Introduction
Cancer is one of the greatest public health burdens
worldwide, causing abnormal cell growth through DNA mutation that
often leads to death (1).
Metastatic tumors, in particular, result in 90% of human cancer
deaths (2). Cancer metastasis
contributes to both morbidity and mortality, and it is the most
challenging target of effective cancer treatment (3). Metastatic cells detach from the
primary tumor, travel to other sites through the circulatory or
lymphatic system, and adhere and proliferate in distant organs,
resulting in highly incurable and fatal diseases (3,4).
Recent research has focused on understanding the precise mechanism
of metastasis in order to prevent various steps in the process
(5).
In addition, there is emerging evidence that
telomerase is a promising target for the development of new cancer
therapies (6,7). Elevated telomerase activity has been
found in approximately 90% of cancer cells (8). Cancer cells can be immortalized by
maintaining the telomere length, which is regulated by telomerase.
Reactivation or increased expression of telomerase leads to
continuous proliferation of cancer cells (9).
Natural products have been used in human medicine
since ancient times. For several decades, plants and plant-derived
compounds have been used to combat cancer often with fewer side
effects than traditional treatments (10). Since plants have been a useful
source of approved anticancer agents, many efforts have been
undertaken to investigate the wide range of pharmacological effects
of plants against various types of cancer (11,12).
Myrmecodia (Rubiaceae) is a genus of the
epiphytic myrmecophytes or ant-plant, which is locally known as
Sarang Semut. This genus is native to Southeast Asia, but it can
also be found in Myanmar, Indochina, and northern Queensland in
Australia (13). Myrmecodia
plants contain a highly specialized tuber and modified stems, which
are used by ant colonies (14).
Myrmecodia species have traditionally been used to treat
diseases such as ulcers, tumors, cancer, hepatitis, and coronary
artery disease (15).
In pharmacological studies, Myrmecodia
pendens and tuberose are both considered to be potential
anticancer agents. Water extracts of Myrmecodia pendens have
been shown to inhibit HeLa and MCM-B2 cancer cell growth (16). Five flavonoids of Myrmecodia
pendens, kaempferol, luteolin, rutin, quercetin, and apigenin,
were identified and quantified, and some or all of these phenolic
compounds may have anticancer properties (17). Ethanol extracts of Myrmecodia
tuberose have been shown to suppress tumor growth and induce
apoptosis of oral carcinoma cells (18). In addition, this plant extract
significantly increased macrophage phagocytosis and lymphocyte
proliferation in doxorubicin-induced immunosuppressed rats
(19). Since one of the main side
effects of doxorubicin in cancer chemotherapy is immunosuppression,
this research supports the notion that Myrmecodia tuberose
could be used as a complementary agent for cancer treatment by
improving immune responses (20,21).
Although Myrmecodia platytyrea Becc. is
commonly used for cancer treatment by local residents throughout
Southeast Asia, there has been little scientific investigation
about its pharmacological activity. Bioactive compounds such as
stigmasterol, morindolide, and flavonoids have been found in the
tuber of the plant (22). The
tuber extract has demonstrated toxic effects on HepG2 cells without
affecting normal monkey kidney cells, suggesting this extract
inhibits the growth of human hepatocellular carcinoma (HCC)
(23). However, very little is
known about the biological activity of Myrmecodia platytyrea
Becc. leaves. This study investigated the potential anticancer
activity of the methanol extract of Myrmecodia platytyrea
Becc. leaves (MMPL) in HCC cells and the mechanism underlying these
effects.
Materials and methods
MMPL preparation
The methanol extract of Myrmecodia platytyrea
Becc. leaves (Voucher no. KRIBB0047070) was purchased from the
International Biological Material Research Center in Korea Research
Institute of Bioscience and Biotechnology (KRIBB, http://www.ibmrc.re.kr, Daejeon, Korea). A voucher
specimen (KRIBB0047070) was deposited at KRIBB. The extract was
diluted in dimethyl sulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO,
USA) and added directly to the culture media. To avoid cell damage,
the final concentration of DMSO never exceeded 1%.
Cell culture and reagents
Human hepatoma cell lines with different metastatic
potentials, including SK-Hep1 with high metastatic potential and
Huh7 with low metastatic potential (Korean Cell Line Bank, Seoul,
Korea), were maintained in Dulbecco's modified Eagle's medium
(DMEM) or Roswell Park Memorial Institute 1640 (RPMI-1640)
supplemented with 10% fetal bovine serum (FBS), 50 U/ml penicillin
and 50 µg/ml streptomycin (Gibco-BRL, Grand Island, NY, USA)
at 37°C in a humidified 5% CO2 atmosphere.
Rabbit polyclonal anti-signal transducer and
activator of transcription 3 (STAT3, cat no. sc-482), rabbit
polyclonal anti-Akt (cat no. sc-8312), rabbit polyclonal
anti-phospho (p)-Akt (Ser473, cat no. sc-7985), mouse monoclonal
antic-Jun N-terminal kinase (JNK, cat no. sc-7345), rabbit
polyclonal anti-inhibitor of κBα (IκBα, cat no. sc-371), and mouse
monoclonal anti-α-tubulin (cat no. sc-8035) antibodies were
obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
Rabbit polyclonal anti-uPAR (cat no. 9692), rabbit polyclonal
anti-p-STAT3 (Tyr705, cat no. 9131), rabbit polyclonal anti-p-p38
(Thr180/Tyr182, cat no. 9211), rabbit polyclonal anti-p38 (cat no.
9212), rabbit polyclonal anti-extrcellular signal-regulated kinase
(ERK, cat no. 9102), mouse monoclonal anti-p-ERK (Thr202/Tyr204,
cat no. 9106), and rabbit polyclonal anti-p-JNK (Thr183/Tyr185, cat
no. 9251) were purchased from Cell Signaling Technology Inc.
(Danvers, MA, USA). 5-Fluorouracil (5-FU) was from Sigma-Aldrich
and BIBR1532 was from ApexBio Technology (Houston, TX, USA). Emodin
was purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo,
Japan).
Cell viability assay
Cells were seeded in 24-well plates
(1–1.5×105 cells/well) and treated with various
concentrations of MMPL for either 24 h or 48 h with media
containing 1% or 10% FBS. Cell viability was evaluated using
EZ-Cytox solution (Daeil Lab, Seoul, Korea) according to the
manufacturer's instructions. Briefly, EZ-Cytox solution was added
to each well and the plate was incubated for 1 h at 37°C. The
optical density (OD) of the supernatants was measured at 450 nm
using a Synergy H1 Microplate Reader (BioTek Instruments, Winooski,
VT, USA).
Wound healing assay
SK-Hep1 and Huh7 cells were seeded in 6-well plates
(0.5–1×106 cells/well) and cultured until they reached
90% confluence. The wound area was created using a scratcher tip
(0.5 mm; SPL Life Sciences, Gyeonggi-do, Korea). The detached cells
were removed with phosphate-buffered saline (PBS) supplemented with
media containing 1% FBS and treated with the indicated
concentration of MMPL for 24 h. The cells that migrated into the
wound surface were observed using a JuLI Stage Real-Time Cell
History Recorder (NanoEnTek Inc., Seoul, Korea). The change in
wound closure is represented as the percent of wound recovery. All
experiments were performed in triplicate.
Transwell invasion and migration
assay
SK-Hep1 cells (5×104 cells/well) in 250
µl of media containing 1% FBS were added to the upper
chamber with or without MMPL, and the lower chamber was filled with
500 µl of media containing 10% FBS. The plates were
incubated at 37°C and 5% CO2 for 18 h. For the Matrigel
invasion assay, 24-Transwell plates (pore size, 8 µm) were
coated with 30 µl of diluted Matrigel (0.5 mg/ml; BD
Biosciences, Franklin Lakes, NJ, USA) for 2 h, and the cells were
seeded onto the insert with or without MMPL for 18 h. The membranes
to which cells migrated were fixed in 3.7% paraformaldehyde in PBS,
and cells adhering to the upper surface of the membrane were
removed with a cotton swab. The invasive cells were stained with
0.5% crystal violet. After being dried, five random fields of the
membrane were observed and counted by averaging the total number of
cells at ×100 magnification.
Luciferase assay
A luciferase reporter plasmid containing the uPAR
(−682/+27) promoter region (pcDNA3.1-uPAR (−682/+27)-luc) was
constructed with the insertion of a 709 bp polymerase chain
reaction (PCR) product (using the specific primers: sense, 5′-GGG
GCT AGA TCT GAT TCT CCT GCC TCA GCC TC-3′ with BglII site
and antisense, 5′-CCG GAA TTC GGG TCC CTG CAC GTC TTC TC-3′ with
EcoRI site) into the BglII and EcoRI sites of the plasmid
pcDNA3.1-luc, which contains the luciferase gene. For luciferase
assays, the cells were co-transfected with pcDNA3.1-uPAR
(−682/+27)-luc along with a gWIZ-green fluorescence protein (GFP)
plasmid using polyethylenimine (PEI, Polysciences, Inc.,
Warrington, PA, USA); transfected cells were then divided into
12-well plates (1×105 cells/well). After 24 h of
incubation, cells were treated with various concentrations of MMPL
for 24 h. Cells were then lysed with passive lysis buffer (Promega,
Madison, WI, USA) and promoter activity was assayed according to
the manufacturer′s instructions. Cell lysates were assayed for GFP
expression and the relative luciferase activity was normalized
against the levels of GFP expression.
Gelatin zymography
The activity of matrix metalloproteinases (MMP-2 and
MMP-9) was assayed by gelatin zymography (24). Briefly, SK-Hep1 cells
(3.5×106 cells/well) were seeded in 6-well culture
plates and incubated in serum-free medium in the presence of
indicated concentrations of MMPL for 24 h, and the cultured media
were collected. The samples were mixed with 5X sample buffer [312.5
mM Tris-Cl (pH 6.8), 5% sodium dodecyl sulfate (SDS), 50% glycerol,
and 0.05% bromophenol blue] and separated by 10% SDS-polyacrylamide
gel electrophoresis (PAGE) containing 0.1% gelatin. The gels were
washed with wash buffer [50 mM Tris-HCl (pH 7.5), 0.2 M NaCl, 5 mM
CaCl2, 0.1 µM ZnCl, and 2.5% Triton X-100) twice
for 30 min at room temperature (RT) and then incubated in substrate
buffer [50 mM Tris-HCl (pH 7.5), 0.2 M NaCl, 5 mM CaCl2,
0.1 µM ZnCl2, 0.016% NaN3, and 1%
Triton X-100] at 37°C for 18 h. The gels were fixed with fixing
solution (40% methanol and 10% acetic acid) for 30 min and then
stained with staining solution (0.25% Coomassie brilliant blue
R-250, 45% methanol, and 10% acetic acid) for 1 h, followed by
destaining with destaining solution (5% methanol and 8% acetic
acid). The zymography gels were scanned using an HP Photosmart
image scanner (HP Development Co., L.P., Palo Alto, CA, USA). The
band intensities were quantified using LabWorks Analysis software
(UVP, LLC, Upland, CA, USA).
Telomerase repeat amplification protocol
(TRAP) assay
Telomerase activity was measured by a modified TRAP
method (25). SK-Hep1 cells
(3.5×106 cells/well) were seeded in 6-well culture
plates and incubated with media containing 1% FBS in the presence
of indicated concentrations of MMPL for 24 h. Cells were lysed with
CHAPS lysis buffer [0.5% CHAPS, 10 mM Tris-HCl (pH 7.5), 1 mM
MgCl2, 1 mM ethylenediaminetetraacetic acid (EDTA), 0.1
mM phenylmethylsulfonyl fluoride (PMSF), 5 mM β-mercaptoethanol,
and 10% glycerol] on ice for 30 min. Cell lysates were centrifuged
at 13,000 × g for 30 min at 4°C, and the supernatants were used for
the TRAP assays.
For the direct telomerase activity assay with
endogenous telomerase, cell lysates were mixed with different
concentrations of MMPL for 30 min on ice before the TRAP assay was
performed as previously described (26). Cell extracts (2 µg) were
mixed with 5 µl of 10X TRAP reaction buffer [200 mM Tris-HCl
(pH 8.3), 15 mM MgCl2, 630 mM KCl, 0.5% Tween-20 and 10
mM EDTA], 400 µg/ml BSA, 2.5 mM dNTP, 150 ng of TS
(telomerase substrate oligonucleotide) primer (5′-AAT CCG TCG AGC
AGA GTT-3′), 100 ng of ACX reverse primer [5′-GCG CGG (CTT ACC)3
CTA ACC-3′], 150 ng of NT internal control primer (5′-ATC GCT TCT
CGG CCT TTT-3′), 0.1 pM TSNT (substrate for 36 bp internal standard
control) primer (5′-AAT CCG TCG AGC AGA GTT AAA AGG CCG AGA AGC
GAT-3′), and 5 U of Taq polymerase (Dynebio Inc., Seoul, Korea).
The incubation was performed at 25°C for 30 min. PCR was then
performed (95°C for 5 min followed by 20 cycles at 95°C for 30 sec,
52°C for 30 sec and 72°C for 30 sec). The reaction mixture (total
volume of 50 µl) mixed with 5 µl of loading solution
(0.25% bromophenol blue, 50% glycerol, and 50 mM EDTA) was
separated on a 12% non-denaturing PAGE gel for 45 min at 200 V.
Gels were stained with ethidium bromide staining solution for 3 min
and visualized with a UV illuminator.
For the expression of human telomerase catalytic
unit (hTERT) and human telomerase RNA (hTR), reverse-transcription
polymerase chain reaction (RT-PCR) was carried out. Total RNA was
extracted using Accuzol solution (Bioneer, Daejeon, Korea) and 1
µg of total RNA was used for cDNA synthesis. Relative mRNA
expression levels were normalized to the expression levels of
glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The PCR products
were separated on a 2% agarose gel and visualized with a UV
illuminator. PCR was performed on the cDNA using the following
primers; hTERT, 5′-CGG AAG AGT GTC TGG AGC AA-3′ (forward)
and 5′-GGA TGA AGC GGA GTC TGG A-3′ (reverse) (27); hTR, 5′-ACC CTA ACT GAG AAG
GGC GT-3′ (forward) and 5′-GCC AGC AGC TGA CAT TTT TT-3′ (reverse)
(28); GAPDH, 5′-GTC TTC
ACC ACC ATG GAG AAG G-3′ (forward) and 5′-CCT GCT TCA CCA CCT TCT
TGA T-3′ (reverse).
Immunoblotting analysis
SK-Hep1 cells were seeded with 10% FBS-containing
media. When cells reached 80% confluence, they were treated with
the indicated concentrations of MMPL for 24 h. Total proteins were
extracted in lysis buffer containing 20 mM Tris-HCl (pH 7.5), 150
mM NaCl, 1 mM EDTA, 0.5% Triton X-100, 0.5% IGEPAL, 10% glycerol, 1
mM dithiothreitol, and 1 mM PMSF. Concentrations of soluble cell
lysates were measured using Bradford protein assay kit (Bio-Rad,
Hercules, CA, USA) and the samples were boiled at 100°C for 5 min.
Next, 20–40 µg of cell lysates were separated on a 10%
polyacrylamide gel and transferred onto a nitrocellulose membrane
as described (29). The membrane
was blocked in 5% non-fat dry milk for 1 h at RT and incubated with
appropriate antibodies with 5% BSA or 5% non-fat dry milk, followed
by incubation with a secondary antibody conjugated to horseradish
peroxidase. The immunoreactive bands were visualized using an ECL
system (Pierce, Rockford, IL, USA) and a cooled charge-device
camera system (AE-9150; ATTO Technology, Tokyo, Japan). The band
intensities were quantified using LabWorks Analysis software.
Statistical analysis
All data represent three independent experiments and
are expressed as means ± standard error of the mean (SEM).
Statistical analyses were performed with a one-way ANOVA of
Dunnett's Multiple Comparison method using Prism 3.0 (GraphPad
Software, San Diego, CA, USA) and p<0.05, p<0.01, and
p<0.001 were considered to indicate statistically
significant.
Results
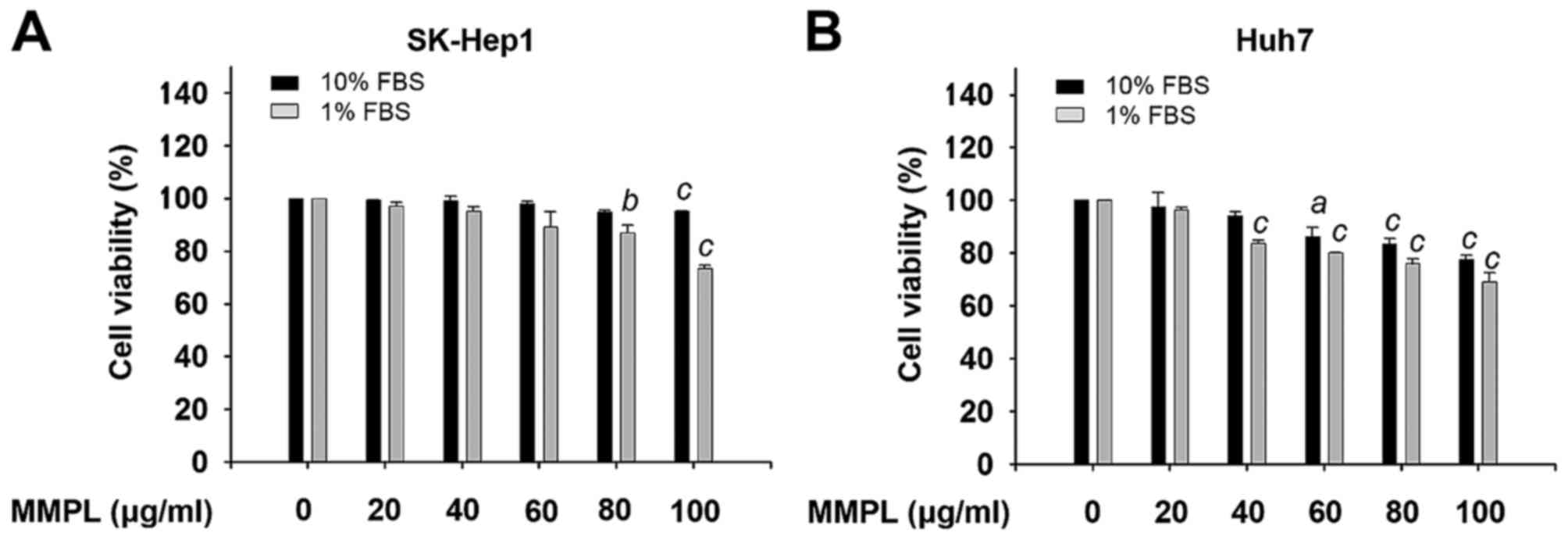
Effects of MMPL on cell viability
To investigate the cytotoxic effect of MMPL on
hepatoma cells, SK-Hep1 and Huh7 cells were incubated with various
concentrations of MMPL (0, 20, 40, 60 and 100 µg/ml) for 24
h in cell culture media with 1% or 10% FBS. Cell viability was
examined using EZ-Cytox solution and the effect of MMPL on cell
viability is expressed as a percentage of the control (Fig. 1). For SK-Hep1 cells, the results
showed little or no effect on cell viability in cell culture media
with 1% and 10% FBS and ≤80 µg/ml of MMPL (Fig. 1A). For Huh7 cells, MMPL slightly
inhibited cell viability in a dose-dependent manner but it did not
induce cytotoxicity until the concentration was >40 µg/ml
(Fig. 1B). Therefore, MMPL
concentrations ≤80 µg/ml in SK-Hep1 cells and 40
µg/ml in Huh7 cells were used throughout this study to
eliminate the effect of MMPL-induced cytotoxicity on cell migration
and invasion.
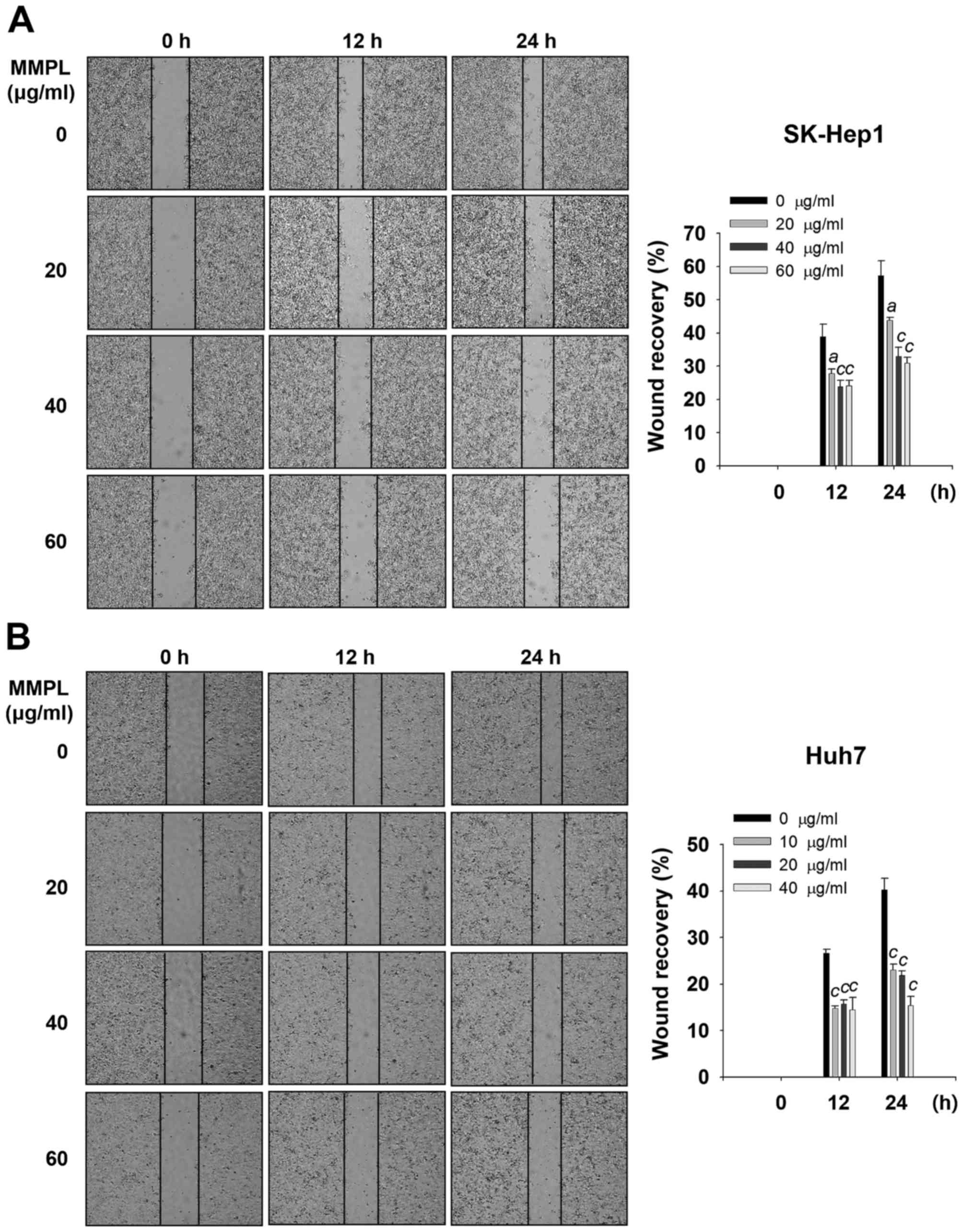
Inhibitory effects of MMPL on cell
migration and invasion
Tumor progression requires cells to migrate and
induce degradation of the extracellular matrix (ECM) or basement
membrane (BM) for efficient cell invasion (30). Wound healing assays were performed
to evaluate the effect of MMPL on cell migration. Cells were
treated with various concentrations of MMPL and the cell confluence
within the wound regions was measured using a Real-Time Cell
History Recorder. The results showed that MMPL significantly
suppressed wound closure in a dose- and time-dependent manner
(Fig. 2A and B). When SK-Hep1
cells were treated with MMPL for 24 h, the percentage of wound
recovery was decreased from 57.2±4.6% (0 µg/ml) to 30.9±1.8%
(60 µg/ml) (Fig. 2A). For
Huh7 cells, MMPL also inhibited wound recovery in a dose-dependent
manner from 40.3±2.3% (0 µg/ml) to 15.4±1.9% (40
µg/ml) (Fig. 2B).
To further investigate the effect of MMPL on the
motility of SK-Hep1 cells, migration and invasion assays were
performed using a Transwell chamber. As shown in Fig. 2C, the result indicated that MMPL
inhibited the migration of SK-Hep1 cells in a dose-dependent manner
compared to the control group; the motility decreased by 54.8±9.5%
after treatment with 60 µg/ml MMPL.
The BM and ECM environments, composed of collagen,
fibronectin, and laminin, are the first barriers that cancer cells
must overcome for invasion (31).
Thus, invasion assays were conducted using a Matrigel-coated
Transwell chamber to determine whether MMPL reduces the invasive
potency of SK-Hep1 cells. MMPL significantly inhibited the invasion
of SK-Hep1 cells in a dose-dependent manner (Fig. 2C). Results showed that cell
invasion decreased by 54.7±5.1% after treatment with 60
µg/ml MMPL. Taken together, these results suggest that MMPL
has an inhibitory effect on the metastatic properties of hepatoma
cell lines.
Inhibitory effects of MMPL on MMP-2 and
MMP-9 activity
In cancer progression, MMPs are essential for the
facilitation of cellular metastasis (32). Through the urokinase-type
plasminogen activator (uPA)-uPAR signaling pathway, the expression
and activation of MMPs are promoted, which enhances ECM degradation
(33). Among MMPs, MMP-2 and MMP-9
are highly activated type IV collagenases which play critical roles
in tumor invasion and metastasis by facilitating enzymatic
digestion of the BM (34–36). Thus, the enzymatic activity of
MMP-2 and MMP-9 was measured using gelatin zymography after SK-Hep1
cells were treated with various concentrations of MMPL (0, 20, 40
and 60 µg/ml) for 24 h. As shown in Fig. 3A, the constitutive secretion of low
levels of MMP-2 and high levels of MMP-9 by untreated SK-Hep1 cells
was observed. When cells were treated with MMPL, the activity of
MMP-2 and MMP-9 was significantly inhibited in a dose-dependent
manner. These results indicate that the inhibitory effect of MMPL
on cell migration and invasion might be due to the downregulation
of MMP-2 and MMP-9 activity.
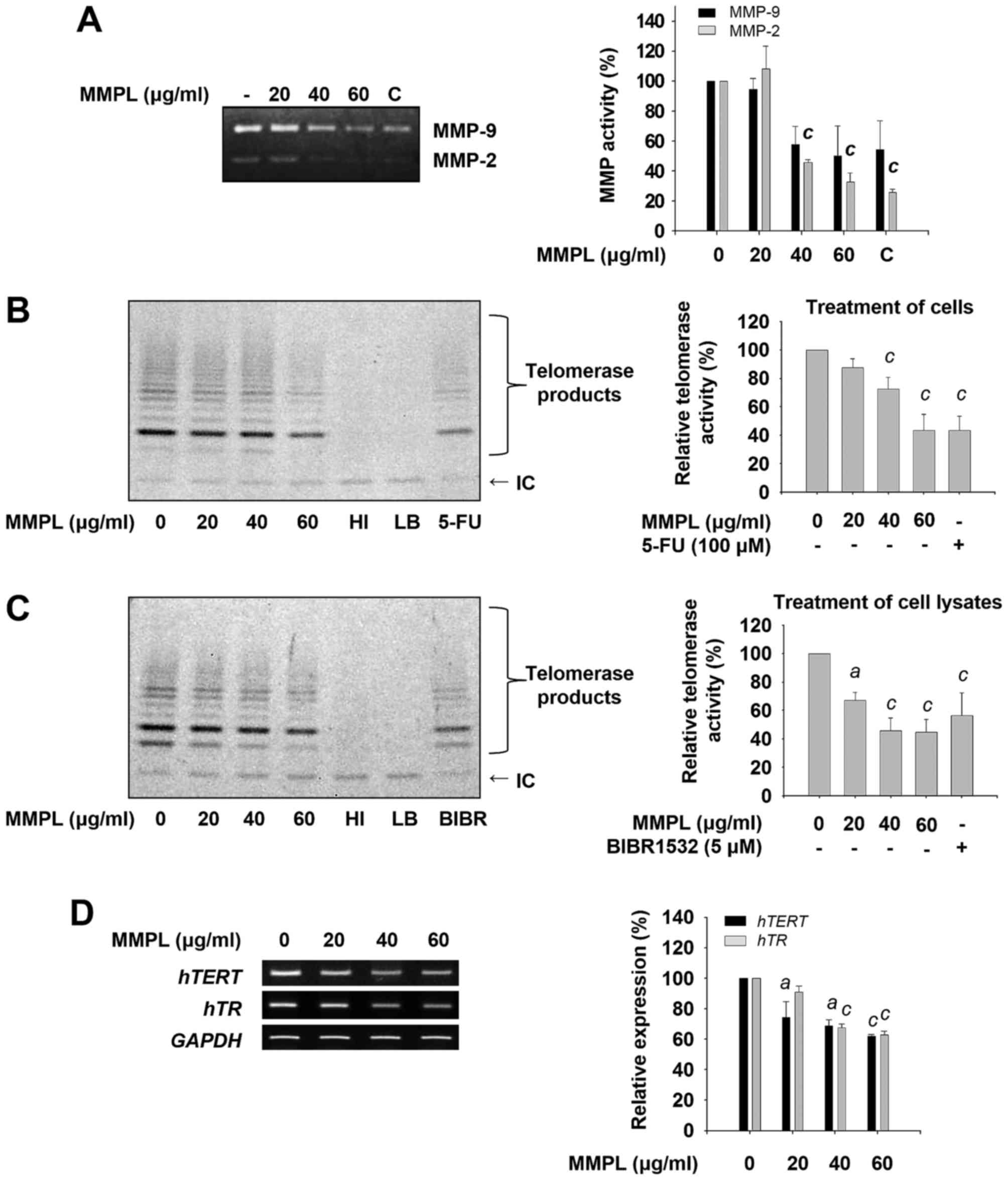 | Figure 3Effects of MMPL on MMP-2, MMP-9, and
telomerase activity. (A) SK-Hep1 cells were treated with MMPL (0,
20, 40 and 60 µg/ml) in serum-free media for 24 h. The
cultured media were collected and the activity of secreted MMP-2
and MMP-9 was determined using gelatin zymography as described in
Materials and methods. Emodin (10 µM) was used as a positive
control (C). (B) SK-Hep1 cells were treated with MMPL (0, 20, 40
and 60 µg/ml) in media containing 1% FBS for 24 h.
Telomerase activity was determined by performing a TRAP assay with
cell lysates. (C) To detect the direct effect of MMPL on telomerase
activity, cell lysates were incubated with indicated concentrations
of MMPL for 3 h and then the TRAP assay was performed in a
cell-free condition. (D) SK-Hep1 cells were treated with MMPL (0,
20, 40 and 60 µg/ml) in media containing 1% FBS for 24 h.
RT-PCR was carried out to measure the expression of hTERT
and hTR. Expression of GAPDH was used as an internal
control. Data shown are a representative of three experiments and
expressed as the means ± SEM (n=3). ap<0.05,
bp<0.01, and cp<0.001 relative to the
MMPL-untreated control group. IC, internal control; HI, heat
inactivation; LB, lysis buffer; 5-FU, 5-fuorouracil. |
Inhibitory effects of MMPL on telomerase
activity
Recently, telomerase has been considered critical
for carcinogenesis (9,37). To investigate whether MMPL inhibits
telomerase activity, SK-Hep1 cells were treated with MMPL.
Subsequently, the TRAP assay was performed using cell lysates to
determine telomerase activity. As shown in Fig. 3B, telomerase activity was
suppressed by MMPL in a dose-dependent manner. 5-FU, which was used
as a positive control (38), also
inhibited telomerase activity.
To determine whether MMPL can directly suppress the
enzymatic activity of telomerase, a telomerase activity assay was
carried out after cell lysates were treated with MMPL. The results
presented in Fig. 3C show a
significant decrease in telomerase activity by MMPL compared with
the known telomerase inhibitor BIBR1532 (39).
Telomerase has two core components, hTERT and hTR
(40). When SK-Hep1 cells were
treated with MMPL, the mRNA levels of hTERT and hTR
were also inhibited in a dose-dependent manner (Fig. 3D). Collectively, MMPL can directly
inhibit the enzymatic activity of telomerase and indirectly
diminish its activity through the downregulation of hTERT
and hTR expression, suggesting telomerase is a potential
target of MMPL in hepatoma cells.
Inhibitory effects of MMPL on uPAR,
STAT3, and ERK signaling pathways
Since increased expression of uPAR has been reported
in several types of cancer, uPAR is an attractive therapeutic
target for the treatment of cancer metastasis (41). uPAR regulates ECM proteolysis by
binding to uPA and promotes cell migration, invasion, and
metastasis at cellular surfaces (42). Since MMPL significantly blocked
cell motility and the activity of MMPs, reporter assays using a
luciferase reporter plasmid containing the uPAR (−682/+27) promoter
region were carried out in SK-Hep1 and Huh7 cells. Cells were
transfected with pcDNA3.1-uPAR (−682/+27)-luc and then treated with
indicated concentrations of MMPL (0, 20, 40, 60 and 80
µg/ml) for 24 h. As shown in Fig. 4A, uPAR promoter activity was
suppressed by MMPL in a dose-dependent manner in SK-Hep1 and Huh7
cells. Immunoblotting analysis with cell lysates obtained from
MMPL-treated SK-Hep1 cells was performed to further investigate the
effect of MMPL on the level of uPAR protein. As shown in Fig. 4B and C, uPAR protein expression
decreased in a dose-dependent manner. Taken together, these results
suggest that MMPL can attenuate transactivation of uPAR in hepatoma
cells.
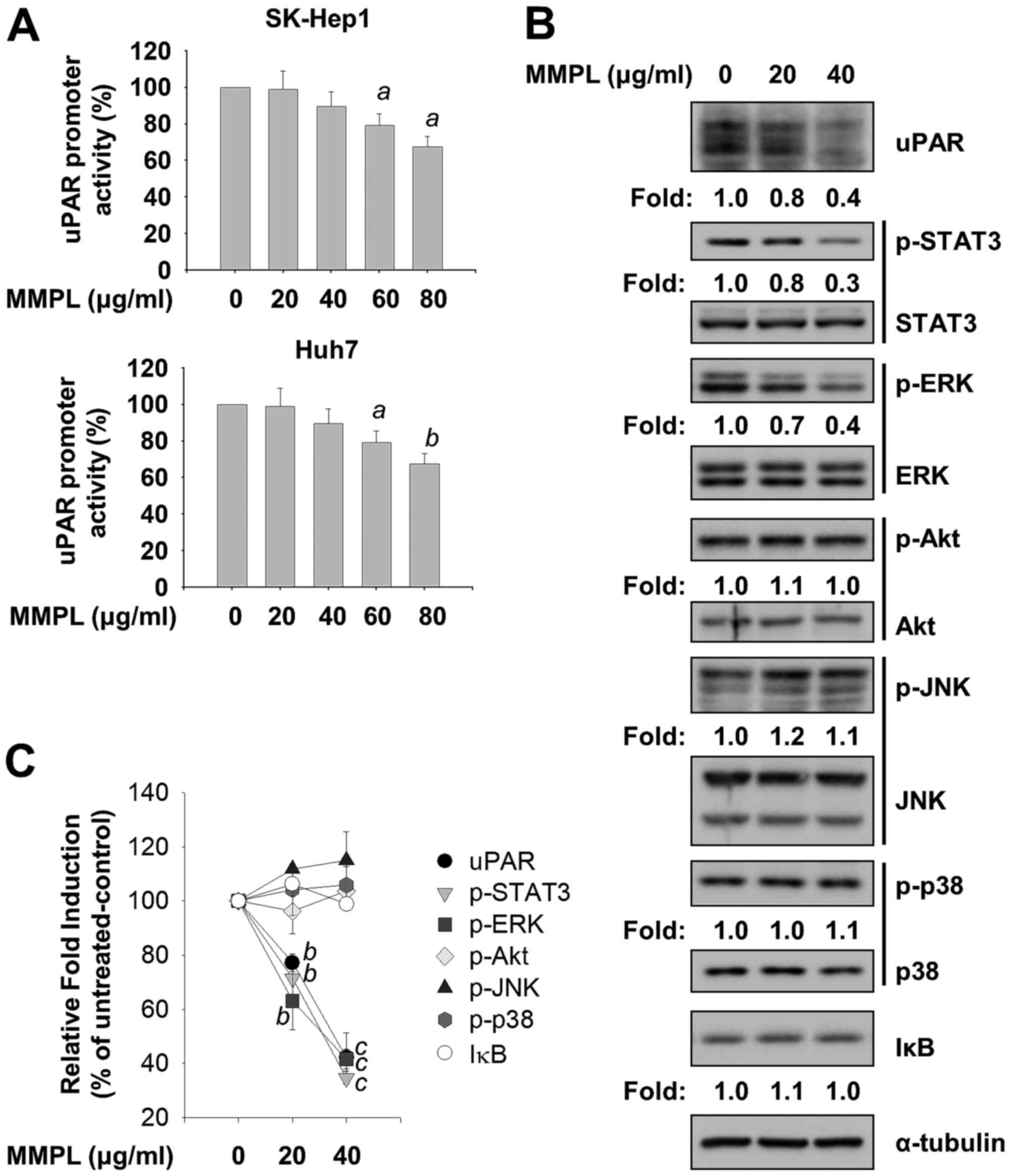 | Figure 4Effects of MMPL on uPAR, ERK, and
STAT3 signaling pathway. (A) Following transfection with
pcDNA3.1-uPAR (−682/+27)-luc and gWIZ-GFP as an internal control,
SK-Hep1 and Huh7 cells were treated with MMPL (0, 20, 40, 60 and 80
µg/ml) in media containing 10% FBS for 24 h. Total cell
lysates were prepared using passive lysis buffer and uPAR
luciferase activity was measured using a microplate reader after
reacting the lysates with luciferin as a substrate. (B) SK-Hep1
cells were treated with MMPL (0, 20 and 40 µg/ml) in media
containing 1% FBS for 24 h. Total cell lysates were harvested and
separated by SDS-PAGE as described in Materials and methods. The
expression levels of uPAR, p-STAT3 (Tyr705), STAT3, p-ERK
(Thr202/Tyr204), ERK, p-Akt (Ser473), Akt, p-JNK (Thr183/Tyr185),
JNK, p-p38 (Thr180/Tyr182), p38, and IκB were detected by specific
antibodies. α-tubulin was used as a loading control. (C) The graph
illustrates the quantitative analysis data of immunoblot band
intensities shown in (B). Data shown are normalized to α-tubulin
expression and expressed as the means ± SEM (n=3).
ap<0.05, bp<0.01, and
cp<0.001 relativeto the MMPL-untreated control
group. |
Many signaling pathways, such as uPA/uPAR, Janus
kinase (JAK)/STAT, phosphatidylinositol-3-kinase (PI3K)/Akt,
mitogen-activated protein kinase (MAPK), and nuclear factor kappa-B
(NF-κB), are involved in human cancer progression (43–46).
To examine how MMPL suppresses the metastatic ability of hepatoma
cells by targeting signaling pathways, the expression levels of
phosphorylated STAT3, MAPKs, Akt, and inhibitor of κBα (IκBα) were
measured using immunoblotting analysis after incubating SK-Hep1
cells with indicated concentrations of MMPL (0, 20 and 40
µg/ml) for 24 h. As shown in Fig. 4B and C, phosphorylation levels of
STAT3 at Tyr705 were dose-dependently suppressed by MMPL in SK-Hep1
cells without changing the total protein levels of STAT3. In
addition, among three conventional MAPKs (ERK, JNK, and p38), MMPL
inhibited the phosphorylation levels of ERK at Thr202/Tyr204 in a
dose-dependent manner without changing the total protein levels of
ERK. To examine the regulation of PI3K/Akt, one of the most
frequently altered pathways in human cancer (47), we investigated the change in
phosphorylation levels of Akt at Ser473 by MMPL. However, no
significant change was found. Since NF-κB activity is involved in
the regulation of uPAR expression (48) and because its transcriptional
activity is regulated by the phosphorylation dependent-proteasomal
degradation of IκB (49), the
expression levels of IκBα protein were also measured. We found that
MMPL did not affect the levels of IκBα. Taken together, these
results suggest that the anticancer effects of MMPL in hepatoma
cells are regulated through inhibition of the STAT3 and ERK
pathways.
Discussion
HCC, a primary malignancy of the liver, is now the
third leading cause of cancer death worldwide (50). There has been increasing interest
in characterizing the molecular mechanisms of HCC in order to
develop novel therapeutic approaches (51). In this study, the suppressive
action of MMPL as a potential anticancer agent was explored in
hepatoma cells.
Recently, the development and design of drugs
affecting multiple targets or pathways have emerged for cancer
treatment (52). Natural products
such as curcumin, berberine, and baicalein are rich reservoirs due
to their structural and chemical diversity; therefore, the
discovery of drug candidates by screening natural products is
considered to be an effective strategy for the development of new
anticancer drugs (53,54). Natural products-derived
chemotherapeutic drugs have shown cancer chemopreventive activity
whereas many of common chemotherapeutic drugs such as doxorubicin,
5-FU, bleomycin, and cyclophosphamide cause toxic side-effects as a
results of chemotherapeutic treatments (55–59).
Therefore, the discovery of drug candidates by screening natural
products is considered to be an effective strategy for the
development of new anticancer drugs. This study found that MMPL
inhibits cell migration and invasion by inhibiting MMPs and uPAR
via the regulation of the ERK and STAT3 pathways; MMPL may
therefore be a good candidate to improve HCC treatment.
In addition, the toxicity of many anticancer agents
on normal tissues and organs often limits their use. Since side
effects occur with almost all anticancer agents (60), novel anticancer agents must
overcome this obstacle. In previous studies, normal monkey kidney
Vero cells were not susceptible to the cytotoxicity of the methanol
extract of Myrmecodia platytyrea Becc. with an
IC50 value of 0.76 mg/ml, and oral administration of the
extract was not harmful to mice. However, the extract was toxic to
human hepatoma HepG2 cells with an IC50 value of 0.07
mg/ml, suggesting Myrmecodia platytyrea Becc. has the
potential to treat liver cancer without affecting normal cells
(23,61).
Proteolytic enzymes, including metallo-, serine-,
aspartyl-, and cysteine proteases, play critical roles in cancer
progression and metastasis (62).
uPA, a serine protease, binds to uPAR with high affinity and
facilitates the conversion of plasminogen to plasmin; plasmin then
degrades the ECM and activates other proteases, such as MMPs,
leading to tissue remodeling for cancer invasion and progression
(62). It has been shown that high
expression levels of MMPs and uPA correlate with aggressive
behavior of HCC growth and metastasis (35,63).
Therefore, suppressing the expression or activity of these
proteases has been considered a valuable target against HCC. In
this study, MMPL clearly inhibited the migration and invasion of
SK-Hep1 cells (Fig. 2).
Furthermore, MMPL suppressed protein expression levels of uPAR as
well as the enzymatic activity of MMP-2 and MMP-9 in a
dose-dependent manner (Figs. 3 and
4). This indicated that MMPL
inhibits the metastatic activity of hepatoma cells through the
downregulation of uPAR expression, thereby contributing to the
decrease in MMP activity. Further studies are needed to confirm
whether the reduction of MMP-2 and MMP-9 activity is due to their
decreased protein expression.
Cancer metastasis is regulated by complex molecular
mechanisms. Recent studies have demonstrated that PI3K/Akt and
MAPKs, such as ERK, JNK, and p38, are involved in regulating the
expression of MMPs, uPA, as well as uPAR. Furthermore, the STAT3
signaling pathway plays a critical role in the regulation of MMPs
and uPAR. These studies imply that the inhibition of these pathways
represents a potential target for new anti-metastasis therapies. In
the present study, MMPL inhibited the phosphorylation levels of ERK
at Thr202/Tyr204 and STAT3 at Tyr705 in a dose-dependent manner,
but it did not inhibit phosphorylation of JNK at Thr183/Tyr185 or
p38 at Thr180/Tyr182 (Fig. 4B).
Collectively, these results suggest that the anti-metastatic
activity of MMPL is mediated through the inhibition of the ERK and
STAT3 pathways.
In most normal cells, telomerase activity is minimal
after embryonic differentiation except in germ cells, stimulated
lymphocytes, and stem cells (8,64,65).
Therefore, the length of telomeres decreases with age and normal
cells have a limited capacity to divide. However, upregulated
telomerase activity has been detected in most cancer cells and
contributes to the proliferation of cancer (9). In addition, cancer initiating cells
are likely to have short telomeres compared with normal cells,
suggesting cancer cells are more susceptible to telomerase
inhibition (66). Therefore,
telomerase is considered to be a useful target in cancer treatment.
Two major components of telomerase, hTERT and hTR, remain suitable
targets since their expression and activity are essential for the
proper functioning of telomerase (67,68).
Many signaling pathways such as PI3K/Akt/mTOR, MAPK,
and JAK/STAT are involved in the regulation of telomerase
expression and activity (69–71).
It has been reported that ERK1/2 promotes the expression and
activation of TERT by stimulating Ets-1, Sp-1, and c-Myc
transcriptional factors (69,72).
Furthermore, STAT3 activation induces TERT expression by binding to
the TERT gene promoter region in various cancer cell lines
(71). In the present study, MMPL
suppressed telomerase activity and mRNA levels of hTR and
hTERT (Fig. 3). In
addition, the phosphorylation levels of ERK and STAT3 were
inhibited by MMPL (Fig. 4B and C).
Taken together, these data suggest that MMPL inhibits telomerase
expression and activity by suppressing the ERK and STAT3 pathways.
Moreover, MMPL can directly inhibit telomerase activity (Fig. 3C). MMPL might represent a promising
new candidate for anti-telomerase therapies.
Phytochemical investigation of Myrmecodia
platytyrea Becc. has been poorly studied. The plant has been
reported to possess several compounds such as liquiritigenin,
stigmasterol, morindolide, calycosin, 2-(2-methylbutyryl)
phloroglucinol glucoside, and an acylated flavanone (22). Liquiritigenin is known to
downregulate MMP-2/9 and PI3K/AKT signaling pathway that are
involved in cancer metastasis (73). Calycosin also inhibits breast
cancer cell growth by regulating p38 and AKT signaling pathways
(74). In addition, stigmasterol
induces apoptosis in human hepatoma cells (75). Since MMPL exhibits anticancer
activity by inhibiting ERK and STAT3 signaling pathways rather than
regulating p38 and AKT activities, further studies are needed to
identify the possible active ingredient of the plant for anticancer
effects.
Taken together, the results of this study suggest
that MMPL has anticancer and anti-metastatic properties in HCC;
therefore, MMPL can serve as an effective therapeutic agent for
treating human liver cancer.
Acknowledgments
This study was supported by the National Research
Foundation of Korea (NRF) grant funded by the Ministry of Science,
ICT and Future Planning (NRF-2015R1A2A2A11001446, and
2015R1A5A1008958) and the Chung-Ang University Excellent Student
Scholarship in 2013.
References
|
1
|
Krishnamurthi K: Screening of naturla
products for anticancer and antidiabetic properties. Health
Administrator. XX:69–75. 2000.
|
|
2
|
Steeg PS: Tumor metastasis: Mechanistic
insights and clinical challenges. Nat Med. 12:895–904. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Seyfried TN and Huysentruyt LC: On the
origin of cancer metastasis. Crit Rev Oncog. 18:43–73. 2013.
View Article : Google Scholar :
|
|
4
|
Wells A, Grahovac J, Wheeler S, Ma B and
Lauffenburger D: Targeting tumor cell motility as a strategy
against invasion and metastasis. Trends Pharmacol Sci. 34:283–289.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Steeg PS and Theodorescu D: Metastasis: A
therapeutic target for cancer. Nat Clin Pract Oncol. 5:206–219.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Agrawal A, Dang S and Gabrani R: Recent
patents on anti-telomerase cancer therapy. Recent Patents
Anticancer Drug Discov. 7:102–117. 2012. View Article : Google Scholar
|
|
7
|
Holysz H, Lipinska N, Paszel-Jaworska A
and Rubis B: Telomerase as a useful target in cancer fighting-the
breast cancer case. Tumour Biol. 34:1371–1380. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim NW, Piatyszek MA, Prowse KR, Harley
CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL and Shay
JW: Specific association of human telomerase activity with immortal
cells and cancer. Science. 266:2011–2015. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jafri MA, Ansari SA, Alqahtani MH and Shay
JW: Roles of telomeres and telomerase in cancer, and advances in
telomerase-targeted therapies. Genome Med. 8:69–88. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Demain AL and Vaishnav P: Natural products
for cancer chemotherapy. Microb Biotechnol. 4:687–699. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Norikura T, Kojima-Yuasa A, Shimizu M,
Huang X, Xu S, Kametani S, Rho SN, Kennedy DO and Matsui-Yuasa I:
Mechanism of growth inhibitory effect of Blumea balsamifera extract
in hepatocellular carcinoma. Biosci Biotechnol Biochem.
72:1183–1189. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Machana S, Weerapreeyakul N, Barusrux S,
Thumanu K and Tanthanuch W: Synergistic anticancer effect of the
extracts from Polyalthia evecta caused apoptosis in human hepatoma
(HepG2) cells. Asian Pac J Trop Biomed. 2:589–596. 2012. View Article : Google Scholar
|
|
13
|
Huxley CR: The ant-plants Myrmecodia and
Hydnophytum (Rubiaceae), and the relationships between their
morphology, ant occupants, physiology and ecology. New Phytol.
80:231–268. 1978. View Article : Google Scholar
|
|
14
|
Lok AFSL and Tan HTW: Tuberous, epiphytic,
rubiaceous myrmecophytes of singapore. Nat Singap. 2:231–236.
2009.
|
|
15
|
Subroto MA and Saputro H: Gempur Penyakit
Dengan Sarang Semut. Jakarta Penebar Swadaya. 11–22. 2006.In
Indonesian.
|
|
16
|
Soeksmanto A, Subroto MA, Wijaya H and
Simanjuntak P: Anticancer activity test for extracts of Sarang
semut plant (Myrmecodya pendens) to HeLa and MCM-B2 cells. Pak J
Biol Sci. 13:148–151. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Engida AM, Kasim NS, Tsigie YA, Ismadji S,
Huynh LH and Ju Y-H: Extraction, identification and quantitative
HPLC analysis of flavonoids from sarang semut (Myrmecodia pendan).
Ind Crops Prod. 41:392–396. 2013. View Article : Google Scholar
|
|
18
|
Yuletnawati SE, Meiyanto E and Agustina D:
High antitumor activity of ethanolic extracts of Papua's ant nest
plant (Myrmecodia tuberosa) on an oral carcinoma (KB) cell line.
Int J Sci Res. 5:1619–1623. 2016.
|
|
19
|
Sumardi, Hertiani T and Sasmito E: Ant
plant (Myrmecodia tuberosa) hypocotyl extract modulates
TCD4+ and TCD8+ cell profile of
doxorubicin-induced immune-suppressed sprague dawley rats in vivo.
Sci Pharm. 81:1057–1069. 2013. View Article : Google Scholar
|
|
20
|
Zhang XY, Li WG, Wu YJ and Gao MT:
Amelioration of doxorubicin-induced myocardial oxidative stress and
immunosuppression by grape seed proanthocyanidins in tumour-bearing
mice. J Pharm Pharmacol. 57:1043–1052. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sultana R, Di Domenico F, Tseng M, Cai J,
Noel T, Chelvarajan RL, Pierce WD, Cini C, Bondada S, Clair DK St,
et al: Doxorubicin-induced thymus senescence. J Proteome Res.
9:6232–6241. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mohamad Haris NF, Nik Hasan MK, Wahab IA,
Hasan MH, Ponto T and Adam A: Compounds from the antioxidant active
fraction of M. platytyrea. J Sains Kesihatan Malaysia. 14:23–29.
2016. View Article : Google Scholar
|
|
23
|
Mizaton HH, Samad MA, Wahab IA, Mohamad
Haris NF, Ponto T and Adam A: Toxicological evaluation of
Myrmecodia platytyrea. 2010 International Conference on Science and
Social Research; Kuala Lumpur. 2010, View Article : Google Scholar
|
|
24
|
Lai KC, Huang AC, Hsu SC, Kuo CL, Yang JS,
Wu SH and Chung JG: Benzyl isothiocyanate (BITC) inhibits migration
and invasion of human colon cancer HT29 cells by inhibiting matrix
metalloproteinase-2/-9 and urokinase plasminogen (uPA) through PKC
and MAPK signaling pathway. J Agric Food Chem. 58:2935–2942. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang L, Suwa T, Wright WE, Shay JW and
Hornsby PJ: Telomere shortening and decline in replicative
potential as a function of donor age in human adrenocortical cells.
Mech Ageing Dev. 122:1685–1694. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pascolo E, Wenz C, Lingner J, Hauel N,
Priepke H, Kauffmann I, Garin-Chesa P, Rettig WJ, Damm K and
Schnapp A: Mechanism of human telomerase inhibition by BIBR1532, a
synthetic, non-nucleosidic drug candidate. J Biol Chem.
277:15566–15572. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nakamura TM, Morin GB, Chapman KB,
Weinrich SL, Andrews WH, Lingner J, Harley CB and Cech TR:
Telomerase catalytic subunit homologs from fission yeast and human.
Science. 277:955–959. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Heine B, Hummel M, Demel G and Stein H:
Demonstration of constant upregulation of the telomerase RNA
component in human gastric carcinomas using in situ hybridization.
J Pathol. 185:139–144. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Le HTT, Cho YC and Cho S: Inhibition of
protein tyrosine phosphatase non-receptor type 2 by PTP inhibitor
XIX: Its role as a multiphosphatase inhibitor. BMB Rep. 50:329–334.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Friedl P and Wolf K: Tumour-cell invasion
and migration: Diversity and escape mechanisms. Nat Rev Cancer.
3:362–374. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gattazzo F, Urciuolo A and Bonaldo P:
Extracellular matrix: A dynamic microenvironment for stem cell
niche. Biochim Biophys Acta. 1840:2506–2519. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ellerbroek SM and Stack MS: Membrane
associated matrix metalloproteinases in metastasis. BioEssays.
21:940–949. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Danø K, Rømer J, Nielsen BS, Bjørn S, Pyke
C, Rygaard J and Lund LR: Cancer invasion and tissue
remodeling--cooperation of protease systems and cell types. APMIS.
107:120–127. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Giannelli G, Bergamini C, Marinosci F,
Fransvea E, Quaranta M, Lupo L, Schiraldi O and Antonaci S:
Clinical role of MMP-2/TIMP-2 imbalance in hepatocellular
carcinoma. Int J Cancer. 97:425–431. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yeh HC, Lin SM, Chen MF, Pan TL, Wang PW
and Yeh CT: Evaluation of serum matrix metalloproteinase (MMP)-9 to
MMP-2 ratio as a biomarker in hepatocellular carcinoma.
Hepatogastroenterology. 57:98–102. 2010.PubMed/NCBI
|
|
36
|
Chen R, Cui J, Xu C, Xue T, Guo K, Gao D,
Liu Y, Ye S and Ren Z: The significance of MMP-9 over MMP-2 in HCC
invasiveness and recurrence of hepatocellular carcinoma after
curative resection. Ann Surg Oncol. 19(Suppl 3): S375–S384. 2012.
View Article : Google Scholar
|
|
37
|
Bernardes de Jesus B and Blasco MA:
Telomerase at the intersection of cancer and aging. Trends Genet.
29:513–520. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Padmanabhan P, Otero J, Ray P, Paulmurugan
R, Hoffman AR, Gambhir SS, Biswal S and Ulaner GA: Visualization of
telomerase reverse transcriptase (hTERT) promoter activity using a
trimodality fusion reporter construct. J Nucl Med. 47:270–277.
2006.PubMed/NCBI
|
|
39
|
Barma DK, Elayadi A, Falck JR and Corey
DR: Inhibition of telomerase by BIBR 1532 and related analogues.
Bioorg Med Chem Lett. 13:1333–1336. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Podlevsky JD, Bley CJ, Omana RV, Qi X and
Chen JJL: The telomerase database. Nucleic Acids Res. 36(Database):
D339–D343. 2008. View Article : Google Scholar :
|
|
41
|
Ulisse S, Baldini E, Sorrenti S and
D'Armiento M: The urokinase plasminogen activator system: A target
for anti-cancer therapy. Curr Cancer Drug Targets. 9:32–71. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Smith HW and Marshall CJ: Regulation of
cell signalling by uPAR. Nat Rev Mol Cell Biol. 11:23–36. 2010.
View Article : Google Scholar
|
|
43
|
Helbig G, Christopherson KW II,
Bhat-Nakshatri P, Kumar S, Kishimoto H, Miller KD, Broxmeyer HE and
Nakshatri H: NF-kappaB promotes breast cancer cell migration and
metastasis by inducing the expression of the chemokine receptor
CXCR4. J Biol Chem. 278:21631–21638. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sen B and Johnson FM: Regulation of SRC
family kinases in human cancers. J Signal Transduct.
2011:8658192011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Noh H, Hong S and Huang S: Role of
urokinase receptor in tumor progression and development.
Theranostics. 3:487–495. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Steelman LS, Chappell WH, Abrams SL, Kempf
RC, Long J, Laidler P, Mijatovic S, Maksimovic-Ivanic D, Stivala F,
Mazzarino MC, et al: Roles of the Raf/MEK/ERK and
PI3K/PTEN/Akt/mTOR pathways in controlling growth and sensitivity
to therapy-implications for cancer and aging. Aging (Albany NY).
3:192–222. 2011. View Article : Google Scholar
|
|
47
|
Fresno Vara JA, Casado E, de Castro J,
Cejas P, Belda-Iniesta C and González-Barón M: PI3K/Akt signalling
pathway and cancer. Cancer Treat Rev. 30:193–204. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chang HJ, Kim MH, Baek MK, Park JS, Chung
IJ, Shin BA, Ahn BW and Jung YD: Triptolide inhibits tumor
promoter-induced uPAR expression via blocking NF-kappaB signaling
in human gastric AGS cells. Anticancer Res. 27A:3411–3417.
2007.
|
|
49
|
Oeckinghaus A and Ghosh S: The NF-kappaB
family of transcription factors and its regulation. Cold Spring
Harb Perspect Biol. 1:a0000342009. View Article : Google Scholar
|
|
50
|
McGlynn KA and London WT: The global
epidemiology of hepatocellular carcinoma: Present and future. Clin
Liver Dis. 15:223–243. vii–x. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chen C and Wang G: Mechanisms of
hepatocellular carcinoma and challenges and opportunities for
molecular targeted therapy. World J Hepatol. 7:1964–1970. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lu JJ, Pan W, Hu YJ and Wang YT:
Multi-target drugs: The trend of drug research and development.
PLoS One. 7:e402622012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Tan W, Lu J, Huang M, Li Y, Chen M, Wu G,
Gong J, Zhong Z, Xu Z, Dang Y, et al: Anti-cancer natural products
isolated from chinese medicinal herbs. Chin Med. 6:272011.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Srinivas NR: Baicalin, an emerging
multi-therapeutic agent: Pharmacodynamics, pharmacokinetics, and
considerations from drug development perspectives. Xenobiotica.
40:357–367. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Desai AG, Qazi GN, Ganju RK, El-Tamer M,
Singh J, Saxena AK, Bedi YS, Taneja SC and Bhat HK: Medicinal
plants and cancer chemoprevention. Curr Drug Metab. 9:581–591.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Avilés A, Arévila N, Díaz Maqueo JC, Gómez
T, García R and Nambo MJ: Late cardiac toxicity of doxorubicin,
epirubicin, and mitoxantrone therapy for Hodgkin's disease in
adults. Leuk Lymphoma. 1:275–279. 1993. View Article : Google Scholar
|
|
57
|
Macdonald JS: Toxicity of 5-fluorouracil.
Oncology (Williston Park). 13(Suppl 3): 33–34. 1999.
|
|
58
|
Adamson IY: Pulmonary toxicity of
bleomycin. Environ Health Perspect. 16:119–126. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Fraiser LH, Kanekal S and Kehrer JP:
Cyclophosphamide toxicity. Characterising and avoiding the problem.
Drugs. 42:781–795. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Liang XJ, Chen C, Zhao Y and Wang PC:
Circumventing tumor resistance to chemotherapy by nanotechnology.
Methods Mol Biol. 596:467–488. 2010. View Article : Google Scholar
|
|
61
|
OECD: Acute oral toxicity. Acute oral
toxic class method guideline 423 adopted 23.03.1996. Eleventh
Addendum to the, OECD, guidelines for the testing of chemicals
organisation for economical co-operation and development Paris:
June. 2000
|
|
62
|
Skrzydlewska E, Sulkowska M, Koda M and
Sulkowski S: Proteolytic-antiproteolytic balance and its regulation
in carcinogenesis. World J Gastroenterol. 11:1251–1266. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Sakamoto Y, Mafune K, Mori M, Shiraishi T,
Imamura H, Mori M, Takayama T and Makuuchi M: Overexpression of
MMP-9 correlates with growth of small hepatocellular carcinoma. Int
J Oncol. 17:237–243. 2000.PubMed/NCBI
|
|
64
|
Shay JW and Bacchetti S: A survey of
telomerase activity in human cancer. Eur J Cancer. 33:787–791.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Wright WE, Piatyszek MA, Rainey WE, Byrd W
and Shay JW: Telomerase activity in human germline and embryonic
tissues and cells. Dev Genet. 18:173–179. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Shay JW and Keith WN: Targeting telomerase
for cancer therapeutics. Br J Cancer. 98:677–683. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Collado M, Blasco MA and Serrano M:
Cellular senescence in cancer and aging. Cell. 130:223–233. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Meyerson M, Counter CM, Eaton EN, Ellisen
LW, Steiner P, Caddle SD, Ziaugra L, Beijersbergen RL, Davidoff MJ,
Liu Q, et al: hEST2, the putative human telomerase catalytic
subunit gene, is up-regulated in tumor cells and during
immortalization. Cell. 90:785–795. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Maida Y, Kyo S, Kanaya T, Wang Z, Yatabe
N, Tanaka M, Nakamura M, Ohmichi M, Gotoh N, Murakami S, et al:
Direct activation of telomerase by EGF through Ets-mediated
transactivation of TERT via MAP kinase signaling pathway. Oncogene.
21:4071–4079. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Kawauchi K, Ogasawara T, Yasuyama M,
Otsuka K and Yamada O: Regulation and importance of the
PI3K/Akt/mTOR signaling pathway in hematologic malignancies.
Anticancer Agents Med Chem. 9:1024–1038. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Konnikova L, Simeone MC, Kruger MM,
Kotecki M and Cochran BH: Signal transducer and activator of
transcription 3 (STAT3) regulates human telomerase reverse
transcriptase (hTERT) expression in human cancer and primary cells.
Cancer Res. 65:6516–6520. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Bermudez Y, Yang H, Cheng JQ and Kruk PA:
Pyk2/ERK 1/2 mediate Sp1- and c-Myc-dependent induction of
telomerase activity by epidermal growth factor. Growth Factors.
26:1–11. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Shi H, Wu Y, Wang Y, Zhou M, Yan S, Chen
Z, Gu D and Cai Y: Liquiritigenin potentiates the inhibitory
effects of cisplatin on invasion and metastasis via downregulation
MMP-2/9 and PI3 K/AKT signaling pathway in B16F10 melanoma cells
and mice model. Nutr Cancer. 67:761–770. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Chen J, Hou R, Zhang X, Ye Y, Wang Y and
Tian J: Calycosin suppresses breast cancer cell growth via
ERβ-dependent regulation of IGF-1R, p38 MAPK and PI3K/Akt pathways.
PLoS One. 9:e912452014. View Article : Google Scholar
|
|
75
|
Kim YS, Li XF, Kang KH, Ryu B and Kim SK:
Stigmasterol isolated from marine microalgae Navicula incerta
induces apoptosis in human hepatoma HepG2 cells. BMB Rep.
47:433–438. 2014. View Article : Google Scholar :
|