Introduction
Cancer has long been one of the most malignant
diseases and a leading cause of mortality, worldwide (1). In recent years, with the accelerated
pace of life, the deterioration of the environment and the
increased work pressures, the incidence of cancer increase year by
year (2). In the past, surgery and
radiotherapy has been the most common treatment method (3). Although there is now a new era of
molecular targeted therapy, these traditional therapies often bring
numerous unwanted side effects and problems, and do not always
improve the symptoms (4). Due to
the limited efficacy traditional therapeutics, it is important to
identify novel treatment strategies with reduced side effects.
In recent years, in Japan and worldwide, the
interest in the biological activities of compounds from marine
organisms is increasing (5).
Various compounds with biological activities have been investigated
and several have been developed into herbal medicines that are
commercially available (6). A
novel polysaccharide derived from algae extract has been previously
investigated. The biological activity of this novel polysaccharide
was first investigated using retinal pigment epithelial cells
(7), and the effect in inhibiting
human gastric carcinoma MKN45 cells was also reported recently
(8). The current study aimed to
determine if this extract affects other types of cancers, and the
signaling pathways involved. Human MCF-7 breast cancer cells were
used to investigate the effect of the novel polysaccharide on MCF-7
proliferation and migration, and determine the mechanisms involved
in the process.
Abnormal proliferation and migration are critical
physiological processes for cancer cell invasion (9). Induction of cell apoptosis is a
useful mechanism to inhibit cell proliferation (10). In addition to apoptosis, cell cycle
arrest is another cause of proliferation inhibition (11). It is well established that
mitogen-activated protein kinase (MAPK) signaling pathways are
involved in cell cycle, proliferation and migration (12). Jun N-terminal kinase (JNK), a
member of MAPK family, is associated with cell proliferation
inhibition (13). Phosphorylation
of JNK activates downstream tumor suppressors, p53, caspase-9 and
caspase-3, followed by apoptosis and cell cycle arrest (14). p38 MAPK, another member of MAPK
family, increases matrix metalloproteinase-9 (MMP-9)/MMP-2 activity
and induces cell migration (15).
Based on the conclusions of our previous experiments
using MKN45 cells (8), in the
current study, MCF-7 cells were used to investigate the effect of
the novel polysaccharide on the development of cancer, and to
understand the mechanisms involved in the processes. The novel
polysaccharide derived from algae extract suppressed MCF-7 cell
proliferation by inducing apoptosis and cell cycle arrest, and
activating the JNK signal pathway involving p53, caspase-9 and
caspase-3. By contrast, this polysaccharide did not affect
migration and did not change p38 MAPK signaling and the downstream
MMP-9/MMP-2.
Materials and methods
Preparation of the novel
polysaccharide
The novel polysaccharide was obtained from Toyo
Medicine Institute (Ashikita, Japan). The novel polysaccharide was
extracted from a type of Phaeophyceae (Sargassum), which is
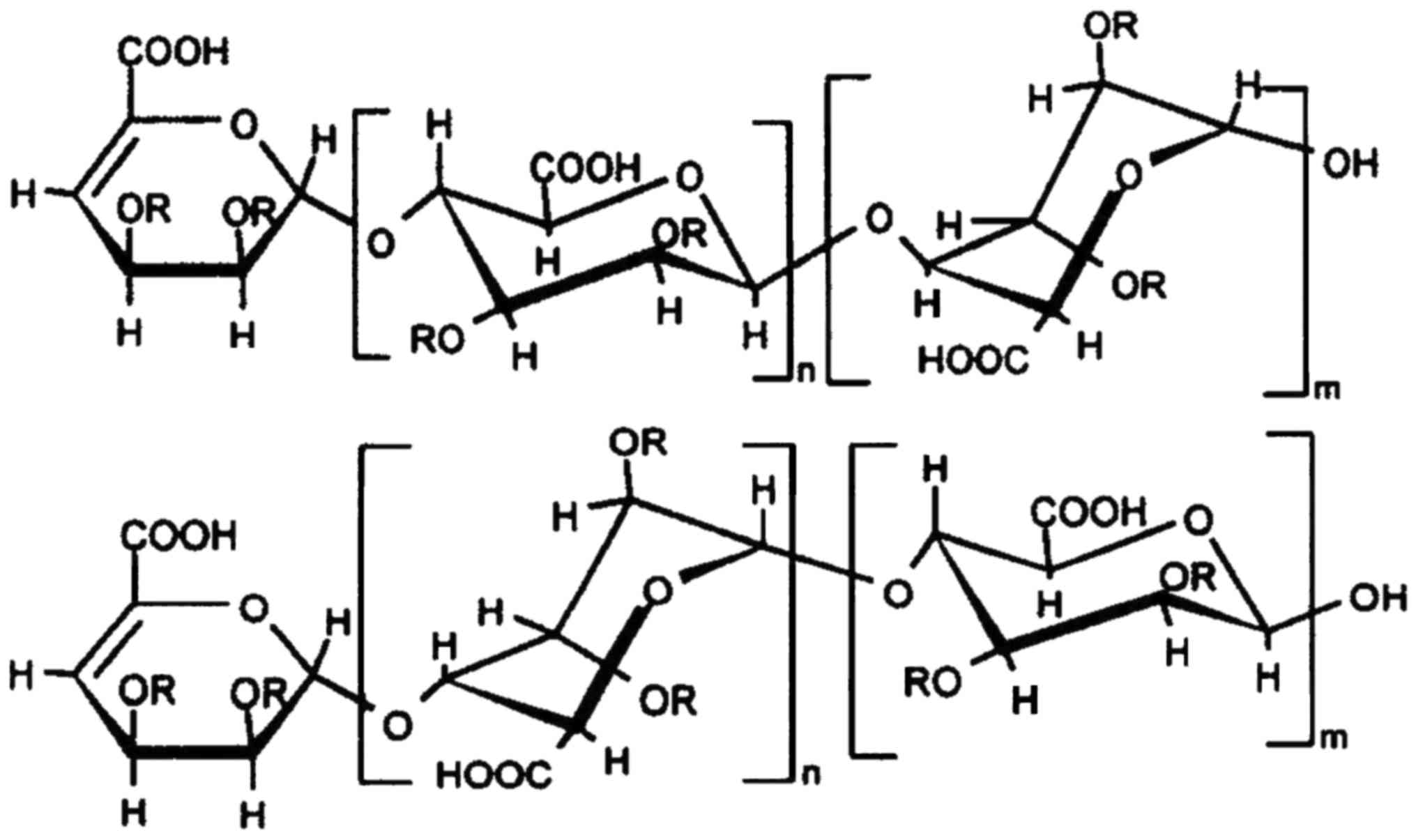
rich in hydrion and sulfate. The structure (Fig. 1) of the novel polysaccharide is
modified by sulfate and phosphate groups (represented by R), with a
typical sugar chain made of polymeride of disaccharides. According
to the method described previously (16,17),
the novel polysaccharide was extracted using chloroform, ethyl
acetate and n-butyl alcohol. Following isolation by column
chromatography on silica gel and Sephadex LH-20 columns (GE
Healthcare Bio-Sciences, Pittsburgh, PA, USA), polysaccharide was
purified on a macroporous absorption resin column, and then
sulfonated by sulfuric acid. The molecular weight of the
polysaccharide was 11680. The molecular weight was used for
calculation of molar concentration (μM) (18).
Cell culture
MCF-7 cells expressing the fluorescent
ubiquitination-based cell cycle indicator (Fucci) probes
(MCF-7-Fucci cells) were purchased from RIKEN BioResource Center
(Tsukuba, Japan). MCF-7-Fucci cells were cultured in RPMI-1640
medium supplemented with 10% fetal bovine serum (FBS) and
antibiotics (100 U/ml penicillin and 100 mg/ml streptomycin) (all
from Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). HeLa cells
(RIKEN BioResource Center) were cultured in Dulbecco's modified
Eagle's medium (Sigma-Aldrich; Merck KGaA) supplemented with 10%
FBS, 100 U/ml penicillin and 100 mg/ml streptomycin. MCF-7 cells
and HeLa cells were incubated in 5% CO2 at 37°C for all
experiments.
MTT assay
Following pretreatment with SP600125 (5 μM)
for 1 h or no treatment, MCF-7 cells or HeLa cells were plated at a
density of 5×103 cells/well in a 96-well plate and
exposed to polysaccharide (100 μg/ml) for 48 h. According to
the method described by Yuan et al (19), the viability of cells was
determined by a colorimetric MTT assay. Absorbance at 550 and 690
nm was determined by an MTP-800 microplate reader (Corona Electric,
Co., Ltd., Tokyo, Japan). The percentage of viable cell number was
calculated as: Optical density (OD) of treated sample/OD of
untreated control cells ×100.
Fluorescence activated cell sorting
(FACS) analysis
MCF-7 cells were incubated in a 6-well plate
(1×105 cells/well) in RPMI medium. After treatment with
the polysaccharide (100 μg/ml) for another 48 h, MCF-7 cells
were washed twice with PBS (Sigma-Aldrich; Merck KGaA). To detect
the apoptosis of cell, 10,000 individual cells were collected for
each sample and Annexin V-Biotin Apoptosis kit was used following
the manufacturer's instructions (BioVision, Inc., Milpitas, CA,
USA). Apoptotic cells were analyzed using a FACSCalibur™ flow
cytometer (BD Biosciences, San Jose, CA, USA) with CellQuest
software (version 6.1; BD Biosciences).
Cell cycle analysis
Cell cycle analysis was performed by flow cytometry
using a FACSCalibur™ and CellQuest software, as previously
described (20). Briefly, MCF-7
cells (1×105 cells/well) were exposed to polysaccharide
(100 μg/ml) for 48 h, washed and re-suspended in PBS (420
μl) following trypsinization and fixed in 99% ethanol at
−20°C for 2 h. Subsequently, samples were incubated in 50 μl
10 mg/ml RNase A (Sigma-Aldrich; Merck KGaA) at 37°C for 30 min,
and then incubated with propidium iodide (20 μl 0.2 mg/ml
solution) at room temperature for another 10 min. Subsequently, DNA
content was evaluated by FACS.
Nuclear staining
MCF-7 cells or HeLa cells were cultured in 6-well
plates (1×105 cells/well) for 24 h. Following treatment
with the polysaccharide (100 μg/ml) for another 48 h, cells
were washed with PBS, and fixed in 4% paraformaldehyde
(Sigma-Aldrich; Merck KGaA) for 30 min. Cells were stained with
Hoechst 33342 (20 mg/ml) at room temperature in the dark for 15
min. Then cell morphological changes were assessed by fluorescence
microscopy.
Fucci system
MCF-7 cells were plated at a density of
1×105 cells/well in a 6-well plate and treated with
polysaccharide (100 μg/ml) for 48 h. The MCF-7 cells used
expressed two Fucci probes, emitting red fluorescence
(SCFSkp2) in G1/G0 phase and green fluorescence
(APCCdh1) in S/G2/M phases (21). A FV10i-DOC confocal laser-scanning
microscope with a UPLSAPO ×60 Wobjective lens (Olympus Corporation,
Tokyo, Japan) was used to observe the cellular fluorescence and
obtain phase contrast images as previously described (22).
Migration assay
A 48-well chamber migration assay kit with
polycarbonate membrane (Whatman® Nuclepore™;
Sigma-Aldrich; Merck KGaA) was used for a migration assay according
to the method previously described (23). Briefly, the upper wells were coated
with 0.01% collagen for 30 min at 37°C. MCF-7 cells were treated
with polysaccharide (100 μg/ml) for 48 h at 37°C, then MCF-7
cells (5×104 cells/well) were seeded on the upper
chamber of the Transwell in serum-free RPMI medium. As chemotactic
medium, RPMI containing 10% fetal calf serum (Sigma-Aldrich; Merck
KGaA) was added to the lower wells. After 24 h at 37°C, the cells
that migrated towards to the lower filter surface were fixed with
4% paraformaldehyde for 10 min at room temperature and then stained
with crystal violet for 10 min at room temperature. The number of
migrated cells was counted under a ×100 microscope (Olympus
Optical, Co., Ltd., Tokyo, Japan).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
MCF-7 cells were treated with TRIzol reagent (Life
Technologies; Thermo Fisher Scientific, Inc.) for 2–3 min to
completely dissolve cells. Total RNA was extracted from MCF-7
cells. RT was performed using a Transcriptor First Strand cDNA
Synthesis kit (Roche Applied Science, Madison, WI, USA), with
incubation at 37°C for 20 min, then 75°C for 10 min. The relative
mRNA quantification was performed by ABI 7300 Fast real-time PCR
system (Applied Biosystems, Foster City, CA, USA) and normalized to
GAPDH. SYBR Premix Ex Taq II (Takara Bio, Inc., Otsu, Japan) was
used and the thermocycling conditions were: 95°C for 30 sec for
pre-denaturation, then 40 cycles of 95°C for 3 sec for
denaturation, 60°C for 31 sec for annealing and 72°C for 60 sec for
elongation, and finally 72°C for 5 min for re-elongation. RT-qPCR
results were analyzed by 2−ΔΔCq method described by
Livak and Schmittgen (24).
Certified™ PCR Agarose (Bio-Rad Laboratories, Inc., Hercules, CA,
USA) and ethidium bromide (Sigma-Aldrich; Merck KGaA) staining were
used to separate PCR products. The following primers (Hokkaido
System Science Co., Ltd., Sapporo, Japan) were used: MMP-9,
forward, 5′-CTTCACTTTCCTGGGTAAG-3′ and reverse,
5′-CACTTCTTGTCGCTGTCAAA-3′; MMP-2, forward,
5′-GACATACATCTTTGCTGGAGAC-3′ and reverse,
5′-TTCAGGTAATAGGCACCCTT-3′; and GAPDH, forward,
5′-TGCACCACCAACTGCTTAGC-3′ and reverse,
5′-GGCATGGACTGTGGTCATGAG-3′.
Gelatin zymography
MCF-7 cells (1×105 cells/well) were
pretreated with 100 μg/ml polysaccharide in RPMI medium for
48 h. As described previously (25), supernatants of culture medium of
MCF-7 cells (10 μl) were collected and subjected to
electrophoresis (10% SDS-polyacrylamide gel copolymerized with 0.1%
gelatin for substrate reaction). After washing in 2.5% Triton X-100
to remove SDS, gels were then incubated with developing buffer (50
mM Tris-HCl pH 7.4, 200 mM NaCl, 5 mM CaCl2 and 0.02%
Briji-35) at 37°C for >12 h. Gels were then stained with 0.5%
Coomassie Brilliant Blue R-250 for 2 min for band observation. The
intensities of bands were quantified with ImageJ software (National
Institutes of Health, Bethesda, MA, USA). The sum of MMP-9 and
MMP-2 bands was determined as activity.
Western blot analysis
The western blot analysis was performed as described
previously (26). Proteins were
extracted using lysis buffer (1 M Tris-HCl, pH 7.4; 1 M NaCl; 20%
Triton X100; 10% SDS; and 0.5 M EDTA). Protein concentration was
determined by bicinchoninic acid method as described previously
(27). A total of 20 μg
protein was loaded per lane of a 12% polyacrylamide gel. The
polyvinylidene fluoride membrane (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) was treated with Block Ace™ (4%) for 30 min at
22°C. The first reaction was performed using rabbit immunoglobulin
(Ig)G antibodies against JNK (cat. no. J4500; 1:2,000), phospho-JNK
(cat. no. 07-175; 2 μg/ml), p53 (cat. no. SAB4503015;
1:500), caspase-9 (cat. no. C7729; 1:300), caspase-3 (cat. no.
C9598; 1:3,000) and p38 MAPK (cat. no. SAB4500492; 1:500) (all from
Sigma-Aldrich; Merck KGaA) in PBS containing 0.03% Tween-20 for 1 h
at room temperature. Following washing in the same buffer three
times, the second reaction was performed using horseradish
peroxidase-conjugated anti-rabbit goat IgG (cat. no. A0545; 20
ng/ml; Sigma-Aldrich; Merck KGaA) for 30 min at room temperature.
Following washing, enhanced chemiluminescence (ECL) was used to
incubate the membrane and visualized using the ECL Plus Western
Blotting Detection System™ (GE Healthcare Life Sciences, Little
Chalfont, UK). ImageJ (version 1.49v; National Institutes of
Health, Bethesda, MD, USA) was used for the densitometry analysis
of western blots.
Detection of intracellular reactive
oxygen species (ROS)
Intracellular accumulation of ROS was estimated
using the fluorescent dye H2-dichlorofluorescin diacetate (DCFDA;
Life Technologies; Thermo Fisher Scientific, Inc.), which is
converted to a membrane impermeable and highly fluorescent
compound, dichlorofluorescin (DCF), in the presence of ROS. The
MCF-7 cells were seeded in a 6-well plate at the density of
1×105 cells/well. Following treatment with the
polysaccharide (100 μg/ml) or SP600125 (5 μM), MCF-7
cells (1×105 cells/well) were further incubated for 48
h. The cells were rinsed with serum-free medium and were incubated
in 5 μM H2-DCFDA for 60 min at 37°C. The cells were then
examined under a fluorescence microscope (C1-T-SM; Nikon
Corporation, Tokyo, Japan), collected and subjected to a
fluorescence spectrophotometry (F-2500; Hitachi, Ltd., Tokyo,
Japan) to detect the DCF fluorescence inside cells (excitation, 488
nm; emission, 521 nm) as described (8).
Statistical analysis
Analyses were performed using SPSS 19.0 (IBM Corp.,
Armonk, NY, USA). All data were presented as the mean ± standard
deviation of three independent experiments. Normality of
distribution was analyzed by D'Agostino and Pearson omnibus
normality test. Multiple comparisons between groups were performed
using one-way analysis of variance and post hoc test (Dunnett's
test). P<0.05 was considered to indicate a statistically
significant difference.
Results
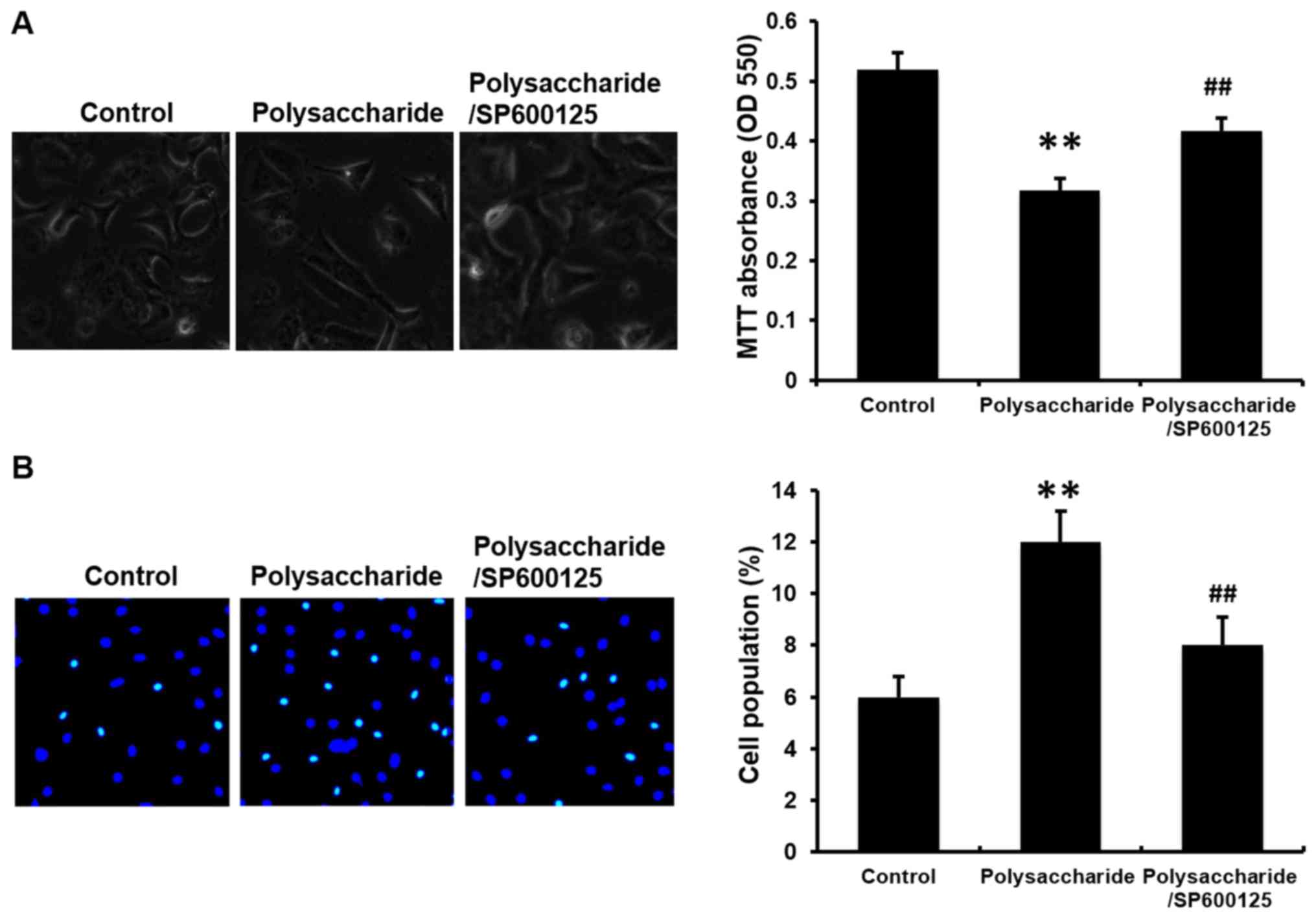
Novel polysaccharide suppresses cell
proliferation, induces cell apoptosis and cell cycle arrest in
MCF-7 cells
Recently, we reported that the polysaccharide
inhibited the invasion ability of human MKN45 gastric carcinoma
cells (8). To better understand
whether the polysaccharide has similar efficacy on other types of
cancer cells, the viability of human MCF-7 breast cancer cells was
determined. MCF-7 cells were exposed to 100 μg/ml
polysaccharide for 48 h and the cellular viability measured by
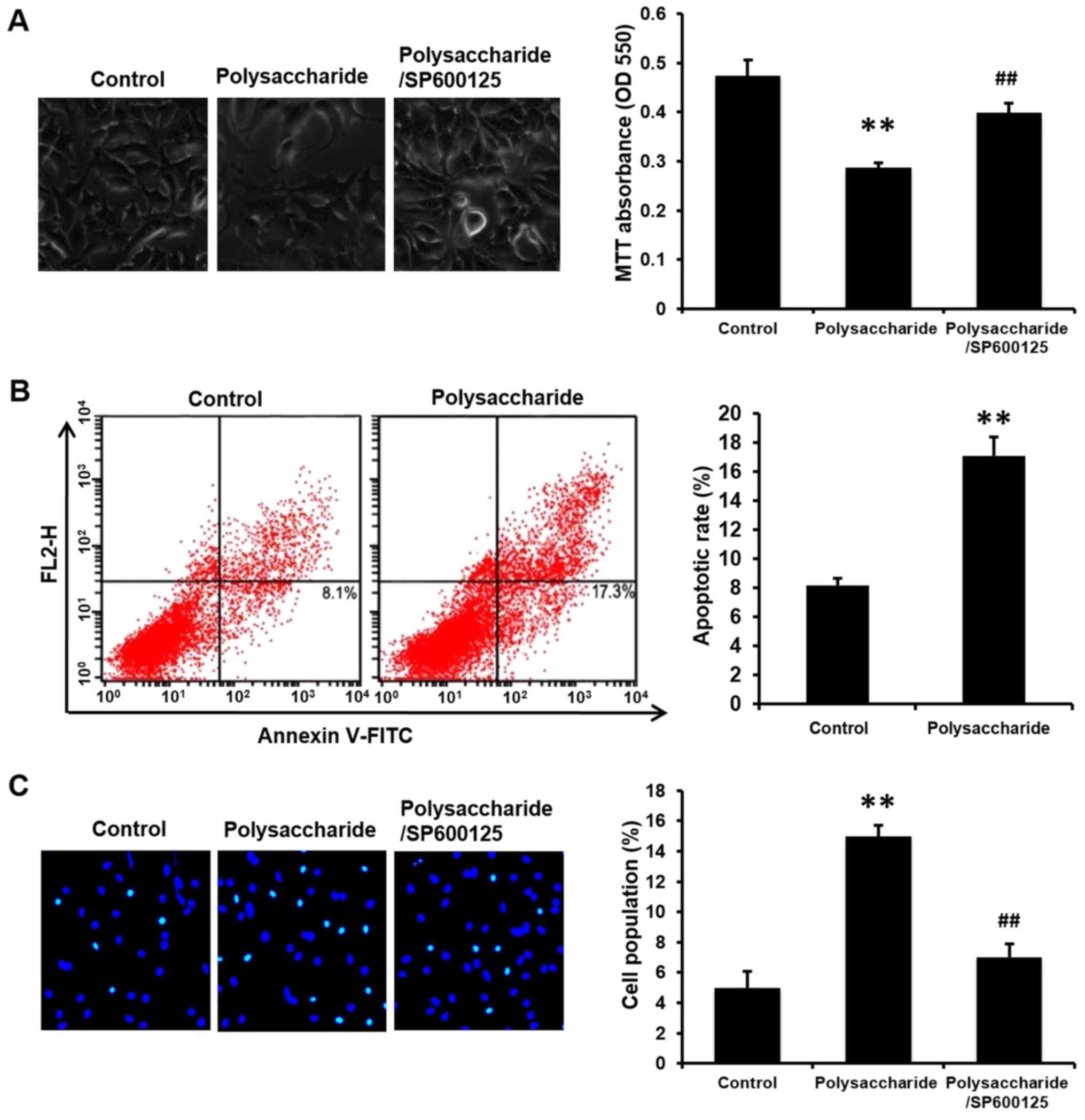
colorimetric MTT assay. The viability of MCF-7 cells was reduced by
the polysaccharide treatment (Fig.
2A). As the inhibition of viability is typically caused by
increased cellular apoptosis, FACSCalibur™ flow cytometry and
nuclear staining were performed to determine cell apoptosis. The
polysaccharide induced cell apoptosis compared with the cells
without polysaccharide treatment (Fig.
2B and C). Considering that the suppressed cell growth may be
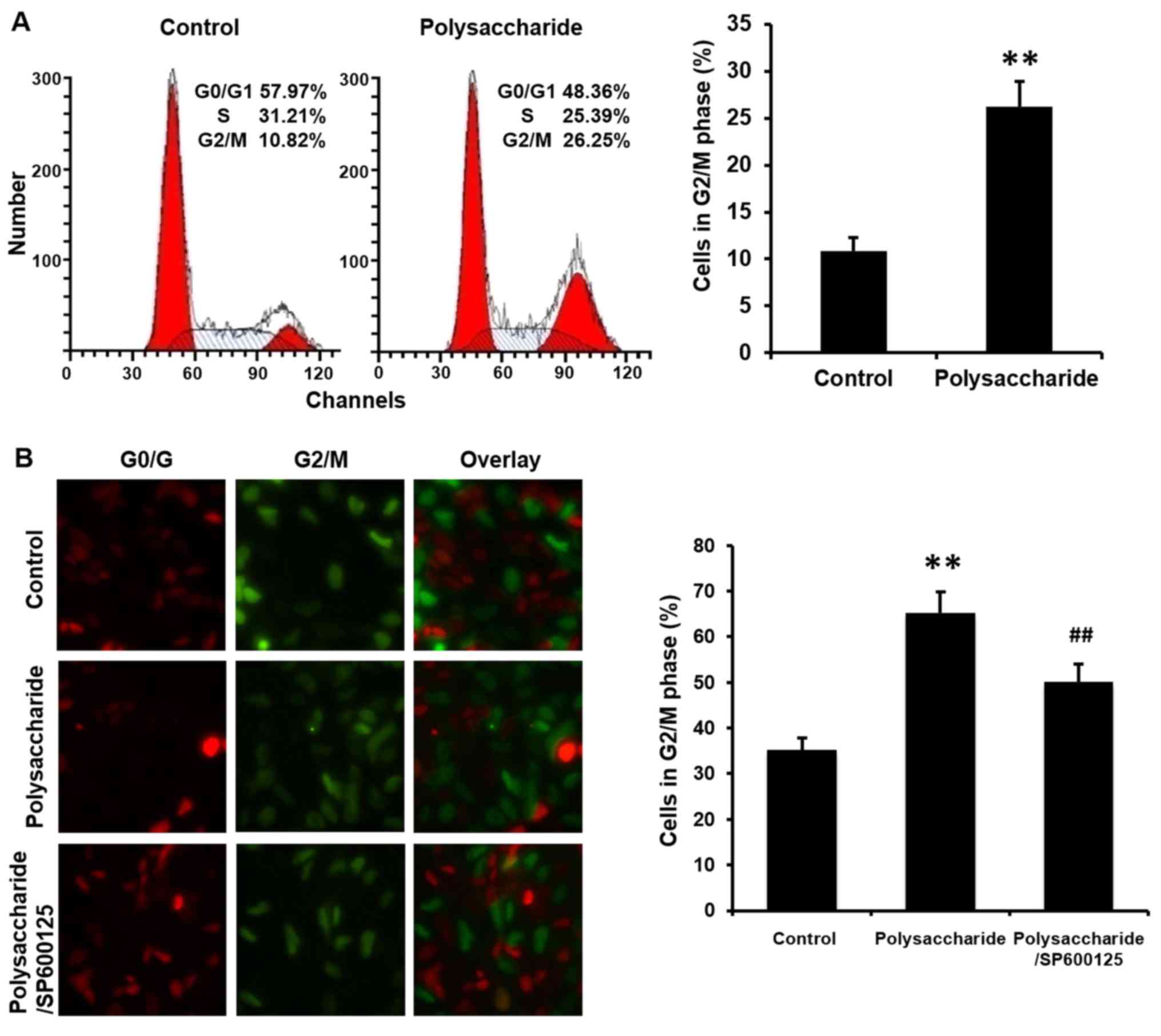
due to the cell cycle arrest, flow cytometry and a Fucci system
were used to analyze the cell cycle. The polysaccharide arrested
the cell cycle at G2/M (Fig. 3).
These results indicate that the novel polysaccharide reduced MCF-7
cells viability, and induces apoptosis and cell cycle arrest, which
are consistent with the results in our previous study (8).
Lack of inhibitory effects of the novel
polysaccharide on migration or MMP-9/MMP-2 expression in MCF-7
cells
Migratory capacity is another characteristic of
cancer cells. In order to understand whether the polysaccharide
affects MCF-7 cells by inhibiting cell migration, we used a
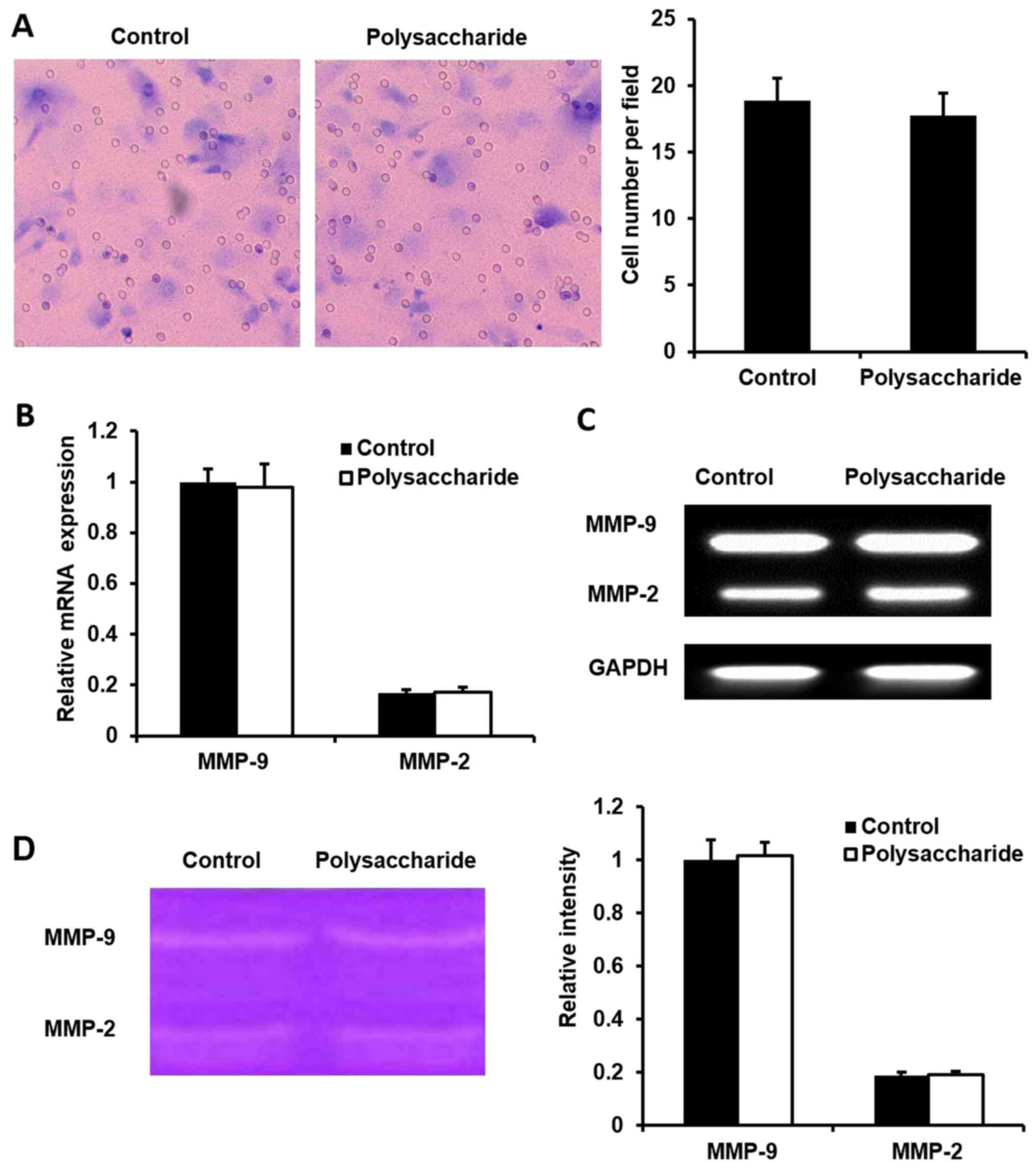
migration assay kit to determine the cell migration. There was no
difference in the number migrated cells between the
polysaccharide-treated and non-treated MCF-7 cells (Fig. 4A). Furthermore, the MMP-9/MMP-2
mRNA expression was measured, which was reported to be important
for the migration of cancer cells. MMP-9/MMP2 mRNA expression
(Fig. 4B and C) and the MMP
activity (Fig. 4D) were not
changed in polysaccharide-treated MCF-7 cells compared with control
cells. These results suggested that the polysaccharide does not
affect the migration of MCF-7 cells.
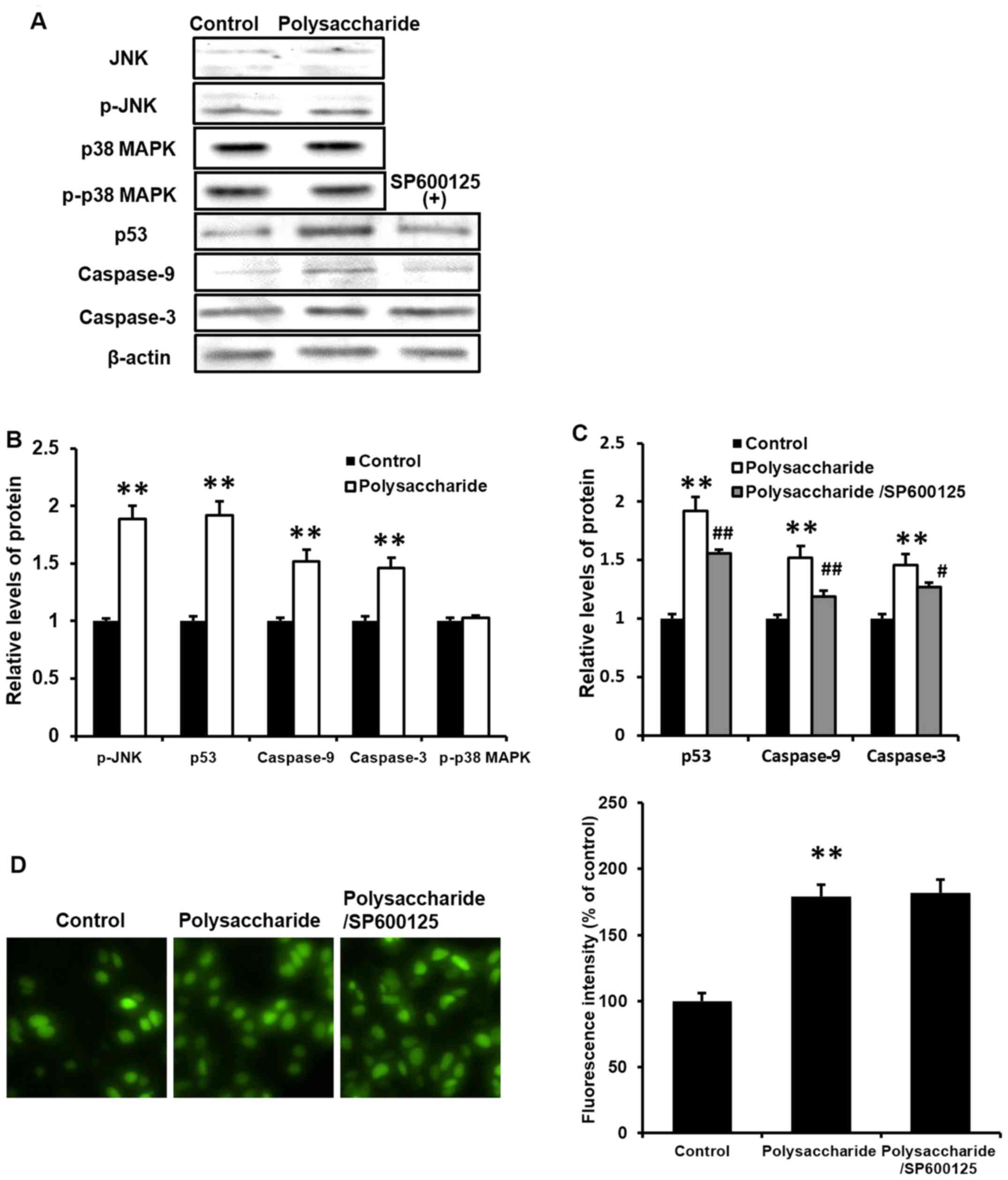
Novel polysaccharide induces the
phosphorylation of JNK, and expression of p53, caspase-9 and
caspase-3, with no effect on p38 MAPK phosphorylation in MCF-7
cells
The results of the current study demonstrated that
the polysaccharide inhibited MCF-7 cell viability, induced cell
apoptosis and cell cycle arrest, without affecting the migration of
MCF-7 cells. To further determine the potential signaling pathways
involved in this process, western blot analysis was performed to
detect the phosphorylation of JNK and p38 MAPK, and expression of
p53, caspase-9 and caspase-3. The novel polysaccharide upregulated
the phosphorylation of JNK, and the expression of p53, caspase-9
and caspase-3 (P<0.01), however, there was no effect on p38 MAPK
phosphorylation in MCF-7 cells (Fig.
5A and B). These results indicate that, the novel
polysaccharide inhibits MCF-7 cell viability, and induces cell
apoptosis and cell cycle arrest via JNK signaling, whereas there
was no effect on cancer cell or p38 MAPK phosphorylation.
SP600125 inhibits the effects of the
novel polysaccharide on cell viability, apoptosis and cell cycle
arrest in MCF-7 cells
In order to establish whether JNK signaling pathway
is necessary for this process, MCF-7 cells were pretreated with
SP600125, an inhibitor of JNK (5 μM) for 1 h. Notably,
SP600125 significantly blocked the polysaccharide-induced reduction
in cell viability (Fig. 2A) and
prevented polysaccharide induced cell apoptosis (Fig. 2C) and cell cycle arrest (Fig. 3B; P<0.01).
SP600125 prevents the novel
polysaccharide-induced p53, caspase-9 and caspase-3 in MCF-7
cells
To further clarify whether JNK signaling is
necessary in the potential processes induced by the polysaccharide,
cells were treated with SP600125 prior to western blot analysis of
various proteins. SP600125 significantly prevented the
polysaccharide-induced expression of p53, caspase-9 and caspase-3
in MCF-7 cells (Fig. 5A and C;
P<0.05). These results were consistent with our previous finding
(8) and indicated that JNK
signaling is crucial and necessary in this process.
SP600125 does not affect the novel
polysaccharide-induced ROS generation in MCF-7 cells
Previously, MKN45 cells were used to investigate the
mechanisms of JNK/ROS (8). As the
activation of JNK is associated with ROS generation, ROS generation
was analyzed in MCF-7 cells in the current study. MCF-7 cells were
pretreated with 5 μM SP600125 (a JNK inhibitor) for 1 h prior to
the polysaccharide (100 μg/ml) treatment. Subsequently, the
cells were incubated further for 48 h. Intracellular accumulation
of ROS was estimated using the fluorescent dye H2-DCFDA and flow
cytometry. The novel polysaccharide significantly induced ROS
generation in MCF-7 cells (Fig.
5D; P<0.01). However, pretreatment with SP600125 did not
affect the polysaccharide-induced ROS generation in MCF-7 cells,
suggesting that the effects on ROS are upstream of JNK.
SP600125 prevents the novel
polysaccharide-induced cell proliferation and apoptosis in human
cervical cancer cell line (HeLa cells)
The biological activity of the novel polysaccharide
in HeLa cells was also investigated. The novel polysaccharide
inhibited cell viability and induced cell apoptosis in HeLa cells.
SP600125 significantly prevented the cell viability inhibition and
cell apoptosis induction by the polysaccharide. (Fig. 6; P<0.01).
Discussion
Cancer is a major cause of mortality globally
(28). Surgery and radiotherapy
are the most common therapies (2);
however, due to the accompanied side-effects (1,29),
it is necessary and crucial to develop novel treatment strategies
for cancer with reduced side-effects. In recent years, research has
focused on molecular-targeted treatment for cancers (30) and the interest in biological
activities of compounds from marine organisms has intensified
(6). Numerous compounds have been
investigated, and some have been developed into herbal medicine in
Japan and elsewhere (6). A novel
polysaccharide derived from algae extract was investigated in this
study. The biological activity of this compound on human MKN45
gastric carcinoma cells via activating ROS/JNK signaling pathway
was reported previously (8), and
in the current study, another type of human cancer cell was used,
human MCF-7 breast cancer cells, to investigate the polysaccharide
for anticancer activity and the mechanisms involved. As described
in our previous study (8), the
effect of this novel polysaccharide in inhibiting human gastric
carcinoma MKN45 cells was measured in pre-experiments, where the
effects were dose-dependent (1, 10, 100 and 1,000 μg/ml) and
time-dependent (12, 24 and 48 h). A significant difference was
reached at 100 μg/ml and at 48 h. Thus, 100 μg/ml of
polysaccharide and 48 h of treatment was used in the current
study.
Abnormal proliferation and migration have important
physiological roles in the process of cancer invasion (8). Apoptosis and programmed cell death
are crucial mechanisms of proliferation inhibition (10). The majority of chemotherapeutic
agents inhibit cancer development by inducing the mechanisms of
apoptosis and programmed cell death (31). In accordance with the conclusions
of previous experiments using MKN45 cells (8), the present study demonstrated that
the novel polysaccharide reduced cell viability and induced
apoptosis in MCF-7 cells. In addition to apoptosis, cell cycle
arrest is another cause of proliferation inhibition (11). Various anticancer drugs inhibit
cell cycle progression at the G0/G1, S or G2/M phases (32). Abnormal cell cycle regulation has
been linked with cancer progression, and cell cycle arrest is an
effective method to block cancer cell proliferation (33,34).
Various anticancer drugs synchronize tumor cells in M phase, which
is the most radiosensitive stage of the cell cycle (35), appropriate timing of administration
results in optimal radio-sensitization (36). With the use of the Fucci system in
the present study confirmed that the novel polysaccharide arrested
MCF-7 cells at G2/M phase. These results again demonstrated the
potential ability of the novel polysaccharide in blocking the
development of cancers.
The development to cancer is often associated with
JNK, p38 MAPK and other signaling pathways. The MAPK family
includes extracellular signal-regulated kinase (ERK), p38, JNK and
ERK5 (37). MAPK signaling
pathways are the most widespread mechanisms of eukaryotic cell
regulation (38), including cell
cycle, proliferation and migration regulation (12). JNK, an important member of MAPK
family, is reported to be associated with cell proliferation
inhibition (13). The activated
phospho-JNK induces the expression of downstream tumor suppressors
(14). p53, a tumor suppressor, is
involved in coordinating apoptosis to preserve genomic stability
and prevent tumor formation. Previous studies have also suggested
the involvement of p53 in the autophagic pathway (39). p53 induces cell cycle arrest and
leads to self-mediated apoptosis (40). In addition, p53 also induces the
expression of several factors involved in apoptosis, including
caspase-9 and caspase-3 (41). The
activation of caspase-9 and caspase-3 induces proteolysis and leads
to the damage of cell structure and functional disorder (42). Phosphorylated JNK activates p53,
caspase-9 and caspase-3, consequently leading to apoptosis and cell
cycle arrest (14). The current
study demonstrated that the novel polysaccharide induced the
phosphorylation of JNK, and increased the expression of p53,
caspase-9 and caspase-3, suggesting the involvement of JNK
activation, and p53, caspase-9 and caspase-3 expression in the
inhibitory effects of the novel polysaccharide.
p38 MAPK is another member of the MAPK family. p38
MAPK signaling is the main pathway involved in inducing cell
migration (43). Notably, in the
current study, the novel polysaccharide did not affect the
migration of MCF-7 cells. MMP-9 and MMP-2 are key enzymes involved
in tumor metastasis (44). p38
MAPK signaling increases the activity of MMP-9 and MMP-2 and
induces cell migration (15). To
better understand whether the p38 MAPK signaling pathway is
involved in the effects of the polysaccharide, the expression of
p38 MAPK, MMP-9 and MMP-2 were examined in the present study. The
polysaccharide did not affect the mRNA expression or activity of
MMP-9 and MMP-2, or the phosphorylation of p38 MAPK. These results
suggested that the p38 MAPK signaling pathway and its downstream
cascades were not involved in the inhibitory effects of the algae
polysaccharide.
To understand the role of JNK in the effects of the
novel polysaccharide, the JNK inhibitor SP600125 was used in
further experiments. SP600125 significantly blocked the reduced
MCF-7 cell viability cause by the novel polysaccharide, prevented
the induction of cell apoptosis and cell cycle arrest.
Additionally, SP600125 prevented the novel polysaccharide-induced
expression of p53, caspase-9 and caspase-3 in MCF-7 cells. The
association between activation JNK and ROS generation was reported
in our previous study (8); thus,
in the current study, the, generation of ROS was determined in
MCF-7 cells. The novel polysaccharide significantly induced ROS
generation in MCF-7 cells; however, pretreatment with SP600125 did
not affect the polysaccharide-induced ROS generation in MCF-7
cells, suggesting that the effect on ROS is upstream of JNK.
Currently, three types of cancer cells have been
used to investigate the biological activities of the novel
polysaccharide. MKN45 cells were used in our previous study
(8) and MCF-7 cells were used in
the current study. In order to support the findings, experiments
were also performed in HeLa cells, with the novel polysaccharide
exerting similar effects on cell viability and cell apoptosis.
SP600125 significantly inhibited the reduced cell viability and
increased cell apoptosis induced by the polysaccharide.
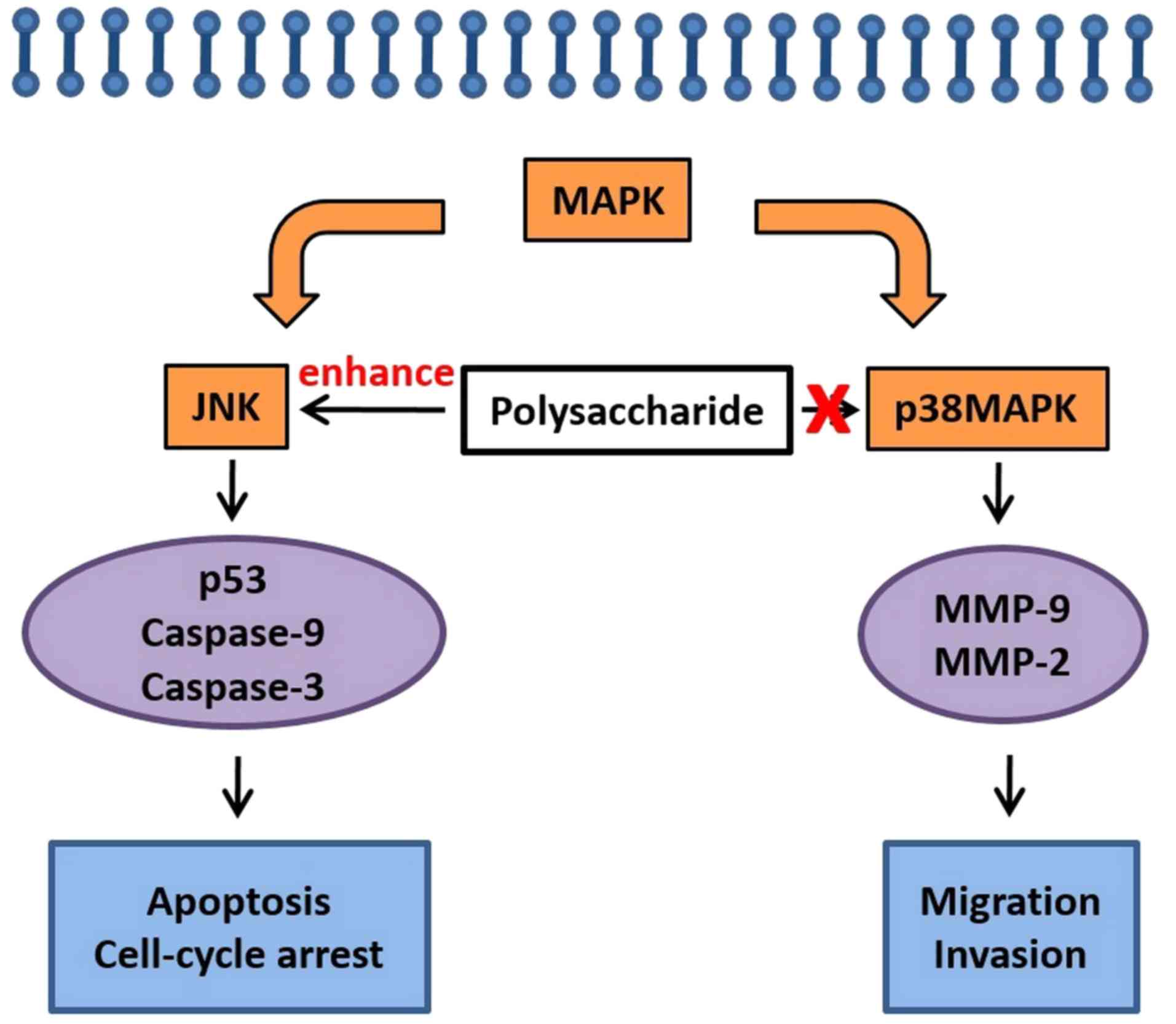
A proposed pathway summary is presented in Fig. 7. The novel polysaccharide derived
from algae extract upregulates the phosphorylation of JNK,
activates the downstream cascades of p53, caspase-9 and caspase-3,
and the leads to the inhibition of cancer cell growth, and induces
cell apoptosis and cell cycle arrest. By contrast, the
polysaccharide did not alter cancer cell migration, which is
typically mediated through p38 MAPK signaling pathway or
MMP-9/MMP-2 downstream.
The findings of the current study demonstrated that
the novel polysaccharide suppressed cancer cell proliferation,
induced cancer cell apoptosis and arrested the cell cycle via JNK
signaling, whereas cancer cell migration was not inhibited and
there was no effect on p38 MAPK signaling pathway. The application
of the polysaccharide derived from algae extract may provide a key
insight into the development of novel clinical treatment for
cancers with reduced side-effects. However, in addition to JNK and
p38 MAPK, many signaling pathways are involved in the processes of
cancer development; the deeper and more complicated mechanisms will
be examined in further investigations.
Acknowledgments
Not applicable.
Notes
[1]
Funding
No funding was received.
[2] Availability
of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
[3] Authors'
contributions
PX was a major contributor in performing experiments
and writing the manuscript. FH was an assistant for experiments. IF
provided technical assistance. JZ and MS provided the novel
polysaccharide and technical assistance. MM was the leader of this
study. All authors read and approved the final manuscript.
[4] Ethics
approval and consent to participate
Not applicable.
[5] Consent for
publication
Not applicable.
[6] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen L, Jiang Z, Ma H, Ning L, Chen H, Li
L and Qi H: Volatile oil of Acori Graminei Rhizoma-induced
apoptosis and autophagy are dependent on p53 status in human glioma
cells. Sci Rep. 6:211482016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu S, Powers S, Zhu W and Hannun YA:
Substantial contribution of extrinsic risk factors to cancer
development. Nature. 529:43–47. 2016. View Article : Google Scholar :
|
|
3
|
Brody JG, Rudel RA, Michels KB, Moysich
KB, Bernstein L, Attfield KR and Gray S: Environmental pollutants,
diet, physical activity, body size, and breast cancer: Where do we
stand in research to identify opportunities for prevention? Cancer.
109(Suppl 12): 2627–2634. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang H, Wei L, Li C, Zhou J and Li Z:
CDK5RAP1 deficiency induces cell cycle arrest and apoptosis in
human breast cancer cell line by the ROS/JNK signaling pathway.
Oncol Rep. 33:1089–1096. 2015. View Article : Google Scholar
|
|
5
|
Onofrejová L, Vasícková J, Klejdus B,
Stratil P, Misurcová L, Krácmar S, Kopecký J and Vacek J: Bioactive
phenols in algae: The application of pressurized-liquid and
solid-phase extraction techniques. J Pharm Biomed Anal. 51:464–470.
2010. View Article : Google Scholar
|
|
6
|
Abdjul DB, Yamazaki H, Kanno S, Takahashi
O, Kirikoshi R, Ukai K and Namikoshi M: Structures and biological
evaluations of agelasines isolated from the Okinawan marine sponge
Agelas nakamurai. J Nat Prod. 78:1428–1433. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xie P, Fujii I, Zhao J, Shinohara M and
Matsukura M: A novel polysaccharide compound derived from algae
extracts protects retinal pigment epithelial cells from high
glucose-induced oxidative damage in vitro. Biol Pharm Bull.
35:1447–1453. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xie P, Fujii I, Zhao J, Shinohara M and
Matsukura M: A novel polysaccharide derived from algae extract
induces apoptosis and cell cycle arrest in human gastric carcinoma
MKN45 cells via ROS/JNK signaling pathway. Int J Oncol.
49:1561–1568. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peng R, Li Z, Lin Z, Wang Y, Wang W, Hu B,
Wang X, Zhang J, Wang Y, Zhou R, et al: The HSP90 inhibitor 17-PAG
effectively inhibits the proliferation and migration of
androgen-independent prostate cancer cells. Am J Cancer Res.
5:3198–3209. 2015.PubMed/NCBI
|
|
10
|
Pal D, Sharma U, Singh SK, Kakkar N and
Prasad R: Over-expression of telomere binding factors (TRF1 &
TRF2) in renal cell carcinoma and their inhibition by using siRNA
induce apoptosis, reduce cell proliferation and migration in vitro.
PLoS One. 10:e01156512015. View Article : Google Scholar
|
|
11
|
Tian X, Yang N, Li B, Zhang J, Xu X, Yue
R, Li H, Chen L, Shen Y and Zhang W: Inhibition of HL-60 cell
growth via cell cycle arrest and apoptosis induction by a
cycloartane-labdane heterodimer from Pseudolarix amabilis. Org
Biomol Chem. 14:2618–2624. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Deng L, Yang J, Chen H, Ma B, Pan K, Su C,
Xu F and Zhang J: Knockdown of TMEM16A suppressed MAPK and
inhibited cell proliferation and migration in hepatocellular
carcinoma. Onco Targets Ther. 9:325–333. 2016.PubMed/NCBI
|
|
13
|
Uchakina ON, Ban H and McKallip RJ:
Targeting hyaluronic acid production for the treatment of leukemia:
Treatment with 4-methylumbelliferone leads to induction of
MAPK-mediated apoptosis in K562 leukemia. Leuk Res. 37:1294–1301.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Leber B, Geng F, Kale J and Andrews DW:
Drugs targeting Bcl-2 family members as an emerging strategy in
cancer. Expert Rev Mol Med. 12:e282010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang L, Shu T, Liang Y, Gu W, Wang C, Song
X, Fan C and Wang W: GDC–0152 attenuates the malignant progression
of osteosarcoma promoted by ANGPTL2 via PI3K/AKT but not p38 MAPK
signaling pathway. Int J Oncol. 46:1651–1658. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Z, Yu G, Guan H, Zhao X, Du Y and
Jiang X: Preparation and structure elucidation of alginate
oligosaccharides degraded by alginate lyase from Vibro sp. 510.
Carbohydr Res. 339:1475–1481. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang Z, Yu G, Zhao X, Liu H, Guan H,
Lawson AM and Chai W: Sequence analysis of alginate-derived
oligosaccharides by negative-ion electrospray tandem mass
spectrometry. J Am Soc Mass Spectrom. 17:621–630. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang D, Fujii I, Lin C, Ito K, Guan H,
Zhao J, Shinohara M and Matsukura M: The stimulatory activities of
polysaccharide compounds derived from algae extracts on insulin
secretion in vitro. Biol Pharm Bull. 31:921–924. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yuan Z, Feng W, Hong J, Zheng Q, Shuai J
and Ge Y: p38 MAPK and ERK promote nitric oxide production in
cultured human retinal pigmented epithelial cells induced by high
concentration glucose. Nitric Oxide. 20:9–15. 2009. View Article : Google Scholar
|
|
20
|
Pozarowski P and Darzynkiewicz Z: Analysis
of cell cycle by flow cytometry. Methods Mol Biol. 281:301–311.
2004.PubMed/NCBI
|
|
21
|
Sakaue-Sawano A, Kurokawa H, Morimura T,
Hanyu A, Hama H, Osawa H, Kashiwagi S, Fukami K, Miyata T, Miyoshi
H, et al: Visualizing spatiotemporal dynamics of multicellular
cell-cycle progression. Cell. 132:487–498. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Roccio M, Hahnewald S, Perny M and Senn P:
Cell cycle reactivation of cochlear progenitor cells in neonatal
FUCCI mice by a GSK3 small molecule inhibitor. Sci Rep.
5:178862015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Falk W, Goodwin RH Jr and Leonard EJ: A
48-well micro chemotaxis assembly for rapid and accurate
measurement of leukocyte migration. J Immunol Methods. 33:239–247.
1980. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
25
|
Guo L, Ning W, Tan Z, Gong Z and Li X:
Mechanism of matrix metalloproteinase axis-induced neointimal
growth. J Mol Cell Cardiol. 66:116–125. 2014. View Article : Google Scholar
|
|
26
|
Sasaki A, Arawaka S, Sato H and Kato T:
Sensitive western blotting for detection of endogenous
Ser129-phosphorylated α-synuclein in intracellular and
extracellular spaces. Sci Rep. 5:142112015. View Article : Google Scholar
|
|
27
|
Krieg RC, Dong Y, Schwamborn K and
Knuechel R: Protein quantification and its tolerance for different
interfering reagents using the BCA-method with regard to 2D SDS
PAGE. J Biochem Biophys Methods. 65:13–19. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lacroix M, Abi-Said D, Fourney DR,
Gokaslan ZL, Shi W, DeMonte F, Lang FF, McCutcheon IE, Hassenbusch
SJ, Holland E, et al: A multivariate analysis of 416 patients with
glioblastoma multiforme: Prognosis, extent of resection, and
survival. J Neurosurg. 95:190–198. 2001. View Article : Google Scholar
|
|
29
|
Messaoudi K, Clavreul A and Lagarce F:
Toward an effective strategy in glioblastoma treatment. Part I:
Resistance mechanisms and strategies to overcome resistance of
glioblastoma to temozolomide. Drug Discov Today. 20:899–905. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ho JW, Leung YK and Chan CP: Herbal
medicine in the treatment of cancer. Curr Med Chem Anticancer
Agents. 2:209–214. 2002. View Article : Google Scholar
|
|
31
|
Cooper WA, Kohonen-Corish MR, Zhuang L,
McCaughan B, Kennedy C, Screaton G, Sutherland RL and Lee CS: Role
and prognostic significance of tumor necrosis factor-related
apoptosis-inducing ligand death receptor DR5 in nonsmall-cell lung
cancer and precursor lesions. Cancer. 113:135–142. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gamet-Payrastre L, Li P, Lumeau S, Cassar
G, Dupont MA, Chevolleau S, Gasc N, Tulliez J and Tercé F:
Sulforaphane, a naturally occurring isothiocyanate, induces cell
cycle arrest and apoptosis in HT29 human colon cancer cells. Cancer
Res. 60:1426–1433. 2000.
|
|
33
|
Drexler HG: Review of alterations of the
cyclin-dependent kinase inhibitor INK4 family genes p15, p16, p18
and p19 in human leukemia-lymphoma cells. Leukemia. 12:845–859.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Snoek BC, de Wilt LH, Jansen G and Peters
GJ: Role of E3 ubiquitin ligases in lung cancer. World J Clin
Oncol. 4:58–69. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Terasima T and Tolmach LJ: Changes in
x-ray sensitivity of HeLa cells during the division cycle. Nature.
190:1210–1211. 1961. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Terasima T and Tolmach LJ: Growth and
nucleic acid synthesis in synchronously dividing populations of
HeLa cells. Exp Cell Res. 30:344–362. 1963. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Johnson GL, Dohlman HG and Graves LM: MAPK
kinase kinases (MKKKs) as a target class for small-molecule
inhibition to modulate signaling networks and gene expression. Curr
Opin Chem Biol. 9:325–331. 2005. View Article : Google Scholar
|
|
38
|
Tesh VL: Activation of cell stress
response pathways by Shiga toxins. Cell Microbiol. 14:1–9. 2012.
View Article : Google Scholar
|
|
39
|
An HK, Kim KS, Lee JW, Park MH, Moon HI,
Park SJ, Baik JS, Kim CH and Lee YC: Mimulone-induced autophagy
through p53-mediated AMPK/mTOR pathway increases caspase-mediated
apoptotic cell death in A549 human lung cancer cells. PLoS One.
9:e1146072014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lane DP: Cancer. p53, guardian of the
genome. Nature. 358:15–16. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang Q, Su L, Liu N, Zhang L, Xu W and
Fang H: Cyclin dependent kinase 1 inhibitors: A review of recent
progress. Curr Med Chem. 18:2025–2043. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lee K, Hart MR, Briehl MM, Mazar AP and
Tome ME: The copper chelator ATN-224 induces caspase-independent
cell death in diffuse large B cell lymphoma. Int J Oncol.
45:439–447. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Odagiri H, Kadomatsu T, Endo M, Masuda T,
Morioka MS, Fukuhara S, Miyamoto T, Kobayashi E, Miyata K, Aoi J,
et al: The secreted protein ANGPTL2 promotes metastasis of
osteosarcoma cells through integrin α5β1, p38 MAPK, and matrix
metalloproteinases. Sci Signal. 7:ra72014. View Article : Google Scholar
|
|
44
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: Regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|