Introduction
Colon cancer is one of the most common malignancies.
In the last decades, the diagnosis and treatment for colon cancer
have been improved greatly, especially with the screening at the
early stage (1,2). However, the prognosis is still far
from satisfactory and the incidence of colon cancer is increasing,
even though the targeted therapy agents have been introduced to
clinic (3). The challenges for the
treatment of colon cancer include the serious adverse effects of
the traditional chemotherapy and the metastasis to disseminate the
cancer cells to other organs or tissues. Therefore, there is still
a great clinical need to provide new effective reagents for
ameliorating the treatment of colon cancer.
The natural products or their derivate is one of the
important sources for anticancer drugs (4). Multiple drugs from this source have
been used for cancer treatment for a few decades, such as
camptothecin (5), paclitaxel
(6) and vincristine (7). Tetrandrine (Tet), an alkaloid of
bis-benzylisoquinoline, originates from the dried root of herbal
medicine Stephania tetrandra S. Moore (Chinese herb hang
fang ji) (8). Tet possesses
various pharmacological effects, so it can be used for
anti-inflammatory, immunosuppression and anti-hypertension
(9–11). Tet has essential anticancer effects
in prostate cancer, lung cancer, bladder cancer, gastric and colon
cancer (8,12–15).
It has been reported that several signaling pathways or critical
factors have been involved in this activity, such as inhibition of
PI3K/Akt, ERK and induction of p53 (16–18).
Our previous study indicated that Tet can inhibit Wnt/β-catenin
signaling in human colon cancer cells (14,19).
However, the detail molecular mechanism remains unclear.
The transforming growth factor β (TGF-β) is a
super-family, which plays an important role in regulating many
cellular physiological processes, such as proliferation,
differentiation, apoptosis and other functions (20). TGF-β functions as ligand binding to
the type II receptor, which recruits and phosphorylates the type I
receptor. The phosphorylated type I receptor then phosphorylates
the receptor-regulated SMADs (R-SMADs), and then the R-Smads binds
with the coSmad (SMAD4).
The R-Smad/coSmad complexes translocate to the
nucleus where they act as transcriptional factors to regulate the
expression of downstream targets (20). Therefore, the appropriate TGF-β
signaling is critical for the maintenance of homeostasis (21). Three sub-types of TGF-β are found
in this family, including TGF-β1, TGF-β2 and TGF-β3. In reference
to cancer, all the three isoforms of TGF-β are involved in cancer
progression (22–24). For colon cancer, the dysfunction or
loss of TGF-β signaling will promote progression of cancer
(25,26). Although TGF-β1 has been
demonstrated to be associated with colon cancer (27), its role in colon cancer remains
controversial. It can promote or suppress the growth of colon
cancer cells. These may be dependent on the microenvironment, or
have genetic defects (28).
Evidence indicated that Tet may be a powerful anticancer agent for
colon cancer treatment (14,19).
It has been reported that Tet can inhibit the transcription of
TGF-β1 to suppress the reproductive activity of cells (29). To date, it remains unclear whether
TGF-β1 is associated with the anticancer activity of Tet in colon
cancer.
In this investigation, we studied the effect of Tet
on TGF-β1 in HCT116 cells, to evaluate the effect of TGF-β1 on the
anticancer activity of Tet. We found that Tet can substantially
upregulate TGF-β1 in HCT116 cells, and TGF-β1 may mediate the
anti-proliferation activity of Tet through inactivating PI3K/Akt
signaling partly.
Materials and methods
Chemicals and drug preparations
Tetrandrine (Tet) was from Hao-xuan Bio-tech (Xi'an,
China). The HCT116 cells was purchased from American Type Culture
Collection (ATCC, Manassas, VA, USA). All primary antibodies were
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). LY294002 and LY364947 was from Targetmol Co., Ltd. (Shanghai,
China). Cells were maintained in Dulbecco's modified Eagle's medium
(DMEM) with 10% fetal bovine serum (FBS), 100 U/ml of penicillin
and 100 µg/ml of streptomycin at 37°C and 5%
CO2.
Recombinant adenoviral constructs for
TGF-β1, GFP and siRNA for PTEN
The recombinant adenovirual vectors were constructed
following the AdEasy system (30).
Briefly, the coding sequence (CDS) of human TGF-β1 and green
fluorescent protein (GFP) were amplified, the siRNA fragments for
PTEN knockdown was synthesized commercially. These fragments were
cloned into the shuttle vector pAdTrace, respectively. Then, the
shuttle vectors were linearized and transfected into HEK293 cells
for the package of the recombinant adenoviruses, which were
designated as AdTGF-β1 and AdsiPTEN. The recombinant adenoviruses
mediated the over-expression were tagged with green GFP or red
fluorescent protein (RFP) for knockdown to track the viruses, and
the recombinant adenovirus expressing GFP (AdGFP) only was used as
vehicle control.
Cell viability assay
The cell viability was determined with Cell Counting
Kit-8 (CCK-8). In brief, HCT116 cells were seeded in 96-well plates
with a density of 3×103 cells/well. Then, the cells were
treated with different concentrations of Tet, recombinant
adenovirus or DMSO for 24, 48 and 72 h. At the scheduled
time-point, 10 µl of CCK-8 was added into each well and
incubated for 4 h. The absorbance was determined at 450 nm with an
microplate reader. Each test was conducted in triplicate.
Clonogenic assay
The clonogenic assay was introduced to determine the
ability of cells to undergo unlimited division and form colonies in
a given population. Briefly, cells were pre-treated with different
concentrations of Tet for 24 h, and then re-plated the cells to
12-well plates with a density of 2000 or 200 cells per well. Cells
were maintained without Tet treatment up to 14 days until the
colonies were formed. Finally, the plates were gently washed with
PBS and incubated with 0.25% crystal violet formalin solution at
room temperature for 20 min, and finally washed with tap water and
air-dried. The assay was conducted in triplicate independently.
Flow cytometric analysis for cell cycle
and apoptosis
Cells were seeded in 6-well plates and treated with
different concentrations of Tet for 48 h. For cell cycle analysis,
cells were harvested and washed with phosphate buffered saline
(PBS, 4°C), fixed with cold (4°C) 70% ethanol, washed with 50% and
30% ethanol, and PBS. Finally, cells were stained with 1 ml of
propidium iodide (PI, 20 mg/ml) containing RNase (1 mg/ml) in PBS
for 30 min, followed by flow cytometry analysis. For apoptosis
analysis, cells were harvested and washed with PBS (4°C), followed
by incubating with Annexin V-EGFP (#KGA104, KeyGen Biotech,
Nanjing, China) and PI. Finally, the cells were analyzed with
fluorescence activated cell sorting (FACS). Each assay was done in
triplicate.
Reverse transcription (RT) and real-time
polymerase chain reaction (PCR) analysis
The cells were plated in T25 flask and treated with
different concentration of Tet. At the scheduled time-point, total
RNA were extracted with TRIzol reagent (Invitrogen), and followed
by RT reaction to generate cDNA. Then, the cDNA products were used
as templates for real-time PCR to detect the expression level of
target genes. The data of each sample were normalized with the
corresponding expression level of glyceraldehyde-3-phosphate
dehydrogenase (GAPDH). The primer sequences for this investigation
are presented in Table I.
 | Table IThe primers used for PCR assay. |
Table I
The primers used for PCR assay.
| Gene | Primer sequence
(5′-3′) |
|---|
| GAPDH | F:
CAACGAATTTGGCTACAGCA
R: AGGGGAGATTCAGTGTGGTG |
| PCNA | F:
GGCTCTAGCCTGACAAATGC
R: GCCTCCAACACCTTCTTGAG |
| TGF-β1 | F:
CCCACAACGAAATCTATGACAA
R: AAGATAACCACTCTGGCGAGTC |
| TGF-β2 | F:
ACTACGCCAAGGAGGTTTACAA
R: TCTGAACTCTGCTTTCACCAAA |
| TGF-β3 | F:
TGGTTAGAGGAAGGCTGAACTC
R: ATGAGCAAATCCAACCTCAGAT |
| PTEN | F:
TAAAGGCACAAGAGGCCCTA
R: CGCCACTGAACATTGGAATA |
Western blot assay
Sub-confluent HCT116 cells were seeded in 6-well
plates, and then treated with different concentrations of Tet
and/or combined with corresponding recombinant adenovirus. At the
scheduled time point, cell lysates were collected and boiled for 10
min. All samples were subjected to electrophoresis with SDS-PAGE
and transfered to polyvinylidene fluoride membranes, blotted with
primary antibodies and corresponding secondary antibodies
conjugated with horseradish peroxidase successively. Finally, the
target bands were developed with SuperSignal West Femto Substrate
(#34095, Thermo Scientific, Rockford, IL, USA). Each assay was done
in triplicate.
Immunocytochemical staining
Cells were plated into 48-well plates and treated as
the experimental design. At the scheduled time point, cells were
fixed with cold (4°C) methanol for 15 min, and washed with cold PBS
and permeablized with 0.5% Triton X-100. Cells were blocked with 5%
BSA at room temperature for 1 h, followed by incubation with
p-Akt1/2/3, or PTEN antibody, the corresponding IgG were used as
control, followed by incubation with rhodamine conjugated
corresponding secondary antibodies for 30 min. Finally, cells were
stained with DAPI (1 µg/ml). The fluorescence images were
recorded under an inverted microscope. Each assay was done in
triplicate.
Statistical analysis
Microsoft Excel was employed to calculate the
standard deviations. The differences were analyzed using the
Student's t-test.
Results
Effects of Tet on the proliferation of
HCT116 cells
Tet has been shown to have powerful anticancer
activity in various types of cancer cells. In this study, we tested
the function of Tet in human colon cancer cell line HCT116. The
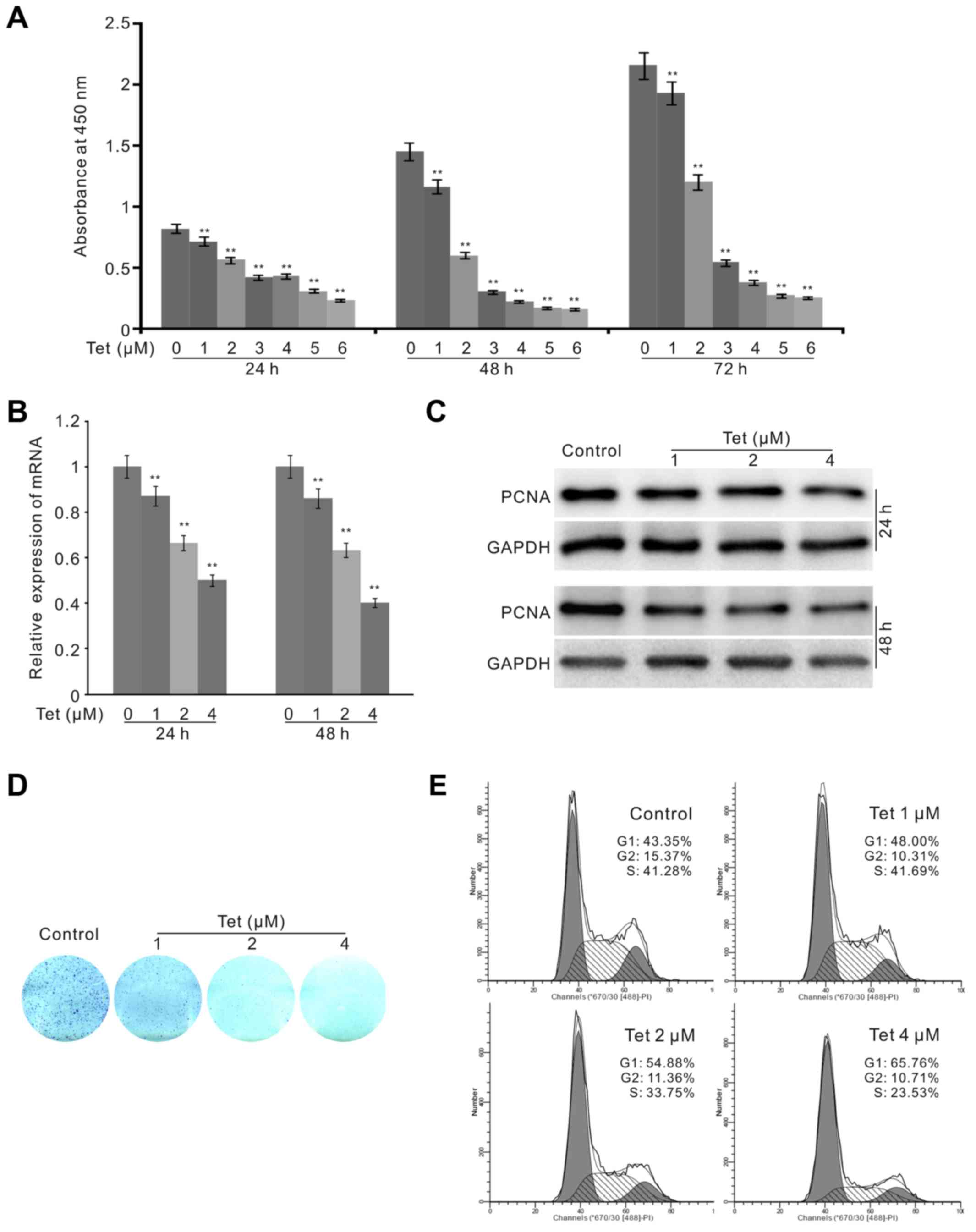
CCK-8 assay results revealed that Tet can inhibit the proliferation
of HCT116 cells concentration-dependently (Fig. 1A). The PCR assay results showed
that the expression of PCNA was decreased substantially by the
treatment of Tet (Fig. 1B), and
western blot analysis results recaptured the same effect of Tet on
PCNA in HCT116 cells (Fig. 1C).
The colony formation assays demonstrated that the proliferation
ability of HCT116 cells was greatly reduced by Tet (Fig. 1D). Finally, we introduced flow
cytometry analysis to determine the effect of Tet on the cell
cycle. The results showed that Tet arrested the cell cycle at G1
phase and obviously reduced the percentage of S phase in HCT116
cells (Fig. 1E). These data
suggested that Tet can effectively suppress the proliferation of
HCT116 cells.
Effects of Tet on apoptosis in HCT116
cells
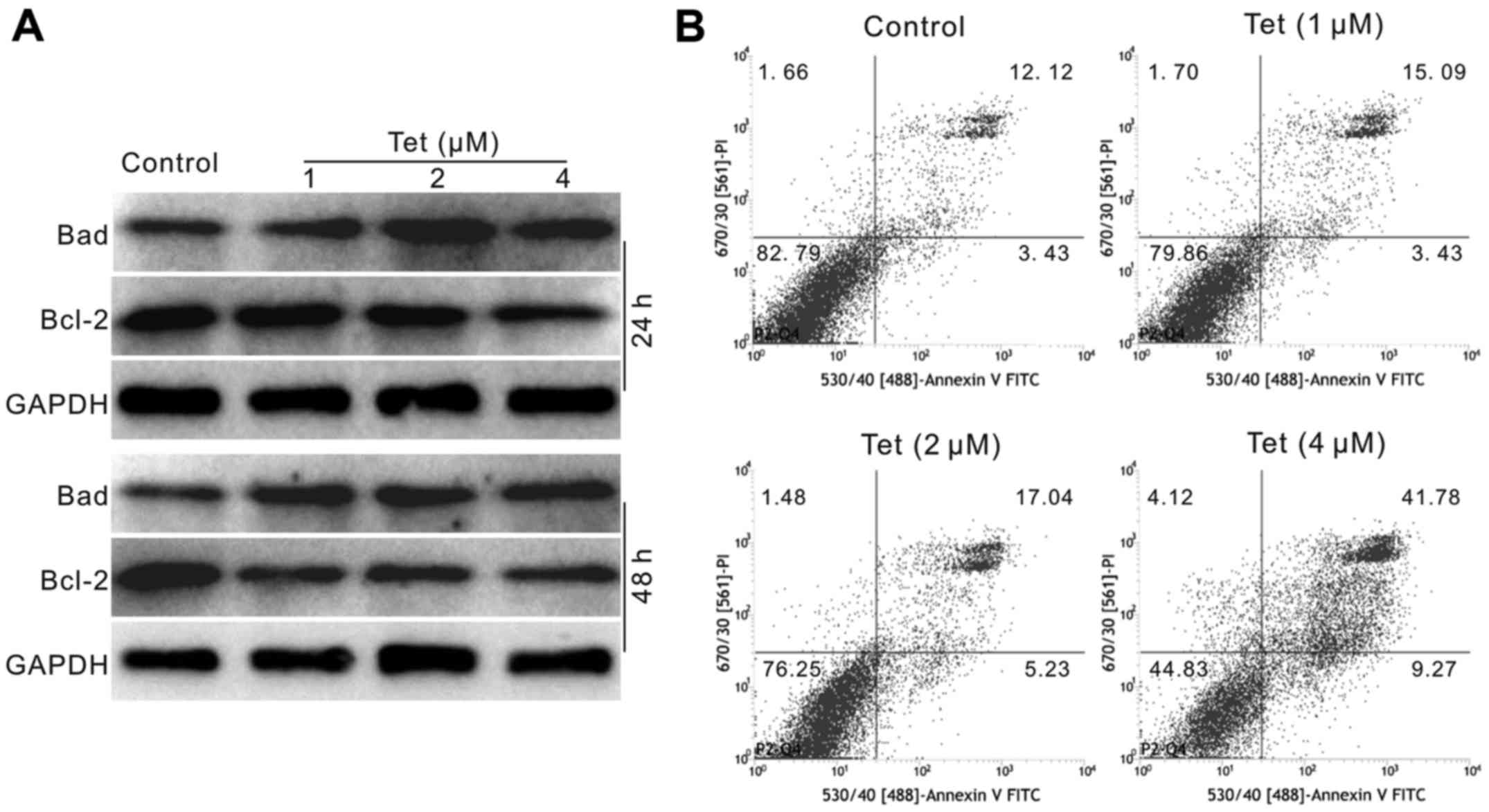
As apoptosis-inducing is one of the important
properties of drugs for chemotherapy to show the anticancer
activity, we next investigated the effect of Tet on the apoptosis
in HCT116 cells. Western blot assay results showed that Tet
decreases the level of Bcl-2, but increases the level of Bad in
HCT116 cells (Fig. 2A). The flow
cytometric analysis results showed that Tet increases the
percentage of apoptotic cells in HCT116 cells (Fig. 2B). These results suggested that Tet
may be an effective apoptosis inducer for cancer cells.
Effects of Tet on the expression of
TGF-β1 in HCT116 cells
The above data demonstrated that Tet can effectively
suppress the growth and induce apoptosis in HCT116 cells, but the
specific molecular mechanisms corresponding to these effects
remains unclear. TGF-β has been reported to be involved in the
cause of colon cancer (24,26).
Therefore, TGF-β may be one of the potential candidate targets for
colon cancer treatment, although its role in colon cancer remains
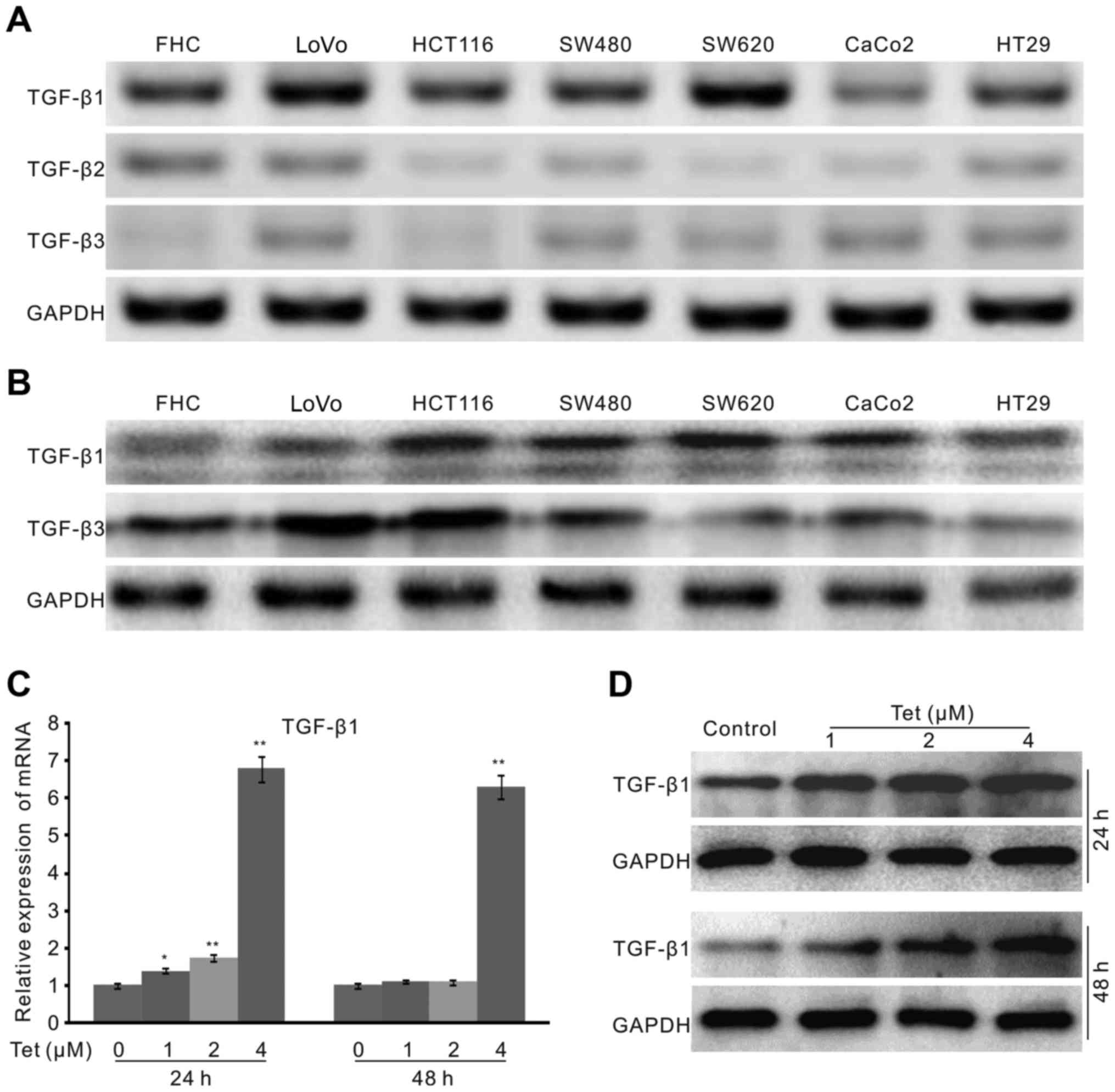
unclear. Hence, we detected the endogenous expression of the three
sub-types of TGF-β in the available colon cancer cell lines and FHC
cells. PCR results showed that the expression level of TGF-β1 is
higher than that of TGF-β2 and TGF-β3 in these cells (Fig. 3A). As reports showed that TGF-β1
and TGF-β3 were related with colon cancer (24,27),
we checked the endogenous protein level of these two types of
TGF-β, the results showed that both the protein of TGF-β1 and
TGF-β3 are detectable in these cells, and the level of TGF-β1 in
FHC cells is much lower than that of TGF-β3. However, the protein
level of TGF-β1 in cancer cells is much higher than that of FHC
cells (Fig. 3B). These data may
imply that TGF-β1 is more associated with colon cancer cells.
Hence, we focus on TGF-β1 in the following experiments. Firstly, we
checked the effect of Tet on the mRNA expression of TGF-β1 in
HCT116 cells. The results showed that Tet greatly increases the
expression of TGF-β1 (Fig. 3C).
Western blot assay confirmed that Tet increases the level of TGF-β1
obviously in HCT116 cells (Fig.
3D). These data implied that TGF-β1 may be involved in the
anticancer activity of Tet in HCT116 cells.
Effects of TGF-β1 on the
anti-proliferation and apoposis-inducing effect of Tet in HCT116
cells
As Tet greatly increases the level of TGF-β1 in
colon cancer cells and the endogenous level of TGF-β1 in colon
cancer cells is much higher than that of FHC cells, we next
investigated the effect of TGF-β1 on the anticancer activity of Tet
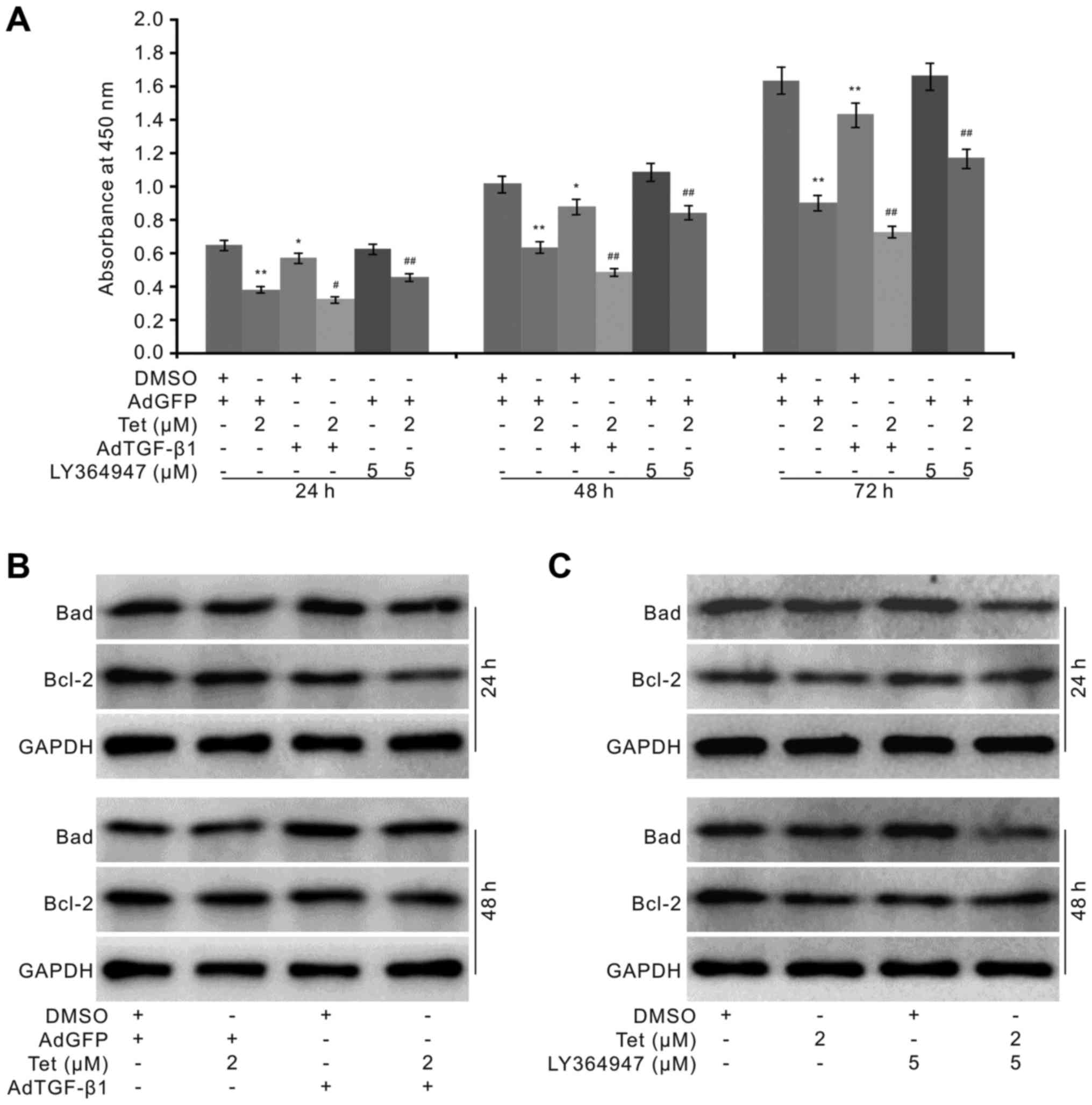
in HCT116 cells. We introduced the recombinant adenovirus to
mediate the exogenous expression of TGF-β1, and specific inhibitor
to block the TGF-β1 signaling. The results showed that the
exogenous TGF-β1 partly reduces the survival and apparently
potentiates the anti-proliferation effect Tet in HCT116 cells,
while the TGF-β1 specific inhibitor increases the proliferation and
partly reverses the anti-proliferation effect of Tet in HCT116
cells (Fig. 4A). Moreover, the
exogenous TGF-β1 increases the level of Tet-induced Bad and
decreases the level of Bcl-2 induced by Tet (Fig. 4B). The TGF-β1 inhibitor apparently
decreases the level of Tet-induced Bad, and increases the level of
Bcl-2 which was decreased by Tet in HCT116 cells (Fig. 4C). These results suggested that
TGF-β1 may play an important role in mediating the anticancer
activity of Tet in HCT116 cells, although the explicit mechanism
underlying this effect remains unknown.
Effects of TGF-β1 on the PI3K/Akt
signaling in HCT116 cells
TGF-β1 ligand binding with type II receptor activate
TGF-β signaling, but our pilot tests demonstrated that Tet can not
increase the phosphorylation of Smad2/3 (data are not shown).
Hence, TGF-β1 may mediate the anticancer effect of Tet through
non-canonical TGF-β signaling, such as PI3K/Akt pathway. PI3K/Akt
signaling is one of the essential pathways for the regulation of
cell survival and differentiation, and drugs have been developed
and targeted on this signaling to inhibit the proliferation of
cancer cells. It has been reported that Tet can induce cell cycle
arrest through inhibiting the PI3K/Akt pathway in HT29 cells
(31). However, it is unclear
whether the anti-proliferation effect of Tet in HCT116 cells can
also be mediated through downregulating this pathway. Hence, we
determined the effect of Tet on PI3K/Akt pathway in HCT116 cells.
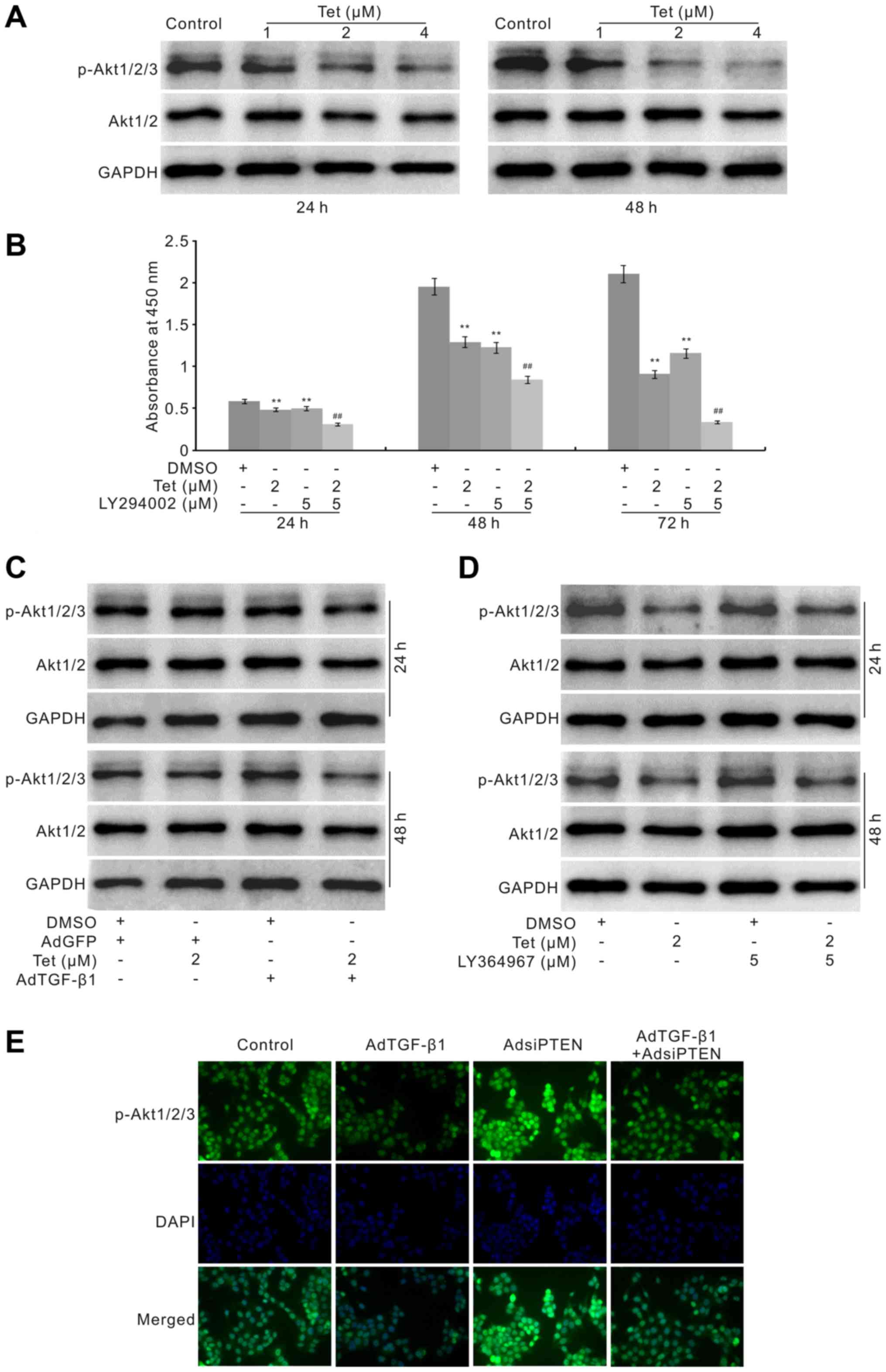
Western blot analysis showed that Tet markedly reduces the level of
phosphorylated Akt1/2/3 (p-Akt1/2/3), although no obvious effect
was seen on the total level of Akt1/2 (Fig. 5A). The CCK-8 assay results showed
that PI3K inhibitor markedly potentiates the anti-proliferation
effect of Tet in HCT116 cells (Fig.
5B). These data indicated that the anti-proliferation effect of
Tet in HCT116 cells may be mediated through the inactivation of
PI3K/Akt signaling, but how Tet suppresses this pathway remains
unclear. As Tet substantially increases the level of TGF-β1 in
HCT116 cells, we speculated that the inactivation of PI3K/Akt
signaling by Tet may be associated with the Tet-induced increase of
TGF-β1 in HCT116 cells.
Western blot assay showed that exogenous TGF-β1
promotes the effect of Tet by decreasing the level of p-Akt1/2/3
but no obvious effect on the level of total Akt1/2 (Fig. 5C). The TGF-β1 inhibitor increases
the level of p-Akt1/2/3 and partly reverses the Tet-induced
decrease of p-Akt1/2/3 (Fig. 5D).
In order to confirm the effect of TGF-β1 on the p-Akt1/2/3 in
HCT116 cells, we constructed the recombinant adenovirus for siRNA
fragments of PTEN, a well-known negative regulator for PI3K/Akt
signaling. The immunofluorescence staining results showed that
knockdown of PTEN greatly increases the level of p-Akt1/2/3, but
this effect can be thoroughly eliminated by the exogenous TGF-β1 in
HCT116 cells (Fig. 5E). All these
data suggested that the anti-proliferation effect of Tet in HCT116
cells may be mediated by inactivating PI3K/Akt signaling through
upregulating the expression of TGF-β1.
Effects of TGF-β1 on the PTEN affected by
Tet in HCT116 cells
PI3K/Akt signaling is finely regulated by various
factors, one of which is PTEN. PTEN dephosphorylates PIP3 to form
PIP2, which makes the inactivation of PI3K/Akt signaling. The above
mentioned data showed that Tet decreases the level of p-Akt1/2/3 in
HCT116 cells, which may be mediated by upregulating TGF-β1. As PTEN
is a critical negative regulator for PI3K/Akt signaling, it implied
that the effect TGF-β1 on PI3K/Akt signaling may result from the
upregulation of PTEN in HCT116 cells. With this hypothesis, we
determined the effect of TGF-β1 on PTEN and phosphorylated PTEN
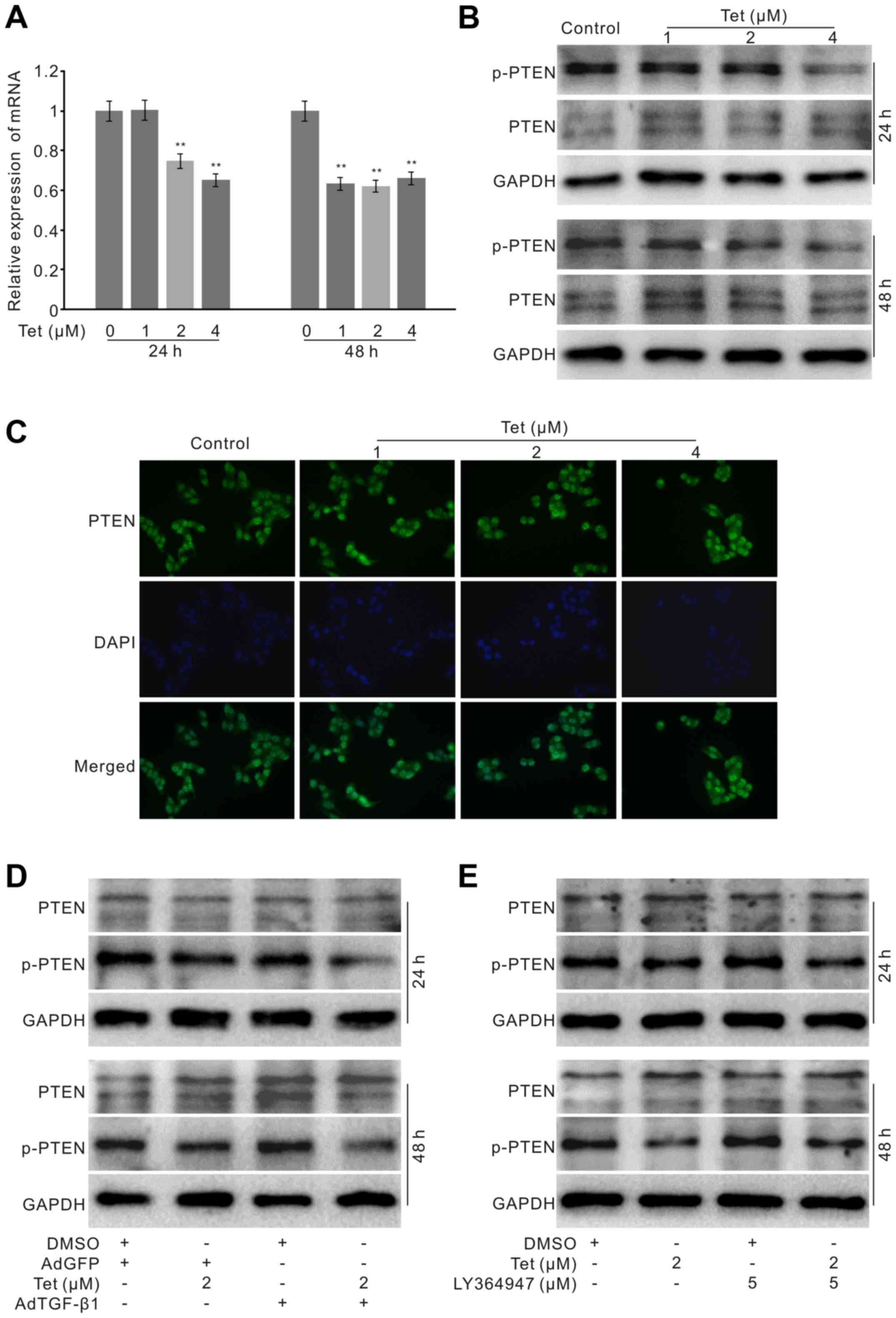
(p-PTEN). The PCR assay results showed that Tet suppresses the mRNA
expression of PTEN in HCT116 cells (Fig. 6A). However, western blot analysis
results showed that Tet exerts no substantial effect on the total
protein level of PTEN, but decreases the level of p-PTEN (Fig. 6B). The immunofluorescence staining
results confirmed that Tet exerts no obvious effect on the level of
PTEN in HCT116 cells (Fig. 6C).
Further analysis showed that exogenous TGF-β1 can partly decrease
the level of p-PTEN and synergistically reduces the level of p-PTEN
induced by Tet in HCT116 cells (Fig.
6D). However, the TGF-β1 inhibitor increases the level of
p-PTEN and partly reverses the Tet-induced decrease of p-PTEN
(Fig. 6E). These data indicated
that the upregulation of TGF-β1 may mediate the anticancer effect
of Tet in HCT116 cells through partly suppressing the
phosphorylation of PTEN.
Discussion
Colon cancer is one of the leading malignancies in
gastrointestinal system and the unsatisfactory prognosis need to be
greatly ameliorated. Tet has been demonstrated as a powerful
reagent with anticancer activity. We investigated the anticancer
activity of Tet in HCT116 cells and found that the effect of Tet in
colon cancer cells may be mediated by upregulating TGF-β1 to
inactivate the PI3K/Akt signaling through partly decreasing the
phosphorylation of PTEN.
The treatment of colon cancer includes chemotherapy,
and surgery. The prognosis remains undesirable even though the
targeted therapy has been introduced to the clinic (3). The challenge for treatment of cancer
includes the serious side effects of the traditional chemotherapy
drugs, metastasis and the drug resistance of cancer cells. There is
a great clinical need to explore new effective drugs for the
treatment of colon cancer. Natural products or their derivates is
one of the essential resources for anticancer drugs. With regard to
colon cancer, camptothecin or its deriveate irinotecan have been
clinically used for decades (32,33).
Tet is a plant-derived bisbenzylisoquinoline alkaloid, mostly
extracted from the root of medicine herb Stephania tetrandra
S Moore (8).
It is a well-know calcium blocker and possesses
multi-pharmacological activities, so Tet can be used widely for the
treatment of hypertension, asthma, tuberculosis, dysentery and
hyperglycemia (34). Increasing
evidence supports that Tet is also a potent anticancer reagent, it
exhibits efficacious anticancer activity to many kinds of cancer
cells, such as prostate cancer, lung cancer, bladder cancer,
gastric and colon cancer (8,12–15).
Our previous study also suggested that Tet may be a chemotherapy
and/or chemoprevention drug for colon cancer (14,19).
Consequently, Tet may be a promising anticancer candidate agent for
colon cancer treatment. In terms of the mechanism, various targets
have been reported to be related with the anticancer activity of
Tet, such as the inhibition of Wnt/β-catenin signaling, PI3K/Akt
signaling, ERK and inducing p53 (16–19).
So far, the specific molecular mechanism remains unclear, and
further thorough investigation need to be carried out for unveiling
this process.
TGF-β super-family includes secreted proteins, such
as TGF-β, bone morphogenetic proteins (BMPs), activins, and Nodal
(35). These proteins act as
ligand to bind with TGF-β type II receptor, and followed by the
activation of TGF-β type I receptor. Finally, it interacts with
different Smads to regulate multiple downstream targets, which are
involved in a plethora of physiological processes. Therefore, the
aberrant TGF-β signaling has been referred to in many diseases
(36,37). For TGF-β, there are three isoforms,
including TGF-β1, TGF-β2 and TGF-β3, and they are all expressed in
mammalian cells. The role of TGF-β is still unclear in tumor
initiation and progression, as it can act as a tumor suppressor or
promoter (36). The dual role of
TGF-β may be context and stage of cancer-dependent. In epithelial
cells, TGF-β usually functions as a tumor suppressor though
inhibiting proliferation and inducing apoptosis; in other tissues,
TGF-β may promote the progression of cancer, such as increasing the
invasion and metastasis of tumor cells (38).
TGF-β may also suppress the tumor at the early stage
and act as a promoter at the late stage (39). In term of colon cancer, TGF-β1 has
been reported to increase the progression of colon cancer by
upregulating the expression of Human Cripto-1 (CR-1) (40), and promoting the migration of
cancer cells (41). It remains
unknown whether TGF-β1 can also exert anti-proliferation activity
in colon cancer cells. Our previous study demonstrated the
potential anti-proliferation effect of Tet in colon cancer
(14,19). It has been reported that Tet can
decrease the mRNA level of TGF-β1 in the nitrofen-induced
congenital diaphragmatic hernia (42), but its effect on the expression of
TGF-β1 in cancer cells remains unclear.
In this study, we found that the isoforms of TGF-β
all are detectable in the colon cancer and FHC cells. However, the
protein level of TGF-β1 in FHC cells is much lower than any of the
cancer cell lines. For TGF-β3, its level in FHC cells are higher
than that of other cancer cell lines. These data suggested that
TGF-β1 and TGF-β3 may function differently for colon cancer. With
PCR and western blot analysis we found that Tet can obviously
increase the expression of TGF-β1 in HCT116 cells. Hence, we
speculated that the anti-proliferation activity of Tet in HCT116
cells may be associated with the upregulation of TGF-β1. With
further analysis, we found that exogenous expression of TGF-β1
enhances the anti-proliferation effect of Tet, and TGF-β1 inhibitor
partly reverses the Tet-induced suppression of proliferation.
Exogenous expression of TGF-β1 enhances the effect of Tet on the
increase of Bad and reduces Bcl-2. On the contrary, TGF-β1
inhibitor decreases the effect of Tet on increasing the level of
Bad and partly reverses the Tet-induced decrease of Bcl-2 in HCT116
cells. Herein, our data suggested that the anti-proliferation
activity of Tet may be partly mediated by upregulating TGF-β1 in
HCT116 cells.
One of the important functions of TGF-β is to
regulate cell proliferation, apoptosis and differentiation through
the canonical TGF-β pathway, by binding with their receptor, or the
non-canonical TGF-β pathway, such as Wnt/β-catenin and PI3K/Akt
signaling. TGF-β prevents osteogenic differentiation by inhibiting
BMP and Wnt/β-catenin signaling (43). TGF-β has also been reported to
active PI3K/Akt signaling to promote the migration and invasion of
prostate cancer cells, and the differentiation of radial glia into
astrocyte (44,45). Our results demonstrated that Tet
can not increase the phosphorylation of Smad2/3, instead of
reducing it (data are not shown). Thus, TGF-β1 may mediate the
anti-proliferation effect of Tet through non-canonical TGF-β
signaling pathway. It was reported that Tet can inactivate PI3K/Akt
signaling to induce cell cycle arrest and apoptosis in HT29 cells
(31).
Our results indicated that Tet can also decrease the
phosphorylation of Akt1/2/3 (p-Akt1/2/3) in HCT116 cells, although
it did not exert any obvious effect on the total level of Akt1/2.
Thus, it is possible for Tet to inactivate PI3K/Akt signaling
through the upregulation of TGF-β1 in HCT116 cells. Further
analysis revealed that exogenous expression of TGF-β1 potentiates
the inhibitory effect of Tet on the level of p-Akt1/2/3, while
TGF-β1 inhibitor partly reverses the Tet-induced decrease of
p-Akt1/2/3 in HCT 116 cells. Our data also showed that exogenous
expression of TGF-β1 substantially diminishes the level of
p-Akt1/2/3 induced by knockdown of the phosphatase and tensin
homolog deleted on chromosome ten (PTEN) in HCT116 cells.
Therefore, the evidence suggested that Tet may inhibit the
phosphorylation of Akt1/2/3 through up regulating TGF-β1 in colon
cancer cells.
PTEN is a well-known tumor suppressor, as
phosphatase negatively regulates the phosphatidylinositol-3-kinase
(PI3K) signaling pathway by catalyzing phosphatidylinositol
3,4,5-triphosphate (PIP3) dephosphorylates to PI-4,5-bisphosphate
(PIP2). The PI3K/Akt signaling is critical for cell survival and
differentiation. Accordingly, the dysfunction of PTEN will make the
PI3K/Akt signaling over-activated and the cell growth out of
control. Therefore, it is also considered as the potential target
for cancer treatment (46).
Various factors may lead to the function loss of PTEN, such as
inherited mutations, epigenetic and/or transcriptional silence, and
post-transcriptional modification (47). In colon cancer, the dysfunction of
PTEN, may result from genetic and/or epigenetic changes, and is one
of the main causes for the initiation and progression of cancer
(48). PTEN may be used as a
prognostic and predictive factor for colon cancer, and a target for
the treatment of colon cancer. In this study, we demonstrated that
Tet decreases the phosphorylation of Akt1/2/3 in HCT116 cells from
upregulating TGF-β1. Thus, we speculated that this effect of TGF-β1
on the phosphorylation of Akt1/2/3 may result from the regulation
of PTEN. With PCR, we found that Tet decreases the mRNA expression
of PTEN rather than increasing it. However, western blot assay
results showed that Tet substantially decreases the phosphorylation
level of PTEN, although has no obvious effect on the total level of
PTEN. Exogenous expression of TGF-β1 increases the inhibitory
effect of Tet on the phosphorylation of PTEN (p-PTEN), but the
TGF-β1 inhibitor promotes the phosphorylation of PTEN and partly
reverses the Tet-induced decrease of p-PTEN. However, how TGF-β1
regulates the phorphorylation of PTEN need to be further
elucidated.
Taken together, our data strongly suggested that Tet
may be a potential candidate for colon cancer treatment. The
anticancer activity of Tet in HCT116 cells may be partly mediated
by upregulating the expression of TGF-β1, which may inactivate the
PI3K/Akt signaling through partly decreasing the phosphorylation of
PTEN.
Acknowledgments
We would like to thank Professor T.C. He of the
University of Chicago Medical Center (Chicago, IL, USA) for his
kind provision of the recombinant adenoviruses. This work was
supported by research grant from Chongqing Science and Technology
Commision (grant no. cstc2015jcyjA10046 to K.W.) and the National
Natural Science Foundation of China (grant no. NSFC 81372120 to
B.-C.H.).
References
|
1
|
Winder T and Lenz HJ: Molecular predictive
and prognostic markers in colon cancer. Cancer Treat Rev.
36:550–556. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Anderson JC and Shaw RD: Update on colon
cancer screening: Recent advances and observations in colorectal
cancer screening. Curr Gastroenterol Rep. 16:4032014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Karanikas M and Esebidis A: Increasing
incidence of colon cancer in patients <50 years old: A new
entity? Ann Transl Med. 4:1642016. View Article : Google Scholar :
|
|
4
|
Dinic J, Podolski-Renic A, Stankovic T,
Bankovic J and Pesic M: New approaches with natural product drugs
for overcoming multidrug resistance in cancer. Curr Pharm Des.
21:5589–5604. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chazin EL, Reis RR, Junior WT, Moor LF and
Vasconcelos TR: An overview on the development of new potentially
active camptothecin analogs against cancer. Mini Rev Med Chem.
14:953–962. 2014. View Article : Google Scholar
|
|
6
|
Baird RD, Tan DS and Kaye SB: Weekly
paclitaxel in the treatment of recurrent ovarian cancer. Nat Rev
Clin Oncol. 7:575–582. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xie X, Tang B, Zhou J, Gao Q and Zhang P:
Inhibition of the PI3K/Akt pathway increases the chemosensitivity
of gastric cancer to vincristine. Oncol Rep. 30:773–782.
2013.PubMed/NCBI
|
|
8
|
Qin R, Shen H, Cao Y, Fang Y, Li H, Chen Q
and Xu W: Tetrandrine induces mitochondria-mediated apoptosis in
human gastric cancer BGC-823 cells. PLoS One. 8:e764862013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin YC, Chang CW and Wu CR:
Anti-nociceptive, anti-inflammatory and toxicological evaluation of
Fang-Ji-Huang-Qi-Tang in rodents. BMC Complement Altern Med.
15:102015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ho LJ, Juan TY, Chao P, Wu WL, Chang DM,
Chang SY and Lai JH: Plant alkaloid tetrandrine downregulates
IkappaBalpha kinases-IkappaBalpha-NF-kappaB signaling pathway in
human peripheral blood T cell. Br J Pharmacol. 143:919–927. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang J, Yu B, Zhang XQ, Sheng ZF, Li SJ,
Wang ZJ, Cui XY, Cui SY and Zhang YH: Tetrandrine, an
antihypertensive alkaloid, improves the sleep state of
spontaneously hypertensive rats (SHRs). J Ethnopharmacol.
151:729–732. 2014. View Article : Google Scholar
|
|
12
|
Kou B, Liu W, He W, Zhang Y, Zheng J, Yan
Y, Zhang Y, Xu S and Wang H: Tetrandrine suppresses metastatic
phenotype of prostate cancer cells by regulating Akt/mTOR/MMP-9
signaling pathway. Oncol Rep. 35:2880–2886. 2016.PubMed/NCBI
|
|
13
|
Lin Y, Wang Y and Liu X, Yan J, Su L and
Liu X: A novel derivative of tetrandrine (H1) induces endoplasmic
reticulum stress-mediated apoptosis and prosurvival autophagy in
human non-small cell lung cancer cells. Tumour Biol.
37:10403–10413. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu K, Zhou M, Wu QX, Yuan SX, Wang DX, Jin
JL, Huang J, Yang JQ, Sun WJ, Wan LH, et al: The role of IGFBP-5 in
mediating the anti-proliferation effect of tetrandrine in human
colon cancer cells. Int J Oncol. 46:1205–1213. 2015.
|
|
15
|
Zhang Y, Liu W, He W, Zhang Y, Deng X, Ma
Y, Zeng J and Kou B: Tetrandrine reverses epithelial-mesenchymal
transition in bladder cancer by downregulating Gli-1. Int J Oncol.
48:2035–2042. 2016.PubMed/NCBI
|
|
16
|
Wei N, Liu GT, Chen XG, Liu Q, Wang FP and
Sun H: H1, a derivative of Tetrandrine, exerts anti-MDR activity by
initiating intrinsic apoptosis pathway and inhibiting the
activation of Erk1/2 and Akt1/2. Biochem Pharmacol. 82:1593–1603.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dang Y, Xu Y, Wu W, Li W, Sun Y, Yang J,
Zhu Y and Zhang C: Tetrandrine suppresses
lipopolysaccharide-induced microglial activation by inhibiting
NF-κB and ERK signaling pathways in BV2 cells. PLoS One.
9:e1025222014. View Article : Google Scholar
|
|
18
|
Meng LH, Zhang H, Hayward L, Takemura H,
Shao RG and Pommier Y: Tetrandrine induces early G1 arrest in human
colon carcinoma cells by down-regulating the activity and inducing
the degradation of G1-S-specific cyclin-dependent kinases and by
inducing p53 and p21Cip1. Cancer Res. 64:9086–9092. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
He BC, Gao JL, Zhang BQ, Luo Q, Shi Q, Kim
SH, Huang E, Gao Y, Yang K, Wagner ER, et al: Tetrandrine inhibits
Wnt/β-catenin signaling and suppresses tumor growth of human
colorectal cancer. Mol Pharmacol. 79:211–219. 2011. View Article : Google Scholar :
|
|
20
|
Bellomo C, Caja L and Moustakas A:
Transforming growth factor β as regulator of cancer stemness and
metastasis. Br J Cancer. 115:761–769. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lampropoulos P, Zizi-Sermpetzoglou A,
Rizos S, Kostakis A, Nikiteas N and Papavassiliou AG: TGF-beta
signalling in colon carcinogenesis. Cancer Lett. 314:1–7. 2012.
View Article : Google Scholar
|
|
22
|
Zarzynska JM: Two faces of TGF-beta1 in
breast cancer. Mediators Inflamm. 2014:1417472014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ouhtit A, Madani S, Gupta I,
Shanmuganathan S, Abdraboh ME, Al-Riyami H, Al-Farsi YM and Raj MH:
TGF-β2: A novel target of CD44-promoted breast cancer invasion. J
Cancer. 4:566–572. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Laverty HG, Wakefield LM, Occleston NL,
O'Kane S and Ferguson MW: TGF-beta3 and cancer: A review. Cytokine
Growth Factor Rev. 20:305–317. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yu M, Trobridge P, Wang Y, Kanngurn S,
Morris SM, Knoblaugh S and Grady WM: Inactivation of TGF-β
signaling and loss of PTEN cooperate to induce colon cancer in
vivo. Oncogene. 33:1538–1547. 2014. View Article : Google Scholar
|
|
26
|
Slattery ML, Herrick JS, Lundgreen A and
Wolff RK: Genetic variation in the TGF-β signaling pathway and
colon and rectal cancer risk. Cancer Epidemiol Biomarkers Prev.
20:57–69. 2011. View Article : Google Scholar
|
|
27
|
Kim YH, Kim G, Kwon CI, Kim JW, Park PW
and Hahm KB: TWIST1 and SNAI1 as markers of poor prognosis in human
colorectal cancer are associated with the expression of ALDH1 and
TGF-β1. Oncol Rep. 31:1380–1388. 2014.PubMed/NCBI
|
|
28
|
Principe DR, DeCant B, Staudacher J,
Vitello D, Mangan RJ, Wayne EA, Mascariñas E, Diaz AM, Bauer J,
McKinney RD, et al: Loss of TGFβ signaling promotes colon cancer
progression and tumor-associated inflammation. Oncotarget. Jun
4–2016.Epub ahead of print.
|
|
29
|
Zunwen L, Shizhen Z, Dewu L, Yungui M and
Pu N: Effect of tetrandrine on the TGF-β-induced smad signal
transduction pathway in human hypertrophic scar fibroblasts in
vitro. Burns. 38:404–413. 2012. View Article : Google Scholar
|
|
30
|
Luo J, Deng ZL, Luo X, Tang N, Song WX,
Chen J, Sharff KA, Luu HH, Haydon RC, Kinzler KW, et al: A protocol
for rapid generation of recombinant adenoviruses using the AdEasy
system. Nat Protoc. 2:1236–1247. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen XL, Ren KH, He HW and Shao RG:
Involvement of PI3K/AKT/GSK3beta pathway in tetrandrine-induced G1
arrest and apoptosis. Cancer Biol Ther. 7:1073–1078. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Goldwasser F, Bae I, Fornace AJ Jr and
Pommier Y: Differential GADD45, p21CIP1/WAF1, MCL-1 and
topoisomerase II gene induction and secondary DNA fragmentation
after camptothecin-induced DNA damage in two mutant p53 human colon
cancer cell lines. Oncol Res. 8:317–323. 1996.PubMed/NCBI
|
|
33
|
Chen MC, Lee NH, Ho TJ, Hsu HH, Kuo CH,
Kuo WW, Lin YM, Tsai FJ, Tsai CH and Huang CY: Resistance to
irinotecan (CPT-11) activates epidermal growth factor
receptor/nuclear factor kappa B and increases cellular metastasis
and autophagy in LoVo colon cancer cells. Cancer Lett. 349:51–60.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bhagya N and Chandrashekar KR: Tetrandrine
- A molecule of wide bioactivity. Phytochemistry. 125:5–13. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Watabe T and Miyazono K: Roles of TGF-beta
family signaling in stem cell renewal and differentiation. Cell
Res. 19:103–115. 2009. View Article : Google Scholar
|
|
36
|
Neuzillet C, Tijeras-Raballand A, Cohen R,
Cros J, Faivre S, Raymond E and de Gramont A: Targeting the TGFβ
pathway for cancer therapy. Pharmacol Ther. 147:22–31. 2015.
View Article : Google Scholar
|
|
37
|
Fabregat I, Fernando J, Mainez J and
Sancho P: TGF-beta signaling in cancer treatment. Curr Pharm Des.
20:2934–2947. 2014. View Article : Google Scholar
|
|
38
|
Smith AL, Robin TP and Ford HL: Molecular
pathways: Targeting the TGF-β pathway for cancer therapy. Clin
Cancer Res. 18:4514–4521. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Morrison CD, Parvani JG and Schiemann WP:
The relevance of the TGF-β Paradox to EMT-MET programs. Cancer
Lett. 341:30–40. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mancino M, Strizzi L, Wechselberger C,
Watanabe K, Gonzales M, Hamada S, Normanno N, Salomon DS and Bianco
C: Regulation of human Cripto-1 gene expression by TGF-beta1 and
BMP-4 in embryonal and colon cancer cells. J Cell Physiol.
215:192–203. 2008. View Article : Google Scholar
|
|
41
|
Han S, Bui NT, Ho MT, Kim YM, Cho M and
Shin DB: Dexamethasone inhibits TGF-β1-induced cell migration by
regulating the ERK and AKT pathways in human colon cancer cells via
CYR61. Cancer Res Treat. 48:1141–1153. 2016. View Article : Google Scholar
|
|
42
|
Xu C, Liu W, Chen Z, Wang Y, Xiong Z and
Ji Y: Effect of prenatal tetrandrine administration on transforming
growth factor-beta1 level in the lung of nitrofen-induced
congenital diaphragmatic hernia rat model. J Pediatr Surg.
44:1611–1620. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Guerrero F, Herencia C, Almadén Y,
Martínez-Moreno JM, Montes de Oca A, Rodriguez-Ortiz ME,
Diaz-Tocados JM, Canalejo A, Florio M, López I, et al: TGF-β
prevents phosphate-induced osteogenesis through inhibition of BMP
and Wnt/β-catenin pathways. PLoS One. 9:e891792014. View Article : Google Scholar
|
|
44
|
Vo BT, Morton D Jr, Komaragiri S, Millena
AC, Leath C and Khan SA: TGF-β effects on prostate cancer cell
migration and invasion are mediated by PGE2 through activation of
PI3K/AKT/mTOR pathway. Endocrinology. 154:1768–1779. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Stipursky J, Francis D and Gomes FC:
Activation of MAPK/PI3K/SMAD pathways by TGF-β(1) controls
differentiation of radial glia into astrocytes in vitro. Dev
Neurosci. 34:68–81. 2012. View Article : Google Scholar
|
|
46
|
Ciuffreda L, Falcone I, Incani UC, Del
Curatolo A, Conciatori F, Matteoni S, Vari S, Vaccaro V, Cognetti F
and Milella M: PTEN expression and function in adult cancer stem
cells and prospects for therapeutic targeting. Adv Biol Regul.
56:66–80. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Dillon LM and Miller TW: Therapeutic
targeting of cancers with loss of PTEN function. Curr Drug Targets.
15:65–79. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Molinari F and Frattini M: Functions and
regulation of the PTEN gene in colorectal cancer. Front Oncol.
3:3262014. View Article : Google Scholar : PubMed/NCBI
|