|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bang YJ, Van Cutsem E, Feyereislova A,
Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T,
et al ToGA Trial Investigators: Trastuzumab in combination with
chemotherapy versus chemotherapy alone for treatment of
HER2-positive advanced gastric or gastro-oesophageal junction
cancer (ToGA): A phase 3, open-label, randomised controlled trial.
Lancet. 376:687–697. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
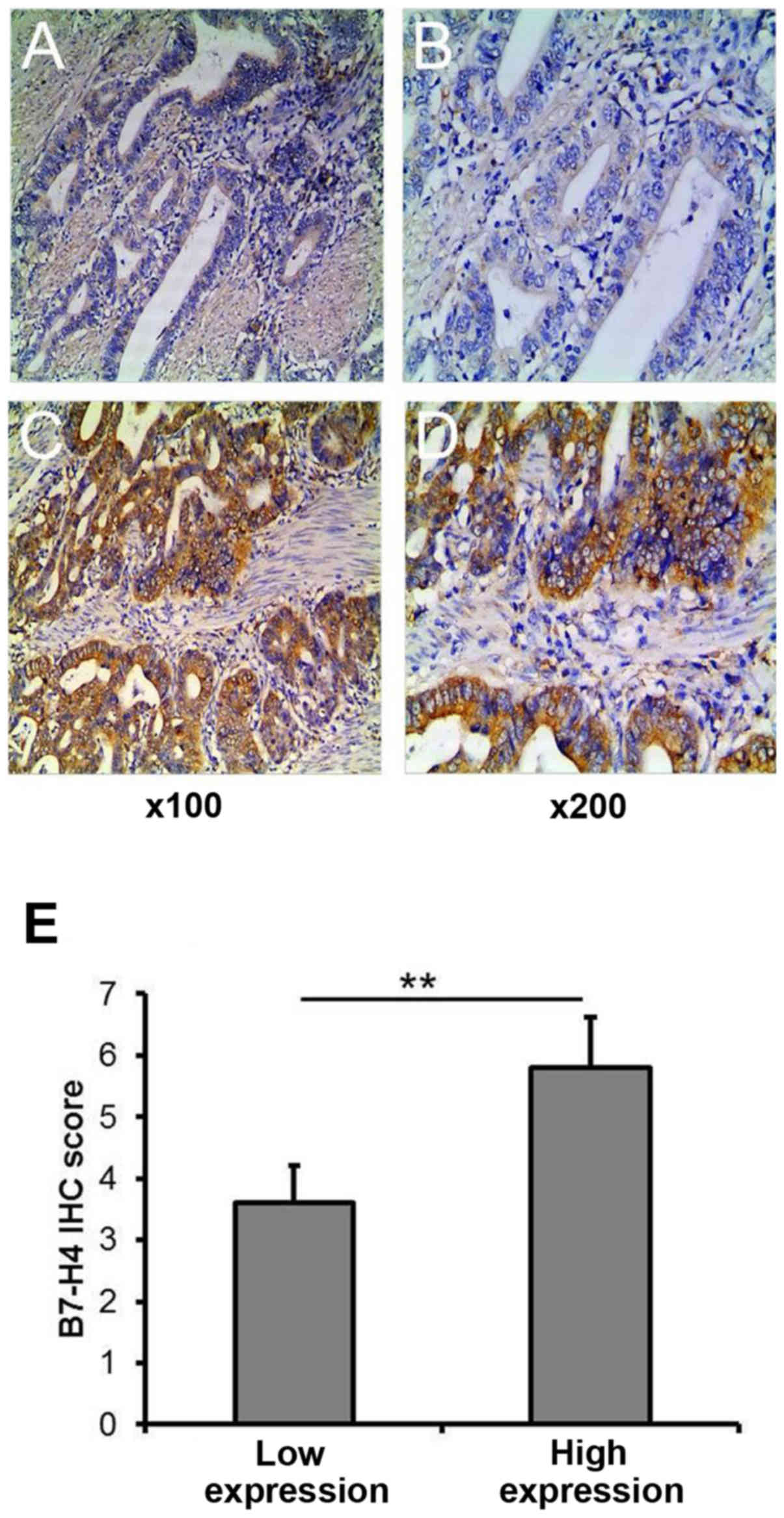
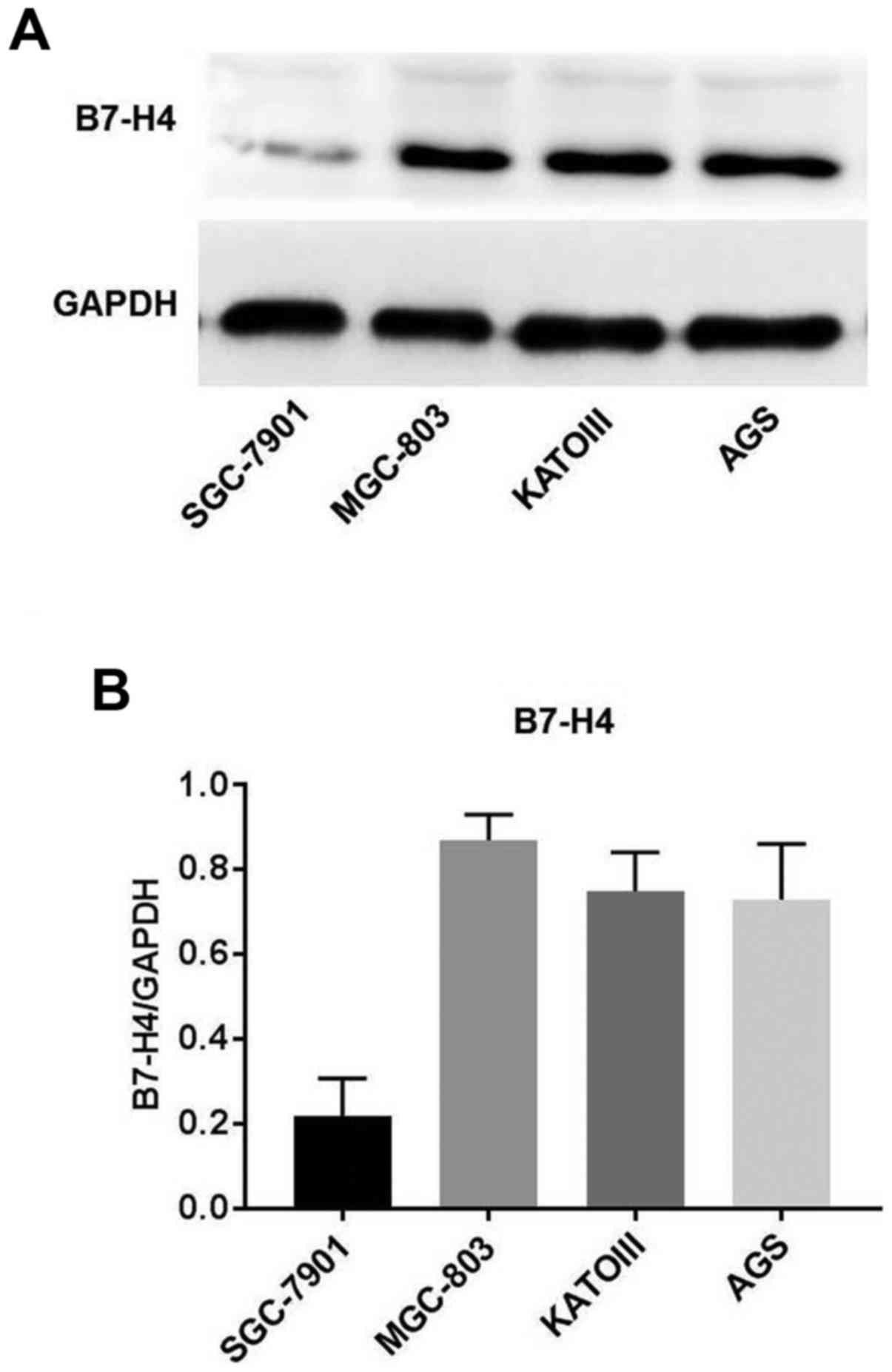
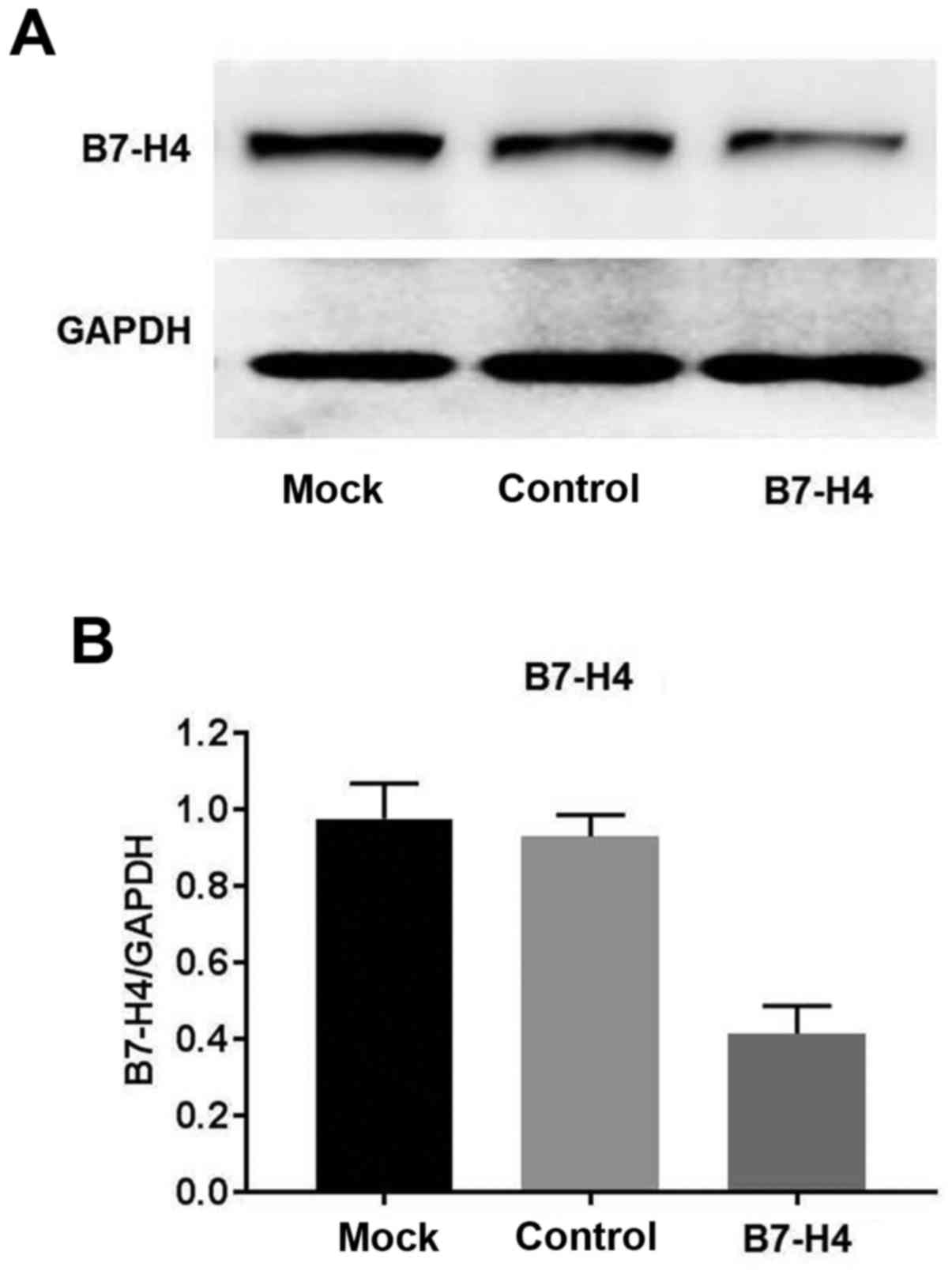
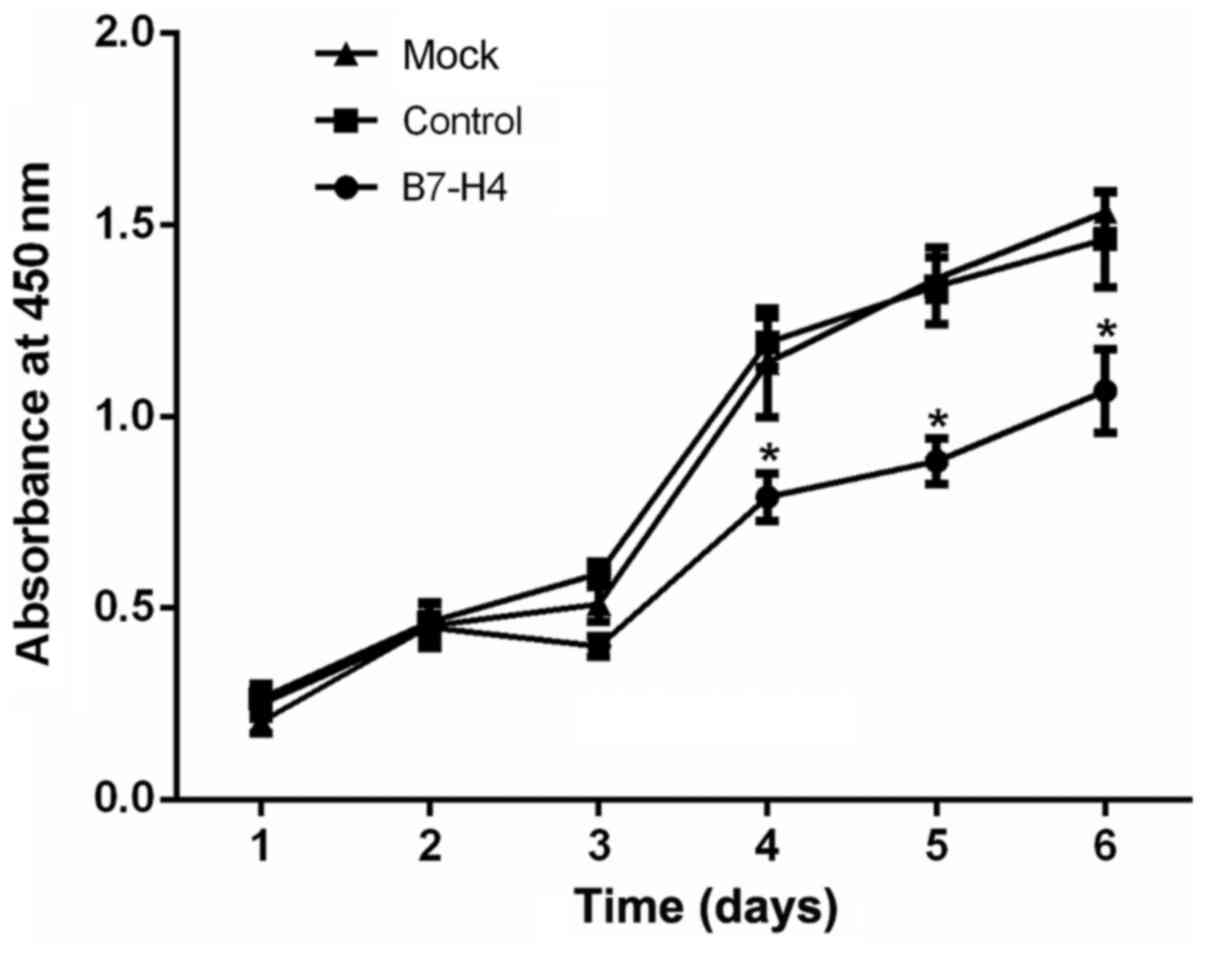
|
Lennerz JK, Kwak EL, Ackerman A, Michael
M, Fox SB, Bergethon K, Lauwers GY, Christensen JG, Wilner KD,
Haber DA, et al: MET amplification identifies a small and
aggressive subgroup of esophagogastric adenocarcinoma with evidence
of responsiveness to crizotinib. J Clin Oncol. 29:4803–4810. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shah MA, Wainberg ZA, Catenacci DV,
Hochster HS, Ford J, Kunz P, Lee FC, Kallender H, Cecchi F, Rabe
DC, et al: Phase II study evaluating 2 dosing schedules of oral
foretinib (GSK1363089), cMET/VEGFR2 inhibitor, in patients with
metastatic gastric cancer. PLoS One. 8:e540142013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kryczek I, Zou L, Rodriguez P, Zhu G, Wei
S, Mottram P, Brumlik M, Cheng P, Curiel T, Myers L, et al: B7-H4
expression identifies a novel suppressive macrophage population in
human ovarian carcinoma. J Exp Med. 203:871–881. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Krambeck AE, Thompson RH, Dong H, Lohse
CM, Park ES, Kuntz SM, Leibovich BC, Blute ML, Cheville JC and Kwon
ED: B7-H4 expression in renal cell carcinoma and tumor vasculature:
Associations with cancer progression and survival. Proc Natl Acad
Sci USA. 103:10391–10396. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Simon I, Zhuo S, Corral L, Diamandis EP,
Sarno MJ, Wolfert RL and Kim NW: B7-h4 is a novel membrane-bound
protein and a candidate serum and tissue biomarker for ovarian
cancer. Cancer Res. 66:1570–1575. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Arigami T, Uenosono Y, Hirata M, Hagihara
T, Yanagita S, Ishigami S and Natsugoe S: Expression of B7-H4 in
blood of patients with gastric cancer predicts tumor progression
and prognosis. J Surg Oncol. 102:748–752. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Thompson RH, Zang X, Lohse CM, Leibovich
BC, Slovin SF, Reuter VE, Cheville JC, Blute ML, Russo P, Kwon ED,
et al: Serum-soluble B7x is elevated in renal cell carcinoma
patients and is associated with advanced stage. Cancer Res.
68:6054–6058. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun Y, Wang Y, Zhao J, Gu M, Giscombe R,
Lefvert AK and Wang X: B7-H3 and B7-H4 expression in non-small-cell
lung cancer. Lung Cancer. 53:143–151. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Quandt D, Fiedler E, Boettcher D, Marsch
WC and Seliger B: B7-h4 expression in human melanoma: Its
association with patients' survival and antitumor immune response.
Clin Cancer Res. 17:3100–3111. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tringler B, Zhuo S, Pilkington G, Torkko
KC, Singh M, Lucia MS, Heinz DE, Papkoff J and Shroyer KR: B7-h4 is
highly expressed in ductal and lobular breast cancer. Clin Cancer
Res. 11:1842–1848. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Choi IH, Zhu G, Sica GL, Strome SE,
Cheville JC, Lau JS, Zhu Y, Flies DB, Tamada K and Chen L: Genomic
organization and expression analysis of B7-H4, an immune inhibitory
molecule of the B7 family. J Immunol. 171:4650–4654. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sica GL, Choi IH, Zhu G, Tamada K, Wang
SD, Tamura H, Chapoval AI, Flies DB, Bajorath J and Chen L: B7-H4,
a molecule of the B7 family, negatively regulates T cell immunity.
Immunity. 18:849–861. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Prasad DV, Richards S, Mai XM and Dong C:
B7S1, a novel B7 family member that negatively regulates T cell
activation. Immunity. 18:863–873. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zang X, Loke P, Kim J, Murphy K, Waitz R
and Allison JP: B7x: a widely expressed B7 family member that
inhibits T cell activation. Proc Natl Acad Sci USA.
100:10388–10392. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dangaj D, Lanitis E, Zhao A, Joshi S,
Cheng Y, Sandaltzopoulos R, Ra HJ, Danet-Desnoyers G, Powell DJ Jr
and Scholler N: Novel recombinant human b7-h4 antibodies overcome
tumoral immune escape to potentiate T-cell antitumor responses.
Cancer Res. 73:4820–4829. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Salceda S, Tang T, Kmet M, Munteanu A,
Ghosh M, Macina R, Liu W, Pilkington G and Papkoff J: The
immunomodulatory protein B7-H4 is overexpressed in breast and
ovarian cancers and promotes epithelial cell transformation. Exp
Cell Res. 306:128–141. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jiang J, Zhu Y, Wu C, Shen Y, Wei W, Chen
L, Zheng X, Sun J, Lu B and Zhang X: Tumor expression of B7-H4
predicts poor survival of patients suffering from gastric cancer.
Cancer Immunol Immunother. 59:1707–1714. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Arigami T, Uenosono Y, Ishigami S,
Hagihara T, Haraguchi N and Natsugoe S: Clinical significance of
the B7-H4 coregulatory molecule as a novel prognostic marker in
gastric cancer. World J Surg. 35:2051–2057. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou D, Pan G, Zheng C, Zheng J, Yian L
and Teng X: Expression of the RON receptor tyrosine kinase and its
association with gastric carcinoma versus normal gastric tissues.
BMC Cancer. 8:3532008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hao L, Zhang C, Qiu Y, Wang L, Luo Y, Jin
M and Zhang Y, Guo TB, Matsushima K and Zhang Y: Recombination of
CXCR4, VEGF, and MMP-9 predicting lymph node metastasis in human
breast cancer. Cancer Lett. 253:34–42. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Qian Y, Hong B, Shen L, Wu Z, Yao H and
Zhang L: B7-H4 enhances oncogenicity and inhibits apoptosis in
pancreatic cancer cells. Cell Tissue Res. 353:139–151. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Walker PR, Kwast-Welfeld J, Gourdeau H,
Leblanc J, Neugebauer W and Sikorska M: Relationship between
apoptosis and the cell cycle in lymphocytes: Roles of protein
kinase C, tyrosine phosphorylation, and AP1. Exp Cell Res.
207:142–151. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
American Joint Committee on Cancer: AJCC
Cancer Staging Manual. 6th edition. Springer-Verlag; New York, NY:
2002
|
|
26
|
Washington K: 7th edition of the AJCC
cancer staging manual: Stomach. Ann Surg Oncol. 17:3077–3079. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kryczek I, Wei S, Zhu G, Myers L, Mottram
P, Cheng P, Chen L, Coukos G and Zou W: Relationship between B7-H4,
regulatory T cells, and patient outcome in human ovarian carcinoma.
Cancer Res. 67:8900–8905. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cheng L, Jiang J, Gao R, Wei S, Nan F, Li
S and Kong B: B7-H4 expression promotes tumorigenesis in ovarian
cancer. Int J Gynecol Cancer. 19:1481–1486. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang L, Wu H, Lu D, Li G, Sun C, Song H,
Li J, Zhai T, Huang L, Hou C, et al: The costimulatory molecule
B7-H4 promote tumor progression and cell proliferation through
translocating into nucleus. Oncogene. 32:5347–5358. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schultz DR and Harrington WJ Jr:
Apoptosis: Programmed cell death at a molecular level. Semin
Arthritis Rheum. 32:345–369. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Korsmeyer SJ, Shutter JR, Veis DJ, Merry
DE and Oltvai ZN: Bcl-2/Bax: A rheostat that regulates an
anti-oxidant pathway and cell death. Semin Cancer Biol. 4:327–332.
1993.PubMed/NCBI
|
|
32
|
Würstle ML, Laussmann MA and Rehm M: The
central role of initiator caspase-9 in apoptosis signal
transduction and the regulation of its activation and activity on
the apoptosome. Exp Cell Res. 318:1213–1220. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cohen GM: Caspases: The executioners of
apoptosis. Biochem J. 326:1–16. 1997. View Article : Google Scholar : PubMed/NCBI
|