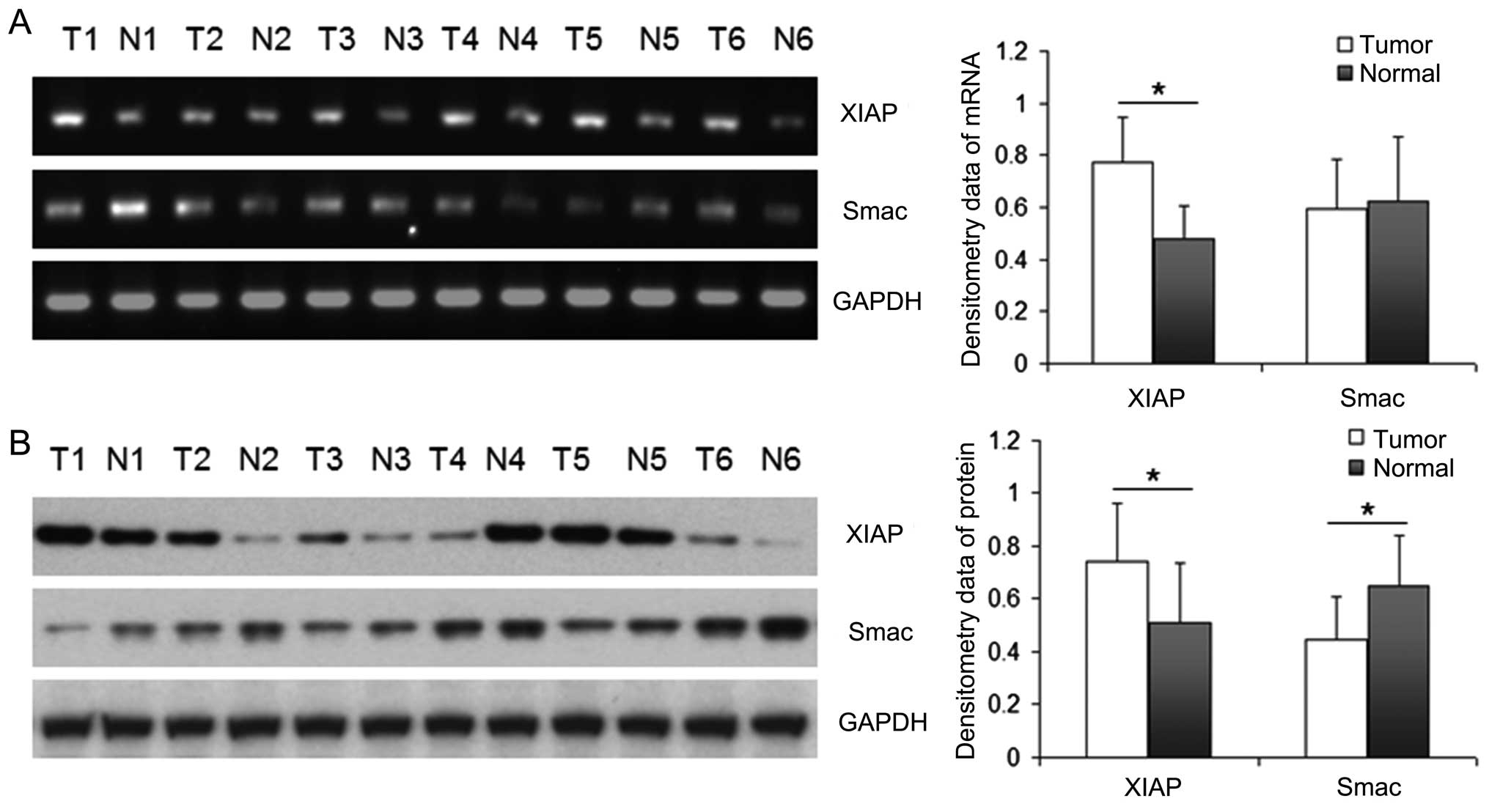
Expression of XIAP and Smac in NSCLC
RT-PCR and western blotting were performed to detect
the levels of mRNA and protein of Smac and XIAP in 26 NSCLC tissues
and their matched para-tumor tissues. Representative result is
shown in Fig. 1. The bands were
quantified by densitometry and normalized to GAPDH. The data in
different groups were normal distribution and equality of
variances, so we used paired-samples t-test. The mRNA of XIAP in
NSCLC tissues was significantly higher than that in the matched
para-tumor tissues (P<0.05), while the mRNA of Smac was not
significantly different between cancer tissues and para-tumor
tissues. The protein expression of XIAP in NSCLC tissues was also
significantly higher than that in the matched lung tissues
(P<0.05), which corresponded with the mRNA level. However, the
protein expression of Smac was significantly higher in para-tumor
tissues than that in NSCLC tissues (P<0.05). There was a
negative correlation between Smac and XIAP only on the level of
protein (r=−0.59, P<0.05) but not on the level of mRNA in the
cancer samples of NSCLC patients (P>0.05).
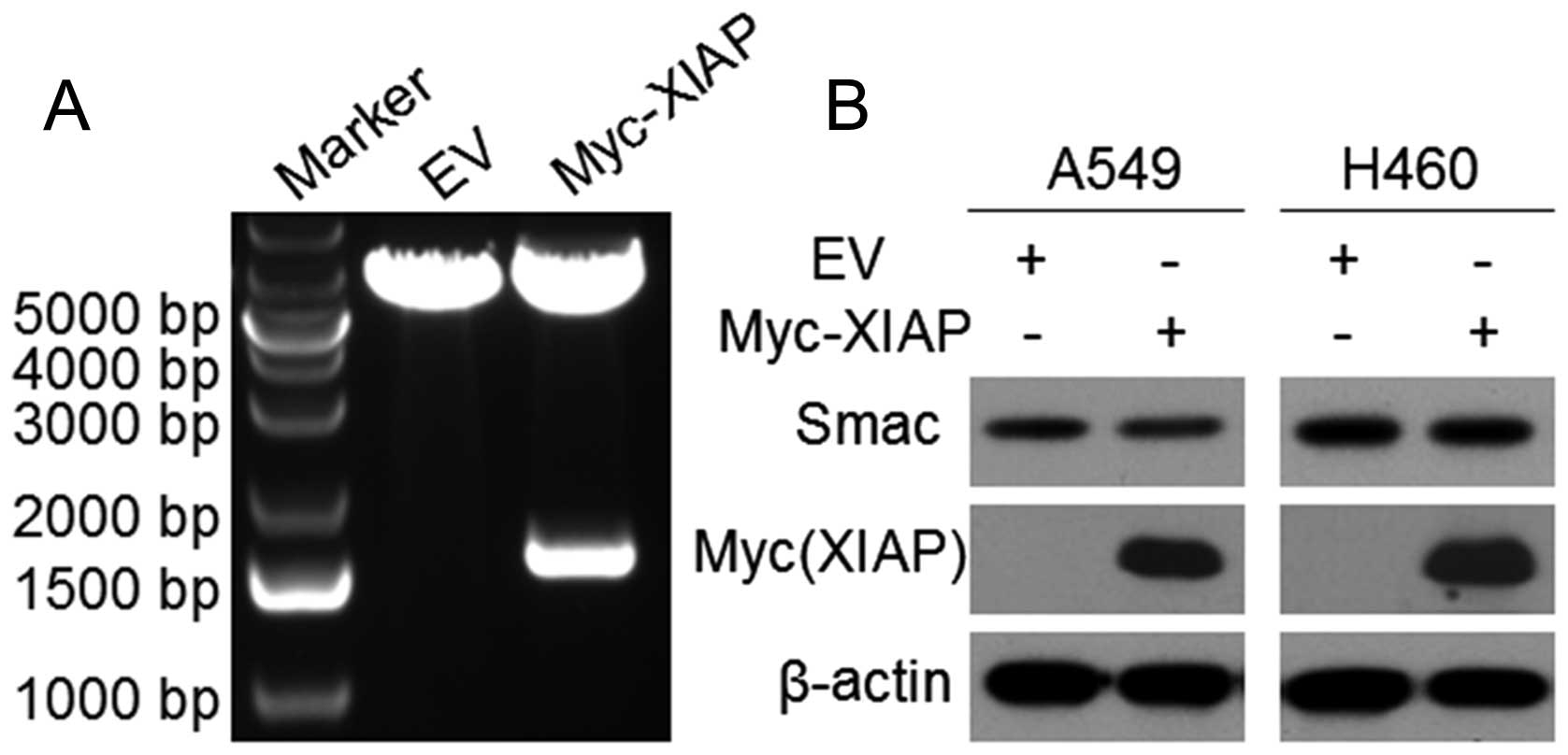
XIAP could not degrade endogenous Smac in
lung cancer cell lines
We constructed plasmid pcDNA3-Myc-XIAP and confirmed
it by sequencing and double digestion (Fig. 2A). To investigate the effects of
XIAP to endogenous Smac, we transfected Myc-XIAP or empty vector
into A549 and H460 cells for 48 h. Then, western blotting confirmed
that the expression of XIAP in the XIAP-transfected cells was
higher than that in the control cells. However, the expression of
Smac did not change prominently after XIAP overexpression, thus,
XIAP could not degrade endogenous Smac in NSCLC cells (Fig. 2B).
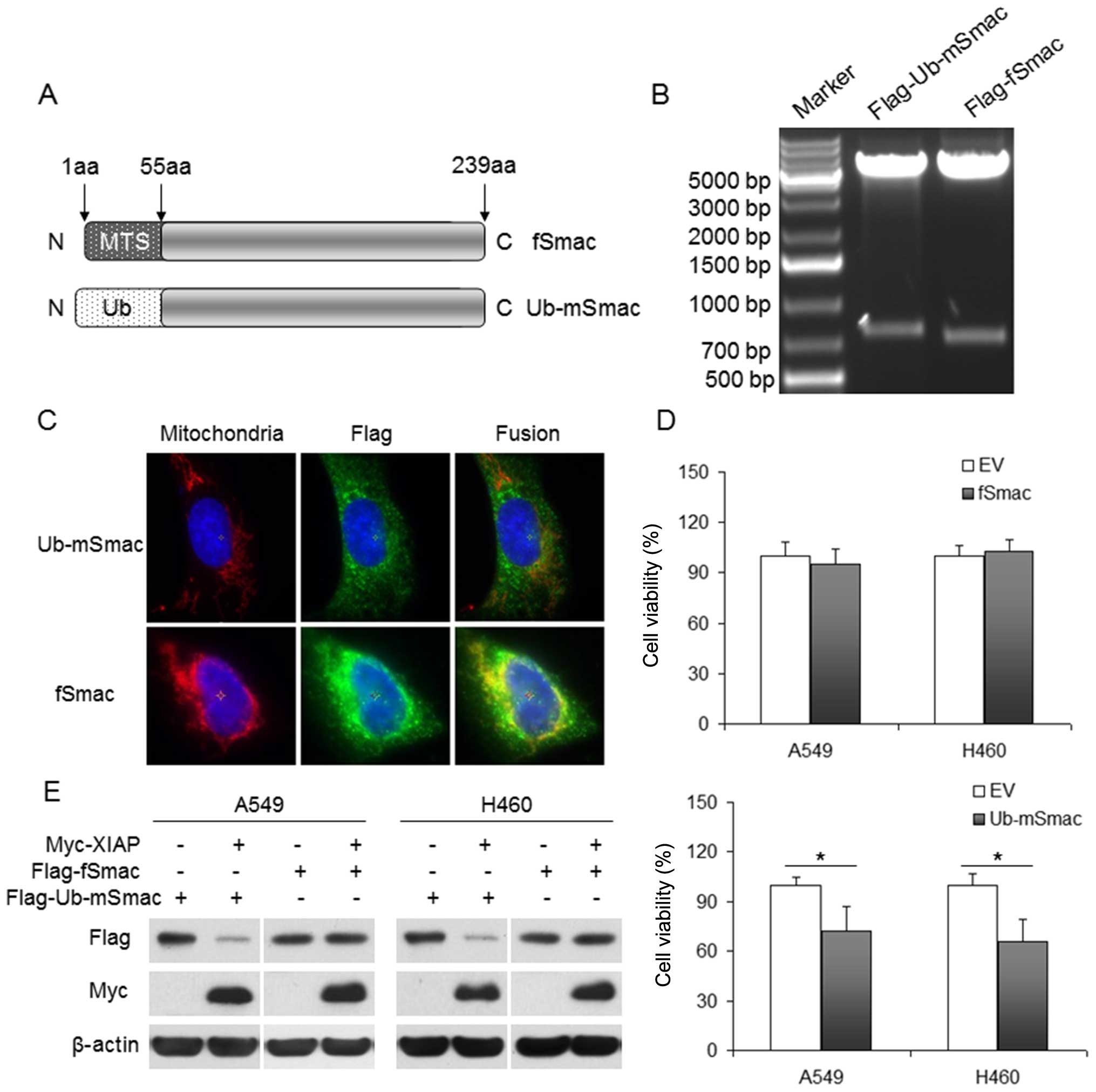
The location and function of Smac and
mature Smac
The first 55 amino acids at the N terminal of Smac
constitute a mitochondrial targeting signal (MTS) peptide and are
cleaved to generate mature Smac. We constructed plasmids
pcDNA3-Flag-fSmac and pcDNA3-Flag-Ub-mSmac. Their structures are
shown in the Fig. 3A and their
digestion results were confirmed by sequencing and double digestion
(Fig. 3B). To further identify the
expression and the location of fSmac and mSmac, we transfected
Flag-fSmac or Flag-Ub-mSmac into U2OS cells, which are commonly
used to detect protein localization because their appropriate
nucleus to cytoplasm ratio. By immunofluorescence, we found
Flag-fSmac was mainly located in the mitochondria and Flag-Ub-mSmac
was located in the cytoplasm (Fig.
3C).
After transfection with Flag-fSmac or Flag-Ub-mSmac
into lung cancer A549 and H460 cells for 48 h, cell viability was
assessed using an MTT assay. We found that mSmac but not fSmac
inhibited cell viability (Fig.
3D). Cells were co-transfected with Myc-XIAP and Flag-fSmac or
Flag-Ub-mSmac for 48 h, and exogenous Smac and XIAP were evaluated
by western blotting, which showed XIAP inhibited the expression of
mSmac but not fSmac (Fig. 3E).
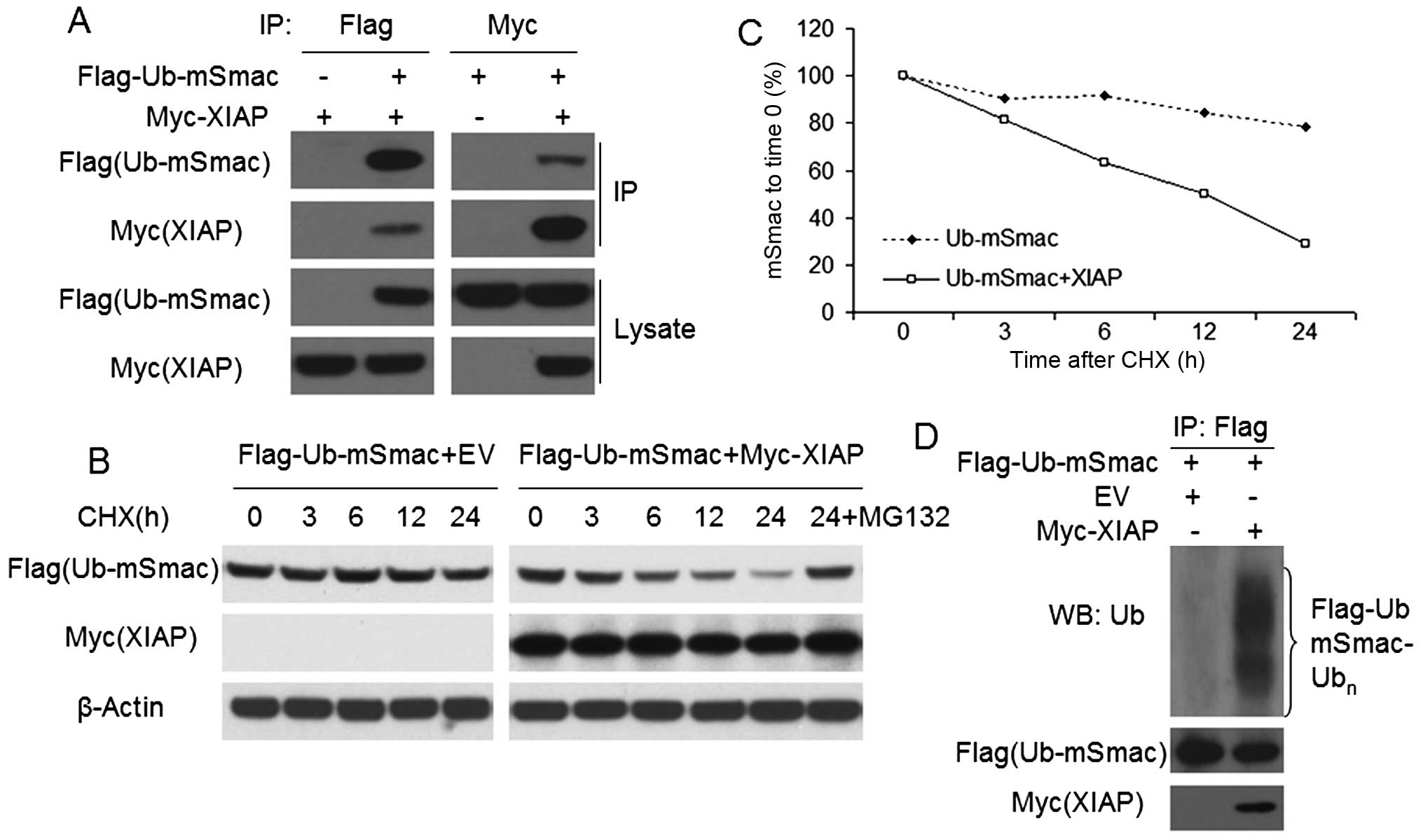
XIAP degrades mSmac through ubiquitin
pathway
To study how XIAP degrades mSmac in NSCLC, we
transfected Flag-Ub-mSmac and/or Myc-XIAP into H460 cells for 48 h.
Reciprocal co-immunoprecipitation was done by using anti-Flag or
anti-Myc antibodies to confirm the interaction between XIAP and
mSmac (Fig. 4A). Then, CHX chase
assays was done by cell treatment with a protein synthesis
inhibitor, cycloheximide (CHX), for 0–24 h to analyze the XIAP
mediated downregulation of mSmac in Flag-Ub-mSmac with/without
Myc-XIAP transfected H460 cells. In the course of time, the
half-life of mSmac was prominently decreased in co-transfected
cells, compared with that in the cells with mSmac transfection
alone (Fig. 4B and C). Besides,
the downregulation of mSmac was prevented by MG132, a proteasome
inhibitor (Fig. 4B). Moreover,
ubiquitin assay was done by western blotting using anti-ubiquitin
antibody in cells transfected with Flag-Ub-mSmac with/without
Myc-XIAP. As shown in Fig. 4D,
XIAP overexpression substantially increased the ubiquitination of
mSmac in H460 cells.
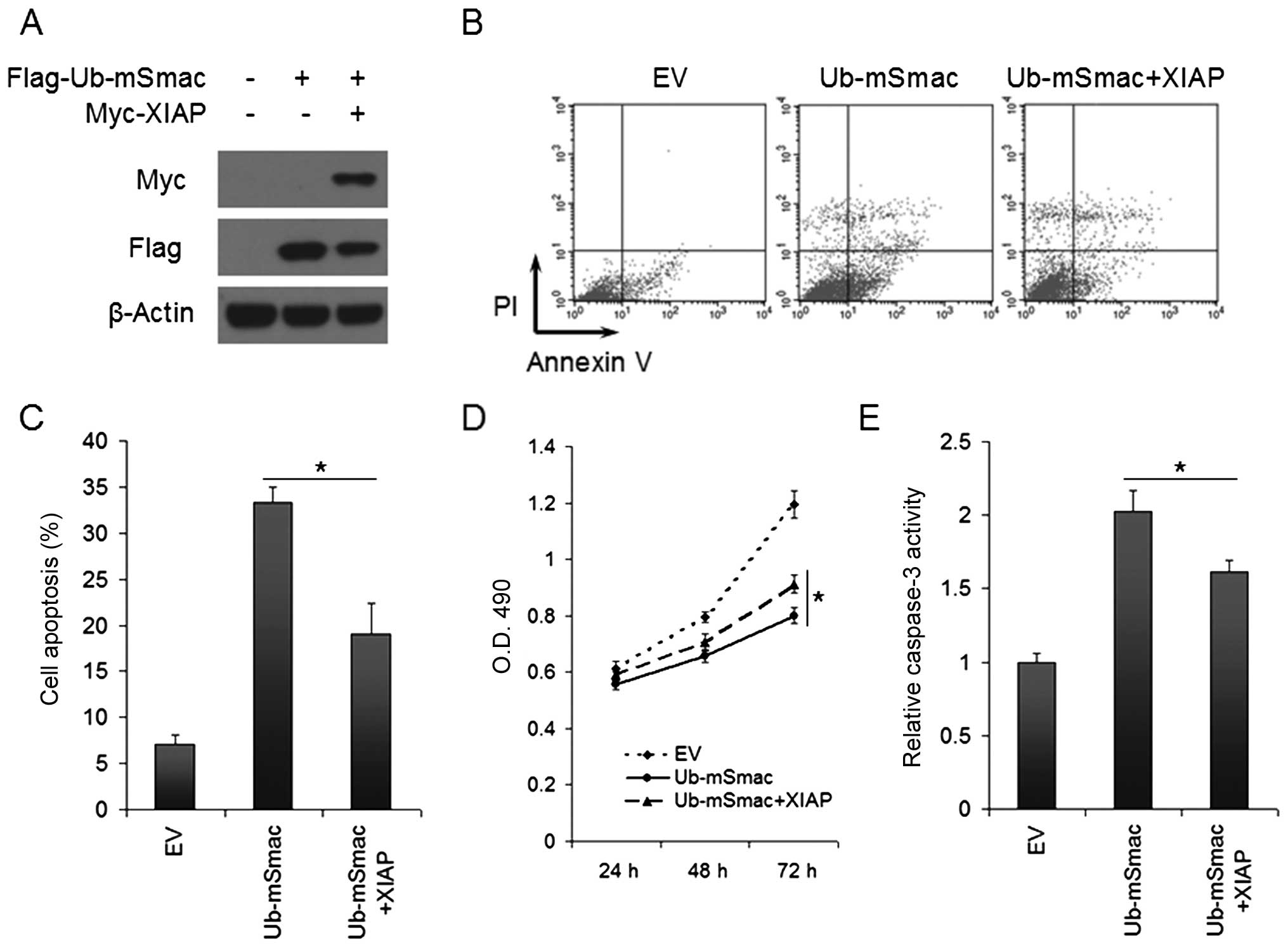
The function of XIAP and mSmac in vitro
and in vivo
To explore the function of XIAP and mSmac in lung
cancer cells, we transfected Flag-Ub-mSmac and/or Myc-XIAP into
H460 cells for 48 h. Western blotting was done to confirm that
exogenous XIAP can degrade mSmac (Fig.
5A). Cell apoptosis and viability were evaluated by Annexin
V/PI analysis and MTT assays separately. Mature Smac promoted
apoptosis and inhibited cell viability in lung cancer H460 cells,
while XIAP partially reverted the effect of exogenous mSmac,
leading to a significantly decreased cell apoptosis and increased
cell viability (P<0.05, Fig.
5B–D). Furthermore, caspase assay was performed to show that
mSmac-induced apoptosis was due to the increased caspase-3
activation, and the reversion of apoptosis by XIAP was caused by
decreasing the activation of caspase-3 (Fig. 5E).
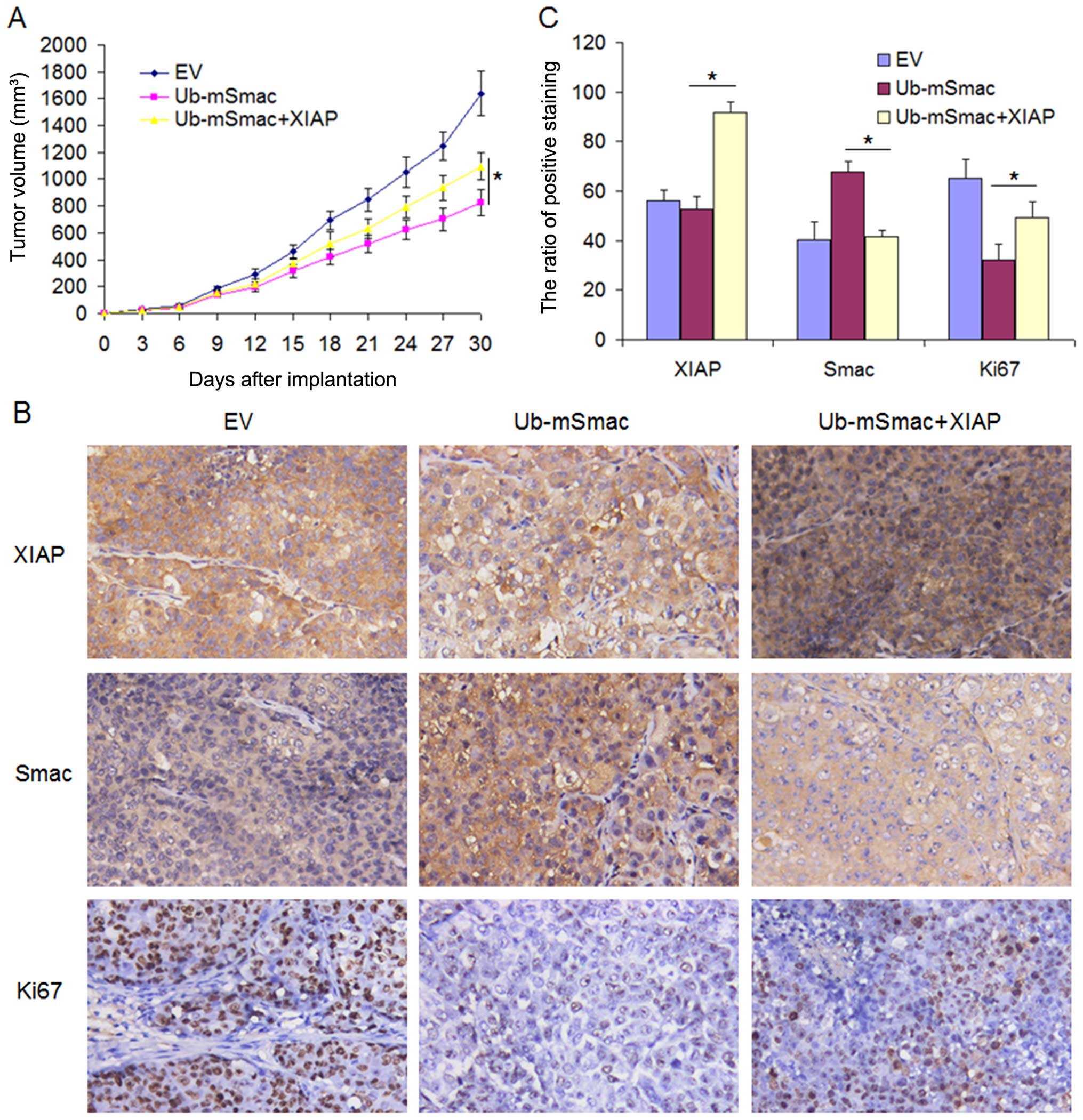
To further confirm this result in vivo, we
stably transfected Flag-Ub-mSmac with/without Myc-XIAP into H460
cells and implanted cells into nude mice via subcutaneous
injection. Tumor growth curve demonstrated that mSmac slowed down
H460 tumor growth in mice, while XIAP partially restored tumor
growth for mSmac overexpressed H460 cells (Fig. 6A). After one month growth, the mice
were sacrificed and the tumor tissues from the mice were detected
by immunohistochemistry. As expected, the expression of mSmac was
downregulated by XIAP overexpression. Overexpression of mSmac
inhibited Ki-67 expression, while XIAP reversed this inhibition by
degrading mSmac. Taken together, these data indicate that mature
Smac inhibited tumor cell proliferation, but this effect was
partially reversed by XIAP (Fig.
6B and C).
Discussion
In renal cell carcinoma, breast, bladder cancer and
other solid tumors, the expression of XIAP is higher with a later
TMN stage, worse tumor differentiation and higher recurrence rate.
The overall survival of patient with high XIAP expression is much
shorter than those with low XIAP expression (15–17).
In contrast, as a pro apoptotic factor, Smac expression is lower in
cancer tissues than that in normal tissues in hepatocellular
carcinoma or testicular germ tumors, and its high expression
indicates a good prognosis (18,19).
However, the level of Smac varies in different tumors. For example,
in cervical cancer, the expression of Smac in cancer tissue is
higher than in normal cervical tissue, especially in mRNA level
(23). In this study, we found
that the mRNA level of XIAP in lung cancer tissues was
significantly higher than that in the corresponding normal tissues,
while the mRNA level of Smac had no significant difference between
normal and cancer tissues. However, the protein of XIAP in lung
cancer tissue was significantly higher than that in the normal
tissue, while the protein of Smac was significantly higher than
that in normal tissue. Smac and XIAP were negatively correlated in
protein levels but not in mRNA levels. It was possibly because the
degradation of Smac by XIAP was at the level of protein.
Overexpression of full length of Smac increases
chemo- or radiotherapy-induced cell apoptosis in lung, breast,
ovarian cancer (24–27). In our previous studies, we found
overexpression of Smac increased cisplatin and indomethacin-induced
apoptosis because Smac released from mitochrondria into cytosol
under the treatment of the drug, and then performed its function in
lung cancer and esophageal cancer (28,29).
In this study, fSmac was not degraded by XIAP, and it did not
promote apoptosis, whereas mSmac could be degraded by XIAP and
induced apoptosis. It was because fSmac was localized in
mitochondria, while mSmac was localized in the cytoplasm. In
addition, Smac promoted apoptosis only if it was located in the
cytoplasm, similarly, it bound to and degraded by XIAP only in the
cytoplasm. The Ub and mature Smac fusion protein, which was the
expressed production of the Flag-Ub-mSmac plasmid, can directly
localized in the cytoplasm (30).
It was easier to distinguish the speed of Smac degradation in the
presence from absence of XIAP by using Flag-Ub-mSmac and Myc-XIAP
plasmids.
IAPs play the role of E3 ligase by their RING
domains, which are closely related to their self-ubiquitination
(3,31). Mature Smac in the cytoplasm
participates in the self-ubiquitination of the IAP family members.
Some studies reported that IAP proteins, such as XIAP, c-IAP1,
c-IAP2 and Apollon, degraded Smac through the ubiquitin pathway
(32–34), while others reported that Smac
promoted the degradation of survivin, cIAP1, cIAP2, but not XIAP
and livin (35,36). To clarify, in this study, we found
that mature Smac in the cytoplasm combined with XIAP, which
accelerated mature Smac ubiquitination. However, the expression of
XIAP was relatively stable in the half-life experiment, even in the
cells co-transfected with Smac. It is highly possible that Smac
promoted IAP self-ubiquitination, at the same time, IAP transfered
ubiquitin to Smac by its E3 ubiquitin ligase and degraded Smac.
Therefore, both of them were degraded faster after combination. But
different IAP family members had different stability. XIAP is not
easy to degrad, while c-IAP1, c-IAP2 are relatively unstable.
Therefore, previous studies demonstrated that XIAP, c-IAP1 and
c-IAP2 degraded Smac, while Smac degraded cIAP1, and c-IAP2, but
not XIAP.
In the present study, we found that the mSmac in the
cytoplasm increased apoptosis, and XIAP inhibited mSmac induced
apoptosis by degradation of mSmac. It was clear that mSmac was
unstable in the cell cytoplasm because it was easily degraded by
XIAP or other IAP family members. Therefore, the function of
endogenous mSmac is limited. Ala-Val-Pro-Ile, a four N-terminal
residue of Smac, competed with caspases for binding to the BIR
domain of IAP proteins, sequentially releasing caspases and causing
cell apoptosis. A novel drug, Smac mimetics, was designed to treat
cancer by mimicking this residue. Preclinical studies showed that
Smac mimetics activated caspases and degraded cIAP1,2 to induce
apoptosis. Moreover, Smac mimetics displayed their anticancer
effectiveness in phase I and II clinical trials. Further study of
this drug shows promising future in the field of apoptosis-related
drug development.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China, approved ID no. 81402506.
References
|
1
|
Evan GI and Vousden KH: Proliferation,
cell cycle and apoptosis in cancer. Nature. 411:342–348. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cotter TG: Apoptosis and cancer: The
genesis of a research field. Nat Rev Cancer. 9:501–507. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fulda S and Vucic D: Targeting IAP
proteins for therapeutic intervention in cancer. Nat Rev Drug
Discov. 11:109–124. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schimmer AD, Dalili S, Batey RA and Riedl
SJ: Targeting XIAP for the treatment of malignancy. Cell Death
Differ. 13:179–188. 2006. View Article : Google Scholar
|
|
5
|
Kaufmann T, Strasser A and Jost PJ: Fas
death receptor signalling: Roles of Bid and XIAP. Cell Death
Differ. 19:42–50. 2012. View Article : Google Scholar :
|
|
6
|
Van den Abeele P and Bertrand MJ: The role
of the IAP E3 ubiquitin ligases in regulating pattern-recognition
receptor signalling. Nat Rev Immunol. 12:833–844. 2012. View Article : Google Scholar
|
|
7
|
Qin S, Yang C, Li S, Xu C, Zhao Y and Ren
H: Smac: Its role in apoptosis induction and use in lung cancer
diagnosis and treatment. Cancer Lett. 318:9–13. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Morizane Y, Honda R, Fukami K and Yasuda
H: X-linked inhibitor of apoptosis functions as ubiquitin ligase
toward mature caspase-9 and cytosolic Smac/DIABLO. J Biochem.
137:125–132. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Flanagan L, Sebastià J, Tuffy LP, Spring
A, Lichawska A, Devocelle M, Prehn JH and Rehm M: XIAP impairs Smac
release from the mitochondria during apoptosis. Cell Death Dis.
1:e492010. View Article : Google Scholar
|
|
10
|
Li S, Sun J, Yang J, Zhang L, Wang L, Wang
X and Guo Z: XIAP expression is associated with pancreatic
carcinoma outcome. Mol Clin Oncol. 1:305–308. 2013.
|
|
11
|
Yi XP, Han T, Li YX, Long XY and Li WZ:
Simultaneous silencing of XIAP and survivin causes partial
mesenchymal-epithelial transition of human pancreatic cancer cells
via the PTEN/PI3K/Akt pathway. Mol Med Rep. 12:601–608.
2015.PubMed/NCBI
|
|
12
|
Zhao YC, Wang Y, Ni XJ, Li Y, Wang XM, Zhu
YY and Luo CY: Clinical significance of Smac and survivin
expression in breast cancer patients treated with
anthracycline-based neoadjuvant chemotherapy. Mol Med Rep.
9:614–620. 2014.
|
|
13
|
Shintani M, Sangawa A, Yamao N and
Kamoshida S: Smac/DIABLO expression in human gastrointestinal
carcinoma: Association with clinicopathological parameters and
survivin expression. Oncol Lett. 8:2581–2586. 2014.PubMed/NCBI
|
|
14
|
Qu Y, Xia P, Zhang S, Pan S and Zhao J:
Silencing XIAP suppresses osteosarcoma cell growth, and enhances
the sensitivity of osteosarcoma cells to doxorubicin and cisplatin.
Oncol Rep. 33:1177–1184. 2015.PubMed/NCBI
|
|
15
|
Li M, Song T, Yin ZF and Na YQ: XIAP as a
prognostic marker of early recurrence of nonmuscular invasive
bladder cancer. Chin Med J (Engl). 120:469–473. 2007.
|
|
16
|
Mizutani Y, Nakanishi H, Li YN, Matsubara
H, Yamamoto K, Sato N, Shiraishi T, Nakamura T, Mikami K, Okihara
K, et al: Overexpression of XIAP expression in renal cell carcinoma
predicts a worse prognosis. Int J Oncol. 30:919–925.
2007.PubMed/NCBI
|
|
17
|
Zhang Y, Zhu J, Tang Y, Li F, Zhou H, Peng
B, Zhou C and Fu R: X-linked inhibitor of apoptosis positive
nuclear labeling: A new independent prognostic biomarker of breast
invasive ductal carcinoma. Diagn Pathol. 6:492011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bao ST, Gui SQ and Lin MS: Relationship
between expression of Smac and Survivin and apoptosis of primary
hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int.
5:580–583. 2006.PubMed/NCBI
|
|
19
|
Kempkensteffen C, Jäger T, Bub J, Weikert
S, Hinz S, Christoph F, Krause H, Schostak M, Miller K and Schrader
M: The equilibrium of XIAP and Smac/DIABLO expression is gradually
deranged during the development and progression of testicular germ
cell tumours. Int J Androl. 30:476–483. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yan Y, Mahotka C, Heikaus S, Shibata T,
Wethkamp N, Liebmann J, Suschek CV, Guo Y, Gabbert HE, Gerharz CD,
et al: Disturbed balance of expression between XIAP and Smac/DIABLO
during tumour progression in renal cell carcinomas. Br J Cancer.
91:1349–1357. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shibata T, Mahotka C, Wethkamp N, Heikaus
S, Gabbert HE and Ramp U: Disturbed expression of the apoptosis
regulators XIAP, XAF1, and Smac/DIABLO in gastric adenocarcinomas.
Diagn Mol Pathol. 16:1–8. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qin S, Xu C, Li S, Wang X, Sun X, Wang P,
Zhang B and Ren H: Hyperthermia induces apoptosis by targeting
Survivin in esophageal cancer. Oncol Rep. 34:2656–2664.
2015.PubMed/NCBI
|
|
23
|
Arellano-Llamas A, Garcia FJ, Perez D,
Cantu D, Espinosa M, De la Garza JG, Maldonado V and
Melendez-Zajgla J: High Smac/DIABLO expression is associated with
early local recurrence of cervical cancer. BMC Cancer. 6:2562006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fandy TE, Shankar S and Srivastava RK:
Smac/DIABLO enhances the therapeutic potential of chemotherapeutic
drugs and irradiation, and sensitizes TRAIL-resistant breast cancer
cells. Mol Cancer. 7:602008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
McNeish IA, Bell S, McKay T, Tenev T,
Marani M and Lemoine NR: Expression of Smac/DIABLO in ovarian
carcinoma cells induces apoptosis via a caspase-9-mediated pathway.
Exp Cell Res. 286:186–198. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Maas C, Verbrugge I, de Vries E, Savich G,
van de Kooij LW, Tait SW and Borst J: Smac/DIABLO release from
mitochondria and XIAP inhibition are essential to limit
clonogenicity of Type I tumor cells after TRAIL receptor
stimulation. Cell Death Differ. 17:1613–1623. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mao HL, Liu PS, Zheng JF, Zhang PH, Zhou
LG, Xin G and Liu C: Transfection of Smac/DIABLO sensitizes
drug-resistant tumor cells to TRAIL or paclitaxel-induced apoptosis
in vitro. Pharmacol Res. 56:483–492. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Qin S, Yang C, Wang X, Xu C, Li S, Zhang B
and Ren H: Overexpression of Smac promotes Cisplatin-induced
apoptosis by activating caspase-3 and caspase-9 in lung cancer A549
cells. Cancer Biother Radiopharm. 28:177–182. 2013. View Article : Google Scholar
|
|
29
|
Qin S, Xu C, Li S, Yang C, Sun X, Wang X,
Tang SC and Ren H: Indomethacin induces apoptosis in the EC109
esophageal cancer cell line by releasing second
mitochondria-derived activator of caspase and activating caspase-3.
Mol Med Rep. 11:4694–4700. 2015.PubMed/NCBI
|
|
30
|
Hunter AM, Kottachchi D, Lewis J, Duckett
CS, Korneluk RG and Liston P: A novel ubiquitin fusion system
bypasses the mitochondria and generates biologically active
Smac/DIABLO. J Biol Chem. 278:7494–7499. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Vucic D, Dixit VM and Wertz IE:
Ubiquitylation in apoptosis: A post-translational modification at
the edge of life and death. Nat Rev Mol Cell Biol. 12:439–452.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hu S and Yang X: Cellular inhibitor of
apoptosis 1 and 2 are ubiquitin ligases for the apoptosis inducer
Smac/DIABLO. J Biol Chem. 278:10055–10060. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hao Y, Sekine K, Kawabata A, Nakamura H,
Ishioka T, Ohata H, Katayama R, Hashimoto C, Zhang X, Noda T, et
al: Apollon ubiquitinates SMAC and caspase-9, and has an essential
cytoprotection function. Nat Cell Biol. 6:849–860. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
MacFarlane M, Merrison W, Bratton SB and
Cohen GM: Proteasome-mediated degradation of Smac during apoptosis:
XIAP promotes Smac ubiquitination in vitro. J Biol Chem.
277:36611–36616. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
McNeish IA, Lopes R, Bell SJ, McKay TR,
Fernandez M, Lockley M, Wheatley SP and Lemoine NR: Survivin
interacts with Smac/DIABLO in ovarian carcinoma cells but is
redundant in Smac-mediated apoptosis. Exp Cell Res. 302:69–82.
2005. View Article : Google Scholar
|
|
36
|
Yang QH and Du C: Smac/DIABLO selectively
reduces the levels of c-IAP1 and c-IAP2 but not that of XIAP and
livin in HeLa cells. J Biol Chem. 279:16963–16970. 2004. View Article : Google Scholar : PubMed/NCBI
|