Introduction
In the most recent 5 years, delay-adjusted cancer
incidence rates declined by 1.8% per year in men and were stable in
women, while cancer death rates nationwide decreased by 1.8% per
year in men and by 1.4% per year in women in the United States
(1). However, breast cancer is
still one of the most frequently diagnosed cancers in women and the
leading cause of cancer-related death among women worldwide,
including China, and also one of the leading causes of
disease-associated death among women. Despite the great progress
that has been made in breast cancer research and treatment,
measures for efficient targeting triple-negative breast cancer
(TNBC) are still unavailable. Colony-stimulating factor 1 (CSF1),
also known as macrophage colony-stimulating factor (M-CSF) is a
secreted cytokine which influences hematopoietic stem cells to
differentiate into macrophages or other related cell types, and is
involved in the proliferation, differentiation and survival of
monocytes, macrophages and bone marrow progenitor cells (2,3). The
active form of CSF1 is found extracellularly as a disulfide-linked
homodimer, and has been reported to be correlated with poor
prognosis in many cancers (4). It
is reported that about one in eight women in the United States will
develop invasive breast cancer over the course of their lifetime,
and TNBC is one subtype with the poorest prognosis, which accounts
of 15–25% breast cancers (1).
Metastasis is a far from known, complex, multi-step process,
including at least these six steps: tumor cell invasion of the
basement membrane, intravasation into the vascular or lymphatic
system, survival in the blood circulation or lymph nodes,
attachment to the blood vessel wall and extravasation to the target
organ, followed by subsequent colonization and aggressive growth to
form a macrometastasis under a favorable microenvironment (5). Each step makes cancer metastasis an
almost impossible mission. However, in clinical practice, a number
of subjects with early-stage breast cancer even die from relapse or
metastasis, regardless of the intensive adjuvant therapy given.
Substantial efforts have been made in understanding the molecular
mechanisms of the disease and applying adjuvant therapy to decrease
the probabilities of relapse and metastasis, such as targeted
therapy and endocrine therapy; however, there is no such an
effective measure to patients with TNBC. The role of tumor
microenvironment (TME) during the initiation and progression of
breast cancer is now realized to be of great importance, both for
the understanding of breast cancer biology and exploiting new
molecular targets for breast cancers. Macrophage is a major
component of TME, and its infiltration is not an uncommon
phenomenon in cancer tissues. Normally, these macrophages are
called tumor-associated macrophages (TAMs), which share properties
of alternatively activated macrophage (M2) phenotype (6). Reports indicate that the prevalence
of TAM infiltration correlates with poor prognosis in some cancer
patients indicating a macrophage-supporting role for tumor
progression (7,8). However, the exact mechanisms involved
are still ambiguous.
Macrophage originates from the mononuclear
phagocytic lineage, whose polarization is dependent on the
cytokines in the microenvironment. The classical activation (M1) is
triggered by T helper 1 (Th1) cytokines, such as interferon-γ,
bacterial lipopolysaccharide (LPS) and TNF-α. In contrast, the
alternative activation (M2) is induced by T helper 2 (Th2)
cytokines, like IL-4, IL-13 and CSF1 (M-CSF) (9,10).
Classical or M1 activation mediates the defense against bacterial
pathogens, and secretes high levels of IL-12 and low levels of
IL-10, whilst alternative or M2 activation has a ‘pro-tumorigenic’
effect by producing high levels of IL-10, TGF-β and low levels of
IL-12. TAMs present the M2 phenotype in TME, and seem to actively
promote tumor growth (11,12). TAMs differentiate from circulating
monocytes that leave the vasculature and enter the tumor tissue in
response to a variety of cues secreted from the tumor and also in
response to TME. Once there, they have been shown to promote
angiogenesis, tumor growth, invasion and metastasis through
secretion of cytokines to coordinate tumor-promoting immune
responses as well as through secretion of tissue-remodeling
cysteine cathepsin proteases (13).
In this study, the objective was to examine whether
CSF1 level and TAM infiltration correlates with breast cancer
hormone receptor status, which influences treatment measures and
cancer prognosis. Further, to clarify the mechanism involved may
usher in an era of new treatment for CSF1 expression breast cancer,
especially for those hormone-independent TNBCs with no
well-established targeted therapy available.
Materials and methods
Ethics statement
The study involving human participants was approved
by the Ethics Committee of Fudan University Shanghai Cancer Center.
Written informed consent was obtained from all the patients before
the enrollment. All animal protocols were approved by the Animal
Care Committee of Shanghai Institutes for Biological Sciences,
Chinese Academy of Sciences (permit no. IBS14-0806). Animals were
kept under stable temperature and humidity conditions. All surgery
was performed by cervical dislocation to minimize suffering.
Immunohistochemistry
Paraffin-embedded blocks were obtained from the
Breast Malignancy Database established by the Department of Breast
Surgery, Fudan University Shanghai Cancer Center (Shanghai, China).
All of the enrolled patients have fully detailed
clinicopathological information and follow-up results. Written
informed consent was obtained from the patients before enrollment.
We are authorized to use the tissues for research only and have
reported the database information previously (14). For immunohistochemical analysis, 89
paraffin-embedded blocks were cut into 5-μm serial sections, and
following the confirmation of breast cancer diagnosis by H&E
staining, immunohistochemistry was performed following standard
procedures. CD163 antibody (clone 10D6; Novocastra/Leica
Microsystems, Newcastle, UK) used for immunostaining was titered to
find the optimal concentration (1:100). Sections were
counterstained with hematoxylin for the identification of the
nuclei. Detection was performed using the Dako EnVision System.
Images were captured under a microscope with a CCD camera.
Cell culture
Breast cancer cell lines MDA-MB-231, MD-MB-468 and
MCF-7 were originally obtained from the American Type Culture
Collection (ATCC; Manassas, VA, USA); the human promonocytic cell
line U937 originating from the ATCC was kindly provided by
Professor Duan Ma (Key Laboratory of Molecular Medicine, Ministry
of Education, Shanghai Medical College, Fudan University). All
cells were maintained in RPMI-1640 supplemented with 10% fetal
bovine serum (FBS) and 100 U/ml penicillin/streptomycin
(Gibco/Invitrogen, Carlsbad, CA, USA) at 37°C in a humidified
atmosphere of 5% CO2.
Lentivirus infected stable cell line
generation
MDA-MB-231, MD-MB-468, MCF-7 and U937 cells were
transfected with green or red fluorescence protein (GFP or RFP)
expressing lentiviral particles (cat. nos. LVP340 and LVP299;
GenTarget, Inc., San Diego, CA, USA) according to the
manufacturer's instructions. MCF-7 was transfected with GFP with
puromycin resistance (GFP-puro)-tagged CSF1-overexpressing
lentiviral particles (cat. no. GCK970139; GeneChem Co., Ltd.,
Shanghai, China), and MDA-MB-231 was transfected with
GFP-puro-tagged shRNA lentiviral particles specifically targeting
CSF1 (cat. no. ST3071-A; Shanghai SunBio Medical Biotechnology Co.,
Ltd., Shanghai, China). Briefly, for adhesive cells the cells were
seeded in complete medium at appropriate density (at the time of
transduction, cells should be 50–75% confluent) and incubated
overnight, the medium was removed and fresh warm complete medium
was added with appropriate amount of pre-made lentivirus to obtain
the desired MOI, then incubated for another 72 h at 37°C in a
humidified atmosphere of 5% CO2. For suspension cells,
the cells were seeded in complete medium at appropriate density and
incubated until density reaching 3×106 cells/ml, then
cells were diluted into 1×106 cells/ml with fresh warm
complete medium and appropriate amount of pre-made lentivirus was
added to obtain the desired MOI, incubated for 24 h at 37°C in a
humidified atmosphere of 5% CO2 with a shaking flask,
then an equal amount of fresh medium containing relevant
antibiotics was added for another 48 h. Positive transduction of
cells were visualized by fluorescence microscopy and isolated by
fluorescence-activated cell sorting (FACS) followed by puromycin or
neomycin selection for 2 weeks.
Transwell assay with or without
extracellular matrix barrier
For the co-culture assay, MDA-MB-231, MDA-MB-468,
MCF-7 and U937 cells cultured in RPMI-1640 supplemented with 10%
FBS and 100 U/ml penicillin/streptomycin at a final concentration
of 5×105/ml using 6-well insert system with 0.4-μm pores
(cat. no. 3450; Corning, Inc., Corning, NY, USA). Breast cancer
cell suspension (1 ml) was added to each insert well and 1 ml of
U937 cell suspension was added to each lower well. After a 72-h
incubation, U937 cells in the lower well were harvested for further
examinations. Microscopy images were captured for each well at five
randomly picked fields under a microscope with a 10X objective
lens, and the number of adhesive cells for each field was
quantified. The number of adhesive cells was determined by
comparing different breast cancer cell lines and blank control,
which was used as reference.
For the Transwell migration assay, GFP expression
MDA-MB-231, MCF-7 stable cells were cultured in complete RPMI-1640
medium and RFP expression U937 stable cells cultured in serum-free
RPMI-1640 using a 24-well insert system with 5-μm pores (cat. no.
3421; Corning, Inc.). A total of 0.5 ml of 5×105/ml
breast cancer cell suspension was added to each lower well, and
after a 24-h incubation, 0.5 ml of 5×105/ml U937 cell
suspension was added to each insert well. The breast cancer
conditioned medium in the lower chamber served as a
chemoattractant. After a 72-h incubation, migratory cells were
visualized by fluorescence microscopy and images were captured
under a fluorescence microscope with a CCD camera. Microscopy
images were captured for each well at five randomly picked fields
with a 10X objective lens, and the number of migratory cells for
each field was quantified. The number of migratory cells was
determined by comparing different breast cancer cell lines and
blank control, which was used as reference.
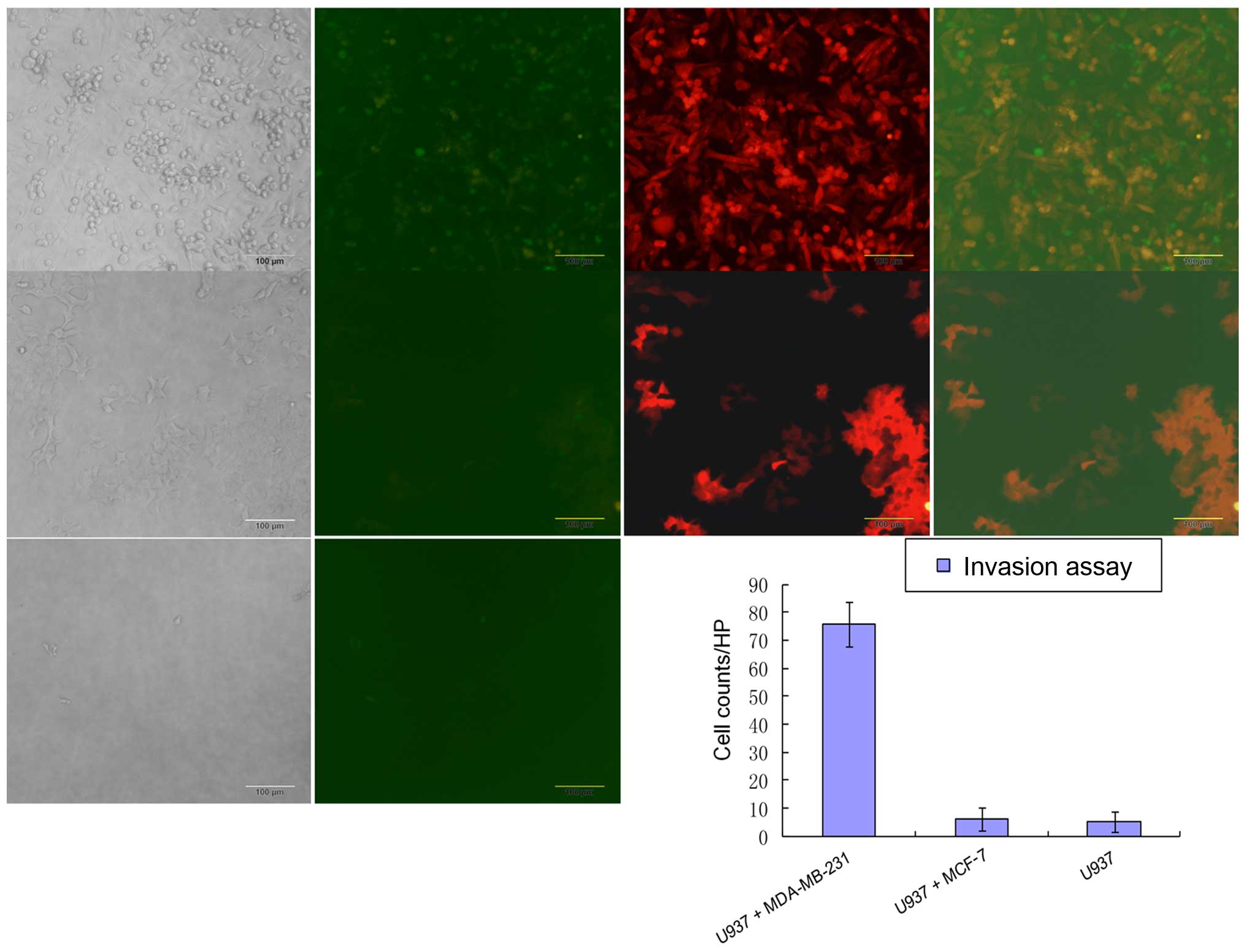
The invasion assay was performed similarly to
migration assay using 24-well insert system with 8-μm pores (cat.
no. 3422; Corning, Inc.) except the inside of the insert was
precoated with a thin layer of Matrigel™ Basement Membrane Matrix
(BD Biosciences) diluted with serum-free medium to a final
concentration of 6 mg/ml. A total of 0.5 ml of 5×105/ml
breast cancer cell suspension was added to each lower well, and
after a 24-h incubation, 0.5 ml of 5×105/ml U937 cell
suspension was added to each insert well. The breast cancer
conditioned medium in the lower chamber served as a
chemoattractant. After a 72-h incubation, invaded cells were
visualized by fluorescence microscopy and images were captured
under a fluorescence microscope with a CCD camera. Microscopy
images were captured for each well at five randomly picked fields
with a 10X objective lens, and the number of invaded cells for each
field was quantified. The number of invaded cells was determined by
comparing different breast cancer cell lines and blank control,
which was used as reference.
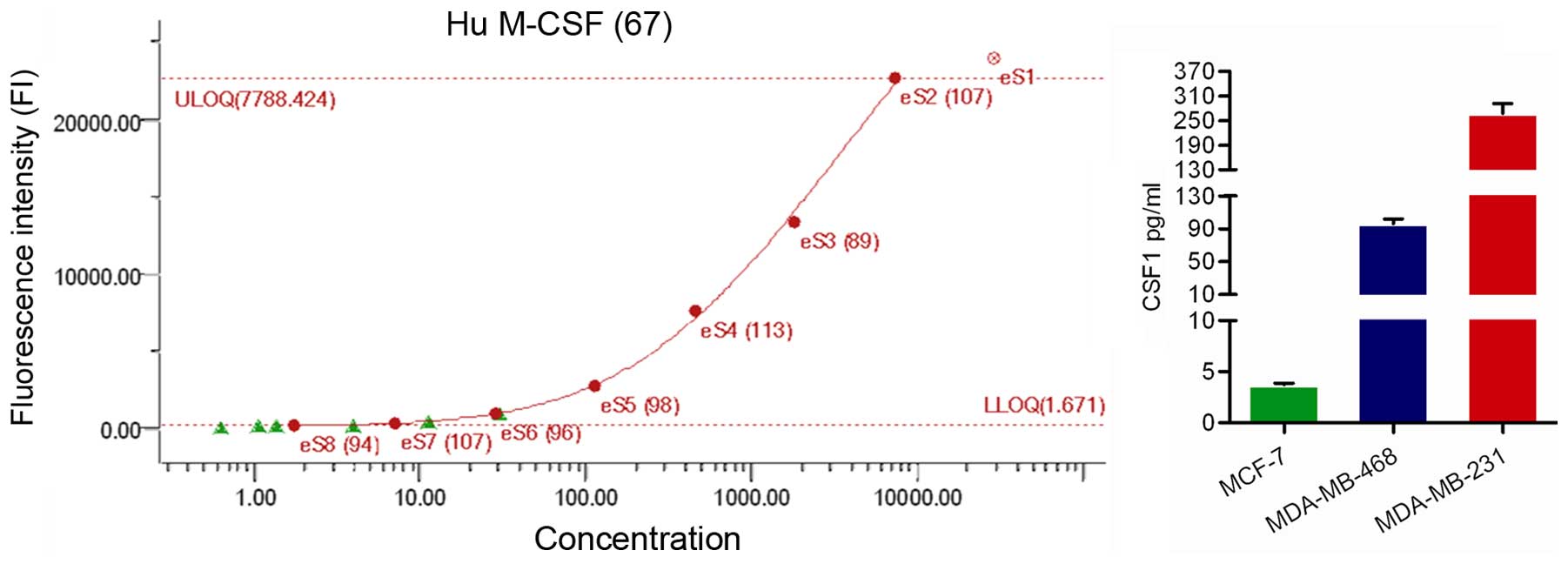
Measurement of cytokine production
Cytokine secretion was quantified by
Bio-Plex® cytokine assay. Briefly, conditioned medium
samples were obtained after centrifugation to remove cells and
their debris and stored at −80°C until cytokine profiling. We used
the fluorescent bead-based detection assay system, with an array of
beads in liquid suspension, each containing different ratios of two
spectrally distinct fluorophores, thereby assigning a unique
spectral identity. The beads, which had been conjugated with a
monoclonal antibody specific for a target protein, were incubated
with the samples to be tested, washed, followed by addition of a
biotinylated detection antibody, washed again, and finally
incubated with streptavidin-PE. A wide range of standards
(1.78–29,107.00 pg/ml) was used to enable quantitation of the
individual cytokines using a Bio-Plex array reader with a dual
laser detector and real-time digital signal processing. Cytokine
levels were measured using a Multiplex kit (Bio-Rad Laboratories,
Inc., San Diego, CA, USA) according to the manufacturer's
instructions. Standard curves for each cytokine were generated
using the reference concentrations provided in the kit. The plate
was run on a Luminex 200 Bio-Plex Instrument (Bio-Rad, Hercules,
CA, USA). Raw fluorescence data were analyzed with software using
the 5-parameter logistic method. Detailed procedures of the
Bio-Plex® Suspension Array System have been described
elsewhere (15–17).
Reverse transcription-PCR analysis
Total RNA was isolated from 1×106 cells
using TRIzol (Invitrogen) according to the manufacturer's
instructions. One microgram of total RNA was reverse transcribed
using PrimeScript® RT-PCR Kit (Takara Bio, Dalian,
China) primed with oligo(dT). One microliter of cDNA was subjected
to PCR amplification using gene-specific primers. PCR products were
resolved on a 2% agarose gel and visualized by ethidium bromide
staining. Quantitative PCR was carried out using the
SYBR®-Green PCR Master Mix Kit (Takara Bio). Primer
sequences are detailed in Table I.
The quantification of gene expression was normalized to the
expression of GAPDH.
 | Table ICharacteristics of PCR primer sets
and products. |
Table I
Characteristics of PCR primer sets
and products.
| Gene | Size of PCR product
(bp) | Primer sequences
(5′→3′) |
|---|
| GAPDH | 200 | F
acccagaagactgtggatgg
R tctagacggcaggtcaggtc |
| CD163 | 146 | F
cgagttaacgccagtaagg
R gaacatgtcacgccagc |
| CD204 | 366 | F
ccagggacatgggaatgcaa
R ccagtgggacctcgatctcc |
| CSF1 | 88 | F
tagccacatgattgggagtgga
R ctcaaatgtaatttggcacgaggtc |
Western blotting assay
For immunoblot analysis, cell lysates containing 50
μg of total protein were separated by electrophoresis on 10% sodium
dodecyl sulfate-polyacrylamide gels and electroblotted onto
polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA,
USA). After blocking of the unoccupied sites with 5% skim milk-PBS,
the membranes were probed with the desired primary antibodies at
4°C overnight, followed by incubation with the appropriate
HRP-conjugated secondary antibodies, according to the
manufacturer's instructions. The primary antibodies were
anti-β-actin, anti-CSF1, and the proper dilution of them was
according to the instructions. The signal was visualized by ECL
Western Blotting Detection Reagents (Pierce Biotechnology, Inc.)
and photographed with ImageQuant LAS 4000 (GE Healthcare,
Piscataway, NJ, USA). β-actin was used as a loading control.
Breast cancer xenografts
To assess the tumorigenicity in vivo,
8-week-old female non-obese diabetic/severe combined
immunodeficient (NOD/SCID) mice were used. Eighteen mice were
assigned to six groups equally. A total of
5×105 cells suspended in 100 μl PBS were
injected orthotopically into the right lower mammary fat pad
according to standard injection procedures. Once tumors were
palpable, tumor volume (1/2 × length × width2) was
monitored every other day for 8 weeks. Tumor growth curves were
plotted.
Statistical analysis
The quantitative results are presented as the mean ±
standard error of at least three independent experiments.
Significant differences were determined with Student's t-test using
Excel or GraphPad Prism 5 Demo software (GraphPad Software, Inc.,
San Diego, CA, USA). P<0.05 was considered statistically
significant.
Results
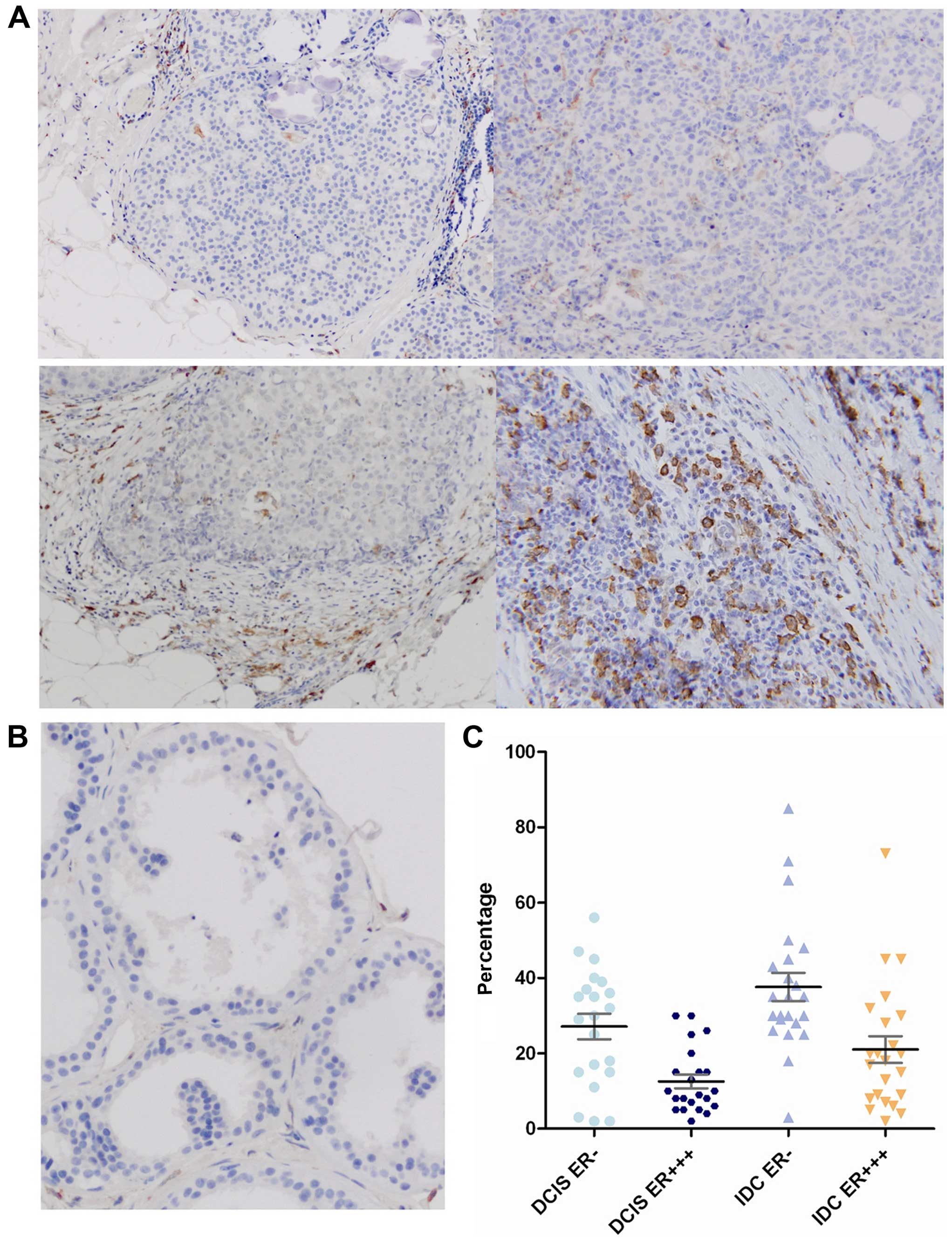
The prevalence of TAM infiltration is
significantly higher in hormone-independent breast cancer samples
compared with hormone-dependent counterparts
TAMs play an important role in the growth and
progression of cancers. CD163 has been reported as a specific
surface antigen of M2 macrophages (12). Herein, we first detected CD163
expression in breast cancer tissues or non-cancer breast tissues by
immunohistochemistry. The results showed that the percentage of
CD163-positive cells was significantly higher in
hormone-independent breast cancers than that in hormone-dependent
counterparts and normal breast tissues, in some cases, TAMs may
make up as much as 50% of tumor volume. Moreover, the difference
was also significant between hormone-independent DCIS and IDC,
whereas CD163 expression was hardly detectable in normal breast
tissues (Fig. 1). However, we did
not observe a significant difference among tumor size, HER2
expression, lymph node involvement, proliferation index (Ki67) and
menstrual status (Table II).
 | Table IITAM infiltration in relation to
clinicopathological characteristics of the breast cancer
patients. |
Table II
TAM infiltration in relation to
clinicopathological characteristics of the breast cancer
patients.
| CD163+
macrophages | |
|---|
|
| |
|---|
| Low n (%) | High n (%) | P-value |
|---|
| No. of
patients | 22 (24.7) | 67 (75.3) | |
| Tumor size
(cm) | | | |
| ≤2 | 12 (27.3) | 32 (72.7) | 0.667 |
| >2 | 10 (23.3) | 33 (76.7) | |
| ER status | | | |
| + | 18 (39.1) | 27 (60.9) | 0.001 |
| − | 4 (9.1) | 40 (90.9) | |
| HER2
overexpression | | | |
| Yes | 3 (20.0) | 12 (80.0) | 0.642 |
| No | 19 (25.7) | 55 (74.3) | |
| Lymph node
involvement | | | |
| Yes | 3 (21.4) | 11 (78.6) | 0.756 |
| No | 19 (25.3) | 56 (74.7) | |
| Proliferation index
(Ki67) (%) | | | |
| >15 | 18 (24.3) | 56 (75.7) | 0.848 |
| <15 | 4 (26.7) | 11 (73.3) | |
| Menstrual
status | | | |
| Premenopausal | 9 (20.5) | 35 (79.5) | 0.356 |
|
Postmenopausal | 13 (28.9) | 32 (71.1) | |
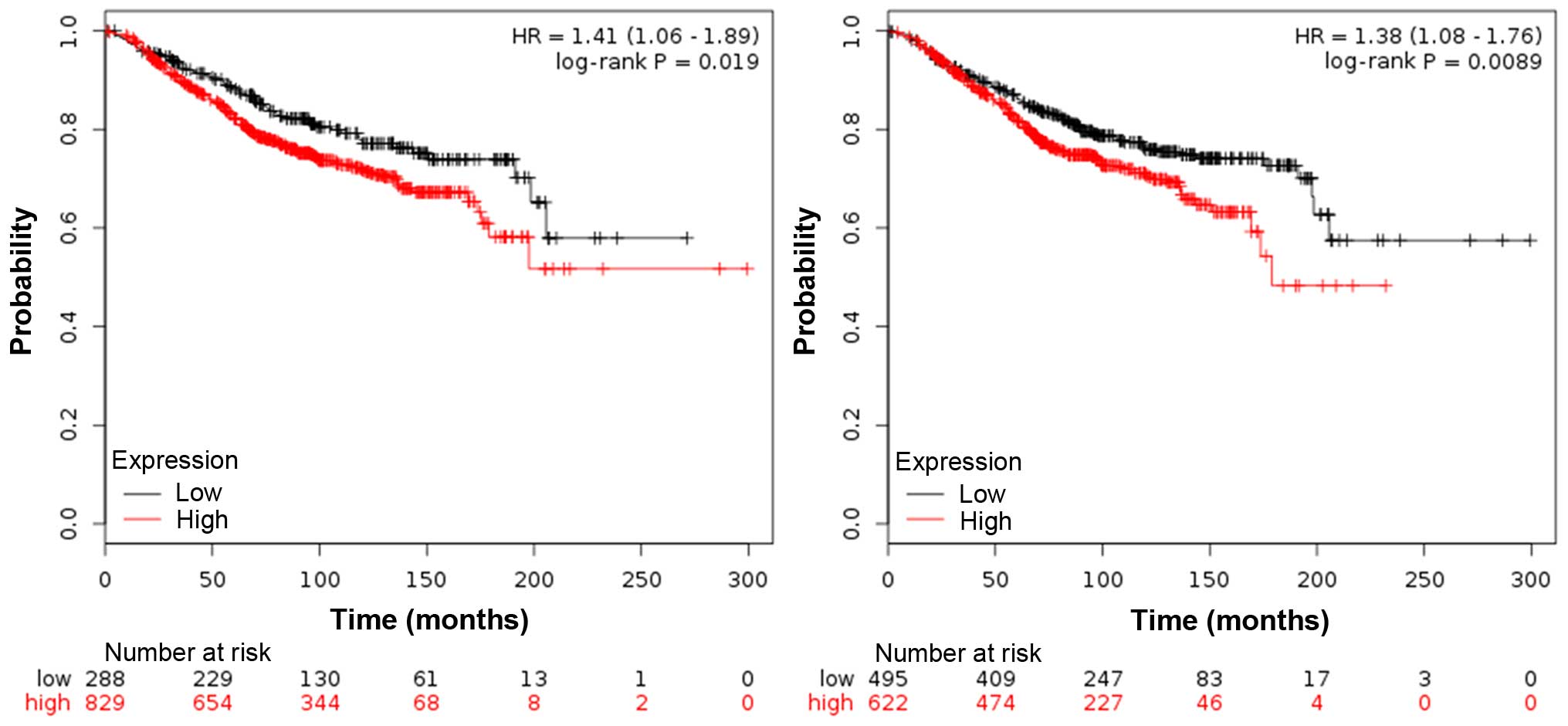
Impact of TAM infiltration on breast
cancer survival
The impact of TAM infiltration on overall survival
(OS) was evaluated in breast cancer patients. Both CD163 and CD204
are specific surface antigens of M2 macrophages, as previously
reported (18,19). We assessed CD163 and CD204 mRNA
expression level in a cohort of 1,117 breast cancer tissue samples
(containing tumor stroma) based on publically available gene
expression datasets (20). For
CD163+ macrophage frequency, patients were grouped as
‘high’ or ‘low’ using the lower quartile as a cut-off point; while
for CD204+ macrophage frequency, patients were grouped
as ‘high’ or ‘low’ using the median as a cut-off point. Increased
expression of CD163 and CD204 was observed in ER-negative patients,
and the patients with high density of CD163+ and
CD204+ macrophages also showed a poor OS, which was an
independent poor prognostic factor for OS (Fig. 2).
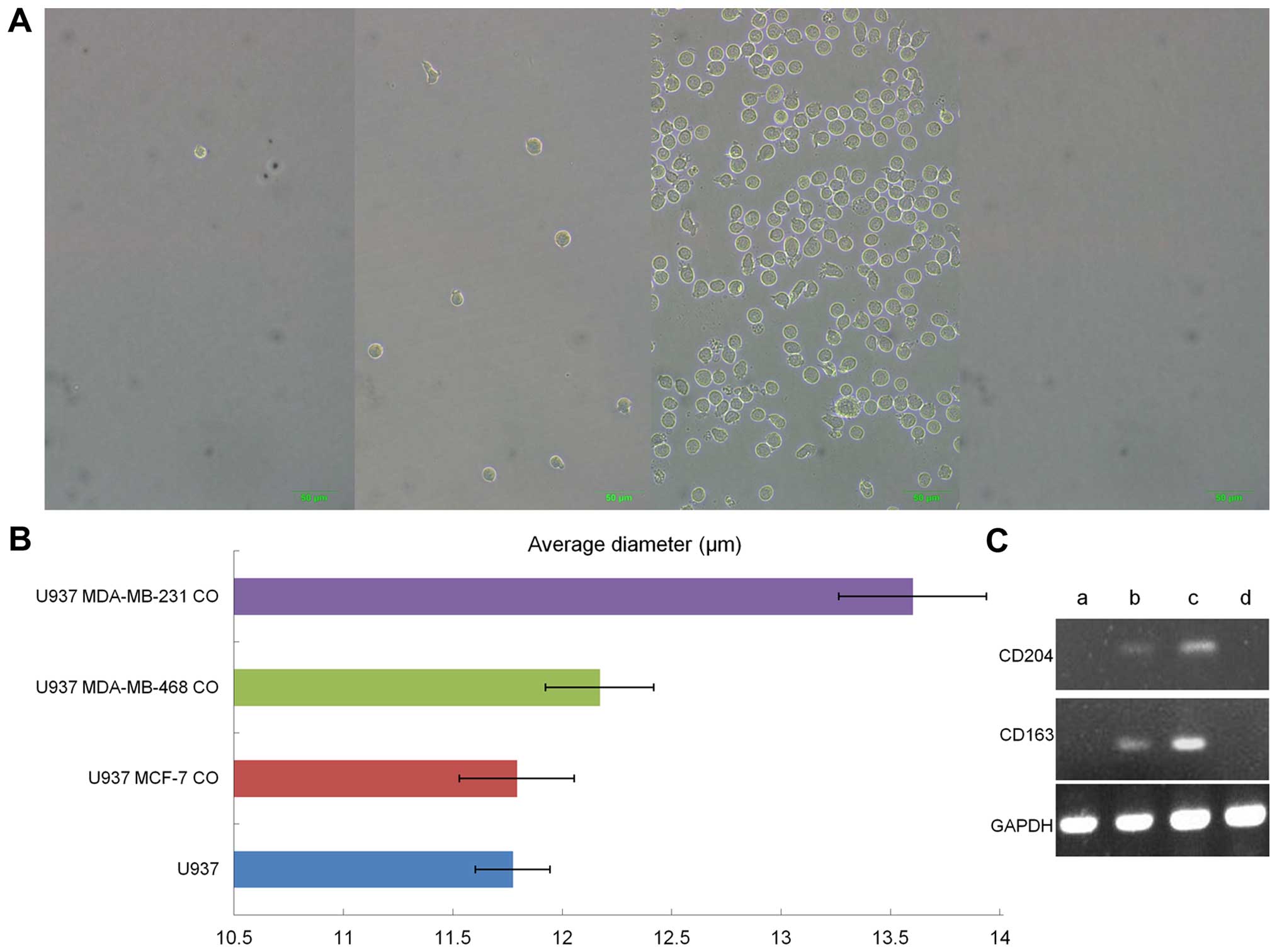
Hormone-independent breast cancer cells
influence monocyte polarization toward TAMs
The human promonocytic U937 cell line was used as a
model to simulate the interaction between monocytes and breast
cancer cells in vitro. U937 cells normally grow in
suspension and have a smooth surface, as previously reported
(21). However, after co-cultured
with hormone-independent breast cancer cell lines MDA-MB-231 and
MDA-MB-468 in a 6-well insert system with 0.4-μm pores, they
differentiated into M2 macrophages, as evidenced by a thorny
morphology, large cell volume and increase in adherence, and
increasing expression of surface antigen CD163 and CD204, all of
which are characteristics of M2 macrophages. Whereas, no further
obvious changes were observed when co-cultured with
hormone-dependent MCF-7 cell line (Fig. 3).
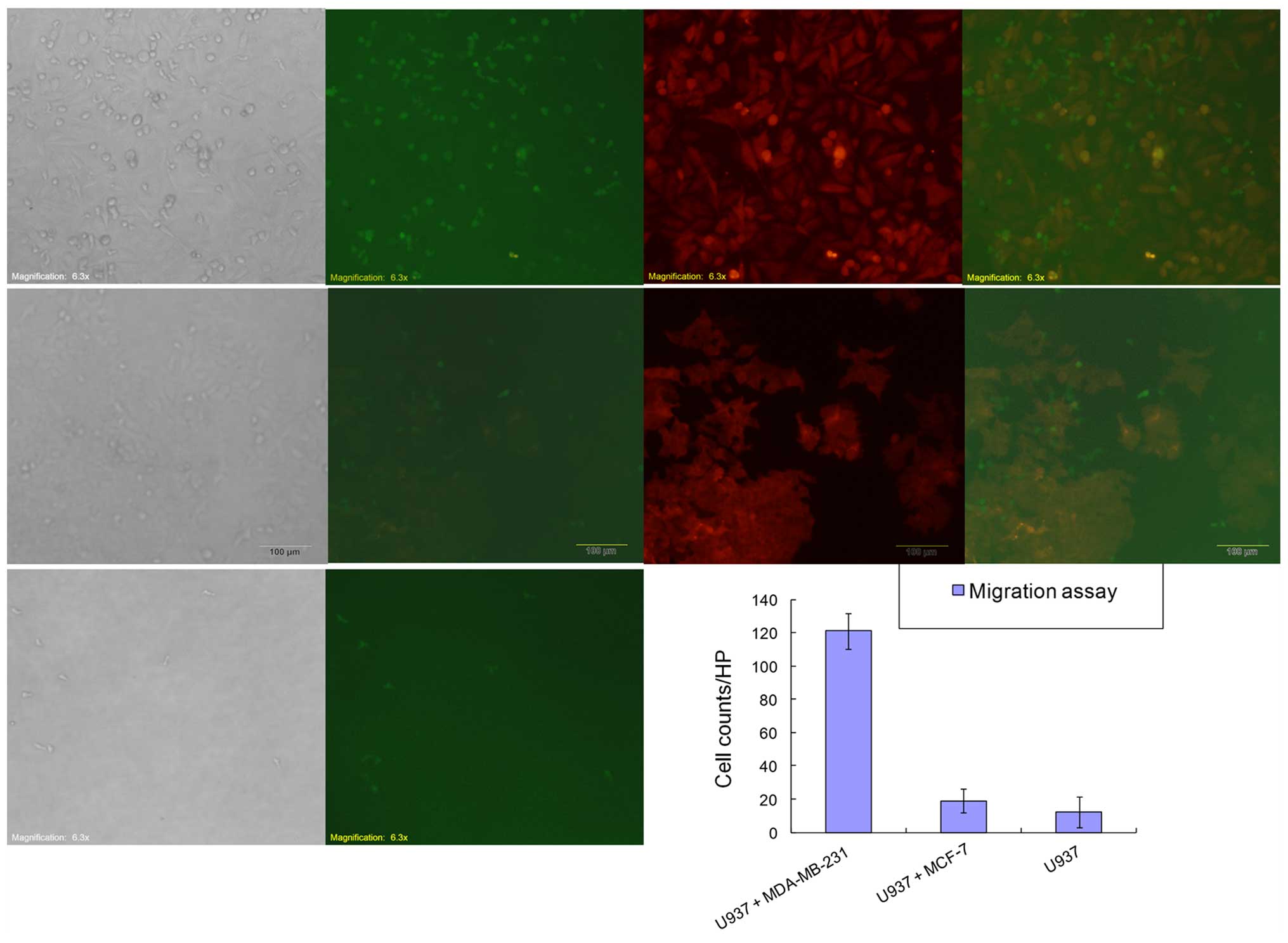
MDA-MB-231 cancer cells recruit monocyte
homing in vitro
To determine the factors that contributed to
monocyte differentiation leaving the vasculature and entering the
tumor tissue, we generated stably expressing GFP-puro MDA-MB-231,
MCF-7 and RFP with neomycin resistance (RFP-neo) U937 cell lines
using lentiviral particles according to the protocol.
We performed the Transwell assay without
extracellular matrix barrier to study the capability of breast
cancer cells to recruit monocytes using a 24-well insert system
with 5-μm pores. After co-cultured for 72 h, migratory cells were
visualized by fluorescence microscopy and images were captured
under a fluorescence microscope. Microscopy images were captured
for each well at five randomly picked fields with a 10X objective
lens, and the number of migratory cells for each field was
quantified (Fig. 4). We further
mimicked the in vivo circumstance applying the Transwell
assay with extracellular matrix barrier (BD Biosciences) using a
24-well insert system with 8-μm pores. Our results showed that
co-cultured MDA-MB-231 with U937 caused an increase in migration
and invasion compared with MCF-7, which indicated that
hormone-independent breast cancer cells recruited more monocytes
than hormone-dependent breast cancer cells (Fig. 5).
Hormone-independent breast cancer cells
secrete larger amount of monocyte differentiation-related cytokine
CSF1
The fluorescent bead-based detection assay is a
highly sensitive method for profiling multiple cytokines, which has
an advantage over conventional enzyme-linked immunosorbent assay
(ELISA), which enables analysis of a number of analytes
simultaneously (22). The strong
effects of the MDA-MB-231 cells in monocyte recruitment and
differentiation led us to inspect their conditioned media (CM) for
dissimilarly secreted monocyte chemotaxis and differentiating
factors. Among the cytokines tested, there was a significant
difference in CSF1 secretion level among different breast cancer
cell lines. We found that mean CSF1 level was much higher in
MDA-MB-231 cell conditioned medium compared to that of MCF-7
(Fig. 6).
Overexpression of CSF1 in MCF-7 fails to
rebuild its aggressiveness both in vitro and in vivo while genetic
inhibition of CSF1 abrogates TAM infiltration and consequently
reduces tumorigenesis in MDA-MB-231
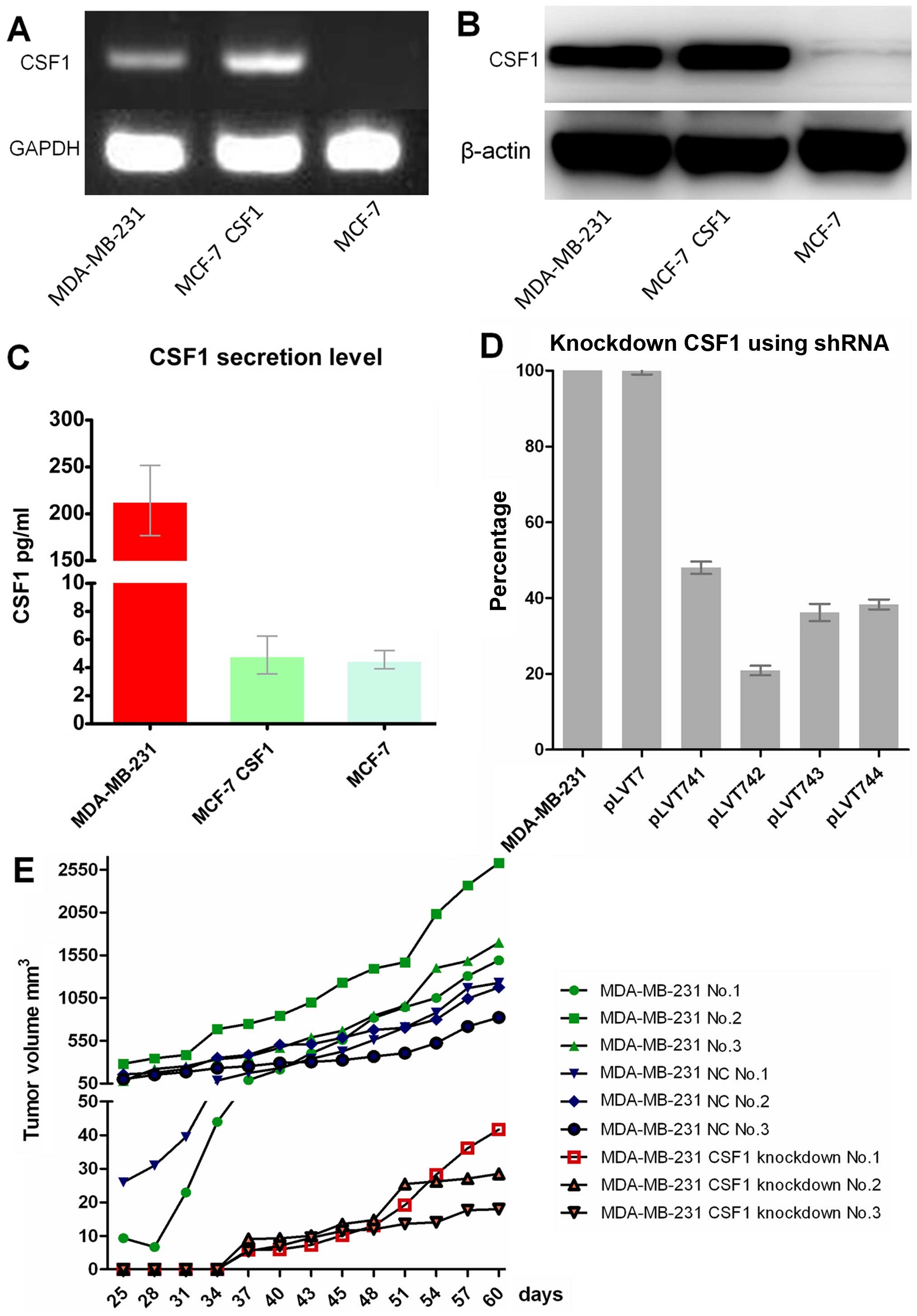
In order to further study the correlation of CSF1
secretion level with monocyte differentiation and homing, and with
tumorigenic ability, we overexpressed CSF1 in MCF-7 cells and
generated the stable cell line using lentivirus particles following
the protocol. The CSF1-overexpressing MCF-7 stable cells were
identified by RT-PCR and western blotting, which showed that CSF1
was overexpressed in MCF-7 artificially (Fig. 7A and B). However, CSF1 level was
not increased in MCF-7 CM (Fig.
7C).
We co-cultured CSF1-overexpressing MCF-7 stable
cells with U937 cells to identify its ability to induce monocyte
differentiation using Transwell assay in vitro. We observed
that CSF1-overexpressing MCF-7 stable cells did not make U937 cells
to differentiate into M2 macrophage (data not shown). This seems to
be contradictory superficially, however, as mentioned previously,
the active form of CSF1 is an extracellular disulfide-linked
homodimer, which is evidenced by our data. No obvious increased
CSF1 secretion profile in CSF1-overexpressing MCF-7 stable cell
conditioned medium, since we did not envelope the CSF1 plasmid with
a proper signal peptide.
Subsequently, we knocked down CSF1 in MDA-MB-231
cells using a series of siRNA sequences (Table III) specifically targeting the
CSF1 gene using shRNA lentivirus particles following the protocol.
The CSF1-knockdown MDA-MB-231 cells were identified by real-time
PCR, which showed that CSF1 was knocked down somewhat in MDA-MB-231
artificially (Fig. 7D). We applied
the most satisfactory shRNA lentivirus particles (cat. nos.
ST3071-A and pLVT742) to generate a stable knockdown cell line for
further study. Our data suggested that CSF1-knockdown stable
MDA-MB-231 cells secrete much less CSF1 in its CM and thus failed
to induce U937 cell differentiation into M2 macrophages.
 | Table IIIInformation on shRNA and target
sequences. |
Table III
Information on shRNA and target
sequences.
| Marker | Gene | Gene ID | TargetSeq | GC (%) |
|---|
| pLVT741 | CSF1 | NM_000757 |
CCTCGTGCCAAATTACATT | 42.1 |
| pLVT742 | CSF1 | NM_000757 |
CCATGCGCTTCAGAGATAA | 47.4 |
| pLVT743 | CSF1 | NM_000757 |
GCCAAGATGTGGTGACCAA | 52.6 |
| pLVT744 | CSF1 | NM_000757 |
GGATGACAGACAGGTGGAA | 52.6 |
| pLVT7 | NC | |
TTCTCCGAACGTGTCACGT | 52.6 |
In an in vivo tumorigenic ability study,
5×105 cells suspended in 100 μl PBS were injected
orthotopically into the mammary fat pad of 8-week-old female
NOD/SCID mice. Similarly, only MDA-MB-231 cell lines form a
palpable xenograft tumor successfully in 6 weeks, while being
sacrificed in 8 weeks was confirmed again (Fig. 7E). Both the results of in
vitro and in vivo studies confirmed that CSF1 played its
role in promoting tumor progression by inducing the differentiation
of monocytes.
Discussion
Many pre-clinical and clinical studies demonstrate
an inverse correlation between TAM infiltration and patient
prognosis indicating a macrophage-supporting role for tumor
progression (23–25). The recruitment of host stromal
cells, such as macrophages and mesenchymal stem cells (MSCs), to
the primary tumor is a vital step towards cancer malignancy.
Breast cancer metastasis transforms a local disease
which is cured by surgical excision into a systemic disease which
responds poorly to available therapies and is the major cause of
patient mortality (26). Multiple
stromal cell types, including TAMs, are recruited to the TME and
play their roles in metastasis (27,28).
TAM infiltration is quite common in breast cancer, and sometimes
outnumbers the cancer cells in certain cases (29,30).
The abundance of TAMs in primary breast cancer biopsies is
correlated with metastasis and patient mortality (30–32).
In mouse models, CSF1 secreted by breast cancer cells binds to
their cognate receptors, CSF1 receptor (CSF1R) and CCR2 on TAMs,
leading to their recruitment to the TME, where they interact with
cancer cells to promote invasion and metastasis (33–36).
In breast cancer, it is reported that the density of
macrophage infiltration and abundance of genes associated with
macrophage infiltration are part of a molecular signature that
heralds negative prognosis in node-negative, tamoxifen-treated
breast carcinomas (37). Though
the expression of M2 macrophage-specific antigen CD163 varied
significantly in primary breast cancer, its prevalence has a
prognostic impact on OS (38).
In this study, we applied the U937 cell line as a
model to investigate the correlation between monocytes and breast
cancer cells. Although the use of human peripheral blood monocyte
primary cells should allow stronger conclusions to be drawn, the
enrichment of human peripheral blood monocytes involves many
ethical issues in our institute. U937 cells have been widely used
in macrophage research and have been shown to closely model primary
macrophages extracted from whole blood (39–41).
Moreover, we demonstrated an increased TAM infiltration within
ER-negative breast cancer, and confirmed that the density of
macrophage infiltration is associated with poor prognosis.
In in vitro models, using co-culture
experiments, we also successfully established the differentiation
and polarization of monocytes in breast cancer CM as confirmed by
morphology and gene expression profile. Our data showed that
hormone-independent breast cancer cell CM can induce U937 cell
differentiation and then recruit it, whilst in hormone-dependent
breast cancer cells no such phenomenon was observed using a panel
of representing breast cancer cell lines MCF-7, MDA-MB-231 and
MDA-MB-468. Despite this, in the presence of MDA-MB-231 cells, U937
cells significantly increased invasive ability compared to in the
presence of MCF-7. This suggests that the specialized M2
macrophages secrete a set of specific proteins that directly
destroy the basement, which contribute to cancer invasion and
metastasis. We further found that there was a much higher level of
CSF1 secretion in MDA-MB-231 CM than in MCF-7, which is required
for monocyte differentiation and homing. Consequently, we
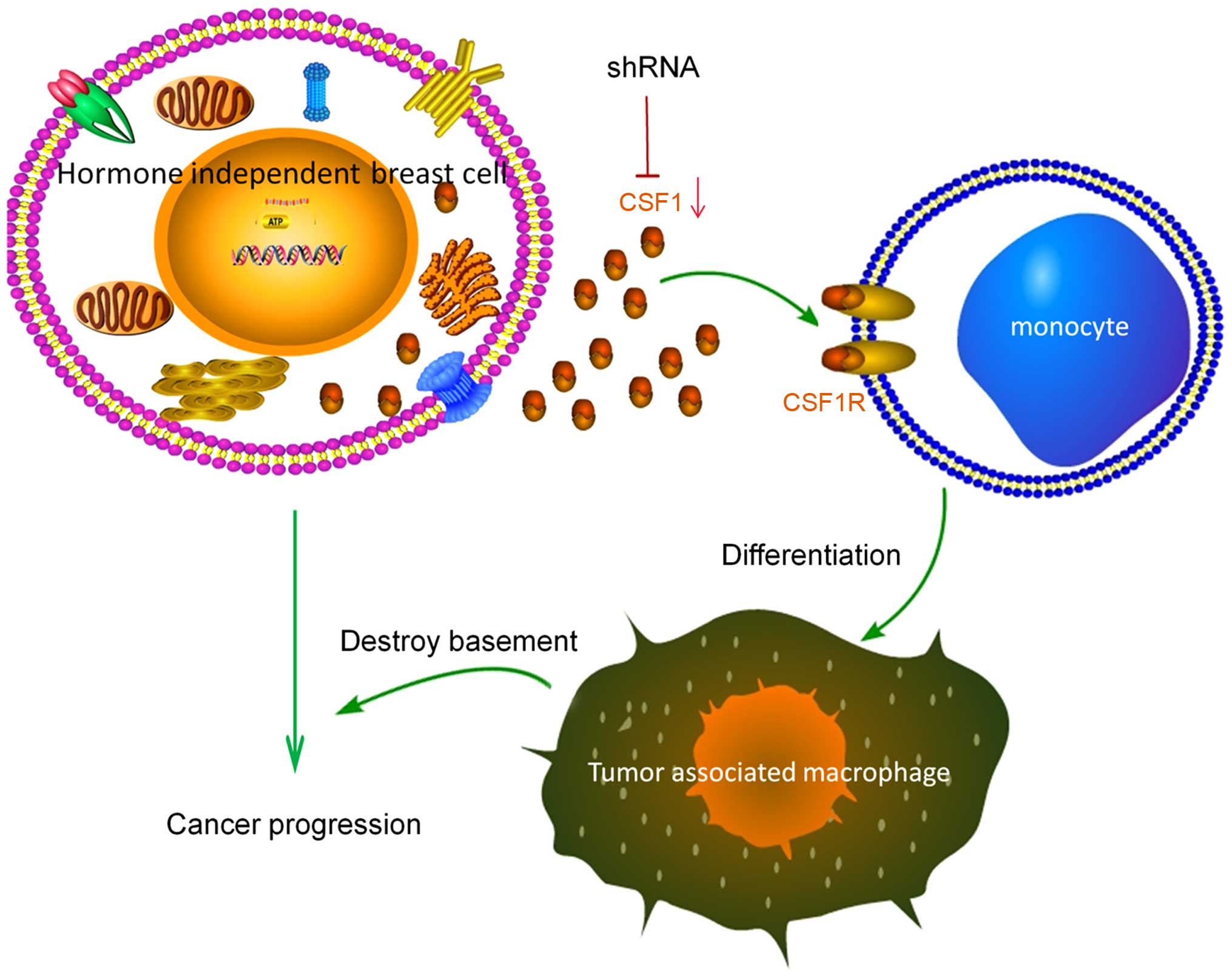
tentatively depict the progression of hormone-independent breast
cancer in Fig. 8. Identification
of this specific protein will aid in developing novel drugs to
treat the pro-tumorigenic microenvironment for TNBC. Using our
model we have identified a potential treatment to overcome the
pro-tumorigenic effects of the macrophage microenvironment.
To the best of our knowledge, this is the first
report demonstrating direct experimental evidence that
hormone-independent breast cancers demonstrate more aggressively
than hormone-dependent breast cancers through secreting CSF1 to
educate and recruit monocytes and rebuilding a pro-tumorigenic
microenvironment.
In conclusion, TAMs play a critically important role
in hormone-independent breast cancer progression, and circulating
monocytes are inclined to being recruited and differentiating into
TAMs in special tumor niche. New strategies for cancer treatment
could emerge from a better understanding of the reciprocal
paracrine loop between breast cancer and the microenvironment.
Consequently, preventative treatment based on the suppression of
monocyte differentiation and homing might be possible. These
findings suggest the future possibility of using TAMs as a novel
therapeutic target in patients with anti-estrogen resistance and
primary TNBC, a tumor type defined by lack of estrogen receptor,
progesterone receptor and ERBB2 gene amplification with no
effective therapeutic measures at present, although representing
~16% of all breast cancers.
Acknowledgements
We thank Dr Ping Zhang of the Fudan University
Cancer Institute Research Center for help with FACS, Dr Wentao Yang
of the Clinical Pathology Center for the histological evaluation,
and Professor Duan Ma of the Key Laboratory of Molecular Medicine
of the Ministry of Education for providing the U937 cell line. This
study was supported in part by the Jiangxi Provincial Health and
Family Planning Commission [2014]18/20154010, and the Organization
Department of the Central Committee of the Communist Party of China
2015 ‘Sunshine of the West’ visiting scholar program, and also the
National Basic Research Program of China (2010CB834305,
2010CB834301).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stanley ER, Berg KL, Einstein DB, Lee PS,
Pixley FJ, Wang Y and Yeung YG: Biology and action of
colony-stimulating factor-1. Mol Reprod Dev. 46:4–10. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stanley ER, Berg KL, Einstein DB, Lee PS
and Yeung YG: The biology and action of colony stimulating
factor-1. Stem Cells. 12(Suppl 1): 15–24. 1994.PubMed/NCBI
|
|
4
|
Sapi E: The role of CSF-1 in normal
physiology of mammary gland and breast cancer: An update. Exp Biol
Med (Maywood). 229:1–11. 2004.
|
|
5
|
Chambers AF, Groom AC and MacDonald IC:
Dissemination and growth of cancer cells in metastatic sites. Nat
Rev Cancer. 2:563–572. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hughes R, Qian BZ, Rowan C, Muthana M,
Keklikoglou I, Olson OC, Tazzyman S, Danson S, Addison C, Clemons
M, et al: Perivascular M2 macrophages stimulate tumor relapse after
chemotherapy. Cancer Res. 75:3479–3491. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sugimura K, Miyata H, Tanaka K, Takahashi
T, Kurokawa Y, Yamasaki M, Nakajima K, Takiguchi S, Mori M and Doki
Y: High infiltration of tumor-associated macrophages is associated
with a poor response to chemotherapy and poor prognosis of patients
undergoing neoadjuvant chemotherapy for esophageal cancer. J Surg
Oncol. 111:752–759. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sasayama T, Tanaka K, Mizowaki T,
Nagashima H, Nakamizo S, Tanaka H, Nishihara M, Mizukawa K, Hirose
T, Itoh T, et al: Tumor-associated macrophages associate with
cerebrospinal fluid interleukin-10 and survival in primary central
nervous system lymphoma (PCNSL). Brain Pathol. 26:479–487. 2016.
View Article : Google Scholar
|
|
9
|
Gordon S: Alternative activation of
macrophages. Nat Rev Immunol. 3:23–35. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Svensson J, Jenmalm MC, Matussek A,
Geffers R, Berg G and Ernerudh J: Macrophages at the fetal-maternal
interface express markers of alternative activation and are induced
by M-CSF and IL-10. J Immunol. 187:3671–3682. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wynn TA, Chawla A and Pollard JW:
Macrophage biology in development, homeostasis and disease. Nature.
496:445–455. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Geissmann F, Manz MG, Jung S, Sieweke MH,
Merad M and Ley K: Development of monocytes, macrophages, and
dendritic cells. Science. 327:656–661. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Park KY, Li G and Platt MO:
Monocyte-derived macrophage assisted breast cancer cell invasion as
a personalized, predictive metric to score metastatic risk. Sci
Rep. 5:138552015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu KD, Li JJ, Di GH, Wu J, Shen ZZ and
Shao ZM: A straight-forward but not piecewise relationship between
age and lymph node status in Chinese breast cancer patients. PLoS
One. 5:e110352010. View Article : Google Scholar
|
|
15
|
Hannan NJ, Bambang K, Kaitu'u-Lino TJ,
Konje JC and Tong S: A bioplex analysis of cytokines and chemokines
in first trimester maternal plasma to screen for predictors of
miscarriage. PLoS One. 9:e933202014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee KS, Chung JH, Lee KH, Shin MJ, Oh BH
and Hong CH: Bioplex analysis of plasma cytokines in Alzheimer's
disease and mild cognitive impairment. Immunol Lett. 121:105–109.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim YW, Kim SK, Kim CS, Kim IY, Cho MY and
Kim NK: Association of serum and intratumoral cytokine profiles
with tumor stage and neutrophil lymphocyte ratio in colorectal
cancer. Anticancer Res. 34:3481–3487. 2014.PubMed/NCBI
|
|
18
|
Shigeoka M, Urakawa N, Nakamura T, Nishio
M, Watajima T, Kuroda D, Komori T, Kakeji Y, Semba S and Yokozaki
H: Tumor associated macrophage expressing CD204 is associated with
tumor aggressiveness of esophageal squamous cell carcinoma. Cancer
Sci. 104:1112–1119. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kaku Y, Imaoka H, Morimatsu Y, Komohara Y,
Ohnishi K, Oda H, Takenaka S, Matsuoka M, Kawayama T, Takeya M, et
al: Overexpression of CD163, CD204 and CD206 on alveolar
macrophages in the lungs of patients with severe chronic
obstructive pulmonary disease. PLoS One. 9:e874002014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Györffy B, Lanczky A, Eklund AC, Denkert
C, Budczies J, Li Q and Szallasi Z: An online survival analysis
tool to rapidly assess the effect of 22,277 genes on breast cancer
prognosis using microarray data of 1,809 patients. Breast Cancer
Res Treat. 123:725–731. 2010. View Article : Google Scholar
|
|
21
|
Hass R, Bartels H, Topley N, Hadam M,
Köhler L, Goppelt-Strübe M and Resch K: TPA-induced differentiation
and adhesion of U937 cells: Changes in ultrastructure, cytoskeletal
organization and expression of cell surface antigens. Eur J Cell
Biol. 48:282–293. 1989.PubMed/NCBI
|
|
22
|
Fulton RJ, McDade RL, Smith PL, Kienker LJ
and Kettman JR Jr: Advanced multiplexed analysis with the
FlowMetrix system. Clin Chem. 43:1749–1756. 1997.PubMed/NCBI
|
|
23
|
Schmieder A, Michel J, Schönhaar K, Goerdt
S and Schledzewski K: Differentiation and gene expression profile
of tumor-associated macrophages. Semin Cancer Biol. 22:289–297.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Obeid E, Nanda R, Fu YX and Olopade OI:
The role of tumor-associated macrophages in breast cancer
progression (Review). Int J Oncol. 43:5–12. 2013.PubMed/NCBI
|
|
25
|
Kennedy BC, Showers CR, Anderson DE,
Anderson L, Canoll P, Bruce JN and Anderson RC: Tumor-associated
macrophages in glioma: Friend or foe? J Oncol. 2013:4869122013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Marino N, Woditschka S, Reed LT, Nakayama
J, Mayer M, Wetzel M and Steeg PS: Breast cancer metastasis: Issues
for the personalization of its prevention and treatment. Am J
Pathol. 183:1084–1095. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Quail DF and Joyce JA: Microenvironmental
regulation of tumor progression and metastasis. Nat Med.
19:1423–1437. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hanahan D and Coussens LM: Accessories to
the crime: Functions of cells recruited to the tumor
microenvironment. Cancer Cell. 21:309–322. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kelly PM, Davison RS, Bliss E and McGee
JO: Macrophages in human breast disease: A quantitative
immunohistochemical study. Br J Cancer. 57:174–177. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Medrek C, Pontén F, Jirström K and
Leandersson K: The presence of tumor associated macrophages in
tumor stroma as a prognostic marker for breast cancer patients. BMC
Cancer. 12:3062012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tsutsui S, Yasuda K, Suzuki K, Tahara K,
Higashi H and Era S: Macrophage infiltration and its prognostic
implications in breast cancer: The relationship with VEGF
expression and microvessel density. Oncol Rep. 14:425–431.
2005.PubMed/NCBI
|
|
32
|
Chaturvedi P, Gilkes DM, Takano N and
Semenza GL: Hypoxia-inducible factor-dependent signaling between
triple-negative breast cancer cells and mesenchymal stem cells
promotes macrophage recruitment. Proc Natl Acad Sci USA.
111:E2120–E2129. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lin EY, Nguyen AV, Russell RG and Pollard
JW: Colony-stimulating factor 1 promotes progression of mammary
tumors to malignancy. J Exp Med. 193:727–740. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wyckoff J, Wang W, Lin EY, Wang Y, Pixley
F, Stanley ER, Graf T, Pollard JW, Segall J and Condeelis J: A
paracrine loop between tumor cells and macrophages is required for
tumor cell migration in mammary tumors. Cancer Res. 64:7022–7029.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pollard JW: Tumour-educated macrophages
promote tumour progression and metastasis. Nat Rev Cancer. 4:71–78.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Qian BZ, Li J, Zhang H, Kitamura T, Zhang
J, Campion LR, Kaiser EA, Snyder LA and Pollard JW: CCL2 recruits
inflammatory monocytes to facilitate breast-tumour metastasis.
Nature. 475:222–225. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Paik S, Shak S, Tang G, Kim C, Baker J,
Cronin M, Baehner FL, Walker MG, Watson D, Park T, et al: A
multigene assay to predict recurrence of tamoxifen-treated,
node-negative breast cancer. N Engl J Med. 351:2817–2826. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shabo I, Stål O, Olsson H, Doré S and
Svanvik J: Breast cancer expression of CD163, a macrophage
scavenger receptor, is related to early distant recurrence and
reduced patient survival. Int J Cancer. 123:780–786. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lin JA, Lin FY and Chen TL: Bone
components downregulate expression of toll-like receptor 4 on the
surface of human monocytic U937 cells: A cell model for
postfracture immune dysfunction. Mediators Inflamm.
2015:8965762015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Martin-Manso G, Galli S, Ridnour LA,
Tsokos M, Wink DA and Roberts DD: Thrombospondin 1 promotes tumor
macrophage recruitment and enhances tumor cell cytotoxicity of
differentiated U937 cells. Cancer Res. 68:7090–7099. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yang XV, Banerjee Y, Fernández JA, Deguchi
H, Xu X, Mosnier LO, Urbanus RT, de Groot PG, White-Adams TC,
McCarty OJ, et al: Activated protein C ligation of ApoER2 (LRP8)
causes Dab1-dependent signaling in U937 cells. Proc Natl Acad Sci
USA. 106:274–279. 2009. View Article : Google Scholar : PubMed/NCBI
|