Introduction
In eutherian mammals, the chromosomal sex is
determined at fertilization and sexual differences begin after the
7th week in humans when the sex determining region of the Y
chromosome (SRY) is activated. The next stage of sex
differentiation, Müllerian duct regression and Wolffian duct
development, rely on two hormones: testosterone, secreted by Leydig
cells and Müllerian inhibiting substance, also named anti-Müllerian
hormone (MIS/AMH), produced by Sertoli cells (1). MIS/AMH was first suggested by Alfred
Jost in the late 1940s (2) showing
that a testicular product different from testosterone which he
named 'Müllerian inhibitor', was responsible for the regression of
Müllerian ducts in the male fetus (3).
MIS/AMH is a member of the transforming growth
factor-β super-family of growth and differentiation response
modifiers through binding two similar type I and type II receptors
(4). MIS/AMH from testicular
Sertoli cells, causes regression of the Müllerian ducts that are
the precursors to the Fallopian tubes, the surface epithelium of
the ovaries, the uterus, the cervix, and the upper third of the
vagina in male embryos (5). It is
expected to inhibit the growth of gynecological cancers, because
the three most common gynecological cancers, ovarian, endometrial
(uterine) and cervical cancer, originate from Müllerian
duct-derived tissues (6–8). MIS/AMH type II receptors
(MISRII/AMHRII) which bind MIS/AMH, have been shown to be expressed
in gynecological cancers, whreas their expression is low in normal
tissues (9,10). It was previously shown that MIS/AMH
inhibited growth of gynecological cancer cells by regulating cell
cycle, apoptosis and Wnt signaling pathways (11–13).
From among these pathways, cell cycle arrest is regarded as playing
a major role in MIS/AMH-mediated signal transduction cascades in
gynecological cancer. MIS/AMH upregulates expression of p16,
pRB-related proteins, and some E2F family members, and induces G1
arrest and subsequent apoptosis (5,11,13,14).
Furthermore, Wnt signaling pathway also has an important role in
both embryonic development and tumorigenesis (15,16).
β-catenin, a key component of the Wnt signaling pathway, interacts
with the TCF/LEF family of transcription factors and activates
transcription of Wnt target genes which in turn regulates
proliferation, polarity, adhesion and motility (17). It was also demonstrated in human
epithelial ovarian cancer cell lines, that ovcar-8 expresses highly
MISRII/AMHRII and shows high susceptibility to MIS/AMH (13,18).
In our previous study in endometrial cancer, we
showed upregulation by MIS/AMH treatment of the β-catenin
interacting protein (ICAT) (11)
which was found to negatively regulate the Wnt signaling pathway by
inhibiting the interaction between β-catenin and TCF family members
(19). To understand how MIS/AMH
regulates Wnt/β-catenin in gynecological cancers, we show that
MIS/AMH upregulates ICAT expression which results in ovarian cancer
growth by disruption of the β-catenin-dependent Wnt signaling
pathway.
Materials and methods
Recombinant human MIS/AMH
Recombinant human MIS/AMH was purified and its
biological activity was confirmed in the Pediatric Surgical
Research Laboratories at the Massachusetts General Hospital
(Boston, MA, USA) from serum-free and serum containing conditioned
media as previously described (20).
Cells and cell culture
The human ovarian cancer cell line ovcar-8
(Pediatric Surgical Research Laboratories, Massachusetts General
Hospital) was maintained in Dulbecco's modified Eagle's medium and
10% fetal bovine serum (FBS), 1% penicillin/streptomycin and 1%
L-glutamine for no more than 8 passages and subcultures were
initiated at 80% confluence. The cultures were maintained in a
humidified atmosphere of 5% CO2 at 37°C.
Transfection of siRNA
The small interfering RNA (siRNA) targeted against
human ICAT gene silencer (acceccion number NM_001012329), siRNA
transfection reagent, and serum-free transfection medium were
purchased from Qiagen (Mansfield, MA, USA). The day before the
transfection, 1×105 cells were seeded in each well of
6-well cell culture plates in antibiotic-free medium. The next day,
cells were washed with transfection medium and transfection
complexes were prepared using ICAT siRNA, siRNA transfection
reagent, and transfection medium according to the manufacturer's
instructions and were delivered to cell monolayers in 1 ml fresh
media with 20 nM final concentration of siRNA. The siRNAs used were
as follows: ICAT was silenced in ovcar-8 (siICAT/ovcar-8) using
FlexiTube siRNA (Hs_CTNNBIP1_2); the target sequence is
5′-TCCCTTCAGACTGGCCCTTAA-3′ (Qiagen cat. no. SI00125734) as
previously shown. Non-silencing negative control (con/ovcar-8) used
AllStars Neg Control siRNA (Qiagen cat. no. I027281).
Methylthiazol tetrazolium (MTT)
assay
Three thousand cells/well were seeded in 96-well
plates. After 24 h the cells were exposed to vehicle control or 10
μg/ml) of MIS/AMH for 48 h. Cells were washed with
phosphate-buffered saline (PBS) and 100 μl of MTT solution
(5 mg/ml MTT stock in PBS diluted to 1 mg/ml with 10% DMEM) was
added to each well. Cells were incubated for 4 h at 37°C at the end
of which time 200 μl dimethyl sulfoxide (DMSO;
Sigma-Aldrich, St. Louis, MO, USA) was added and incubated further
for 30 min at room temperature in the dark. Optical densities at
550 nm were measured using an ELISA plate reader (BioTek
Instruments, Winooski, VT, USA).
Scratch wound migration assay
One hundred percent confluent ovcar-8 monolayers
were used and the scratch assay performed using a sterile
200-μl pipette tip to scratch the cells to form a cell-free
gap. The cells were then cultured in 10% FBS medium contained
vehicle or 10 μg/ml of MIS/AMH and fixed with formalin.
Migration of wounded cells was evaluated at 0 and 48 h with an
inverted Olympus phase-contrast microscope. The six different wells
were scratched at the same time in two independent experiments and
migration was determined using the ImageJ program as an average
closed area of the wound relative to the initial scratch area at 48
h after wounding.
Cell cycle analysis
Ovcar-8 cells were exposed to 10% FBS medium with 10
μg/ml MIS/AMH or vehicle control buffer for 48 h and the
cells were collected by trypsinization. The cells were fixed with
100% methanol and stored for 30 min at 20°C and washed with PBS.
Following centrifugation the cells were re-suspended in 1 ml DNA
staining solution (20 μg/ml propidium iodide, 200
μg/ml DNase free RNase) and incubated in the dark at 37°C
for 30 min. The cells were analyzed on a FACSVantage SE flow
cytometer (Becton-Dickinson, San Jose, CA, USA). The forward
scatter and red fluorescence above 600 nm were measured and the
results analyzed using CellQuest™ software (Verity Software House,
Inc., Topsham, ME, USA).
Annexin V analysis
The MIS/AMH treated cells were stained for Annexin V
and propidium iodide (PI) using the Annexin V-FITC apoptosis
detection kit I (556547; BD Biosciences, San Diego, CA, USA)
according to the manufacturer's protocol. Briefly, following drug
treatment for 48 h, 1×105 cells were pelleted and washed
once with PBS and re-suspended in 100 ml of binding buffer [10 mM
HEPES (pH 7.4), 150 mM NaCl, 5 mM potassium chloride, 1 mM
MgCl2 and 2 mM calcium chloride]. Subsequently, 5
μl of Annexin V-FITC and PI was added to the cells that were
then incubated for 15 min at room temperature in the dark. After
this incubation, 400 μl of binding buffer was added and
cells were analyzed using a FACSVantage SE flow cytometer
(Becton-Dickinson). Data analyses were conducted using CellQuest
software.
Western blot analysis
Proteins from cells treated with 10 μg/ml
MIS/AMH were harvested in RIPA buffer (150 mM NaCl, 1% NP-40, 0.5%
sodium deoxycholate, 0.1% SDS and 50 nM Tris-HCl) with 1 μM
PMSF and the protein concentration was determined by BCA protein
assay reagent (23225; Thermo Fisher Scientific, Waltham, MA, USA).
Equal amounts of protein were separated on SDS-polyacrylamide gels
(50 μg per lane) and transferred to PVDF membrane. The blots
were blocked in TBS-T (20 mM Tris-HCl, pH 7.6, 137 mM NaCl, 0.1%
Tween-20) containing 5% powdered milk for 1 h and then incubated in
5% powdered milk TBS-T at 4°C overnight with the primary
antibodies, ICAT (1:100, sc-99240; Santa Cruz Biotechnology, Santa
Cruz, CA, USA), caspase-3 (1:200, 9668; Cell Signaling Technology,
Inc., Boston, MA, USA), Apaf-1 (1:100, sc-8339; Santa Cruz
Biotechnology), E2F1 (1:200, 3742; Cell Signaling Technology), p107
(1:100, sc-318; Santa Cruz Biotechnology), c-Myc (1:200, 5605; Cell
Signaling Technology), phospho-c-Jun (1:200, 3270; Cell Signaling
Technology), β-catenin (1:200, 9562; Cell Signaling Technology),
beclin-1 (1:200, 3495; Cell Signaling Technology) and LC3A/B
(1:200, 12741; Cell Signaling Technology). Blots were then washed
three times with 1% TBS-T and incubated with the corresponding
horseradish peroxidase-conjugated secondary antibody, diluted to
1:5,000 in 1% non-fat dry milk TBS-T. Blots were detected using the
Pierce ECL western blotting substrate (Thermo Fisher
Scientific).
Statistical analysis
MTT results are presented as percentage of control,
which was calculated using the following equation: (mean absorbance
of treated cells/mean absorbance of control cells) × 100. Data are
expressed as mean ± SD from nine independent experiments. A
P<0.05 was considered statistically significant when compared
with corresponding vehicle control cells. Cell cycle distribution
after exposure to MIS/AMH in ovarian cancer cells are presented as
histograms of the mean ± SD from three independent experiments.
Annexin V analysis was done for evaluation of apoptosis. Quadrant
rectangular dot grams from a representative of three independent
experiments are shown. Western blotting results were presented as
mean ± SD from three independent experiments. Statistical
comparisons between two experimental groups were performed using
Student's t-test (paired) whilst multiple group comparisons were
performed using analysis of variance (ANOVA). Data were regarded as
being significant at P<0.05.
Results
ICAT siRNA reverses the inhibition effect
of MIS/AMH on ovcar-8 cells
There were no significant differences between
siRNA-transfected cells and untransfected controls (data not
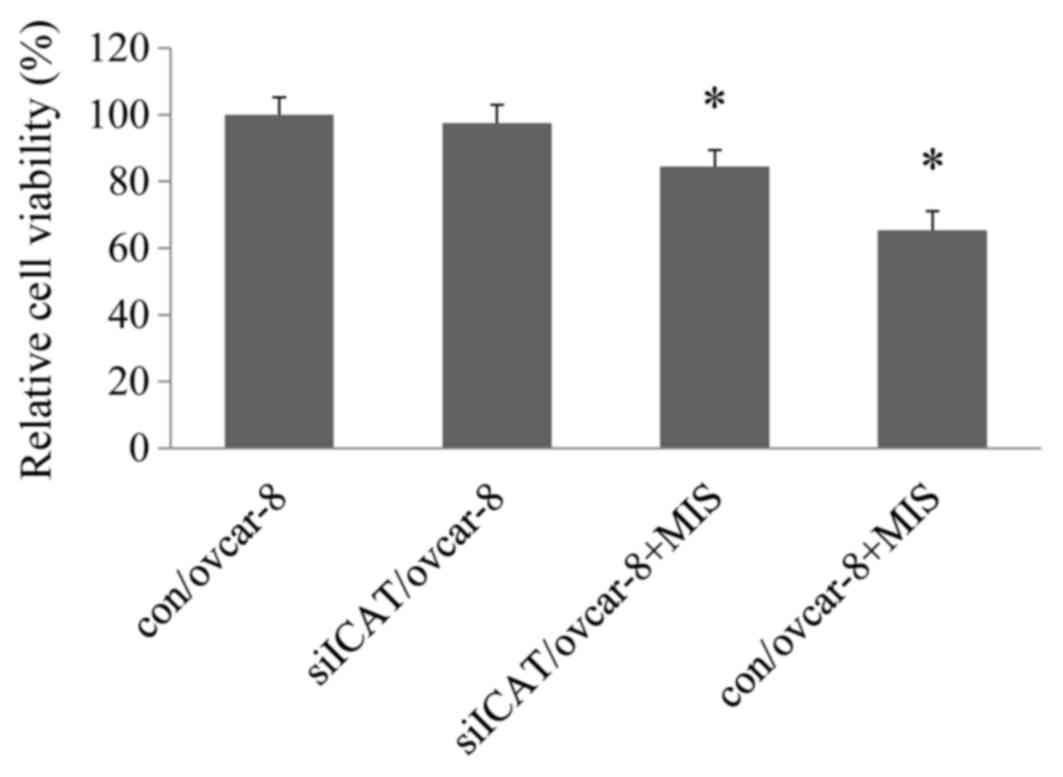
shown). Upon MIS/AMH expose, the viability of OVCAR-8 cells
decreased by ~65.34% relative to MIS/AMH untreated control and
siICAT/ovcar-8 cells, but when treated with ICAT-specific siRNA
inhibition was reduced to 84.51% in MIS/AMH treated siICAT/ovcar-8
cells. The inhibitory effect of MIS/AMH on ovcar-8 cell viability
was reduced by 19.17% in the siCAT/ovcar-8 group compared to the
con/ovcar-8 group (Fig. 1).
ICAT siRNA reduces the effect of MIS/AMH
on migration
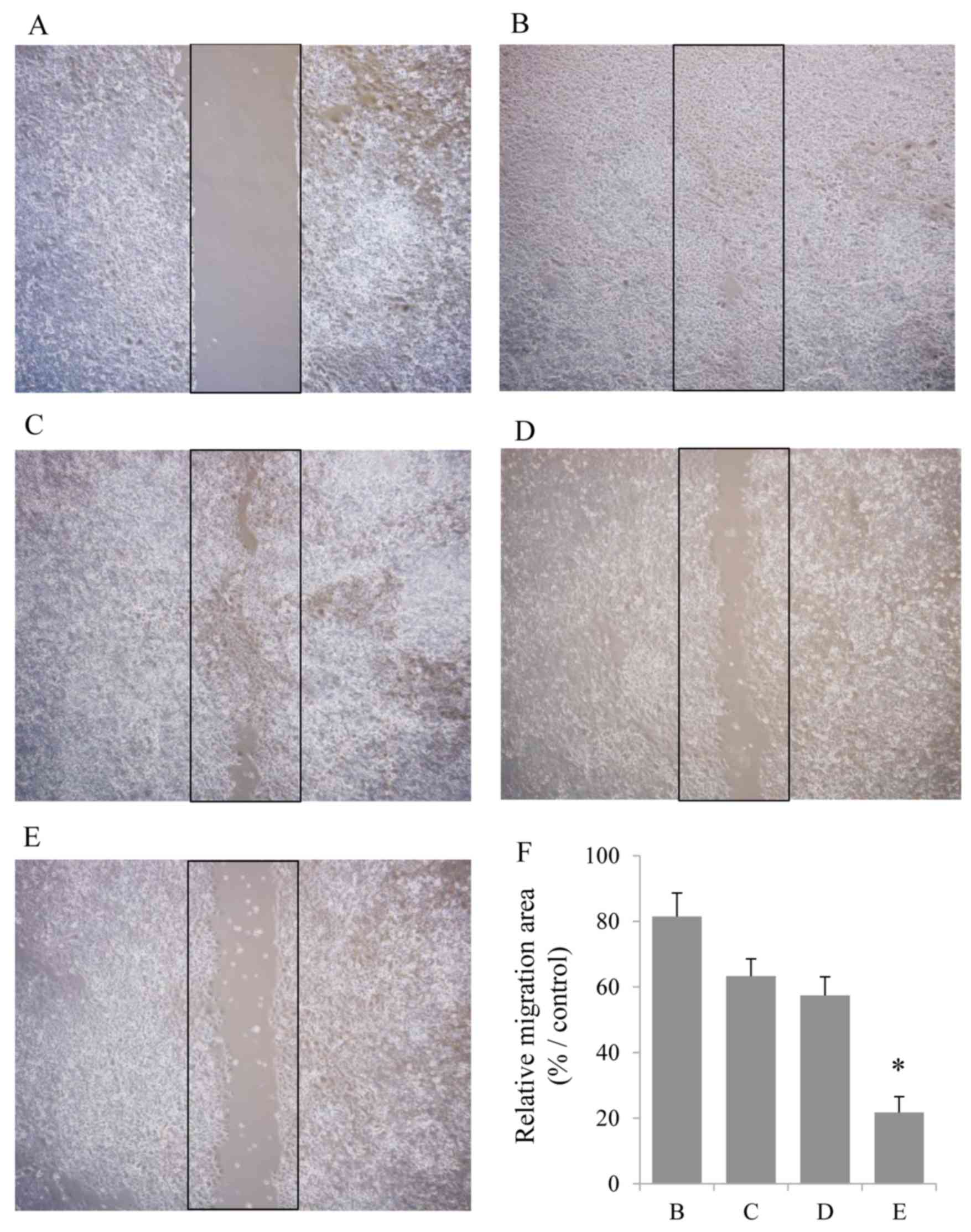
The scratch wound migration assay was performed on
con/ovcar-8 and siICAT/ovcar-8 cells to study ICAT effect on cell
migration (Fig. 2). The scratch
area was almost the same size in each experimental group at 0 h.
The area of migration cells in the MIS/AMH untreated control and
ICAT gene silenced group had reached 81 and 63% at 48 h. The area
of migration cells in the MIS/AMH treated control and ICAT gene
silenced group had decreased 22 and 57% at 48 h. Compared with the
control and ICAT gene silencing groups, cell migration was
significantly reduced only by MIS/AMH treatment (P<0.05). The
results showed that ICAT gene expression negatively correlated with
ovcar-8 cell migration ability, and MIS/AMH suppressed ovcar-8 cell
migration, which may be a result of the upregulation of ICAT gene
expression.
ICAT siRNA inhibits apoptosis induced by
MIH/AMH, but had no effect on cell cycle arrest
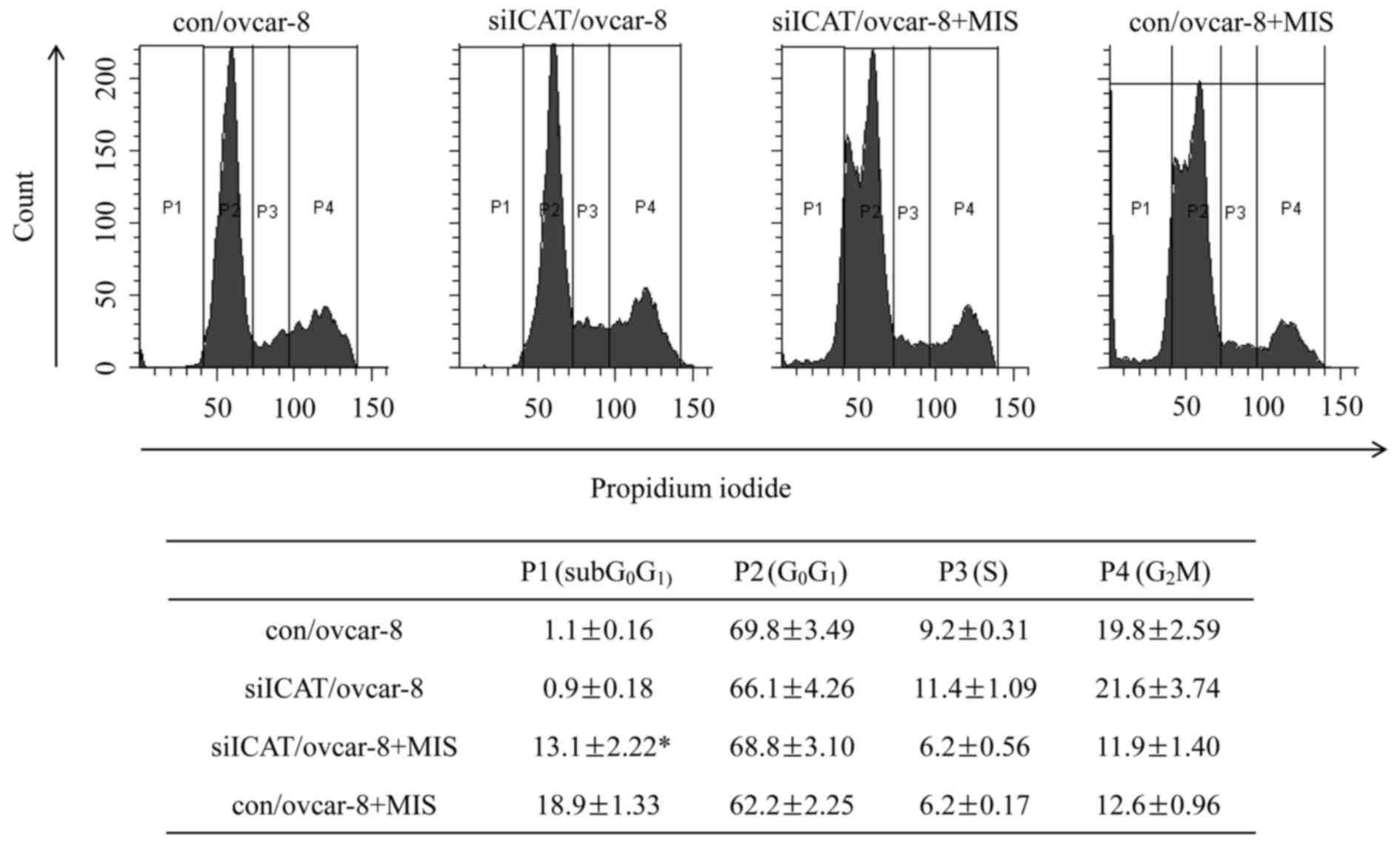
Flow cytometry clearly confirmed the reversal of
ICAT on the apoptosis effect of MIS/AMH. After treatment of control
cells with MIS/AMH, the cell population in
subG0G1 and G0G1 phase
changed from 1.1 to 18.9 and 69.8 to 62.2%, respectively which was
accompanied by a decrease from 9.1 to 6.2 and 19.8 to 12.5% in S
and G2 phases. Exposure to MIS/AMH cells after ICAT
siRNA silencing led to an increase in G0G1 to
only 13.1% and a decrease of G0G1 to 68.8%
while the S and G2 phases decreased 6.2 and 11.9%
(Fig. 3). It showed significant
difference only in the subG0G1 phage
(P=0.003) and showed no significant change in the other phages. We
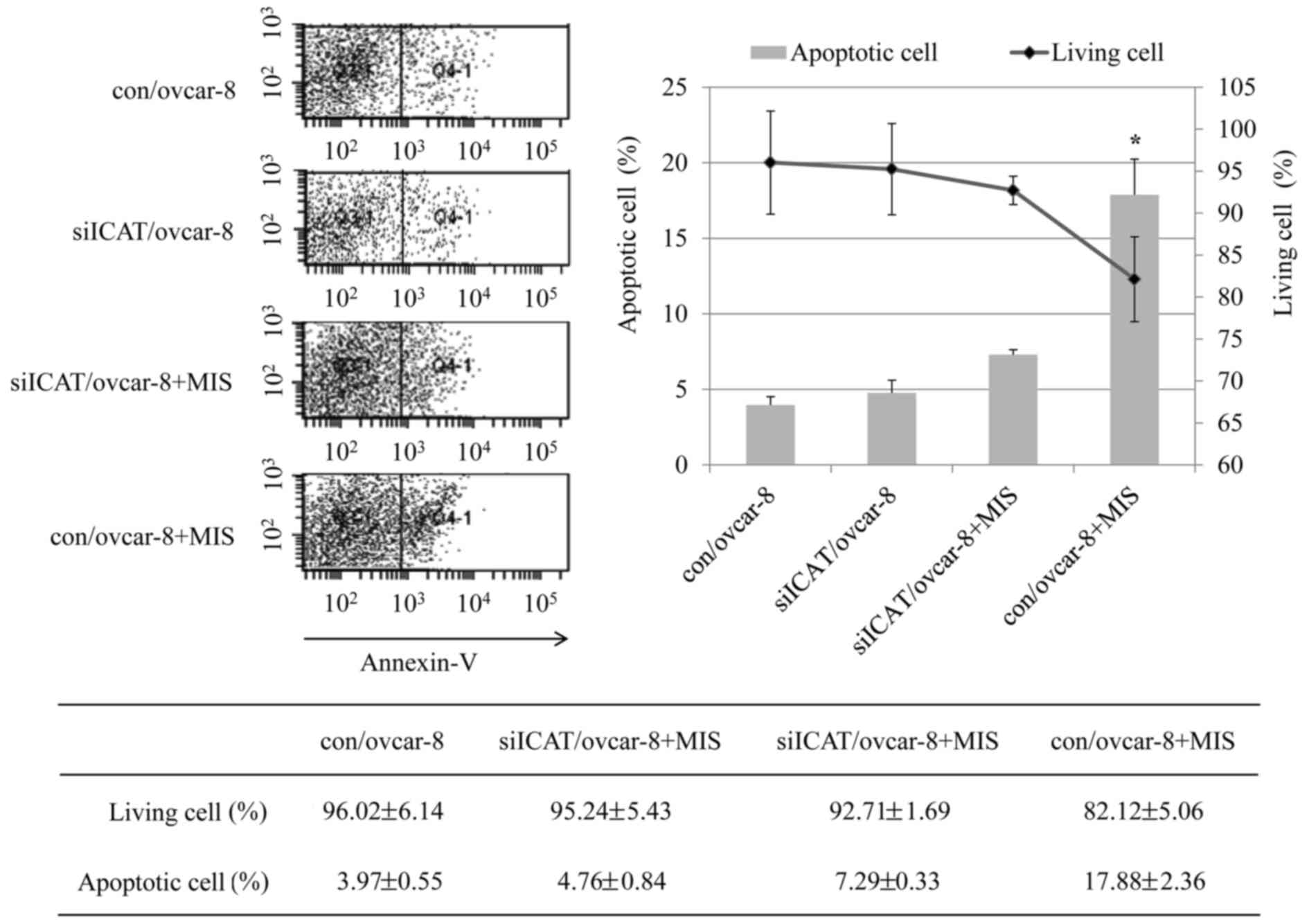
evaluated the occurrence of apoptosis by Annexin V/PI double
staining apoptosis detection kit. As shown in Fig. 4, the percentage of apoptotic cells
in the control group was 3.97% while the con/ovcar-8 cells treated
with MIS/AMH had an apoptotic rate of 17.88% and the siICAT/ovcar-8
cells treated with MIS/AMH showed 7.29% quantitative results
revealed a significant difference between con/ovcar-8 and
siICAT/ovcar-8 cells treated MIS/AMH cells.
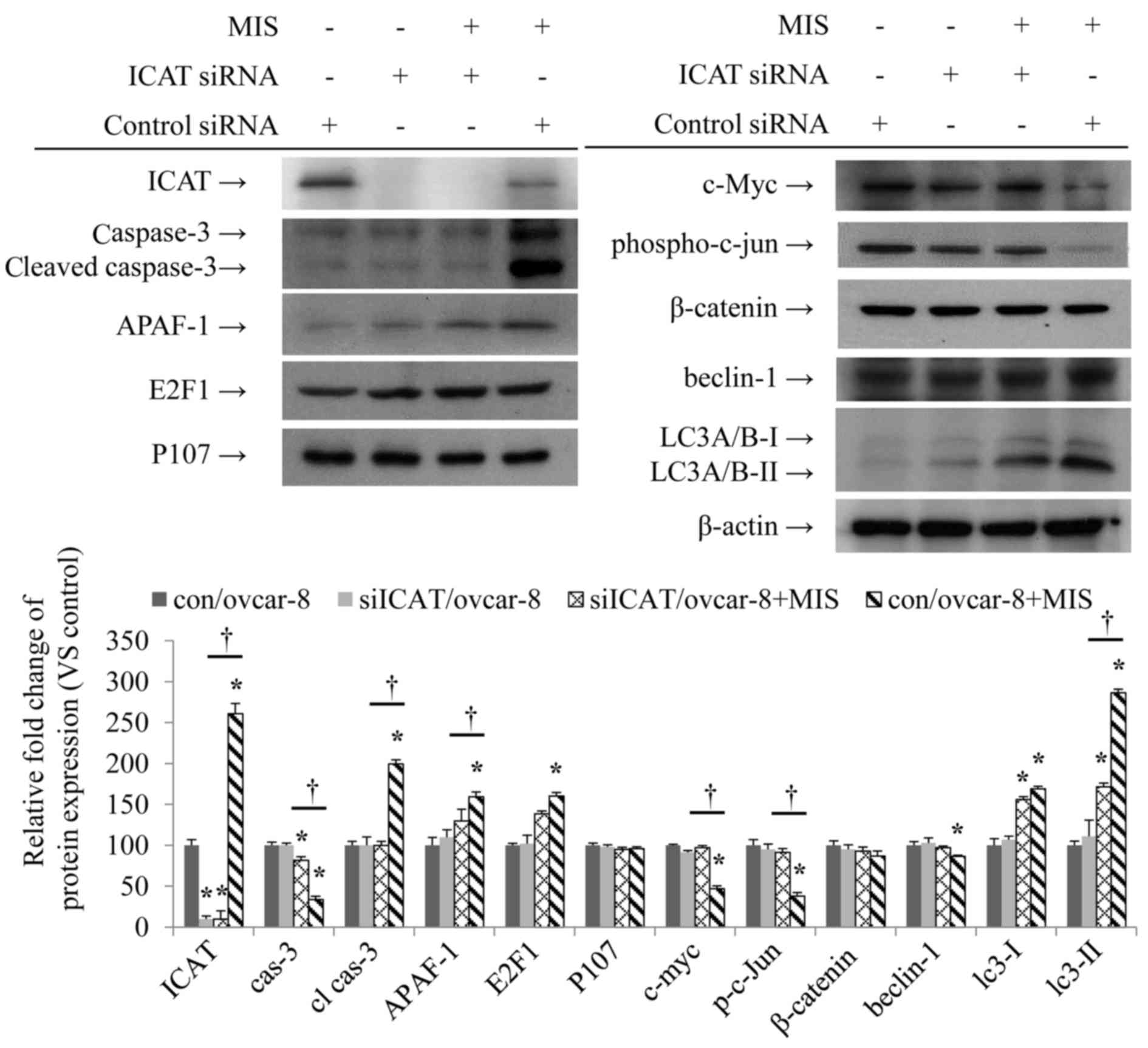
Verification of the related protein by
western blot analysis
To assess further the effect of ICAT on the MIS/AMH
treated ovcar-8 cells we performed western blots on the related
proteins (Fig. 5). The expression
of ICAT was increased after MIS/AMH treatment of con/ovcar-8 but
reversed by siICAT/ovcar-8 whether MIS/AMH treated or not.
Conversely, the decrease of apoptosis related protein pro-caspase-3
caused by MIS/AMH was reduced when siICAT/ovcar-8 was added. This
was accompanied by a concomitant decrease in cleaved caspase-3 and
APAF-1. In contrast there was no change in the MIS/AMH inducement
of E2F1. Similarly, p107 was uneffected by either treatment. The
Wnt signaling pathway related protein, c-myc and phospho-c-Jun
decrease by MIS/AMH treatment was unaffected by addition of
siICAT/ovcar-8. β-catenin was slightly decreased by MIS/AMH, but
ICAT siRNA increased to the control level again, this change was
not statistically significant. However, there was no effect on the
autophagy related protein, beclin-1 and ICAT siRNA had no effect on
the MIS/AMH induction of LC3-I but significantly decreased LC3-II
in ovcar-8 cells.
Discussion
A recent large-scale sequencing project (The Cancer
Genome Atlas) profiled genetic alteration in 20 malignancies and
identified signaling pathways. The Wnt signaling pathway was
revealed as one of the key signaling pathways affected by
tumorigenesis in three major gynecological cancers (21–23).
Wnt signaling regulates developmental processes and cell growth and
differentiation through β-catenin import into the nucleus where it
activates transcription of target genes including cyclin D1
and c-myc (24). Within the
Wnt signaling pathway, β-catenin and TCF/Lef-1 complex represent
primer targets for screening anticancer drugs as their deregulation
is common in cancers (25).
Expression of β-catenin and the TCF/Lef-1 complex was found to be
increased in ovarian cancer, compared to the normal ovary
suggesting a functional role for Wnt signaling in accelerating
tumorigenesis (26). β-catenin
interacting protein 1 (CTNNBIP1), also known as ICAT
(inhibitor of β-catenin and TCF4), functions as a crucial node to
mediate the cross-talk between E2F1 and β-catenin signaling. ICAT
is a direct transcriptional target of E2F1, and activation of
ICAT by E2F1 is required for E2F1 to inhibit β-catenin
activity (19,27). ICAT inhibits ovarian cancer cell
proliferation and invasion, by inducing cell apoptosis and arrests
cell cycle progression (28).
MIS/AMH inhibits cell growth and induces autophagy
in gynecological cancer cell lines (29). A recent study shows that
MIS/AMH-treated cells accumulated in the G1 phase of the
cell cycle and subsequently underwent apoptosis in human epithelial
ovarian cancer cells. Prolonged treatment with MIS/AMH
downregulated the Rb-related protein, p107 and increased the Rb
family-regulated transcription factor E2F1, overexpression of which
inhibited growth (13). During
MIS/AMH exposure, ICAT is upregulated by the proteins of E2F1 and
the outcome of death, usually depends on the balance between the
positive and negative apoptosis. Another study shows a number of
major pathways included metabolism, signal transduction, cell
growth and apoptosis in ovarian cancer cells (30). Among these pathways MIS/AMH is
mainly responsible for the suppressive effect on cell cycle by
regulating cyclin-dependent kinase (CDK) inhibitors and CDKs.
In the present study, we investigated whether
MIS/AMH may regulate the Wnt/β-catenin signaling pathway. The
importance of Wnt-mediated growth, migration and invasion also was
appreciated when cancer cell autophagy was observed after blocking
Wnt/β-catenin signaling pathway in breast and prostate cancer cells
(31,32) since autophagy is considered as a
key mechanism of cell death in ovarian, cervical and endometrial
cancers (33). In the present
study, we demonstrated that the ICAT is upregulated in ovarian
cancer cells when exposed to MIS/AMH where it reduces cell
viability and induces cell cycle arrest, apoptosis and autophagy.
ICAT downregulation by siRNA reversed the decrease in cell
viability, migration and apoptosis induced by MIS/AMH. ICAT siRNA,
however, had little effect on cell cycle or autophagy. The
β-catenin, which is key molecule in the Wnt signaling pathway, was
not significantly changed by treatment with MIS/AMH or ICAT siRNA.
However, it has been reported that MIS/AMH cause β-catenin to
accumulate in the cytoplasm (34).
In other words, according to the results demonstrated in this
experiment β-catenin complex is inhibited with the TCF/Lef-1 by
ICAT. β-catenin, which could not go to the nucleus, does not act as
a transcription factor and the expression of c-myc and c-jun is
reduced, making it difficult to avoid apoptosis and this is
confirmed by the increase of cleaved caspase-3 and APAF-1 by
MIS/AMH. MIS/AMH induced ICAT led to apoptosis, thus, implicating
the Wnt signaling pathway without affecting the cell cycle and
autophagy related proteins, p107, beclin-1 and LC3-I. As LC3-II was
significantly reduced by ICAT siRNA compared to MIS/AMH treated
cells, additional studies are required to explore the role of ICAT
on LC3-II. E2F1, an important transcriptional factor affecting cell
cycle, was increased by MIS/AMH in both E2F1 and ICAT. However, no
change in the treatment of ICAT siRNA could be found. Despite these
results, it is still difficult to apply the mechanism that controls
MIS/AMH to clinical practice. We are planning on creating ovarian
cancer cell lines from patient-derived ovarian cancer samples and
will proceed with research that further enhances the clinical
approach. In other words, the data suggest that clinical studies
should be evaluated in future to elucidate regulation of gene
expression for MIS/AMH in ovarian cancer cases.
In summary, ICAT may serve as a tumor-suppressor in
human gynecological cancer, suggesting it as a promising pathway
that could be activated to suppress gynecological cancer. MIS/AMH
inhibits the growth of ovarian cancer cell lines in vitro,
suggesting a key role for this hormone in the biology human
epithelial ovarian cancer. The present study implicates the Wnt
signaling pathway as part of the downstream pathway mediated by
MIS/AMH. The results of this study also suggest that MIS/AMH could
synergize with therapies developed to inactivate the Wnt pathway,
particularly in MIS/AMH receptor expressing cells such as ovarian
cancer.
Glossary
Abbreviations
Abbreviations:
|
MIS/AMH
|
Müllerian inhibiting
substance/anti-Müllerian hormone
|
|
MISRII
|
MIS type II receptor
|
|
ICAT
|
β-catenin interacting protein
|
|
siRNA
|
interfering RNAs
|
|
SRY
|
sex determining region of the Y
chromosome
|
|
CDK
|
cyclin dependent kinase
|
References
|
1
|
Miyamoto Y, Taniguchi H, Hamel F,
Silversides DW and Viger RS: A GATA4/WT1 cooperation regulates
transcription of genes required for mammalian sex determination and
differentiation. BMC Mol Biol. 9:442008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rey R, Lukas-Croisier C, Lasala C and
Bedecarrás P: AMH/MIS: What we know already about the gene, the
protein and its regulation. Mol Cell Endocrinol. 211:21–31. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jost A: Problems of fetal endocrinology:
The gonadal and hypophyseal hormones. Recent Prog Horm Res.
8:379–418. 1953.
|
|
4
|
Baarends WM, van Helmond MJ, Post M, van
der Schoot PJ, Hoogerbrugge JW, de Winter JP, Uilenbroek JT, Karels
B, Wilming LG, Meijers JH, et al: A novel member of the
trans-membrane serine/threonine kinase receptor family is
specifically expressed in the gonads and in mesenchymal cells
adjacent to the müllerian duct. Development. 120:189–197.
1994.PubMed/NCBI
|
|
5
|
Barbie TU, Barbie DA, MacLaughlin DT,
Maheswaran S and Donahoe PK: Mullerian inhibiting substance
inhibits cervical cancer cell growth via a pathway involving p130
and p107. Proc Natl Acad Sci USA. 100:15601–15606. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Donahoe PK, Fuller AF Jr, Scully RE, Guy
SR and Budzik GP: Mullerian inhibiting substance inhibits growth of
a human ovarian cancer in nude mice. Ann Surg. 194:472–480. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fuller AF Jr, Guy S, Budzik GP and Donahoe
PK: Mullerian inhibiting substance inhibits colony growth of a
human ovarian carcinoma cell line. J Clin Endocrinol Metab.
54:1051–1055. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ferlay J, Steliarova-Foucher E,
Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D and
Bray F: Cancer incidence and mortality patterns in Europe:
Estimates for 40 countries in 2012. Eur J Cancer. 49:1374–1403.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bakkum-Gamez JN, Aletti G, Lewis KA,
Keeney GL, Thomas BM, Navarro-Teulon I and Cliby WA: Müllerian
inhibiting substance type II receptor (MISIIR): A novel,
tissue-specific target expressed by gynecologic cancers. Gynecol
Oncol. 108:141–148. 2008. View Article : Google Scholar
|
|
10
|
Song JY, Chen KY, Kim SY, Kim MR, Ryu KS,
Cha JH, Kang CS, MacLaughlin DT and Kim JH: The expression of
Müllerian inhibiting substance/anti-Müllerian hormone type II
receptor protein and mRNA in benign, borderline and malignant
ovarian neoplasia. Int J Oncol. 34:1583–1591. 2009.PubMed/NCBI
|
|
11
|
Chung YJ, Kim HJ, Park SH, Yoon JH, Kim
MR, Nam SW, MacLaughlin DT, Donahoe PK and Kim JH: Transcriptome
analysis reveals that Müllerian inhibiting substance regulates
signaling pathways that contribute to endometrial carcinogenesis.
Int J Oncol. 46:2039–2046. 2015.PubMed/NCBI
|
|
12
|
Tanwar PS, Commandeur AE, Zhang L, Taketo
MM and Teixeira JM: The Müllerian inhibiting substance type 2
receptor suppresses tumorigenesis in testes with sustained
β-catenin signaling. Carcinogenesis. 33:2351–2361. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ha TU, Segev DL, Barbie D, Masiakos PT,
Tran TT, Dombkowski D, Glander M, Clarke TR, Lorenzo HK, Donahoe
PK, et al: Mullerian inhibiting substance inhibits ovarian cell
growth through an Rb-independent mechanism. J Biol Chem.
275:37101–37109. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Namkung J, Song JY, Jo HH, Kim MR, Lew YO,
Donahoe PK, MacLaughlin DT and Kim JH: Mullerian inhibiting
substance induces apoptosis of human endometrial stromal cells in
endometriosis. J Clin Endocrinol Metab. 97:3224–3230. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Anastas JN and Moon RT: WNT signalling
pathways as therapeutic targets in cancer. Nat Rev Cancer.
13:11–26. 2013. View
Article : Google Scholar
|
|
16
|
Madan B and Virshup DM: Targeting Wnts at
the source - new mechanisms, new biomarkers, new drugs. Mol Cancer
Ther. 14:1087–1094. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chien AJ, Conrad WH and Moon RT: A Wnt
survival guide: From flies to human disease. J Invest Dermatol.
129:1614–1627. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Masiakos PT, MacLaughlin DT, Maheswaran S,
Teixeira J, Fuller AF Jr, Shah PC, Kehas DJ, Kenneally MK,
Dombkowski DM, Ha TU, et al: Human ovarian cancer, cell lines, and
primary ascites cells express the human Mullerian inhibiting
substance (MIS) type II receptor, bind, and are responsive to MIS.
Clin Cancer Res. 5:3488–3499. 1999.PubMed/NCBI
|
|
19
|
Tago K, Nakamura T, Nishita M, Hyodo J,
Nagai S, Murata Y, Adachi S, Ohwada S, Morishita Y, Shibuya H, et
al: Inhibition of Wnt signaling by ICAT, a novel
beta-catenin-interacting protein. Genes Dev. 14:1741–1749.
2000.PubMed/NCBI
|
|
20
|
Lorenzo HK, Teixeira J, Pahlavan N,
Laurich VM, Donahoe PK and MacLaughlin DT: New approaches for
high-yield purification of Müllerian inhibiting substance improve
its bioactivity. J Chromatogr B Analyt Technol Biomed Life Sci.
766:89–98. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
McConechy MK, Ding J, Senz J, Yang W,
Melnyk N, Tone AA, Prentice LM, Wiegand KC, McAlpine JN, Shah SP,
et al: Ovarian and endometrial endometrioid carcinomas have
distinct CTNNB1 and PTEN mutation profiles. Mod Pathol. 27:128–134.
2014. View Article : Google Scholar :
|
|
22
|
Liu Y, Patel L, Mills GB, Lu KH, Sood AK,
Ding L, Kucherlapati R, Mardis ER, Levine DA, Shmulevich I, et al:
Clinical significance of CTNNB1 mutation and Wnt pathway activation
in endometrioid endometrial carcinoma. J Natl Cancer Inst.
106:1062014. View Article : Google Scholar
|
|
23
|
Ford CE, Henry C, Llamosas E, Djordjevic A
and Hacker N: Wnt signalling in gynaecological cancers: A future
target for personalised medicine? Gynecol Oncol. 140:345–351. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Duchartre Y, Kim YM and Kahn M: The Wnt
signaling pathway in cancer. Crit Rev Oncol Hematol. 99:141–149.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen HJ, Hsu LS, Shia YT, Lin MW and Lin
CM: The β-catenin/TCF complex as a novel target of resveratrol in
the Wnt/β-catenin signaling pathway. Biochem Pharmacol.
84:1143–1153. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rask K, Nilsson A, Brännström M, Carlsson
P, Hellberg P, Janson PO, Hedin L and Sundfeldt K: Wnt-signalling
pathway in ovarian epithelial tumours: Increased expression of
beta-catenin and GSK3beta. Br J Cancer. 89:1298–1304. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu Z, Zheng S, Li Z, Tan J and Yu Q: E2F1
suppresses Wnt/β-catenin activity through transactivation of
β-catenin interacting protein ICAT. Oncogene. 30:3979–3984. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ono M, Yin P, Navarro A, Moravek MB, Coon
JS V, Druschitz SA, Gottardi CJ and Bulun SE: Inhibition of
canonical WNT signaling attenuates human leiomyoma cell growth.
Fertil Steril. 101:1441–1449. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Borahay MA, Lu F, Ozpolat B, Tekedereli I,
Gurates B, Karipcin S and Kilic GS: Mullerian inhibiting substance
suppresses proliferation and induces apoptosis and autophagy in
endometriosis cells in vitro. ISRN Obstet Gynecol. 2013:3614892013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nam SW, Jo YS, Eun JW, Song JY, Ryu KS,
Lee JY, Lee JM, Maclaughlin DT and Kim JH: Identification of
large-scale characteristic genes of Müllerian inhibiting substance
in human ovarian cancer cells. Int J Mol Med. 23:589–596.
2009.PubMed/NCBI
|
|
31
|
Li P, Guo Y, Bledsoe G, Yang Z, Chao L and
Chao J: Kallistatin induces breast cancer cell apoptosis and
autophagy by modulating Wnt signaling and microRNA synthesis. Exp
Cell Res. 340:305–314. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lin R, Feng J, Dong S, Pan R, Zhuang H and
Ding Z: Regulation of autophagy of prostate cancer cells by
β-catenin signaling. Cell Physiol Biochem. 35:926–932. 2015.
View Article : Google Scholar
|
|
33
|
Orfanelli T, Jeong JM, Doulaveris G,
Holcomb K and Witkin SS: Involvement of autophagy in cervical,
endometrial and ovarian cancer. Int J Cancer. 135:519–528. 2014.
View Article : Google Scholar
|
|
34
|
Xavier F and Allard S: Anti-Müllerian
hormone, beta-catenin and Müllerian duct regression. Mol Cell
Endocrinol. 211:115–121. 2003. View Article : Google Scholar : PubMed/NCBI
|