Introduction
Cancer of the uterine cervix is one of the most
prevalent malignant neoplasms diagnosed, and remains the fourth
main cause of cancer-related mortality among women worldwide
(1). Globally, ~529,800 new cases
of cervical carcinoma are diagnosed each year, resulting in
~275,100 deaths annually, especially in developing countries
(2). Infection with high-risk
human papillomavirus (HPV) types is the common cause of cervical
cancer, which leads to activation of oncogenes and deactivation of
tumor suppressor genes (3).
Smoking, multiple sexual partners, and poor sanitation are
additional risk factors closely associated with cervical cancer
development (4). Although advances
in vaccinations, Pap smear screening programs, and therapeutic
methods (including surgery techniques, chemotherapy and
radiotherapy) have decreased the morbidity and mortality of
cervical carcinoma (5,6), these prevention, diagnostic tools and
treatments have their limitations. Moreover, patients with lymph
node metastasis, lymphovascular space involvement, and higher
differentiation grade or FIGO stage show poorer clinical outcomes
(7–9). While these prognostic indicators have
been correlated with biomarkers (such as C14ORF166, B3GNT3, URG4
and CISD2) (10–14), we still require novel prevention
and treatment strategies to improve the outcomes of patients with
cervical cancer.
One potential therapeutic or prognostic target is
TIMELESS, a 138,658-Da protein that was first characterized in
Drosophila melanogaster as a core component of the circadian
clock (15). Since then, TIMELESS
has been shown to be a highly conserved protein that is also
involved in the cell cycle checkpoint system, cell survival after
damage or DNA replication stress, increasing DNA polymerase epsilon
activity, maintaining telomere length, epithelial cell
morphogenesis and in the formation of branching tubules (16,17).
Recently, circadian rhythm disruption was hypothesized to explain
an increased susceptibility to certain malignancies (18). Indeed, previous studies report a
relationship between TIMELESS and human cancers of many different
organs, including lung, breast, liver, prostate, colon, kidney,
bladder, pancreas and blood (19–31).
However, the characteristics of TIMELESS expression in human
cervical cancer have not been investigated and its clinical
significance remains largely unknown.
In the present study, we sought to investigate the
expression of TIMELESS in cervical cancer and analyze its
clinicopathologic and prognostic value in 189 archival early-stage
cervical carcinoma specimens.
Materials and methods
Cell lines
Eight cervical cancer cell lines (HeLa229, MS751,
ME180, SiHa, HeLa, CasKi, C33A and HCC94) were purchased from the
American Type Culture Collection (ATCC; Rockville, MD, USA) and
maintained in the Department of Experimental Research, Sun Yat-sen
University Cancer Center. They were grown in RPMI-1640 medium
(Gibco-BRL, Grand Island, NY, USA) supplemented with 10% fetal
bovine serum (FBS; HyClone Laboratories, Logan, UT, USA) and 1%
antibiotics (100 U/ml penicillin and 100 µg/ml
streptomycin).
Samples and patient cohorts
Written informed consent was obtained from each of
the cervical cancer patients and the study was approved by the
research ethics committee of the Cancer Center of Sun Yat-sen
University for the use of these clinical materials for study
purposes. The Institutional Review Board (IRB) at the Sun Yat-sen
University approved all experimental methods. Included in the
present study were tumor specimens from 189 consecutive patients
undergoing surgical treatment for cervical cancer at the Department
of Sun Yat-sen University Cancer Center from 2006 to 2010. The
tumor staging and clinicopathological classification of the tumors
were accessed in accordance with the International Federation of
Gynecologists and Obstetricians (FIGO, 2009) criteria. The
classification of the histological types was based on the World
Health Organization (WHO) recommendations.
The 189 patients were in Ib1-IIa2 stage cervical
cancer and all received a radical hysterectomy and lymphadenectomy.
Patients had not undergone anticancer treatment, such as
immunotherapy, chemotherapy, hormone therapy or radiotherapy,
before specimen collection. Patients who received postoperative
chemotherapy and/or radiotherapy were those with high-risk factors,
including: pelvic lymph node metastasis (PLNM), high
differentiation grade, positive parametrial involvement, positive
lymphovascular space involvement, positive surgical margin, deep
stromal invasion and large tumor size (>4 cm). Patients with
only deep stromal invasion or positive surgical margins received
postoperative radiotherapy, while patients with only lymphovascular
space involvement, high differentiation grade, or large tumor size
(>4 cm) received postoperative chemotherapy. No patients
received immunotherapy after surgery.
In total, 189 early-stage cervical cancer patients
were included (aged 23–68 years, with a median age of 46 years).
The media age is used to determine the age gap of patients. Thus,
we separated the groups by a 46-year age gap in the present study.
Demographic and clinicopathological information were collected from
impatient medical records (Table
I). In addition, six pairs of cervical cancer tissues and the
matched tumor-adjacent morphologically normal tissues, which were
collected from early-stage cervical cancer patients who underwent
surgery in 2015, were frozen in liquid nitrogen at −80°C until
further use.
 | Table IClinicopathological characteristics
and tumor expression of TIMELESS in patients with early-stage
cervical cancer. |
Table I
Clinicopathological characteristics
and tumor expression of TIMELESS in patients with early-stage
cervical cancer.
|
Characteristics | No. of cases,
(%) |
|---|
| Age (years) | |
| ≤46 | 98
(51.9) |
| >46 | 91
(48.1) |
| FIGO stage | |
| Ib1 | 83
(43.9) |
| Ib2 | 28
(14.8) |
| IIa1 | 55
(29.1) |
| IIa2 | 23
(12.2) |
| Histological
type | |
| Squamous
carcinoma | 179 (94.7) |
|
Adenocarcinoma | 10 (5.3) |
| Tumor size, cm | |
| <4 | 142 (75.1) |
| ≥4 | 47
(24.9) |
| Squamous cell
carcinoma antigen, ng/ml | |
| ≤1.5 | 91
(48.1) |
| >1.5 | 98
(51.9) |
| HPV infection | |
| No | 37
(19.6) |
| Yes | 152 (80.4) |
| Pelvic lymph node
metastasis | |
| No | 134 (70.9) |
| Yes | 55
(29.1) |
| Tumor
recurrence | |
| No | 160 (84.7) |
| Yes | 29
(15.3) |
| Vital status (at
last follow-up) | |
| Alive | 152 (80.4) |
| Dead | 37
(19.6) |
| Differentiation
grade | |
| G1 | 62
(32.8) |
| G2 | 111 (58.7) |
| G3 | 16 (8.5) |
| Myometrium
invasion | |
| <1/2 | 69
(36.5) |
| ≥1/2 | 120 (63.5) |
| Property of
surgical margin | |
| No | 177 (93.7) |
| Yes | 12 (6.3) |
| Infiltration of
parauterine organ | |
| No | 179 (94.7) |
| Yes | 10 (5.3) |
| Lymphovascular
space involvement | |
| No | 163 (86.2) |
| Yes | 26
(13.8) |
| Chemotherapy | |
| No | 84
(44.4) |
| Yes | 105 (55.6) |
| Radiation
therapy | |
| No | 168 (88.9) |
| Yes | 21
(11.1) |
| Concurrent
chemotherapy and radiotherapy | |
| No | 173 (91.5) |
| Yes | 16 (8.5) |
| TP
chemotherapy | |
| No | 27
(14.3) |
| Yes | 93
(49.2) |
| Radical
hysterectomy | |
| No | 9 (4.8) |
| Yes | 180 (95.2) |
| Expression of
TIMELESS protein | |
| Low or none | 65
(34.4) |
| High | 124 (65.6) |
Quantitative and real-time RT-PCR
analysis
To explore the role of TIMELESS in the development
of cervical cancer, we evaluated its mRNA expression pattern in
cervical cancer cell lines and tissues. RNA extraction from the
cultured cell lines and surgically dissected tissue samples was
carried out using the TRIzol reagent (Invitrogen) following the
manufacturer's guidelines. Agilent Bioanalyzer 2100 was used to
evaluate the RNA quality (RIN, 2.4–8.8; median 5.9). For
PCR-mediated synthesis and amplification of TIMELESS cDNA, an
initial amplification reaction using TIMELESS-specific primers was
performed with a denaturation step at 95°C for 10 min, followed by
30 denaturation cycles at 95°C for 60 sec, primer annealing at 55°C
for 30 sec, and primer extension at 72°C for 30 sec. A final
extension at 72°C for 5 min was carried out before the reaction was
stopped and stored at 4°C on completion of the cycling steps.
TIMELESS-specific primers were designed using the Primer Express v.
2.0 software (Applied Biosystems) as follows: forward
5′-CCATACATCAGTGG ACCAACC-3′ and reverse 5′-CTCCTCCGGGCTTCTGA-3′.
To control the variability in expression levels, expression data of
TIMELESS were normalized to the expression of the housekeeping
gene, GADPH. Primers for GADPH were: forward
5′-TTGAGGTCAATGAAGGGGTC-3′ and reverse 5′-GAAGGTGAAGGTCGGAGTCA-3′.
Quantitative RT-PCR (qRT-PCR) was performed in a total volume of 10
µl using the LightCycler 480 instrument (Roche Diagnostics,
Penzberg, Germany) with the following conditions: 95°C for 5 min,
followed by 45 cycles of 95°C for 30 sec, 55°C for 30 sec and 72°C
for 15 sec, and a final extension step of 72°C for 5 min. The
relative quantitative value was expressed by the 2−ΔΔCt
method, where Ct represents the threshold cycle for each
transcript. All experiments were repeated three times.
Western blot analysis
We also evaluated TIMELESS protein expression in
cervical cancer cell lines and tissues. The cervical tissues and
cell lines were prepared and lysed using the cell total protein
extraction kits, on ice, according to the manufacturer's
instructions (Millipore, Billerica, MA, USA). Western blots were
performed according to standard methods in our previous publication
(32). Briefly, we separated equal
amounts of protein samples (30 µg) on 9% SDS polyacrylamide
gels and transferred them to PVDF membranes (Immobilon P;
Millipore, Bedford, MA, USA). Anti-TIMELESS rabbit monoclonal
(1:1,000; Abcam, Cambridge, MA, USA) and anti-rabbit (1:2,000;
Santa Cruz Biotechnology, Santa Cruz, CA, USA) antibodies were used
to detect TIMELESS protein. After detection, the blotted membranes
were stripped, and anti-GADPH was detected using a mouse monoclonal
antibody (1:2,000; Sigma-Aldrich, St. Louis, MO, USA). The
secondary antibody (anti-mouse antibody; Santa Cruz Biotechnology)
was diluted 1:2,000 in both samples. TIMELESS signals were examined
on X-ray film using an ECL prime Western blotting detection reagent
(Amersham Biosciences).
Immunohistochemical staining and
assessment
Paraffin-embedded specimens were obtained from 189
early-stage cervical cancer patients for immunohistochemical
analysis. The procedures of immunostaining were carried out with
standard protocols. In detail, paraffin tissue slides (4 µm)
were dried at 60°C for 1 h, deparaffinized for 10 min in xylene
(twice), and rehydrated through a series of incubations in graded
ethanol solutions (100, 100, 95, 90 and 80%) for 5 min,
respectively. Endogenous peroxidase activity was blocked with 3%
hydrogen peroxide in methanol for 15 min at room temperature to
quench endogenous peroxidase activity. Following this, all
deparaffinized sections were immersed and boiled in buffered
ethylenediaminetetraacetic acid (pH 8.0) for 2 min in a pressure
cooker to retrieve antigen, and then cooled to room temperature.
Then, sections were immersed with 1% bovine serum albumin (BSA) to
avoid non-specific binding. Subsequently, the slides were
immunostained with a primary antibody against TIMELESS (Abcam)
diluted at 1:250 at 4°C overnight in a moist chamber.
Phosphate-buffered saline (PBS) was used as a negative control.
After washing with PBS buffer, the slides were incubated with
prediluted anti-rabbit secondary antibody (Abcam) and then
incubated with streptavidin-horseradish-peroxidase complex (Abcam).
The tissue sections were immersed in 3-amino-9-ethylcarbazole,
counterstained with 10% Mayer's haematoxylin, dehydrated and
mounted in Crystal Mount. For visualization, tissue slides were
stained with DAB (3,3-diaminobenzidine) for 1 min at room
temperature. Finally, the tissue specimens were counterstained with
hematoxylin, dehydrated and mounted using neutral balsam
(Sigma-Aldrich).
All immunostained tissue slides were assessed
independently by two observers without prior knowledge of clinical
data. To evaluate IHC expression of TIMELESS, we applied a scoring
system based on multiplying the proportion of positively stained
tumor cells and the intensity of staining. The staining intensity
(SI) was graded according to the following criteria: 0, no
staining; 1, weak staining; 2, modest staining; and 3, strong
staining. The proportion of positive tumor cells was scored as
follows: 0 (no positive tumor cells); 1 (<10% positive tumor
cells); 2 (10–50% positive tumor cells); 3 (51–80% positive tumor
cells), and 4 (>80% positive tumor cells). The slides were
rescored if the difference between the two pathologists was >3.
The optimal cut-off values were distinguished and categorized by
the median of the immunoreactive score (IRS) results: an SI score
of 6 was used to identify tumors with high TIMELESS expression and
an SI score of 4 was used for low TIMELESS expression.
Statistical analysis
All calculations were carried out using the SPSS
version 18.0 software package (SPSS, Inc., Chicago, IL, USA). The
relationship of TIMELESS protein expression levels with clinical
and histomorphological characteristics were determined using a
Chi-squared test. The overall survival (OS) and disease-free
survival (DFS) curves in association with TIMELESS expression were
plotted using the Kaplan-Meier method and the difference between
the groups was tested by a log-rank test. Multivariate analysis was
conducted independently for each biomarker, including only
significant clinical/histomorphological factors from the univariate
analysis. Univariate and multivariate survival analyses were
carried out using the Cox regression model. A statistically
significant difference was determined by a two-sided P-value of
<0.05.
Results
Expression of TIMELESS is upregulated in
human cervical cancer
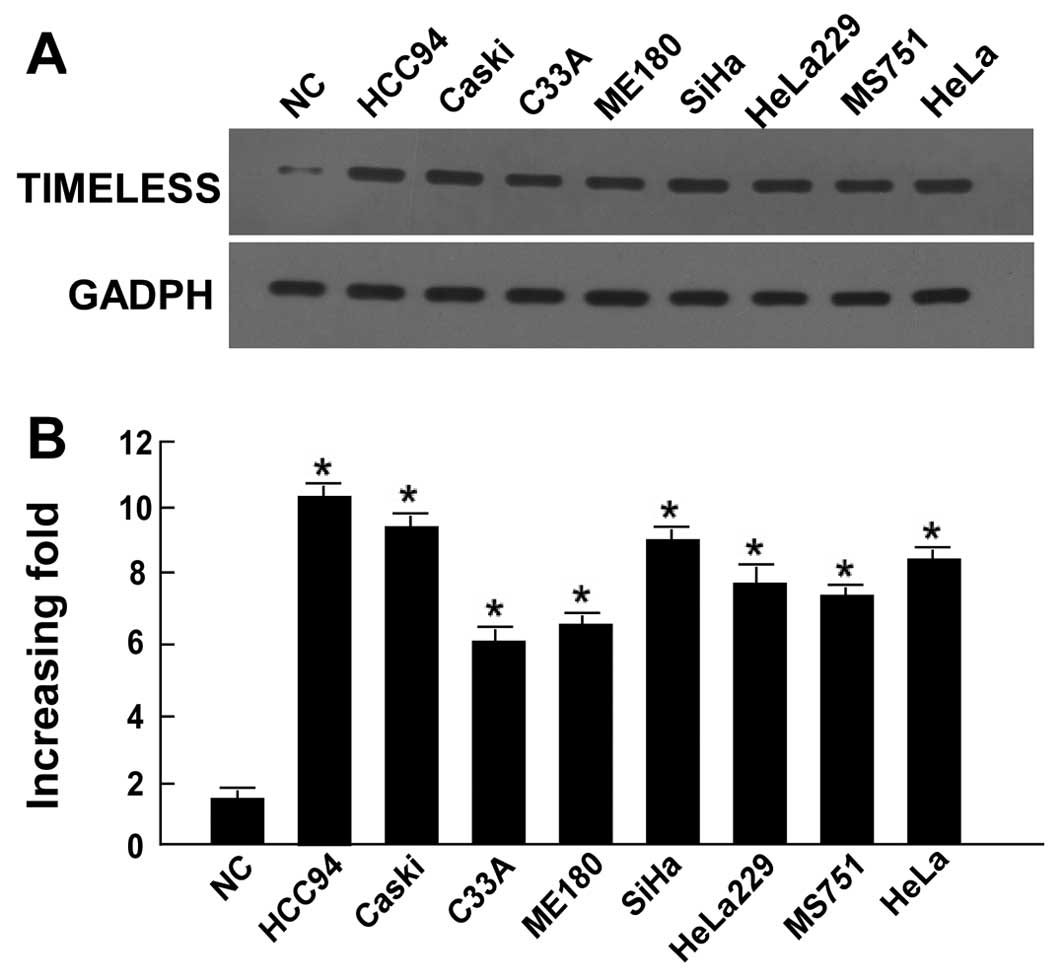
Significantly higher TIMELESS protein expression and
mRNA levels were observed in all eight cervical cancer cell lines
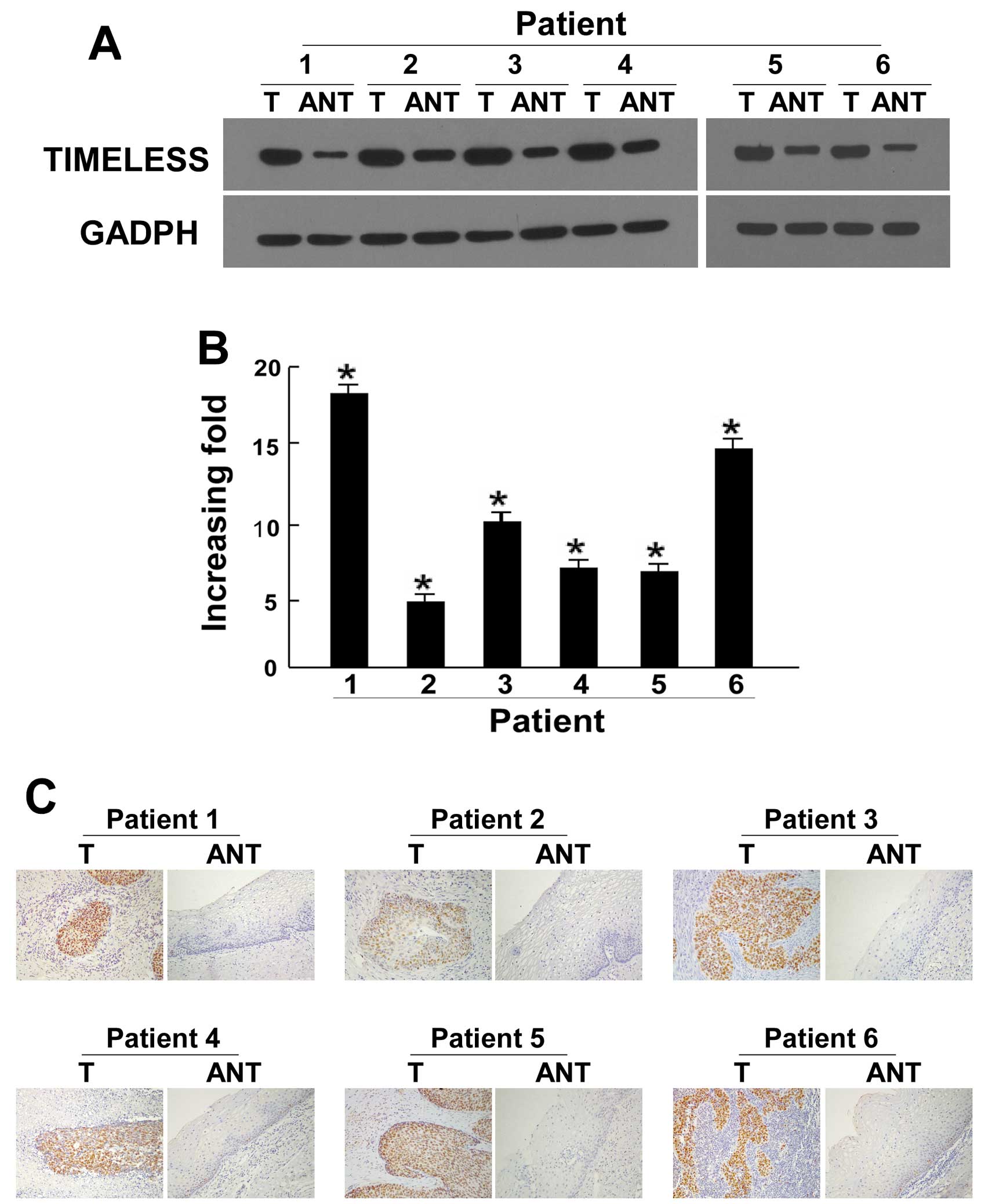
compared to the normal cervical tissue sample (P<0.05; Fig. 1). TIMELESS protein expression was
also significantly upregulated in the six cervical cancer cases
compared with matched adjacent non-cancerous cervical tissues
(P<0.05; Fig. 2A). Similarly,
qRT-PCR data also indicated that TIMELESS mRNA was significantly
upregulated in cervical cancer specimens compared to their matched
adjacent non-cancerous regions (P<0.05; Fig. 2B). By real-time RT-PCR evaluation,
the tumor/adjacent non-cancerous (T/N) ratio of TIMELESS mRNA
expression was >5-fold in all cases, with the highest ratio
being ~18-fold (Fig. 2B). In
agreement with the results of the western blot analysis and
real-time RT-PCR assay, IHC showed high expression of TIMELESS
protein in cervical cancer lesions (Fig. 2C). Together, our results showed
that TIMELESS expression was elevated at both the transcriptional
level and translational levels in the cervical cancer cell lines
and tissues.
TIMELESS overexpression is correlated
with clinicopathological variables of early-stage cervical
cancer
We collected 189 paraffin-embedded early-stage
cervical cancer tissue samples for IHC, including 83 cases of stage
IB1, 28 cases of stage IB2, 55 cases of stage IIA1 and 23 cases of
stage IIA2 tumors. Following IHC staining, TIMELESS expression was
assessed as positive in 124/189 (65.6%) cases and weakly positive
or negative in 65 cases (34.4%). TIMELESS expression was mainly
observed in the nucleus and rarely in the cytoplasm of epithelial
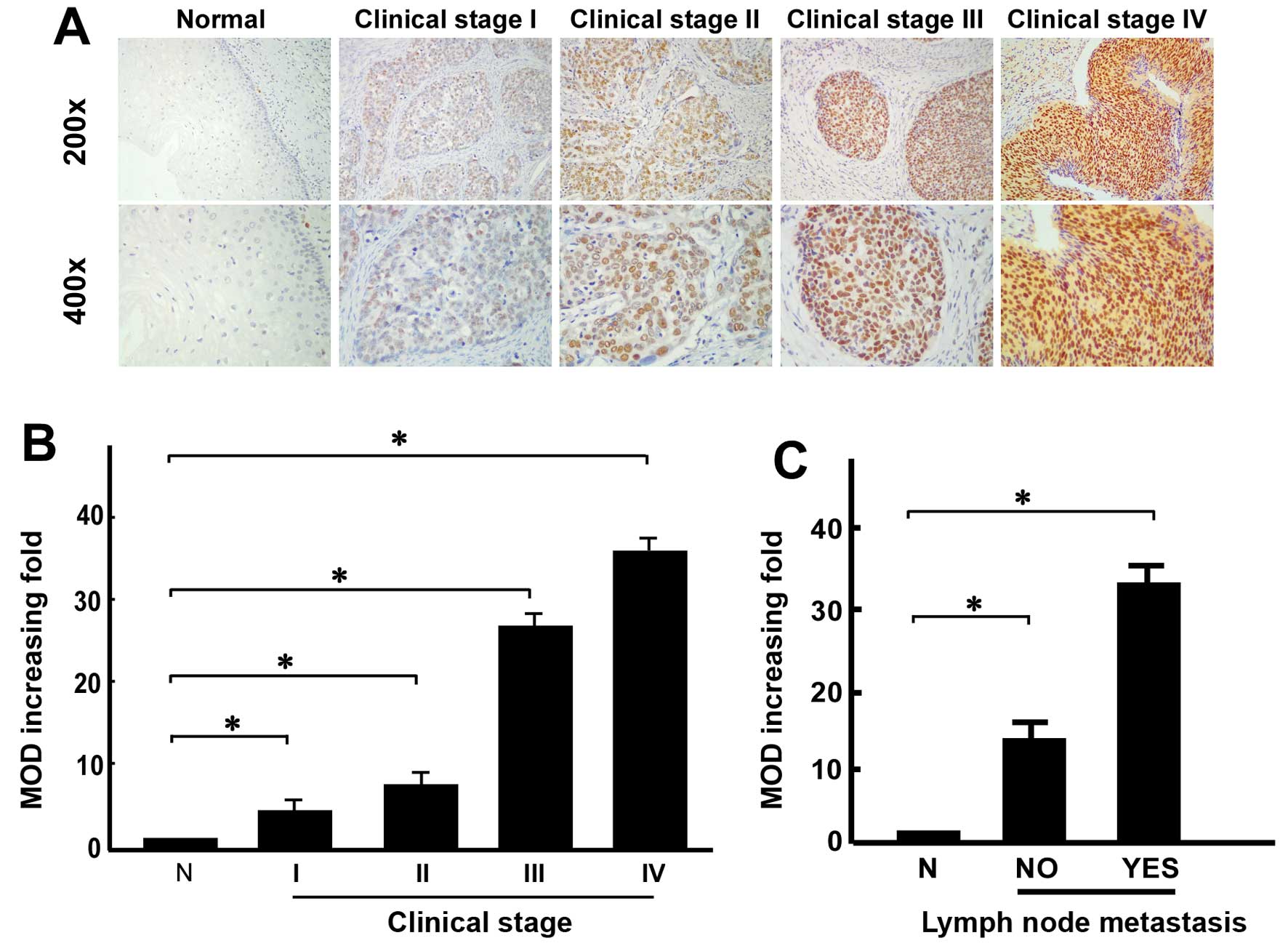
cells (Fig. 3A). Notably, TIMELESS
expression was found in most cancerous lesions in the primary
cervical tumors, but was reduced in the adjacent normal cervical
regions. TIMELESS protein expression was generally negative in
normal cervical tissues, weak in stage IB1 and IB2 cervical cancer
tissues, and strong in stage IIA1 and IIA2 cervical cancer tissues;
TIMELESS expression obviously increased with the progression of
tumor grades (P<0.05; Fig. 3A).
Mean optical density (MOD) represents the average response strength
of all positive cells in the field of vision. Quantitative IHC data
revealed that the MOD values of TIMELESS were significantly
associated with the cervical cancer tumor grades (Fig. 3B), and were higher in cervical
cancer tissues with PLNM than in those without PLNM (Fig. 3C).
Next, we examined the relationship between TIMELESS
expression and established clinicopathological and investigative
parameters using the Chi-squared test and Fisher's exact test
(Table II). In patients with
early-stage cervical cancer, high TIMELESS expression was markedly
associated with age (P=0.011), clinical stage (P<0.001), PLNM
(P<0.001), squamous cell carcinoma (SCC) antigen (P=0.003),
tumor recurrence (P=0.015), vital status (P<0.001),
differentiation grade (P<0.001), property of surgical margin
(P=0.036) and lymphovascular space involvement (P=0.001; Table III). Moreover, patients with
increased TIMELESS protein expression showed strong tendencies to
receive postoperative radiotherapy (P=0.002). However, no
significant correlations between TIMELESS expression and the
histological type, HPV infection, tumor size, myometrium invasion,
or infiltration of the parauterine organ were observed (P>0.05;
Table III). Patients with
aberrant TIMELESS protein expression also showed no obvious
tendency to receive chemotherapy, concurrent chemotherapy and
radiotherapy, chemotherapy with TP regimen (paclitaxel plus
cisplatin), or radical hysterectomy (P>0.05; Table III). TP chemotherapy is the main
chemotherapy of cervical cancer patients which combines paclitaxel
with cisplatin.
 | Table IICorrelation between TIMELESS protein
expression and the clinicopathological features of early-stage
cervical cancer. |
Table II
Correlation between TIMELESS protein
expression and the clinicopathological features of early-stage
cervical cancer.
|
Characteristics | Total | No or weak TIMELESS
expression | Moderate or strong
TIMELESS expression | Chi-squared
test
(P-value) | Fisher's exact
test
(P-value) |
|---|
| Age (years) | | | | | |
| ≤46 | 98 | 42 (22.2) | 56 (29.6) | 0.011 | 0.014 |
| >46 | 91 | 23 (12.2) | 68 (36.0) | | |
| Histological
type | | | | | |
|
Adenocarcinoma | 10 | 2 (1.1) | 8 (4.2) | 0.325 | 0.498 |
| Squamous cell
carcinoma | 179 | 63 (33.3) | 116 (61.4) | | |
| HPV infection | | | | | |
| No | 40 | 14 (7.4) | 23 (12.2) | 0.623 | 0.700 |
| Yes | 153 | 51 (27.0) | 101 (53.4) | | |
| FIGO stage | | | | | |
| Ib1 | 83 | 41 (21.7) | 42 (22.2) | <0.001 | - |
| Ib2 | 28 | 13 (6.9) | 15 (7.9) | | |
| IIa1 | 55 | 7 (3.7) | 48 (25.4) | | |
| IIa2 | 23 | 4 (2.1) | 19 (10.1) | | |
| Pelvic lymph node
metastasis | | | | | |
| Absent | 134 | 60 (31.7) | 74 (39.2) | <0.001 | <0.001 |
| Present | 55 | 5 (2.6) | 50 (26.5) | | |
| Squamous cell
carcinoma antigen, ng/ml | | | | | |
| ≤1.5 | 91 | 41 (21.7) | 50 (26.5) | 0.003 | 0.004 |
| >1.5 | 98 | 24 (12.7) | 74 (39.1) | | |
| Tumor size
(cm) | | | | | |
| <4 | 142 | 50 (26.5) | 92 (48.7) | 0.680 | 0.726 |
| ≥4 | 47 | 15 (7.9) | 32 (16.9) | | |
| Tumor
recurrence | | | | | |
| No | 160 | 63 (33.3) | 106 (56.1) | 0.015 | 0.014 |
| Yes | 29 | 2 (1.1) | 18 (9.5) | | |
| Vital status (at
last follow-up) | | | | | |
| Alive | 158 | 63 (33.3) | 89 (47.1) | <0.001 | <0.001 |
| Dead | 35 | 2 (1.1) | 35 (18.5) | | |
| Differentiation
grade | | | | | |
| G1 | 62 | 38 (20.1) | 24 (12.7) | <0.001 | - |
| G2 | 111 | 26 (13.8) | 85 (45.0) | | |
| G3 | 16 | 1 (0.5) | 15 (7.9) | | |
| Chemotherapy | | | | | |
| No | 84 | 32 (16.9) | 52 (27.5) | 0.338 | 0.358 |
| Yes | 105 | 33 (17.5) | 72 (38.1) | | |
| Radiation
therapy | | | | | |
| No | 168 | 64 (33.9) | 104 (55.0) | 0.002 | 0.001 |
| Yes | 21 | 1 (0.5) | 20 (10.6) | | |
| Concurrent
chemotherapy and radiotherapy | | | | | |
| No | 177 | 62 (32.8) | 111 (58.7) | 0.169 | 0.270 |
| Yes | 16 | 3 (1.6) | 13 (6.9) | | |
| TP
chemotherapy | | | | | |
| No | 27 | 11 (9.2) | 16 (13.3) | 0.167 | 0.232 |
| Yes | 93 | 25 (20.8) | 68 (56.7) | | |
| Radical
hysterectomy | | | | | |
| No | 9 | 1 (0.5) | 8 (4.2) | 0.132 | 0.168 |
| Yes | 180 | 64 (33.9) | 116 (61.4) | | |
| Myometrium
invasion | | | | | |
| <1/2 | 69 | 30 (15.9) | 39 (20.6) | 0.046 | 0.057 |
| ≥1/2 | 120 | 35 (18.5) | 85 (45.0) | | |
| Property of
surgical margin | | | | | |
| No | 177 | 64 (33.9) | 113 (59.8) | 0.036 | 0.037 |
| Yes | 12 | 1 (0.5) | 11 (5.8) | | |
| Infiltration of
parauterine organ | | | | | |
| No | 179 | 61 (32.3) | 118 (62.4) | 0.701 | 0.739 |
| Yes | 10 | 4 (2.1) | 6 (3.2) | | |
| Lymphovascular
space involvement | | | | | |
| No | 163 | 63 (33.3) | 100 (52.9) | 0.001 | 0.001 |
| Yes | 26 | 2 (1.1) | 24 (12.7) | | |
 | Table IIISpearman correlation analysis of
TIMELESS protein expression vs. clinicopathological factors. |
Table III
Spearman correlation analysis of
TIMELESS protein expression vs. clinicopathological factors.
| Variables | TIMELESS protein
expression
|
|---|
| Spearman's
correlation coefficient | P-value |
|---|
| Age | 0.185 | 0.011 |
| Histological
type | 0.072 | 0.327 |
| HPV infection | 0.036 | 0.625 |
| Clinical
staging | 0.326 | <0.001 |
| Pelvic lymph node
metastasis | 0.341 | <0.001 |
| Squamous cell
carcinoma antigen, ng/ml | 0.216 | 0.003 |
| Tumor size | 0.030 | 0.682 |
| Recurrence | 0.177 | 0.015 |
| Vital status | 0.301 | <0.001 |
| Differentiation
grade | 0.404 | <0.001 |
| Survival time | −0.526 | <0.001 |
| Chemotherapy | 0.070 | 0.340 |
| Radiation
therapy | 0.221 | 0.002 |
| Concurrent
chemotherapy and radiotherapy | 0.100 | 0.170 |
| TP
chemotherapy | 0.126 | 0.169 |
| Radical
hysterectomy | −0.110 | 0.133 |
| Myometrium
invasion | 0.145 | 0.046 |
| Property of
surgical margin | 0.153 | 0.036 |
| Infiltration of
parauterine organ | −0.028 | 0.703 |
| Lymphovascular
space involvement | 0.232 | 0.001 |
Association coefficient analyses (Spearman's
correlation) further confirmed the above-mentioned relationships
between the TIMELESS protein expression levels and the
clinicopathological features (Table
III).
High TIMELESS expression is associated
with poor prognosis of patients with early-stage cervical
cancer
We examined whether patients with TIMELESS-positive
samples had a poorer survival rate than those with
TIMELESS-negative samples. Survival time was markedly different
between patients with TIMELESS-positive and TIMELESS-negative
samples based on the log-rank test. Patients with higher TIMELESS
expression had shorter OS (log-rank, P<0.001; Fig. 4A) and DFS (log-rank, P=0.001;
Fig. 4B) than those with lower
TIMELESS expression. When examining the whole study cohort,
patients with high TIMELESS expression had a significantly lower
cumulative 5-year survival rate than those with low TIMELESS
protein expression (73.1 vs. 96.8%, respectively; P<0.05).
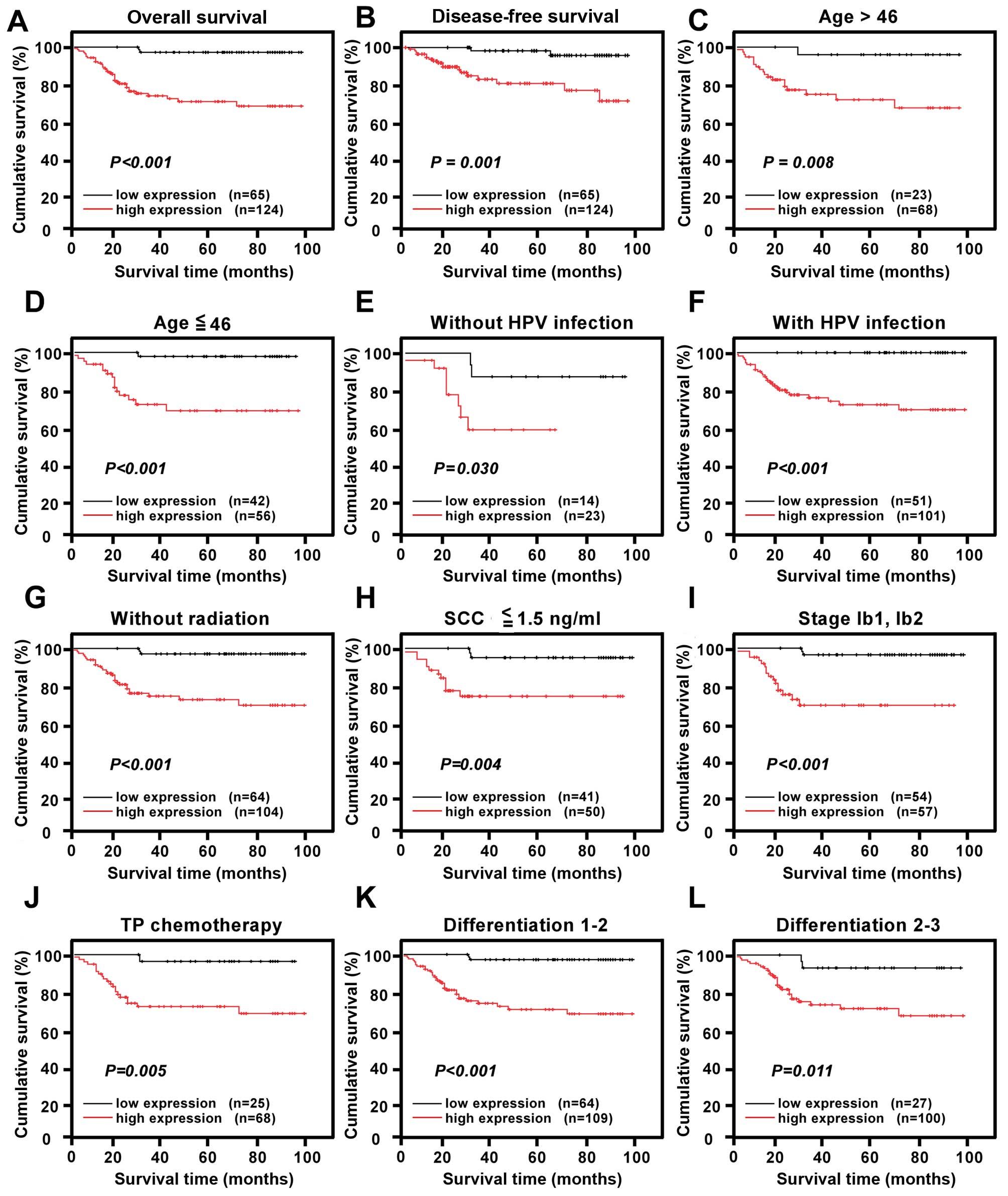 | Figure 4Kaplan-Meier curves of univariate
analysis data (log-rank test). The overall survival (OS) and
disease-free survival (DFS) for the patients with low TIMELESS
expression vs. high TIMELESS expression (A and B). Survival curves
for the patients (C) aged >46 years, (D) aged ≤46 years, (E)
without HPV infection, (F) with HPV infection, (G) without
radiation, (H) with SCC ≤1.5 ng/ml, (I) at stages Ib1-Ib2, (J) with
TP chemotherapy, (K) at differentiation grades 1 and 2, (L) at
differentiation grades 2 and 3. (P<0.05, respectively). |
We also explored the prognostic value of TIMELESS
protein expression in different subgroups of patients with
early-stage cervical cancer. Subgroups of patients were stratified
according to age, HPV infection, FIGO stage, PLNM, SCC antigen,
tumor size, differentiation grade, chemotherapy, radiotherapy,
concurrent chemotherapy and radiotherapy, TP chemotherapy, radical
hysterectomy, myometrium invasion, properties of the surgical
margin, infiltration of parauterine organ and lymphovascular space
involvement. TIMELESS protein expression was strongly associated
with the OS duration of patients aged >46 years (log-rank test;
P=0.008), aged ≤46 years (log-rank test; P<0.001), with HPV
infection (log-rank test; P<0.001), without HPV infection
(log-rank test; P=0.030), without radiotherapy (log-rank test;
P<0.001), with SCC antigen ≤1.5 ng/ml (log-rank test; P=0.004),
with clinical stage IB1-IB2 (log-rank test; P<0.001), with TP
chemotherapy (log-rank test; P=0.005), with histological
differentiation grade (grades 1 and 2, log-rank test, P<0.001;
grades 2 and 3, log-rank test; P=0.011), with tumor size (tumor
size <4 cm, log-rank test; P<0.001; tumor size ≥4 cm,
log-rank test; P=0.033), with radical hysterectomy (log-rank test;
P<0.001), without infiltration of parauterine organ (log-rank
test; P<0.001), without PLNM (log-rank test; P<0.001), with
SCC (log-rank test; P<0.001), without lymphovascular space
involvement (log-rank test; P<0.001), without properties of the
surgical margin (log-rank test; P<0.001), without myometrium
invasion (log-rank test; P=0.001), with myometrium invasion
(log-rank test; P=0.001) and with chemotherapy (log-rank test,
P<0.001; Figs. 4 and 5).
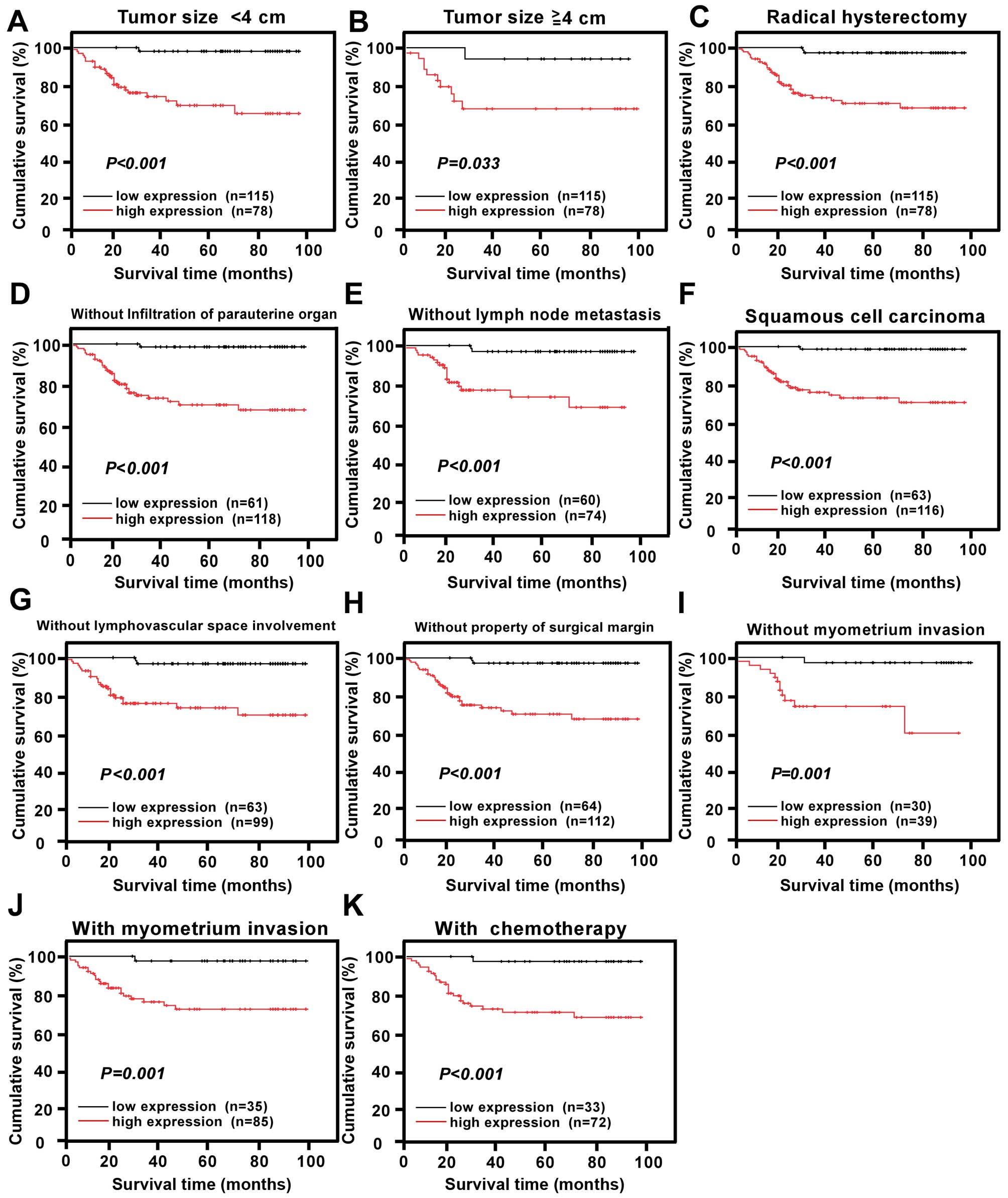 | Figure 5Survival curves for the patients in
select patient subgroups (log-rank test). OS rates for patients (A)
with tumor size <4 cm, (B) with tumor size ≥4 cm, (C) with
radical hysterectomy, (D) without infiltration of parauterine
organ, (E) without lymph node metastasis, (F) with pathologic type
of squamous cell carcinoma, (G) without lymphovascular space
involvement, (H) without property of surgical margin, (I) without
myometrium invasion, (J) with myometrium invasion, (K) with
chemotherapy. (P<0.05, respectively). |
Univariate analysis based on the Cox regression
model demonstrated that upregulation of TIMELESS protein expression
was a significant prognostic biomarker of poor OS (P<0.001;
Table IV). In addition,
early-stage cervical carcinoma patients with PLNM, with
lymphovascular space involvement, and those who had undergone
radiation therapy, showed poorer prognosis (Table IV). In our multivariate analysis,
only TIMELESS protein expression and tumor recurrence remained
significant (Table IV), and
served as independent prognostic factors for poor OS in cervical
cancer patients (P=0.002, 95% CI, 2.285–42.639; and P<0.001, 95%
CI, 2.430–10.673, respectively).
 | Table IVUnivariate and multivariate analyses
of prognostic factors in early-stage cervical cancer using a
Cox-regression model. |
Table IV
Univariate and multivariate analyses
of prognostic factors in early-stage cervical cancer using a
Cox-regression model.
| Univariate analysis
| Multivariate
analysis
|
|---|
| No. of
patients | P-value | Regression
coefficient (SE) | P-value | Relative risk | 95% CI |
|---|
| TIMELESS | | <0.001 | 13.712 (0.729) | 0.002 | 9.871 | 2.285–42.639 |
| Low
expression | 65 | | | | | |
| High
expression | 124 | | | | | |
| Pelvic lymph node
metastasis | | 0.015 | 2.237 (0.330) | 0.607 | 1.203 | 0.595–2.434 |
| Absent | 134 | | | | | |
| Present | 55 | | | | | |
| Radiation
therapy | | 0.041 | 2.372 (0.422) | 0.537 | 1.330 | 0.537–3.291 |
| No | 168 | | | | | |
| Yes | 21 | | | | | |
| Lymphovascular
space involvement | | 0.005 | 2.748 (0.360) | 0.994 | 0.997 | 0.450–2.209 |
| No | 163 | | | | | |
| Yes | 26 | | | | | |
| Recurrence | | <0.001 | 7.148 (0.348) | <0.001 | 5.092 | 2.430–10.673 |
| No | 160 | | | | | |
| Yes | 29 | | | | | |
Discussion
The present study demonstrated that TIMELESS is
abundantly expressed at both transcriptional and translational
levels in human cervical cancer compared with normal cervical
tissues. This elevation in TIMELESS expression was strongly
associated with age, clinical stage, PLNM, SCC antigen, tumor
recurrence, vital status, differentiation grade, property of
surgical margin and lymphovascular space involvement. Patients with
upregulated TIMELESS protein expression were also more likely to
receive postoperative radiotherapy. Furthermore, our survival
analysis revealed that high TIMELESS expression was an independent
biomarker associated with poorer clinical outcomes in cervical
cancer patients.
Similar to our findings, recent reports have shown
the importance of TIMELESS in many different types of human cancers
(19–31). TIMELESS was overexpressed in a
panel of human lung cancer cell lines and clinical lung cancer
specimens compared to normal lung controls (19). Associations between TIMELESS and
breast cancer risk have also been demonstrated, with TIMELESS being
reported as promising marker of tamoxifen resistance in women with
estrogen receptor α-positive breast tumors (20–22).
In colorectal cancer, high TIMELESS expression was significantly
associated with the clinical and pathological features (25). TIMELESS expression is also closely
related to the clinicopathological factors in bladder cancer
(29). On the other hand, TIMELESS
is downregulated in kidney cancer cells (28), and TIMELESS transcription was
significantly lower in pancreatic ductal adenocarcinoma compared to
adjacent tissue and benign tissue (30). As we know, it is quite common that
some gene may act as an oncogenic role in several types of cancers
while it plays an anti-oncogenic role in other types of cancers.
For example, our previous finding showed that B3GNT3 was
upregulated in cervical cancer and is correlated with the
progression of cervical cancer (11). Moreover, several reports suggested
that B3GNT3 plays an oncogenic role in certain types of cancer such
as colon and pancreatic cancer (33,34).
However, it is found that B3GNT3 might play a critical role in
suppressing the malignant properties of neuroblastoma (35).
With regards to its mechanism of action, TIMELESS
appears to regulate the many signaling pathways involved in tumor
proliferation, apoptosis, metastasis and the cell cycle. Indeed,
TIMELESS has been implicated in cell proliferation in leukemia stem
cells (31). It has also been
shown to activate the eeF1a2/PI3K/aKt/mtoR axis to contribute to
the migratory phenotype in hepatocellular carcinoma, and may also
activate the protumorigenic eeF1a2/aKt/mDm4 axis (23). Moreover, TIMELESS was previously
shown to be essential for Rad3-related-checkpoint kinase 1
(ATR-Chk1)-mediated and ataxia telangiectasia mutated-checkpoint
kinase 2 (ATM-Chk2)-mediated signaling and S-phase arrest (25). While our results also indicate that
TIMELESS is important in the development, progression and
metastasis of cervical cancer, further studies are warranted to
identify its specific role in the related signaling pathways.
Our results also indicate that overexpression of
TIMELESS might be an effective biomarker for identifying patients
with more aggressive risk factors, such as those with higher
clinical stage, with PLNM, with tumor recurrence, higher
differentiation grade, with property of surgical margin and with
lymphovascular space involvement. In our experience, several
clinical characteristics play important roles in the prognosis of
cervical cancer and can affect patients' optimal treatment
selections. For example, PLNM has been previously shown to be an
important biomarker for the clinical outcome of cervical cancer
patients (36), as well as well as
for determining the optimal treatment. In the clinic, patients
found to have PLNM before surgery will receive chemoradiotherapy
instead of undergoing an operation. While many tests (e.g.,
physical examination, magnetic resonance imaging, biomarkers, among
others) are currently available to assess PLNM, they are still
prone to errors. Thus, more precise molecular marker are required
to avoid misdiagnosis and improper treatment. PLNM also determines
the optimal postoperative therapy. For example, after radical
hysterectomy plus lymphadenectomy, patients with PLNM will receive
postoperative chemotherapy and radiation.
In the present study, we found that TIMELESS
expression is clearly associated with PLNM. Thus, TIMELESS may be
an effective biomarker for PLNM and for determining the optimal
treatment in cervical cancer patients. In previous reports,
elevated TIMELESS levels were suggested to play an oncogenic role
in hepatocellular carcinoma and promote tumor cell migration
(23), and were also associated
with tumor metastasis in prostate cancer (24). While our present results are in
agreement with these previous studies, we failed to show that
TIMELESS is an independent prognostic marker of PLNM, most likely
due to the small sample size. In our next study, we aim to detect
the utility of TIMELESS as an independent prognostic biomarker of
PLNM by examining a larger sample size of early-stage cervical
cancer patients. Furthermore, we found a strong correlation between
poorer OS and aberrant TIMELESS protein expression in patients
without lymph node metastasis. Thus, TIMELESS may be an outstanding
marker of PLNM and a good prognostic marker for cervical cancer
patients without PLNM, although further studies are required.
Lymphovascular space involvement has also been
identified as an important high-risk factor that affects prognosis
and determines postoperative treatment (37). Patients with lymphovascular space
involvement may face poorer clinical outcomes and should receive
more aggressive treatment, such as postoperative chemotherapy
(38). Our data illustrated that
TIMELESS protein overexpression is markedly correlated with
lymphovascular space involvement and unfavorable survival rates.
This indicates that patients with lymphovascular space involvement
should undergo more aggressive therapy to reduce the mortality
rates.
Past evidence has demonstrated that high
differentiation grade is an essential indicator for aggressiveness
and worse outcomes in cervical cancer (38). Cervical cancer cells of high
differentiation grade show strong proliferative and migratory
ability. Furthermore, differentiation grade is critical for the
selection of therapy in cervical cancer. According to our treatment
guidelines, after resection of the tumor of uterine cervix,
patients of high differentiation grade cervical cancer should
receive postoperative chemotherapy. Here we showed that the
expression of TIMELESS is associated with the differentiation grade
of cervical cancer and thus, may be used to evaluate prognosis and
guide treatments.
The standard treatments for early-stage cervical
cancer patients are currently radical hysterectomy plus
lymphadenectomy or chemoradiotherapy. Patients with high-risk
factors, such as myometrium invasion, will receive postoperative
radiation therapy. On the other hand, patients with high-risk
factors, such as lymphovascular space involvement, high grade of
tumor differentiation, or a tumor size >4 cm will receive
postoperative chemotherapy. In addition, patients with PLNM,
property of surgical margin, infiltration of the parauterine organ,
or lymphovascular space involvement should receive postoperative
chemoradiotherapy. Previous reports have proved that postoperative
radiation and chemotherapy contribute to the prognosis of patients
with high risk factors (39). In
the present study, we found that patients with high TIMELESS
protein expression showed an increased tendency to receive
postoperative radiation therapy. Interestingly, we observed that
TIMELESS protein overexpression was significantly correlated with
OS in patients with radical hysterectomy, with chemotherapy, with
TP chemotherapy, and without postoperative radiation therapy. Thus,
TIMELESS protein expression may be useful in identifying patients
potentially with shorter OS in these subgroups, who may wish to
undertake more aggressive treatments.
While tumor recurrence remains a major issue for
cervical cancer mortality (40),
it remains difficult to diagnose as the lesions are too small to be
seen using available imaging methods. Moreover, no effective
techniques or biomarkers are available for predicting tumor
recurrence. Our findings demonstrated that TIMELESS protein
over-expression is strongly associated with tumor recurrence and is
closely correlated with poorer DFS. Thus, TIMELESS protein
expression is a useful prognostic biomarker of tumor recurrence in
early-stage cervical cancer. Despite our positive results, whether
TIMELESS protein or mRNA overexpression could be detected in blood
serum and was strongly correlated with tumor recurrence requires
further investigation.
Together our results indicate that TIMELESS may be a
new therapeutic target for the treatment of cervical cancer. For
example, TIMELESS depletion was previously shown to increase
sensitivity to doxorubicin, an anticancer drug (26,27).
Thus, TIMELESS may represent a novel therapeutic target. However,
we must first unravel its exact mechanism of action in cervical
cancer to confirm our hypothesis.
In conclusion, our present data demonstrated that
TIMELESS expression was upregulated in cervical cancer and was
significantly associated with age, clinical stage, PLNM, SCC
antigen, tumor recurrence, vital status, differentiation grade,
radiation therapy, property of surgical margin and lymphovascular
space involvement. Furthermore, TIMELESS protein overexpression was
identified as an independent prognostic biomarker for predicting
the poor clinical outcome of early-stage cervical cancer patients.
However, further investigation into the molecular mechanisms behind
the TIMELESS-mediated potential tumorigenic effects in cervical
cancer are required.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar
|
|
3
|
Gu Y, Ma C, Zou J, Zhu Y, Yang R, Xu Y and
Zhang Y: Prevalence characteristics of high-risk human
papillomaviruses in women living in Shanghai with cervical
precancerous lesions and cancer. Oncotarget. 7:24656–24663.
2016.PubMed/NCBI
|
|
4
|
Mayadev J, Lim J, Durbin-Johnson B,
Valicenti R and Alvarez E: Smoking decreases survival in locally
advanced cervical cancer treated with radiation. Am J Clin Oncol.
Jan 22–2016.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Santesso N, Mustafa RA, Schünemann HJ,
Arbyn M, Blumenthal PD, Cain J, Chirenje M, Denny L, De Vuyst H,
Eckert LO, et al Guideline Support Group: World Health Organization
Guidelines for treatment of cervical intraepithelial neoplasia 2–3
and screen-and-treat strategies to prevent cervical cancer. Int J
Gynaecol Obstet. 132:252–258. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Crafton SM and Salani R: Beyond
chemotherapy: An overview and review of targeted therapy in
cervical cancer. Clin Ther. 38:449–458. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun F, Xiong Y, Zhou XH, Li Q, Xiao L,
Long P, Li LJ, Cai MY, Wei YX, Ma YL, et al: Acylglycerol kinase is
over-expressed in early-stage cervical squamous cell cancer and
predicts poor prognosis. Tumour Biol. 37:6729–6736. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hou T, Liang D, Xu L, Huang X, Huang Y and
Zhang Y: Atypical chemokine receptors predict lymph node metastasis
and prognosis in patients with cervical squamous cell cancer.
Gynecol Oncol. 130:181–187. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hou T, Liang D, Yang D, He J, Huang Y and
Zhang Y: High expression of CRAM correlates with poor prognosis in
patients with cervical carcinoma. Int J Clin Exp Pathol.
7:1060–1068. 2014.PubMed/NCBI
|
|
10
|
Zhang W, Ou J, Lei F, Hou T, Wu S, Niu C,
Xu L and Zhang Y: C14ORF166 overexpression is associated with
pelvic lymph node metastasis and poor prognosis in uterine cervical
cancer. Tumour Biol. 37:369–379. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang W, Hou T, Niu C, Song L and Zhang Y:
B3GNT3 Expression is a novel marker correlated with pelvic lymph
node metastasis and poor clinical outcome in early-stage cervical
cancer. PLoS One. 10:e01443602015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang L, Huang H, Zhang L, Hou T, Wu S,
Huang Q, Song L and Liu J: URG4 overexpression is correlated with
cervical cancer progression and poor prognosis in patients with
early-stage cervical cancer. BMC Cancer. 14:8852014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu L, Xia M, Wang J, Zhang W, Zhang Y and
He M: CISD2 expression is a novel marker correlating with pelvic
lymph node metastasis and prognosis in patients with early-stage
cervical cancer. Med Oncol. 31:1832014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hou T, Zhang W, Tong C, Kazobinka G, Huang
X, Huang Y and Zhang Y: Putative stem cell markers in cervical
squamous cell carcinoma are correlated with poor clinical outcome.
BMC Cancer. 15:7852015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Unsal-Kacmaz K, Mullen TE, Kaufmann WK and
Sancar A: Coupling of human circadian and cell cycles by the
timeless protei. Mol Cell Biol. 25:3109–3116. 2005. View Article : Google Scholar
|
|
16
|
Gotter AL, Suppa C and Emanuel BS:
Mammalian TIMELESS and Tipin are evolutionarily conserved
replication fork-associated factors. J Mol Biol. 366:36–52. 2007.
View Article : Google Scholar
|
|
17
|
Urtishak KA, Smith KD, Chanoux RA,
Greenberg RA, Johnson FB and Brown EJ: Timeless maintains genomic
stability and suppresses sister chromatid exchange during
unperturbed DNA replication. J Biol Chem. 284:8777–8785. 2009.
View Article : Google Scholar :
|
|
18
|
Stevens RG: Circadian disruption and
breast cancer: From melatonin to clock genes. Epidemiology.
16:254–258. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yoshida K, Sato M, Hase T, Elshazley M,
Yamashita R, Usami N, Taniguchi T, Yokoi K, Nakamura S, Kondo M, et
al: TIMELESS is overexpressed in lung cancer and its expression
correlates with poor patient survival. Cancer Sci. 104:171–177.
2013. View Article : Google Scholar
|
|
20
|
Fu A, Leaderer D, Zheng T, Hoffman AE,
Stevens RG and Zhu Y: Genetic and epigenetic associations of
circadian gene TIMELESS and breast cancer risk. Mol Carcinog.
51:923–929. 2012. View
Article : Google Scholar
|
|
21
|
Dong H, Claffey KP, Brocke S and Epstein
PM: Expression of phosphodiesterase 6 (PDE6) in human breast cancer
cells. Springerplus. 2:6802013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tozlu-Kara S, Roux V, Andrieu C, Vendrell
J, Vacher S, Lazar V, Spyratos F, Tubiana-Hulin M, Cohen P, Dessen
P, et al: Oligonucleotide microarray analysis of estrogen receptor
a-positive postmenopausal breast carcinomas: Identification of
HRPAP20 and TIMELESS as outstanding candidate markers to predict
the response to tamoxifen. J Mol Endocrinol. 39:305–318. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Elgohary N, Pellegrino R, Neumann O,
Elzawahry HM, Saber MM, Zeeneldin AA, Geffers R, Ehemann V,
Schemmer P, Schirmacher P, et al: Protumorigenic role of Timeless
in hepato-cellular carcinoma. Int J Oncol. 46:597–606. 2015.
|
|
24
|
Chiang YT, Gout PW, Collins CC and Wang Y:
Prostate cancer metastasis-driving genes: Hurdles and potential
approaches in their identification. Asian J Androl. 16:545–548.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mazzoccoli G, Panza A, Valvano MR, Palumbo
O, Carella M, Pazienza V, Biscaglia G, Tavano F, Di Sebastiano P,
Andriulli A, et al: Clock gene expression levels and relationship
with clinical and pathological features in colorectal cancer
patients. Chronobiol Int. 28:841–851. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kemp MG, Akan Z, Yilmaz S, Grillo M,
Smith-Roe SL, Kang TH, Cordeiro-Stone M, Kaufmann WK, Abraham RT,
Sancar A, et al: Tipin-replication protein A interaction mediates
Chk1 phosphorylation by ATR in response to genotoxic stress. J Biol
Chem. 285:16562–16571. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang X, Wood PA and Hrushesky WJ:
Mammalian TIMELESS is required for ATM-dependent CHK2 activation
and G2/M checkpoint control. J Biol Chem. 285:3030–3034. 2010.
View Article : Google Scholar :
|
|
28
|
Mazzoccoli G, Piepoli A, Carella M, Panza
A, Pazienza V, Benegiamo G, Palumbo O and Ranieri E: Altered
expression of the clock gene machinery in kidney cancer patients.
Biomed Pharmacother. 66:175–179. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schepeler T, Lamy P, Hvidberg V, Laurberg
JR, Fristrup N, Reinert T, Bartkova J, Tropia L, Bartek J,
Halazonetis TD, et al: A high resolution genomic portrait of
bladder cancer: Correlation between genomic aberrations and the DNA
damage response. Oncogene. 32:3577–3586. 2013. View Article : Google Scholar
|
|
30
|
Relles D, Sendecki J, Chipitsyna G, Hyslop
T, Yeo CJ and Arafat HA: Circadian gene expression and
clinicopathologic correlates in pancreatic cancer. J Gastrointest
Surg. 17:443–450. 2013. View Article : Google Scholar
|
|
31
|
Gerber JM, Gucwa JL, Esopi D, Gurel M,
Haffner MC, Vala M, Nelson WG, Jones RJ and Yegnasubramanian S:
Genome-wide comparison of the transcriptomes of highly enriched
normal and chronic myeloid leukemia stem and progenitor cell
populations. Oncotarget. 4:715–728. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang W, Niu C, He W, Hou T, Sun X, Xu L
and Zhang Y: Upregulation of centrosomal protein 55 is associated
with unfavorable prognosis and tumor invasion in epithelial ovarian
carcinoma. Tumour Biol. 37:6239–6254. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shiraishi N, Natsume A, Togayachi A, Endo
T, Akashima T, Yamada Y, Imai N, Nakagawa S, Koizumi S, Sekine S,
et al: Identification and characterization of three novel beta
1,3-N-acetylglucosaminyltransferases structurally related to the
beta 1,3-galactosyltransferase family. J Biol Chem. 276:3498–3507.
2001. View Article : Google Scholar
|
|
34
|
He M, Wu C, Xu J, Guo H, Yang H, Zhang X,
Sun J, Yu D, Zhou L, Peng T, et al: A genome wide association study
of genetic loci that influence tumour biomarkers cancer antigen
19–9, carcinoembryonic antigen and α fetoprotein and their
associations with cancer risk. Gut. 63:143–151. 2014. View Article : Google Scholar
|
|
35
|
Ho WL, Che MI, Chou CH, Chang HH, Jeng YM,
Hsu WM, Lin KH and Huang MC: B3GNT3 expression suppresses cell
migration and invasion and predicts favorable outcomes in
neuroblastoma. Cancer Sci. 104:1600–1608. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang S, Liu Y, Xia B, Deng J, Liu T, Li Q,
Yang Y, Wang Y, Ning X, Zhang Y, et al: DLL4 as a predictor of
pelvic lymph node metastasis and a novel prognostic biomarker in
patients with early-stage cervical cancer. Tumour Biol.
37:5063–5074. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Koh WJ, Greer BE, Abu-Rustum NR, Apte SM,
Campos SM, Cho KR, Chu C, Cohn D, Crispens MA, Dorigo O, et al:
Cervical Cancer, Version 2.2015. J Natl Compr Canc Netw.
13:395–404; quiz 404. 2015.PubMed/NCBI
|
|
38
|
Pol FJ, Zusterzeel PL, van Ham MA,
Kuijpers DA, Bulten J and Massuger LF: Satellite lymphovascular
space invasion: An independent risk factor in early stage cervical
cancer. Gynecol Oncol. 138:579–584. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lan ML, Yu X, Xiao H, Zhou P, Hu N, Li J
and Wang G: Clinical outcomes and toxicity of postoperative
intensity-modulated versus three-dimensional conformal radiation
therapy in patients with cervical cancer. Asia Pac J Clin Oncol.
Feb 28–2016.Epub ahead of print. View Article : Google Scholar
|
|
40
|
Ikeda Y, Furusawa A, Kitagawa R, Tokinaga
A, Ito F, Ukita M, Nomura H, Yamagami W, Tanabe H, Mikami M, et al:
Practice patterns of adjuvant therapy for intermediate/high
recurrence risk cervical cancer patients in Japan. J Gynecol Oncol.
27:e292016. View Article : Google Scholar : PubMed/NCBI
|