Introduction
Malignant pleural mesothelioma (mesothelioma) is an
aggressive form of cancer that originates in the mesothelial cells
of the pleura of the lung and is closely associated with asbestos
exposure (1). Mesothelioma
incidence has increased over the last several decades with deaths
approximately 43,000 worldwide annually; furthermore, decline is
not expected by 2020 (2). Combined
treatment with surgery and chemotherapy has improved survival and
quality of life for mesothelioma patients; however, most patients
survive <12–18 months (3) and
long-term survival is dismal (4).
Therefore, more effective treatments and early detection strategies
for mesothelioma patients are urgently needed.
Receptor tyrosine kinases (RTKs) have a pivotal role
in tumor growth and metastasis, and RTKs have been associated with
controlling the signaling cascades that result in cell
transformation, cell proliferation, and invasion of the transformed
cells (5). MET protein, encoded by
MET proto-oncogene, is an RTK and a hepatocyte growth factor
(HGF) receptor (6). MET is
responsible for the modulation of cell growth and is activated in a
number of human mesothelioma cell lines and tissues (7,8).
Previous studies have demonstrated that HGF induced mesothelioma
cell proliferation through activation of PI3K/ERK5/Fra-1 pathway
and HGF has also been shown to phosphorylate ERK5 in human
mesothelioma cells (9). ERK5, a
mitogen-activated protein kinase (MAPK), is activated upon a double
phosphorylation by the unique MAPK-ERK kinase 5 (MEK5). The
MEK5/ERK5 pathway is essential for blood vessel and cardiac
development (10). Moreover, the
MEK5/ERK5 pathway is important in regulating the proliferation and
survival of endothelial cells (11,12)
and mesothelioma cells (13).
Doublecortin-like kinase 1 (DCLK1) belongs to the
family of calmodulin-dependent kinases and binds to micro-tubules.
It is a marker for tuft cells in the small intestine and pancreas
(14–16). DCLK1 is classified as a pancreatic
cancer stem-cell marker and its expression is also upregulated in
pancreatic ductal adenocarcinoma (14,17).
It has been suggested that DCLK1 marks the tumor-initiating cells
in numerous tumor types (18–20).
Moreover, DCLK1 is also known to act as a regulator for several
known oncogenes such as KRAS, NOTCH1 and c-Myc (17,21,22).
Although DCLK1 has been linked to various cancers,
little is known about its role in the growth of human mesothelioma.
Furthermore, the relationship between MET, ERK5, and DCLK1 in human
mesothelioma is unknown. In this study, we sought to evaluate the
efficacy of MET, ERK5, and DCLK1 as possible biomarkers and
potential targets for therapeutic development for human
mesothelioma.
Materials and methods
Reagents
Monoclonal rabbit anti-human MET [EP1454Y],
polyclonal rabbit anti-human MET (phospho Y1230 + Y1234 + Y1235)
and polyclonal rabbit anti-human DCLK1 were purchased from Abcam,
Inc. (Cambridge, MA, USA). Polyclonal rabbit anti-human ERK5 and
polyclonal rabbit anti-human phospho-ERK5 (Thr218/Tyr220) were
purchased from Cell Signaling, Inc. (Danvers, MA, USA).
Cabozantinib (XL184) and XMD8-92 were purchased from Selleck
Chemicals LLC (Houston, TX, USA).
Cell lines and cell culture
Human mesothelioma cell lines 211H, H2052, and H28
were purchased from American Type Culture Collections (ATCC,
Manassas, VA, USA). H290 and MS-1 were purchased from NIH
(Frederick, MD, USA). H513 and human normal mesothelial cell line
LP9 were purchased from the Cell Culture Core Facility at Harvard
University (Boston, MA, USA). The mesothelioma cell lines were
maintained in RPMI-1640 medium supplemented with 10% (v/v)
heat-inactivated FBS, penicillin (100 IU/ml) and streptomycin (100
µg/ml). LP9 was maintained in medium consisting of a 1:1
composition of M199 and M106 medium (Life Technologies)
supplemented with 15% (v/v) newborn calf serum (Fisher
Scientific/Hyclone), 10 ng/ml EGF and 0.4 µg/ml
hydrocortisone, penicillin (100 IU/ml), and streptomycin (100
µg/ml).
Tissue samples and
immunohistochemistry
Fresh mesothelioma and adjacent normal pleural
tissues were obtained from patients with malignant plural
mesothelioma who underwent surgical resection of the primary tumor
at University of California, San Francisco Medical Center from July
1997 to December 2008. Primary human mesothelioma samples from 73
patients were fixed in formalin and embedded in paraffin in
4-µm tissue microarray sections. In eight of these patients,
a small amount of normal pleural tissue had been obtained
simultaneously to serve as controls. We only have complete
information of 45 of the patients. For the other 28 patients, the
information is incomplete. The average age of these 45 patients is
68.13 years, and the median age is 69 years. The mean following up
period for these patients is 2 years based on their registered last
visit date. The end-point record relies on the notification of the
deaths of these registered patients to the medical center and the
obituary information. All human tissue samples were obtained and
analyzed in accordance with procedures approved by the
institutional review board of the University of California, San
Francisco (IRB H8714-22 942-01), and written informed consent was
obtained from all subjects. All the methods were carried out in
accordance with the approved guidelines.
The sections were immunostained as previously
described (23). The sections and
mesothelioma cell lines H290, H513, H28, 211H, MS-1 and H2052 and
mesothelial cell line LP9 were immunostained stained with the
properly diluted antibodies: anti-MET (ab51067) at 1:400; anti-ERK
at 1:100; anti-DCLK1 (ab31704) at 1:300.
The following scoring system was used: −, no stain;
+, weak staining (≥10% stained cellularity considered as positive);
++, moderate staining (≥30% stained cellularity considered as
positive); +++, strong staining (≥50% stained cellularity
considered as positive). The scoring was done under a low power
objective lens (×10) with a Zeiss Axioscop 2 microscope (Carl Zeiss
inc., germany). Images were taken under a ×10 or ×20 or ×40
objective lens.
Cell viability assay
Cells were cultured in a 96-well plate and treated
with different doses of XL184 and XMD8-92 (XL184: 0, 0.001, 0.003,
0.01, 0.03, 0.1, 0.3,1, 3, 10 and 30 µM; XMD8-92: 0, 0.003,
0.01, 0.03, 0.1, 0.3,1, 3, 10, 30 and 100 µM). After 48 h of
incubation, cells were lysed and luminescent signaling was
generated by a CellTiter-Glo Luminescent Cell Viability assay
reagent (Promega). Luminescent signaling was measured on the
GloMax-96 Microplate Luminometer. Proportional cell viability was
analyzed with GraphPad Prism 6 software (GraphPad Software, Inc.,
La Jolla, CA, USA), which was used to calculate dose-response
curves and IC50.
Western blot analysis
Total protein was extracted from cell lines using
M-PER Mammalian Protein Extraction reagent (Thermo Fisher
Scientific, Waltham, MA, USA) supplied with Complete Protease
Inhibitor Cocktails (Roche, Lewes, UK), according to the
manufacturer's protocols. The protein concentrations were measured
with the Pierce BCA Protein assay kit (Thermo Fisher Scientific). A
total of 30 µg of proteins were run on 4–20% gradient
SDS-polyacrylamide gels (Bio-Rad Laboratories, Inc., Hercules, CA,
USA) and transferred to Immobilon-P nitrocellulose membranes
(Millipore, Bellerica, MA, USA). The membranes were blocked in 5%
BSA and then probed with the primary antibodies overnight at 4°C.
The membranes were incubated with appropriate secondary antibodies,
either peroxidase-conjugated anti-mouse or anti-rabbit (Santa Cruz
Biotechnology, Santa Cruz, CA, USA). GAPDH was used as a loading
control. Then the antigen-antibody complexes were detected by using
an ECL blotting analysis system (Amersham Pharmacia Biotech,
Piscataway, NJ, USA).
Quantitative real-time PCR
Total RNA was extracted from cells using the Rneasy
Mini kit (Qiagen, Valencia, CA, USA). The cDNA was transcribed from
1 µg of total RNA using iScript cDNA Synthesis kits
(Bio-Rad), according to the manufacturer's protocol. The cDNA was
used as the template for real-time PCR detection using TaqMan
Technology on an Applied Biosystems 7900HT sequence detection
system (Applied Biosystems, Foster City, CA, USA). Expression of
MET, ERK5, and DCLK1 genes, and endogenous control gene
β-glucuronidase (GUSB) were detected by using probes commercially
(Applied Biosystems) and analyzed using Relative Quantification
Software SDS 2.4 (Applied Biosystems). The relative mRNA expression
level of DCLK1 in each sample was calcu lated using the comparative
expression level 2−ΔΔCt method. All experiments were
carried out in triplicate for each data point.
Transwell invasion assay
The Transwell invasion assay was performed in a
6-well plate Transwell system (Corning Inc., NY, USA). The
Transwell inserts were coated with 300 µl Matrigel and
incubated at 37°C for half an hour. Cells (3×105) of
H290 and H2052 were harvested and resuspended in serum-free medium
supplemented with 1.0 µM XL184, 1.0 µM XMD8-92 or
DMSO (0.1%) to the upper chamber of the Transwell. The lower
chamber was infused with 2.6 ml complete growth medium (10% FBS).
The Transwell was incubated at 37°C for 20 h, and then the gel and
cells in the upper chamber were removed. After formalin fixation,
the membrane was stained by crystal violet (Sigma, St. Louis, MO,
USA) for 20 min. Phase contrast images were taken and the cells on
the lower side of the membrane were counted in six random visual
fields under a 20× objective lens.
Tumorsphere assays
Number (1×103) of H290 single-cell
suspensions after treated with 0.3 µM XL184, 1.0 µM
XL184, 0.3 µM XMD8-92, 1.0 µM XMD8-92 or DMSO (0.1%)
for 48 h were plated in 24-well ultra-low attachment plates
(Corning Inc.) in StemPro MSC SFM Basal Medium CTS + StemPro MSC
SFM Supplement CTS (Life Technologies, Grand Island, NY, USA), 2 NM
L-glutamine, and penicillin (100 IU/ml). Tumorspheres were cultured
for 7 days. Tumorspheres formed in non-adherent cultures were
counted under a 10× objective lens. The cut-off size for the
spheres counted is 60 µm.
Statistical analysis
Data are expressed as mean ± standard deviation (SD)
from three independent experiments. The Chi-square independence
test was used to compare IHC results between the staining intensity
of MET, ERK5, and DCLK1 in the same mesothelioma tumors. The other
statistical analyses were performed using the GraphPad Prism
(Version 6.0; GraphPad Software, San Diego, CA, USA). Student's
t-test was used for comparison between two groups. One-way ANOVA
followed by Scheffe multiple comparisons were used to compare the
differences among multiple groups. Survival analysis in these 45
patients was performed by Kaplan-Meier analysis with GraphPad Prism
(Version 6.0; GraphPad software). A significant difference was
considered when the P-value from a two-tailed test was
<0.05.
Results
MET, ERK5, and DCLK1 are overexpressed in
human mesothelioma tumors
To investigate MET, ERK5, and DCLK1 expression in
human mesothelioma cells, primary human mesothelioma tissue samples
from 73 patients were analyzed using immunohistochemistry. Tables I and II indicated the positive or negative
results for MET, ERK5, and DCLK1 staining in tissue microarray
sections of human mesothelioma and normal pleural samples.
 | Table IPositive and negative number and
ratio of MET, ERK5, and DCLK1 in normal pleural samples. |
Table I
Positive and negative number and
ratio of MET, ERK5, and DCLK1 in normal pleural samples.
| − Number
(ratio) | + Number
(ratio) | ++ Number
(ratio) | +++ Number
(ratio) |
|---|
| MET | 4 (50.0%) | 3 (37.5%) | 1 (12.5%) | 0 (0%) |
| ERK5 | 5 (62.5%) | 3 (37.5%) | 0 (0%) | 0 (0%) |
| DCLK1 | 6 (75%) | 2 (25%) | 0 (0%) | 0 (0%) |
 | Table IIPositive and negative number and
ratio of MET, ERK5, and DCLK1 in mesothelioma samples. |
Table II
Positive and negative number and
ratio of MET, ERK5, and DCLK1 in mesothelioma samples.
| − Number
(ratio) | + Number
(ratio) | ++ Number
(ratio) | +++ Number
(ratio) |
|---|
| MET | 6 (8.2%) | 18 (24.7%) | 39 (53.4%) | 10 (13.7%) |
| ERK5 | 14 (19.2%) | 24 (32.8%) | 28 (38.4%) | 7 (9.6%) |
| DCLK1 | 14 (19.2%) | 22 (30.1%) | 26 (35.6%) | 11 (15.1%) |
MET was stained in the cytoplasm of mesothelioma
samples (Fig. 1A). In the eight
normal pleural tissue samples, 50.0% was negative, 37.5% was weak,
12.5% was moderate, and none of the samples had strong MET staining
(Table I). In the 73 human
mesothelioma samples, 8.2% was negative, 24.7% was weak, 53.4% was
moderate, and 13.7% had strong MET staining (Table II). Our results showed that
moderate to strong staining for MET was detected in 67.1% of the
analyzed mesothelioma tumor samples but in only 12.5% of the normal
pleural tissues. The results of MET staining in mesothelioma cell
lines H290, H513, H28, 211H, MS-1, and H2052 and normal mesothelial
cell line LP9 are shown in Fig.
1B. Four of the tested mesothelioma cell lines H290, H513, H28
and 211H showed strong positive staining for MET, whereas MS-1,
H2052 and normal mesothelial cell line LP9 showed negative staining
for MET, indicating a lack of expression (Fig. 1B).
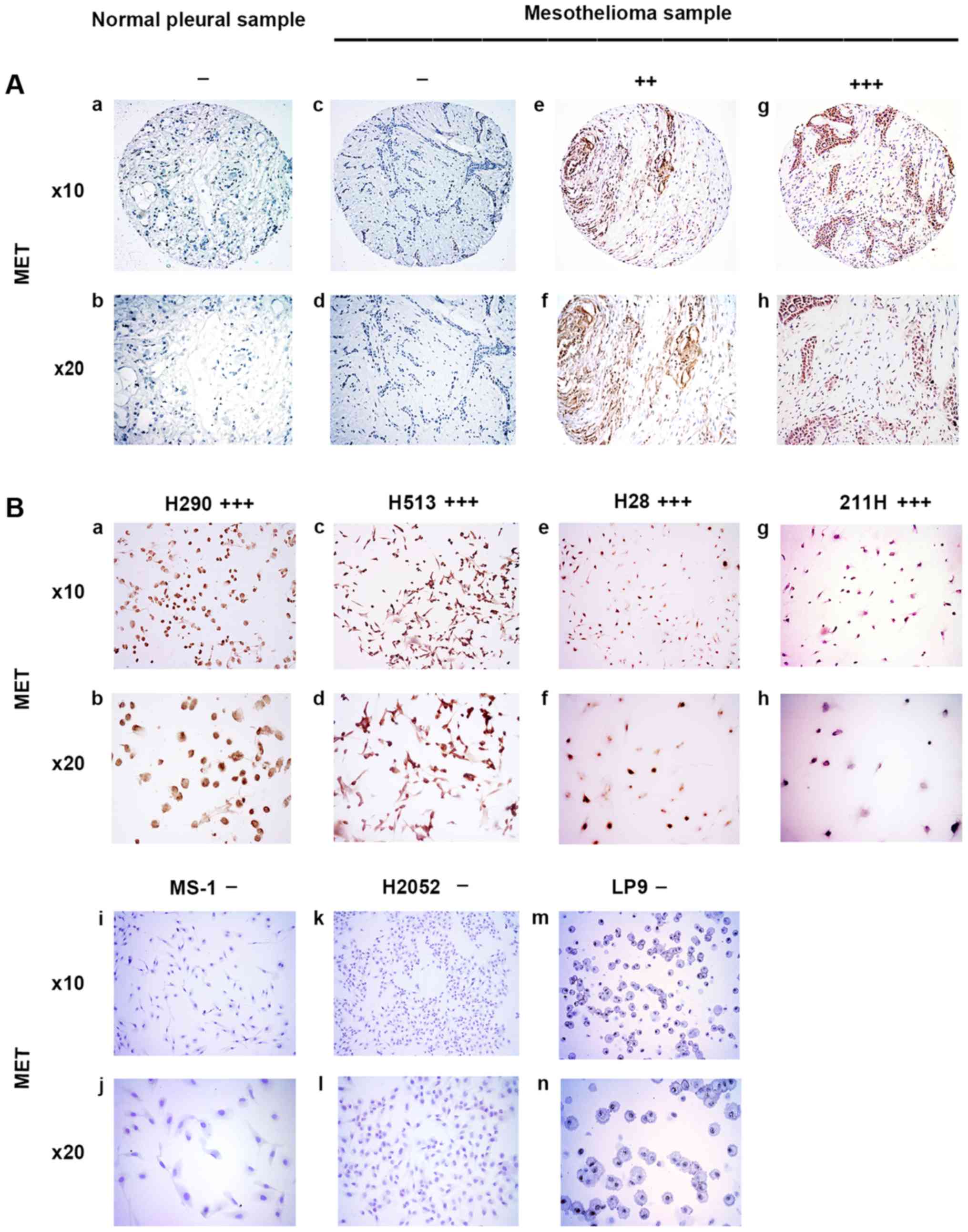 | Figure 1Immunohistochemistry of MET in normal
pleural and mesothelioma samples. (A) a and b, normal pleura
sample. c–h, mesothelioma samples. c and d, negative. e and f,
moderate stain. g and h, strong stain. (B) Mesothelioma cell lines.
a and b, H290, strong stain. c and d, H513, strong stain. e and f,
H28, strong stain. g and h, 211H, strong stain. i and j, MS-1,
negative. k and l, H2052, negative. m and n, normal mesothelial
cell line LP9, negative. |
ERK5 was stained in the cytoplasm or nuclei of
mesothelioma samples (Fig. 2A). In
the eight normal pleural tissue samples, 62.5% was negative, 37.5%
was weak, and none of the samples had moderate or strong ERK5
staining (Table I). In the 73
human mesothelioma samples, 19.2% was negative, 32.8% was weak,
38.4% was moderate, and 9.6% had strong ERK5 staining (Table II). Our results showed that
moderate to strong staining for ERK5 was detected in 48% of the
analyzed mesothelioma tumor samples but not in the normal pleural
tissues. The results of ERK5 staining in mesothelioma cell lines
H290, H513, H28, 211H, MS-1, and H2052 and normal mesothelial cell
line LP9 are shown in Fig. 2B. The
H290 cell line also showed strong positive staining for ERK5,
whereas the H513 and H28 cell lines showed moderately positive
staining for ERK5. Cell lines 211H, MS-1, H2052 and normal
mesothelial cell line LP9 showed negative staining for ERK5
(Fig. 2B).
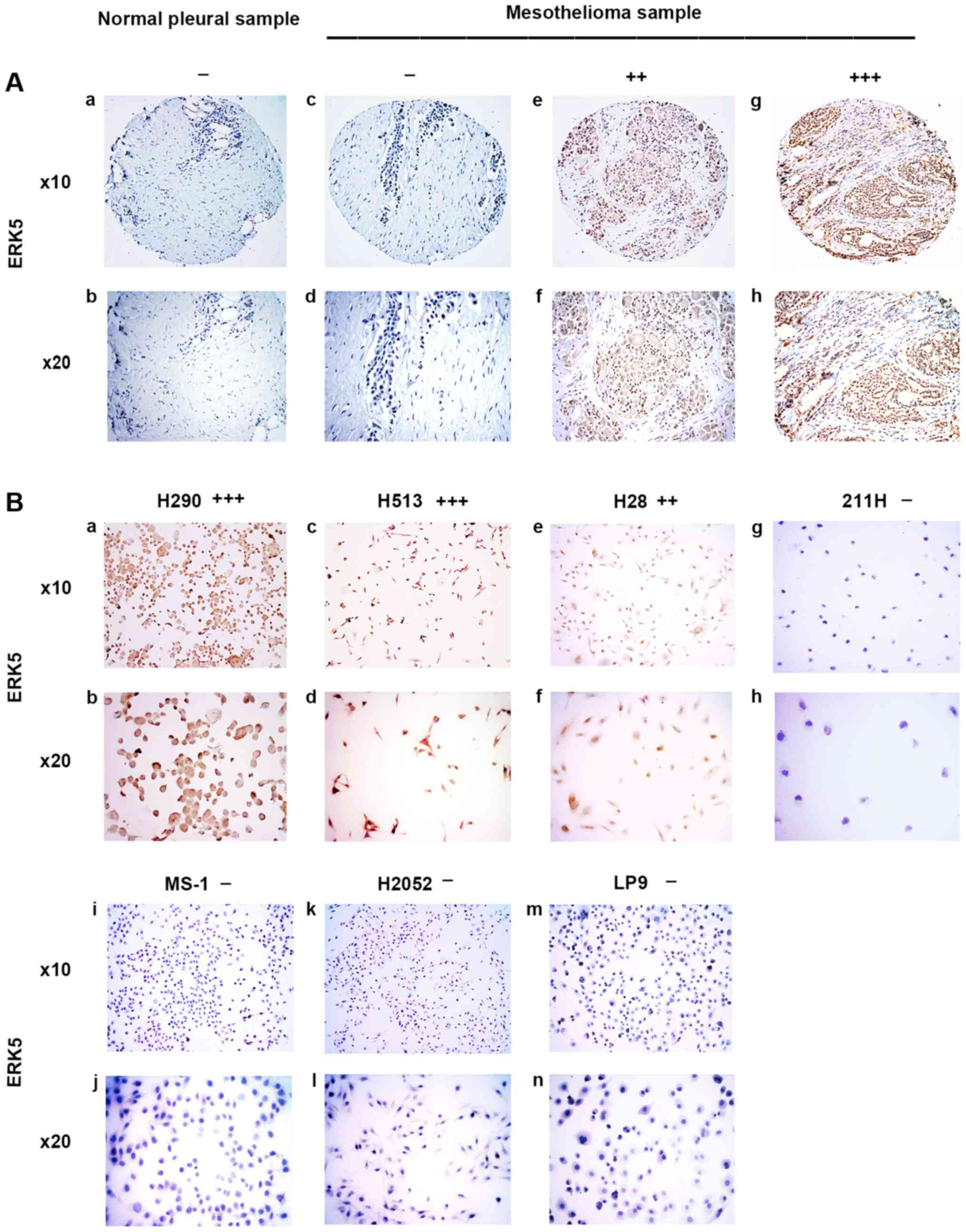 | Figure 2Immunohistochemistry of ERK5 in
normal pleural and mesothelioma samples. (A) a and b, normal pleura
sample. c–h, mesothelioma samples. c and d, negative. e and f,
moderate stain. g and h, strong stain. (B) Mesothelioma cell lines.
a and b, H290, strong stain. c and d, H513, moderate stain. e and
f, H28, moderate stain. g and h, 211H, negative; i and j, MS-1,
negative. k and l, H2052, negative. m and n, normal mesothelial
cell line LP9, negative. |
DCLK1 was stained in the nuclei of normal pleural
samples and mesothelioma samples (Fig.
3A). In the eight normal pleural tissue samples, 75% was
negative, 25% was weak, and none of the samples had moderate or
strong DCLK1 staining (Table I).
In the 73 human mesothelioma samples, 19.2% was negative, 30.1% was
weak, 35.6% was moderate, and 15.1% had strong DCLK1 staining
(Table II). Our results showed
that moderate to strong staining for DCLK1 was detected in 50.7% of
the analyzed mesothelioma tumor samples but not in the normal
pleural tissues. The results of DCLK1 staining in mesothelioma cell
lines H290, H513, H28, 211H, MS-1, H2052 and normal mesothelial
cell line LP9 are shown in Fig.
3B. H290 and H28 cell lines showed strong positive staining for
DCLK1 and the H513 cell line showed moderate staining for DCLK1.
The cell lines 211H, MS-1, H2052, and normal mesothelial cell line
LP9 were all negative for DCLK-1 (Fig.
3B).
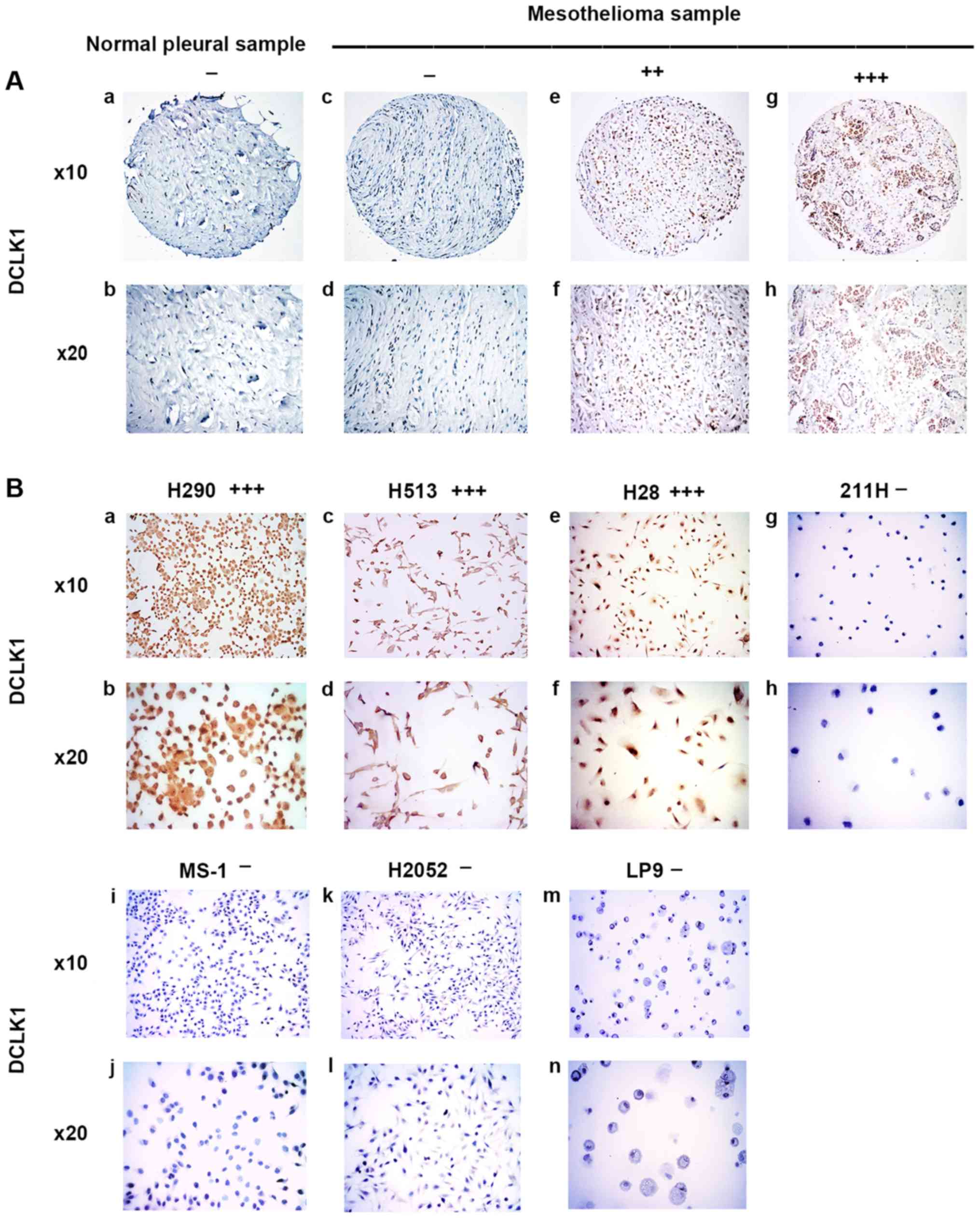 | Figure 3Immunohistochemistry of DCLK1 in
normal pleural and mesothelioma samples. (A) a and b, normal pleura
sample. c-h, mesothelioma samples. c and d, negative. e and f,
moderate stain. g and h, strong stain. (B) Mesothelioma cell lines.
a and b, H290, strong stain. c and d, H513, moderate stain. e and
f, H28, strong stain. g and h, 211H, negative. i and j, MS-1,
negative. k and l, H2052, negative. m and n, normal mesothelial
cell line LP9, negative. |
Our analysis also showed that, in 38.4% of the
mesothelioma tumor samples, moderate to strong expression of all
three proteins was detected (n=28) (Fig. 4A and Tables I and II). Statistical analysis revealed a
significant association between MET, ERK5, and DCLK1 expression in
these mesothelioma samples (P<0.05, Chi-square test) (Tables III–V). We analyzed the IHC staining score of
MET, ERK5, and DCLK1 of mesothelioma samples and normal pleural
tissues obtained from 8 patients (Fig.
4B–D). Statistical analysis indicated a significant higher
expression of MET, ERK5 and DCLK1 in mesothelioma samples compared
to their expressions in normal pleural tissues obtained from the
sample 8 patients.
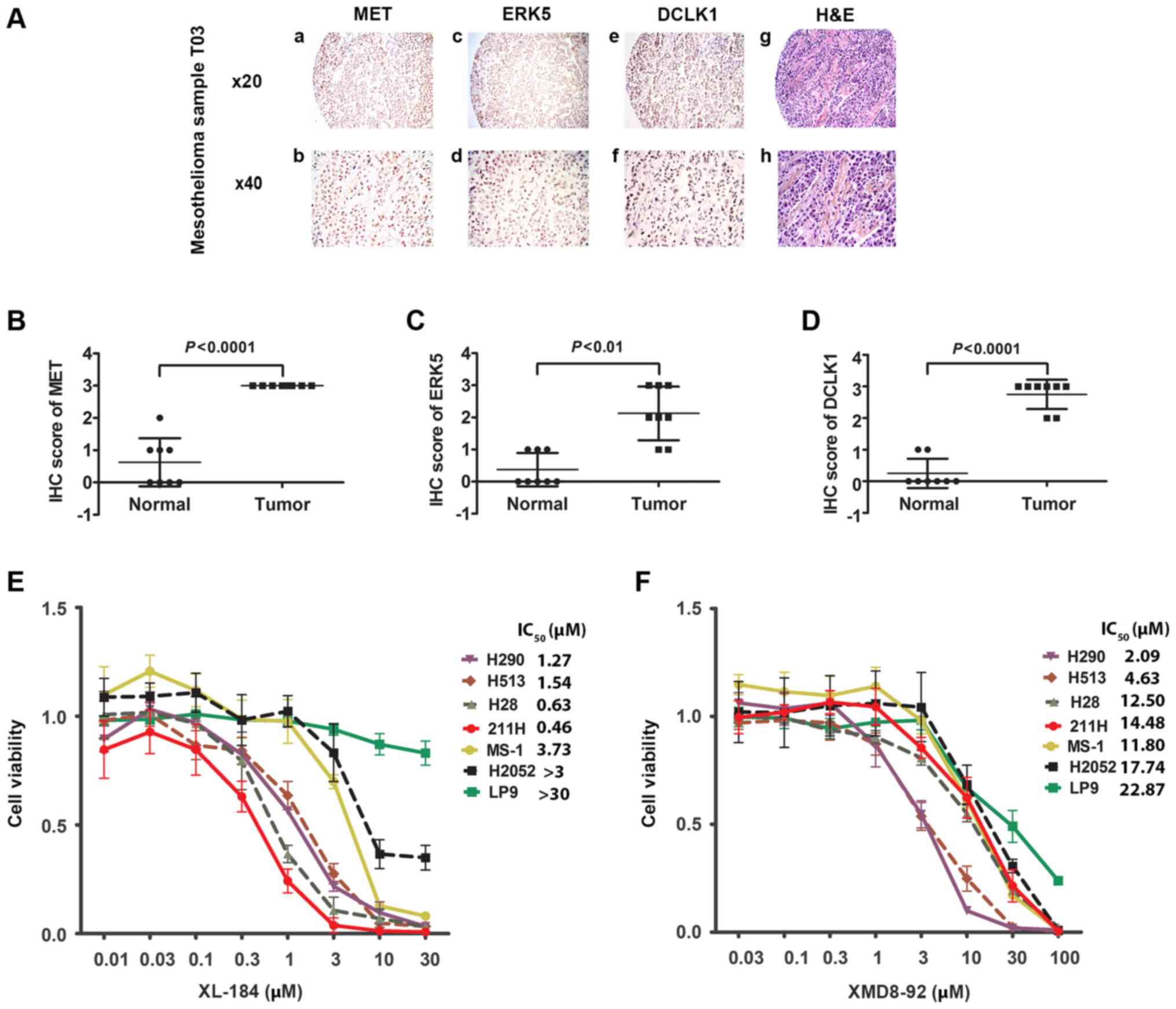 | Figure 4Immunohistochemistry of MET, ERK5,
and DCLK1, and H&E staining in one mesothelioma sample and cell
viability assay of XL184 and XMD8-92. (A) Immunohistochemistry of
MET (a and b), ERK5 (c and d) and DCLK1 (e and f) and H&E (g
and h) staining in T03 mesothelioma sample. (B) Scatter plot of IHC
score of MET from mesothelioma tissues and normal tissues from 8
patients (P<0.0001, Student's t-test). (C) Scatter plot of IHC
score of MET from mesothelioma tissues and normal tissues from 8
patients (P<0.001, Student's t-test). (D) Scatter plot of IHC
score of MET from mesothelioma tissues and normal tissues from 8
patients (P<0.0001, Student's t-test). (E) Cell viability assay
of six mesothelioma cell lines (H290, H513, H28, 211H, MS-1 and
H2052) and one normal mesothelial cell line LP9 after treatment
with the MET inhibitor XL184. (F) Cell viability assay of six
mesothelioma cell lines (H290, H513, H28, 211H, MS-1 and H2052) and
one normal mesothelial cell line LP9 after treatment with the ERK5
inhibitor XMD8-92. |
 | Table IIIChi-square test for association
analysis of MET and ERK5 expression in mesothelioma tumors
(P=0.00000212; P<0.05, Chi-square). |
Table III
Chi-square test for association
analysis of MET and ERK5 expression in mesothelioma tumors
(P=0.00000212; P<0.05, Chi-square).
| ERK5 (−/+)
(%)(n/total) | ERK5 (++/+++) (%)
(n/total) |
|---|
| ERK5 (−/+) | 30.1 (22/73) | 21.9 (16/73) |
| ERK5 (++/+++) | 2.8 (2/73) | 45.2 (33/73) |
 | Table VChi-square test for association
analysis of ERK5 and DCLK1 expression in mesothelioma tumors
(P=0.00001429; P<0.05, Chi-square). |
Table V
Chi-square test for association
analysis of ERK5 and DCLK1 expression in mesothelioma tumors
(P=0.00001429; P<0.05, Chi-square).
| ERK5 (−/+)
(%)(n/total) | ERK5 (++/+++) (%)
(n/total) |
|---|
| DCLK1 (−/+) | 38.3 (28/73) | 11 (8/73) |
| DCLK1 (++/+++) | 13.7 (10/73) | 37 (27/73) |
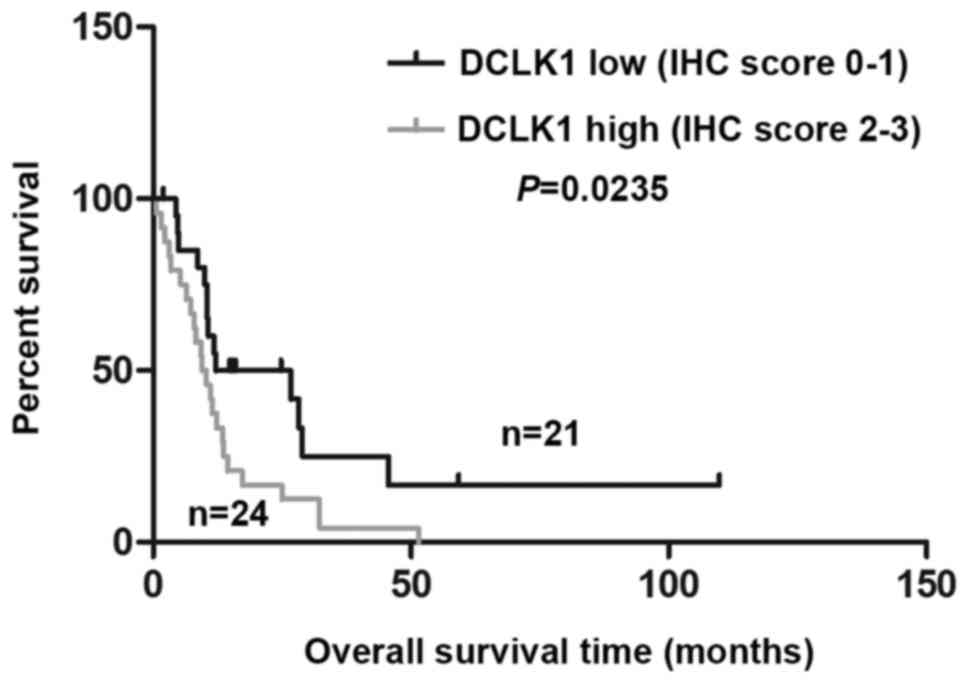
DCLK1 upregulation correlates with poor
prognosis in mesothelioma
DCLK1 expression was significantly higher in MPM
patients' tissues than normal pleural tissues (Table VI) (P=0.000409), suggesting a
possible oncogenic role of DCLK1 in MPM. Analyzing the associations
between DCLK1 expression and clinicopathologic characteristics, we
did not find significantly correlations between DCLK1 and age,
gender, smoke status or TNM stage (Table VII). We further evaluated the
correlation between DCLK1 expression and overall survival (OS) in
MPM patients using Kaplan-Meier analysis. As shown in Fig. 5, the OS times with high DCLK1
staining (2–3) is significantly shorter than those
with low DCLK1 staining (0–1) (24 vs. 21, P=0.0235), which
indicated DCLK1 level is a potential indicator of OS survival of
MPM patients.
 | Table VIResults of immunohistochemistry
staining of DCLK1 in normal pleural and mesothelioma samples. |
Table VI
Results of immunohistochemistry
staining of DCLK1 in normal pleural and mesothelioma samples.
| Histologic
classification | Level of DCLK1
expression
| Total | P-value |
|---|
| − | + | ++ | +++ |
|---|
| Normal pleural | 6 (75%) | 2 (25%) | 0 (0%) | 0 (0%) | 8 | |
| Mesothelioma | 14 (19.2%) | 22 (30.1%) | 26 (35.6%) | 11 (15.1%) | 73 | 0.000986 |
 | Table VIIAssociation between
clinicopathological characteristics and DCLK1 protein expression in
45 cases of primary mesothelioma tissues with available
clinicopathological information. |
Table VII
Association between
clinicopathological characteristics and DCLK1 protein expression in
45 cases of primary mesothelioma tissues with available
clinicopathological information.
| Clinical
information | Total | Level of DCLK1
expression
| P-value |
|---|
| Low | High |
|---|
| Age | | | | |
| <70 | 23 | 12 (52.2%) | 11 (47.8%) | 0.449 |
| ≥70 | 22 | 9 (40.9%) | 13 (59.1%) | |
| Gender | | | | |
| Male | 34 | 18 (52.9%) | 16 (47.1%) | 0.926 |
| Female | 11 | 6 (54.5%) | 5 (45.5%) | |
| Smokers | | | | |
| Never | 17 | 8 (47.1%) | 9 (51.9%) | 0.722 |
| Past | 25 | 14 (56.0%) | 11 (44.5%) | |
| Current | 3 | 2 (66.7%) | 1 (33.3%) | |
| TNM stagea | | | | |
| I | 6 | 4 (66.7%) | 2 (33.3%) | 0.363 |
| II | 9 | 2 (22.2%) | 7 (77.8%) | |
| III | 12 | 6 (50.0%) | 6 (50.0%) | |
| IV | 5 | 2 (40.0%) | 3 (60.0%) | |
MET and ERK5 inhibitors suppress cell
viability of human mesothelioma cells
We tested the effects of MET and ERK5 inhibitors on
six mesothelioma cell lines H290, H513, H28, 211H, MS-1, and H2052
and on the normal human mesothelial cell line LP9 by treating them
with MET inhibitor XL184 and ERK5 inhibitor XMD8-92 at different
doses for 48 h. Cell viability was assayed and IC50 of
each cell line was calculated based on the dose-response curves
(Fig. 4E and F and Table VIII). A higher dose of XL184 and
XMD8-92 resulted in lower viability of the mesothelioma cell lines.
The IC50 values indicate different levels of inhibitory
effects of XL184 and XMD8-92 among the cell lines and are displayed
in Fig. 4B and C, respectively.
Importantly, four of the mesothelioma cell lines (H290, H513, H28
and 211H), in which strong positive staining with anti-MET antibody
was detected by immunohistochemistry, showed relatively high
sensitivity to the MET inhibitor XL184 compared to the cell lines
with weak staining of anti-MET. In contrast, XL184 had minimal
inhibitory effects on the viability of the normal human mesothelial
cell line LP9. Three cell lines (H290, H513 and H28) stained
positive with anti-ERK5 antibody, which also showed relative high
sensitivity to the ERK5 inhibitor XMD8-92.
 | Table VIIIIC50 values of XL184 and
XMD8-92 in six mesothelioma cell lines and a normal pleural cell
line. |
Table VIII
IC50 values of XL184 and
XMD8-92 in six mesothelioma cell lines and a normal pleural cell
line.
| H290 | H513 | H28 | 211H | MS-1 | H2052 | LP-9 |
|---|
| IC50
values of XL184 (µM) | 1.27 | 1.54 | 0.63 | 0.46 | 3.73 | >3 | >30 |
| IC50
values of XMD8-92 (µM) | 2.09 | 4.63 | 12.50 | 14.48 | 11.80 | 17.74 | 22.87 |
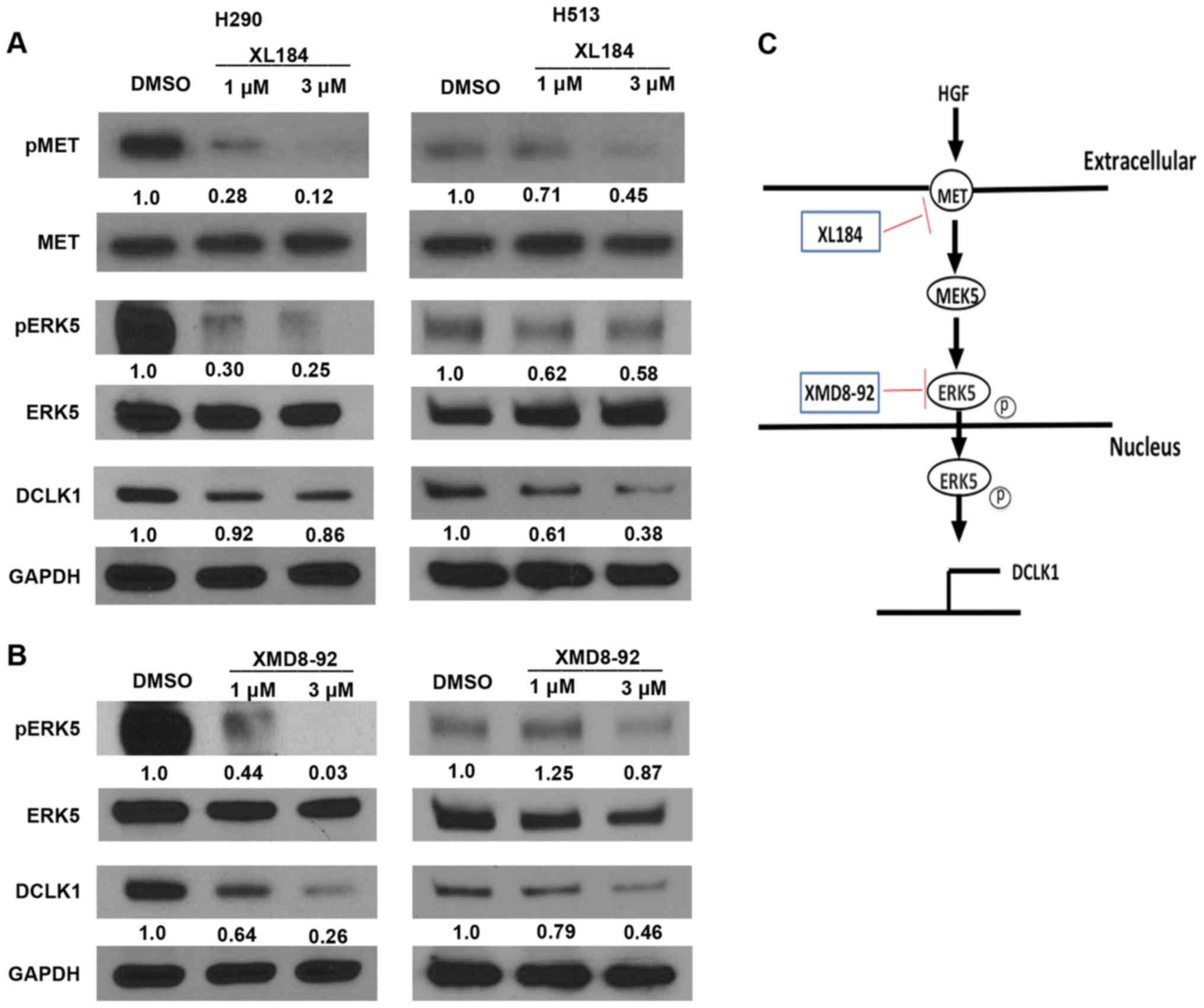
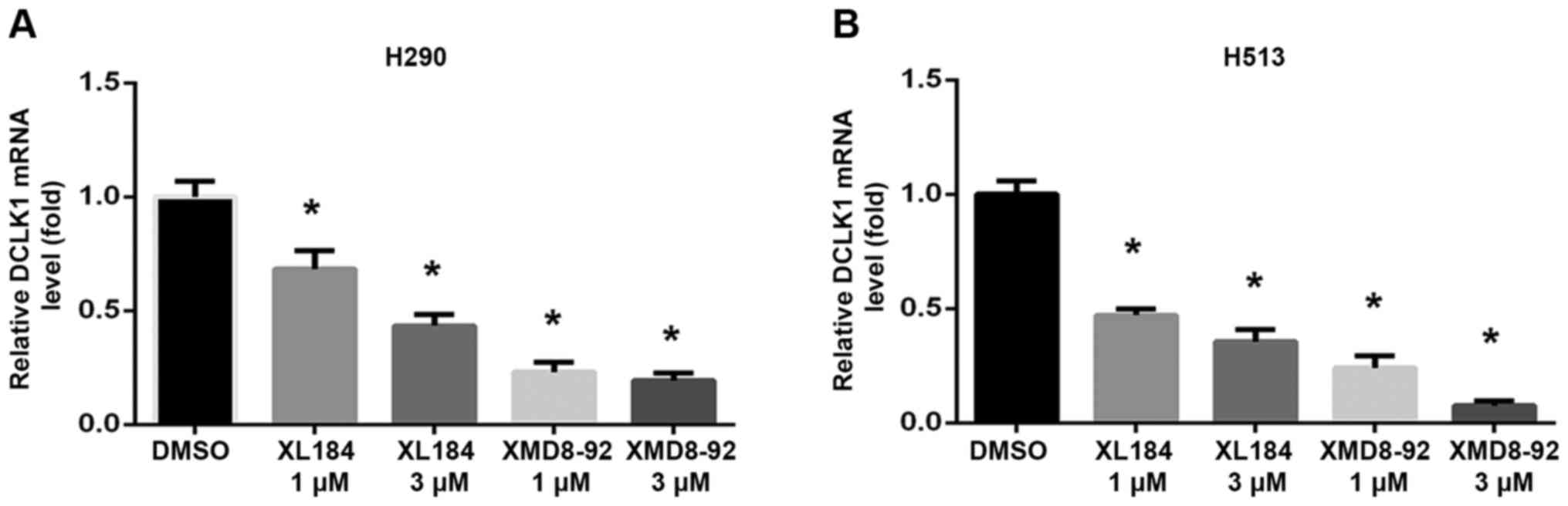
MET or ERK5 inhibition causes
downregulation of MET/ERK5/DCLK1 signaling
To assess whether inhibiting MET or ERK5 affects
MET/ERK5/DCLK1 signaling activity, we analyzed phospho-MET, MET,
phospho-ERK5, ERK5 and DCLK1 expression in H290 and H513 cell lines
treated with the MET inhibitor XL184 or the ERK5 inhibitor XMD8-92.
We found that phosphor-MET, phospho-ERK5 and DCLK1 protein level
decreased after treatment with XL184 (Fig. 6A) or XMD8-92 (Fig. 6B) in the mesothelioma cells, as
compared to what occurred in cells treated with DMSO. MET and ERK5
protein level was not decreased after the treatments (Fig. 6A and B). Significant decrease in
mRNA levels of DCLK1 was detected in both H290 and H513 cells after
XL184 or XMD8-92 treatments analyzed by quantitative real-time PCR
(Fig. 7). These results suggest
that MET or ERK5 inhibition decreased the expression of
phospho-ERK5 and DCLK1 in H290 and H513 cells. Our proposed schema
for MET/ERK5/DCLK1 signaling in human mesothelioma is shown in
Fig. 6C. The use of specific
inhibitors, XL184 and XMD8-92, one targeting MET signaling and the
other targeting ERK5, decreased the mRNA levels and protein levels
of DCLK1 in human mesothelioma cells, suggesting that DCLK1 lies
downstream of both MET and ERK5 signaling.
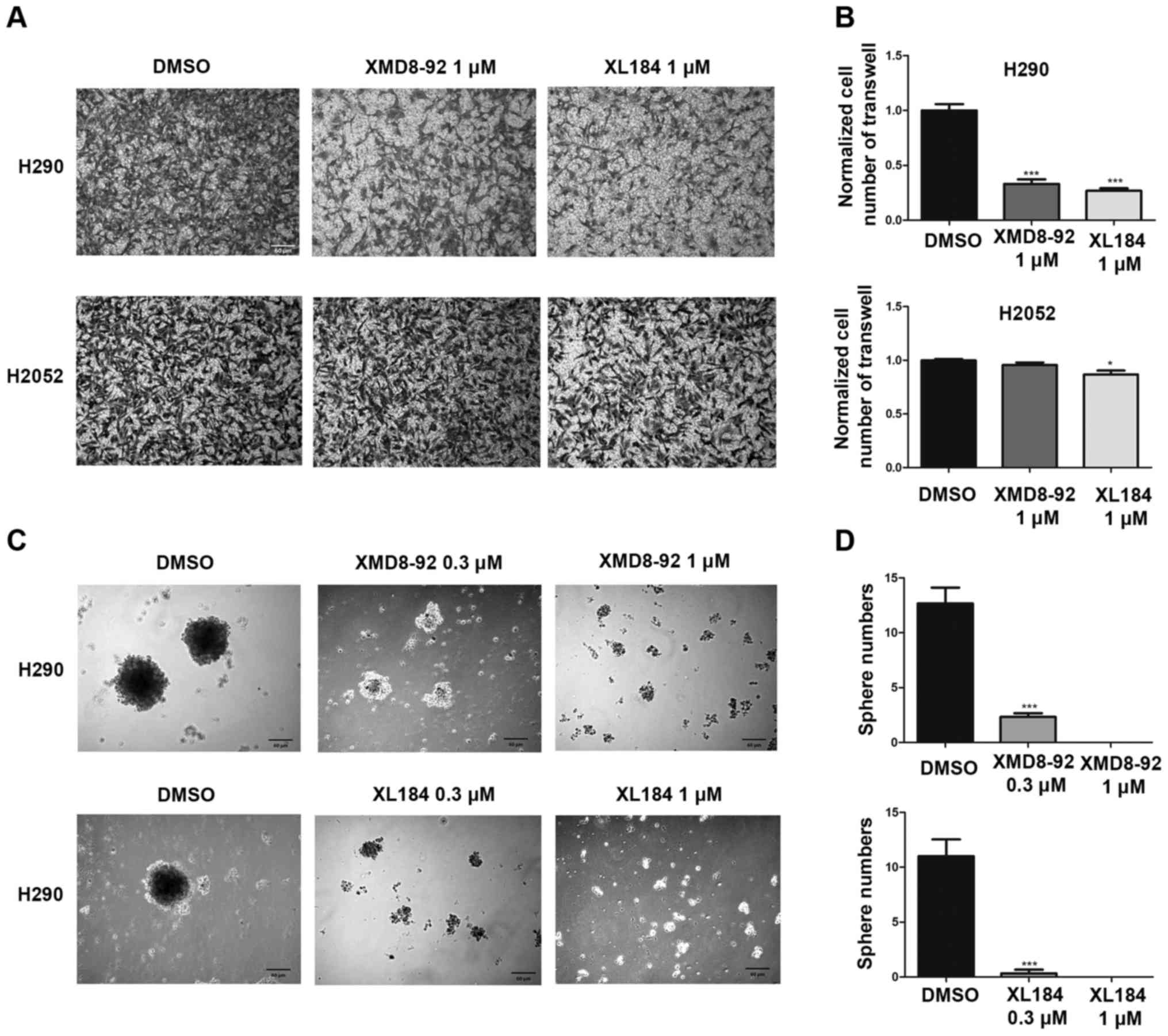
MET or ERK5 inhibition impairs invasion
and tumor sphere formation ability of mesothelioma H290 cells
To test the effect of MET or ERK5 inhibition on the
invasion ability of mesothelioma cells, a Transwell assay was
performed using H290 cells and H2052 (Fig. 8A and B). After 20 h of 1.0
µM XL184, 1.0 µM XMD8-92 or 0.1% DMSO treatment, the
number of the H290 cells that invaded to the lower side of the
membrane decreased significantly compared to that in the control
group treated with DMSO. However, 20 h of 1.0 µM XMD8-92
treatment did not affect the invasion of the H2052 cells compared
to that in the control DMSO group. Twenty hours of 1.0 µM
XL184 treatment slightly deceased the number of the H2052 cells
invaded to the lower side of the membrane compared to that in the
control DMSO group. The cell viability of H290 treated with 1.0
µM XL184 and 1.0 µM XMD8-92 for 20 h were 84.9±1.0
and 95.2±1.5% respectively, compared to cells treated with 0.1%
DMSO. The cell viability of H2052 was not affected by 1.0 µM
XL184 and 1.0 µM XMD8-92 treatment for 20 h compared to
cells treated with 0.1% DMSO.
To measure the effect of EMT or ERK5 inhibition on
the self-renewal of cancer stem cells in mesothelioma, we used a
tumorsphere assay. Under our experimental conditions, H2052 and
MS-1 cells could not form compact spheres after 1-week incubation.
Currently, we do not know the reason for this. However, H290 cells
nicely formed compact spheres. Tumorsphere formation efficiency
decreased significantly in a dose-dependent manner in H290 after
(0.3 and 1.0 µM) XL184 or (0.3 and 1.0 µM) XMD8-92
treatment (Fig. 8C and D).
Discussion
In our study to evaluate MET, ERK5, and DCLK1 as
potential biomarkers and targets for therapeutic development
against mesothelioma, we investigated whether MET, or its
downstream signaling partners ERK5 and DCLK1, were overexpressed in
human mesothelioma cell lines and tissue samples.
MET amplification has been observed in
mesothelioma patients, suggesting that MET is an oncogenic driver
and a potential therapeutic target for mesothelioma (24,25).
Altered HGF/MET signaling has previously been reported in human
mesotheliomas and this pathway plays an important role in tumor
invasion and metastasis (26,27).
MET receptor activation targets cellular functions involving
cell-cell interactions, migration, and remodeling of extracellular
matrix. Moreover, paracrine activation of the MET receptor via HGF
regulates tumorstroma interactions. Deregulated activity of MET can
lead to many different cancers (28). Our study shows that MET was
overexpressed in most of the human mesothelioma samples and it was
expressed at low levels in most normal pleural tissues.
Furthermore, MET was highly expressed in four (H290, H513, H28 and
211H) of six mesothelioma cell lines tested. Negative expression of
MET was found in the other two mesothelioma cell lines (MS-1,
H2052) and one normal human mesothelial cell line LP9. Different
expression of MET in mesothelioma cell lines was also detected by
Kawaguchi et al (29). The
MET pathway is one of the most frequently dysregulated pathways in
human cancer (30). To further
analyze the interaction between the MET pathway and cell growth, we
used the multi-target MET inhibitor XL184. XL184, which also
affects the VEGF receptor 2 (31),
and has significant inhibitory activity against numerous solid
tumors including melanoma, non-small cell lung cancer, breast
cancer, liver cancer, ovarian cancer, and prostate cancer (32–35).
The US FDA approved XL184 for the treatment of medullary thyroid
cancer in November 2012 (36) and
for the treatment of advanced renal cell carcinoma in April 2016
(37). Interestingly, four
analyzed human mesothelioma cell lines (H290, H513, H28 and 211H)
which had high expression of MET showed relative high sensitivity
(IC50, H290-1.27 µM; H513-1.54 µM;
H28-0.62 µM; 211H-0.46 µM) to the MET inhibitor
XL184. XL184 showed only minimal inhibitory effects on the
viability of the normal human mesothelial cell line LP9. The
prognosis for mesothelioma patients is poor with current surgery
and chemotherapy. Our study demonstrated the specific targeting and
high sensitivity of XL184 to mesothelioma cells, which indicated
that XL184 alone or in combination with other drugs could be worth
to performing clinical trials on mesothelioma patients.
Furthermore, XL184 significantly inhibited the invasion and
tumorsphere formation ability of H290. This supported XL184 may
prevent tumor invasion and metastasis. Our data suggested that MET
is a potential therapeutic biomarker, and MET inhibitors such as
XL184 may be effective therapeutics for patients with mesothelioma.
However, in the present study we cannot rule out the possible
contributions by the inhibition of other targets of XL184 such as
VEGFR2 and RET. Further studies are needed to validate the
potential of MET as an effective therapeutic target and XL184 as an
effective therapy for patients with malignant mesothelioma in the
future.
ERK5 and its upstream kinases enhance cell
proliferation, angiogenesis, invasion, and metastasis (38). In a mouse model, ERK5 knockdown
increases tumor epithelialization and suppresses intravascular
invasion by reducing the generation of circulating tumor cells
(CTCs) and the formation of lung metastases in human breast cancer
cells (39). ERK5 has also been
associated with cancer cell proliferation and survival, and
activation by growth factor stimuli (40,41).
Studies have shown that HGF promotes proliferation of human
mesothelioma cells via ERK5 activation (9). We also observed ERK5 expression in
human mesothelioma tumor samples. ERK5 was highly expressed in
three cell lines (H290, H513 and H28) of six mesothelioma cell
lines. To further investigate the role of the MAPK/ERK pathway,
which is important for cancer cell growth and proliferation
(42), we used XMD8-92, which is a
small molecule inhibitor of ERK5 (43,44).
The same three cell lines also showed relative high sensitivity to
the ERK5 inhibitor XMD8-92. However, the sensitivity of these cell
lines to XMD8-92 is less than the sensitivity to XL184 based on the
IC50 of these two inhibitors. Like XL184, XMD8-92
significantly inhibited the invasion and tumorsphere formation
ability of H290. Our data suggested that ERK5 may be a potential
therapeutic target for patients with mesothelioma.
The role of DCLK1 in cancer has only recently been
investigated (45). DCLK1 is
overexpressed in several forms of cancer including colon and
pancreatic cancer (46). DCLK1
kinase activity may play a critical role in several important
pathways involving cell proliferation, cell cycle regulation,
epithelial mesenchymal transition (EMT), and stem-cell like
properties in cancer (45). DCLK1
is a cancer stem cell (CSC) marker and a potential therapeutic
target for pancreatic ductal adenocarcinoma treatment (47), however, its role in human
mesotheliomas is unclear. Our study demonstrated that DCLK1 was
overexpressed in 47.9% of the analyzed human mesothelioma tissue
samples and was highly expressed in three (H290, H513 and H28) of
the six analyzed mesothelioma cell lines, indicating an important
role of DCLK1 in malignant mesothelioma. The statistical analysis
of the immunohistochemistry results showed significant association
between MET, ERK5 and DCLK1 expression in mesothelioma tissue
arrays of the 73 tumor samples. MET, ERK5, and DCLK1 were all
highly expressed in three cell lines (H290, H513 and H28) of six
mesothelioma cell lines studied. These results suggest that MET,
ERK5, and DCLK1 could be closely regulated in human mesothelioma.
Moreover, DCLK1 expression was negatively associated with overall
survival time of MPM patients, indicating DCLK1 may serve as a
predictor for prognosis of MPM patients.
Constitutive activation of ERK5 in various malignant
mesothelioma cell lines could be a consequence of activated MET
(48). Our results showed that
treatment with MET inhibitor XL184 downregulated the protein levels
of phospho-ERK5 and DCLK1 (Fig.
6A). After XL184 treatment, the DCLK1 mRNA levels also deceased
in a dose-dependent manner (Fig.
7). Regulation of ERK5 expression is important in cancer cells
(49,50). The ERK5 inhibitor XMD8-92 can
suppress the activity of ERK5 in the vasculature integrity of
animals (51) and inhibits
DCLK1-mediated kinase activity (44). XMD8-92-mediated DCLK1 inhibition
results in suppression of downstream oncogenic signaling, i.e.,
EMT, angiogenesis, pluripotency, and anti-apoptotic activity
(52). We found that XMD8-92 not
only decreased the protein levels of phospho-ERK5, but also
decreased the protein levels of DCLK1 in mesothelioma cells
(Fig. 6B). After XMD8-92
treatment, the DCLK mRNA levels also deceased in a dose-dependent
manner (Fig. 7). Our results show
that this MET/ERK5/DCLK1 signaling (Fig. 6C) activity could be important for
cell growth in mesotheliomas and these small molecule inhibitors
could be investigated for their therapeutic potential against
mesotheliomas.
In conclusion, we found that MET, ERK5, and DCLK1
are overexpressed in several human mesothelioma cell lines and
human mesothelioma tumor tissues. DCLK1 may serve as a predictor
for prognosis of MPM patients. Moreover, our results suggest that
DCLK1 is regulated by MET/ERK5 signaling in human mesothelioma, and
MET and ERK5 could be further developed into a promising
therapeutic target against mesothelioma.
Acknowledgments
This study was supported by the National Institutes
of Health (NIH; grant no. R01 CA140654, to L.Y.). We are grateful
for support from the Kazan McClain Partners' Foundation. We thank
Pamela Derish in the UCSF Department of Surgery for editorial
assistance with the manuscript.
References
|
1
|
La Vecchia C and Boffetta P: Role of
stopping exposure and recent exposure to asbestos in the risk of
mesothelioma. Eur J Cancer Prev. 21:227–230. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Buikhuisen WA, Hiddinga BI, Baas P and van
Meerbeeck JP: Second line therapy in malignant pleural
mesothelioma: A systematic review. Lung Cancer. 89:223–231. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mossman BT, Shukla A, Heintz NH,
Verschraegen CF, Thomas A and Hassan R: New insights into
understanding the mechanisms, pathogenesis, and management of
malignant mesotheliomas. Am J Pathol. 182:1065–1077. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Campbell NP and Kindler HL: Update on
malignant pleural mesothelioma. Semin Respir Crit Care Med.
32:102–110. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Blume-Jensen P and Hunter T: Oncogenic
kinase signalling. Nature. 411:355–365. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Maulik G, Shrikhande A, Kijima T, Ma PC,
Morrison PT and Salgia R: Role of the hepatocyte growth factor
receptor, c-Met, in oncogenesis and potential for therapeutic
inhibition. Cytokine Growth Factor Rev. 13:41–59. 2002. View Article : Google Scholar
|
|
7
|
Mukohara T, Civiello G, Davis IJ, Taffaro
ML, Christensen J, Fisher DE, Johnson BE and Jänne PA: Inhibition
of the met receptor in mesothelioma. Clin Cancer Res. 11:8122–8130.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cacciotti P, Libener R, Betta P, Martini
F, Porta C, Procopio A, Strizzi L, Penengo L, Tognon M, Mutti L, et
al: SV40 replication in human mesothelial cells induces HGF/Met
receptor activation: A model for viral-related carcinogenesis of
human malignant mesothelioma. Proc Natl Acad Sci USA.
98:12032–12037. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ramos-Nino ME, Blumen SR, Sabo-Attwood T,
Pass H, Carbone M, Testa JR, Altomare DA and Mossman BT: HGF
mediates cell proliferation of human mesothelioma cells through a
PI3K/MEK5/Fra-1 pathway. Am J Respir Cell Mol Biol. 38:209–217.
2008. View Article : Google Scholar
|
|
10
|
Regan CP, Li W, Boucher DM, Spatz S, Su MS
and Kuida K: Erk5 null mice display multiple extraembryonic
vascular and embryonic cardiovascular defects. Proc Natl Acad Sci
USA. 99:9248–9253. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hayashi M, Kim SW, Imanaka-Yoshida K,
Yoshida T, Abel ED, Eliceiri B, Yang Y, Ulevitch RJ and Lee JD:
Targeted deletion of BMK1/ERK5 in adult mice perturbs vascular
integrity and leads to endothelial failure. J Clin Invest.
113:1138–1148. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Roberts OL, Holmes K, Müller J, Cross DA
and Cross MJ: ERK5 is required for VEGF-mediated survival and
tubular morphogenesis of primary human microvascular endothelial
cells. J Cell Sci. 123:3189–3200. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rovida E, Spinelli E, Sdelci S, Barbetti
V, Morandi A, Giuntoli S and Dello Sbarba P: ERK5/BMK1 is
indispensable for optimal colony-stimulating factor 1
(CSF-1)-induced proliferation in macrophages in a Src-dependent
fashion. J Immunol. 180:4166–4172. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sureban SM, May R, Lightfoot SA, Hoskins
AB, Lerner M, Brackett DJ, Postier RG, Ramanujam R, Mohammed A, Rao
CV, et al: DCAMKL-1 regulates epithelial-mesenchymal transition in
human pancreatic cells through a miR-200a-dependent mechanism.
Cancer Res. 71:2328–2338. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bailey JM, Alsina J, Rasheed ZA,
McAllister FM, Fu YY, Plentz R, Zhang H, Pasricha PJ, Bardeesy N,
Matsui W, et al: DCLK1 marks a morphologically distinct
subpopulation of cells with stem cell properties in preinvasive
pancreatic cancer. Gastroenterology. 146:245–256. 2014. View Article : Google Scholar
|
|
16
|
May R, Riehl TE, Hunt C, Sureban SM, Anant
S and Houchen CW: Identification of a novel putative
gastrointestinal stem cell and adenoma stem cell marker,
doublecortin and CaM kinase-like-1, following radiation injury and
in adenomatous polyposis coli/multiple intestinal neoplasia mice.
Stem Cells. 26:630–637. 2008. View Article : Google Scholar
|
|
17
|
Sureban SM, May R, Qu D, Weygant N,
Chandrakesan P, Ali N, Lightfoot SA, Pantazis P, Rao CV, Postier
RG, et al: DCLK1 regulates pluripotency and angiogenic factors via
microRNA-dependent mechanisms in pancreatic cancer. PLoS One.
8:e739402013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nakanishi Y, Seno H, Fukuoka A, Ueo T,
Yamaga Y, Maruno T, Nakanishi N, Kanda K, Komekado H, Kawada M, et
al: Dclk1 distinguishes between tumor and normal stem cells in the
intestine. Nat Genet. 45:98–103. 2013. View Article : Google Scholar
|
|
19
|
Vega KJ, May R, Sureban SM, Lightfoot SA,
Qu D, Reed A, Weygant N, Ramanujam R, Souza R, Madhoun M, et al:
Identification of the putative intestinal stem cell marker
doublecortin and CaM kinase-like-1 in Barrett's esophagus and
esophageal adenocarcinoma. J Gastroenterol Hepatol. 27:773–780.
2012. View Article : Google Scholar :
|
|
20
|
Sureban SM, May R, Ramalingam S,
Subramaniam D, Natarajan G, Anant S and Houchen CW: Selective
blockade of DCAMKL-1 results in tumor growth arrest by a Let-7a
MicroRNA-dependent mechanism. Gastroenterology. 137:649–659.
659.e641–642. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sureban SM, May R, Mondalek FG, Qu D,
Ponnurangam S, Pantazis P, Anant S, Ramanujam RP and Houchen CW:
Nano-particle-based delivery of siDCAMKL-1 increases microRNA-144
and inhibits colorectal cancer tumor growth via a Notch-1 dependent
mechanism. J Nanobiotechnology. 9:402011. View Article : Google Scholar
|
|
22
|
Ali N, Allam H, May R, Sureban SM, Bronze
MS, Bader T, Umar S, Anant S and Houchen CW: Hepatitis C
virus-induced cancer stem cell-like signatures in cell culture and
murine tumor xenografts. J Virol. 85:12292–12303. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li T, Li H, Wang Y, Harvard C, Tan JL, Au
A, Xu Z, Jablons DM and You L: The expression of CXCR4, CXCL12 and
CXCR7 in malignant pleural mesothelioma. J Pathol. 223:519–530.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Varesano S, Salvi S, Boccardo S, Ravetti
JL, Ferro P, Canessa PA, Fedeli F, Pistillo MP and Roncella S:
Amplification of MET in a Patient with Malignant Pleural
Mesothelioma. J Thorac Oncol. 10:e103–e104. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kawakami H, Okamoto I, Okamoto W, Tanizaki
J, Nakagawa K and Nishio K: Targeting MET amplification as a new
oncogenic driver. Cancers (Basel). 6:1540–1552. 2014. View Article : Google Scholar
|
|
26
|
Matsumoto K and Nakamura T: NK4
(HGF-antagonist/angiogenesis inhibitor) in cancer biology and
therapeutics. Cancer Sci. 94:321–327. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Carbone M and Yang H: Molecular pathways:
targeting mechanisms of asbestos and erionite carcinogenesis in
mesothelioma. Clin Cancer Res. 18:598–604. 2012. View Article : Google Scholar
|
|
28
|
Davis IJ, McFadden AW, Zhang Y, Coxon A,
Burgess TL, Wagner AJ and Fisher DE: Identification of the receptor
tyrosine kinase c-Met and its ligand, hepatocyte growth factor, as
therapeutic targets in clear cell sarcoma. Cancer Res. 70:639–645.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kawaguchi K, Murakami H, Taniguchi T,
Fujii M, Kawata S, Fukui T, Kondo Y, Osada H, Usami N, Yokoi K, et
al: Combined inhibition of MET and EGFR suppresses proliferation of
malignant mesothelioma cells. Carcinogenesis. 30:1097–1105. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu X, Newton RC and Scherle PA:
Developing c-MET pathway inhibitors for cancer therapy: Progress
and challenges. Trends Mol Med. 16:37–45. 2010. View Article : Google Scholar
|
|
31
|
Yakes FM, Chen J, Tan J, Yamaguchi K, Shi
Y, Yu P, Qian F, Chu F, Bentzien F, Cancilla B, et al: Cabozantinib
(XL184), a novel MET and VEGFR2 inhibitor, simultaneously
suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer
Ther. 10:2298–2308. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Trump DL: Commentary on 'Cabozantinib in
patients with advanced prostate cancer: results of a phase II
randomized discontinuation trial'. Smith DC, Smith MR, Sweeney C,
Elfiky AA, Logothetis C, Corn PG, Vogelzang NJ, Small EJ, Harzstark
AL, Gordon MS, Vaishampayan UN, Haas NB, Spira AI, Lara PN Jr, Lin
CC, Srinivas S, Sella A, SchoffskiSchöffski P, Scheffold C,
Weitzman AL and Hussain M: University of Michigan; Ann Arbor,
MI:
J Clin Oncol. 2013.31(4): 412–9.
View Article : Google Scholar : Epub 2012 Nov 19.
Urol Oncol. 31:18482013. View Article : Google Scholar
|
|
33
|
Dai J, Zhang H, Karatsinides A, Keller JM,
Kozloff KM, Aftab DT, Schimmoller F and Keller ET: Cabozantinib
inhibits prostate cancer growth and prevents tumor-induced bone
lesions. Clin Cancer Res. 20:617–630. 2014. View Article : Google Scholar :
|
|
34
|
Smith DC, Smith MR, Sweeney C, Elfiky AA,
Logothetis C, Corn PG, Vogelzang NJ, Small EJ, Harzstark AL, Gordon
MS, et al: Cabozantinib in patients with advanced prostate cancer:
Results of a phase II randomized discontinuation trial. J Clin
Oncol. 31:412–419. 2013. View Article : Google Scholar
|
|
35
|
Vaishampayan U: Cabozantinib as a novel
therapy for renal cell carcinoma. Curr Oncol Rep. 15:76–82. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mughal A, Aslam HM, Sheikh A, Khan AM and
Saleem S: c-Met inhibitors. Infect Agent Cancer. 8:132013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
U.S. Food and Drug Administration (FDA):
Cabozantinib. CABOMETYX. 2016, https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm497483.htm.
|
|
38
|
Whyte J, Bergin O, Bianchi A, Mcnally S
and Martin F: Key signalling nodes in mammary gland development and
cancer. Mitogen-activated protein kinase signalling in experimental
models of breast cancer progression and in mammary gland
development. Breast Cancer Res. 11:2092009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Javaid S, Zhang J, Smolen GA, Yu M,
Wittner BS, Singh A, Arora KS, Madden MW, Desai R, Zubrowski MJ, et
al: MAPK7 regulates EMT features and modulates the generation of
CTCs. Mol Cancer Res. 13:934–943. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kato Y, Tapping Ri, Huang S, Watson MH,
Ulevitch RJ and Lee JD: Bmk1/Erk5 is required for cell
proliferation induced by epidermal growth factor. Nature.
395:713–716. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kato Y, Kravchenko VV, Tapping RI, Han J,
Ulevitch RJ and Lee JD: BMK1/ERK5 regulates serum-induced early
gene expression through transcription factor MEF2C. EMBO J.
16:7054–7066. 1997. View Article : Google Scholar
|
|
42
|
Dhillon AS, Hagan S, Rath O and Kolch W:
MAP kinase signalling pathways in cancer. Oncogene. 26:3279–3290.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Deng X, Yang Q, Kwiatkowski N, Sim T,
McDermott U, Settleman JE, Lee JD and Gray NS: Discovery of a
benzo[e] pyrimido-[5,4-b][1,4]diazepin-6(11H)-one as a potent and
selective inhibitor of Big MAP kinase 1. ACS Med Chem Lett.
2:195–200. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yang Q, Deng X, Lu B, Cameron M, Fearns C,
Patricelli MP, Yates JR III, Gray NS and Lee JD: Pharmacological
inhibition of BMK1 suppresses tumor growth through promyelocytic
leukemia protein. Cancer Cell. 18:258–267. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Weygant N, Qu D, Berry WL, May R,
Chandrakesan P, Owen DB, Sureban SM, Ali N, Janknecht R and Houchen
CW: Small molecule kinase inhibitor LRRK2-IN-1 demonstrates potent
activity against colorectal and pancreatic cancer through
inhibition of doublecortin-like kinase 1. Mol Cancer. 13:1032014.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Thu KL, Radulovich N, Becker-Santos DD,
Pikor LA, Pusic A, Lockwood WW, Lam WL and Tsao MS: SOX15 is a
candidate tumor suppressor in pancreatic cancer with a potential
role in Wnt/β-catenin signaling. Oncogene. 33:279–288. 2014.
View Article : Google Scholar
|
|
47
|
Qu D, Johnson J, Chandrakesan P, Weygant
N, May R, Aiello N, Rhim A, Zhao L, Zheng W, Lightfoot S, et al:
Doublecortin-like kinase 1 is elevated serologically in pancreatic
ductal adenocar-cinoma and widely expressed on circulating tumor
cells. PLoS One. 10:e01189332015. View Article : Google Scholar
|
|
48
|
Shukla A, Miller JM, Cason C, Sayan M,
MacPherson MB, Beuschel SL, Hillegass J, Vacek PM, Pass HI and
Mossman BT: Extracellular signal-regulated kinase 5: A potential
therapeutic target for malignant mesotheliomas. Clin Cancer Res.
19:2071–2083. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Boguslawski G, Mcglynn PW, Harvey KA and
Kovala AT: SU1498, an inhibitor of vascular endothelial growth
factor receptor 2, causes accumulation of phosphorylated ERK
kinases and inhibits their activity in vivo and in vitro. J Biol
Chem. 279:5716–5724. 2004. View Article : Google Scholar
|
|
50
|
McCubrey JA, Steelman LS, Chappell WH,
Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M,
Tafuri A, et al: Roles of the Raf/MEK/ERK pathway in cell growth,
malignant transformation and drug resistance. Biochim Biophys Acta.
1773:1263–1284. 2007. View Article : Google Scholar
|
|
51
|
Yang Q and Lee JD: Targeting the BMK1 MAP
kinase pathway in cancer therapy. Clin Cancer Res. 17:3527–3532.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sureban SM, May R, Weygant N, Qu D,
Chandrakesan P, Bannerman-Menson E, Ali N, Pantazis P, Westphalen
CB, Wang TC, et al: XMD8-92 inhibits pancreatic tumor xenograft
growth via a DCLK1-dependent mechanism. Cancer Lett. 351:151–161.
2014. View Article : Google Scholar : PubMed/NCBI
|