Introduction
SMAD proteins are distributed in the nucleus and
cytoplasm. The SMAD4 gene is located on chromosome 18q21 (1), a putative location for other
tumor-suppressor genes (2). Loss
of Smad4 plays a causal role in initiating squamous cell carcinomas
of skin and upper digestive tract as well as adenocarcinomas of
gastrointestinal tract, SMAD4 inactivation is associated with a
poor prognosis (3).
Schwarte-Waldhoff and and Schmiegel (4) used restoration of Smad4 in deficient
cancer cells as an impartial approach to reveal the Smad4 tumor
suppressor functions. However, stable re-expression of Smad4 in
human colon and pancreatic cancer cells potently suppressed tumor
growth in vivo in nude mice. However, it was not sufficient
to suppress tumor cell growth in vitro, nor did it restore
TGF-β responsiveness. Rather, Smad4 restoration influenced
angiogenesis by decreasing expression of vascular endothelial
growth factor and increasing expression of thrombospondin-1. These
findings suggest that Smad4 not only inhibits the uncontrolled
proliferation of epithelial cells, but also mediates tumor
promotion predominately through the surrounding stroma (such as
endothelial cells) other than the precancerous epithelial cells
themselves (5).
Mutations in TGF-β pathway gene SMAD4, have been
admitted as genetic causes of a vascular malformation syndrome,
hereditary hemorrhagic telangiectasia (HHT) (6–8).
Infants and children with a family history of HHT are at risk for
sudden and catastrophic intracranial hemorrhage (ICH) (9). However, there is not much research on
the role of Smad4 in the cancer blood endothelial cells (BECs).
In this study, we identified that SMAD4 expression
is decreased in the vessel ECs of the ovarian cancer. In
vitro and in vivo assays revealed that loss SMAD4 could
reduce angiogenesis but increase vessel hyperpermeability and tumor
invasion, by modulating the FYN signaling pathway. Taken together,
these data highlight the possibility that SMAD4 could be as a
therapeutic target in combating ovarian cancer in the future.
Materials and methods
Cell
The human ovarian cancer cell line C13K was a gift
from the Department of Obstetrics and Gynecology, Ottawa
University, Department of Cell and Molecular Research Center. SKOV3
and A2780 and breast cancer line MDA-MB-231 were purchased from
ATCC and cultured according to their guidelines. All the above cell
lines used in this study were cultured in RPMI-1640 medium
supplemented with 5% fetal bovine serum. The cells were used for
the experiments within 20 passages. Human umbilical vein
endothelial cells (HUVECs) were purchased from Collection of
Biological Center, Wuhan University and cultured in endothelial
cell medium (ECM; ScienCell) with 5% FBS and endothelial growth
medium supplements.
Cell transfection
The Smad4-siRNA sequences used were as follows:
GUACUUCAUACCAUGCCGATT and UCGGCAUGGUAUGAAGUACTT. Alternatively,
Lipofectamine (Invitrogen)-mediated Smad4-siRNA Oligo transfection
was used to knock down Smad4 in HUVECs. Cells were harvested 2 days
later for expression analysis or in vitro tube
formation.
Animals
Female athymic nude (nu/nu) mice (4-week-old) were
purchased from SLAC Laboratory Animal Co. Ltd. (Shanghai, China)
for studies approved by the Committee on the Ethics of Animal
Experiments of Tongji Medical College. The mice were maintained in
the accredited animal facility of Tongji Medical College. C13K
tumor cells (3×106) and 1×106 HUVEC were
washed, suspended in 50 µl of PBS, and co-injected
subcutaneously into nude mice. Tumor volumes were measured using a
slide caliper every 3 days according to the formula: volume =
(larger diameter) × (smaller diameter)2 / 2 (10).
Immunohistochemistry
Specimens from normal ovary (14 cases), ovarian
carcinoma (19 cases) and normal endometrium (7 cases) were acquired
by surgeries as approved by the Ethics Committee of the Medical
Faculty of Tongji Medical College (Wuhan, China). The tumor
specimens were acquired from patients with cancer who had not
undergone preoperative radiotherapy or chemotherapy. Tissue
sections were subjected to immunohistochemical (IHC) analysis using
the avidin-biotin complex (ABC) Vectastain kit (Zsgb-Bio, Beijing,
China) according to the manufacturer's protocol. Anti-human CD34
(Abcam, ab81289), and anti-human SMAD4 (R&D Systems, AF2097)
antibodies were used as primary antibodies. Briefly, slides were
scanned at low power and the areas with the highest density of
CD34-positive vessels were identified. The pathological analyses
were done double-blinded.
Western blot analysis
Cells were pre-treated with siRNA as needed. Total
proteins were harvested with RIPA buffer. Immunoblotting was
performed according to manufacturer's instructions. The relative
expression level was quantified using Image-Pro Plus.
In vitro tube formation assay
HUVECs and primary pericytes were mixed and replated
to 48-well plates precoated with a thin layer of Matrigel (BD
Biosciences) in culture medium containing 5% FCS, and allowed to
form tube-like structures for 4–6 h. Measurement was performed as
described (11).
In vitro permeability assay
In vitro permeability assay were performed as
described (12). The concentration
of FITC-conjugated dextran (MW40,000, Sigma) was determined with an
EnVision fluorescence multiwell plate reader (BD) using a
fluorescein filter pair [Ex (l) 480 nm; Em (l)535 nm]. The
percentages of control were quantified.
Real-time PCR and microarray
analysis
RNA was extracted from HUVECs by TRIzol reagent
(Invitrogen) and reverse transcribed by using an mRNA selective PCR
kit (Takara). Real-time PCR was performed with Roche LightCycler
2.0 system using a SYBR Green assay. Primers used were as follows:
smad4 sense, 5′-CAGGATCAGTAGGTGGAAT AGC-3′; antisense,
5′-TCTTTGATGCTCTGTCTTGGG-3′. INSR sense,
5′-GGAAGTTACGTCTGATTCGAGG-3′; antisense,
5′-TGAGTGATGGTGAGGTTGTG-3′. FYN sense, 5′-ACTATGAAGCACGGACAGAAG-3′;
antisense, 5′-TGCTGGGAATGTAACCTGTC-3′. PTPRM sense,
5′-CGATGAGGTGAAGGTGTTAGG-3′; antisense, 5′-ACTGGAAGGT
AGCAAACTGG-3′. PTPRJ sense, 5′-CTAGTCCAATTCCTGACCCTTC-3′;
antisense, 5′-AGCTTTCACCATCCTCACTG-3′. VCAN sense,
5′-CACTCTAATCCCTGTCGTAATGG-3′; antisense,
5′-ATGTCTCGGTATCTTGCTCAC-3′. C CND2 sense,
5′-TGAGGAACAGAAGTGCGAAG-3′; antisense, 5′-TGGTCTCTTTGAGTTTGGAGG-3′.
Control-siRNA and Smad4-siRNA HUVECs were subjected to microarray
analysis, which was performed using human Gene 1.0 ST array
(Affymetrix).
Statistical methods
Data were evaluated using a Student's t-test,
two-tailed. p<0.05 and p<0.01 was considered statistically
significant. The error bars on graphs represent the mean ± standard
deviation (SD).
Results
Loss of SMAD4 in the blood vessel ECs of
the ovarian cancer
To identify the molecular differences between
tumor-associated BECs and their normal BEC counterparts, blood
vessels were isolated using in situ laser capture
microdissection and verified by the detection of the mRNA of
specific markers. Then, the gene expression profiles of tumor BECs
and normal BECs were analyzed using a cDNA microarray as described
(13). Since we focused on smad in
this study, SMAD4, among the top 10 genes, was chosen for further
investigation.
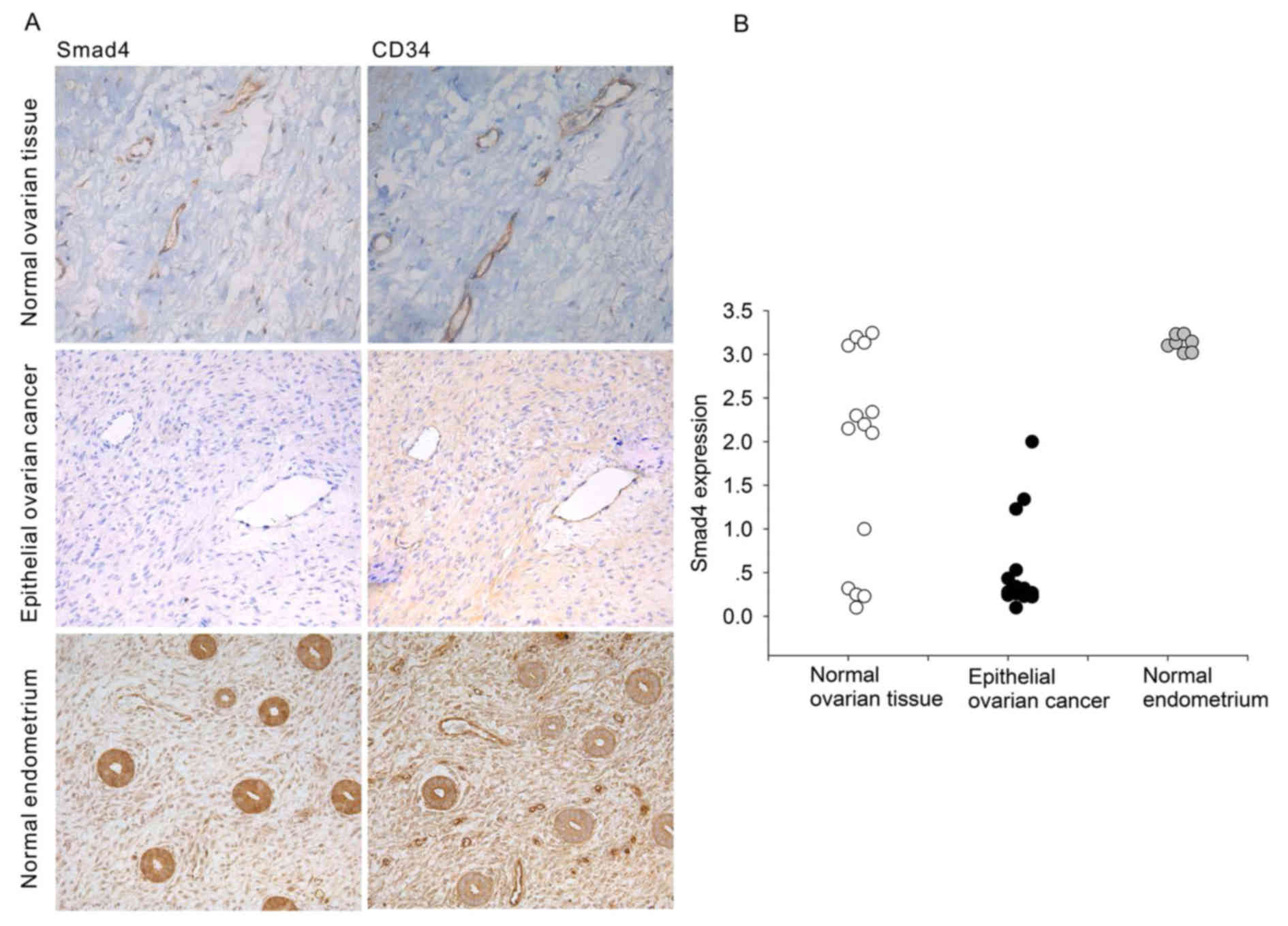
Immunohistochemical analysis of serial sections
showed that SMAD4 colocalized with the blood vessel marker CD34.
(Fig. 1A). Compared with blood
vessels in normal tissues (normal ovary and normal endometrium),
the expression of SMAD4 protein in tumor-associated blood vessels
was significantly decreased (Fig.
1A). In addition, we showed SMAD4 expression intensity in
Fig. 1B). These results suggest
that tumor blood vessels might functionally differ from normal
blood vessels due to loss of SMAD4 expression.
Effect of SMAD4 deletion in HUVECs in
vitro
Research on SMAD4 in ovarian cancer is scarce.
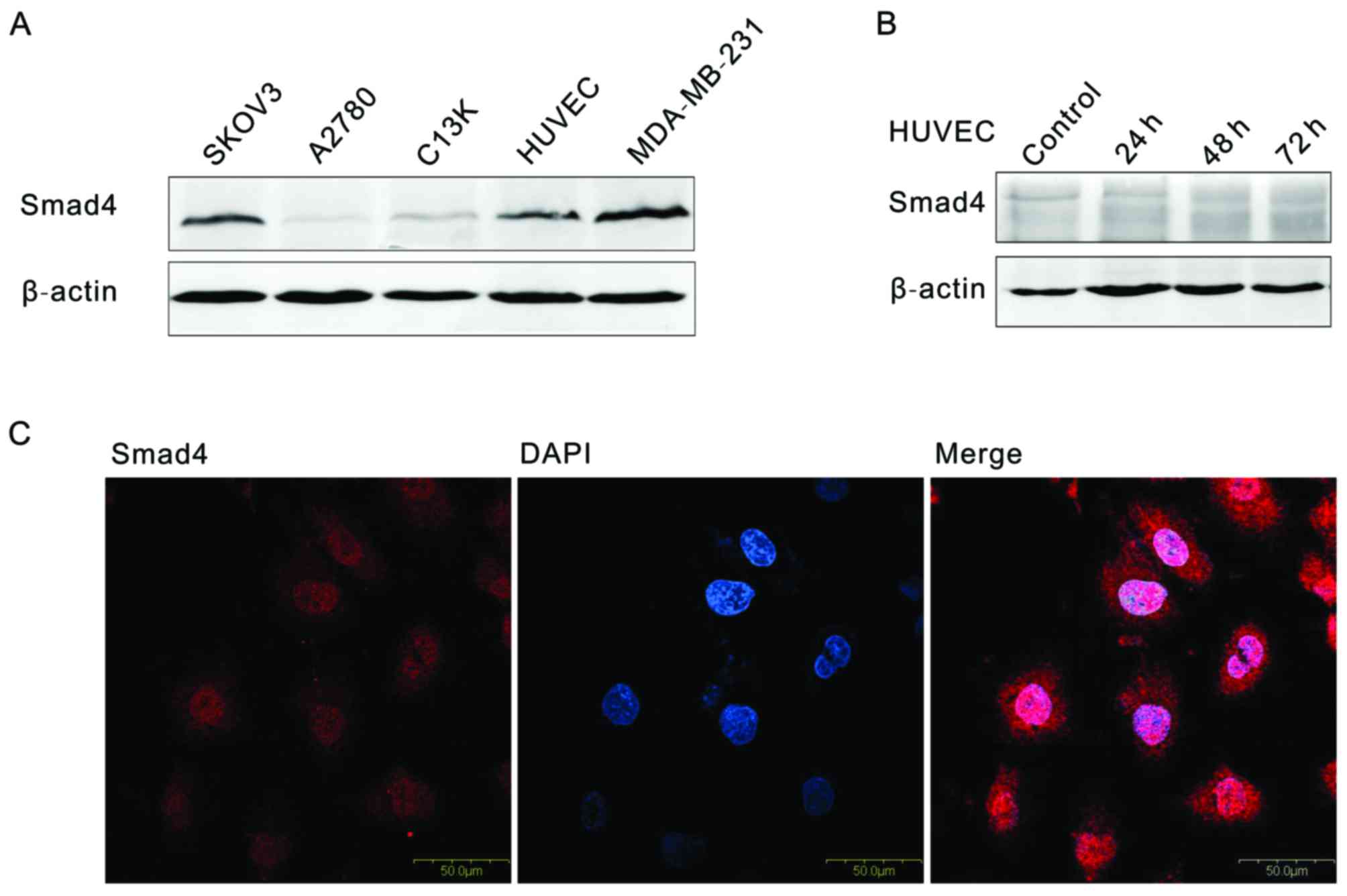
Western blotting and immunofluorescence assays were used to
detected SMAD4 expression. As shown in Fig. 2A, SMAD4 is expressed universally in
3 ovarian tumor cell lines (SKOV3, A2780 and C13K), human umbilical
vein endothelial cells (HUVECs) and one breast cancer cell line
(MDA-MB-231) (Fig. 2A).
Immunofluorescence analysis of SMAD4 protein are distributed in the
nuclear and cytoplasm in HUVEC. In order to simulate the low
expression of SMAD4, siRNA was used to interfere the SMAD4
expression in HUVEC, at 48 h, compared with control, the SMAD4
expression was decreased 90% (Fig.
2B).
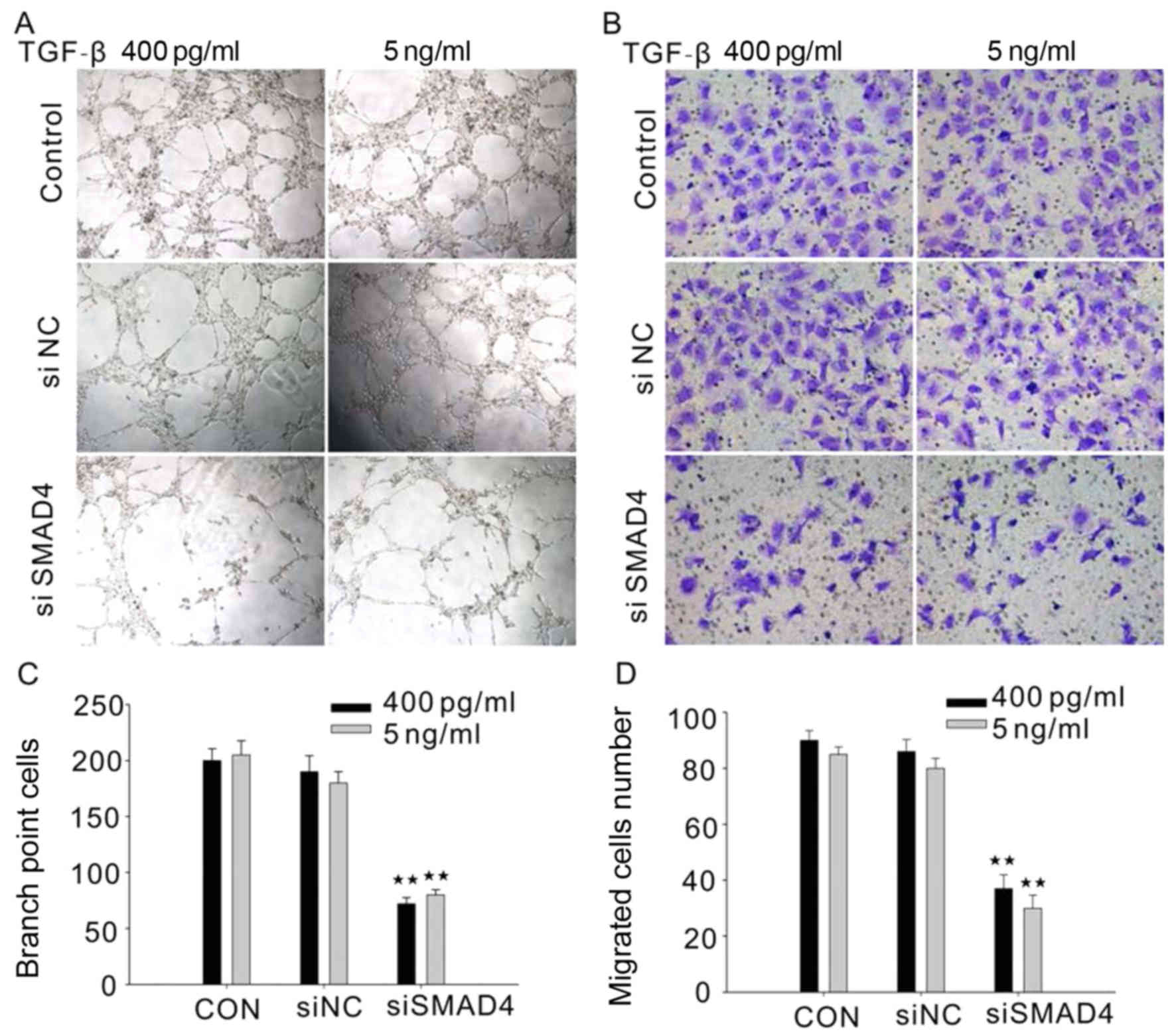
We next investigated the role of SMAD4 in regulation
of HUVEC tube formation and migration. TGF-β (5 ng/ml or 400 pg/ml)
treated HUVECs with siRNA targeting SMAD4 exhibit a decrease in
tube formation and migration (Fig. 3A
and B). This result raised the possibility that SMAD4 plays a
very important role in angiogenesis.
Effect of SMAD4 deletion in HUVECs in
vivo
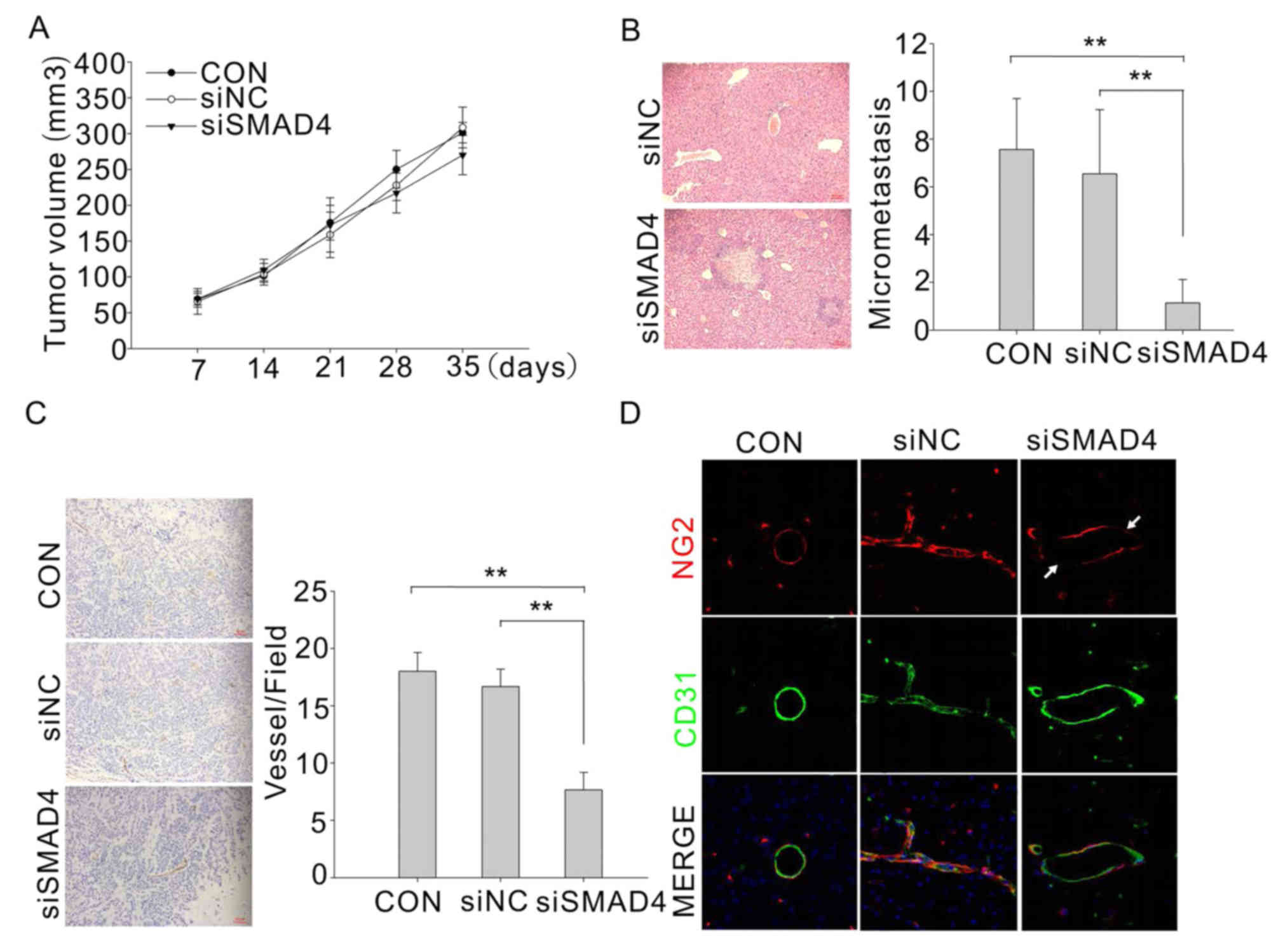
As SMAD4 deletion decrease the angiogenesis in
vitro, we next determined whether the effect also occur in
vivo. We transduced HUVECs with SMAD4-siRNA or control-siRNA
and co-inject with C13K subcutaneous in nude mice. Tumor volume was
periodically measured, and tumor weight was determined upon
dissection at the end of the experiment. Tumor xenografts with
SMAD4-deficient HUVECs did not display a significant reduction in
volume and tumor burden (Fig. 4A),
but they were unexpected associated with a remarkable increase of
spontaneous metastatic dissemination to the liver (Fig. 4B). The metastatic potential was
quantified by scoring micrometastasis in the liver. Moreover, we
found that blood vessels in the tumor with SMAD4-deficient HUVECs
were obviously reduced (Fig. 4C).
We further investigated whether there was any defect in mural cell
coverage. As shown in Fig. 4D, the
control tumor vasculature was completely enveloped by mural cells,
which were identified by NG2 immunostaining. In comparison, a local
smooth muscle cell-coating deficiency was observed in the vessel of
Smad4-deficient tumors. These results clearly showed that loss of
Smad4 in tumor BECs resulted in defective EC-mural cell contact,
which in turn might contribute to decreased mechanical stability,
then allow the tumor cells to cross the blood vessel barrier easier
and consequently tumor metastasis.
Smad4-deficient HUVECs show decreased
barrier function and three-dimensional tube formation in
coculture
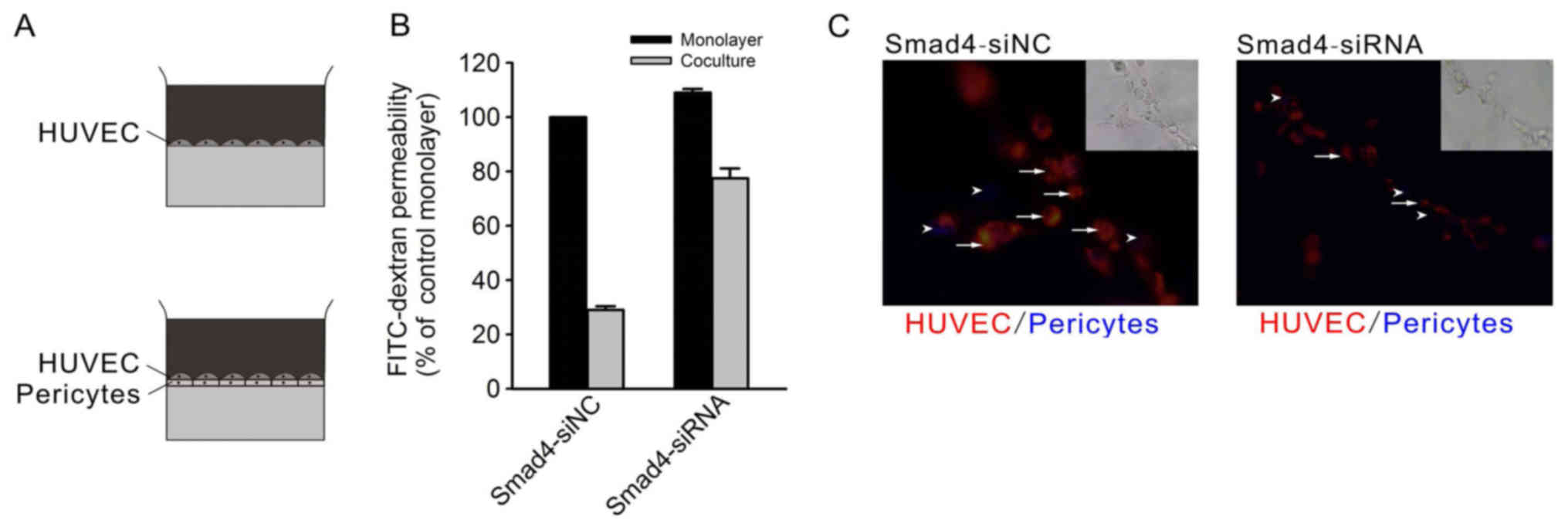
To explore EC-pericyte interaction in vitro,
we used an in vitro BBB model (13) by coculturing HUVECs with the
primary pericytes. When monolayer HUVECs were seeded on the
Transwell membrane in tracer permeability assays, there was no
apparent difference between siSMAD4 and siNC HUVECs (Fig. 5A and B). Coculture with pericytes
reduced the permeability in the control HUVECs to a greater degree
than in Smad4-deficient HUVECs, thus, the Smad4-deficient EC
barrier supported by pericytes was notably weaker than that of the
control.
To further validate the defect in EC-pericyte
interaction, we performed an in vitro three-dimensional
coculture system, in which both ECs and pericytes are
morphologically stretched and coordinately formed capillary-like
structures (14). With the same
primary pericytes in the coculture, Smad4-siRNA HUVECs demonstrated
significantly impaired tube-forming capacity compared to the siNC
HUVECs. Morphologically, Smad4-siRNA HUVECs showed inefficient
elongation and connection with pericytes (Fig. 5C). These results strengthen the
opinion that loss of Smad4 in ECs impairs EC-pericyte
interaction.
FYN facilitates EC-pericyte interactions
mediated by endothelial Smad4
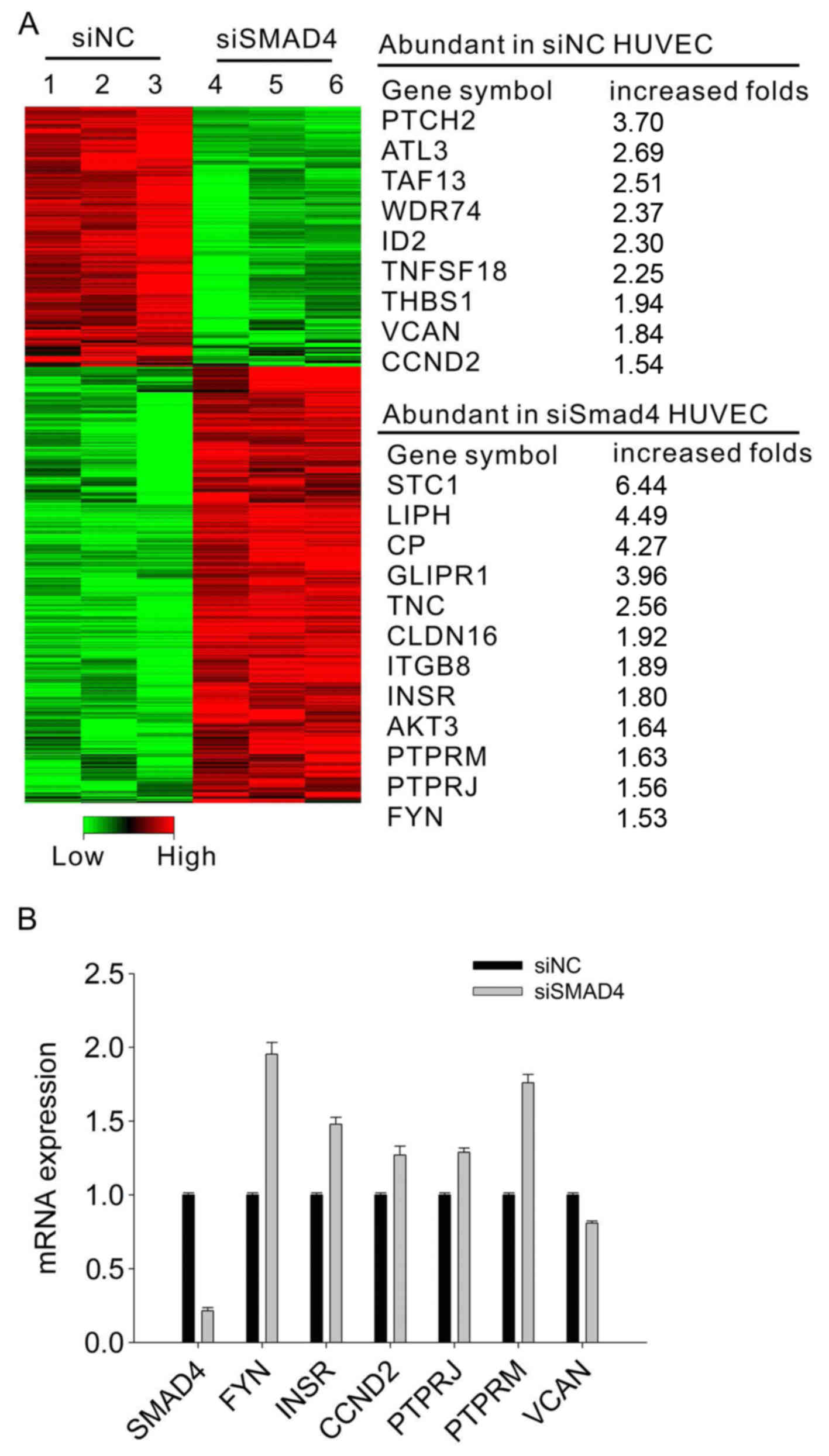
To identify potential Smad4 target genes that
regulate blood vascular integrity in ovarian tumor ECs, a
microarray assay was performed to compare the gene expression
profiles of HUVECs cells transfected with Smad4-siRNA and
control-siRNA. Using GO function enrichment and pathway enrichment
analysis, we further verified some expression changes of the genes
involving PTCH2, ATL3, PTPR and FYN (Fig. 6A). Of particular interest was the
remarkable increase in FYN in Smad4-siRNA HUVECs. We further
examined the FYN expression by real-time PCR. As shown in Fig. 6B, the decrease of SMAD4 in the
HUVECs induced a remarkably increased FYN expression. These results
demonstrated that impaired EC-pericyte interaction in
Smad4-deficient ECs might be largely due to the increased FYN
expression.
Discussion
The present study reveals an essential role for
endothelial Smad4 in the maintenance of ovarian tumor vascular
integrity. We show that endothelial Smad4-mediated signaling is
required for stabilizing the interaction between vascular ECs and
pericytes. Furthermore, we provide a mechanism between SMAD4 and
FYN signaling in maintaining ovarian tumor vascular integrity,
which has important implications for the treatment in combating
ovarian tumor.
Invading cancer cells could enter the circulation by
migrating directly through blood vessel walls (intravasation),
which requires the disruption of endothelial junctions. Factors
that locally reduce endothelial barrier function, such as
transforming growth factor-β (TGFβ) or vascular endothelial growth
factor (VEGF), increase the number of cancer cells entering into
blood vessels, increase metastasis (15) and contribute to extravasation.
While in this study, we found that the SMAD4 expression is reduced
in ovarian tumor vessel ECs, which could weaken cell-cell junctions
directly.
Pericytes are required for maintaining vascular
integrity (16,17), and TGFβ signaling has been
identified as an important signal pathway in the differentiation of
vascular smooth muscle cells/pericytes at mid-gestation, as
revealed by gene knockout research on the signal components,
including TGF-β1, Tgfbr2, Alk1, Alk5, endoglin, Smad5, and Smad4
(11,18–22).
When an inflammatory agonist (such as TGF-β1) binds to its
respective receptor expressed on the endothelial surface, multiple
cascades of intracellular signalling reactions are initiated, such
as Rho GTPases, MAP kinases and protein kinases (23).
Multiple cascades of intracellular signaling
reactions are initiated, such as Rho GTPases, MAP kinases and
protein kinases, when an inflammatory agonist binds to its
respective receptor expressed on the endothelial surface. Both in
the Rho GTPases and MAP kinases signaling activation, the Src
protein is playing a pivotal role (23). Also, Src family PTKs (c-Src, Blk,
Fgr, Fyn, Hck, Lck, Lyn, Yes and Yrk) have been implicated in the
regulation of vascular permeability in vitro (24,25)
as well as in vivo (26,27).
Pharmacological inhibition of Src family PTKs has been associated
with reduction of vascular permeability in response to several
agonists including VEGF (28).
Similarly, a role for Fyn has been described for increasing
transcellular permeability of microvascular endothelial cells to
albumin (29,30).
In this study, we identified that SMAD4 expression
is reduced in the vessel ECs of the ovarian cancer. Also, we found
that inactivation of SMAD4 could reduce angiogenesis but increase
vessel hyperpermeability and tumor invasion in vitro and
in vivo. Use of Gene chip screening of differentially
expressed genes, GO function enrichment and pathway enrichment
analysis, we discovered that SMAD4 could regulate the FYN
expression contrarily. Maybe loss of SMAD4 induced vessel barrier
dysfunction by activation of FYN, which will be studied in more
detail in the future. Taken together, these data highlight the
possibility that SMAD4 could be a therapeutic target in ovarian
cancer treatment in the future.
Acknowledgments
This study was supported by the '973' Program of
China (no. 2015CB553903), National Science-Technology Supporting
Projects (2015BAI13B05), Chinese National Key Plan of Precision
Medicine Research (2016YFC0902901) and National Science Foundation
of China (81472783, 81230038 and 81201639) and Tongji Hospital
(2201101877).
References
|
1
|
Ramachandra M, Atencio I, Rahman A,
Vaillancourt M, Zou A, Avanzini J, Wills K, Bookstein R and Shabram
P: Restoration of transforming growth factor beta signaling by
functional expression of smad4 induces anoikis. Cancer Res.
62:6045–6051. 2002.PubMed/NCBI
|
|
2
|
Lefter LP, Furukawa T, Sunamura M, Duda
DG, Takeda K, Kotobuki N, Oshimura M, Matsuno S and Horii A:
Suppression of the tumorigenic phenotype by chromosome 18 transfer
into pancreatic cancer cell lines. Genes Chromosomes Cancer.
34:234–242. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yatsuoka T, Sunamura M, Furukawa T,
Fukushige S, Yokoyama T, Inoue H, Shibuya K, Takeda K, Matsuno S
and Horii A: Association of poor prognosis with loss of 12q, 17p,
and 18q, and concordant loss of 6q/17p and 12q/18q in human
pancreatic ductal adenocarcinoma. Am J Gastroenterol. 95:2080–2085.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schwarte-Waldhoff I and Schmiegel W: Smad4
transcriptional pathways and angiogenesis. Int J Gastrointest
Cancer. 31:47–59. 2002. View Article : Google Scholar
|
|
5
|
Radisky DC and Bissell MJ: Cancer. Respect
thy neighbor. Science. 303:775–777. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Berg JN, Gallione CJ, Stenzel TT, Johnson
DW, Allen WP, Schwartz CE, Jackson CE, Porteous ME and Marchuk DA:
The activin receptor-like kinase 1 gene: Genomic structure and
mutations in hereditary hemorrhagic telangiectasia type 2. Am J Hum
Genet. 61:60–67. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gallione CJ, Repetto GM, Legius E, Rustgi
AK, Schelley SL, Tejpar S, Mitchell G, Drouin E, Westermann CJ and
Marchuk DA: A combined syndrome of juvenile polyposis and
hereditary haemorrhagic telangiectasia associated with mutations in
MADH4 (SMAD4). Lancet. 363:852–859. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
McAllister KA, Grogg KM, Johnson DW,
Gallione CJ, Baldwin MA, Jackson CE, Helmbold EA, Markel DS,
McKinnon WC, Murrell J, et al: Endoglin, a TGF-beta binding protein
of endothelial cells, is the gene for hereditary haemorrhagic
telangiectasia type 1. Nat Genet. 8:345–351. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Morgan T, McDonald J, Anderson C, Ismail
M, Miller F, Mao R, Madan A, Barnes P, Hudgins L and Manning M:
Intracranial hemorrhage in infants and children with hereditary
hemorrhagic telangiectasia (Osler-Weber-Rendu syndrome).
Pediatrics. 109:E122002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu S, Yu M, He Y, Xiao L, Wang F, Song C,
Sun S, Ling C and Xu Z: Melittin prevents liver cancer cell
metastasis through inhibition of the Rac1-dependent pathway.
Hepatology. 47:1964–1973. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang X, Bai X, Cao Y, Wu J, Huang M, Tang
D, Tao S, Zhu T, Liu Y, Yang Y, et al: Lymphoma endothelium
preferentially expresses Tim-3 and facilitates the progression of
lymphoma by mediating immune evasion. J Exp Med. 207:505–520. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lan Y, Liu B, Yao H, Li F, Weng T, Yang G,
Li W, Cheng X, Mao N and Yang X: Essential role of endothelial
Smad4 in vascular remodeling and integrity. Mol Cell Biol.
27:7683–7692. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nakagawa S, Deli MA, Nakao S, Honda M,
Hayashi K, Nakaoke R, Kataoka Y and Niwa M: Pericytes from brain
microvessels strengthen the barrier integrity in primary cultures
of rat brain endothelial cells. Cell Mol Neurobiol. 27:687–694.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Darland DC and D'Amore PA: TGF beta is
required for the formation of capillary-like structures in
three-dimensional cocultures of 10T1/2 and endothelial cells.
Angiogenesis. 4:11–20. 2001. View Article : Google Scholar
|
|
15
|
Anderberg C, Cunha SI, Zhai Z, Cortez E,
Pardali E, Johnson JR, Franco M, Páez-Ribes M, Cordiner R, Fuxe J,
et al: Deficiency for endoglin in tumor vasculature weakens the
endothelial barrier to metastatic dissemination. J Exp Med.
210:563–579. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Daneman R, Zhou L, Kebede AA and Barres
BA: Pericytes are required for blood-brain barrier integrity during
embryogenesis. Nature. 468:562–566. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lindahl P, Johansson BR, Levéen P and
Betsholtz C: Pericyte loss and microaneurysm formation in
PDGF-B-deficient mice. Science. 277:242–245. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dickson MC, Martin JS, Cousins FM,
Kulkarni AB, Karlsson S and Akhurst RJ: Defective haematopoiesis
and vasculogenesis in transforming growth factor-beta 1 knock out
mice. Development. 121:1845–1854. 1995.PubMed/NCBI
|
|
19
|
Larsson J, Goumans MJ, Sjöstrand LJ, van
Rooijen MA, Ward D, Levéen P, Xu X, ten Dijke P, Mummery CL and
Karlsson S: Abnormal angiogenesis but intact hematopoietic
potential in TGF-beta type I receptor-deficient mice. EMBO J.
20:1663–1673. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li DY, Sorensen LK, Brooke BS, Urness LD,
Davis EC, Taylor DG, Boak BB and Wendel DP: Defective angiogenesis
in mice lacking endoglin. Science. 284:1534–1537. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Oh SP, Seki T, Goss KA, Imamura T, Yi Y,
Donahoe PK, Li L, Miyazono K, ten Dijke P, Kim S, et al: Activin
receptor-like kinase 1 modulates transforming growth factor-beta 1
signaling in the regulation of angiogenesis. Proc Natl Acad Sci
USA. 97:2626–2631. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang X, Castilla LH, Xu X, Li C, Gotay J,
Weinstein M, Liu PP and Deng CX: Angiogenesis defects and
mesenchymal apoptosis in mice lacking SMAD5. Development.
126:1571–1580. 1999.PubMed/NCBI
|
|
23
|
Kumar P, Shen Q, Pivetti CD, Lee ES, Wu MH
and Yuan SY: Molecular mechanisms of endothelial hyperpermeability:
Implications in inflammation. Expert Rev Mol Med. 11:e192009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nwariaku FE, Liu Z, Zhu X, Turnage RH,
Sarosi GA and Terada LS: Tyrosine phosphorylation of vascular
endothelial cadherin and the regulation of microvascular
permeability. Surgery. 132:180–185. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tinsley JH, Ustinova EE, Xu W and Yuan SY:
Src-dependent, neutrophil-mediated vascular hyperpermeability and
beta-catenin modification. Am J Physiol Cell Physiol.
283:C1745–C1751. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Paul R, Zhang ZG, Eliceiri BP, Jiang Q,
Boccia AD, Zhang RL, Chopp M and Cheresh DA: Src deficiency or
blockade of Src activity in mice provides cerebral protection
following stroke. Nat Med. 7:222–227. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Weis S, Shintani S, Weber A, Kirchmair R,
Wood M, Cravens A, McSharry H, Iwakura A, Yoon YS, Himes N, et al:
Src blockade stabilizes a Flk/cadherin complex, reducing edema and
tissue injury following myocardial infarction. J Clin Invest.
113:885–894. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Eliceiri BP, Paul R, Schwartzberg PL, Hood
JD, Leng J and Cheresh DA: Selective requirement for Src kinases
during VEGF-induced angiogenesis and vascular permeability. Mol
Cell. 4:915–924. 1999. View Article : Google Scholar
|
|
29
|
Mehta D and Malik AB: Signaling mechanisms
regulating endothelial permeability. Physiol Rev. 286:279–367.
2006. View Article : Google Scholar
|
|
30
|
Gong P, Angelini DJ, Yang S, Xia G, Cross
AS, Mann D, Bannerman DD, Vogel SN and Goldblum SE: TLR4 signaling
is coupled to SRC family kinase activation, tyrosine
phosphorylation of zonula adherens proteins, and opening of the
paracellular pathway in human lung microvascular endothelia. J Biol
Chem. 283:13437–13449. 2008. View Article : Google Scholar : PubMed/NCBI
|