Introduction
In medicinal chemistry, many compounds with high
biological activities derive from scaffolds, and their unique
bioactivity may depend on substituent group (1). In addition, designing and
transforming the substituent group of small molecule compounds have
become an effective way for drug discovery. Benzothiazole is a
heterocyclic scaffold with a benzene ring fused with a
five-membered thiazole ring, and it is recognized as an important
basis of the nucleus for drug synthesis (2,3).
Benzothiazole derivatives have a wide range of biological
activities (4), such as anticancer
(5,6), antimicrobial (7) and immunosuppressive activity
(8), benzothiazole analogues are
also biologically active compounds in the central nervous system
(9). In addition, benzothiazole
derivatives as commercial drugs are extensively used in the
clinical treatment of numerous diseases (10,11).
Our research team first reported a novel
benzothiazole derivative BD750
[2-(2-benzothiazoleyl)-4,5,6,7-tetrahydro-2H-indazol-3-ol,
C14H13N3OS, MW: 271.3]. BD750 has significantly shown
immunosuppressive activity by inhibiting T cell proliferation
(8). However, its poor solubility
in water is unsatisfactory. In addition, the poor water solubility
may make it difficult in clinical application. Thus, our research
team was committed to design, synthesize, screen and biological
evaluate of novel water-soluble benzothiazole derivatives based on
BD750. Then we find BD926, a new water-soluble benzothiazole
derivative in which the H-2 of benzothiazol is replaced by
4,5,6,7-tetrahydro-2H-indazol-3-olate group and it has shown good
water solubility of approximately 25 mg/ml in water (12).
In the present study, we investigated the antitumor
biological activity of BD926 in the human Ramos B-lymphoma cell
line, and explored its potential mechanisms. Our findings aim to
clarify that BD926 may be a potential antitumor drug.
Materials and methods
Drug and reagents
BD926, Sodium
2-(2-benzothiazoleyl)-4,5,6,7-tetrahydro-2H-indazol-3-olate
(Fig. 1A), was synthesized
previously by our team and the structure was confirmed by 1H-NMR,
13C-NMR and HRMS (ESI). Purity (85%) was measured by HPLC analysis
(12). BD926 was dissolved in
ultrapure water at a stock concentration of 10 mM and stored at
−20°V.
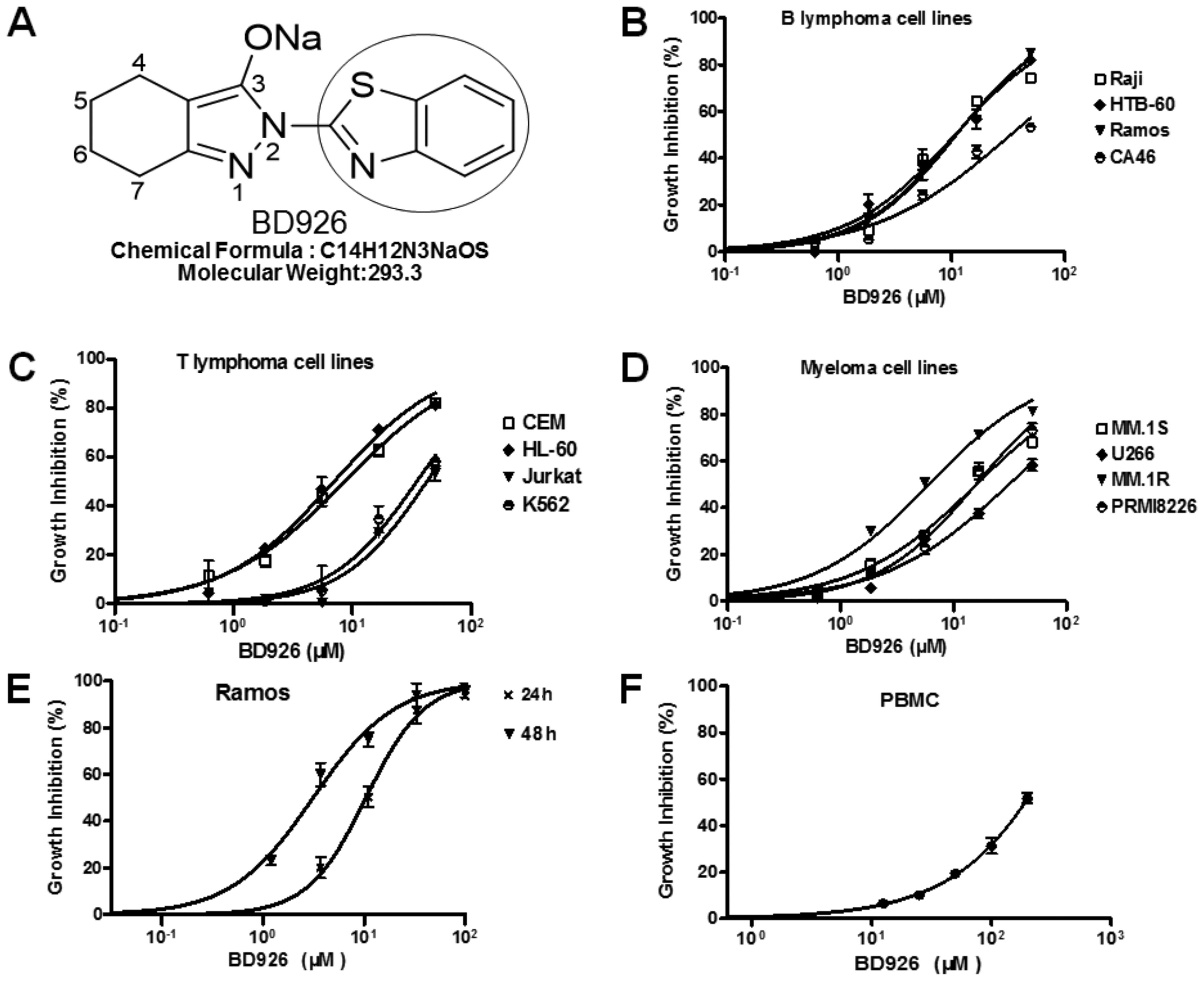 | Figure 1BD926 inhibits proliferation of PBMC
and tumor cells in vitro. The growth inhibition effects of
BD926 in PBMC and tumor cells were determined by CCK-8 assay. (A)
Molecular structure of BD926, in which the H-2 of benzothiazol was
replaced by 4,5,6,7-tetrahydro-2H-indazol-3-olate group. (B) BD926
inhibited the proliferation of B lymphoma cell lines in a
dose-dependent manner with IC50 ~10.9 μM in Ramos, 10.8
μM in Raji, 10.7 μM in HTB-60 and 33.2 μM in CA46. (C) BD926
inhibited the proliferation of T lymphoma cell lines in a
dose-dependent manner with IC50 ~8.6 μM in CEM, 7.0 μM
in HL-60, 42.2 μM in Jurkat and 34.9 μM in K562. (D) BD926
inhibited the proliferation of myeloma cell lines in a
dose-dependent manner with IC50 ~15.9 μM in MM.1S, 6 μM
in MM.1R, 30.9 μM in U266 and 15.8 μM in PRMI-8226. (E) BD926
inhibited the proliferation of Ramos cells in a time- and
dose-dependent manner with IC50 ~10.9 μM for 24 h and 3
μM for 48 h. (F) BD926 exhibited low toxicity in PBMC with
CC50 ~200 μM for 48 h. The results are presented as the
means ± SD (n=3) and were analyzed with the GraphPad Prism
software. |
The antibodies against GAPDH, PARP, Bcl-xl, Bcl-2,
Bid, caspase-3, caspase-8, caspase-9 were purchased from Cell
Signaling Technology. The caspase-12 antibody was purchased from
Abcam. Primers were synthesized by the Shanghai Biological
Engineering Company of China. All other chemicals were of
analytical grade.
Cell culture
B lymphoma cell lines Ramos, Raji, HTB-60 and CA46,
T lymphoma cell lines CEM, HL-60, Jurkat and K562, myeloma cell
lines MM.1S, MM.1R, U266 and PRMI-8226 were saved by our
laboratory. The cells were propagated under humidified conditions
with 5% CO2 at 37°C in RPMI-1640 medium and supplemented
with 10% fetal bovine serum (FBS; both from Gibco, Gaithersburg,
MD, USA), 100 units/ml penicillin and streptomycin. To ensure that
there was no mycoplasma contamination present, antibiotic treatment
was performed regularly.
Human peripheral blood mononuclear cells (PBMC) were
isolated from three healthy donors by density-gradient
centrifugation using lymphocyte separation liquid (Nycomed AS,
Oslo, Norway) and cultivated in the complete medium of RPMI-1640
containing 10% FBS. The blood donors were healthy individuals who
provided informed consent.
Cell proliferation assay
Cell viability was performed by the CCK-8 assay
(Dojindo Laboratories, Kumamoto, Japan). Briefly, the exponentially
growing cells (1E4 cells/well) were seeded in a 96-well flat bottom
microtiter plate and treated with BD926 for 24 or 48 h. Then, 20 μl
of CCK-8 solution was added to each well and incubated for another
4 h at 37°C. The absorbance of each well was measured with Spectra
microplate spectrophotometer (BioTek Instruments, Winooski, VT,
USA) at 450 nm wavelength and the median inhibitory concentration
(IC50) was calculated with the GraphPad Prism software.
Three replicate wells were used for each analysis. The results were
obtained from three separate experiments.
Cell cycle analysis
After treated with BD926 for 12 h, the cells were
harvested and washed briefly in cold PBS, then fixed in 75%
ice-cold ethanol overnight. The samples were concentrated after
removal of ethanol. Propidium iodide (PI) staining solution (1%
Triton X-100, 0.01% RNase, 0.05% PI) (Sigma-Aldrich, St. Louis, MO,
USA) was added to the samples to stain cellular DNA at 4°C for 30
min in dark. The cell cycle distribution was measured and analyzed
by flow cytometry (FCM) (BD FACS Accuri C6; BD Biosciences, San
Jose, CA USA).
Morphological observation under phase
contrast microscope
Ramos cells were seeded in 12-well plates and
treated with BD926 for 24 h. Then, the morphology of Ramos cells
were observed under a phase contrast microscope (Olympus, Tokyo,
Japan).
Apoptosis analysis by flow cytometry
Annexin V-FITC/PI apoptosis detection kit (Roche
Diagnostics, Indianapolis, IN, USA) was carried out to detect the
apoptotic cells. Briefly, after treated with different
concentrations of BD926 for 12 h, Ramos cells were harvested and
washed with cold PBS. The cells were then stained with Annexin
V-FITC/PI then were analyzed by FCM.
Mitochondrial membrane potential
testing
JC-1 is a fluorescent probe for the detection of
mitochondrial membrane potential (MMP). After treated with BD926
for 12 h, changes in mitochondrial transmembrane potential (ΔΨm)
were evaluated by staining cells with JC-1 (Roche Diagnostics).
Cell culture and drug treatment were done as described above. The
harvested Ramos cells were washed with cold PBS, incubated with
JC-1 (5 mg/ml) at 37°C for 30 min in the dark, then measured by
FCM.
Cytochrome c detecting
Ramos cells were treated with BD926 for 12 h, washed
with PBS, fixed (BD™ Phosflow Fix Buffer) and permeabilized (BD
Phosflow™ Perm Buffer), then stained with anti-cytochrome c/FITC
(BD Biosciences) antibody. The resistance type with FITC labeled
rat lgG0/G1 antibodies were used for comparison. FCM was used to
detect cytochrome c content of the cytoplasm in Ramos
cells.
Western blot analysis
After treated with BD926 for 24 h, Ramos cells were
lysed in RIPA buffer (Bioteke Corp., Beijing, China) and the lysate
was centrifuged at 13,000 × g at 4°C for 15 min. The supernatant
was harvested and the protein concentration was measured by BCA
method (Bioteke). Equal amounts of total proteins were subjected to
12% SDS-PAGE and transferred onto polyvinylidene fluoride membranes
(Millipore Corp., Bedford, MA, USA). After electrophoresis, the
membranes were blocked at room temperature for 1.5 h and incubated
in the respective primary antibodies at 4°C overnight, then the
bound antibodies were detected with horseradish peroxidase
(HRP)-conjugated secondary antibody (Cell Signaling Technology,
Danvers, MA, USA). The immunostaining signal was visualized by
enhanced chemiluminescence (Millipore).
Real-time quantitative RT-PCR
Total RNA was extracted from the treatment cells
using the acid guanidinium thiocyanate-phenol-chloroform method
(TRIzol; Takara Bio, Dalian, China), and cDNAs were synthesized
with the PrimeScript™ RT reagent kit (Takara Bio). Quantitative PCR
was performed using the Bio-Rad CFX96 Real-Time PCR detection
system with the TaqMan probe method (Roche Diagnostics). The
primers are 5′-cagatgaaaatgggggtaccta-3′ (F) and 5′-tcaagagt
ggtgaagatttttgat-3′ (R) for CHOP; 5′-agctgtagcgtatggtgctg-3′ (F)
and 5′-aaggggacatacatcaagcagt-3′ (R) for GRP78.
The mRNA expression levels were calculated with the
2−ΔΔCT method and expressed in relative quantification
units. A control without cDNA was run in parallel with each assay.
Each reaction was amplified in triplicate, and relative mRNA levels
were normalized to GAPDH.
ROS measurement
To assess the generation of ROS, BD926-treated cells
(1×105) were incubated with 10 μM
2,7-dichlorofluorescein diacetate (DCFH-DA). Within the cells,
DCFH-DA is converted to DCFH, which can be oxidized to the
fluorescent compound DCF in the presence of ROS. Cell culture and
drug treatment were done as described above, and Ramos cells were
washed with PBS after incubated with DCFH-DA (Bi Yun Tian Corp.,
Kuaidamao, China) at 25°C for 30 min in the dark. Then the
expression of cell fluorescence signal was detected by FCM.
Statistical analysis
The results of the statistical analysis used
GraphPad Prism 5 software with one-way ANOVA and Dunnett's test,
analysis, comparison between group differences. Descriptive
statistics were calculated with mean ± standard deviation, and
P<0.05 showed a statistically significant difference.
Results
BD926 inhibits the proliferation of tumor
cells
In order to confirm the anticancer activity of BD926
in cell models, we examined the growth inhibition effect of BD926
on tumor cell lines including B lymphoma cells, T lymphoma cells
and myeloma cells. Cells were treated with BD926 for 24 h and then
the cell viability was assayed by CCK-8. The results showed that
BD926 significantly inhibited the cell proliferation with
IC50 from 6.0 to 42.2 μM depending on different tumor
cell lines (Fig. 1B–D).
The present study aimed to clarify the anticancer
effect and mechanism of BD926 based on Ramos cells. The result
showed, BD926 exhibited a significant cell proliferation inhibition
with IC50 ~3 μM for 48 h and 10.9 μM for 24 h in Ramos
cells (Fig. 1E). This indicated
that BD926 treatment decreased the viability of Ramos cells in a
time- and dose-dependent manner. The 50% inhibition concentration
of cytotoxicity (CC50) of BD926 in resting human PBMC
was as high as 200 μM for 48 h (Fig.
1F). This result suggested that BD926 had almost no cytotoxic
effect on resting human PBMC under the effective inhibition
concentration of tumor cells, which indicated that BD926 could
effectively inhibit tumor proliferation and exhibited low toxicity
in PBMC.
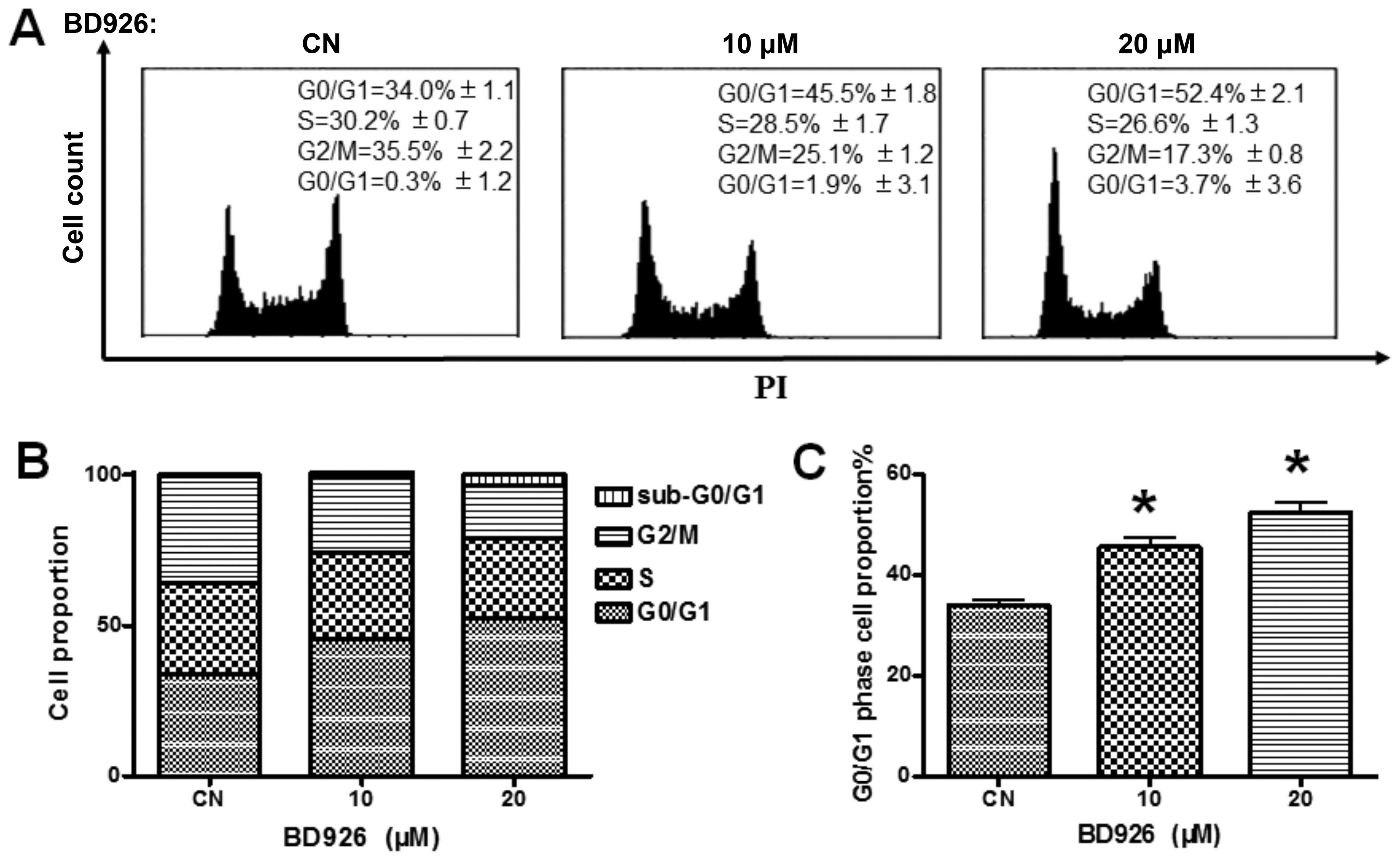
BD926 induces Ramos cell cycle arrest at
G0/G1 phase
Because the proliferation of Ramos cells was
suppressed by BD926, we determined how BD926 blocks the cell cycle
progression by FCM analysis. The Ramos cells were treated by BD926
for 12 h, the cellular DNA was stained with PI and analysed via
FCM. The results are shown in Fig.
2. A dose-dependent increase in the cell population of G0/G1
phase was observed. BD926 treatment increased the percentage of
G0/G1 cells from 34.0% in non-treated group to 52.4% in 20 μM
BD926-treated group in Ramos cells. The results indicated that
BD926 induced Ramos cell cycle arrest at G0/G1 phase.
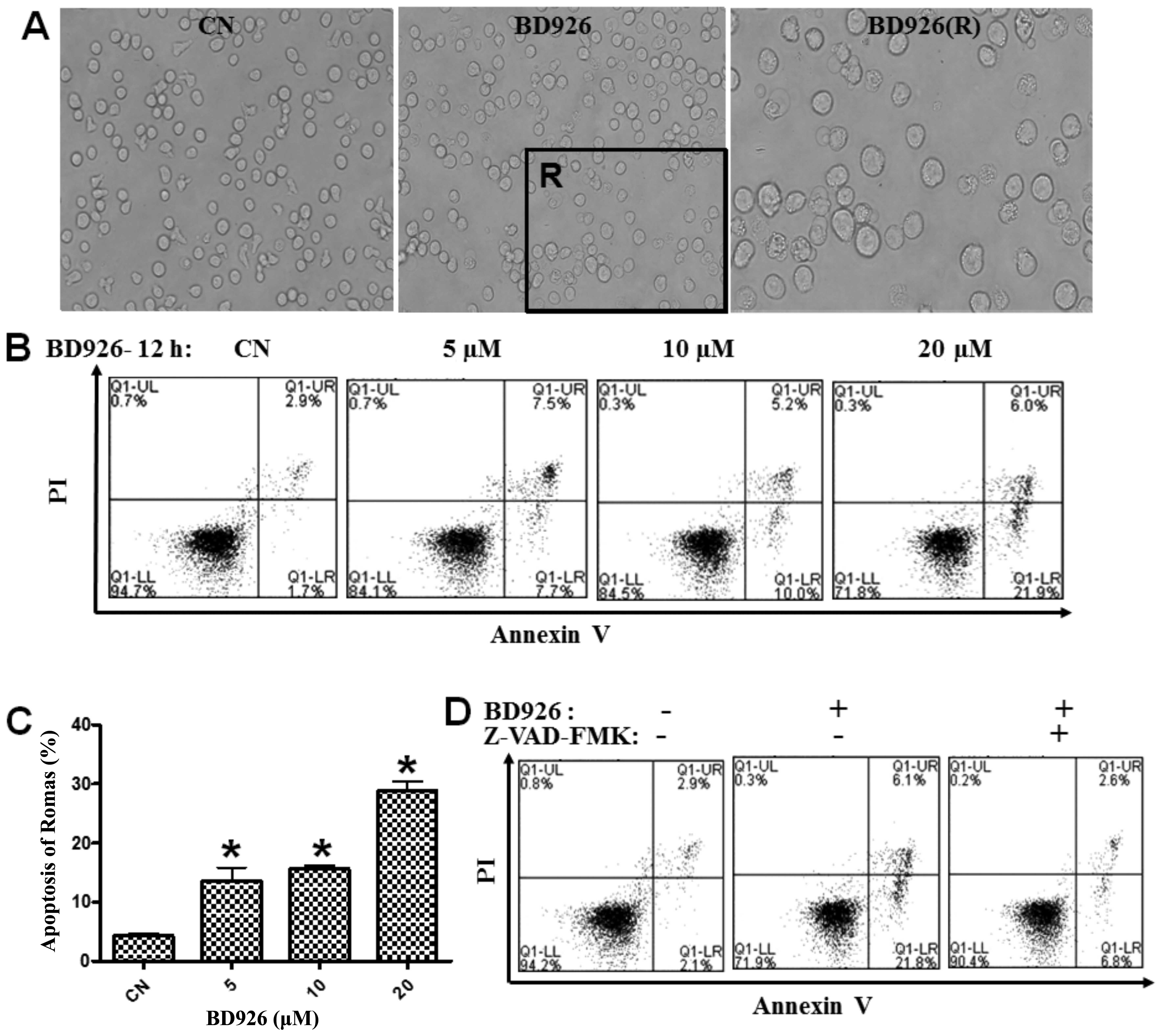
BD926 induces apoptosis of Ramos
cells
To obtain BD926-induced apoptosis information of
Ramos cells, cell morphological changes were observed by phase
contrast microscopy. BD926 induced significant apoptotic
morphological changes in Ramos cells. Cells treated with BD926
became deformed, smaller, cell membrane integrity and foaming
phenomenon was seen along with apoptotic bodies (Fig. 3A).
To confirm this cell death, we also used Annexin
V-FITC and PI fluorescence staining to detect the apoptosis of
Ramos cells by FCM. A dose-dependent increase in the population of
apoptotic cells was observed, and the percentage of apoptotic cells
reached 27.9% at the concentration of 20 μM BD926 compared with
4.6% in the negative control (Fig.
3). To explore whether BD926-induced apoptosis was specifically
associated with caspase activation, we examined whether Z-VAD-FMK
(a general caspase inhibitor) could affect apoptosis. As shown in
Fig. 3D, treatment with 20 μM
BD926 combined with 20 mM Z-VAD-FMK decreased the percentage of
apoptotic cells compared with BD926 treatment alone.
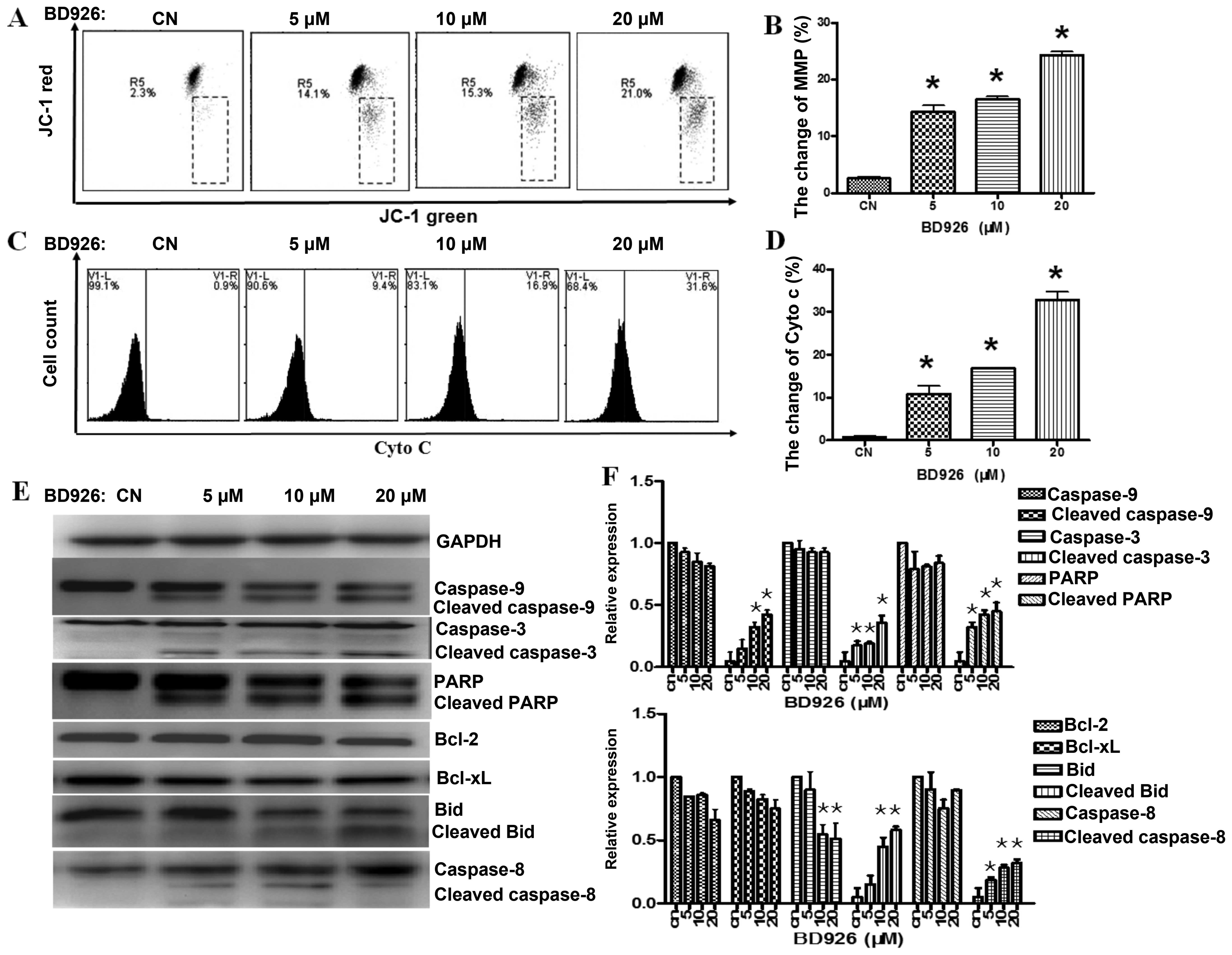
BD926 induces the Ramos cell apoptosis
via mitochondrial pathway
Effect of BD926 on mitochondrial
membrane potential (MMP)
Mitochondria are involved in the regulation of
apoptotic cell death (13), and
the change of mitochondrial membrane potential (ΔΨm) is known to be
one of the important factors for mitochondrial dysfunction.
Therefore, we examined ΔΨm with JC-1 staining by FCM, on account of
the dissipated MMP preventing the accumulation of JC-1 in the
mitochondria, and made a shift from red (JC-1 aggregates) to green
fluorescence (JC-1 monomers). As shown in Fig. 4A, BD926 depolarized the MMP in a
dose-dependent manner. In addition, BD926 at a concentration of 20
μM induced 21.0% decrease of ΔΨm in Ramos cells compared with 2.3%
in the negative control.
Effect of BD926 on cytochrome c
A limited step in the intrinsic apoptotic pathway is
related to the release of cytochrome c from mitochondria
into the cytosol. To observe the change in Ramos cells,
anti-cytochrome c/FITC antibody was used. As shown in
Fig. 4C, BD926 induced the release
of cytochrome c in dose-dependent manner. BD926 at a
concentration of 20 μM induced the release of cytochrome c
reaching 31.6% in Ramos cells compared with 0.9% in the negative
control.
Apoptotic protein detection
To evaluate the contribution of active caspases in
BD926 induced-apoptosis, we characterized the activation of
caspase-9/-8/-3 and PARP by western blot analysis. As shown in
Fig. 4E, a decrease in
pro-caspases-9/-8/-3 and an increase in the levels of their cleaved
forms were observed in Ramos cells. The activation of Bid and PARP
protein were also observed, but the change of Bcl-2 and Bcl-xl were
not significant.
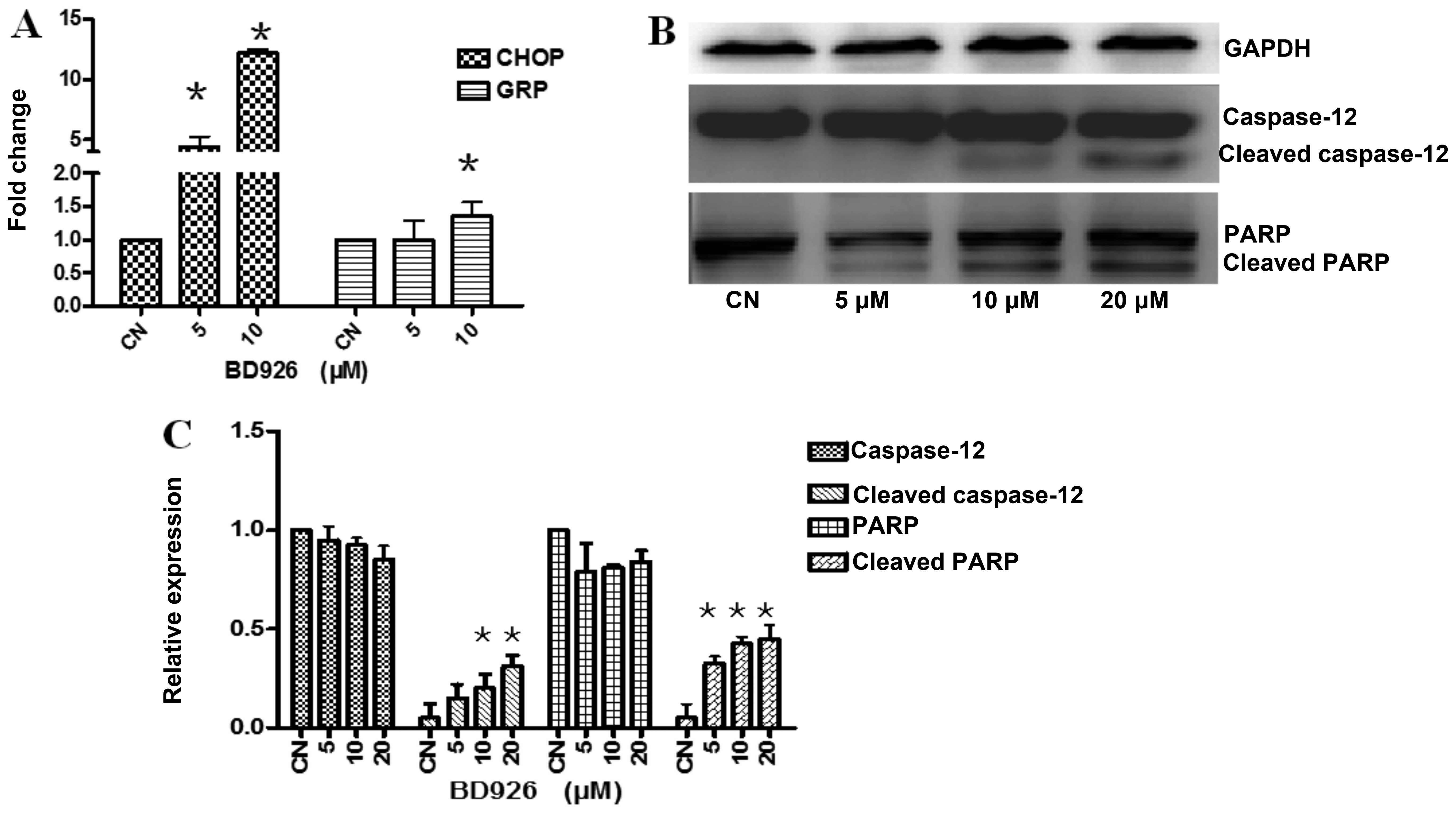
Effect of BD926 on the endoplasmic
reticulum apoptosis pathway
Endoplasmic reticulum (ER) stress have shown to be
involved in the cell apoptosis (14,15).
To confirm whether BD926 induced apoptosis by ER stress signaling,
we examined the mRNA transcription of the markers in ER apoptosis
pathway using real-time reverse transcription PCR. As shown in
Fig. 5A, BD926-treated cells
significantly increased the mRNA expression levels of ER
stress-related molecules, including glucose regulated protein 78
(Grp78) which is a major protective player of the unfolded protein
response (UPR) and pro-apoptotic transcriptional regulator C/EBP
homologous protein GADD153/CHOP.
Caspase-12, an ER resident caspase, is localized on
the ER and activated during apoptosis induced by ER stress
(16). Different from
caspase-7/-8/-9, caspase-12 is a specific medium which associated
with ER stress-induced apoptosis (17). As the results showed, BD926 induced
the activation of caspase-12 and the cleaved caspase-12 expression
levels were significantly increased by BD926 (Fig. 5B). Moreover, the poly(ADP-ribose)
polymerase (PARP) cleavage was also increased significantly. These
results indicated that BD926 induced ER stress-associated
apoptosis.
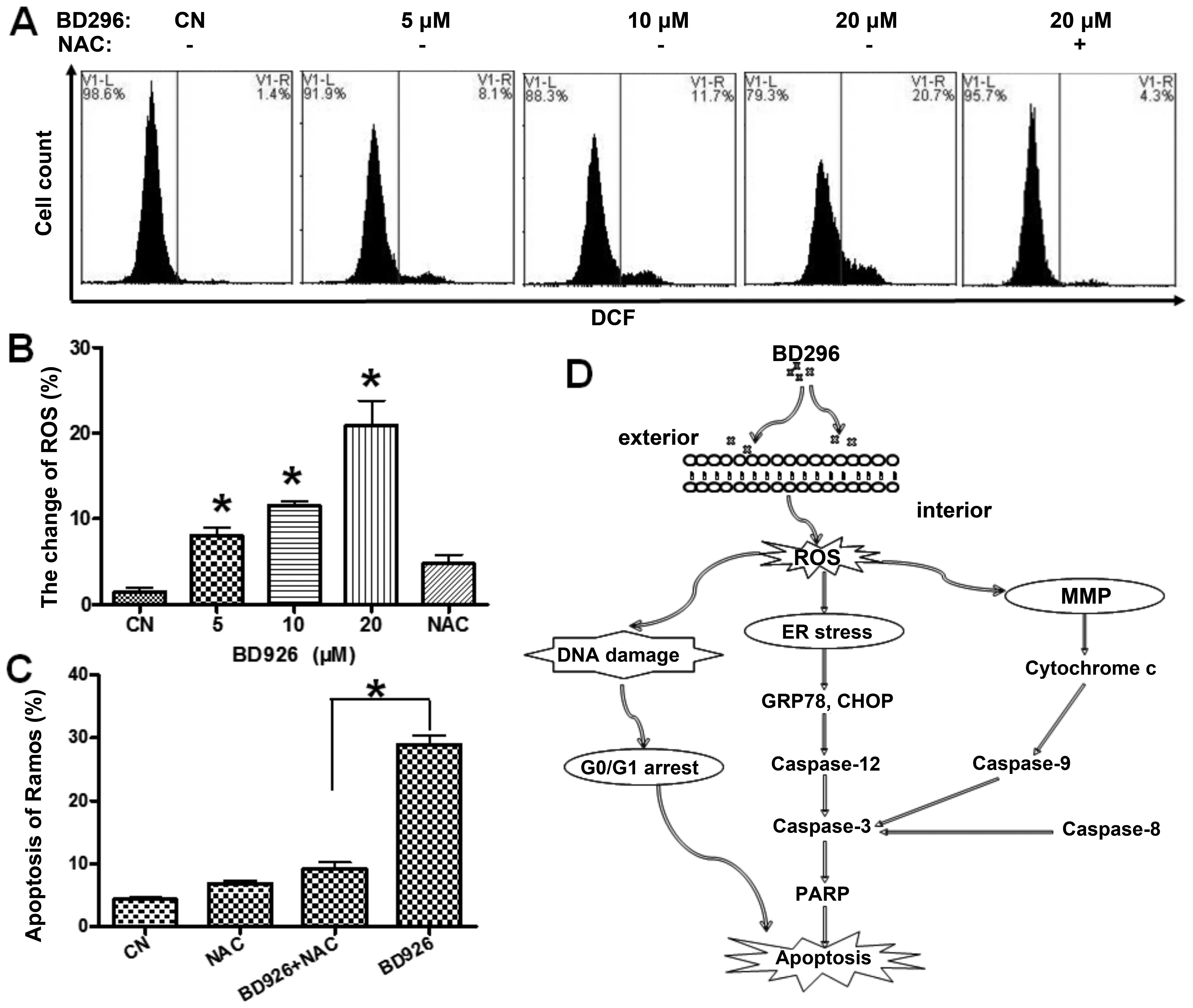
Reactive oxygen species (ROS) plays a
crucial role in BD926-induced apoptosis
ROS is a mediator of intracellular signaling
cascades. The excessive generation of ROS can induce oxidative
stress, loss of cell functioning and apoptosis (18). To confirm whether BD926-induced
apoptosis was triggered by ROS accumulation, the intracellular ROS
level was measured using the ROS-detecting fluorescence dye
DCFH-DA. As shown in Fig. 6A,
according to the ratio of DCF-positive cells we judged that the
level of ROS was significantly increased in the cells treated with
BD926. In addition, BD926 at a concentration of 20 μM induced 20.7%
DCF-positive cells in Ramos cells compared with 1.4% in the
negative control. We also found ROS inhibitor NAC attenuated
BD926-induced apoptosis (Fig. 6C).
This finding suggested that ROS mediated BD926-induced
apoptosis.
Discussion
The chemotherapeutic agents enhancing oxidative
stress are toxic to the cancer cells because they are involved in
the biological processes including cell cycle arrest, DNA repair
and apoptosis. ROS is a well known mediator of intracellular
signaling of cascades. The excessive generation of ROS can induce
oxidative stress, loss of cell functioning and apoptosis (14). It is also reported that ROS
accumulation could lead to mitochondrial dysfunction via
depolarizing the mitochondrial membrane potential (18). Our findings indicate that BD926
induces apoptosis in human Ramos B-lymphoma cells, accompanying
with ROS generation. ROS inhibitor NAC significantly reduces the
BD926-induced ROS production and attenuates the apoptosis in Ramos
cells, suggesting that ROS may play a key role in BD926-induced the
apoptosis of Ramos cells.
The results showed that BD926 enhanced the
disruption of MMP, induced the release of cytochrome c, then
increased the expression of cleaved-Bid, cleaved caspase-9/-3 and
its downstream target cleaved-PARP leading to activation of the
mitochondrial apoptotic pathway. The increased mRNA expression of
CHOP and the activated caspase-12 are also observed, which
indicated that BD926 induced ER-associated apoptosis. Moreover,
BD926 also resulted in cell cycle arrest at the G0/G1 stage. In
brief, we propose that BD926 activates apoptosis by increasing ROS
production, which triggers the ER stress and the mitochondrial
membrane dysfunction in Ramos cells, accompanied by DNA damage and
cell cycle arrest (Fig. 6D).
BD926, a new water-soluble benzothiazole derivative
in which the H-2 of benzothiazol was replaced by
4,5,6,7-tetra-hydro-2H-indazol-3-olate group. In this study, we
found that BD926 showed a growth inhibition effect against human
tumor cell lines including B lymphoma cells, T lymphoma cells and
myeloma cells. BD926 induced apoptosis of human Ramos B-lymphoma
cells in a dose- and time-dependent manner. Importantly, BD926 had
almost no cytotoxic effect on resting human PBMC under the
effective inhibition concentration on tumor cells, which indicated
that BD926 was able to effectively inhibit tumor proliferation and
maintain low toxicity, a prerequisite for a lead compound.
The present study provides important insights into
molecular mechanisms of the anticancer biological activities of
BD926 in cell models, and the potential value of BD926 as a novel
candidate antitumor drug. H-2 of benzothiazol was replaced by
4,5,6,7-tetrahydro-2H-indazol-3-olate group contributing to improve
the biological activity and water solubility, which will promote
the clinical application process of the benzothiazole derivatives
BD926. Our findings suggest that BD926 may be a promising lead
compound, design and development of new benzothiazole derivatives
based on BD926 have wide reaching prospects in cancer
chemotherapy.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (nos. 81301919 and 81273530),
the Applied Basic Research Programs of Science and Technology
Department of Sichuan Province (no. 2015JY0205), the Scientific
Research Fund of Sichuan Provincial Education Department (no.
13ZB0220), the Research Fund of Chengdu Medical College (no.
CYZ11-005), the Scientific Research Fund of Sichuan Provincial
Health Department (nos. 130302 and 130298), the National
Undergraduates Innovating Experimentation Project (nos.
201313705007, 201313705003 and 201413705005).
References
|
1
|
Sun D and Gündisch D: Editorial:
Privileged Scaffolds in Natural Products and Drug Discovery. Curr
Top Med Chem. 16:11992016. View Article : Google Scholar
|
|
2
|
Keri RS, Patil MR, Patil SA and Budagumpi
S: A comprehensive review in current developments of
benzothiazole-based molecules in medicinal chemistry. Eur J Med
Chem. 89:207–251. 2015. View Article : Google Scholar
|
|
3
|
Sharma PC, Sinhmar A, Sharma A, Rajak H
and Pathak DP: Medicinal significance of benzothiazole scaffold: An
insight view. J Enzyme Inhib Med Chem. 28:240–266. 2013. View Article : Google Scholar
|
|
4
|
Gill RK, Rawal RK and Bariwal J: Recent
advances in the chemistry and biology of benzothiazoles. Arch Pharm
(Weinheim). 348:155–178. 2015. View Article : Google Scholar
|
|
5
|
Singh M and Singh SK: Benzothiazoles: How
relevant in cancer drug design strategy? Anticancer Agents Med
Chem. 14:127–146. 2014. View Article : Google Scholar
|
|
6
|
Noolvi MN, Patel HM and Kaur M:
Benzothiazoles: Search for anticancer agents. Eur J Med Chem.
54:447–462. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sharma PC, Bansal KK, Deep A and Pathak M:
Benzothiazole derivatives as potential anti-infective agents. Curr
Top Med Chem. 16:12016.
|
|
8
|
Liu Y, Yang T, Li H, Li MH, Liu J, Wang
YT, Yang SX, Zheng J, Luo XY, Lai Y, et al: BD750, a benzothiazole
derivative, inhibits T cell proliferation by affecting the
JAK3/STAT5 signalling pathway. Br J Pharmacol. 168:632–643. 2013.
View Article : Google Scholar :
|
|
9
|
Hroch L, Aitken L, Benek O, Dolezal M,
Kuca K, Gunn-Moore F and Musilek K: Benzothiazoles - scaffold of
interest for CNS targeted drugs. Curr Med Chem. 22:730–747. 2015.
View Article : Google Scholar
|
|
10
|
Kamal A, Syed MA and Mohammed SM:
Therapeutic potential of benzothiazoles: A patent review
(2010–2014). Expert Opin Ther Pat. 25:335–349. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Seth S: A comprehensive review on recent
advances in synthesis & pharmacotherapeutic potential of
benzothiazoles. Antiinflamm Antiallergy Agents Med Chem. 14:98–112.
2015. View Article : Google Scholar
|
|
12
|
Liu Y, Lai Y, Li H, Liu J, Luo XY, Li MH,
Yang T, Wang YT, Yang SX, Li LM, et al: A novel water-soluble
benzothiazole derivative BD926 inhibits human activated T cell
proliferation by down-regulating the STAT5 activation. Eur J
Pharmacol. 761:36–43. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bhola PD and Letai A: Mitochondria-judges
and executioners of cell death sentences. Mol Cell. 61:695–704.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Farooqi AA, Li KT, Fayyaz S, Chang YT,
Ismail M, Liaw CC, Yuan SS, Tang JY and Chang HW: Anticancer drugs
for the modulation of endoplasmic reticulum stress and oxidative
stress. Tumour Biol. 36:5743–5752. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chaudhari N, Talwar P, Parimisetty A,
Lefebvre d'Hellencourt C and Ravanan P: A molecular web:
Endoplasmic reticulum stress, inflammation, and oxidative stress.
Front Cell Neurosci. 8:2132014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu D, Zhang M and Yin H: Signaling
pathways involved in endoplasmic reticulum stress-induced neuronal
apoptosis. Int J Neurosci. 123:155–162. 2013. View Article : Google Scholar
|
|
17
|
Szegezdi E, Fitzgerald U and Samali A:
Caspase-12 and ER-stress-mediated apoptosis: The story so far. Ann
NY Acad Sci. 1010:186–194. 2003. View Article : Google Scholar
|
|
18
|
Yang Y, Karakhanova S, Hartwig W, D'Haese
JG, Philippov PP, Werner J and Bazhin AV: Mitochondria and
mitochondrial ROS in cancer: Novel targets for anticancer therapy.
J Cell Physiol. Feb 19–2016.(Epub ahead of print). View Article : Google Scholar
|