Introduction
Cancer is one of the leading human health problems
worldwide. Since the term includes an extensive variety of
different diseases, there are still many types of cancer with a
dismal prognosis. Breast cancer (BrC) is the most common cause of
cancer in working age women. Since most cases are diagnosed at
advanced stages, BrC mortality is particularly high in
underdeveloped countries (1,2).
Similar to most cancers, BrC is a highly heterogeneous disease, and
although we currently have several classification and staging
strategies to distinguish BrC sub-types with variable clinical
outcomes, diseases originally thought as non-aggressive are often
treatment resistant or relapse with highly aggressive
characteristics (3). BrC cells
also exhibit a large genetic and epigenetic intra-tumoral
heterogeneity, conferring individual cells with specific
immunosuppressive, proliferative, survival, metabolic, and invasive
characteristics, that signal specific tumor clones with cancer
dissemination and treatment resistance capacities (4). Furthermore, tumor clones are
surrounded by a highly dynamic microenvironment formed by
tumor-associated macrophages, neutrophils, T-cells, NK cells,
fibroblasts, among other cells (the tumor microenvironment or TME).
Although, in recent years we have seen a hefty increase in our
understanding of critical interactions between tumor cells and TME
cells, that has propelled the design of therapeutic molecular
inhibitors (5), we significantly
lack behind in our comprehension of relevant interactions between
tumor clones with different aggressive features and the impact of
these interactions on disease progression and prognosis.
Miller et al in a 1983 hallmark study
observed that there is cooperation between metastatic and
non-metastatic tumor clones. This group reported in a syngeneic
mouse model that the presence of a metastatic subpopulation enabled
non-mobile subpopulations to metastasize (6). More recently, a similar observation
was also made by Calbo et al (7). Experiments using the Drosophila
melanogaster (fruit fly) in which different cells were
engineered to express either RASV12 or
scrib− common oncogenic mutations, revealed
intra-clonal cooperation that promoted tumor growth and invasion
(8). Similarly, Cleary et
al observed in a mouse model of BrC that two different cellular
clones had to be transferred to propagate the tumor in new mice,
one clone with an Hras genetic mutation and the other with
the capacity to secrete high levels of the Wnt1 signaling molecule
but harboring a wild-type Hras (9). Soluble factors secreted by
chemoresistant tumor cells and also by cancer stem cells (CSCs)
promote resistance of chemo-sensitive cancer cells (10). Moreover, Mukherjee et al
showed that non-migratory CSCs confer metastatic potential to
non-CSCs (11).
Understanding the origin of intra-tumoral
heterogeneity is one of the greatest challenges nowadays. There is
evidence supporting tumor cell plasticity to microenvironmental
stimuli and to genetic and epigenetic changes. Differentiated tumor
cells seem able to acquire stem cell-like properties, and
conversely, CSCs can lose stemness and form more differentiated
populations (12). This
bi-directionality among highly adaptable cells shapes the tumor
with highly organized cell populations that directly impact disease
evolution and prognosis (13). The
epithelial to mesenchymal transition (EMT) is a conserved embryonic
developmental process that also occurs in cancer. During EMT,
epithelial cells lose their typical adhesive characteristics while
gaining properties more related to mesenchymal mobile cells
(14). The best-understood
biomolecule associated with triggering EMT is TGF-β (transforming
growth factor-β), and mounting evidence supports a TGF-β role in
cancer cell invasion, metastasis, chemoresistance and relapse
(15). EMT has been shown to
correlate with acquisition of a CSC-like phenotype (16,17),
and circulating BrC cells often share characteristics of both
stem-like cells and of EMT cells (18).
In this study, we report dynamic interactions
between BrC cells with different aggressive potential leading to
lateral transmission of aggressive features. We used four BrC cell
lines, two characterized by an epithelial phenotype and the
inability to induce metastasis in mice (MCF-7 and T47D; identified
therein as non-aggressive or NA-BrC cells) and two with a
mesenchymal phenotype and highly metastatic potential (HS578T and
MDA-MB-231; identified as highly aggressive or HA-BrC cells). We
found that aggressive cells promoted an EMT/CSC-like and invasive
phenotype in non-aggressive cells. Altogether, the experimental
observations fit within a molecular regulatory network in which
G-CSF, GM-CSF, IL-8 and MCP-1 inflammatory cytokines induce a
stem-like invasive phenotype in NA-BrC cells, which respond
increasing the activity of the CXCL12/CXCR4/CXCR7 chemokine
signaling axis.
Materials and methods
Cell culture
All cell lines were obtained from the American Type
Culture Collection (ATCC). Culture media and supplements were
obtained from Gibco BRL Life Technologies. BrC cells were estrogen
receptor (ER)-positive cells MCF-7 and T47D, and triple-negative
HS578T and MDA-MB-231. MCF-7 (HTB-22) and HS578T (HTB-126) were
cultured in Dulbecco's modified Eagle's medium (DMEM) with High
Glucose (4.5 g/l) (ref. 11965-092), T47D (HTB-133) with RPMI-1640
medium (ref. 11875-093) and MDA-MB-231 (CRM-HTB-26) were cultured
in Dulbecco's modified Eagle's medium with Nutrient Mixture F-12
(DMEM/F12, ref. 11039-021), the media were supplemented with 10%
fetal bovine serum (FBS) (ref. 16000-044), 100 U/ml penicillin, 100
µg/ml streptomycin, and 0.25 µg/ml fungizone (ref.
15240-062) and were cultured at 37°C in 5% CO2. All BrC
cell lines were submitted to short tandem repeat analysis to verify
the authenticity of each cell line. To obtain conditioned media
2×106 cells of each cell line were plated in 182
cm2 flasks in their standard supplemented medium.
Supernatants were discarded when cultures reached 80% of
confluence, cells were rinsed with PBS 1X (Phosphate Buffered
Saline, Gibco, ref. 20012-027), and then 30 ml of their respective
culture media without FBS was added. Conditioned media were
harvested after incubation for an additional 48 h, centrifuged at
1500 rpm/5 min, aliquoted, and stored at −20°C until use.
Sera from breast cancer patients
Sera from BrC patients and healthy blood donors
(controls) were obtained from the tissues and sera bank of the
Unidad de Investigación en Virología y Cáncer, Hospital Infantil de
México Federico Gómez. This study was approved by the Scientific,
Ethical and Biosecurity Review Boards of the Hospital Infantil de
México Federico Gómez (Comité de Investigación, Comité de Ética en
Investigación and Comité de Bioseguridad). All patients and healthy
controls were prospectively enrolled and were informed about the
nature of the study. Those willing to participate signed a written
informed consent prior to specimen collection and were treated
according to the ethical guidelines and best clinical practice of
our institution. The identity of the participants' names was
anonymized for the duration of the study. Patients were on average
55.5 years old (range: 37-88), 22 were diagnosed with invasive
ductal carcinoma, 2 with lobular and 4 with mixed carcinoma, and
they had not received neoadjuvant therapy at the time of sample
collection. All patients were classified as luminal A, luminal B,
Her-2 positive and triple-negative and as clinical stages I, II,
and III, except for 4 patients for whom we could not obtain this
information. Consecutive numbers were given to the samples as they
were collected.
Induction of the invasive/stemness
phenotype
BrC cells were plated at a density of
3×105 cells/ml/well in 6-well flat bottom culture
plates. Once cells attached to the plate, supernatants were
discarded, cells were rinsed with PBS 1X, and then cultured with 3
ml of conditioned media or with their respective media supplemented
with 5, 10 or 20 ng/ml of human recombinant TGF-β (ref. 100-21) or
100 ng/ml of the following cytokines: G-CSF (ref. 300-23), GM-CSF
(ref. 300-03), IL-8 (ref. 200-08) and MCP-1 (ref. 300-04) (all
cytokines were from PeproTech) individually or all combined in a
cocktail of cytokines. After incubation for 72 h cells were
harvested for analyses. To neutralize the biological activity of
TGF-β of the HA-CMs, 2 µg/ml of the rabbit anti-human
TGF-β1, anti-β2, and anti-β3 neutralizing antibody (anti-TGF-β,
R&D Systems, Inc., ref. MAB1835) were added according to the
guideline provided by the manufacturer.
Immunofluorescence
Cells (3×104) were seeded on coverslips
for 24 h, the invasive/stemness phenotype was experimentally
induced as explained above, after which cells were fixed with
paraformaldehyde 4% for 10 min, and permeabilized with 0.2% Triton
X-100 in PBS for 20 min. Cells were blocked for 1 h and then
stained overnight at 4°C with the primary antibodies: mouse
monoclonal anti-E-cadherin (1:100, Clone: 36/E-cadherin, BD
Biosciences. ref. 610181), rabbit monoclonal anti-vimentin-Alexa
Fluor-594 (1:1000, Clone: EPR3776. ref. ab154207), rabbit
polyclonal anti-Oct4 (1:100, ref. ab18976) or rabbit polyclonal
anti-Sox2 (1:100, ref. ab97959); all antibodies were from Abcam.
After that, cells were incubated for 30 min with the secondary
antibodies: goat anti-mouse-IgG-FITC (1:500 Sigma-Aldrich Co., ref.
F0257) or donkey anti-rabbit-FITC (1:500, Jackson ImmunoResearch
Laboratories, ref. 711-095-152). Finally, nuclei were stained with
DAPI (4′,6-diamidino-2-phenylindole dihydrochloride; Thermo Fisher
Scientific, ref. D1306) for 25 min. Cells were observed using a
fluorescence microscope Olympus BX51 and images were acquired with
a digital camera (Camedia C4040, Olympus). FITC staining intensity
was quantified using the Image Pro Plus software, and the
integrated optical density (IOD) of green cells was obtained.
Flow cytometry
For extracellular staining 3×105 cells
were blocked with an unspecific IgG antibody 1:100 diluted in PBS
1X supplemented with 3% FBS for 15 min and then incubated for 30
min with mouse monoclonal anti-human CXCR3-PE-Cy7 (1:50, Clone:
1C6/CXCR3, ref. 560831), CXCR4-PE-Cy7 (1:50, Clone: 12G5, ref.
560669), CXCR7-APC (1:50, Clone: 10D1-J16, ref. 391406), CCR7-Alexa
647 (1:50, Clone: 150503, ref. 560816), CD44-PE (1:50, Clone:
G44-26, 555479) (all antibodies were from BD Biosciences; except
for anti-CXCR7 that was from BioLegend). Finally, cells were
incubated with 7AAD (7-amino-actinomycin, BD Biosciences, ref.
559925). For intracellular staining cells were blocked with an
unspecific IgG antibody 1:100, diluted in PBS 1X supplemented with
3% FBS, then cells were washed with Phosflow Perm/Wash Buffer I
(1X) (PWB 1X; BD Biosciences, ref. 557885), and fixed and
permeabilized using Cytofix/Cytoperm solution (BD Biosciences, ref.
554722). To block intracellular Fc receptors the cells were again
incubated with the unspecific IgG antibody, and then incubated for
1 h with mouse monoclonal anti-human Sox-2-Alexa 488 (Clone:
245610, BD Biosciences, ref. 560301). All acquisitions were
performed on a FACSAria flow cytometer (Becton Dickinson). Analysis
of flow cytometry data was performed on viable 7-AAD negative cells
(except for Sox-2 staining) using FlowJo V10 software (Tree Star,
Inc.).
Invasion assay
Cells (2×104) were resuspended in 200
µl of their respective media with or without FBS and placed
in the upper chamber of a Transwell [6.5-mm diameter, 8 µm
pore size (Corning Inc.) filled with Matrigel (Corning Inc., ref.
356237)]. Then, the Transwells were placed in a 24-well culture
dish containing 800 µl of their respective media
supplemented with any of the following chemoattractants: 10% FBS,
the different CMs or 100 ng/ml of CXCL12 (C-X-C motif chemokine
ligand 12, also known as SDF-1α, PeproTech, ref. 300-28A). To
neutralize the biological activity of CXCL12 of the HA-CMs, 0.5
µg/ml of the goat anti-human CXCL12/SDF-1 neutralizing
antibody (R&D Systems, Inc., ref. AF-310-NA) were added
according to the guideline provided by the manufacturer. After 24 h
of incubation at 37°C, invasive cells were stained with crystal
violet and observed using a microscope Motic AE31 and images were
acquired with a digital camera (Moticam 5.0 MP). When cells invaded
in groups, reliable cell counts were not possible, in those cases
crystal violet staining intensity was quantified using the Image
Pro Plus software, and the IOD of invading cells was reported.
Analysis of TGF-β and cytokine
profile
The level of TGF-β in the BrC CMs was analyzed with
the human TGF-β Quantikine ELISA kit (R&D Systems, ref. DY240)
according to the protocol provided by the manufacturer. To
determine the cytokine profile of CMs and sera from patients with
BrC, the concentration (in pgs/ml) of G-CSF, GM-CSF, IL-1β, IL-2,
IL-4, IL-6, IL-8, IL-10, IL-17, IL-12 p70, Interferon-α2 (INF-α2),
MCP-1, RANTES, EGF, VEGF, CXCL12 (also known as SDF-1), and
metalloproteinases MMP-1, MMP-2, MMP-7, MMP-9, and MMP-10 were
determined with the MILLIPLEX HCYTOMAG-60K kit (EMD Millipore
Corp.) following the manufacturer's recommended procedure. The
analysis of data was performed in the xPONENT®
Software.
Tumorsphere forming assay
After induction of the invasive/stemness phenotype,
single-cell suspensions were plated in 96-well ultra-low attachment
plates (Costar; Corning Inc.) at 100 cells in 100 µl of
MammoCult medium plus growth factors (Stem Cell Technologies, ref.
05621). Cultures were grown for 7 days. Tumorspheres were observed
and photographed using Motic AE31 microscope with a digital camera
(Moticam 5.0 MP). Spheres >50 µm were counted and
graphed.
Statistical analysis
The Prism software version 5.01 (GraphPad) was used
for statistical analysis. A one-way analysis of variance (ANOVA)
test with the Tukey as post hoc test was applied to more than two
groups of data, and the non-parametric Kruskal-Wallis test with
Dunnett's as post hoc test was applied to more than two groups in
which the data lack normality and/or homogeneity of variance.
Significant P-values are indicated as follows: ≤0.05 by one
asterisk *, ≤0.01 by two asterisks ** and ≤
0.001 by three asterisks ***.
Results
Aggressive breast cancer cells promote
loss of E-cadherin and invasive features in non-aggressive
cells
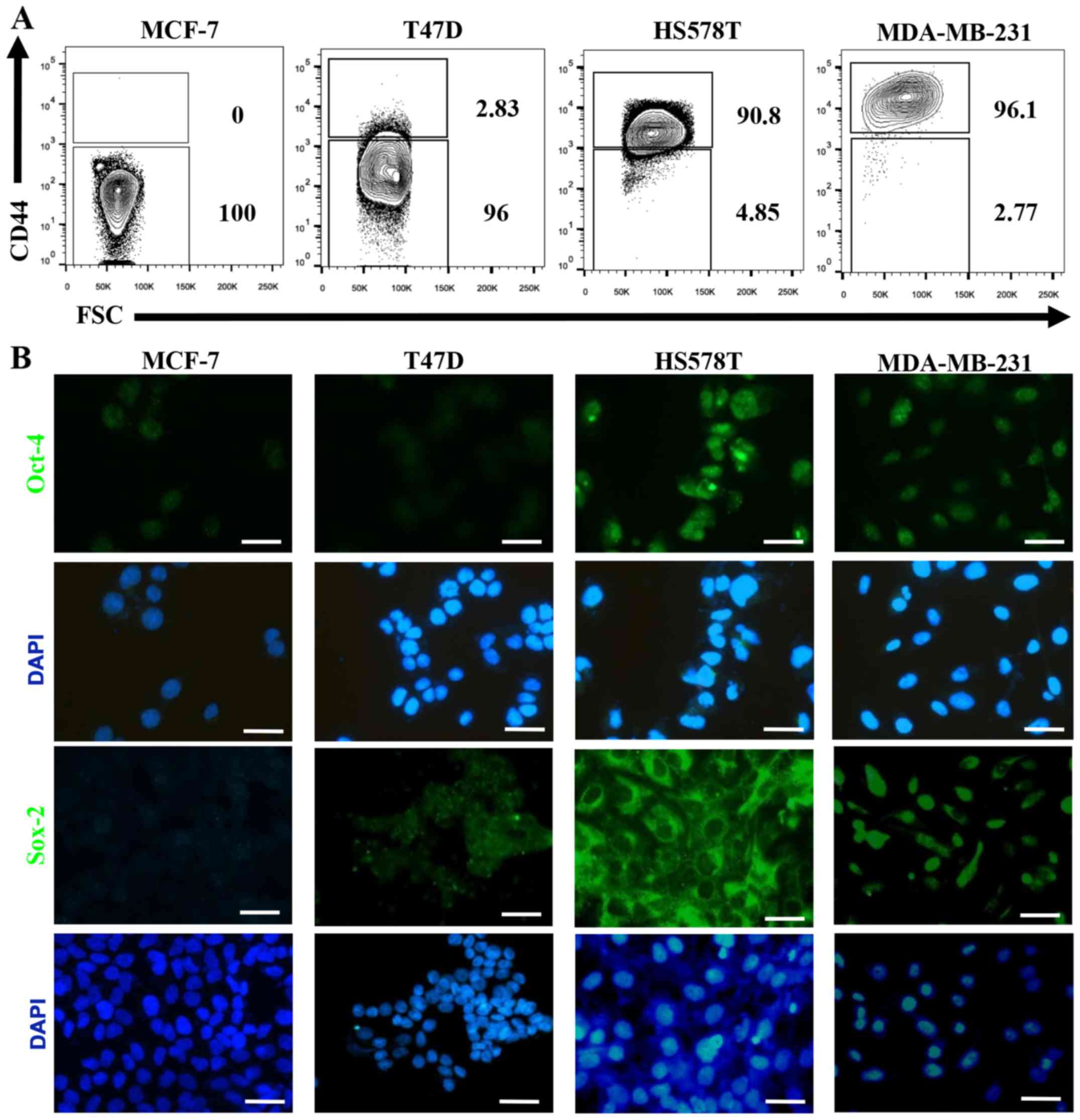
We defined MCF-7 and T47D ER-positive cells as
NA-BrC cells and HS578T and MDA-MB-231 triple-negative cells as
HA-BrC cells based on expression of the EMT markers E-cadherin and
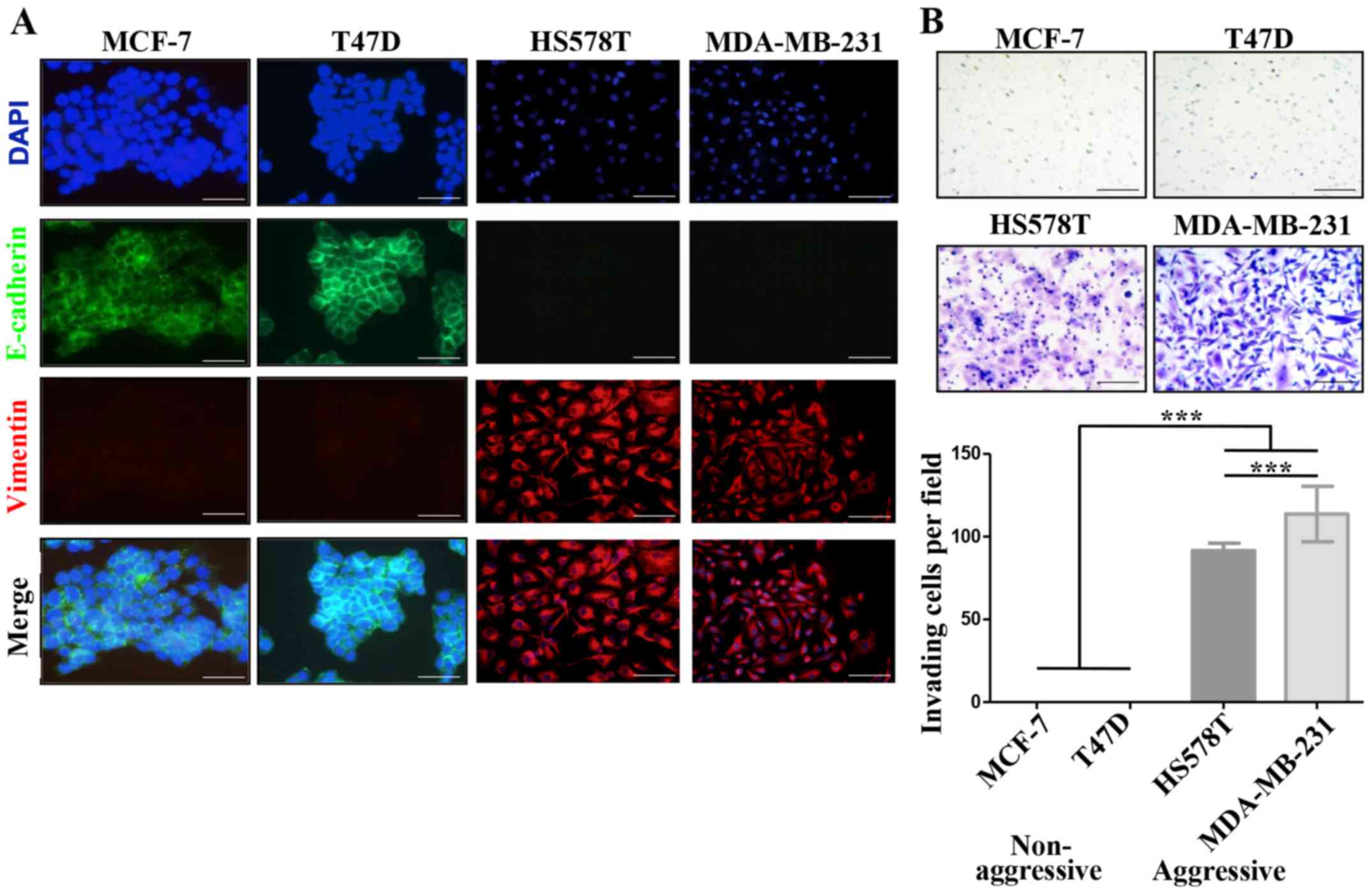
vimentin and their invasive properties (Fig. 1A and B). In agreement, the former
cell lines are non-metastatic in mouse, while the latter are highly
metastatic in similar experimental conditions (19). Since during metastasis cellular
communication is importantly mediated by secreted factors (20), we assessed whether NA-BrC cells
cultured with conditioned media (CM) of HA-BrC cells, and
vice-versa, modify each others aggressive characteristics. Fig. 1C shows that MCF-7 cells cultured
with the HS578T CM reduced E-cadherin levels from a basal IOD of
22,000 to an IOD of 8,000, while with the CM of MDA-MB-231 cells
E-cadherin levels were undetectable. This reduction of E-cadherin
levels correlated well with an acquired capacity of cells to
invade, observing an average of 10 and 30 MCF-7 invading
cells/field when cultured in HS578T and MDA-MB-231 CMs,
respectively. The same was true for T47D cells, finding a reduction
of the E-cadherin IOD from 50,000 to <500, and 15–20 invading
cells/field after culture with CM from aggressive cells (Fig. 1D). On the contrary, no significant
changes were observed in HA-BrC cells cultured with CM derived from
NA-BrC cells (data no shown). Thus, these results support the
capacity of aggressive tumor cells to laterally transmit aggressive
features into non-aggressive cells, characterized by a partial EMT
phenotype with reduction of E-cadherin but not significant
induction of vimentin, and increased invasiveness.
CXCL12-CXCR4/CXCR7 axis in the
inducible-invasive phenotype
CXCL12 is an essential chemokine often involved in
the migration of BrC cells (21);
similarly CXCL12 receptors CXCR4 and CXCR7, but also CXCR3 and CCR7
mediate metastasis of BrC cells (22). To further explore the mechanism by
which HA-BrC cells induce the migration of NA-BrC cells, we
analyzed the concentration of CXCL12 in the CM of the BrC cells and
the expression levels of the chemokine receptors on the NA-BrC
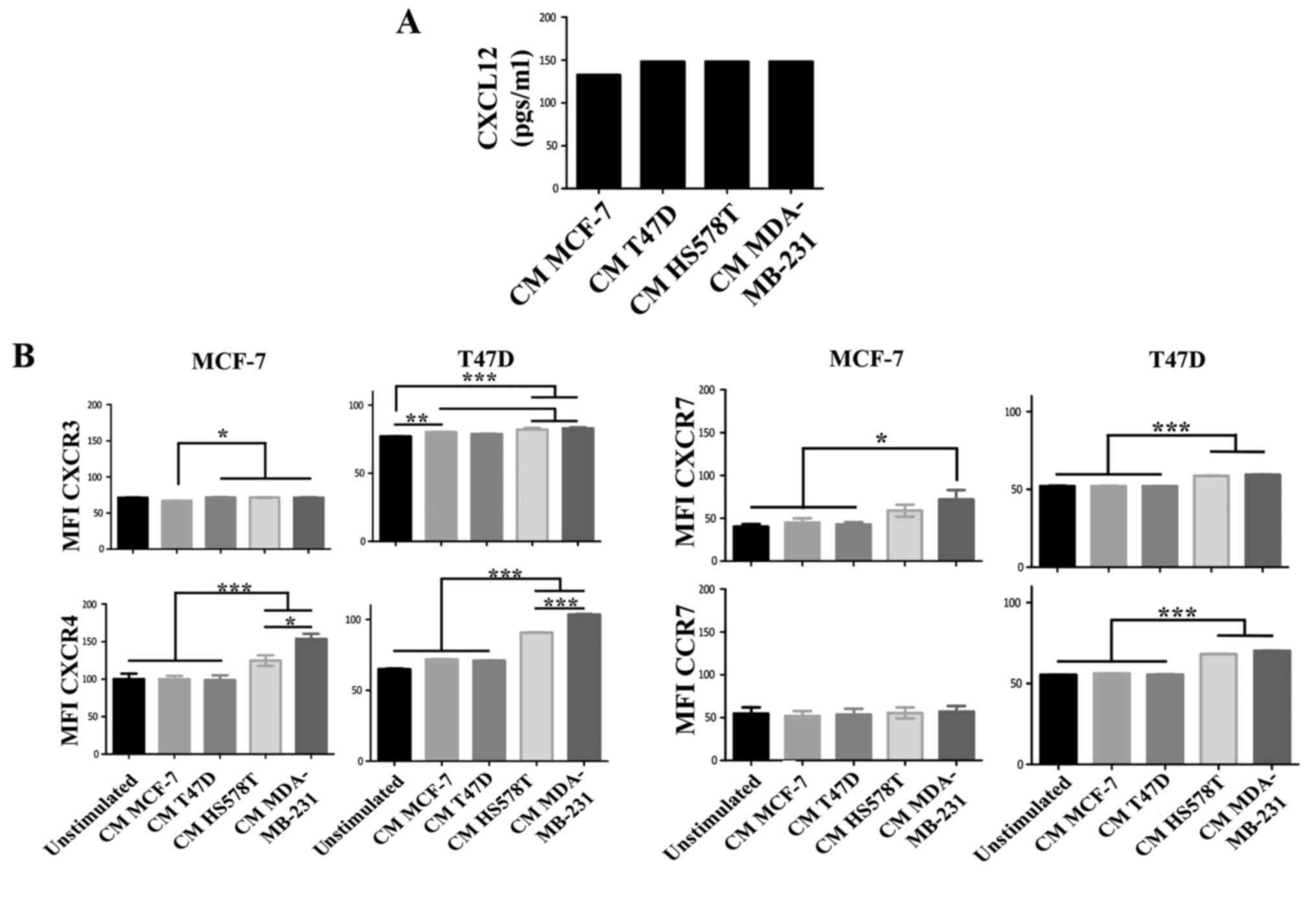
cells treated with the CM of HA-BrC cells. Although high levels of
CXCL12 were found in the CM of the BrC cell lines, no significant
differences were found between aggressive and non-aggressive cells
(Fig. 2A). Of the chemokine
receptors, we found a discrete and more heterogeneous result with
CXCR3, contrary to CXCR4, CXCR7 and CCR7 that significantly
increased expression solely in response to HA-BrC CMs; CXCR4 and
CXCR7 changed in both NA-BrC cells, while CCR7 only on induced T47D
cells (Fig. 2B). Since CXCR4 and
CXCR7 are CXCL12 receptors, CXCL12 was tested as chemoattractant in
invasion assays.
We found that both induced invasive NA-BrC cells
migrated in response to this chemokine (Fig. 2C). To address whether the CM of
aggressive cells could also attract cells, NA-BrC cells were
cultured with the HA-BrC CMs as before, and then subjected to an
invasive assay in which the same CM used to stimulate them was
placed as chemoattractant in the lower chamber of the transwell
cameras. We observed that the induced invasive MCF-7 and T47D cells
were also migrating in response to the HA-BrC CMs, with MCF-7 cells
seeming more responsive than T47D cells. In addition, the CM of
MDA-MB-231 cells gave a more potent response than the CM of HS578T
cells, with an average IOD/field of 900,000 for MCF-7 and 320,000
for T47D invading cells (Fig. 2D).
Cells did not invade in the absence of CXCL12 (data not shown). To
confirm that CXCL12 had a role in the invasive phenotype, invasion
assays were performed on the induced invasive MCF-7 and T47D cells
using the CM of the BrC cell lines, either alone or plus a CXCL12
neutralizing antibody. We observed that the stimulated NA-BrC cells
decreased their invasiveness in the presence of the inhibitory
antibody (Fig. 2E). These data
support that the CM of HA-BrC cells induce migratory properties on
NA-BrC cells together with upregulation of CXCR4 and CXCR7
expression, and that these induced invasive NA-BrC cells then move
in response to CXCL12.
A TGF-β independent but G-CSF, GM-CSF,
IL-8 and MCP-1 dependent response
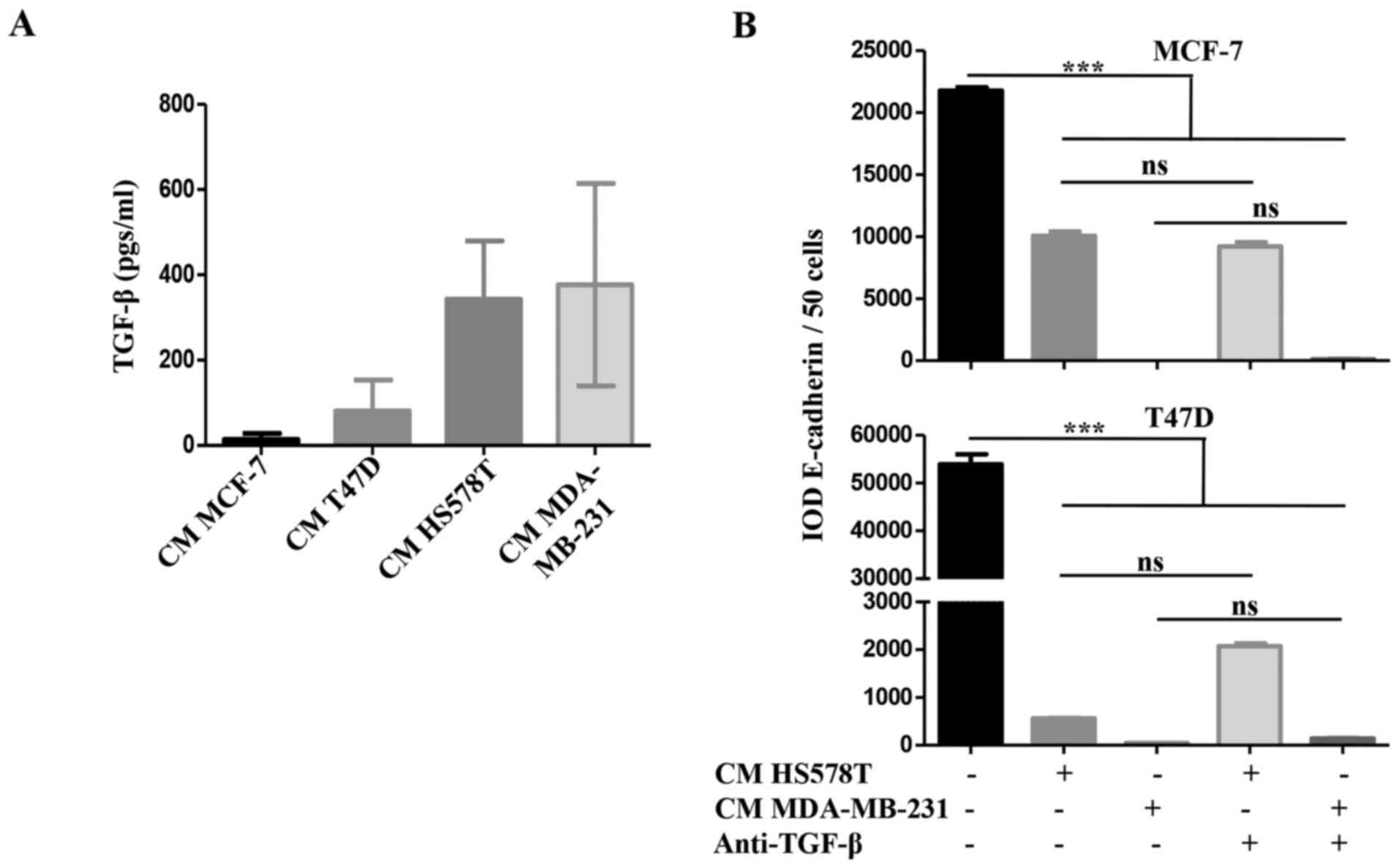
We addressed whether TGF-β was responsible for the
inducible-invasive phenotype. When we quantified TGF-β levels in
the CM of the BrC cell lines, we found a higher concentration of
this cytokine in the CM of the HA-BrC cell lines (average of 343
and 377 pgs/ml in HS578T and MDA-MB-231 cells, respectively) than
in the NA-BrC CMs (80 and 14 pgs/ml in T47D and MCF-7 cells,
respectively) (Fig. 3A). We then
used a TGF-β neutralizing antibody to assess whether this cytokine
was responsible for the inducible-invasive phenotype of NA-BrC
cells. To do this, NA-BrC cells were stimulated for 72 h with CM
from HA-BrC cells in the presence of the TGF-β neutralizing
antibody. Surprisingly, when expression of E-cadherin was assessed
we still observed a significant reduction of the E-cadherin IOD
promoted by the HA-BrC CMs (Fig.
3B). Similarly, the stimulated NA-BrC cells remain invasive in
spite of the presence of the neutralizing antibody (Fig. 3C). Additionally, we added exogenous
TGF-β at various concentrations to MCF-7 and T47D cells for 72 h,
with no observed variations in E-cadherin or vimentin expression.
Accordingly, we did not observe a TGF-β-induced invasiveness
(Fig. 3D). TGF-β is known to
induce its own expression in Caski cervical cancer cells (23). Exogenous TGF-β increased its own
gene expression in Caski cells, and the neutralizing antibody
abolished this induction, indicating that both recombinant TGF-β
and the anti-TGF-β antibody were functional (data no shown).
We then analyzed for the presence of the following
growth factors, cytokines, chemokines and matrix metalloproteinases
(MMPs) in the CM of the BrC cell lines, which have been previously
associated with tumor cell invasion and/or EMT (24,25):
Granulocyte-colony stimulating factor (G-CSF), Granulocyte
macrophage-colony stimulating factor (GM-CSF), IL-1β, IL-2, IL-4,
IL-6, IL-8, IL-10, IL-17, IL-12p70, Interferon-α2 (INF-α2),
Monocyte chemoattractant protein-1 (MCP-1), Regulated on
activation, normal T-cell expressed and secreted (RANTES, also
known as CCL5), epidermal growth factor (EGF), Vascular endothelial
growth factor (VEGF), MMP-1, MMP-2, MMP-7, MMP-9 and MMP-10.
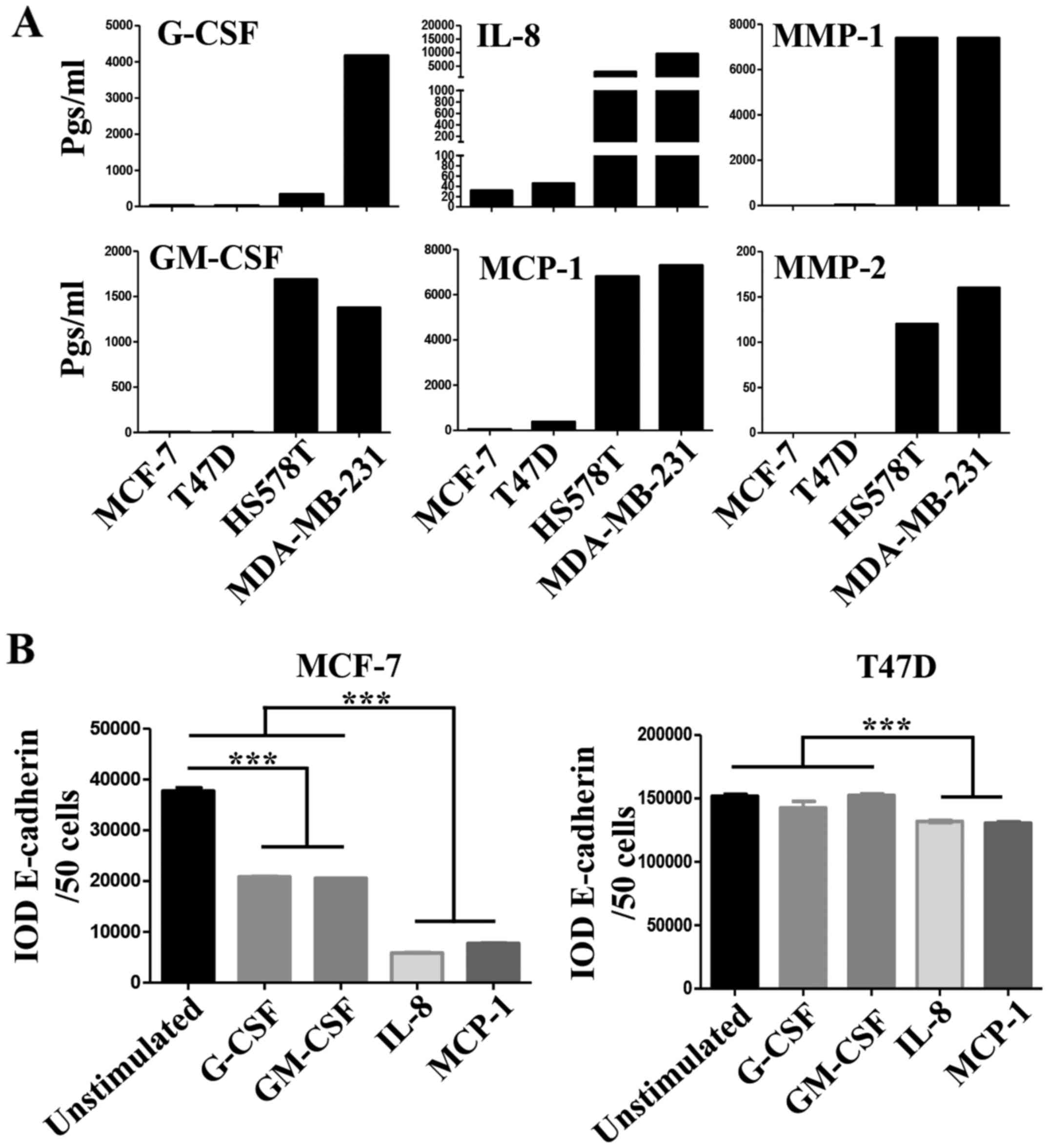
Important differences between the CMs of NA- and HA-BrC cells were
found in the levels of G-CSF, GM-CSF, IL-8 and MCP-1 (Fig. 4A). These cytokines are known to
induce differentiation, proliferation and activation of myeloid
cells recruited to the tumor microenvironment, and often, they also
induce autocrine activation of tumor cells (26-28).
To assess whether these factors participate in the
inducible-invasive phenotype, MCF-7 and T47D cells were cultured in
media supplemented with G-CSF, GM-CSF, IL-8 and MCP-1 for 72 h. We
found that MCF-7 cells were highly responsive to these cytokines
with G-CSF and GM-CSF inducing a partial loss, and IL-8 and MCP-1
an almost complete loss of E-cadherin levels (Fig. 4B left plot), we also observed that
the expression of CXCR4 significantly increased in response to all
cytokines while CXCR7 increased only in response to IL-8 and MCP-1
(Fig. 4C left plots), which
closely correlated with induction of invasiveness (Fig. 4D left panels). Overall, these data
show a great correlation between IL-8/MCP-1-induction of CXCR7 and
E-cadherin reduction/increased invasion.
Somehow the results obtained with T47D cells were
more complex. We only observed a very discreet but significant
reduction of E-cadherin levels on T47D cells, and specifically upon
stimulation with IL-8 and MCP-1 (Fig.
4B right plot). We also observed an increase of CXCR4
expression in response to all cytokines, while CXCR7 increased
expression only in response to IL-8 and MCP-1 (Fig. 4C right plots). However, the T47D
invasion assay was not as clear, since G-CSF, GM-CSF and MCP-1 were
able to induce invasion but IL-8 was not (Fig. 4D right panels). Of note, FBS was a
more powerful chemoattractant of cytokine-activated T47D cells
(Fig. 4E), while CXCL12 was for
MCF-7 cells (Fig. 4D), even in
cells stimulated with IL-8. A cocktail with all four cytokines did
not further promote E-cadherin loss or increased invasion of NA-BrC
cells (data not shown). Vimentin expression was not induced in
these experimental conditions as we have previously observed with
the HA-BrC CMs (data not shown and Fig. 1C and D). Overall these data support
an important role for GM-CSF, G-CSF, IL-8 and MCP-1 on the
inducible-invasive phenotype of NA-BrC cells, with an IL-8 and
MCP-1 stronger activity on MCF-7 cells and a more variegated effect
on T47D cells. Of note, these data point out that even though MCF-7
and T47D cells are highly plastic reacting to signals communicated
by aggressive tumor cells, there are significant differences
between the mechanisms of induced-aggressive behavior displayed by
each cell line. We tested the concentration of CXCL12, G-CSF,
GM-CSF, IL-8 and MCP-1 cytokines and of MMP-1 and MMP-2
metalloproteinases in sera of BrC patients (see Table I for the clinical characteristics
of patients) and healthy controls, finding detectable levels of all
analytes tested. Since we could only obtain five sera of each BrC
subtype no statistical analysis was performed (Fig. 4F).
 | Table IClinical characteristics of the
patients. |
Table I
Clinical characteristics of the
patients.
| Serum from
patients | Histological
subtype | TNM staging | Clinical stage | Molecular
classification |
|---|
| UIVC-IDC-13 | IDC | T2, N0, M0 | IIA |
Triple-negative |
| UIVC-IDC-14 | IDC | T1, N0, M0 | I | Luminal B |
| UIVC-IDC-15 | IDC | T1, N0, M0 | I | Luminal A |
| UIVC-IDC-16 | IDC | T1, N0, M0 | I |
Triple-negative |
| UIVC-IDC-17 | IDC | WD | WD | Her-2 |
| UIVC-IDC-18 | IDC | WD | WD | Luminal A |
| UIVC-IDC-19 | IDC | T2, N0, M0 | IIA |
Triple-negative |
| UIVC-IDC-20 | IDC | WD | WD | Luminal B |
| UIVC-IDC-21 | IDC | WD | WD | Her-2 |
| UIVC-IDC-22 | IDC | T1, N0, M0 | I | Luminal A |
| UIVC-IDC-23 | IDC | T1, N1, M0 | IIA | Luminal A |
| UIVC-IDC-24 | IDC | T1, N0, M0 | I | Luminal A |
| UIVC-IDC-25 | IDC | T2, N0, M0 | IIA |
Triple-negative |
| UIVC-IDC-26 | IDC | T2, N0, M0 | IIA | Her-2 |
| UIVC-IDC-27 | IDC | T2, N0, M0 | IIA | Luminal A |
| UIVC-IDC-28 | IDC | T2, N1, M0 | IIB | Her-2 |
| UIVC-IDC-29 | IDC | T1, N0, M0 | I | Luminal A |
| UIVC-IDC-30 | IDC | T3, N0, M0 | IIB | Luminal A |
| UIVC-IDC-31 | IDC | T2, N0, M0 | IIA | Luminal A |
| UIVC-IDC-32 | IDC | T1, N0, M0 | I | Her-2 |
| UIVC-IDC-33 | IDC | T3, N1, M0 | IIIA | Luminal A |
| UIVC-IDC-34 | IDC | T2, N0, M0 | IIA | Luminal B |
| UIVC-LC-2 | ILC | T3, N1, M0 | IIIA |
Triple-negative |
| UIVC-LC-3 | ILC | T2, N1, M0 | IIB | Luminal A |
| UIVC-MC-2 | MC | T1, N1, M0 | IIA | Luminal B |
| UIVC-MC-3 | MC | T3, N1, M0 | IIIA | Luminal B |
| UIVC-MC-4 | MC | T2, N0, M0 | IIA | Her-2 |
| UIVC-MC-5 | MC | T2, N0, M0 | IIA | Luminal A |
CD44, Oct4 and Sox2 are upregulated in
induced-invasive cells
The hyaluronan receptor (also known as CD44) has
been widely associated with a CSC-like phenotype, and Oct-4 and
Sox-2 are essential transcription factors involved in maintenance
of pluripotency during embryogenesis, and their induced-expression
has been evidenced in several types of cancer upon acquisition of
metastatic potential and EMT (29,30).
When we evaluated the expression of these CSC markers on the BrC
cell lines we found that CD44 positive cells were not (MCF-7=0% of
positive cells) or rarely represented (T47D=2.83%) on NA-BrC cells,
while they were highly represented on HS578T (90.8%) and MDA-MB-231
(96.1%) HA-BrC cells (Fig. 5A). A
similar observation was made for Oct-4 and Sox-2 (Fig. 5B). We addressed whether the
inducible-invasive trait correlated with acquisition of these stem
markers. We found that both the frequency of CD44 positive cells
and the MFI of CD44 expression were induced upon treatment with the
HA-BrC CMs in both MCF-7 and T47D NA-BrC cells (Fig. 5C and D, respectively). T47D cells
were the most sensitive cells to stimulation as more than half of
the cells expressed CD44 upon treatment with the MDA-MB-231 CM. We
also observed a significantly increased expression of Oct-4 and
Sox-2 in MCF-7 cells upon stimulation with the CM from HA-BrC cell
lines (Fig. 5E). T47D cells
significantly changed the expression of Sox-2 upon stimulation,
while the levels of Oct-4 remained low (Fig. 5F).
A more quantitative analysis of the frequency of
Sox-2 expressing cells by flow cytometry confirmed the results
obtained with the immunofluorescence analyses (Fig. 5G). Since the formation of
tumorspheres is a model of CSC seeding and expansion (31), we addressed the BrC cell lines
basal and induced potential of formation of tumorspheres. We
observed that all the cell lines exhibited similar capacities of
formation of tumorospheres (Fig.
5H), the only difference found was the morphology of the
spheres, with HA-BrC cells forming less adherent spheres that
resemble cell aggregates, typical of aggressive cell lines with low
expression of adhesion proteins (32). When NA-BrC cells were treated with
the CM of HA-BrC cells, the frequency and morphology of the
tumor-spheres remained unchanged; however, spheres of increased
size were observed (Fig. 5I).
Taken together all these data show that the inducible-invasive
trait correlates with acquisition of a CSC-like phenotype defined
by expression of CD44, Oct-4 and Sox-2 stemness markers, and also
with the formation of larger tumorspheres in low adherent
plates.
Discussion
The original conception of cancer initiation and
progression highlighted the importance of accumulation of genetic
mutations, with tumors exhibiting greater genetic heterogeneity
also exhibiting an increased risk to display more aggressive
features. More recent studies have also highlighted the capacity of
the tumor to communicate with non-tumor cells within the tumor
microenvironment as the source of aggression (5). In this study, we evidenced a
potential mechanism of paracrine communication between tumor cells
that results in lateral transmission of aggressive features. The
induced aggressive characteristics consist of acquisition of a
partial EMT phenotype with loss of E-cadherin but without gain of
vimentin expression, gain of CSC markers CD44, Sox-2 and Oct-4,
increased expression of CXCL12 receptors CXCR4 and CXCR7, and
increased invasiveness in response to CXCL12. Altogether, these
mechanisms of tumor communication may facilitate the appearance of
new clones with novel functions, further extending the clonal
heterogeneity that increases the tumor aggressive potential without
relying on genetic irreversible mutations.
The clonal evolution model of tumors points out that
distinct genetic clones exhibit differential fitness, and only
those clones with specific advantages and under particular
selective pressures will be maintained, favoring the progression of
disease in a kind of Darwinian evolution competition (33). In 2014, Marusyk et al
proposed that interactions between rare and affluent tumor clones
favored the emergence of clones with novel phenotypes and functions
allowing the tumor to adapt to microenvironmental changes (34). Other studies support intra-clonal
communication and cooperation, particularly among metastatic and
non-metastatic clones (6,7,11),
adding another layer of complexity to the origin and evolution of
tumors. The plasticity of the tumor cell has been extensively
studied, with the EMT at the center of this plasticity. More recent
evidence support that cancer cells undergoing EMT also increase
expression of stem markers, and tumors in which the EMT/stemness
programs are active, are also more invasive and metastatic denoting
cancers with the worst clinical outcomes (16,17,24,35,36).
How the EMT and stemness programs support tumor heterogeneity and
intra-clonal coexistence to facilitate tumor maintenance remains as
one of the most challenging puzzles in cancer biology.
Interestingly, although TGF-β is one of the best characterized EMT
triggers (15), we could not find
any evidence of a TGF-β participation in the inducible invasive
stem-like phenotype.
To our knowledge, Mani et al in 2008 were the
first to describe a strong correlation between EMT and stemness
(16), laying the bases for a
novel understanding of tumor plasticity and tumor aggression.
Today, mounting evidence supports that association; for instance,
BrCs with a high density of CD44- positive cells are specifically
associated with reduced disease-free survival (37). Expression of Oct-4 and Sox-2 is
also associated with poor clinical outcomes in BrC patients
(38,39). While we were working in this study,
Mukherjee et al reported that CSCs with different capacities
of migration co-exist within primary tumors and in MCF-7
mamospheres. Low migrating inner core CSCs have the capacity to
induce migratory properties into outer core non-CSCs through
paracrine secretion of EGF, TGF-β1, VEGF and IL-6 (11). In agreement with our study, induced
non-CSCs showed increased expression of stemness markers CD44,
Oct-4 and Sox-2, and invasion was marked by upregulation of
CXCR4. We observed an increased expression of CXCR4 and
CXCR7 on induced-NA-BrC cell lines, which promoted cell migration
in response to CXCL12, particularly in induced MCF-7 cells. In the
case of T47D there is not a clear correlation that could be
explained because contrary to MCF-7 cells, T47D cells also
upregulate CXCR3 and CCR7 expression, as it can be observed in
Fig. 2B. Still, the
CXCR4/CXCR7/CXCL12 axis is widely documented as a potent promoter
of invasion and metastasis in several types of cancer, and most
likely also has an important role in the promotion of invasion of
the induced-NA BrC cells (40,41).
Tumors with high density of CSCs are characterized
by high expression of inflammatory mediators (42,43).
We found that HA-BrC cell lines secret high levels of G-CSF,
GM-CSF, MCP-1 and IL-8. Of them, MCP-1 and IL-8 were potent
inducers of invasiveness of MCF-7 cells, and G-CSF, GM-CSF, IL-8
and MCP-1 of T47D cells. MCP-1 and IL-8 are considered critical
pro-tumoral cytokines mainly because of their capacity to shape the
TME by attracting immune cell populations (44,45).
We have also observed that aggressive tumor cells are particularly
proficient to attract monocytes/macrophages through secretion of
GM-CSF and MCP-1, and monocyte/macrophage co-cultivation with tumor
cells further increase secretion of IL-8 and IL-1β (46). Importantly, this study supports the
capacity of tumor cells to secrete pro-inflammatory cytokines
promoting stemness and invasion independent of other cellular
components of the tumor stroma. In agreement, other studies support
that autocrine overexpression of MCP-1 and IL-8 promotes tumor cell
proliferation, migration, chemoresistance, metastasis and disease
relapse (27,28,47).
In patients with breast and prostate cancers MCP-1 overexpression
correlates with poor prognosis (48,49),
and autocrine regulation of MCP-1 is associated with EMT,
immunosuppression and metastasis (50). Blocking MCP-1 in triple-negative
BrC decreased the frequency and self-renewal potential of breast
CSCs (51). Other studies support
an association between IL-8, MCP-1 and MMPs, and induction of CSCs
and metastasis (24,51,52).
Of note, we also observed high levels of MMP-1 and MMP-2 secreted
by HA-BrC cells.
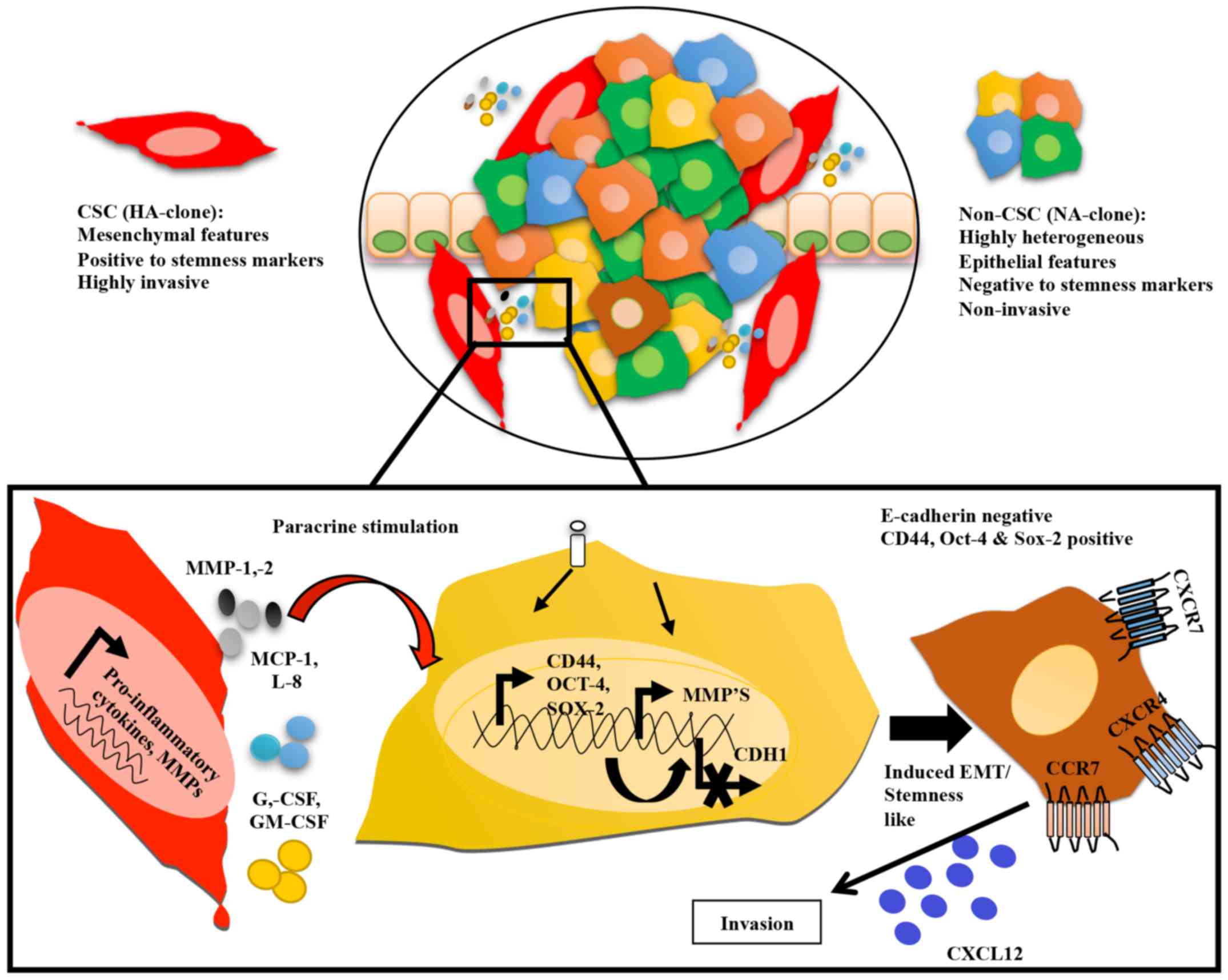
In conclusion, our results support a model in which
in a heterogeneous disease like cancer, highly aggressive tumor
clones communicate with less aggressive clones through paracrine
mediators, such as IL-8, MCP-1, G-CSF, GM-CSF and MMPs,
transferring aggressive features in line with a CSC-like phenotype
and increased potential for invasion (Fig. 6). Although TGF-β remains the
best-understood signal accountable for tumor cell plasticity, we
could not find TGF-β participation in the inducible-invasive
phenotype. Further studies should look into these pro-inflammatory
factors in carefully staged BrC patients, comparing metastatic
tumors against non-metastatic tumors or comparing other relevant
clinical parameters, such as resistance to treatment, disease
relapse and overall survival. We were unable to obtain the clinical
data in our series of patients. Almost all the patients included in
this study were classified as stage I and II, with only three
patients in stage III and none in stage IV. Understanding the
mechanisms guiding intra-tumoral heterogeneity, cell plasticity and
tumor aggressiveness will provide better targets for diagnosis,
prognosis and therapeutic strategies.
Acknowledgments
N.A.E.-S. is a doctoral student from Programa de
Doctorado en Ciencias Biomédicas, Universidad Nacional Autónoma de
México (UNAM) and received fellowship 231663 from CONACYT.
N.A.E.-S. also acknowledges the financial support provided by the
Mexican Institute of Social Security (IMSS, ref. 2010-017). J.C.B.
acknowledges the scholarship provided by CONACyT and IMSS. This
work was supported by CONACyT FONSEC SSA/IMSS/ISSSTE Project no.
233061 and by Fondo de Apoyo a la investigación, Hospital Infantil
de México Federico Gómez (Project no. HIM-2014-053) to E.M.F.-P.
The authors wish to thank Dr Abigail Morales Sánchez for her input
during the statistical analysis.
References
|
1
|
Bray F, Ren JS, Masuyer E and Ferlay J:
Global estimates of cancer prevalence for 27 sites in the adult
population in 2008. Int J Cancer. 132:1133–1145. 2013. View Article : Google Scholar
|
|
2
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar
|
|
3
|
Sørlie T, Perou CM, Tibshirani R, Aas T,
Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey
SS, et al: Gene expression patterns of breast carcinomas
distinguish tumor subclasses with clinical implications. Proc Natl
Acad Sci USA. 98:10869–10874. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ng CKY, Pemberton HN and Reis-Filho JS:
Breast cancer intratumor genetic heterogeneity: Causes and
implications. Expert Rev Anticancer Ther. 12:1021–1032. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chimal-Ramírez GK, Espinoza-Sánchez NA and
Fuentes-Pananá EM: Protumor activities of the immune response:
Insights in the mechanisms of immunological shift, oncotraining,
and oncopromotion. J Oncol. 2013:8359562013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Miller FR, Miller BE and Heppner GH:
Characterization of metastatic heterogeneity among subpopulations
of a single mouse mammary tumor: Heterogeneity in phenotypic
stability. Invasion Metastasis. 3:22–31. 1983.PubMed/NCBI
|
|
7
|
Calbo J, van Montfort E, Proost N, van
Drunen E, Beverloo HB, Meuwissen R and Berns A: A functional role
for tumor cell heterogeneity in a mouse model of small cell lung
cancer. Cancer Cell. 19:244–256. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu M, Pastor-Pareja JC and Xu T:
Interaction between Ras(V12) and scribbled clones induces tumour
growth and invasion. Nature. 463:545–548. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cleary AS, Leonard TL, Gestl SA and
Gunther EJ: Tumour cell heterogeneity maintained by cooperating
subclones in Wnt-driven mammary cancers. Nature. 508:113–117. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bose D, Zimmerman LJ, Pierobon M,
Petricoin E, Tozzi F, Parikh A, Fan F, Dallas N, Xia L, Gaur P, et
al: Chemoresistant colorectal cancer cells and cancer stem cells
mediate growth and survival of bystander cells. Br J Cancer.
105:1759–1767. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mukherjee S, Manna A, Bhattacharjee P,
Mazumdar M, Saha S, Chakraborty S, Guha D, Adhikary A, Jana D,
Gorain M, et al: Non-migratory tumorigenic intrinsic cancer stem
cells ensure breast cancer metastasis by generation of CXCR4(+)
migrating cancer stem cells. Oncogene. 35:4937–4948. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chaffer CL, Marjanovic ND, Lee T, Bell G,
Kleer CG, Reinhardt F, D'Alessio AC, Young RA and Weinberg RA:
Poised chromatin at the ZEB1 promoter enables breast cancer cell
plasticity and enhances tumorigenicity. Cell. 154:61–74. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shackleton M, Quintana E, Fearon ER and
Morrison SJ: Heterogeneity in cancer: Cancer stem cells versus
clonal evolution. Cell. 138:822–829. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Massagué J: TGFbeta in cancer. Cell.
134:215–230. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Santisteban M, Reiman JM, Asiedu MK,
Behrens MD, Nassar A, Kalli KR, Haluska P, Ingle JN, Hartmann LC,
Manjili MH, et al: Immune-induced epithelial to mesenchymal
transition in vivo generates breast cancer stem cells. Cancer Res.
69:2887–2895. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Aktas B, Tewes M, Fehm T, Hauch S, Kimmig
R and Kasimir-Bauer S: Stem cell and epithelial-mesenchymal
transition markers are frequently overexpressed in circulating
tumor cells of metastatic breast cancer patients. Breast Cancer
Res. 11:R462009. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lacroix M and Leclercq G: Relevance of
breast cancer cell lines as models for breast tumours: An update.
Breast Cancer Res Treat. 83:249–289. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
McAllister SS and Weinberg RA: The
tumour-induced systemic environment as a critical regulator of
cancer progression and metastasis. Nat Cell Biol. 16:717–727. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Luker KE and Luker GD: Functions of CXCL12
and CXCR4 in breast cancer. Cancer Lett. 238:30–41. 2006.
View Article : Google Scholar
|
|
22
|
Ali S and Lazennec G: Chemokines: Novel
targets for breast cancer metastasis. Cancer Metastasis Rev.
26:401–420. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
García-Rocha R, Moreno-Lafont M,
Mora-García ML, Weiss-Steider B, Montesinos JJ, Piña-Sánchez P and
Monroy-García A: Mesenchymal stromal cells derived from cervical
cancer tumors induce TGF-β1 expression and IL-10 expression and
secretion in the cervical cancer cells, resulting in protection
from cytotoxic T cell activity. Cytokine. 76:382–390. 2015.
View Article : Google Scholar
|
|
24
|
Chimal-Ramírez GK, Espinoza-Sánchez NA and
Fuentes-Pananá EM: A role for the inflammatory mediators Cox-2 and
metalloproteinases in cancer stemness. Anticancer Agents Med Chem.
15:837–855. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mukaida N, Sasaki S and Baba T: Chemokines
in cancer development and progression and their potential as
targeting molecules for cancer treatment. Mediators Inflamm.
2014:1703812014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Aliper AM, Frieden-Korovkina VP, Buzdin A,
Roumiantsev SA and Zhavoronkov A: A role for G-CSF and GM-CSF in
nonmyeloid cancers. Cancer Med. 3:737–746. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Soria G, Ofri-Shahak M, Haas I,
Yaal-Hahoshen N, Leider-Trejo L, Leibovich-Rivkin T, Weitzenfeld P,
Meshel T, Shabtai E, Gutman M, et al: Inflammatory mediators in
breast cancer: Coordinated expression of TNFα & IL-1β with CCL2
& CCL5 and effects on epithelial-to-mesenchymal transition. BMC
Cancer. 11:1302011. View Article : Google Scholar
|
|
28
|
Schadendorf D, Möller A, Algermissen B,
Worm M, Sticherling M and Czarnetzki BM: IL-8 produced by human
malignant melanoma cells in vitro is an essential autocrine growth
factor. J Immunol. 151:2667–2675. 1993.PubMed/NCBI
|
|
29
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schoenhals M, Kassambara A, De Vos J, Hose
D, Moreaux J and Klein B: Embryonic stem cell markers expression in
cancers. Biochem Biophys Res Commun. 383:157–162. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Weiswald LB, Bellet D and Dangles-Marie V:
Spherical cancer models in tumor biology. Neoplasia. 17:1–15. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Manuel Iglesias J, Beloqui I,
Garcia-Garcia F, Leis O, Vazquez-Martin A, Eguiara A, Cufi S, Pavon
A, Menendez JA, Dopazo J, et al: Mammosphere formation in breast
carcinoma cell lines depends upon expression of E-cadherin. PLoS
One. 8:e772812013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Greaves M and Maley CC: Clonal evolution
in cancer. Nature. 481:306–313. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Marusyk A, Tabassum DP, Altrock PM,
Almendro V, Michor F and Polyak K: Non-cell-autonomous driving of
tumour growth supports sub-clonal heterogeneity. Nature. 514:54–58.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kasimir-Bauer S, Hoffmann O, Wallwiener D,
Kimmig R and Fehm T: Expression of stem cell and
epithelial-mesenchymal transition markers in primary breast cancer
patients with circulating tumor cells. Breast Cancer Res.
14:R152012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Oon ML, Thike AA, Tan SY and Tan PH:
Cancer stem cell and epithelial-mesenchymal transition markers
predict worse outcome in metaplastic carcinoma of the breast.
Breast Cancer Res Treat. 150:31–41. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
McFarlane S, Coulter JA, Tibbits P,
O'Grady A, McFarlane C, Montgomery N, Hill A, McCarthy HO, Young
LS, Kay EW, et al: CD44 increases the efficiency of distant
metastasis of breast cancer. Oncotarget. 6:11465–11476. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang D, Lu P, Zhang H, Luo M, Zhang X, Wei
X, Gao J, Zhao Z and Liu C: Oct-4 and Nanog promote the
epithelial-mesenchymal transition of breast cancer stem cells and
are associated with poor prognosis in breast cancer patients.
Oncotarget. 5:10803–10815. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ben-Porath I, Thomson MW, Carey VJ, Ge R,
Bell GW, Regev A and Weinberg RA: An embryonic stem cell-like gene
expression signature in poorly differentiated aggressive human
tumors. Nat Genet. 40:499–507. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
40
|
Singh AK, Arya RK, Trivedi AK, Sanyal S,
Baral R, Dormond O, Briscoe DM and Datta D: Chemokine receptor
trio: CXCR3, CXCR4 and CXCR7 crosstalk via CXCL11 and CXCL12.
Cytokine Growth Factor Rev. 24:41–49. 2013. View Article : Google Scholar
|
|
41
|
Guo F, Wang Y, Liu J, Mok SC, Xue F and
Zhang W: CXCL12/CXCR4: A symbiotic bridge linking cancer cells and
their stromal neighbors in oncogenic communication networks.
Oncogene. 35:816–826. 2016. View Article : Google Scholar
|
|
42
|
Liu H, Patel MR, Prescher JA, Patsialou A,
Qian D, Lin J, Wen S, Chang YF, Bachmann MH, Shimono Y, et al:
Cancer stem cells from human breast tumors are involved in
spontaneous metastases in orthotopic mouse models. Proc Natl Acad
Sci USA. 107:18115–18120. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sheridan C, Kishimoto H, Fuchs RK,
Mehrotra S, Bhat-Nakshatri P, Turner CH, Goulet R Jr, Badve S and
Nakshatri H: CD44+/CD24− breast cancer cells
exhibit enhanced invasive properties: An early step necessary for
metastasis. Breast Cancer Res. 8:R592006. View Article : Google Scholar
|
|
44
|
Palena C, Hamilton DH and Fernando RI:
Influence of IL-8 on the epithelial-mesenchymal transition and the
tumor microenvironment. Future Oncol. 8:713–722. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chen W, Gao Q, Han S, Pan F and Fan W: The
CCL2/CCR2 axis enhances IL-6-induced epithelial-mesenchymal
transition by cooperatively activating STAT3-Twist signaling.
Tumour Biol. 36:973–981. 2015. View Article : Google Scholar
|
|
46
|
Espinoza-Sánchez NA, Chimal-Ramírez GK,
Mantilla A and Fuentes-Pananá EM: IL-1β, IL-8 and matrix
metalloproteinases -1, -2 and -10 are enriched upon monocyte-breast
cancer cell co-cultivation in a Matrigel-based three dimensional
system. Front Immunol. 8:2052017. View Article : Google Scholar
|
|
47
|
Ning Y, Manegold PC, Hong YK, Zhang W,
Pohl A, Lurje G, Winder T, Yang D, LaBonte MJ, Wilson PM, et al:
Interleukin-8 is associated with proliferation, migration,
angiogenesis and chemosensitivity in vitro and in vivo in colon
cancer cell line models. Int J Cancer. 128:2038–2049. 2011.
View Article : Google Scholar :
|
|
48
|
Saji H, Koike M, Yamori T, Saji S, Seiki
M, Matsushima K and Toi M: Significant correlation of monocyte
chemoattractant protein-1 expression with neovascularization and
progression of breast carcinoma. Cancer. 92:1085–1091. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lu Y, Cai Z, Xiao G, Liu Y, Keller ET, Yao
Z and Zhang J: CCR2 expression correlates with prostate cancer
progression. J Cell Biochem. 101:676–685. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kudo-Saito C, Shirako H, Ohike M,
Tsukamoto N and Kawakami Y: CCL2 is critical for immunosuppression
to promote cancer metastasis. Clin Exp Metastasis. 30:393–405.
2013. View Article : Google Scholar
|
|
51
|
Fang WB, Yao M, Brummer G, Acevedo D,
Alhakamy N, Berkland C and Cheng N: Targeted gene silencing of CCL2
inhibits triple negative breast cancer progression by blocking
cancer stem cell renewal and M2 macrophage recruitment. Oncotarget.
7:49349–49367. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Velasco-Velázquez MA, Popov VM, Lisanti MP
and Pestell RG: The role of breast cancer stem cells in metastasis
and therapeutic implications. Am J Pathol. 179:2–11. 2011.
View Article : Google Scholar : PubMed/NCBI
|