Introduction
Gastric cancer is one of the most common
malignancies in the United States. There is a decline in gastric
cancer occurrence partly due to a lower prevalence of
Helicobacter pylori infection because of improved hygiene
and a higher consumption of vegetables and lower salt intake
(1). However, it is estimated that
28,000 new cases and 10,960 deaths will occur in 2017 in the US
(1). In China 679,100 new gastric
cancer cases occurred in 2015, being one of the four most common
cancers diagnosed (2). Notably,
gastric cancer is the second leading cause of cancer death
worldwide. Current therapies in clinic mainly include surgery,
chemotherapy, and chemoradiation for gastric cancer (3). Although surgery remains the curative
therapy, chemotherapy is the important treatment for gastric cancer
(4). Due to intrinsic or acquired
resistance to chemotherapeutic drug, chemotherapy often fail to
achieve effective treatment benefit for gastric cancer patients.
Therefore, it is important to determine the molecular mechanism of
drug resistance in gastric cancer (5).
Cisplatin has been known as an effective
chemotherapeutic drug for the treatment of human malignancies
including gastric cancer (6).
Cisplatin and fluoropyrimidine-based chemotherapy and trastuzumab
have been widely used for advanced stage patients with epidermal
growth factor 2 (EGFR2) positivity (7). Unfortunately, cisplatin resistance
often occurred during chemotherapeutic treatment (8). Emerging evidence has suggested that
drug resistant tumor cells acquired epithelial-mesenchymal
transition (EMT) (9). During EMT
progress, epithelial cells transit to mesenchymal phenotype,
leading to loss of epithelial cell-cell junction and actin
cytoskeleton reorganization (10).
Subsequently, the expression of epithelial marker E-cadherin was
downregulated, but the expression of mesenchymal markers was
upregulated, including Vimentin, Snail, Slug, ZEB1 (zinc-finger
E-box binding homeobox 1), and ZEB2 (11). A study revealed that
chemoresistance to cisplatin induced EMT in human lung
adenocarcinoma cells (12).
Similarly, another study identified that EMT is associated with
cisplatin resistance in gastric cancer cells (13). Furthermore, EMT transcription
factor Snail and Slug directly contribute to cisplatin resistance
in ovarian cancer cells (14).
Although these studies indicated the cisplatin resistance molecular
basis, the precise mechanisms of cisplatin resistance are still
elusive.
TAZ (transcriptional co-activator with PDZ-binding
motif) has been characterized to be involved in gastric
tumorigenesis (15). It is known
that YAP1 (Yes-associated protein 1) and its paralog TAZ are key
factors which are negatively regulated by the Hippo pathway
(16). TAZ exerts its oncogenic
function through interaction with TEAD transcription factors.
Notably, TAZ was highly expressed in gastric cancer samples
(17). Strikingly, the expression
of TAZ had a close correlation with lymphatic metastasis and tumor
TNM (tumor, node, metastasis) stage in gastric cancer (18). Several studies have revealed that
TAZ was critically involved in drug resistance in a variety of
human cancers (19,20). For instance, taxol resistance is
mediated by TAZ and its downstream transcriptional targets Cyr61
and CTGF (connective tissue growth factor) in breast cancer cells
(21). Moreover, TAZ promoted EMT
and cancer stem cell maintenance in oral cancer (22). Due to TAZ playing a key role in
drug resistance and EMT, it is important to determine whether TAZ
could regulate drug resistance and EMT in gastric cancer. In the
present study, we investigated whether cisplatin-resistant (CR)
cells exhibit EMT features in gastric cancer. We also elucidate the
role of TAZ in CR-mediated EMT in gastric cancer.
Materials and methods
Reagents
MTT [3-(4,5-dimethythiazol- 2-yl)-2,5-diphenyl
tetrazolium bromide] was obtained from Sigma (St. Louis, MO, USA).
Primary antibodies including anti-TAZ, anti-Vimentin, anti-Snail,
anti-Slug, anti-ZO1, anti-E-cadherin, anti-β-catenin, and
anti-Tubulin were purchased from Santa Cruz Biotechnology (Santa
Cruz, CA, USA). Lipofectamine 2000 and (Roswell Park Memorial
Institute) RPMI-1640 medium were purchased from Invitrogen
(Carlsbad, CA, USA). The non-specific control siRNA and TAZ siRNA
oligonucleotides were purchased from GenePharma (Shanghai, China).
Tumor Invasion Assay System was obtained from BD Biosciences
(Bedford, MA, USA). Protein assay kit was purchased from Bio-Rad
Laboratories (Hercules, CA, USA).
Cell culture
Human gastric cancer cell lines MGC803 and SGC7901
were cultured in RPMI-1640 medium supplemented with 5% FBS (fetal
bovine serum), penicillin, and streptomycin. Cells were maintained
in a humidified 5% CO2 incubator at 37°C. Cells were
cultured in medium with increasing concentrations of cisplatin for
more than 6 months to establish CR cell lines (23).
MTT assay
The gastric cancer cells were seeded in each well of
the 96-well plates. After 24 h incubation, the cells were treated
with different concentrations of cisplatin for 72 h. MTT assay was
conducted as previously described (24).
Transwell invasion assay
Cell invasion was detected using 24-well inserts
with matrigel in 8-µm pores as previously described
(24). The invasive activity of
cells was detected by using Transwell Invasion kit following the
protocol from the manufacturer as previously described (24). Briefly, cells were cultured in the
upper chamber of the inserts and RPMI-1600 medium with 10% FBS was
added in the lower chamber. After 12 h at 37°C, cells on the upper
side of the Transwell were removed. The invading cells on the lower
side were fixed and stained with Giemsa solution as well as
photographed under a microscope.
Cell attachment and detachment assay
Cell attachment and detachment assays were performed
as previously described (25).
Briefly, for attachment assay, gastric cancer cells were added in
24-well plates. After 1 h of incubation, we removed the unattached
cells and counted the attached cells. For cell detachment assay,
after 24 h incubation, the detached cells by 0.05% trypsin
digestion for 2 min were counted. The remaining attached cells were
trypsinized and counted. The results are presented as a percentage
of the attached or detached cells to total cells.
Wound healing assay
The cells were seeded in 6-well plate. After the
cells reached >90% confluency, the scratch wound was generated
by a pipette tip. After 16 h, photographic images were taken
(26).
Reverse transcription-PCR (polymerase
chain reaction) analysis for gene expression
The total RNA was isolated with TRIzol (Invitrogen)
according to the manufacturer's protocols. The real-time PCR
reactions and the primers used in PCR reaction were previously
described (24).
Western blot analysis: Cells were lysed
with RIPA buffer supplemented with protease inhibitors
The protein concentrations were measured by the
Bio-Rad protein assay kit. The same amount of protein samples were
separated by SDS-PAGE (sodium dodecyl sulfate polyacrylamide gel
electrophoresis) and then electrotransferred to the membranes. The
membranes were immunoblotted with primary antibodies for western
blotting as previously described (27).
Transfection
Gastric cells were seeded in 6-well plates and
transfected with TAZ siRNA, or control siRNA (GenePharma) using
Lipofectamine 2000 as previously described (24). After the transfection, the cells
were applied for further investigation as described under the
results section.
Statistical analysis
Statistical analyses were performed to evaluate
significance between different groups by GraphPad Prism 5.0 (Graph
pad Software, La Jolla, CA, USA). All data were expressed as mean ±
SD (standard deviation). P<0.05 was considered to indicate a
statistical significant difference.
Results
Establishment of cisplatin-resistant
gastric cancer cell lines
To determine the acquired function of drug resistant
gastric cancer cells, the cisplatin-resistant (CR) gastric cancer
cells were established. MGC803 and SGC7901 cells were exposed to
low concentration of cisplatin for several days. The dead cells
were removed and increasing concentrations of cisplatin were added
in culture medium. After gastric cancer cells were cultured for
more than 6 months with increasing concentrations of cisplatin, CR
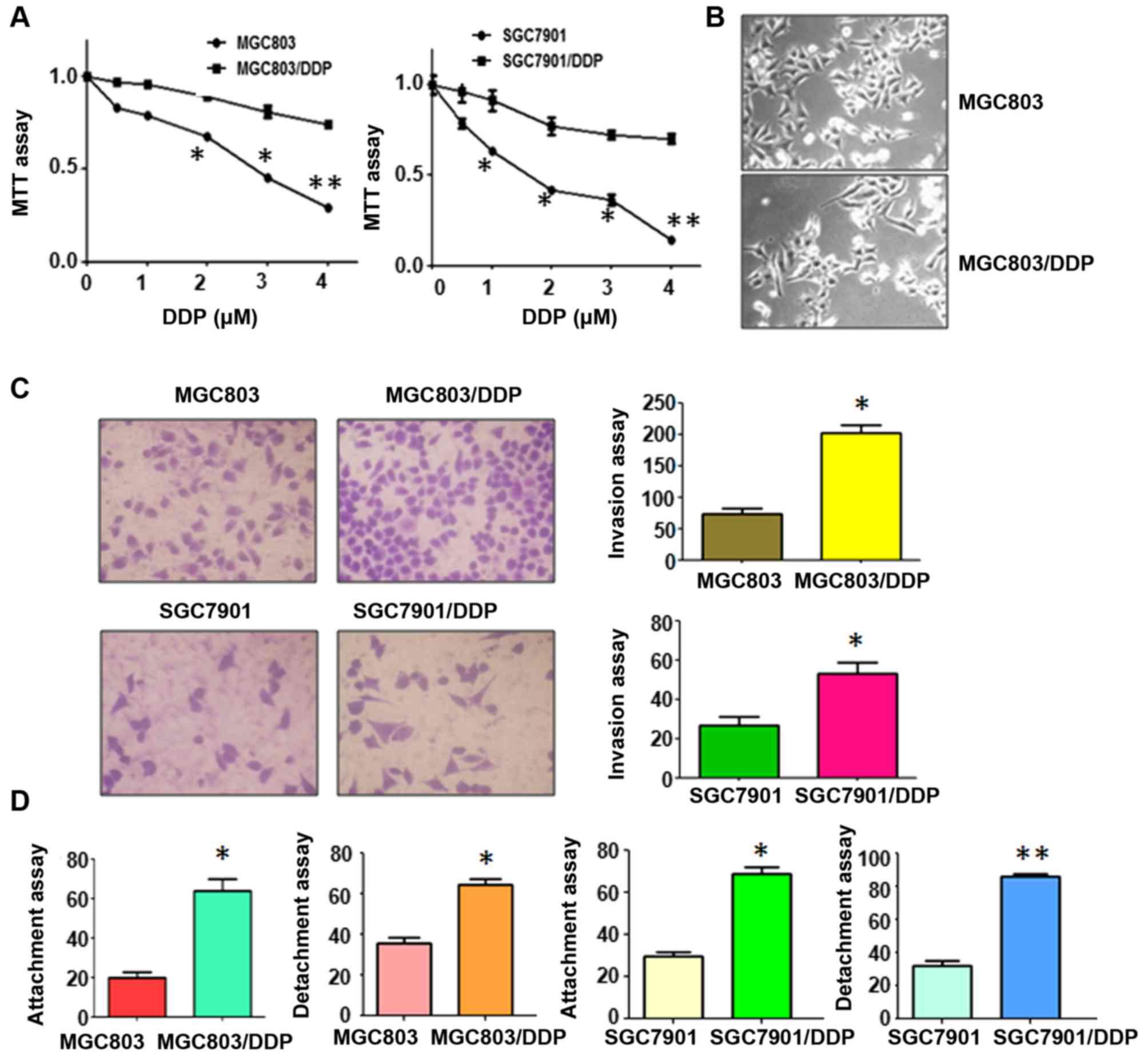
cells (MGC803/DDP and SGC7901/DDP) cells were established (23). Our MTT assay showed that 3
µM cisplatin led to approximately 50% cell growth inhibition
in both MGC803 and SGC7901 cells (Fig.
1A). Moreover, 4 µM cisplatin treatment caused more than
60% and 80% growth inhibition in MGC803 and SGC7901 cells,
respectively (Fig. 1A). However, 2
µM cisplatin did not inhibit cell growth in both CR cells
(Fig. 1A). The resistant cells
were continuously cultured in medium with 2 µM cisplatin for
the following study.
CR cells exhibit EMT features
It has been documented that drug resistant cells
acquired EMT phenotype (28,29).
In line with this concept, MGC803/DDP and SGC7901/DDP cells
displayed morphologic changes, such as EMT phenotype. Both
MGC803/DDP and SGC7901/DDP cells become elongated, and
fibroblastoid in morphology, whereas MGC803 and SGC7901 were a
rounded shape (Fig. 1B). EMT-type
cells often have aggressive characteristics. Our Transwell invasion
assay showed that the numbers of invasive cells were increased in
CR cells compared with parental cells (Fig. 1C). Consistently, CR cells exhibited
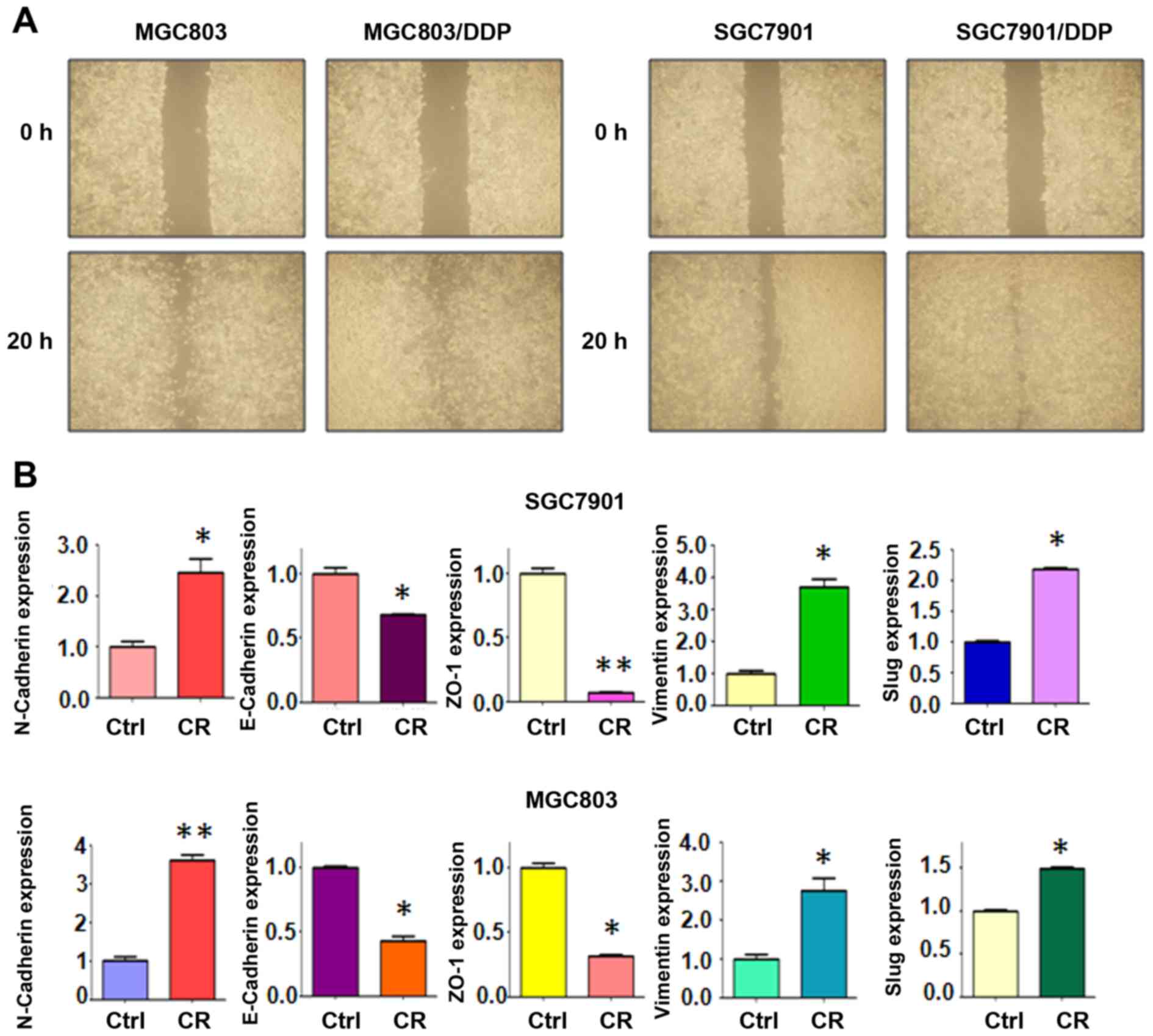
enhanced capacity of detachment and attachment (Fig. 1D). Similarly, our wound healing
assay results demonstrated that CR cells acquired increased
motility activity (Fig. 2A).
Altogether, CR cells acquired EMT characteristics.
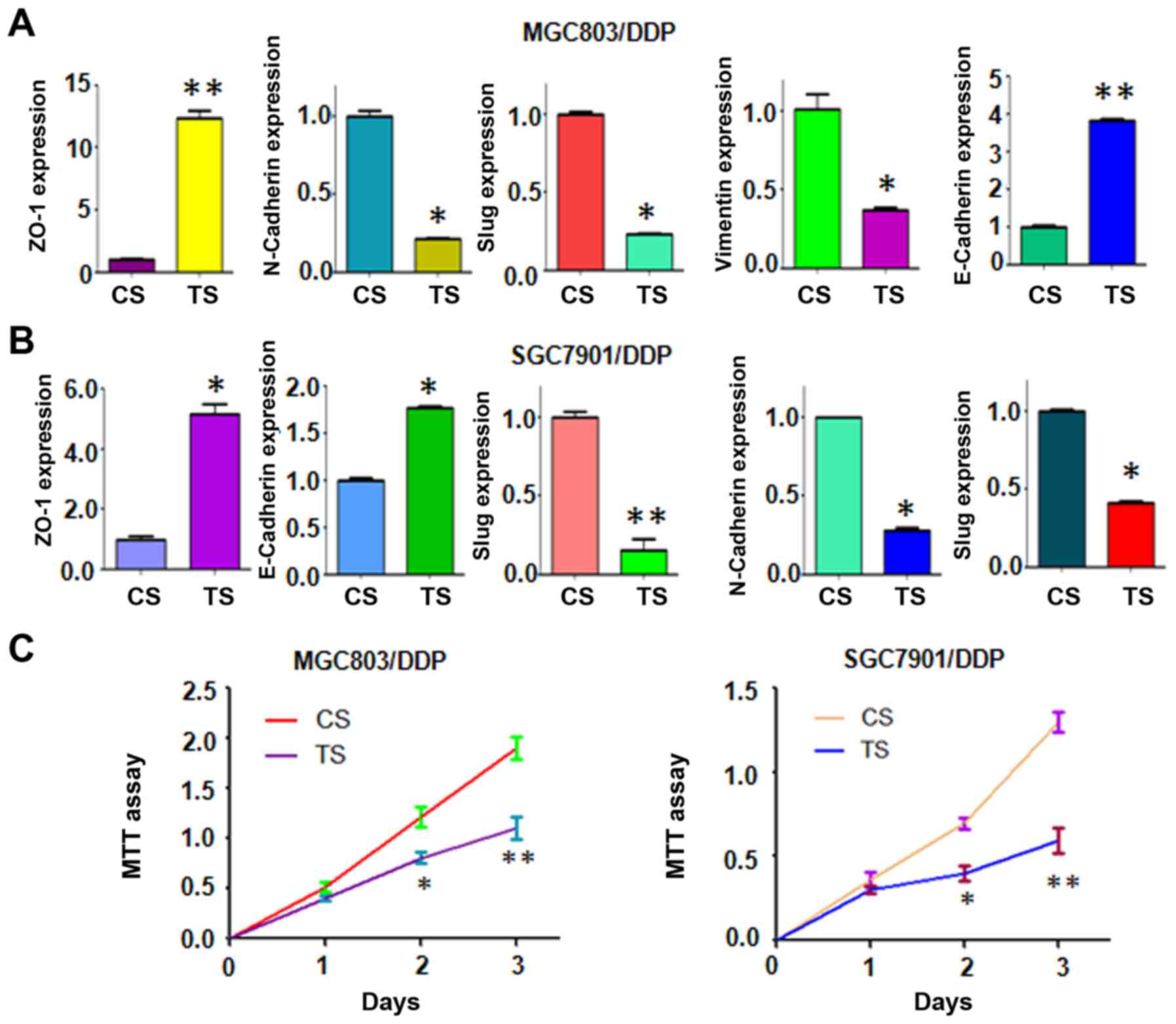
CR cells have EMT molecular marker
changes
To further confirm whether CR cells acquired EMT, we
detected the expression of EMT molecular markers in CR cells and
their parental cells, including N-cadherin, E-cadherin, Vimentin,
Slug, and ZO-1. Our real-time RT-PCR results indicated that the
expression of E-cadherin and ZO-1 was downregulated in CR cells,
whereas the levels of N-cadherin, Vimentin, and Slug were
upregulated in CR cells (Fig. 2B).
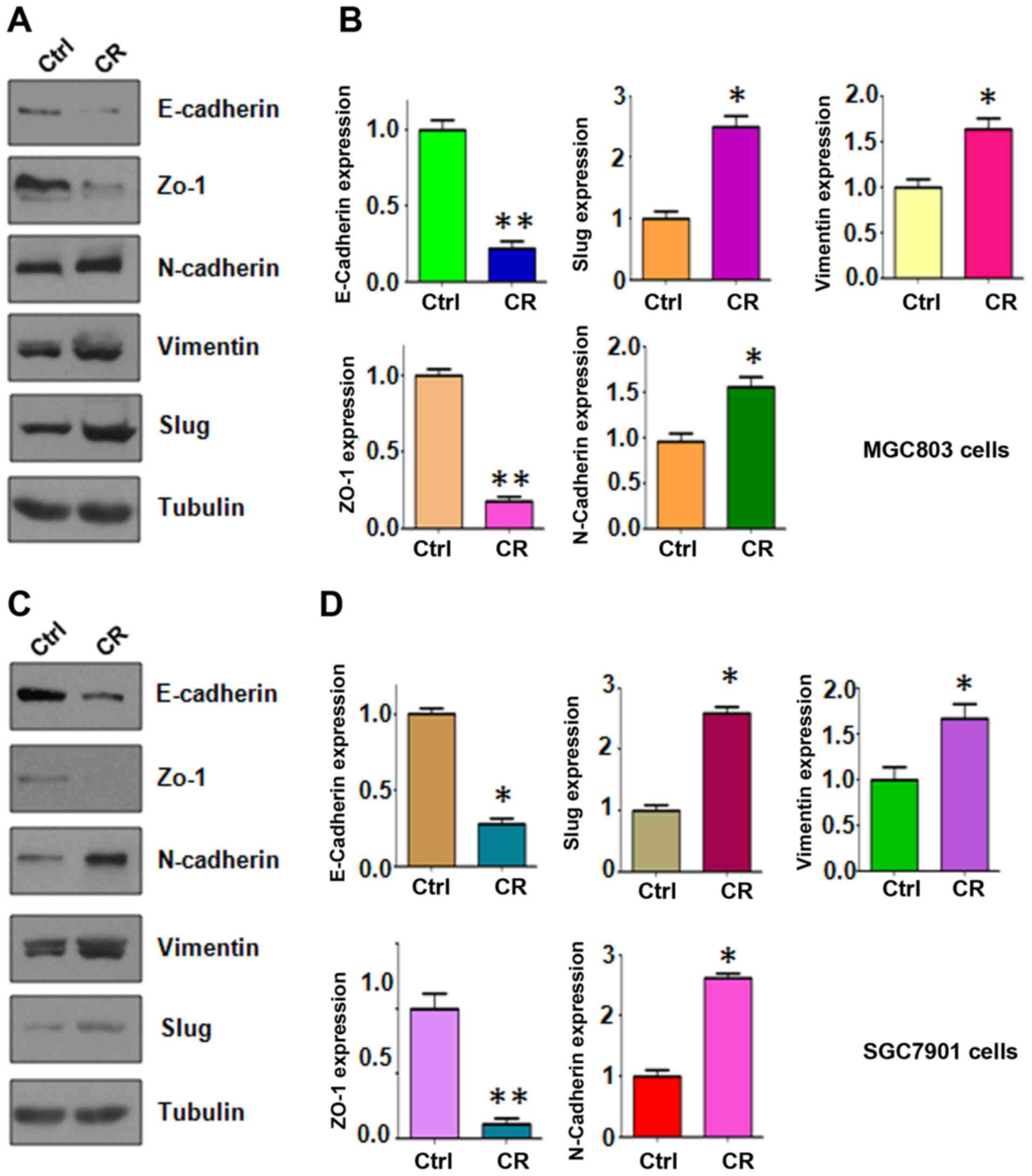
Importantly, our western blotting data validated that epithelial
molecules were decreased in CR cells, but mesenchymal markers were
increased in CR cells (Fig. 3).
These findings suggest that CR cells acquired a mesenchymal
feature.
Overexpression of TAZ in CR cells
Several studies have revealed that TAZ is associated
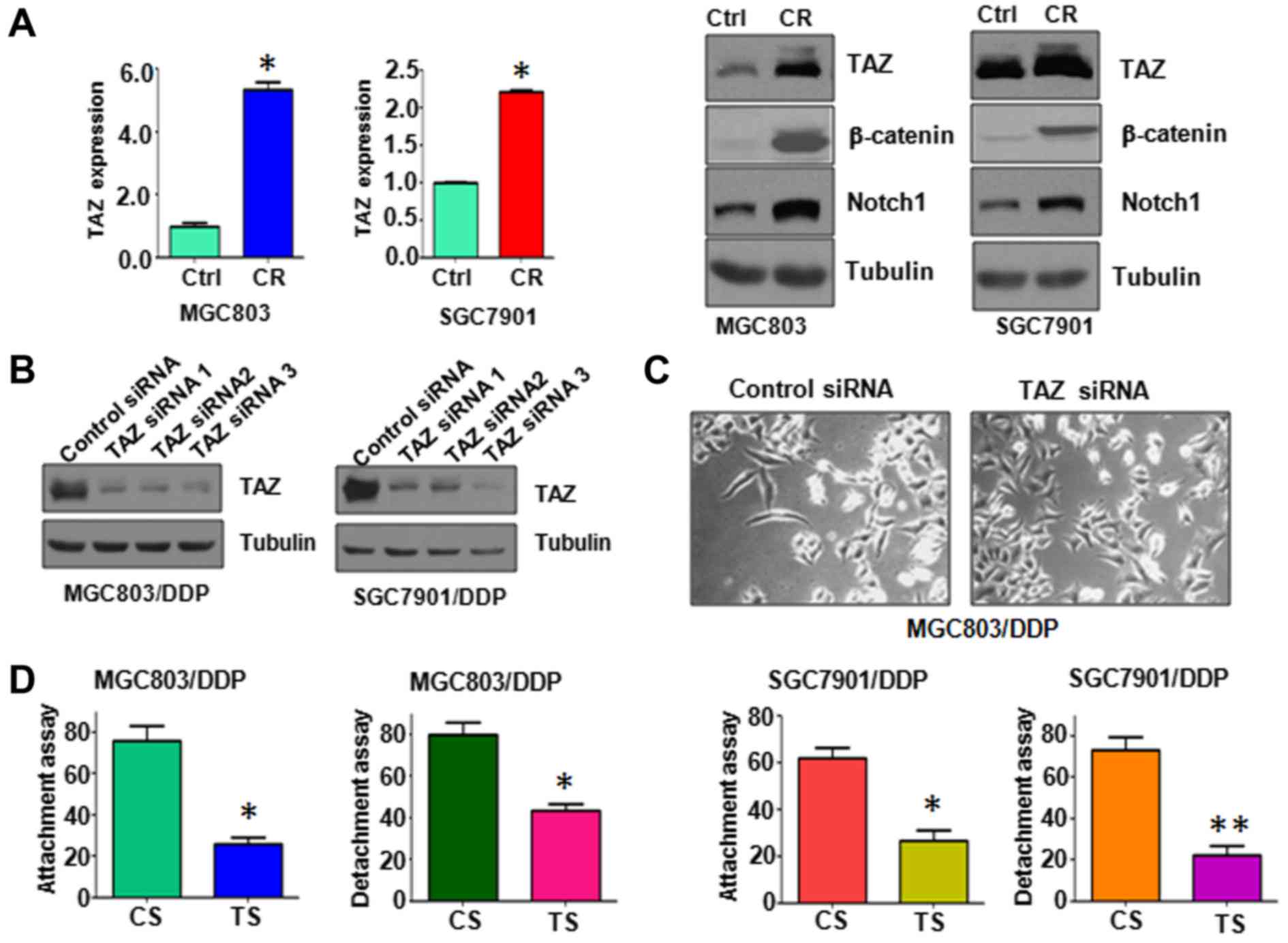
with EMT in cancers (30,31). To determine whether TAZ was
involved in CR-induced EMT process, real-time RT-PCR and western
blotting were performed to measure the transcription and
translation levels of TAZ in CR cells. We observed that both mRNA
and protein levels of TAZ were significantly upregulated in CR
cells (Fig. 4A). Notably, Notch1,
a target of TAZ, was also increased (Fig. 4A). We also found that β-catenin is
highly expressed in CR cells (Fig.
4A). Therefore, our results displayed that the acquisition of
EMT might be due to higher expression of TAZ in CR cells.
Depletion of TAZ reverses EMT to MET in
CR cells
To further dissect the role of TAZ in CR-triggered
EMT, we knocked down the expression of TAZ in CR cells. Our TAZ
siRNAs significantly downregulated the expression of TAZ in CR
cells (Fig. 4B). We selected TAZ
siRNA3 for the following study. We found that depletion of TAZ
reverses EMT to mesenchymal-epithelial transition (MET) in CR cells
(Fig. 4C). Noteworthy,
downregulation of TAZ decreased cell attachment and detachment
activities (Fig. 4D). These data
suggest that TAZ plays an essential role in CR-induced EMT.
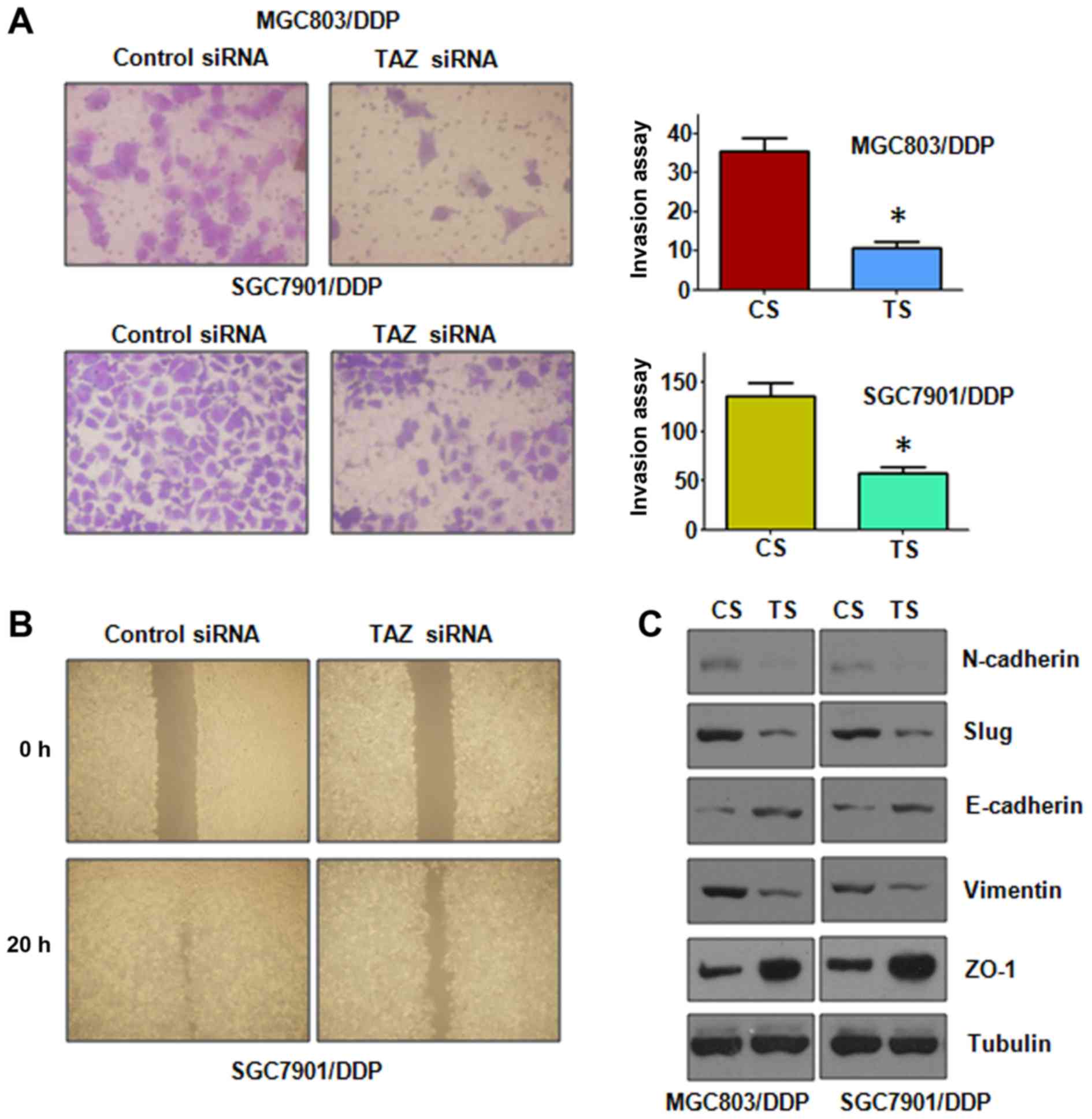
Depletion of TAZ retards motility and
invasion in CR cells
To deeper define the function of TAZ in CR cells,
Transwell invasion assay was conducted. Our invasion results
displayed that downregulation of TAZ significantly inhibited cell
invasion in CR cells (Fig. 5A). In
support of this, our wound healing assay showed that depletion of
TAZ reduced cell motility in CR cells (Fig. 5B). Therefore, TAZ plays an
important role in regulation of cell migration and invasion in CR
cells.
Depletion of TAZ regulates expression of
EMT markers
Next, we explore whether knockdown of TAZ could
regulate the expression of EMT markers. To achieve this goal, the
mRNA and protein levels of EMT markers were measured by real-time
RT-PCR and western blotting, respectively. We found that
downregulation of TAZ increased the expression of epithelial
markers, but decreased the level of mesenchymal markers (Figs. 5C and 6A and B). Therefore, these results
further confirmed the role of TAZ in regulation of EMT in CR
cells.
Downregulation of TAZ enhances CR cells
to cisplatin sensitivity
To determine whether depletion of TAZ expression
enhances CR cells to cisplatin sensitivity, MTT assay was used to
detect the cell growth in TAZ siRNA transfected CR cells. We
observed that downregulation of TAZ significantly attenuated cell
growth inhibition approximately 20–30% induced by 2 µM
cisplatin (Fig. 6C). These MTT
results implied that downregulation of TAZ could enhance CR cells
to cisplatin-induced cell growth inhibition.
Discussion
Gastric cancer is the fourth most common cancer
worldwide. Since chemotherapy often has treatment failure due to
drug resistance, it is pivotal to explore the mechanisms of
chemotherapy resistance in gastric cancer. It is clear that reasons
of drug resistance are complex and multifactorial. Thus, it is
necessary to define the mechanisms of chemoresistance in gastric
cancer (23). In the present
study, we aimed to elucidate the molecular basis of chemoresistance
in gastric cancer. We observed that cisplatin-resistant (CR)
gastric cancer cells obtained EMT features, leading to enhanced
motility and invasion. Mechanistically, higher expression of TAZ
was found in CR cells. Moreover, depletion of TAZ reversed
CR-induced EMT to MET. Our findings demonstrated that TAZ plays a
critical role in chemoresistance and EMT in gastric cancer cells.
Therefore, inhibition of TAZ could be a promising approach to
overcome cisplatin resistance in gastric cancer.
Emerging evidence has demonstrated that
chemoresistant cancer cells are associated with EMT (32). For example, gemcitabine-resistant
pancreatic cancer cells displayed EMT via upregulation of Notch
signaling pathway (33). Another
study showed that gemcitabine-resistant hepatocellular carcinoma
cells underwent EMT process through activation of PDGF-D pathway
(24). Cisplatin resistance in
gastric cancer cells is correlated with Her2 upregulation-induced
EMT (34). CR cervical cancer
cells exhibited EMT phenotype and overexpression of Sema4C
(35). Consistent with these
findings, our current study revealed that CR gastric cancer cells
acquired EMT phenotype with decreased E-cadherin and increased
expression of mesenchymal markers including Snail, Slug, Vimentin,
and N-cadherin. Notably, EMT-type cells exhibited higher activities
of migration and invasion. We confirmed that CR gastric cancer
cells are associated with EMT.
Multiple studies have elucidated that some signaling
pathways controlled cisplatin-induced chemoresistance. Sun et
al found that NF-κB signaling played irreplaceable roles in
cisplatin-induced chemoresistance and tumor progression in bladder
cancer (36). Liu et al
reported that cisplatin resistance is associated with increased
motility and stem-like properties through upregulation of
STAT3/Snail axis in tumor cells (37). One study showed that SET-mediated
NDRG1 inhibition is involved in cisplatin resistance and EMT in
human lung cancer cells (38).
Another study demonstrated that Fbw7 upregulation promoted
cisplatin cytotoxicity in non-small cell lung cancer cells
(39). Wang et al reported
that Akt/β-catenin/Snail signaling pathway was associated with CR
induced EMT in lung cancer cells (40). Downregulation of Par-4 conferred
cisplatin resistance through PI3K (phosphoinositide 3-kinase)/Akt
pathway-dependent EMT in pancreatic cancer cells (41). Connexin 43 has been found to
reverse the resistance of lung adenocarcinoma cells to cisplatin
through inhibition of EMT (42).
Further study revealed that prolonged pemetrexed pretreatment
enhanced persistence of cisplatin-induced DNA damage and eliminated
tumor cells with EMT and cancer stem-like features in lung cancer
cells (43). Our study identified
that TAZ governed the CR-mediated EMT in gastric cancer cells.
Further investigation is required to explore how TAZ controls
CR-induced EMT in gastric cancer.
Accumulating evidence has demonstrated that miRNAs
play an essential role in drug resistance and EMT in human cancers.
For example, it has been observed that miR-20a induced cisplatin
resistance via targeting CYLD in human gastric cancer cells
(44). Zhao et al found
that miR-181a suppressed autophagy and sensitized gastric cancer
cells to cisplation (45). In
addition, miR-26a enhanced the sensitivity of gastric cancer cells
to cisplatin through targeting NRAS and E2F2 (46). Several miRNAs regulated the
cisplatin resistance of human gastric cancer cells. Importantly,
Chen et al reported that miR-206 regulated cisplatin
resistance and EMT partly by targeting MET in human lung cancer
cells (12). Wang et al
found that miR-30a modulated EMT and cisplatin sensitivity in
gastric cancer cells (13). Zuo
et al identified that miR-141 inhibited tumor growth and
metastasis via directly targeting TAZ in gastric cancer studies
(47). These reports indicated
that regulation of these miRNAs could be helpful to govern
cisplatin-induced EMT.
In summary, our study validated that CR gastric
cells acquired EMT in part due to overexpression of TAZ. In
addition, consistent with other study (26,48),
TAZ was found to increase the expression of Notch-1 and β-catenin.
Notch-1 and Wnt/β-catenin have been characterized as the drivers to
induce EMT (33,49). Therefore, we believe that
TAZ-induced EMT could be partly through upregulation of Notch-1 and
β-catenin. Our results also indicated that downregulation of TAZ
could be a useful strategy for restoring sensitivity to cisplatin.
Indeed, one study has shown that knockdown of TAZ modified breast
cancer cell sensitivity to EGFR inhibitors (50). In line with this, we found that
depletion of TAZ sensitized CR cells to cisplatin treatment.
Interestingly, one natural compound, curcumin, has been reported to
inhibit the expression of TAZ in bladder cancer cells (51) and pancreatic cancer cells (48). Due to non-toxic nature of natural
agents, inhibition of TAZ by these compounds could be a safe and
effective approach for the prevention and the treatment of gastric
cancer.
Acknowledgments
We thank our colleague for their critical
reading.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hayakawa Y, Sethi N, Sepulveda AR, Bass AJ
and Wang TC: Oesophageal adenocarcinoma and gastric cancer: Should
we mind the gap? Nat Rev Cancer. 16:305–318. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lordick F and Janjigian YY: Clinical
impact of tumour biology in the management of gastroesophageal
cancer. Nat Rev Clin Oncol. 13:348–360. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wadhwa R, Song S, Lee JS, Yao Y, Wei Q and
Ajani JA: Gastric cancer-molecular and clinical dimensions. Nat Rev
Clin Oncol. 10:643–655. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Petrelli F, Zaniboni A, Coinu A, Cabiddu
M, Ghilardi M, Sgroi G and Barni S: Cisplatin or not in advanced
gastric cancer: A systematic review and meta-analysis. PLoS One.
8:e830222013. View Article : Google Scholar
|
|
7
|
Orditura M, Galizia G, Sforza V,
Gambardella V, Fabozzi A, Laterza MM, Andreozzi F, Ventriglia J,
Savastano B, Mabilia A, et al: Treatment of gastric cancer. World J
Gastroenterol. 20:1635–1649. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen DJ, Chen W, Jiang H, Yang H, Wang YC
and Chen JH: Downregulation of DOCK1 sensitizes bladder cancer
cells to cisplatin through preventing epithelial-mesenchymal
transition. Drug Des Devel Ther. 10:2845–2853. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Du B and Shim JS: Targeting
epithelial-mesenchymal transition (EMT) to overcome drug resistance
in cancer. Molecules. 21:212016. View Article : Google Scholar
|
|
10
|
De Craene B and Berx G: Regulatory
networks defining EMT during cancer initiation and progression. Nat
Rev Cancer. 13:97–110. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen QY, Jiao DM, Wang J, Hu H, Tang X,
Chen J, Mou H and Lu W: miR-206 regulates cisplatin resistance and
EMT in human lung adenocarcinoma cells partly by targeting MET.
Oncotarget. 7:24510–24526. 2016.PubMed/NCBI
|
|
13
|
Wang LL, Zhang XH, Zhang X and Chu JK:
MiR-30a increases cisplatin sensitivity of gastric cancer cells
through suppressing epithelial-to-mesenchymal transition (EMT). Eur
Rev Med Pharmacol Sci. 20:1733–1739. 2016.PubMed/NCBI
|
|
14
|
Haslehurst AM, Koti M, Dharsee M, Nuin P,
Evans K, Geraci J, Childs T, Chen J, Li J, Weberpals J, et al: EMT
transcription factors snail and slug directly contribute to
cisplatin resistance in ovarian cancer. BMC Cancer. 12:912012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kang W, Cheng AS, Yu J and To KF: Emerging
role of Hippo pathway in gastric and other gastrointestinal
cancers. World J Gastroenterol. 22:1279–1288. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hong AW, Meng Z and Guan KL: The Hippo
pathway in intestinal regeneration and disease. Nat Rev
Gastroenterol Hepatol. 13:324–337. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yue G, Sun X, Gimenez-Capitan A, Shen J,
Yu L, Teixido C, Guan W, Rosell R, Liu B and Wei J: TAZ is highly
expressed in gastric signet ring cell carcinoma. BioMed Res Int.
2014:3930642014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou GX, Li XY, Zhang Q, Zhao K, Zhang CP,
Xue CH, Yang K and Tian ZB: Effects of the hippo signaling pathway
in human gastric cancer. Asian Pac J Cancer Prev. 14:5199–5205.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim MH and Kim J, Hong H, Lee SH, Lee JK,
Jung E and Kim J: Actin remodeling confers BRAF inhibitor
resistance to melanoma cells through YAP/TAZ activation. EMBO J.
35:462–478. 2016. View Article : Google Scholar :
|
|
20
|
Tian T, Li A, Lu H, Luo R, Zhang M and Li
Z: TAZ promotes temozolomide resistance by upregulating MCL-1 in
human glioma cells. Biochem Biophys Res Commun. 463:638–643. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lai D, Ho KC, Hao Y and Yang X: Taxol
resistance in breast cancer cells is mediated by the hippo pathway
component TAZ and its downstream transcriptional targets Cyr61 and
CTGF. Cancer Res. 71:2728–2738. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li Z, Wang Y, Zhu Y, Yuan C, Wang D, Zhang
W, Qi B, Qiu J, Song X, Ye J, et al: The Hippo transducer TAZ
promotes epithelial to mesenchymal transition and cancer stem cell
maintenance in oral cancer. Mol Oncol. 9:1091–1105. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang Q, Huang J, Wu Q, Cai Y, Zhu L, Lu X,
Chen S, Chen C and Wang Z: Acquisition of epithelial-mesenchymal
transition is associated with Skp2 expression in
paclitaxel-resistant breast cancer cells. Br J Cancer.
110:1958–1967. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu Q, Wang R, Yang Q, Hou X, Chen S, Hou
Y, Chen C, Yang Y, Miele L, Sarkar FH, et al: Chemoresistance to
gemcitabine in hepatoma cells induces epithelial-mesenchymal
transition and involves activation of PDGF-D pathway. Oncotarget.
4:1999–2009. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kong D, Wang Z, Sarkar SH, Li Y, Banerjee
S, Saliganan A, Kim HR, Cher ML and Sarkar FH: Platelet-derived
growth factor-D overexpression contributes to
epithelial-mesenchymal transition of PC3 prostate cancer cells.
Stem Cells. 26:1425–1435. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao Z, Zheng N, Wang L, Hou Y, Zhou X and
Wang Z: Rottlerin exhibits antitumor activity via down-regulation
of TAZ in non-small cell lung cancer. Oncotarget. 8:7827–7838.
2017.
|
|
27
|
Tan Y, Qin S, Hou X, Qian X, Xia J, Li Y,
Wang R, Chen C, Yang Q, Miele L, et al: Proteomic-based analysis
for identification of proteins involved in 5-fluorouracil
resistance in hepatocellular carcinoma. Curr Pharm Des. 20:81–87.
2014. View Article : Google Scholar
|
|
28
|
Wang Z, Li Y, Ahmad A, Banerjee S, Azmi
AS, Kong D and Sarkar FH: Pancreatic cancer: Understanding and
overcoming chemoresistance. Nat Rev Gastroenterol Hepatol. 8:27–33.
2011. View Article : Google Scholar
|
|
29
|
Mallini P, Lennard T, Kirby J and Meeson
A: Epithelial-to-mesenchymal transition: What is the impact on
breast cancer stem cells and drug resistance. Cancer Treat Rev.
40:341–348. 2014. View Article : Google Scholar
|
|
30
|
Xie D, Cui J, Xia T, Jia Z, Wang L, Wei W,
Zhu A, Gao Y, Xie K and Quan M: Hippo transducer TAZ promotes
epithelial mesenchymal transition and supports pancreatic cancer
progression. Oncotarget. 6:35949–35963. 2015.PubMed/NCBI
|
|
31
|
Xiao H, Jiang N, Zhou B, Liu Q and Du C:
TAZ regulates cell proliferation and epithelial-mesenchymal
transition of human hepatocellular carcinoma. Cancer Sci.
106:151–159. 2015. View Article : Google Scholar :
|
|
32
|
Piskareva O, Harvey H, Nolan J, Conlon R,
Alcock L, Buckley P, Dowling P, Henry M, O'Sullivan F, Bray I, et
al: The development of cisplatin resistance in neuroblastoma is
accompanied by epithelial to mesenchymal transition in vitro.
Cancer Lett. 364:142–155. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang Z, Li Y, Kong D, Banerjee S, Ahmad A,
Azmi AS, Ali S, Abbruzzese JL, Gallick GE and Sarkar FH:
Acquisition of epithelial-mesenchymal transition phenotype of
gemcitabine-resistant pancreatic cancer cells is linked with
activation of the notch signaling pathway. Cancer Res.
69:2400–2407. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Huang D, Duan H, Huang H, Tong X, Han Y,
Ru G, Qu L, Shou C and Zhao Z: Cisplatin resistance in gastric
cancer cells is associated with HER2 upregulation-induced
epithelial-mesenchymal transition. Sci Rep. 6:205022016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Song J and Li Y: miR-25-3p reverses
epithelial-mesenchymal transition via targeting Sema4C in
cisplatin-resistance cervical cancer cells. Cancer Sci. 108:23–31.
2017. View Article : Google Scholar
|
|
36
|
Sun Y, Guan Z, Liang L, Cheng Y, Zhou J,
Li J and Xu Y: NF-κB signaling plays irreplaceable roles in
cisplatin-induced bladder cancer chemoresistance and tumor
progression. Int J Oncol. 48:225–234. 2016.
|
|
37
|
Liu WH, Chen MT, Wang ML, Lee YY, Chiou
GY, Chien CS, Huang PI, Chen YW, Huang MC, Chiou SH, et al:
Cisplatin-selected resistance is associated with increased motility
and stem-like properties via activation of STAT3/Snail axis in
atypical teratoid/rhabdoid tumor cells. Oncotarget. 6:1750–1768.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu H, Gu Y, Yin J, Zheng G, Wang C, Zhang
Z, Deng M, Liu J, Jia X and He Z: SET-mediated NDRG1 inhibition is
involved in acquisition of epithelial-to-mesenchymal transition
phenotype and cisplatin resistance in human lung cancer cell. Cell
Signal. 26:2710–2720. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yu HG, Wei W, Xia LH, Han WL, Zhao P, Wu
SJ, Li WD and Chen W: FBW7 upregulation enhances cisplatin
cytotoxicity in non-small cell lung cancer cells. Asian Pac J
Cancer Prev. 14:6321–6326. 2013. View Article : Google Scholar
|
|
40
|
Wang H, Zhang G, Zhang H, Zhang F, Zhou B,
Ning F, Wang HS, Cai SH and Du J: Acquisition of
epithelial-mesenchymal transition phenotype and cancer stem
cell-like properties in cisplatin-resistant lung cancer cells
through AKT/β-catenin/Snail signaling pathway. Eur J Pharmacol.
723:156–166. 2014. View Article : Google Scholar
|
|
41
|
Tan J, You Y, Xu T, Yu P, Wu D, Deng H,
Zhang Y and Bie P: Par-4 downregulation confers cisplatin
resistance in pancreatic cancer cells via PI3K/Akt
pathway-dependent EMT. Toxicol Lett. 224:7–15. 2014. View Article : Google Scholar
|
|
42
|
Yu M, Zhang C, Li L, Dong S, Zhang N and
Tong X: Cx43 reverses the resistance of A549 lung adenocarcinoma
cells to cisplatin by inhibiting EMT. Oncol Rep. 31:2751–2758.
2014.PubMed/NCBI
|
|
43
|
Tièche CC, Peng RW, Dorn P, Froment L,
Schmid RA and Marti TM: Prolonged pemetrexed pretreatment augments
persistence of cisplatin-induced DNA damage and eliminates
resistant lung cancer stem-like cells associated with EMT. BMC
Cancer. 16:1252016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhu M, Zhou X, Du Y, Huang Z, Zhu J, Xu J,
Cheng G, Shu Y, Liu P, Zhu W, et al: miR-20a induces cisplatin
resistance of a human gastric cancer cell line via targeting CYLD.
Mol Med Rep. 14:1742–1750. 2016.PubMed/NCBI
|
|
45
|
Zhao J, Nie Y, Wang H and Lin Y: MiR-181a
suppresses autophagy and sensitizes gastric cancer cells to
cisplatin. Gene. 576:828–833. 2016. View Article : Google Scholar
|
|
46
|
Wen L, Cheng F, Zhou Y and Yin C: MiR-26a
enhances the sensitivity of gastric cancer cells to cisplatin by
targeting NRAS and E2F2. Saudi J Gastroenterol. 21:313–319. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zuo QF, Zhang R, Li BS, Zhao YL, Zhuang Y,
Yu T, Gong L, Li S, Xiao B and Zou QM: MicroRNA-141 inhibits tumor
growth and metastasis in gastric cancer by directly targeting
transcriptional co-activator with PDZ-binding motif, TAZ. Cell
Death Dis. 6:e16232015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhou X, Su J, Feng S, Wang L, Yin X, Yan J
and Wang Z: Antitumor activity of curcumin is involved in
down-regulation of YAP/TAZ expression in pancreatic cancer cells.
Oncotarget. 7:79076–79088. 2016.PubMed/NCBI
|
|
49
|
Zhang J, Tian XJ and Xing J: Signal
transduction pathways of EMT induced by TGF-β, SHH, and WNT and
their crosstalks. J Clin Med. 5:52016. View Article : Google Scholar
|
|
50
|
Guo L, Zheng J, Zhang J, Wang H, Shao G
and Teng L: Knockdown of TAZ modifies triple-negative breast cancer
cell sensitivity to EGFR inhibitors by regulating YAP expression.
Oncol Rep. 36:729–736. 2016.PubMed/NCBI
|
|
51
|
Gao Y, Shi Q, Xu S, Du C, Liang L, Wu K,
Wang K, Wang X, Chang LS, He D, et al: Curcumin promotes KLF5
proteasome degradation through downregulating YAP/TAZ in bladder
cancer cells. Int J Mol Sci. 15:15173–15187. 2014. View Article : Google Scholar : PubMed/NCBI
|