1. Cancer
Cancer is a syndrome of diseases wherein the cells
upon aberrant mutations/environmental stresses witness either the
activation of proto-oncogenes or the suppression of tumor
suppressor proteins. Resultantly, they undergo uncontrolled
infinite replication, lose their functional characteristics and
exploit their physiological neighbors for nutrients and space. The
process is often accompanied with malignant transformation and
metastatic spread, EMT (1),
especially when the availability of resources at a particular
cancer niche shortens. Various mechanisms like autocrine and
endocrine regulation of growth factors, platelet adhesion,
quiescence, cytoskeleton rearrangements, cancer cell
differentiation, micrometastasis, EMT, angiogenesis and niche
formation determine the outcome. WHO data states that the global
cancer burden is likely to rise to 22 million by 2030 (2), due to a variety of homeostatic
insults. Cancer cells operate via various molecular mechanisms,
popularly categorized as the cell signaling pathways. We provide an
outline of the major tumor suppressor and oncogenic pathways, which
regulate the process of human carcinogenesis.
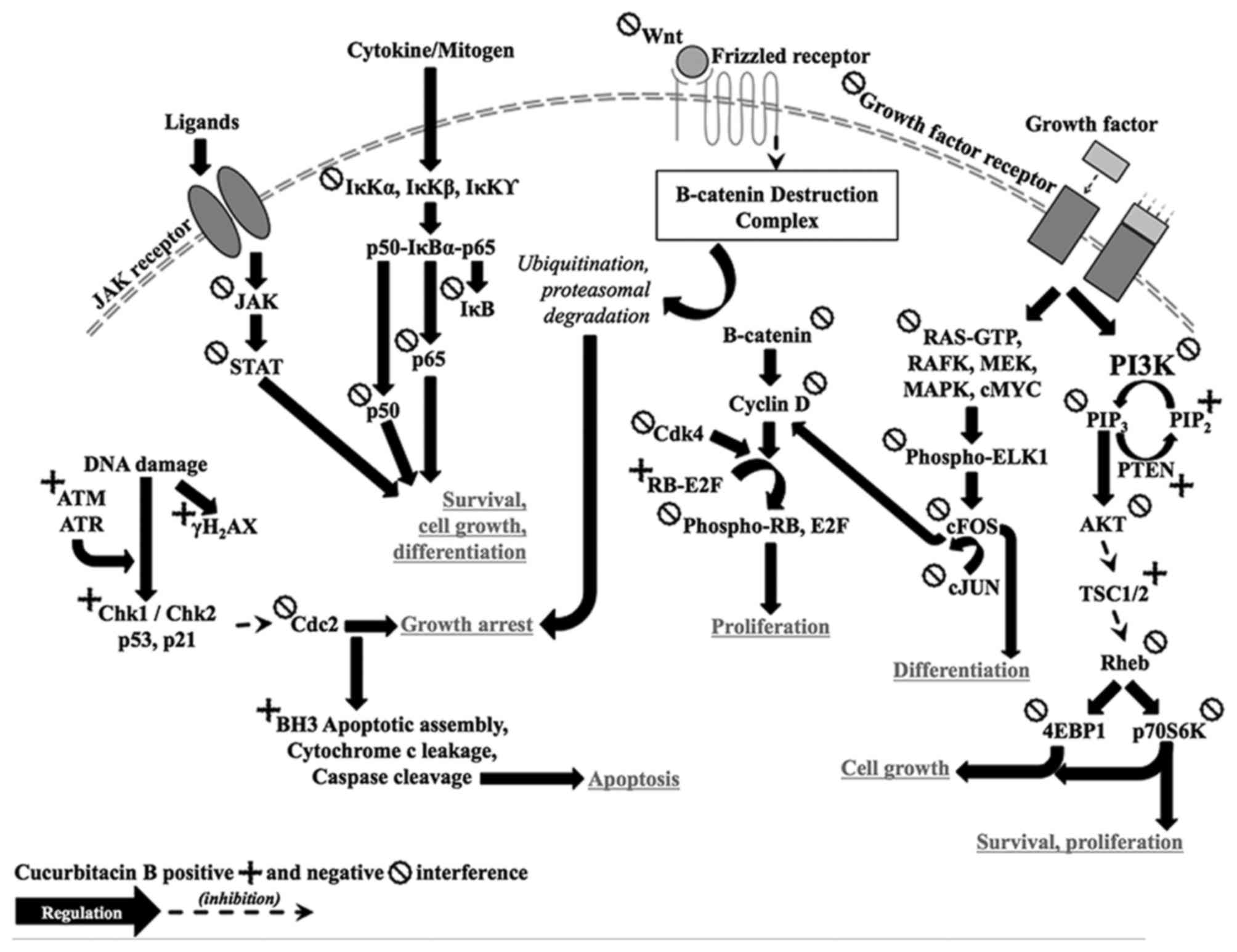
Tumor suppressor p53 protein (Fig. 1) is known as the guardian of the
genome (3–5). External or internal cellular injury
to a normal cell activates the p53 tumor suppressor pathway. It
directs the cell into growth arrest and apoptotic state via
activation of several downstream effectors including growth arrest
(p21WAF1 and others) and apoptosis (BAX, PUMA and
others) inducing proteins. Retinoblastoma (RB) protein (Fig. 1) is coded by RB1 tumor suppressor
gene (6–9). At the G1-S checkpoint,
escalated cyclin D binds with CDK4 leading to pRB phosphorylation,
abrogation of pRB-E2F complexes and free E2F, which translocates to
nucleus and activates genetic transcription via polymerase
secretion, which is crucial for proliferation.
Wnt protein interacts with frizzled receptor
extracellularly and deactivates β-catenin-destruction complex,
which is made up of GSK3, Axin, CSK1α, PP2A and APC, coded by their
respective genes (10). The
complex binds to β-catenin present in the cytoplasm and degrades it
via phosphorylation-ubiquitination-proteasomal degradation.
Mutations in any of them (ultimately resulting into cancer), most
frequently in APC, result in faulty complex structure, which fails
to bind to and degrade β-catenin. Excess β-catenin translocates to
the nucleus, binds with galectin-3, interacts with T-cell
transcription and lymphoid enhancing transcription factors, and
promotes genes, which code for cyclin D protein essential for
G1/S cell cycle transition. STAT3 is a latent
transcriptional factor in the cytoplasm, which is regulated by JAK
phosphorylation (Fig. 1) (11–18).
It directly or indirectly upregulates genes associated with tumor
proliferation and survival, and constitutes an important
intracellular signal transduction pathway in cancer, which
regulates cancer cell growth and differentiation. Inactivation or
de-phosphorylation of the protein forces cells to undergo growth
arrest and apoptosis.
Cancer tissue is fast growing and evading, central
mass of which often suffers hypoxia. Emergency mechanisms such as
HIF signaling get activated, which trigger angiogenesis, metastatic
spread of disease and resistance to chemotherapy. HIF-1 monomer is
continuously transcribed and translated, and degraded via ubiquitin
mediated proteasomal degradation under normoxic conditions. In
hypoxic condition, this degradation is downregulated, resulting
into accumulation of monomer. Accumulated monomers translocate to
the nucleus and dimerize with HIF-1β to form a complex, which
modulates transcription and translation of the target genes. NF-κB
proteins function as dimeric transcription factors and regulate the
expression of genes influencing immunological, stress and
inflammatory response (19). NF-κB
pathway proteins are normally bound to IκB and remain in an
inactive state. Pro-inflammatory cytokines and other mitogens
activate IKK complex (IKKβ-IKKα-NEMO), which phosphorylates IκB
proteins for its ubiquitination and proteasomal degradation. Free
and active NF-κB upon further phosphorylation, acetylation and
glycosylation, translocate to nucleus to induce target gene
expression.
Activated dimer of GFR phosphorylates RAS-GDP
complex to RAF-GTP, which via series of downstream phosphorylation
activates RAF kinase, MEK, MAP kinase and cMYC in cytosol and ELK1
in the nucleus (Fig. 1) (20). ELK1 further activates cFOS
transcription, which dimerize with cJUN. Activated cMYC and
cJUN-cFOS complex triggers subsequent transcription, which are
essential for DNA replication, such as cyclin D. Receptor tyrosine
kinase activates PI3K, a complex of p85 and p110 proteins (21). Activated PI3K phosphorylates PIP2
into PIP3, which via PDK1 activation phosphorylates AKT. After
downstream signaling involving TSC1/2, Rheb and mTORC1 it activates
p70S6K and 4EBP1, which via estrogen receptor further translates to
cell survival, growth, proliferation, differentiation, migration
and metabolism. Telomeres are specialized chromosome ends in the
cells consisting of tightly held and repetitive (10–15 kb) TTAGGG
sequence, and protein caps, which protect chromosomes from terminal
fusion (22,23). Every time a normal cell divides a
small portion of the telomeric end gets eroded of ~50–200 base
pairs until the bare chromosomal ends are left. At this stage, cell
enters an irreversible and non-proliferative phase called
replicative senescence. Most cancer cells acquire the ability to
synthesize enzyme telomerase, which helps to regenerate telomeric
ends. Autophagy is a conservation-indicated destructive process,
wherein the non-functional or stressed cytoplasmic organelles and
other constituents are delivered to lysosomes (24). Lysosome engulfs and digests the
material, and release energy and other important elements, which
may be utilized in cellular metabolism. It is frequently found to
take place as a response to cellular stress, but is skeptical as it
may be either protective or discretely destructive. It is
characterized by the appearance of large cytoplasmic vacuoles or
vesicles with upregulation of ATG family proteins viz., LC3II/I,
ULK1/2 and Beclin1. Stress stimuli, nutritional imbalance, hypoxia
and other chemical mediators like ROS and insults to intracellular
homeostasis may lead to induction of autophagy response.
Prevention of production of ROS or catabolic
destruction of ROS is explicitly defined as the antioxidant
mechanism. ROS are charged super-ions, continually generated and
released in the cells, presenting with vacuolization in cytosol and
reaction with biomolecules like cell organelles and genetic
material to generate peroxides and malondehyde. These alter
membrane potential and signal transduction, and may induce cell
offing mechanisms like necrosis, apoptosis and autophagy. ROS
generation increases as a result of environment insults like
pollution, radiation and infestation. Prevention and control of
cancer is one of the most expensive and least prolific healthcare
investments, on top of which it is also burdened with toxic adverse
effects associated with chemotherapy and radiotherapy. Use of
natural medicines could not only lower the expenditure towards the
disease extensively, but may also bring down the adverse effects
rate in clinical patients. Holistic approach (consumption of
natural and related molecules in the ratio prepared by nature) for
the treatment of cancer, could be adopted. Kaefer and Milner
(25) enumerated a list of
benefits if the treatment of the disease is mainly herbal, and
their probable role in cancer. Many biologically active molecules
from the herbal sources have been identified in the last few
decades, which in experiment have potential to prevent the disease,
control its growth, and possibly eradicated it completely.
Cucurbitacin B is one of the most extensively studied natural
bioactives.
2. Cucurbitacin B
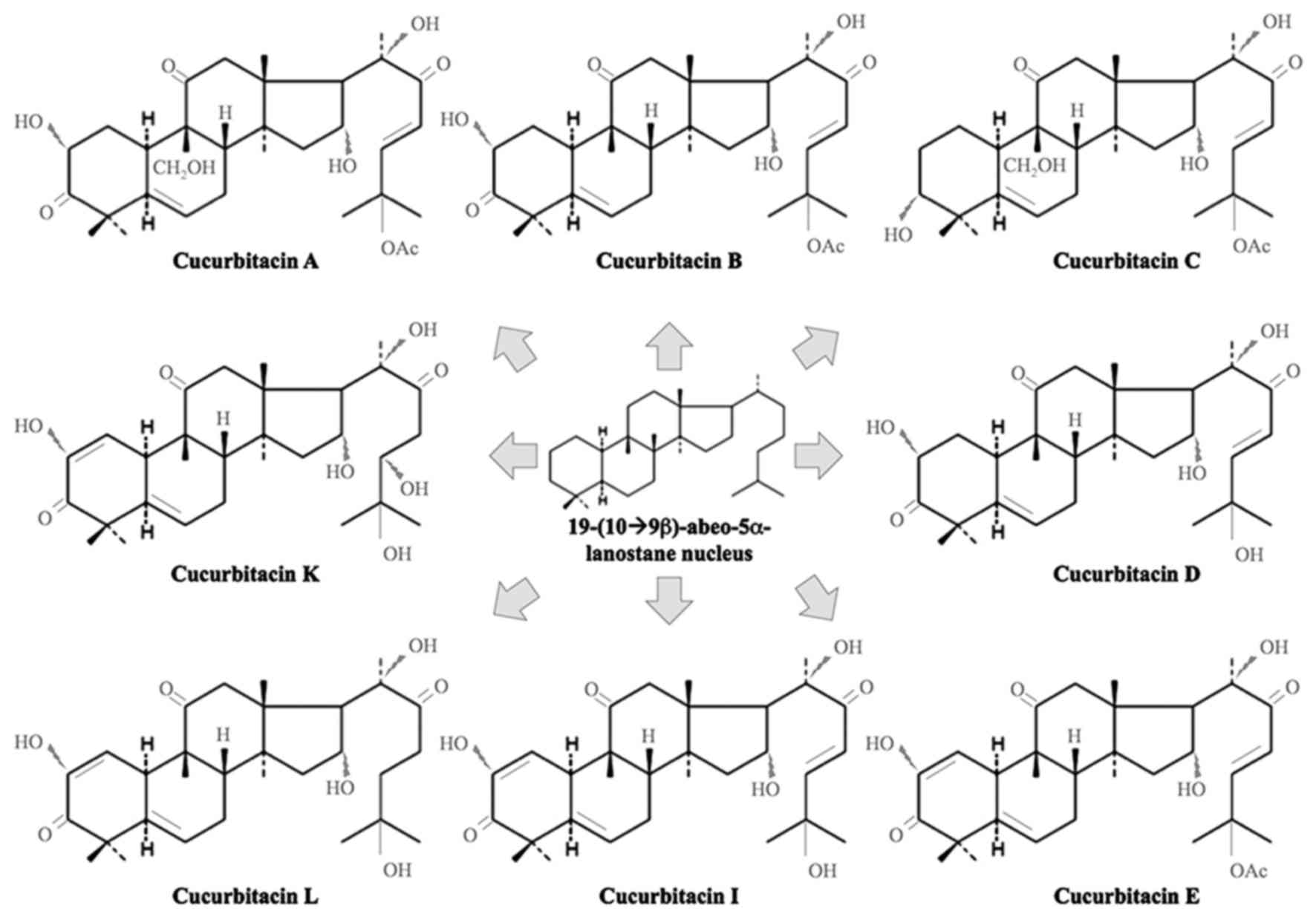
Cucurbitacins are chemically characterized by
tetracyclic cucurbitane (triterpene hydrocarbon) nucleus skeleton
19-(10➝9β)-abeo-5α-lanostane base, varied by the positional
substitution of an oxygen atom (Fig.
2) (8,26). There are mainly 40 known species of
cucurbitacins and their derivatives, divided into 12 groups namely
A, B, C, D, E, I, H, Q, R, dihydrocucurbitacin B (27). These are usually crystalline in
nature, purgative, hydrophobic and easily soluble in organic
solvents with absorption maxima for ultraviolet light ranging from
228 to 234 nm, having exceptions such as cucurbitacin H, which is
usually an amorphous solid. Various studies have been conducted to
examine the effects of these compounds in various cell lines in
vitro and in vivo against different cancer subtypes
(A/B/E/D/I/Q, lung; B/E/D/I/dihydrocucurbitacin B, breast,
neurological, colon and hepatocellular; E/I, prostate; E, ovarian;
D/dihydrocucurbitacin B, leukemia; D, lymphoma; Q, murine).
Cucurbitacin B has been one of the most explored for its role in
biological systems. Cucurbitacin B
(C32H46O8, molecular weight
558.712 g/mol, 19-(10➝9β)-abeo-10-lanost-5-ene triterpene), found
in the plants of cucurbitaceae and other plant families like
Brassicaceae, is classified as a steroid with peculiar bitter taste
and cytotoxic properties. Its bitterness could offer it protection
against predators and parasites (28). It has previously been reported to
be effective against various illnesses notably generalized
inflammation and algesia, carbon chloride-induced hepatotoxicity
and profound cholestasis, CD18-mediated disorders, infestation of
insects, cell adhesion and leukemic disorders, immune mediated and
angiogenic disorders (29–31). Dantas et al (32) analyzed few derivatives of
cucurbitacin obtained from the seeds of Cayaponia racemosa
for cytotoxic potential. Nearly all were lethal to brine shrimp and
inhibited cancer cell proliferation. However, their toxicity did
not reach the order to inhibit the biological development of sea
urchin or mouse erythrocytic lysis. The authors credited this to
the chemical structure of cucurbitacins, and postulated that such
differences in toxicity profile could be attributed to the position
of carbonyl group at carbon-11 and absence of a double bond between
carbon-23 and carbon-24. In another study, authors isolated a
chemical substitute of cucurbitacin B from the fruiting bodies of
mushroom Leucopaxillus gentianeus, which differed from
cucurbitacin B in lacking an oxygen substituent at carbon-16, and
screened for antitumor toxicity with respect to chemical structure
variation (30). This minute
substitution might be responsible for its significant (~6-fold)
antitumor profile. It has high affinity for glycosides and, not
rare to find cucurbitacin B in a naturally bound form. Hatam et
al (33) isolated and
performed NMR studies on cucurbitacin glycosides derived from the
fruit of Citrullus colocynthis L. Schrad. Cucurbitacin B
showed strong affinity to human serum albumin via hydrophobic and
electrostatic forces and increased the binding of ibuprofen to
albumin (34). This indicated the
catalytic role of cucurbitacin B in NSAIDs-pharmacokinetics.
3. Cucurbitacin B - sources
Cucurbitacin B has been extracted from plants of
various families and genera around the world for research purpose
(compiled in Table I). We examined
the effect of cucurbitacin B, derived from Helicteres
angustifolia (35), on a
variety of cancer and normal cells and discovered its cytotoxic
potential in bone osteosarcoma cells at nanomolar doses.
Cucurbitacin B fractionated from the methanolic extract of
Licaniaintra petiolaris was analyzed for toxicity via
kenacid acid cytotoxicity assay (36) and, caused, at the dose <0.01
μg/ml, >50% cell death in human oral epidermoid carcinoma
cells. Cucurbitacin B obtained in both pure and glycoside form from
the fractionation from root extract of Casearia arborea
showed potent cytotoxicity against gliosarcoma and melanoma cell
lines (37); from the
chloroform-methanol extracts of Cucumis prophetarum it
showed cytotoxicity against tumor derived and virally transformed
mouse cancer cells (38); from the
fruiting bodies of the fungal mushroom Leucopaxillus
gentianeus it showed very strong cytotoxicity against NSCLC,
RCC, HCC and HER2−/ER+ breast carcinoma cells
(28); from the stems of
Cucumis melo it showed significant cytotoxicity against
NSCLC and HCC in vitro via activation of phospho-STAT3
(39); from the fruiting bodies of
the fungal mushroom Leucopaxillus gentianeus it showed
cytotoxicity against NSCLC, RCC, HCC and
HER2−/ER+ breast carcinoma cells (28); and from the methanol extract of
Trichosanthes kirilowii Cucurbitacin B and its close
relatives demonstrated potent anticancer activities mediated
through HIF-1α and NF-κB suppression (40). From the air-dried rhizomes of
Begonia nantoensis it not only showed cytotoxicity against
NSCLC, HER2−/ER+ breast carcinoma, gastric
adenocarcinoma and non-neoplastic nasopharyngeal epithelial cell
carcinoma cells, but also inhibited HIV replication in human
T-lymphocytes, flaunting its potential as an anti-viral molecule
(41).
 | Table IPotency of cucurbitacin B against
various diseases. |
Table I
Potency of cucurbitacin B against
various diseases.
| Year | Source | Presenting
condition | Activity
explored | Refs. |
|---|
| 1988 | Ecballium
elaterium | Inflammation | Vascular
permeability | (42) |
| 1989 | Citrullus
colocynthis | – | – | (33) |
| 2000 | Casearia
arborea | Brain and skin
cancer | Cytotoxicity | (37) |
| 2001 | Licania
intrapetiolaris | Oral epidermoid
cancer | Cytotoxicity | (36) |
| 1999 | Leukopaxillus
gentianeus | Multiple
cancers | Cytotoxicity | (28) |
| 2004 | Begonia
nantoensis | Multiple
cancers | Cytotoxicity | (41) |
| 2006 | Cayaponia
racemosa | – | – | (32) |
| 2006 | Leucopaxillus
gentianeus | Lung cancer | Cytotoxicity | (30) |
| 2007 | Citrullus
colocynthis | Breast cancer | Cytotoxicity | (45) |
| 2008 | Trichosanthes
cucumerina | Multiple
cancers | Cytotoxicity | (81) |
| 2009 | Cucumis
melo | Multiple
cancers | Cytotoxicity | (39) |
| 2010 | Trichosanthes
cucumerina | Breast cancer | Cytotoxicity | (47) |
| 2010 | Trichosanthes
kirilowii | Liver cancer | Cytotoxicity | (40) |
| 2011 | Cucumis
prophetarum | Embryonic
cancer | Cytotoxicity | (38) |
| 2012 | Trichosanthes
cucumerina | Breast cancer | Cytotoxicity | (10) |
| 2012 | Trichosanthes
cucumerina | Breast cancer | Cytotoxicity | (49) |
| 2012 | Luffa
operculata | Lung cancer | – | (60) |
| 2013 | Trichosanthes
cucumerina | Breast cancer | Cytotoxicity | (50) |
| 2014 | Cucurbita
pepo | Inflammation | ROS scavenging and
COX-1/2 inhibition | (43) |
| 2014 | Lagenaria
siceraria | | | |
| 2014 | Luffa
cylindrical | | | |
| 2014 | Trichosanthes
kirilowii | Multiple
cancers | Cytotoxicity | (92) |
| 2015 | Luffa
graveolense | Lung cancer | Cytotoxicity | (64) |
| 2015 | Ecballium
elaterium | Liver inujry |
Hepatoprotection | (126) |
| 2016 | Helicteres
angustifolia | Bone cancer | Cytotoxicity | (35) |
| 2016 | Luffa
graveolense | Breast cancer | Cytotoxicity | (59) |
| 2016 | Wilbrandia
ebracteata | Lung cancer | Cytotoxicity | (65) |
| 2016 | Luffa
graveolense | Lung cancer | Cytotoxicity | (66) |
| 2017 | Ecballium
elaterium | Multiple
cancers | Cytotoxicity | (76) |
Many studies have shed light on other medicinal
properties of cucurbitacin B. Yesilada et al (42) showed potent anti-inflammatory
activity in a fraction from the freeze-dried fruit juice of
squirting cucumber Ecballium elaterium L. Rich. Rawat et
al (43) demonstrated the
anti-oxidant and anti-inflammatory potential of extracts of
cucurbits Lagenaria siceraria, Cucurbita pepo and
Luffa cylindrical. Overall, in last 50 years, cucurbitacin B
has been shown to possess therapeutic value for ailments such as
cancer, HIV-AIDS and inflammatory disorders. Indeed, methods for
preparing cucurbitacin with longer shelf life have been developed
(44). More recently, several
studies, using in vitro and in vivo approaches, have
reported its mechanism of action.
4. Cucurbitacin B - anticancer activity
Many researchers around the world have based their
research questions on the activity of cucurbitacin B in cancer
cells (compiled in Table II).
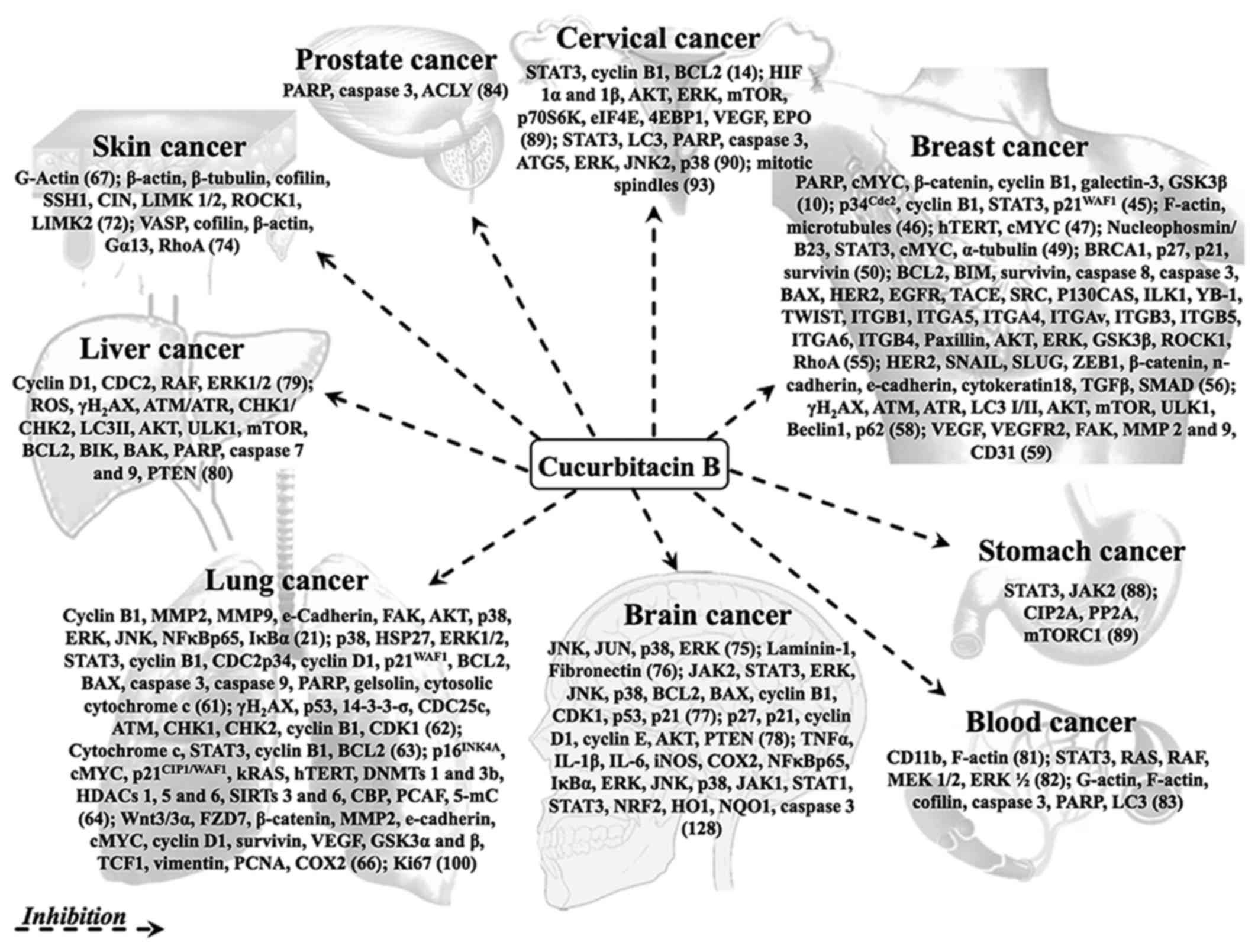
Cucurbitacin B has been shown to possess strong anticancer activity
in a variety of cancers in vitro and in vivo
(Fig. 3).
 | Table IIMolecular targets of cucurbitacin B
in cancer signaling pathways. |
Table II
Molecular targets of cucurbitacin B
in cancer signaling pathways.
| Year | Source | Cancer cell
lines | Target | Refs. |
|---|
| 2007 | Citrullus
colocynthis | MCF7,
MDA-MB-231 | p34Cdc2,
cyclin B1, STAT3, p21WAF1 | (45) |
| 2008 | Commercially
procured | MCF7, MDA-MB-231,
MDA-MB-453, BT474, SKBR3, ZR751 T47D, | F-actin,
microtubules | (46) |
| 2015 | Commercially
procured | U87, T98G, U118,
U343, U373 | JNK, JUN, p38,
ERK | (75) |
| 2008 | Commercially
procured | Hep2 | STAT3, cyclin B1,
BCL2 | (14) |
| 2008 | Trichosanthes
cucumerina | HL60, U937, THP1,
NB4, K562, BALL1, Reh, RCH, Daudi, LY4, MD901, SP49, Jeko1,
NCEB1 | CD11b, F-actin | (81) |
| 2010 | Trichosanthes
cucumerina | T47D, SKBR3, MCF7,
HBL100 | hTERT, cMYC | (47) |
| 2010 | Commercially
procured | BEL7402 | Cyclin D1, CDC2,
RAF, ERK1/2 | (79) |
| 2010 | Commercially
procured | K562 | STAT3, RAS, RAF,
MEK 1/2, ERK ½ | (82) |
| 2011 | Commercially
procured | B16F10 | G-actin | (67) |
| 2012 | Trichosanthes
cucumerina | T47D, SKBR3, MCF7,
HBL100 | PARP, cMYC,
β-catenin, cyclin B1, galectin-3, GSK3β | (10) |
| 2012 | Trichosanthes
cucumerina | MDA-MB-231,
MCF7 | Nucleophosmin/B23,
STAT3, cMYC, α-Tubulin | (49) |
| 2012 | Luffa
operculata | A549 | – | (60) |
| 2012 | Commercially
procured | Jurkat cells | G-actin, F-actin,
cofilin, caspase-3, PARP, LC3 | (83) |
| 2012 | Commercially
procured | HeLa, MCF7, HCT116,
U87, ARO, TPC-1 MEFs | STAT3, LC3, PARP,
caspase-3, ATG5, ERK, JNK2, p38 | (94) |
| 2013 | Trichosanthes
cucumerina | MCF7, MDA-MB-231,
HCC1937, MDA-MB-436 | BRCA1, p27, p21,
survivin | (50) |
| 2013 | Commercially
procured | H1299, H661,
A549 | p38, HSP27, ERK1/2,
STAT3, cyclin B1, CDC2, p34, cyclin D1, p21WAF1, BCL2,
BAX, caspase-3, caspase-9, PARP, gelsolin, cytosolic, cytochrome
c | (61) |
| 2013 | Commercially
procured | A375, B16F10 | β-actin, β-Tubulin,
cofilin, SSH1, CIN, LIMK 1/2, ROCK1, LIMK2 | (72) |
| 2014 | Commercially
procured | MDA-MB-231, MCF7,
SKBR3 | BCL2, BIM,
survivin, caspase-8, caspase-3, BAX, HER2, EGFR, TACE, SRC,
P130CAS, ILK1, YB-1, TWIST, ITGB1, ITGA5, ITGA4, ITGAv, ITGB3,
ITGB5, ITGA6, ITGB4, Paxillin, AKT, ERK, GSK3β, ROCK1, RhoA | (55) |
| 2014 | Commercially
procured | MDA-MB-231, MCF7,
4T1, MDA-MB-231-HER2-overexpressed, MCF7-HER2-overexpressed | HER2, SNAIL, SLUG,
ZEB1, β-catenin, N-cadherin, E-cadherin, cytokeratin18, TGFβ,
SMAD | (56) |
| 2014 | Commercially
procured | A549, MCF7 | γH2AX,
p53, 14-3-3-σ, CDC25c, ATM, CHK1, CHK2, cyclin B1, CDK1 | (62) |
| 2014 | Commercially
procured | A549 | Cytochrome
c, STAT3, cyclin B1, BCL2 | (63) |
| 2014 | Commercially
procured | A375, B16F10 | VASP, cofilin,
β-actin, Gα13, RhoA | (74) |
| 2014 | Commercially
procured | PC3, LNCaP,
PrEC | PARP, caspase-3,
ACLY | (84) |
| 2014 | Commercially
procured | SH-SY5Y | JAK2, STAT3, ERK,
JNK, p38, BCL2, BAX, cyclin B1, CDK1, p53, p21 | (77) |
| 2014 | Commercially
procured | SHSY5Y | p27, p21, cyclin
D1, cyclin E, AKT, PTEN | (78) |
| 2014 | Trichosanthes
kirilowii | HeLa, SK-Hep1,
Hep3B, HEK293, AGS | HIF 1α and 1β, AKT,
ERK, mTOR, p70S6K, eIF4E, 4EBP1, VEGF, EPO | (92) |
| 2014 | Commercially
procured | 3T3-L1 | STAT3, KLF5, PPARγ,
aP2, C/EBPα, adiponectin, CD36, cyclin D1 | (130) |
| 2015 | Commercially
procured | MCF7 | γH2AX,
ATM, ATR, LC3 I/II, AKT, mTOR, ULK1, Beclin1, p62 | (58) |
| 2015 | Luffa
graveolense | H1299, A549,
H1650 |
p16INK4A, cMYC,
p21CIP1/WAF1, kRAS, hTERT, DNMTs 1 and 3b, HDACs 1, 5
and 6, SIRTs 3 and 6, CBP, PCAF, 5-mC | (64) |
| 2015 | Ecballium
elaterium | Wistar rats | Liver-blood and
histological parameters | (126) |
| 2015 | Commercially
procured | Balb/c mice | Keratinocyte
proliferation, psoriatic cytokines, NF-κBp65, IκBα, STAT3,
Ki-67 | (127) |
| 2015 | Commercially
procured | TLR 2/4
agonists-stimulated microglia | TNFα, IL-1β, IL-6,
iNOS, COX2, NF-κBp65, IκBα, ERK, JNK, p38, JAK1, STAT1, STAT3,
NRF2, HO1, NQO1, caspase-3 | (128) |
| 2016 | Luffa
graveolense | MDA-MB-231, 4T1,
HUVEC | VEGF, VEGFR2, FAK,
MMP 2 and 9, CD31 | (59) |
| 2012 | Wilbrandia
ebracteata | A549 | Cyclin B1, MMP2,
MMP9, E-cadherin, FAK, AKT, p38, ERK, JNK, NF-κBp65, IκBα | (21) |
| 2016 | Luffa
graveolense | A549, H1299,
HUVECs | Wnt3/3α, FZD7,
β-catenin, MMP2, E-cadherin, cMYC, cyclin D1, survivin, VEGF, GSK3α
and β, TCF1, vimentin, PCNA, COX2 | (66) |
| 2016 | Commercially
procured | BEL7402 | ROS,
γH2AX, ATM/ATR, CHK1/CHK2, LC3II, AKT, ULK1, mTOR, BCL2,
BIK, BAK, PARP, caspase-7 and 9, PTEN | (80) |
| 2016 | Commercially
procured | MKN45 | STAT3, JAK2 | (88) |
| 2017 | Ecballium
elaterium | U87, HT1080, HT29,
K562, HMEC-1 | Laminin-1,
fibronectin | (76) |
| 2017 | Commercially
procured | Sprague Dawley
rats | Pulmonary gas and
histological parameters, cytokines, proteins and inflammatory
mediators | (125) |
| 2017 | Commercially
procured | SGC7901/DDP | CIP2A, PP2A,
mTORC1 | (89) |
| 2017 | Commercially
procured | U-2 OS | JAK2, STAT3, MAPK,
BH3 domain family, caspase-3, MMP2, MMP9, VEGF | (90) |
| 2017 | Commercially
procured | A2780/Taxol | p53, p21, BCL2,
caspase-3, P-glycoprotein-1 | (91) |
| 2017 | Commercially
procured | U-2 OS, HeLa | Mitotic
spindles | (93) |
Breast cancer
Tannin-Spitz et al (45) isolated cucurbitacin glycosides from
the leaves of Citrullus colocynthis and found that the
combination (cucurbitacin B/E glycosides 1:1) inhibited the growth
of breast cancer cells in a dose- and time-dependent fashion, and
forced the cells into G2/M phase cell cycle arrest
furthering into apoptosis. Growth arrest was elucidated by the
downregulation of p34Cdc2/cyclin B1 protein complex and
upregulation of phospho-STAT3 and p21WAF1. Breast cancer
cells treated with the combination of cucurbitacin B and glycosides
showed remarkable annular appearance representative of actin
filament disruption. This led to ER status-independent inhibition
of PKB protein phosphorylation and reduction in the expression of
survivin, contributing to apoptosis. Wakimoto et al
(46) confirmed that cucurbitacin
B induced dose-dependent disruption of actin microtubules and
filaments to illustrate rapid morphological changes in breast
cancer cells, as soon as 15–20 min after exposure to the compound.
These were due to the disruption of F-actin within 3–5 min after
exposure, followed by disruption of microtubules over 15–20 min.
These findings were verified using nude mice, in which tri-weekly
orthotopic 1 mg/kg intra-peritoneal injections of cucurbitacin B
resulted in ~50% tumor size growth suppression.
Duangmano et al (47) reported that cucurbitacin B
inhibited telomerase activity in the breast cancer cells. Telomere
erosion and telomerase inhibition was supported with the inhibition
of hTERT and cMYC proteins and consequential cell cycle arrest into
G2/M phase (47,48).
Two years later they reported downregulation of cyclin B1 (key cell
cycle regulatory protein) by cucurbitacin B (10). They also showed inhibition of
phosphorylation of GSK-3β, resulting in enhanced activity of the
β-catenin-degradation complex, accompanied with downregulation of
galectin-3. These results indicated an over obstruction of
canonical Wnt/β-catenin signaling axis. They also reported the role
of neucleophosmin/B23 in breast cancer cells (49). Nucleophosmin/B23 protein plays an
important role in controlling cellular cycling activities, and
continuously patrols from nucleolus to cytoplasm and back.
Microtubules are cytoskeleton builders consisting of α-tubulin and
β-tubulin subunit chains, and have fundamental role in maintaining
cell structure, motility, trafficking and division. Shortage of
nucleophosmin/B23 may lead to disruption of tubulin subunit
polymerization. The authors found that cucurbitacin B treatment
caused strong anti-proliferative and apoptotic effects in breast
cancer cells through downregulation of nucleophosmin/B23 and
disruption of microtubule polymerization. They investigated the
effectiveness of cucurbitacin B in BRCA1-defective breast cancer
cells (50), where it
counterpoised uncontrolled proliferation and drug resistance of
cancer cells. Additionally, it caused upregulation in the
expression of cell cycle inhibitors p21WAF1 and
p27KIP1, driving the cells into apoptosis.
HER2 status and integrins play a vital role in the
carcinogenesis in breast cancer cells. HER2/neu, a proto-oncogenic
protein, is a mitogenic tyrosine kinase receptor, which directly
participates in cell transformation, survival and proliferation.
Integrins are a large family of extracellular matrix (tumor cell
microenvironment governing) receptor proteins, composed of two α-
and two β-glycoprotein units linked with non-covalent bonds. There
are ~24 popular integrin dimers, distinctly mended by at least 18α
and 8β subunits. Integrins ITGAVB3 and ITGA6B4 specifically foster
survival and cell motility (51–53),
whereas ITGB1 and ITGB3 induce apoptosis (integrin-mediated cell
death) (54). Gupta and Srivastava
(55,56) reported that cucurbitacin B
treatment inhibited HER2 receptor and cell adhesion integrins, and
triggered integrin-mediated cell death via B1 and B3 upregulation
through a dose- and time-dependent BAX and caspase-8 cleavage. They
also reported that cucurbitacin B treatment in HER2+
breast cancer cells reduced their invasiveness by EMT reversal.
HER2+ cells acquire mesenchymal properties via
upregulation of TGFβ and downregulation of E-cadherin (57). Cucurbitacin B inhibited the
expression of TGFβ, SMAD, SNAIL, SLUG, ZEB1 and N-cadherin, and
abrogated the expression of E-cadherin in HER2+ breast
cancer cells. Cucurbitacin B treatment induced autophagy in breast
cancer cells (58). It caused DNA
damage in HER2-breast cancer cells via ROS mediated upregulation of
γH2AX and phosphorylation of ATM/ATR resulting in
p53-dependent cell apoptosis. Autophagy induction was confirmed by
the appearance of autophagic vacuoles in monodansylcadaverine (MDC)
staining and upregulation of Beclin1, ULK1 and LC3 in a time- and
dose-dependent manner. The effects of ROS reversed if the cells
were pretreated with NAC. Cucurbitacin B also reduced the
metastatic potential of the breast cancer cells (59). At the non-apoptotic dose,
cucurbitacin B delayed migration and invasion potential of the
breast cancer and human vascular endothelial cells in a time- and
dose-dependent manner. It was further accompanied by inhibition of
angiogenesis potential and a dose-dependent suppression of
VEGF/FAK/MMP9 signaling axis. These results were validated via
retrograde inhibition of FAK using its inhibitor FI14 and genetic
silencing, and in vivo studies.
Lung cancer
Lang et al (60) tested the effect of cucurbitacin B
on NSCLC cells. They showed that the highly oxygenated bitter
triterpene (cucurbitacin B) caused moderate to potent cytotoxicity
with IC50 0.13 μM in 48 h and 0.04 μM in
72 h. Thiols are one of the crucial redox regulators for
cytoskeleton remodeling, cell cycle progression, mitochondrial
apoptosis and regulation of redox sensitive signal transducers and
activators of transcriptors. Kausar et al (61) showed that the thiols act as the
intermediate between Cucurbitacin B and its toxicity in lung cancer
cells. All cytotoxic effects were attenuated with thiol
anti-oxidant NAC pretreatment, whereas exacerbated with GSH
synthesis inhibitor BSO. They demonstrated the physical interaction
of cucurbitacin B with NAC and glutathione (GSH) extracellularly
using ultraviolet (UV) and FTICR mass spectrometry. Furthermore,
Guo et al (62) reported
that cucurbitacin B in lung cancer cells also caused DNA damage via
ROS formation and let cells into G2/M cell cycle arrest.
ROS caused double stranded DNA breaks, which led to ATM-mediated
activation of Chk1-Cdc25C-Cdk1 and p53-14-3-3-σ-CDC-25C-CDK1
pathways leading to mitotic interruption in parallel. Lung cancer
when treated with cucurbitacin B inhibited phosphorylation of STAT3
to cause growth arrest and apoptosis in dose- and time-dependent
manner (63). It inhibited STAT3
pathway leading to downregulation of cyclin B1 and BCL2. Shukla
et al (64) reported global
DNA methylation-associated epigenetic modifications due to
cucurbitacin B treatment in highly aggressive NSCLC cells. They
reported that the altered histone modifications at the respective
promoter sites caused upregulation of p16INK4A and
p21WAF1 and downregulation of hTERT to cause replicative
senescence, growth arrest and apoptosis. They also found that
cucurbitacin B caused downregulation of cMYC/kRAS at only protein
and not transcriptional level. 5-mC reduction was accompanied with
downregulation of DNMTs, HDACs and SIRT and upregulation of CBP and
PCAF. The results were validated using carcinogen NNK-induced lung
tumorigenesis in mice, advocating epigenetic modulation to be a
significant anticancer mechanism of cucurbitacin B.
Silva et al (65) reported PI3Kinase and MAPK pathway
inhibition due to cucurbitacin B treatment. The treatment delayed
cell migration, alleviated invasion potential and caused dose- and
time-dependent apoptosis. It inhibited MMP release and FAK
activation, and upregulated (activated) phospho-AKT, -ERK, -JNK and
-NF-κBp65. In another study, cucurbitacin B exhibited a strong
anti-metastatic potential in the NSCLC cells via downregulation of
canonical Wnt/β-catenin signaling axis (66). In both moderately aggressive and
highly aggressive NSCLC cell lines in vitro, cucurbitacin B
treatment delayed migration and impeded invasion with
downregulation of Wnt3/Wnt3α, FZD7, β-catenin, MMP2, cMYC, cyclin
D1, survivin and VEGF and upregulation of E-cadherin proteins. It
also showed potent anti-angiogenic effects. These effects regressed
upon the treatment with EMT inducer TGFβ or by retrograde genetic
knockdown of Wnt3/Wnt3α. All the results were validated using
NNK-induced lung tumorigenesis in mice. Intraperitoneal injections
of cucurbitacin B at 0.1 mg/kg body weight, administered thrice a
week, reduced the tumor incidence, frequency and volume by 80–90%
compared to vehicle group.
Skin cancer
In malignant melanoma, cucurbitacin B inhibited cell
proliferation as well as their migratory potential (67). Treatment in a mouse melanoma cell
line caused cell membrane blebbing and deformation and multiploidy,
via ROS induction resulting in dose-dependent G2/M phase
growth arrest. There was significant depletion and aggregation of
G-actin protein from the cellular cytoskeleton, reversed using NAC.
NAC is an N-acetyl derivative of L-cysteine and an
established potent antioxidant. Inhibition of growth of
subcutaneously implanted melanoma cells was validated using female
C57BL/6 mice. Cofilin is an actin-depolymerization factor. It is
regulated by phosphatidylinositol phosphate, pH variations, redox
balance and competition with tropomyosins (68), activated by dephosphorylation at
its serine residue 3 by chronotropin or slingshot phosphatases, and
inactivated by phosphorylation via LIM or TES kinases (69). Rho-associated kinases indirectly
modulate the stress fibers and focal adhesions (70). Cofilin and actin form rods and are
implicated in the pathogenesis of neurogenerative disorders
(71). Cucurbitacin B dramatically
activated cofilin via thiol oxidation, which formed rod-like
structures co-localizing with the actin (tandem rods) in melanoma
cells (72). Rho kinase and LIMK
were downregulated, which were reversed by the treatment of
thiol-ROS-scavenger NAC. Cucurbitacin B induced slingshot homolog-1
dependent hyper activation of cofilin and formation of
cofilin-actin rods downstream of actin aggregation. VASP, an
F-actin barbed end binding protein facilitates actin filament
elongation through interaction with G-actin, profilin and other
actin regulatory factors (73). It
directly controls the assembly of the actin-filament mesh,
transforms the morphology and behavior of membrane protrusion
structures and also contributes to cell migration and adhesion.
Cucurbitacin B induced VASP phosphorylation (deactivation) at
serine 157 (74). It was mediated
by cAMP independent activation of Gα13/RhoA/PKA pathway, to
generate VASP clumps. These clumps co-localized with amorphous
actin aggregates, prior to the formation of highly ordered
cofilin-actin rods in melanoma cells.
Brain cancer
Cucurbitacin B inhibited growth of human
glioblastoma multiforme cells by intense disturbance in its
internal cytoskeleton (75).
Within 15–30 min of treatment, it caused loss of pseudopodia,
multi-nucleation and skirting up of cells associated with
disruption of actin and microtubules, but was followed by the
activation of cell survival pathways. It also inhibited cell
proliferation, decreased migration and caused cell cycle arrest in
G2/M phase and apoptosis. After 30 min of treatment, it
caused upregulation of phospho-p38, JNK, JUN and ERK in a
time-dependent manner. This was validated using SP600125, a
broad-spectrum anthrapyrazolone inhibitor of JNK. SP600125 blocked
the effects of cucurbitacin B. The treated glioblastoma multiforme
cells exhibited marked disintegration and rearrangement of
filamentous actin and β-tubulin. Touihri-Barakati et al
(76) recently found that the
proliferation, adhesion and migration inhibition due to
cucurbitacin B may be mediated through α5β1 integrins. Laminins and
fibronectin are extracellularly present high molecular weight cell
adhesion proteins, and constitute major component of basal lamina
contributing to cell adherence, dissemination and differentiation.
Their upregulation is commonly found in EMT. Authors found
significant downregulation of Laminin-1 and fibronectin in human
glioblastoma cells. Further introspection in microvasculature
endothelial cells demonstrated potent anti-angiogenic activity.
Cucurbitacin B also showed remarkable anticancer effects in human
neuroblastoma cancer cell lines (77). It induced G2/M arrest
and apoptosis supported with upregulation of p53, p21 and BAX, and
downregulation of BCL2. This was accompanied with the
downregulation of proteins (CDK1 and cyclin B1) essential for cell
cycle progression. It also inhibited JAK2/STAT3 signaling cascades
and induced JNK/p38MAPK-activation/ERK-inactivation. Shang et
al (78) reported that PTEN
activation is crucial and adds positively to the anti-proliferative
effects of cucurbitacin B. Apart from proliferation inhibition,
cell cycle restraining and apoptotic trend, they dissected the
possible mechanism of inhibition of AKT signaling pathway in human
neuroblastoma cells. Although they found that some of the key
down-stream factors in the pathway viz., NF-κB, AMPK and p38 did
not alter; genetically activating AKT using plasmid vector carrying
myr-AKT locus reversed the toxic effects of cucurbitacin B.
Silencing of activated PTEN resulted in AKT phosphorylation and
upregulation of the proapoptotic markers and repression of
cyclins.
Liver cancer
Dat et al (40) isolated cucurbitacin B and its close
relatives, which demonstrated potent anticancer activities mediated
through HIF-1α and NF-κB inhibition in hepatic carcinoma cells.
Cucurbitacin B inhibited the activation of HIF-1α in an
artificially-induced hypoxia cell-based reporter assay and
inhibited NF-κB in secreted alkaline phosphatase reporter assay.
VEGF-specific ELISA validated its anti-angiogenic potential. Chan
et al (79) explored the
activity of cucurbitacin B in human hepatocellular carcinoma in
vitro and in vivo, where they reported that it caused
dose- and time-dependent clonogenic inhibition via STAT3
independent S-phase arrest. This phenomenon occurred probably via
ERK1/2 activation and suppression of cyclin D1, CDC2, and c-RAF
phosphorylation. Cucurbitacin B induced ROS-mediated DNA damage
leading to apoptosis and autophagy in hepatocellular carcinoma
cells (80). It was supported with
increased upregulation of γH2AX, phosphorylation of
ATM/ATR and CHK1/CHK2, modulations in BCL2 family proteins and
cleavage of caspases-7 and -9. They also ascertained cucurbitacin
B-induced autophagy, which was marked by vacuolization,
upregulation of LC3II and ULK1, downregulation of AKT and mTOR
proteins and activation of PTEN. They proposed that the
contemporary activation of PTEN protein, which probably led to
AKT/mTOR inhibition, had indirect correlation with autophagy. They
used silencing RNA to genetically knock down PTEN gene to confirm
inhibition of AKT and upregulation of autophagy specific
proteins.
Blood cancer
Cucurbitacin B with variable IC50 value
induced cell cycle arrest and cellular differentiation in acute
promyelocytic and myeloid leukemia (81). It induced S phase growth arrest,
swollen morphology of cells, altered cytoskeleton due to rapid and
haphazard polymerization of filamentous actin into globular
aggregates, and decreased clonogenicity in the immature blasts. It
also caused upregulation of monocytic-/granulocytic-specific CD11b,
signifying cell differentiation. Cucurbitacin B suppressed the
activated MAPK/ERK pathway and inhibited STAT3 activation in
various human chronic myeloid leukemia cells (82). Protein expression analysis showed
activation of STAT3, RAF, MEK and ERK proteins was inhibited. STAT3
activation modulation ensued through a crosstalk mechanism, which
also resulted into G2/M arrest and apoptosis. Similar
findings were noted in human leukemic Jurkat cells (83). Cucurbitacin B caused cofilin
dephosphorylation and actin dynamics intrusion, prompting cell
volume increase and simultaneous multi-nucleation. It also induced
autophagosomes and LC3II upregulation as a compensatory protective
response. Constitutive suppression of autophagy led to dramatic
increase in caspase-3 mediated autophagy.
Others
Gao et al (84) reported the anticancer effects of
cucurbitacin B in prostate cancer cells. Cucurbitacin B caused
selective toxicity in human epithelial type prostate cancer cells.
ACLY is an enzyme, which metabolically converts mitochondrial
citrate into acetyl coenzyme A (85,86).
Acetyl coenzyme A is a major precursor and constituent responsible
for synthesis of fatty acids and mevalonate, which promote
carcinogenesis. Cucurbitacin B downregulated the expression of
acetyl coenzyme A, fatty acid synthesis and mevalonate,
supplemented with mitochondrial ROS production. It was suggested
that the inhibition of the acetyl coenzyme A signaling might be one
of the downstream mechanisms of ROS-mediated intracellular insults
leading into apoptosis. Cucurbitacin B exhibited significant
toxicity in human laryngeal cancer cells leading to G2/M
phase arrest (87). This was
supported with downregulation of cyclin B1, phospho-STAT3 and BCL2,
associated with chromatin condensation, nuclear fragmentation, and
appearance of apoptotic bodies in a dose- and time-dependent
manner. Cucurbitacin B inhibited proliferation and induced
G0/G1 cell cycle arrest in a dose- and
time-dependent manner in gastric cancer cells (88). It suppressed the expression of
cyclin D1, cyclin E, CDK4 and CDK2 and upregulated the expression
of p27, all of which are indicated in inhibition of cell cycle
progression. It also inhibited phosphorylation of JAK2 and STAT3
and caused apoptosis. It also induced caspase-dependent apoptosis
and autophagy in SGC7901/DDP DDP-resistant gastric cancer cells
(89). It suppressed cell
proliferation and induced apoptosis and autophagy via inhibition of
activation of CIP2A-PP2A-mTORC1 axis. Cucurbitacin B was shown to
cause cytotoxicity to a variety of bone cancer and normal cells at
nanomolar doses (35). It forced
osteoblastoma and normal lung fibroblasts into G2/M
phase growth arrest, and unanimously activated p53 and pRB tumor
suppressor pathways, leading to characteristic senescence-like
growth arrest and/or apoptosis. At 20–100 μM dose it
suppressed cell proliferation and induced apoptosis in U-2OS human
osteosarcoma cells (90). It
inhibited JAK2/STAT3 and MAPK pathways leading to BH3 domain and
caspase family protein modulation to induce cell cycle arrest,
along with MMP2, MMP9, VEGF inhibition adding up to delay in
migration potential of the cells and angiogenesis inhibition.
Cucurbitacin B showed anticancer activity in paclitaxel-resistant
A2780/Taxol ovarian cancer cells (91). It caused growth inhibition in
G2/M phase and apoptosis via upregulation of p53 and
p21, downregulation of BCL2, activation of caspase-3, and
suppression of P-gp, suggesting potential role against multidrug
resistance clinical cancer. Ma et al (92) monitored hypoxia-induced signaling
and modulation by cucurbitacin B in various cell lines in
vitro. They found that the activation of HIF-1α-monomer was
suppressed due to cucurbitacin B treatment in a dose-dependent
manner. They also found that this effect co-existed and correlated
with strong dephosphorylation of mTOR, p70S6K, 4EBP1 and ERK1/2
proteins. It also downregulated the HIF-1 targets, VEGF and
erythropoietin. Contemporary treatment with a known proteasomal
inhibitor MG-132 overshadowed the hypoxia signaling suppression,
implying that cucurbitacin B did not affect transcriptional coding,
but regressed its ubiquitin-mediated proteasomal degradation. It
also altered microtubule structures in human cervical cancer HeLa
and osteosarcoma U-2OS cells (93). It affected mitotic spindles and
induced G2/M cell cycle arrest. Zhang et al
(94) showed that cucurbitacin B
induced caspase-independent autophagy reflux in a variety of cell
lines. It suppressed cell growth and proliferation, and inhibited
interleukin-6 mediated phosphorylation of STAT3 in a dose-dependent
manner. Increase in mitochondrial-derived ROS and activated ERK1/2
and JNK proteins were also observed. It induced autophagy as
evidenced by appearance of autophagosomes and upregulation of
lipidated LC3 II. However, inhibition or knockdown of ATG5 and
Beclin1 proteins did not rescue the cells from death. Instead, it
forced cells to undergo caspase-dependent apoptosis, suggesting a
competitive cross-relation between autophagy and apoptosis.
5. Cucurbitacin B - as combination
therapy
Cucurbitacin B and its derivatives have shown
synergism with other anticancer agents (reviewed in Table III). Marostica et al
(95) presented DACE, a novel
semisynthetic derivative of cucurbitacin B and tested its effect in
NSCLC cells as individually and in combination with either
cisplatin (9 μM), irinotecan (4.25 μM) or paclitaxel
(0.125 μM) (96).
Cucurbitacin B (20 mg) was made to react with
p-toluenesulfonyl chloride (34 mg) and DABCO (20 mg) in
dichloromethane (200 ml) to obtain tosylated intermediate (20 mg),
which was then made to undergo nucleophilic substitution using
sodium azide (18.2 mg) in dimethylformamide (250 μl) to
finally attain DACE. Cisplatin, an inorganic water-soluble platinum
complex, reacts with and alters DNA after undergoing hydrolysis to
produce crosslinks. Crosslinks impair DNA replication, and forces
the cells into G2 phase arrest. Irinotecan is a
topoisomerase inhibitor. Paclitaxel is a cyclodecane isolated from
the bark of Taxus brevifolia. It stabilizes microtubules
when they are in polymerized form, which causes cell death. In
combination with either one of three accomplices, DACE (0.125
μM) reduced the risk of drug resistance, which is one of the
major hurdles today in anticancer therapy by synthetic agents. It
also showed selective toxicity to the cancer cells, forced them
into G2/M phase cell cycle arrest via STAT3 activation
suppression and AKT downregulation, filamentous actin aggregation,
nuclear fragmentation, inactivated cofilin1 suppression and
E-cadherin substantiation. DACE also interfered with the activation
of EGFR and its downstream factors AKT, ERK, STAT3 and survivin,
contributing to growth arrest and caspase-3-mediated apoptosis.
Efficacy in combination with cisplatin has also been checked in
cutaneous squamous cell carcinoma (97). Cucurbitacin B (0.1–2.5 μM)
with cisplatin (5–20 μM) was pulse exposed to the cells
i.e., exposed for a limited duration followed by culture in usual
growth medium. The combination synergistically downregulated cyclin
B1 and CDC2 expressions, with reduction in migratory and invasive
potential of the cells. In laryngeal squamous cell carcinoma, the
combination (0.1 μM cucurbitacin B, 10 μM cisplatin)
interdependently suppressed activation of STAT3 and translational
expression of cyclin B1 and BCL2 (98). In ovarian cancer, cucurbitacin B
sensitized cells to cisplatin (99). Cucurbitacin B (2 μM)
pretreated with cisplatin (10 μM) resulted in enhanced
cytotoxicity than that by cisplatin alone in both cisplatin
sensitive and insensitive ovarian cancer cells, via depletion of
glutathione and increase in ROS production. It downregulated
Dyrk1B, phosphorylated ERK1/2 and STAT3 and cyclin B1, leading to
G2/M phase growth arrest. It also downregulated BCL2 and
APAF1 and upregulated caspase-9 indicating apoptotic induction.
These effects afforded by the combination had cucurbitacin B at the
dose of just a fourth of its IC50. Cucurbitacin B (1
mg/kg) in combination with paclitaxel (10 mg/kg) injected
intravenously suppressed tumor growth in Balb/c nude mice (100). Treatment was well tolerated by
the animals and no evident damage in liver and kidneys was
observed.
 | Table IIISynergism of Cucurbitacin B with
other molecules. |
Table III
Synergism of Cucurbitacin B with
other molecules.
| Year | Combination
with | Cancer cell
lines | Cancer type | Target | Refs. |
|---|
| 2008 | Docetaxel | Hep-2 | Laryngeal squamous
cell carcinoma | STAT3, BCL2, cyclin
B1 | (87) |
| 2009 | Gemcitabine | MiaPaCa2, Panc1,
PL45 | Pancreatic
cancer | JAK2, STAT 3 and 5,
cyclin A and B1, BCL-XL, p21WAF1, caspases | (103) |
| 2010 | Cisplatin | SRB1, SRB12, SRB13,
COLO16 | Cutaneous squamous
cell carcinoma | Cyclin B1,
CDC2 | (97) |
| 2010 | Cisplatin | Hep2 | Laryngeal squamous
cell carcinoma | STAT3, cyclin B1,
BCL2 | (98) |
| 2010 | Gemcitabine | Panc1 | Pancreatic
cancer | STAT3, JAK2, cMYC,
BCL2, BCL-XL, caspases-3 and -9 | (104) |
| 2011 | Methotrexate | MG63, Saos2 | Osteosarcoma | ERK1/2, JUN, FOS,
mTOR, p70S6K, 4EBP1, cyclin A1 and D1, p21WAF1, PARP,
surviving | (106) |
| 2011 | Valproic acid | B16F10 | Malignant
melanoma | Ac-HI3, eIF2α,
LC3B, JNK, γH2AX, BCL2, survivin, caspase-3 | (112) |
| 2012 | Radiation | MDA-MB-231,
MCF7 | HER2 - breast
cancer | p21 | (119) |
| 2013 | Docetaxel,
gemcitabine | MDA-MB-231 | Breast cancer | Liver and blood
parameters | (101) |
| 2015 | Cisplatin,
irinotecan, paclitaxel | A549 | Non-small cell lung
cancer | Survivin, p53, AKT,
STAT3, cofilin1, MMP2 and 9, E-cadherin, F-actin | (95) |
| 2016 | Gefitinib | HT29, HCT116 | Colorectal
cancer | BCL2, BAX, BAK,
p27KIP1, STAT3, EGFR, cyclin D1 | (105) |
| 2015 | Curcumin | BEL7402/5-Fu | Hepatocellular
carcinoma | Caspase-3, ATP | (117) |
| 2016 | Cisplatin | A2780, A2780CP | Ovarian cancer | Cyclin B1, NK-κB,
BAX, BCL2, APAF1, caspase-3, PARP, BAX, caspase-9, Dyrk1B, ERK1/2,
STAT3 | (99) |
| 2017 | Doxorubicin | 8505C, CAL62 | Anaplastic thyroid
carcinoma | BCL-XL, BCL2, BAX,
BID, PARP, survivin, JAK2, STAT3, ERK1/2, COX2 | (109) |
| 2017 | Doxorubicin | MM1.S, MM1.R,
RPMI8226, U266 | Multiple
myeloma | Caspase-3 and -8,
PARP, p38MAPK, JNK, aurora A, H3, p53, STAT3, cofilin, cytochrome
c | (110) |
| 2017 | Paclitaxel | Balb/c mice | Non-small cell lung
cancer | Ki-67 | (100) |
Docetaxel is a microtubule inhibitor. It prevents
formation of microtubules leading to failure of growth and cell
division. Gemcitabine, a nucleoside metabolic inhibitor, prevents
the synthesis of genetic element, hence preventing DNA replication.
Cucurbitacin B (0.001–0.05 μM) synergistically enhanced the
anti-proliferative and apoptosis effects of docetaxel (0.001–0.005
μM) or gemcitabine (0.001–0.1 μM) in breast cancer
cells in vitro and in vivo, at much lower dose of
either drug than that used in chemotherapy, indicating minimum
adverse effects (101). However,
even with combination, but at only high dose, myelosuppression,
hepatotoxicity and leukopenia with gemcitabine combination was
encountered. In another study, combination of cucurbitacin B (1
μM) and docetaxel (0.025 μM) caused cell cycle arrest
at G2/M phase and apoptosis via synergistic suppression
of phospho-STAT3, BCL2 and cyclin B1 in laryngeal cancer cells
(102). Thoennissen et al
(103) reported synergism of
cucurbitacin B with gemcitabine in pancreatic cancer. Cucurbitacin
B (0.01–0.1 μM) and gemcitabine (0.001–0.01 μM)
combination caused dose-dependent multi-nucleation, G2/M
arrest and apoptosis, associated with inhibition of activated JAK2,
STAT 3/5, cyclin A/B1 and BCL-XL and activation of
p21WAF1 and caspases, more than cucurbitacin B (0.05–0.2
μM) alone. The same group in another study in 2010 showed
that this combination also inhibited BCL2 and cMYC adding up to
apoptosis in pancreatic cancer cells in vitro. These effects
were positively validated in vivo with only mild toxicity
(104). Protein kinase activity
is crucial for a majority of intra-cellular signaling pathways.
Prevention of protein kinase results into cell growth arrest.
Gefitinib is a protein kinase inhibitor and known to be orally
active selective inhibitor of EGFR, which inhibits
autophosphorylation of intracellular tyrosine residues.
Cucurbitacin B at safe dose (0.5 μM) offered synergistic
anti-proliferative affects to benign dose of gefitinib (10
μM) against human colorectal cancer cells in vitro
(105), as validated via LDH
assay. There was more significant downregulation of BCL2, cyclin
D1, and phospho-EGFR and STAT3, and upregulation of BAX, BAK and
p27KIP1, indicating stronger growth arrest and apoptotic signaling.
Thymidylate is one of the important building blocks of DNA. Its
synthesis is mainly stimulated by tetrahydrofolate. Methotrexate,
an antineoplastic antimetabolite with immunosuppressant properties,
is an inhibitor of tetrahydrofolate dehydrogenase. Cucurbitacin B
(0.07 μM) and methotrexate (0.05 μM) combination
caused G2/M growth arrest and apoptosis in human
osteosarcoma cells (106). This
was corroborated by inhibition of protein expression of ERK, AKT
and mTOR in JAK/STAT inactive cells. Further in vivo
validation exhibited tumor suppression by combination (cucurbitacin
B at 0.5 mg/kg body weight, methotrexate at 150 mg/kg body weight)
by 60–80%, which held at 66% after methotrexate withdrawal.
Doxorubicin, a topoisomerase inhibiting anthracycline, intercalates
DNA and exerts its anticancer activity by the suppression of DNA
polymerase, topoisomerase II, and methyltransferase (107). Doxorubicin-resistant cells are
known to entail survivin super-expression (108). Cucurbitacin B (0.2–1 μM)
and doxorubicin (1–5 μM) showed synergism against the
progression of anaplastic thyroid cancer (109). They synergistically enhanced ROS
production, with consequential upregulation of cleaved PARP, COX2
and phospho-ERK1/2, and downregulation of BCL2, survivin and
JAK/STAT-signaling axis. In a recent study, cucurbitacin B (up to
0.2 μM) with doxorubicin (up to 0.2 μM) showed
synergistic antitumor activity via inhibition of aurora A leading
to G2/M phase cell cycle arrest, and IL10-induced STAT3
phosphorylation inhibition (110). It also induced cofilin
dephosphorylation and caspase-mediated apoptosis.
Valproic acid, popular anti-convulsion and mood
stabilizing fatty acid, is inhibited by histone deacetylase (drug
resistance). Histone deacetylase activates tumor suppressors p53,
p21 and CDKs, and increase histone acetylation, which suppress the
activation of many proto-oncogenes (111). Cucurbitacin B caused
dose-dependent toxicity in mouse melanoma cells, followed by
multiploidy, autophagy and BCL2 upregulation for cell survival
(112). This demonstrated the
prosurvival role of autophagy in cancer cells after treatment with
cucurbitacin B (1 μM). When administered in combination with
valproic acid (1–5 mM), it showed synergism to sensitize the cells
to the toxicity due to valproic acid. It henceforth caused cell
apoptosis and tapered multiploidization. Curcumin, obtained mainly
from Curcuma longa, a yellow-orange phytopolyphenolic
pigment commonly known as turmeric, is known for its medicinal
properties such as anti-oxidant (prevention of ROS production),
anti-inflammatory (COX inhibition) and anticancer (suppression of
protein kinase C). It showed potency against alcoholic liver injury
and insult due to lipopolysaccharide, D-(+)-galactosamine and heavy
metals (113–115). It fundamentally operates via
mitochondrial invasion and is relatively safe to the healthy tissue
(116). Combination of
cucurbitacin B (143.2 μM) and curcumin (108.6 μM)
showed synergism dose- and time-dependently against the human
hepatocellular carcinoma (117),
forcing the cells into G0/G1 phase arrest and
apoptosis. It decreased the sensitivity of the cells to
P-glycoprotein and reversed the resistance to 5-fluorouracil,
indicating reversal of multidrug resistance. In vivo
validation using cucurbitacin B (10 mg/kg body weight) and curcumin
(5 mg/kg body weight) combination resulted in greater suppression
in tumor growth than with either molecule alone, with enhanced
caspase-3 activity and reduced ATP contents. Cucurbitain B in
combination with glycosides exhibited excellent antioxidant
properties in a dose-dependent manner (118). The combination inhibited hydroxyl
radical-dependent DEPMPO-hydroxyl radical adduct formation,
superoxide radical anion-dependent DEPMPO-hydroxyl radical addict
formation and singlet oxygen-dependent TEMP-singlet oxygen addict
generated in the photoradiationporphin system. Duangmano et
al (119) reported that
cucurbitacin B increased the sensitivity of HER2-negative breast
cancer variants to radiotherapy, and caused dose-dependent
G2/M phase arrest and apoptosis in vitro via
progressive upregulation of the p21 mRNA. Further augmentation with
irradiation resulted in rapid cell death. Cucurbitacins, Picracin
and deacetylpicracin from Picrorhiza scrophulariiflora
inhibited mitogen-induced T cell proliferation and IL-2 (120). Authors regarded picracin as more
active in inducing apoptosis of non-stimulated T cells than the
others.
6. Cucurbitacin B - other biological
systems
Potential of cucurbitacin B against cardiac, liver,
lung, neuronal and skin injuries, systemic infections,
inflammation, sex-related behavior and adipocyte differentiation
has also been explored. Cucurbitacins exhibit a broad range of
pharmacological properties such as anti-inflammatory, antioxidant,
antiviral, antipyretic, analgesic and anti-malarial (121–123). Cucurbitacin B (0.2 mg/kg) offered
protection against pressure-overload cardiac hypertrophy with
potent anti-hypertrophy and anti-fibrosis effects (124). Male C57/BL6 mice were given
surgical aortic banding to induce cardiac hypertrophy. Cucurbitacin
B fed mice demonstrated a significant reduction in heart weight,
myocardial cell cross sectional area and interstitial fibrosis.
These effects were further accompanied with inhibition of
hypertrophy in phenylephrine stimulated cardiomyocytes attributed
to inhibition of AKT/mTOR/FOX03A axis and autophagy. Cucurbitacin B
may be indicated in sepsis-induced acute lung injury (ALI)
(125). A rat ALI model following
abdominal septic puncture was treated with cucurbitacin B
intraperitoneally. Cucurbitacin B-treated rats showed an increase
in arterial oxygen partial pressure, and reduction in lung
effusion, protein content, neutrophils and lymphocytes, and
cytokines in a dose-dependent manner, with 3- to 5-fold increment
in survival. Histological examination revealed that the pulmonary
destruction was alleviated with cucurbitacin B treatment, endorsing
its anti-oxidant and anti-inflammatory properties. Cucurbitacin B
offered hepatoprotective and anti-inflammatory effects in
artificially induced acute liver damage in outbred albino male
Wistar rats (126).
Flower-squeezed-sterile-juice of cucurbitacin B administered orally
at 200–700 μl/kg body weight for three days before
artificial induction of carbon chloride mediated hepatotoxicity
resulted in mild and reversible damage in the liver parenchyma and
liver function tests. However, higher dose showed signs of
irreversible hepatotoxicity. Psoriasis is a chronic keratinocyte
inflammatory adaptive immune-mediated disease, involving Th1-type
immune cells and cytokines, encompassing ~0.5–3% human population
globally. It is characterized by hyperproliferation, inflammation
and abnormal keratinocyte differentiation. Keratinocytes act as the
first line barrier in immune surveillance, and govern innate
immunity via inflammation-associated molecules such as NF-κB.
Imiquimod is an analog of adenosine, a chemical stress inducer.
Topical application of cucurbitacin B inhibited imiquimod-induced
pathogenesis of psoriatic dermatitis (127). It caused growth inhibition of
keratinocytes, which was mediated by inhibition of NF-κB and STAT3
signaling leading to reduced expression of psoriatic IL8 and CCL20
cytokines, thus, reducing epidermal hyperplasia and psoriatic
pathogenesis. Yesilada et al (42) indexed anti-inflammatory property of
cucurbitacin B using Whittle method, using a fraction from the
freeze-dried fruit juice of squirting cucumber Ecballium
elaterium. They estimated dye leakage from the vascular tissue
in orally fed mice. Vascular leakage corresponded to permeability
and inflammatory response. Authors recorded an increase in
permeability with cucurbiatcin B dose; however, 400 mg/kg body
weight (highest) also imposed severe toxicity to the animals.
Likewise, the ameliorative anti-oxidant effects of
cucurbitacin B and its relatives were shown as beneficial against
the neuronal injury (128). It
significantly inhibited neuroinflammatory mediators in TLR2/4
agonist-stimulated microglia. Excess TLR activated cytokine and
complement proteins may result into neuronal injury and
consequential cell death, mediated through pro-inflammatory
cytokines (TNFα and IL1β/6), and JAK/STAT and NF-κB pathways.
Pretreatment with cucurbitacin B suppressed the phosphorylation of
JAK1, STAT1 and STAT3, NF-κB transactivation and the cytokine
release, resulting into attenuation of expression of iNOS and COX2.
It enhanced the activity of Nrf2/ARE pathway to upregulate HO1 and
NQO1, and inhibited caspase activity to offer significant
neuroprotection. Rawat et al (43) also evaluated the anti-oxidant and
anti-inflammatory potential of cucurbitacin B containing plant
extracts. Extracts of cucurbits from L. siceraria, C.
pepo and L. cylindrical by means of superoxide radical
scavenging activity assay and catalase activity assay in the
presence of xylene orange and Amplex® Red demonstrated
potent radical scavenging activity. They also inhibited
inflammation specific cytokines COX-1 and COX-2 in
lipopolysaccharide-induced mouse serum. Cucurbitacun B with
cucurbitacin E in the dichloromethanolic extract from the roots of
Wilbrandia ebracteata Cogn. protected against inflammation
and algesia in rats (129). Male
Wistar rats were injected with inflammatory agent carrageenan in
paws, which developed into inflammation. Inflammation was
significantly less in rats fed with the extract. Furthermore, pain
felt with acetic acid application in the inflamed area was
significantly less as compared to the vehicle group.
Cucurbitacin B reduced the likelihood of onset of
hepatocellular carcinoma by preventing STAT3 phosphorylation and
consequent adipocyte accumulation (130). Phospho-STAT3 stimulates the
regulation of pro-proliferative cyclin D1 and adipogenesis markers
PPARγ, aP2, C/EBPα, adiponectin and CD36 proteins, and inhibits
adipocyte differentiation inhibitor KLF5. Seo et al
(130) showed that cucurbitacin B
suppressed the activation of STAT3, henceforth suppressing
adipocyte differentiation and accumulation. Prevention of
adipogenesis and lipid accumulation by 0.2–0.3 μM
cucurbitacin B was more promising than by 20 mM resveratrol
(standardized anti-adipogenic compound). Genetic silencing of STAT3
significantly attenuated the anti-adipogenic activity. Cucurbitacin
B showed synergistic antibacterial and antiviral potential against
Staphylococcus aureus and Herpes simplex virus-1 (131). Within a range of dose,
cucurbitacin B in synergism with other antibiotics such as
tetracycline and oxacillin inhibited the growth of clinical drug
resistant variant of Staphylococcus aureus, as determined by
microdilution method and checkboard assay. By means of plaque
number reduction assay, it showed potent anti-HSV-1 potential,
comparable to acyclovir. Cucurbitacin B treatment, however, led to
decline in the sexual hormone and behavior in male moth Agrotis
ipsilon (132). Cucurbitacin
B as an antagonist of 20E/EcR/USP complex and signaling pathway
staging crucial for sexual behavior from the male antennal lobe
reduced the amount of AipsEcR and AipsUSP in a dose-dependent
manner, which inhibited central pheromone processing in the adult
sexually mature male moth.
7. Cucurbitacin B - toxicity
Cucurbitacin B to the best of our knowledge has not
been included in toxic or restricted use by any of the drug and
safety bureau across the world, nor is there any report claiming
its toxic role in vivo across the world (133), except an FDA report from 1955 and
follow-up in 1983, where the authors reported that the median
lethal dose of cucurbitacin B via intraperitoneal route was ~1
mg/kg in male albino mice at the end of three doses (134). Cause of death was acute pulmonary
edema and respiratory arrest. Smit in 2000 (135) reported that in rabbits the median
lethal dose of cucurbitacin B was 0.5 mg/kg body weight after
intravenous administration (136). Ferguson et al (137) also reported poisoning after human
consumption of 700 g of commercially produced Cucurbita pepo
L. within 6-h period. Toxic symptoms included bitter taste in
mouth, abdominal cramps, diarrhea, and occasional vertigo and
syncope. Australian Therapeutic Goods Administration has listed
cucurbitacin B free from restricted use and encourages its use in
combination with other agents (138).
Cucurbitacin B in its pure form had median lethal
dose of ~5 mg/kg (oral route) (139) and 1 mg/kg (intraperitoneal)
(134) in mice, 0.5 mg/kg
(intravenous) (140) in rabbit,
and 0.32 mg/kg (intravenous) (141) in cat, recorded so far. Deaths at
this dose were accompanied with symptoms such as pulmonary edema,
however, with no mention of additional symptoms. Furthermore,
severe toxicity leading to death following oral administration were
reported in various studies reporting the use of plant and extracts
- Ecballium elaterium in rabbits caused nervousness,
restlessness, seizures, anorexia, tachycardia, tachypnea, dyspnea,
cyanosis, and diarrhea eventually leading to death (142,143); Cucurbita texana in Swiss
Webster mice caused severe anemia followed by death (144); Citrullus colosynthis and
Lagenaria siceraria in goats caused restlessness, anorexia,
diarrhea, dehydration, anemia, dyspnea, kinetic latency, internal
hemorrhages, pulmonary emphysema, enteritis, multi-organ failure,
and eventually death (145); and
Citrullus colocythis in Bovans-type chicks led to hepatic
fatty changes and toxicity, catarrhal enteritis, pulmonary
emphysema and renal tubular necrosis, but these symptoms reversed
after 4 weeks of withdrawal (146).
Various human toxicity complaints have been
reported from around the world after accidental or intentional
consumption of fruit juice of zucchini squash (142,147,148). Symptoms reported were similar to
those in the in vivo studies pivoting digestive and
neurological systems. However, following topical application with
the same extract the human subjects remained asymptomatic (149). Since the median lethal dose via
oral route was significantly higher than the parenteral, possibly
digestion, emergence of metabolites and their absorption from the
gut before reaching systemic circulation is crucial to alleviate
its toxic effects and liberate its medicinal potency. Moreover,
oral feeding led toxicities and deaths involved components
additional to cucurbitacin B, advocating bail for cucurbitacin B
off severely toxic profile. Therefore, oral administration of
purified form of cucurbitacin B as an independent executor may be
justified, as long as the dosage is conscientiously tailored and
the symptoms are cautiously checked.
8. Cucurbitacin B - biological supply chain
and future scope
The natural origin and synthetic production of
cucurbitacin B have been extensively studied over the last few
decades. Jung and Lui (150)
presented the artificial convergent edifice efforts. Razavilar and
Choi (151) found that the
diffusivity of cucurbitacin B depends on wiggling motion of the
block copolymers to diffuse while the contemporaneous water
molecules diffusion via a hopping mechanism. Toker et al
(152) proposed a novel method to
naturally increase the yield of cucurbitacin B using Ecballium
elaterium callus culture technique. They showed that the
subculture calluses of stem explants incubated in medium
supplemented with 1 mg/l benzyl adenine and 0.1 mg/l naphthalene
acetic acid could competently accrue the compound. Mei et al
(153) suggested a process for
cucurbitacin B bioproduction from one of its parent glycoside using
a specific Streptomyces sp., by enzyme broth incubation and
extraction with ethyl acetate. The process was mild, simple and
prolific; nevertheless, its hydrophobic and electrostatic structure
left it prognostically difficult. Quick exploitation followed by
attenuated performance of cucurbitacin B may abete solid doubts
about the treatment modality and dosage in practical use.
Cucurbitacin B is hydrophobic, hence suffers in situ
absorption. Its absorption from carboxymethyl chitosan films loaded
with phospholipid-bile salt-mixed micelles as mucoadhesive buccal
films resulted in 2.69-fold increase in bioavailability (154), with no toxic effect on buccal
mucosa. Molavi et al (155) prepared polymeric micelles of less
than 90 nanometers by encapsulating cucurbitacin B in
PEO-b-PCL and PEO-b-PBCL. PEO-b-PCL micelles showed
superiority in maintaining a sustained release of hydrophobic
cucurbitacin B, thereby limiting the rate of STAT3 activation in
murine cancer cell line. Patel et al (156) with the help of molecular dynamics
simulation studies predicted that the increasing PCL/PEO ratio in
PEO-b-PCL-cucurbitacin B micelles would reduce the drug release
rate, due to polar intermolecular interactions. Docking energy
analysis showed that the increase in ratio would favor additional
hydrogen bond formation between the molecule and
poly(ε-caprolactone). Cheng et al (157) prepared berberine hydrochloride
modified phospholipid complex loaded cucurbitacin B (CUB-PLC-BER)
as a formulation and tested its delivery and efficacy against
cholangiocarcinoma in 2015. Berberine hydrochloride is known to
stimulate bile release, hence was believed to contribute to
sustained and prolonged drug release. Cucurbitacin B loaded with
phospholipids and berberine hydrochloride had higher drug delivery
rate, and was more cytotoxic to the cancer cells in both in
vitro and in vivo. Wang et al (158) encapsulated cucurbitacin B in
glycosylated solid lipid nanoparticles. Administration of the
formulation resulted into significant increase in targeted
cytotoxicity, with overall target specific efficiency of nearly
64%, where the conventional formulations showed only 23–26%.
You et al (159) predicted the direct relation
between the STAT3 protein localization and autophagy. STAT3 in
cytoplasm inhibited autophagy via sequestration of EIF2AK2 and
interaction with FOXO1/3. Cytoplasmic localization initiated direct
protein-to-protein interaction and impeded autophagy by
countervailing autophagy related proteins. Nuclear STAT3 attuned
autophagy via transcriptional regulation of autophagy-related genes
such as the BCL2 family. It is mediated at the genetic level where
STAT3 activation may prompt genetic remodeling and attenuation of
autophagy. Mitochondrial translocation of STAT3 constitutively
suppressed autophagy. Studies reported that cucurbitacin B and
derivatives interfered with the activation of STAT3 (61,63,74,82,87,88,94,95,98,99,
102–105,109,110,119,130). Autophagy signaling is a fairly
new subject. Cucurbitacin B and its role in autophagy is mostly
unexplored, hence the field is wide blank to fill in.
9. Summary
Cucurbitacins, steroids derived from triterpene
hydrocarbon, found in a number of plant families, are some of the
principle compounds, which attribute medicinal qualities to the
plant. Cucurbitacin B, the bitter toxin, in previous few decades
has splendidly verified its cytotoxic potential against cancer and
other medical conditions. Through various mechanisms, it suppressed
the pathogenesis and inhibited the spread of cancer in vitro
and in vivo (Figs. 1 and
3). It did so via onset of tumor
suppressor p53 pathway and inhibition of tumor progression
Wnt/β-catenin, phospho-RB, NF-κB, JAK/STAT, PI3K-AKT, and MAPK/ERK
pathways. Treatment with cucurbitacin B consistently upregulated
DNA damage and pro-apoptotic protein markers, while downregulated
pro-survival markers, growth receptors and cell cycle progressors
at either transcriptional level or via their ubiquitination and
proteasomal degradation. Consequently, it caused growth arrest,
apoptosis, cellular differentiation, and inhibition of
proliferation in cancer cells. It also showed significant efficacy
against the developing detrimental effects of cardiac, pulmonary,
neurological and hepatic injuries, psoriasis, infections and
lipogenesis. It also caused STAT3 dependent autophagy.
Bio-production, efficacy and metabolism of the compound may be
improved by means of slight chemical modulation such as the use of
hydroponics, bio-transformers, micelles and nanomaterials. It is
not difficult to categorize cucurbitacin B as an effective
anticancer molecule. It has the potential to prevent cancer, delay
its progression, and might be able to completely cure it. Since it
is procured from the natural sources, it could significantly
economize the expenditure against the continuously growing disease.
Surprisingly, not one human study to explore the efficacy of the
molecule has been conducted so far. Clinical trials for
cucurbitacin B as the mainline-targeted anticancer therapy either
as an independent effector or as a supplement is warranted.
Acknowledgments
The present study was supported by grants from the
Department of Biotechnology (Government of India) and the National
Institute of Advanced Industrial Science and Technology, Japan.
References
|
1
|
Massagué J and Obenauf AC: Metastatic
colonization by circulating tumour cells. Nature. 529:298–306.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
World Health Organization: International
Agency for Research on Cancer: World cancer factsheet. Cancer
Research UK. http://gicr.iarc.fr/public/docs/20120906-WorldCancerFactSheet.pdf.
Accessed June 20, 2017.
|
|
3
|
Cheung EC and Vousden KH: The role of p53
in glucose metabolism. Curr Opin Cell Biol. 22:186–191. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Engelmann D and Pützer BM: Emerging from
the shade of p53 mutants: N-terminally truncated variants of the
p53 family in EMT signaling and cancer progression. Sci Signal.
7:re92014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hengartner MO: The biochemistry of
apoptosis. Nature. 407:770–776. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kamijo T, Zindy F, Roussel MF, Quelle DE,
Downing JR, Ashmun RA, Grosveld G and Sherr CJ: Tumor suppression
at the mouse INK4a locus mediated by the alternative reading frame
product p19ARF. Cell. 91:649–659. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Korenjak M and Brehm A: E2F-Rb complexes
regulating transcription of genes important for differentiation and
development. Curr Opin Genet Dev. 15:520–527. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sharpless NE, Alson S, Chan S, Silver DP,
Castrillon DH and DePinho RA: p16INK4a and p53
deficiency cooperate in tumorigenesis. Cancer Res. 62:2761–2765.
2002.PubMed/NCBI
|
|
9
|
Stott FJ, Bates S, James MC, McConnell BB,
Starborg M, Brookes S, Palmero I, Ryan K, Hara E, Vousden KH, et
al: The alternative product from the human CDKN2A locus,
p14ARF, participates in a regulatory feedback loop with
p53 and MDM2. EMBO J. 17:5001–5014. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dakeng S, Duangmano S, Jiratchariyakul W,
U-Pratya Y, Bögler O and Patmasiriwat P: Inhibition of Wnt
signaling by cucurbitacin B in breast cancer cells: Reduction of
Wnt-associated proteins and reduced translocation of
galectin-3-mediated β-catenin to the nucleus. J Cell Biochem.
113:49–60. 2012. View Article : Google Scholar
|
|
11
|
Nefedova Y and Gabrilovich DI: Targeting
of Jak/STAT pathway in antigen presenting cells in cancer. Curr
Cancer Drug Targets. 7:71–77. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hung MH, Tai WT, Shiau CW and Chen KF:
Downregulation of signal transducer and activator of transcription
3 by sorafenib: A novel mechanism for hepatocellular carcinoma
therapy. World J Gastroenterol. 20:15269–15274. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hossain DM, Pal SK, Moreira D, Duttagupta
P, Zhang Q, Won H, Jones J, D'Apuzzo M, Forman S and Kortylewski M:
TLR9-targeted STAT3 silencing abrogates immunosuppressive activity
of myeloid-derived suppressor cells from prostate cancer patients.
Clin Cancer Res. 21:3771–3782. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu H, Ren G, Wang T, Chen Y, Gong C, Bai
Y, Wang B, Qi H, Shen J, Zhu L, et al: Aberrantly expressed Fra1 by
IL6/STAT3 transactivation promotes colorectal cancer aggressiveness
through epithelial mesenchymal transition. Carcinogenesis.
36:4594682015. View Article : Google Scholar
|
|
15
|
Peyser ND, Freilino M, Wang L, Zeng Y, Li
H, Johnson DE and Grandis JR: Frequent promoter hypermethylation of
PTPRT increases STAT3 activation and sensitivity to STAT3
inhibition in head and neck cancer. Oncogene. 35:1163–1169. 2016.
View Article : Google Scholar
|
|
16
|
Wen W, Wu J, Liu L, Tian Y, Buettner R,
Hsieh MY, Horne D, Dellinger TH, Han ES, Jove R, et al: Synergistic
anti-tumor effect of combined inhibition of EGFR and JAK/STAT3
pathways in human ovarian cancer. Mol Cancer. 14:1002015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yao X, Liu H, Zhang X, Zhang L, Li X, Wang
C and Sun S: Cell surface GRP78 accelerated breast cancer cell
proliferation and migration by activating STAT3. PLoS One.
10:e01256342015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yoon J, Ko YS, Cho SJ, Park J, Choi YS,
Choi Y, Pyo JS, Ye SK, Youn HD, Lee JS, et al: Signal transducers
and activators of transcription 3-induced metastatic potential in
gastric cancer cells is enhanced by glycogen synthase kinase-3β.
APMIS. 123:373–382. 2015. View Article : Google Scholar
|
|
19
|
Royds JA, Dower SK, Qwarnstrom EE and
Lewis CE: Response of tumour cells to hypoxia: Role of p53 and
NF-κB. Mol Pathol. 51:55–61. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bulavin DV and Fornace AJ Jr: p38 MAP
kinase's emerging role as a tumor suppressor. Adv Cancer Res.
92:95–118. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pal I and Mandal M: PI3K and Akt as
molecular targets for cancer therapy: Current clinical outcomes.
Acta Pharmacol Sin. 33:1441–1458. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Harley CB: Aging of cultured human skin
fibroblasts. Methods Mol Biol. 5:25–32. 1990.PubMed/NCBI
|
|
23
|
Hayflick L: The limited in vitro lifetime
of human diploid cell strains. Exp Cell Res. 37:614–636. 1965.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kimmelman AC and White E: Autophagy and
tumor metabolism. Cell Metab. 25:1037–1043. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kaefer CM and Milner JA: Herbs and Spices
in Cancer Prevention and Treatment. Herbal Medicine: Biomolecular
and Clinical Aspects. 2nd edition. CRC Press/Taylor & Francis;
2011, View Article : Google Scholar
|
|
26
|
Kaushik U, Aeri V and Mir SR:
Cucurbitacins - An insight into medicinal leads from nature.
Pharmacogn Rev. 9:12–18. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Alghasham AA: Cucurbitacins - a promising
target for cancer therapy. Int J Health Sci (Qassim). 7:77–89.
2013. View Article : Google Scholar
|
|
28
|
Clericuzio M, Mella M, Vita-Finzi P, Zema
M and Vidari G: Cucurbitane triterpenoids from Leucopaxillus
gentianeus. J Nat Prod. 67:1823–1828. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen JC, Chiu MH, Nie RL, Cordell GA and
Qiu SX: Cucurbitacins and cucurbitane glycosides: Structures and
biological activities. Nat Prod Rep. 22:386–399. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Clericuzio M, Tabasso S, Bianco MA,
Pratesi G, Beretta G, Tinelli S, Zunino F and Vidari G: Cucurbitane
triterpenes from the fruiting bodies and cultivated mycelia of
Leucopaxillus gentianeus. J Nat Prod. 69:1796–1799. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wiart C: The definition and significance
of Cucurbitacin B a STAT3 inhibitors. Cancer Lett. 328:1882013.
View Article : Google Scholar
|
|
32
|
Dantas INF, Gadelha GCM, Chaves DC, Monte
FJQ, Pessoa C, de Moraes MO and Costa-Lotufo LV: Studies on the
cytotoxicity of cucurbitacins isolated from Cayaponia racemosa
(Cucurbitaceae). Z Naturforsch C. 61:643–646. 2006.PubMed/NCBI
|
|
33
|
Hatam NAR, Whiting DA and Yousif NJ:
Cucurbitacin glycosides from Citrullus colocynthis. Phytohemistry.
28:1268–1271. 1989. View Article : Google Scholar
|
|
34
|
Abou-Khalil R, Jraij A, Magdalou J, Ouaini
N, Tome D and Greige-Gerges H: Interaction of cucurbitacins with
human serum albumin: Thermodynamic characteristics and influence on
the binding of site specific ligands. J Photochem Photobiol B.
95:189–195. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li K, Yu Y, Sun S, Liu Y, Garg S, Kaul SC,
Lei Z, Gao R, Wadhwa R and Zhang Z: Functional characterization of
anticancer activity in the aqueous extract of Helicteres
angustifolia L. roots. PLoS One. 11:e01520172016. View Article : Google Scholar
|
|
36
|
Oberlies NH, Burgess JP, Navarro HA, Pinos
RE, Soejarto DD, Farnsworth NR, Kinghorn AD, Wani MC and Wall ME:
Bioactive constituents of the roots of Licania intrapetiolaris. J
Nat Prod. 64:497–501. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Beutler JA, McCall KL, Herbert K, Herald
DL, Pettit GR, Johnson T, Shoemaker RH and Boyd MR: Novel cytotoxic
diterpenes from Casearia arborea. J Nat Prod. 63:657–661. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ayyad SEN, Abdel-Lateff A, Basaif SA and
Shier T: Cucurbitacins-type triterpene with potent activity on
mouse embryonic fibroblast from Cucumis prophetarum, cucurbitaceae.
Pharmacognosy Res. 3:189–193. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen C, Qiang S, Lou L and Zhao W:
Cucurbitane-type triterpenoids from the stems of Cucumis melo. J
Nat Prod. 72:824–829. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dat NT, Jin X, Hong YS and Lee JJ: An
isoaurone and other constituents from Trichosanthes kirilowii seeds
inhibit hypoxia-inducible factor-1 and nuclear factor-kappaB. J Nat
Prod. 73:1167–1169. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wu KJ, Grandori C, Amacker M, Simon-Vermot
N, Polack A, Lingner J and Dalla-Favera R: Direct activation of
TERT transcription by c-MYC. Nat Genet. 21:220–224. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yesilada E, Tanaka S, Sezik E and Tabata
M: Isolation of an anti-inflammatory principle from the fruit juice
of Ecballium elaterium. J Nat Prod. 51:504–508. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Rawat I, Sharma D and Goel HC: Antioxidant
and anti-inflammatory potential of some dietary cucurbits. Oxid
Antioxid Med Sci. 3:65–72. 2014. View Article : Google Scholar
|
|
44
|
Schabort JC and Potgieter JJ: A thin-layer
and an improved paper-chromatographic methods for the separation of
Cucurbitacin B and 23,24-dihydrocucurbitacin B. J Chromatog.
31:235–237. 1967. View Article : Google Scholar
|
|
45
|
Tannin-Spitz T, Grossman S, Dovrat S,
Gottlieb HE and Bergman M: Growth inhibitory activity of
cucurbitacin glucosides isolated from Citrullus colocynthis on
human breast cancer cells. Biochem Pharmacol. 73:56–67. 2007.
View Article : Google Scholar
|
|
46
|
Wakimoto N, Yin D, O'Kelly J, Haritunians
T, Karlan B, Said J, Xing H and Koeffler HP: Cucurbitacin B has a
potent antiproliferative effect on breast cancer cells in vitro and
in vivo. Cancer Sci. 99:1793–1797. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Duangmano S, Dakeng S, Jiratchariyakul W,
Suksamrarn A, Smith DR and Patmasiriwat P: Antiproliferative
effects of cucurbitacin B in breast cancer cells: Down-regulation
of the c-Myc/hTERT/telomerase pathway and obstruction of the cell
cycle. Int J Mol Sci. 11:5323–5338. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wu PL, Lin FW, Wu TS, Kuoh CS, Lee KH and
Lee SJ: Cytotoxic and anti-HIV principles from the rhizomes of
Begonia nantoensis. Chem Pharm Bull (Tokyo). 52:345–349. 2004.
View Article : Google Scholar
|
|
49
|
Duangmano S, Sae-Lim P, Suksamrarn A,
Domann FE and Patmasiriwat P: Cucurbitacin B inhibits human breast
cancer cell proliferation through disruption of microtubule
polymerization and nucleophosmin/B23 translocation. BMC Complement
Altern Med. 12:1852012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Promkan M, Dakeng S, Chakrabarty S, Bögler
O and Patmasiriwat P: The effectiveness of cucurbitacin B in BRCA1
defective breast cancer cells. PLoS One. 8:e557322013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Boone JJ, Bhosle J, Tilby MJ, Hartley JA
and Hochhauser D: Involvement of the HER2 pathway in repair of DNA
damage produced by chemotherapeutic agents. Mol Cancer Ther.
8:3015–3023. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Friedrichs K, Ruiz P, Franke F, Gille I,
Terpe HJ and Imhof BA: High expression level of alpha 6 integrin in
human breast carcinoma is correlated with reduced survival. Cancer
Res. 55:901–906. 1995.PubMed/NCBI
|
|
53
|
Jones JL, Royall JE, Critchley DR and
Walker RA: Modulation of myoepithelial-associated alpha6beta4
integrin in a breast cancer cell line alters invasive potential.
Exp Cell Res. 235:325–333. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Desgrosellier JS and Cheresh DA: Integrins
in cancer: Biological implications and therapeutic opportunities.
Nat Rev Cancer. 10:9–22. 2010. View Article : Google Scholar
|
|
55
|
Gupta P and Srivastava SK: Inhibition of
Integrin-HER2 signaling by Cucurbitacin B leads to in vitro and in
vivo breast tumor growth suppression. Oncotarget. 5:1812–1828.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Gupta P and Srivastava SK: HER2 mediated
de novo production of TGFβ leads to SNAIL driven
epithelial-to-mesenchymal transition and metastasis of breast
cancer. Mol Oncol. 8:1532–1547. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
de Herreros AG, Peiró S, Nassour M and
Savagner P: Snail family regulation and epithelial mesenchymal
transitions in breast cancer progression. J Mammary Gland Biol
Neoplasia. 15:135–147. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ren G, Sha T, Guo J, Li W, Lu J and Chen
X: Cucurbitacin B induces DNA damage and autophagy mediated by
reactive oxygen species (ROS) in MCF-7 breast cancer cells. J Nat
Med. 69:522–530. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Sinha S, Khan S, Shukla S, Lakra AD, Kumar
S, Das G, Maurya R and Meeran SM: Cucurbitacin B inhibits breast
cancer metastasis and angiogenesis through VEGF-mediated
suppression of FAK/MMP-9 signaling axis. Int J Biochem Cell Biol.
77(Pt A): 41–56. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Lang KL, Silva IT, Zimmermann LA, Machado
VR, Teixeira MR, Lapuh MI, Galetti MA, Palermo JA, Cabrera GM,
Bernardes LS, et al: Synthesis and cytotoxic activity evaluation of
dihydrocucurbitacin B and cucurbitacin B derivatives. Bioorg Med
Chem. 20:3016–3030. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kausar H, Munagala R, Bansal SS, Aqil F,
Vadhanam MV and Gupta RC: Cucurbitacin B potently suppresses
non-small-cell lung cancer growth: Identification of intracellular
thiols as critical targets. Cancer Lett. 332:35–45. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Guo J, Wu G, Bao J, Hao W, Lu J and Chen
X: Cucurbitacin B induced ATM-mediated DNA damage causes G2/M cell
cycle arrest in a ROS-dependent manner. PLoS One. 9:e881402014.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zhang M, Bian ZG, Zhang Y, Wang JH, Kan L,
Wang X, Niu HY and He P: Cucurbitacin B inhibits proliferation and
induces apoptosis via STAT3 pathway inhibition in A549 lung cancer
cells. Mol Med Rep. 10:2905–2911. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Shukla S, Khan S, Kumar S, Sinha S, Farhan
M, Bora HK, Maurya R and Meeran SM: Cucurbitacin B alters the
expression of tumor-related genes by epigenetic modifications in
NSCLC and inhibits NNK-induced lung tumorigenesis. Cancer Prev Res
(Phila). 8:552–562. 2015. View Article : Google Scholar
|
|
65
|
Silva IT, Geller FC, Persich L, Dudek SE,
Lang KL, Caro MS, Durán FJ, Schenkel EP, Ludwig S and Simões CM:
Cytotoxic effects of natural and semisynthetic cucurbitacins on
lung cancer cell line A549. Invest New Drugs. 34:139–148. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Shukla S, Sinha S, Khan S, Kumar S, Singh
K, Mitra K, Maurya R and Meeran SM: Cucurbitacin B inhibits the
stemness and metastatic abilities of NSCLC via downregulation of
canonical Wnt/β-catenin signaling axis. Sci Rep. 6:218602016.
View Article : Google Scholar
|
|
67
|
Zhang Y, Ouyang D, Xu L, Ji Y, Zha Q, Cai
J and He X: Cucurbitacin B induces rapid depletion of the G-actin
pool through reactive oxygen species-dependent actin aggregation in
melanoma cells. Acta Biochim Biophys Sin (Shanghai). 43:556–567.
2011. View Article : Google Scholar
|
|
68
|
Bamburg JR and Bernstein BW: Roles of
ADF/cofilin in actin polymerization and beyond. F1000 Biol Rep.
2:622010.PubMed/NCBI
|
|
69
|
Wang W, Eddy R and Condeelis J: The
cofilin pathway in breast cancer invasion and metastasis. Nat Rev
Cancer. 7:429–440. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Van Troys M, Huyck L, Leyman S, Dhaese S,
Vandekerkhove J and Ampe C: Ins and outs of ADF/cofilin activity
and regulation. Eur J Cell Biol. 87:649–667. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Bamburg JR, Bernstein BW, Davis RC, Flynn
KC, Goldsbury C, Jensen JR, Maloney MT, Marsden IT, Minamide LS,
Pak CW, et al: ADF/Cofilin-actin rods in neurodegenerative
diseases. Curr Alzheimer Res. 7:241–250. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Zhang YT, Ouyang DY, Xu LH, Zha QB and He
XH: Formation of cofilin-actin rods following
cucurbitacin-B-induced actin aggregation depends on Slingshot
homolog 1-mediated cofilin hyperactivation. J Cell Biochem.
114:2415–2429. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Trichet L, Sykes C and Plastino J:
Relaxing the actin cytoskeleton for adhesion and movement with
Ena/VASP. J Cell Biol. 181:19–25. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Zhang YT, Xu LH, Lu Q, Liu KP, Liu PY, Ji
F, Liu XM, Ouyang DY and He XH: VASP activation via the
Gα13/RhoA/PKA pathway mediates cucurbitacin-B-induced actin
aggregation and cofilin-actin rod formation. PLoS One.
9:e935472014. View Article : Google Scholar
|
|
75
|
Yin D, Wakimoto N, Xing H, Lu D, Huynh T,
Wang X, Black KL and Koeffler HP: Cucurbitacin B markedly inhibits
growth and rapidly affects the cytoskeleton in glioblastoma
multiforme. Int J Cancer. 123:1364–1375. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Touihri-Barakati I, Kallech-Ziri O, Ayadi
W, Kovacic H, Hanchi B, Hosni K and Luis J: Cucurbitacin B purified
from Ecballium elaterium (L.) A. Rich from Tunisia inhibits α5β1
integrin-mediated adhesion, migration, proliferation of human
glioblastoma cell line and angiogenesis. Eur J Pharmacol.
797:153–161. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Zheng Q, Liu Y, Liu W, Ma F, Zhou Y, Chen
M, Chang J, Wang Y, Yang G and He G: Cucurbitacin B inhibits growth
and induces apoptosis through the JAK2/STAT3 and MAPK pathways in
SH-SY5Y human neuroblastoma cells. Mol Med Rep. 10:89–94. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Shang Y, Guo XX, Li WW, Rao W, Chen ML, Mu
LN and Li SJ: Cucurbitacin-B inhibits neuroblastoma cell
proliferation through up-regulation of PTEN. Eur Rev Med Pharmacol
Sci. 18:3297–3303. 2014.PubMed/NCBI
|
|
79
|
Chan KT, Meng FY, Li Q, Ho CY, Lam TS, To
Y, Lee WH, Li M, Chu KH and Toh M: Cucurbitacin B induces apoptosis
and S phase cell cycle arrest in BEL-7402 human hepatocellular
carcinoma cells and is effective via oral administration. Cancer
Lett. 294:118–124. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Niu Y, Sun W, Lu JJ, Ma DL, Leung CH, Pei
L and Chen X: PTEN activation by DNA damage induces protective
autophagy in response to cucurbitacin B in hepatocellular carcinoma
cells. Oxid Med Cell Longev. 2016:43132042016. View Article : Google Scholar
|
|
81
|
Haritunians T, Gueller S, Zhang L, Badr R,
Yin D, Xing H, Fung MC and Koeffler HP: Cucurbitacin B induces
differentiation, cell cycle arrest, and actin cytoskeletal
alterations in myeloid leukemia cells. Leuk Res. 32:1366–1373.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Chan KT, Li K, Liu SL, Chu KH, Toh M and
Xie WD: Cucurbitacin B inhibits STAT3 and the Raf/MEK/ERK pathway
in leukemia cell line K562. Cancer Lett. 289:46–52. 2010.
View Article : Google Scholar
|
|
83
|
Zhu JS, Ouyang DY, Shi ZJ, Xu LH, Zhang YT
and He XH: Cucurbitacin B induces cell cycle arrest, apoptosis and
autophagy associated with G actin reduction and persistent
activation of cofilin in Jurkat cells. Pharmacology. 89:348–6.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Gao Y, Islam MS, Tian J, Lui VWY and Xiao
D: Inactivation of ATP citrate lyase by Cucurbitacin B: A bioactive
compound from cucumber, inhibits prostate cancer growth. Cancer
Lett. 349:15–25. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Zaidi N, Swinnen JV and Smans K:
ATP-citrate lyase: A key player in cancer metabolism. Cancer Res.
72:3709–3714. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Zaytseva YY, Rychahou PG, Gulhati P,
Elliott VA, Mustain WC, O'Connor K, Morris AJ, Sunkara M, Weiss HL,
Lee EY, et al: Inhibition of fatty acid synthase attenuates
CD44-associated signaling and reduces metastasis in colorectal
cancer. Cancer Res. 72:1504–1517. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Liu T, Zhang M, Zhang H, Sun C and Deng Y:
Inhibitory effects of cucurbitacin B on laryngeal squamous cell
carcinoma. Eur Arch Otorhinolaryngol. 265:1225–1232. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Xie YL, Tao WH, Yang TX and Qiao JG:
Anticancer effect of cucurbitacin B on MKN-45 cells via inhibition
of the JAK2/STAT3 signaling pathway. Exp Ther Med. 12:2709–2715.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Liu X, Duan C, Ji J, Zhang T, Yuan X,
Zhang Y, Ma W, Yang J, Yang L, Jiang Z, et al: Cucurbitacin B
induces autophagy and apoptosis by suppressing CIP2A/PP2A/mTORC1
signaling axis in human cisplatin resistant gastric cancer cells.
Oncol Rep. 38:271–278. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Zhang ZR, Gao MX and Yang K: Cucurbitacin
B inhibits cell proliferation and induces apoptosis in human
osteosarcoma cells via modulation of the JAK2/STAT3 and MAPK
pathways. Exp Ther Med. 14:805–812. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Qu Y, Cong P, Lin C, Deng Y, Li-Ling J and
Zhang M: Inhibition of paclitaxel resistance and apoptosis
induction by cucurbitacin B in ovarian carcinoma cells. Oncol Lett.
14:145–152. 2017.PubMed/NCBI
|
|
92
|
Ma J, Zi Jiang Y, Shi H, Mi C, Li J, Xing
Nan J, Wu X, Joon Lee J and Jin X: Cucurbitacin B inhibits the
translational expression of hypoxia-inducible factor-1α. Eur J
Pharmacol. 723:46–54. 2014. View Article : Google Scholar
|
|
93
|
Wang X, Tanaka M, Peixoto HS and Wink M:
Cucurbitacins: Elucidation of their interactions with the
cytoskeleton. PeerJ. 5:e33572017. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Zhang T, Li Y, Park KA, Byun HS, Won M,
Jeon J, Lee Y, Seok JH, Choi SW, Lee SH, et al: Cucurbitacin
induces autophagy through mitochondrial ROS production which
counteracts to limit caspase-dependent apoptosis. Autophagy.
8:559–576. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Marostica LL, Silva IT, Kratz JM, Persich
L, Geller FC, Lang KL, Caro MSB, Durán FJ, Schenkel EP and Simões
CM: Synergetic antiproliferative effects of a new cucurbitacin B
derivative and chemotherapy drugs on lung cancer cell line A549.
Chem Res Toxicol. 28:1949–1960. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Silva IT, Carvalho A, Lang KL, Dudek SE,
Masemann D, Durán FJ, Caro MSB, Rapp UR, Wixler V, Schenkel EP, et
al: In vitro and in vivo antitumor activity of a novel
semisynthetic derivative of cucurbitacin B. PLoS One.
10:e01177942015. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Chen W, Leiter A, Yin D, Meiring M, Louw
VJ and Koeffler HP: Cucurbitacin B inhibits growth, arrests the
cell cycle, and potentiates antiproliferative efficacy of cisplatin
in cutaneous squamous cell carcinoma cell lines. Int J Oncol.
37:737–743. 2010.PubMed/NCBI
|
|
98
|
Liu T, Peng H, Zhang M, Deng Y and Wu Z:
Cucurbitacin B, a small molecule inhibitor of the Stat3 signaling
pathway, enhances the chemosensitivity of laryngeal squamous cell
carcinoma cells to cisplatin. Eur J Pharmacol. 641:15–22. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
El-Senduny FF, Badria FA, El-Waseef AM,
Chauhan SC and Halaweish F: Approach for chemosensitization of
cisplatin-resistant ovarian cancer by cucurbitacin B. Tumour Biol.
37:685–698. 2016. View Article : Google Scholar
|
|
100
|
Marostica LL, de Barros ALB, Oliveira J,
Salgado BS, Cassali GD, Leite EA, Cardoso VN, Lang KL, Caro MSB,
Durán FJ, et al: Antitumor effectiveness of a combined therapy with
a new cucurbitacin B derivative and paclitaxel on a human lung
cancer xenograft model. Toxicol Appl Pharmacol. 329:272–281. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Aribi A, Gery S, Lee DH, Thoennissen NH,
Thoennissen GB, Alvarez R, Ho Q, Lee K, Doan NB, Chan KT, et al:
The triterpenoid cucurbitacin B augments the antiproliferative
activity of chemotherapy in human breast cancer. Int J Cancer.
132:2730–2737. 2013. View Article : Google Scholar
|
|
102
|
Liu T, Zhang M, Zhang H, Sun C, Yang X,
Deng Y and Ji W: Combined antitumor activity of cucurbitacin B and
docetaxel in laryngeal cancer. Eur J Pharmacol. 587:78–84. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Thoennissen NH, Iwanski GB, Doan NB,
Okamoto R, Lin P, Abbassi S, Song JH, Yin D, Toh M, Xie WD, et al:
Cucurbitacin B induces apoptosis by inhibition of the JAK/STAT
pathway and potentiates antiproliferative effects of gemcitabine on
pancreatic cancer cells. Cancer Res. 69:5876–5884. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Iwanski GB, Lee DH, En-Gal S, Doan NB,
Castor B, Vogt M, Toh M, Bokemeyer C, Said JW, Thoennissen NH, et
al: Cucurbitacin B, a novel in vivo potentiator of gemcitabine with
low toxicity in the treatment of pancreatic cancer. Br J Pharmacol.
160:998–1007. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Yar Saglam AS, Alp E, Elmazoglu Z and
Menevse S: Treatment with cucurbitacin B alone and in combination
with gefitinib induces cell cycle inhibition and apoptosis via EGFR
and JAK/STAT pathway in human colorectal cancer cell lines. Hum Exp
Toxicol. 35:526–543. 2016. View Article : Google Scholar
|
|
106
|
Lee DH, Thoennissen NH, Goff C, Iwanski
GB, Forscher C, Doan NB, Said JW and Koeffler HP: Synergistic
effect of low-dose cucurbitacin B and low-dose methotrexate for
treatment of human osteosarcoma. Cancer Lett. 306:161–170. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Tacar O, Sriamornsak P and Dass CR:
Doxorubicin: An update on anticancer molecular action, toxicity and
novel drug delivery systems. J Pharm Pharmacol. 65:157–170. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Zhang Z, Zhang Y, Lv J and Wang J: The
survivin suppressant YM155 reverses doxorubicin resistance in
osteosarcoma. Int J Clin Exp Med. 8:18032–18040. 2015.
|
|
109
|
Kim SH, Kang JG, Kim CS, Ihm SH, Choi MG,
Yoo HJ and Lee SJ: Doxorubicin has a synergistic cytotoxicity with
cucurbitacin B in anaplastic thyroid carcinoma cells. Tumour Biol.
39:10104283176922522017.PubMed/NCBI
|
|
110
|
Yang T, Liu J, Yang M, Huang N, Zhong Y,
Zeng T, Wei R, Wu Z, Xiao C, Cao X, et al: Cucurbitacin B exerts
anti-cancer activities in human multiple myeloma cells in vitro and
in vivo by modulating multiple cellular pathways. Oncotarget.
8:5800–5813. 2017.
|
|
111
|
Di Gennaro E, Bruzzese F, Pepe S, Leone A,
Delrio P, Subbarayan PR, Avallone A and Budillon A: Modulation of
thymidilate synthase and p53 expression by HDAC inhibitor
vorinostat resulted in synergistic antitumor effect in combination
with 5FU or raltitrexed. Cancer Biol Ther. 8:782–791. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Ouyang D, Zhang Y, Xu L, Li J, Zha Q and
He X: Histone deacetylase inhibitor valproic acid sensitizes B16F10
melanoma cells to cucurbitacin B treatment. Acta Biochim Biophys
Sin (Shanghai). 43:487–495. 2011. View Article : Google Scholar
|
|
113
|
Lee HI, McGregor RA, Choi MS, Seo KI, Jung
UJ, Yeo J, Kim MJ and Lee MK: Low doses of curcumin protect
alcohol-induced liver damage by modulation of the alcohol metabolic
pathway, CYP2E1 and AMPK. Life Sci. 93:693–699. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
García-Niño WR and Pedraza-Chaverrí J:
Protective effect of curcumin against heavy metals-induced liver
damage. Food Chem Toxicol. 69:182–201. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Cerný D, Lekić N, Váňová K, Muchová L,
Hořínek A, Kmoníčková E, Zídek Z, Kameníková L and Farghali H:
Hepatoprotective effect of curcumin in
lipopolysaccharide/-galactosamine model of liver injury in rats:
Relationship to HO-1/CO antioxidant system. Fitoterapia.
82:786–791. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Aggarwal BB, Kumar A and Bharti AC:
Anticancer potential of curcumin: Preclinical and clinical studies.
Anticancer Res. 23(1A): 363–398. 2003.PubMed/NCBI
|
|
117
|
Sun Y, Zhang J, Zhou J, Huang Z, Hu H,
Qiao M, Zhao X and Chen D: Synergistic effect of cucurbitacin B in
combination with curcumin via enhancing apoptosis induction and
reversing multidrug resistance in human hepatoma cells. Eur J
Pharmacol. 768:28–40. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Tannin-Spitz T, Bergman M and Grossman S:
Cucurbitacin glucosides: Antioxidant and free-radical scavenging
activities. Biochem Biophys Res Commun. 364:181–186. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Duangmano S, Sae-Lim P, Suksamrarn A,
Patmasiriwat P and Domann FE: Cucurbitacin B causes increased
radiation sensitivity of human breast cancer cells via G2/M cell
cycle arrest. J Oncol. 2012:6016822012. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Smit HF, van den Berg AJJ, Kroes BH,
Beukelman CJ, Quarles van Ufford HC, van Dijk H and Labadie RP and
Labadie RP: Inhibition of T-lymphocyte proliferation by
cucurbitacins from Picrorhiza scrophulariaeflora. J Nat Prod.
63:1300–1302. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Chen JC, Chiu MH, Nie RL, Cordell GA and
Qiu SX: Cucurbitacins and cucurbitane glycosides: Structures and
biological activities. Nat Prod Rep. 22:386–399. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Jayaprakasam B, Seeram NP and Nair MG:
Anticancer and antiinflammatory activities of cucurbitacins from
Cucurbita andreana. Cancer Lett. 189:11–16. 2003. View Article : Google Scholar
|
|
123
|
Miro M: Cucurbitacins and their
pharmacological effects. Phytother Res. 9:159–168. 1995. View Article : Google Scholar
|
|
124
|
Xiao Y, Yang Z, Wu QQ, Jiang XH, Yuan Y,
Chang W, Bian ZY, Zhu JX and Tang QZ: Cucurbitacin B: Cucurbitacin
B protects against pressure overload induced cardiac hypertrophy. J
Cell Biochem. 118:3899–3910. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Hua S, Liu X, Lv S and Wang Z: Protective
effects of Cucurbitacin B on acute lung injury induced by sepsis in
rats. Med Sci Monit. 23:1355–1362. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
El Naggar MB, Chalupová M, Pražanová G,
Parák T, Švajdlenka E, Žemlička M and Suchý P: Hepatoprotective and
proapoptotic effect of Ecballium elaterium on
CCl4-induced hepatotoxicity in rats. Asian Pac J Trop
Med. 8:526–531. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Li ZJ, Shin JM, Choi DK, Lim SK, Yoon TJ,
Lee YH, Sohn KC, Im M, Lee Y, Seo YJ, et al: Inhibitory effect of
cucurbitacin B on imiquimod-induced skin inflammation. Biochem
Biophys Res Commun. 459:673–678. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Park SY, Kim YH and Park G: Cucurbitacins
attenuate microglial activation and protect from neuroinflammatory
injury through Nrf2/ARE activation and STAT/NF-κB inhibition.
Neurosci Lett. 609:129–136. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Peters RR, Farias MR and Ribeiro-do-Valle
RM: Anti-inflammatory and analgesic effects of cucurbitacins from
Wilbrandia ebracteata. Planta Med. 63:525–528. 1997. View Article : Google Scholar
|
|
130
|
Seo CR, Yang DK, Song NJ, Yun UJ, Gwon AR,
Jo DG, Cho JY, Yoon K, Ahn JY, Nho CW, et al: Cucurbitacin B and
cucurbitacin I suppress adipocyte differentiation through
inhibition of STAT3 signaling. Food Chem Toxicol. 64:217–224. 2014.
View Article : Google Scholar
|
|
131
|
Hassan STS, Berchova-Bimova K, Petras J
and Hassan KTS: Cucurbitacin B interacts synergistically with
antibiotics against Staphylococcus aureus clinical isolates and
exhibits antiviral activity against HSV-1. S Afr J Bot. 108:90–94.
2017. View Article : Google Scholar
|
|
132
|
Duportets L, Maria A, Vitecek S, Gadenne C
and Debernard S: Steroid hormone signaling is involved in the
age-dependent behavioral response to sex pheromone in the adult
male moth Agrotis ipsilon. Gen Comp Endocrinol. 186:58–66. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Mezher M: The essential list of regulatory
authorities in Asia. Regulatory Affairs Professional Society.
published April 4, 2015. http://www.raps.org/Regulatory-Focus/News/Databases/2015/04/06/21908/The-Essential-List-of-Regulatory-Authorities-in-Asia/.
Accessed Sep 29, 2017.
|
|
134
|
David A and Vallance DK: Bitter principles
of Cucurbitaceae. J Pharm Pharmacol. 7:295–296. 1955. View Article : Google Scholar
|
|
135
|
Baxter H, Harborne JB and Moss GP:
Phytochemical Dictionary: A Handbook of Bioactive Compounds from
Plants. 2nd edition. Taylor & Francis Ltd; London, UK: pp.
7861999
|
|
136
|
World Health Organization: WHO Monograph
on Selected Medicinal Plants. 4:2662009.
|
|
137
|
Ferguson JE, Fischer DC and Metcalf RL: A
report of Cucurbitacin poisonings in humans. Rep Cucurbit Genet
Coop. 6:73–74. 1983.
|
|
138
|
Therapeutic Goods Association: Health
Safety Regulation - Substances that may be used in listed medicines
in Australia. Government of Australia. 862011.
|
|
139
|
Le Men J, Buffard G, Provost J, Tiberghien
R, Forgacs P, Lagrange E, Albert O and Aurousseau M: Relations
entre la structure de quelques cucurbitacines, leur toxicité et
leur activité laxative. Chim Therapeutique. 4:459–465. 1969.In
French.
|
|
140
|
Enslin PR: Bitter principles of the
Cucurbitaceae I - observations on the chemistry of cucurbitacin A.
J Sci Food Agric. 5:410–416. 1954. View Article : Google Scholar
|
|
141
|
Gry J, Soborg I and Anderson HC: Identity,
physical and chemical properties, analytical methods. Cucurbitacins
in plant food. Ekspressen Tyrk & Kopicenter, Copenhagen,
Denmark. 182006.
|
|
142
|
Sezik E: Research on the Turkish medicinal
plant Ecballium elaterium. Chem Nat Compd. 33:541–542. 1997.
View Article : Google Scholar
|
|
143
|
Steyn DG: The toxicity of bitter-tasting
cucurbitaceous vegetables (vegetable marrow, watermelons, etc) for
man. S Afr Med J. 24:713–715. 1950.PubMed/NCBI
|
|
144
|
Stoewsand GS, Jaworski A, Shannon S and
Robinson RW: Toxicologic response in mice fed Cucurbita fruit. J
Food Prot. 48:50–51. 1985. View Article : Google Scholar
|
|
145
|
Barri MES, Onsa TO, Elawad AA, Elsayed NY,
Wasfi IA, Abdul-Bari EM and Adam SEI: Toxicity of five Sudanese
plants to young ruminants. J Comp Pathol. 93:559–575. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Bakhiet AO and Adam SE: An estimation of
Citrullus colocynthis toxicity for chicks. Vet Hum Toxicol.
37:356–358. 1995.PubMed/NCBI
|
|
147
|
Rymal KS, Chambliss OL, Bond MD and Smith
DA: Squash containing toxic Cucurbitacin compounds occurring in
California and Alabama. J Food Prot. 47:270–271. 1984. View Article : Google Scholar
|
|
148
|
Pilegaard K and Søborg I: Squash med
bitter smag. Nyt Levnedsmiddelstyrelsen Nr. 23:1995.In Danish.
|
|
149
|
Raikhlin-Eisenkraft B and Bentur Y:
Ecbalium elaterium (squirting cucumber) - remedy or poison? J
Toxicol Clin Toxicol. 38:305–308. 2000. View Article : Google Scholar
|
|
150
|
Jung ME and Lui RM: Studies toward the
total syntheses of cucurbitacins B and D. J Org Chem. 75:7146–7158.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Razavilar N and Choi P: Molecular dynamics
study of the diffusivity of a hydrophobic drug Cucurbitacin B in
pseudo-poly(ethylene oxide-b-caprolactone) micelle environments.
Langmuir. 30:7798–7803. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Toker G, Memişoğlu M, Toker MC and
Yeşilada E: Callus formation and cucurbitacin B accumulation in
Ecballium elaterium callus cultures. Fitoterapia. 74:618–623. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Mei J, Li S, Jin H, Tang L, Yi Y, Wang H
and Ying G: A biotransformation process for the production of
cucurbitacin B from its glycoside using a selected Streptomyces sp.
Bioprocess Biosyst Eng. 39:1435–1440. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Lv Q, Shen C, Li X, Shen B, Yu C, Xu P, Xu
H, Han J and Yuan H: Mucoadhesive buccal films containing
phospholipid-bile salts-mixed micelles as an effective carrier for
cucurbitacin B delivery. Drug Deliv. 22:351–358. 2015. View Article : Google Scholar
|
|
155
|
Molavi O, Ma Z, Mahmud A, Alshamsan A,
Samuel J, Lai R, Kwon GS and Lavasanifar A: Polymeric micelles for
the solubilization and delivery of STAT3 inhibitor cucurbitacins in
solid tumors. Int J Pharm. 347:118–127. 2008. View Article : Google Scholar
|
|
156
|
Patel SK, Lavasanifar A and Choi P: Roles
of nonpolar and polar intermolecular interactions in the
improvement of the drug loading capacity of PEO-b-PCL with
increasing PCL content for two hydrophobic Cucurbitacin drugs.
Biomacromolecules. 10:2584–2591. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Cheng L, Xu PH, Shen BD, Shen G, Li JJ,
Qiu L, Liu CY, Yuan HL and Han J: Improve bile duct-targeted drug
delivery and therapeutic efficacy for cholangiocarcinoma by
cucurbitacin B loaded phospholipid complex modified with berberine
hydrochloride. Int J Pharm. 489:148–157. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Wang W, Zhao X, Hu H, Chen D, Gu J, Deng Y
and Sun J: Galactosylated solid lipid nanoparticles with
cucurbitacin B improves the liver targetability. Drug Deliv.
17:114–122. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
You L, Wang Z, Li H, Shou J, Jing Z, Xie
J, Sui X, Pan H and Han W: The role of STAT3 in autophagy.
Autophagy. 11:729–739. 2015. View Article : Google Scholar : PubMed/NCBI
|