Introduction
The amniotic membrane is the innermost layer of
fetal membrane that encloses the amniotic fluid and the fetus
during pregnancy. Human amniotic epithelial cells (hAECs) can be
easily obtained from amnion membrane with enzymatic digestion after
being separated from chorion (1).
hAECs are reported to have the ability to differentiate into all
three germ layers in vitro (2). In addition, hAECs express markers of
embryonic or germ cells (SSEA-3/4, TRA 1–60, and TRA 1–81), and
some transcription factors of pluripotent stem cells (Oct-4, Sox-2,
and Nanog) (3). Owning to their
easy isolation, low-immunogenicity, anti-inflammatory properties,
no tumorigenicity, and no ethical consideration, hAECs have gained
increasing attention in regenerative medicine therapy, such as
treating primary ovarian insufficiency (4), alveolar defect (5), skin regeneration (6) and so on.
Some unique traits of hAECs have attracted
increasing attention about the potential anticancer properties of
hAECs, such as induction apoptosis in lymphocytes and inhibition
angiogenesis in a rat dorsal skinfold chamber model (7). Niknejad et al reported that
hAEC-conditioned medium (hAEC-CM) could induce apoptosis in HeLa
cells and MDA-MB-231 cells in vitro (8). Kang et al showed that hAECs
displayed anticancer activity in a breast cancer-bearing nude mouse
model through both cell-to-cell contact and paracrine way (9). However, Mamede et al revealed
adverse effects of amniotic membrane-extracted proteins on human
cancer cell lines tested by MTT assay in vitro (10).
The effects of hAECs on human epithelial ovarian
cancer (EOC) have not been reported before. To characterize whether
hAECs have innate antitumor effects on EOC cells in vivo, we
established an SK-OV-3/hAECs co-injected xenografted BALB/c nude
mouse model. In addition, we used hAEC-CM or Transwell co-culture
system to detect the influences of hAEC-secreted factors on
proliferation and cell cycle progression of EOC cells in
vitro. Immunohistochemistry (IHC) was used to test the effects
of hAECs on regulators of cell cycle progression, including
p16INK4A, p21, phospho-c-Jun N-terminal kinases (JNK)
and phospho-pRB (Ser807) (phospho-retinoblastoma protein) in tumor
tissues. Cytokine array was conducted to discover
anticancer-related cytokines released from hAECs, which showed that
TGF-β1 was the most abundant cell cycle-regulatory cytokine
secreted from hAECs. Recombinant human TGF-β1 (rhTGF-β1) was used
to treat EOC cells and then cellular viability and cell cycle were
analyzed. TGF-β1 antibody was added in the Transwell system to
neutralize hAEC-secreted TGF-β1 and then cell cycle of EOC cells
was analyzed.
Materials and methods
Isolation and culture of human amniotic
epithelial cells
Human placentas were obtained at term pregnancy
during uncomplicated Caesarean sections with written and informed
consent from women who were negative for HIV-I, and hepatitis B and
C. This study has been approved by the Institutional Ethics
Committee of the International Peace Maternity and Child Health
Hospital, and written informed consent was obtained from all
participants. The protocol of hAECs isolation was described
previously (11). hAECs was
cultured in DMEM/F12 (Thermo Fisher Scientific) medium supplemented
with 10% fetal bovine serum (FBS; Thermo Fisher Scientific),
streptomycin (100 U/ml; Thermo Fisher Scientific), penicillin (100
U/ml; Thermo Fisher Scientific), 1× non-essential amino acids
(Thermo Fisher Scientific), and 1 mM of sodium pyruvate (Thermo
Fisher Scientific), and incubated at 37°C in an incubator
containing 5% CO2. Once the density of cells reached
80–90% confluency, cells were collected for subsequent experiments.
hAECs (5×106) were seeded in 100 mm diameter dishes
(Corning, USA) in complete culture medium for 72 h. The hAEC-CM
were then collected, 0.22 mm filtered, and used in subsequent
experiments.
Immunofluorescence
hAECs were identified by using the indirect
immunofluorescent labeling technique to detect the expression of
CK-7 and vimentin. Cells were fixed with 4% paraformaldehyde (PFA),
and then incubated with the following primary antibodies at 4°C
overnight: CK-7 (1:200 dilution; Boster, Wuhan, China) and vimentin
(1:200 dilution; Boster). After that, cells were incubated with
secondary antibody conjugated with Alexa Fluor® 488
(1:200; Thermo Fisher Scientific). Cells were counterstained with
DAPI (Thermo Fisher Scientific) and examined under the fluorescence
microscope (Leica, Germany).
Cancer cell lines culture
The EOC cell lines, SK-OV-3 and A2780 were obtained
from Shanghai Cell Bank of Chinese Academy of Sciences and cultured
in DMEM/high glucose (Thermo Fisher Scientific) medium supplemented
with 10% FBS, streptomycin (100 U/ml) and penicillin (100 U/ml).
Cells were incubated at 37°C in an incubator containing 5%
CO2. Once the density of cells reached 80–90%
confluency, cells were collected for subsequent experiments.
CCK-8 assay
Cell viability was detected with cell counting kit-8
(CCK8; Dojindo Molecular Technologies, Japan) according to the
manufacturer's protocol. EOC cells (7,000 cells per well) were
seeded in 96-well plate with complete culture medium or hAEC-CM or
mixture of complete culture medium and hAEC-CM at 1:1 ratio or
different concentration of rhTGF-β1 for 48 h.
Co-culturing ovarian cancer cell lines
and hAECs
Transwell co-culture system (Corning) was used to
evaluate the effects of hAEC-secreted factors on EOC cells. hAECs
(1×106 cells) were seeded on Transwell inserts and EOC
cells (2×106 cells) were cultured in 6-well culture
plates at the lower compartment. Cells were cultured in DMEM/high
glucose medium supplemented with 10% FBS, streptomycin (100 U/ml)
and penicillin (100 U/ml). All groups were incubated at 37°C in a
5% CO2 incubator for 4 days.
RNA extraction and real-time polymerase
chain reaction (PCR)
Total RNA was extracted from cultured EOC cells by
TRIzol (Thermo Fisher Scientific) according to the manufacturer's
instructions. Real-time quantitative PCR reactions were performed
in triplicate, using the SYBR Green Real-time PCR Master Mix
(Takara, Japan). PCR primers were designed according to cDNA
sequences in the NCBI database. The following primers were used:
GAPDH, 5′-AGGTCGGTGTGAACGGATTTG-3′ and 5′-GGGGTCGTTGATGGCAACA-3′;
PCNA, 5′-TTCCTGTGCAAAAGACGGAG-3′ and 5′-TCACCGTTGAAGAGAGTGGA-3′;
Ki-67, 5′-GAGGCAAATCATCCGAACCC-3′ and 5′-TTATTTTGGCGTCTGGAGCG-3′.
Cycling conditions for the PCR machine were as follows: 95°C 5 sec,
60°C 30 sec and 72°C 30 sec for 40 cycles. All reactions were
performed in a 10-µl volume. Gene expression levels were
evaluated using the ΔΔCt method, standardized to levels of GAPDH
amplification.
Establishment of xenografted nude mouse
models
Twelve female BALB/c nude mice (4-week-old) were
obtained from Shanghai Jiao Tong University School of Medicine
(Shanghai, China). All experimental protocols were approved by the
Ethics Committee of the School of Medicine of Shanghai Jiao Tong
University, and were in accordance with the approved guidelines set
by the Institutional Animal Care and Use Committee. The mice were
housed under a laminar flow hood in an isolated room and were
maintained under pathogen-free conditions. Five mice per group were
subcutaneously injected with 2×106 SK-OV-3 or
SK-OV-3/hAECs (2×106:1×106). Tumor volume was
measured every two weeks and tumor weight was measured at the
endpoint of the experiment. Animals were sacrificed by cervical
dislocation under anesthetic status after 28 days. Tumor volume was
calculated using the formula: tumor volume (mm3) = 0.52
× [width (mm2)] × [length (mm)] (12). To localize the hAECs in the tumor
microenvironment, we transfected hAECs with green fluorescent
protein (GFP) by lentivirus which was kindly provided by the
Professor Lijian Hui (Chinese Academic of Sciences, Shanghai,
China). Two mice were used to establish SK-OV-3/hAECsGFP
(2×106:1×106) xenografts and were sacrificed
after 28 days.
Immunofluorescence (IF) staining and
immunohistochemistry (IHC) staining
Tissue sections were deparaffinized and dehydrated.
The slides were then incubated in a 3% H2O2
solution to block the endogenous peroxidase (IF staining can skip
this procedure), followed by rinsing in PBS. To retrieve the
antigenicity, sections were then treated with heated antigen
retrieval solution containing ethylene diamine tetraacetic acid
(EDTA; Wuhan Goodbio Technology, Wuhan, China). After being
incubated with 5% bull serum albumin for 30 min to block the
non-specific antibody binding sites, the samples were then
incubated with the following primary antibodies at 4°C overnight:
PCNA (1:6,000 dilution; CST, USA), Ki-67 (1:200 dilution; Arigo
Biolaboratories, Taiwan, China), p16INK4A (1:300
dilution; Wanleibio, China), p21 (1:300 dilution; Wanleibio),
phospho-JNK (1:300 dilution; Wanleibio), phospho-pRB (Ser807)
(1:500 dilution; Abcam, UK). For IHC analysis, horseradish
peroxidase (HRP)-labelled anti-mouse/rabbit second antibodies and
diaminobenzidine were used according to the manufacturer's
instructions (Wuhan Goodbio Technology). Slides were counterstained
with hematoxylin, differentiated with 0.1% hydrochloric acid
alcohol, and washed by water. They were then dehydrated through a
graded ethanol series, immersed in xylene and mounted with
permount™ mounting medium. For IF analysis for GFP, tissues were
stained with secondary antibody conjugated with Alexa Fluor 488
(1:200; Thermo Fisher Scientific). Then tissues were counterstained
with DAPI (Thermo Fisher Scientific) and examined under the
fluorescence microscope (Leica).
For the semi-quantitative evaluation of p21,
p16INK4A and phospho-JNK expressions, we used a scoring
method described by Budwit-Novotny et al (13). The evaluation of PCNA, Ki-67 and
phospho-pRB (Ser807) were measured by the percentage of cells with
positive signals in the nucleus.
Cytokine array
A human antibody array 1000 (a combination of human
L-507 and human L-493) (RayBiotech, Inc., USA) was performed to
detect the expression of anticancer-associated cytokines in hAECs.
Samples of hAEC-CM were collected as previously described. Fresh
DMEM/F12 culture medium was used as the control. The cytokine array
was performed according to the instructions. The intensities of
signals were quantified by densitometry.
Cell cycle analysis
EOC cells were cultured alone or in the presence of
hAEC-CM. After 48 h, cancer cells were harvested by 0.25%
trypsin/ethylene diamine tetraacetic acid, then being washed with
phosphate-buffered solution, and fixed with 70% cold-ethanol for 24
h at −20°C. The cells were treated with 50 µg/ml RNase A
(Tiangen, China) for 30 min at 37°C and then stained with 100
µg/ml propidium iodide (PI; Thermo Fisher Scientific) for 30
min at 4°C. The cells were analyzed using a Cytomics™ FC500 flow
cytometer (Beckman Coulter, Brea, CA, USA). Data were analyzed
using Beckman Coulter CXP software.
Enzyme-linked immunosorbent assay
(ELISA)
The ELISA kit was used to test the efficiency of
TGF-β1 antibody added to Transwell system to neutralize
hAEC-secreted TGF-β1 at 48 h (R&D Systems, USA) and was
conducted according to the manufacturer's instructions.
Statistical analysis
Results from three independent experiments are
reported as the mean ± SEM. Kolmogorov-Smirnov test was used to
test whether data accorded with Gaussian distribution. Homogeneity
of variance test was conducted by Bartlett's test. Two-tail
Student's t-test or ordinary one-way analysis of variance (ANOVA)
with Tukey's multiple comparisons test or Mann-Whitney U test
(non-parametric test) or Kruskal-Wallis test (non-parametric test)
was used to evaluate statistical differences via GraphPad Prism
version 6 (GraphPad Software). Differences were considered
significant at p-value <0.05.
Results
hAECs inhibit proliferation of EOC cells
in vitro in a paracrine manner
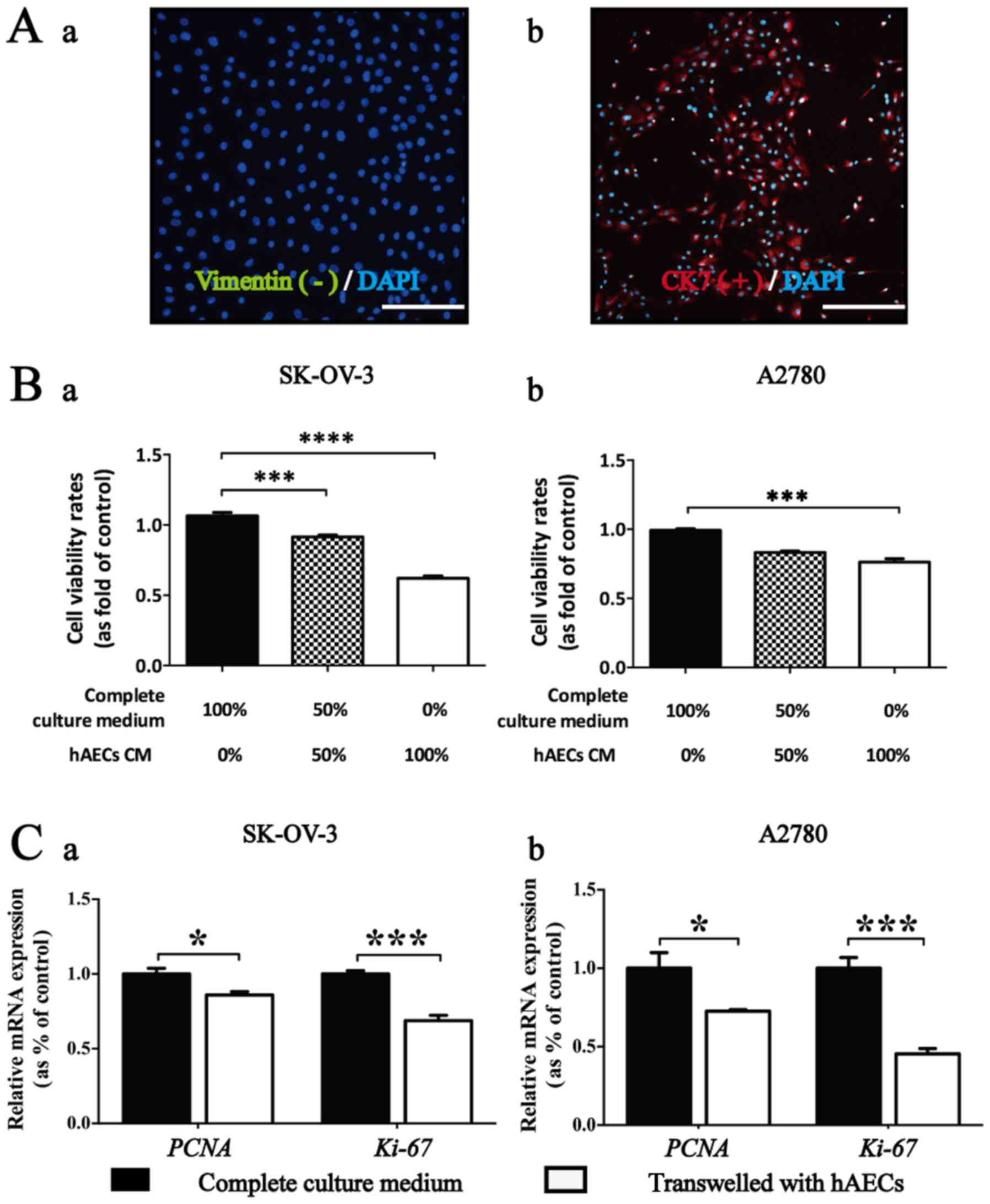
The hAECs represented a cobblestone-like morphology.
These cells were positive for epithelial marker CK7, and negative
for mesenchymal marker vimentin using IF analysis (Fig. 1A).
CCK-8 assay was conducted to test whether
hAEC-secreted factors could influence the viability of EOC cells.
Results showed that significant vitality inhibition in EOC cells
was induced by hAEC-CM in different concentration compared with the
control group at 48 h (n=6). Moreover, the growth inhibition
induced by hAEC-CM on EOC cells was significantly dose-dependent
(Fig. 1B).
Results from real-time PCR showed that the
expression levels of PCNA and Ki-67, two genes relating to cellular
proliferation, were significantly downregulated within EOC cells
co-cultured with hAECs compared with the control group (Fig. 1C; n=3).
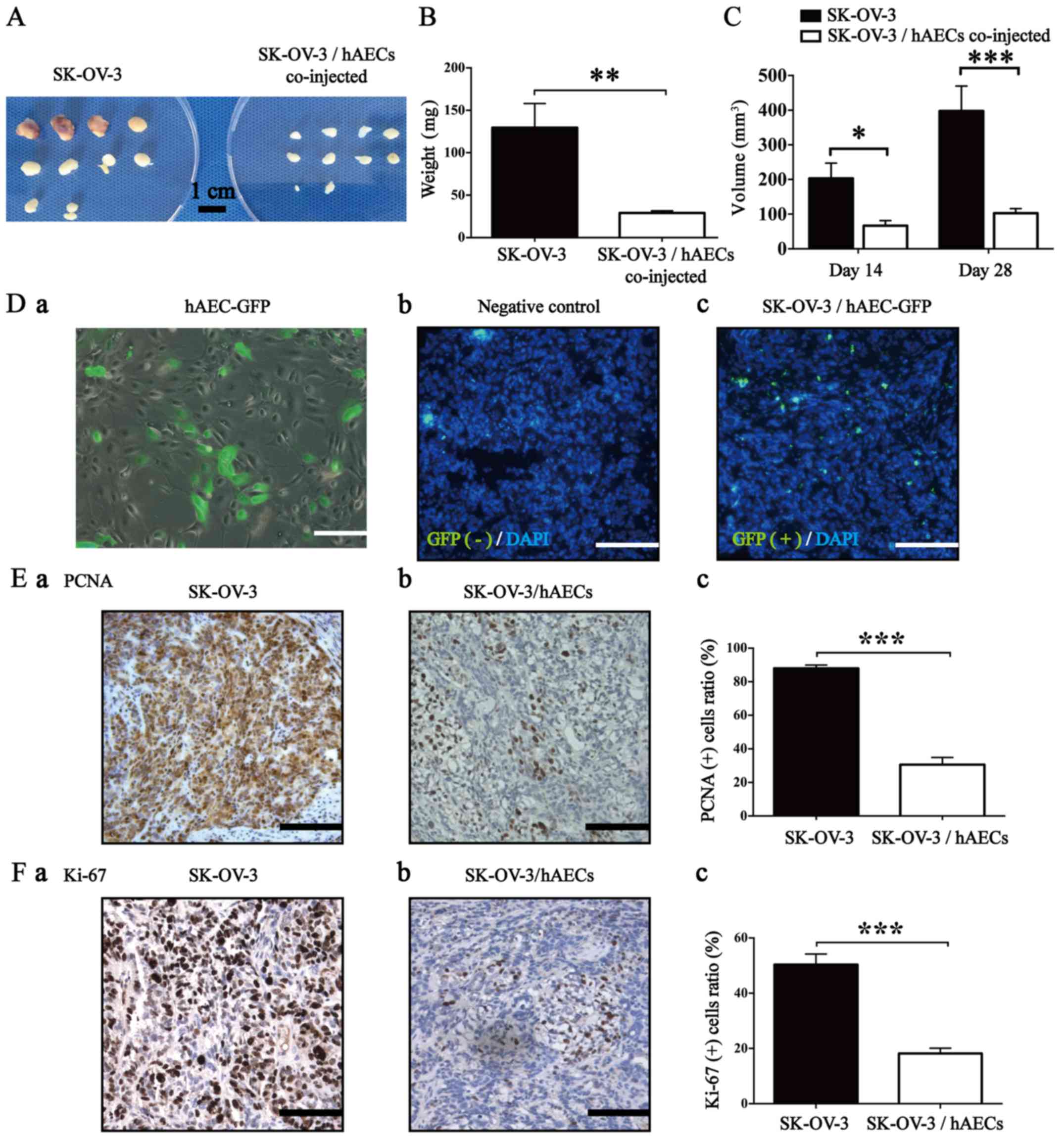
hAECs inhibit growth of SK-OV-3 cells in
a tumor-bearing nude mouse model
To explore the effects of hAECs on EOC cells in
vivo, we established a tumor-bearing nude mouse model by
subcutaneously co-injecting SK-OV-3 cells and hAECs at 2:1 ratio
(2×106:1×106 cells; five mouse per group) at
both sides of the scapular region. Four weeks after injection, we
observed that hAECs significantly decreased the average weight and
average volume of xenografted tumors compared with the control
group (Fig. 2A–C; n=10). To
further trace the location of hAECs in the tumor tissues, we
labelled hAECs with GFP (Fig.
2D–a) and established a xenografted mouse model by
subcutaneously co-injecting SK-OV-3 and hAECGFP+ cells
at 2:1 ratio (2×106:1×106 cells; two mice
were used) at both sides of the scapular region. After 28 days,
hAECGFP+ cells were found in the stromal area of
xenografted tumor tissues and no positive signal was observed in
the negative control using IF assay (Fig. 2D–b and –c).
Results from IHC assay showed that hAECs
significantly decreased the positive rates of both PCNA and Ki-67
in the SK-OV-3/hAECs co-injected tumor tissues compared with that
in the only SK-OV-3 injected tumor tissues (Fig. 2E and F; n=5), which was consistent
with the ex vivo results.
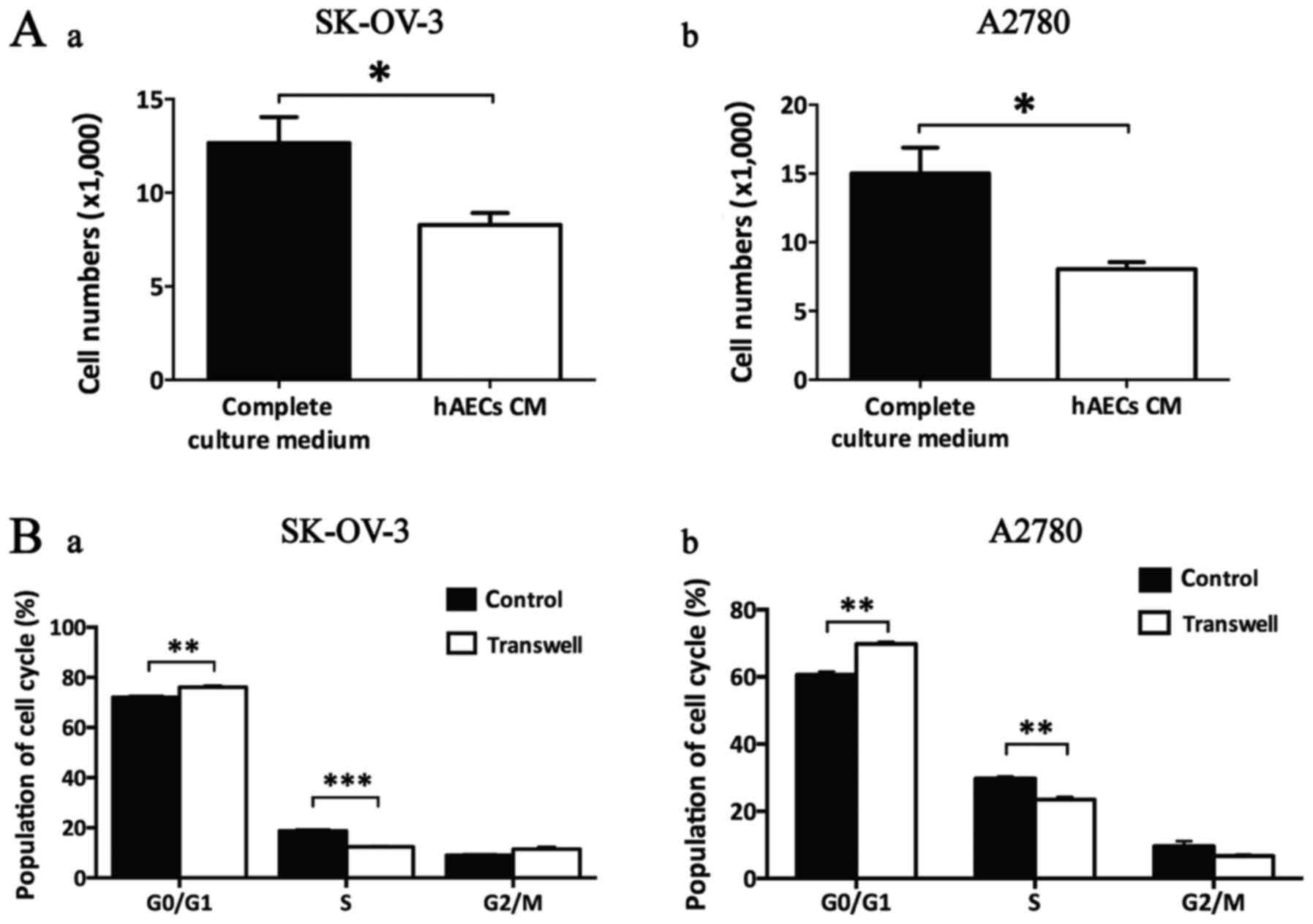
hAECs induce G0/G1 cell cycle arrest in
EOC cells in a paracrine manner
Cell count assay showed that hAEC-CM significantly
decreased the cell numbers of both SK-OV-3 and A2780 cells at 48 h,
indicating hAEC-secreted factors influenced the division of EOC
cells (Fig. 3A; n=3).
By using Transwell system, we observed that hAECs
significantly induced G0/G1 cell cycle arrest in both SK-OV-3 and
A2780 cells with a significant decrease in S phase using flow
cytometry analysis (Fig. 3B;
n=3).
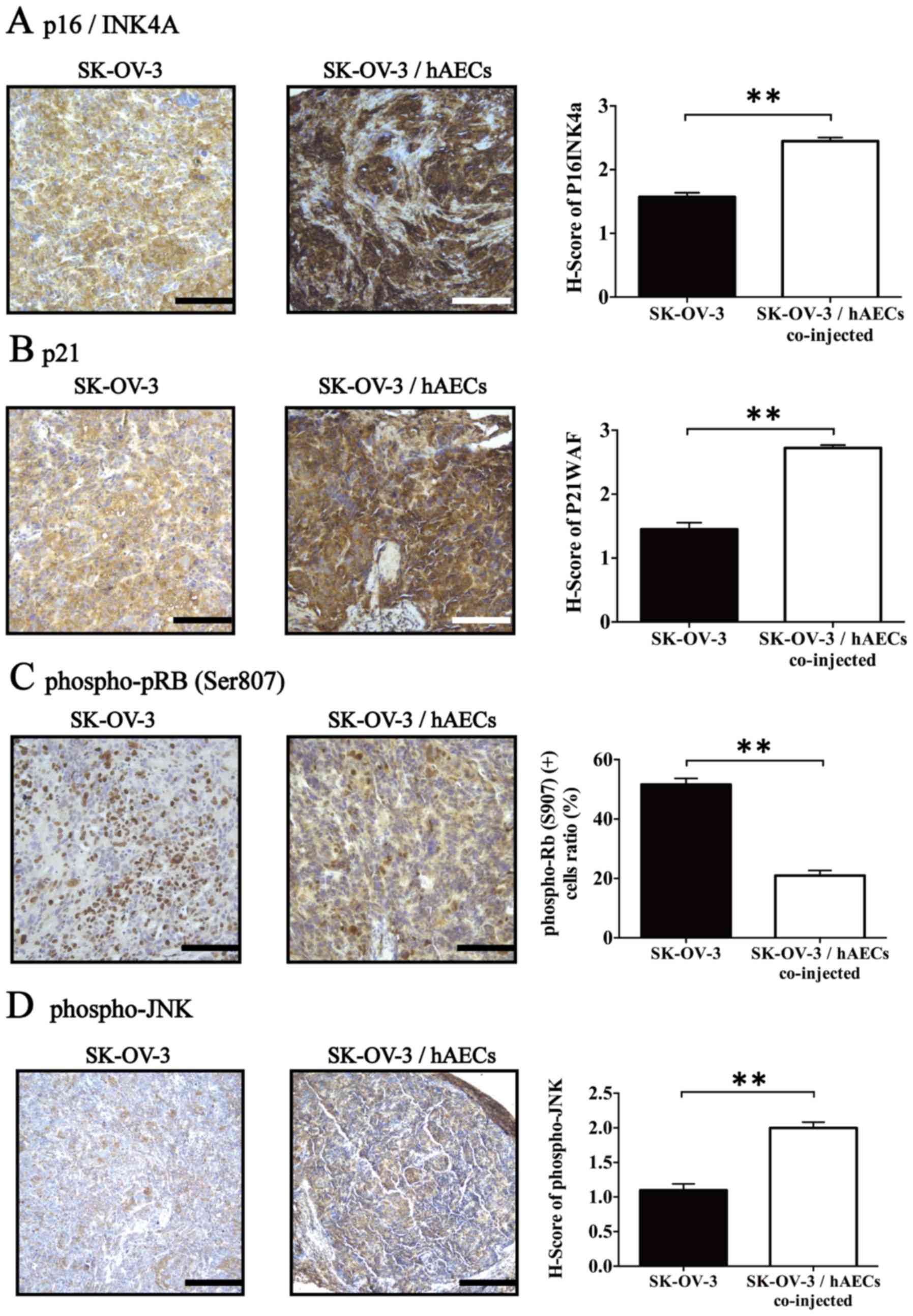
hAECs influence expression of cell
cycle-regulatory proteins in vivo
We further tested the effects of hAECs on the
expression levels of three negative regulators of cell cycle
progression, p16INK4A and p21, and phospho-JNK, which
was associated with TGF-β1-induced growth inhibition, in the tumor
tissues (14–16). IHC assay revealed that
SK-OV-3/hAECs co-injected tumor tissues exhibited significantly
higher expressions of p16INK4A, p21 and phospho-JNK
compared with tumor tissues obtained from SK-OV-3 injected group
(Fig. 4A–C; n=5). Furthermore, the
level of phospho-pRB (Ser807), a major regulator controlling cell
division cycle from G1 to S phase, was detected in the tumor
tissues using IHC. Results showed that hAECs significantly
inhibited phosphorylation of pRB (Ser807) within tumor tissues
obtained from xenografts compared with control group (Fig. 4D; n=5).
TGF-β1 is enriched in hAEC-released cell
cycle-regulatory cytokines and induces G0/G1 cell cycle arrest in
EOC cells
The results from cytokine array revealed that hAECs
express various anticancer-related cytokines, including TGF-β1,
granulocyte/macrophage colony-stimulating factor (GM-CSF),
interleukin-8 (IL-8), IL-6, IL-1, interferon-γ (IFN-γ), tumor
necrosis factor-α (TNF-α), TGF-β2 and Smad 7 (Fig. 5A–a). The relative expression of
cytokines was defined as the ratio of certain cytokine to Smad 7.
We found that the level of hAEC-secreted TGF-β1 was the most
abundant among these cytokines which were reported to be able to
negatively regulate cell cycle progression (Fig. 5A–b).
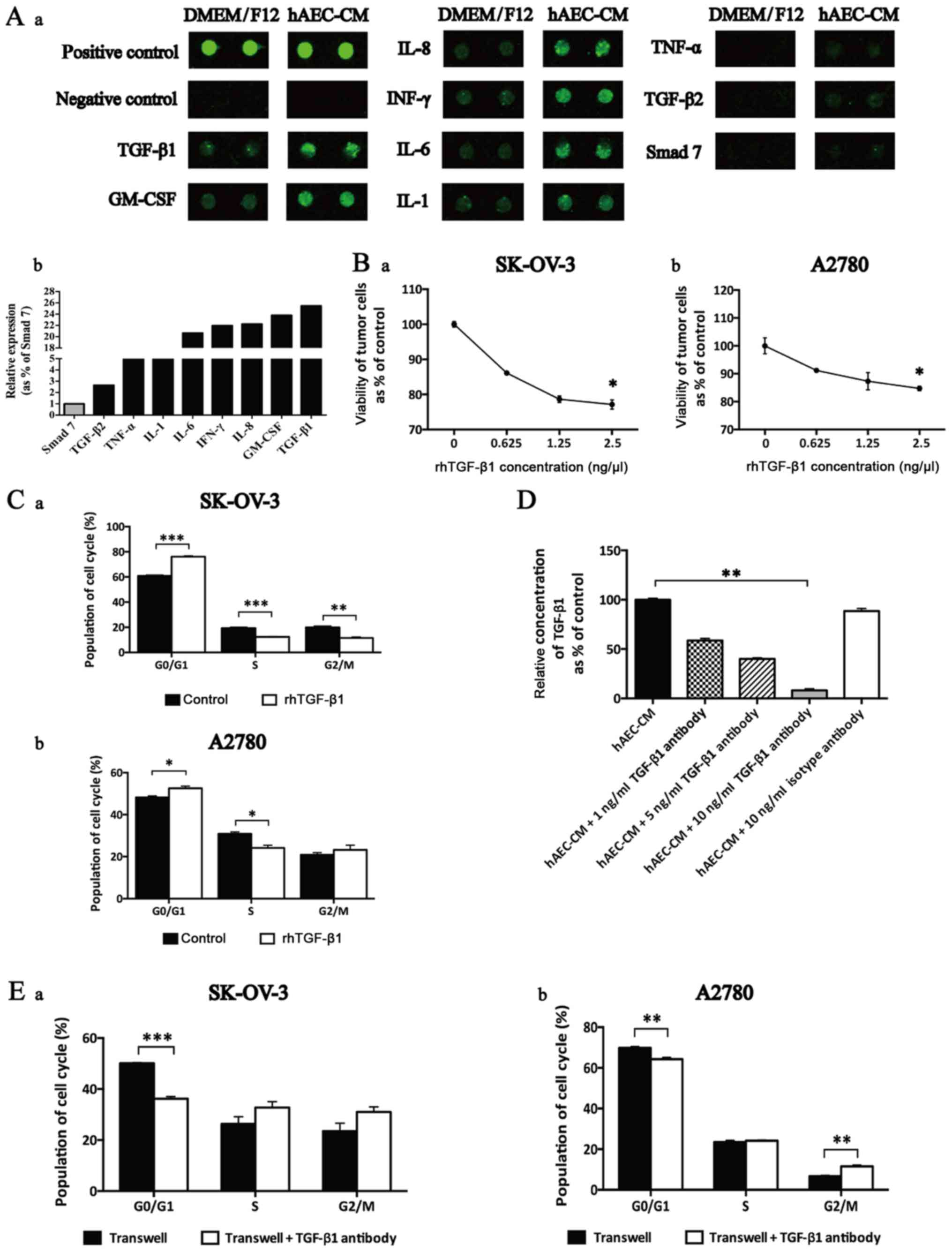 | Figure 5TGF-β1 was enriched in hAEC-released
cell cycle-regulatory cytokines and induced G0/G1 cell cycle arrest
in EOC cells. (A) (a) Selective map of the human antibody array
1000. hAECs released multiple cell cycle-regulatory cytokines,
including TGF-β1, GM-CSF, IL-8, IFN-γ, IL-6, IL-1, TNF-α, TGF-β2
and Smad 7. (b) The intensities of the signals were quantified by
densitometry and the expression of Smad 7 was regarded as control.
(B) CCK-8 cell viability assay used to test the effects of rhTGF-β1
in different concentrations on the viability of EOC cells at 48 h
(n=6; performed in triplicate). Data were analyzed by Mann-Whitney
U test. (C) After being treated with rhTGF-β1, cell cycle of EOC
cells was analyzed by flow cytometry. (D) ELISA was used to test
the efficiency of TGF-β1 antibody added to neutralize the function
of hAEC-secreted TGF-β1. Data were analyzed by Kruskal-Wallis test.
(E) After being treated with TGF-β1 antibody in the Transwell
system, cell cycle of EOC cells was analyzed by flow cytometry.
Data are presented as means ± SEM. *p<0.05,
**p<0.01 and ***p<0.001. |
To illustrate the effects of TGF-β1 on cell cycle
progression within EOC cells, we conducted CCK-8 cell viability
assay and found that the proliferative inhibition mediated by
rhTGF-β1 was dose-dependent (Fig.
5B; n=6). Further we added rhTGF-β1 to treat EOC cells and then
cell cycle progression was evaluated by flow cytometry. Results
showed that rhTGF-β1 induced a significant increase in G0/G1 phase
and a decrease in S phase in both cell lines (Fig. 5C; n=3).
To evaluate the neutralization efficiency of
antibody used to attenuate the function of hAEC-secreted TGF-β1, an
ELISA kit was used. Results indicated that 10 ng/ml TGF-β1 antibody
showed the best efficiency of reducing the amount of hAEC-secreted
TGF-β1 in the supernatant (Fig.
5D).
Finally, we added TGF-β1 antibody into the Transwell
system and evaluated the cell cycle progression of EOC cells at 48
h. We observed that TGF-β1 antibody reversed hAEC-induced G0/G1
cell cycle arrest and increased the population in S phase and G2/M
phase (Fig. 5E; n=3).
Discussion
hAECs show potent value in regenerative medicine. We
have reported that both hAECs and hAEC-CM endowed ability of
restoring fertility in mouse models of chemotherapy-induced POI/POF
(premature ovarian failure and insufficiency) via inhibiting
apoptosis in granular cells (11),
transdifferentiating into granular cells (17), and enhancing angiogenesis (4). Before further clinical research and
application, it is necessary to evaluate the safety of hAEC-based
therapy in malignant situation, including the effects of hAECs on
epithelial ovarian cancer which remains the leading cause of
gynecological cancer-related death (18).
Many studies showed that hAECs might be a promising
source for cell-based therapy for cancer, because of their ability
to induce apoptosis (8) and
stimulating cell cycle arrest (19). It is known that proliferation of
cancer cells depends in part on cell cycle progression (20). hAEC-secreted factors have been
shown to have the ability to induce cell cycle arrest in several
cancer cells (19,21). However, the specific factors and
mechanisms responsible for the cell cycle-regulatory roles of hAECs
on cancer cells still remain unclear. In this study, results from a
cytokine array revealed that hAECs released various cytokines which
are able to induce G0/G1 cell cycle arrest, including TGF-β1
(22), GM-CSF (23), IL-8 (24), IL-6 (25), IL-1 (26), IFN-γ, TNF-α (27), TGF-β2 (28) and Smad 7 (29). Our results showed that the
secretion of TGF-β1 derived from hAECs was abundant, this is
consistent with the report of Kang et al showing the amount
of TGF-β1 ranked first among those anticancer-associated factors
(9). We used recombinant human
TGF-β1 to imitate the function of hAEC-secreted TGF-β1 on EOC
cells. We observed that rhTGF-β1 could inhibit the viability of EOC
cells dose-dependently and induced G0/G1 cell cycle arrest in both
cell lines. In addition, after confirming the concentration of
TGF-β1 antibody used to neutralize hAEC-secreted TGF-β1 in the
supernatant via ELISA, we found that hAEC-secreted factors-induced
G0/G1 cell cycle arrest in Transwell system could be partially
reversed by adding excess neutralizing TGF-β1 antibody, indicating
that hAEC-secreted TGF-β1 play an important role in the induction
of cell cycle arrest in EOC cells.
The cell cycle progression is well-organized by
cyclin-dependent kinase (CDK) and CDK inhibitors. CDK activity is
regulated by two families of inhibitors: INK4 proteins, including
p16INK4A, p15INK4B, p18INK4C and
p19INK4D and the Cip and Kip family, consisting of p21,
p27 and p57 (30). Many studies
reported that TGF-β1 could increase the expression of
p16INK4A (14) and p21
(15), which functioned as
negative regulators of cell cycle progression and might be
associated with TGF-β1-mediated G0/G1 cell cycle arrest.
Schlosshauer et al reported that the loss of
p16INK4A might attribute to tumor progression from
ovarian borderline tumors to micropapillary tumors and low-grade
carcinomas through overcoming the senescence barrier and/or
indirect p53 downregulation (31).
Kudoh et al suggested that deletion of p16INK4A
and/or p15INK4B was a potential indicator for poor
chemotherapy response and adverse prognosis in ovarian cancer
patients (32). Consistently, we
found that lower expression of p16INK4A was observed in
larger xenografts obtained from SK-OV-3 injected group compared
with tumor tissues obtained from SK-OV-3/hAECs co-injected group.
In addition, our results showed that hAECs upregulated expression
of p21 in tumor tissues and p21 overexpression was considered to
indicate a better prognostic factor in epithelial ovarian cancer
(33,34). JNKs coordinate cell responses to
stress and influence regulation of cell growth and transformation,
whose phosphorylation is associated with TGF-β1-induced growth
inhibition (35) and protein
stabilization of p21 (16). Herein
our result was consistent with it and showed that hAECs upregulated
level of phospho-JNK in xenografted model.
TGF-β is a growth suppressing cytokine which
maintains pocket proteins (p107, p130 and pRB) in an active,
hypo-phosphorylated state through directly inducing expressing of
CDK inhibitor proteins (36),
including p16INK4A and p21. By using a Rb1 knockout
mouse model, Francis et al proved that hypophosphorylated
pRB is required to inhibit expression of activator E2Fs (E2F1, E2F2
and E2F3) target genes, including positive regulators of cell cycle
(PCNA and cyclin E1) (37). In
this study, we found that hAECs decreased level of phospho-pRB
(Ser807) in tumor tissues with lower expression of PCNA and higher
levels of p16INK4A, p21 and phospho-JNK, indicating
hAECs attenuated the malignant progression of EOC cells through
halting cell cycle arrest in EOC cells.
In conclusion, we demonstrated the inhibitory
effects of hAECs on epithelial ovarian cancer cells in vivo
and in vitro. TGF-β1 secreted from hAECs plays an important
role in hAEC-mediated growth inhibition in epithelial ovarian
cancer cells through inducing G0/G1 cell cycle arrest in cancer
cells. This study provides a potential application of hAEC-based
strategies against epithelial ovarian cancer. In addition, it
suggests that hAEC-based therapy may be safe, but still needs
further studies.
Acknowledgments
This study was supported by grants from National
Natural Science Foundation of China (no. 81370678), Shanghai
Municipal Council for Science and Technology (no. 14411961500),
Shanghai Municipal Education Commission-Gaofeng Clinical Medicine
(no. 20152236), Shanghai Municipal Health Bureau (no. Y20140241),
and Shanghai Jiao Tong University Medicine-Engineering Fund (no. YG
2014QN12).
Abbreviations:
|
hAECs
|
human amniotic epithelial cells
|
|
EOC
|
epithelial ovarian cancer
|
|
TGF-β1
|
transforming growth factor-β1
|
|
PCR
|
real-time polymerase chain
reaction
|
|
IHC
|
immunohistochemistry
|
|
pRB
|
retinoblastoma protein
|
|
JNK
|
phospho-c-Jun N-terminal kinases
|
|
hAEC-CM
|
hAEC-conditioned medium
|
|
FBS
|
fetal bovine serum
|
|
PFA
|
paraformaldehyde
|
|
H&E
|
hematoxylin and eosin
|
|
PCNA
|
proliferating cell nuclear antigen
|
|
GFP
|
green fluorescent protein
|
|
HRP
|
horseradish peroxidase
|
|
ANOVA
|
analysis of variance
|
|
POI/POF
|
premature ovarian failure and
insufficiency
|
|
GM-CSF
|
granulocyte/macrophage
colony-stimulating factor
|
|
IL
|
interleukin
|
|
IFN-γ
|
interferon-γ
|
|
TNF-α
|
tumor necrosis factor-α
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
References
|
1
|
Barbati A, Grazia Mameli M, Sidoni A and
Di Renzo GC: Amniotic membrane: Separation of amniotic mesoderm
from amniotic epithelium and isolation of their respective
mesenchymal stromal and epithelial cells. Curr Protoc Stem Cell
Biol Chapter. 1:82012.
|
|
2
|
Ilancheran S, Michalska A, Peh G, Wallace
EM, Pera M and Manuelpillai U: Stem cells derived from human fetal
membranes display multilineage differentiation potential. Biol
Reprod. 77:577–588. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Miki T, Lehmann T, Cai H, Stolz DB and
Strom SC: Stem cell characteristics of amniotic epithelial cells.
Stem Cells. 23:1549–1559. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yao X, Guo Y, Wang Q, Xu M, Zhang Q, Li T
and Lai D: The paracrine effect of transplanted human amniotic
epithelial cells on ovarian function improvement in a mouse model
of chemotherapy-induced primary ovarian insufficiency. Stem Cells
Int. 2016:41489232016. View Article : Google Scholar
|
|
5
|
Si JW, Zhang JJ, Dai JW, Yu DD, Yu HB, Shi
J, Wang XD, Shen SGF and Guo LH: Osteogenic differentiation of
human amniotic epithelial cells and its application in alveolar
defect restoration. Stem Cells Transl Med. 3:1504–1513. 2014.
View Article : Google Scholar
|
|
6
|
Jiang LW, Chen H and Lu H: Using human
epithelial amnion cells in human de-epidermized dermis for skin
regeneration. J Dermatol Sci. 81:26–34. 2016. View Article : Google Scholar
|
|
7
|
Niknejad H, Paeini-Vayghan G, Tehrani FA,
Khayat-Khoei M and Peirovi H: Side dependent effects of the human
amnion on angiogenesis. Placenta. 34:340–345. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Niknejad H, Khayat-Khoei M, Peirovi H and
Abolghasemi H: Human amniotic epithelial cells induce apoptosis of
cancer cells: A new anti-tumor therapeutic strategy. Cytotherapy.
16:33–40. 2014. View Article : Google Scholar
|
|
9
|
Kang NH, Yi BR, Lim SY, Hwang KA, Baek YS,
Kang KS and Choi KC: Human amniotic membrane-derived epithelial
stem cells display anticancer activity in BALB/c female nude mice
bearing disseminated breast cancer xenografts. Int J Oncol.
40:2022–2028. 2012.PubMed/NCBI
|
|
10
|
Mamede AC, Laranjo M, Carvalho MJ,
Abrantes AM, Pires AS, Brito AF, Moura P, Maia CJ and Botelho MF:
Effect of amniotic membrane proteins in human cancer cell lines: An
exploratory study. J Membr Biol. 247:357–360. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang Q, Xu M, Yao X, Li T, Wang Q and Lai
D: Human amniotic epithelial cells inhibit granulosa cell apoptosis
induced by chemotherapy and restore the fertility. Stem Cell Res
Ther. 6:1522015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu T, Zhao L, Zhang Y, Chen W, Liu D, Hou
H, Ding L and Li X: Ginsenoside 20(S)-Rg3 targets HIF-1α to block
hypoxia-induced epithelial-mesenchymal transition in ovarian cancer
cells. PLoS One. 9:e1038872014. View Article : Google Scholar
|
|
13
|
Budwit-Novotny DA, McCarty KS, Cox EB,
Soper JT, Mutch DG, Creasman WT, Flowers JL and McCarty KS Jr:
Immunohistochemical analyses of estrogen receptor in endometrial
adenocarcinoma using a monoclonal antibody. Cancer Res.
46:5419–5425. 1986.PubMed/NCBI
|
|
14
|
Jin G, Cao Z, Sun X, Wang K, Huang T and
Shen B: Protein O-glucosyltransferase 1 overexpression
downregulates p16 in BT474 human breast cancer cells. Oncol Lett.
8:594–600. 2014.PubMed/NCBI
|
|
15
|
Datto MB, Yu Y and Wang XF: Functional
analysis of the transforming growth factor beta responsive elements
in the WAF1/Cip1/p21 promoter. J Biol Chem. 270:28623–28628. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim GY, Mercer SE, Ewton DZ, Yan Z, Jin K
and Friedman E: The stress-activated protein kinases p38 alpha and
JNK1 stabilize p21 (Cip1) by phosphorylation. J Biol Chem.
277:29792–29802. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang F, Wang L, Yao X, Lai D and Guo L:
Human amniotic epithelial cells can differentiate into granulosa
cells and restore folliculogenesis in a mouse model of
chemotherapy-induced premature ovarian failure. Stem Cell Res Ther.
4:1242013. View
Article : Google Scholar :
|
|
18
|
Jayson GC, Kohn EC, Kitchener HC and
Ledermann JA: Ovarian cancer. Lancet. 384:1376–1388. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Di Germanio C, Bernier M, Petr M, Mattioli
M, Barboni B and de Cabo R: Conditioned medium derived from rat
amniotic epithelial cells confers protection against inflammation,
cancer, and senescence. Oncotarget. 7:39051–39064. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dickson MA: Molecular pathways: CDK4
inhibitors for cancer therapy. Clin Cancer Res. 20:3379–3383. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Magatti M, De Munari S, Vertua E and
Parolini O: Amniotic membrane-derived cells inhibit proliferation
of cancer cell lines by inducing cell cycle arrest. J Cell Mol Med.
16:2208–2218. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen X, Zheng S, Gao Y, Dai H, Mou H and
Yang H: Growth regulation of ovarian cancer cell line HO-8910 by
transforming growth factor beta 1 in vitro. Chin Med J (Engl).
111:546–550. 1998.
|
|
23
|
Ketley NJ, Allen PD, Kelsey SM and Newland
AC: Modulation of idarubicin-induced apoptosis in human acute
myeloid leukemia blasts by all-trans retinoic acid, 1,25(OH)2
vitamin D3, and granulocyte-macrophage colony-stimulating factor.
Blood. 90:4578–4587. 1997.PubMed/NCBI
|
|
24
|
Schinke C, Giricz O, Li W, Shastri A,
Gordon S, Barreyro L, Bhagat T, Bhattacharyya S, Ramachandra N,
Bartenstein M, et al: IL8-CXCR2 pathway inhibition as a therapeutic
strategy against MDS and AML stem cells. Blood. 125:3144–3152.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Resnitzky D, Tiefenbrun N, Berissi H and
Kimchi A: Interferons and interleukin 6 suppress phosphorylation of
the retinoblastoma protein in growth-sensitive hematopoietic cells.
Proc Natl Acad Sci USA. 89:402–406. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Muthukkumar S, Sells SF, Crist SA and
Rangnekar VM: Interleukin-1 induces growth arrest by
hypophosphorylation of the retinoblastoma susceptibility gene
product RB. J Biol Chem. 271:5733–5740. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lillibridge CD and O'Connell BC: In human
salivary gland cells, overexpression of E2F1 overcomes an
interferon-gamma- and tumor necrosis factor-alpha-induced growth
arrest but does not result in complete mitosis. J Cell Physiol.
172:343–350. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu DT, Bitzer M, Ju W, Mundel P and
Böttinger EP: TGF-beta concentration specifies differential
signaling profiles of growth arrest/differentiation and apoptosis
in podocytes. J Am Soc Nephrol. 16:3211–3221. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kitamura K, Aota S, Sakamoto R, Emori T
and Okazaki K: Smad7 induces G0/G1 cell cycle arrest in mesenchymal
cells by inhibiting the expression of G1 cyclins. Dev Growth
Differ. 47:537–552. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Malumbres M and Barbacid M: Cell cycle,
CDKs and cancer: A changing paradigm. Nat Rev Cancer. 9:153–166.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schlosshauer PW, Deligdisch L,
Penault-Llorca F, Fatemi D, Qiao R, Yao S, Pearl M, Yang Z, Sheng T
and Dong J: Loss of p16I NK4A expression in low-grade ovarian
serous carcinomas. Int J Gynecol Pathol. 30:22–29. 2011. View Article : Google Scholar
|
|
32
|
Kudoh K, Ichikawa Y, Yoshida S, Hirai M,
Kikuchi Y, Nagata I, Miwa M and Uchida K: Inactivation of p16/CDKN2
and p15/MTS2 is associated with prognosis and response to
chemotherapy in ovarian cancer. Int J Cancer. 99:579–582. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Buchynska LG, Nesina IP, Yurchenko NP,
Bilyk OO, Grinkevych VN and Svintitsky VS: Expression of p53,
p21WAF1/CIP1, p16INK4A and Ki-67 proteins in serous ovarian tumors.
Exp Oncol. 29:49–53. 2007.PubMed/NCBI
|
|
34
|
Bali A, O'Brien PM, Edwards LS, Sutherland
RL, Hacker NF and Henshall SM: Cyclin D1, p53, and p21Waf1/Cip1
expression is predictive of poor clinical outcome in serous
epithelial ovarian cancer. Clin Cancer Res. 10:5168–5177. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Khalil N, Xu YD, O'Connor R and Duronio V:
Proliferation of pulmonary interstitial fibroblasts is mediated by
transforming growth factor-beta1-induced release of extracellular
fibroblast growth factor-2 and phosphorylation of p38 MAPK and JNK.
J Biol Chem. 280:43000–43009. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Henley SA and Dick FA: The retinoblastoma
family of proteins and their regulatory functions in the mammalian
cell division cycle. Cell Div. 7:102012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Francis SM, Bergsied J, Isaac CE, Coschi
CH, Martens AL, Hojilla CV, Chakrabarti S, Dimattia GE, Khoka R,
Wang JY, et al: A functional connection between pRB and
transforming growth factor beta in growth inhibition and mammary
gland development. Mol Cell Biol. 29:4455–4466. 2009. View Article : Google Scholar : PubMed/NCBI
|