Introduction
Bladder cancer is a significant cause of morbidity
and mortality in the UK with ~10,000 new cases diagnosed and 5,000
deaths registered each year (1).
The most common pathological subtype is transitional cell carcinoma
(TCCU) (90% of cases) (2) with
~50% of all cases being stage II or beyond at the time of diagnosis
(1). Standard of care for
localized, muscle invasive TCCU is radical cystectomy, which
historically, i.e., without modern neoadjuvant combination
chemotherapy, leads to 5-year overall survival in the approximate
range of 50–60% (3).
A meta-analysis including 11 trials and 3,005
patients confirmed the value of preoperative cisplatin based
combination chemotherapy which was shown to reduce the risk of
relapsing disease and conferring a 5-year absolute overall survival
advantage of 5% (4). A recent
meta-analysis comprising 945 patients and nine clinical trials
similarly demonstrated the benefits of postoperative cisplatin
based combination chemotherapy (5). There is thus significant evidence
supporting the use of chemotherapy in localized disease. However,
not all patients are eligible for curative-intent multimodal
therapy, and about one in five patients present with stage IV
disease (i.e., locally extending to other organs, to the pelvic or
abdominal wall, and/or with evidence of distant metastases) at the
time of diagnosis (1).
Patients with relapse following primary treatment,
or with advanced disease at presentation, confer a significant
challenge, and even among those fit for optimal platinum-based
combination chemotherapy the median overall survival does not
exceed the range of 12–15 months (6,7). The
recommended first line chemotherapy for these patients are
cisplatin based combinations and either MVAC (methotrexate,
vinblastine, doxorubicin, and cisplatin) or GC (gemcitabine and
cisplatin) (6,7) although the GC regimen is often
preferred due to a milder toxicity profile (7). For patients with acceptable
performance status and preserved organ functions, and where the
relapse occurs later than 12 months following neoadjuvant/adjuvant
cisplatin-based combination chemotherapy, change of platinum based
regimen may be a feasible option (8). In selected cases the addition of
paclitaxel to gemcitabine and cisplatin may be considered (9). For patients unfit for cisplatin
combinations alternative although potentially less efficient
combination regimens have been proposed, either with alternative
platinum agents [oxaliplatin (10)
or carboplatin (11)] or a
platinum-free combination of paclitaxel and gemcitabine (12). In patients deemed ineligible for
standard cisplatin based treatment, combination treatment with
split dose cisplatin and gemcitabine has reported encouraging
results (13).
Following failure of first line chemotherapy, be it
early relapse following platinum based neoadjuvant/adjuvant
chemotherapy, or progressive disease during palliative first-line
chemotherapy, treatment options have so far been limited. Studies,
mostly phase II and retrospective series, have reported activity
with taxanes and pemetrexed (14).
Until recently there were no randomized studies to confirm the
benefit of second line chemotherapy for patients with metastatic
TCCU. Vinflunine (Javlor®), a microtubule inhibitor of
the vinca-alkaloid family of anticancer agents (15), is the first drug to obtain European
Medicine Agency (EMA) approval for use in TCCU (2009) due to
evidence of efficacy from phase II (16,17)
and phase III trials (18,19). Considering the multiple challenges
in the second-line setting, with declining performance status due
to progressive disease, persistent side effects or complications
from earlier treatments, and primary or acquired chemo resistance
after primary chemotherapy, the safety profile and efficacy data
from the recent vinflunine reports are encouraging. In the phase
III trial (18,19) median overall survival was 6.9
months in the vinflunine plus best supportive care compared to 4.3
months in the best supportive care only population.
Further empirical studies have confirmed vinflunine
to be a safe and effective second line approach in Spain [n=66
(20)], France [n=134 (21)] and Germany [n=77, Hegele (22)] with reported overall survival of
7.7–10.4 months. Based on the accumulating evidence, the ESMO
guidelines now suggest vinflunine as the recommended second-line
therapy in advanced bladder cancer (23).
Vinflunine as a second line therapy is not currently
recommended for UK practice, nor is it available to the NHS
patients through the approved list of drugs on cancer drug fund. It
has however been made available through the Free of Charge Program
(FOCP) sponsored by Pierre Fabre. Here we evaluate the outcome of
patients treated with vinflunine as a second line therapy in this
program.
Patients and methods
Data were collected retrospectively on patients with
advanced metastatic TCCU diagnosed between 6th June 1999 and 20th
June 2013. Data were collected via a pre-defined CRF adopted for
the study and sent to local investigators for population. All
patients who received vinflunine as a second line therapy following
failure of first line therapy were eligible for inclusion.
All patients received vinflunine as a second-line
therapy through the free of charge program (FOCP) and received at
least one dose of vinflunine. All patients were included in the
analysis, irrespective of any dose reductions or toxicities. The
dose of vinflunine was 320/280/250 mg/m2 every 3 weeks
as per the SPc. The aim was to document the toxicity, radiological
RR and OS for patients treated with vinflunine in real life setting
within the FOCP.
Demographic data were collected on patient gender,
age, height, weight and performance status as well as the site of
the disease recurrence and their hemoglobin level at the time that
the decision to prescribe vinflunine was made. Details on the type
of first-line therapy were requested as was whether this was in the
neo-adjuvant or adjuvant setting.
Continuous data are presented as medians (IQR) and
categorical data are expressed as frequencies of counts. The
primary outcome measure of interest is overall survival (OS) which
is measured as the time from intention to treat with vinflunine
until death by any cause. Survival estimates obtained via method of
Kaplan and Meier. Progression-free survival (PFS) is measured as
the time from first vinflunine administration until progression or
death by any cause. Objective response rate is defined using RECIST
criteria (version 1.1) and determined at each local site.
Univariate analyses are carried out to assess patient demographics
as prognostic factors for overall survival using Cox proportional
hazards models. Hazard ratios (HR) are presented with associated
95% confidence intervals (CI). No multivariate analyses were
carried out. All P-values are considered significant at the 5%
level. All analysis were carried out using R (version 3.1.2).
Reported toxicities are defined using CTCAE (version
4) and are reported based on being either grade 1–2 or 3–4,
respectively.
Results
Data are provided on 49 patients from nine
contributing sites throughout the UK and Ireland (CCC 15; Christie
5; Cork 6; Glasgow 9; St. George's 1; Royal Free London 2; Royal
Marsden 6; Northampton 1; Newcastle 4) diagnosed with advanced
metastatic TCCU between 6th June 1999 and 20th June 2013. All
patients have progression of disease after first-line platinum
based therapy. Patient demographics are provided in Table I. The patient group consists of 67%
(33/49) males and have a median (IQR) age of 64 (57–70). As per the
summary of product characteristics (SPC) for vinflunine, patients
with a 0/1 performance status (ECOG) or a hemoglobin (Hb) level
>10 g/dl were targeted. Thirteen patients (27%) had an ECOG
performance status of 2 or a hemoglobin level <10. Sixteen
patients (33%) had either bone or liver recurrence. Two patients
(4%) had chronic constipation at presentation and two patients (4%)
had coronary artery disease.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Category | Level | n=49 |
|---|
| Gender | Female | 16 (33%) |
| Male | 33 (67%) |
| Weight, kg | Median (IQR) | 71 (63–83) |
| Height, cm | Median (IQR) | 168 (160–175) |
| Age, years | Median (IQR) | 64 (57–70) |
| Performance status
(n=46) | 0 | 12 (26%) |
| 1 | 30 (65%) |
| 2 | 4 (9%) |
| Site of disease
recurrence (n=46) | Bone | 8 (17%) |
| Liver | 11 (24%) |
| Lung | 15 (33%) |
| Other visceral | 8 (17%) |
| Other
non-visceral | 3 (7%) |
| None | 1 (2%) |
| Hemoglobin
(g/dl) | <10 | 11 (28%) |
| 10–12 | 20 (50%) |
| >12 | 9 (22%) |
Information on first-line therapy is available for
48/49 (98%) of all participating patients. First line neo-adjuvant
chemotherapy was given to 60% (27/48) and consisted of
gemcitabine/cisplatin (74%; 20/27), gemcitabine/carboplatin (22%,
6/27), or gemcitabine/cisplatin switched to carboplatin in view of
worsening renal functions (4%, 1/27). In the metastatic setting
patients received platinum regimens consisting of either
gemcitabine/cisplatin (30%, 13/43), gemcitabine/carboplatin (37%,
16/43), gemcitabine/cisplatin switched to carboplatin (2%, 1/43),
or some other combination (30%, 13/43).
Following first-line therapy, patient best response
rates were complete response 15% (7/48), partial response 48%
(23/48), stable disease 17% (8/48) and progressive disease 19%
(9/48). Best response was not available for one patient. The
overall response rate for first-line therapy was 63% (30/48) and
98% (43/44) of patients demonstrated disease progression with 77%
(34/44) demonstrating bone, liver, or lung metastases.
Table II gives the
details of vinflunine administrations. At the point of data
collection 248 cycles of vinflunine had been administered. The
starting dose was 250 mg/m2 (4%, 2/49), 280
mg/m2 (76%, 37/49) or 320 mg/m2 (20%, 10/49).
Three patients (6%) had a dose escalation and 10 (21%) had dose
reduction. The median (IQR) number of cycles given was 3.5
(2–6.25). Eight patients (17%) had only a single cycle, while the
maximum number of cycles at the time of analysis was 18.
 | Table IIVinflunine administration. |
Table II
Vinflunine administration.
| Category | Level | |
|---|
| Starting dose
(mg/m2) | 250 | 2 (4%) |
| 280 | 37 (75%) |
| 320 | 10 (21%) |
| Escalation
(n=47) | No | 44 (94%) |
| yes | 3 (6%) |
| Reduction (n=47) | No | 37 (79%) |
| Yes | 10 (21%) |
| Cycles | Median (IQR) | 3.5 (2–6.25) |
Toxicity
Toxicities are reported by sites as being either
grade 1/2 or grade 3/4 hematological or non-hematological. Results
are given in Table III. Overall
31% (15/49) of patients reported at least one grade 3/4 adverse
event. The most common grade 3/4 toxicities are constipation
reported by 8% (4/49) and asthenia/fatigue reported by 6% (3/49) of
patients.
 | Table IIIReported hematological and
non-hematological toxicities. |
Table III
Reported hematological and
non-hematological toxicities.
| Toxicity | Grade
|
|---|
| 1–2 | 3–4 |
|---|
| Hematological | | |
| Anemia | 5 | 2 |
| Neutropenia | 13 | 1 |
| Neutropenic
infection | 0 | 2 |
|
Thrombocytopenia | 2 | 0 |
| Febrile
infection | 1 | 0 |
| Leukopenia | 1 | 1 |
| Other | 0 | 2 |
|
Non-hematological | | |
| Constipation | 9 | 4 |
|
Asthenia/fatigue | 20 | 3 |
| Vomiting | 8 | 0 |
| Abdominal
pain | 2 | 0 |
| Other | 0 | 2 |
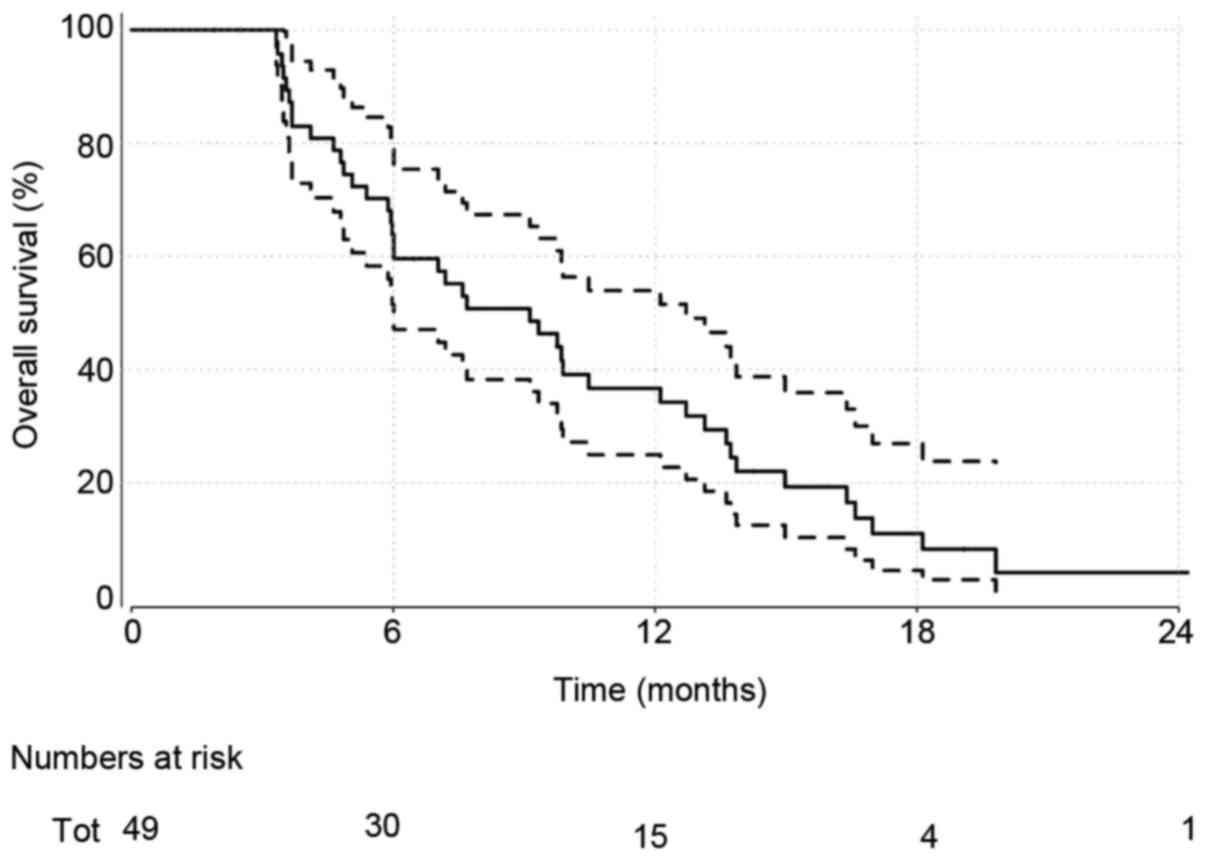
Overall survival
Patients were followed up for a median of 9.1
months. At the time of analysis, 41 (84%) patients had died.
Overall survival estimates obtained via the method of Kaplan-Meier
(Fig. 1) give a median (95%
confidence interval) of 9.1 months (6.0–12.7). ECOG performance
measures were investigated further as a key prognostic indicator of
interest (Fig. 2). Median survival
estimates of 13.1 (6.0, NA) months and 7.6 (5.98–10.5) months were
observed for ECOG performance measures of 0 and 1–2, respectively.
This was not significant statistically [HR (95% CI): 1.62 (0.74,
3.55); P=0.23].
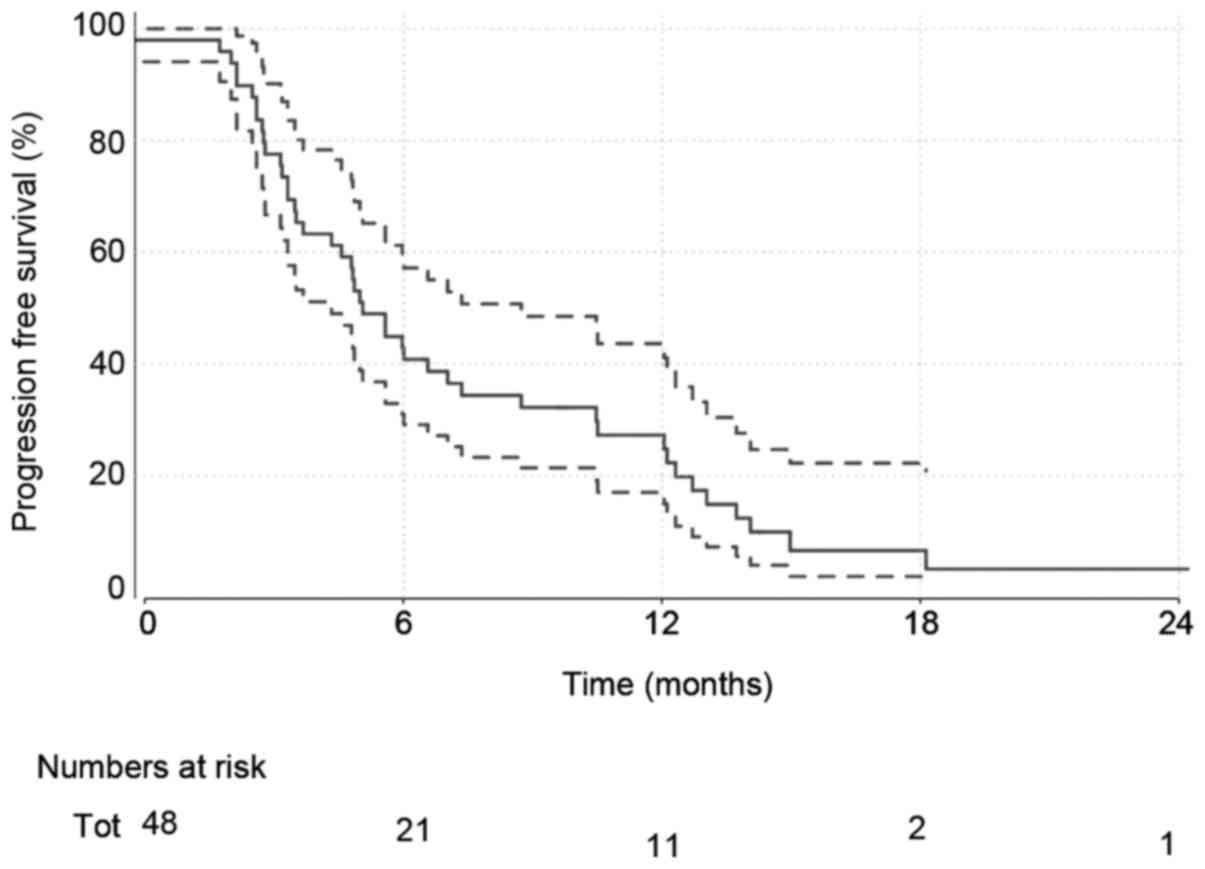
Disease progression
A total of 45 (92%) of patients had disease
progression at the time of analysis. Median progression-free
survival estimates (Fig. 2) are
5.1 months (4.3–8.7).
Patient response
Forty-eight patients were evaluable for overall
response (Table IV). One patient
did not provide any response data.
 | Table IVOverall best response for vinflunine
administration. |
Table IV
Overall best response for vinflunine
administration.
| Best response |
|---|
| CR | 0 |
| PR | 14 (29%) |
| SD | 10 (21%) |
| PD | 19 (40%) |
| NE | 4
(8%) |
Sensitivity analyses report the result only on
patients who meet the SPC (ECOG <2, HB >10). Here 35 patients
gave an estimated OS of 9.9 months (7.6–16.4) with an estimated PFS
of 6.0 months (4.8–12.1) and a partial response rate of 30%.
Discussion
The prognosis for patients with advanced metastatic
TCCU who have progressed after first-line therapy remains dismal,
and whilst previously published randomised trials and empirical
evidence have favoured the use of vinflunine in this patient group,
the results have not been clear enough to favour its wide spread
use in the UK. As a consequence, many patients will end antitumour
therapy or proceed onto palliative care once the first-line regimen
has failed.
We performed a retrospective observational study in
this clinical setting to assess the effects of vinflunine in terms
of both efficacy and toxicity. Data on 49 patients from sites
across the UK and Ireland were collected and analysed. The dose of
and number of cycles were consistent with previous studies, with a
median of 3.5 cycles being similar to the number recorded in the
phase III trial (18,19). The most common starting dose was
280 g/m2 (75%) and the dose was relatively well
tolerated with only 10 (29%) patients requiring a reduction. There
was no evidence of any correlation between the starting dose and
dose reduction.
Further to this, the present median overall survival
of 9.1 months (6.0–12.7) is consistent with or even slightly better
than the 6.9 months of the vinflunine treated arm of the Bellmunt
phase III study (18,19). Further, the present numbers compare
favourably with the empirical 'real life' results obtained from
Spain (16) Germany (22) and France (21) which all report median survivals
between 7.7–10.4 months in similar patient populations.
Toxicity rates observed in our study also show
little cause for concern with 39% (19/49) patients reporting a
grade 3+ adverse event, the most common being constipation (4
patients) and fatigue (3 patients). Toxicity can be further
improved with the use of prophylactic laxative and oral antibiotics
and should provide little concern in the prescribing of
vinflunine.
Further inspection of study efficacy shows that
patients with a baseline ECOG PS of 0 display a median OS of 13.1
(6.0, NA) months, while patients of PS 1–2 have a poorer prognosis
of 7.6 (6.0–10.5) months. This is consistent with the data of ref.
20 reporting survival estimates
of 13.2 and 6.7 months, respectively, for the same patient groups
and emphasise baseline PS as a key prognostic predictor although
not ruling out the potential benefits even in the PS 1–2
population.
This study is limited by the retrospective nature of
the data collection and the lack of a randomised comparative arm
against which to compare the efficacy, which ultimately open the
study results to possible bias. In saying that, however, the study
analysis includes patients who did not meet the SPC for vinflunine
as well as patients who only received a single dose. Coupled with
the high rate of ECOG >0 and the distribution of metastases this
suggests a patient population which is representative of general
clinical practice rather than a narrowly selected clinical trial
population. Recently there has been much interest in the role of
immune checkpoint inhibitors in urothelial cancer with durable
responses have been noted with PD1 [e.g., pembrolizumab (24) and PDL1 atezolizumab (25)]. The results of recently completed
trials comparing anti-PD1/PDL1 antibodies, with standard of care
chemotherapy of institution's choice that includes vinflunine in
the 2nd line setting (NCT 02256436) are awaited with interest.
In conclusion, the empirical results obtained from
UK and Ireland practice reflect what has been observed in
randomised trials of vinflunine as well as other retrospective
studies throughout the European Union. The accumulating evidence
puts forward vinflunine single drug therapy as an efficient, safe
and reasonably tolerable second-line therapy in patients with
advanced TCCU.
References
|
1
|
Cancer Research UK: Statistics 2012–2013.
http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/bladder-cancer.
|
|
2
|
Amin MB, McKenney JK, Paner GP, Hansel DE,
Grignon DJ, Montironi R, Lin O, Jorda M, Jenkins LC, Soloway M, et
al International Consultation on Urologic Disease-European
Association of Urology Consultation on Bladder Cancer 2012:
ICUD-EAU international consultation on bladder cancer 2012:
Pathology. Eur Urol. 63:16–35. 2013. View Article : Google Scholar
|
|
3
|
Madersbacher S, Hochreiter W, Burkhard F,
Thalmann GN, Danuser H, Markwalder R and Studer UE: Radical
cystectomy for bladder cancer today -a homogeneous series without
neoadjuvant therapy. J Clin Oncol. 21:690–696. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Advanced Bladder Cancer Meta-analysis
Collaboration: Neoadjuvant chemotherapy for invasive bladder
cancer. Cochrane Database Syst Rev. 12004.
|
|
5
|
Leow JJ, Martin-Doyle W, Rajagopal PS,
Patel CG, Anderson EM, Rothman AT, Cote RJ, Urun Y, Chang SL,
Choueiri TK, et al: Adjuvant chemotherapy for invasive bladder
cancer: A 2013 updated systematic review and meta-analysis of
randomized trials. Eur Urol. 66:42–54. 2014. View Article : Google Scholar
|
|
6
|
Loehrer PJ Sr, Einhorn LH, Elson PJ,
Crawford ED, Kuebler P, Tannock I, Raghavan D, Stuart-Harris R,
Sarosdy MF, Lowe BA, et al: A randomized comparison of cisplatin
alone or in combination with methotrexate, vinblastine, and
doxorubicin in patients with metastatic urothelial carcinoma: A
cooperative group study. J Clin Oncol. 10:1066–1073. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
von der Maase H, Hansen SW, Roberts JT,
Dogliotti L, Oliver T, Moore MJ, Bodrogi I, Albers P, Knuth A,
Lippert CM, et al: Gemcitabine and cisplatin versus methotrexate,
vinblastine, doxorubicin, and cisplatin in advanced or metastatic
bladder cancer: Results of a large, randomized, multinational,
multicenter, phase III study. J Clin Oncol. 18:3068–3077. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Necchi A, Pond GR, Giannatempo P, Di
Lorenzo G, Eigl BJ, Locke J, Pal SK, Agarwal N, Poole A,
Vaishampayan UN, et al: Cisplatin-based first-line therapy for
advanced urothelial carcinoma after previous perioperative
cisplatin-based therapy. Clin Genitourin Cancer. 13:178–184. 2015.
View Article : Google Scholar :
|
|
9
|
Bellmunt J, von der Maase H, Mead GM,
Skoneczna I, De Santis M, Daugaard G, Boehle A, Chevreau C,
Paz-Ares L, Laufman LR, et al: Randomized phase III study comparing
paclitaxel/cisplatin/gemcitabine and gemcitabine/cisplatin in
patients with locally advanced or metastatic urothelial cancer
without prior systemic therapy: EORTC Intergroup Study 30987. J
Clin Oncol. 30:1107–1113. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Carles J, Esteban E, Climent M, Font A,
Gonzalez-Larriba JL, Berrocal A, Garcia-Ribas I, Marfa X, Fabregat
X, Albanell J, et al Spanish Oncology Genito Urinary Group Study
Group: Gemcitabine and oxaliplatin combination: A multicenter phase
II trial in unfit patients with locally advanced or metastatic
urothelial cancer. Ann Oncol. 18:1359–1362. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
De Santis M, Bellmunt J, Mead G, Kerst JM,
Leahy M, Maroto P, Skoneczna I, Marreaud S, de Wit R and Sylvester
R: Randomized phase II/III trial assessing gemcitabine/carboplatin
and methotrexate/carboplatin/vinblastine in patients with advanced
urothelial cancer 'unfit' for cisplatin-based chemotherapy: Phase
II -results of EORTC study 30986. J Clin Oncol. 27:5634–5639. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Calabrò F, Lorusso V, Rosati G, Manzione
L, Frassineti L, Sava T, Di Paula ED, Alonso S and Sternberg CN:
Gemcitabine and paclitaxel every 2 weeks in patients with
previously untreated urothelial carcinoma. Cancer. 115:2652–2659.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hussain SA, Stocken DD, Riley P, Palmer
DH, Peake DR, Geh JI, Spooner D and James ND: A phase I/II study of
gemcitabine and fractionated cisplatin in an outpatient setting
using a 21-day schedule in patients with advanced and metastatic
bladder cancer. Br J Cancer. 91:844–849. 2004.PubMed/NCBI
|
|
14
|
Bambury RM, Benjamin DJ, Chaim JL, Zabor
EC, Sullivan J, Garcia-Grossman IR, Regazzi AM, Ostrovnaya I,
Apollo A, Xiao H and Voss: The safety and efficacy of single-agent
pemetrexed in platinum-resistant advanced urothelial carcinoma: A
large single-institution experience. Oncologist. 20:508–515. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bennouna J, Delord JP, Campone M and
Nguyen L: Vinflunine: A new microtubule inhibitor agent. Clin
Cancer Res. 14:1625–1632. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Culine S, Theodore C, De Santis M, Bui B,
Demkow T, Lorenz J, Rolland F, Delgado FM, Longerey B and James N:
A phase II study of vinflunine in bladder cancer patients
progressing after first-line platinum-containing regimen. Br J
Cancer. 94:1395–1401. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vaughn DJ, Srinivas S, Stadler WM, Pili R,
Petrylak D, Sternberg CN, Smith DC, Ringuette S, de Wit E, Pautret
V, et al: Vinflunine in platinum-pretreated patients with locally
advanced or metastatic urothelial carcinoma: Results of a large
phase 2 study. Cancer. 115:4110–4117. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bellmunt J, Théodore C, Demkov T, Komyakov
B, Sengelov L, Daugaard G, Caty A, Carles J, Jagiello-Gruszfeld A,
Karyakin O, et al: Phase III trial of vinflunine plus best
supportive care compared with best supportive care alone after a
platinum-containing regimen in patients with advanced transitional
cell carcinoma of the urothelial tract. J Clin Oncol. 27:4454–4461.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bellmunt J, Fougeray R, Rosenberg JE, von
der Maase H, Schutz FA, Salhi Y, Culine S and Choueiri TK:
Long-term survival results of a randomized phase III trial of
vinflunine plus best supportive care versus best supportive care
alone in advanced urothelial carcinoma patients after failure of
platinum-based chemotherapy. Ann Oncol. 24:1466–1472. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Castellano D, Puente J, de Velasco G,
Chirivella I, López-Criado P, Mohedano N, Fernández O,
García-Carbonero I, González MB and Grande E: Safety and
effectiveness of vinflunine in patients with metastatic
transitional cell carcinoma of the urothelial tract after failure
of one platinum-based systemic therapy in clinical practice. BMC
Cancer. 14:7792014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Medioni J, Guillot A, Spaeth D, Di Palma M
and Theodore C: Historical data in real life from patients treated
by vinflunine for an advanced or metastatic urothelial carcinoma:
Results of the CURVE study. Eur J Cancer. 49:S646–S647. 2013.
|
|
22
|
Hegele A, De Geeter P, Goebell P, Matz U,
De Schultz W and Retz M: Vinflunine in routine practice for the
treatment of advanced or metastatic urothelial cell carcinoma in
Germany. Eur J Cancer. 49:669. 2013.
|
|
23
|
Bellmunt J, Orsola A, Maldonado X and
Kataja V; ESMO Guidelines Working Group: Bladder cancer: ESMO
Practice Guidelines for diagnosis, treatment and follow-up. Ann
Oncol. 21(Suppl 5): v134–v136. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Plimack ER, Bellmunt J, Gupta S, Berger R,
Montgomery RB, Heath K, Juco J, Emancipator K, Pathiraja K,
Lunceford JK, et al: Pembrolizumab (MK-3475) for advanced
urothelial cancer: Updated results and biomarker analysis from. J
Clin Oncol. 33:45022015.
|
|
25
|
Hoffman-Censits JH, Grivas P, Van Der
Heijden MS, Dreicer R, Loriot Y, Retz M, Vogelzang NJ, Perez-Gracia
JL, Rezazadeh A, Bracarda S, et al: IMvigor 210, a phase II trial
of atezolizumab (MPDL3280A) in platinum-treated locally advanced or
metastatic urothelial carcinoma (mUC). Genitourinary cancer
meeting: 2016 Genitourinary cancers symposium. Welcome and general
session 4: Immunotherapy for urothelial carcinoma. J Clin Oncol.
34(Suppl 2): 3552016. View Article : Google Scholar
|