Introduction
Kidney cancer is the ninth and fourteenth most
common cancer in men and women, respectively, and the sixteenth
most common cause of cancer-related mortality worldwide. In Japan,
the estimated age-standardized incidence of kidney cancer among
both sexes was 5.3 per 100,000 population (http://gco.iarc.fr/today/online-analysis-map?mode=cancer&mode_population=continents&population=900&sex=0&cancer=29&type=0&statistic=0&prevalence=0&color_palette=default&projection=natural-earth)
(1,2). Renal cell carcinoma (RCC) is the most
common type of adult epithelial kidney cancer (2). The 2016 World Health Organization
(WHO) classification describes 13 distinct morphotypes (including
unclassified ones) in RCC. Among those subtypes, clear cell RCC
(ccRCC) is the most common type of renal parenchymal tumor,
accounting for 63–83% of RCC cases, and its outcome is
significantly poorer when compared with the outcome of other
subtypes, such as papillary RCC or chromophobe RCC (3–5). The
International Society of Urological Pathology (ISUP) 2012 Consensus
Conference also reported new recommendations for classification.
There was a strong consensus (98%) that the main morphotypes of RCC
have prognostic significance, and that the cancer-specific survival
(CSS) rate of ccRCC is significantly lower compared with that of
papillary or chromophobe RCC at comparable stages (6).
In addition to tumor morphotype, other pathological
parameters were suggested as being potential prognostic factors at
the 2012 ISUP Conference, including sarcomatoid differentiation
(SD), rhabdoid differentiation (RD), tumor necrosis (TN), novel
nuclear ISUP grade and microvascular invasion (MVI) (6). In the present study, we focused on
TN, ISUP grade and MVI as potential prognostic factors. TN is often
encountered in RCC and has been reported in 27–32% of ccRCC cases
(7,8). The presence of TN has been correlated
with not only high-risk clinicopathological parameters, but also
poor disease-specific survival (9–13).
The 2012 ISUP Conference recommended the use of the ISUP grading
system rather than the Fuhrman grading system. The ISUP grading
system for ccRCC is defined only by nucleolar prominence and has
exhibited a stronger association with patient outcome compared with
that exhibited by the Fuhrman grading system (14). MVI is controversial as a prognostic
factor in some studies.
To date, there have been several reports on the
assessment of the ISUP recommendations in Western countries;
however, there are few reports from East Asia, and no reports on
the Japanese population. As the Japanese are a homogeneous race,
the validity of the research results in Western countries require
verification in a Japanese cohort.
The aim of the present study was to review all
pathological slides of localized RCC from Japanese patients who
underwent radical surgery at our institution over the past 10
years, and reclassify them by morphotype, TN, ISUP grade and MVI,
according to the consensus of the 2012 ISUP Conference and,
subsequently, on the basis of these results, to identify strong
predictive factors affecting recurrence-free survival (RFS) and
CSS. To the best of our knowledge, this study is the first to
report on a Japanese population with postoperative localized or
locally advanced ccRCC assessed according to the 2012 ISUP
recommendations.
Materials and methods
Patient selection
The postoperative prognostic and clinicopathological
data were retrospectively analyzed in accordance with a protocol
approved by Tokai University Institutional Review Board. Between
January 2004 and December 2014, a total of 631 patients with
localized or locally advanced RCC underwent radical or partial
nephrectomy at the Department of Urology of Tokai University
Hospital (Isehara, Japan). Patients with synchronous bilateral
renal tumors or synchronous metastases (to lymph nodes or other
organs) were excluded from the analysis. All pathological slides of
the patients were revised on the basis of the 2016 WHO
classification and the TNM system by a single pathologist (C.I.).
Among all 631 tumors, 514 were confirmed as being of the
morphological ccRCC type. Finally, 406 patients who could be
observed for >2 years after surgery were included in the
analysis. The median age of the 406 patients was 62 years (range,
27–85 years). The male:female ratio was ~3:1. Radical nephrectomy
was performed in 268 patients, and partial nephrectomy in 138
patients. The median postoperative follow-up period was 59 months
(range, 3–137 months). A total of 48 patients had recurrent tumors
during the follow-up period. The characteristics of the patients
are summarized in Table I.
 | Table IClinicopathological characteristics of
the 406 patients with ccRCC. |
Table I
Clinicopathological characteristics of
the 406 patients with ccRCC.
| Characteristics | No. |
|---|
| Median age, years
(range) | 62 (27–85) |
| Sex |
| Male | 309 |
| Female | 97 |
| Side |
| Left | 192 |
| Right | 214 |
| Nephrectomy type |
| Radical | 268 |
| Partial | 138 |
| Median postoperative
follow-up, months (range) | 59 (3–137) |
| Pathological T
stage |
| T1a | 272 |
| T1b | 76 |
| T2a | 12 |
| T2b | 3 |
| T3a | 16 |
| T3b | 26 |
| T4 | 1 |
| Tumor size, median mm
(range) | 32 (9–250) |
| Fuhrman grade |
| 1 | 3 |
| 2 | 343 |
| 3 | 38 |
| 4 | 22 |
| ISUP grade |
| 1 | 4 |
| 2 | 227 |
| 3 | 153 |
| 4 | 22 |
| Microvascular
invasion |
| Positive | 77 |
| Negative | 329 |
| Tumor necrosis |
| Positive | 43 |
| Negative | 363 |
| Sarcomatoid
differentiation |
| Positive | 9 |
| Negative | 397 |
| Rhabdoid
differentiation |
| Positive | 17 |
| Negative | 389 |
Pathological review
A single pathologist (C.I.) reviewed all tumors of
the 631 patients and reassigned the tumors by histological subtype,
pathological T stage, nuclear grade, TN, SD, RD and MVI.
Histological subtype and pathological T stage were reassigned
according to the 2016 WHO classification. TN, SD, RD, and MVI were
assessed according to the 2012 ISUP Consensus Conference
recommendations (6). The presence
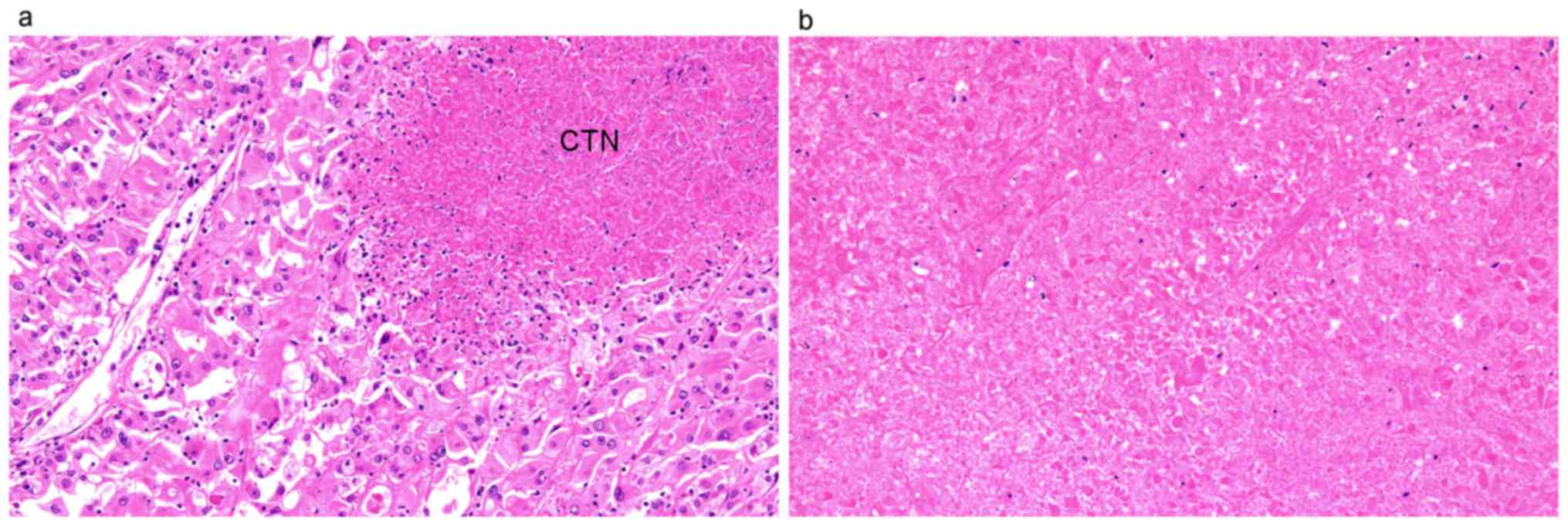
of TN was defined as microscopic coagulative tumor necrosis in the
tumor section (Fig. 1a). Fibrosis,
hyalinization, hemorrhage and ischemic necrosis (Fig. 1b) within a tumor were distinguished
from coagulative tumor necrosis to avoid misdiagnosis of tumor
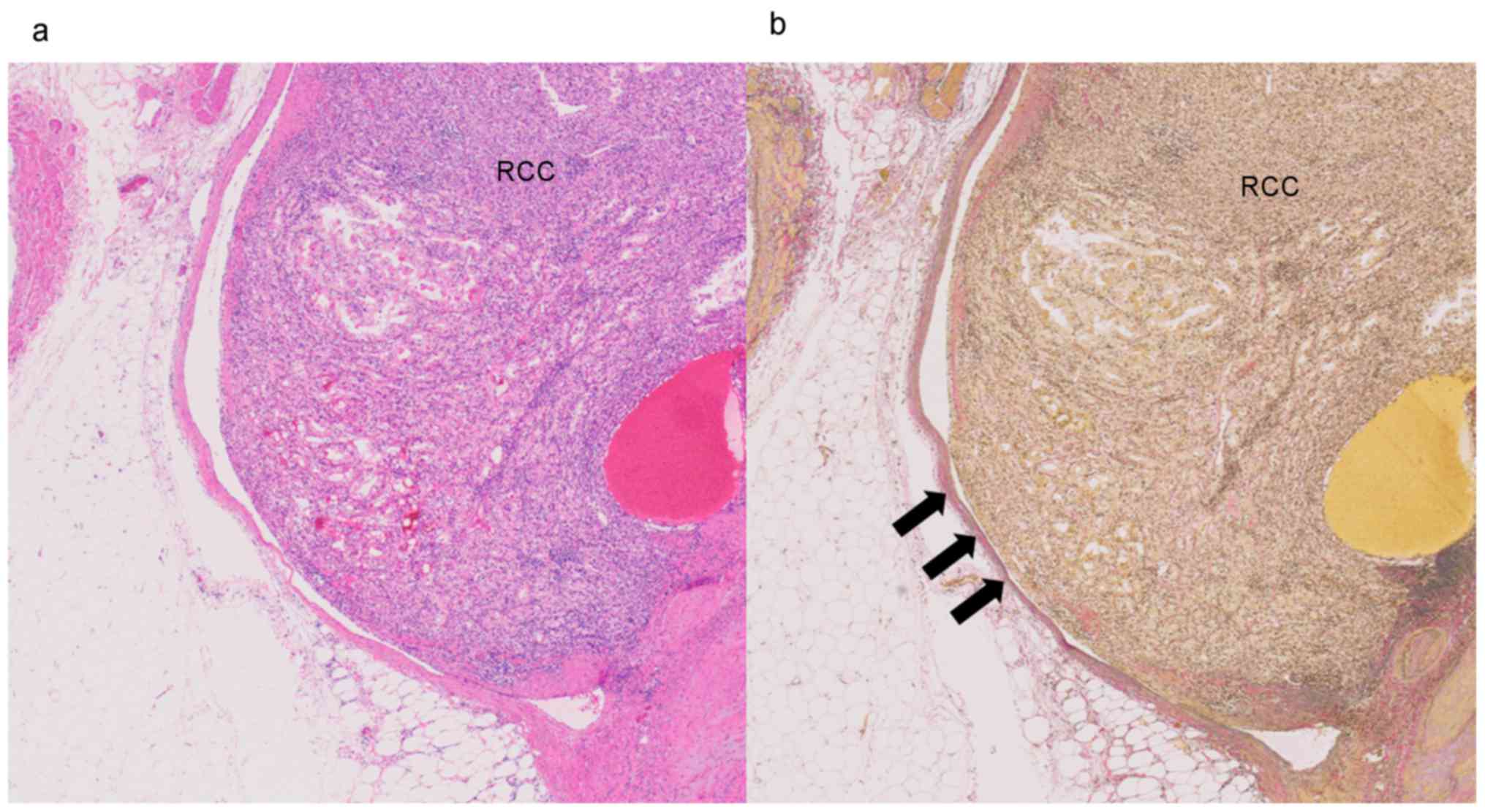
necrosis. MVI was defined as a lump of tumor cells inside a vessel
lined by one or more layers of vascular endothelial cells. If the
presence of endothelial cells made the image unclear, elastin
staining was performed to identify the vessel wall (Fig. 2a and b). Nuclear grade was
reassigned according to both the Fuhrman and ISUP grading systems.
The group that was reclassified by the Fuhrman grading system was
named group Fuhrman, and the group that was reclassified by the
ISUP grading system was named group ISUP. Cases with questionable
pathological diagnoses were reviewed by another pathologist (H.K.).
If the two pathologists had different opinions regarding the
diagnosis, they examined the slides together and consensus was
reached through discussion. The pathologists were blinded to the
clinical outcome. The pathological findings are summarized in
Table I. The Fuhrman and ISUP
grading systems are defined as in Table II.
 | Table IIFuhrman and ISUP grading systems. |
Table II
Fuhrman and ISUP grading systems.
| Fuhrman grading
system | ISUP grading
system |
|---|
| Grade 1 | Grade 1 |
| Tumors were
composed of cells with small (~10 µm) round uniform nuclei
with inconspicuous or absent nucleoli | Nucleoli are
inconspicuous or absent at a magnification of ×400 |
| Grade 2 | Grade 2 |
| Tumor cells had
larger (~15 µm) nuclei that exhibited irregularities in the
outline and nucleoli when examined under high-power magnification
(×400) | Nucleoli are
clearly visible at high-power magnification, but are not
prominent |
| Grade 3 | Grade 3 |
| Tumor cells had
even larger nuclei (~20 µm) with an obviously irregular
outline and prominent large nucleoli even at low-power
magnification (×100) | Nucleoli are
prominent and are easily visualized at low-power magnification |
| Grade 4 | Grade 4 |
| Tumor cells exhibit
characteristics similar to those of grade 3 tumors with the
addition of bizarre, often multilobed nuclei and heavy chromatin
clumps. These tumors often display areas of spindled-shaped cells
resembling sarcomas | Presence of tumor
giant cells and/or marked nuclear pleomorphism and/or with rhabdoid
or sarcomatoid differentiation |
Statistical analysis
The RFS interval was defined as the time from the
day of surgery until detection of recurrence. The CSS interval was
defined as the time from the day of surgery until death from RCC.
Data from patients who remained alive without recurrence at the
last evaluation or who died of other causes were censored. RFS and
CSS for clinical and pathological factors were calculated according
to the Kaplan-Meier method and compared with the log-rank test.
Clinical and pathological factors were defined as follows: Age (≤62
vs. >62 years), sex (male vs. female), pT stage (≤pT2 vs. ≥pT3),
Fuhrman grade (1/2 vs. 3/4), ISUP grade (1/2 vs. 3/4), TN (absence
or presence) and MVI (absence or presence). SD and RD were
excluded, as these factors were included in one of the factors of
grade 4 of the ISUP grading system. The median age of 62 years was
used to separate younger from older patients. Multivariate analyses
were performed using a Cox proportional hazards model. P-values of
<0.05 were considered to indicate statistically significant
difference. Clinical outcomes (RFS and CSS) were separately
calculated in group Fuhrman and group ISUP. To discriminate the
predictive accuracy for clinical outcomes between groups Fuhrman
and ISUP, the concordance index (c-index) was used. A value of 1.0
represents perfect predictive models, and a value of 0.5 is
equivalent by chance.
We also specifically evaluated the clinical outcomes
for patients whose cancers were reclassified to a different grade
by the ISUP grading system. All the statistical analyses were
performed with JMP® version 12.0.1 (SAS Institute, Cary,
NC, USA) and Stata 14 (StataCorp LP, College Station, TX, USA).
Results
Results of pathological review based on
the 2016 WHO classification and the 2012 ISUP Consensus
Conference
Table I shows the
histopathological characteristics of all 406 patients with ccRCC
reviewed on the basis of the 2016 WHO classification and the 2012
ISUP recommendations. The nuclear grade according to the Fuhrman
grading system was 1 in 3 tumors, 2 in 343 tumors, 3 in 38 tumors,
and 4 in 22 tumors. In the same population, the ISUP grade was 1 in
4 tumors, 2 in 227 tumors, 3 in 153 tumors, and 4 in 22 tumors. The
number of cases whose nuclear grading differed between the Fuhrman
and the ISUP grading systems are presented in Table III. The percentage of positivity
of the adverse pathological characteristics of TN and MVI was 10.6
and 19.0%, respectively.
 | Table IIINumber of cases whose nuclear grading
changed between the Fuhrman and ISUP systems. |
Table III
Number of cases whose nuclear grading
changed between the Fuhrman and ISUP systems.
| ISUP grade | Fuhrman grade
|
|---|
| 1 | 2 | 3 | 4 | Total |
|---|
| 1 | 2 | 2 | 0 | 0 | 4 |
| 2 | 1 | 224 | 2 | 0 | 227 |
| 3 | 0 | 117 | 36 | 0 | 153 |
| 4 | 0 | 0 | 0 | 22 | 22 |
| Total | 3 | 343 | 38 | 22 | 406 |
Statistical analysis of the impact of
clinicopathological factors on RFS and CSS
The 5-year RFS and CSS rates for all 406 patients
with ccRCC estimated by the Kaplan- Meier method were 88.1 and
96.6%, respectively. A total of 48 patients developed recurrence,
and 18 patients had succumbed to RCC at the last follow-up. Among
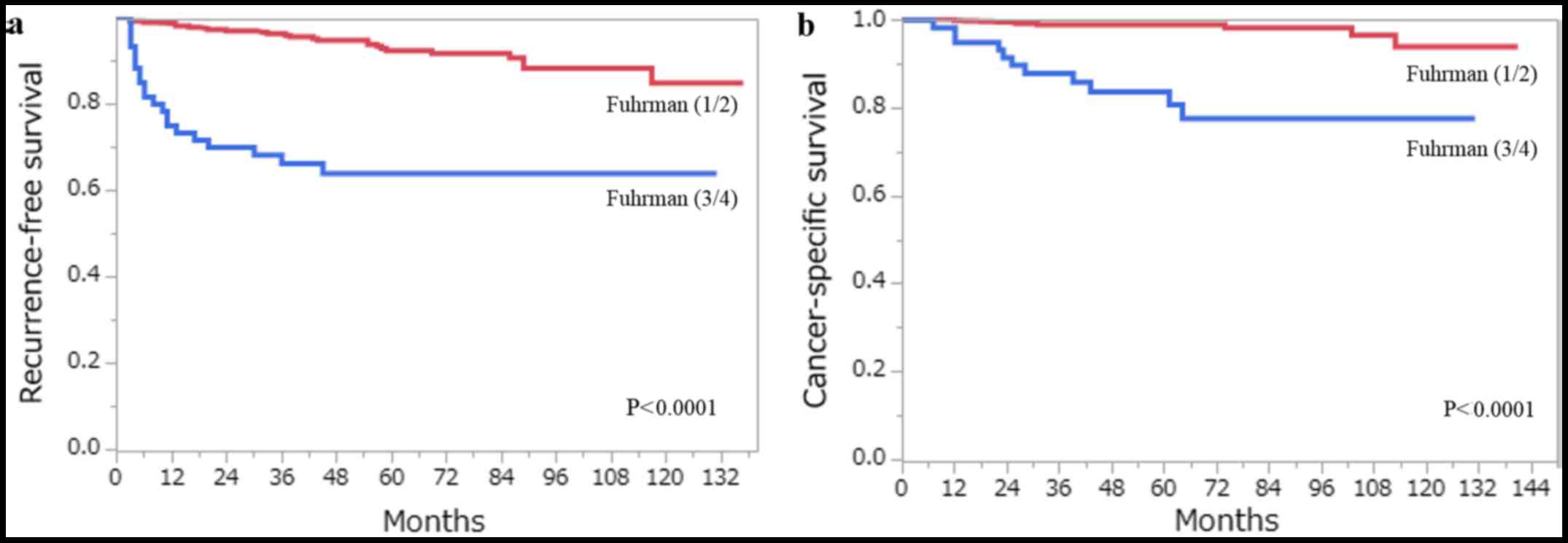
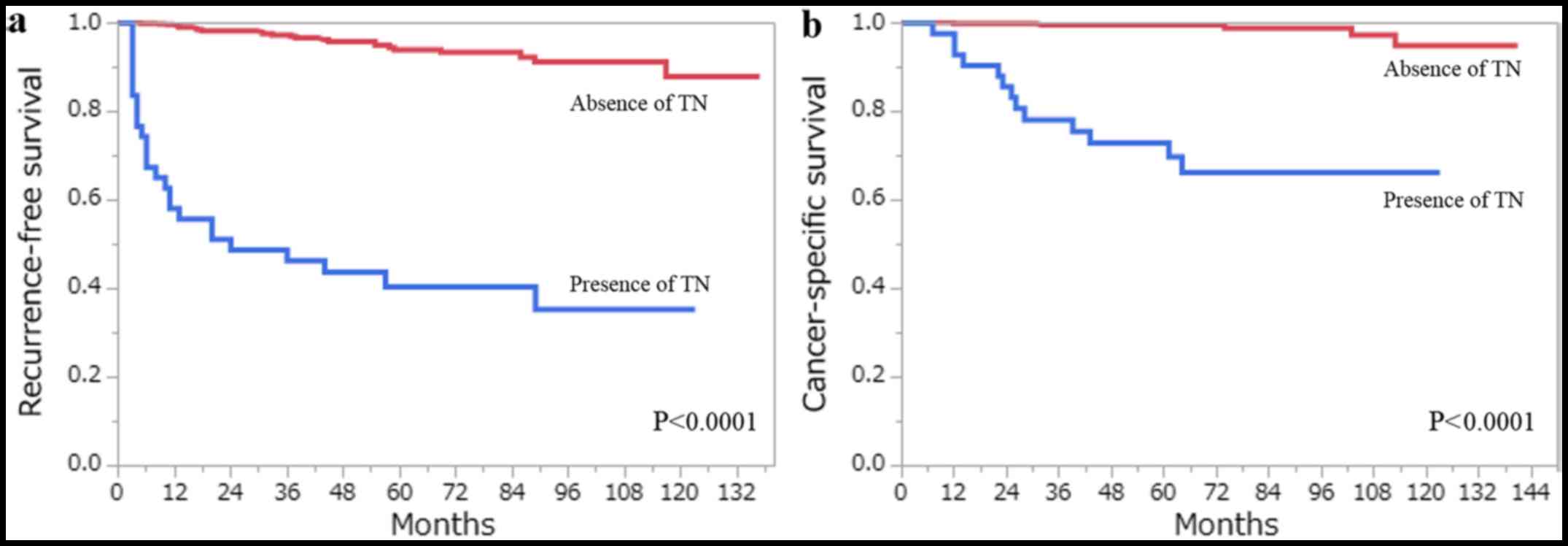
the clinicopathological factors that were assessed by the log-rank
test, age (>62 years), pT stage (≥pT3), Fuhrman grade (3/4),
ISUP grade (3/4), the presence of TN and the presence of MVI, were
all associated with RFS and CSS on the univariate analysis
(Figs. 3 and 4). Using multivariate analysis, we
analyzed independent prognostic factors for RFS and CSS for the
Fuhrman grade (group Fuhrman) and ISUP grade (group ISUP). The
former group, consisting of age, pT stage, Fuhrman grade, TN and
MVI, was incorporated into a multivariate analysis using the Cox
proportional hazards model. The latter group, consisting of age, pT
stage, ISUP grade, TN and MVI, was incorporated into a multivariate
analysis using the Cox proportional hazards model. In group
Fuhrman, TN (P<0.0001) and MVI (P=0.0057) were independent risk
factors for postoperative recurrence, while age (P=0.0059), Fuhrman
grade (P=0.0497) and TN (P=0.0007) were independent risk factors
for CSS (Table IV). In group
ISUP, TN (P<0.0001) and MVI (P=0.0057) were independent risk
factors for postoperative recurrence, while age (P=0.0327), ISUP
grade (P=0.0355) and TN (P=0.0003) were independent risk factors
for CSS (Table V). The predictive
accuracy for RFS using group Fuhrman and group ISUP was 0.8637 and
0.8642, respectively, whereas for CSS using group Fuhrman and group
ISUP was 0.9366 and 0.9478, respectively.
 | Table IVRisk factors for predicting
postoperative recurrence and cancer-specific survival in ccRCC
using the Fuhrman grading system. |
Table IV
Risk factors for predicting
postoperative recurrence and cancer-specific survival in ccRCC
using the Fuhrman grading system.
| Factors | Recurrence-free
survival
| Cancer-specific
survival
|
|---|
Univariate
| Multivariate
| Univariate
| Multivariate
|
|---|
| P-value | HR (95% CI) | P-value | P-value | HR (95% CI) | P-value |
|---|
| Age (years) | 0.0246 | | 0.0580 | 0.0018 | | 0.0059 |
| ≤62 | | | | | | |
| >62 | | 1.78
(0.99–3.31) | | | 4.74
(1.51–20.9) | |
| Sex | 0.5708 | | | 0.4909 | | |
| Female | | | | | | |
| Male | | | | | | |
| pT stage | <0.0001 | | 0.4687 | <0.0001 | | 0.8612 |
| ≤pT2 | | | | | | |
| ≥pT3 | | 1.35
(0.61–3.10) | | | 1.12
(0.33–4.42) | |
| Fuhrman grade | <0.0001 | | 0.1666 | <0.0001 | | 0.0497 |
| 1/2 | | | | | | |
| 3/4 | | 1.62
(0.82–3.23) | | | 2.97
(1.00–9.37) | |
| TN | <0.0001 | | <0.0001 | <0.0001 | | 0.0007 |
| Absence | | | | | | |
| Presence | | 6.62
(3.11–13.9) | | | 8.90
(2.45–34.8) | |
| MVI | <0.0001 | | 0.0057 | <0.0001 | | 0.2171 |
| Absence | | | | | | |
| Presence | | 2.96
(1.34–6.27) | | | 2.41
(0.58–9.21) | |
 | Table VRisk factors for predicting
postoperative recurrence and cancer-specific survival in ccRCC
using the ISUP grading system. |
Table V
Risk factors for predicting
postoperative recurrence and cancer-specific survival in ccRCC
using the ISUP grading system.
| Factors | Recurrence-free
survival
| Cancer-specific
survival
|
|---|
Univariate
| Multivariate
| Univariate
| Multivariate
|
|---|
| P-value | HR (95% CI) | P-value | P-value | HR (95% CI) | P-value |
|---|
| Age (years) | 0.0246 | | 0.0630 | 0.0018 | | 0.0327 |
| ≤62 | | | | | | |
| >62 | | 1.77
(0.98–3.28) | | | 3.91
(1.27–17.0) | |
| Sex | 0.5708 | | | 0.4909 | | |
| Female | | | | | | |
| Male | | | | | | |
| pT stage | <0.0001 | | 0.4095 | <0.0001 | | 0.8970 |
| ≤pT2 | | | | | | |
| ≥pT3 | | 1.41
(0.63–3.26) | | | 1.09
(0.32–4.33) | |
| ISUP grade | <0.0001 | | 0.8596 | <0.0001 | | 0.0355 |
| 1/2 | | | | | | |
| 3/4 | | 1.07
(0.53–2.19) | | | 9.31
(1.73–172) | |
| TN | <0.0001 | | <0.0001 | <0.0001 | | 0.0003 |
| Absence | | | | | | |
| Presence | | 8.01
(3.91–16.5) | | | 8.56
(2.80–29.9) | |
| MVI | <0.0001 | | 0.0057 | <0.0001 | | 0.2185 |
| Absence | | | | | | |
| Presence | | 2.97
(1.33–6.30) | | | 2.32
(0.56–8.67) | |
Clinical outcomes of ccRCC with Fuhrman
grade 2
A total of 343 cases with Fuhrman grade 2 were
identified, which accounted for the majority of our study
population. The accuracy of the ISUP grading system in predicting
clinical outcomes was compared with that of the Fuhrman grading
system. On reclassifying the 343 Fuhrman grade 2 cases using the
ISUP grading system, 224 were classified as ISUP grade 2, while 117
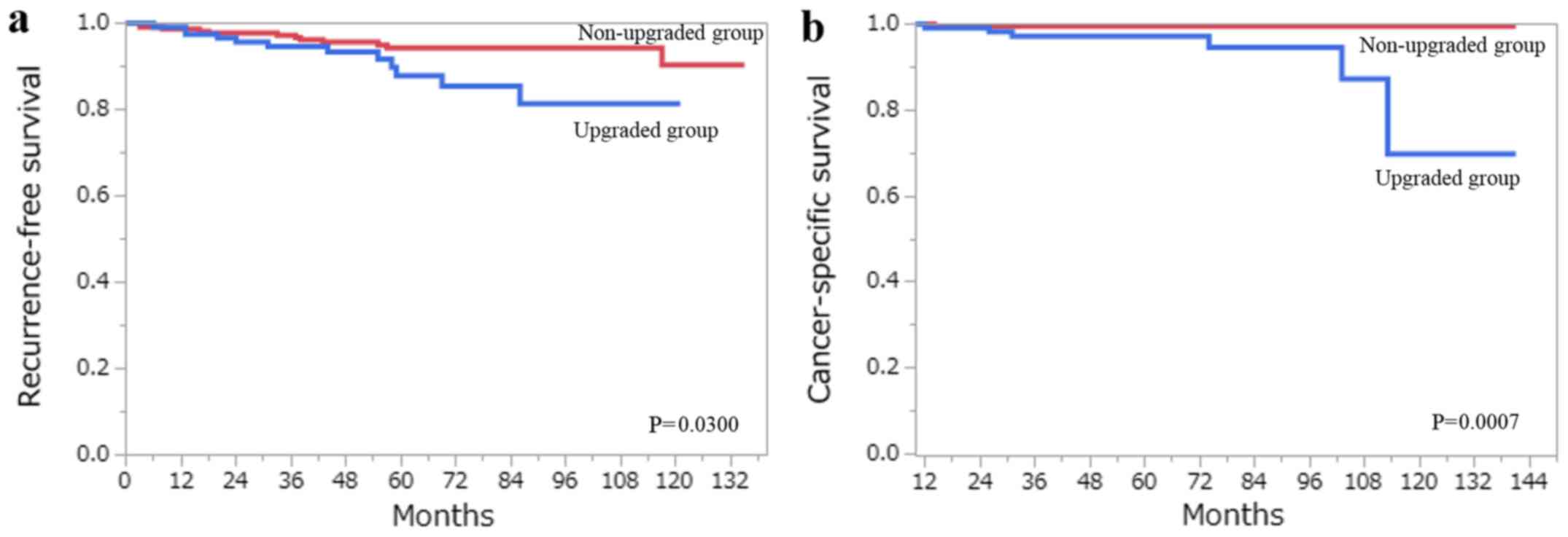
were upgraded to ISUP grade 3. The RFS at 10 years was 90.4% in
tumors that were revised from Fuhrman grade 2 to ISUP grade 2
(non-upgraded group), and 81.5% in the tumors that were upgraded
from Fuhrman grade 2 to ISUP grade 3 (upgraded group) (Fig. 5a). The CSS at 10 years was 99.6 and
69.9% in the non-upgraded and upgraded groups, respectively
(Fig. 5b). Multivariate analysis
using this cohort demonstrated that upgrading from Fuhrman grade 2
to ISUP grade 3 was an independent predictor of CSS (Table VI).
 | Table VIRisk factors for predicting
postoperative recurrence and cancer-specific survival in ccRCC with
Fuhrman grade 2. |
Table VI
Risk factors for predicting
postoperative recurrence and cancer-specific survival in ccRCC with
Fuhrman grade 2.
| Factors | Recurrence-free
survival
| Cancer-specific
survival
|
|---|
Univariate
| Multivariate
| Univariate
| Multivariate
|
|---|
| P-value | HR (95% CI) | P-value | P-value | HR (95% CI) | P-value |
|---|
| Age (years) | 0.2070 | | | 0.0325 | | 0.2167 |
| ≤62 | | | | | | |
| >62 | | | | | 3.45
(0.52–67.8) | |
| Sex | 0.6169 | | | 0.5200 | | |
| Female | | | | | | |
| Male | | | | | | |
| pT stage | <0.0001 | | 0.0582 | 0.0283 | | 0.1785 |
| ≤pT2 | | | | | | |
| ≥pT3 | | 3.06
(0.96–9.55) | | | 0.23
(0.02–1.94) | |
| Fuhrman grade
2 | 0.0300 | | 0.7038 | 0.0007 | | 0.0389 |
| Non-upgraded
group | | | | | | |
| Upgraded
group | | 1.19
(0.49–2.88) | | | 7.20
(1.09–143) | |
| TN | <0.0001 | | 0.0349 | <0.0001 | | 0.0444 |
| Absence | | | | | | |
| Presence | | 3.33
(1.09–9.75) | | | 7.58
(1.06–47.2) | |
| MVI | <0.0001 | | 0.0047 | 0.0001 | | 0.0168 |
| Absence | | | | | | |
| Presence | | 4.65
(1.63–12.8) | | | 9.48
(1.54–74.1) | |
Discussion
At the ISUP 2012 Consensus Conference, tumor
morphotype, SD, RD, TN, the ISUP grading system and MVI were
recommended as potential prognostic factors of RCC. Among the major
morphological subtypes, namely ccRCC, papillary RCC and chromophobe
RCC, ccRCC accounts for ~60–70% of malignant kidney epithelial
tumors and has the poorest prognosis (3–5,15).
Therefore, in the present study, these recommended factors were
evaluated with the focus being ccRCC. To evaluate the validity of
these factors in Japanese patients with localized ccRCC, 406 ccRCC
pathological slides were reviewed and reassigned pT stage, SD, RD,
TN, nuclear grade and MVI, according to the 2012 ISUP
recommendations and the 2016 WHO classification. For each potential
risk factor, postoperative RFS and CSS were calculated by the
Kaplan-Meier method; independent associations with RFS and CSS were
calculated by the Cox regression analysis. Nuclear grade was
classified by the Fuhrman and ISUP grading systems and then RFS and
CSS were calculated for each classified group. Multivariate
analysis indicated that TN and MVI were significantly associated
with RFS, while age, nuclear grade and TN were significantly
associated with CSS in both groups Fuhrman and ISUP. The c-index
for RFS using group ISUP (c-index, 0.8642) indicated that its
predictive ability is equivalent to that using group Fuhrman
(c-index, 0.8637), whereas there was a possibility that the
predictive ability for CSS using group ISUP (c-index, 0.9478) may
be superior to that using group Fuhrman (c-index, 0.9366).
In the present study, the presence of TN affected
both RFS and CSS as an independent risk factor. Although a number
of studies have reported the correlation of TN with established
clinicopathological parameters, such as high T stage, large tumor
size, poor tumor grade, and CSS or overall survival, there are only
a few reports on the association of the presence of TN with RFS,
particularly in the East Asian population (3,10,13,16–18).
In our study population of Japanese patients with localized ccRCC,
TN, one of the new potential prognostic factors, was found to be
the strongest predictive prognostic factor for both RFS and
CSS.
The Fuhrman grading system has been widely adopted
worldwide, including Japan; however, certain problems have been
identified in the literature, such as the inclusion of various
tumor types that differ morphologically and genetically, and poor
reproducibility due to multiple parameters, including nuclear size
and appearance (19–23). The ISUP grading system assesses
only nucleolar prominence, is simple and reproducible, and is
correlated with clinical outcome (14,24).
In our study cohort, multivariate analysis revealed that the ISUP
grade was not significantly associated with RFS, but it was
significantly associated with CSS. In particular, cases that were
subsequently upgraded to ISUP grade 3 from the Fuhrman grade 2
group exhibited a worse prognosis compared with those in the
non-upgraded group. The ISUP grading system was found to be
superior to the Fuhrman grading system due to its diagnostic
reproducibility and its ability to predict clinical outcomes.
The predictive ability of MVI is controversial. Some
reports have shown MVI to be correlated with prognosis; however,
others have reported no such correlation (25–30).
In the present study, the multivariate analysis demonstrated that
MVI was independently associated with RFS of both group Fuhrman and
group ISUP.
To the best of our knowledge, this study is the
first to prove the correlation between coagulative TN and clinical
outcome (RFS and CSS), as well as demonstrate the advantages of the
ISUP grading system when compared with Fuhrman grading in Japanese
ccRCC patients. Based on the results of our study, as well as the
report of the 2012 ISUP Consensus Conference, we recommend closer
surveillance or adjuvant therapy for patients with the presence of
TN and higher ISUP grade (ISUP grade >3) (6).
The present study is limited by its retrospective
design. Another limitation is that the majority of our cases lacked
clinical data on risk factors that are considered to be associated
with the prognosis of RCC, such as smoking and hypertension.
Moreover, some patients dropped out of follow-up shortly after
surgery, resulting in loss of prognostic data. In the present
study, the unclear histological subtypes were excluded, but we
included various stages of ccRCC, which may have resulted in
confounding bias. To strengthen the reliability of the results of
this study, prospective multicenter studies must be designed to
confirm these pathological parameters in a larger population of
consecutive patients with RCC.
In conclusion, to the best of our knowledge, this
retrospective study is the first to report on a Japanese population
with RCC to investigate the correlations between the postoperative
prognosis of ccRCC and the pathological parameters recommended by
the 2012 ISUP Consensus Conference. Adapting the ISUP
recommendations and the 2016 WHO classification for a Japanese
ccRCC population is likely to be of value in clinical practice.
Acknowledgments
Not applicable.
Notes
[1]
Funding
No funding was received.
[2] Availability
of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
[3] Authors'
contributions
HKi, CI and TU collected the data and wrote the
manuscript. CI and HKa reviewed the slides and confirmed the
pathological diagnosis. HF and TK analyzed and interpreted the
patient data regarding the clinical outcomes. HKo, NN and AM
provided the study concept and design, and revised the manuscript.
The final version of the manuscript was read and approved by all
authors.
[4] Ethics
approval and consent to participate
The postoperative prognostic and clinicopathological
data were retrospectively analyzed in accordance with a protocol
approved by the Tokai University Institutional Review Board
(15R-065).
[5] Consent for
publication
Not applicable.
[6] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar
|
|
2
|
Znaor A, Lortet-Tieulent J, Laversanne M,
Jemal A and Bray F: International variations and trends in renal
cell carcinoma incidence and mortality. Eur Urol. 67:519–530. 2015.
View Article : Google Scholar
|
|
3
|
Moch H, Gasser T, Amin MB, Torhorst J,
Sauter G and Mihatsch MJ: Prognostic utility of the recently
recommended histologic classification and revised TNM staging
system of renal cell carcinoma: A Swiss experience with 588 tumors.
Cancer. 89:604–614. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Amin MB, Amin MB, Tamboli P, Javidan J,
Stricker H, de-Peralta Venturina M, Deshpande A and Menon M:
Prognostic impact of histologic subtyping of adult renal epithelial
neoplasms: An experience of 405 cases. Am J Surg Pathol.
26:281–291. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ficarra V, Martignoni G, Galfano A, Novara
G, Gobbo S, Brunelli M, Pea M, Zattoni F and Artibani W: Prognostic
role of the histologic subtypes of renal cell carcinoma after slide
revision. Eur Urol. 50:786–793; discussion 793–794. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Delahunt B, Cheville JC, Martignoni G,
Humphrey PA, Magi-Galluzzi C, McKenney J, Egevad L, Algaba F, Moch
H, Grignon DJ, et al: Members of the ISUP Renal Tumor Panel: The
International Society of Urological Pathology (ISUP) grading system
for renal cell carcinoma and other prognostic parameters. Am J Surg
Pathol. 37:1490–1504. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee SE, Byun SS, Oh JK, Lee SC, Chang IH,
Choe G and Hong SK: Significance of macroscopic tumor necrosis as a
prognostic indicator for renal cell carcinoma. J Urol.
176:1332–1337; discussion 1337–1338. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Katz MD, Serrano MF, Grubb RL III,
Skolarus TA, Gao F, Humphrey PA and Kibel AS: Percent microscopic
tumor necrosis and survival after curative surgery for renal cell
carcinoma. J Urol. 183:909–914. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Frank I, Blute ML, Cheville JC, Lohse CM,
Weaver AL and Zincke H: An outcome prediction model for patients
with clear cell renal cell carcinoma treated with radical
nephrectomy based on tumor stage, size, grade and necrosis: The
SSIGN score. J Urol. 168:2395–2400. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pichler M, Hutterer GC, Chromecki TF,
Jesche J, Kampel-Kettner K, Rehak P, Pummer K and Zigeuner R:
Histologic tumor necrosis is an independent prognostic indicator
for clear cell and papillary renal cell carcinoma. Am J Clin
Pathol. 137:283–289. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lam JS, Shvarts O, Said JW, Pantuck AJ,
Seligson DB, Aldridge ME, Bui MH, Liu X, Horvath S, Figlin RA, et
al: Clinicopathologic and molecular correlations of necrosis in the
primary tumor of patients with renal cell carcinoma. Cancer.
103:2517–2525. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sengupta S, Lohse CM, Leibovich BC, Frank
I, Thompson RH, Webster WS, Zincke H, Blute ML, Cheville JC and
Kwon ED: Histologic coagulative tumor necrosis as a prognostic
indicator of renal cell carcinoma aggressiveness. Cancer.
104:511–520. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ficarra V, Martignoni G, Lohse C, Novara
G, Pea M, Cavalleri S and Artibani W: External validation of the
Mayo Clinic Stage, Size, Grade and Necrosis (SSIGN) score to
predict cancer specific survival using a European series of
conventional renal cell carcinoma. J Urol. 175:1235–1239. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Delahunt B, Sika-Paotonu D, Bethwaite PB,
William Jordan T, Magi-Galluzzi C, Zhou M, Samaratunga H and
Srigley JR: Grading of clear cell renal cell carcinoma should be
based on nucleolar prominence. Am J Surg Pathol. 35:1134–1139.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cheville JC, Lohse CM, Zincke H, Weaver AL
and Blute ML: Comparisons of outcome and prognostic features among
histologic subtypes of renal cell carcinoma. Am J Surg Pathol.
27:612–624. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim H, Cho NH, Kim DS, Kwon YM, Kim EK,
Rha SH, Park YW, Shim JW, Lee SS, Lee SN, et al: Genitourinary
Pathology Study Group of the Korean Society of Pathologists: Renal
cell carcinoma in South Korea: A multicenter study. Hum Pathol.
35:1556–1563. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Thompson RH, Leibovich BC, Lohse CM,
Cheville JC, Zincke H, Blute ML and Frank I: Dynamic outcome
prediction in patients with clear cell renal cell carcinoma treated
with radical nephrectomy: The D-SSIGN score. J Urol. 177:477–480.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Isbarn H, Patard JJ, Lughezzani G,
Rioux-Leclercq N, Crépel M, Cindolo L, de la Taille A, Zini L,
Villers A, Shariat SF, et al: Limited prognostic value of tumor
necrosis in patients with renal cell carcinoma. Urology.
75:1378–1384. 2010. View Article : Google Scholar
|
|
19
|
Fuhrman SA, Lasky LC and Limas C:
Prognostic significance of morphologic parameters in renal cell
carcinoma. Am J Surg Pathol. 6:655–663. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Delahunt B: Advances and controversies in
grading and staging of renal cell carcinoma. Mod Pathol. 22(Suppl
2): S24–S36. 2009.PubMed/NCBI
|
|
21
|
Goldstein NS: Grading of renal cell
carcinoma. Urol Clin North Am. 26:637–642, vii. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lang H, Lindner V, de Fromont M, Molinié
V, Letourneux H, Meyer N and Martin M: Jacqmin D. Multicenter
determination of optimal interobserver agreement using the Fuhrman
grading system for renal cell carcinoma: Assessment of 241 patients
with > 15-year follow-up. Cancer. 103:625–629. 2005. View Article : Google Scholar
|
|
23
|
Lanigan D, Conroy R, Barry-Walsh C, Loftus
B, Royston D and Leader M: A comparative analysis of grading
systems in renal adenocarcinoma. Histopathology. 24:473–476. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Delahunt B, Bethwaite PB and Nacey JN:
Outcome prediction for renal cell carcinoma: Evaluation of
prognostic factors for tumours divided according to histological
subtype. Pathology. 39:459–465. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pichler M, Hutterer GC, Chromecki TF,
Jesche J, Groselj-Strele A, Kampel-Kettner K, Pummer K and Zigeuner
R: Prognostic value of the Leibovich prognosis score supplemented
by vascular invasion for clear cell renal cell carcinoma. J Urol.
187:834–839. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cho HJ, Kim SJ, Ha US, Hong SH, Kim JC,
Choi YJ and Hwang TK: Prognostic value of capsular invasion for
localized clear-cell renal cell carcinoma. Eur Urol. 56:1006–1012.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sevinç M, Kirkali Z, Yörükoğlu K, Mungan U
and Sade M: Prognostic significance of microvascular invasion in
localized renal cell carcinoma. Eur Urol. 38:728–733. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Van Poppel H, Vandendriessche H, Boel K,
Mertens V, Goethuys H, Haustermans K, Van Damme B and Baert L:
Microscopic vascular invasion is the most relevant prognosticator
after radical nephrectomy for clinically nonmetastatic renal cell
carcinoma. J Urol. 158:45–49. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Roos FC, Weirich J, Victor A, Elsässer A,
Brenner W, Biesterfeld S, Hampel C and Thüroff JW: Impact of
several histopathological prognosticators and local tumour
extension on oncological outcome in pT3b/c N0M0 renal cell
carcinoma. BJU Int. 104:461–469. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lang H, Lindner V, Letourneux H, Martin M,
Saussine C and Jacqmin D: Prognostic value of microscopic venous
invasion in renal cell carcinoma: Long-term follow-up. Eur Urol.
46:331–335. 2004. View Article : Google Scholar : PubMed/NCBI
|