Introduction
The annual incidence of head and neck cancer (HNC)
is estimated to be between 400,000 and 600,000 new cases, and the
mortality rate is between 223,000 and 300,000 deaths per year
(1). In Japan, the number of
patients affected by HNC was above 24,000 in 2012, and has
gradually increased every year (2). Surgery, radiotherapy, chemotherapy
and radio-chemotherapy are major therapies in HNC. The combination
of 5-fluorouracil (5-FU) and cisplatin (CDDP) (FP treatment) is
most frequently used in unresectable recurrent and distant
metastatic cases (3–6). In addition, a synergetic effect of FP
treatment combined with anti-epidermal growth factor receptor
(EGFR) antibody, cetuximab, has been reported. It was thus
demonstrated that cetuximab plus platinum-fluorouracil chemotherapy
prolonged both overall survival and progression-free survival as
compared with FP therapy alone (7). Despite improvement of the clinical
effects, recurrence and distant metastasis occurred in 30 and 25%
of the patients, respectively, and resistance to CDDP was seen in
some of the patients (6,8–10).
The 5-year survival rate was less than 50% in advanced cases
(6,8). Therefore, development of novel
therapies are needed.
Chemical drugs are known to not only have direct
killing effects on cancer cells but also to sensitize the target
cells to cytotoxic T-lymphocytes (CTL) and favorably modulate the
immune system (11–14). For example, it has been shown that
5-FU upregulates the expression of tumor antigens, HLA-class I
molecules, or FAS, on the surface of target cells such as cell
lines derived from breast carcinoma (15), colon carcinoma (16,17),
melanoma (18) and oral squamous
cell carcinoma (OSCC) (19), which
results in enhancement of the killing activity by tumor
antigen-specific CTL toward these tumor cells. The platinum-based
drugs cisplatin, carboplatin, and oxaliplatin enhance engulfment of
the apoptotic tumor cells by dendritic cells (DCs) through
calreticulin exposed on the plasma membrane of the apoptotic tumor
cells (20), and ATP released from
dying tumor cells promotes chemo-attractive recruitment of DCs to
tumor sites (21), and activation
and crosspresentation of DCs by recognition of high-mobility group
protein box-1 (HMGB-1) with toll like receptor 4 (TLR4) on the DCs
(22). Moreover, it has been
demonstrated that paclitaxel, cisplatin, and doxorubicin enhance
tumor cell susceptibility to CTL-mediated killing, which is
mediated via upregulation of mannose-6-phosphate receptors on the
surface of tumor cells (23).
Furthermore, several drugs have been reported to cause a reduction
of immunosuppressive cells such as regulatory T-cells (Tregs), M2
macrophages and myeloid-derived suppressive cells (MDSCs) (14). Therefore, it is likely that
continuous immune responses, induced by chemotherapy, to cancer are
related to long durable responses. Thus, the development of
combined therapies with chemical drugs and immunotherapies for many
types of advanced cancers have become recent, challenging issues
(24).
In our hospital, adoptive immunotherapy using
T-cells activated by autologous cancer tissue has been conducted in
the past decade for several types of advanced cancers, including
HNC, and has shown objective responses in 3 of 10 patients with
HNC, including 2 complete remissions (25). Based on these studies, we are
attempting to obtain basic research data that can be utilized in
development of combined immunotherapy with T-cell infusion and
chemical drugs in advanced HNC, especially in CDDP-resistant cases
and in physically weak patients who cannot withstand strong drug
side effects. We investigated in this study the influence of FP
treatment on T-cell functions such as proliferation, cytokine
release response and cytotoxicity in vitro using
cytomegalovirus (CMV) pp65 antigen-specific cytotoxic T-lymphocytes
(CMVpp65-CTLs). The main reason why we used CMVpp65-CTLs instead of
tumor specific-CTLs is that it is very difficult to prepare enough
tumor specific-CTLs for study. However, it is reliable to use
CMVpp65-CTLs in an in vitro model like the one in this study
because it is easy to prepare enough CMVpp65-CTLs in one batch,
and, in general, the molecular mechanisms involved in killing
target cells by the virus-CTLs, including CMVpp65-CTLs, are the
same as those used by tumor specific-CTLs (26–28).
Materials and methods
Antibodies, MHC-tetramers and flow
cytometry
PerCP-conjugated anti-CD8 monoclonal antibody (mAb)
was purchased from eBioscience Inc. (San Diego, CA, USA).
Allophycocyanin (APC)-conjugated HLA-A*24:02 CMV pp65
tetramer-QYDPVAALF, APC-conjugated HLA-A*02:01 CMV pp65
tetramer-NLVPMVATV, FITC-conjugated anti-HLA-A2 mAb and
FITC-conjugated anti HLA-A24 mAb were purchased from MBL Co.
(Nagoya, Japan). For intracellular IFN-γ staining, the induced
CMVpp65-CTLs were co-cultured with or without the cognate CMVpp65
synthetic peptides at 37°C in 5% CO2 for 2 h in the
presence of 1 µg/ml brefeldin A (BD Biosciences, San Jose,
CA, USA). Subsequently, they were fixed in 10% formaldehyde and
stained with FITC-conjugated anti-IFN-γ mAb (45.15; Beckman
Coulter, Fullerton, CA, USA) with 0.25% saponin for 30 min at room
temperature. CMVpp65 antigen was detected by anti-CMVpp65 mAb
(Virusys, Inc., Taneytown, MD, USA). The cells were fixed with 10%
formaldehyde for 30 min at 4°C, and were washed 2 times. The cells
were reacted with 5 µg/ml of mouse anti-CMVpp65 mAb
(Virusys, Inc.) including 0.25% saponin, and were subsequently
reacted with FITC-conjugated anti-mouse IgG (MBL Co.). Cells were
analyzed on a FACSCanto II (BD Biosciences) with the aid of Flow Jo
software (Tree Star, Inc., Ashland, OR, USA).
Reagents
CDDP (Randa) was obtained from Nippon Kayaku Co.,
Ltd. (Tokyo, Japan). 5-FU was obtained from Kyowa Hakko Kirin Co.,
Ltd. (Tokyo, Japan). Premix WST-1 Cell Proliferation Assay System
was purchased from Takara Bio Inc. (Otsu, Japan).
Cells and culture media
Peripheral blood mononuclear cells (PBMCs) were
isolated by centrifugation on a Ficoll density gradient. Blood
samples were collected after obtaining written informed consent,
and the study was approved by the Institutional Review Board of
Aichi Medical University. Primary T-cell lines were induced in
RPMI-1640 (Sigma-Aldrich, St. Louis, MO, USA) supplemented with 25
mM HEPES, 10% autologous plasma, gentacine, and 2 mM L-glutamine
(referred to as T-cell medium). Epstein-Barr virus-transformed B
cells (B-LCL) were established by infecting an aliquot of PBMCs
with B95-8 supernatant. Human OSCC cell lines (HSC-2, HSC-3, and
HSC-4) were obtained from the Japanese Collection of Research
Bioresource Cell Bank. Cells were maintained in DMEM (Gibco,
Paisley, UK) supplemented with 10% fetal bovine serum (FBS)
(HyClone Laboratories, Inc. South Logan, UT, USA) and 1%
penicillin-streptomycin (Gibco, Grand Island, NY, USA) at 37°C in
5% CO2 humidified air. Adherent cells were dissociated
from a 25 cm2 flask by using trypsin and seeded in
96-well plates for the experiments.
Preparation of CMVpp65-CTLs
CMVpp65-CTLs were prepared by mixed lymphocyte
peptide cultures (MLPCs) followed by the CD137-guided CTL isolation
method (29). MLPCs were performed
as follows: 0.1 µM HLA-A*24:02- or HLA-A*02:01-restricted
synthetic CMV pp65 T-cell epitope peptides [QYDPVAALF aa 341-349
(30) or NLVPMVATV aa 495-503
(31)], respectively, (MBL Co.)
were directly added to PBMCs suspended in 1 ml T lymphocyte medium
at a cell concentration of 2.0×106/ml, and the cultures
were maintained in 15 ml round bottom tubes at 37°C and 5%
CO2 for 2 days. On day 2, 1 ml of T lymphocyte medium
supplemented with 100 IU/ml of IL-2 was added. Starting on day 7,
half of the medium supernatant was removed and an equal volume of
ALyS505N medium (Cell Science & Technology Institute, Inc.,
Sendai, Japan) supplemented with 100 IU/ml of IL-2 was added. Until
day 14, the cells were cultured with appropriate medium (ALyS505N
with 100 IU/ml of IL-2). Then, a CD137-guided CTL isolation was
performed. CMV-CTLs prepared by MLPCs were re-stimulated for 2 days
by the cognate peptide-pulsed autologous B-LCLs, and isolated by
the anti-human CD137 MicroBeads kit (Miltenyi Biotec Inc., Bergisch
Gladbach, Germany). The isolated cells were propagated for 7 days
in ALyS505N medium supplemented with 100 IU/ml of IL-2. Cultures
were fed by changing half of the supernatant twice a week. Viable
cell counts were determined using the trypan blue assay.
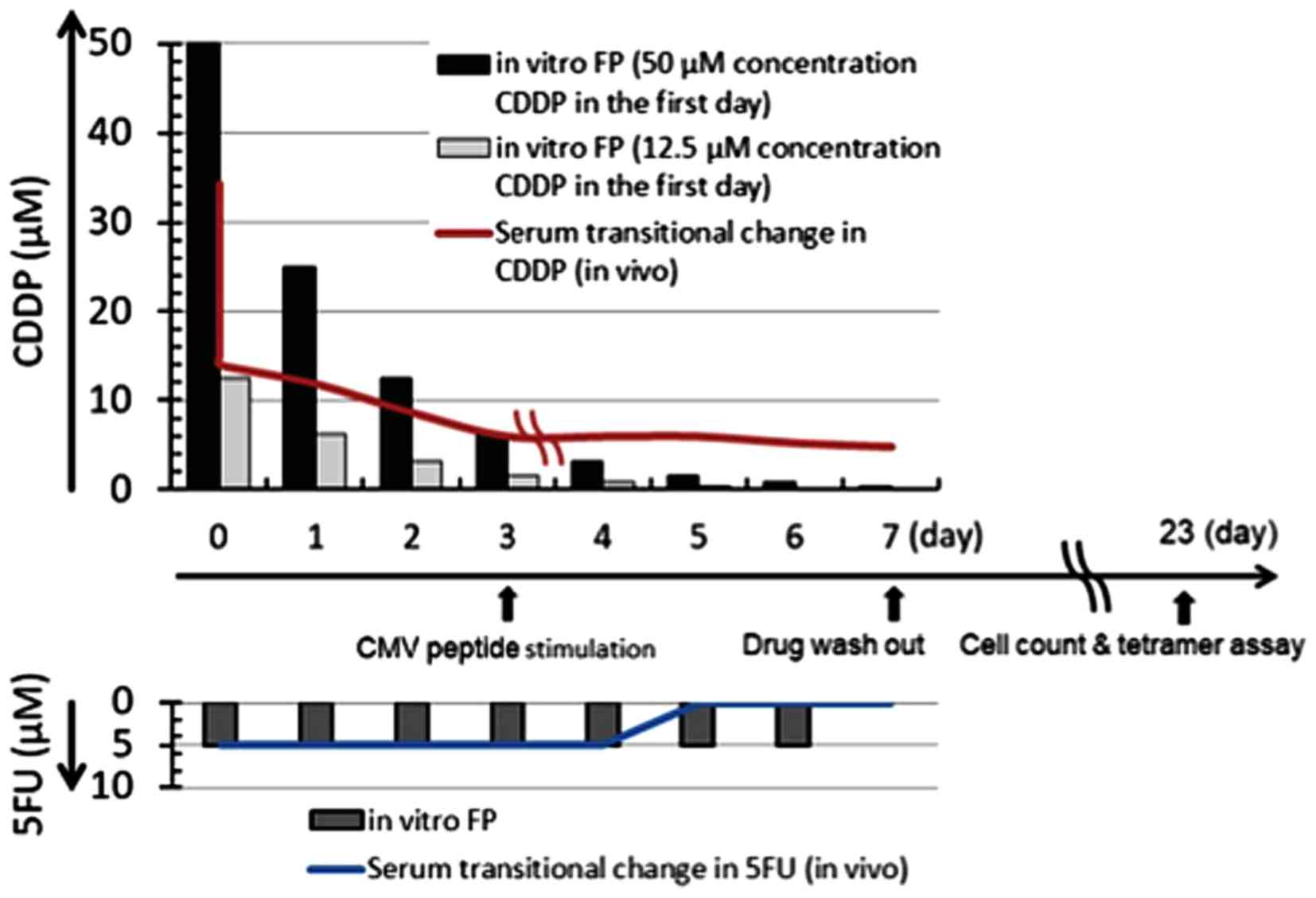
Effect of FP treatment on CMVpp65-CTL
induction
PBMC from a healthy volunteer who was HLA-A*24:02
positive were suspended in T-cell medium at a cell concentration of
106/ml, and 1 ml cell suspensions were dispensed into 15
ml round-bottom tubes in duplicate, and CDDP and 5-FU were added to
each of the duplicate tubes at final concentrations of 12.5
µM or 50 µM, respectively, for CDDP and 5 µM
for 5-FU, and the cells were cultured at 37°C in 5% CO2.
CDDP concentrations were reduced by half every day until day 5,
while the 5-FU concentration was maintained at 5 µM, in
order to simulate the changing drug concentration in the plasma of
patients during FP treatment (Fig.
1). On day 7, the drugs were washed out by washing the cells 3
times. HLA-A*24:02-restricted CMVpp65 T-cell epitope peptide
QYDPVAALF (MBL Co.) was added on day 3 at a final concentration of
0.1 µM, and 2 days after addition of the peptide, IL-2 was
added at a final concentration of 50 IU/ml, and the cells were
cultured for 5 days. Then, half of the medium supernatants were
removed and equal volumes of ALyS505N medium (Cell Science &
Technology Institute, Inc.) supplemented with 100 IU/ml of IL-2
were added. The cells were cultured until day 23 in the appropriate
medium (ALyS505N with 100 IU/ml of IL-2). Viable cell counts were
determined using the trypan blue assay.
Construction of CMVpp65
antigen-expressing lenti-viral vector and infection
Lentiviral vectors encoding full-length human
CMVpp65 antigen fused with the fluorescent protein, Tomato, in the
c-terminus (pLVSIN-CMV Neo_pp65-TMT), and the MOCK vector encoding
Tomato (pLVSIN-CMV Neo_TMT) were constructed by recombination of
each of the synthesized cDNAs with the lentiviral vector,
pLVSIN-CMV Neo (Takara Bio Inc.). pLVSIN-CMV Neo_pp65-TMT or
pLVSIN-CMV Neo_TM) were co-transfected with Lentiviral High Titer
Packaging Mix (Takara Bio Inc.) into 293T/17 cells (ATCC, Manassas,
VA, USA). Forty-eight hours after the co-transfection, the culture
supernatant, including virus particles, was used for infection of
OSCC cell lines with the lentivirus. The infected OSCC cells were
cultured in DMEM supplemented with 250 µg/ml of G418, and
the Tomato-expressing cells were isolated by FACSAria III (Becton
Dickinson). CMVpp65 expression by the infected cells was analyzed
by flow cytometry using anti-CMVpp65 mAb.
Establishment of CDDP-resistant subline
from the HSC-3
A CDDP-resistant subline was raised over 3 months by
continuous exposure to 1.25 µM concentration of CDDP, and
cultured in DMEM medium. Subsequently, the CDDP resistance of the
surviving cells was increased over 3 months by continuous exposure
to 2.5 µM concentration of CDDP. The resistant subline was
then considered established and was named HSC-3/CDDP-R1.
Drug sensitivity and sensitivity to
CTL-mediated killing of OSCC cell lines
Two days after OSCC cells were seeded in 96-well
plates at 20,000 cells per well, serial concentrations of drugs and
serial numbers of CMVpp65-CTLs were added in triplicate and
cultured for 4-7 days. Then, each well was washed with 150
µl of PBS 3 times to remove the dead cells and the CTL
cells, and the cell viability assay was performed by incubating
with WST-1, a highly water-soluble disulfonated tetrazolium salt,
4-[3-(2-methoxy-4-nitrophenyl)-2-(4-nitrophenyl)-2H-5-tetra-zolio]-l,3-benzene
disulfonate sodium salt (32). The
absorbance (OD) was measured with a micro-plate reader (SpectraMAX
M5 spectrophotometer, Molecular Devices, Sunnyvale, CA, USA) at a
wavelength of 450 nm. Cell viability was calculated according to
the following formula: % viability = 100 × (E − S)/(M − S), where E
is the absorbance of experimental well, M is the absorbance in the
absence of CTL and/or drugs (cells were incubated with medium
alone), and S is that of medium alone. An average of triplicate
measurements is shown.
Results
Effect of FP treatment on CTL
induction
The CMVpp65 tetramer positivity in each well of
duplicate cultures of PBMC stimulated with the epitope peptide in
the absence of the drugs on day 23 was increased to 12.6 and 13.4%,
respectively, from 0.004% on day 0, whereas the cultures stimulated
with 5-FU (5 µM) were 7.3 and 16.2%, respectively. The
cultures stimulated with FP treatment [5-FU (5 µM)+CDDP
(12.5 µM)] were 2.2 and 3.9%, respectively (average, 3.05%)
(Fig. 2A). Since the numbers of
cells were extremely reduced, cultures treated with FP at a high
concentration of CDDP [5-FU (5 µM)+CDDP (50 µM)]
could not be measured. The total cell numbers and tetramer-positive
cell numbers in each well of duplicate culture of PBMC stimulated
with the epitope peptide on day 23 in the absence of the drugs were
increased to 28.5×105 and 37.8×105 (growth
rate; 3.32-fold) and 35.9×104 and 50.7×104
(growth rate; 10,825-fold), respectively, from 10.0×105
and 0.4×102 on day 0, respectively. Cultures that were
treated with a single treatment of 5-FU (5 µM) were
increased to 24.6×105 and 33.0×105 (growth
rate; 2.28-fold) and 1.8×105 and 5.3×105
(growth rate; 8,925-fold), respectively, and in cultures receiving
FP treatment of 5-FU [5 µM)+CDDP (12.5 µM)], the
total cell numbers in each well were increased to
2.5×105 and 3.0×105 (growth rate; 0.28-fold),
respectively, and the tetramer-positive cell numbers in each well
were increased to 0.6×104 and 1.2×104 (growth
rate; 215-fold), respectively (Fig.
2B). Summing up these observations, although the effect on
CMVpp65-CTL induction in the 5-FU (5 µM) single-treatment
cultures was minimal, there was a partial effect in the FP-treated
cultures that was especially remarkable at a high concentration of
CDDP [5-FU (5 µM)+CDDP (50 µM)].
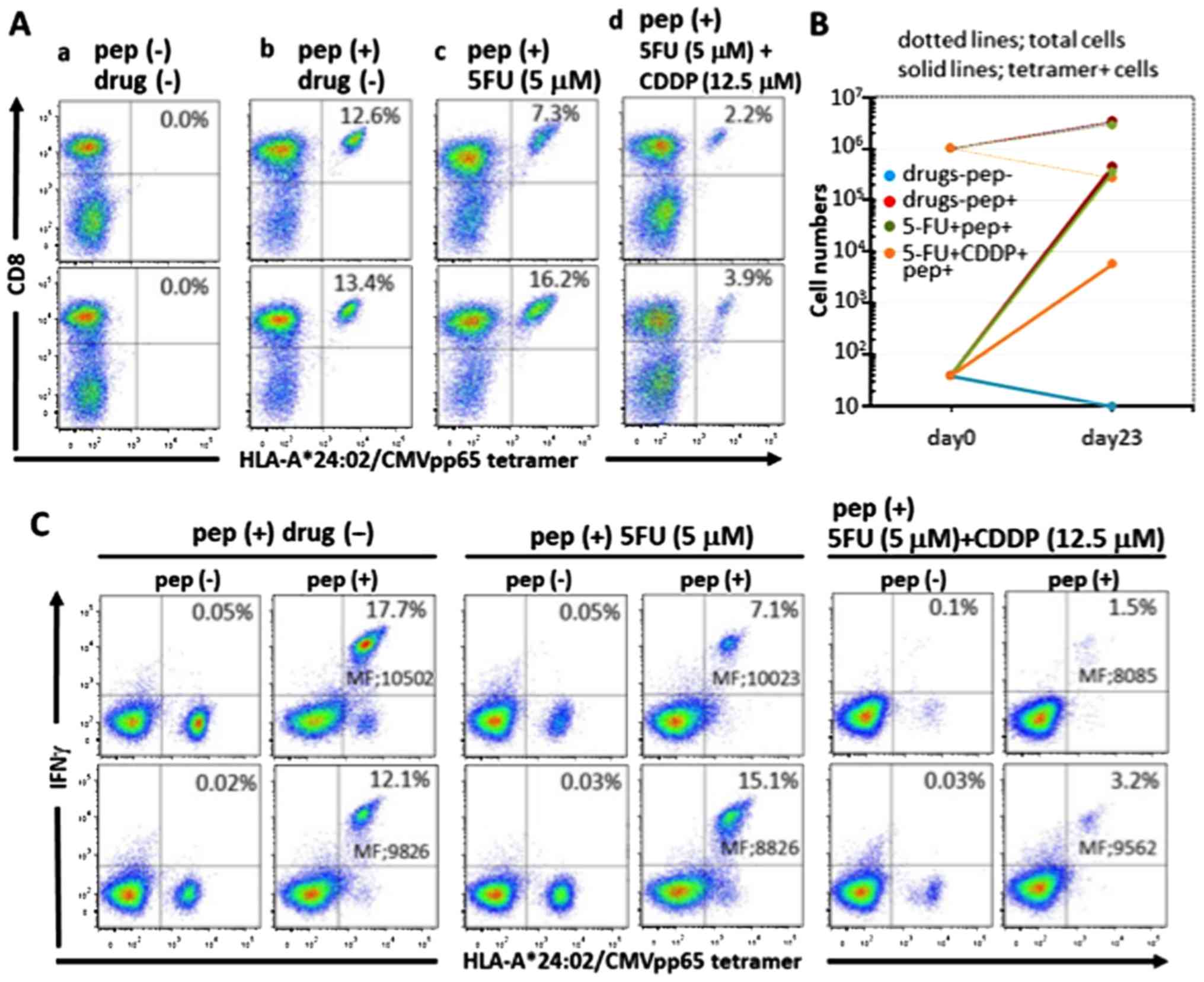 | Figure 2Induction of CMVpp65-CTLs in the
presence of the drugs. (A) HLA-A*24:02-restricted CMVpp65-CTLs were
induced in duplicate from PBMC of a healthy donor by culturing for
23 days under different conditions: incubation in the absence of
the drugs and the peptide (a), incubation in the presence of the
epitope peptide only (b), incubation with 5 µM 5-FU and the
epitope peptide (c) and incubation with 5 µM 5-FU+12.5
µM CDDP and the epitope peptide (d). The cells were stained
for CMVpp65 tetramer and CD8 and analyzed by flow cytometry. The
viable cell population was determined by FSC and SSC levels, and
the data are plotted to show CD8 and HLA-A*24:02 CMVpp65
tetramer-positivity. Percentages relative to the entire viable cell
population of both CD8 and HLA-A*24:02 CMVpp65 tetramer-positive
cells are indicated in each panel. (B) Expansion of the total cell
numbers and the tetramer-positive CMVpp65-CTL cell numbers: the
total cell numbers and the tetramer-positive CMVpp65-CTL cell
numbers on day 0 and 23, respectively, were plotted.
HLA-A*24:02-restricted CMVpp65-CTLs were induced in duplicate as
described below. Solid lines and dotted lines indicate expansion of
the total cells and the tetramer-positive cells, respectively. Each
color signifies the following: blue, no drugs and no peptide; red,
epitope peptide only; green, 5 µM 5-FU and the epitope
peptide; orange, 5 µM 5-FU+12.5 µM CDDP and the
epitope peptide. (C) Intracellular IFN-γ assay of the induced
CMVpp65-CTLs: the induced CTLs under different conditions as
described in (A): incubation in the presence of the epitope peptide
only (a), incubation with 5 µM 5-FU and the epitope peptide
(b) and incubation with 5 µM 5-FU+12.5 µM CDDP and
the epitope peptide (c), were cultured with the cognate peptide
[pep (+)] or no peptide [pep (−)] in the presence of brefeldin A
for 2 h, and stained with HLA-A*24:02 CMVpp65 tetramer and
anti-IFN-γ mAb and analyzed by flow cytometry. Percentages relative
to the entire viable cell population of both IFN-γ and HLA-A*24:02
CMVpp65 tetramer-positive cells are indicated in each panel. MF in
the panels indicates mean fluorescence intensity of IFN-γ and
HLA-A*24:02 CMVpp65 tetramer-positive cells. |
The immune response of the induced CMVpp65-CTL in
the presence of drugs to the cognate epitope peptide was evaluated
by an intracellular IFN-γ assay (Fig.
2C). IFN-γ production was preferentially shown in the
tetramer-positive cells in every well, and the mean fluorescence
intensity of IFN-γ was almost equal between wells receiving
non-treatment, 5-FU single-treatment and FP treatment. This
observation indicates that the IFN-γ production per cell was not
inhibited by these drugs. Thus, these drug treatments did not
affect the specific response of CMVpp65-CTL.
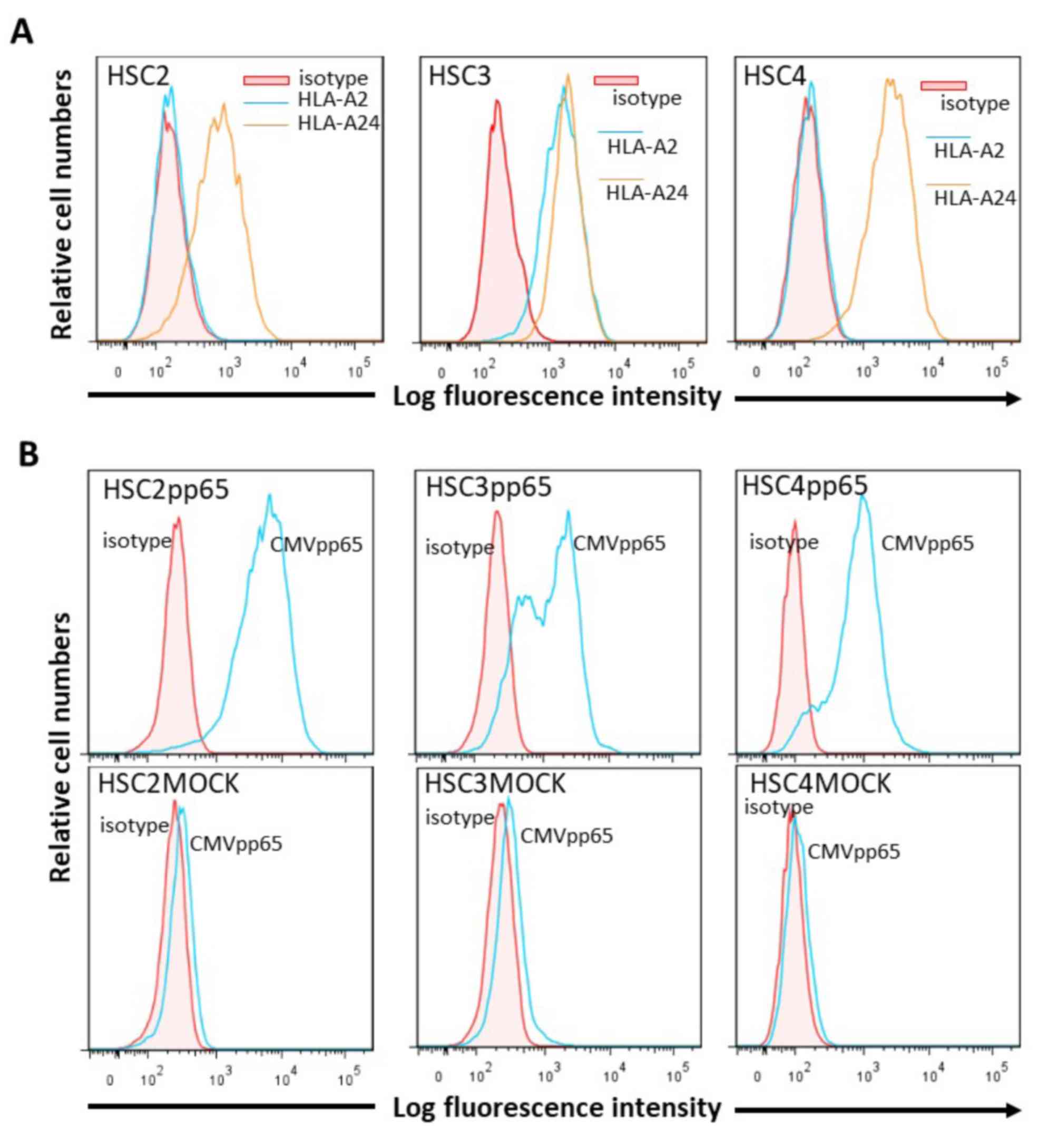
Expression of HLA class I molecules and
CMVpp65 antigen in OSCC cells
Flow cytometric analysis showed that HLA-A24 was
expressed on all of the OSCC cells, HSC-2, HSC-3 and HSC-4, that
were used in this study; and HLA-A2 was expressed on HSC-3, but not
on HSC-2 and HSC-4. CMVpp65 was specifically expressed in
CMVpp65-transfected OSCC cells but not in the MOCK cells (Fig. 3).
Preparation of CMV-CTLs
We successfully prepared highly purified
HLA-A24-restricted CMV-CTLs (A24-CMV-CTLs) from donor 1 and
HLA-A2-restricted CMV-CTLs (A2-CMV-CTLs) from donor 2 with 90.2 and
97.1% MHC-tetramer positivity, respectively. These CMV-CTLs showed
specific full cytotoxic activity toward pp65-expressing OSCC cells
in an HLA-type-restricted manner. The cytotoxicity was shown even
at a very low E/T ratio (1/128) (Fig.
4).
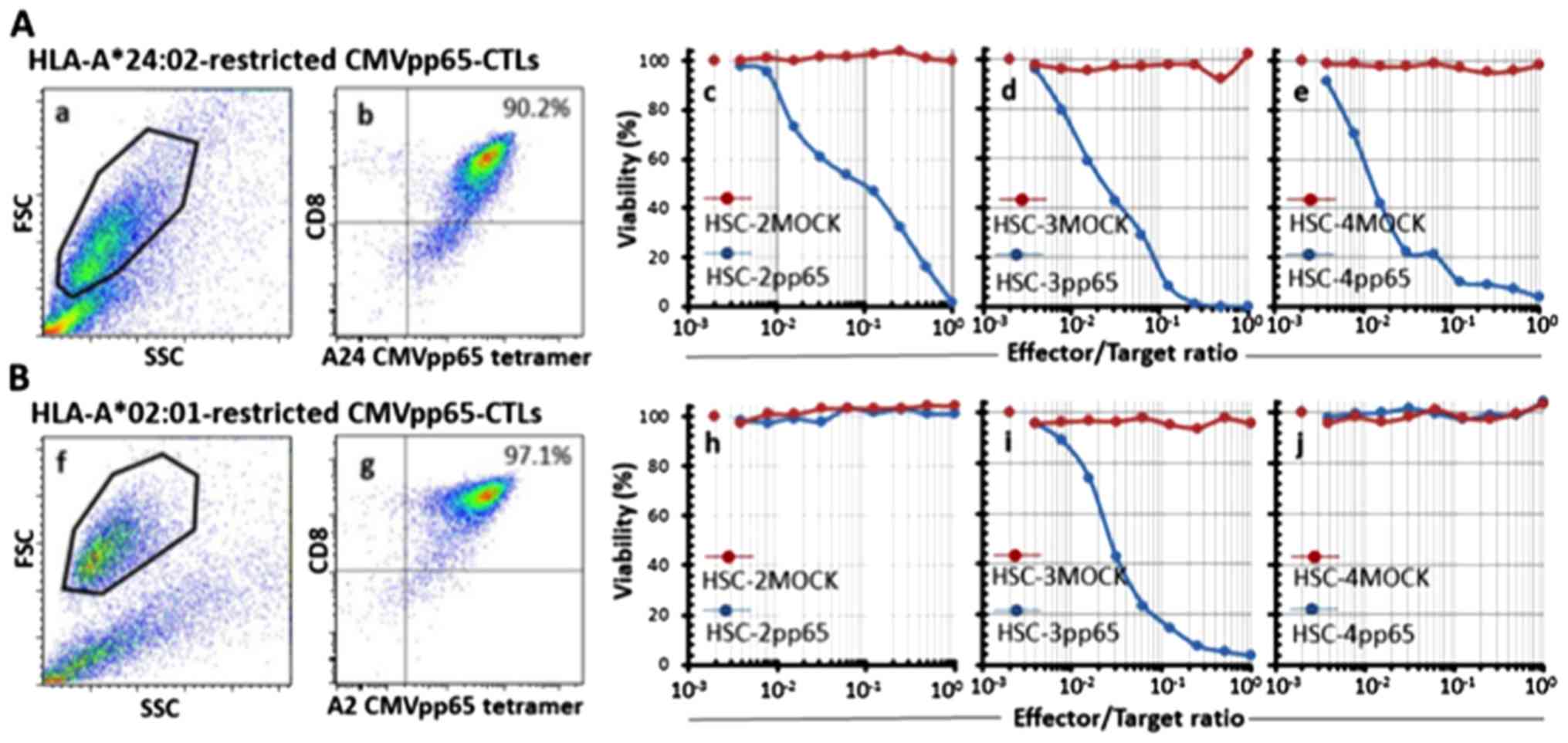 | Figure 4Purity and specificity of the
prepared CMVpp65-CTLs. HLA-A*24:02-restricted CMVpp65-CTLs (A) and
HLA-A*02:01-restricted CMVpp65-CTLs (B) were prepared from an
HLA-A24-positive healthy donor and an HLA-A2-positive healthy
donor, respectively, and were evaluated for purity and specificity.
The cells were stained for CMVpp65 tetramer and CD8 and analyzed by
flow cytometry. The viable cell population was determined by FSC
and SSC levels (a and f, respectively) and the data are plotted to
show CD8 and HLA-A*24:02 or HLA-A*02:01/CMVpp65 tetramer-positivity
(b and g, respectively). Percentages relative to the entire viable
cell population of both CD8 and HLA-A*24:02 or HLA-A*02:01/CMVpp65
tetramer-positive cells are indicated in each panel. The
cytotoxicity of the CMVpp65-CTLs were indirectly evaluated by the
WST-1 assay. HLA-A*24:02-restricted CMVpp65-CTLs or
HLA-A*02:01-restricted CMVpp65-CTLs were co-cultured with
lentivirus-infected OSCC cell lines as the target cells for 7 days.
When the target cells were co-cultured with HLA-A*24:02-restricted
CMVpp65-CTLs, the viability of HSC-2pp65, HSC-3pp65 or HSC-4pp65
(blue lines in c-e, respectively) were reduced in an E/T
ratio-dependent manner, but the viability of HSC-2MOCK, HSC-3MOCK
or HSC-4MOCK (red lines in c-e, respectively) were not reduced. In
contrast, when the target cells were co-cultured with
HLA-A*02:01-restricted CMVpp65-CTLs, the viability of HSC-3pp65 but
not HSC-2pp65 and HSC-4pp65 (blue lines in h-j, respectively) were
reduced in an E/T ratio-dependent manner, but the viability of
HSC-2MOCK, HSC-3MOCK or HSC-4MOCK (red lines in h-j, respectively)
were not reduced. Each of the plots at the left side of the images
that are not associated with a line shows the E/T ratio of 0. |
Effect of 5-FU and/or CDDP on
cytotoxicity of CMVpp65-CTLs toward OSCC cells
HLA subtypes are different between these cell lines.
HSC-3 is HLA-A24+/HLA-A2+, HSC-2 and HSC-4
are HLA-A24+/HLA-A2−. Synergistic effects of
5-FU and/or CDDP to antigen-specific cytotoxicity of CTL in a
HLA-subtype-restricted manner can be exactly investigated by using
target cell lines that have different HLA subtypes.
The sensitivity of OSCC cells to CMVpp65-CTL was
measured at serial E/T ratios in combination with serial
concentrations of 5-FU and/or CDDP in order to evaluate the effect
of 5-FU and/or CDDP on the cytotoxicity of CMVpp65-CTLs using the
WST-1 assay. When CMVpp65-CTLs were co-cultured with the
HLA-type-matched CMVpp65-expressing OSCC cells, a remarkable
reduction of the cell viability was observed in an E/T
ratio-dependent manner at all drug concentrations (panels a, b and
c in Figs. 5A and 6A, panels b in Figs. 5B and 6B), and the 'E/T 50' (E/T ratio
indicating 50% cell viability) was shifted to a low ratio in a drug
concentration-dependent manner (panels b and c in Fig. 5A; panels a, b and c in Fig. 6A; panel b in Figs. 5B and 6B). Conversely, a remarkable reduction of
the cell viability was also observed in a drug
concentration-dependent manner at all E/T ratios, and the
IC50 of these drugs was shifted to a low value in an E/T
ratio-dependent manner (panels a, b and c in Fig. 7, panels a and b in Fig. 8). These results indicate that
neither 5-FU nor CDDP affected the cytotoxicity of CTL, but that
CTL treatment in combination with 5-FU and/or CDDP generated a
synergistic killing effect. Especially, 5-FU clearly sensitized
HSC-3pp65 and HSC-4pp65 to CTL cytotoxicity. The cell viability was
drastically reduced by CTLpp65-CTL cytotoxicity even at a low E/T
ratio in combination with 5-FU (panels b and c in Fig. 5A; panel b in Fig. 5B).
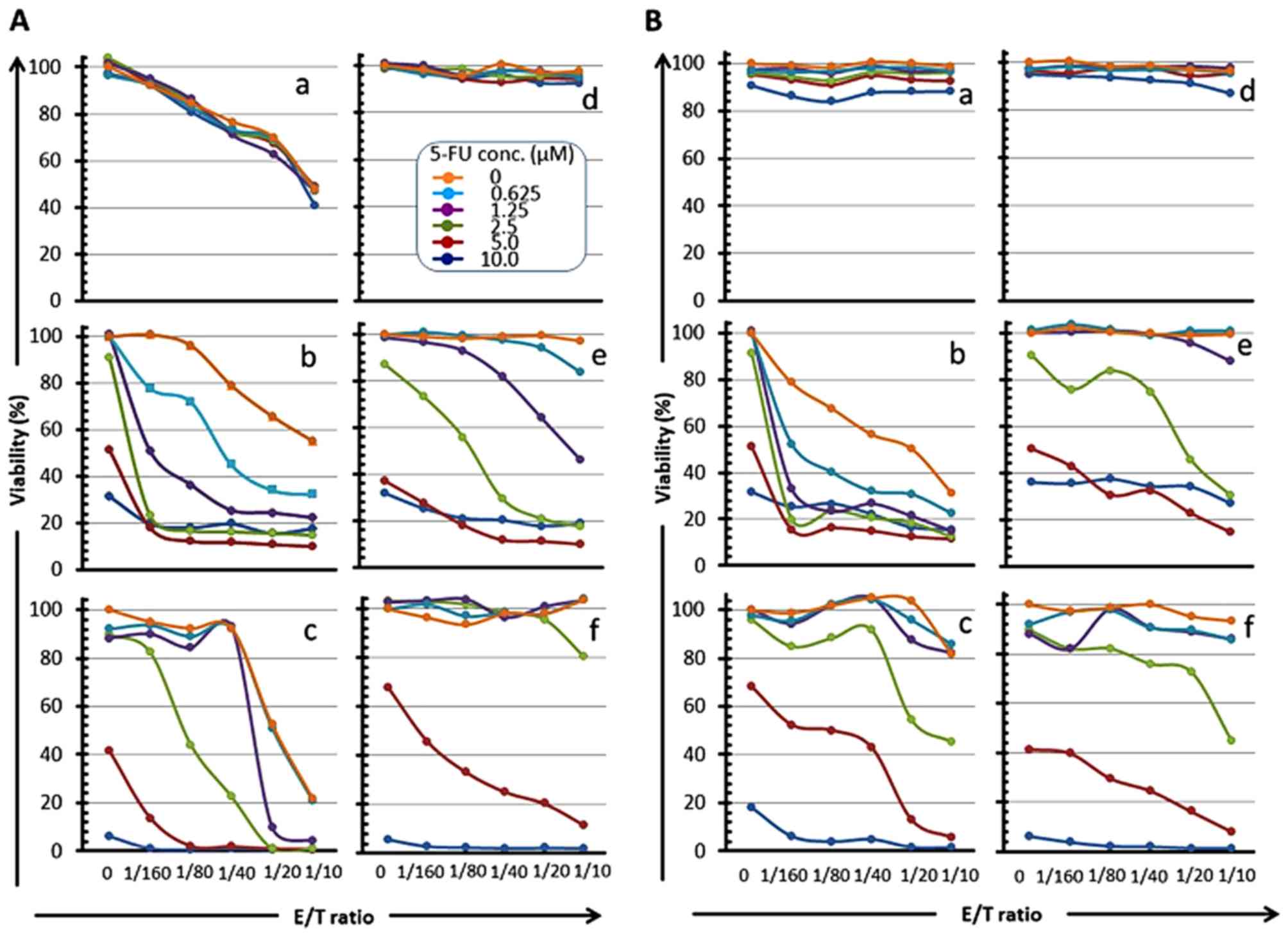 | Figure 5Reduction of OSCC cell viability by
CMVpp65-CTLs in an E/T ratio-dependent manner in combination with
5-FU. CMVpp65-overexpressed OSCC cells (a, HSC-2pp65; b, HSC-3pp65;
c, HSC-4pp65) or the MOCK cells (d, HSC-2MOCK; e, HSC-3MOCK; f,
HSC-4MOCK) were co-cultured with HLA-A*24:02-restricted
CMVpp65-CTLs (A) or HLA-A*02:01-restricted CMVpp65-CTLs (B) for 7
days in the presence of serial concentrations of 5-FU (0, 0.625,
1.25, 2.5, 5, 10 µM, as shown in A-d legend) and the
viabilities of the target cells were measured by the WST-1
assay. |
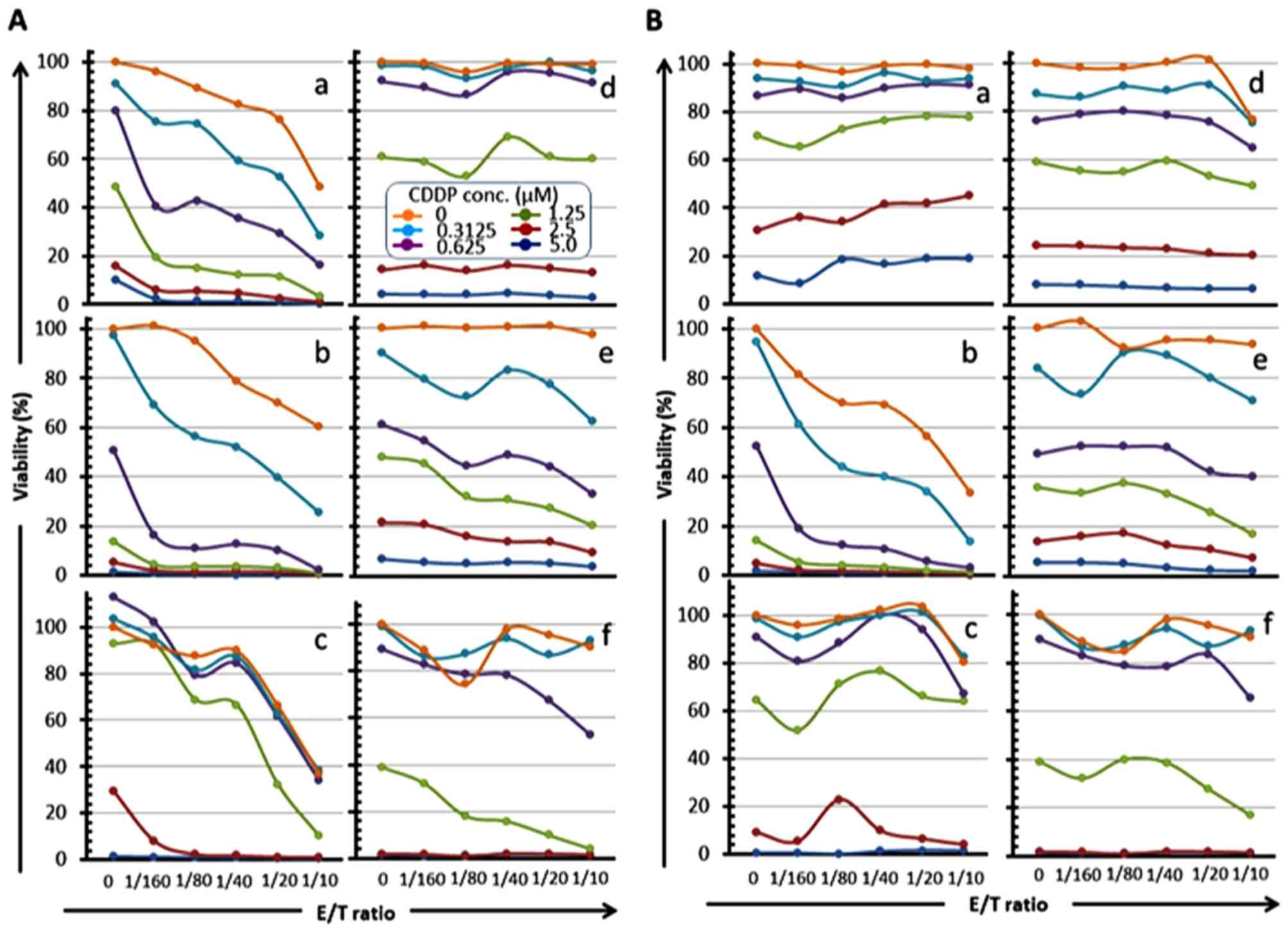 | Figure 6Reduction of OSCC cell viability by
CMVpp65-CTLs in an E/T ratio-dependent manner in combination with
CDDP. CMVpp65-overexpressed OSCC cells (a, HSC-2pp65; b, HSC-3pp65;
c, HSC-4pp65) or the MOCK cells (d, HSC-2MOCK; e, HSC-3MOCK; f,
HSC-4MOCK) were co-cultured with HLA-A*24:02-restricted
CMVpp65-CTLs (A) or HLA-A*02:01-restricted CMVpp65-CTLs (B) for 7
days in the presence of serial concentrations of CDDP (0, 0.3125,
0.625, 1.25, 2.5, 5 µM, as shown in A-d legend) and the
viabilities of the target cells were measured by the WST-1
assay. |
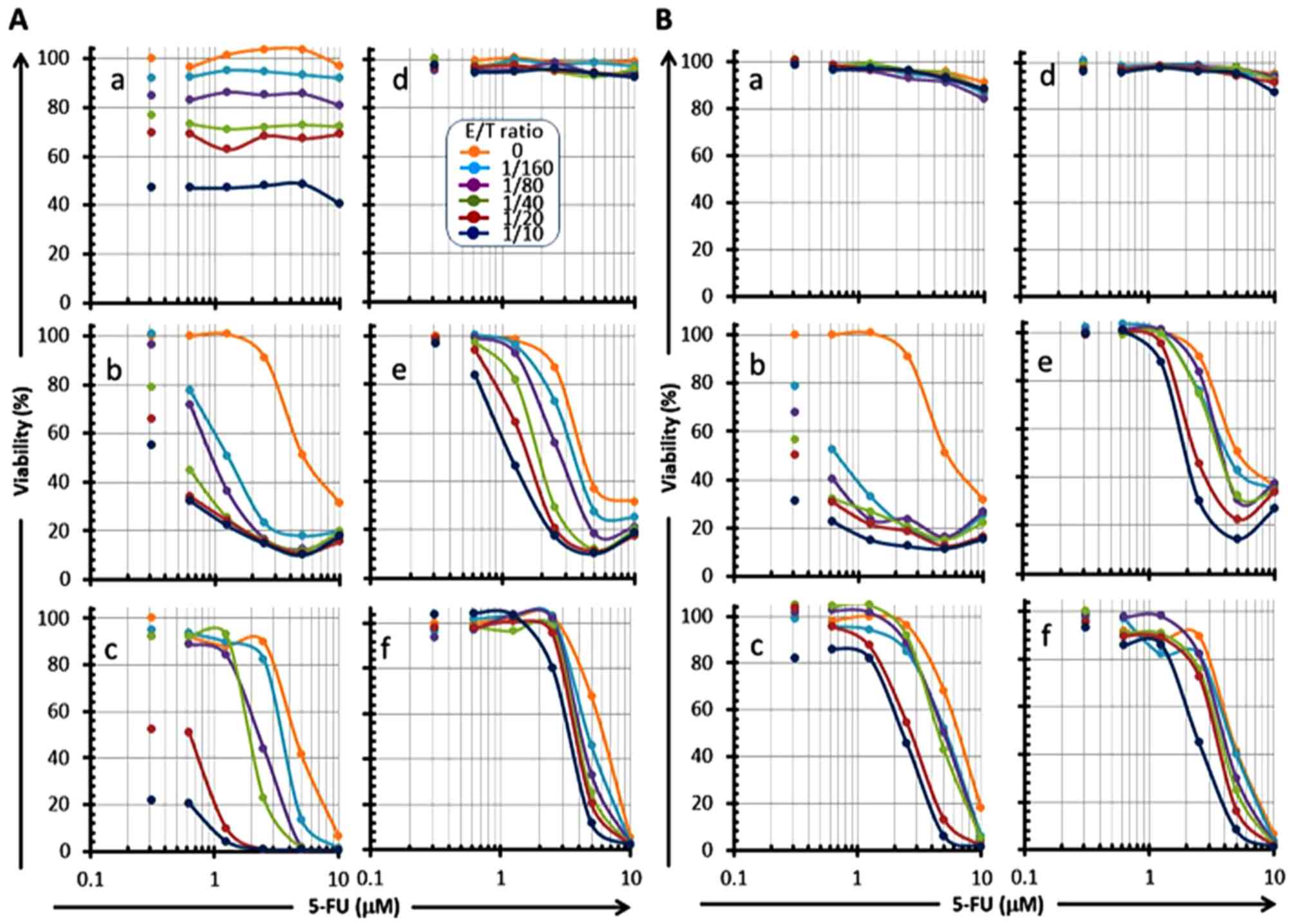 | Figure 7Reduction of OSCC cell viability in a
5-FU concentration-dependent manner in combination with serial E/T
ratios of CMVpp65-CTLs. CMVpp65-overexpressed OSCC cells (a,
HSC-2pp65; b, HSC-3pp65; c, HSC-4pp65) or the MOCK cells (d,
HSC-2MOCK; e, HSC-3MOCK; f, HSC-4MOCK) were co-cultured with 5-FU
in the presence of serial E/T ratios (0, 1/160, 1/80, 1/40, 1/20,
1/10, as shown in A-d legend) of HLA-A*24:02-restricted
CMVpp65-CTLs (A) or HLA-A*02:01-restricted CMVpp65-CTLs (B) for 7
days, and the viabilities of the target cells were measured by the
WST-1 assay. Each of the left-side plots that are not associated
with a line shows 0 µM of 5-FU. |
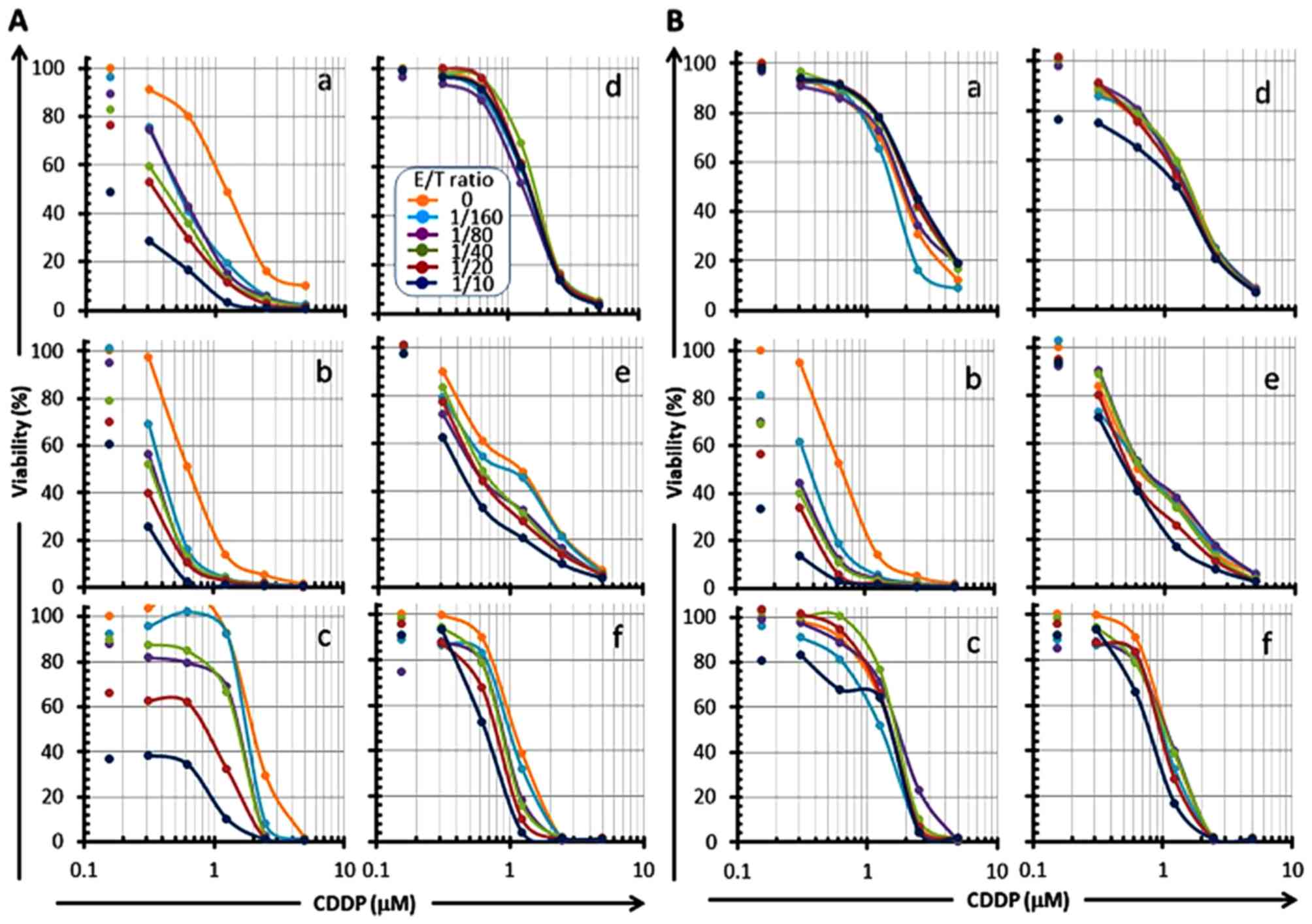 | Figure 8Reduction of OSCC cell viability by
CMVpp65-CTLs in an E/T ratio-dependent manner in combination with
CDDP. CMVpp65-overexpressed OSCC cells (a, HSC-2pp65; b, HSC-3pp65;
c, HSC-4pp65) or the MOCK cells (d, HSC-2MOCK; e, HSC-3MOCK; f,
HSC-4MOCK) were co-cultured with CDDP in the presence of serial E/T
ratios (0, 1/160, 1/80, 1/40, 1/20, 1/10, as shown in A-d legend)
of HLA-A*24:02-restricted CMVpp65-CTLs (A) or
HLA-A*02:01-restricted CMVpp65-CTLs (B) for 7 days, and the
viabilities of the target cells were measured by the WST-1 assay.
Each of the left-side plots that are not associated with a line
shows 0 µM of CDDP. |
It is of interest to note that a slight reduction in
cell viability was shown in an E/T ratio-dependent manner when MOCK
OSCC cells, which do not express CMVpp65 antigen, were co-cultured
with CMVpp65-CTLs in the presence of serial concentrations of the
drugs. CMVpp65-CTLs did not reduce the cell viability without the
presence of any drugs, even at a high E/T ratio (1/10). For
example, when the HSC-3MOCK or HSC-4MOCK cells were co-cultured
with CMVpp65-CTLs, a significant reduction of cell viability was
noted in an E/T ratio-dependent manner in the presence of 2.5
µM or 5.0 µM of 5-FU, although CMVpp65-CTLs did not
reduce the viability of HSC-3MOCK or HSC-4MOCK cells without the
presence of any drugs (panels e and f in Fig. 5A and B). Moreover,
HLA-A2-restricted CMVpp65-CTLs reduced the viability of both the
HLA-type-mismatched HSC-4pp65 and HSC-4MOCK cells in an E/T
ratio-dependent manner in the presence of 2.5 or 5.0 µM of
5-FU, although the viability was not reduced without the presence
of any drugs (panel c and f in Fig.
5B). These observations suggest that 5-FU sensitizes the HSC-3
and HSC-4 cells to the cytotoxicity of CMVpp65-CTLs in an
antigen-non-specific as well as antigen-specific manner.
Susceptibility to 5-FU is different between these
cell lines. HSC-2 cells were judged resistant to 5-FU because the
cell viability was barely reduced even at a high 5-FU concentration
(10.0 µM). The susceptibility of HSC-2 to 5-FU is lower than
those of HSC-3 and HSC-4, which may suggest one reason why the
susceptibility of HSC-2 to CTL cytotoxicity is not enhanced by 5-FU
in comparison with HSC-3 and HSC-4. We suggest that 5-FU did not
sensitize the 5-FU resistant cells to the cytotoxicity of
CMVpp65-CTLs (panels a and d in Fig.
5A and B; panels a and d in Fig.
7A and B).
Cytotoxicity of CMVpp65-CTLs toward
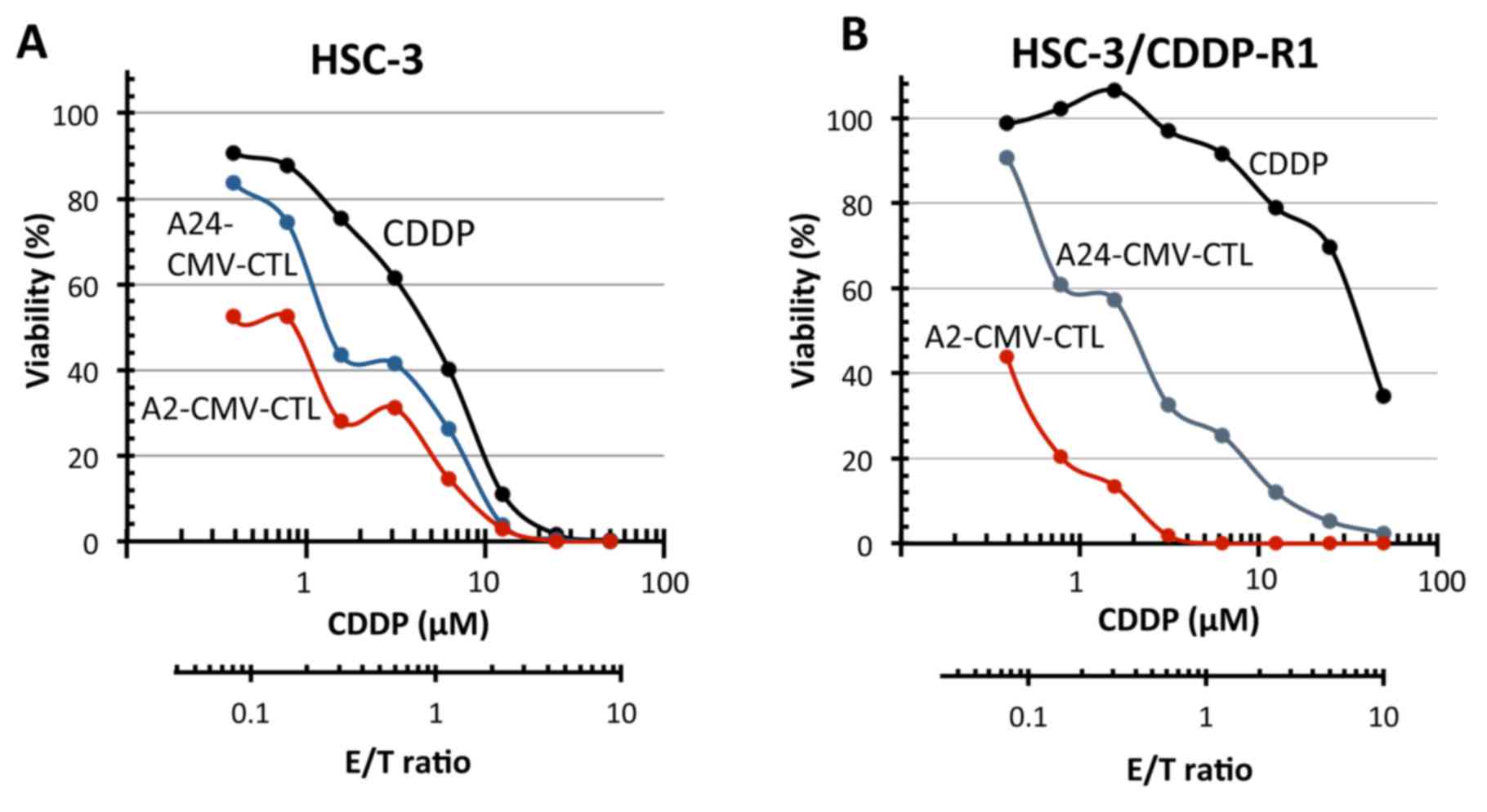
CDDP-resistant HSC-3 cells
The IC50 of CDDP to the parental HSC-3
and the CDDP-resistant HSC-3 cells, which were established in this
study, were 5 and 20 µM, respectively, while the E/T50 of
CMVpp65-CTLs toward the CMVpp65 T-cell epitope peptide-pulsed cells
were both 1/10 (Fig. 9). Thus, it
may be possible that the CTL treatment could also be effective in
CDDP-resistant cases.
Discussion
Although chemoimmunotherapies are expected to
improve the therapeutic effects of cancer treatment, the direct
effect of chemical drugs on the functions of antigen-specific CTLs
has not been clarified. Since most chemical drugs arrest the cell
cycle, it is of concern that the immune responses of T-cells to
tumor antigens may be inhibited during chemo-immunotherapy. We have
studied the effects of FP treatment on the antigen-specific T-cell
immune responses and functions using CMVpp65-CTLs in order to
evaluate a potential immunotherapy in combination with FP treatment
of HNC. CMVpp65-CTLs were used in this study because they are
easier to handle in in vitro experiments than tumor-specific
CTLs because, in general, the frequency of memory T-cells to
CMVpp65 antigen in PBMC in healthy donors is very high (30). Moreover, CMVpp65-CTLs can be
efficiently induced and proliferated by a simple technique using
stimulation with CMVpp65 antigen T-cell epitope peptide. Therefore,
antigen-specific T-cell responses and proliferation can be easily
monitored in the culture by an intracellular IFN-γ assay and an
MHC-tetramer assay. Since a highly purified CMV-CTL line can be
prepared by our CD137-guided isolation method (Fig. 4), antigen-specific cytotoxic
activity can be evaluated exactly. In addition, although the
affinity of the T-cell receptor for tumor antigen is considered to
be lower than that for CMVpp65 antigen, the recognition and killing
mechanisms of CMVpp65-CTLs are the same as those of tumor
antigen-specific CTLs. Thus, an experimental study using
CMVpp65-CTLs is very useful for evaluation of drug effects on
T-cell responses and functions.
First, the effects of CDDP and 5-FU on CMV-CTL
induction were investigated in vitro in culture conditions
in which the concentrations of the drugs were changed over time in
order to simulate in vivo drug concentrations (Fig. 1). Inhibition of CMVpp65-CTL
proliferation in vitro was limited in the presence of only
5-FU; in contrast, the proliferation was inhibited by the FP
treatment, especially at a high concentration of CDDP (Fig. 2). However, the proliferation was
not inhibited completely, and the IFN-γ release response of the
CMV-CTLs, which were induced in the presence of 5-FU and/or CDDP,
was not inhibited at all. We suggest that 5-FU is adequate as a
combination partner in immunotherapy, and that CDDP must be used at
a low concentration when FP treatment is used in combination with
immunotherapy, such as in a vaccine.
Second, we investigated the effects of 5-FU and CDDP
on CMVpp65-CTL cytotoxicity using the CMVpp65 antigen-transfected
OSCC cell lines, HSC-2pp65, HSC-3pp65 and HSC-4pp65 as the targets.
The drugs did not affect the cytotoxicity against any of the three
target cells. It is important to note that a synergistic killing
effect of CMVpp65-CTLs with 5-FU and/or CDDP was observed even at a
very low E/T ratio (less than 1/100). Especially, the
IC50 value of 5-FU against HSC-3pp65 was drastically
reduced in the presence of CMVpp65-CTLs even at the low E/T ratio
of 1/160 (panels b and c in Fig.
7A, panel b in Fig. 7B). Also
noteworthy, a slight reduction of the IC50 of 5-FU
against HSC-3MOCK was also observed in the presence of CMVpp65-CTLs
(panels e and f in Fig. 7A and B).
These observations were shown in both of the two CMVpp65-CTL lines
used in this study. It is possible that 5-FU sensitized the target
cells for CMVpp65-CTLs not only in an antigen-specific manner but
also in a non-specific manner. It has been reported previously that
CDDP and 5-FU have not only direct killing ability toward cancer
cells but that they also can enhance the susceptibility of cancer
cells to CTL by modulation of related molecules involved in
apoptosis, sensitization to CTLs, and activation of DCs. Although
upregulation of tumor antigen, Fas and MHC class I expression have
been suggested as potential mechanisms for target cell
sensitization by 5-FU (15,18),
upregulation of such molecules was not observed in our experiments
(data not shown). Other mechanisms are being considered as related
to the sensitization. 5-FU did not sensitize HSC-2 which is
indicated as being 5-FU-resistant (panel a in Figs. 5A and 7A). This observation suggests that
molecules, related to the apoptosis-signaling cascade downstream of
5-FU are important for the sensitization. We are now planning to
explore the molecules related to the sensitization in a protein
array system.
Third, we tested the CMV-CTL killing activity toward
CDDP-resistant HSC-3 cells in comparison with the activity toward
the parental cells. Both of the CMVpp65-CTL lines showed a high
killing activity toward CDDP-resistant HSC-3 cells that was the
same as that toward parental HSC-3 cells (Fig. 9). Although CDDP has been clinically
validated as being effective in a broad spectrum of cancers, the
patients frequently acquire resistance and show heavy side effects.
Therefore, combination therapy of CDDP with other cancer drugs has
been applied as novel therapeutic strategies. Immunotherapy is
considered to be an attractive combination with CDDP because the
molecular mechanisms of action of CDDP (33) are different from the CTL killing
mechanism (34), and the molecules
related to CDDP resistance, such as membrane transporters, heat
shock proteins, small GTPases, transcription factors, and DNA
repair enzymes (35) do not affect
the CTL killing activity. Our results suggest, therefore, a
possible combination of immunotherapy with CDDP.
Several studies have been published on combinational
vaccine therapy with CDDP and/or 5-FU. It was thus demonstrated
that adenovirus encoding ovalbumin (Ad5-OVA) together with a low
dose of 5-FU had a synergistic impact on survival in
tumor-challenged mice. Enhancement of OVA-specific CTLs combined
with elimination of tumor bulk were observed (36). It has also been demonstrated in
mice that 5-FU combined with thymidine synthase (TS) peptide
vaccine prevented the occurrence of tumors formed by inoculation of
autologous (TS+)EL-4/HHD lymphoma cells (37). Our results may support these
observations. In addition, in a clinical study, an increase was
demonstrated in the immunological response in cases of personalized
peptide vaccination combined with UFT and UZEL in metastatic
colorectal cancer patients, and increases in immunological
responses were also reported in advanced gastric or colorectal
carcinoma patients receiving personalized peptide vaccine in
combination with TS-1 (38,39).
Furthermore, it was reported that a peptide vaccine in combination
with 5-FU and CDDP, or with chemo-radiation combined with 5-FU and
CDDP effectively induced the peptide-specific CTLs (40,41).
Collectively, our results and previous studies
indicate that adoptive immunotherapy, conducted in our hospital
using T-cells activated by autologous cancer tissue combined with
FP treatment, might be possible by optimization of the CDDP
concentration. This treatment is expected to have improved clinical
effects, although FP treatment with a high dose of CDDP inhibited
the induction of antigen-specific-CTLs. In addition, the treatment
may be expected to be effective in patients with CDDP-resistant
tumors and in those who cannot withstand the strong side effects by
CDDP.
Recently, it has been shown that immune checkpoint
systems and immunosuppressive cells, such as regulatory T-cells, M2
macrophages and MDSC, play important roles in creating
immunosuppressive microenvironments in cancer, which result in
tumor progression (24). The
immune checkpoint inhibitors, ipilimumab (anti-CTLA4), nivolumab
and pembrolizumab (anti-PD-1) have demonstrated excellent clinical
effects beyond the conventional chemotherapy or molecular target
therapy (38,42,43).
It has also been reported that depletion of Tregs by mogamulizumab
(anti-CCR4) enhances cancer immune responses both in an in
vitro study (44) and in a
clinical study (45). Presently,
modulation and regulation of immunosuppressive microenvironments in
cancer have become major directions for development of cancer
therapy. Since chemical drugs are effective not only in direct
cytotoxicity but also in modulation of immune microenvironments in
cancer, the development of combined therapies with chemical drugs
and immunotherapies for many types of advanced cancers have become
recent challenging issues. Adoptive immunotherapy developed in our
hospital showed clinical effects with long-term survival even in
stage IV patients with oral carcinomas, but 75% of the patients
were non-responders (25).
Immunosuppressive microenvironments in the cancer tissues might be
considered as one of the reasons for poor responses. Development of
comprehensive combined therapy with FP treatment, including immune
checkpoint blockade and/or regulation of immunosuppressive cells,
is considered to be necessary for improving the clinical effects of
adoptive immunotherapy for HNC.
In conclusion, although the specific proliferation
of CMVpp65-CTL by stimulation with CMVpp65 epitope peptide was
partially affected by FP treatment, it was not affected by 5-FU,
and the cytokine release response to CMVpp65 T-cell epitope peptide
was not affected by FP treatment. Moreover, the cytotoxicity of
CMVpp65-CTL toward OSCC cell lines overexpressing CMVpp65 antigen
was not affected by CDDP and/or 5-FU, and it was identical toward
CDDP-resistant cells and parent cells. These observations indicate
that immunotherapy combined with FP treatment is effective in
advanced HNC including CDDP-resistant cases and in physically weak
patients who cannot withstand the strong side effects of CDDP.
Abbreviations:
|
5-FU
|
5-fluorouracil
|
|
B-LCL
|
Epstein-Barr virus-transformed B
cell
|
|
CDDP
|
cisplatin
|
|
CMV
|
cytomegalovirus
|
|
CTL
|
cytotoxic T-lymphocyte
|
|
DC
|
dendritic cell
|
|
EGFR
|
epidermal growth factor receptor
|
|
HLA
|
human leucocyte antigen
|
|
HNC
|
head and neck cancer
|
|
mAb
|
monoclonal antibody
|
|
MDSC
|
myeloid-derived suppressive cell
|
|
MLPC
|
mixed lymphocyte peptide culture
|
|
MHC
|
major histocompatibility complex
|
|
OSCC
|
oral squamous cell cancer
|
|
PBMC
|
peripheral blood mononuclear cell
|
|
Treg
|
regulatory T-cell
|
References
|
1
|
Chaturvedi AK, Anderson WF,
Lortet-Tieulent J, Curado MP, Ferlay J, Franceschi S, Rosenberg PS,
Bray F and Gillison ML: Worldwide trends in incidence rates for
oral cavity and oropharyngeal cancers. J Clin Oncol. 31:4550–4559.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hori M, Matsuda T, Shibata A, Katanoda K,
Sobue T and Nishimoto H; Japan Cancer Surveillance Research Group:
Cancer incidence and incidence rates in Japan in 2009: A study of
32 population-based cancer registries for the Monitoring of Cancer
Incidence in Japan (MCIJ) project. Jpn J Clin Oncol. 45:884–891.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gibson MK, Li Y, Murphy B, Hussain MH,
DeConti RC, Ensley J and Forastiere AA; Eastern Cooperative
Oncology Group: Randomized phase III evaluation of cisplatin plus
fluorouracil versus cisplatin plus paclitaxel in advanced head and
neck cancer (E1395): An intergroup trial of the Eastern Cooperative
Oncology Group. J Clin Oncol. 23:3562–3567. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Forastiere AA, Goepfert H, Maor M, Pajak
TF, Weber R, Morrison W, Glisson B, Trotti A, Ridge JA, Chao C, et
al: Concurrent chemotherapy and radiotherapy for organ preservation
in advanced laryngeal cancer. N Engl J Med. 349:2091–2098. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Adelstein DJ, Li Y, Adams GL, Wagner H Jr,
Kish JA, Ensley JF, Schuller DE and Forastiere AA: An intergroup
phase III comparison of standard radiation therapy and two
schedules of concurrent chemoradiotherapy in patients with
unresectable squamous cell head and neck cancer. J Clin Oncol.
21:92–98. 2003. View Article : Google Scholar
|
|
6
|
Cooper JS, Pajak TF, Forastiere AA, Jacobs
J, Campbell BH, Saxman SB, Kish JA, Kim HE, Cmelak AJ, Rotman M, et
al Radiation Therapy Oncology Group 9501/Intergroup: Postoperative
concurrent radiotherapy and chemotherapy for high-risk
squamous-cell carcinoma of the head and neck. N Engl J Med.
350:1937–1944. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vermorken JB, Mesia R, Rivera F, Remenar
E, Kawecki A, Rottey S, Erfan J, Zabolotnyy D, Kienzer HR, Cupissol
D, et al: Platinum-based chemotherapy plus cetuximab in head and
neck cancer. N Engl J Med. 359:1116–1127. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Laramore GE, Scott CB, al-Sarraf M,
Haselow RE, Ervin TJ, Wheeler R, Jacobs JR, Schuller DE, Gahbauer
RA, Schwade JG, et al: Adjuvant chemotherapy for resectable
squamous cell carcinomas of the head and neck: Report on Intergroup
Study 0034. Int J Radiat Oncol Biol Phys. 23:705–713. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bernier J, Domenge C, Ozsahin M,
Matuszewska K, Lefèbvre JL, Greiner RH, Giralt J, Maingon P,
Rolland F, Bolla M, et al European Organization for Research and
Treatment of Cancer Trial 22931: Postoperative irradiation with or
without concomitant chemotherapy for locally advanced head and neck
cancer. N Engl J Med. 350:1945–1952. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brockstein B, Haraf DJ, Rademaker AW, Kies
MS, Stenson KM, Rosen F, Mittal BB, Pelzer H, Fung BB, Witt ME, et
al: Patterns of failure, prognostic factors and survival in
locoregionally advanced head and neck cancer treated with
concomitant chemoradiotherapy: A 9-year, 337-patient,
multi-institutional experience. Ann Oncol. 15:1179–1186. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
de Biasi AR, Villena-Vargas J and
Adusumilli PS: Cisplatin-induced antitumor immunomodulation: A
review of preclinical and clinical evidence. Clin Cancer Res.
20:5384–5391. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hato SV, Khong A, de Vries IJ and
Lesterhuis WJ: Molecular pathways: The immunogenic effects of
platinum-based chemotherapeutics. Clin Cancer Res. 20:2831–2837.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Apetoh L, Ladoire S, Coukos G and
Ghiringhelli F: Combining immunotherapy and anticancer agents: The
right path to achieve cancer cure? Ann Oncol. 26:1813–1823. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Alizadeh D and Larmonier N:
Chemotherapeutic targeting of cancer-induced immunosuppressive
cells. Cancer Res. 74:2663–2668. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Correale P, Aquino A, Giuliani A,
Pellegrini M, Micheli L, Cusi MG, Nencini C, Petrioli R, Prete SP,
De Vecchis L, et al: Treatment of colon and breast carcinoma cells
with 5-fluorouracil enhances expression of carcinoembryonic antigen
and susceptibility to HLA-A(*)02.01 restricted,
CEA-peptide-specific cytotoxic T cells in vitro. Int J Cancer.
104:437–445. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Correale P, Del Vecchio MT, Di Genova G,
Savellini GG, La Placa M, Terrosi C, Vestri M, Urso R, Lemonnier F,
Aquino A, et al: 5-fluorouracil-based chemotherapy enhances the
antitumor activity of a thymidylate synthase-directed polyepitopic
peptide vaccine. J Natl Cancer Inst. 97:1437–1445. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bergmann-Leitner ES and Abrams SI:
Treatment of human colon carcinoma cell lines with anti-neoplastic
agents enhances their lytic sensitivity to antigen-specific
CD8+ cytotoxic T lymphocytes. Cancer Immunol Immunother.
50:445–455. 2001. View Article : Google Scholar
|
|
18
|
Yang S and Haluska FG: Treatment of
melanoma with 5-fluorouracil or dacarbazine in vitro sensitizes
cells to antigen-specific CTL lysis through perforin/granzyme- and
Fas-mediated pathways. J Immunol. 172:4599–4608. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Iwase M, Watanabe H, Kondo G, Ohashi M and
Nagumo M: Enhanced susceptibility of oral squamous cell carcinoma
cell lines to FAS-mediated apoptosis by cisplatin and
5-fluorouracil. Int J Cancer. 106:619–625. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Panaretakis T, Kepp O, Brockmeier U,
Tesniere A, Bjorklund AC, Chapman DC, Durchschlag M, Joza N,
Pierron G, van Endert P, et al: Mechanisms of pre-apoptotic
calreticulin exposure in immunogenic cell death. EMBO J.
28:578–590. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Elliott MR, Chekeni FB, Trampont PC,
Lazarowski ER, Kadl A, Walk SF, Park D, Woodson RI, Ostankovich M,
Sharma P, et al: Nucleotides released by apoptotic cells act as a
find-me signal to promote phagocytic clearance. Nature.
461:282–286. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Apetoh L, Ghiringhelli F, Tesniere A,
Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E,
Saulnier P, et al: Toll-like receptor 4-dependent contribution of
the immune system to anticancer chemotherapy and radiotherapy. Nat
Med. 13:1050–1059. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ramakrishnan R, Assudani D, Nagaraj S,
Hunter T, Cho HI, Antonia S, Altiok S, Celis E and Gabrilovich DI:
Chemotherapy enhances tumor cell susceptibility to CTL-mediated
killing during cancer immunotherapy in mice. J Clin Invest.
120:1111–1124. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Suzuki S, Ishida T, Yoshikawa K and Ueda
R: Current status of immunotherapy. Jpn J Clin Oncol. 46:191–203.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ohtani T, Yamada Y, Furuhashi A, Ohmura Y,
Nakamura S, Kato H, Yoshikawa K and Kazaoka Y: Activated cytotoxic
T-lymphocyte immunotherapy is effective for advanced oral and
maxillofacial cancers. Int J Oncol. 45:2051–2057. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mescher MF: Molecular interactions in the
activation of effector and precursor cytotoxic T lymphocytes.
Immunol Rev. 146:177–210. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Halle S, Halle O and Förster R: Mechanisms
and dynamics of T cell-mediated cytotoxicity in vivo. Trends
Immunol. 38:432–443. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Klenerman P and Oxenius A: T cell
responses to cytomegalovirus. Nat Rev Immunol. 16:367–377. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Watanabe K, Suzuki S, Kamei M, Toji S,
Kawase T, Takahashi T, Kuzushima K and Akatsuka Y: CD137-guided
isolation and expansion of antigen-specific CD8 cells for potential
use in adoptive immunotherapy. Int J Hematol. 88:311–320. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ishiyama M, Miyazono Y, Sasamoto K, Ohkura
Y and Ueno K: A highly water-soluble disulfonated tetrazolium salt
as a chromogenic indicator for NADH as well as cell viability.
Talanta. 44:1299–1305. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kuzushima K, Hayashi N, Kimura H and
Tsurumi T: Efficient identification of HLA-A*2402-restricted
cytomegalovirus-specific CD8(+) T-cell epitopes by a computer
algorithm and an enzyme-linked immunospot assay. Blood.
98:1872–1881. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wills MR, Carmichael AJ, Mynard K, Jin X,
Weekes MP, Plachter B and Sissons JG: The human cytotoxic
T-lymphocyte (CTL) response to cytomegalovirus is dominated by
structural protein pp65: Frequency, specificity, and T-cell
receptor usage of pp65-specific CTL. J Virol. 70:7569–7579.
1996.PubMed/NCBI
|
|
33
|
Dasari S and Tchounwou PB: Cisplatin in
cancer therapy: Molecular mechanisms of action. Eur J Pharmacol.
740:364–378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Galandrini R, Capuano C and Santoni A:
Activation of lymphocyte cytolytic machinery: Where are we? Front
Immunol. 4:3902013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shen DW, Pouliot LM, Hall MD and Gottesman
MM: Cisplatin resistance: A cellular self-defense mechanism
resulting from multiple epigenetic and genetic changes. Pharmacol
Rev. 64:706–721. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Geary SM, Lemke CD, Lubaroff DM and Salem
AK: The combination of a low-dose chemotherapeutic agent,
5-fluorouracil, and an adenoviral tumor vaccine has a synergistic
benefit on survival in a tumor model system. PLoS One.
8:e679042013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Robert C, Thomas L, Bondarenko I, O'Day S,
Weber J, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ, et al:
Ipilimumab plus dacarbazine for previously untreated metastatic
melanoma. N Engl J Med. 364:2517–2526. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hattori T, Mine T, Komatsu N, Yamada A,
Itoh K, Shiozaki H and Okuno K: Immunological evaluation of
personalized peptide vaccination in combination with UFT and UZEL
for metastatic colorectal carcinoma patients. Cancer Immunol
Immunother. 58:1843–1852. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sato Y, Fujiwara T, Mine T, Shomura H,
Homma S, Maeda Y, Tokunaga N, Ikeda Y, Ishihara Y, Yamada A, et al:
Immunological evaluation of personalized peptide vaccination in
combination with a 5-fluorouracil derivative (TS-1) for advanced
gastric or colorectal carcinoma patients. Cancer Sci. 98:1113–1119.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Masuzawa T, Fujiwara Y, Okada K, Nakamura
A, Takiguchi S, Nakajima K, Miyata H, Yamasaki M, Kurokawa Y, Osawa
R, et al: Phase I/II study of S-1 plus cisplatin combined with
peptide vaccines for human vascular endothelial growth factor
receptor 1 and 2 in patients with advanced gastric cancer. Int J
Oncol. 41:1297–1304. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Iinuma H, Fukushima R, Inaba T, Tamura J,
Inoue T, Ogawa E, Horikawa M, Ikeda Y, Matsutani N, Takeda K, et
al: Phase I clinical study of multiple epitope peptide vaccine
combined with chemoradiation therapy in esophageal cancer patients.
J Transl Med. 12:842014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Borghaei H, Paz-Ares L, Horn L, Spigel DR,
Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al:
Nivolumab versus docetaxel in advanced nonsquamous non-small-cell
lung cancer. N Engl J Med. 373:1627–1639. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Motzer RJ, Escudier B, McDermott DF,
George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G,
Plimack ER, et al: CheckMate 025 Investigators: Nivolumab versus
everolimus in advanced renal-cell carcinoma. N Engl J Med.
373:1803–1813. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sugiyama D, Nishikawa H, Maeda Y, Nishioka
M, Tanemura A, Katayama I, Ezoe S, Kanakura Y, Sato E, Fukumori Y,
et al: Anti-CCR4 mAb selectively depletes effector-type
FoxP3+CD4+ regulatory T cells, evoking
antitumor immune responses in humans. Proc Natl Acad Sci USA.
110:17945–17950. 2013. View Article : Google Scholar
|
|
45
|
Kurose K, Ohue Y, Wada H, Iida S, Ishida
T, Kojima T, Doi T, Suzuki S, Isobe M, Funakoshi T, et al: Phase Ia
Study of FoxP3+ CD4 Treg depletion by infusion of a
humanized anti-CCR4 antibody, KW-0761, in cancer patients. Clin
Cancer Res. 21:4327–4336. 2015. View Article : Google Scholar : PubMed/NCBI
|