Introduction
Angiogenesis, the formation of new blood vessels
from pre-existing vessels, is required for a variety of normal
physiological functions such as embryonic development, wound
healing and tissue or organ regeneration (1,2).
However, angiogenesis is also involved in the pathogenesis of
several diseases, including age-related macular degeneration,
diabetic retinopathy, psoriasis, rheumatoid arthritis and cancer
(3). Particularly, upregulation of
angiogenesis is a central step in sustained tumor growth and
metastasis. The new blood vessels grow and infiltrate into the
tumor, providing it with essential nutrients and oxygen, and a
route for tumor metastasis (4,5).
Therefore, antiangiogenesis has become an important strategy for
the treatment of cancer.
Chemical stimulation of angiogenesis is performed by
various angiogenic proteins, including vascular endothelial growth
factor (VEGF) (6). VEGF stimulates
cellular responses by binding to VEGF receptor 2 (VEGFR2) on the
cell surface, causing them to dimerize and become activated through
transphosphorylation. Activation of VEGFR2 leads to phosphorylation
of specific downstream signal transduction mediators, including
extracellular signal-regulated kinases (ERK) and AKT. Signaling
from VEGFR2 consequently promotes the proliferation, migration and
differentiation of endothelial cells (7,8).
Therefore, VEGFR2 has been recognized as the most important target
for the antiangiogenesis therapy of cancer. Bevacizumab
(Avastin®), sunitinib malate (Sutent®,
SU11248), and sorafenib (Nexavar®, BAY 43-9006) that
were developed for antiangiogenic actions have been approved by the
United States Food and Drug Administration (FDA) for treatment of
patients with specific types of cancer. All three agents inhibit
VEGF signaling by blocking VEGF ligand or VEGF receptor function
(9,10). But most of angiogenesis inhibitors
have some adverse effects, including hypertension and proteinuria,
which emphasizes that discovery of novel VEGFR2 inhibitors with
better safety and efficacy in treating human cancer is still needed
(11,12).
In general, rapid growth of tumor cells causes
hypoxia in tumor tissues, which drives angiogenesis to improve the
influx of oxygen. Thus, the hypoxic microenvironment can stimulate
the expression of VEGF via hypoxia inducible factor-1 (HIF-1), the
transcription factor which binds the regulatory region of VEGF gene
and induces its transcription during hypoxia (13,14).
HIF-1 is a heterodimeric transcription factor composed of an
oxygen-regulated α-subunit (HIF-1α) and a constitutively expressed
β-subunit (HIF-1β). HIF-1α plays a key role in the regulation of
the expression of many genes involved in metabolic adaptation to
low oxygen, survival and angiogenesis. Under normoxic condition,
HIF-1α is rapidly degraded by proteasome after post-translational
modification, whereas, under hypoxic condition, it remains stable
and binds with HIF-1β to activate the transcription of a large
number of genes (15). It has been
reported that overexpression of HIF-1α was associated with poor
prognosis of many human cancers (16). Given the crucial role of
VEGF-mediated signaling in promoting tumor angiogenesis, dual
inhibition of VEGFR2 and HIF-1α activities could potentiates
antiangiogenic therapy in cancer treatment.
There has been increasing interest in the research
on flavonoids, a large class of plant metabolites, because of their
multifaceted health benefits (17,18).
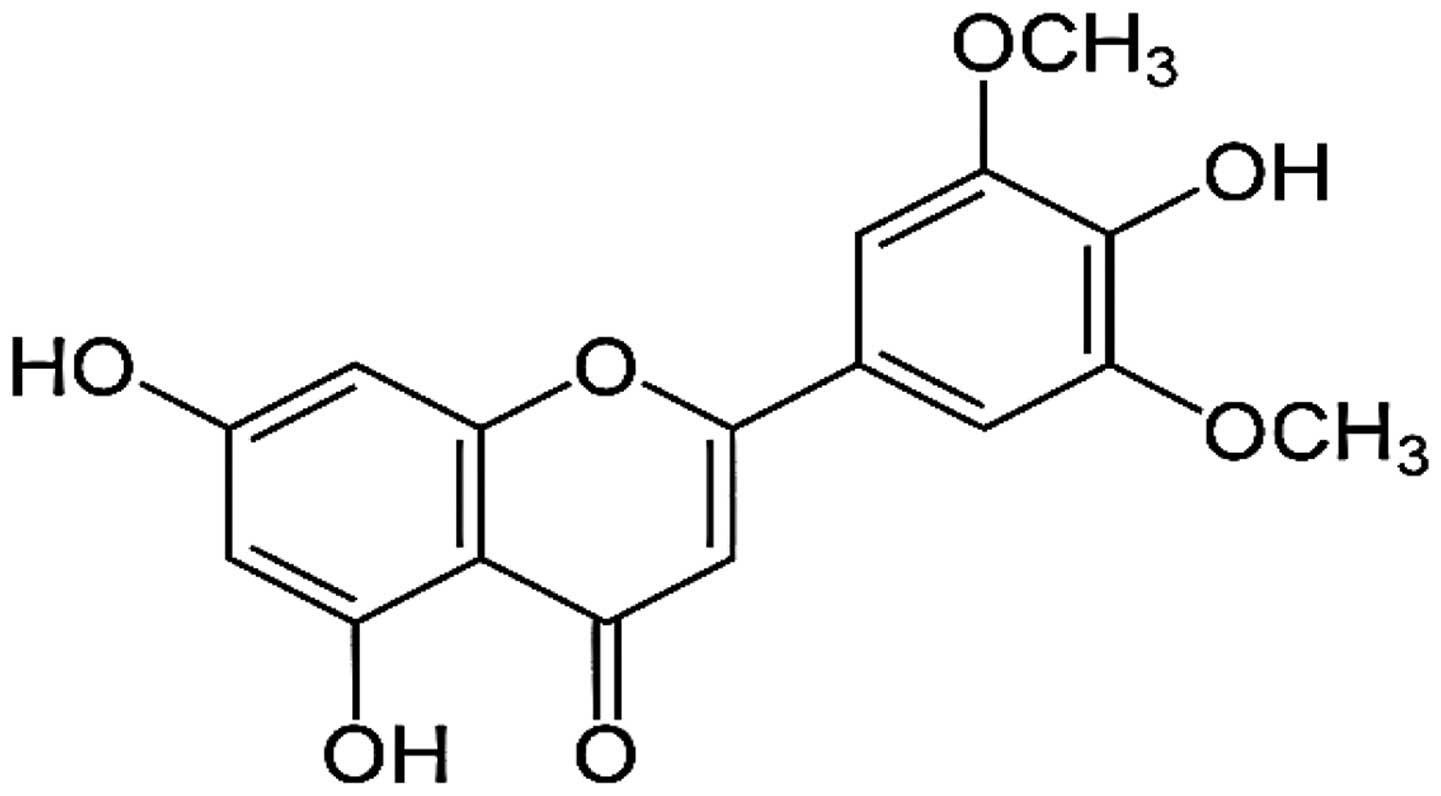
The flavonoid, tricin (4′,5,7-trihydroxy-3′,5′-dimethoxyflavone)
was reported as a valuable anticancer agent having a
pharmacokinetic advantage (Fig. 1)
(19–21). However, the antiangiogenic
potential of tricin has not been explored. In the present study, we
investigated the in vitro antiangiogenic effect and the
molecular mechanisms of tricin. Our results demonstrated that
tricin could efficiently suppress tumor angiogenesis by
downregulating both VEGFR2 signaling and HIF-1α activity.
Materials and methods
Materials
Tricin was purchased from ChemFaces (Wuhan, China).
Endothelial growth medium-2 (EGM-2) was obtained from Lonza
(Walkersville, MD, USA). Dulbecco’s modified Eagle’s medium (DMEM),
minimum essential medium (MEM), RPMI-1640 medium, and fetal bovine
serum (FBS) were purchased from Invitrogen (Grand Island, NY, USA).
Recombinant human vascular endothelial growth factor (VEGF),
Matrigel and Transwell chamber systems were obtained from Koma
Biotech (Seoul, Republic of Korea), BD Biosciences (San Jose, CA,
USA) and Corning Costar (Acton, MA, USA), respectively.
Anti-hypoxia inducible factor-1α (HIF-1α) antibody was purchased
from BD Biosciences. Anti-phospho-VEGFR2, anti-VEGFR2,
anti-phospho-AKT, anti-AKT, anti-phospho-ERK1/2, anti-ERK1/2 and
anti-β-actin antibodies were purchased from Cell Signaling
Technology (Beverly, MA, USA).
Cell culture and hypoxic conditions
Human umbilical vein endothelial cells (HUVECs) and
U87MG (human glioblastoma) cells were grown in EGM-2 and MEM
supplemented with 10% FBS, respectively. AGS (human gastric
carcinoma) and HCT116 (human colon carcinoma) cells were maintained
in RPMI-1640 medium containing 10% FBS. HeLa (human cervical
carcinoma) and HepG2 (human hepatocarcinoma) cells were grown in
DMEM supplemented with 10% FBS. All cells were maintained at 37°C
in a humidified 5% CO2 incubator. For hypoxic
conditions, cells were incubated in a hypoxic chamber (Forma
Scientific, Marietta, OH, USA) under 5% CO2 and 1%
O2 balanced with N2.
Cell proliferation assay
HUVECs (3×103 cells/well) and various
cancer cells (2×103 cells/well) were seeded in 96-well
culture plates and then treated with various concentrations of
tricin for 72 h. Cell proliferation was measured using a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
colorimetric assay.
Cell viability assay
HUVECs were seeded at a density of 1×105
cells/well in 12-well culture plates. Tricin (1–20 μM) was added to
each well and the cells were incubated for up to 72 h. After 72 h,
the cells were stained with Trypan blue and counted using a
hemocytometer.
Chemoinvasion assay
The invasiveness of HUVECs was investigated using a
Transwell chamber system with polycarbonate filter inserts with a
pore size of 8.0 μm. Briefly, the lower side of the filter was
coated with 10 μl gelatin (1 mg/ml) and the upper side was coated
with 10 μl Matrigel (3 mg/ml). Serum-starved HUVECs
(8×104 cells) were placed in the upper chamber of the
filter and tricin (2.5–10 μM) was added to the lower chamber in the
presence of VEGF (30 ng/ml). The chamber was incubated at 37°C for
18 h, and then the cells were fixed with methanol and stained with
hematoxylin and eosin (H&E). The total number of cells that
invaded the lower chamber of the filter was counted using an
optical microscope (Olympus, Center Valley, PA, USA) at a ×100
magnification.
Capillary tube formation assay
Serum-starved HUVECs (8×104 cells) were
inoculated on a surface containing Matrigel (10 mg/ml) and were
incubated with tricin (2.5–10 μM) for 6 h in the presence of VEGF
(30 ng/ml). Morphological changes of the cells and tube formation
were visualized under a microscope and photographed at a ×100
magnification (Olympus). Tube formation was quantified by counting
the total number of branched tubes in randomly selected fields at a
×100 magnification.
Chorioallantoic membrane (CAM) assay
Fertilized chick eggs were maintained in a
humidified incubator at 37°C for 3 days. Approximately 6 ml egg
albumin was removed with a hypodermic needle, allowing the CAM and
yolk sac to drop away from the shell membrane. After 2 days, the
shell was punched out and peeled away. Thermanox coverslips (Nalge
Nunc International, Rochester, NY, USA) with or without tricin were
air-dried and applied to the CAM surface. Two days later, 2 ml of
10% fat emulsion (Greencross Co., Yongin, Republic of Korea) were
injected into the chorioallantois and the CAM was observed under a
microscope.
Tumor cell-induced angiogenesis
assay
Tumor-induced angiogenesis was assessed using an
in vitro co-culture system based on the chemoinvasion assay
(22). U87MG cells were plated in
the lower chamber and treated with tricin (2.5–10 μM) for 24 h. And
then the medium in each lower chamber was replaced with fresh
medium without tricin, and serum-starved HUVECs (8×104
cells) were placed in the upper chamber. The chamber was incubated
at 37°C for 18 h, and HUVECs that invaded the lower chamber of the
filter were analyzed using the same procedure as described in the
chemoinvasion assay. To further verify the activity of tricin to
tumor cell-induced angiogenesis, a conditioned medium was collected
from U87MG cells and used as the angiogenic stimuli for the tube
formation of HUVECs (22).
Briefly, U87MG cells were treated with tricin (2.5–10 μM) for 24 h,
and then the medium was replaced with fresh medium without tricin.
The conditioned medium was used in the in vitro tube
formation assay.
Western blot analysis
Cell lysates were separated by 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and the
separated proteins were transferred to polyvinylidene difluoride
(PVDF) membranes (Millipore, Billerica, MA, USA) using standard
electroblotting procedures. The blots were blocked and
immunolabeled with primary antibodies against phospho-VEGFR2,
VEGFR2, phospho-AKT, AKT, phospho-ERK1/2, ERK1/2, HIF-1α and
β-actin overnight at 4°C. Immunolabeling was detected with an
enhanced chemiluminescence (ECL) kit (Bio-Rad Laboratories,
Hercules, CA, USA), according to the manufacturer’s
instructions.
Reactive oxygen species (ROS)
measurement
ROS levels were detected with
2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA;
Molecular Probes, Eugene, OR, USA). For the assay, serum-starved
HUVECs seeded at a density of 1×105 cells/well in
96-black well culture plates were pretreated with tricin (2.5–10
μM) for 3 h. After incubation with H2DCFDA (10 μM) for 5
min, the cells were stimulated to VEGF (30 ng/ml) for 5 min. The
fluorescence intensity of DCF was detected using a multimode
microplate reader (Thermo Fisher Scientific, Vantaa, Finland) at
the excitation and emission wavelengths of 495 and 529 nm,
respectively.
Measurement of VEGF by enzyme-linked
immunosorbent assay (ELISA)
VEGF concentration in the media from the
tricin-treated cells was determined using a VEGF immunoassay kit
(R&D Systems, Minneapolis, MN, USA) according to the
manufacturer’s instructions. The results were expressed as the
concentration of VEGF relative to the total amount of protein from
each well.
Statistical analysis
The results are expressed as the mean ± standard
error (SE). Student’s t-test was used to determine statistical
significance between the control and the test groups. A P-value of
<0.05 was considered to indicate a statistically significant
result.
Results
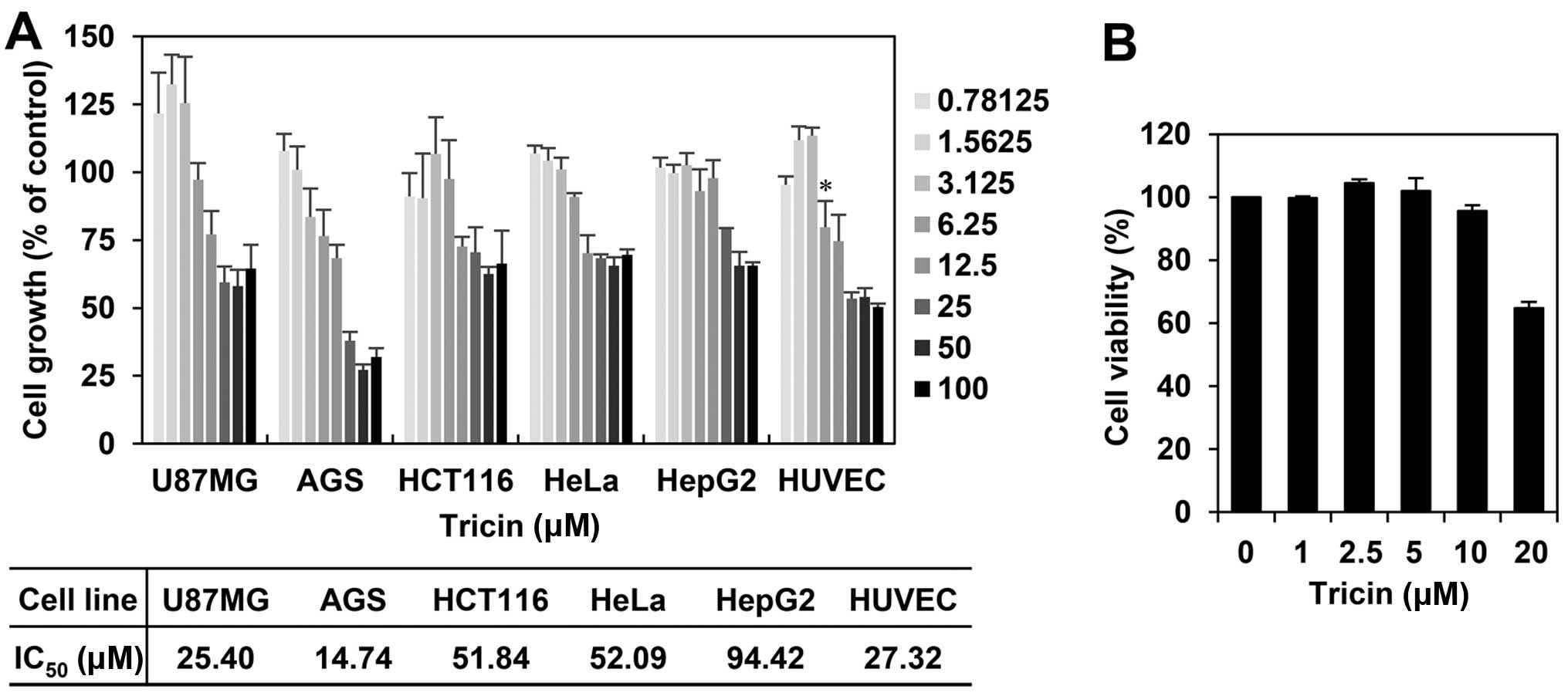
The effect of tricin on the proliferation
of human umbilical vein endothelial cells
We first investigated the effect of tricin on the
growth of various cell lines, including several cancer cells and
human umbilical vein endothelial cells (HUVECs), using the MTT
colorimetric assay. As shown in Fig.
2A, tricin inhibited the proliferation of each cell line with a
different sensitivity to growth inhibition. Notably, tricin showed
comparatively better inhibition effect on the growth of HUVECs with
an IC50 of 27.32 μM among the tested cell lines. To
further evaluate whether the endothelial cell growth inhibition by
tricin was due to cytotoxic or cytostatic activity, a viability
assay was performed using the Trypan blue exclusion method. As
shown in Fig. 2B, the viability of
HUVECs was not affected up to 10 μM of tricin treatment, indicating
that the antiproliferative activity of tricin shown at range of
<10 μM is not due to mere cytotoxicity of the compound.
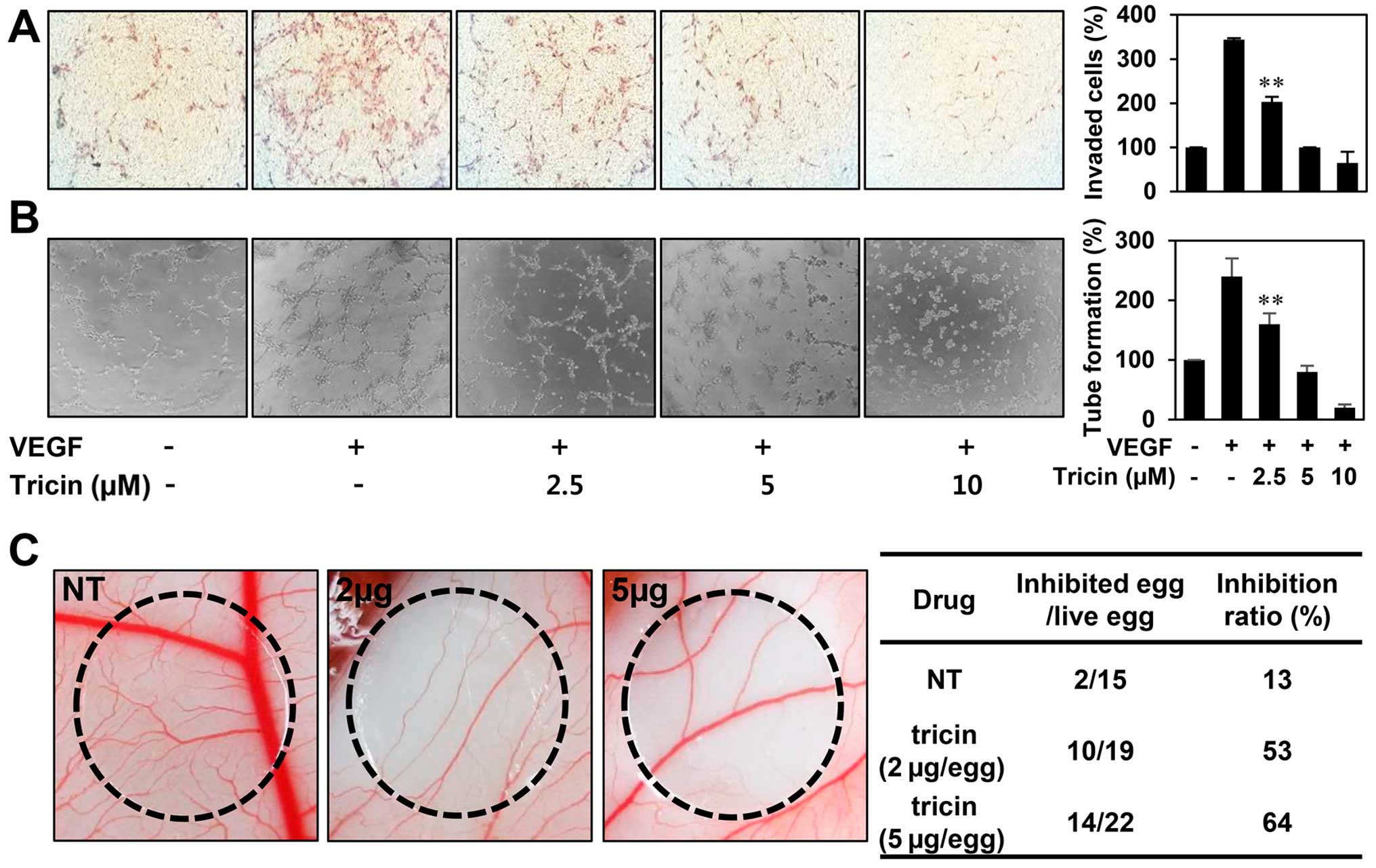
The in vitro antiangiogenic activity of
tricin
We next examined the effect of tricin on key
angiogenic phenotypes such as endothelial cell invasion and tube
formation. The in vitro angiogenesis assays were conducted
in a non-cytotoxic concentration range of tricin (2.5–10 μM). To
elucidate the inhibitory activity of tricin on VEGF-induced
angiogenesis, serum starved HUVECs were stimulated by VEGF with or
without tricin. As shown in Fig. 3A
and B, tricin significantly decreased the VEGF-induced
invasiveness and tube forming ability of HUVECs in a dose-dependent
manner.
Furthermore, the antiangiogenic activity of tricin
was validated using a chorioallantoic membrane (CAM) assay.
Coverslips containing tricin were placed on the CAM surface, and
angiogenesis zones were observed under a microscope. As shown in
Fig. 3C, the inhibition of
neovascularization on control coverslips was 13% (n=15), whereas
tricin much more potently inhibited the angiogenesis of the CAM
(53% at 2 μg/egg, n=19; 64% at 5 μg/egg, n=22) without toxicity
against pre-existing vessels. These results demonstrate that tricin
significantly inhibited angiogenesis without exhibiting
cytotoxicity on endothelial cells in vitro.
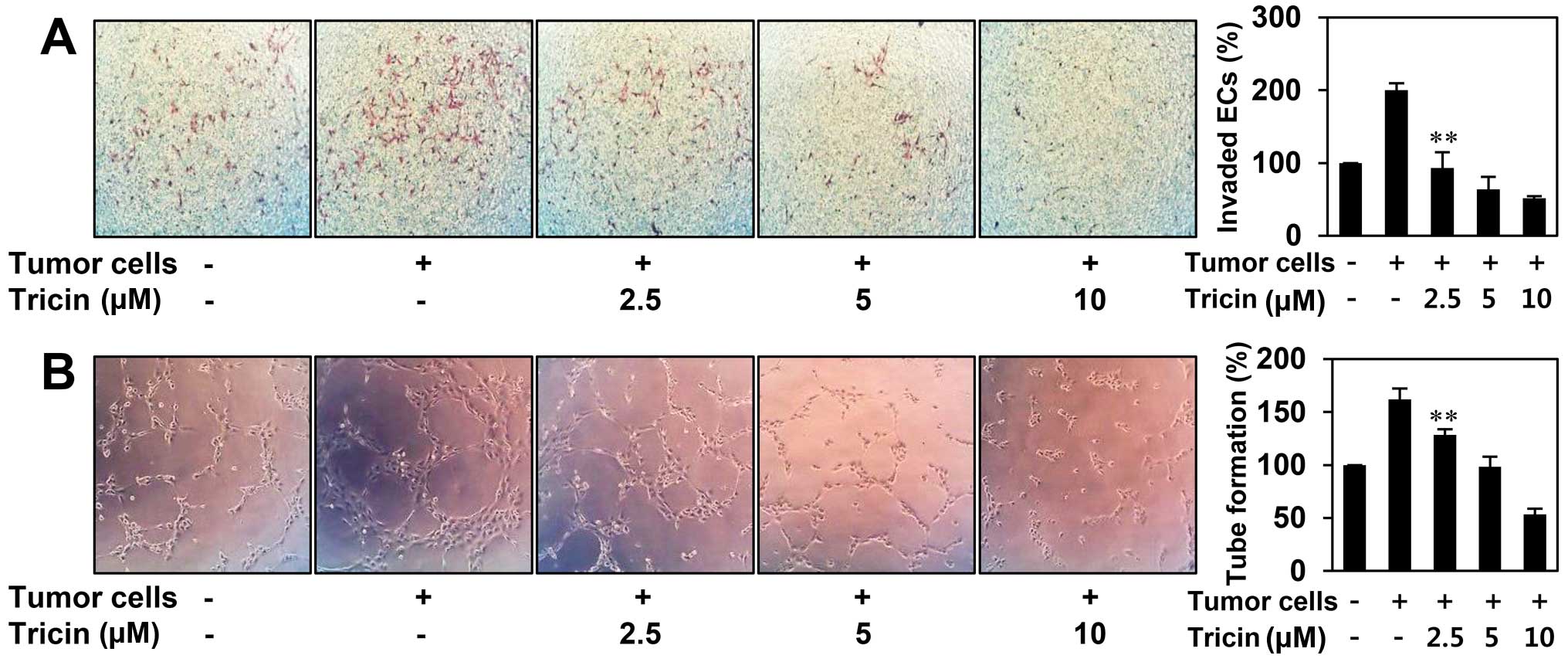
The inhibitory effect of tricin on tumor
cell-induced angiogenesis
Angiogenesis is recognized as a crucial step in the
transition of tumors from a dormant condition to a malignant state
by inducing tumor growth and metastasis (4,5). To
evaluate whether tricin inhibits tumor cell-induced angiogenesis,
we investigated the effect of tricin on HUVEC invasion induced by
U87MG glioblastoma cells using a coculture assay. As shown in
Fig. 4A, HUVECs cocultured with
U87MG cells invaded 2-fold faster compared to HUVECs alone. The
increased invasion of HUVECs was effectively prevented when U87MG
cells were treated with tricin. To further verify its activity to
tumor cell-induced angiogenesis, we also assessed the effect of
tricin on the tube formation of HUVECs induced by U87MG cells. As
shown in Fig. 4B, the conditioned
medium from U87MG cells induced tube formation of HUVECs by
1.6-fold compared to control (medium only). However, tricin-treated
conditioned medium from U87MG cells blocked the stimulated tube
formation of HUVECs in a dose-dependent manner, implying that
tricin could inhibit tumor cell-induced angiogenesis.
Downregulation of VEGFR2 signal
transduction by tricin
VEGFR2 signal transduction leads to the activation
of various downstream signaling substrates that are involved in
proliferation, migration and capillary tube formation of
endothelial cells (7,8). We, thus, evaluated the effect of
tricin on VEGF-mediated VEGFR2 signaling pathways in HUVECs. As
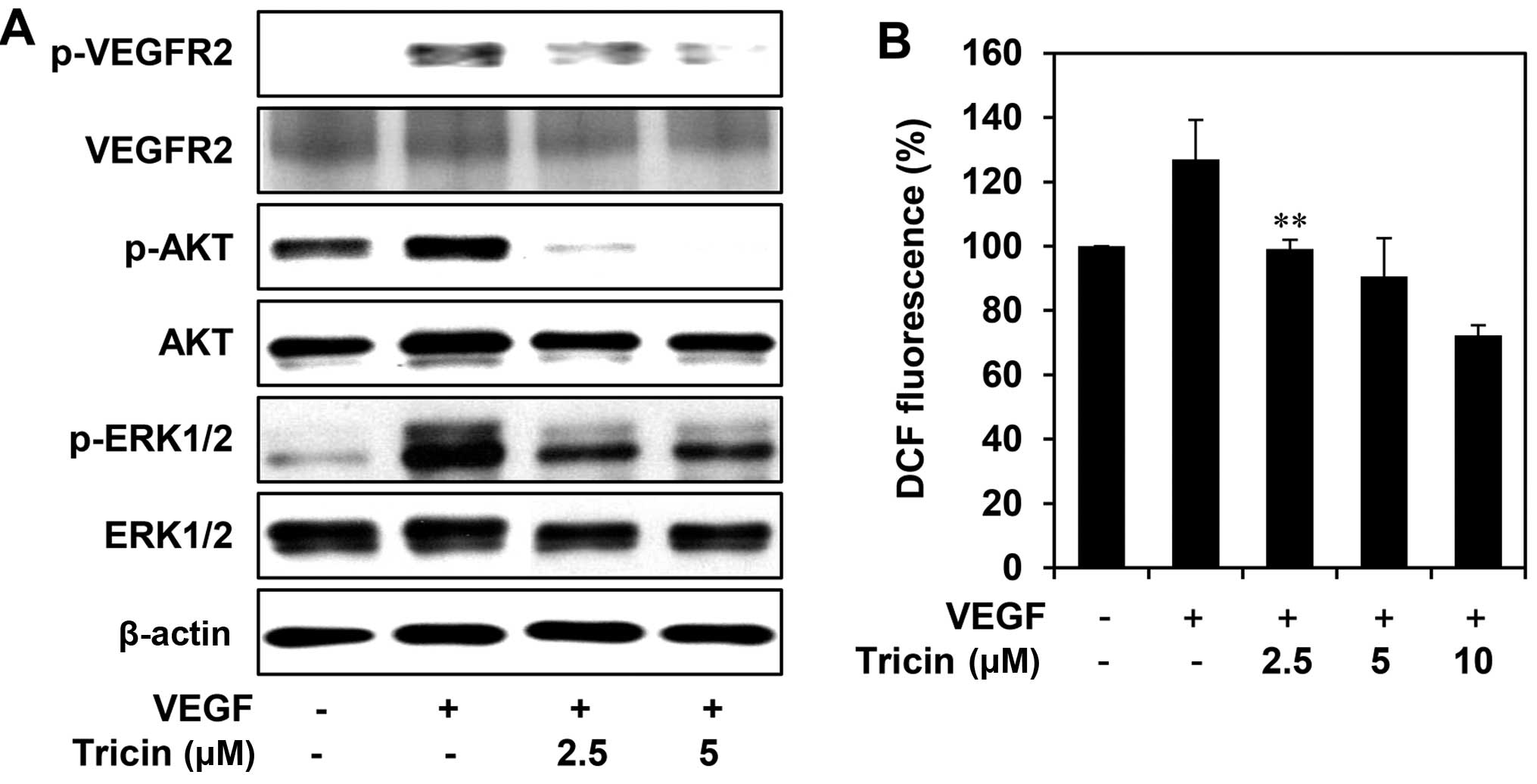
shown in Fig. 5A, tricin
efficiently suppressed the phosphorylation of VEGFR2, AKT and
ERK1/2 induced by VEGF, without affecting the total protein levels,
suggesting that tricin exhibits the antiangiogenic activity by
inhibiting VEGFR2-mediated downstream signaling cascades. In
addition, we found that tricin dose-dependently reduced the
generation of ROS induced by VEGF in HUVECs (Fig. 5B). It has been previously reported
that VEGF stimulates ROS production and in turn promotes VEGFR2
autophosphorylation by reversibly oxidizing and inactivating
protein tyrosine phosphatases (PTPs) (23,24).
Taken together, these results suggest that tricin may block the
VEGFR2 signaling in HUVECs via the down-regulation of ROS
generation.
The effect of tricin on hypoxia-induced
accumulation of HIF-1α protein
HIF-1α pathway activation in hypoxic tumor cells is
an important stimulus for tumor angiogenesis through the regulation
of the expression of proangiogenic genes such as VEGF (13,14).
To determine the role of HIF-1α in mediating the antiangiogenic
effect of tricin, we evaluated the HIF-1α inhibitory activity of
tricin in the human hepatoma cell line HepG2, a hypervascularized
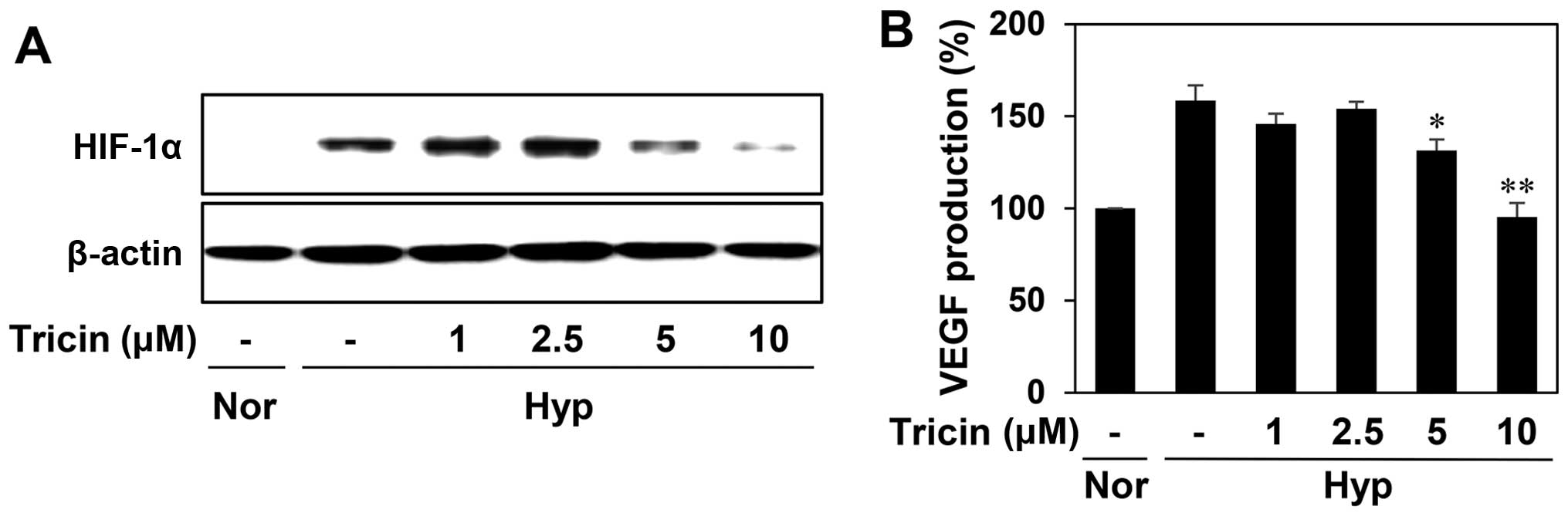
tumor. As shown in Fig. 6A,
tricin-treated HepG2 cells reduced the hypoxia-induced accumulation
of HIF-1α protein in a dose-dependent manner. We further assessed
the effect of tricin on the expression of VEGF induced by hypoxia.
Tricin dose-dependently decreased VEGF production in HepG2 cells
under hypoxic condition (Fig. 6B).
These data indicate that tricin could inhibit tumor angiogenesis by
downregulating HIF-1α and its target gene, VEGF.
The enhanced antiangiogenic effect of
combined treatment with tricin and bevacizumab
Bevacizumab (Avastin) is a monoclonal antibody that
blocks angiogenesis by inhibiting vascular endothelial growth
factor (9). Although the drug has
been used in treatment of various solid tumors in combination with
several anticancer agents, the efficacy of this treatment is
limited due to the development of resistance (25). We, thus, evaluated whether tricin
elevates the antiangiogenic function of the VEGF blocker using a
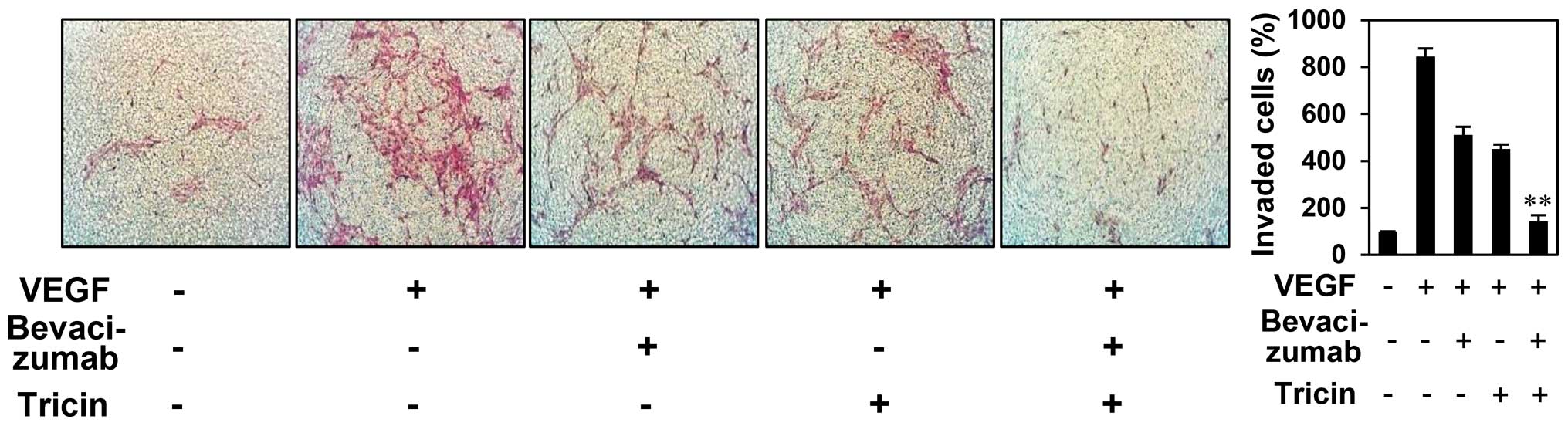
chemoinvasion assay. As shown in Fig.
7, treatment with both 2.5 μM of tricin and 100 ng/ml of
bevacizumab resulted in additive inhibition of VEGF-induced
endothelial cell invasion (inhibition of 45, 53 and 94% with
tricin, bevacizumab, and tricin/bevacizumab combination,
respectively), suggesting that tricin potentiates the
antiangiogenic activity of bevacizumab. Thus, tricin administered
alone or in combination with bevacizumab may overcome the
resistance to bevacizumab for antiangiogenesis.
Discussion
Owing to the crucial role of angiogenesis in the
growth and metastasis of solid tumors, the specific perturbation of
angiogenesis has been considered a powerful strategy for anticancer
therapy. The inhibition of VEGF pathway has become the focus of
antiangiogenesis research since VEGF is a pivotal stimuli of
angiogenesis. Strategies to inhibit the VEGF pathway include the
blockade of VEGFR2-mediated angiogenic signal transduction in
endothelial cells and the prevention of VEGF expression by
suppressing its transcription regulators such as HIF-1α in tumor
cells (26). Therefore, the novel
angiogenesis inhibitors that target both VEGFR2 and HIF-1α
activities could provide more effective therapeutic potential for
the treatment of hypervascularized tumors.
In recent years, research on dietary flavonoids has
become increasingly important with the discovery of their diverse
biological activities at non-toxic concentrations. Previous studies
have revealed that tricin, a naturally occurring flavone, possesses
antiviral, antiinflammatory, antioxidant, antitubercular,
antiulcerogenic, antimelanogenic, antihistaminic and anticancer
effects (27). In addition, the
molecular mechanisms for its biological effects were partly
identified. Tricin reduced inflammatory responses in human
peripheral blood mononuclear cells (hPBMCs) by regulating the
TLR4/NF-κB/STAT, p38MAPK and PI3K/AKT pathways (28,29).
It has been also found that tricin exhibits potent anticancer
effects in various cancer cells including breast and colon cancers
via the inhibition of cyclooxygenase and P-glycoprotein activities
(30,31). The antitumor effect of tricin was
also demonstrated in the present study. Tricin significantly
inhibited the growth of human brain, gastric, colon, cervical and
liver cancer cell lines (Fig. 2A).
Moreover, in recent research, tricin showed pharmacokinetic benefit
compared with apigenin, a known anticancer flavone. The dietary
administration of tricin in mice was more available than apigenin
in blood and tissues (32).
Moreover, tricin did not show genotoxic properties in mice,
suggesting that the safety of tricin may increase its potential
clinical usefulness (33).
However, to the best of our knowledge, no evaluation of the
antiangiogenic activity of tricin has been reported to date.
In the present study, we describe for the first time
the antiangiogenic activity and underlying molecular mechanisms of
tricin. The flavone exhibited potent antiangiogenic activity in
vitro with no obvious cytotoxicity (Fig. 3). We also found that tricin
effectively suppressed tumor cell-induced angiogenesis (Fig. 4). Furthermore, our results
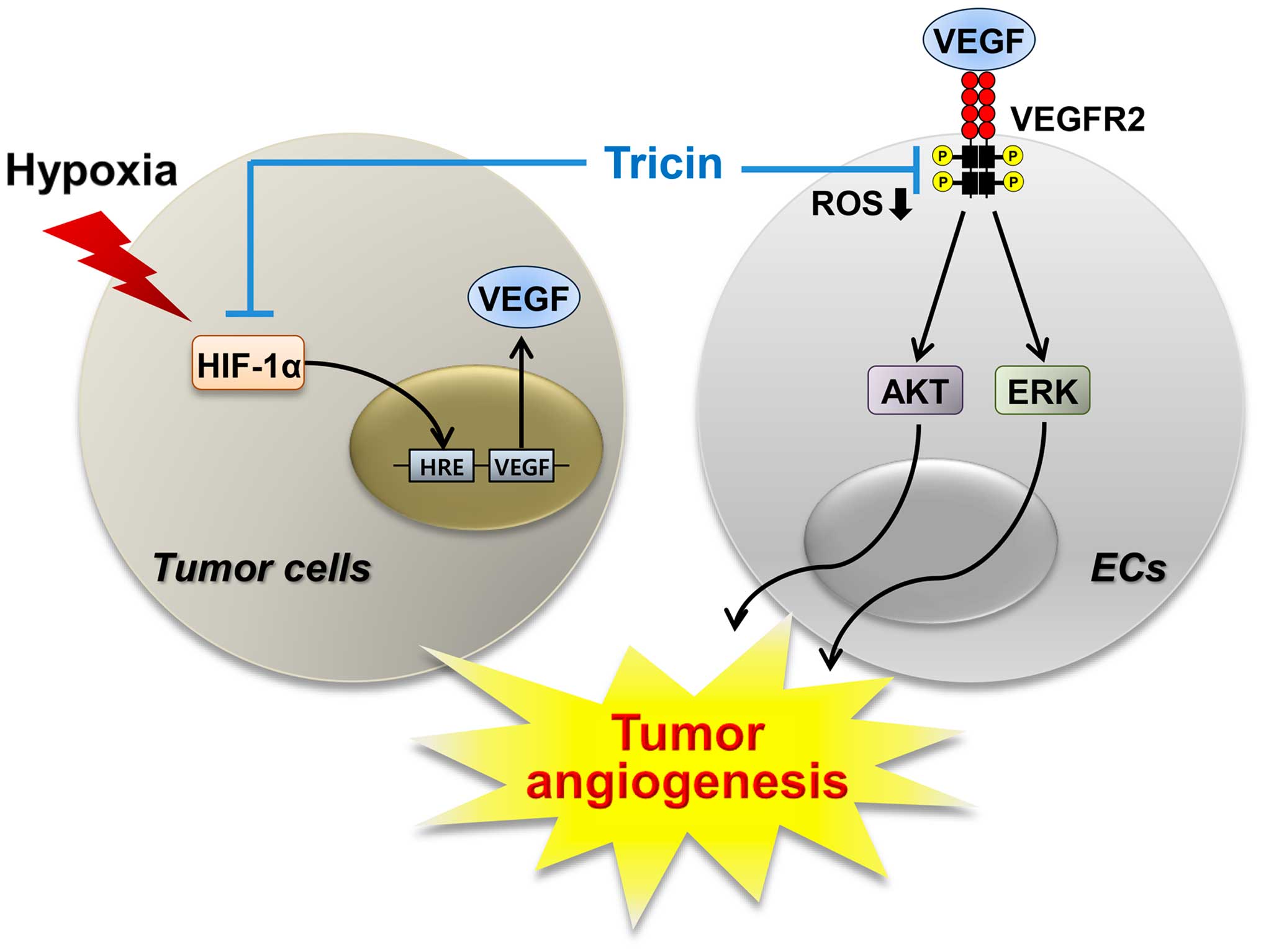
demonstrated that tricin may inhibit tumor angiogenesis by
modulating at least two angiogenic pathways (Fig. 8). In this study, both the VEGFR2
signaling of endothelial cells and the HIF-1α and VEGF expression
levels of tumor cells were downregulated by tricin treatment
(Figs. 5 and 6). In addition, the blockade of VEGFR2
signal transduction may be associated with the reduction of ROS,
generated from NADPH oxidase or mitochondria, by tricin.
An anti-VEGF monoclonal antibody, bevacizumab, has
been approved for combination use with standard chemotherapy in
certain metastatic cancers (9,10).
However, recent clinical results of bevacizumab have revealed its
limited therapeutic efficacy in drug-resistant solid tumors as well
as several adverse effects such as hypertension, proteinuria and
hemorrhage (11,12,25).
We, thus, evaluated whether tricin ameliorates the antiangiogenic
effect of bevacizumab. Combined treatment with tricin and
bevacizumab more effectively inhibited VEGF-induced angiogenesis
compared with single agent treatment (Fig. 7). Therefore, these results suggest
that tricin may have promising therapeutic potential to overcome
the resistance to bevacizumab, alone or in combination with
bevacizumab.
Taken together, our results provide new therapeutic
aspect for tricin as a potent inhibitor of angiogenesis via the
dual blocking of VEGFR2 and HIF-1α pathways. In addition, the known
pharmacokinetic advantages of tricin may contribute to its clinical
application to antiangiogenic therapy for cancer. Although the
inhibitory effect of tricin on the VEGFR2 signaling was partially
associated with the decrease of ROS generation, precise mechanisms
of how tricin controls bidirectionally angiogenic pathways remain
unclear. Further studies to understand the action mechanism of
flavonoids will help the discovery of the upstream cellular
mediators of tumor angiogenesis regulated by tricin.
Acknowledgements
We are grateful to Yonghyo Kim for his help with the
CAM assay. The present study was carried out with the support of
‘Cooperative Research Program for Agriculture Science and
Technology Development (Project No. PJ01188001)’ Rural Development
Administration, Republic of Korea, the Basic Science Research
Program through the National Research Foundation of Korea (NRF)
funded by the Ministry of Education (NRF-2014R1A1A2057902), and the
Brain Korea 21 Plus Project, Republic of Korea.
References
|
1
|
Bussolino F, Mantovani A and Persico G:
Molecular mechanisms of blood vessel formation. Trends Biochem Sci.
22:251–256. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Folkman J: Seminars in Medicine of the
Beth Israel Hospital, Boston. Clinical applications of research on
angiogenesis. N Engl J Med. 333:1757–1763. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Carmeliet P: Angiogenesis in health and
disease. Nat Med. 9:653–660. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bergers G and Benjamin LE: Tumorigenesis
and the angiogenic switch. Nat Rev Cancer. 3:401–410. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
André T, Chastre E, Kotelevets L, Vaillant
JC, Louvet C, Balosso J, Le Gall E, Prévot S and Gespach C: Tumoral
angiogenesis: Physiopathology, prognostic value and therapeutic
perspectives. Rev Med Interne. 19:904–913. 1998.(In French).
View Article : Google Scholar
|
|
6
|
Ferrara N: VEGF and the quest for tumour
angiogenesis factors. Nat Rev Cancer. 2:795–803. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hicklin DJ and Ellis LM: Role of the
vascular endothelial growth factor pathway in tumor growth and
angiogenesis. J Clin Oncol. 23:1011–1027. 2005. View Article : Google Scholar
|
|
8
|
Holmes K, Roberts OL, Thomas AM and Cross
MJ: Vascular endothelial growth factor receptor-2: Structure,
function, intracellular signalling and therapeutic inhibition. Cell
Signal. 19:2003–2012. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ferrara N, Hillan KJ, Gerber HP and
Novotny W: Discovery and development of bevacizumab, an anti-VEGF
antibody for treating cancer. Nat Rev Drug Discov. 3:391–400. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ivy SP, Wick JY and Kaufman BM: An
overview of small-molecule inhibitors of VEGFR signaling. Nat Rev
Clin Oncol. 6:569–579. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen HX and Cleck JN: Adverse effects of
anticancer agents that target the VEGF pathway. Nat Rev Clin Oncol.
6:465–477. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Verheul HM and Pinedo HM: Possible
molecular mechanisms involved in the toxicity of angiogenesis
inhibition. Nat Rev Cancer. 7:475–485. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Höckel M and Vaupel P: Tumor hypoxia:
Definitions and current clinical, biologic, and molecular aspects.
J Natl Cancer Inst. 93:266–276. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Forsythe JA, Jiang BH, Iyer NV, Agani F,
Leung SW, Koos RD and Semenza GL: Activation of vascular
endothelial growth factor gene transcription by hypoxia-inducible
factor 1. Mol Cell Biol. 16:4604–4613. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Semenza GL: HIF-1 and tumor progression:
Pathophysiology and therapeutics. Trends Mol Med. 8(Suppl):
S62–S67. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Semenza GL: Targeting HIF-1 for cancer
therapy. Nat Rev Cancer. 3:721–732. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Harborne JB: Nature, distribution and
function of plant flavonoids. Prog Clin Biol Res. 213:15–24.
1986.PubMed/NCBI
|
|
18
|
Havsteen BH: The biochemistry and medical
significance of the flavonoids. Pharmacol Ther. 96:67–202. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cai H, Hudson EA, Mann P, Verschoyle RD,
Greaves P, Manson MM, Steward WP and Gescher AJ: Growth-inhibitory
and cell cycle-arresting properties of the rice bran constituent
tricin in human-derived breast cancer cells in vitro and in nude
mice in vivo. Br J Cancer. 91:1364–1371. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hudson EA, Dinh PA, Kokubun T, Simmonds MS
and Gescher A: Characterization of potentially chemopreventive
phenols in extracts of brown rice that inhibit the growth of human
breast and colon cancer cells. Cancer Epidemiol Biomarkers Prev.
9:1163–1170. 2000.PubMed/NCBI
|
|
21
|
Oyama T, Yasui Y, Sugie S, Koketsu M,
Watanabe K and Tanaka T: Dietary tricin suppresses
inflammation-related colon carcinogenesis in male Crj: CD-1 mice.
Cancer Prev Res (Phila). 2:1031–1038. 2009. View Article : Google Scholar
|
|
22
|
Ali MA, Choy H, Habib AA and Saha D:
SNS-032 prevents tumor cell-induced angiogenesis by inhibiting
vascular endothelial growth factor. Neoplasia. 9:370–381. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ushio-Fukai M: VEGF signaling through
NADPH oxidase-derived ROS. Antioxid Redox Signal. 9:731–739. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jung HJ, Kim Y, Chang J, Kang SW, Kim JH
and Kwon HJ: Mitochondrial UQCRB regulates VEGFR2 signaling in
endothelial cells. J Mol Med (Berl). 91:1117–1128. 2013. View Article : Google Scholar
|
|
25
|
Bergers G and Hanahan D: Modes of
resistance to anti-angiogenic therapy. Nat Rev Cancer. 8:592–603.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
El-Kenawi AE and El-Remessy AB:
Angiogenesis inhibitors in cancer therapy: Mechanistic perspective
on classification and treatment rationales. Br J Pharmacol.
170:712–729. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou J and Ibrahim RK: Tricin - a
potential multifunctional nutraceutical. Phytochem Rev. 9:413–424.
2010. View Article : Google Scholar
|
|
28
|
Shalini V, Pushpan CK, Sindhu G,
Jayalekshmi A and Helen A: Tricin, flavonoid from Njavara reduces
inflammatory responses in hPBMCs by modulating the p38MAPK and
PI3K/Akt pathways and prevents inflammation associated endothelial
dysfunction in HUVECs. Immunobiology. 221:137–144. 2016. View Article : Google Scholar
|
|
29
|
Shalini V, Jayalekshmi A and Helen A:
Mechanism of anti-inflammatory effect of tricin, a flavonoid
isolated from Njavara rice bran in LPS induced hPBMCs and
carrageenan induced rats. Mol Immunol. 66:229–239. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cai H, Al-Fayez M, Tunstall RG, Platton S,
Greaves P, Steward WP and Gescher AJ: The rice bran constituent
tricin potently inhibits cyclooxygenase enzymes and interferes with
intestinal carcinogenesis in ApcMin mice. Mol Cancer Ther.
4:1287–1292. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jeong YH, Chung SY, Han AR, Sung MK, Jang
DS, Lee J, Kwon Y, Lee HJ and Seo EK: P-glycoprotein inhibitory
activity of two phenolic compounds, (−)-syringaresinol and tricin
from Sasa borealis. Chem Biodivers. 4:12–16. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cai H, Boocock DJ, Steward WP and Gescher
AJ: Tissue distribution in mice and metabolism in murine and human
liver of apigenin and tricin, flavones with putative cancer
chemopreventive properties. Cancer Chemother Pharmacol. 60:257–266.
2007. View Article : Google Scholar
|
|
33
|
Verschoyle RD, Greaves P, Cai H, Borkhardt
A, Broggini M, D’Incalci M, Riccio E, Doppalapudi R, Kapetanovic
IM, Steward WP, et al: Preliminary safety evaluation of the
putative cancer chemopreventive agent tricin, a naturally occurring
flavone. Cancer Chemother Pharmacol. 57:1–6. 2006. View Article : Google Scholar
|