Introduction
Brain tissues consist of neurons and glial cells,
including astrocytes, oligodendrocytes, microglia and ependymal
cells. They differentiate under the control of various
transcription factors (1). Gliomas
generally occur from glial cells or their precursor cells, and
consist of various subtypes with different histologies and natures
(2–4). However, gliomas generally exhibit a
high invasive activity, and expand outward by infiltrating into
normal bain tissues (5). They are
actually resistant to chemotherapy and radiation therapy, and the
presence of brain blood barrier hampers the effects of various
anticancer reagents. On the other hand, surgical resection is not
so efficient, since neural tissues have no regenerative capacity.
Thus, there are no radical therapies to eradicate gliomas, and thus
patients with the disease have a very poor prognosis. In
particular, patients with glioblastomas have the worst prognosis,
i.e., the 1-year survival rate is 51.6% and the 5-year survival
rate is 7.8% (6).
Sialic acid-containing glycosphingolipids,
gangliosides, have been reported to be expressed at very high
levels in neural tissues (7), and
play important roles in the development and functions of the
central nervous system (8). In
particular, glycosphingolipid structures change during brain
development and among various anatomical sites, which strongly
suggests that individual gangliosides play roles depending on the
polymorphic carbohydrate structures (9). During the early stages of
embryogenesis, simple gangliosides, such as GM3 and GD3 are major
structures. On the other hand, more complex gangliosides (GM1,
GD1a, GD1b and GT1b) are mainly expressed during the later stages
of brain formation, such as neurite extension and synaptogenesis
(10).
Some gangliosides have been reported to be
tumor-associated antigens (11,12).
For example, a number of neuro-ectoderm-derived cancers, such as
melanomas (12) and neuroblastomas
(13,14) have been reported to
characteristically express GD3 and GD2, respectively. Recently, a
number of studies have demonstrated that other human cancers also
express GD3 and GD2, such as GD3 in childhood T cell lymphoblastic
malignancies and human leukocytes and leukemia cells (15,16),
as well as GD2 in other human cancers, i.e., neuroblastomas
(13,14), small cell lung carcinomas (17,18),
osteosarcomas (19,20), breast cancers (21) and human lymphotropic type I
virus-infected T cells (22).
As for gliomas, there are some studies on the
expression of gangliosides with relatively short chains (23–29).
Although there have been some reports on GD3 and/or GD2 expression
in gliomas, and on the degree of malignancy increasing along with
the elevated expression of these gangliosides (27), there have been no studies to date
investigating the roles of gangliosides in the malignant properties
of human gliomas, at least to the best of our knowledge.
In this study, we aimed to determine the mechanisms
through which gangliosides are involved in the malignant properties
of gliomas, and to identify the molecules involved in the signaling
pathways mediated by glioma-associated gangliosides. The
nomenclature of gangliosides was based on the study by Svennerholm
(30).
Materials and methods
Immunohistochemistry
Brain tumor samples were obtained for use in the
experiments after obtaining informed consent. This study was
performed in accordance with the code of Ethics of the World
Medical Association, and approved by the Ethics Committee of Nagoya
University Graduate School of Medicine, Nagoya, Japan. The brain
tumors were embedded in OCT compound (Tissue-TEK™; Sakura Finetek
Japan, Tokyo, Japan) immediately after surgical resection, and
frozen sections were prepared using a cryostat ULTRACUT S™
(Reichert, Leica, Wien, Austria) with 7 µm thickness, and
then mounted onto MAS-coated slide glasses. After blocking with 10%
goat serum in phosphate-buffered saline (PBS) at room temperature
for 1 h, staining was performed using monoclonal antibodies (mAbs)
reactive with individual gangliosides. Anti-GD3 antibody (R24;
mouse IgG3) was provided by L.J. Old at Sloan-Kettering Cancer
Center, and anti-GD2 antibody (220-51; mouse IgG1) was as
previously described (31). These
mAbs were used at a 1:200 dilution of ascites. Reactions with these
primary antibodies were carried out overnight at 4°C, and the glass
slides were washed with PBS 3 times, followed by incubation with
the secondary antibody Alexa Fluor 555-labeled goat anti-mouse IgG
(A21422; Invitrogen, Carlsbad, CA, USA) (1:500 dilution) at room
temperature for 1 h. After washing with PBS 3 times, ganglioside
expression was examined under a confocal microscope FV-100-D
(Olympus, Tokyo, Japan).
Cell culture
The cells were cultured in Dulbecco's modified
Eagle's medium (DMEM) containing 10% fetal calf serum (FCS), 0.16%
NaHCO3, 2 mM L-glutamine, 96 U/ml penicillin G, 72 U/ml
streptomycin at 37°C under 5% CO2. The cell lines used
in this study were the following: Human glioma cell lines, U-251MG
(from JCRB Cell Bank, Osaka, Japan), T-98G, U-87MG (both from ATCC,
Manassas, VA, USA), and LN319 (from Y. Kato at Tohoku University,
Sendai, Japan). It should be noted that the U-87MG cell line is not
the original glioblastoma cell line established in 1968 at the
University of Uppsala as previously described (32), but is most probably a glioblastoma
cell line. The LN319 cell line is an anaplastic astrocytoma (mixed
astrocytoma type), and has been shown to be a derivative of the
LN-992 cell line (33). Since
these issues are unlikely to affect the outcomes of our study, we
decided to include these results from these problematic cell lines.
Details can be seen at the following website from the International
Cell Line Authentification Committee (ICLAC), the Database of
Cross-Contaminated or Misidentified Cell Lines (http://iclac.org/databases/cross-contaminations/).
Further information for the U87MG cell line can be found at
http://web.expasy.org/cellosaurus/CVCL_0022, and for
the LN319 cell line at https://web.expasy.org/cello-saurus/CVCL_3958.
Flow cytometry
Following trypsinization of the cells in the dishes,
the cells were washed with PBS (Ca++, Mg++
free) termed PBS(−) at 4°C, then were resuspended in PBS(−) with 1%
FCS. Using ~5.0×104 to 1.0×105 cells, the
primary antibody reaction was performed at 4°C for 1 h using mAbs
R24 or 220-51 as described above, and the cells were then washed
with PBS(−) 3 times. Subsequently, FITC-labeled secondary
antibodies (goat anti-mouse IgG) (AS-28175-05; AnaSpec, Fremont,
CA, USA) (1:200 dilution) were added to the cells, followed by
incubation at 4°C for 1 h. After washing with PBS(−), the labeled
cells were analyzed using a FACSCalibur™ flow cytometer (BD
Biosciences, Franklin Lakes, NJ, USA).
Reverse transcription-quantitative
(real-time) PCR (RT-qPCR)
Total RNA was extracted from the cells using TRIzol™
reagent (Invitrogen) according to the manufacturer's instructions.
Using extracted total RNA, reverse transcription was performed
using M-MLV RT™ (Invitrogen) according to the manufacturer's
instructions. The final products, cDNAs were applied used in the
DNA Engine Opticon2™ system (MJ Research, Waltham, MA, USA) to
quantify the gene expression levels. The primer sequences used were
as follows: GD3 synthase (ST8SIA1) sense,
5′-ttcaacctctctcttcccaca-3′ and antisense,
5′-tcttcttcagaatcccaccatt-3′; and β-actin (ACTINB) sense,
5′-acccactcctccaccttgac-3′ and antisense,
5′-cctgttgctgtagcccaaattcg-3′.
Generation of transfectant cell lines of
GD3 synthase cDNA
The U-251MG cells were transfected with an
expression vector, pMIKneo-GD3S (34) using Lipofectamine 2000™
(Invitorgen) and OPTI-MEM™ (Gibco-BRL, Gaithersburg, MD, USA)
followed by selection with G418 (500 µg/ml). Finally,
G418-resistant GD3-positive cells were applied for limiting
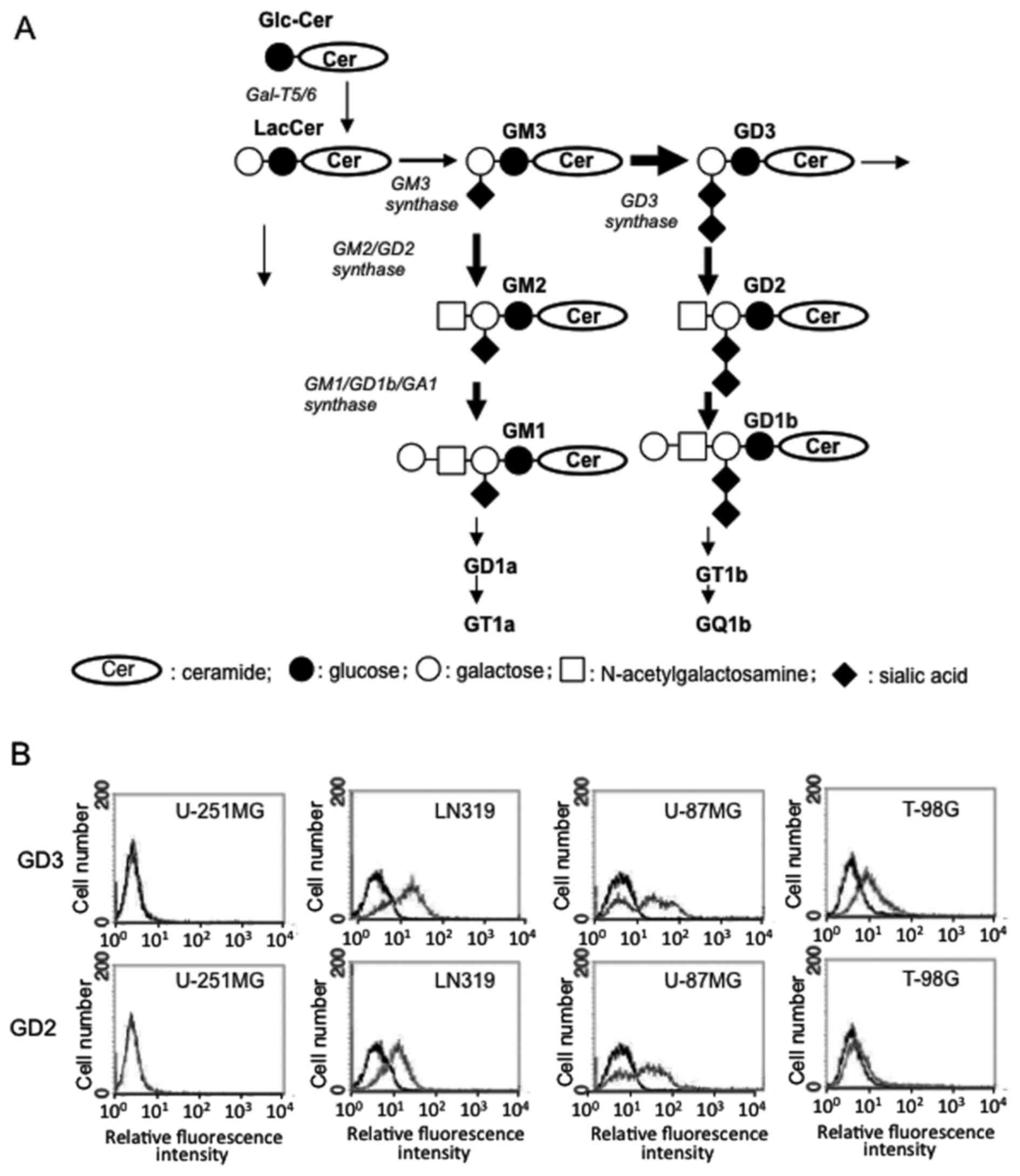
dilution to establish monoclonal cell lines. The synthetic pathway
of gangliosides is shown in Fig.
1A.
Invasion assay
The cell invasive activity was examined using the
Boyden-chamber method as previously described (35). The cells were plated in the upper
chamber in DMEM, and the chamber was placed in 2 ml of 0.1%
FCS-containing DMEM in a 6-well plate. After 24 h, the numbers of
cells that had migrated to the reverse side of the chamber were
counted after being fixed and stained with Giemsa (Wako, Osaka,
Japan). The counting of the cell numbers was carried out randomly
selecting 9 vision fields with no overlapping under an inverted
research microscope (Olympus IX73; Olympus, Tokyo, Japan)
MTT assay
The cells (3.0–5.0×103) were plated in
96-well plates, and incubated for the appropriate amount of time
(12–24 h). Subsequently, MTT solution (5 mg/ml) was added (20
µl/well), followed by incubation for 3 h at 37°C in a 5%
incubator. Acidic isopropyl alcohol (150 µl/well) was added,
and the cell membranes were destroyed by rigorous pipetting.
Subsequently, 100 µl of supernatant were transferred from
each well to another 96-well plate, and the absorption was measured
using an EIA reader at 590 nm (background: 620 nm). The ratio of OD
to the standard one was calculated, and plotted by the function of
time.
Western blot analysis
Cell lysates were prepared by scraping the cells
following the addition of lysis buffer to the dishes on ice, and
the collected lysates were treated by sonication at 4°C, then
centrifuged at 20,630 × g at 4°C. The supernatants were used as
cell lysates.
To prepare the fraction of lysates, trypsinized
cells were collected and resuspended in buffer A [400 mM Tris-HCl
(pH 7.4), 10 mM NaCl, 1 mM EDTA, 0.5% DTT, 1 mM sodium
orthovanadate, 1 mM NaF, 100 nM Okadaic acid and 1X proteinase
inhibitor mixture (Calbiochem, San Diego, CA, USA)]. These cells
were gently treated with a syringe with 23G ca 10 times to destroy
the cell membrane, and centrifuged at 1,000 × g for 10 min at 4°C.
The resulting supernatant was used as a cytoplasmic fraction. When
the trypsinized cells were treated in buffer B [400 mM Tris-HCl (pH
7.4), 150 mM NaCl, 1 mM EDTA, 0.2% Triton X-100, 0.5% DTT, 1 mM
sodium orthovanadate, 1 mM NaF, 100 nM Okadaic acid, and 1X
proteinase inhibitor mixture], the lysates were vortexed 5 times (1
min each), and left for 10 min at 4°C. Finally, the lysates were
centrifuged at 16,000 × g for 25 min at 4°C, and the supernatant
was used as solubilized proteins.
These lysates were treated with a 4X SDS sample
buffer [125 mM Tris-HCl (pH 6.8), 4% SDS, 20% glycerol, bromphenol
blue (BPB) and 2% 2-mercaptoethanol] and boiled for 5 min. The
proteins were then separated by SDS-PAGE, and blotted onto PVDF
membranes (Immobilon-PT™; Merck Millipore, Tokyo, Japan) under wet
condition for 240 min at 80 V. After blotting, the PVDF membranes
were soaked in PBST buffer [20 mM Tris-HCl (pH 7.5), 140 mM NaCl
and 0.05% Tween-20], containing 5% skim milk for 1 h at room
temperature. The reaction with the primary antibody was performed
at 4°C overnight. The membranes were then washed with PBST buffer 4
times, and the reaction with the secondary antibody was carried out
for 1 h at room temperature. After washing, antibody binding was
detected using ECL™ (GE Healthcare Japan, Tokyo, Japan). Antibodies
used in western blotting were as follows: anti-focal adhesion
kinase (FAK) antibody (SC558, rabbit IgG, 1:1,000), anti-p130Cas
(SC860, rabbit IgG, 1:1,000) purchased from Santa Cruz
Biotechnology (Santa Cruz, CA, USA), anti-phospho-FAK (Tyr577,
3281, rabbit IgG, 1:1,000), anti-phospho-p130Cas (Tyr410, 4011S,
rabbit IgG, 1:1,000), anti-paxillin antibody (total paxillin,
2542S, rabbit IgG, 1:1,000; Tyr118, 2541, rabbit IgG, 1:1,000),
anti-phospho-p38 antibody (9211S, rabbit IgG, 1:1,000), anti-p38
antibody (9212S, rabbit IgG, 1:1,000), anti-phospho-SAPK/JNK
antibody (9251S, rabbit IgG, 1:1,000), anti-SAPK/JNK antibody
(9252S, rabbit IgG, 1:1,000), anti-p16 antibody (4824, mouse IgG,
1:1,000), anti-p21 antibody (2946S, mouse IgG, 1:1,000),
anti-phospho-p53 (Ser20) antibody (9287, rabbit IgG, 1:500),
anti-p53 antibody (9282S, rabbit IgG, 1:1,000), anti-phospho-Erk1/2
antibody (9101S, rabbit IgG, 1:1,000), anti-Erk1/2 antibody (9102S,
IgG, 1:1,000), anti-phospho-Akt antibody (Thr308, 9275S, rabbit
IgG, 1:1,000; Ser473, 9271S, rabbit IgG, 1:1,000) and anti-Akt
antibody (9272S, rabbit IgG, 1:1,000), anti-total mTOR (45175,
mouse IgG, 1:1,000) and anti-phospho-mTOR (29715, rabbit IgG,
1:1,000) (all purchased from Cell Signaling Technology, Danvers,
MA, USA). Anti-phospho-paxillin (Tyr31, 44-720G, rabbit IgG,
1:1,000) was purchased from Invitrogen. As the secondary
antibodies, HRP-labeled anti-mouse IgG (NA931, 1:500) and
HRP-labeled anti-rabbit IgG (NA934, 1:500) antibodies were
purchased from GE Healthcare.
Analysis of apoptosis
Following trypsinization, the cells were treated
with FITC-labeled Annexin V and propiodium iodide (PI) and
incubated on ice for 30 min, and then examined using a FACSCalibur™
flow cytometer (BD Biosciences) to obtain the 2-D staining pattern
of FITC and PI as previously described (36).
Cell cycle analysis
Following detachment of the cells by trypsinization,
1 mM EDTA, 0.2% Triton X-100, 50 µg/ml RNase were added
followed by incubation for 30 min. After staining the DNA with PI,
the fluorescence intensity of PI was measured using a FACSCalibur™
flow cytometer (BD Biosciences), and the cell cycle was analyzed
using ModFiT LT™ software (Verity Software House, Topsham, ME,
USA).
Real-time cell electron sensoring
(RT-CES)
The cells (1.0×104) were plated in the
well of a RT-CES™ 16× E-plate (ACEA Biosciences, Inc; San Diego,
CA, USA), and the cell index was measured according to the attached
protocol provided by the company.
Statistical analysis
Data are presented as the means ± SD. The data were
analyzed by one-way ANOVA with the Tukey-Kramer post hoc test, or
by two-way ANOVA with the Bonferroni post-hoc test except for
Fig. 6 (Student's t-test), and as
indicated in the individual figure legends.
Results
GD3/GD2 are definitely expressed as shown
by the immunohistochemical staining of glioma tissues
Frozen sections of primary brain tumor tissues were
applied for immunohistochemistry using anti-GD3 and anti-GD2
antibodies (data not shown). The definite expression of GD3 and GD2
in brain tumors was observed.
GD2/GD3 expression is found on the cell
surface of glioma cells
Using the U-251MG, T-98G, LN319 and U-87MG cell
lines, the surface expression of GD2/GD3 was analyzed by flow
cytometry (Fig. 1B). Although the
U-251MG cells did not express either GD3 or GD2, the T-98G cells
expressed GD3, and the LN319 and U-87MG cells expressed both GD3
and GD2.
mRNA expression of GD3 synthase in
cultured glioma cell lines
Using 4 glioma lines, U-251MG, T-98G, LN319 and
U-87MG, the mRNA expression levels of the GD3 synthase gene were
examined by RT-qPCR. The U-87MG cells exhibited high levels of GD3
synthase mRNA, while the U-251MG, T-98G and LN319 cells exhibited
low expression levels (data not shown). Since the U-251MG cells
repeatedly expressed almost no GD3 synthase gene corresponding with
no expression of GD3 or GD2 (Fig.
1B), this cell line was deemed suitable for the transfection of
GD3 synthase cDNA and the generation of a stable transfectant cell
line to be used for the analysis of phenotypic changes and the
functions of newly expressed gangliosides. Thus, the U-251MG cells
were used for the remodeling of gangliosides hereafter.
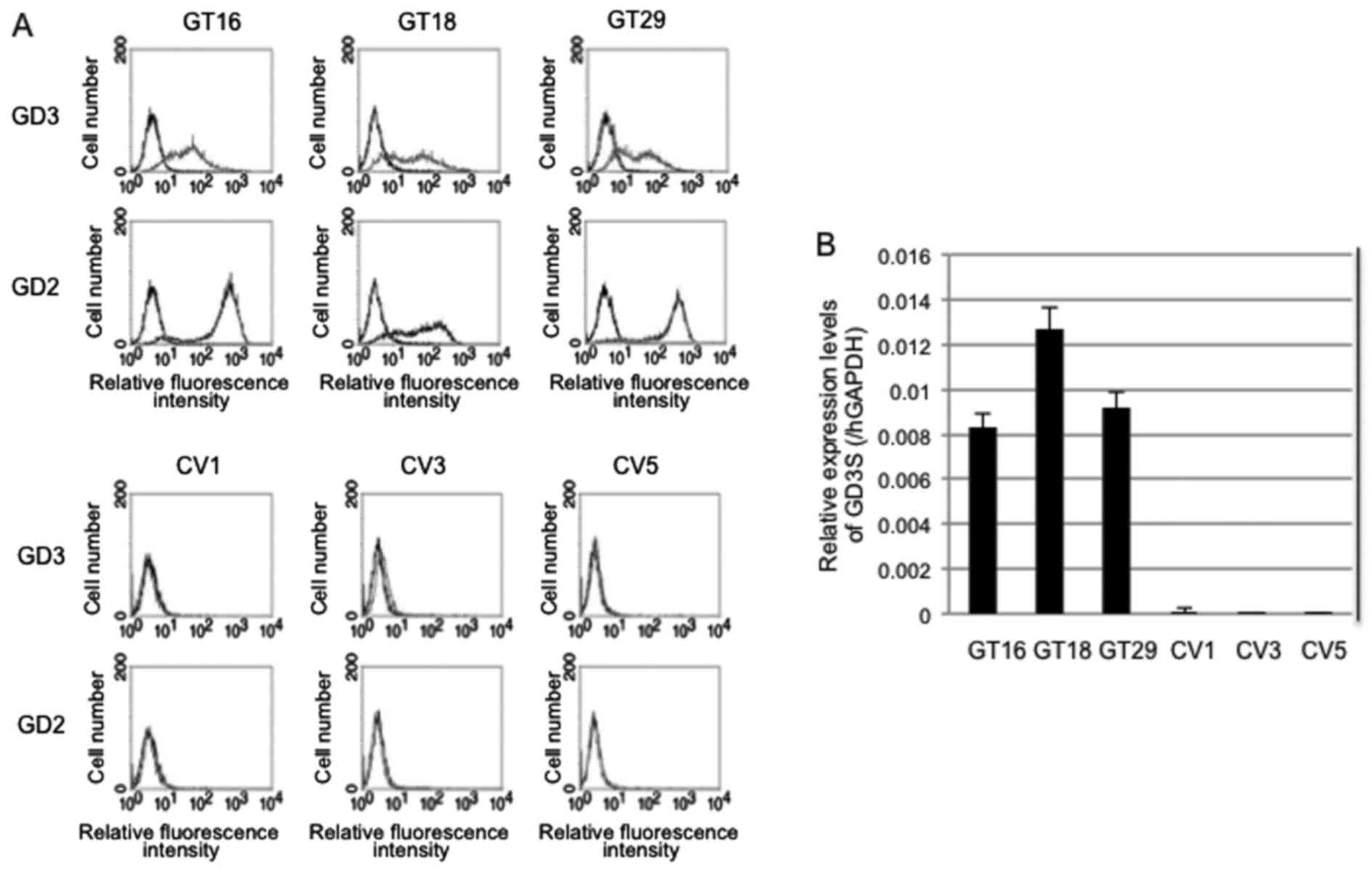
Generation of stable transfectant cells
of GD3 synthase cDNA using the U-251MG cells
Three clones each of U-251MG cells expressing GD3
[GD3(+)] and not expressing GD3 [GD3(−)] were generated by
transfection with the expression vector, pMIKneo-GD3S suing
Lipofectamine™ and subsequent limiting dilution. GD3(+) clones were
designated as GT16, GT18 and GT29 [GD3(+) cells], while
transfectants with the vector alone were designated as CV1, CV3 and
CV5 [GD3(−) cells]. The cell surface expression of GD3/GD2 on these
transfectant cells was analyzed by flow cytometry (Fig. 2A). All the GT16, GT18 and GT29
clones repeatedly exhibited a high expression of GD3/GD2. However,
the expression of GD2 was stronger than that of GD3 in all GD3(+)
cells, exhibiting similar patterns as observed in the
immunohistochemistry of primary brain tumors.
The mRNA expression of GD3 synthase in these cell
lines was confirmed by RT-qPCR (Fig.
2B). The GT16, GT18 and GT29 clones had a strong expression,
whereas CV1, CV3, and CV5 had no expression, as was expected.
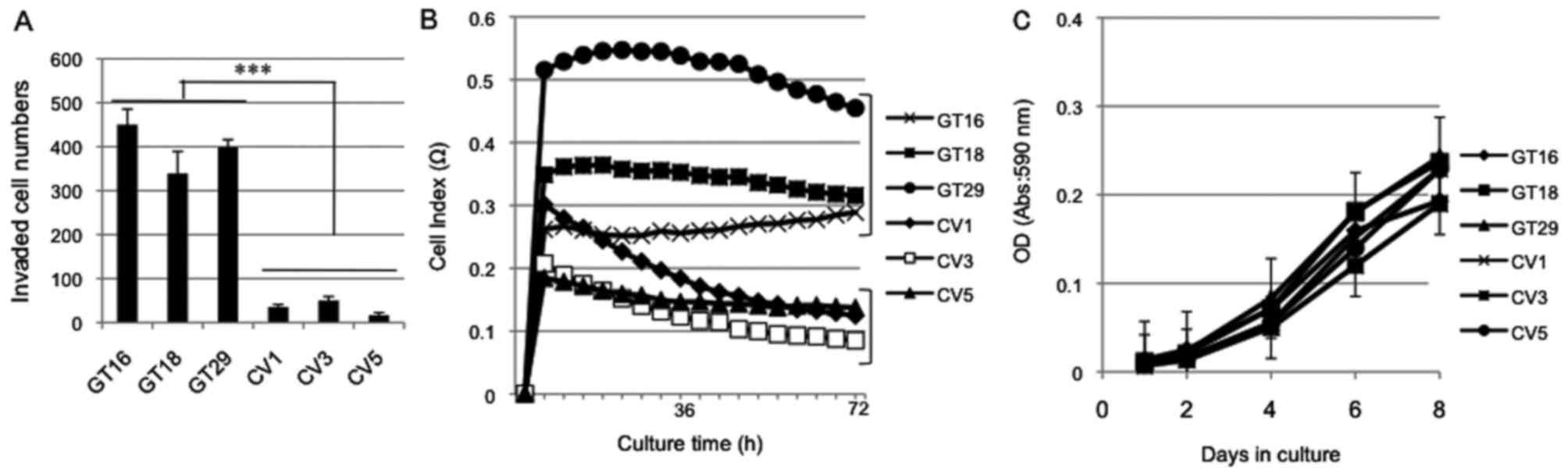
Increased invasion and motility of GD3(+)
cells
In order to examine effects of GD3/GD2 expression on
the representative malignant properties, the cell invasive activity
(Fig. 3A), cell adhesion (Fig. 3B) and cell growth (Fig. 3C) were analyzed. The cell invasion
activity was analyzed by Boyden-chamber assay, showing that the
GD3(+) cells had a greater invasion activity than the GD3(−) cells
(Fig. 3A). Using RT-CES, cell
adhesion and subsequent cell expansion were examined with the
GD3(+) cells and GD3(−) cells, and the GD3(+) cells had a greater
adhesion/expansion activity than the GD3(−) cells (Fig. 3B). We also compared the growth
rates of these cells, resulting in the almost equivalent cell
growth under regular culture conditions (Fig. 3C).
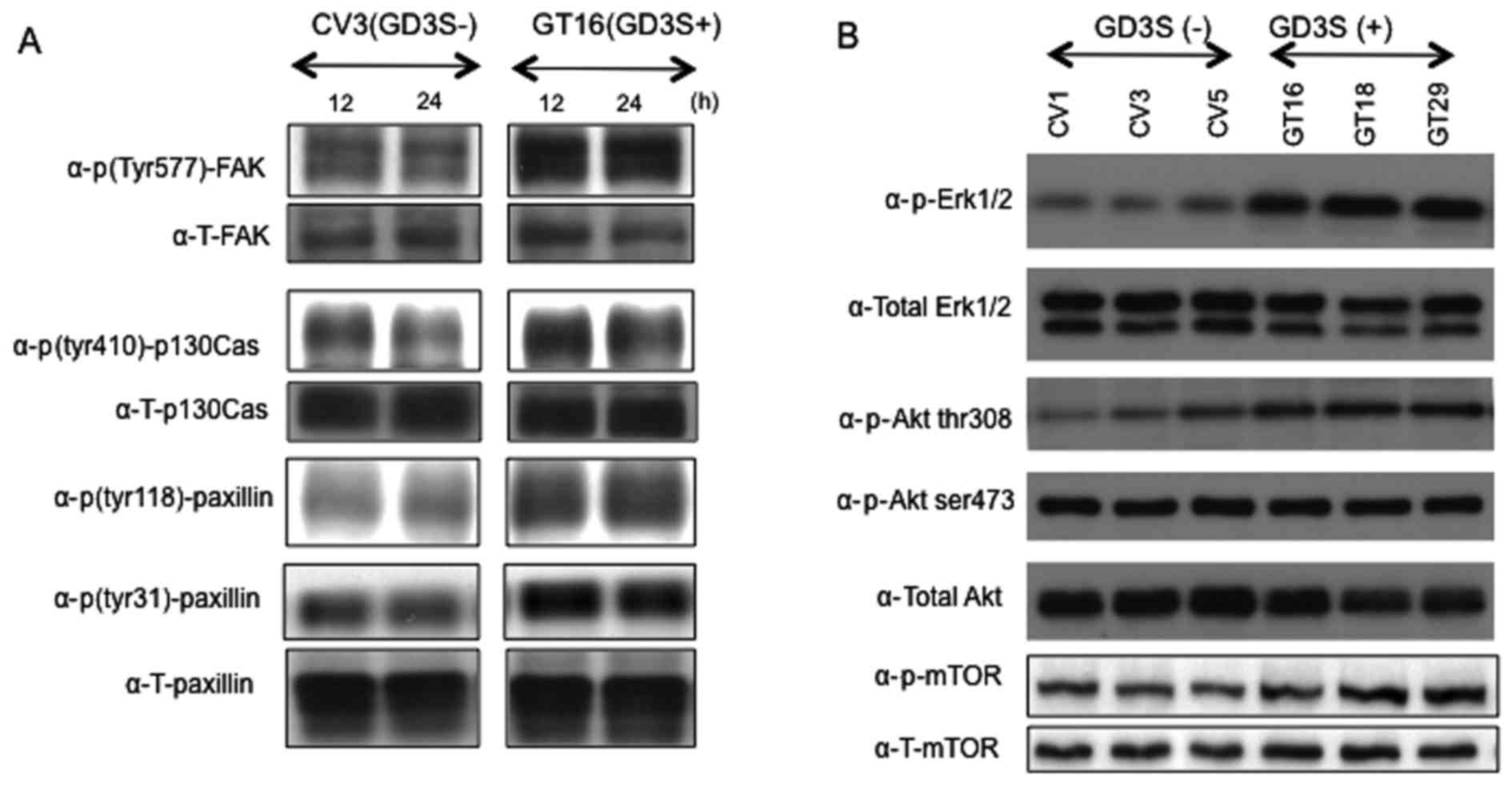
Subsequently, the phosphorylation levels of FAK,
paxillin and p130Cas, that have been reported to be involved in
invasion and motility (35), were
analyzed. Increased phosphorylation levels of FAK thr577, paxillin
Tyr477 and p130Cas Tyr118 were observed in the GD3(+) cells at 12
and 24 h after plating (Fig. 4A).
Furthermore, the activation levels of main signaling molecules
involved in cell proliferation, i.e., Erk1/2 and Akt were analyzed.
Consequently, western blot analysis of phosphorylated Erk1/2 and
Akt revealed increased phosphorylation levels of both Erk1/2 and
Akt thr308 in the GD3(+) transfectant lines (Fig. 4B). There were no apparent
differences in the band intensities of either total mTOR or
p-mTOR.
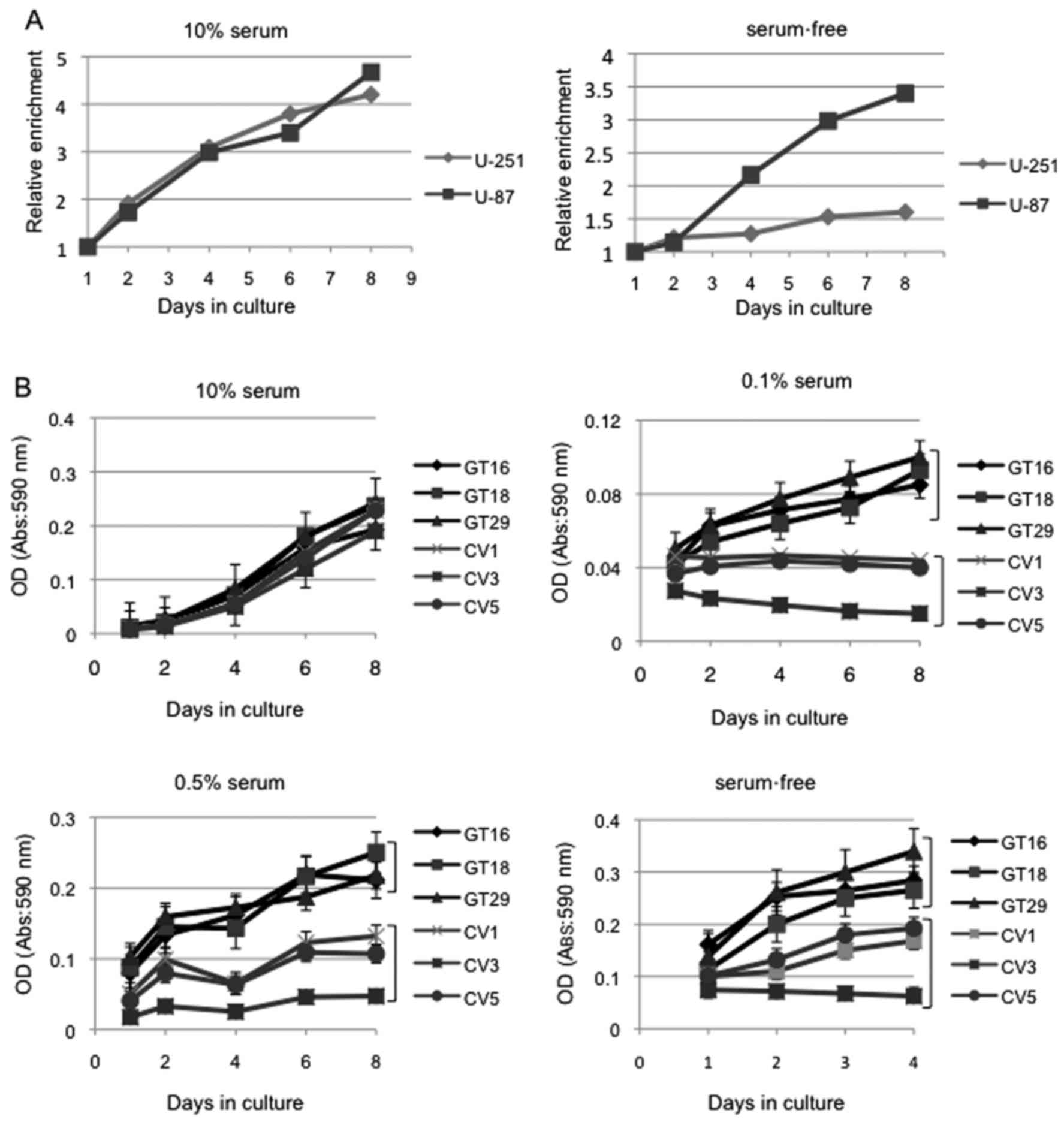
Cell growth under low-serum
conditions
Since there was no significant difference in growth
between the GD3/GD2-high U-87MG and GD3/GD2-negative U-251MG cells
when cultured under regular conditions containing 10% FCS (Fig. 5A, left panel), we compared the
growth of these cells cultured under serum-free conditions.
Consequently, the U-87MG cells cultured in serum-free conditions
exhibited rapid growth compared with the U-251MG cells (Fig. 5A, right panel). Thus, the
expression of GD3/GD2 seemed to be crucially involved in cell
growth under serum-free conditions.
To further analyze this point, cell growth was
compared by culturing the GD3(+) cells and GD3(−) cells in medium
containing 10, 0.5, 0.1 or 0% of serum. The results indicated that
the GD3(+) cells had a definitely increased cell growth compared
with the GD3(−) cells when cultured under FCS concentrations of
0.5, 0.1 and 0%, while these cells showed no significant
differences when cultured in medium with 10% FCS (Fig. 5B). These results suggested that
GD3/GD2 expression confers the ability to cells to grow under
conditions of reduced serum concentrations by modulating signaling
molecules involved in cell growth.
Since the GD3(+) cells had significantly higher
growth rates than the GD3(−) cells when cultured under conditions
with 0.1% FCS, underlying mechanisms that are involved in the
different growth under the condition with 0.1% FCS were
investigated, focusing on apoptosis and the cell cycle.
Consequently, no apoptosis was observed in both types of cells, as
analyzed by 2-D flow cytometry with PI and FITC-Annexin V (data not
shown).
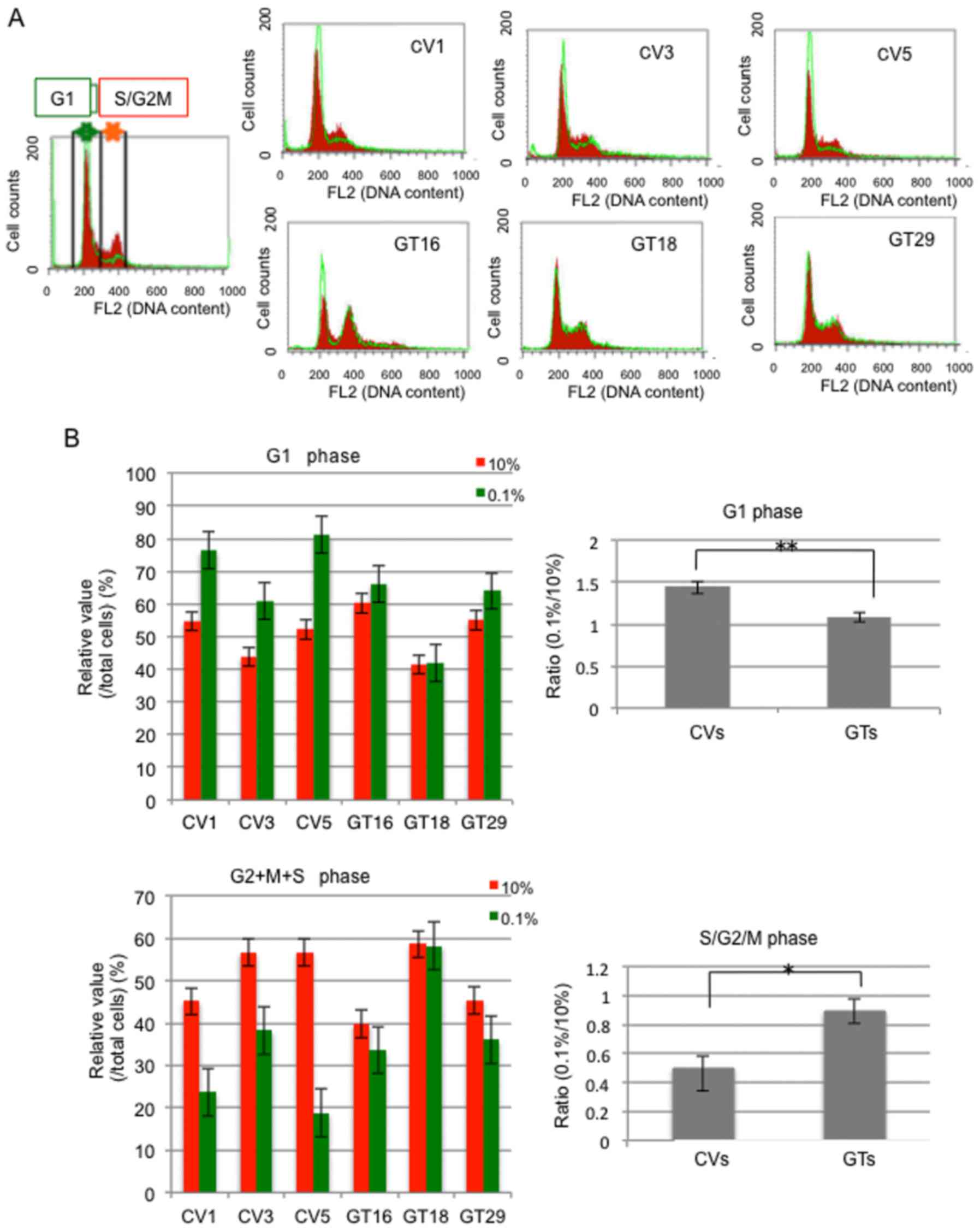
Cell cycle analysis under low serum
conditions
Cell cycle distribution was analyzed, as shown in
Fig. 6A. Cell cycle analysis
revealed that the number of GD3(−) cells (CVs) in the G1 phase
markedly increased as compared with the GD3(+) cells (GTs) when the
serum concentration was reduced (Fig.
6). In turn, the numbers of GD3(−) cells in the G2/M and S
phases markedly decreased, while the GD3(+) cells exhibited minimal
changes (Fig. 6B). Of note, the
GD3(−) cells seemed to stay mainly at the G1 phase under conditions
with 0.1% FCS. These results corresponded with the results of MTT
assay in which the GD3(−) cells exhibited a much slower growth rate
than the GD3(+) cells under culture conditions with 0.1% FCS.
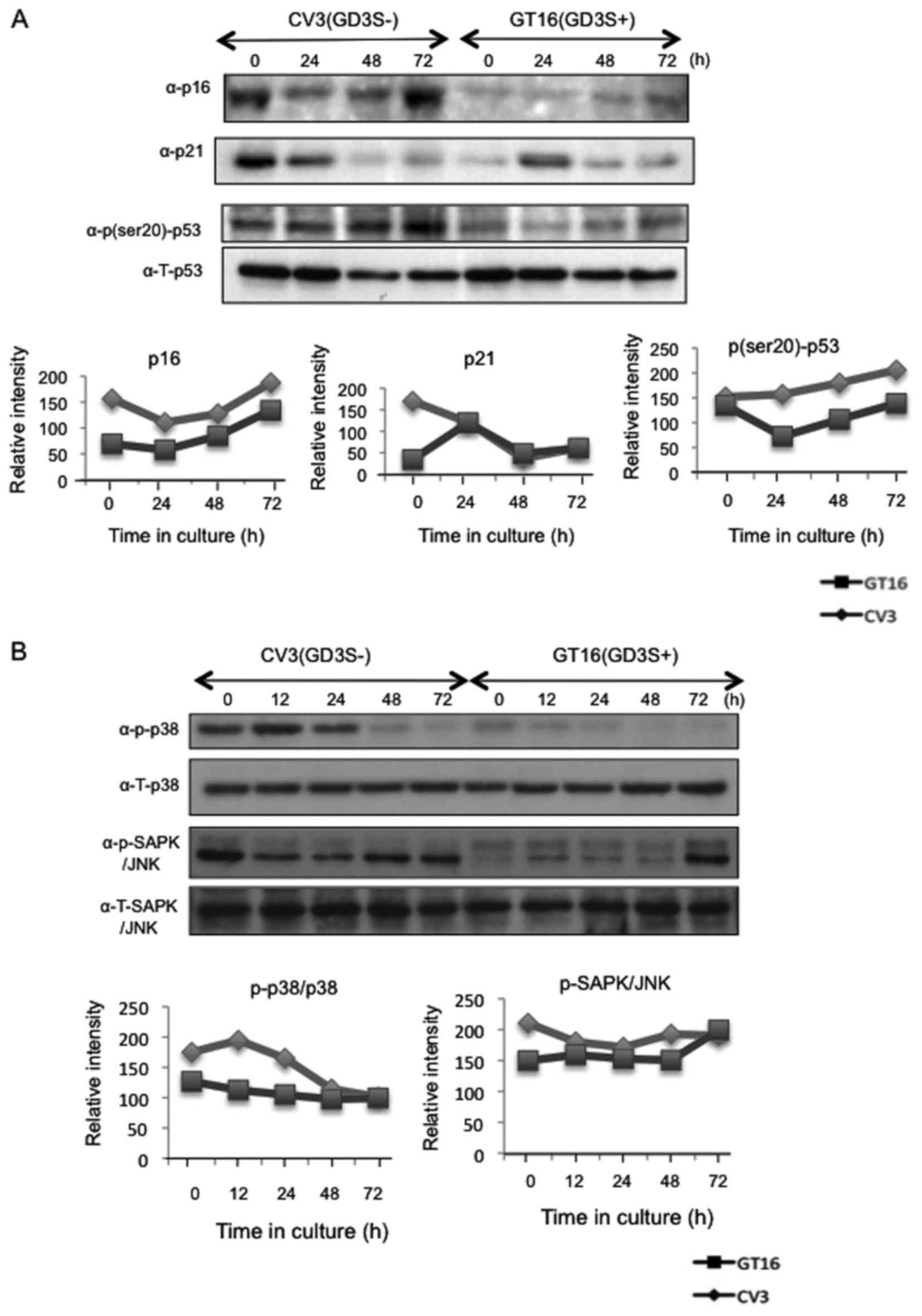
Expression of cell cycle-related
proteins
Since the results of cell cycle analysis suggested
that the GD3(−) cells arrested at the G1 phase when the serum
concentration was reduced, the expression of p16 and p21, and the
phosphorylation of p53 (Ser20) were analyzed by western blot
analysis. Consequently, the induction and/or accumulation of p16
and p21 was found in the GD3(−) cells compared with the GD3(+)
cells (Fig. 7A). Moreover, the
phosphorylation of p53 (Ser20) was also increased in the GD3(−)
cells. Taken together, the activation of p53 may have induced the
accumulation of p16 and p21, leading to cell cycle arrest at the G1
phase in the GD3(−) cells. As for SAPK/JNK and p38, western blot
analysis revealed that a stronger phosphorylation of p38 and
SAPK/JNK was induced in the GD3(−) cells than in the GD3(+) cells
(Fig. 7B).
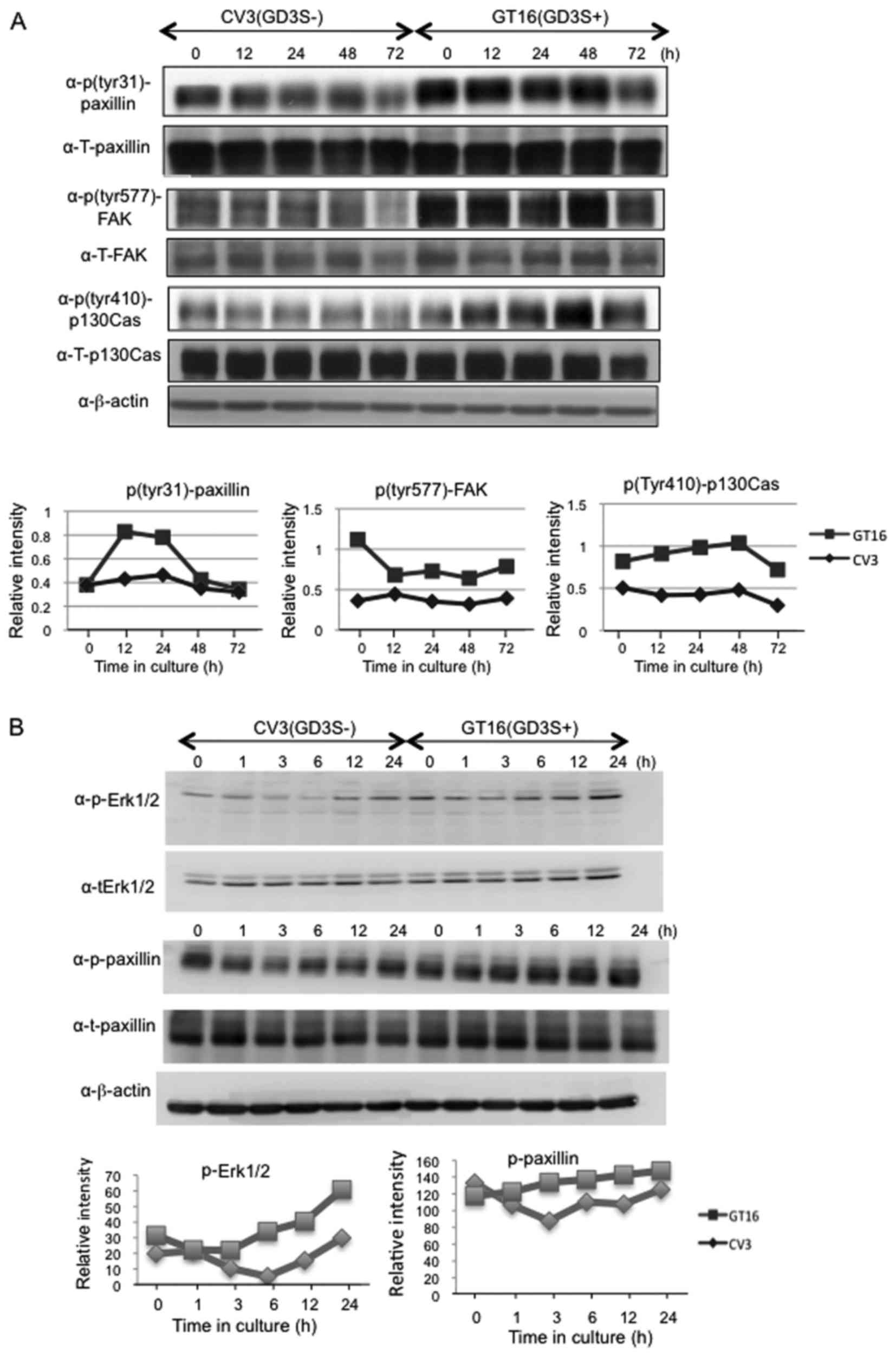
The posphorylation levels of FAK, paxillin and
p130Cas were fairly well maintained in the GD3(+) cells. By
contrast, these levels showed a gradual reduction in the GD3(−)
cells following serum removal, while they recovered ~12–24 h after
the reduction of serum (Fig.
8).
Discussion
One of the poor prognostic factors of gliomas, is
the fact that glioma cells are characterized by an invasive
behavior and ability to infiltrate into the surrounding normal
tissues (37). Indeed, the border
between tumor tissues and normal tissues is vague, thereby
rendering the radical resection of tumors very difficult. In this
study, it was clearly demonstrated that the U-251MG cells
expressing high levels of GD3/GD2 exhibited increased invasion
activity and enhanced motility. Furthermore, the elevation of the
phosphorylation of Erk1/2 and Akt, and the increased
phosphorylation levels of FAK, p130Cas and paxillin were observed.
Thus, gangliosides GD3 and GD2 may be involved in the enhancement
of the malignant properties of glioma cells, such as invasion and
motility, as reported in malignant melanomas (35). In this study, their involvment in
cell adhesion in gliomas was also clearly demonstrated in the
RT-CES system (Fig. 3C).
The fact that the U-251MG cells expressing high
levels of GD3 [GD3(+) cells] did not undergo cell cycle arrest, and
proliferated even under no serum conditions suggested that rapidly
growing cells become large in population, and GD3(+) tumor cells
can overcome the difficulties in their survival, when competition
between growth factors, nutrition and oxygen become severe among
tumor cells. In this situation, GD3 and GD2 may be involved in the
better adaptation of tumor cells to severe microenvironments.
First, GD3 and GD2 should assemble each other and
form a cluster in glycolipid-enriched microdomains (GEM)/rafts
(38). This may also recruit
various membrane molecules, such as growth factor receptors and
adhesion receptors to GEM/rafts, and modulate their functions to
efficiently transduce cell signals to down-stream molecules and
transcription factors (39–41).
There have been a number of reports on the roles of GD3/GD2 in the
enhancement of cell signaling mediated by epidermal growth factor
receptor (42), TrkA (43), Met (21), integrins (44).
In melanomas, we have demonstrated that GD3
expression enhanced the activation levels of FAK, p130Cas and
paxillin (35,45), and adhesion signals via integrins
is also required to induce the activation of these signaling
molecules (46,47). Moreover, we recently reported that
the neogenin molecule is associated with GD3 on the cell surface of
melanoma cells (48). γ-secretase
is also recruited to GEM/rafts under GD3 expression, thereby
cleaving intracytoplasmic region of neogenin to generate neogenin-
intracytoplasmic domain (Ne-ICD). Intriguingly, Ne-ICD appeared to
play as a transcription factor by translocating into the nuclei and
to promote expression of various target genes (48). Similar functions of GD3/GD2 may be
also present to make glioma cells potentiated and grow better under
culture condition with low serum concentration as shown in this
study. Therefore, we should search ganglioside-associated molecules
in glioma cells, too, and clarify roles of those molecules in the
enhancement of malignant properties in gliomas by forming molecular
complexes with GD3/GD2.
In recent studies, it has been shown that molecules
located in the signaling pathway of receptor tyrosin kinases
(RTKs)/RAS/PI3K frequently undergo genetic mutations, leading to
ligand-independent activation of PTKs and the transduction of
abnormal signals, and finally abnormal tumor growth and invasion
into surrounding tissues (49,50).
In addition, the transcription factors AREB6 and Elk-1 are located
at down-stream of RTK/RAS/PI3K signaling pathway, and both of these
have been reported to positively regulate GD3 synthase gene
(51). Thus, it may be possible
that GD3/GD2 highly expressed in glioma tissues recruit mutated
RTKs to GEM/rafts, and activate RTKs in a ligand-independent
manner, leading to the increased malignant properties of gliomas.
On the other hand, activated AREB6 and Elk-1 by RTK/RAS/PI3K
signals may enhance expression of GD3/GD2 by forming a positive
feed back loop (51).
Gliomas are generally resistant to current
chemotherapy and radiation therapy. Therefore, it is necessary to
develop novel approaches in which cancer cells can be selectively
destroyed without damage to normal tissues. Therefore, GD3/GD2 and
their associating molecules on the cell surface may be promising
targets in the construction of therapeutic strategy for patients
with glioma. However, this study has limitations due to in
vitro analysis. Therefore, we have performed animal
experiments, and some of these results have already already
published (29); Further studies
using mouse gliomas generated in GD3 synthase-lacking mice are
ongoing. As for clinical analysis, we aim to carry out studies
using materials from patients with glioma. Thus, supportive results
for our conclusions reported herein will be obtained in the near
future.
Glossary
Abbreviations
Abbreviations:
|
GD3S
|
GD3 synthase
|
|
mAb
|
monoclonal antibody
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
FCS
|
fetal calf serum
|
|
RT-qPCR
|
reverse transcription-quantitative
PCR
|
|
FAK
|
focal adhesion kinase
|
|
RTK
|
receptor tyrosine kinase
|
Acknowledgments
The authors would like to thank Mrs. T. Mizuno, Mrs.
Y. Nakayasu (at Nagoya University) and Mrs. S. Yamamoto (at Chubu
University) for providing technical assistance.
Notes
[1]
Funding
This study was supported by Grants-in-Aid from the
Ministry of Education, Culture, Sports and Technology of Japan
(MEXT) (15H04696, 15K15080).
[2] Availability
of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
[3] Authors'
contributions
TI, PZ, YO and YO performed the biochemical and cell
biology experiments. HM, RHB and TW performed the
immunohistochemistry experiments. RHB and KeF performed the cell
cycle analysis. HM, KeF and KoF designed the experiments, and TI,
KeF and KoF wrote the manuscript.
[4] Ethics
approval and consent to participate
Brain tumor samples were obtained for use in the
experiments after obtaining informed consent. This study was
performed in accordance with the code of Ethics of the World
Medical Association, and approved by the Ethics Committee of Nagoya
University Graduate School of Medicine, Nagoya, Japan.
[5] Consent for
publication
Not applicable.
[6] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Greene LA, Lee HY and Angelastro JM: The
transcription factor ATF5: Role in neurodevelopment and neural
tumors. J Neurochem. 108:11–22. 2009. View Article : Google Scholar :
|
|
2
|
Schiffer D, Annovazzi L, Caldera V and
Mellai M: On the origin and growth of gliomas. Anticancer Res.
30:1977–1998. 2010.PubMed/NCBI
|
|
3
|
Huse JT and Holland EC: Targeting brain
cancer: Advances in the molecular pathology of malignant glioma and
medulloblastoma. Nat Rev Cancer. 10:319–331. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zong H, Verhaak RG and Canoll P: The
cellular origin for malignant glioma and prospects for clinical
advancements. Expert Rev Mol Diagn. 12:383–394. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xie Q, Mittal S and Berens ME: Targeting
adaptive glioblastoma: An overview of proliferation and invasion.
Neuro Oncol. 16:1575–1584. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Woehrer A, Bauchet L and Barnholtz-Sloan
JS: Glioblastoma survival: Has it improved? Evidence from
population-based studies. Curr Opin Neurol. 27:666–674.
2014.PubMed/NCBI
|
|
7
|
Wiegandt H: Gangliosides. Glycolipids.
Wiegandt H: 10. Elsevier Science Publishers; Amsterdam: pp.
199–260. 1985, View Article : Google Scholar
|
|
8
|
Yu RK, Bieberich E, Xia T and Zeng G:
Regulation of ganglioside biosynthesis in the nervous system. J
Lipid Res. 45:783–793. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schengrund CL: Gangliosides:
Glycosphingolipids essential for normal neural development and
function. Trends Biochem Sci. 40:397–406. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yates AJ: Gangliosides in the nervous
system during development and regeneration. Neurochem Pathol.
5:309–329. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hakomori S: Philip Levine award lecture:
Blood group glycolipid antigens and their modifications as human
cancer antigens. Am J Clin Pathol. 82:635–648. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lloyd KO: Humoral immune responses to
tumor-associated carbohydrate antigens. Semin Cancer Biol.
2:421–431. 1991.PubMed/NCBI
|
|
13
|
Schulz G, Cheresh DA, Varki NM, Yu A,
Staffileno LK and Reisfeld RA: Detection of ganglioside GD2 in
tumor tissues and sera of neuroblastoma patients. Cancer Res.
44:5914–5920. 1984.PubMed/NCBI
|
|
14
|
Saito M, Yu RK and Cheung NK: Ganglioside
GD2 specificity of monoclonal antibodies to human neuroblastoma
cell. Biochem Biophys Res Commun. 127:1–7. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Merritt WD, Casper JT, Lauer SJ and Reaman
GH: Expression of GD3 ganglioside in childhood T-cell lymphoblastic
malignancies. Cancer Res. 47:1724–1730. 1987.PubMed/NCBI
|
|
16
|
Siddiqui B, Buehler J, DeGregorio MW and
Macher BA: Differential expression of ganglioside GD3 by human
leukocytes and leukemia cells. Cancer Res. 44:5262–5265.
1984.PubMed/NCBI
|
|
17
|
Cheresh DA, Rosenberg J, Mujoo K,
Hirschowitz L and Reisfeld RA: Biosynthesis and expression of the
disialoganglioside GD2, a relevant target antigen on small cell
lung carcinoma for monoclonal antibody-mediated cytolysis. Cancer
Res. 46:5112–5118. 1986.PubMed/NCBI
|
|
18
|
Yoshida S, Fukumoto S, Kawaguchi H, Sato
S, Ueda R, Furukawa K and Ganglioside G: Ganglioside G(D2) in small
cell lung cancer cell lines: Enhancement of cell proliferation and
mediation of apoptosis. Cancer Res. 61:4244–4252. 2001.PubMed/NCBI
|
|
19
|
Azuma K, Tanaka M, Uekita T, Inoue S,
Yokota J, Ouchi Y and Sakai R: Tyrosine phosphorylation of paxillin
affects the metastatic potential of human osteosarcoma. Oncogene.
24:4754–4764. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shibuya H, Hamamura K, Hotta H, Matsumoto
Y, Nishida Y, Hattori H and Furukawa K, Ueda M and Furukawa K:
Enhancement of malignant properties of human osteosarcoma cells
with disialyl gangliosides GD2/GD3. Cancer Sci. 103:1656–1664.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cazet A, Bobowski M, Rombouts Y, Lefebvre
J, Steenackers A, Popa I, Guérardel Y, Le Bourhis X, Tulasne D and
Delannoy P: The ganglioside G(D2) induces the constitutive
activation of c-Met in MDA-MB-231 breast cancer cells expressing
the G(D3) synthase. Glycobiology. 22:806–816. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Furukawa K, Akagi T, Nagata Y, Yamada Y,
Shimotohno K, Cheung NK, Shiku H and Furukawa K: GD2 ganglioside on
human T-lymphotropic virus type I-infected T cells: Possible
activation of beta-1,4-N-acetylgalactosaminyltransferase gene by
p40tax. Proc Natl Acad Sci USA. 90:1972–1976. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fredman P, von Holst H, Collins VP, Ammar
A, Dellheden B, Wahren B, Granholm L and Svennerholm L: Potential
ganglioside antigens associated with human gliomas. Neurol Res.
8:123–126. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wikstrand CJ, Fredman P, Svennerholm L and
Bigner DD: Detection of glioma-associated gangliosides GM2, GD2,
GD3, 3′-isoLM1 3′,6′-isoLD1 in central nervous system tumors in
vitro and in vivo using epitope-defined monoclonal antibodies. Prog
Brain Res. 101:213–223. 1994. View Article : Google Scholar
|
|
25
|
Gaini SM, Riboni L, Cerri C, Grimoldi N,
Sganzerla EP and Berra B: Ganglioside content and composition in
human gliomas. Acta Neurochir Suppl (Wien). 43:126–129. 1988.
|
|
26
|
Kawai K, Takahashi H, Watarai S, Ishizu H,
Fukai K, Tanabe Y, Nose S and Kuroda S: Occurrence of ganglioside
GD3 in neoplastic astrocytes. An immunocytochemical study in humans
Virchows Arch. 434:201–205. 1999.
|
|
27
|
Wagener R, Röhn G, Schillinger G, Schröder
R, Kobbe B and Ernestus RI: Ganglioside profiles in human gliomas:
Quantification by microbore high performance liquid chromatography
and correlation to histomorphology and grading. Acta Neurochir
(Wien). 141:1339–1345. 1999. View Article : Google Scholar
|
|
28
|
Vukelić Z, Kalanj-Bognar S, Froesch M,
Bîndila L, Radić B, Allen M, Peter-Katalinić J and Zamfir AD: Human
gliosarcomaassociated ganglioside composition is complex and
distinctive as evidenced by high-performance mass spectrometric
determination and structural characterization. Glycobiology.
17:504–515. 2007. View Article : Google Scholar
|
|
29
|
Ohkawa Y, Momota H, Kato A, Hashimoto N,
Tsuda Y, Kotani N, Honke K, Suzumura A, Furukawa K, Ohmi Y, et al:
Ganglioside GD3 enhances invasiveness via Yes activation by forming
a complex of GD3/PDGFRα/Yes in gliomas. J Biol Chem.
290:16043–16058. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Svennerholm L: Chromatographic separation
of human brain gangliosides. J Neurochem. 10:613–623. 1963.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao J and Furukawa K, Fukumoto S, Okada
M, Miyazaki H, Shiku H, Aizawa S, Matsuyama M and Furukawa K:
Attenuation of the interleukin 2 signals in complex
gangliosidelacking mice. J Biol Chem. 274:13744–13747. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Allen M, Bjerke M, Edlund H, Nelander S
and Westermark B: Origin of the U87MG glioma cell line: Good news
and bad news. Sci Transl Med. 8:354re32016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bady P, Diserens AC, Castella V, Kalt S,
Heinimann K, Hamou MF, Delorenzi M and Hegi ME: DNA fingerprinting
of glioma cell lines and considerations on similarity measurements.
Neuro Oncol. 14:701–711. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Haraguchi M, Yamashiro S, Yamamoto A and
Furukawa K, Takamiya K, Lloyd KO, Shiku H and Furukawa K: Isolation
of GD3 synthase gene by expression cloning of GM3
alpha-2,8-si-alyltransferase cDNA using anti-GD2 monoclonal
antibody. Proc Natl Acad Sci USA. 91:10455–10459. 1994. View Article : Google Scholar
|
|
35
|
Hamamura K, Furukawa K, Hayashi T, Hattori
T, Nakano J, Nakashima H, Okuda T, Mizutani H, Hattori H, Ueda M,
et al: Ganglioside GD3 promotes cell growth and invasion through
p130Cas and paxillin in malignant melanoma cells. Proc Natl Acad
Sci USA. 102:11041–11046. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Aixinjueluo W and Furukawa K, Zhang Q,
Hamamura K, Tokuda N, Yoshida S, Ueda R and Furukawa K: Mechanisms
for the apoptosis of small cell lung cancer cells induced by
anti-GD2 monoclonal antibodies: Roles of anoikis. J Biol Chem.
280:29828–29836. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rao JS: Molecular mechanisms of glioma
invasiveness: The role of proteases. Nat Rev Cancer. 3:489–501.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hakomori SI: Cell adhesion/recognition and
signal transduction through glycosphingolipid microdomain.
Glycoconj J. 17:143–151. 2000. View Article : Google Scholar
|
|
39
|
Kim OS, Park EJ, Joe EH and Jou I:
JAK-STAT signaling mediates gangliosides-induced inflammatory
responses in brain microglial cells. J Biol Chem. 277:40594–40601.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gabellini N, Facci L, Milani D, Negro A,
Callegaro L, Skaper SD and Leon A: Differences in induction of
c-fos transcription by cholera toxin-derived cyclic AMP and
Ca2+ signals in astrocytes and 3T3 fibroblasts. Exp Cell
Res. 194:210–217. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Song N, Kim SJ, Kwon HY, Son SW, Kim KS,
Ahn HB and Lee YC: Transcriptional activation of human GM3 synthase
(hST3Gal V) gene by valproic acid in ARPE-19 human retinal pigment
epithelial cells. BMB Rep. 44:405–409. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang J and Yu RK: Interaction of
ganglioside GD3 with an EGF receptor sustains the self-renewal
ability of mouse neural stem cells in vitro. Proc Natl Acad Sci
USA. 110:19137–19142. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Fukumoto S, Mutoh T, Hasegawa T, Miyazaki
H, Okada M, Goto G and Furukawa K, Urano T and Furukawa K: GD3
synthase gene expression in PC12 cells results in the continuous
activation of TrkA and ERK1/2 and enhanced proliferation. J Biol
Chem. 275:5832–5838. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ohkawa Y, Miyazaki S, Miyata M, Hamamura K
and Furukawa K and Furukawa K: Essential roles of integrin-mediated
signaling for the enhancement of malignant properties of melanomas
based on the expression of GD3. Biochem Biophys Res Commun.
373:14–19. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hamamura K, Tsuji M, Ohkawa Y, Nakashima
H, Miyazaki S, Urano T, Yamamoto N, Ueda M and Furukawa K and
Furukawa K: Focal adhesion kinase as well as p130Cas and paxillin
is crucially involved in the enhanced malignant properties under
expression of ganglioside GD3 in melanoma cells. Biochim Biophys
Acta. 1780:513–519. 2008. View Article : Google Scholar
|
|
46
|
Valentino LA and Ladisch S: Tumor
gangliosides enhance alpha2 beta1 integrin-dependent platelet
activation. Biochim Biophys Acta. 1316:19–28. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ohkawa Y, Miyazaki S, Hamamura K, Kambe M,
Miyata M, Tajima O, Ohmi Y, Yamauchi Y and Furukawa K and Furukawa
K: Ganglioside GD3 enhances adhesion signals and augments malignant
properties of melanoma cells by recruiting integrins to
glycolipid-enriched microdomains. J Biol Chem. 285:27213–27223.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kaneko K, Ohkawa Y, Hashimoto N, Ohmi Y,
Kotani N, Honke K, Ogawa M, Okajima T and Furukawa K and Furukawa
K: Neogenin defined as a GD3-associated molecule by enzyme-mediated
activation of radical sources confers malignant properties via
intra-cytoplasmic domain in melanoma cells. J Biol Chem.
291:16630–16643. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Guo G, Gong K, Wohlfeld B, Hatanpaa KJ,
Zhao D and Habib AA: Ligand-independent EGFR signaling. Cancer Res.
75:3436–3441. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Comoglio PM, Boccaccio C and Trusolino L:
Interactions between growth factor receptors and adhesion
molecules: Breaking the rules. Curr Opin Cell Biol. 15:565–571.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Dae HM, Kwon HY, Kang NY, Song NR, Kim KS,
Kim CH, Lee JH and Lee YC: Isolation and functional analysis of the
human glioblastoma-specific promoter region of the human GD3
synthase (hST8Sia I) gene. Acta Biochim Biophys Sin (Shanghai).
41:237–245. 2009. View Article : Google Scholar
|