Introduction
Stomach cancer, also known as gastric cancer,
develops from the lining of the stomach (1). The cancer may spread from the stomach
to other parts of the body, especially the lungs, liver, bones,
lining of the abdomen and lymph nodes (2). World-wide, stomach cancer is the
fifth leading cause of cancer as well as the third leading cause of
death from cancer making up 7% of cases and 9% of deaths. Gastric
cancer is a disease due to multiple factors (3,4).
Additionally, the pathogenesis of gastric cancer represents a
classic example of gene-environment interactions (5). Cancer of the stomach is difficult to
cure unless it is found at an early stage before it has begun to
spread. Because early stomach cancer causes few symptoms, the
disease is usually advanced when the diagnosis is made (6). Thus, finding effective target and
strategy for therapies is necessary.
MicroRNAs (miRNAs) are endogenous, small 18–25
nucleotides, and non-coding RNAs that negatively regulate gene
expression at the post-transcriptional level through binding to the
3′-untranslated region (UTR) belonging to a specific messenger RNAs
(mRNAs) (7–9). With sequences partially complementary
to their target mRNAs, miRNAs play critical roles in moderating
(mostly suppressing) gene expression in a variety of organisms
(10,11). Moreover, studies have confirmed
that miRNAs participate in various biological processes, including
cell growth, development, proliferation and metabolism, via
inhibition of mRNA translation (12,13).
Recently, studies revealed that ~20–30% of human genes are
regulated by miRNAs, affecting gene expression (14). miR-370 has been suggested to play
an inhibition role in many cancers or tumors, including colon
cancer and HCC, breast cancer as well as gastric cancer (15). Because modulation of miR-370 is
common to a number of cancers, it has been hypothesized that
miR-370 may play an essential role in tumor growth, development and
tumorigenesis. However, the exact role of miR-370 on tumor
progression remains unclear, especially in gastric cancer.
Previous studies indicated that miR-370 suppress
solid tumor progression originated from cancer cell lines (16,17).
It is of interest that miR-370 might be a potential target for the
inhibition of gastric cancer. However, there is not sufficient
research on the role, effect, and significance of miR-370 at the
clinical level in cervical cancer growth. Thus further study is
required to confirm the effects of miR-370 in the inhibition of
gastric cancer.
Materials and methods
Tissue specimens and cell cultures
Human gastric cancer and the adjacent normal
non-tumor tissues were acquired from patients undergoing surgical
resection in the Department of Gastric Cancer and Soft Tissue
Sarcomas, Quanzhou Affiliated First Hospital of Fujian Medical
University, Fujian, China, from January 2009 to December 2012 for
quantitative RT-PCR analysis. All tissue samples were immediately
frozen in the liquid nitrogen and then stored at −80°C for the
following studies.
Paraffin-embedded tumor tissues collected from
consecutive patients with gastric cancer between January 2009 and
December 2012 were performed for tissue assays. Clinical data
collection and postoperative follow-up procedures were done based
on a uniform guideline of the Fujian Cancer Center of Fujian
Medical University. All samples were collected and analyzed with
the prior written and informed consents, which were obtained from
all patients, and the study was approved by the Clinical Research
Ethics Committee.
Cell culture
Human gastric cancer cell lines, including NCI-N87,
MKN74, NUGC-3, MGC-803 and BGC-823, and the normal gastric RGM-1
cells were purchased from American Type Culture Collection, the
Cell Resource Center, Shanghai Institute of Biochemistry and Cell
Bank at the Chinese Academy of Sciences. Cell lines were routinely
authenticated by DNA-fingerprinting and isoenzyme analyses and
checked for contamination by mycoplasma using Hoechst staining. All
cell lines were maintained in Roswell Park Memorial Institute
(RPMI)-1640, Dulbecco’s modified Eagle’s medium or minimum
essential medium, containing 10% fetal bovine serum (FBS) and were
incubated at 37°C with 5% CO2.
Oligonucleotides
RNA oligos were synthesized chemically and purified
by Genepharma Co. Ltd., (Shanghai, China). The sense sequence of
human miR-370 mimics was 5′-GUG CCU GGG AGG CAC CAU AG-3′ and
antisense sequence was 5′-UGC UAG GUA GCC GUC CCU CCC A-3′.
Negative control oligonucleotides was 5′-AAC AAG UCC UUG UGU ACT
T-3′ and 5′-GCA UGA AUT GUA AUU CGG T-3′. The final concentration
of miRNA-370 was 50 nM.
Plasmids and transfection
The production of a miR-370 expression vector was
carried out, a 235-bp genomic fragment which covers the region
coding for pri-miR-370 and its upstream and downstream regions was
PCR amplified and then cloned onto the pLvthm vector (Addgene,
USA). The full length of PTEN-2 3′-UTR is 4,629 bp long. The
miR-370 binding site in PTEN-2 3′-UTR is located at 4,335–4,344 bp.
The region of the human PTEN-2 3′-UTR from 4,302 to 4,381 bp was
generated by PCR amplification and subcloned into the sites of
pGL3-basic luciferase reporter plasmid (Promega, USA). The miR-370
mimics, and negative control were purchased from Genecopoeia
(Genecopoeia Co. Ltd., USA) and then transfected into gastric
cancer cells with Lipofectamine 2000 reagent (Invitrogen, USA),
based on the manufacturer’s instructions.
Gene expression knockdown or inhibition
of PTEN
PTEN expression in gastric cancer cells was silenced
through transfecting the targeted siRNA sequences as follows
(Sangon Biotech, China) with Lipofectamine 2000 (Invitrogen); AGA
TAC GTT CTC TAC GCT CAG. The control siRNAs were produced through
introducing 4 base substitutions in PTEN targeting sequence (GAG
TCG GTA GCT ACA CTC). Forty-eight hours after transfection, PTEN
expression was examined by immunoblotting.
Luciferase reporter assays
The gastric cancer cells, seeded in a 48-well plate,
were co-transfected with 50 nM single-stranded miRNA mimics, or
negative control oligonucleotides, 50 ng of firefly luciferase
reporter and 10 ng of pRL-TK (Promega) with the JetPRIME reagent.
Cells were collected 36 h after the final transfection, and then
analyzed via Dual-Luciferase Reporter Assay system (Promega).
Western blot analysis
Cell proteins were extracted using T-PER Tissue
Protein Extraction Reagent kit (Thermo) according to the
manufacturer’s instructions. Protein concentrations were determined
by BCA protein assay kit, and equal amounts of protein were loaded
per well on a 10% sodium dodecyl sulphatepolyacrylamide gel.
Subsequently, proteins were transferred onto polyvinylidene
difluoride membrane. The resulting membrane was blocked with
Tris-buffered saline containing 0.05% Tween-20 (TBS-T),
supplemented with 5% skim milk (Sigma, USA) at room temperature for
2 h on a rotary shaker, and followed by TBS-T washing. The specific
primary antibody, diluted in TBST, was incubated with the membrane
at 4°C overnight. Subsequently, the membrane was washed with TBS-T
followed by incubation with the peroxidase-conjugated secondary
antibody at room temperature for 1 h. The immunoactive proteins
were detected by using an enhanced chemiluminescence western
blotting detection kit. Western blot bands were observed using GE
Healthcare ECL Western Blotting Analysis system and exposed to
X-ray film (Kodak). The primary antibodies are shown in Table I.
 | Table IThe primary antibodies performed in
western blotting. |
Table I
The primary antibodies performed in
western blotting.
| Primary
antibodies | Dilution ratio | Corporation |
|---|
| Rabbit
anti-P53 | 1:1,000 | Cell Signaling
Technology |
| Rabbit
anti-MDM2 | 1:1,000 | Abcam |
| Rabbit
anti-p-MDM2 | 1:1,000 | Abcam |
| Rabbit
anti-mTOR | 1:1,000 | Abcam |
| Rabbit
anti-p-mTOR | 1:1,000 | Abcam |
| Rabbit
anti-p-GSK3β | 1:1,000 | Cell Signaling
Technology |
| Mouse
anti-PTEN | 1:1,000 | Abcam |
| Rabbit
anti-caspase3 | 1:1,000 | Cell Signaling
Technology |
| Rabbit
anti-Bax | 1:200 | Santa Cruz
Biotechnology |
| Rabbit
anti-Bcl-2 | 1:1,000 | Cell Signaling
Technology |
| Rabbit
anti-AKT | 1:1,000 | Cell Signaling
Technology |
| Rabbit
anti-p-AKT | 1:1,000 | Cell Signaling
Technology |
| GAPDH | 1:500 | Santa Cruz
Biotechnology |
RNA isolation, reverse transcription (RT)
and real-time PCR (RT-PCR)
Total RNA from tissue samples and cultured cells was
isolated through the mirVana miRNA Isolation kit (Ambion) based on
the manufacturer’s instructions. Then the cDNA was synthesized from
total RNA with the TaqMan miRNA reverse transcription kit (Applied
Biosystems, USA). Real-time PCR was conducted using the Applied
Biosystems 7500 Sequence Detection system with iQ™ SYBR Green
Supermix (Bio-Rad Laboratories, USA) containing 5 ng cDNA and 10 pM
of each primer. The data were normalized to the geometric mean of
housekeeping gene GAPDH or U6 small nuclear RNA expression and
calculated as 2−ΔΔCT method. Sequences of the primers
are summarized as follows: miR-370 forward, 5′-GCA TCG TTC CTT CAA
GCC GAT CT-3′ and reverse, 5′-TGG GTG AGT CGT TCG G-3′; U6 forward,
5′-GTC CTG GCA GAT ATA CAC TAA ACA T-3′ and reverse, 5′-CTC ACG CTT
GAA TTC ATG CGG CTT-3′; PTEN forward, 5′-TGT TTG GCA GAT CTT CCT
TG-3′ and reverse, 5′-CTC GGT CGT CGC TCA TAT-3′; GAPDH forward,
5′-CAT TCA AGA CCG GAC AGA GG-3′ and reverse, 5′-ACA TAC TCA GCA
CCA GCA TCA CC-3′.
Cell proliferation analysis
The transfected gastric cancer cells were seeded
into 96-well plates at a density of 1×104 cells per
well. Twenty microliters of 5 mg/ml MTT solution was administered
to the cultures for a total volume of 200 μl and then incubated for
4 h at 37°C. After discarding the culture medium, the remaining
crystals were then dissolved in DMSO. Finally, the absorbance of
560 nm was determined.
Colony-forming, migration and invasion
assay
Gastric cancer cells were suspended in 0.9%
methylcellulose-based semisolid medium MethoCult H4100 (StemCell,
Beijing, China). After 14 days, individual primary clones (450
cells) were trypsinized and re-plated in the same conditions to
examine the secondary colony-forming ability for self-renewal. For
the Transwell migration assays, 10×104 cells were placed
in the top chamber with a non-coated membrane. For the invasion
assays, 2×105 cells were placed in the top chamber with
a Matrigel-coated membrane. For both assays, the cells were seeded
in a serum-free medium, and a medium with 10% serum was used as a
chemoattractant in the lower chamber. The cells were then incubated
for 16 h at 37°C and 5% CO2 in a tissue culture
incubator. After 16 h, the non-migrated/non-invading cells were
removed from the upper sides of the Transwell membrane filter
inserts with cotton-tip swabs. The migrated/invaded cells on the
lower sides of the inserts were then stained with Giemsa, and
finally the cells were counted.
Establishment of xenograft tumor
models
The mouse experiments were conducted in the Animal
Laboratory Center. NUGC-3 cells (1×107 cells) treated
with miR-370 mimics were suspended in 100 μl serum-free medium and
injected subcutaneously into the left flank of 4- to 6-week old
male BALB/c nu/nu nude mice. Tumor size was measured with digital
caliper and calculated every week. Tumor volume was measured every
seven days and at the end of ~6 weeks after treatment, mice were
sacrificed. Tumors were excised, weighed, fixed in 10% neutral
formalin, and embedded in paraffin for histological analysis.
Immunohistochemistry
The cells seeded on the plates were dewaxed and
rehydrated with xylene and a graded alcohol series. Endogenous
peroxidase activity was blocked with 3% hydrogen peroxide for 15
min at room temperature. After washing in water, non-specific
binding sites were blocked with 5% bovine serum in
phosphate-buffered saline (PBS) for 30 min at room temperature. The
samples were incubated with primary monoclonal antibody PTEN, P53
and caspase-3 (1:200) at 4°C overnight. The slide was then gently
rinsed with PBS and developed by the Envision system/HRP for 30 min
and substrate-chromogen for 15 min at room temperature. The nuclei
were counterstained with Mayer’s hematoxylin.
Immunofluorescence assays
After induction by conditioned culture medium, the
cells were fixed in 4% paraformaldehyde, permeabilized with 0.1%
Triton X-100 in PBS containing 0.5% BSA (PBS-BSA) for 30 min. The
cells were subsequently incubated with PTEN for 30 min, followed by
labeling with Alexa Fluor 488-conjugated rabbit anti-mouse or goat
anti-rabbit IgG antibody. The cells were viewed under a fluorescent
microscope.
Flow cytometry assays
Flow cytometric assay was used to clarify the
apoptotic cells and the cell cycle arrest. The gastric cancer cells
were collected with trypsinisation and then washed twice with PBS,
and fixed in cold 80% ethanol, and finally stored at 4°C overnight.
The cells were washed with PBS twice and RNase A (10 mg/ml) was
administered for analysis. Propidium iodide was then added to tubes
at a concentration of 0.05 mg/ml and then incubated for 20 min at
4°C in the dark. FITC-labeled Annexin V/PI staining was applied
based on the manufacturer’s instructions (Keygen, China). In brief,
1×106 cells in each well were suspended with buffer
containing FITC-conjugated Annexin V/PI. Samples were then analyzed
by flow cytometry.
Statistical analysis
The differences of the data are presented as the
means ± SEM. The treated tissue and the corresponding controls were
compared using Graph Pad Prism (version 6.0; Graph Pad Software,
USA) by one-way ANOVA with Dunn’s least significant difference
tests. Differences between groups were considered significant at
p<0.05.
Results
miR-370 is decreased in the tissues and
cell lines of gastric cancer
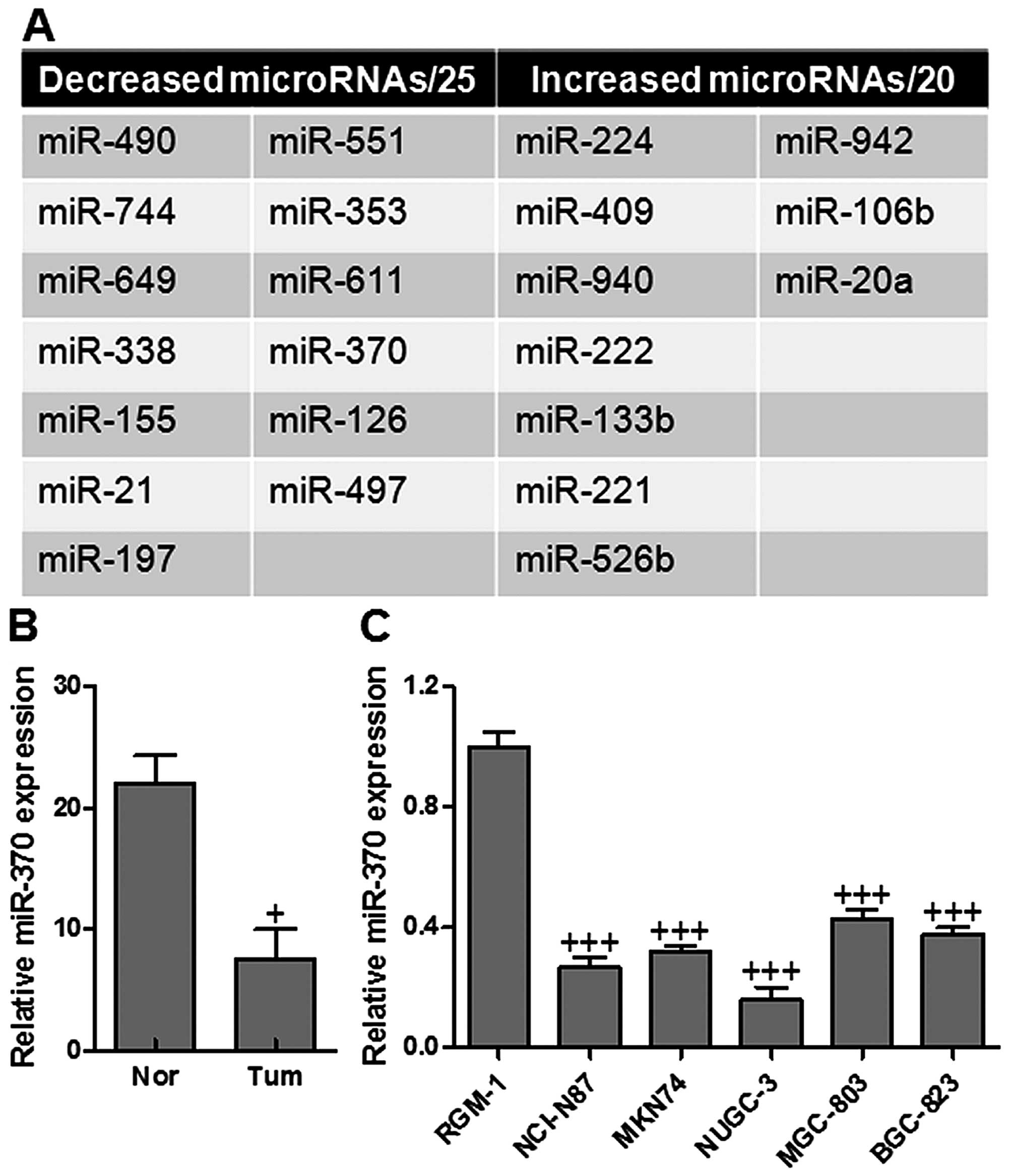
To be included in our analysis, the protein target
of PTEN had to be predicted concordantly by at least three
prediction tools and the following were used in our study: PicTar,
miRanda, TargetScan as well as miRDB. Based on the released version
of January 2012, we found 8 microRNAs which potentially targeted
PTEN. We explored several miRNAs expressed abnormally in gastric
cancer with a gene chip (Fig. 1A).
Based on the results of predictions above, miR-370 was an important
upstream target of PTEN. To determine the expression levels of
miR-370 in gastric cancer samples and cell lines, total RNAs were
extracted from gastric cancer tissues and cell lines, and the
expression levels of miR-370 were analyzed by RT-PCR and normalized
to endogenous control (U6 RNA). As shown in Fig. 1B, miR-370 was significantly
decreased in gastric cancer tissues in comparison with adjacent
normal tissues. It was also shown that miR-370 was downregulated in
gastric cancer cell lines, compared with normal gastric RGM-1 cells
(Fig. 1C). The data suggested that
miR-370 was decreased in the tissues and cell lines of gastric
cancer.
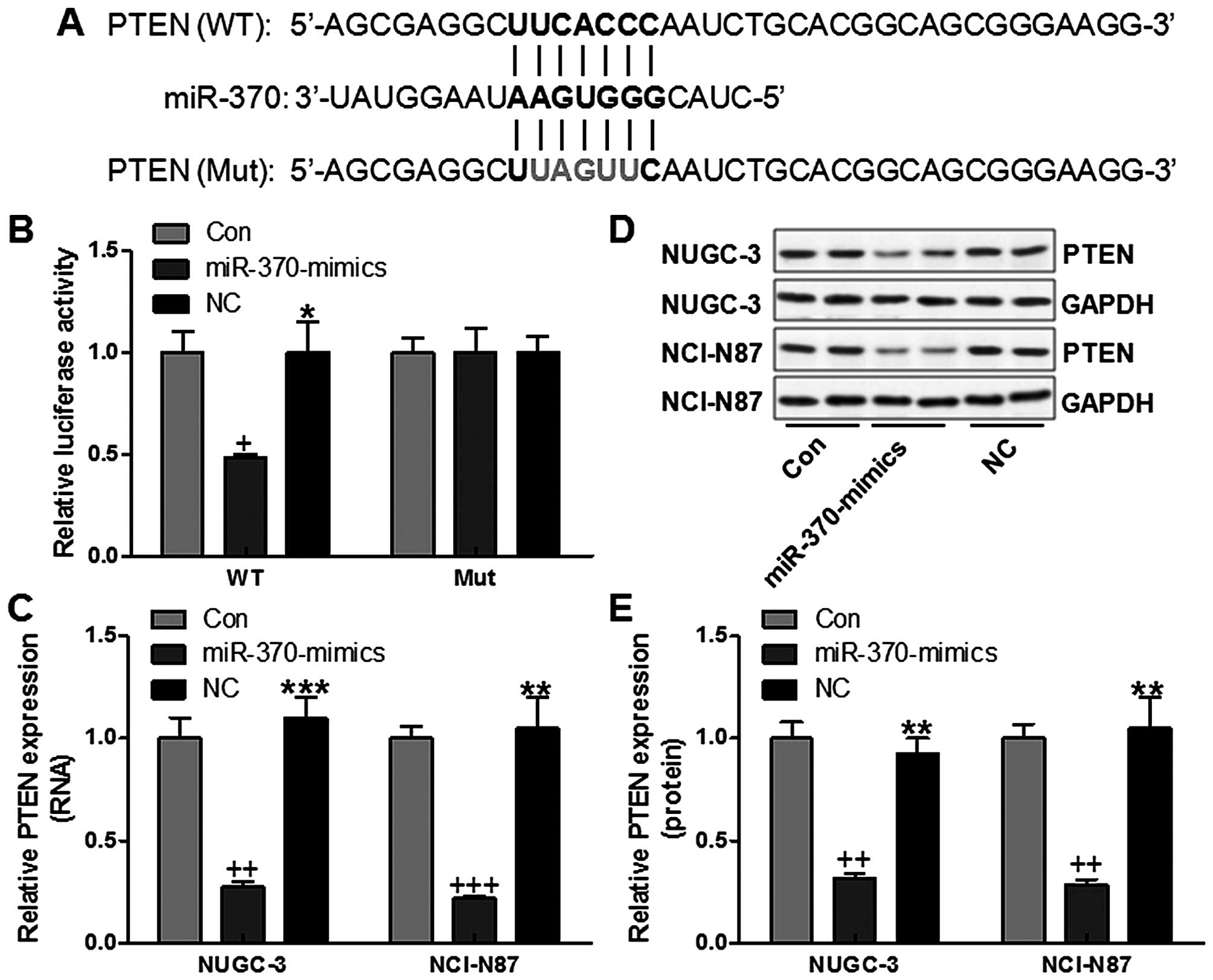
miR-370 directly targets and regulates
PTEN expression in gastric cancer cells
miRNAs mainly function via its regulation of target
genes, and the target gene of miR-370 was further analyzed. After
checking the newly published CLASH data, ~408 genes were targeted
by miR-370 in NUGC-3 cells. Among these genes, PTEN, a key
regulator in apoptosis and proliferation, modulating cellular
processes, was focused on in our study. In order to confirm whether
miR-370 could affect the expression of PTEN, we performed
luciferase reporter assays in NUGC-3 cells. We then created a
luciferase reporter plasmid with wild-type (WT) or mutant (Mut)
targeting sequence of PTEN mRNA (Fig.
2A), which were cotransfected with miR-370 mimics or the
negative control (NC) oligonucleotides into NUGC-3 cells for 48 h,
and determined luciferase activity in the transfected cancer cells.
Our results indicated that the reporter plasmid with WT targeting
sequence of PTEN mRNA caused a significant downregulation of
luciferase activity in cells transfected with miR-370 compared with
the control group without any treatment and negative group, whereas
reporter plasmid with mutant sequence of PTEN produced no
alteration of luciferase activity (Fig. 2B). NUGC-3 and NCI-N87 gastric
cancer cells were transfected with miR-370 mimics, or negative
control oligonucleotides, and PTEN mRNA and protein levels were
examined by RT-PCR and western blot analysis, respectively. PTEN
mRNA expression was low by miR-370 mimics in NUGC-3 and NCI-N87
cells, respectively (Fig. 2C). The
level of PTEN protein was consistently and substantially
downregulated by miR-370 in NUGC-3 gastric cancer cells,
respectively (Fig. 2D and E).
Also, similar expression levels were further confirmed in NCI-N87
gastric cancer cells (Fig. 2D and
E). The results suggested that PTEN might be a direct target of
miR-370 in gastric cancer cells.
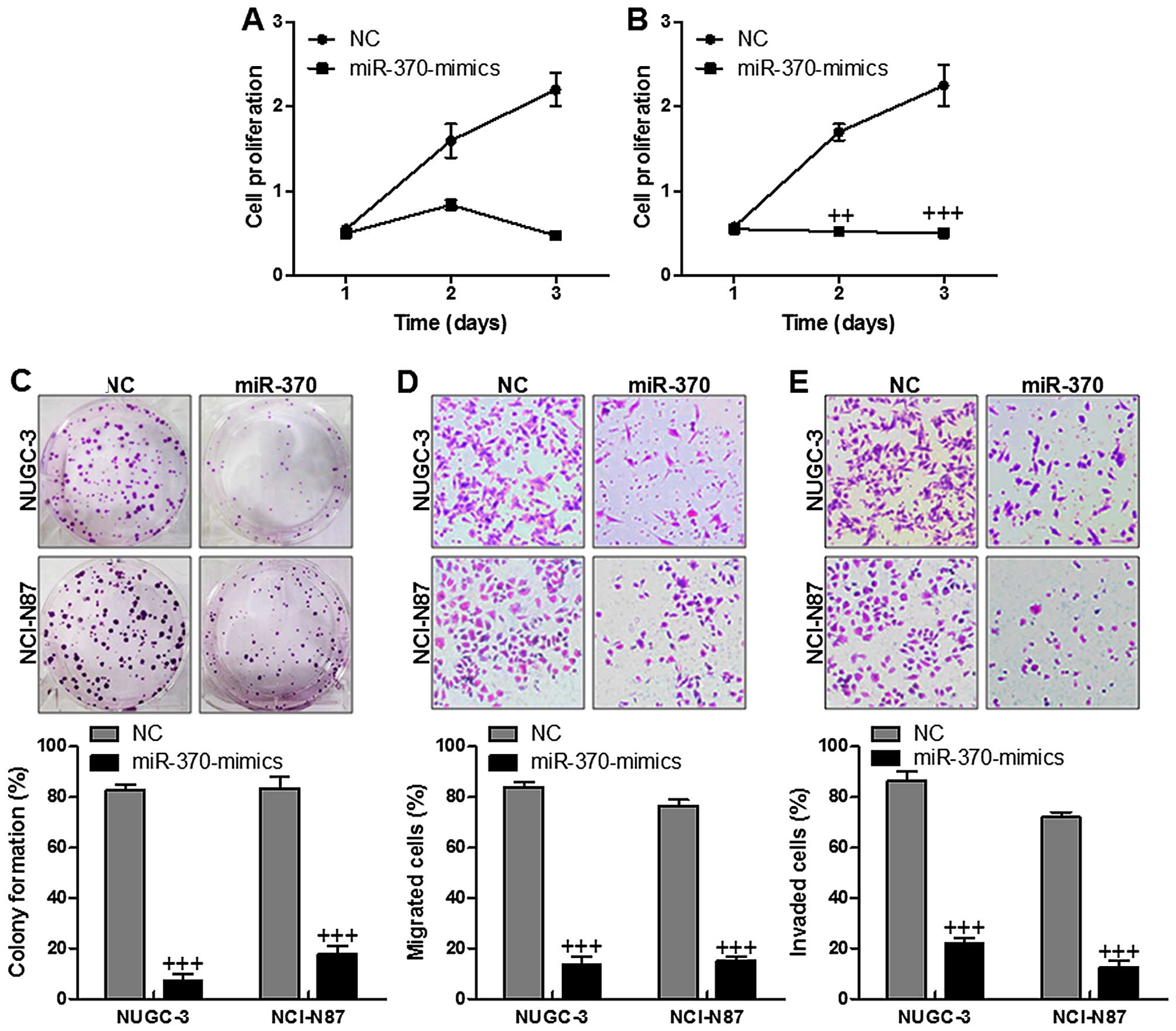
miR-370 downregulates gastric cancer cell
growth and proliferation
The gastric cancer cells with the enhanced miR-370
expression exhibited downregulated cell proliferation significantly
in comparison to the cells of NC group in both NUGC-3 (Fig. 3A) and NCI-N87 cell lines (Fig. 3B). Cell function assays displayed
that upregulation of miR-370 revealed lower number of colony
formation in both NUGC-3 and NCI-N87 gastric cancer cell lines
(Fig. 3C). In addition, impact of
miR-370 on cell migration (Fig.
3D) and invasion (Fig. 3E)
across a Transwell chamber showed that increasing of miR-370
ameliorated migration and invasion capacity of both gastric cancer
cell lines, while upregulation of miR-370 inhibited migration and
invasion capacity of both gastric cancer cell lines. In conclusion,
the data suggested that miR-370 plays a potentially inhibiting role
in gastric cancer growth and progression via the inhibition of cell
proliferation, migration and invasion.
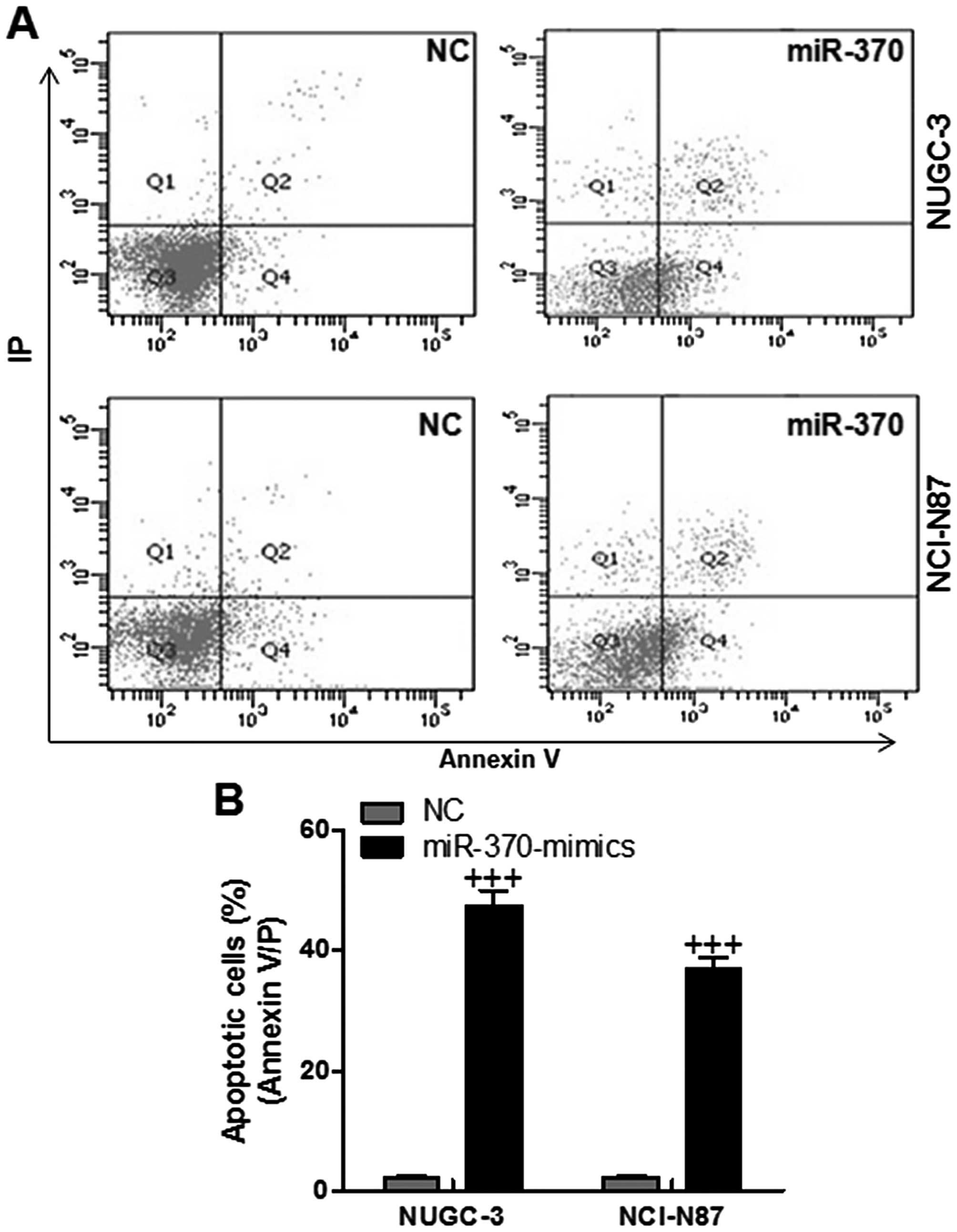
The effects of miR-370 on apoptosis in
the gastric cancer cell lines via flow cytometry assays
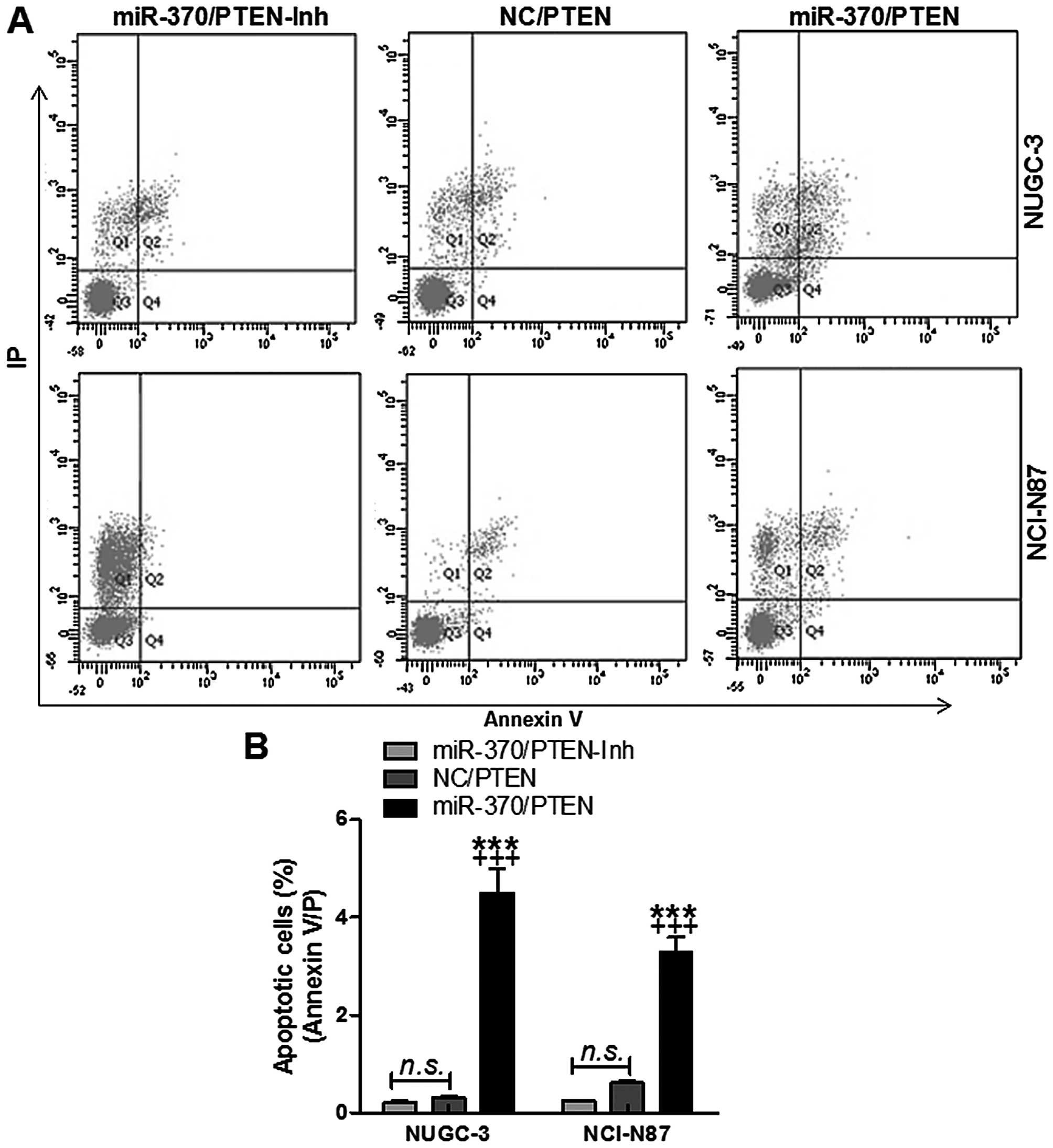
In order to further confirm that miR-370 could
target PTEN, resulting in apoptosis in gastric cancer cells and
ameliorating gastric cancer progression, flow cytometry was used to
evaluate the apoptotic levels in NUGC-3 and NCI-N87 cells. As shown
in Fig. 4, the number of apoptotic
cells were higher in miR-370-mimic treated groups, indicating that
miR-370 had a potential role in enhancing apoptosis in gastric
cancer. We found that gastric cancer cell apoptosis was
downregulated in cells transfected with miR-370 mimics with PTEN
inhibition. However, the number of apoptotic cells was enhanced in
the cells with forced miR-370 and PTEN restoration in both NUGC-3
and NCI-N87 cell lines (Fig. 5).
The data indicated that miR-370 regulated apoptosis in gastric
cancer cells via PTEN regulation.
The effects of miR-370 on cell cycle in
the gastric cancer cell lines via flow cytometry assays
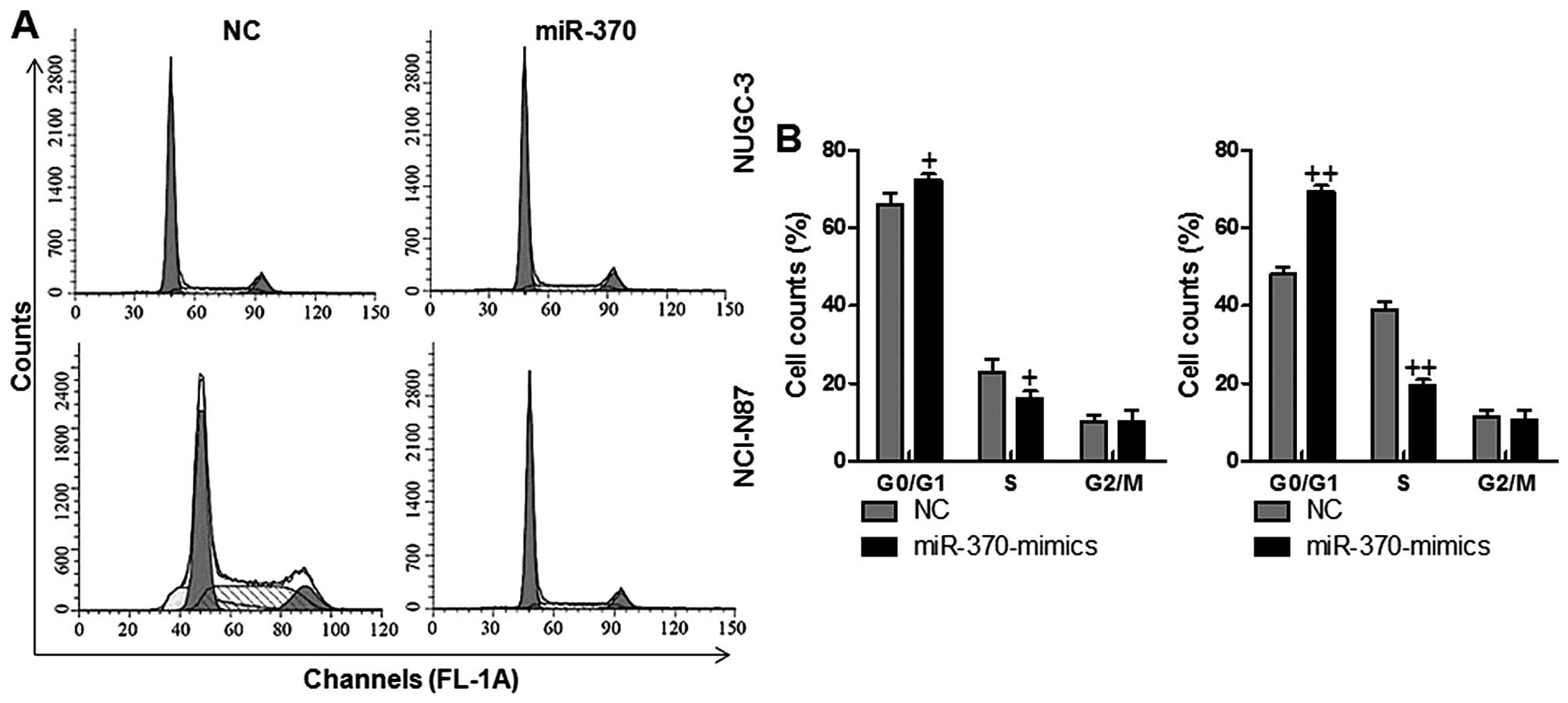
In order to determine if the reduction of cell
growth in NUGC-3 and NCI-N87 cells regulated by miR-370 was linked
with the alterations of cell cycle arrest, the gastric cancer cells
were transfected with miR-370. Subsequently, flow cytometry was
performed to determine the cell cycle. Our results showed that
miR-370 mimics caused an increased population of G0/G1 and
decreased population of S phase cells, suggesting that miR-370 had
a potential ability to arrest the cervical cancer cell cycle in the
G0/G1 and S phase (Fig. 6). No
alteration was found in G2/M phase in the two groups in the gastric
cancer cellss NUGC-3 and NCI-N87. Collectively, these data
indicated that the inhibitory role of miR-370 on gastric cancer
cell growth was related to induction of G0/G1 and S phase
arrest.
miR-370 induces gastric cancer cell line
apoptosis via p-AKT and Bcl-2 inactivation and caspase-3
activation
Loss of PTEN leads to the P13K/AKT signaling pathway
to be hyperactive, resulting in cell survival and resistance to
therapeutics in various cancers, including liver cancer (18). The Bcl-2 protein family includes
major regulators of cell survival, including Bax, which promotes or
suppresses apoptosis progression. Additionally, the caspase
signaling pathway was partly regulated by Bcl-2 (19). The data above indicated that
restoration of miR-370 could suppress the growth and proliferation
of gastric cancer cells and PTEN might be a potential target of
miR-370. So we hypothesized that miR-370 regulated cell growth in
gastric cancer cells by targeting PTEN and its possible related
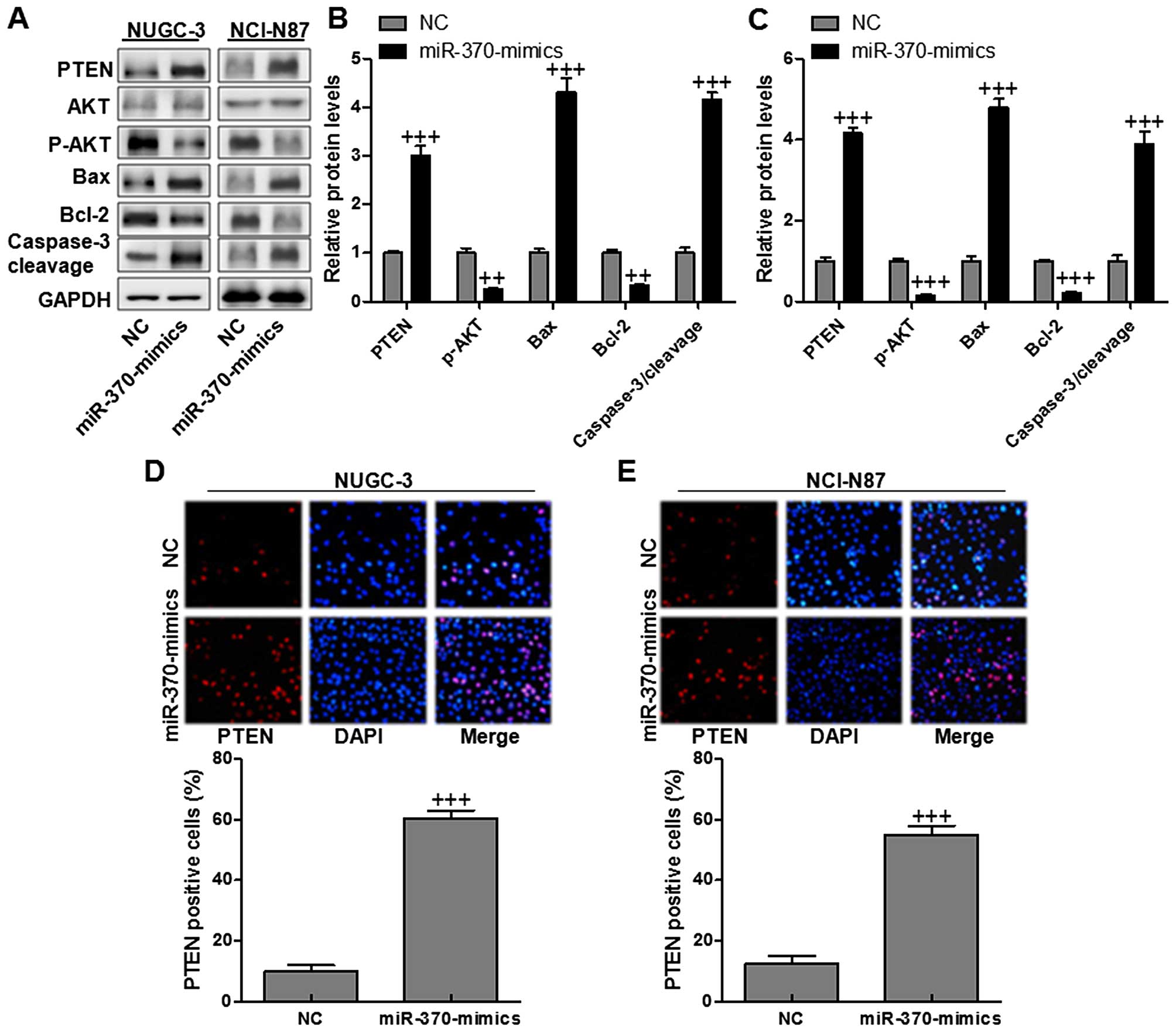
signals. As expected, PTEN in NUGC-3 and NCI-N87 cells was reduced
significantly (Fig. 7A–C). In
addition, AKT was downregulated with Bcl-2 decreasing in cells with
high level of miR-370. However, Bax was markedly activated, leading
to the increasing of caspase-3 cleavage. Finally, apoptosis was
enhanced due to caspase-3 activity. PTEN expression levels were
determined via immunofluorescent assays. In NUGC-3 and NCI-N87
gastric cancer cells (Fig. 7D and
E), PTEN was highly expressed in miR-370 mimic-treated group in
comparison to the NC group. The data illustrated that miR-370 had a
possible effect on accelerating apoptosis in gastric cancer cell
growth and progression via caspase-3 activation related with PTEN
activation.
miR-370 regulates gastric cancer cell
line proliferation and growth via p53 and mTOR signaling pathway
through PTEN modulation
The murine double minute 2 (MDM2) protein is
involved in the regulation of growth, survival, and invasion. p53,
as an important tumor suppressor, was regulated by MDM2. MDM2/P53
signaling pathway plays an essential role in cell cycle arrest,
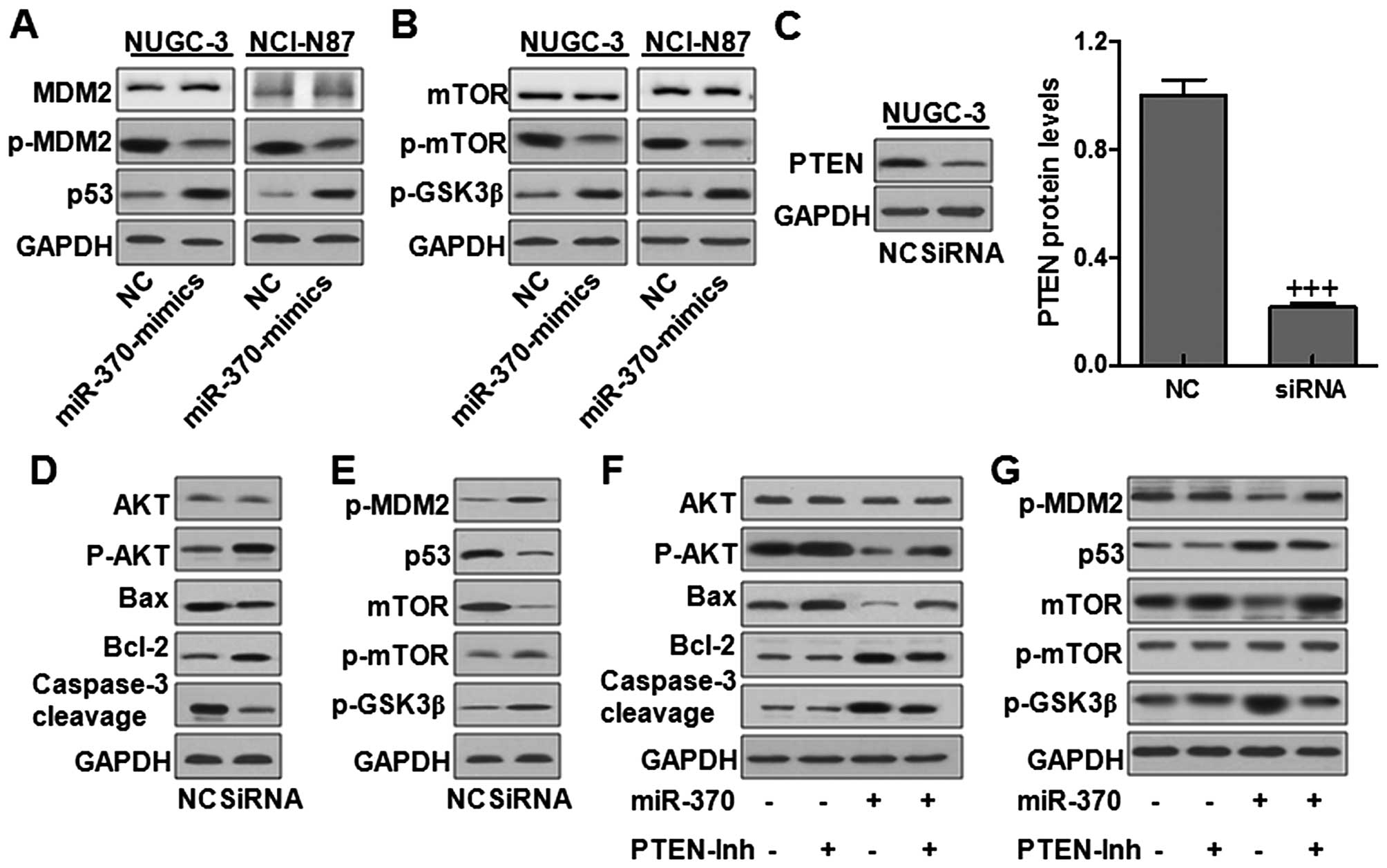
regulating cell proliferation (20). Here we found that MDM2 was
phosphorylated significantly in the NC group and miR-370 reduced
this effect. Subsequently, p53 was upregulated, reaching the effect
of gastric cancer cell growth (Fig.
8A). mTOR and GSK3β play important roles in cell growth
translation and cell cycle. In our study, we found that mTOR was
inhibited due to the high levels of miR-370 in both gastric cancer
cells. While GSK3β was found to be enhanced in miR-370 mimics
treatment (Fig. 8B). The data
suggested that miR-370 could regulate gastric cancer cell
proliferation and growth via MDM2 and mTOR inactivation and p53 and
GSK3β activation. Next, PTEN was knocked down via siRNA treatment.
As shown in Fig. 8C, PTEN was
downregulated significantly in the NUGC-3 cells. Then the AKT,
caspase-3, MDM2, mTOR and GSK3β signals were investigated. Fig. 8D shows that AKT activity was
enhanced due to PTEN knockdown, resulting in Bcl-2 upregulation and
Bax downregulation, and caspase-3 was reduced accordingly. PTEN
knockdown promoted MDM2 phosphorylation and p53 decreased. In
addition, mTOR was activated combined with the upregulation of
GSK3β, further indicating that gastric cancer progression was, at
least partly, related with PTEN expression levels (Fig. 8E). In order to further explore the
effects of miR-370 and its target PTEN on gastric cancer cell
growth, we combined miR-370 and PTEN-inhibitor together. As shown
in Fig. 8F, we found that it was
the combination of miR-370 and PTEN that had a significant role in
reducing AKT activation and caspase-3 promotion, leading to
apoptosis in gastric cancer cells. Also, the combined miR-370 and
PTEN could reduce p-MDM2 while increase p53 and GSK3β levels
(Fig. 8G). The data further
confirmed that PTEN was a direct target for miR-370-regulated
gastric cancer development.
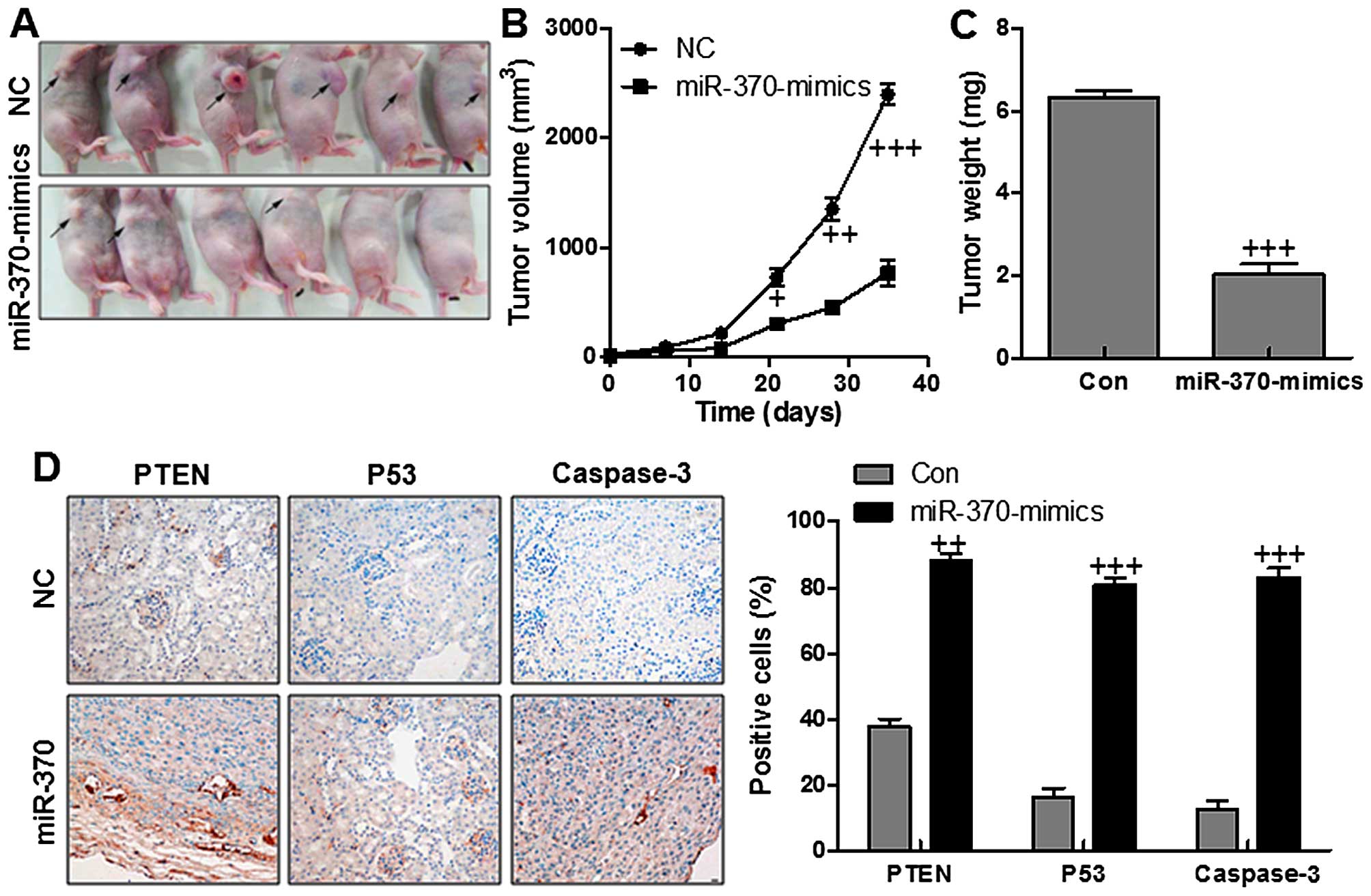
miR-370 inhibites tumor growth and
progression in vitro
To understand whether miR-370 is involved in
cervical cancer tumorgenesis in vivo, we engineered NUGC-3
cells to stably overexpress miR-370. The control cells, and
miR-370-over-expressing cells were subcutaneously inoculated into
nude mice, respectively. As shown in Fig. 9A and B, the tumors in the NC group
grew more rapidly than the tumors in miR-370 group. Also, the tumor
weight was higher in the NC group in comparison to the miR-370
group with significant difference (Fig. 9C). The results indicated that
miR-370 could inhibit cervical cancer growth in vivo.
Consistently, gastric cancer tissues with low miR-744 showed much
lower expression of PTEN, P53 and caspase-3, compared with the
normal gastric cancer tissues displaying high level of miR-370
(Fig. 9D). Taken together, the
results demonstrated that PTEN might be a potential target of
miR-370 in inhibiting gastric cancer cells in vivo.
Discussion
Gastric cancer is one of the most frequent of all
cancers world-wide (21). Most of
the patients are diagnosed in the advanced stage and no more than
one-half of these patients survive for over five years. Thus,
searching for a more effective therapeutic strategy for detecting
gastric cancer in early-stage is necessary for gastric cancer
diagnosis and treatment (22).
miRNAs are small non-coding RNAs, binding to sites in the
3′-untranslated region of targeting mRNAs (23), leading to mRNA degradation and
translational repression (24).
Previous studies have reported a number of miRNAs, which have
essential effects on cancer progression, especially in the
progression of tumor invasion as well as metastasis (25,26).
Downregulation of microRNA-370 has been suggested in various
cancers. microRNA-370 can play as an oncogene or a tumor inhibitor
gene. However, the clinicopathological importance of miR-370
expressed levels in gastric cancer has not been revealed. miR-370
expression was found to be frequently reduced in glioma tissues. In
addition, its expression was negatively correlated with the
malignant degree of many tumors. To our knowledge, miR-370
expression in gastric cancer has not been reported. This study was
conducted to explore the miR-370 expression in gastric cancer
tissues.
We reported that miR-370 was downregulated in
gastric cancer tissues compared to the matched adjacent tissues of
the tumor, and also the result was confirmed in gastric cancer cell
lines. Decreased expression of miR-370 was related to
clinicopathological features of gastric cancer significantly,
including small tumor size, inhibition of cell migration,
proliferation and invasion. Functionally, reduced expression of
miR-370 promoted proliferation, colony formation as well as cell
cycle progression in gastric cancer cells. Further, we found that
PTEN was a novel direct target of miR-370. The functional role of
miR-370 alteration in gastric cancer cells was related to PTEN.
Furthermore, the PTEN expression was decreased in gastric cancer
cells and its expression level was correlated with miR-370.
Additionally, miR-370 was found to be related with G0/G1 and S
phase arrest induction. Together, our results indicated that PTEN
is a direct target of miR-370, which could inhibit gastric cancer
development and progression.
AKT is a serine/threonine kinase-activated
downstream of integrin (27). It
is a receptor for different bioactive substances, pro-proliferation
and the extracellular matrix receptor. AKT activation often results
in tumorigenesis and plays an important role in cell motility
regulation, which is essential for local invasion as well as
metastasis. In the PTEN heterozygous knockout mice, PI3K/AKT
signaling pathway is continually activated, contributing to tumor
formation (28–30). Amplification and mutations of AKT
gene lead to AKT activation in a variety of hematological neoplasms
and solid carcinomas, and PTEN mutation has a negative effect on
PI3K/AKT activation, causing the abnormal AKT activation (31). In our study, we found that PTEN was
reduced significantly in gastric cancer cells with miR-370
downregulation. Then, abnormal AKT activation was caused, which was
consistent with previous studies. The results here suggested that
miR370-regulated PTEN alteration displayed regulation role in AKT
activity. AKT regulates cancer cell proliferation by modulating
Bcl-2, Bax and caspase-3, which are linked to apoptosis. Studies
have suggested that phosphorylated AKT could enhance Bcl-2 while
suppressing Bax expression (32,33).
As an anti-apoptotic factor, Bcl-2 was found to be downregulated in
gastric cancer cells with forced miR-370. In contrast, Bax as a
pro-apoptotic factor was found to be enhanced after AKT
inactivation induced by PTEN promotion with miR-370 enhancement. Of
note, the cleaved caspase-3 was discovered with high expression
with PTEN and Bax increasing as well as p-AKT and Bcl-2 decreasing
regulated by miR-370, which led to apoptosis in gastric cancer
cells. The data above revealed that miR-370-targeted PTEN could
inhibit gastric cancer progression and development via apoptosis
promotion through inhibiting AKT and activating caspase-3.
Under normal physiological situations, P53, an
important tumor suppressor gene, suppresses cell proliferation,
inhibits cell division, and promotes the damaged DNA repair
(34,35). Also, p53 initiates apoptosis
formation in case of failed DNA repair (36). In the absence of normal p53
protein, cells are known to be sensitive to the S phase entry with
damaged DNA and the genetic alterations, leading to change of cell
malignant and tumor formation (37). As a negative regulator of p53, mdm2
interacts with p53 protein to inhibit the transcriptional
activation of p53, leading to cell proliferation in a tumor
(38). In our study, we found that
miR-370 could inhibit MDM2 activation, and subsequently, p53 was
stimulated, performing its role in suppression of gastric cancer
cell proliferation. The data in this regard illustrated that the
miR-370-suppressed gastric cancer progression, at least partly was
related to p53 activation and MDM2 inhibition.
mTOR is a serine/threonine kinase that is activated
by AKT (39). The PI3K-AKT-mTOR
pathway has been reported to be activated in many malignant tumors
due to abnormalities in various genes. These fundamental findings
suggest that the PI3K-AKT-mTOR pathway is important as a target for
anti-neoplastic agents (40,41).
In our study, phosphorylated mTOR was found to be downregulated in
gastric cancer cells with miR-370 enhancement. GSK3β was in
contrast upregulated in the cells with high levels of miR-370,
suppressing the cell cycle and growth.
In this study, we found that miR-370 could inhibit
gastric cancer progression via targeting PTEN, which inactivated
AKT. Subsequently, caspase-3 was enhanced, causing apoptosis.
Further, p53 signaling pathway was stimulated due to increasing
miR-370, displaying antitumor effects on gastric cancer. Also, mTOR
inhibition as well as GSK3β promotion were discovered in gastric
cancer cells expressing high level of miR-370. Of note, we found
that it was the combination of miR-370 and PTEN that performed the
role in gastric cancer suppression. Our results suggested that
promoting miR-370 might be a therapeutic strategy in the gastric
cancer treatment.
References
|
1
|
Karpińska-Kaczmarczyk K, Lewandowska M,
Białek A, Ławniczak M and Urasińska E: Gastric hyperplastic polyps
coexisting with early gastric cancers, adenoma and neuroendocrine
cell hyperplasia. Pol J Pathol. 67:33–38. 2016. View Article : Google Scholar
|
|
2
|
Jung Y, Park J, Bang YJ and Kim TY: Gene
silencing of TSPYL5 mediated by aberrant promoter methylation in
gastric cancers. Lab Invest. 88:153–160. 2008. View Article : Google Scholar
|
|
3
|
Ushijima T and Sasako M: Focus on gastric
cancer. Cancer Cell. 5:121–125. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Smith MG, Hold GL, Tahara E and El-Omar
EM: Cellular and molecular aspects of gastric cancer. World J
Gastroenterol. 12:2979–2990. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Panani AD: Cytogenetic and molecular
aspects of gastric cancer: Clinical implications. Cancer Lett.
266:99–115. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Maxwell AW, Wood S and Dupuy DE: Primary
extraskeletal Ewing sarcoma of the stomach: A rare disease in an
uncommon location. Clin Imaging. 40:843–845. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Iorio MV and Croce CM: MicroRNAs in
cancer: Small molecules with a huge impact. J Clin Oncol.
27:5848–5856. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hobert O: Gene regulation by transcription
factors and microRNAs. Science. 319:1785–1786. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Glud M, Rossing M, Hother C, Holst L,
Hastrup N, Nielsen FC, Gniadecki R and Drzewiecki KT:
Downregulation of miR-125b in metastatic cutaneous malignant
melanoma. Melanoma Res. 20:479–484. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ueda T, Volinia S, Okumura H, Shimizu M,
Taccioli C, Rossi S, Alder H, Liu CG, Oue N, Yasui W, et al:
Relation between microRNA expression and progression and prognosis
of gastric cancer: A microRNA expression analysis. Lancet Oncol.
11:136–146. 2010. View Article : Google Scholar
|
|
11
|
Croce C: Introduction to the role of
microRNAs in cancer diagnosis, prognosis, and treatment. Cancer J.
18:213–214. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nikitina EG, Urazova LN and Stegny VN:
MicroRNAs and human cancer. Exp Oncol. 34:2–8. 2012.PubMed/NCBI
|
|
13
|
Holley CL and Topkara VK: An introduction
to small non-coding RNAs: miRNA and snoRNA. Cardiovasc Drugs Ther.
25:151–159. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Baroni D and Arrigo P: MicroRNA target and
gene validation in viruses and bacteria. Methods Mol Biol.
1107:223–231. 2014. View Article : Google Scholar
|
|
15
|
Sim J, Ahn H, Abdul R, Kim H, Yi KJ, Chung
YM, Chung MS, Paik SS, Song YS and Jang K: High MicroRNA-370
expression correlates with tumor progression and poor prognosis in
breast cancer. J Breast Cancer. 18:323–328. 2015. View Article : Google Scholar
|
|
16
|
Duan N, Hu X, Yang X, Cheng H and Zhang W:
MicroRNA-370 directly targets FOXM1 to inhibit cell growth and
metastasis in osteosarcoma cells. Int J Clin Exp Pathol.
8:10250–10260. 2015.PubMed/NCBI
|
|
17
|
Chen T, Gao F, Feng S, Yang T and Chen M:
MicroRNA-370 inhibits the progression of non-small cell lung cancer
by down-regulating oncogene TRAF4. Oncol Rep. 34:461–468.
2015.PubMed/NCBI
|
|
18
|
Cantley LC and Neel BG: New insights into
tumor suppression: PTEN suppresses tumor formation by restraining
the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci USA.
96:4240–4245. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yo YT, Shieh GS, Hsu KF, Wu CL and Shiau
AL: Licorice and licochalcone-A induce autophagy in LNCaP prostate
cancer cells by suppression of Bcl-2 expression and the mTOR
pathway. J Agric Food Chem. 57:8266–8273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lev Bar-Or R, Maya R, Segel LA, Alon U,
Levine AJ and Oren M: Generation of oscillations by the p53-Mdm2
feedback loop: A theoretical and experimental study. Proc Natl Acad
Sci USA. 97:11250–11255. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yoshikawa T, Aoyama T, Tanabe K, Nishikawa
K, Ito Y, Hayashi T, Cho H, Miyashita Y, Tsuburaya A and Sakamoto
J: Feasibility and safety of transhiatal approach and D2 total
gastrectomy after neoadjuvant chemotherapy for adenocarcinoma of
the esophago-gastric junction: A subset analysis of the COMPASS
trial. Dig Surg. 33:424–430. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Burkitt MD, Varro A and Pritchard DM:
Importance of gastrin in the pathogenesis and treatment of gastric
tumors. World J Gastroenterol. 15:1–16. 2009. View Article : Google Scholar :
|
|
23
|
Kozomara A and Griffiths-Jones S: miRBase:
Integrating microRNA annotation and deep-sequencing data. Nucleic
Acids Res. 39(Database): D152–D157. 2011. View Article : Google Scholar :
|
|
24
|
Wojcicka A, Swierniak M, Kornasiewicz OW,
Maciag M, Kolanowska M, Kotlarek M, Gornicka B, Koperski L,
Niewinski G, et al: Next generation sequencing reveals microRNA
isoforms in liver cirrhosis and hepatocellular carcinoma. Int J
Biochem Cell Biol. 53:208–217. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nielsen CB, Shomron N, Sandberg R,
Hornstein E, Kitzman J and Burge CB: Determinants of targeting by
endogenous and exogenous microRNAs and siRNAs. RNA. 13:1894–1910.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jazdzewski K, Boguslawska J, Jendrzejewski
J, Liyanarachchi S, Pachucki J, Wardyn KA, Nauman A and de la
Chapelle A: Thyroid hormone receptor beta (THRB) is a major target
gene for microRNAs deregulated in papillary thyroid carcinoma
(PTC). J Clin Endocrinol Metab. 96:E546–E553. 2011. View Article : Google Scholar
|
|
27
|
Courtney KD, Corcoran RB and Engelman JA:
The PI3K pathway as drug target in human cancer. J Clin Oncol.
28:1075–1083. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Song MS, Salmena L and Pandolfi PP: The
functions and regulation of the PTEN tumour suppressor. Nat Rev Mol
Cell Biol. 13:283–296. 2012.PubMed/NCBI
|
|
29
|
Downes CP, Perera N, Ross S and Leslie NR:
Substrate specificity and acute regulation of the tumour suppressor
phosphatase, PTEN. Biochem Soc Symp. 74:69–80. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kong D and Yamori T: Advances in
development of phosphatidylinositol 3-kinase inhibitors. Curr Med
Chem. 16:2839–2854. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Roy S, Yu Y, Padhye SB, Sarkar FH and
Majumdar AP: Difluorinated-curcumin (CDF) restores PTEN expression
in colon cancer cells by down-regulating miR-21. PLoS One.
8:e685432013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kunze D, Wuttig D, Fuessel S, Kraemer K,
Kotzsch M, Meye A, Grimm MO, Hakenberg OW and Wirth MP: Multitarget
siRNA inhibition of antiapoptotic genes (XIAP, BCL2, BCL-X(L)) in
bladder cancer cells. Anticancer Res. 28B:2259–2263. 2008.
|
|
33
|
Wang YB, Qin J, Zheng XY, Bai Y, Yang K
and Xie LP: Diallyl trisulfide induces Bcl-2 and
caspase-3-dependent apoptosis via downregulation of Akt
phosphorylation in human T24 bladder cancer cells. Phytomedicine.
17:363–368. 2010. View Article : Google Scholar
|
|
34
|
Bouska A, Lushnikova T, Plaza S and
Eischen CM: Mdm2 promotes genetic instability and transformation
independent of p53. Mol Cell Biol. 28:4862–4874. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Clegg HV, Itahana K and Zhang Y: Unlocking
the Mdm2-p53 loop: Ubiquitin is the key. Cell Cycle. 7:287–292.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yamashita SI, Masuda Y, Yoshida N,
Matsuzaki H, Kurizaki T, Haga Y, Ikei S, Miyawaki M, Kawano Y,
Chujyo M, et al: p53AIP1 expression can be a prognostic marker in
non-small cell lung cancer. Clin Oncol (R Coll Radiol). 20:148–151.
2008. View Article : Google Scholar
|
|
37
|
Iwakuma T and Lozano G: MDM2, an
introduction. Mol Cancer Res. 1:993–1000. 2003.
|
|
38
|
Günther T, Schneider-Stock R, Häckel C,
Kasper HU, Pross M, Hackelsberger A, Lippert H and Roessner A: Mdm2
gene amplification in gastric cancer correlation with expression of
Mdm2 protein and p53 alterations. Mod Pathol. 13:621–626. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Granville CA, Memmott RM, Gills JJ and
Dennis PA: Handicapping the race to develop inhibitors of the
phosphoinositide 3-kinase/Akt/mammalian target of rapamycin
pathway. Clin Cancer Res. 12:679–689. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Markman B, Atzori F, Pérez-García J,
Tabernero J and Baselga J: Status of PI3K inhibition and biomarker
development in cancer therapeutics. Ann Oncol. 21:683–691. 2010.
View Article : Google Scholar
|
|
41
|
Karar J and Maity A: PI3K/AKT/mTOR pathway
in angiogenesis. Front Mol Neurosci. 4:512011. View Article : Google Scholar : PubMed/NCBI
|