Introduction
Bladder cancer is one of the most common
genitourinary cancers (1). More
than 90% of bladder cancer cases are diagnosed as bladder
urothelial carcinoma (2).
Approximately 75–85% of the patients harbored superficial bladder
cancer (3). Despite transurethral
resection of bladder tumor and intravesical therapy, 1–45% of cases
progress to invasive bladder cancer, known as muscle-invasive
bladder cancer, within 5 years (4). Up until now, radical cystectomy is
the mainstay therapy for muscle-invasive bladder cancer (5). However, the exact molecular
mechanisms of bladder tumor formation and progression are not yet
completely understood. Thus, genetic and molecular factors may both
play an important role in the progression of bladder cancer, which
might be an effective target for bladder cancer treatment in the
future.
The Rb gene is a recessive gene, located on
chromosome 13q14. It is the prototype of a tumor suppressor gene
and is well described (6,7). Although the gene was named for its
prominent role in the genesis of retinoblastoma, Rb-inactivation
seems to be crucial for the development of a variety of other
cancers and tumors, including liver cancer, breast cancer and lung
cancer (8). Inactivation of the Rb
gene, caused by mutations of the coding region or promoter region,
as well as the loss of heterozygosity have been reported, which was
an important factor, contributing to tumor or cancer progression
via p53 and E2F3 modulation (9).
However, whether Rb could be a therapeutic target for bladder
cancer for future is not clear. Hence, we investigated the role of
Rb in bladder cancer.
Autophagy is a process for major intracellular
degradation, occuring when the cells undergo stress conditions,
including exposure of radiation, nutrient starvation, or cytotoxic
compounds, and suffering from cancer, to enhance cell survival or
to result in the type II programmed cell death (10). Beclin1 and microtubule-associated
protein 1A/1B-light chain 3 (LC3), which are two hallmarks of
autophagy, modulating the initialization of mammalian autophagy
(11). Beclin1 plays an important
role involving in the signaling pathway for autophagy induction and
in the onset of the autophagosome formation (12). In addition, apoptosis (programmed
cell death), is instead an important physiological process, which
occurs in cells during development and normal cellular processes
(13). Apoptosis is induced by
several cellular signals which alter mitochondrial permeability,
leading to a cascade of events such as the release of apoptosis
activators from mitochondria (14). Rb has been reported before to be
associated with autophagy and apoptosis modulation.
Hence, in the study, Rb knockout mice were used to
investigate the role of Rb in urinary bladder cancer progression.
Our results indicated that Rb was directly involved in the progress
of bladder cancer via suppression of autophagy and apoptosis
through p53 and caspase-3 signaling pathways. Rb deficiency is able
to accelerate urinary bladder cancer development by inhibiting
autophagy and apoptosis and promoting cancer cell
proliferation.
Materials and methods
Animals
Thirty male, 6-week-old B6 (body weight, 20±20 g)
were purchased from Experimental Animal Center of Laboratory Animal
Center of Fudan University. The thirty male, 6-week-old B6 Rb
knockout mice (Rbtm3Tyj) (body weight, 20±20 g) were
obtained from The Jackson Laboratory (Bar Harbor, ME, USA). All the
mice were carefully maintained at room tempera ture on a 12-h
light-dark cycle, with free access to chow and water. This study
was approved by the Ethics Committee on Animal Research at the
Tangdu Hospital, The Fourth Military Medical University (Shaanxi,
China). The mice were randomly divided into 2 groups: i) the
Control-WT group; and ii) the Control-Rb−/−
(Rbtm3Tyj) group. BIU87 cells (1×107 cells)
were suspended in 100 µl serum-free medium and injected
subcutaneously into the left flank of the 6-week old male B6 mice.
Tumor size was measured with digital caliper and calculated. Tumor
volume were measured every seven days and at the end of ~7 weeks,
mice were sacrificed. Tumors were excised, weighed, fixed in 10%
neutral formalin, and embedded in paraffin for histological and
western blot analysis.
Cells culture
The bladder cancer BIU87 cells were obtained from
American Type Culture Collection (ATCC, Rockville, MD, USA). They
were cultured at the permissive temperature (37°C) in DMEM medium
(GibcoBRL Life Technologies, Grand Island, NY, USA) containing 10%
fetal bovine serum (FBS) and supplemented with 1%
penicillin-streptomycin-neomycin provided by GibcoBRL Life
Technologies with a humidified incubator in 5% CO2
atmosphere. Additional introduction of Rb or a control vector into
BIU87 cells were administered.
ELISA analysis
The levels of autophagy-related signals, including
Beclin1 and MAP1LC3B levels in serum from animals were determined
by ELISA, following the manufacturer's instructions (R&D
Systems, Inc., Minneapolis, MN, USA). Briefly, polyclonal mouse
anti-rabbit antibodies were used as capturing antibodies and
biotinylated polyclonal mouse anti-rabbit for detection, and the
standard curve of these signals was created. Color changes were
determined at 450 nm.
Colony formation assays
One hundred bladder cancer BIU87 cells after the
vector control or siRb treatment per well in 60-mm plates were
cultured in 10% FBS DMEM for 24 h. After another 7 days of
incubation, the cell colonies were washed twice with PBS, fixed
with 4% paraformaldehyde for 15 min and then stained by Giemsa for
30 min. Each clone with >50 cells was evaluated. Clone forming
efficiency for cells was calculated based on colonies/number of
inoculated cells × 100%.
Wound width assays
Wound-healing assays were carried out using
migration culture dish inserts. Bladder cancer cells of BIU87 after
the vector control or siRb treatment were seeded in the chambers of
the culture dish insert and transfected. Twenty-four hours after
transfection, the insert was removed and fresh culture medium was
added to start the migration process. Images were acquired after 0
and 24 h using a Zeiss Axiovert 24 light microscope and an Axiocam
MRc camera.
Transwell migration and invasion
assay
Bladder cancer cells after treatment were seeded
into the upper chamber of a Transwell insert pre-coated with 5
µg/ml fibronectin for migration or a BD™ Matrigel invasion
chamber for invasion. Medium with 10% serum was put in the lower
chamber as a chemo-attractant, and cells were then incubated for 4
h of migration. Non-migratory cells were removed from the upper
chamber by a cotton bud. The cells on the lower insert surface were
stained with Diff-Quick. Cells were evaluated as the number of
cells observed in five different microscopic fields of two
independent inserts. For invasion assay, 5×104 cells
were placed on the upper chamber of each insert coated with 150 mg
Matrigel. The lower chamber of Transwell was then filled with DMEM
medi um with 20% FBS. After incubation for invasion assays, the
upper surface of the membrane was wiped with a cotton tip and cells
attached to the lower surface were stained with crystal violet. The
invaded cells were captured and counted in five random fields.
Terminal deoxynucleotidyl
transferase-mediated dUTP nick end labeling (TUNEL) assays
Apoptosis assay of samples was determined by TUNEL
used an In Situ Cell Death Detection kit, Fluorescein (Roche
Applied Science, USA) according to the manufacturer's protocol. The
number of TUNEL-positive cells was counted under a fluorescence
microscope. The percentage of apoptotic cells was calculated.
Tissue sections were counter-stained with hematoxylin.
Western blot analysis
The bladder cancer cells and tumor tissue samples
were homogenized into 10% (wt/vol) hypo-tonic buffer (25 mM
Tris-HCl, pH 8.0, 1 mM EDTA, 5 µg/ml leupeptin, 1 mM
Pefabloc SC, 50 µg/ml aprotinin, 5 µg/ml soybean
trypsin inhibitor, 4 mM benzamidine) to yield a homogenate. Then
the final supernatants were obtained by centrifugation at 12,000
rpm for 20 min. Protein concentration was determined by BCA protein
assay kit (Thermo Fisher Scientific, USA) with bovine serum albumin
as a standard. The total protein extract will be used for western
blot analysis. Equal amounts of total protein of tissues were
subjected to 10 or 12% SDS-PAGE followed by immunoblotting using
the following primary polyclonal antibodies: rabbit anti-GAPDH
(Cell Signaling Technology), rabbit anti-Beclin1 (Cell Signaling
Technology), rabbit anti-PARP (Cell Signaling Technology), rabbit
anti-E2F3 (Abcam, USA), rabbit anti-Bax (Abcam), rabbit
anti-caspase-3 (Abcam), mouse anti-Bcl-2 (Cell Signaling
Technology), rabbit anti-P-Rb (Cell Signaling Technology), rabbit
anti-LC3-I (Cell Signaling Technology), rabbit anti-LC3-II (Cell
Signaling Technology), rabbit anti-Rb (Cell Signaling Technology),
mouse anti-Bak (Abcam), rabbit anti-mdm2 (Cell Signaling
Technology), mouse anti-Bid (Abcam), mouse anti-Apaf (Abcam),
rabbit anti-p53 (Cell Signaling Technology), rabbit anti-PTEN (Cell
Signaling Technology), rabbit anti-PI3K (Cell Signaling
Technology), rabbit anti-p-AKT (Cell Signaling Technology), rabbit
anti-AKT (Cell Signaling Technology), mouse anti-p21 (Abcam), mouse
anti-MAP1 (Abcam) and mouse anti-Cyto-c (Abcam).
Immunoreactive bands were visualized by ECL Immunoblot Detection
system (Pierce Biotechnology, Inc., Rockford, IL, USA) and exposed
to Kodak (Eastman Kodak Co., USA) X-ray film. Each protein
expression level was defined as grey value (Version 1.4.2b, Mac OS
X, ImageJ, National Institutes of Health, USA) and standardized to
housekeeping genes (GAPDH) and expressed as a fold of control.
Real-time RT-qPCR
Total RNA from bladder cancer cells and tumors were
isolated using TRIzol (Invitrogen, USA) following the
manufacturer's instructions. The cDNA was synthesized using
SuperScript II reverse transcriptase (Thermo Fisher Scientific).
Quantitative PCR was performed with SYBR Green Real-Time PCR Master
mix (Thermo Fisher Scientific). Finally, the quantitative
expression data were collected and analyzed by a 7900 Real-time PCR
system (Applied Biosystems, USA). Primers were designed to
determine endogenous genes showing as follows and GAPDH using as
the endogenous control. Bax forward, 5′-CAG ACG TAG CAA GAC GTT
AC-3′; reverse 5′-GTG AGA GTT GCT GGC TTG ATA-3′. Bak forward,
5′-CCA GAC TTC GTA TCT ACG AGG TCG-3′; reverse 5′-GCT CAA TGC ATA
GAG TAC TTT TAC-3′. Rb forward, 5′-AAC CCA GGA AGG AAT GGC T-3′;
reverse 5′-CTG CGT TCA GGT GAT TGA TG-3′. p53 forward, 5′-CTA CTG
CCT GCT TTG CGG CGT-3′; reverse, 5′-GAA GCG GCG TAG GTG CTG AG-3′.
Apaf forward, 5′-CGC CAC CGC CAT CTT CTC CA-3′; reverse, 5′-GCA CAA
GGC AGC CAG AAG GC-3′. Bid forward, 5′-AGG ATC GCG CTT AGC ATA CTT
G-3′; reverse 5′-AAC TGT TCA ATC TCT GTG CTC CGT-3′. GAPDH forward,
5′-CTA AGT CGA ACG CAG ACA GTC AG-3′; reverse, 5′-AAC ATA CCA TCC
ACG ACA CGC TC-3′.
Immunofluorescence assays
The tumor tissue in each group was fixed with 10%
buffered formalin, imbedded in paraffin and sliced into 4 µm
to 5 µm thick sections. Immunofluorescent assay of Cyto-c
and caspase-3 were performed according to the manufacturer's
instructions. After induction by conditioned culture medium, the
cells were fixed in 4% paraformaldehyde, permeabilized with 0.1%
Triton X-100 in PBS containing 0.5% BSA (PBS-BSA) for 30 min. The
cells were subsequently incubated with LC3-II, Beclin1 and MAP1 for
30 min, followed by labeling with Alexa Fluor 488- and
594-conjugated rabbit anti-mouse or goat anti-rabbit IgG antibody.
The cells were viewed under a fluorescent microscope.
Immunohistochemistry analysis
Human bladder or bladder cancer tissue samples and
animal model bladder cancer tissue specimans were fixed in
paraformaldehyde and embedded in paraffn. For hematoxylin and eosin
staining (H&E staining), the bladder tumor sections were
incubated in a hematoxylin solution for 15 min and then
counterstained with eosin for 5 min. After 3 µm thickness
sectioning, paraffn-embedded bladder cancer tissues were
immunostained with p21, p53, E2F3, caspase-3, Bcl-2 and Ki-67
antibodies. All of the slides were finally observed with ×200
magnification by a microscope.
Statistical analysis
Every experiment in our study was conducted at least
three times. All data present the mean ± SEM. from three
independent experiments. Student's t-test was used for statistical
analysis.
Results
Rb-knockout was involved in urinary
bladder tumor development
Rb is well known to be involved in many cellular
processes, including cell proliferation, apoptosis, invasion,
autophagy and migration (15).
Previous studies have confirmed that Rb suppression resulted in
tumor progression in different cancers, including liver cancer,
lung cancer and ovarian cancer (16). Thus, here we attempted to clarify
if Rb was involved in urinary bladder cancer progression. Rb
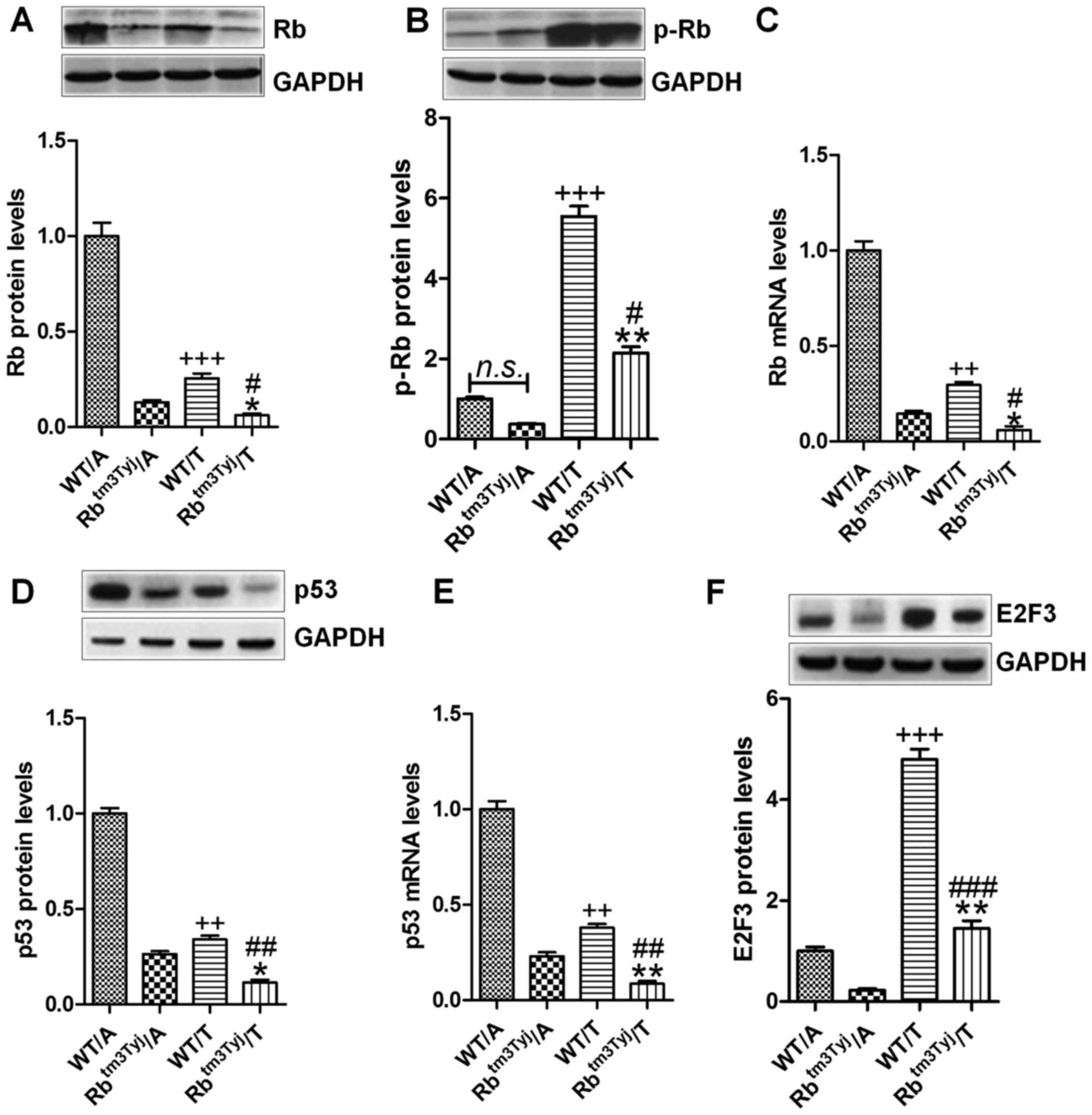
knockout mice were used in our study. Fig. 1A shows that, Rb protein levels were
highly expressed in the adjacent tumor tissues of the wild-type
(WT), while downregulated in the tumor tissue samples in normal
mice. In Rb mutant mice (Rbtm3Tyj), Rb was significantly
downregulated in the tumor tissue compared to the adjacent tissue
samples of Rbtm3Tyj mice. Of note, Rb levels were lower
in the tumor tissue samples compared to the wild-type of mice. In
contrast, phosphorylated Rb was expressed highly in tumor tissue
compared to the adjacent tissues in the wild-type mice. Similarly,
the highly phosphorylated Rb was observed in tumor tissue samples
of Rb-knockout mice (Fig. 1B).
Also, the mRNA levels showed similar trends at lower levels in the
tumor tissue of WT mice, while the least in tumor tissue from the
Rb knockout mice (Fig. 1C). p53,
as an important tumor suppressor, was well investigated in previous
studies, inhibiting cancer progression associated with Rb
alteration (17). p53 protein and
mRNA levels were lower levels in the tumor tissue of wild-type mice
compared to the adjacent parts of the mice (Fig. 1D and E). Also, in the Rb knockout
mice, p53 protein and mRNA levels were much lower in the tumor
tissue. E2F3 was investigated, and it was expressed highly in the
tumor tissue of wild-type mice, while being significantly
upregulated in Rb-knockout mice, suggesting that Rb deficiency
might enhance E2F3 expression, promoting urinary bladder cancer
progression (Fig. 1F).
Collectively, the data above suggested that Rb might be a key
target for urinary bladder cancer progression.
Rb-deficiency promotes urinary bladder
tumor growth in nude mice
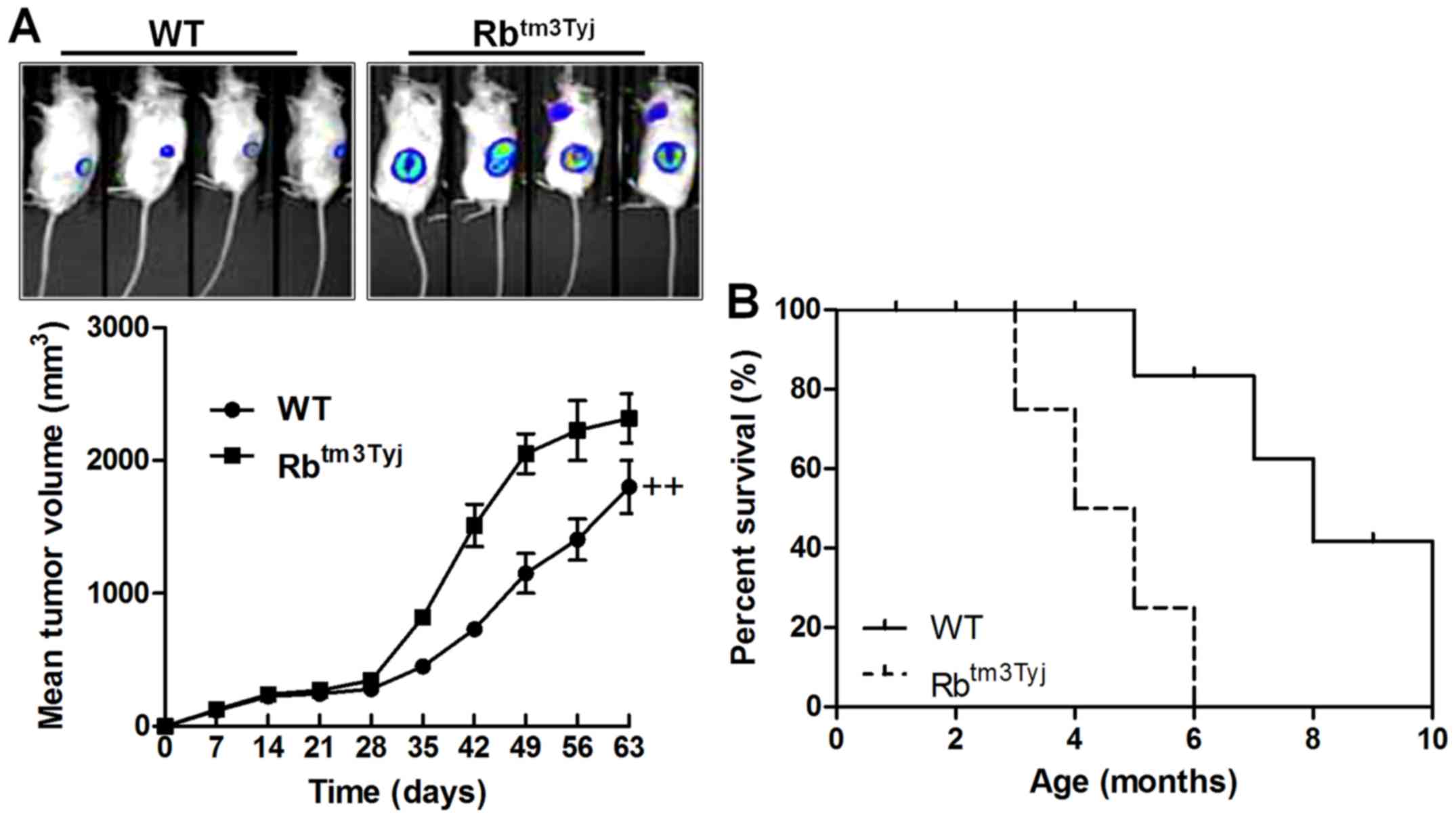
To confirm whether Rb could directly influence
urinary bladder cancer progression, we calculated the tumor size in
the wild-type mice and Rb-knockout mice. We found that the tumor
size was much higher than that in the WT group, suggetsing that Rb
was, at least partly, involved in the urinary bladder cancer
development (Fig. 2A). Also, the
survival rate indicated that Rb knockout promote the animal death,
further suggesting that Rb was of importance in urinary bladder
cancer progression (Fig. 2B).
Rb-deficiency-induced urinary tumor
growth is related to autophagy and apoptosis
Autophagy and apoptosis are two main molecular
mechanism, which regulate growth and progression of many tumors
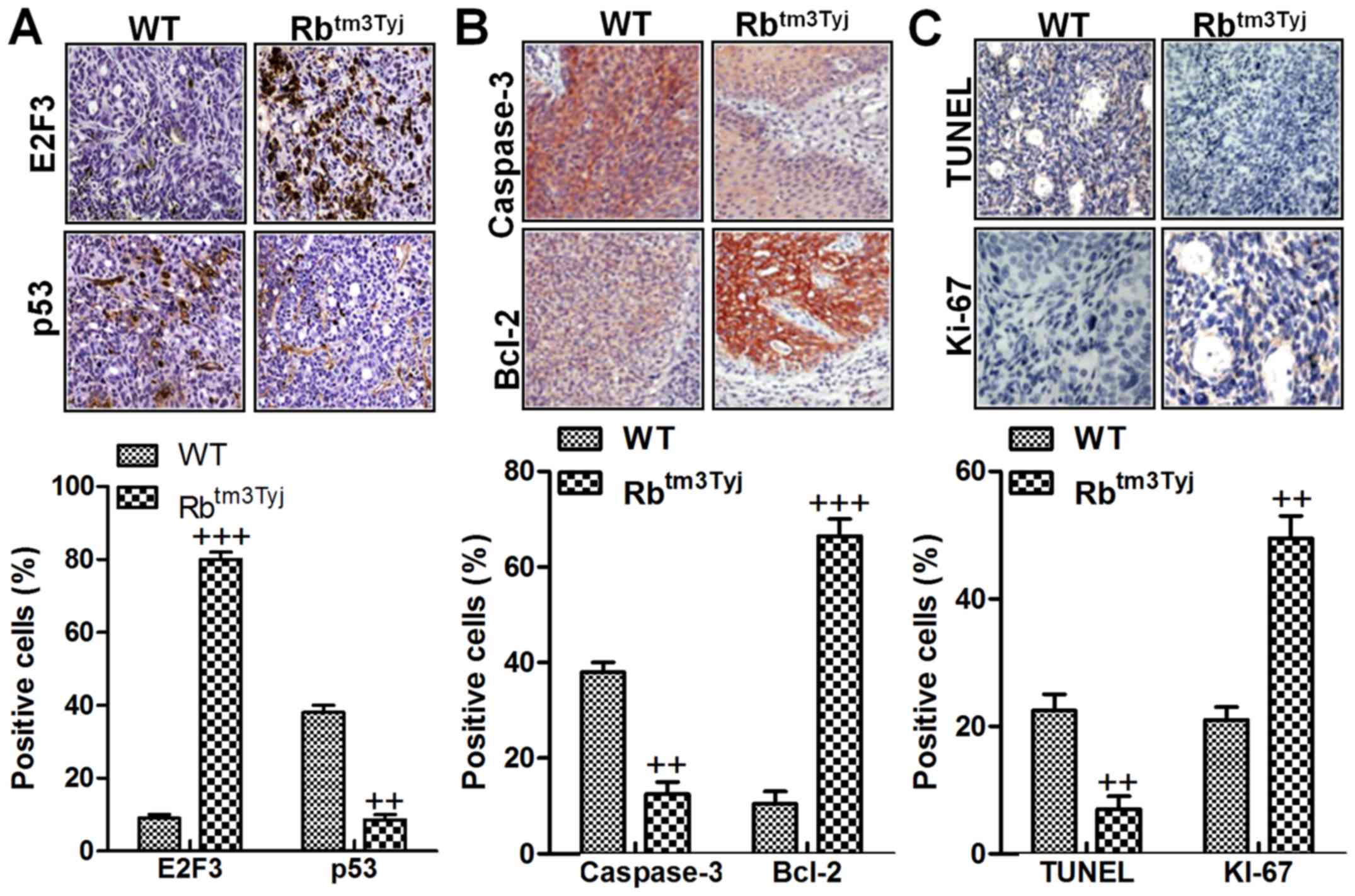
(18). Immunohistochemical
analysis suggested that E2F3 was highly expressed in Rb knockout
mice in comparison to the wild-type, while p53 was downregulated in
mice in the absence of Rb compared to the wild-type ones (Fig. 3A). Additionally, caspase-3 was
significantly reduced for Rb deficiency, inhibiting apoptotic
response in mice with urinary bladder cancer. In contrast, Bcl-2
was highly expressed in tumor mice without Rb expression (Fig. 3B). Bcl-2, as an important
anti-apoptotic factor, is always overexpressed in tumor tissue
samples (19). Finally, TUNEL and
Ki-67 were evaluated in both two tumor tissue samples from the
wild-type mice and Rb-deficient mice. As shown in Fig. 3C, TUNEL levels were downregulated
in Rb-deficient mice, indicating that apoptosis was suppressed for
Rb knockout. However, Ki-67, a factor in tumor progression, was
upregulated in tumor tissue samples without Rb expression. The
results indicated that Rb deficiency was the main reason
contributing to autophagy and apoptosis suppression and leading to
urinary bladder cancer development.
Apoptosis suppression was involved in
Re-deficiency mice with urinary bladder cancer
As shown above, we supposed that apoptosis was the
main contributor, regulating bladder cancer development for Rb
absence. Thus, we attempted to investigate which molecular mechnism
was included. Mitochondaria dysfunction-induced apoptosis is the
main protocol, resulting in cell death in many cellular progresses
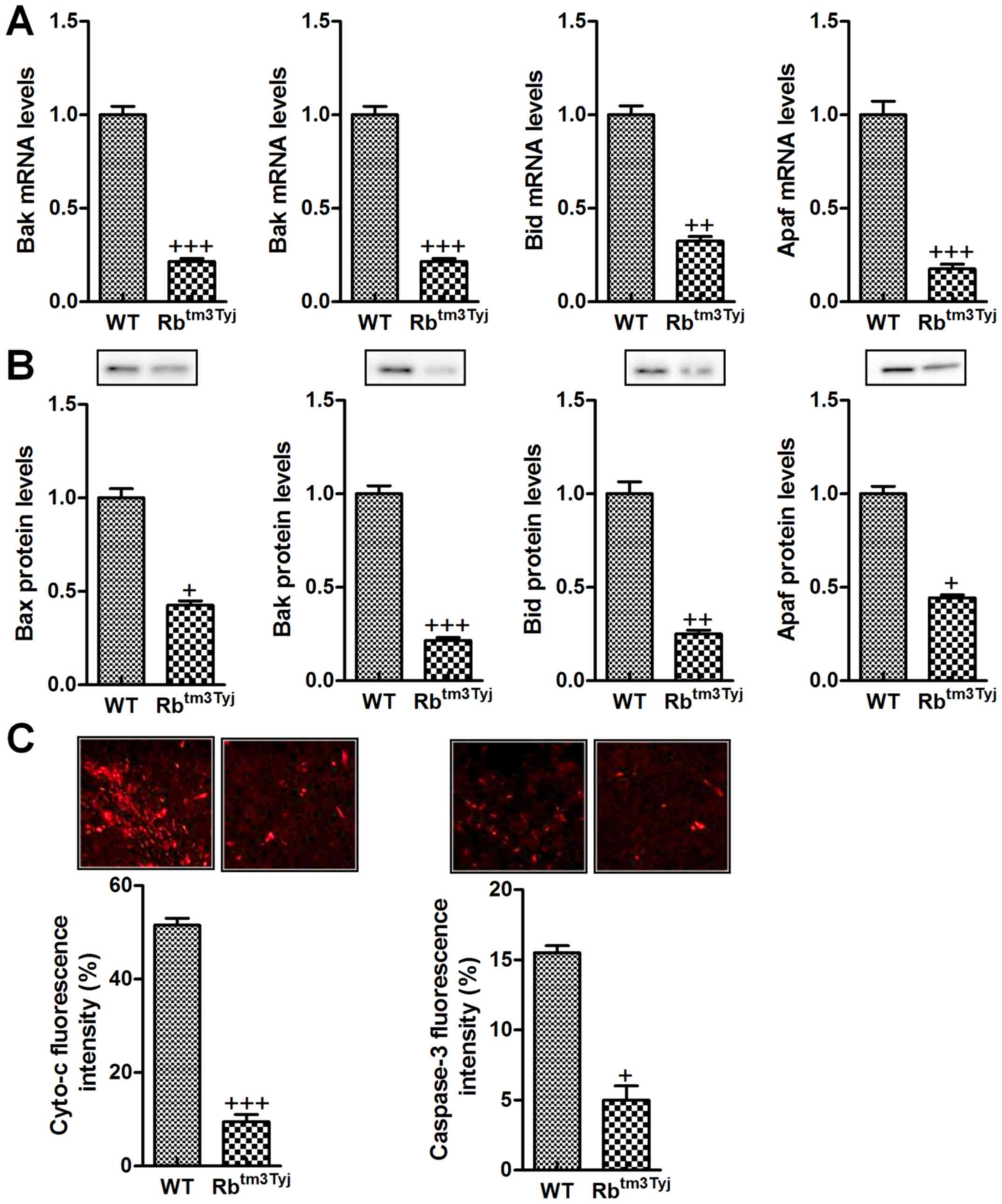
(20). Here, the protein and mRNA
levels of Bax, Bak, Bid and Apaf were found to be reduced in mice
in the absence of Rb, inhibiting apoptotic response in urinary
bladder cancer (Fig. 4A and B).
Furthermore, immunofluorescent analysis indicated that Cyto-c and
caspase-3 were both downregulated in Rb knockout mice, which are
two factors promoting apoptosis in cells and leading to cell death
in tumor treatment (Fig. 4C). The
data above indicated that Rb knockout resulted in urinary bladder
cancer progression was attributed to apoptosis suppression through
caspase-3 signaling pathway disruption.
Autophagy suppression is associated with
urinary bladder cancer progression in Rb-knockout mice
We found that autophagy was the main mechnism
regulating bladder cancer progression. Thus, in order to establish
how Rb modulated urinary bladder cancer progression, the signals
associated with autophagy development were investigated throuh
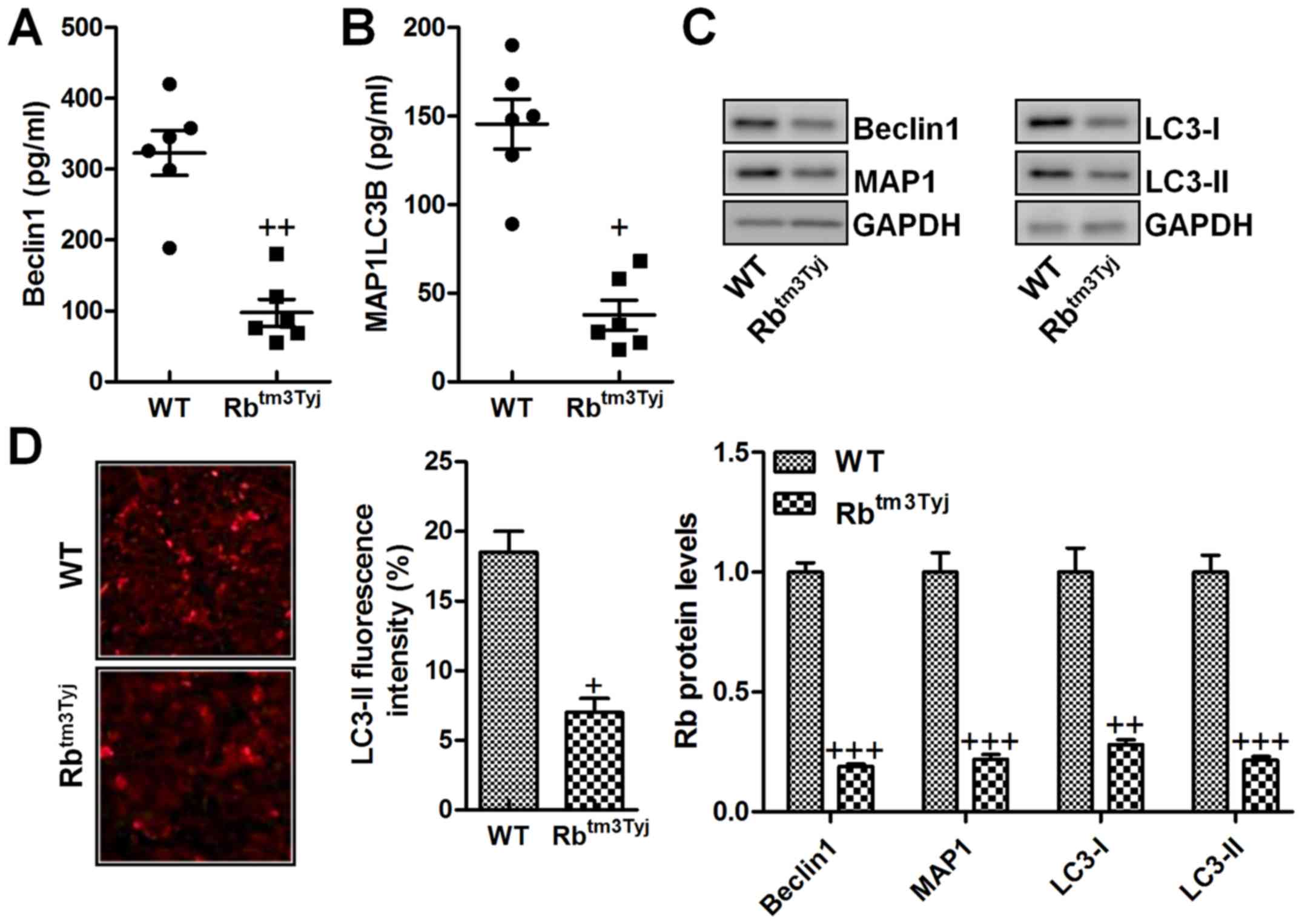
ELISA kits, which suggested that Beclin1 and MAP1LC3B were
significantly downregulated in the serum of mice without Rb
expression (Fig. 5A and B). As
shown in Fig. 5C, western blot
analysis further indicated that Beclin1 and MAP1 were reduced in Rb
knockout. In addition, LC3-I and LC3-II were both decreased in Rb
absence, which have been reported as significant autophagy
induction signals (Fig. 5C). Also,
our data here were in line with the results above that autophagy
was impeded in mice in Rb knockout. Moreover, LC3-II
immuno-fluorescent intensity was also observed with downregulation
in Rb-deficient mice (Fig. 5D).
The data above confirmed that autophagy-related signals were
involved in Rb-regulated urinary bladder cancer.
Rb knockout-induced urinary bladder tumor
progression is dependent on p53 inhibition
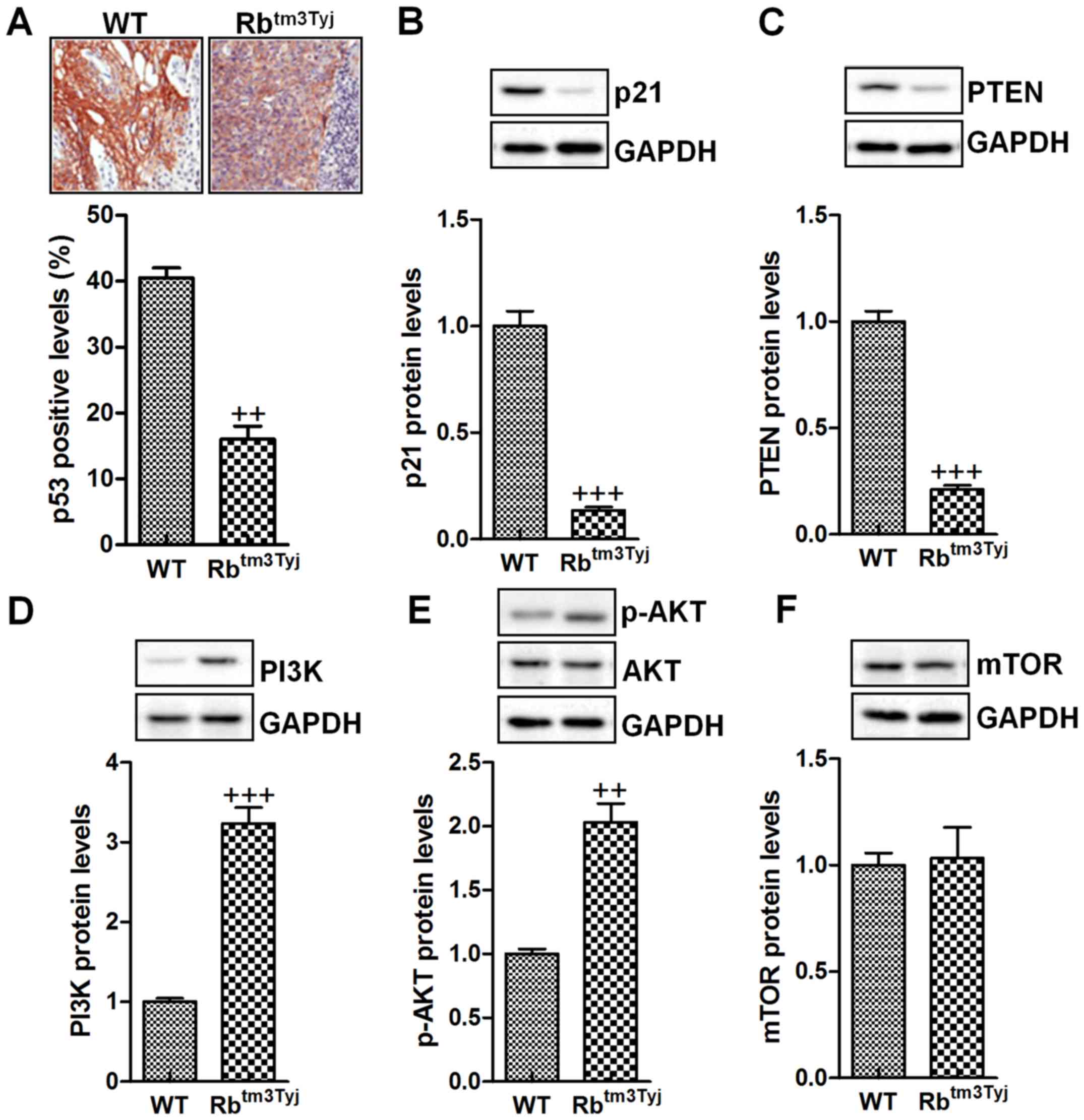
p53 has been considered as a crucial signal
regulating cell proliferation through its downstream signals,
including p21, PTEN and PI3K (21). Fig.
6A shows that p21 was significantly downregulated in Rb
knockout mice. Similarly, the protein levels of p21 were also found
to be downregulated compared to the mice in wild-type group
(Fig. 6B). At the same time, PTEN,
the downstreaming signal of p53, was expressed at low level in Rb
deficiency (Fig. 6C). In contrast,
Rb knockout resulted in PI3K activation, which is important for
tumor growth through AKT phosphorylation (Fig. 6D and E). mdm2 is an important
factor regulated by PI3K/AKT signaling pathway and modulate cell
proliferation (22). However, in
our study, we found that mdm2 was not significantly altered for Rb
deficiency, suggesting that Rb-regulated urinary bladder cancer was
not dependent on mdm2 (Fig. 6F).
Taken together, the results above indicated that p53 and its
related signaling pathway was closely related to Rb-regulated
urinary bladder cancer progression.
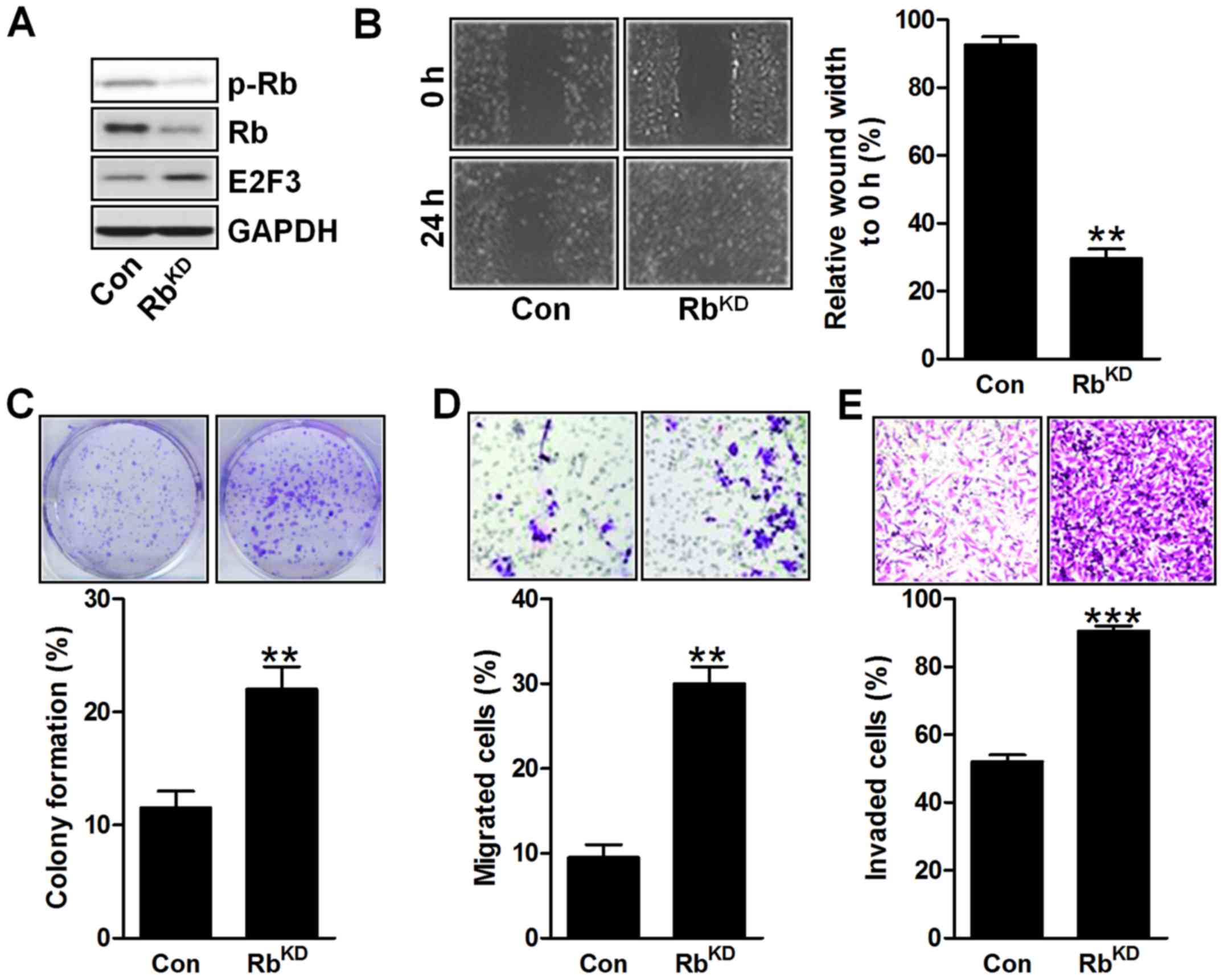
Rb knockdown leads to urinary bladder
cancer cell proliferation in vitro
To further confirm that Rb was a key in urinary
bladder tumor progression, the in vitro study was conducted.
In Fig. 7A, the Rb gene was
silenced in BIU-87 cells, which indicated that Rb was successfully
knocked down, causing its downregulation of phosphorylated Rb and
E2F3 expression in bladder cancer cells. In addition, the results
of wound width to 0 h indicated that the BIU-87 cell proliferation
was enhanced for Rb silence with decreased wound width (Fig. 7B). As shown in Fig. 7C, the colony formation was highly
upregulated in BIU-87 cells for Rb knockdown. Furthermore, much
more migrated and invaded cells of BIU-87 were observed for Rb
silence, which suggested that Rb suppression could result in
urinary bladder cancer cell proliferation (Fig. 7D and E). The data above illustarted
that Rb downregulation indeed caused urinary bladder cancer
development in vitro, which was in line with the in
vivo results as mentioned.
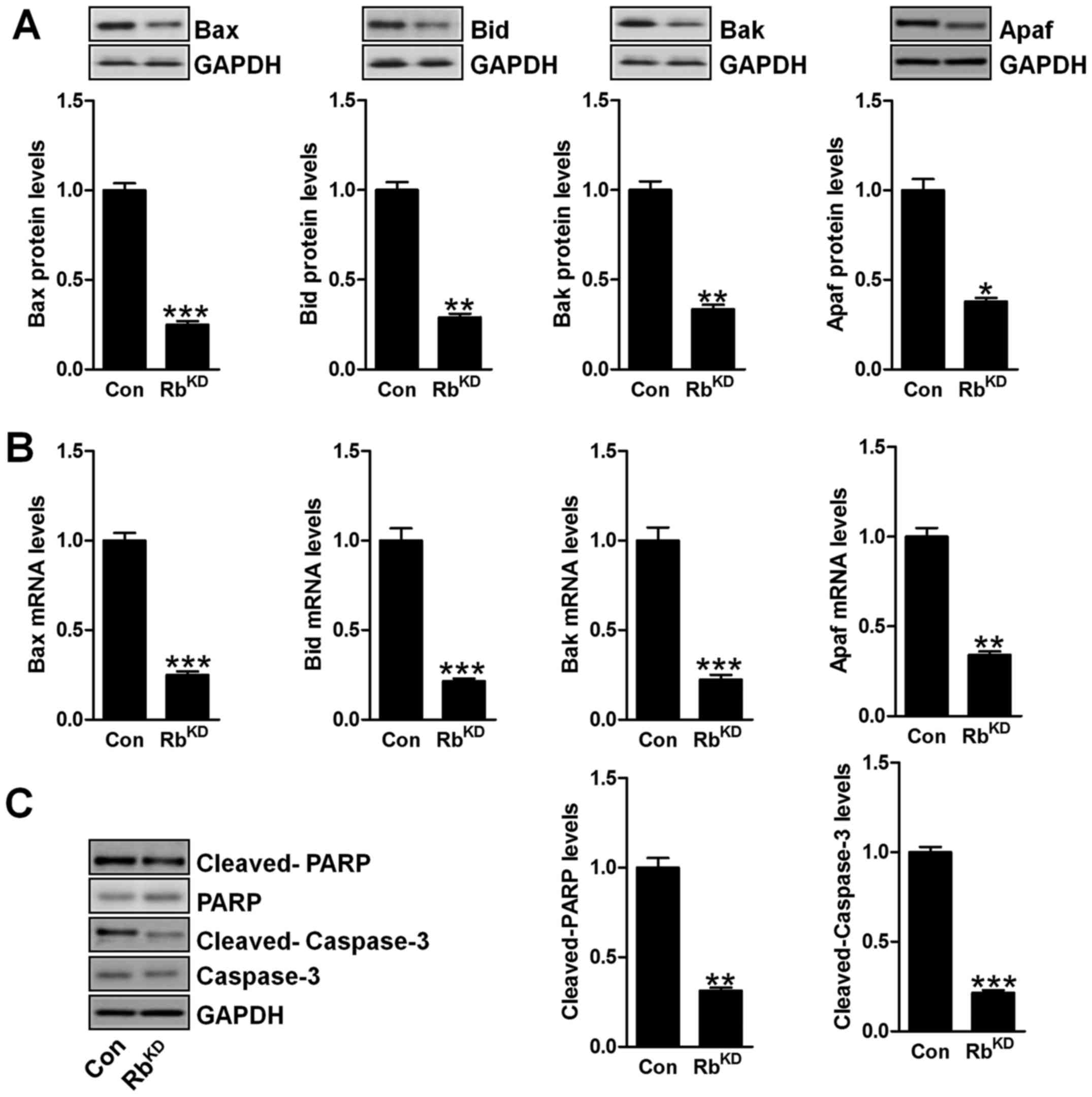
Downregulation of Rb induces apoptosis
and autophagy suppression in vitro
Since the data in vivo implied that apoptosis
inhibition was the main mechnism for bladder cancer progression,
here we further studied the molecular mechnism by which the urinary
bladder cancer was modulated for Rb. As shown in Fig. 8A and B, we found that the protein
and mRNA levels of pro-apoptotic factors, including Bax, Bak, Bid
and Apaf, were significantly downregulated in BIU-87 cells due to
Rb silence. Additionally, the cleaved PARP and caspase-3 were also
discovered with lower levels than the protein levels (Fig. 8C), which was in agreement with the
data in vivo above.
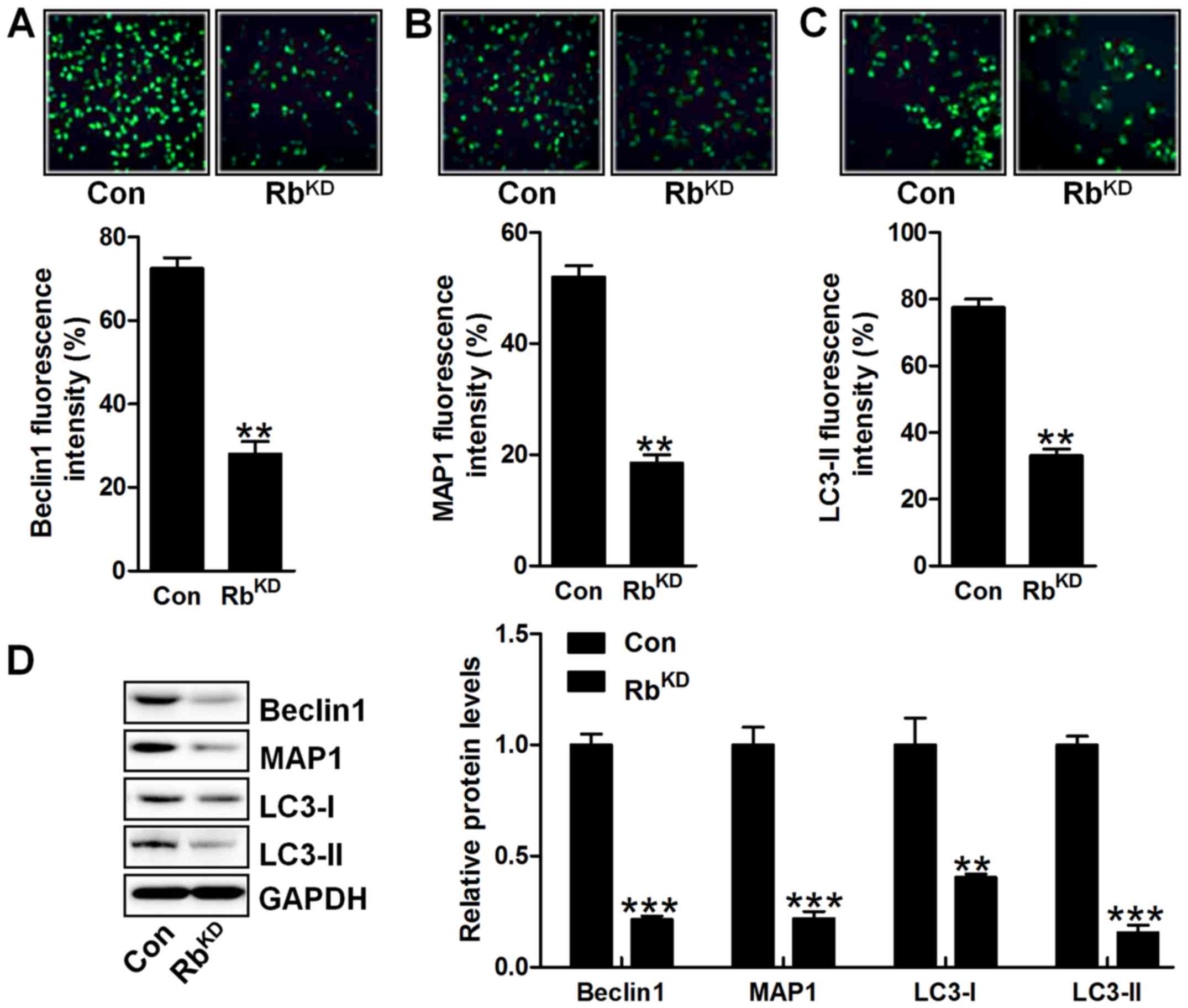
Furthermore, autophagy-related signals of Beclin1,
MAP1 and LC3-II were also observed with downregulated levels via
immunofluorescent analysis in comparison to the Con group (Fig. 9A–C). Western blot analysis also
showed that Beclin1, MAP1, LC3-I and LC3-II were obviously
downregulated for Rb silence, further indicating that autophagy was
suppressed in BIU-87 cells with Rb knockdown (Fig. 9D). Collectively, the data in
vitro confirmed that Rb-regulated urinary bladder cancer
progression was dependent on autophagy alteration.
Rb suppression-induced BIU-87 progression
rely on p53 signaling pathway in vitro
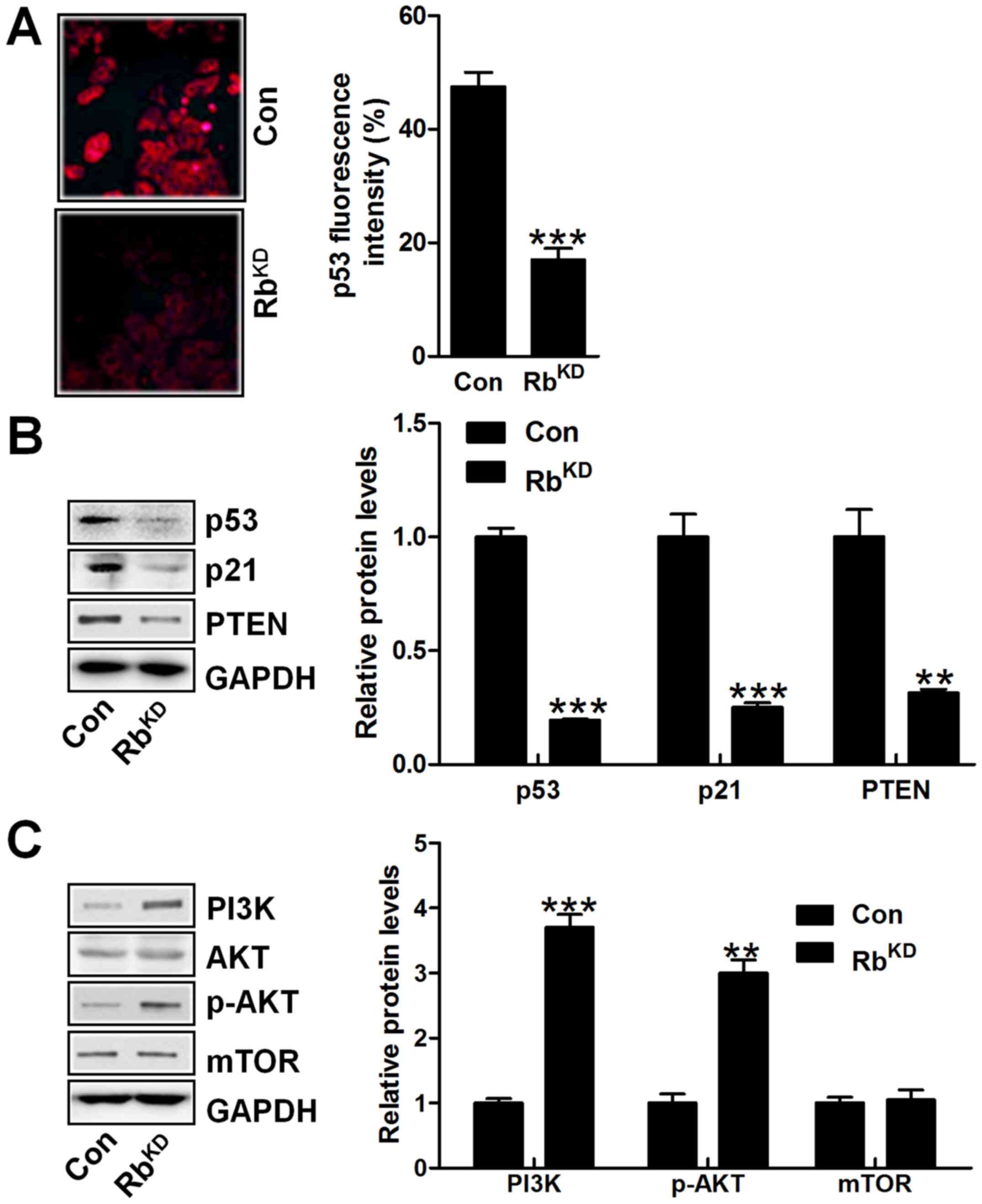
Finally, the p53 signaling pathway was investigated
in vitro with reduced p53 fluorescent intensity in BIU-87
cells after Rb knockdown (Fig.
10A). Similarly, p53, p21 and PTEN were significantly
downregulated in bladder cancer cells with Rb silence (Fig. 10B). However, PI3K/AKT signaling
pathway was activated in Rb-knockdown group, intensifying bladder
cancer progression (Fig. 10C). Of
note, the mdm2 signal was not changed in either group, indicating
that mdm2 might not be involved in Rb-regulated urinary bladder
cancer progression. The data above in vitro further
indicated that p53 was of importance in urinary bladder cancer
progression regulated by Rb.
Discussion
Bladder cancer is one of the most common urological
malignancies and displays a significant reason for morbidity and
mortality across the world (23).
The disease includes two principal forms of cancer, the superficial
and the invasive, with the majority of bladder carcinomas as the
former type at the time of diagnosis (24). The superficial cancers show
papillary and multifocal tumor growth and progression, which
usually recurs following transurethral surgery and progresses to
become an invasive disease occasionally. In contrast, invasive
cancer is often known as nodular, which could metastasize to
distant organs during the early phase of the disease and possesses
a poor prognosis (25). The
treatment used for these cancers or tumors usually includes
transurethral resection of the bladder tumors or a combination of
immunotherapy, chemotherapy and radical cystectomy (26). However, a large number of patients
suffer from the disease recurrence and progression. Thus, a greater
understanding of the molecular mechnism involved in urinary bladder
cancer progression is necessary to find improved and more effective
therapeutic treatments.
Retinoblastoma protein (Rb) is a classical tumor
suppressor for its role in cell cycle checkpoint of G1/S, but
recent data have suggested that Rb participats in many other
cellular functions, such as apoptosis regulation (27). Dysfunction of apoptosis-related
genes are known as a critical mechanism for cancer development
(28), which is recognized as the
most important type of cell death. The B-cell lymphoma 2 protein
(Bcl-2) family display genetic alterations in various cancers,
helping to escape apoptosis through removing pro-apoptotic genes
and promoting anti-apoptotic genes (29). Bad/Bcl-2 heterodimerization
isolates Bcl-2 and results in Bax permeation of both the outer and
inner mitochondrial membranes, leading to the release of cytochrome
c, and the downstream activation of the caspase cascade culminating
in caspase-3 cleavage (30). In
our study, we found that Rb knockout resulted in significant Bcl-2
upregulation, while Bid, Bax and Bak were obviously downregulated,
leading to Apaf decrease. Apaf is known as an important activator
responding to the up-streaming apoptotic factor activation,
activating the downstream signals, such as Cyto-c, contributing to
apoptotic response (31). Caspase
is the executor of apoptosis (32). As the downstream signal of Bcl-2
pathway, we found that caspase-3, consistently, was inactivated in
Rb deficiency or silenced, accompanied with downregulation of
cleaved PARP, which function as important pro-apoptotic genes in
vitro and in vivo studies. These results indicated that
suppressing Rb activation might be the main mechanism by which the
urinary bladder cancer was enhanced through apoptosis
inhibition.
In addition, the tumor suppressor p53, could also
induce cell cycle arrest and apoptosis, resulting in conserved
genome stability integrity responding to cellular stress and DNA
damage (33,34). The expression of p21 has been
investigated in the development of chemotherapeutic drugs, which
could disrupt tumorigenesis via suppressing cell cycle in cancer
cells, contributing to the suppression of cell proliferation. These
results indicated that Rb deficiency downregulated p53 and p21
levels, as well as PTEN, which is known as a significant downstream
signal of p53, helping to suppress cell proliferation (35). Accordingly, with the reduced p53
levels, we found that PTEN was also decreased, further indicating
the role of Rb in regulating urinary bladder cancer development,
knockout of which was the main reason, leading to bladder cancer
progression. E2F3 was invariably disrupted in different human
cancers for its central role in the control of cellular
proliferation. Phosphorylated Rb regulates E2F3 activation, which
is required for the progression into late phase of G1 and S
(36). This sequential regulation
exhibits additional specificity in modulating alternative cell
fates, including differentiation and proliferation, and plays an
important role in tumor development and progression (37). In line with the results of enhanced
colony formation, migrated and invaded cells for Rb suppression, we
found that E2F3 was also upregulated.
Furthermore, as a negative regulator of p53, mdm2
interacts with p53 protein to inhibit the transcriptional
activation of p53, leading to cell proliferation in a tumor
(38). In contrast to p53
alteration, mdm2 was upregulated for Rb inhibition, further
confirming our results of Rb suppression to promote urinary bladder
cancer development. The PI3K-AKT pathway has been reported to be
activated in many malignant tumors due to abnormalities in various
genes (39). Studies have found
that AKT pathway plays an important role in lung cancer, intestine
cancer and pancreatic carcinoma (40). Here, we found that PI3K/AKT
signaling pathway was stimulated for Rb suppression in vitro
and in vivo, which was in agreement with p53 and PTEN
downregulation, enhancing urinary bladder cancer progression.
In conclusion, we demonstrated that Rb was
frequently downregulated in urinary bladder cancer tissues and cell
lines. Rb suppression played a crucial role in the malignant
progression of urinary bladder cancer cells through inactivation of
p53 and caspase-3, inhibiting autophagy and apoptosis. Therefore,
targeting Rb has the potential to be a valuable therapeutic
strategy for urinary bladder cancer.
References
|
1
|
Zhou J, Li J, Wang Z, Yin C and Zhang W:
Metadherin is a novel prognostic marker for bladder cancer
progression and overall patient survival. Asia Pac J Clin Oncol.
8:e42–e48. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Denzinger S, Mohren K, Knuechel R, Wild
PJ, Burger M, Wieland WF, Hartmann A and Stoehr R: Improved
clonality analysis of multifocal bladder tumors by combination of
histopathologic organ mapping, loss of heterozygosity, fluorescence
in situ hybridization, and p53 analyses. Hum Pathol. 37:143–151.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Marr BP, Hung C, Gobin YP, Dunkel IJ,
Brodie SE and Abramson DH: Success of intra-arterial chemotherapy
(chemo-surgery) for retinoblastoma: Effect of orbitovascular
anatomy. Arch Ophthalmol. 130:180–185. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang P, Chen J, Wang L, Na Y, Kaku H,
Ueki H, Sasaki K, Yamaguchi K, Zhang K, Saika T, et al:
Implications of transcriptional factor, OCT-4, in human bladder
malignancy and tumor recurrence. Med Oncol. 29:829–834. 2012.
View Article : Google Scholar
|
|
6
|
Munier FL, Gaillard M-C, Balmer A, Soliman
S, Podilsky G, Moulin AP and Beck-Popovic M: Intravitreal
chemotherapy for vitreous disease in retinoblastoma revisited: From
prohibition to conditional indications. Br J Ophthalmol.
96:1078–1083. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shields CL, Shelil A, Cater J, Meadows AT
and Shields JA: Development of new retinoblastomas after 6 cycles
of chemo-reduction for retinoblastoma in 162 eyes of 106
consecutive patients. Arch Ophthalmol. 121:1571–1576. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shields CL, Palamar M, Sharma P,
Ramasubramanian A, Leahey A, Meadows AT and Shields JA:
Retinoblastoma regression patterns following chemoreduction and
adjuvant therapy in 557 tumors. Arch Ophthalmol. 127:282–290. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ziebold U, Reza T, Caron A and Lees JA:
E2F3 contributes both to the inappropriate proliferation and to the
apoptosis arising in Rb mutant embryos. Genes Dev. 15:386–391.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schleicher SM, Moretti L, Varki V and Lu
B: Progress in the unraveling of the endoplasmic reticulum
stress/autophagy pathway and cancer: Implications for future
therapeutic approaches. Drug Resist Updat. 13:79–86. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Park WH, Kim ES, Kim BK and Lee YY:
Monensin-mediated growth inhibition in NCI-H929 myeloma cells via
cell cycle arrest and apoptosis. Int J Oncol. 23:197–204.
2003.PubMed/NCBI
|
|
12
|
Robinson SM, Tsueng G, Sin J, Mangale V,
Rahawi S, McIntyre LL, Williams W, Kha N, Cruz C, Hancock BM, et
al: Coxsackievirus B exits the host cell in shed microvesicles
displaying autophagosomal markers. PLoS Pathog. 10:e10040452014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nishino I: Autophagic vacuolar myopathy.
Semin Pediatr Neurol. 13:90–95. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Park MA, Zhang G, Martin AP, Hamed H,
Mitchell C, Hylemon PB, Graf M, Rahmani M, Ryan K, Liu X, et al:
Vorinostat and sorafenib increase ER stress, autophagy and
apoptosis via ceramide-dependent CD95 and PERK activation. Cancer
Biol Ther. 7:1648–1662. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang L, Meng Y, Bao C, Liu W, Ma C, Li A,
Xuan Z, Shan G and Jia Y: Robustness and backbone motif of a cancer
network regulated by miR-17-92 cluster during the G1/S
transition. PLoS One. 8:e570092013. View Article : Google Scholar
|
|
16
|
Baldi A, De Luca A, Claudio PP, Baldi F,
Giordano GG, Tommasino M, Paggi MG and Giordano A: The RB2/p130
gene product is a nuclear protein whose phosphorylation is cell
cycle regulated. J Cell Biochem. 59:402–408. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cao C, Subhawong T, Albert JM, Kim KW,
Geng L, Sekhar KR, Gi YJ and Lu B: Inhibition of mammalian target
of rapamycin or apoptotic pathway induces autophagy and
radiosensitizes PTEN null prostate cancer cells. Cancer Res.
66:10040–10047. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
He Z, Zhang Y, Mehta SK, Pierson DL, Wu H
and Rohde LH: Expression profile of apoptosis related genes and
radio-sensitivity of prostate cancer cells. J Radiat Res (Tokyo).
52:743–751. 2011. View Article : Google Scholar
|
|
19
|
Shimizu S, Kanaseki T, Mizushima N, Mizuta
T, Arakawa-Kobayashi S, Thompson CB and Tsujimoto Y: Role of Bcl-2
family proteins in a non-apoptotic programmed cell death dependent
on autophagy genes. Nat Cell Biol. 6:1221–1228. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ondrousková E, Soucek K, Horváth V and
Smarda J: Alternative pathways of programmed cell death are
activated in cells with defective caspase-dependent apoptosis. Leuk
Res. 32:599–609. 2008. View Article : Google Scholar
|
|
21
|
Matsuoka M, Kurita M, Sudo H, Mizumoto K,
Nishimoto I and Ogata E: Multiple domains of the mouse
p19ARF tumor suppressor are involved in p53-independent
apoptosis. Biochem Biophys Res Commun. 301:1000–1010. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Paliwal S, Pande S, Kovi RC, Sharpless NE,
Bardeesy N and Grossman SR: Targeting of C-terminal binding protein
(CtBP) by ARF results in p53-independent apoptosis. Mol Cell Biol.
26:2360–2372. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mitra AP, Hansel DE and Cote RJ:
Prognostic value of cell-cycle regulation biomarkers in bladder
cancer. Semin Oncol. 39:524–533. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Eruslanov E, Neuberger M, Daurkin I,
Perrin GQ, Algood C, Dahm P, Rosser C, Vieweg J, Gilbert SM and
Kusmartsev S: Circulating and tumor-infiltrating myeloid cell
subsets in patients with bladder cancer. Int J Cancer.
130:1109–1119. 2012. View Article : Google Scholar
|
|
25
|
Urquidi V, Kim J, Chang M, Dai Y, Rosser
CJ and Goodison S: CCL18 in a multiplex urine-based assay for the
detection of bladder cancer. PLoS One. 7:e377972012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kaufman DS, Shipley WU and Feldman AS:
Bladder cancer. Lancet. 374:239–249. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tanaka N, Ogi K, Odajima T, Dehari H,
Yamada S, Sonoda T and Kohama G: pRb2/p130 protein expression is
correlated with clinicopathologic findings in patients with oral
squamous cell carcinoma. Cancer. 92:2117–2125. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kunze D, Wuttig D, Fuessel S, Kraemer K,
Kotzsch M, Meye A, Grimm MO, Hakenberg OW and Wirth MP: Multitarget
siRNA inhibition of antiapoptotic genes (XIAP, BCL2, BCL-X(L)) in
bladder cancer cells. Anticancer Res. 28(4B): 2259–2263.
2008.PubMed/NCBI
|
|
29
|
Wang YB, Qin J, Zheng XY, Bai Y, Yang K
and Xie LP: Diallyl trisulfide induces Bcl-2 and
caspase-3-dependent apoptosis via downregulation of Akt
phosphorylation in human T24 bladder cancer cells. Phytomedicine.
17:363–368. 2010. View Article : Google Scholar
|
|
30
|
Yo YT, Shieh GS, Hsu KF, Wu CL and Shiau
AL: Licorice and licochalcone-A induce autophagy in LNCaP prostate
cancer cells by suppression of Bcl-2 expression and the mTOR
pathway. J Agric Food Chem. 57:8266–8273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Siegel PM and Massagué J: Cytostatic and
apoptotic actions of TGF-β in homeostasis and cancer. Nat Rev
Cancer. 3:807–821. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Candé C, Cohen I, Daugas E, Ravagnan L,
Larochette N, Zamzami N and Kroemer G: Apoptosis-inducing factor
(AIF): A novel caspase-independent death effector released from
mitochondria. Biochimie. 84:215–222. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu X, Cai ZD, Lou LM and Zhu YB:
Expressions of p53, c-MYC, BCL-2 and apoptotic index in human
osteosarcoma and their correlations with prognosis of patients.
Cancer Epidemiol. 36:212–216. 2012. View Article : Google Scholar
|
|
34
|
Mojtahedi Z, Hashemi SB, Khademi B, Karimi
M, Haghshenas MR, Fattahi MJ and Ghaderi A: p53 codon 72
polymorphism association with head and neck squamous cell
carcinoma. Braz J Otorhinolaryngol. 76:316–320. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jiang L, Wang C, Lei F, Zhang L, Zhang X,
Liu A, Wu G, Zhu J and Song L: miR-93 promotes cell proliferation
in gliomas through activation of PI3K/Akt signaling pathway.
Oncotarget. 6:8286–8299. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cooper CS, Nicholson AG, Foster C, Dodson
A, Edwards S, Fletcher A, Roe T, Clark J, Joshi A, Norman A, et al:
Nuclear overexpression of the E2F3 transcription factor in human
lung cancer. Lung Cancer. 54:155–162. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Foster CS, Falconer A, Dodson AR, Norman
AR, Dennis N, Fletcher A, Southgate C, Dowe A, Dearnaley D, Jhavar
S, et al: Transcription factor E2F3 overexpressed in prostate
cancer independently predicts clinical outcome. Oncogene.
23:5871–5879. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lin HY, Huang CH, Wu WJ, Chou YH, Fan PL
and Lung FW: Mutation of the p53 tumor suppressor gene in
transitional cell carcinoma of the urinary tract in Taiwan.
Kaohsiung J Med Sci. 21:57–64. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cheng GZ, Park S, Shu S, He L, Kong W,
Zhang W, Yuan Z, Wang LH and Cheng JQ: Advances of AKT pathway in
human oncogenesis and as a target for anti-cancer drug discovery.
Curr Cancer Drug Targets. 8:2–6. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Courtney KD, Corcoran RB and Engelman JA:
The PI3K pathway as drug target in human cancer. J Clin Oncol.
28:1075–1083. 2010. View Article : Google Scholar : PubMed/NCBI
|