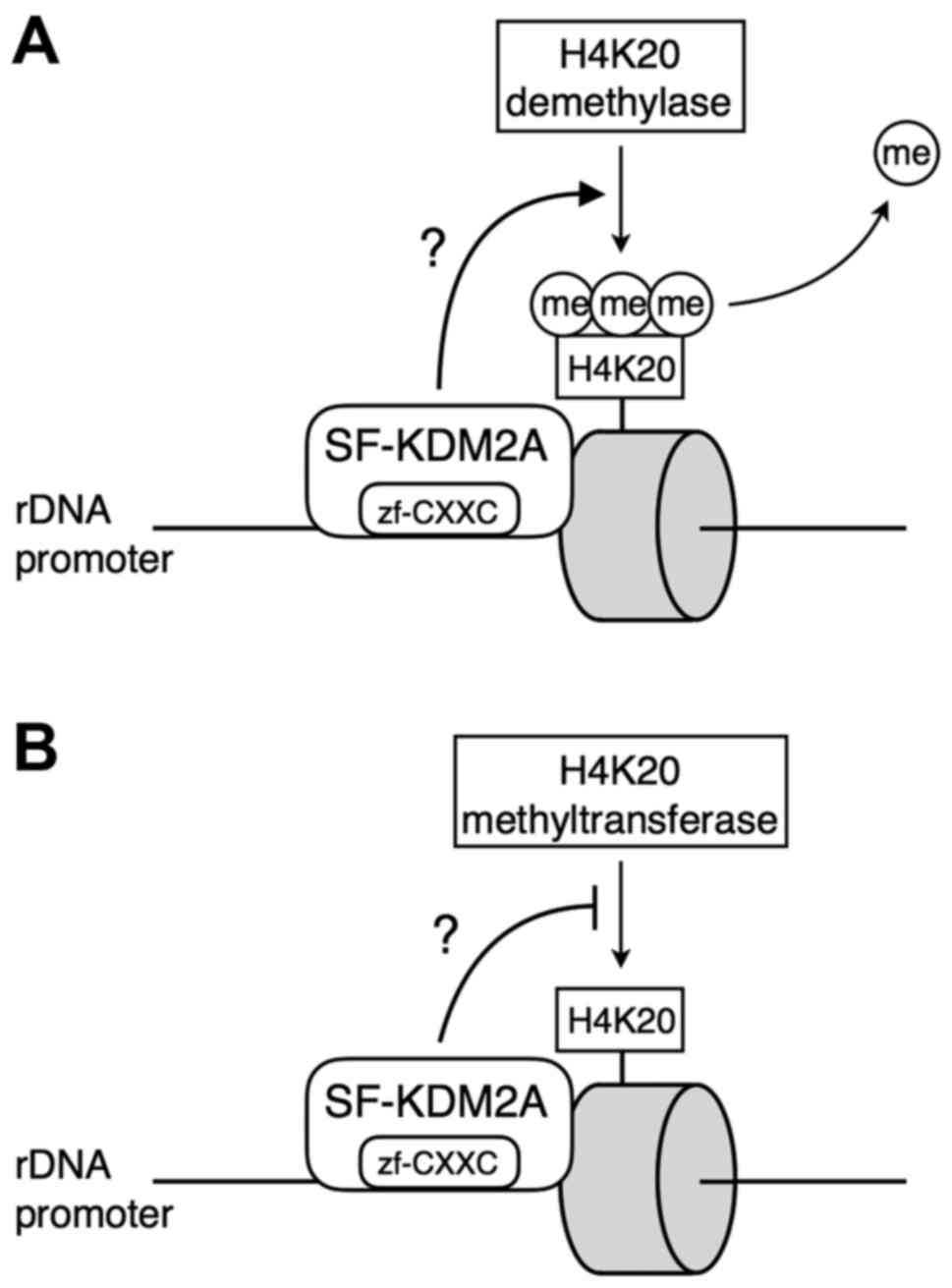
Introduction
Cell growth depends on new ribosome synthesis. RNA
polymerase I (Pol I) transcribes ribosomal RNA (rRNA), and its
activity plays a critical role in the regulation of ribosome
biogenesis (1–3). In eukaryotic cells, a long precursor
transcript called pre-ribosomal RNA (pre-rRNA) is initially
transcribed from the rRNA gene (rDNA) by Pol I, and is processed to
18S, 5.8S and 28S rRNA, which are three of the four structured RNA
molecules constituting the ribosome. Dysregulations of rRNA
transcription are often observed in cancer cells (4). Some oncogene products, such as Myc,
stimulate the transcription of rRNA, and tumor suppressors, such as
PTEN, reduce the transcription of rRNA.
Many discoveries about the relationship between
chromatin structures and transcription have been made during the
past two decades. Several chemical modifications of chromatin
components, including histone methylation, have been identified
(5), and found to be involved in
the regulation of transcription (6). Generally, while methylated Lys4 and
Lys36 of histone H3 function as transcriptionally active signals,
methylated Lys9 and Lys27 of histone H3 and methylated Lys20 of
histone H4 function as transcriptionally repressive signals
(7,8). Histone lysine methylation is
catalyzed by histone methyl transferases (HMTs), and the
demethylation of histones is carried out by histone demethylases
(HDMs) (9,10). The existence of these enzymes
highlights the dynamic nature of the regulation of histone
methylation (6,11).
KDM2A, a member of the JmjC domain-containing
enzymes, has demethylase activity on the mono- and dimethylated
Lys36 of histone H3 (H3K36me1/2) (11,12).
KDM2A contains several domains including the zinc finger CXXC
(zf-CXXC) domain, the PHD finger, F-box and three leucine-rich
repeats (11,12). Previously, we found that KDM2A
localizes in nucleoli and binds to the rDNA promoter through the
zf-CXXC domain, and that glucose starvation induces the demethylase
activity of KDM2A in the rDNA promoter and reduces rRNA
transcription (12–14). The KDM2A gene also produces
a short form of KDM2A (SF-KDM2A), which lacks the JmjC domain on
the N-terminal side (12).
However, the function of SF-KDM2A was not clear.
Recently, it was reported that KDM2A is
frequently amplified and overexpressed, and that elevated
expression of KDM2A is significantly associated with short
survival of breast cancer patients (15). Detailed characterization of the
KDM2A gene revealed that SF-KDM2A is more abundant than
full-length KDM2A in a subset of breast cancers, and may have
oncogenic potential (15).
However, it is unclear how the elevated expression of SF-KDM2A
contributes to tumorigenesis.
In the present study, we found that SF-KDM2A
localized in nucleoli, bound to the rDNA promoter via zf-CXXC
domain, reduced the level of histone H4K20me3 marks in rDNA
promoter and stimulated rRNA transcription.
Materials and methods
Cells and cell culture
The human breast adenocarcinoma cell line MCF-7 was
cultured in RPMI-1640 medium (Nacalai Tesque, Kyoto, Japan)
supplemented with 10% fetal calf serum (FCS) or in Dulbecco's
modified Eagle's medium (DMEM; cat. no. D5796; Sigma-Aldrich Co.,
St. Louis, MO, USA) supplemented with 10% FCS. Cells were cultured
at 37°C in an atmosphere containing 5% CO2 and 100%
humidity. Mammalian expression plasmids were introduced into cells
using FuGENE6 transfection reagent (Promega, Madison, WI, USA)
according to the manufacturer's instructions. MCF-7tet-on cells
(parent) (13) were transfected
with ptetFlag-SF-KDM2A, ptetSF-KDM2A, or ptetmCXXC-SF-KDM2A plus
pAct-Hyg, which confers hygromycin resistance, and cultured in the
presence of 150–250 μg/ml hygromycin and 200 μg/ml
G418. The selected colonies were picked up and cultured for 24 h in
the presence of 1 μg/ml doxycycline (Dox), and the
expression of SF-KDM2A or its mutant proteins was detected by
indirect immunofluorescence and western blotting using an anti-Flag
antibody or an anti-Fbxl11 (KDM2A) antibody. The expression of
SF-KDM2A and mCXXC-SF-KDM2A was induced by adding 1 μg/ml
Dox, or induced by adding Dox at the concentrations indicated in
the figure legends.
siRNA and transfection
Cells were transfected with stealth siRNA using
Lipofectamine RNAiMAX (Thermo Fisher Scientific, Waltham, MA, USA)
according to the manufacturer's instructions. The siRNA
oligonucleotide sequences for SF-KDM2A were
5′-CAGAAUAUUCAAGUAAAUCCGGAUU-3′ (#1 oligo) and
5′-GGCAGAAUAUCUAACUCCUUCAGGA-3′ (#2 oligo). The positions of the
sequence are shown in Fig. 1A. The
siRNA oligonucleotide sequence for KDM2A was
5′-GAACCCGAAGAAGAAAGGAUUCGUU-3′, which was previously described
(11,12). Cells were also transfected with
control stealth RNA (Stealth RNAi Negative Control Medium GC
Duplex; Thermo Fisher Scientific).
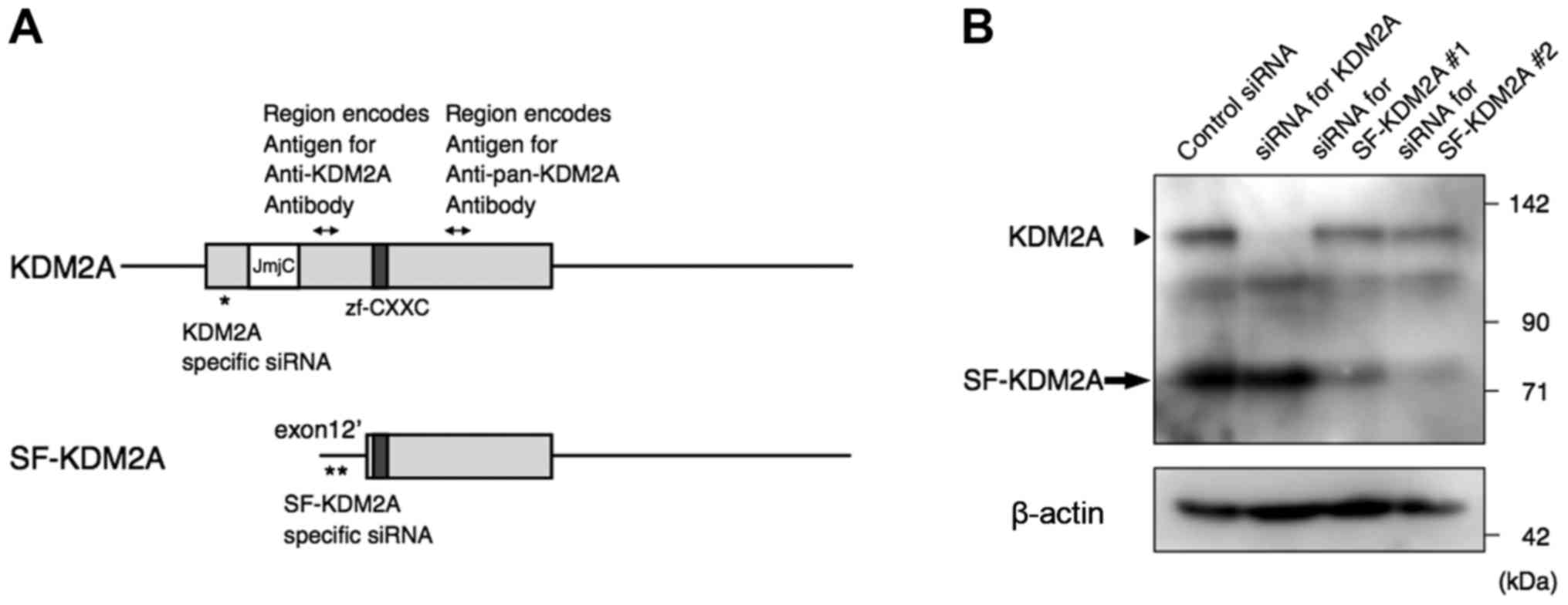 | Figure 1KDM2A and SF-KDM2A. (A) Diagrams of
human KDM2A and SF-KDM2A mRNA. The KDM2A gene codes two
transcripts, KDM2A mRNA (upper bar) and SF-KDM2A mRNA (lower bar).
The bars show untranslated regions and the boxes translated
regions. The white box shows the mRNA region encoding the JmjC
domain, the dark grey box encoding the zf-CXXC domain. The
positions for two siRNAs for SF-KDM2A and siRNA for KDM2A, which
are targeted to sequences only for SF-KDM2A and KDM2A,
respectively, are shown by asterisks. The anti-KDM2A antibody that
recognized only KDM2A was produced against the polypeptide from
Ser360 to Val451 of KDM2A. The anti-pan-KDM2A antibody that
recognized both KDM2A and SF-KDM2A was produced against the
synthetic peptide corresponding to a region between Ser825 and
Ala875 of KDM2A (Ser283 and Ala333 of SF-KDM2A). The regions
encoding the antigens are indicated by double-headed arrows. (B)
Western blot analysis to detect KDM2A and SF-KDM2A proteins. Breast
adenocarcinoma cell line MCF-7 cells were transfected with specific
siRNAs for full-length KDM2A, SF-KDM2A (#1 and #2), or control
siRNA. After 72-h culturing, cells were lysed, and the extracts
were subjected to western blotting using anti-pan-KDM2A and
anti-β-actin antibodies. The positions of KDM2A and SF-KDM2A are
indicated by an arrowhead and an arrow, respectively. The positions
of protein markers with defined molecular weights are indicated on
the right side of the pictures. |
Antibodies
Mouse monoclonal anti-β-actin antibody (AC-15;
Sigma-Aldrich), mouse monoclonal anti-nucleolin antibody, C23
(MS-3, sc-8031; Santa Cruz Biotechnology, Santa Cruz, CA, USA),
rabbit monoclonal anti-flag antibody (SIG1-25; Sigma-Aldrich), goat
anti-rabbit IgG-HRP (sc-2054; Santa Cruz Biotechnology), Alexa
488-conjugated goat anti-mouse IgG (H+L) (A11029; Thermo Fisher
Scientific) and Alexa 568-conjugated goat anti-rabbit IgG (H+L)
(A11011; Thermo Fisher Scientific) were purchased. The rabbit
polyclonal anti-dimethylated histone H3 lys4 antibody (ab7766;
Abcam, Cambridge, UK), rabbit polyclonal anti-trimethylated histone
H3 lys4 antibody (ab8580; Abcam), mouse monoclonal
anti-dimethylated histone H3 lys9 antibody (MABI 0307; Active
Motif, Carlsbad, CA, USA), rabbit polyclonal anti-trimethylated
histone H3 lys9 antibody (ab1186; Abcam), mouse monoclonal
anti-dimethylated histone H3 lys27 antibody (MABI0324; Active
Motif), mouse monoclonal anti-dimethylated histone H3 lys36
antibody (MABI0332; Active Motif), mouse monoclonal
anti-trimethylated histone H3 lys36 antibody (MABI0333, GTX50908;
GeneTex, Inc., Irvine, CA, USA), rabbit polyclonal
anti-trimethylated histone H4 lys20 antibody (07-463; Merck
Millipore, Darmstadt, Germany), rabbit polyclonal anti-acetylated
histone H4 antibody (06-866; Merck Millipore), rabbit polyclonal
anti-histone H3 antibody (ab1791; Abcam), and rabbit polyclonal
anti-histone H4 antibody (ab10158; Abcam) were also purchased. The
anti-Fbxl11 (KDM2A) antibody (ab99242; Abcam) was purchased and
used as the anti-pan-KDM2A antibody. The anti-KDM2A antibody was
previously described (12).
Western blotting and immunofluorescence
staining
Cells were trypsinized and extracted in 3% SDS
solution containing 100 mM Tris-HCl, pH 6.8, 0.1 M DTT and 20%
glycerol. Cell extracts were separated on SDS-PAGE and transferred
to a microporous PVDF membrane (Merck Millipore). After treatment
with antibodies, bands were detected using an Immobilon Western
System (WBKLS0100; Merck Millipore). For indirect
immunofluorescence staining, cells grown on glass coverslips were
fixed in methanol for 30 min at −20°C and incubated in 1% skim milk
in phosphate-buffered saline (PBS). The first antibodies (rabbit
polyclonal antibody and/or mouse monoclonal antibody) were added
and incubated for 60 min at 37°C. After cells were washed three
times in 0.1% skim milk in PBS, Alexa 488-conjugated anti-mouse IgG
and/or Alexa 568-conjugated anti-rabbit IgG were added, incubated
for 60 min at 37°C and washed three times with 0.1% skim milk in
PBS. Finally, cells were embedded in Immunon (Thermo Fisher
Scientific) and observed via confocal fluorescence microscopy (LSM
5 Exciter; Carl-Zeiss, Oberkochen, Germany).
RNA preparation and quantitative reverse
transcriptase-polymerase chain reaction (qRT-PCR)
Total RNA was isolated from cells using a NucleoSpin
RNA II kit (#U0955C; Takara Bio Inc., Otsu, Japan) according to the
manufacturer's instructions. Synthesis of single-strand cDNA was
performed on total RNA (1 μg) by a SuperScript III
First-Strand Synthesis system (Thermo Fisher Scientific) using
random hexamers according to the manufacturer's instructions. The
cDNA products were diluted with distilled water and mixed with
Thunderbird qPCR Mix (#QPS-201; Toyobo, Co., Ltd., Osaka, Japan)
according to the manufacturer's instructions. An Mx3000P QPCR
system (Agilent Technologies, Inc., Santa Clara, CA, USA) or CFX
Connect (Bio-Rad Laboratories, Hercules, CA, USA) is used to
quantify the amount of DNA. The values were normalized using the
amounts for a control mRNA, TATA-binding protein (TBP) mRNA. The
sets of PCR primers used for amplification of the pre-rRNA (a
sequence in the 5′ region 1-155) were 5′-GCTGACACGCTGTCCTCTG-3′ and
5′-TCGGACGCGCGAGAGAAC-3′; for SF-KDM2A, the primers used were
5′-GGATTTTCCCAGAGGCAGA-3′ and 5′-TAACTTGGGATCGCTGTTGG-3′; for
exogenously expressed SF-KDM2A, the primers used were
5′-ATTGCTGGGAATGTCCAAAG-3′ and 5′-ATGGAAGTGGGTGAATGAGG-3′; for TBP,
the primers used were 5′-TGCTGCGGTAATCATGAGGATA-3′ and
5′-TGAAGTCCAAGAACTTAGCTGGAA-3′.
Statistical analysis
P-values were calculated by two-tailed paired
Student's t-test.
Chromatin immunoprecipitation (ChIP)
ChIP assay was performed as previously described
(12,13). To detect specific binding, the
values simultaneously obtained using the control antibody (normal
rabbit IgG) were subtracted from those using specific antibodies.
The values for specific binding were divided by total input (% of
input).
Results
SF-KDM2A is localized in the
nucleoli
KDM2A encodes two proteins, KDM2A and a short
form of KDM2A, SF-KDM2A (12).
While KDM2A contains the JmjC domain, which is critical for histone
demethylase activity, SF-KDM2A lacks it (Fig. 1A). To investigate how SF-KDM2A is
produced from the KDM2A gene, we designed siRNAs against the
sequence of exon 12′ in the KDM2A gene (12), which was found in SF-KDM2A mRNA,
but not KDM2A mRNA (Fig. 1A).
Western blot analysis using anti-pan-KDM2A antibody, which
recognized both KDM2A and SF-KDM2A (Fig. 1A), showed that the siRNAs for exon
12′ (both #1 and #2) reduced the band for SF-KDM2A (lower band) but
not the band for KDM2A (upper band) (Fig. 1B), whereas KDM2A-specific siRNA
reduced the band for KDM2A but not the band for SF-KDM2A (Fig. 1B). These results suggest that the
majority of SF-KDM2A mRNA is not a product processed from the
full-length KDM2A mRNA but is transcribed from the KDM2A
gene separately from KDM2A mRNA. After this, siRNAs for exon 12′
(both #1 and #2) are referred as SF-KDM2A siRNAs.
In order to investigate the subcellular localization
of SF-KDM2A, we established a cell line in which Flag-tagged
SF-KDM2A (Flag-SF-KDM2A) was expressed under the doxycycline
(Dox)-inducible (tet-on) promoter (MCF-7tet-Flag-SF-KDM2A cells).
The cDNA for Flag-SF-KDM2A under the tet-on promoter was introduced
into parental cells, which were MCF-7 cells expressing the tet-on
transcription factor (MCF-7tet-on cells) (13). Western blotting showed that
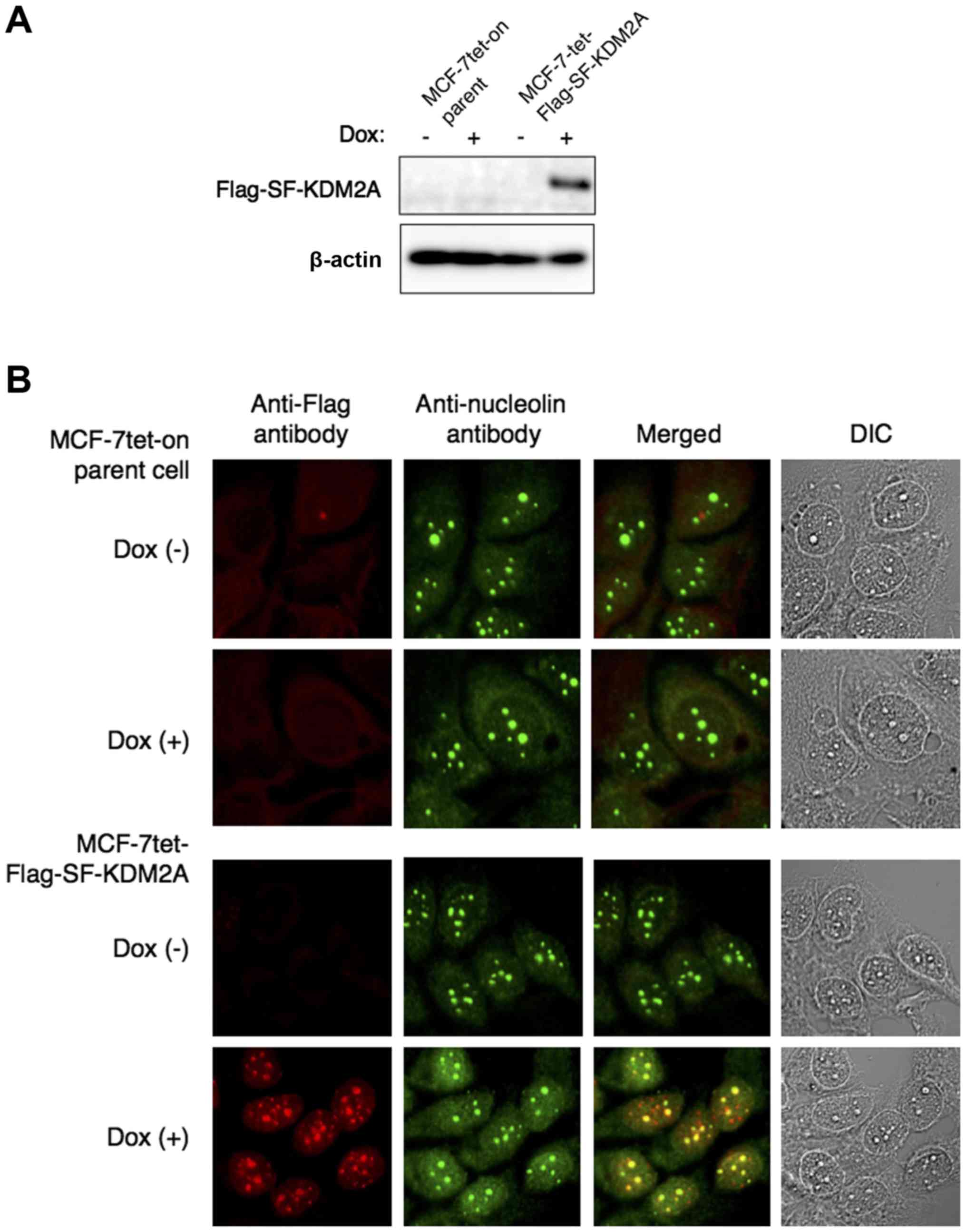
Flag-SF-KDM2A was expressed by adding Dox (Fig. 2A). Immunostaining of
MCF-7tet-Flag-SF-KDM2A cells with anti-Flag antibody showed that
most of the signals for Flag-SF-KDM2A overlapped with those for
nucleolin, suggesting that SF-KDM2A localized in the nucleoli
(Fig. 2B). No signals were
detected in MCF-7tet-on cells either with or without Dox treatment
(Fig. 2B).
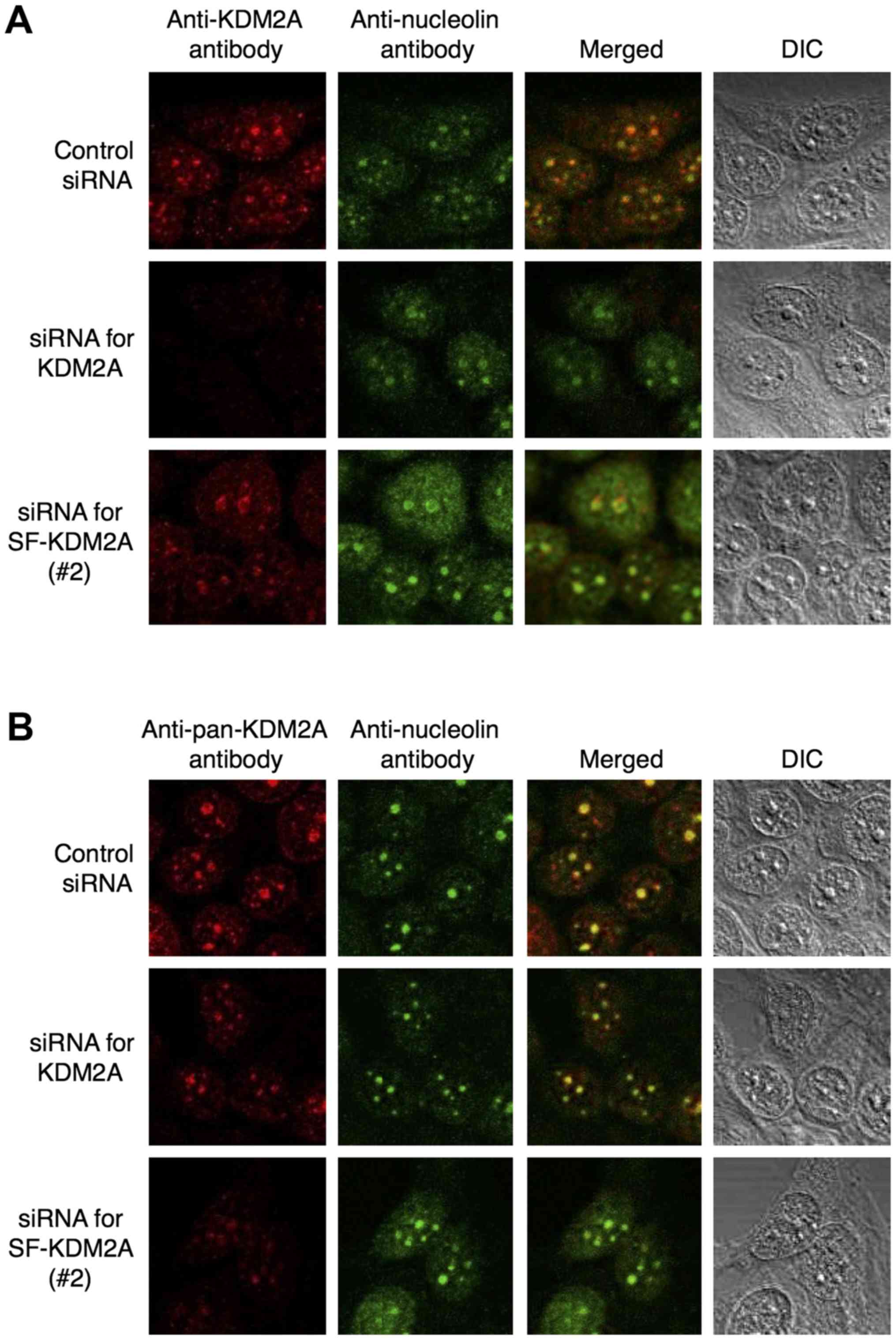
We previously showed that endogenous full-length
KDM2A is localized in nucleoli (12). Immunostaining with anti-KDM2A
antibody, which recognizes only full-length KDM2A, produced signals
in nucleoli, and the siRNA for KDM2A reduced the nucleolar signals
by anti-KDM2A antibody (Fig. 3A),
confirming our previous results. SF-KDM2A siRNAs (#1 and #2) did
not reduce the nucleolar signals by anti-KDM2A antibody (Fig. 3A and data not shown), supporting
our conclusion that full-length KDM2A mRNA was distinct from
SF-KDM2A mRNA. Next, we observed subcellular localization of
endogenous SF-KDM2A. Because the C-terminal side of KDM2A contains
the complete amino acid sequence of SF-KDM2A, it is impossible to
produce an SF-KDM2A-specific antibody. Immunostaining with an
anti-pan-KDM2A antibody, which recognized both SF-KDM2A and KDM2A,
produced signals that overlapped with those of nucleolin (Fig. 3B). The siRNAs specific for KDM2A
partially reduced the signals of the anti-pan-KDM2A antibody
(Fig. 3B), confirming that KDM2A
localized in the nucleoli (12).
SF-KDM2A siRNAs (#1 and #2) also reduced the nucleolar signals by
anti-pan-KDM2A antibody (Fig. 3B
and data not shown). These results indicate that endogenous
SF-KDM2A localizes in the nucleoli.
SF-KDM2A binds to the ribosomal RNA gene
promoter via the zf-CXXC domain
The binding of SF-KDM2A to the rDNA promoter
(Fig. 4A) was tested by chromatin
immunoprecipitation (ChIP). The anti-Flag antibody collected the
fragment of the rDNA promoter when the expression of Flag-SF-KDM2A
(WT) was induced by Dox in MCF-7tet-Flag-SF-KDM2A cells (Fig. 4B), showing that Flag-SF-KDM2A binds
to the rDNA promoter. To confirm the results, we established a cell
line in which SF-KDM2A without the Flag-tag was induced by Dox
(Fig. 4C). The amounts of rDNA
promoter fragment collected by anti-pan KDM2A antibody were
increased when the expression of SF-KDM2A was induced by Dox
(Fig. 4D).
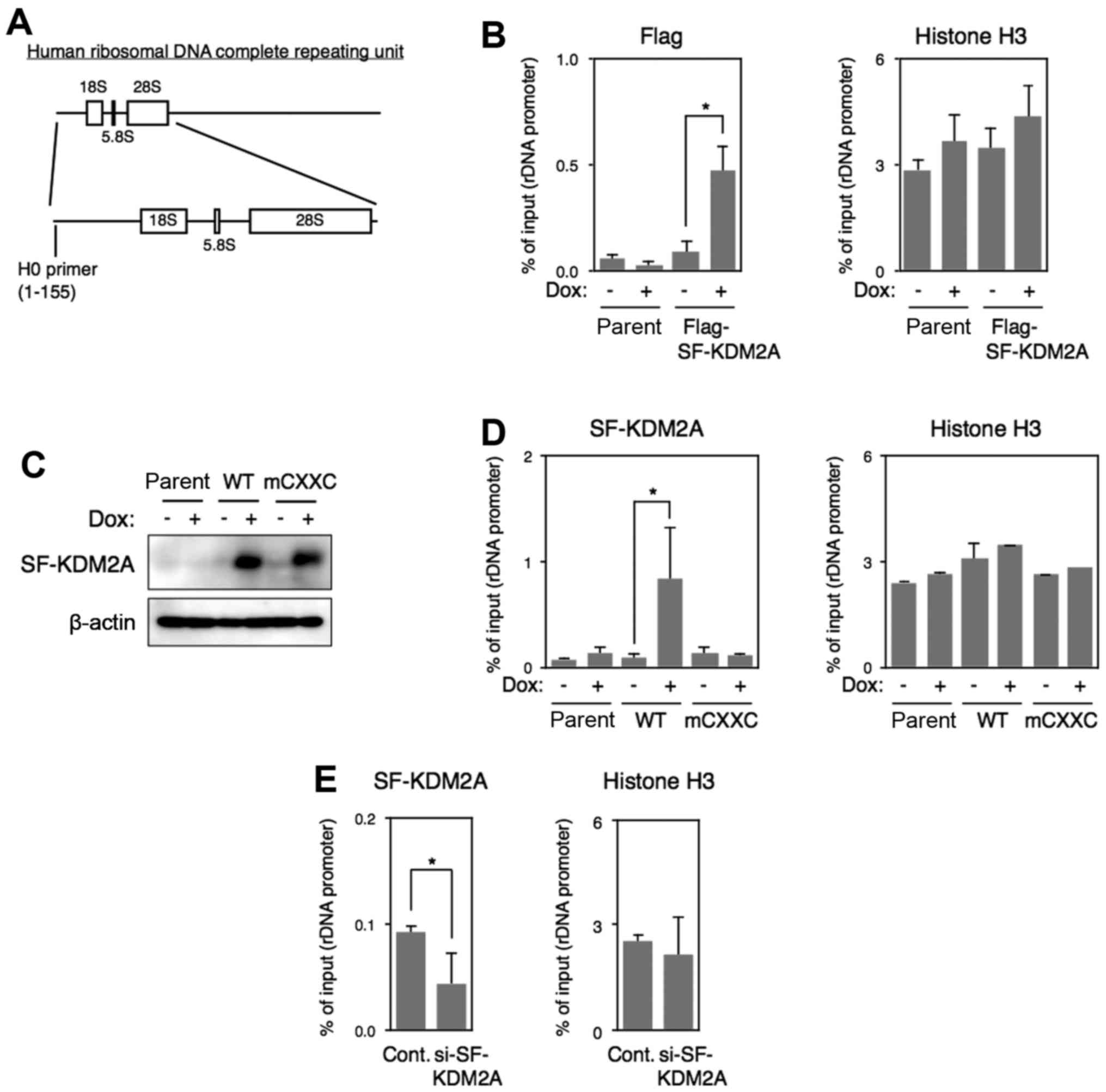 | Figure 4Binding of SF-KDM2A to rDNA promoter
in vivo. (A) A diagram of human rDNA structure. The boxes
show regions encoding rRNA that constitute ribosomes. The position
of PCR primers used in the experiments is shown at the bottom (H0
primer). The numbers in parentheses show nucleotide numbers in
human ribosomal DNA complete repeating unit (GenBank Accession no.
U13369). (B) MCF-7tet-Flag-SF-KDM2A cells (Flag-SF-KDM2A) and
MCF-7tet-on cells (parent) were cultured in the presence or absence
of Dox (1 μg/ml) for 72 h, and chromatin immunoprecipitation
(ChIP) analyses were performed using anti-Flag and histone H3
antibodies to detect Flag-SF-KDM2A and histone H3, respectively, in
the rDNA promoter. The results are shown as the percentage of
input. The experiments were performed three times, and mean values
with standard deviations are indicated. *P<0.05. (C)
Expression of wild-type and zf-CXXC domain-mutant SF-KDM2A
proteins. MCF-7tet-SF-KDM2A cells (WT), MCF-7tet-mCXXC-SF-KDM2A
cells (mCXXC), and MCF-7tet-on cells (parent) were cultured in the
presence or absence of Dox for 72 h. Cells were lysed, and
expression of SF-KDM2A was detected by western blotting using
anti-pan-KDM2A antibody. (D) Cells were cultured as described in
(C), and analyzed by ChIP analyses using pan-KDM2A and histone H3
antibodies. The results are shown as in (B). (E) MCF-7 cells were
transfected with SF-KDM2A siRNAs (#2), or control siRNA. After
72-96 h of culture, cells were harvested and ChIP analyses were
performed using pan-KDM2A and histone H3 antibodies. The results
are shown as in (B). |
SF-KDM2A has the zf-CXXC domain (Fig. 1A), which binds to unmethylated CpG
dinucleotides (13,16) and functions for full-length KDM2A
binding to the rDNA promoter (13). We established a cell line in which
SF-KDM2A with mutations in the zf-CXXC domain (mCXXC-SF-KDM2A) was
expressed under the tet-on system (MCF-7tet-SF-KDM2A-mCXXC cells).
In mCXXC-SF-KDM2A, Cys 29, Cys 32 and Cys 35 in the zf-CXXC domain
were replaced with Ala. Western blotting showed that mCXXC-SF-KDM2A
was expressed at a level comparable to wild-type SF-KDM2A in
MCF-7tet-SF-KDM2A cells in the presence of Dox (Fig. 4C). ChIP analysis showed that the
amount of rDNA promoter fragment collected by the anti-pan-KDM2A
antibody was not increased by the induction of mCXXC-SF-KDM2A
expression (Fig. 4D). The
anti-histone H3 antibody collected the rDNA promoter at similar
levels in all experimental conditions tested here (Fig. 4D). These results indicate that
SF-KDM2A binds to the rDNA promoter through the zf-CXXC domain.
Next, we examined the binding of endogenous SF-KDM2A
to the rDNA promoter in MCF-7 cells. ChIP analysis using the
anti-pan-KDM2A antibody showed that the binding of endogenous
SF-KDM2A to the rDNA promoter was reduced by the treatment with
SF-KDM2A siRNA (Fig. 4E). The
amounts of histone H3 binding to the rDNA promoter was not changed
by SF-KDM2A siRNA (Fig. 4E).
Together, these results demonstrate that SF-KDM2A binds to the rDNA
promoter.
SF-KDM2A stimulates rRNA
transcription
Next, we investigated whether SF-KDM2A regulated
rDNA transcription. When the expression of SF-KDM2A was induced by
Dox in MCF-7tet-SF-KDM2A cells, the expression of SF-KDM2A mRNA and
protein was increased in a Dox concentration-dependent manner
(Fig. 5A and B). The amount of
pre-rRNA was also increased in a Dox concentration-dependent manner
(Fig. 5A). Similarly, in
MCF-7tet-Flag-SF-KDM2A cells, the expression of Flag-SF-KDM2A
increased the amount of pre-rRNA (Fig.
5C). In MCF-7tet-on cells (parent), the Dox treatment increased
neither SF-KDM2A protein nor pre-rRNA levels (Fig. 5A and B). In MCF-7tet-SF-KDM2A-mCXXC
cells, Dox treatment did not change the amount of pre-rRNA, in
spite of the elevation of mCXXC-SF-KDM2A expression (Fig. 5D). When endogenous SF-KDM2A was
reduced using SF-KDM2A siRNA in MCF-7 cells, the amount of rRNA
transcription was decreased (Fig.
5E). Together, these results suggest that SF-KDM2A stimulates
rRNA transcription and the binding of SF-KDM2A via the zf-CXXC
domain to the rDNA promoter is required for the stimulation of rRNA
transcription.
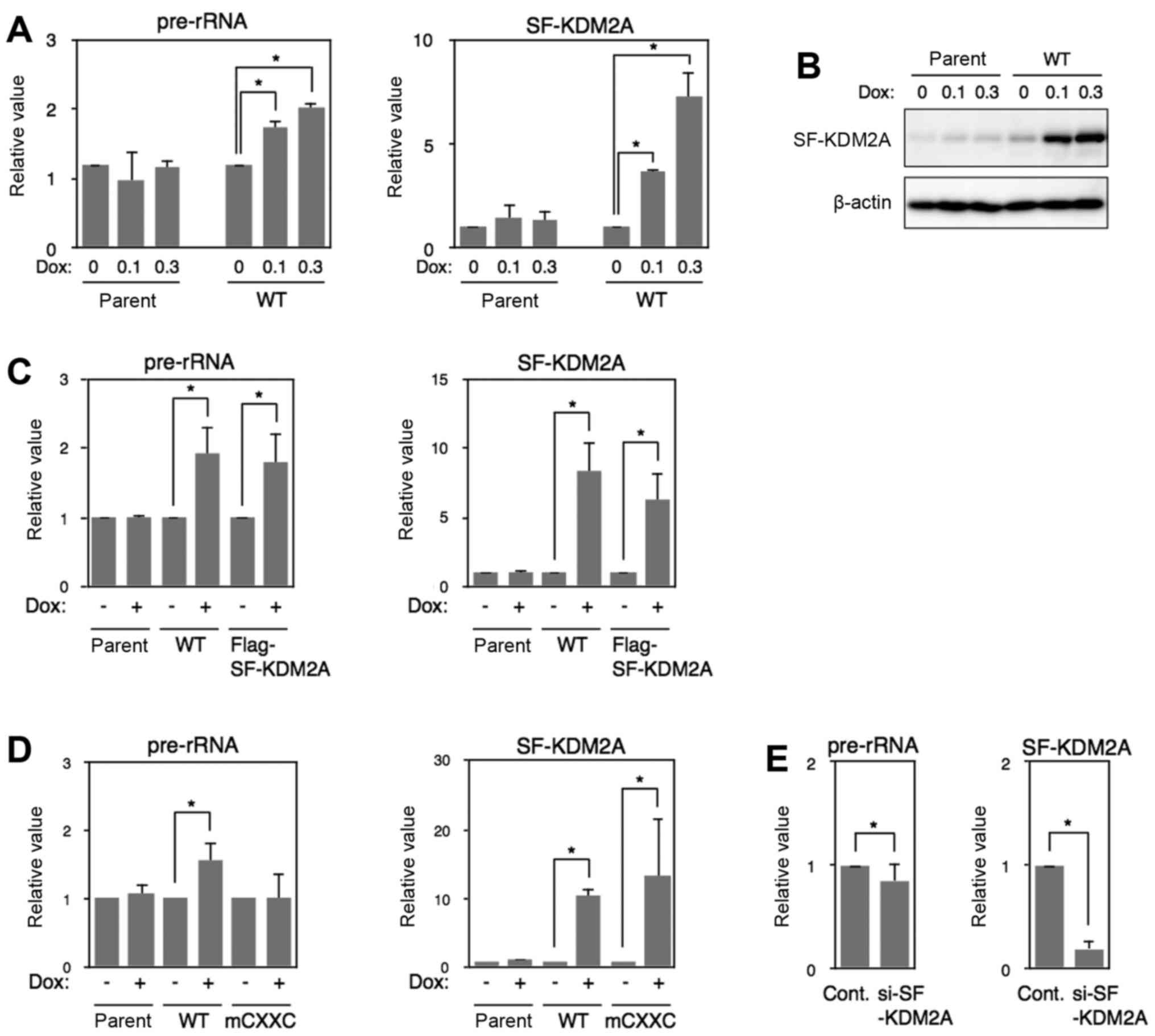 | Figure 5SF-KDM2A increases rRNA
transcription. (A) MCF-7tet-on cells (parent) and MCF-7tet-SF-KDM2A
cells (WT) were cultured in the presence of Dox at the indicated
concentrations (0, 0.1 and 0.3 μg/ml) for 72 h. Total RNA
was isolated, and the amounts of pre-rRNA and SF-KDM2A mRNA were
measured by qRT-PCR. (B) Cells prepared as in (A) were lysed, and
the expression of SF-KDM2A proteins was detected by western
blotting. β-actin was also detected as a loading control. (C)
MCF-7tet-on cells (parent), MCF-7tet-SF-KDM2A cells (SF-KDM2A), and
MCF-7tet-Flag-SF-KDM2A cells (Flag-SF-KDM2A) were cultured in the
presence or absence of Dox (1 μg/ml) for 72 h. Total RNA was
isolated and analyzed by qRT-PCR to detect pre-rRNA, SF-KDM2A mRNA.
(D) MCF-7tet-SF-KDM2A cells (WT), MCF-7tet-mCXXC-SF-KDM2A cells
(mCXXC), and MCF-7tet-on cells (parent), were cultured in the
presence or absence of Dox (1 μg/ml) for 72 h, and the
amounts of pre-rRNA, SF-KDM2A mRNA, were measured by qRT-PCR. (E)
MCF-7 cells were transfected with SF-KDM2A siRNA (#2), or control
siRNA. After culturing for 72–96 h, the amounts of pre-rRNA and
SF-KDM2A mRNA were measured by qRT-PCR. All experiments except (B)
were performed at least three times, and mean values with standard
deviations are indicated. *P<0.05. |
Level of a repressive histone mark
H4K20me3 in the rDNA promoter is reduced by SF-KDM2A
To investigate how SF-KDM2A regulates rRNA
transcription, histone modifications in the rDNA promoter were
investigated by ChIP analyses. In MCF-7tet-SF-KDM2A cells, the
levels of H3K4me2, H3K4me3, H3K9me2, H3K27me2, H3K36me2 and
H3K36me3 marks were hardly changed by Dox treatment, and the level
of H3K9me3 and H4 acetylation marks showed a weak tendency of
increase without statistical significance (Fig. 6A). On the contrary, Dox treatment
significantly reduced a repressive histone mark, H4K20me3, while
not in MCF-7tet-on or MCF-7tet-mCXXC-SF-KDM2A cells (Fig. 6A).
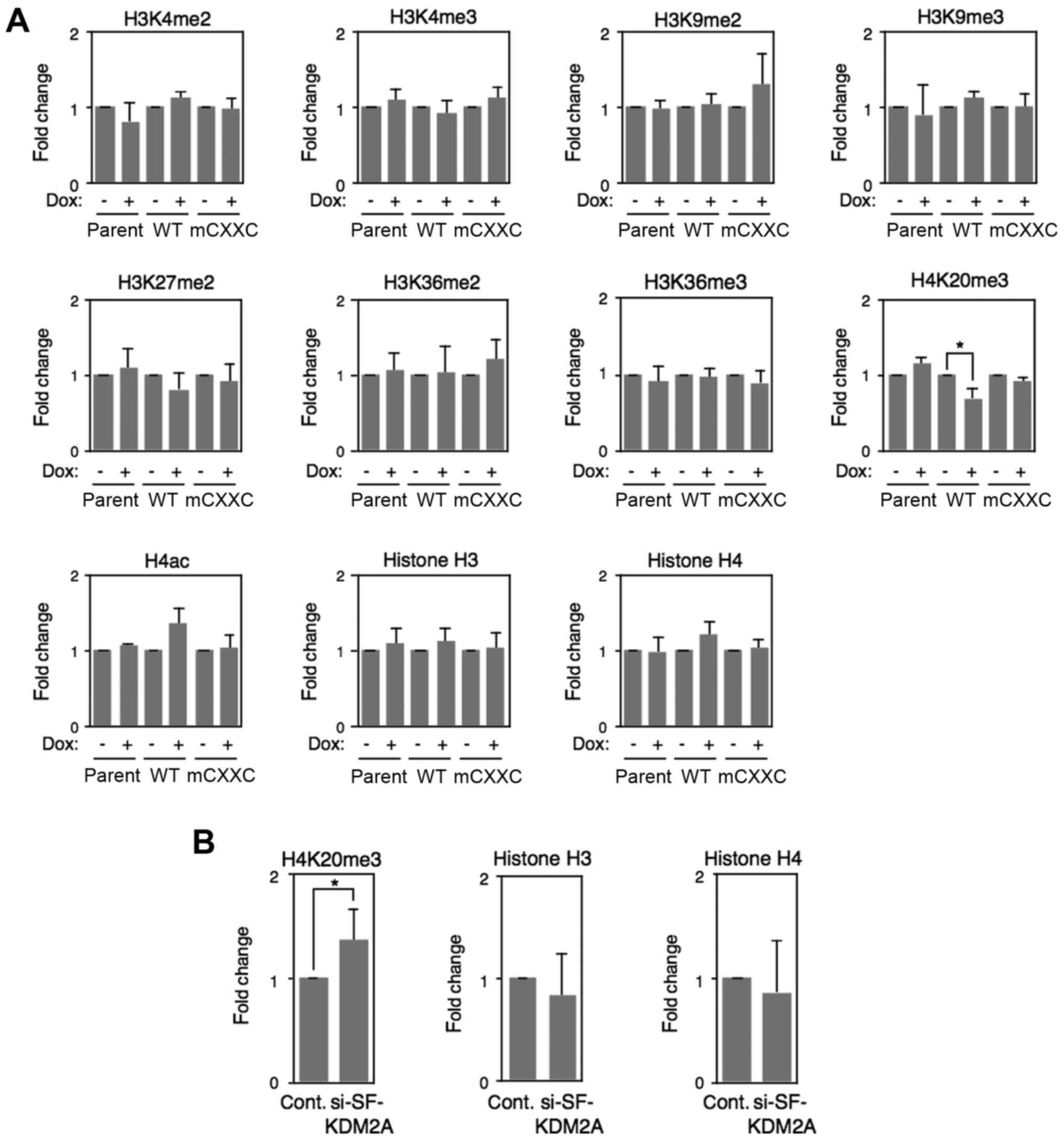 | Figure 6Changes of histone modifications in
rDNA promoter. (A) MCF-7tet-SF-KDM2A cells (WT),
MCF-7tet-mCXXC-SF-KDM2A cells (mCXXC), and MCF-7tet-on cells
(parent) were cultured in the presence or absence of Dox (1
μg/ml) for 72 h. ChIP analyses were performed to detect the
amounts of H3K4me2, H3K4me3, H43K9me2, H3K9me3, H3K27me2, H3K36me2,
H3K36me3, H4K20me3, H4ac, histone H3 and histone H4 in the rDNA
promoter. The results are expressed as fold-changes of the values
without Dox. (B) MCF-7 cells were treated with SF-KDM2A specific
siRNAs (#2), or control siRNA for 72-96 h, and analyzed by ChIP to
detect the amounts of H4K20me3, histone H3 and histone H4 in the
rDNA promoter. The results are expressed as fold-changes of the
values for control siRNA. All experiments were performed three
times and mean values with standard deviations are indicated.
*P<0.05. |
ChIP analyses of MCF-7 cells showed that the
treatment of SF-KDM2A siRNA reduced the amounts of SF-KDM2A
(Fig. 4E) and increased the levels
of H4K20me3 marks in rDNA promoter (Fig. 6B). The amounts of histone H3 and H4
were changed by neither SF-KDM2A siRNA nor control siRNA treatment.
These results suggest that SF-KDM2A reduces the level of H4K20me3
marks in the rDNA promoter.
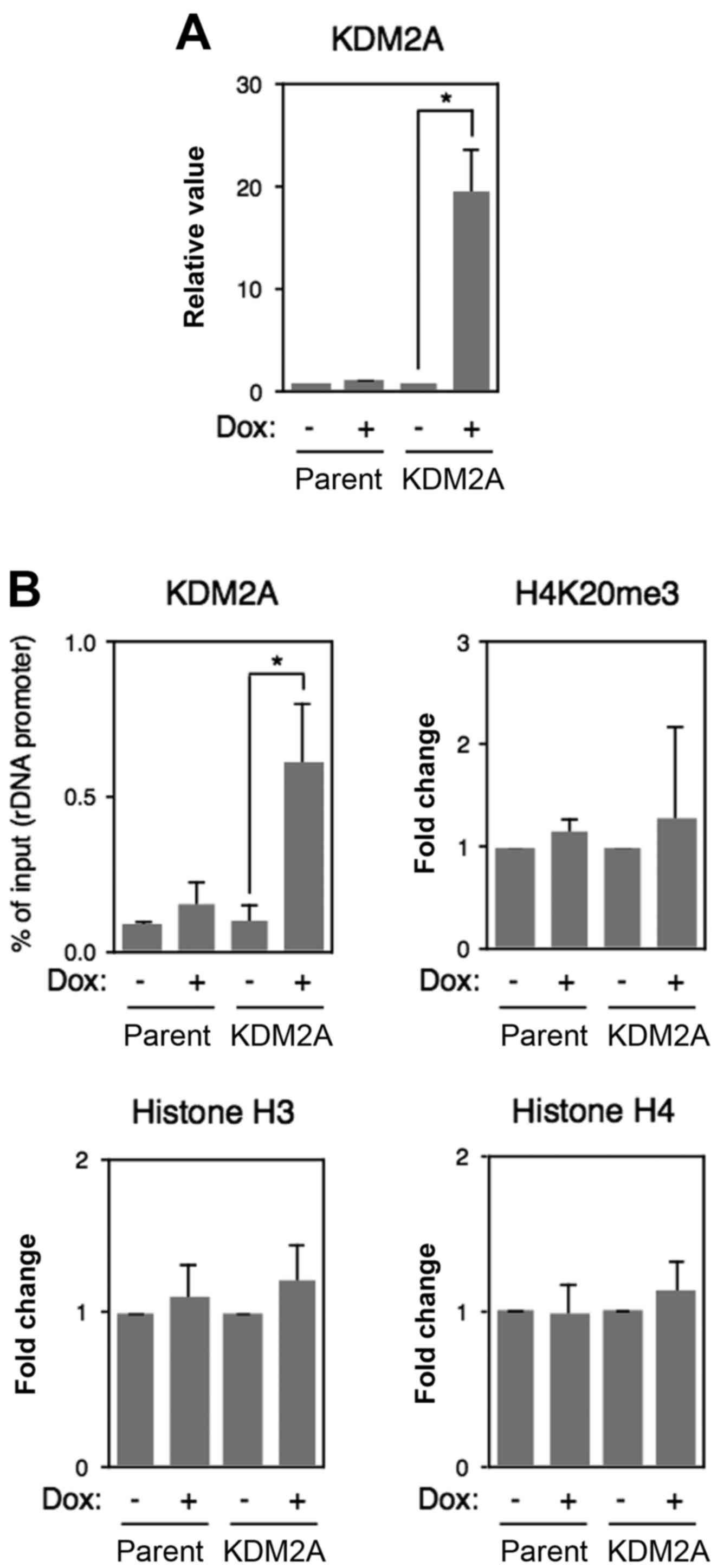
To test whether full-length KDM2A also reduces
H4K20me3 marks in the rDNA promoter, full-length KDM2A was
overexpressed in MCF-7 cells (Fig.
6A). ChIP analyses using anti-pan-KDM2A antibody showed that
KDM2A overexpression produced a level of signals similar to that of
SF-KDM2A overexpression (compare Fig.
7A with 4D, both are indicated by % of input). However, KDM2A
overexpression did not decrease the level of H4K20me3 marks. KDM2A
overexpression did not affect the amounts of histone H3 and histone
H4 in the rDNA promoter (Fig. 7B).
These results suggest that SF-KDM2A has a distinct activity from
KDM2A in the rDNA promoter.
SF-KDM2A is required to maintain cell
proliferation
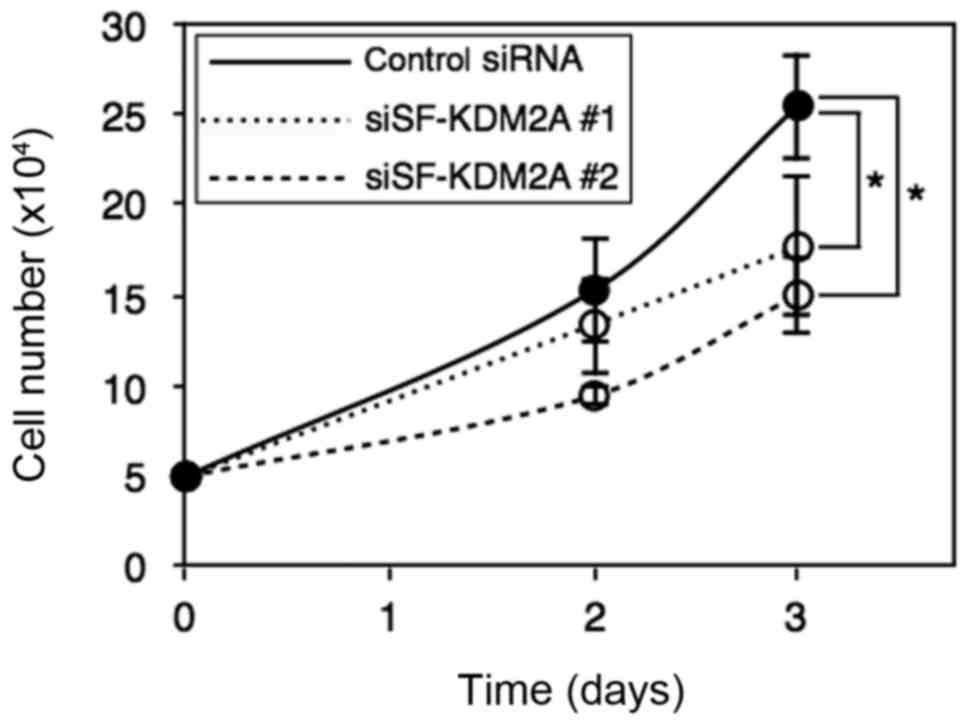
Whether the reduction of SF-KDM2A affected cell
proliferation was investigated. After MCF-7 cells were transfected
with SF-KDM2A siRNA, cell numbers were counted. After three days,
cell numbers were reduced to about a half (Fig. 8). These results suggest that
endogenous SF-KDM2A is required to maintain cell proliferation in
breast cancer cells.
Discussion
In the present study, we investigated the functions
of SF-KDM2A, which is produced by the KDM2A gene, but does
not have the domain for histone demethylase activity (Fig. 1). SF-KDM2A localized in the
nucleoli (Figs. 2 and 3) and bound to the rDNA promoter via its
zf-CXXC domain in a breast cancer cell line MCF-7 (Fig. 3) as full-length KDM2A did (12,13).
Overexpression of SF-KDM2A stimulated rRNA transcription, which
depended on the zf-CXXC motif (Fig.
5). Knockdown of SF-KDM2A reduced rRNA transcription (Fig. 5). Furthermore, we found that
overex-pression of SF-KDM2A reduced the level of a repressive
histone mark, H4K20me3, in the rDNA promoter, and knockdown of
SF-KDM2A increased H4K20me3 marks in the rDNA promoter in MCF-7
cells (Fig. 6). It has been shown
that rRNA transcription levels are associated with cell
proliferation (1-3), and we found that knockdown of
SF-KDM2A reduced cell proliferation (Fig. 8). These results suggest that
SF-KDM2A regulates cell proliferation through controlling rRNA
transcription by regulating the level of H4K20me3 marks in the rDNA
promoter. Induction of SF-KDM2A expression in MCF-7tet-SF-KDM2A
cells did not change cell proliferation (data not shown). These
results suggest that rRNA expression levels supported by endogenous
SF-KDM2A are sufficient for proper cell proliferation, and further
upregulation does not increase cell proliferation in the
experimental conditions tested here.
A recent report suggests that elevated expression of
the KDM2A gene is significantly associated with short
survival of breast cancer patients, that SF-KDM2A is more abundant
than full-length KDM2A in a subset of breast cancers, and thus
SF-KDM2A may have oncogenic potential (15). The functions of KDM2A have been
shown to be associated with its demethylase activity (12–14).
Therefore, SF-KDM2A should affect cell activities in ways different
from KDM2A. We observed that overexpression of KDM2A in MCF-7 cells
did not change the level of H4K20me3 in the rDNA promoter (Fig. 7). These results suggest that the
activities of SF-KDM2A in the rDNA promoter are different from
those of KDM2A. Because there are no domains expected to function
as a histone demethylase in SF-KDM2A, SF-KDM2A may affect other
factors in rDNA promoter to reduce H4K20me3 levels. It was
suggested that a JmjC enzyme PHF2 was associated with rDNA promoter
(17), and that PHF2 reduced
H4K20me3 at TLR4-responsive promoters (18), although the H4K20me3 demethylase
activity of PHF2 was not reported in rDNA promoter. Suv4-20h2 was
detected in rDNA promoter and induced H4K20me3 methylation
(19). Since SF-KDM2A binds to
rDNA promoter, it is possible that SF-KDM2A specifically affects
the activities of these H4K20me3 modifying enzymes to change the
status of H4K20 methylation in rDNA promoter (Fig. 9). The reduced levels of H4K20me3
may relax chromatin compaction to increase gene expression
(19).
Methylation of H4K20 is important for biological
processes (20). H4K20me3 is found
in constitutive hetero-chromatin regions, and is enriched in
regions of chromatin containing silenced genes (21,22).
The levels of H4K20me3 marks are globally decreased in cancer cells
in non-small cell lung and breast cancers (23–27).
In breast carcinomas, the levels of H4K20me3 are associated with
clinicopathological status (27),
and a decreased H4K20me3 level is associated with shorter
disease-free survival in breast cancer patients (27). Therefore, our finding that the
reduction of H4K20me3 observed in SF-KDM2A expressing cells is
consistent with the fact that H4K20me3 is decreased in malignant
breast cancer.
The KDM2B gene, encoding a paralogue of
KDM2A, also produces two forms of KDM2B like the KDM2A gene
(28). The larger one is the
full-length isoform of KDM2B, and the smaller one is a short
isoform of KDM2B lacking a JmjC domain (SF-KDM2B). While the
expression level of full-length KDM2B is elevated only in early
embryos, the short form of KDM2B is ubiquitously expressed during
embryonic development in mice (29). Recent reports suggest that SF-KDM2B
cannot rescue the early differentiation phenotypes induced by KDM2B
knockdown (30). These results
suggest that SF-KDM2B has different functions from full-length
KDM2B, although the function of SF-KDM2B is unclear. FbxL19, a
member of the F-box proteins, contains zf-CXXC, a PHD domain and
three leucine-rich repeats, but not a jmjC domain. This domain
structure of FbxL19 is quite similar to that of SF-KDM2A. FbxL19
functions as E3 ubiquitin ligases. The Skp1-Cullin-F-box complex,
SCFFbxL19, mediates degradation of Rho family proteins
(31,32). It is an open question whether
SF-KDM2A mediates poly-ubiquitination and degradation of
protein.
To the best of our knowledge, there are no published
studies that described the functional roles of SF-KDM2A in human
cancers besides breast cancers. On the other hand, there are some
reports, which described the roles of KDM2A in human malignancies.
Expression of KDM2A was elevated in lung cancer tissues and
overexpression of KDM2A in lung cancer increased tumorigenesis in
its demethylase activity dependent manner (33), probably through epigenetic
repression of expression of dual-specificity phosphatase 3
(33) and HDAC3 (34). The expression level of KDM2A was
increased in gastric cancer tissues. Forced expression of KDM2A in
gastric cancer cells promoted cell growth and migration (35). RUNX3-mediated upregulation of
miR-29b inhibited the proliferation and migration of gastric cancer
cells by targeting KDM2A (36). An
in vitro clonogenic assay showed that knockdown of KDM2A
significantly inhibited the colony forming ability of human
prostate adenocarcinoma (37).
These results suggest that KDM2A have tumor-promoting functions.
However, in some studies PCR primers used to detect KDM2A mRNA can
amplify both KDM2A and SF-KDM2A mRNAs, and KDM2A siRNA that could
reduce expression of both full-length KDM2A and SF-KDM2A was used
to investigate KDM2A functions. It is also sometimes unclear
whether antibody used to detect KDM2A recognized only KDM2A but
also SF-KDM2A. Therefore, it is not clear whether some results
reported as KDM2A functions to date were due to full-length KDM2A,
SF-KDM2A or both. The careful experiments to investigate two
KDM2A gene products separately may clarify the functions of
this gene.
In summary, the KDM2A gene produces KDM2A and
SF-KDM2A. SF-KDM2A shares plural domains with KDM2A except the JmjC
domain. We found that SF-KDM2A bound to the rDNA promoter via a
zf-CXXC motif as full-length KDM2A and is involved in regulation of
rRNA synthesis, but the effects of SF-KDM2A on rRNA transcription
are not the same as those of full-length KDM2A. SF-KDM2A reduces
the transcriptional repressive histone mark H4K20me3 in the rDNA
promoter and activates rRNA transcription. Knockdown of SF-KDM2A
reduces cell proliferation. Our results suggest that SF-KDM2A may
contribute to tumorigenesis through stimulation of rRNA
transcription.
Abbreviations:
|
zf-CXXC
|
zinc finger CXXC
|
|
SF-KDM2A
|
short form-KDM2A
|
|
rDNA
|
rRNA gene
|
|
ChIP
|
chromatin immunoprecipitation
|
Acknowledgments
The present study was supported by the JSPS KAKENHI
grant no. 16K07358. We thank Katherine Ono for proofreading the
manuscript.
References
|
1
|
Grummt I: Life on a planet of its own:
Regulation of RNA polymerase I transcription in the nucleolus.
Genes Dev. 17:1691–1702. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Laferté A, Favry E, Sentenac A, Riva M,
Carles C and Chédin S: The transcriptional activity of RNA
polymerase I is a key determinant for the level of all ribosome
components. Genes Dev. 20:2030–2040. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chédin S, Laferté A, Hoang T, Lafontaine
DL, Riva M and Carles C: Is ribosome synthesis controlled by pol I
transcription? Cell Cycle. 6:11–15. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ruggero D and Pandolfi PP: Does the
ribosome translate cancer? Nat Rev Cancer. 3:179–192. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Berger SL: The complex language of
chromatin regulation during transcription. Nature. 447:407–412.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kustatscher G and Ladurner AG: Modular
paths to 'decoding' and 'wiping' histone lysine methylation. Curr
Opin Chem Biol. 11:628–635. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bannister AJ and Kouzarides T: Reversing
histone methylation. Nature. 436:1103–1106. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Teperino R, Schoonjans K and Auwerx J:
Histone methyl transferases and demethylases; can they link
metabolism and transcription? Cell Metab. 12:321–327. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Agger K, Christensen J, Cloos PA and Helin
K: The emerging functions of histone demethylases. Curr Opin Genet
Dev. 18:159–168. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Accari SL and Fisher PR: Emerging roles of
JmjC domain-containing proteins. Int Rev Cell Mol Biol.
319:165–220. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tsukada Y, Fang J, Erdjument-Bromage H,
Warren ME, Borchers CH, Tempst P and Zhang Y: Histone demethylation
by a family of JmjC domain-containing proteins. Nature.
439:811–816. 2006. View Article : Google Scholar
|
|
12
|
Tanaka Y, Okamoto K, Teye K, Umata T,
Yamagiwa N, Suto Y, Zhang Y and Tsuneoka M: JmjC enzyme KDM2A is a
regulator of rRNA transcription in response to starvation. EMBO J.
29:1510–1522. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tanaka Y, Umata T, Okamoto K, Obuse C and
Tsuneoka M: CxxC-ZF domain is needed for KDM2A to demethylate
histone in rDNA promoter in response to starvation. Cell Struct
Funct. 39:79–92. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tanaka Y, Yano H, Ogasawara S, Yoshioka S,
Imamura H, Okamoto K and Tsuneoka M: Mild glucose starvation
induces KDM2A-mediated H3K36me2 demethylation through AMPK to
reduce rRNA transcription and cell proliferation. Mol Cell Biol.
35:4170–4184. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu H, Liu L, Holowatyj A, Jiang Y and
Yang ZQ: Integrated genomic and functional analyses of histone
demethylases identify oncogenic KDM2A isoform in breast cancer. Mol
Carcinog. 55:977–990. 2016. View
Article : Google Scholar
|
|
16
|
Blackledge NP, Zhou JC, Tolstorukov MY,
Farcas AM, Park PJ and Klose RJ: CpG islands recruit a histone H3
lysine 36 demethylase. Mol Cell. 38:179–190. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shi G, Wu M, Fang L, Yu F, Cheng S, Li J,
Du JX and Wong J: PHD finger protein 2 (PHF2) represses ribosomal
RNA gene transcription by antagonizing PHF finger protein 8 (PHF8)
and recruiting methyltransferase SUV39H1. J Biol Chem.
289:29691–29700. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stender JD, Pascual G, Liu W, Kaikkonen
MU, Do K, Spann NJ, Boutros M, Perrimon N, Rosenfeld MG and Glass
CK: Control of proinflammatory gene programs by regulated
trimethylation and demethylation of histone H4K20. Mol Cell.
48:28–38. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bierhoff H, Dammert MA, Brocks D,
Dambacher S, Schotta G and Grummt I: Quiescence-induced LncRNAs
trigger H4K20 trimethylation and transcriptional silencing. Mol
Cell. 54:675–682. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jørgensen S, Schotta G and Sørensen CS:
Histone H4 lysine 20 methylation: Key player in epigenetic
regulation of genomic integrity. Nucleic Acids Res. 41:2797–2806.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schotta G, Lachner M, Sarma K, Ebert A,
Sengupta R, Reuter G, Reinberg D and Jenuwein T: A silencing
pathway to induce H3-K9 and H4-K20 trimethylation at constitutive
heterochromatin. Genes Dev. 18:1251–1262. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Henckel A, Nakabayashi K, Sanz LA, Feil R,
Hata K and Arnaud P: Histone methylation is mechanistically linked
to DNA methylation at imprinting control regions in mammals. Hum
Mol Genet. 18:3375–3383. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fraga MF, Ballestar E, Villar-Garea A,
Boix-Chornet M, Espada J, Schotta G, Bonaldi T, Haydon C, Ropero S,
Petrie K, et al: Loss of acetylation at Lys16 and trimethylation at
Lys20 of histone H4 is a common hallmark of human cancer. Nat
Genet. 37:391–400. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Van Den Broeck A, Brambilla E,
Moro-Sibilot D, Lantuejoul S, Brambilla C, Eymin B, Khochbin S and
Gazzeri S: Loss of histone H4K20 trimethylation occurs in
preneoplasia and influences prognosis of non-small cell lung
cancer. Clin Cancer Res. 14:7237–7245. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Füllgrabe J, Kavanagh E and Joseph B:
Histone onco-modifications. Oncogene. 30:3391–3403. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chekhun VF, Lukyanova NY, Kovalchuk O,
Tryndyak VP and Pogribny IP: Epigenetic profiling of
multidrug-resistant human MCF-7 breast adenocarcinoma cells reveals
novel hyper- and hypomethylated targets. Mol Cancer Ther.
6:1089–1098. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yokoyama Y, Matsumoto A, Hieda M, Shinchi
Y, Ogihara E, Hamada M, Nishioka Y, Kimura H, Yoshidome K,
Tsujimoto M, et al: Loss of histone H4K20 trimethylation predicts
poor prognosis in breast cancer and is associated with invasive
activity. Breast Cancer Res. 16:R662014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pfau R, Tzatsos A, Kampranis SC,
Serebrennikova OB, Bear SE and Tsichlis PN: Members of a family of
JmjC domain-containing oncoproteins immortalize embryonic
fibroblasts via a JmjC domain-dependent process. Proc Natl Acad Sci
USA. 105:1907–1912. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fukuda T, Tokunaga A, Sakamoto R and
Yoshida N: Fbxl10/Kdm2b deficiency accelerates neural progenitor
cell death and leads to exencephaly. Mol Cell Neurosci. 46:614–624.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
He J, Shen L, Wan M, Taranova O, Wu H and
Zhang Y: Kdm2b maintains murine embryonic stem cell status by
recruiting PRC1 complex to CpG islands of developmental genes. Nat
Cell Biol. 15:373–384. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wei J, Mialki RK, Dong S, Khoo A,
Mallampalli RK, Zhao Y and Zhao J: A new mechanism of RhoA
ubiquitination and degradation: Roles of SCF(FBXL19) E3 ligase and
Erk2. Biochim Biophys Acta. 1833:2757–2764. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhao J, Wei J, Mialki RK, Mallampalli DF,
Chen BB, Coon T, Zou C, Mallampalli RK and Zhao Y: F-box protein
FBXL19-mediated ubiquitination and degradation of the receptor for
IL-33 limits pulmonary inflammation. Nat Immunol. 13:651–658. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wagner KW, Alam H, Dhar SS, Giri U, Li N,
Wei Y, Giri D, Cascone T, Kim JH, Ye Y, et al: KDM2A promotes lung
tumorigenesis by epigenetically enhancing ERK1/2 signaling. J Clin
Invest. 123:5231–5246. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dhar SS, Alam H, Li N, Wagner KW, Chung J,
Ahn YW and Lee MG: Transcriptional repression of histone
deacetylase 3 by the histone demethylase KDM2A is coupled to
tumorigenicity of lung cancer cells. J Biol Chem. 289:7483–7496.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Huang Y, Liu Y, Yu L, Chen J, Hou J, Cui
L, Ma D and Lu W: Histone demethylase KDM2A promotes tumor cell
growth and migration in gastric cancer. Tumour Biol. 36:271–278.
2015. View Article : Google Scholar
|
|
36
|
Kong Y, Zou S, Yang F, Xu X, Bu W, Jia J
and Liu Z: RUNX3-mediated up-regulation of miR-29b suppresses the
proliferation and migration of gastric cancer cells by targeting
KDM2A. Cancer Lett. 381:138–148. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nalla AK, Williams TF, Collins CP, Rae DT
and Trobridge GD: Lentiviral vector-mediated insertional
mutagenesis screen identifies genes that influence androgen
independent prostate cancer progression and predict clinical
outcome. Mol Carcinog. 55:1761–1771. 2016. View Article : Google Scholar
|