Introduction
Ovarian cancer has the highest mortality rate of all
gynecologic malignancies (1),
mainly because most patients present with advanced disease and
widespread metastasis at the time of their diagnosis. The standard
treatment strategy is surgery followed by platinum-based
chemotherapy. Although the majority of patients are initially
sensitive to chemotherapy, most eventually develop drug resistance
or disease recurrence, resulting in treatment failure. This common
outcome makes the investigation of new drugs and therapeutic
modalities imperative. Appropriate animal models of ovarian cancer
are required for screening drugs and preclinical studies. Positive
results are often 'overpredicted' in preclinical studies, with the
highly encouraging preclinical results in mouse models often
failing to show efficacy in phase III clinical trials on patients
with metastatic tumors (2). It is
likely that this disconnect between research success and clinical
outcomes is mainly because mouse models of spontaneous metastatic
or advanced disease have rarely been used in such studies (2,3).
Chemotherapy response varies according to a tumor's organ
environment, as well as between the primary tumor and recurrent or
metastatic tumors (4). Therefore,
models that closely replicate the spontaneous progression of
metastatic ovarian cancer are of great importance.
In studying ovarian cancer, there are four commonly
used animal xenograft models based on the site of tumor
transplantation: subcutaneous, intraperitoneal, subrenal capsule
and orthotopic tumors. The conventional subcutaneous tumor model is
the most widely used model for screening anticancer drugs because
the technique of subcutaneous injection is straightforward.
Although the growth of subcutaneous xenografts can be easily
monitored, spontaneous metastases have been seldom observed. The
intraperitoneal tumor model simulates the process of peritoneal
dissemination and ascites formation in ovarian cancer, and has
generally been used to evaluate the efficacy of intraperitoneal
chemotherapy. However, the relatively short life span of mice and
the absence of primary tumors and spontaneous metastases have
limited its utility. Subrenal capsule models have been used to
determine the responsiveness of patient-derived xenografts (PDXs)
to chemotherapeutic agents because of a high take rate (5,6).
Since ovarian cancers originate in the ovary or fallopian tubes,
none of the three models mentioned above can accurately reflect
clinical disease progression and the therapeutic response of cancer
patients.
In the orthotopic model, tumor cells or tissues are
transplanted into the ovaries. By simulating the microenvironment
of human ovarian cancer, this model can closely replicate the gene
expression profiles, histopathologic features, clinical disease
progression and interactions between cancer cells and the relevant
microenvironment (7). Both primary
solid tumors and spontaneous metastases exist in this model,
allowing for a greater probability of clinical relevance and
translation, especially in predicting subsequent results for
metastatic cancer patients (8).
However, despite the benefits, orthotopic models have technical
difficulties and are time-consuming, and expensive, and
inconvenient monitoring limit their applicability (9).
Since Fu et al reported the first case of PDX
transplantation under the capsule of the nude mouse ovary in 1993
(10), orthotopic models of human
ovarian cancer have greatly advanced in the past two decades. The
rapid development of in vivo imaging technology has made the
visual assessment of orthotopic tumors possible. So far, orthotopic
ovarian cancer models can be established through the following
approaches: 1) genetically engineered mouse models (GEMMs) of
human-like spontaneous mouse tumors (11,12);
2) surgical orthotopic implantation (SOI) of tissue-blocks from
cancer cell lines (13) or from a
patient (10) implanted into the
ovary of an immune-deficient mouse; and 3) an intrabursal injection
of tumor cells into the ovary of an immune-deficient mouse
(cellular orthotopic injection, COI) (14). GEMMs are established in
immune-competent mice by editing the mouse genome through the use
of transgenic technology (inserting extra DNA into the genome to
encode target genes) or knockout/knock-in technology (selectively
modifying specific portions of the mouse genome) (11). Thus, GEMMs are particularly
suitable for assessing immune-based therapies (2,11).
Currently, the application of orthotopic xenograft
tumors in ovarian cancer is less common due to the procedure's
technical challenges and the expensive testing equipment required.
While COI and SOI are the two most common ways of modeling
orthotopic ovarian cancer, there are few studies that compare the
characterizations and applicable situations for COI and SOI. In
several studies of solid tumors, including bladder (15), lung (16,17),
kidney (18) and pancreatic cancer
(19), SOI was found to be more
malignant and clinically relevant than COI. Specifically, SOI
tumors were larger and much more invasive than COI tumors, and the
survival time of the mice in SOI groups was shorter. Yi et
al (20) established an
orthotopic ovarian cancer mouse model using three pathways (cell
suspension; cell suspension isolated from subcutaneous tumor;
tissue-block from subcutaneous tumor) and concluded that a
tissue-block derived transplantation model better simulated tumor
development and invasion. However, the cell line used in the study
was 4T1, a breast cancer cell line, which cannot actually reflect
the features of ovarian cancer. Here, we conducted a head-to-head
comparison of COI and SOI that derived from ovarian cancer cell
lines.
In this study, we utilized continuous dynamic in
vivo bioluminescence imaging to demonstrate the differences in
tumor formation and progression between COI xenografts and SOI
xenografts derived from luciferase-marked human ovarian cancer cell
lines and summarize the advantages and disadvantages of these two
models. We also compared the two models through cellular
experiments and immunohistochemistry of the tumor samples. We
believe that this study can help researchers select the appropriate
mouse model for ovarian cancer research.
Materials and methods
Cell culture
The human ovarian cancer cell lines SKOV3 (a serous
adenocarcinoma cell line) and ES2 (a clear cell carcinoma cell
line) were obtained from American Type Culture Collection and
maintained in DMEM/F12, supplemented with 10% fetal bovine serum
and 1% penicillin and streptomycin. All cells were maintained at
37°C with 5% CO2 in a humidified incubator. Cells were
collected in a logarithmic growth phase and inoculated as soon as
possible.
Lentivirus transfection
Lentivirus transfection was used to establish
ovarian cancer cell lines that were double-marked with green
fluorescent protein (GFP) and luciferase. Briefly, cells were
seeded into a 96-well plate with a quantity of 5000/well and
infected with lentivirus (GenePharma, Shanghai, China) for 72 h.
Then, the cells were screened with 5 μg/ml puromycin for 7
days. Subsequently, single cell-derived clones were established
through serial dilution of the cells into 96-well plates.
Animals
Female Balb/c-nu/nu mice were obtained from the
Beijing HFK Bioscience Co., Ltd., and housed in a specific pathogen
free environment at Laboratory Animal Center, Huazhong University
of Science and Technology. All the procedures were approved by the
Institutional Animal Care and Use Committee at Tongji Medical
College, Huazhong University of Science and Technology. All efforts
were made to minimize suffering. Mice were euthanized when any of
the following conditions occurred: primary tumors reached
approximately 1 cm3 (estimated through palpation); the
mouse experienced significant weight loss (>20%); onset of
cachexia or moribundity (such as massive ascites).
Orthotopic ovarian cancer xenografts
Female nude mice age 5–6 weeks and weighed 17–18 g
were randomized into four groups: SKOV3/SOI, SKOV3/COI, ES2/SOI and
ES2/COI (8 mice/group). For the SOI group, subcutaneous tumors were
established by inoculating 5×106 SKOV3-luc or ES2-luc
cells into the right axillary regions of 2 nude mice aged 4 weeks,
and allowing growth of approximately 0.5 cm3. The
subcutaneous tumors were extracted and cut into 1 mm3
pieces. The mice were anesthetized and placed on their right side.
An 8-mm lateral dorsal incision was made into the fat pad
surrounding the ovary, below the left kidney. After opening the
ovarian capsule, one tissue-block was implanted on the ovary by a
7–0 surgical suture under a stereomicroscope, and then the ovary
was covered using the surrounding fat tissue. Finally, the ovary
was re-inserted, and then the abdomen and skin were closed with a
5–0 surgical suture.
For the COI group, in vitro cultured cells
were re-suspended at a concentration of 5×105 cells/5
μl and injected into the ovarian bursa using a 32 G syringe
needle (Hamilton, Bonaduz, Switzerland).
Animal bioluminescent imaging (BLI)
The BLI was conducted using an in vivo
imaging system (IVIS, Xenogen Corp./Caliper life Science, Alameda,
CA, USA) once a week after implantation. A 150 mg/kg dose of
D-Luciferin potassium salt (Perkin-Elmer, Hopkinton, MA, USA) was
intraperitoneally injected into the mice 10 min before imaging. The
mice were anesthetized using isoflurane and imaged dorsally. The
region of interest (ROI) was selected and the radiance value was
measured by Living Image® 4.3.1 Software (Caliper Life
Science). At the end-point of observation, all the mice were
euthanized immediately after the last in vivo BLI. To detect
intraperitoneal metastases, abdominal organs were obtained within
5–10 min of being euthanized to perform ex vivo BLI.
Tumor size and immunohistochemistry
Tumor diameters were measured by a slide caliper,
and the tumor volume was calculated as Volume = Length x Width x
Height/2. Samples were fixed by 4% paraformaldehyde, followed by
paraffin embedding.
Paraffin-embedded xenograft samples were used for
the immunohistochemical staining of microenvironment-related
markers, including α-smooth muscle actin (α-SMA), CD34, matrix
metallopeptidase 2 (MMP2) and matrix metallopeptidase 9 (MMP9).
α-SMA is a commonly used marker of cancer-associated fibroblasts
(CAFs) (21). CD34 marked
microvessel density (MVD) is a surrogate marker of tumor
angiogenesis (22). MMP2 and MMP9
are two main members of the Zn-dependent proteases family,
metalloproteinase (MMP), which can degrade tissue matrix and basal
membranes, resulting in the migration of cells (23). In addition, E-cadherin and
vimentin, markers of epithelia and mesenchymal cells, respectively,
were assessed to evaluate the occurrence of epithelial-mesenchymal
transition (EMT) in tumor cells (24).
Cell proliferation was determined by nuclear Ki67
staining. After routine deparaffinization and rehydration, paraffin
sections were subjected to heat-mediated antigen retrieval
utilizing pH 6.0 citrate buffer or pH 9.0 Tris-EDTA buffer
according to the manufacturer's instructions of primary antibodies.
The following staining procedures were performed with a rabbit
Biotin-Streptavidin horseradish peroxidase detection system
(SP-9001, ZSGB-BIO, Beijing, China). Primary rabbit antibodies were
diluted as follows: vimentin (ab92547, Abcam, Cambridge, UK;
anti-human, mouse), 1:600; E-cadherin (ab76319, Abcam; anti-human,
mouse), 1:200; α-SMA (23081–1-AP, Proteintech, Wuhan, China;
anti-human, mouse), 1:400; MMP2 (sc-10736, Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA; anti-human, mouse), 1:50;
MMP9 (ab38898, Abcam; anti-human, mouse), 1:200; CD34 (ab81289,
Abcam; anti-human, mouse), 1:400; Ki67 (ab92742, Abcam;
anti-human), 1:500. Sections were counterstained with
hematoxylin.
Images were acquired at a magnification of ×400 and
five regions were selected. Ki67 staining was presented as the
percentage of positive cells (25). MVD was calculated based on CD34
staining (26). All the other
markers were measured by Image-Pro Plus, version 6.0 (Media
Cyberbetics, Bethesda, MD, USA) as Mean Density = Integrated
Optical Density / Area of DAB staining (27).
Primary culture of tumor cells from nude
mouse subcutaneous xenografts
Luciferase-marked human ovarian cancer cells were
isolated from subcutaneous tumor tissues. Briefly, samples were
minced into pieces of 0.1 mm3 and incubated with 1 mg/ml
collagenase type 1 on a thermostat shaker at 37°C for 2 h. After
filtration with a 200-mesh sieve, the cells were centrifuged and
plated in flasks. The primary cells from early passages (1–5) were
used.
Scratch assay
Scratch assay was used to compare the migration
capabilities of the in vitro cultured tumor cells and the
tumor cells primarily isolated from xenografts. The assay was
performed as previous described (28). Images were captured at 0, 6, 12 and
24 h with a magnification of ×40. The average distance of migration
was quantified using Image-Pro Plus version 6.0. The assays were
performed in triplicate and repeated at least three times.
5-Ethynyl-2′-deoxyuridine (EdU)
incorporation assay
Cell proliferation was detected by EdU assay, using
a Cell-Light EdU kit (Ribobio, Guangzhou, China) following the
manufacturer's instructions. Images were acquired at a
magnification of ×100, and the percentage of EdU-positive cells was
calculated. The assays were performed in triplicate and repeated at
least three times.
Statistical analysis
All data are presented as the mean ± standard
deviation. The differences between the two groups were analyzed by
Student's t-test using SPSS version 22.0 (IBM Corp, Armonk, NY,
USA). The results were considered to be statistically significant
at P-values <0.05.
Results
Tumor formation and growth
Xenografts tumor formation was observed in the
ovaries of all of the mice in the ES2/SOI and SKOV3/SOI groups,
while the tumor formation rates in the ES2/COI and SKOV3/COI groups
were 87.5% (7/8). Potential cell leakage would be detected by BLI,
and identified by taking into account the surgery, cell line usage
and the first BLI images. The two main situations we have
encountered were: 1) in highly metastatic cell lines, signals were
evenly distributed throughout the abdomen, with no significant
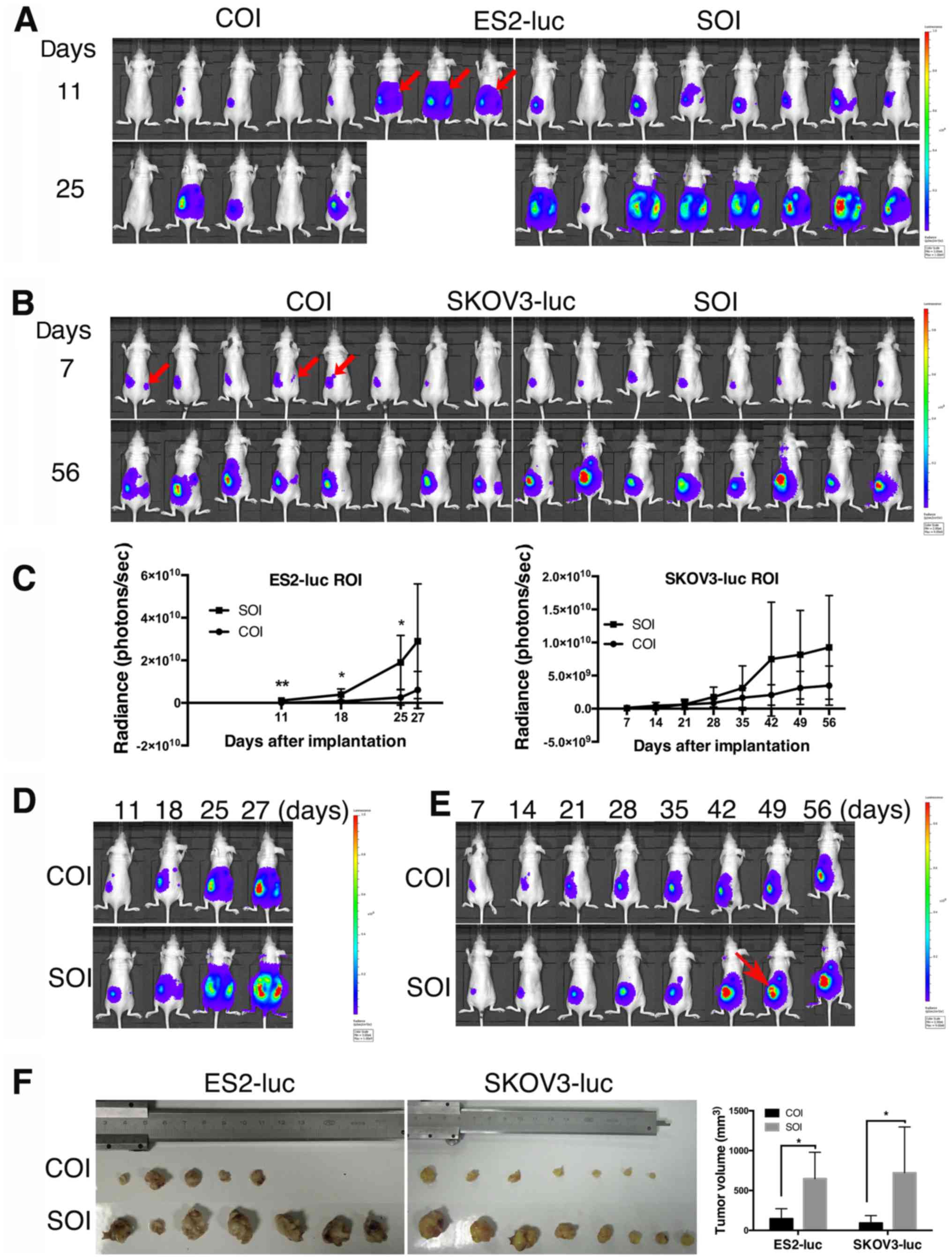
highlight signals in the ovarian portion (Fig. 1A); and 2) in lower metastatic cell
lines, bioluminescent signals were outside the ovarian projection
area on the body's surface (Fig.
1B). Tumor cell leakage occurred in 37.5% (3/8) of the mice in
the COI groups, whereas no cell leakage was observed in the SOI
groups.
The xenografts in the ES2/SOI and SKOV3/SOI groups
progressed more aggressively than in the COI groups. Specifically,
ES2/SOI tumors exhibited extremely rapid growth, and the mice
showed a shorter survival of only 27 days. Since three cases of COI
were found to have cell leakage at 11 days, and quickly progressed
into a moribund state, the data from these three cases were removed
during statistical analysis. One COI mouse failed to form a tumor,
though BLI showed a successful injection at 7 days. COI xenografts
showed retarded growth compared with SOI xenografts (Fig. 1A and D), and significant
differences could be observed at 11 days (P=0.008), 18 days
(P=0.028) and 25 days (P=0.017) (Fig.
1C).
Compared with ES2-luc, the SKOV3-luc implanted mice
formed relatively slow growing tumors and were euthanized at 56
days when tumor volume was estimated to exceed 1 cm3 by
palpation. Additionally, there was one failure case in the COI
group. Although not statistically significant, the bioluminescence
growth curve suggested a relatively faster growing tendency in SOI
tumors (Fig. 1B, C and E). Of
note, through the repeated imaging of each mouse, we found one case
of tumor necrosis in a SOI mouse (Fig.
1E). This may have been caused by rapid tumor growth in
SOI.
The results of tumor volume showed that both ES2-luc
and SKOV3-luc SOI xenografts were significantly larger than
corresponding COI xenografts (PES2=0.010, PSKOV3=0.018;
Fig. 1F). These results suggest
that tumor cells in SOI grow faster than COI and can form
relatively larger primary tumors.
SOI generates xenografts of a more
aggressive phenotype than COI
Consistent with the malignancy of cancer cells,
ES2-derived orthotopic tumors exhibited highly metastatic
properties and generated more metastases than SKOV3-luc cells in
vivo. Three of the five COI mice (60%) and all the SOI mice
(100%, 8/8) developed metastasis. Among them, ascites eventually
appeared in one COI mouse and six SOI mice. By contrast, the rate
of metastasis in SKOV3 group was low. Only 25% (2/8) COI mice and
62.5% (5/8) SOI mice were found to have metastases, and no
formation of ascites was observed.
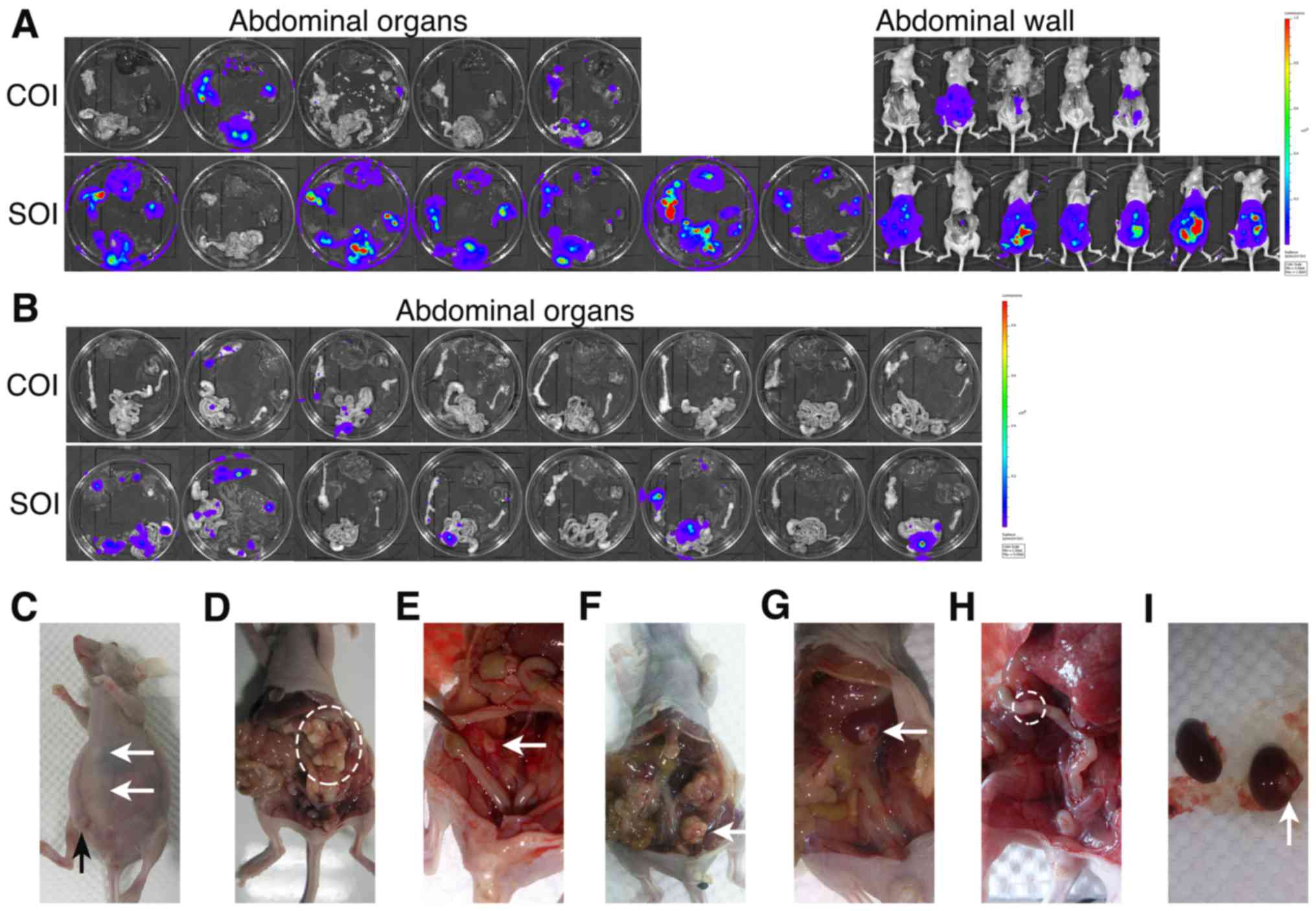
Moreover, we observed markedly more metastases in
mice with SOI xenografts compared with the COI groups (Table I). These results were confirmed by
ex vivo BLI analysis of removed pelvic and abdominal organs
(Fig. 2A and B). Metastases spread
across the abdominal cavities of the mice, including the kidney,
spleen, omentum, intestine, mesentery and the abdominal wall. In
addition, consistent with the progression of human ovarian cancer
were the formation of ascites (Fig.
2C–I), with the omentum and mesentery the most commonly
involved organs. Among mice with metastasis in the SOI groups,
tumor cells mainly metastasized to the omentum (6/7 of ES2, 3/5 of
SKOV3) and mesentery (5/7 of ES2, 4/5 of SKOV3). However, in COI
groups, although mesentery (2/3 of ES2, 1/2 of SKOV3) was still the
main target of metastasis, omentum metastasis (1/3 of ES2, 0/2 of
SKOV3) was rarely observed.
 | Table IMetastasis of the nude mouse groups
with orthotopically implanted human ovarian cancer. |
Table I
Metastasis of the nude mouse groups
with orthotopically implanted human ovarian cancer.
| Groups | Metastasis
rate | Ascites | Organs
|
|---|
| Omentum | Intestine and
mesentery | Liver | Spleen | Kidney | Contralateral
ovary | Peritoneum |
|---|
| ES2-luc-COI | 3/5 | 1/5 | 1/5 | 2/5 | 0/5 | 1/5 | 2/5 | 0/5 | 3/5 |
| ES2-luc-SOIa | 8/8 | 6/8 | 6/7 | 5/7 | 3/7 | 5/7 | 5/7 | 0/7 | 7/7 |
| SKOV3-luc-COI | 2/8 | 0/8 | 0/8 | 1/8 | 0/8 | 1/8 | 0/8 | 0/8 | 2/8 |
| SKOV3-luc-SOI | 5/8 | 0/8 | 3/8 | 4/8 | 2/8 | 0/8 | 1/8 | 0/8 | 4/8 |
The cells that generate SOI and COI
xenografts are different in migration and proliferation
Having established that SOI tumors are more
aggressive than COI tumors, we next investigated whether cell
malignant properties are different between the tumor cells that
form SOI and COI xenografts. Thus, we isolated tumor cells from
subcutaneous tumors (ES2-luc-M and SKOV3-luc-M, the cells that
generate SOI tumors) and compared them with those routinely in
vitro cultured tumor cells (ES2-luc-P and SKOV3-luc-P, the
cells that generate COI tumors) in regards to migration and
proliferation.
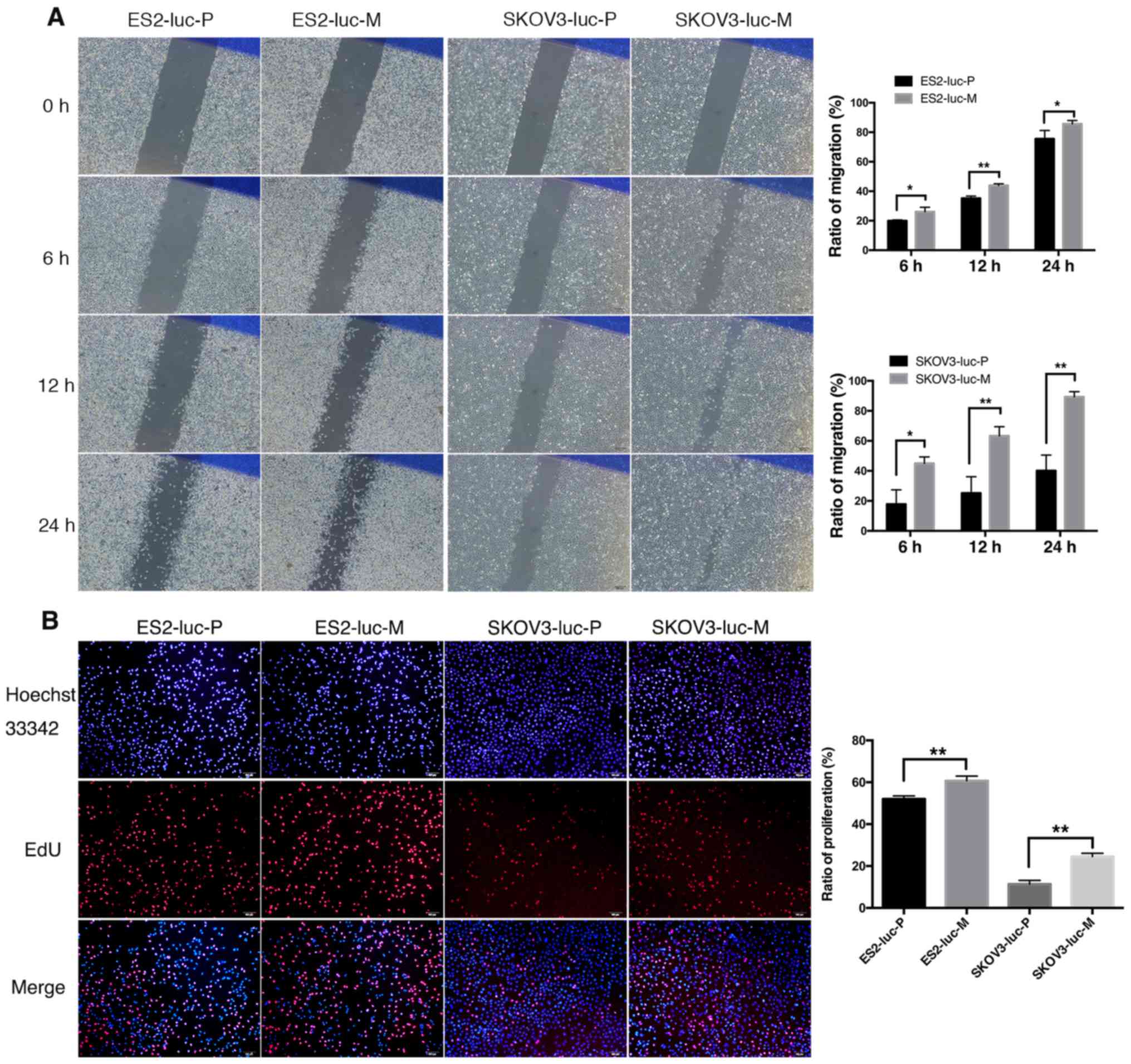
The scratch assay results showed that the primary
cells of ES2-luc subcutaneous tumors have a stronger migration
capability than the parental cells (Fig. 3A). Gap closure in both groups began
within 6 h after the scratch, and significant differences could be
observed (P6 h=0.029). At 12 and 24 h, the migration
areas of ES2-luc-M cells were increased by 25 and 13% (P12
h=0.001; P24 h=0.047). Similar results were
observed in SKOV3-luc-P/M cells. Primary cells showed significant
migration at 6 h and progressed until the gap was nearly invisible
at 24 h. In contrast, this process of SKOV3-luc-P cells was
significantly retarded, and 60% of the initial gap still remained
at 24 h. The migration areas of SKOV3-luc-M cells at 6, 12 and 24 h
were 2.23–2.53-fold to the SKOV3-luc-P groups (P6
h=0.011; P12 h=0.006; P24 h=0.002).
In the EdU assays, significant differences in
EdU-positive cell ratio were observed in both pairs of cell lines:
ES2-luc-M versus ES2-luc-P, 52.0%±1.5% versus 60.7±2.2%, P=0.005;
SKOV3-luc-M versus SKOV3-luc-P, 11.4±1.8% versus 24.5±1.6%, P=0.001
(Fig. 3B). These findings suggest
that primary cells isolated from the subcutaneous tumors that form
SOI tumors have a stronger potential for migration and
proliferation.
Tumor microenvironment in SOI and COI
xenografts
In a similar study of kidney cancer, it is believed
that supportive stromal tissue and cell-cell communication within a
tissue-block, namely the tumor microenvironment, plays an important
role in the full expression of cancer cell spontaneous metastatic
potential (18). Thus, we detected
several tumor microenvironment and metastasis-related markers using
immunohistochemistry staining in the COI and SOI ovarian xenograft
samples.
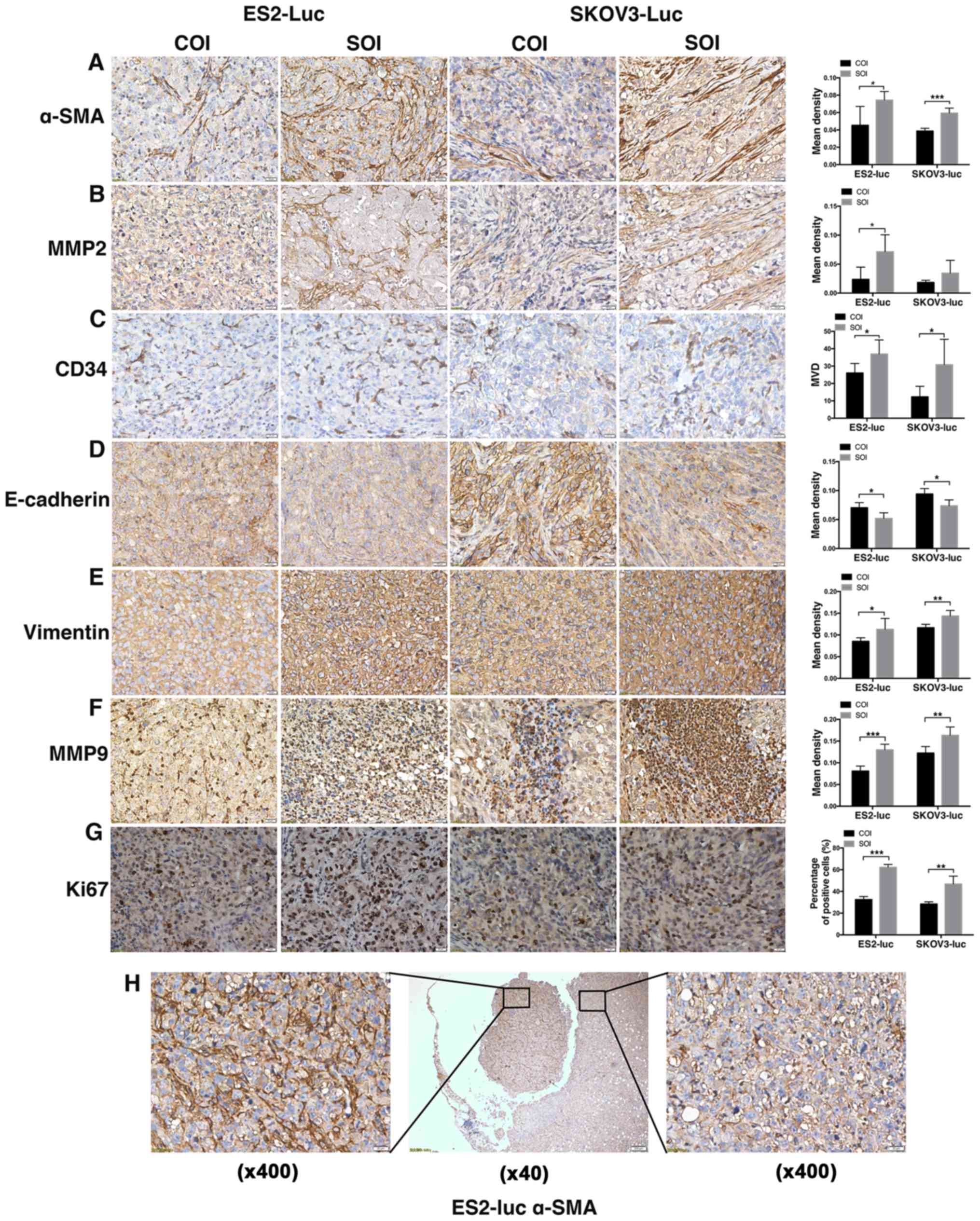
Positive staining of α-SMA was mainly localized to
tumor stroma, and the results (PES2=0.020;
PSKOV3<0.001) showed that there were more CAFs in SOI tumors
(Fig. 4A). CAFs can be induced by
tumor cells to synthesize active MMP2 (29,30).
Through MMP2 staining, we found that the localization and
distribution of MMP2 were very similar to α-SMA, and MMP2's
elevated expression in SOI tumors also confirmed a higher level of
activation in SOI stromal fibroblasts (PES2=0.024;
PSKOV3=0.155; Fig. 4B). The
staining of CD34 revealed that MVD of ES2-SOI group was 1.42-fold
to COI group. The 2.49-fold in SKOV3 tumors also suggests an
enhanced tumor angiogenesis in SOI tumors (PES2=0.049;
PSKOV3=0.046; Fig. 4C).
As the main markers of EMT, the expression of
E-cadherin (PES2=0.017; PSKOV3=0.011) and vimentin
(PES2=0.046; PSKOV3=0.004) indicated that SOI holds a
higher level of EMT (Fig. 4D and
E). Different from MMP2, as another important member of MMP
family, MMP9 is mainly localized to the area of inflammatory cell
infiltration, which was consistent with the theory that
tumor-associated macrophages are the main source of MMP9 (31). In SOI tumors, MMP9 staining
frequently exhibited large block aggregations, while the staining
regions in COI tumors were rather small (PES2<0.001;
PSKOV3=0.006; Fig. 4F). Lastly,
results of Ki67 (PES2<0.001; PSKOV3=0.004) suggested
a higher proliferation capability of cancer cells in SOI tumors
(Fig. 4G).
Additionally, we found a piece of tumor tissue
shedding from the local tumor (Fig.
4H), which was about to form metastasis. Noteworthy, there were
significant differences in the expression of α-SMA between the
shedding tissue and the local tissue (Fig. 4H).
Discussion
In this study, we successfully established an
orthotopic ovarian cancer mouse model by injecting tumor cells
(COI) or transplanting tumor tissue-blocks into the ovary (SOI). By
utilizing ovarian cancer cell lines that stably express GFP and
firefly luciferase, which enabled in vivo
bioluminescent/fluorescent imaging of live tumor cells, the
monitoring of tumor growth was no longer a limiting factor in the
application of orthotopic xenograft models. After a series of
dynamic BLI imaging, we made a comparison of tumor growth and
metastasis between COI and SOI tumors and found that SOI tumors
exhibited a higher take rate, faster growth and more aggressive
phenotype than COI. This conclusion was consistent with previous
studies (15–19).
In recent years, the application of orthotopic
ovarian cancer mouse models has greatly increased, with COI and SOI
the most commonly used methods. Many researchers chose to use COI,
which may have been due to the relative simplicity of the procedure
and its short experimental cycle (no subcutaneous tumor formation
or tissue-block requirement). The take rate of orthotopic tumors
varies depending on researcher experience and experimental
conditions. In most studies, take rates of up to 100% were
achieved, regardless of whether COI or SOI was the method used
(14,32). In our study, all the orthotopic
implantation surgeries were performed by the same person. The take
rate in the SOI group also reached 100%, but it was only 87.5% in
the COI group, even though successful injection was proved by the
first BLI in all mice.
Cell leakage may result in non-spontaneous
metastasis. In our study, none of the SOI mice were found to have
cell leakage, while several COI mice did. We believe that the cells
leak out mainly through the pinhole caused by the injection or due
to an incomplete ovary capsule (if the mouse was too young).
Therefore, SOI is recommended for studies of tumor metastasis,
because COI may exhibit false positive results and cause
experimental variability (18).
When COI is the only choice due to experimental limitations, some
improvements may help to prevent cell leakage: 1) use of semi-solid
medium to fix cells (33); 2) use
of biological glue for closure of the pinhole; 3) the usage of
relatively older mice (aged 6 weeks or more) may be helpful.
Additionally, since the inoculation surgery takes a certain amount
of time (approximately 10 min per mouse, according to the
operator's experience and advanced equipment) and the viability of
suspended cells may decrease over time in COI surgeries of a large
quantity, it is better to use SOI models in this situation. In SOI
surgeries, it is recommended that peripheral tissue of the
subcutaneous tumor be used, which provides better viability. The
advantages and disadvantages of the two models are compared in
Table II.
 | Table IIAdvantages and disadvantages of the
two models. |
Table II
Advantages and disadvantages of the
two models.
| Groups | COI | SOI |
|---|
| Experimental cycle
length | Relatively
short | Relatively long
(because of subcutaneous tumor formation) |
| Technical
requirement | Microscopic
injection | Microscopic
suture |
| Cell leakage | Easy to cause
intraperitoneal dissemination | Seldom cause
artificial metastasis |
| Transient
regulation to cellsa | Able | Unable |
| Take rate | Low | High |
| Growth speed | Slow | Rapid |
| Metastatic
capability | Weak | Strong |
| Stability of
tumorigenesis | Less stable | More stable |
Metastasis can be observed in almost all the studies
of the orthotopic ovarian cancer model. Similar to clinical
disease, peritoneal dissemination is the main pathway of
metastasis. Almost all of the organs in the abdominal cavity may be
involved, including the liver, spleen, kidney, mesentery, omentum
and paraaortic lymph node (13,14,32).
Our results of metastasis showed that SOI tumors were more
metastatic than COI. Consistent with other similar studies, the
incidence of abdominal wall metastasis was the highest: except for
one SKOV3-SOI mouse, all the mice were found to have metastases on
the surface of the peritoneum (32). In spite of the peritoneum,
metastatic patterns of abdominal organs differed between COI and
SOI. Omentum was one of the organs most commonly involved in SOI
metastasis, while omentum metastasis was rarely observed in COI. As
it is understood that the omentum is the main target organ of
ovarian cancer metastasis, up to 80% of epithelial ovarian cancer
patients are found to have omentum metastasis when diagnosed
(34,35). This suggests that the biological
characteristics of cancer cells in the SOI model are more closely
related to clinical disease, thus SOI is more clinically relevant.
In addition, in studying the metastasis of a particular organ,
in vivo selection of highly metastatic cell sub-lines using
the orthotopic model may help to better understand the organ
selectivity of metastasis (14).
Using scratch assay and EdU assay, we confirmed that
the migration and proliferation ability of cancer cells that form
SOI tumors was stronger than the parental cells that form COI
tumors. We think this may be due to the activation of cancer cells
by stroma in the subcutaneous tumor, so we further evaluated tumor
microenvironment markers in COI/SOI xenografts.
Immunohistochemistry showed more CAFs (21) and a higher level of angiogenesis in
SOI, indicating that the tumor micro-environment in SOI is more
conducive to cell proliferation and metastasis. In a previous study
of kidney cancer, the vasculature in the SOI model was also found
to be richer than that in COI model (18). Elevated EMT activation and
expression of Ki67 and stromal MMPs also suggested that tumor
microenvironments in SOI tumors are more pro-proliferative and
pro-metastatic.
In summary, through a comprehensive comparison of
COI and SOI ovarian cancer modeling, we found that SOI is more
malignant and clinically relevant than COI. Furthermore, the pros
and cons of COI/SOI and methods of improvement were summarized. We
believe this study provides useful recommendations and evidence for
modeling orthotopic ovarian cancer. The SOI models can serve as
useful tools for research into the metastasis and microenvironments
of ovarian cancer and more accurately predict the efficacy of
future clinical treatments.
Acknowledgments
We are grateful to Shunchang Zhou (Director of
Laboratory Animal Center, Huazhong University of Science and
Technology) for technical support. This study was supported by the
National Natural Science Foundation of China (81272860, 81472443
and 81572572).
Glossary
Abbreviations
Abbreviations:
|
OCOX
|
ovarian cancer orthotopic
xenograft
|
|
COI
|
cellular orthotopic injection
|
|
SOI
|
surgical orthotopic implantation
|
|
PDXs
|
patient-derived xenografts
|
|
GEMMs
|
genetically engineered mouse
models
|
|
DMEM/F12
|
Dulbecco's modified Eagle's medium:
nutrient mixture F-12
|
|
GFP
|
green fluorescent protein
|
|
BLI
|
bioluminescent imaging
|
|
ROI
|
region of interest
|
|
MMP
|
matrix metallopeptidase
|
|
CAFs
|
cancer-associated fibroblasts
|
|
MVD
|
microvessel density
|
|
EMT
|
epithelial-mesenchymal transition
|
|
EdU
|
5-ethynyl-2′-deoxyuridine
|
|
α-SMA
|
α-smooth muscle actin
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kerbel RS: A decade of experience in
developing preclinical models of advanced- or early-stage
spontaneous metastasis to study antiangiogenic drugs, metronomic
chemotherapy, and the tumor microenvironment. Cancer J. 21:274–283.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Singh M, Lima A, Molina R, Hamilton P,
Clermont AC, Devasthali V, Thompson JD, Cheng JH, Bou Reslan H, Ho
CC, et al: Assessing therapeutic responses in Kras mutant cancers
using genetically engineered mouse models. Nat Biotechnol.
28:585–593. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guerin E, Man S, Xu P and Kerbel RS: A
model of postsurgical advanced metastatic breast cancer more
accurately replicates the clinical efficacy of antiangiogenic
drugs. Cancer Res. 73:2743–2748. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bogden AE, Cobb WR, Lepage DJ, Haskell PM,
Gulkin TA, Ward A, Kelton DE and Esber HJ: Chemotherapy
responsiveness of human tumors as first transplant generation
xenografts in the normal mouse: Six-day subrenal capsule assay.
Cancer. 48:10–20. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee CH, Xue H, Sutcliffe M, Gout PW,
Huntsman DG, Miller DM, Gilks CB and Wang YZ: Establishment of
subrenal capsule xenografts of primary human ovarian tumors in SCID
mice: Potential models. Gynecol Oncol. 96:48–55. 2005. View Article : Google Scholar
|
|
7
|
Céspedes MV, Casanova I, Parreño M and
Mangues R: Mouse models in oncogenesis and cancer therapy. Clin
Transl Oncol. 8:318–329. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Francia G, Cruz-Munoz W, Man S, Xu P and
Kerbel RS: Mouse models of advanced spontaneous metastasis for
experimental therapeutics. Nat Rev Cancer. 11:135–141. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bibby MC: Orthotopic models of cancer for
preclinical drug evaluation: Advantages and disadvantages. Eur J
Cancer. 40:852–857. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fu X and Hoffman RM: Human ovarian
carcinoma metastatic models constructed in nude mice by orthotopic
transplantation of histologically-intact patient specimens.
Anticancer Res. 13:283–286. 1993.PubMed/NCBI
|
|
11
|
Sharpless NE and Depinho RA: The mighty
mouse: Genetically engineered mouse models in cancer drug
development. Nat Rev Drug Discov. 5:741–754. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Howell VM: Genetically engineered mouse
models for epithelial ovarian cancer: Are we there yet? Semin Cell
Dev Biol. 27:106–117. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kiguchi K, Kubota T, Aoki D, Udagawa Y,
Yamanouchi S, Saga M, Amemiya A, Sun FX, Nozawa S, Moossa AR, et
al: A patient-like orthotopic implantation nude mouse model of
highly metastatic human ovarian cancer. Clin Exp Metastasis.
16:751–756. 1998. View Article : Google Scholar
|
|
14
|
Tamada Y, Aoki D, Nozawa S and Irimura T:
Model for paraaortic lymph node metastasis produced by orthotopic
implantation of ovarian carcinoma cells in athymic nude mice. Eur J
Cancer. 40:158–163. 2004. View Article : Google Scholar
|
|
15
|
Fu XY, Theodorescu D, Kerbel RS and
Hoffman RM: Extensive multi-organ metastasis following orthotopic
onplantation of histologically-intact human bladder carcinoma
tissue in nude mice. Int J Cancer. 49:938–939. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang X, Fu X and Hoffman RM: A
patient-like metastasizing model of human lung adenocarcinoma
constructed via thoracotomy in nude mice. Anticancer Res.
12:1399–1401. 1992.PubMed/NCBI
|
|
17
|
Wang X, Fu X, Kubota T and Hoffman RM: A
new patient-like metastatic model of human small-cell lung cancer
constructed orthotopically with intact tissue via thoracotomy in
nude mice. Anticancer Res. 12:1403–1406. 1992.PubMed/NCBI
|
|
18
|
An Z, Jiang P, Wang X, Moossa AR and
Hoffman RM: Development of a high metastatic orthotopic model of
human renal cell carcinoma in nude mice: Benefits of fragment
implantation compared to cell-suspension injection. Clin Exp
Metastasis. 17:265–270. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Morioka CY, Saito S, Ohzawa K and Watanabe
A: Homologous orthotopic implantation models of pancreatic ductal
cancer in Syrian golden hamsters: Which is better for metastasis
research - cell implantation or tissue implantation? Pancreas.
20:152–157. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yi C, Zhang L, Zhang F, Li L, Ling S, Wang
X, Liu X and Liang W: Methodologies for the establishment of an
orthotopic transplantation model of ovarian cancer in mice. Front
Med. 8:101–105. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tommelein J, Verset L, Boterberg T,
Demetter P, Bracke M and De Wever O: Cancer-associated fibroblasts
connect metastasis-promoting communication in colorectal cancer.
Front Oncol. 5:632015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Uzzan B, Nicolas P, Cucherat M and Perret
GY: Microvessel density as a prognostic factor in women with breast
cancer: A systematic review of the literature and meta-analysis.
Cancer Res. 64:2941–2955. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Coussens LM, Fingleton B and Matrisian LM:
Matrix metalloproteinase inhibitors and cancer: Trials and
tribulations. Science. 295:2387–2392. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Micalizzi DS, Farabaugh SM and Ford HL:
Epithelial-mesenchymal transition in cancer: Parallels between
normal development and tumor progression. J Mammary Gland Biol
Neoplasia. 15:117–134. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu B, Jin Q, Zeng J, Yu T, Chen Y, Li S,
Gong D, He L, Tan X, Yang L, et al: Combined tumor- and
neovascular-'dual targeting' gene/chemotherapy suppresses tumor
growth and angiogenesis. ACS Appl Mater Interfaces. 8:25753–25769.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang Y, Tang H, Cai J, Zhang T, Guo J,
Feng D and Wang Z: Ovarian cancer-associated fibroblasts contribute
to epithelial ovarian carcinoma metastasis by promoting
angiogenesis, lymphangiogenesis and tumor cell invasion. Cancer
Lett. 303:47–55. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pei J, Fu W, Yang L, Zhang Z and Liu Y:
Oxidative stress is involved in the pathogenesis of Keshan disease
(an endemic dilated cardiomyopathy) in China. Oxid Med Cell Longev.
2013:4742032013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhu Y, Cai L, Guo J, Chen N, Yi X, Zhao Y,
Cai J and Wang Z: Depletion of Dicer promotes epithelial ovarian
cancer progression by elevating PDIA3 expression. Tumour Biol.
37:14009–14023. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Torng PL, Mao TL, Chan WY, Huang SC and
Lin CT: Prognostic significance of stromal metalloproteinase-2 in
ovarian adenocarcinoma and its relation to carcinoma progression.
Gynecol Oncol. 92:559–567. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hassona Y, Cirillo N, Heesom K, Parkinson
EK and Prime SS: Senescent cancer-associated fibroblasts secrete
active MMP-2 that promotes keratinocyte dis-cohesion and invasion.
Br J Cancer. 111:1230–1237. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang S, Van Arsdall M, Tedjarati S,
McCarty M, Wu W, Langley R and Fidler IJ: Contributions of stromal
metalloproteinase-9 to angiogenesis and growth of human ovarian
carcinoma in mice. J Natl Cancer Inst. 94:1134–1142. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yi XF, Yuan ST, Lu LJ, Ding J and Feng YJ:
A clinically relevant orthotopic implantation nude mouse model of
human epithelial ovarian cancer - based on consecutive observation.
Int J Gynecol Cancer. 15:850–855. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xu X, Ayub B, Liu Z, Serna VA, Qiang W,
Liu Y, Hernando E, Zabludoff S, Kurita T, Kong B, et al:
Anti-miR182 reduces ovarian cancer burden, invasion, and
metastasis: An in vivo study in orthotopic xenografts of nude mice.
Mol Cancer Ther. 13:1729–1739. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nieman KM, Romero IL, Van Houten B and
Lengyel E: Adipose tissue and adipocytes support tumorigenesis and
metastasis. Biochim Biophys Acta. 1831:1533–1541. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lungchukiet P, Sun Y, Kasiappan R, Quarni
W, Nicosia SV, Zhang X and Bai W: Suppression of epithelial ovarian
cancer invasion into the omentum by 1α,25-dihydroxyvitamin D3 and
its receptor. J Steroid Biochem Mol Biol. 148:138–147. 2015.
View Article : Google Scholar
|