Introduction
Lung cancer is known as one of the most common human
cancers across the world (1). Lung
cancer is a disease due to multiple factors (2). Among all lung cancers, the non-small
cell lung cancer (NSCLC) accounts for approximately 80% (3). Despite continuous advances in
treatments, lung cancer remains the main reason for cancer-related
deaths (4). Thus, it is important
to understand the molecular mechanisms and to find effective
therapeutic strategies.
In the last several decades, finding the efficacy of
various natural compounds against different human metabolic
diseases have increased (5,6).
Compounds from many plants belonging to different groups, including
alkaloids, polyphenols and flavonoids evaluated for their role in
cancer-prevention, which have yielded promising data, thus,
supplying a potential therapeutic strategy against deadly diseases
(7). Carnosic acid (Fig. 1A) is known as a natural benzenediol
abietane diterpene detected in rosemary and common sage (8). Carnosic acid is used as a
preservative or antioxidant in food and non-food products,
including toothpaste, mouthwash and chewing gum (9). Presently, carnosic acid has been
suggested to possess some antitumor properties in mammary tumors,
colonic cancer, as well as skin tumors via regulation of cell
growth and apoptosis.
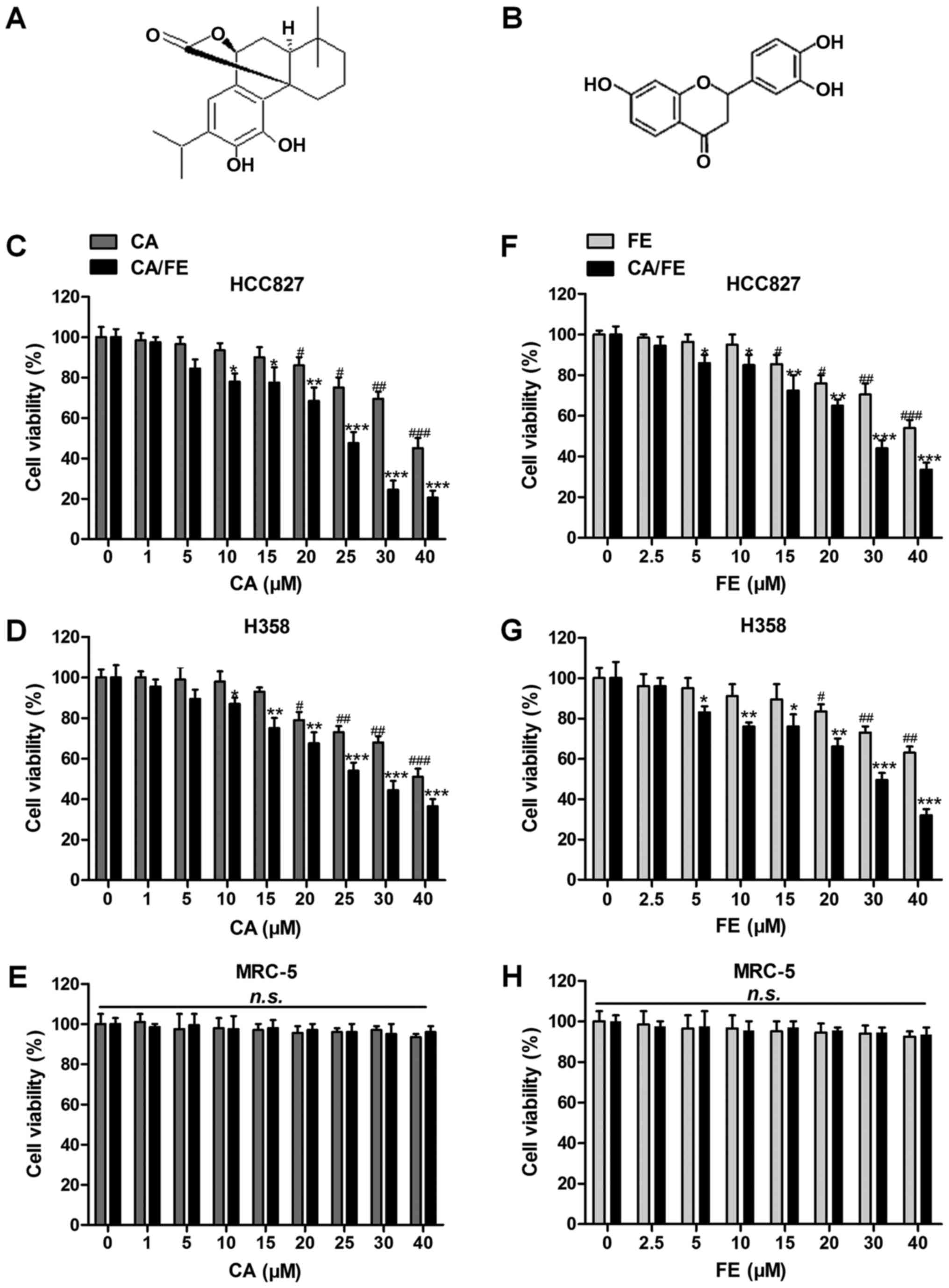 | Figure 1Effects of carnosic acid and fisetin
significantly suppress lung cancer cell proliferation. The chemical
structure of (A) carnosic acid and (B) fisetin is displayed. Left
column, MTT analysis of (C) HCC827, (D) H358 and (E) MRC-5 cells
treated with different concentrations (0, 1, 5, 10, 15, 20, 25, 30
and 40 μM) of CA in the presence or absence of FE (20
μM) for 24 h. Right column, MTT analysis of (F) HCC827, (G)
H358 and (H) MRC-5 cells treated with different concentrations (0,
2.5, 5, 10, 15, 20, 30 and 40 μM) of FE with or without CA
(20 μM) for 24 h. Values are means ± SEM. *P<0.05,
**P<0.01 and ***P<0.001 vs. Con group of CA/FE combination;
#P<0.05, ##P<0.01 and
###P<0.001 vs. Con group of CA or FE monotherapy.
n.s., no significance; CA, carnosic acid; FE, fisetin. |
In addition, the flavonol fisetin
(3,3′,4′,7-tetrahydroxyflavone) (Fig.
1B), in many kinds of fruits and vegetables such as grape,
strawberries, apple, persimmon, onion and cucumber, was suggested
to possess anti-oxidant, anti-microbial, anti-inflammatory and
significantly anti-carcinogenic activity when studied in various
animal model systems and cell cultures. Fisetin is a hydrophobic
compound, penetrating cell membranes in cells to perform its
effects (10,11). For example, it is claimed to be an
orally active neuroprotective and memory-enhancing molecule
(12). Additionally, fisetin could
induce apoptosis in cervical and breast cancer cells (13,14).
Furthermore, fisetin induced cell apoptotic death in human
hepatocellular carcinoma via p21 signaling pathway regulation
(15). Thus, we considered that
fisetin might have effective role in human gastric cancer
progression inhibition.
The present study aimed to calculate the potential
benefit and value of carnosic acid and fisetin in combination for
lung cancer treatment and to explore the possible molecular
mechanism by which the combinational therapy acts in modulating
lung cancer. To the best of our knowledge, this is the first time
that carnosic acid combined with fisetin is used to prevent lung
cancer in vitro and in vivo studies, which might
provide new therapeutic strategy for lung cancer treatment.
Materials and methods
Cells and culture
Human lung caner cell lines, HCC827 and H358 and
human normal lung cells MRC-5 were purchased from the American Type
Culture Collection (ATCC; Manassas, VA, USA). HCC827 and H358 cells
were routinely cultured in RPMI-1640 medium (Gibco, Waltham, MA,
USA), containing 10% fetal bovine serum (FBS; Gibco) and 1%
penicillin/streptomycin. MRC-5 was cultured in Dulbecco's modified
Eagle's medium (DMEM; Gibco) supplemented with 10% FBS, 100 U/ml
penicillin and 100 µg/ml streptomycin. All cells were
cultured in a humidified atmosphere with 5% CO2 and 95%
humidity at 37°C in an incubator. Fisetin and carnosic acid
(>98% purity), used for the treatment of lung cancer, were
purchased from Hangzhou DayangChem, Co., Ltd. (Hangzhou, China),
which was dissolved in dimethyl sulfoxide (DMSO) and stored at
−20°C, and then diluted in medium for experimental treatment. The
final DMSO concentration in the present study is no more than 0.1%
(v/v) in each treatment.
MTT analysis
Cells (5×103) were seeded into a 96-well
plate/well. Carnosic acid (0–40 µM), fisetin (0–40
µM), or the combination of both was added to the medium
after 24 h. The cells were then incubated at 37°C for 24 h, and the
cell viability was detected by the colorimetric MTT assay at 570 nm
(16).
Colony-forming assays
Lung cancer cells (500)/well in 60-mm plates were
cultured in 10% FBS RPMI-1640. Cells were treated with fisetin and
carnosic acid of the indicated concentrations for 24 h. After
another 7 days of incubation, the cell colonies were washed twice
with phosphate-buffered saline (PBS), fixed with 4%
paraformaldehyde for 15 min and then stained by Gimsa for 30 min.
All the clones with over 50 cells were evaluated. Clone forming
efficiency for cells was calculated based on colonies/number of
inoculated cells × 100% (17).
Cell migration assays
Lung cancer cells were seeded into the upper chamber
of a Transwell insert pre-coated with 5 µg/ml fibronectin
for migration or a BD™ Matrigel invasion chamber. Medium with 10%
serum was put in the lower chamber as a chemo-attractant, and cells
were then incubated for 4 h for migration. Non-migratory cells were
removed from the upper chamber by scraping with a cotton bud. The
cells on the lower insert surface were stained with Diff-Quick.
Cells were evaluated as the number of cells observed in five
different microscope fields of three independent inserts (18).
Scratch wound-healing analysis
The lung cancer cells used in this study were seeded
and grown on a 6-well plate overnight. The monolayers of lung
cancer cells were wounded with a pipette tip. Cells were then
washed with PBS to discard cellular debris and subjected to
migration for 24 h. Representative images were taken at 0 and 24 h
after the wounding through an inverted microscope (19).
Caspase-3 and -9 analysis
Caspase-3 and -9 activities were measured by
colorimetric activity assay kits (Clontech Laboratories, Inc.,
Mountain View, CA, USA) following the manufacturer's instructions.
The analysis is according to the chromogenic substrates cleavage,
DEVD-pNA by caspase-3 and LEHD-pNA by caspase-9, respectively.
Cells were dissolved in cold lysis buffer for 10 min and
centrifuged at 10,000 × g for 5 min. Then, solution of caspase
substrate containing specific peptide substrate was added to the
supernatant and grown at 37°C for 2 h before ELISA reader assay at
405 nm.
DNA staining analysis
Hoechst 33258 stain for DAPI staining analysis was
performed for morphological calculation of the nuclei. Lung cancer
cells were incubated with carnosic acid and fisetin and the two
combinations for 24 h. HCC827 and H358 cells were washed with
ice-cold PBS three times in 6-well plate and then stained with 0.5
ml Hoechst 33258 solution for 10 min at 37°C avoiding light. Then,
the cancer cells were washed with ice-cold PBS three times in the
plate. The cells were observed with an inverted fluorescence
microscope (Olympus Corp., Tokyo, Japan) (20).
Apoptosis assays
Apoptosis assay of samples was also determined by
terminal deoxynucleotidyl transferase-mediated dUTP nick end
labeling (TUNEL) using an In Situ Cell Death Detection kit,
Fluorescein (Roche Applied Science, South San Francisco, CA, USA)
according to the manufacturer's protocol. The number of
TUNEL-positive cells was counted under a fluorescence microscope.
The percentages of apoptotic cells were calculated from the ratio
of apoptotic cells to total cells counted. Tissue sections were
counter-stained with hematoxylin. Sections were mounted and
observed under a light microscope. The experiment was performed
independently three times.
Western blot analysis
The lung cancer cells and tumor tissue samples from
mice were homogenized into 10% (wt/vol) hypotonic buffer (25 mM
Tris-HCl, pH 8.0, 1 mM EDTA, 5 μg/ml leupeptin, 1 mM
Pefabloc SC, 50 μg/ml aprotinin, 5 μg/ml soybean
trypsin inhibitor and 4 mM benzamidine) to yield a homogenate.
Then, the final supernatants were obtained by centrifugation at
12,000 rpm for 15 min. Protein concentration was determined by BCA
protein assay kit (Thermo Fisher Scientific, Waltham, MA, USA) with
bovine serum albumin as a standard. The total protein extract was
used for western blot analysis. Equal amounts of total protein of
tissues were subjected to 10% SDS-PAGE followed by immunoblotting
using the following primary polyclonal antibodies (1:1,000): rabbit
anti-GAPDH, Bcl-xl, caspase-9, caspase-8, caspase-3, Bcl-2, Bad and
Bax. Immunoreactive bands were visualized by ECL Immunoblot
Detection system (Pierce Biotechnology, Inc., Rockford, IL, USA)
and exposed to Kodak (Eastman Kodak Company, Rochester, NY, USA)
X-ray film. Each protein expression level was defined as grey value
(Version 1.4.2b, Mac OS X, ImageJ; National Institutes of Health,
Bethesda, MD, USA) and standardized to housekeeping genes (GAPDH)
and expressed as a fold of control.
RT-qPCR assays
qPCR analysis, was performed as previously described
(16,21). Fold induction values were
calculated using the to 2−ΔΔCq method, where ΔCq
represents the differences in cycle threshold number between the
target gene and GAPDH, and ΔΔCq represents the relative change in
the differences between the control and the treatment groups. The
primers used in the study are shown in Table I.
 | Table IPrimer sequences of RT-PCR test. |
Table I
Primer sequences of RT-PCR test.
| Gene | Forward primers
(5′-3′) | Reverse primers
(5′-3′) |
|---|
| DR4 |
TAGGTGAGGTGGAGCTCAGATG |
TGCAACAGCGAAGACCTATTA |
| DR5 |
TATGGGAGCAACCGCTATA |
CGCGAACACAATGGCTATAA |
| TRAIL |
GAAACACGGTGACCACACCC |
CTCACAACGCTGCGGCGA |
| Bcl-xl |
ACAAACACCGCTGGCCA |
GCAGCATTACACAAACCAAGC |
| FADD |
ACAACGCTTCCAGCACC |
CCCGTTATGCGAAACCA |
| Bad |
TCACCAACGTTCGTCGT |
CATTGTCGTTGCAAGTATG |
| Bax |
AGCAAGACAAGGATGCTCG |
CAGCGTTCCATGTCAGTTATGTG |
| Bcl-2 |
GAGGCCAAGACAGGTATAC |
GCGTGGCAATTTAAGTTGTG |
| GAPDH |
CATTCAAGACCGGACAGAGG |
ACATACTCAGCACAGCATCACC |
Athymic nude mouse model
Eight-week-old athymic nude mice were purchased from
the Animal Center of Nanjing Medical University (Nanjing, China)
and kept in a 25±2°C temperature and 50±10% humidity-controlled
environment with a standard 12 h light/dark cycle with food and
water in cages under germ-free conditions. All processes were in
accordance with the Institutional Animal Care and Use Committee of
Huai'an First People's Hospital, Nanjing Medical University.
Briefly, 5×105 HCC827 and H358 cells were subcutaneously
injected into the dorsal flanks of nude mice. Tumor volume was
measured by calculating the two maximum perpendicular tumor
diameters every three days. The tumor-bearing nude mice were
randomly divided into 4 groups: i) control; ii) CA (30 mg/kg); iii)
FE (20 mg/kg); and iv) CA and FE combination every two day for 35
days. Carnosic acid and fisetin were dissolved in DMSO and then
diluted in distilled water. The mice were administered with CA and
FE orally. The control group was given DMSO diluted in water (0.5%
v/v). The body weight was measured twice a week. The tumor volume
was evaluated by a formula 1/2 (L1×L2×H) where L1 is the long
diameter, L2 is the short diameter and H is the height of tumor. At
the end of the present study, the mice were sacrificed. The tumor
tissue samples were removed for molecular mechanism research and
immunohistochemical analysis.
Immunohistochemical (IHC) assays
The tissues in each group were fixed with 10%
buffered formalin, imbedded in paraffin and sliced into 4–5
μm thick sections. Tumor tissues also were subjected to
immunohistochemical (IHC) staining for the analysis of p53
expression. The sections were stained with α-SMA, collagen type I,
collagen type II and MMP-9. All the histological examinations were
carried out according to the standard procedures previously
reported (17,22).
Statistical analysis
Data were expressed as mean ± standard error of the
mean (SEM). Statistical analyses were performed using GraphPad
Prism (version 6.0; GraphPad software) by ANOVA with Dunnet's least
significant difference post-hoc tests. A P<0.05 was considered
statistically significant.
Results
Carnosic acid and fisetin combination
significantly suppresses lung cancer cell proliferation
Before confirming the role of carnosic acid with
fisetin combination (CA/FE) in lung cancer, the possible
cytotoxicity of CA, FE and CA/FE towards lung cancer tumor cells
and normal human lung epithelia cells was explored. As shown in
Fig. 1C and D, at the
concentrations of 15 μM or lower, CA showed no significant
anticancer role in lung cancer cells of HCC827 and H358. Over 20
and 15 μM, CA exhibited remarkable effects on suppressing
HCC827 and H358 cells, respectively, suggesting that CA, to some
degree, possesses inhibitory role in controlling lung cancer cells,
especially combined with FE. However, no significant cytotoxicity
was observed in normal lung epithelia cells of MRC-5 even at the
highest concentration of 40 μM (Fig. 1E). Of note, after combination with
FE, huge anti-proliferation ability of CA and FE was observed.
Significant difference was found at the combination of CA at 10
μM with 20 μM FE and 5 μM CA with 20 μM
FE in HCC827 and H358 cancer cells, respectively, illustrating that
CA combined with FE displayed effective antitumor role in lung
cancer cells. Then, 20 μM CA was used combined with
different concentrations of FE to investigate the monotherapy of FE
and its combination with CA on lung cancer. As seen in Fig. 1F and G, FE alone could also reduce
lung cancer cells viability in a dose-dependent manner. In
addition, significant difference was found with up to 15 or 20
μM in HCC827 and H358 cells, respectively. Compared to FE
alone, CA/FE combination showed strong antitumor effects on HCC827
and H358 proliferation. On the contrary, no cytotoxicity was
observed in MRC-5 cells with the increase of CA treatment (Fig. 1H). The data suggest that CA/FE
possesses huge antitumor role in lung cancer cell proliferation
without causing cytotoxicity in normal cells. In the present study,
the concentrations of CA (20 μM) and FE (20 μM) were
used for combinational therapy in the following investigation.
Carnosic acid and fisetin combination
therapy inhibits lung cancer cell proliferation
In this regard, we attempted to investigate the
effects of CA/FE treatment on lung cancer cells proliferation and
migration. Whether the treatment of CA/FE influenced the clonogenic
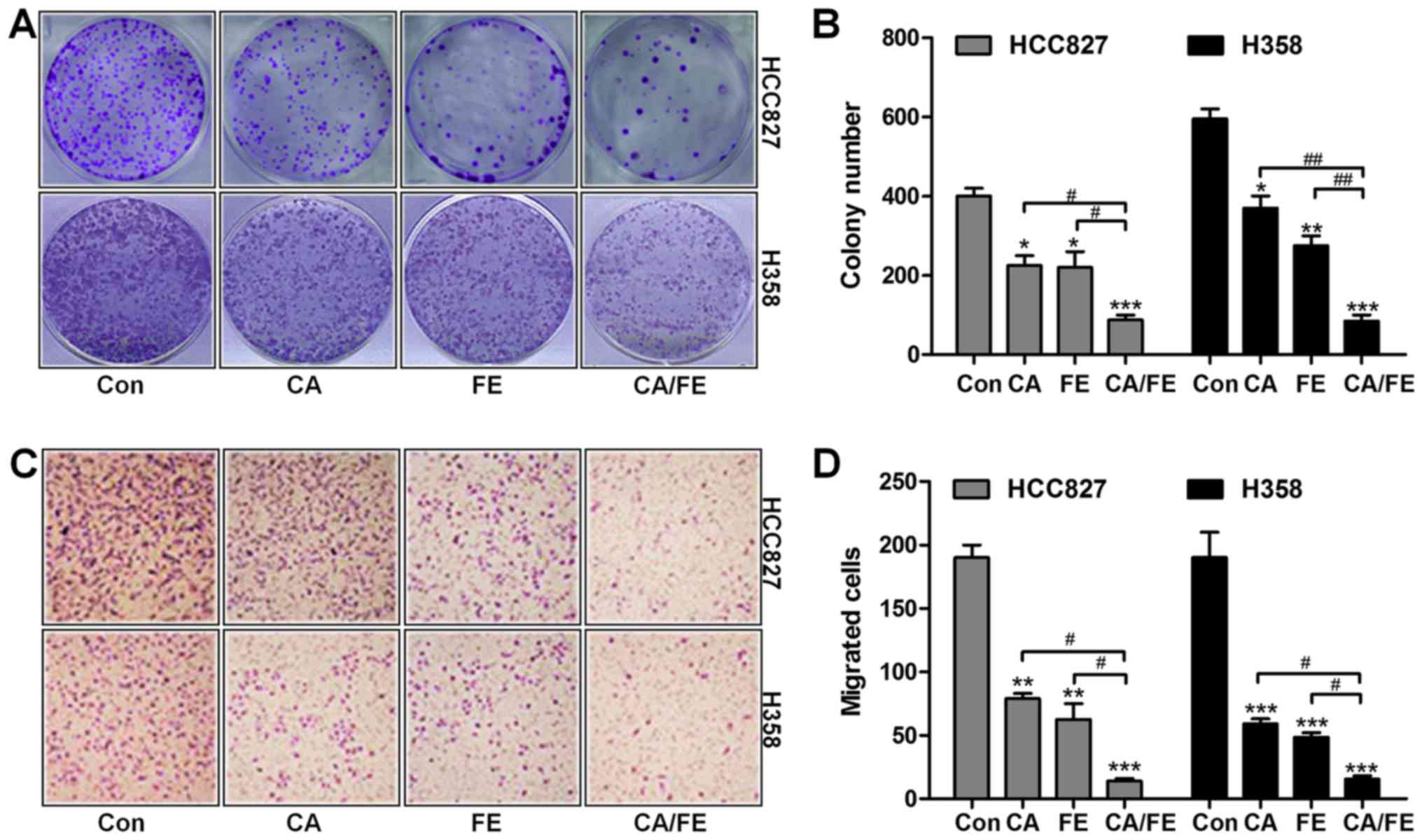
growth of HCC827 and H358, colony-forming analysis was assessed.
Our colony formation assays showed that CA and FE monotherapy
significantly reduced the colony number of cancer cells compared to
the control ones. Notably, combination of CA/FE markedly decreased
the clonogenic growth of lung cancer cells of HCC827 (19.36%) and
H358 (25.87%), which was comparable to CA and FE alone in HCC827
(40.29 and 38.95%) and H358 (71.23 and 56.37%) cells (Fig. 2A and B). In the presence of CA and
FE single therapy, the number of migrated cells of HCC827 and H358
was decreased. However, combination of CA/FE noticeably resulted in
a decreased number of migrated cancer cells of HCC827 (10.58%) and
H358 (11.46%) (Fig. 2C and D).
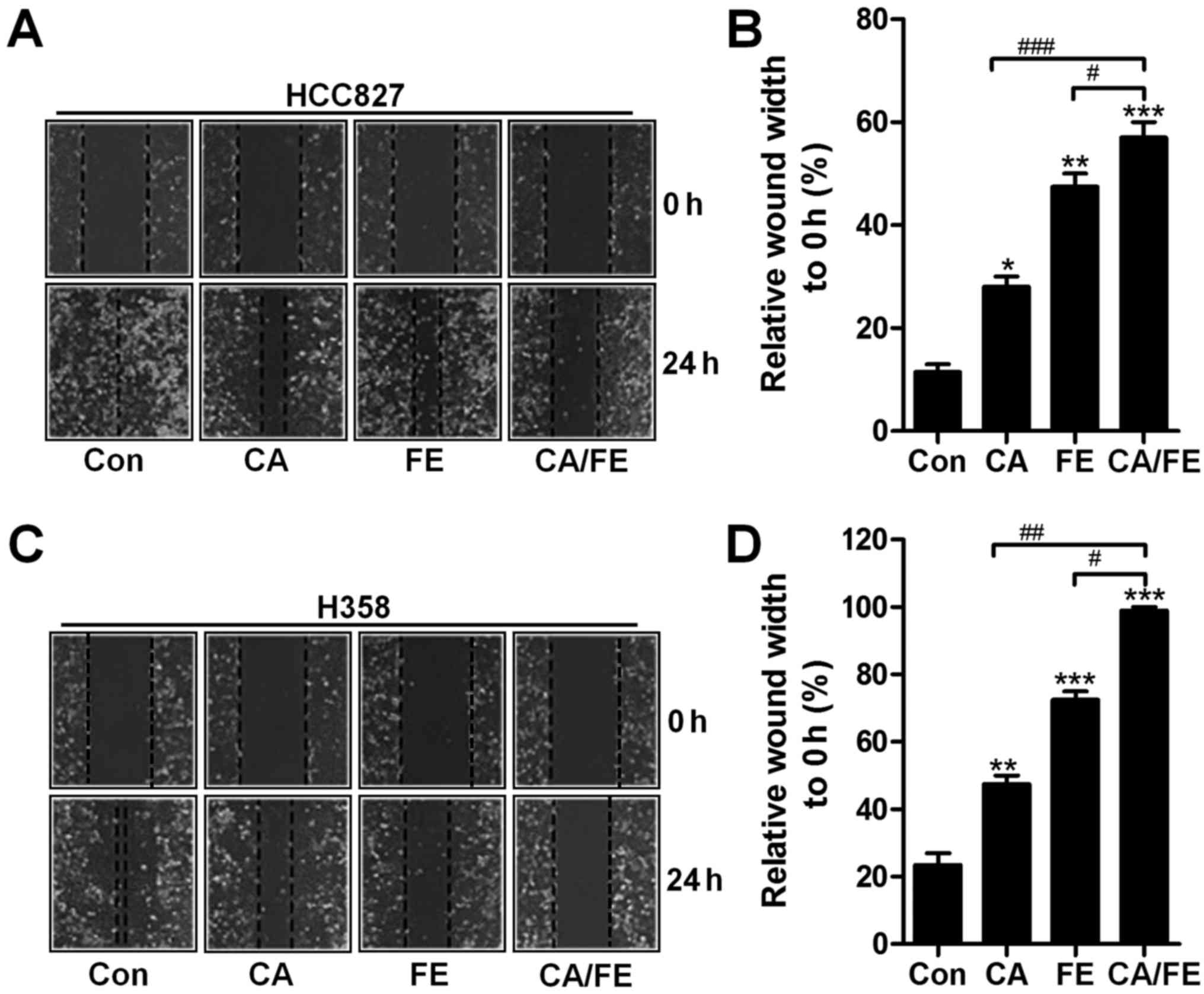
Next, the relative wound width of HCC827 and H358 cells were
detected after different treatments. Fig. 3A and B show that, CA and FE
monotherapy increased in controlling the wound width of HCC827
(27.83 and 46.98%), which was also observed in H358 cells (46.59
and 72.36%), accompanying with remarkable difference compared to
the control (Fig. 3C and D). Also,
in the presence of CA and FE, the wound width to 0 h was found to
be the highest in HCC827 (58.69%) and H358 (93.18%) cells, and
considerable difference was observed in comparison to the CA and FE
single treatment. The results illustrate the capability of CA/FE to
suppress lung cancer cells proliferation and migration which is
apparently stronger than the effect of CA and FE separately in the
present experiments.
Carnosic acid and fisetin co-treatment
significantly induces apoptosis of lung cancer cells
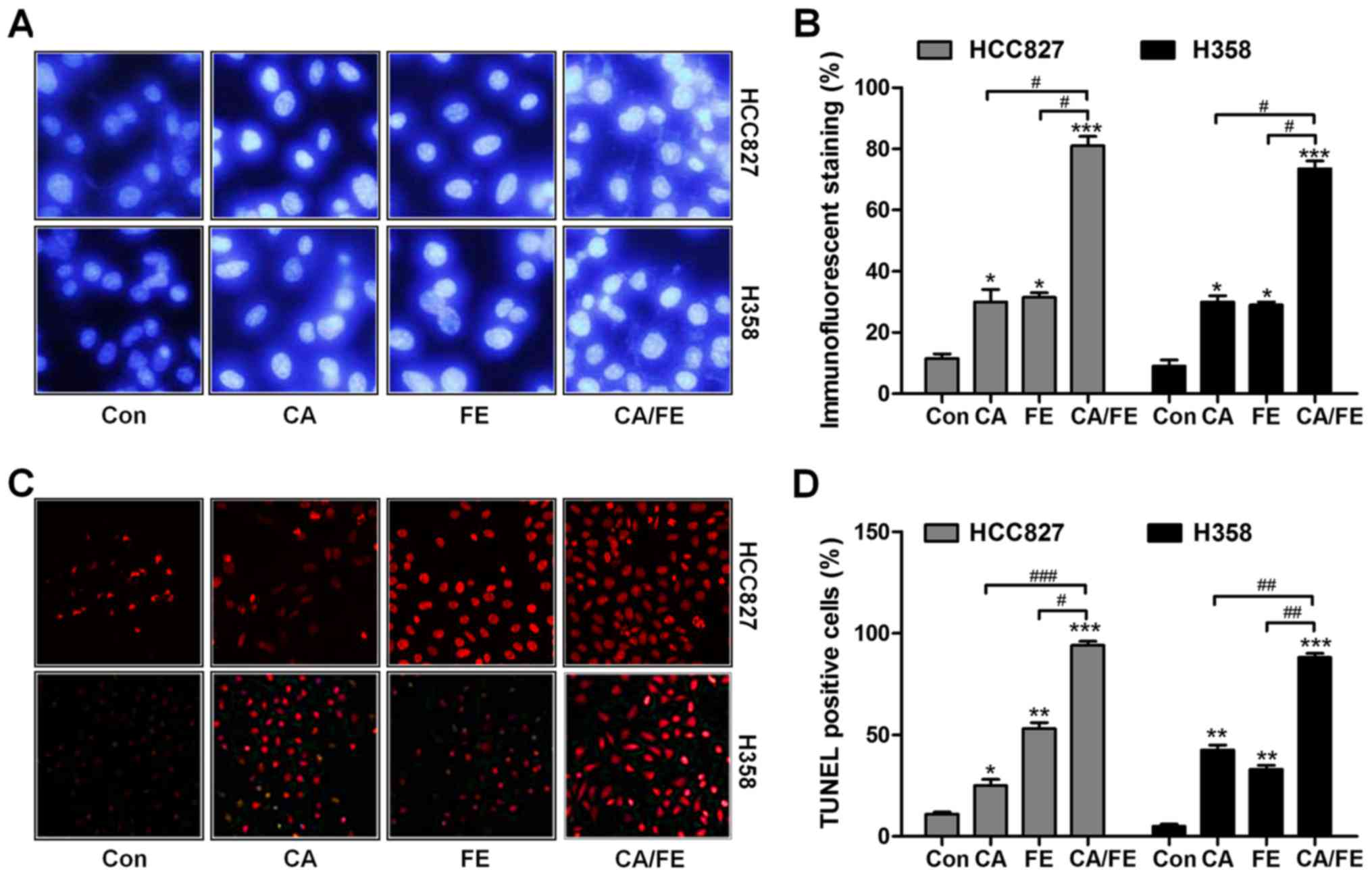
We assessed whether CA/FE co-treatment has any
effects on apoptosis, contributing to lung cancer cells
proliferation suppression and death. As shown in Fig. 4A and B, in comparison to the
control group, CA, FE and CA/FE treatments led to shrunken cancer
cell nuclei and most cell nuclei were apparently condensed and
brightly stained. Nuclear condensation has been considered as a
typical change of morphology for cells experiencing apoptosis
(23). TUNEL assays indicated that
CA and FE single treatment caused higher number of apoptotic cells
in comparison to the control ones, which was further enhanced for
CA/FE combination in HCC827 (92.35%) and H358 (88.27%) cells. Of
note, significant difference was observed between the CA/FE and CA
and FE alone groups both in HCC827 (CA, 24.86%; FE, 55.73%) and
H358 cells (CA, 41.08%; FE, 34.64%) (Fig. 4C and D). The results above suggest
the ability of CA/FE to trigger HCC827 and H358 cell apoptosis is
markedly stronger than CA and FE single therapy.
Carnosic acid and fisetin induce
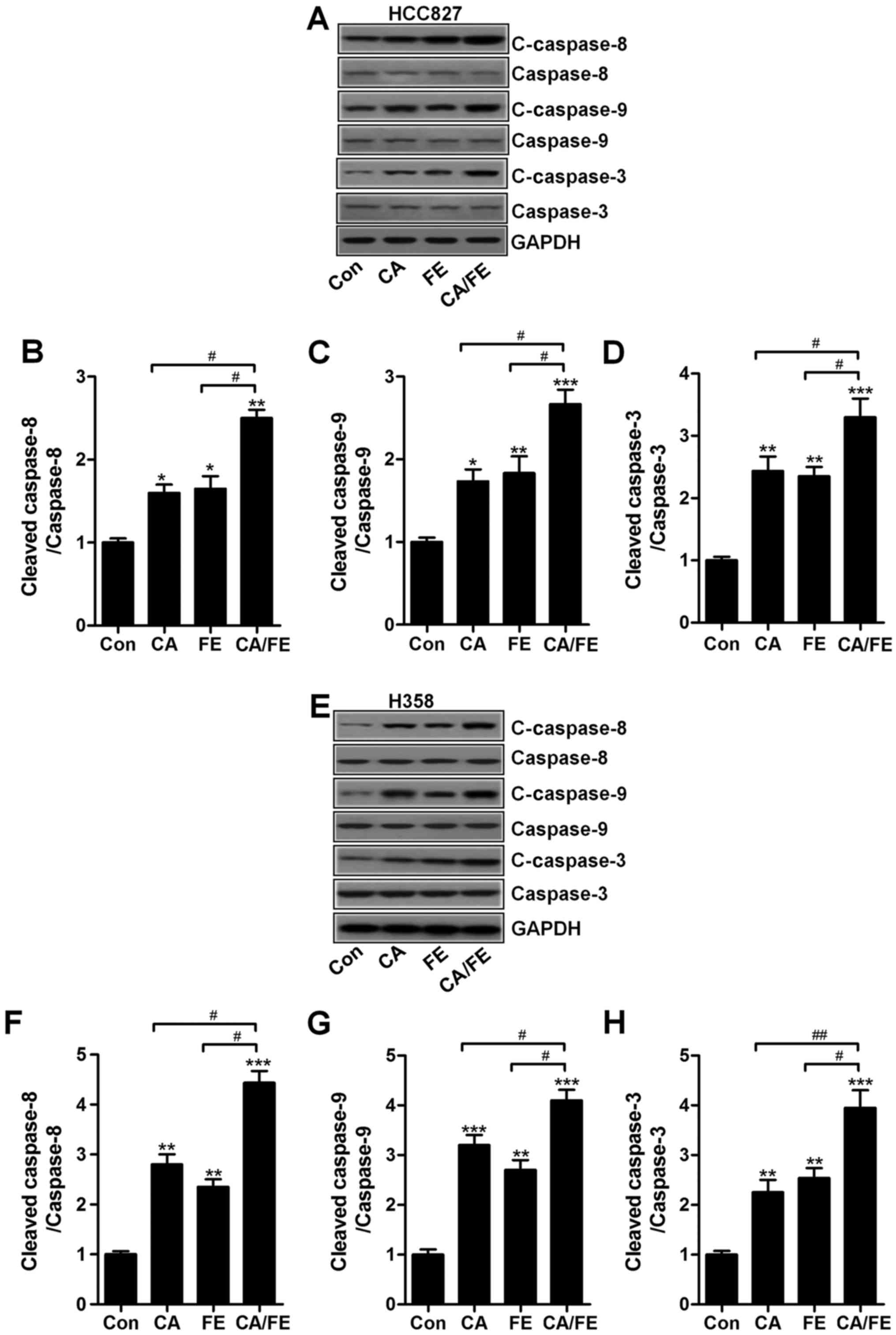
apoptosis in lung cancer cells through caspase-3 activation
The results mentioned above indicated that apoptosis
could be induced for CA, FE especially the CA/FE combination.
Hence, the molecular mechanism was explored. Caspase-8 activation
results in down-stream signals of caspase-9 and caspase-3 activity
(24). Next, the caspase
activation of cancer cells after CA, FE and CA/FE treatment were
determined through western blot analysis. As shown in Fig. 5A, CA and FE markedly induced high
cleavage of caspase-8, leading to caspase-9 activation.
Consequently, caspase-3 was activated and apoptosis was induced.
Significantly, CA/FE combination resulted in an obvious more
intensive caspase-8 (Fig. 5B),
caspase-9 (Fig. 5C) and caspase-3
(Fig. 5D) cleavage in lung cancer
cells of HCC827. Moreover, in H358 cells, cleaved caspase-8
(Fig. 5E and F), caspase-9
(Fig. 5E and G) and caspase-3
(Fig. 5E and H) were markedly
elevated in CA/FE group compared to the CA and FE single
treatment.
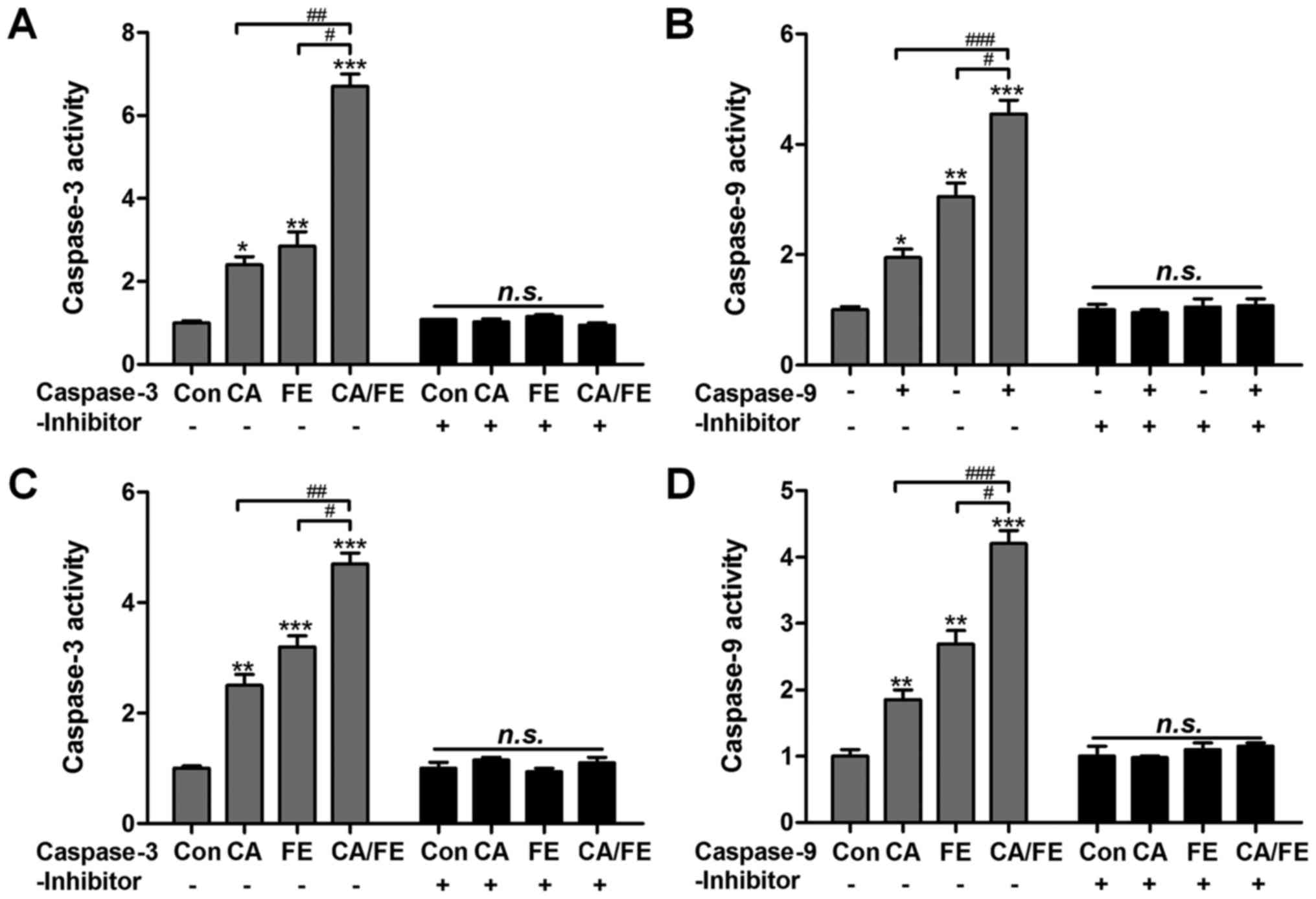
In order to further confirm the role of CA and FE in
caspases activity, caspase-3 and caspase-9 inhibitors were used in
the present study. Fig. 6A, shows
that caspase-3 activity was highly elevated in the CA/FE
combination group with significant difference compared to the CA
and FE single therapy. Caspase-3 inhibitor usage abolished
caspase-3 activity triggered by CA/FE. Also, CA/FE-induced high
caspase-9 activation was also suppressed due to caspase-9 inhibitor
treatment in HCC827 cells (Fig.
6B). In addition, H358 cells after CA/FE co-treatment showed
markedly higher activity of caspase-3 and caspase-9, which was
comparable to the monotherapy-treated groups. Of note, caspase-3
and caspase-9 inhibitors pre-treatment noticeably failed to induce
caspase activation (Fig. 6C and
D). The results above show that caspase signaling pathway
activation is involved in CA/FE-induced apoptosis, which is a main
molecular mechanism by which CA/FE exhibits stronger antitumor
effects.
Carnosic acid and fisetin
combination-induced apoptosis is associated with mitochondrial
pathway
Bcl-2 family members can be divided into the
anti-apoptotic proteins, including Bcl-2 and Bcl-xl, and
pro-apoptotic signals, such as Bax and Bad (25). We further investigated the role of
CA/FE combined therapy in the balance between the pro-apoptotic and
anti-apoptotic members. The combinational treatment of CA/FE on
HCC827 (Fig. 7A) significantly
decreased Bcl-2 and Bcl-xl (Fig. 7C
and D), while Bax (Fig. 7B and
E) and Bad (Fig. 7B and F)
protein levels were markedly increased in NSCLC cells after the
combined H358 cancer cells significantly decreased Bcl-2 (Fig. 7G and I) and Bcl-xl (Fig. 7G and J), while Bax (Fig. 7H and K) and Bad (Fig. 7H and L) protein levels were
markedly increased in NSCLC cells after the combined treatment of
CA/FE.
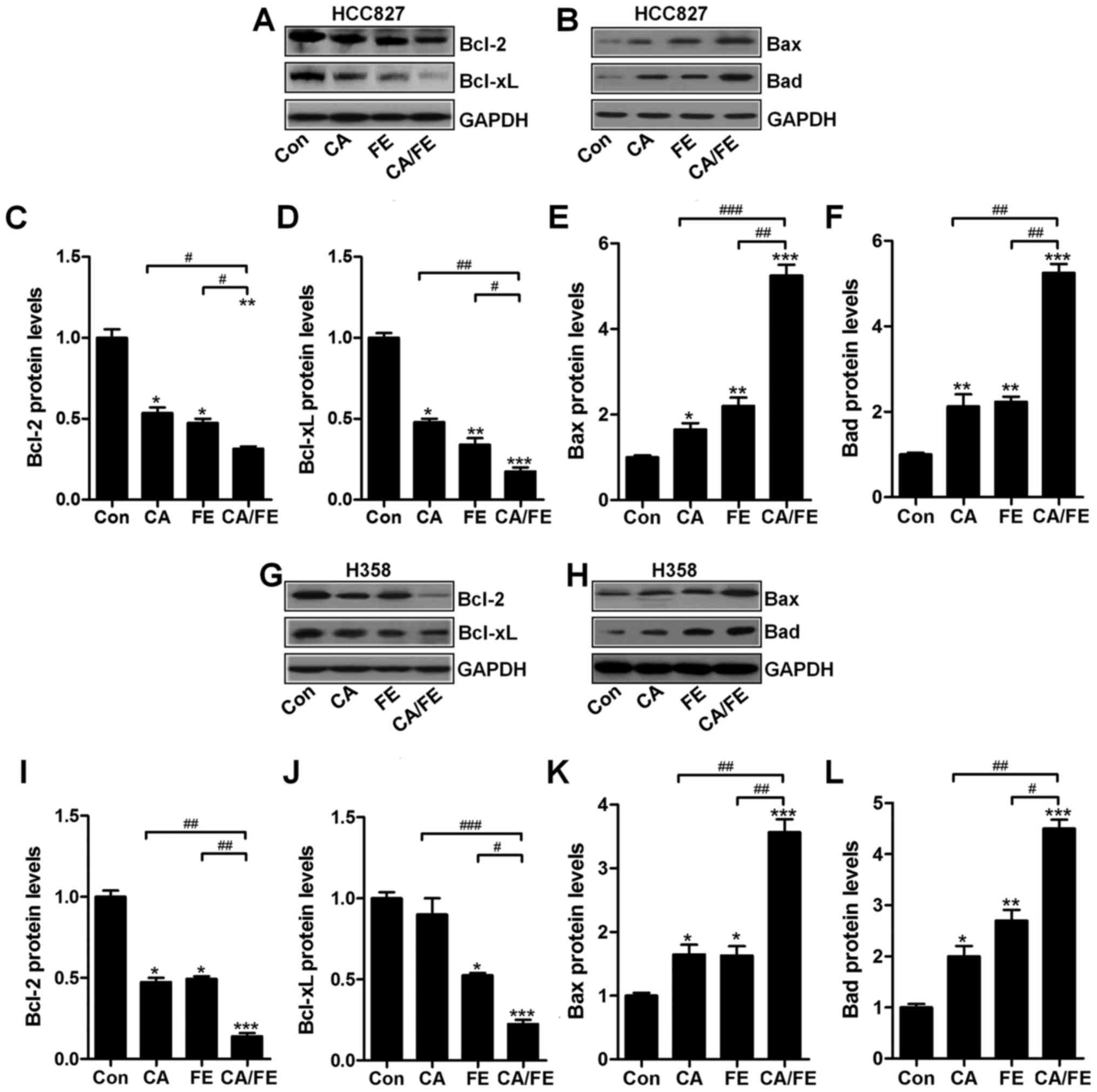 | Figure 7Carnosic acid and fisetin
combination-induced apoptosis is associated with mitochondrial
pathway. Expression of Bcl-2 family members, including (A) Bcl-2,
Bcl-xl, and (B) Bax and Bad, in HCC827 cells under different
experimental conditions were detected through western blot
analysis. The representative images of immunoblot are displayed.
The quantification of (C) Bcl-2, (D) Bcl-xl, (E) Bax, and (F) Bad
based on western blot results in HCC827 cells is shown. Expression
of Bcl-2 family members, including (G) Bcl-2, Bcl-xl and (H) Bax
and Bad, in HCC827 cells under different experimental conditions
were detected through western blot analysis. The representative
images of immunobot are displayed. The quantification of (I) Bcl-2,
(J) Bcl-xl, (K) Bax and (L) Bad protein based on western blot
results in H358 cells is shown. Values are means ± SEM. *P<0.05,
**P<0.01 and ***P<0.001 vs. Con group; #P<0.05,
##P<0.01 and ###P<0.001. |
The effects of carnosic acid and fisetin
combination suppress lung cancer cells through TRAIL signaling
pathway regulation
TRAIL can induce rapid apoptosis in various cancers
(26). TRAIL-induced apoptosis
relies on DRs, leading to the formation of death-inducing signaling
complex. FADD, subsequently, is activated to improve caspase-8
activity (27). In HCC827 and H358
cancer cells under various conditions, RT-qPCR analysis was carried
out to explore how TRAIL, DR4, DR5 and FADD altered after CA and FE
single treatment, or CA/FE co-treatment in HCC827 and H358 cells,
respectively. The results showed that in HCC827 cells, TRAIL
(Fig. 8A), DR4 (Fig. 8B), DR5 (Fig. 8C) and FADD (Fig. 8D) mRNA levels were significantly
augmented by CA and FE monotherapy. Notably, co-treatment of CA/FE
markedly stimulated TRAIL, DR4, DR5 and FADD levels, which was
comparable to the single-treated groups. Furthermore, in H358
cells, TRAIL (Fig. 8E), DR4
(Fig. 8F), DR5 (Fig. 8G) and FADD (Fig. 8H) mRNA levels were apparently
improved by CA and FE single therapy. Of note, co-treatment of
CA/FE markedly stimulated TRAIL, DR4, DR5 and FADD levels, which
was comparable to the CA and FE alone-treated groups. The results
indicate that mitochondrial pathway is involved in CA/FE-induced
apoptosis, which is associated with TRAIL/DRs signaling
pathway.
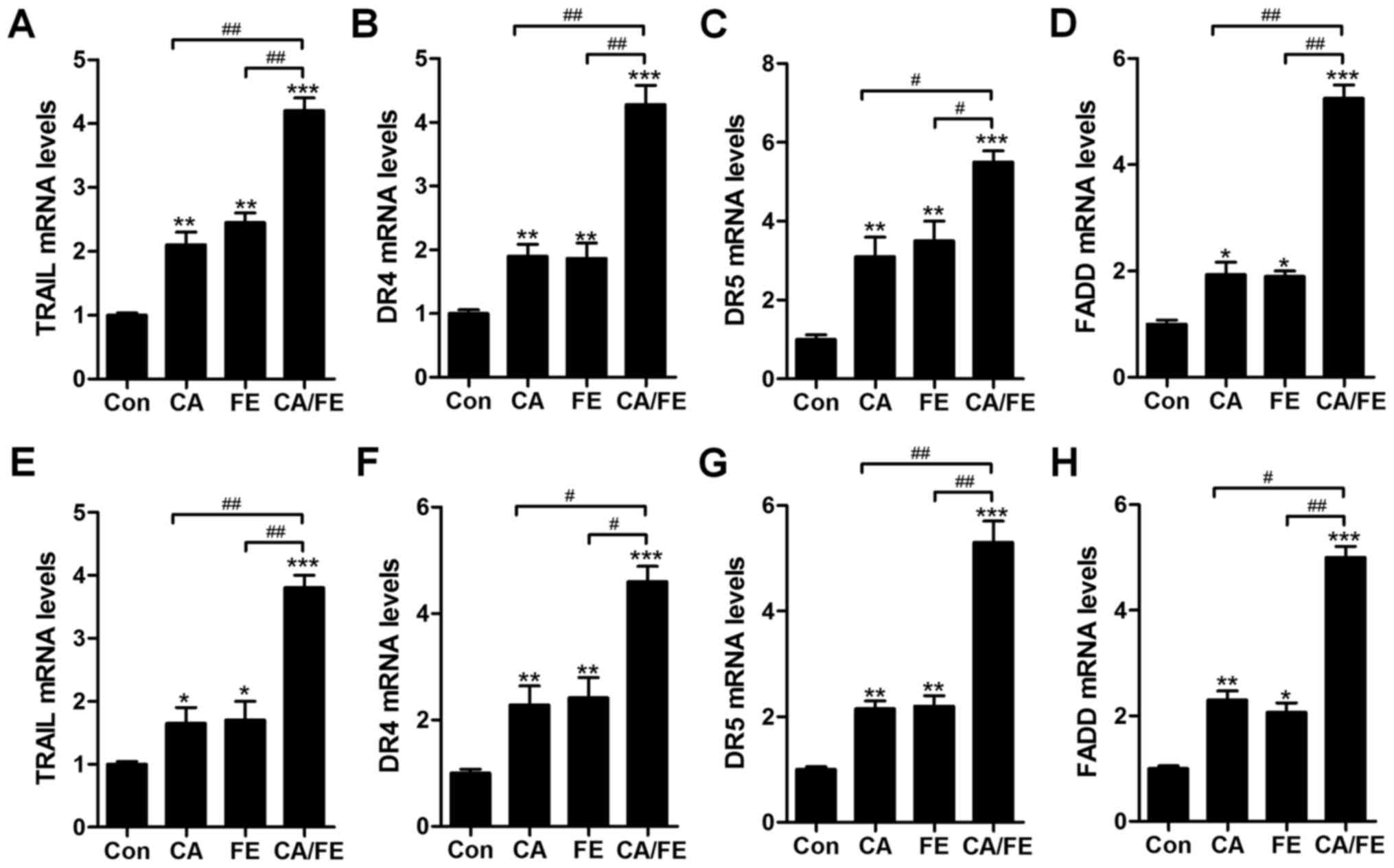 | Figure 8The effects of carnosic acid and
fisetin combination suppressed lung cancer cells through TRAIL
signaling pathway regulation. After different treatments of CA, FE
and the two combinations for 24 h, RT-qPCR was carried out to
determine mRNA levels of (A) TRAIL, (B) DR4, (C) DR5, (D) FADD in
HCC827 cells. Also, in H358 cells under various conditions, (E)
TRAIL, (F) DR4, (G) DR5 and (H) FADD mRNA levels were evaluated by
RT-qPCR. Values are means ± SEM. *P<0.05, **P<0.01 and
***P<0.001 vs. Con group; #P<0.05,
##P<0.01. |
Carnosic acid and fisetin combination
suppresses tumor growth in lung cancer xenograft models in
vivo
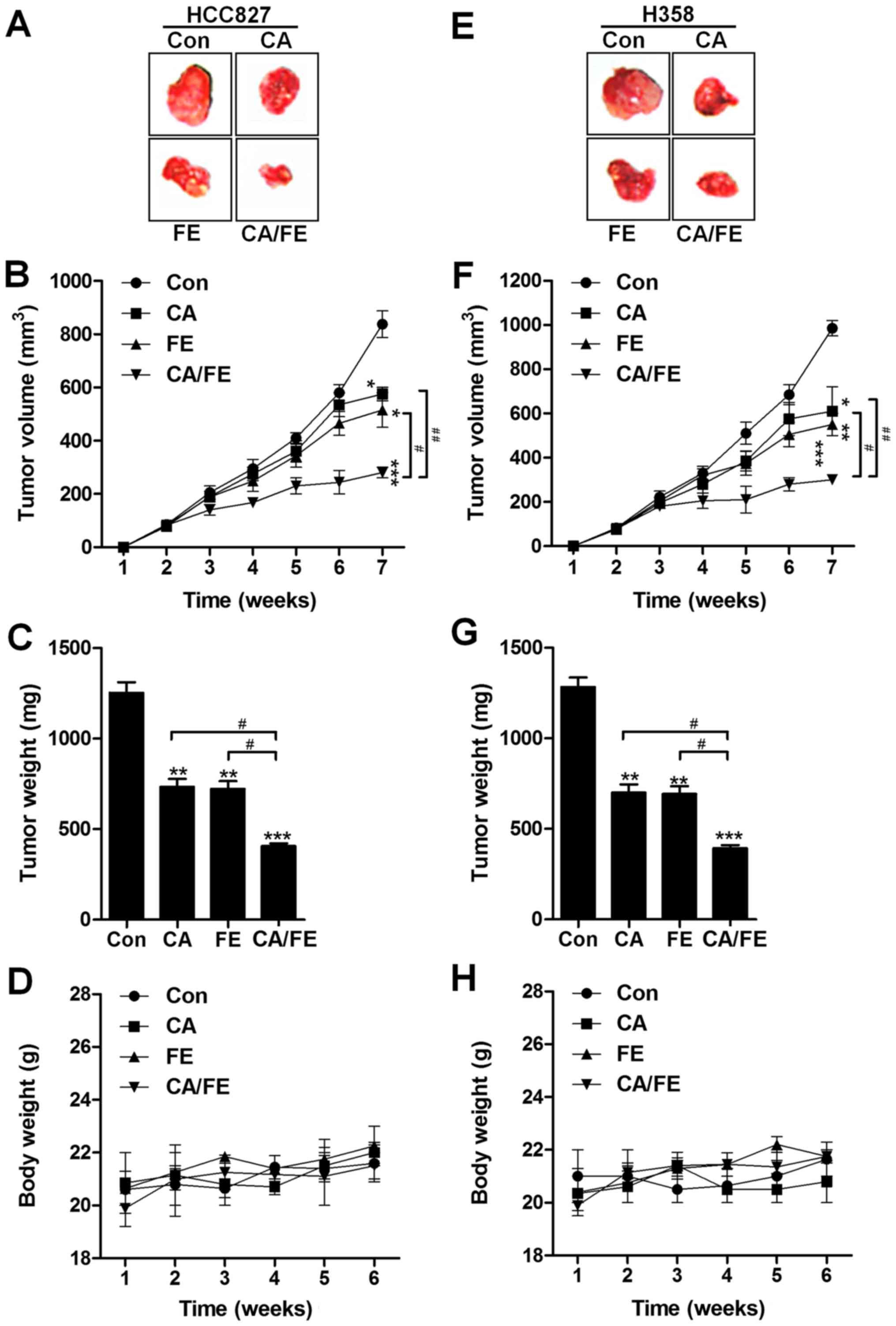
The present study indicated that CA/FE co-treatment
was inhibitory in lung cancer cell proliferation in vitro.
Hence, in order to further investigate the role of CA and FE
monotherapy, and CA/FE combined treatment on tumor growth, the
athymic nude mice bearing the established HCC827 and H358 cells
subcutaneous tumors in the presence of either 30 mg/kg CA, 20 mg/kg
FE or the two combinations were assessed. CA and FE by themselves
significantly reduced tumor volume (Fig. 9A and B) and tumor weight (Fig. 9C) compared to the control group.
Notably, CA/FE combination showed stronger antitumor role in
controlling the tumor volume and weight and marked difference was
observed between the CA/FE and CA- and FE-alone groups.
Additionally, no apparent difference of body weight was found
between different groups in HCC827-transplanted athymic nude mice
(Fig. 9D). Similarly, in H358
subcutaneous nude mice, the tumor volume and tumor weight were
found to be reduced for CA and FE monotherapy, which was further
attenuated for the two combinations with significant difference
(Fig. 9E–G). There was no
difference detected for the body weight among the mice from
different groups (Fig. 9H). The
data indicate that CA, FE and, especially, their combination have
inhibitory role in tumor growth in vivo, consistent with the
results in vitro.
Combination of carnosic acid and fisetin
impedes lung progression through apoptosis induction in vivo
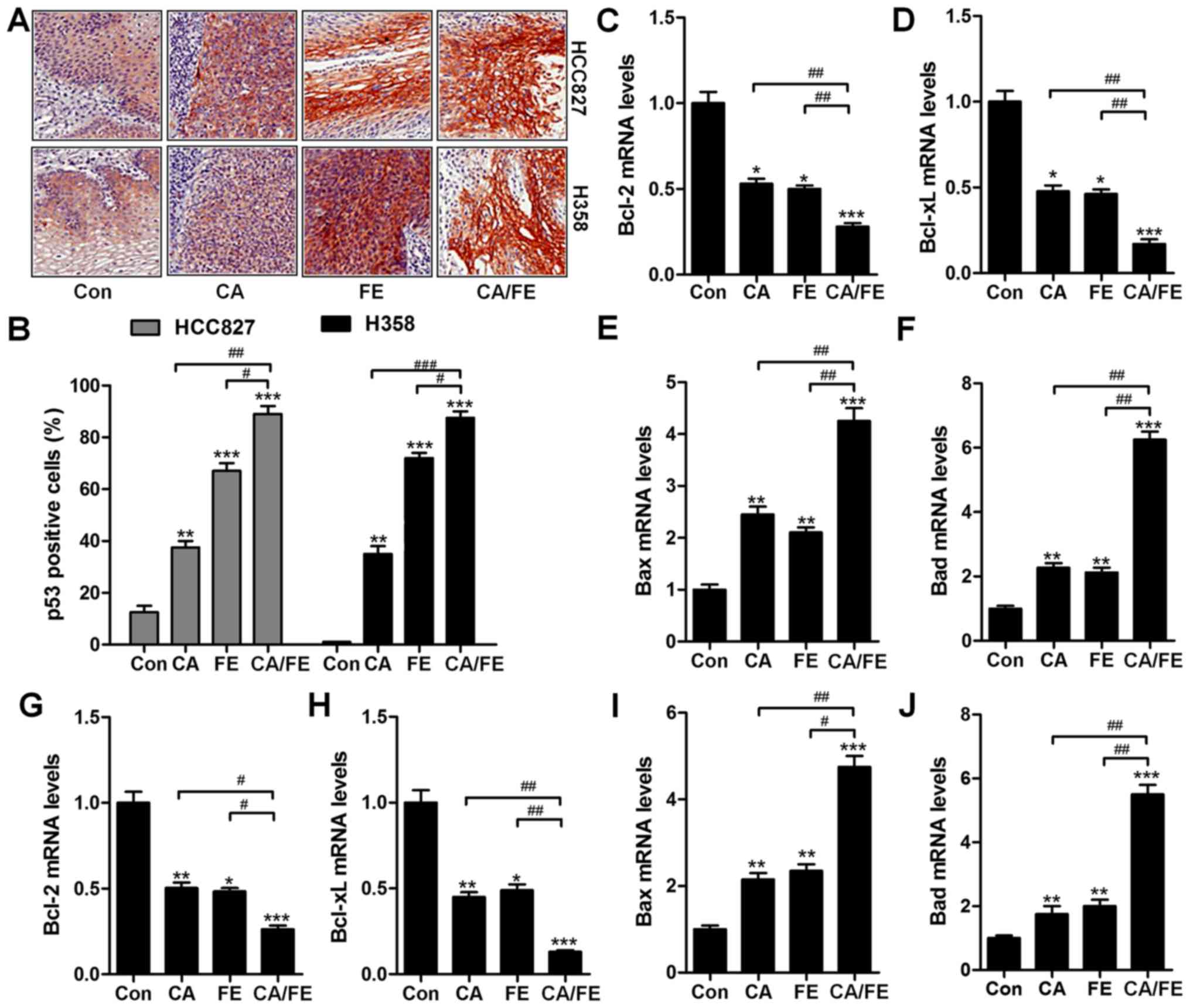
In addition, p53, as previously reported, elevates
DR gene transcription (28). Thus,
p53 is important for TRAIL/DR-induced apoptosis in various tumors
(29). To further confirm our
hypothesis, IHC analysis was performed to calculate p53 levels in
different tumor samples from mice under various conditions. As
shown in Fig. 10A and B, CA and
FE alone treatment could improve the number of p53 positive cells,
being further enhanced for CA/FE in combination. In Fig. 10C and D, RT-qPCR analysis show
lower mRNA levels of Bcl-2 and Bcl-xl in the presence of CA and FE
than that observed in HCC827 tumor tissue samples treated by single
therapy. In contrast, Bax and Bad were significantly upregulated
after CA and FE alone, and were further elevated due to CA/FE
co-treatment (Fig. 10E and F).
Furthermore, RT-qPCR analysis suggested that in H358 tumor tissue
samples, anti-apoptotic members of of Bcl-2 and Bcl-xl were
inhibited from gene levels in CA and FE single treatment, which
were further reduced in the two-drug combinations with significant
difference compared to the CA and FE single group (Fig. 10G and H). Bax and Bad mRNA levels
in tumor samples from H358 subcutaneous mice were enhanced for
CA/FE in combination, which was comparable to the single-treated
ones (Fig. 10I and J). The
results illustrated that the CA/FE in combination suppresses lung
tumor growth through apoptosis induction in vivo, which is
consistent with the results in vitro.
Discussion
Lung cancer is known as the leading cause
contributing to death in human, and its incidence and mortality
will be increasing worldwide. In addition, non-small cell lung
cancer (NSCLC) is the most common type among different lung cancers
(30,31). In recent decades, although progress
has been made in experimental as well as clinical oncology, the
lung cancer prognosis is still far from satisfactory. Also, the
5-year survival rate is approxiamtely 15% (32). Therefore, finding effective
therapeutic strategy and understanding the molecular mechanism of
lung cancer for its progression is still urgently needed to find
better treatments. Carnosic acid is an active component isolated
from plants, which has been reported to suppress various human
cancer progression, such as breast, gastric cancer and liver
disease, through apoptosis induction and cell cycle arrest
(8,9). Fisetin is a naturally flavonoid,
found in many vegetables and fruits, including cucumbers, onions,
grapes, apples, persimmons and strawberries. The anti-oxidative,
anti-inflammatory and neuro-protective activities of fisetin have
been reported (11,12,33).
It has exerted anti-proliferative, pro-apoptotic and
antitumorigenic activities. Also, combination treatment may improve
life quality and prolong survival. Previous studies have reported
that therapy in combination exhibited higher efficiency than those
displayed with monotherapy in various tumors, such as gastric
cancer (34). However, until now,
little is known on whether carnosic acid combined to fisetin could
be worthwhile to prevent lung cancer progression.
In the present study, carnosic acid and fisetin
alone suppressed lung cancer HCC827 and H358 cell proliferation
without cytotoxicity on normal lung cells (Figs. 1 and 2). Significantly, carnosic acid and
fisetin in combination showed stronger anticancer role in
suppressing lung cancer cell proliferation. The present study
provided the effects of carnosic acid and fisetin combination on
lung cancer cell alteration. Detailed study here indicated that
caspase-8, caspase-9, caspase-3, Bax, TRAIL, p53, DR4, DR5 and FADD
were increased (Figs. 5 and
8), while Bcl-2 and Bcl-xl were
decreased for carnosic acid and fisetin in combination (Fig. 7), indicating that these signaling
pathways were involved in carnosic acid/fisetin-regulated lung
cancer development. In addition, the combination of carnosic acid
and fisetin in limiting lung cancer was further confirmed by in
vivo study in nude mice that carnosic acid and fisetin multiple
therapy suppressed tumor growth, which was more significant than
carnosic acid and fisetin single therapy (Fig. 9). Furthermore, MTT assays showed no
significant cell death after carnosic acid, fisetin and their
combination treatment in normal lung cells from human (Fig. 1). Therefore, carnosic acid and
fisetin combination might be a novel option for lung cancer
treatment in future.
Here in the present study, we found that carnosic
acid and fisetin alone treatments suppressed HCC827 and H358 cell
proliferation. Compared to carnosic acid and fisetin single
treatment, the two-combined therapy even in lower concentrations
showed stronger inhibitory role in lung cancer cell proliferation,
triggering considerable apoptosis. Caspases have been reported to
play a significant role in cell apoptosis induction through TRAIL
receptors and the mitochondrial signaling pathways via Bcl-2 and
Bax (35,36). In order to investigate the
molecular mechanism by which carnosic acid and fisetin performed in
lung cancer development suppression, the activation of caspase-8,
caspase-9 and caspase-3 were detected through western blot
analysis. The results indicated that carnosic acid and fisetin in
combination- induced cell apoptosis relied on caspase-8, caspase-9
and caspase-3 activation (Fig. 5).
Next, nuclear condensation was generated for caspase-3 activity,
causing apoptosis in lung cancer cells (37). Caspase-8 is of importance in
mediating apoptosis via mitochondrial signaling pathway. The ratio
of Bax:Bcl-2 is a key in apoptosis modulation via pro-apoptotic and
anti-apoptotic members release (38,39).
Pro-apoptotic molecules inreasing, such as Bax and Bad, helps to
produce apoptosis, while promotion of anti-apoptotic signals,
including Bcl-2 and Bcl-xl, protect cancer cells from experiencing
death (40,41). In this study, carnosic acid and
fisetin combinational treatment downregulated Bcl-2 and Bcl-xl
expression levels, whereas Bax and Bad were upregulated
significantly, upregulating the ratio of Bax:Bcl-2, causing
apoptosis, which was consistent with TUNEL results in lung cancer
cells (Figs. 4 and 7).
TRAIL, belonging to TNF superfamily, leads to rapid
apoptosis through interactions with death receptors (DRs), which
includ DR4 and DR5. TRAIL inhibits cancer cells preferentially over
other normal cells, indicating its possible effects on anticancer
treatment (42,43). DR4 and DR5 activation accumulated
Fas-associated death domain (FADD) and caspase-8, causing caspase-3
activity and apoptosis eventually (44,45).
The present study suggested that carnosic acid and fisetin in
combination significantly upregulated TRAIL, DR4, DR5 and FADD mRNA
levels. p53 activation was also apparently induced by carnosic acid
and fisetin combined treatment (Fig.
8). The results obviously elucidated that carnosic acid and
fisetin combinational treatment is dependent on
TRAIL/caspase-related signaling pathway.
In conclusion, the results above indicated that
carnosic acid and fisetin combination inhibited lung cancer
progression in vitro and in vivo, which is related to
TRAIL/caspase signaling pathway modulation, promoting lung cancer
cell apoptosis without toxicity in normal cells. The results of the
present study revealed that combination of carnosic acid and
fisetin has potential therapeutic role in suppressing human lung
cancer progression.
References
|
1
|
Zhang XY and Zhang P: Sensitization
strategies in lung cancer (Review). Oncol Lett. 12:3669–3673.
2016.PubMed/NCBI
|
|
2
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel R, Ward E, Brawley O and Jemal A:
Cancer statistics, 2011: The impact of eliminating socioeconomic
and racial disparities on premature cancer deaths. CA Cancer J
Clin. 61:212–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Alberg AJ, Ford JG and Samet JM; American
College of Chest Physicians: Epidemiology of lung cancer: ACCP
evidence-based clinical practice guidelines (2nd edition). Chest.
132(Suppl): 29S–55S. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cui Y, Wang G, Li Y, Wang Y, Wang X and Bi
H: Optical coherence tomography and histopathology of macular
uveitis. Optom Vis Sci. 91:1335–1342. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zou ZP, Qiang FY, Zhang T, Sun L, Jia T,
Zhu XC and Xu H: Treatment of experimental autoimmune uveitis in
rats with arsenic trioxide. Chin Ophthalmic Res. 28:306–310.
2010.
|
|
7
|
Mira A, Tanaka A, Tateyama Y, Kondo R and
Shimizu K: Comparative biological study of roots, stems, leaves,
and seeds of Angelica shikokiana Makino. J Ethnopharmacol.
148:980–987. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cheung S and Tai J: Anti-proliferative and
antioxidant properties of rosemary Rosmarinus officinalis. Oncol
Rep. 17:1525–1531. 2007.PubMed/NCBI
|
|
9
|
Steiner M, Priel I, Giat J, Levy J,
Sharoni Y and Danilenko M: Carnosic acid inhibits proliferation and
augments differentiation of human leukemic cells induced by
1,25-dihydroxyvitamin D3 and retinoic acid. Nutr Cancer.
41:135–144. 2001. View Article : Google Scholar
|
|
10
|
Murtaza I, Adhami VM, Hafeez BB, Saleem M
and Mukhtar H: Fisetin, a natural flavonoid, targets chemoresistant
human pancreatic cancer AsPC-1 cells through DR3-mediated
inhibition of NF-kappaB. Int J Cancer. 125:2465–2473. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li J, Cheng Y, Qu W, Sun Y, Wang Z, Wang H
and Tian B: Fisetin, a dietary flavonoid, induces cell cycle arrest
and apoptosis through activation of p53 and inhibition of NF-kappa
B pathways in bladder cancer cells. Basic Clin Pharmacol Toxicol.
108:84–93. 2011. View Article : Google Scholar
|
|
12
|
Ying TH, Yang SF, Tsai SJ, Hsieh SC, Huang
YC, Bau DT and Hsieh YH: Fisetin induces apoptosis in human
cervical cancer HeLa cells through ERK1/2-mediated activation of
caspase-8-/caspase-3-dependent pathway. Arch Toxicol. 86:263–273.
2012. View Article : Google Scholar
|
|
13
|
Khan N, Asim M, Afaq F, Abu Zaid M and
Muhtar H: A novel dietary flavonoid fisetin inhibits androgen
receptor signaling and tumor growth in athymic nude mice. Cancer
Res. 68:8555–8563. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Szliszka E, Helewski KJ, Mizgala E and
Krol W: The dietary flavonol fisetin enhances the
apoptosis-inducing potential of TRAIL in prostate cancer cells. Int
J Oncol. 39:771–779. 2011.PubMed/NCBI
|
|
15
|
Yang PM, Tseng HH, Peng CW, Chen WS and
Chiu SJ: Dietary flavonoid fisetin targets caspase-3-deficient
human breast cancer MCF-7 cells by induction of
caspase-7-associated apoptosis and inhibition of autophagy. Int J
Oncol. 40:469–478. 2012.
|
|
16
|
Liang J, Deng X, Wu FS and Tang YF:
Transcriptomic and proteomic analysis of human hepatic stellate
cells treated with natural taurine. Mol Med Rep. 7:1442–1452.
2013.PubMed/NCBI
|
|
17
|
Li DW, Li JH, Wang YD and Li GR:
Atorvastatin protects endothelial colony-forming cells against
H2O2-induced oxidative damage by regulating
the expression of annexin A2. Mol Med Rep. 12:7941–7948.
2015.PubMed/NCBI
|
|
18
|
Cai JJ, Qi ZX, Chen LC, Yao Y, Gong Y and
Mao Y: miR-124 suppresses the migration and invasion of glioma
cells in vitro via Capn4. Oncol Rep. 35:284–290. 2016.
|
|
19
|
Joshi A, Allen R, Kroetz D, Schaller M,
Dalton J, Kunkel S and Gallagher K: Histone methyltransferase,
Setdb2, regulates wound healing in a diet-induced obesity model of
diabetes (IRM9P 601). J Immunol. 194(Suppl 1): 130.102015.
|
|
20
|
Lyons AB, Blake SJ and Doherty KV: Flow
cytometric analysis of cell division by dilution of CFSE and
related dyes. Curr Protoc Cytom Chapter. 9:112013.
|
|
21
|
Polikepahad S, Knight JM, Naghavi AO, Oplt
T, Creighton CJ, Shaw C, Benham AL, Kim J, Soibam B, Harris RA, et
al: Proinflammatory role for let-7 microRNAS in experimental
asthma. J Biol Chem. 285:30139–30149. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cagin YF, Parlakpinar H, Vardi N, Polat A,
Atayan Y, Erdogan MA and Tanbek K: Effects of dexpanthenol on
acetic acid-induced colitis in rats. Exp Ther Med. 12:2958–2964.
2016.PubMed/NCBI
|
|
23
|
Trussardi-Regnier A, Lavenus S, Gorisse MC
and Dufer J: Thalidomide alters nuclear architecture without ABCB1
gene modulation in drug-resistant myeloma cells. Int J Oncol.
35:641–647. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schultz DR and Harrington WJ Jr:
Apoptosis: Programmed cell death at a molecular level. Semin
Arthritis Rheum. 32:345–369. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li P, Nijhawan D, Budihardjo I,
Srinivasula SM, Ahmad M, Alnemri ES and Wang X: Cytochrome c and
dATP-dependent formation of Apaf-1/caspase-9 complex initiates an
apoptotic protease cascade. Cell. 91:479–489. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ashkenazi A, Holland P and Eckhardt SG:
Ligand-based targeting of apoptosis in cancer: The potential of
recombinant human apoptosis ligand 2/tumor necrosis factor-related
apoptosis-inducing ligand (rhApo2L/TRAIL). J Clin Oncol.
26:3621–3630. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pan Y, Xu R, Peach M, Huang CP,
Branstetter D, Novotny W, Herbst RS, Eckhardt SG and Holland PM:
Evaluation of pharmacodynamic biomarkers in a Phase 1a trial of
dulanermin (rhApo2L/TRAIL) in patients with advanced tumours. Br J
Cancer. 105:1830–1838. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wiezorek J, Holland P and Graves J: Death
receptor agonists as a targeted therapy for cancer. Clin Cancer
Res. 16:1701–1708. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dimberg LY, Anderson CK, Camidge R,
Behbakht K, Thorburn A and Ford HL: On the TRAIL to successful
cancer therapy? Predicting and counteracting resistance against
TRAIL-based therapeutics. Oncogene. 32:1341–1350. 2013. View Article : Google Scholar
|
|
30
|
Dela Cruz CS, Tanoue LT and Matthay RA:
Lung cancer: epidemiology, etiology, and prevention. Clin Chest
Med. 32:605–644. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ganti AK, Siedlik E, Marr AS, Loberiza FR
Jr and Kessinger A: Predictive ability of Charlson comorbidity
index on outcomes from lung cancer. Am J Clin Oncol. 34:593–596.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yao J, Wang YW, Fang BB, Zhang SJ and
Cheng BL: piR-651 and its function in 95-D lung cancer cells.
Biomed Rep. 4:546–550. 2016.PubMed/NCBI
|
|
33
|
Hou DX, Fukuda M, Johnson JA, Miyamori K,
Ushikai M and Fujii M: Fisetin induces transcription of
NADPH:quinone oxidoreductase gene through an antioxidant responsive
element-involved activation. Int J Oncol. 18:1175–1179.
2001.PubMed/NCBI
|
|
34
|
Daliani DD, Tannir NM, Papandreou CN, Wang
X, Swisher S, Wood CG, Swanson DA, Logothetis CJ and Jonasch E:
Prospective assessment of systemic therapy followed by surgical
removal of metastases in selected patients with renal cell
carcinoma. BJU Int. 104:456–460. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang L and Fang B: Mechanisms of
resistance to TRAIL- induced apoptosis in cancer. Cancer Gene Ther.
12:228–237. 2005. View Article : Google Scholar
|
|
36
|
Jin Z, McDonald ER III, Dicker DT and
El-Deiry WS: Deficient tumor necrosis factor-related
apoptosis-inducing ligand (TRAIL) death receptor transport to the
cell surface in human colon cancer cells selected for resistance to
TRAIL-induced apoptosis. J Biol Chem. 279:35829–35839. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kim HS, Lee JW, Soung YH, Park WS, Kim SY,
Lee JH, Park JY, Cho YG, Kim CJ, Jeong SW, et al: Inactivating
mutations of caspase-8 gene in colorectal carcinomas.
Gastroenterology. 125:708–715. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cummins JM, Kohli M, Rago C, Kinzler KW,
Vogelstein B and Bunz F: X-linked inhibitor of apoptosis protein
(XIAP) is a nonredundant modulator of tumor necrosis factor-related
apoptosis-inducing ligand (TRAIL)-mediated apoptosis in human
cancer cells. Cancer Res. 64:3006–3008. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang S, Ong CN and Shen HM: Involvement
of proapoptotic Bcl-2 family members in parthenolide-induced
mitochondrial dysfunction and apoptosis. Cancer Lett. 211:175–188.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kim SL, Trang KT, Kim SH, Kim IH, Lee SO,
Lee ST, Kim DG and Kim SW: Parthenolide suppresses tumor growth in
a xenograft model of colorectal cancer cells by inducing
mitochondrial dysfunction and apoptosis. Int J Oncol. 41:1547–1553.
2012.PubMed/NCBI
|
|
41
|
Carlisi D, D'Anneo A, Angileri L,
Lauricella M, Emanuele S, Santulli A, Vento R and Tesoriere G:
Parthenolide sensitizes hepatocellular carcinoma cells to TRAIL by
inducing the expression of death receptors through inhibition of
STAT3 activation. J Cell Physiol. 226:1632–1641. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Vasilevskaya IA and O'Dwyer PJ:
17-Allylamino-17- demethoxygeldanamycin overcomes TRAIL resistance
in colon cancer cell lines. Biochem Pharmacol. 70:580–589. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Galligan L, Longley DB, McEwan M, Wilson
TR, McLaughlin K and Johnston PG: Chemotherapy and TRAIL-mediated
colon cancer cell death: The roles of p53, TRAIL receptors, and
c-FLIP. Mol Cancer Ther. 4:2026–2036. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Pennarun B, Meijer A, de Vries EG,
Kleibeuker JH, Kruyt F and de Jong S: Playing the DISC: Turning on
TRAIL death receptor- mediated apoptosis in cancer. Biochim Biophys
Acta. 1805:123–140. 2010.
|
|
45
|
Jung YH, Heo J, Lee YJ, Kwon TK and Kim
YH: Quercetin enhances TRAIL-induced apoptosis in prostate cancer
cells via increased protein stability of death receptor 5. Life
Sci. 86:351–357. 2010. View Article : Google Scholar : PubMed/NCBI
|