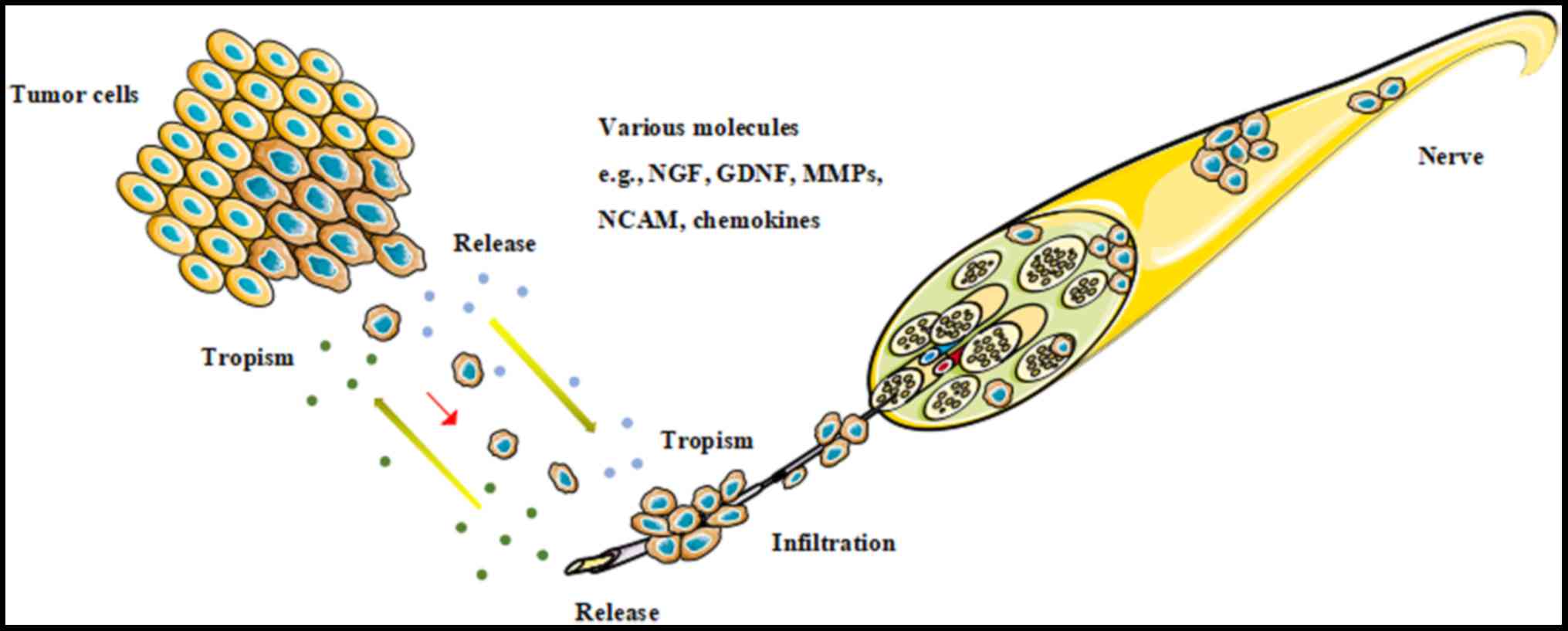
Perineural invasion (PNI) is the existence of cancer
cells along the sides of nerves and/or inside the epineural,
perineural and endoneural spaces of the neuronal sheath (1,2).
Solid tumors disseminate in four well-known ways: Direct invasion
of surrounding tissues, lymphatic spread, hematogenous spread and
seeding along body cavities, with PNI regarded as the fifth route
of cancer spread (1). PNI is
considered as a marker of poor prognosis for numerous malignant
neoplasms, including head and neck (1,3),
pancreatic (4), prostate (5), colorectal (6), gastric (7), salivary (8) and breast (9) cancer. It is closely associated with
increased post-operative locoregional recurrence and a decreased
survival rate.
PNI was first proposed more than 100 years ago, and
a clear shift in the understanding of its pathogenesis has
occurred. The traditional theory for the pathogenesis of PNI was
that tumor cells spread passively along the connective tissues that
covered the nerves or through the perforating vessels of the nerve
beams, where there was least resistance (10). However, recently, studies have
revealed that cancer cells have an innate ability to actively
migrate along nerves in a mechanism called neural tracking, which
is supported by various molecules, including nerve growth factor
(NGF), glial cell line-derived neurotrophic factor (GDNF), neural
cell adhesion molecule, matrix metalloproteinases (MMPs) and
chemokines, which are secreted by tumor cells and other non-tumor
cells in the tumor microenvironment (11). Among these molecules, the role of
chemokines and their receptors in the PNI of malignant neoplasms
have gained a high degree of attention recently (Fig. 1). The present review will evaluate
the biology and the expression of chemokines and their receptors in
cancer type associated with PNI, thoroughly discuss the underlying
molecular mechanisms of chemokines in PNI and identify novel
antitumor targets.
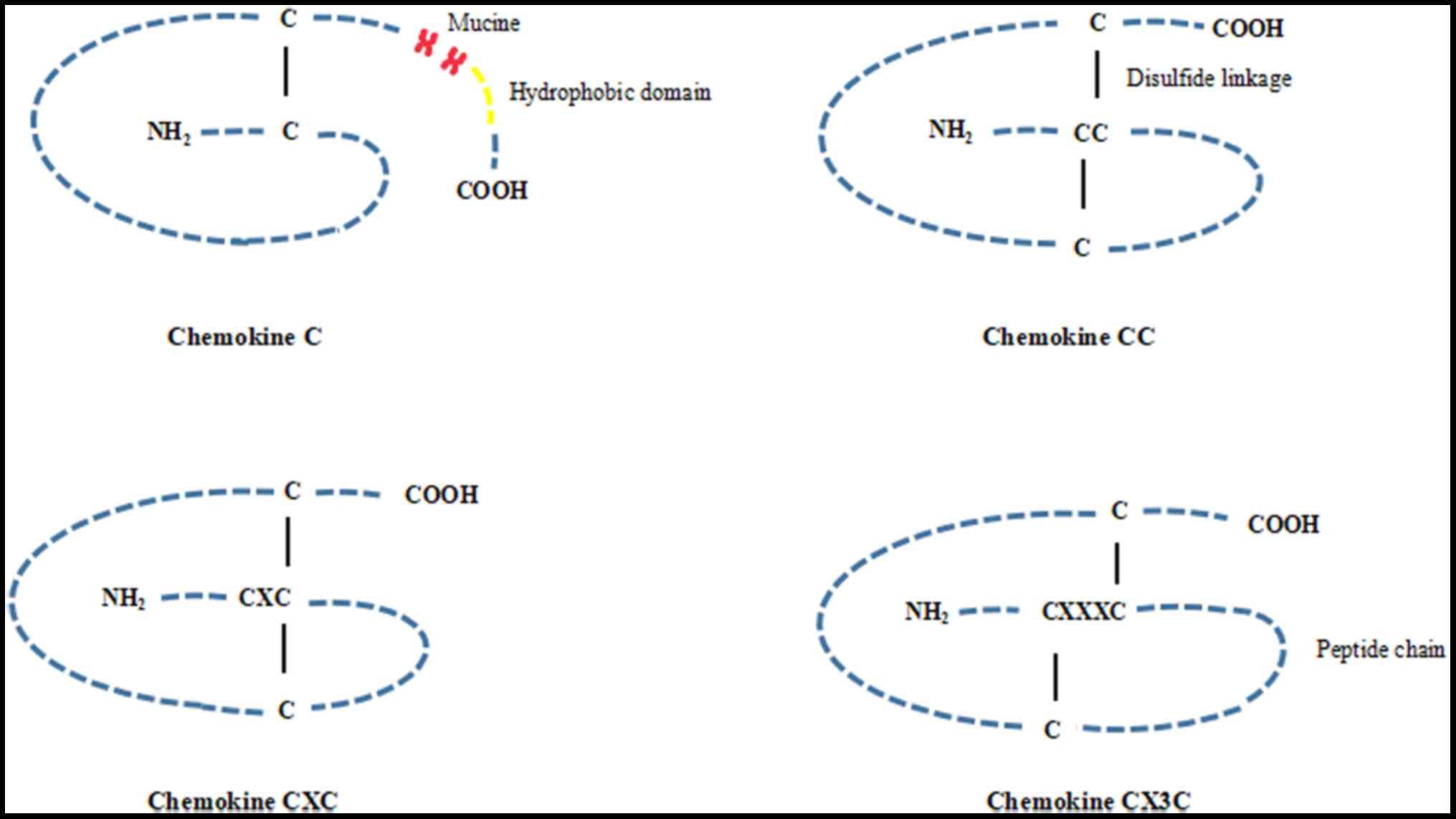
Chemokines are a group of small soluble peptides
(8–14 kDa) secreted by various cell types, including epithelial,
endothelial and immune cells, as well as certain tumor cells
(12–14). Approximately 50 chemokines have
been detected, and they are divided into four chemokine ligand
subtypes, known as the C, CC, CXC, and CX3C subtypes, respectively,
according to the number and relative spatial position of their
conserved cysteine residues in the amino-terminal region of the
peptides (Fig. 2). The majority of
chemokines are composed of the CC and CXC subfamilies, including 28
(CCL1-28) and 16 (CXCL1-16) members, respectively. The C and CX3C
group are two minor subfamilies, and only 2 members (CL1 and CL2)
and 1 member (CX3CL1), respectively, have been described (14–17).
Chemokines are pivotal components and orchestrators during the
process of immune and inflammatory reactions, acting by controlling
the adhesion and cross-endothelial movement of leukocytes,
lymphocytes and monocytes from the circulatory system to
corresponding inflammatory sites. Chemokines are also involved in
other processes, including embryonic growth and development,
homeostasis of the central nervous system, wound healing, and the
occurrence and development of tumors (18,19).
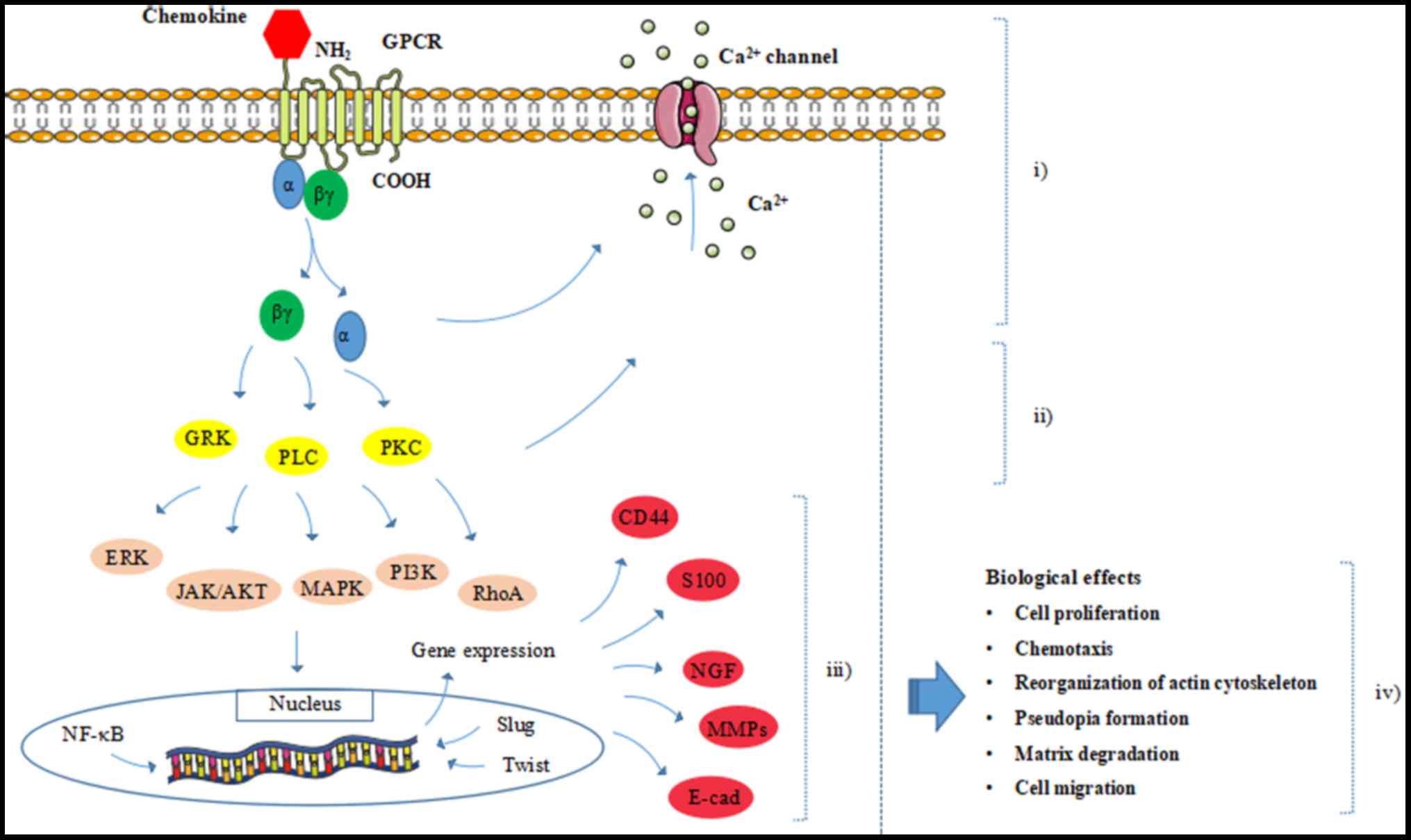
Chemokines unleash their broad biological functions
by combining with their G protein-coupled receptors (GPCRs). These
are transmembrane proteins with an extracellular N-terminal domain,
which is necessary for specific binding and activation of their
corresponding ligands, and an intracellular C-terminus, which is
essential for receptor-mediated signal transduction (15,20)
(Fig. 3). Chemokine receptors can
be divided into C receptor (CR1), CC receptor (CCR1-10), CXC
receptor (CXCR1-7) and CX3C receptor (CX3CR) according to the
subfamilies of chemokine ligands they bind to. In the immune and
inflammatory reactions, several chemokine ligands can interact with
a single receptor, and a single chemokine ligand may also activate
multiple receptors. Conversely, a homeostatic chemokine ligand has
a relatively strict specificity and a single receptor can be
activated by only one or two chemokines typically (21,22).
In general, heterotrimeric GPCR is composed of an α
subunit and a βγ heterodimer. The βγ subunit acts as a Gα inhibitor
by binding to the Gα subunit. Once a chemokine binds to and
activates its receptor, conformational changes in the transmembrane
region of GPCR will be induced, leading to the transition of the Gα
subunit from inactive guanosine diphosphate-bound to active
guanosine triphosphate (GTP)-bound and dissociation from the βγ
heterodimer (23–25). Thus, the downstream signaling
effectors, including G protein-coupled receptor kinases, ion
channels, protein kinase C and phospholipase C can be initiated.
This further brings about signaling cascades, including the
activation of Ras homolog gene family A kinase, extracellular
signal-regulated kinase (ERK), mitogen-activated protein kinase
(MAPK), phosphoinositide 3-kinase (PI3K), protein kinase B (Akt)
and Janus-activated kinase-2, which can promote gene transcription
and evoke various cellular responses, including cell proliferation,
reorganization of the actin cytoskeleton, shape change and
migration (25–28) (Fig.
3).
Studies have demonstrated that chemokines serve an
important role in the progression of tumors (29). Chemokines, as autocrine growth
factors, can accelerate tumor growth via activation of growth
factor receptors. Chemokines also promote the proliferation of
tumor cells by making the tumors insensitive to anti-growth signals
(30,31).
Chemokines can modulate tumor invasion and
metastasis by promoting epithelial mesenchymal transition (EMT),
upregulating the expression of proteases and downregulating the
expression of E-cadherin and integrin through a series of signaling
pathways, including the MAPK/ERK/PI3K/Akt signaling pathways.
Furthermore, chemokine receptors expressed by tumor cells can make
a response to their corresponding chemokine ligands and migrate
directionally towards concentration gradients of chemokine ligands
to achieve organ-specific metastases (32,33).
Another potential mechanism is that the binding of chemokine
ligands to their receptors may induce membrane wrinkling and the
formation of pseudopodia in tumor cells, which facilitates tumor
cells to adhere to and pass through the extracellular matrix (ECM)
and basal membrane to achieve invasion and metastasis (34).
Recently, chemokines have been widely investigated
in the process of PNI, particularly the CXCR4/CXCL12, CCL2/CCR2,
CCL5/CCR5, CXCL13/CXCR5 and CX3CL1/CX3CR1 signaling axes (11,35–37).
The significance and associated mechanisms of these chemokine
signaling axes in cancer with PNI will now be discussed (Table I) (Fig. 3).
The CXCL12/CXCR4 axis is one of the most widely
studied signaling pathways among the chemokine family and their
receptors. CXCR4 functions through combining with its specific
chemokine ligand, CXCL12, also known as chemokine stromal
cell-derived factor 1α (38). This
signaling pathway has been found to serve multiple functions,
including regulating the proliferation of cells, angiogenesis and
EMT (39). This pathway is also
regarded as an important candidate in support of the occurrence,
invasion and metastasis of tumors (40–42).
Furthermore, CXCR4-positive tumor cells can migrate toward distant
organs as a response to CXCL12 concentration gradients (38).
A previous study showed that CXCL12 expression in
prostate cancer was significantly stronger than that in prostate
hyperplasia. CXCR4 was mainly expressed in tumor cells, and CXCL12
was expressed highly in Schwann cells around the tumor. In
vitro, the invasiveness of tumor cells treated with CXCL12
increased significantly, and this invasiveness could be inhibited
by the CXCR4-specific inhibitor AMD3100 (34). Therefore, AMD3100 may be a
potential anti-neoplastic agent. In addition, in another
PNI-associated tumor, pancreatic cancer, CXCR4 was examined in 6
pancreatic cancer lines, CFPAC-1, Panc-1, AsPC-1, SW1990, MiaPaCa-2
and BxPc-3 cells. Weak expression of CXCL12 in pancreatic tumor
cells was detected by ELISA, although it was not detected by
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) or western blot assays. RSC96 Schwann cells and newborn
rat dorsal root ganglia (DRG) showed that the expression of CXCL12
was strong, as determined by immunofluorescence analysis and ELISA
(43). In vitro and in
vivo analyses have revealed that the CXCL12/CXCR4 signaling
axis can promote the chemotactic migration of pancreatic cancer
cells towards nerve cells, which can be inhibited by blocking the
CXCL12/CXCR4 pathway axis using CXCR4 short hairpin RNA and
AMD3100.
The aforementioned studies suggest that tumor cells
express a high level of CXCR4, that the metastasis-targeted organs
and nerves exhibit high expression of CXCL12, and that
CXCR4-positive tumor cells are attracted towards target organs
expressing CXCL12, thus promoting the PNI of the tumor. However,
given the fact that CXCL12 was also detected in prostate and
pancreatic cancer cells, an autocrine or paracrine pathway may also
participate in the process of PNI, mediated by chemokines and their
receptors (34,43).
CX3C motif chemokine ligand 1 (CX3CL1), also known
as fractalkine or neurotactin, is the only member of the CX3C
subfamily of chemokines and forms a high affinity signaling axis by
binding to its chemokine receptor CX3CR1 exclusively (53). CX3CL1 can be abundantly expressed
by activated endothelial cells and neurons, and it can exist in
either a membrane-anchored adhesion molecule or a soluble
chemoattractant form (54,55). Previous studies have shown that the
CX3CL1/CX3CR1 axis serves an important role in the nervous system
by facilitating the information exchange among neurons, glia and
microglia, promoting nerve generation and restricting tissue damage
during the inflammatory response. This occurs by promoting the
mobilization of intracellular Ca2+, chemotaxis and the
inhibition of apoptosis, induced by the Fas signaling pathway or
mediated by the activation of LPS (45,56).
In addition, in nerve-derived tumors, including glioma and
neuroblastoma, CX3CR1 is highly expressed and involved in the
adhesion, transendothelial migration and invasion of tumor cells
(57–59).
Notably, CX3CR1 has also been detected in certain
tumors of non-neural origin, including pancreatic (54,60),
breast (61) and prostate
(62) cancer, and HNSCC (63), which are widely known to invade and
metastasize along peripheral nerves frequently. In a pancreatic
cancer study, up to 90% of the surgical specimens were
CX3CR1-positive, and 56% exhibited a high intensity score according
to immunohistochemical staining (60). CX3CR1 mRNA expression was detected
in pancreatic cancer cell lines by RT-PCR, and the intrapancreatic
nerves were demonstrated to express CX3CL1, while the pancreatic
cancer cells expressed CX3CL1 only weakly (60). In vitro, CX3CL1 expression
could induce the migration of CX3CR1-positive pancreatic cancer
cells in a dose-dependent manner, thus promoting cancer cell
adhesion to nerve cells through activation of GPCRs and
redistribution of β1 integrins and focal adhesion kinase. In
vivo, pancreatic cancer cells expressing CX3CR1 were found to
infiltrate the peripheral nerves when CX3CR1-positive pancreatic
cancer cells were injected into the mice at the middle of the back,
while peripheral nerve invasion was not observed in tumors from
CX3CR1-negative cells (60).
Previous studies demonstrated that 20 out of 21
(95%) specimens with PNI exhibited positive expression of CCR2 in
the cytoplasm in 34 prostate cancer cases assessed by
immunohistochemistry. Just 3 specimens exhibited positive
expression of CCR2 in the 13 cases of prostate cancer without PNI.
The in vitro co-culture model and the in vivo mouse
model demonstrated that CCL2 released by DRG facilitated
CCR2-expressing prostate cancer migration and PNI (11,35).
Western blotting showed that p-MEK1/2 and p-Akt expression was
upregulated in prostate cancer cells. The expression of p-Akt could
be inhibited totally and the expression of p-MEK1/2 could be
decreased by anti-CCL2 antibody. Thus, CCL2 may promote the
invasion and PNI of prostate cancer by activating Akt and MAPK
pathways through CCL2/CCR2 (35).
CCR2 also exhibits high expression in breast and hepatic carcinoma,
and it can promote the motility and invasion of cancer cells by
binding to its ligand CCL2 through mothers against decapentaplegic
homolog3 (Smad3) protein and p42/44 MAPK-dependent mechanisms
(64,65). Thus, Akt, MAPK and Smad3 may be
novel targets of antitumor therapy.
CCR5, receptor of CCL5, is primarily expressed in
prostate cancer and lung adenocarcinoma, and is closely associated
with the invasion and metastasis of these tumors (66,67).
In another PNI tumor, salivary adenoid cystic carcinoma (SACC),
CCR5 and CCL5 expression was detected by flow cytometric analysis
and immunofluorescence analysis. Migration and invasion assays
showed that the CCL5/CCR5 axis can promote the invasion of nerves
in the SACC-83 cell lines by Ca2+ elevation and the
rearrangement of the actin cytoskeleton (36).
In addition, CXCL13 and CXCR5 expression presents
with a significant positive correlation with the occurrence of PNI,
particularly in prostate (68) and
colorectal (69) cancer, where
CXCR5 is overexpressed. CXCL13, the stroma-derived ligand of CXCR5,
can stimulate the expression of CXCR5 in prostate and colorectal
tumor cells, leading to the phosphorylation of ERK and AKT, and the
upregulation of MMPs, thus promoting the PNI of tumor cells
(68–71).
MMPs, a group of zinc-dependent endopeptidases that
can degrade numerous types of components of the ECM, are believed
to promote the invasion and metastasis of tumors (67). Previous studies showed that certain
components secreted by peripheral nerves, including NGF and GDNF,
or the activation of COX-2 and prostaglandin E2 induced by the
combination of tumor necrosis factor-α (TNF-α) and its receptor,
can promote tumor cells to secrete MMPs and then facilitate tumor
cells to invade nerve tissues in a number of tumors, including
cholangiocarcinoma (CCA) and pancreatic cancer (72–74).
Additionally, MMP-2 and MMP-9 can be upregulated by extracellular
MMP inducer (EMMPRIN, CD147), a transmembrane glycoprotein
belonging to the immunoglobulin superfamily, thus achieving PNI in
SACC (75). Blocking of EMMPRIN by
its antibody or silencing of EMMPRIN expression by RNA interference
could effectively inhibit the proliferation and PNI activity of
SACC cells, and reduce the secretion of MMP-2 and MMP-9 in SACC-83
cells (76,77).
Importantly, recent studies have shown that MMPs can
also respond to chemokines and their corresponding chemokine
receptors to promote PNI in certain tumors. The expression and
activity of MMP-2 and MMP-9 in human glioblastoma can be markedly
upregulated by the CCL12/CXCR4 axis, mediated through activation of
the ERK1/2 and Akt signaling pathway (49). In the progression of breast and
pancreatic cancer, the autocrine CCL12-CXCR4 signaling pathway can
promote PNI by increasing the secretion of and promoting the
activation of MMP-2 and MMP-9 (43,45).
The CXCL12/CXCR4 and CXCL13/CXCR5 signaling axes have been
demonstrated to induce prostate cancer cells to secrete MMP-2 and
MMP-9, which degrades the matrix around the tumor and the nerve
tissue, promoting PNI (34,68)
(Fig. 3).
NGF was the first member of the neurotrophic factor
family to be identified, and was widely expressed in tumor tissues,
and involved in tumorigenesis and tumor growth (78). An increasing number of studies have
shown that NGF and its receptor tropomyosin receptor kinase A
(TrkA) are overexpressed, and that the combination of NGF and TrkA
promotes cancer cell growth, increases invasiveness and metastasis,
and eventually causes nerve invasion in a number of human cancer
types, including CCA, and pancreatic and prostate cancer (79–81).
It has been suggested that chemokines can increase
NGF expression, which further accelerates the process of PNI by
receptor binding (34,43). The mechanism of the combination of
NGF and TrkA promoting invasive behaviors acts to increase the
synthesis and release of MMPs via the activation of the p44/42 MAPK
signaling pathway (82,83). The combination of NGF and TrkA can
facilitate nerve cellular axon growth in the direction of the
tumors by supplying chemical tropisms (79,84).
The overexpression of NGF and TrkA also contributes to the PNI of
SACC (85). Additionally, NGF may
upregulate the expression of S100 and reduce the expression of
E-cadherin, leading to decreased adhesion between cancer cells, an
increased ability for metastasis and ultimately, nerve tissue
invasion in SACC (86,87) (Fig.
3).
NF-κB belongs to the transcription factors family,
and the heterodimeric complex of the p65 and p50 subunits is the
predominant form of NF-κB; it is physically confined to the
cytoplasm of normal cells and remains inactive through interaction
with the NF-κB inhibitory protein (88). Following stimulation by various
reagents (e.g., cytokines, viruses, growth factors and DNA damaging
agents), a series of signaling events promote the p65/p50
heterodimer to dissociate from the NF-κB inhibitory protein and
translocate to the nucleus. This causes the activation of the
expression of various genes involved in the prevention of apoptosis
(89), and the promotion of the
invasion and metastasis of cancer cells (90,91).
Slug (Snail2), a member of the Snail family of
zinc-finger transcription factors, is known to serve pivotal roles
in the process of cell migration, ranging from the formation of a
number of tissues during embryonic development to the acquisition
of invasive and metastatic properties in epithelial tumors
(96,97). It was reported that the expression
of Slug was associated with PNI in different tumors, including
human breast cancer (98),
pancreatic cancer (99) and SACC
(100). Slug mediates the PNI of
these tumors mainly by promoting EMT in epithelial cells (100,101). He et al (102) found that Slug promoted PNI and
the metastatic capacity of SACC via the ERK/MAPK signaling
pathways, and MAPK-knockdown reduced the expression of Slug in SACC
cells (103).
Studies demonstrated that CCL18 could trigger the
invasiveness and metastasis of oral squamous cell carcinoma, and
that Slug-knockdown could reverse CCL18-induced EMT (104). The CCL21/CCR7 pathway led to the
occurrence of the EMT process by activating the Slug pathway, and
it promoted migration and invasion in human chondrosarcoma and lung
cancer (105,106). CCL18 promotes the invasion and
metastasis of gastric cancer cells by increasing the expression of
Slug and promoting EMT (107).
The CXCL12/CXCR4 signaling axis has also been shown to be closely
correlated with progression and the metastatic characteristics of
CCA through Slug-induced EMT (108). These results indicated that
Slug-induced EMT is involved in chemokine signaling (Fig. 3); however, whether chemokine
signaling axes may promote tumor PNI via Slug-induced EMT remains
unclear.
Twist, a transcription factor, belongs to the family
of basic helix-loop-helix proteins and can bind to E-box regions in
the promoters of certain genes to activate or inhibit transcription
(109,110). Upregulation of Twist was found in
certain human tumors, including breast cancer (111), prostate cancer (112) and gastric cancer (113). Twist mainly functions by
promoting EMT in malignant tumors (111,114).
Recently, attention has been focused on the
association between chemokine signaling axes and Twist in malignant
tumors. Chen et al (115)
reported that the CCL2/CCR2 axis could enhance EMT by upregulating
the expression of Twist in non-small cell lung cancer.
Low-Marchelli et al (116)
found that CCL2 recruited macrophages to promote tumor progression
by activating Twist signaling. Studies of Yang et al and Xu
et al showed that CXCL12/CXCR4 may upregulate Twist through
ERK and PI3K/AKT signaling, leading to the progression of EMT and
the invasion and metastasis of tumor cells in human glioblastoma
(111,117). Koo et al (118) found that CXCL11 could promote the
invasive capacity of epithelial ovarian cancer via the enhancement
of Twist expression. In addition, the CCR7 pathway upregulated
Twist expression via ERK and PI3K/AKT signaling to manage EMT in
pancreatic ductal adenocarcinoma (119). All the aforementioned studies
showed that chemokines may be involved in tumor PNI through
Twist-induced EMT; however, the concrete mechanisms between Twist
and PNI require further investigation (Fig. 3).
The expression and roles of chemokines and their
receptors in malignant neoplasms can also be regulated by various
microenvironmental factors, including chronic inflammation,
hypoxia, hepatocyte growth factor (HGF) and vascular endothelial
growth factor (VEGF) (44,48). Biphasic effects exist between
chronic inflammation and chemokines. Franciszkiewicz et al
(120) reported that chronic
inflammation, which was partly driven by the chemokine signaling
axis, was closely associated with the development of
gastrointestinal malignancies (120,121). By contrast, chemokine expression
can also be induced by inflammation. For example, CX3CL1 expression
was significantly driven by inflammation in vitro, and
pro-inflammatory TNF-α and interferon-γ could induce a higher
expression level of CX3CL1 mRNA (122).
Hypoxia can influence the occurrence and development
of tumors through the induction of chemokines and their receptors
(123,124). For example, hypoxic conditions
within solid tumors may induce CXCL12 and CXCR4 expression via
hypoxia inducible factor-1 (HIF-1) and VEGF, contributing to the
adhesion, migration and PNI of tumor cells (123). In glioblastoma and breast
carcinoma, necrotic foci and the pseudopalisading regions are
hypoxic and are the main distribution sites of HIF-1 and VEGF,
where overexpression of CXCR4 exists (44,125). Similarly, under hypoxia, the
metastasis of breast cancer was promoted by increasing the
expression of CCR5 and its ligand CCL5 at the metastasis site via
HIF-1 (126). Furthermore,
hypoxia induces increased CCR7 expression in lung cancer cells,
which further promotes the invasion of tumor cells and lymph node
metastasis (127). In prostate
cancer and pancreatic ductal adenocarcinoma, cancer invasion and
metastasis is closely associated with the CX3CR1 expression of
tumor cells, which is induced by hypoxia, and the ligand CX3CL1 is
mainly expressed at the site of metastasis (128,129).
HGF, a multifunctional cytokine secreted by
fibroblasts and other stromal cells in tumors, exerts multiple
functions in tumors, including proliferation, invasion and
metastasis (130–133). HGF can upregulate the expression
of CXCR4 protein in a number of tumors, including breast carcinoma
and glioma, and HGF pre-treatment increases cancer cell migration
toward CXCL12 via NF-κB (44,48).
Hence, HIF-1, HGF and VEGF are considered potential
targets of anticancer therapies. In fact, some of these inhibitors
are in clinical trials as anticancer therapy drugs. For example,
farnesyltransferase inhibitors, HGF binding peptide-1 and
bevacizumab are treated as tumor therapeutic agents targeting
HIF-1, HGF and VEGF, respectively (48,134–136).
PNI occurs in a number of human malignant neoplasms
and is closely associated with postoperative relapse and a reduced
survival rate. Accumulating evidence has indicated that chemokines
and their corresponding receptors serve pivotal roles in the
process of PNI. Although they are discussed in detail in this
review, the precise mechanisms of chemokines in PNI deserve further
investigation. With the development of molecular biology and
advancements in proteomics technology, an increasing number of
pivotal molecular markers associated with chemokines will be found
in cancer with PNI. This should be conducive to improving our
understanding of the mechanism of PNI and highlight novel
chemokine-targeted drugs in the clinic to decrease relapses caused
by the PNI of tumors.
Not applicable.
This study was supported by the National Program on
Key Research Project of China (grant no. 2016YFC0902700), the
National Natural Science Foundation of China (grant nos. 81772891,
81372891, 81672672 and 81572650) and the State Key Laboratory of
Oral Diseases Special Funded Projects (2016).
Not applicable.
MZ and ZZ wrote and edited the manuscript. XG and JW
provided scientific revision of the manuscript. YT and XL prepared
and reviewed the manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Liebig C, Ayala G, Wilks JA, Berger DH and
Albo D: Perineural invasion in cancer: A review of the literature.
Cancer. 115:3379–3391. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lesnik DJ and Boey HP: Perineural invasion
of the facial nerve by a cutaneous squamous cell cancer: A case
report. Ear Nose Throat J 83. 824:826–827. 2004.
|
|
3
|
Gupta A, Veness M, De'Ambrosis B, Selva D
and Huilgol SC: Management of squamous cell and basal cell
carcinomas of the head and neck with perineural invasion. Australas
J Dermatol. 57:3–13. 2016. View Article : Google Scholar
|
|
4
|
Pour PM, Bell RH and Batra SK: Neural
invasion in the staging of pancreatic cancer. Pancreas. 26:322–325.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Feng FY, Qian Y, Stenmark MH, Halverson S,
Blas K, Vance S, Sandler HM and Hamstra DA: Perineural invasion
predicts increased recurrence, metastasis, and death from prostate
cancer following treatment with dose-escalated radiation therapy.
Int J Radiat Oncol Biol Phys. 81:e361–e367. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liebig C, Ayala G, Wilks J, Verstovsek G,
Liu H, Agarwal N, Berger DH and Albo D: Perineural invasion is an
independent predictor of outcome in colorectal cancer. J Clin
Oncol. 27:5131–5137. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Deng J, You Q, Gao Y, Yu Q, Zhao P, Zheng
Y, Fang W, Xu N and Teng L: Prognostic value of perineural invasion
in gastric cancer: A systematic review and meta-analysis. PLoS One.
9:e889072014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zheng SC, Zhang YR, Luo SY and Zhang LP:
The effect of GDNF on matrix-degrading and cell-adhesion during
perineural invasion of salivary adenoid cystic carcinoma. Shanghai
Kou Qiang Yi Xue. 25:212–216. 2016.In Chinese. PubMed/NCBI
|
|
9
|
Figueira RC, Gomes LR, Neto JS, Silva FC,
Silva ID and Sogayar MC: Correlation between MMPs and their
inhibitors in breast cancer tumor tissue specimens and in cell
lines with different metastatic potential. BMC Cancer. 9:202009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Batsakis JG: Nerves and neurotropic
carcinomas. Ann Otol Rhinol Laryngol. 94:426–427. 1985.PubMed/NCBI
|
|
11
|
Amit M, Na'ara S and Gil Z: Mechanisms of
cancer dissemination along nerves. Nat Rev Cancer. 16:399–408.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Abbadie C: Chemokines, chemokine receptors
and pain. Trends Immunol. 26:529–534. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sommer C and Kress M: Recent findings on
how proinflammatory cytokines cause pain: Peripheral mechanisms in
inflammatory and neuropathic hyperalgesia. Neurosci Lett.
361:184–187. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Charo IF and Ransohoff RM: The many roles
of chemokines and chemokine receptors in inflammation. N Engl J
Med. 354:610–621. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Griffith JW, Sokol CL and Luster AD:
Chemokines and chemokine receptors: Positioning cells for host
defense and immunity. Annu Rev Immunol. 32:659–702. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Szekanecz Z, Vegvari A, Szabo Z and Koch
AE: Chemokines and chemokine receptors in arthritis. Front Biosci
(Schol Ed). 2:153–167. 2010. View
Article : Google Scholar
|
|
17
|
Gao YJ and Ji RR: Chemokines,
neuronal-glial interactions, and central processing of neuropathic
pain. Pharmacol Ther. 126:56–68. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rossi D and Zlotnik A: The biology of
chemokines and their receptors. Annu Rev Immunol. 18:217–242. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bonecchi R, Galliera E, Borroni EM, Corsi
MM, Locati M and Mantovani A: Chemokines and chemokine receptors:
An overview. Front Biosci (Landmark Ed). 14:540–551. 2009.
View Article : Google Scholar
|
|
20
|
Bryan SA, Jose PJ, Topping JR, Wilhelm R,
Soderberg C, Kertesz D, Barnes PJ, Williams TJ, Hansel TT and
Sabroe I: Responses of leukocytes to chemokines in whole blood and
their antagonism by novel CC-chemokine receptor 3 antagonists. Am J
Respir Crit Care Med. 165:1602–1609. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Old EA and Malcangio M: Chemokine mediated
neuron-glia communication and aberrant signalling in neuropathic
pain states. Curr Opin Pharmacol. 12:67–73. 2012. View Article : Google Scholar
|
|
22
|
Zlotnik A and Yoshie O: The chemokine
superfamily revisited. Immunity. 36:705–716. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lefkowitz RJ: Seven transmembrane
receptors: A brief personal retrospective. Biochim Biophys Acta.
1768:748–755. 2007. View Article : Google Scholar
|
|
24
|
Hamm HE: The many faces of G protein
signaling. J Biol Chem. 273:669–672. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Violin JD and Lefkowitz RJ:
Beta-arrestin-biased ligands at seven-transmembrane receptors.
Trends Pharmacol Sci. 28:416–422. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Curnock AP, Logan MK and Ward SG:
Chemokine signalling: Pivoting around multiple phosphoinositide
3-kinases. Immunology. 105:125–136. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
DeWire SM, Ahn S, Lefkowitz RJ and Shenoy
SK: Beta-arrestins and cell signaling. Annu Rev Physiol.
69:483–510. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Logothetis DE, Kurachi Y, Galper J, Neer
EJ and Clapham DE: The beta gamma subunits of GTP-binding proteins
activate the muscarinic K+ channel in heart. Nature.
325:321–326. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wilson J and Balkwill F: The role of
cytokines in the epithelial cancer microenvironment. Semin Cancer
Biol. 12:113–120. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Brew R, Erikson JS, West DC, Flanagan BF
and Christmas SE: Interleukin-8 as a growth factor for human
colorectal carcinoma cells in vitro. Biochem Soc Trans.
25:S2641997. View Article : Google Scholar
|
|
31
|
Di Cesare S, Marshall JC, Logan P, Antecka
E, Faingold D, Maloney SC and Burnier MN Jr: Expression and
migratory analysis of 5 human uveal melanoma cell lines for CXCL12,
CXCL8, CXCL1, and HGF. J Carcinog. 6:22007.PubMed/NCBI
|
|
32
|
Liotta LA: An attractive force in
metastasis. Nature. 410:24–25. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Panda S, Padhiary SK and Routray S:
Chemokines accentuating protumoral activities in oral cancer
microenvironment possess an imperious stratagem for therapeutic
resolutions. Oral Oncol. 60:8–17. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang S, Qi L, Li M, Zhang D, Xu S, Wang N
and Sun B: Chemokine CXCL12 and its receptor CXCR4 expression are
associated with perineural invasion of prostate cancer. J Exp Clin
Cancer Res. 27:622008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
He S, He S, Chen CH, Deborde S, Bakst RL,
Chernichenko N, McNamara WF, Lee SY, Barajas F, Yu Z, et al: The
chemokine (CCL2-CCR2) signaling axis mediates perineural invasion.
Mol Cancer Res. 13:380–390. 2015. View Article : Google Scholar :
|
|
36
|
Shen Z, Li T, Chen D, Jia S, Yang X, Liang
L, Chai J, Cheng X, Yang X and Sun M: The CCL5/CCR5 axis
contributes to the perineural invasion of human salivary adenoid
cystic carcinoma. Oncol Rep. 31:800–806. 2014. View Article : Google Scholar
|
|
37
|
Marchesi F, Piemonti L, Mantovani A and
Allavena P: Molecular mechanisms of perineural invasion, a
forgotten pathway of dissemination and metastasis. Cytokine Growth
Factor Rev. 21:77–82. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Müller A, Homey B, Soto H, Ge N, Catron D,
Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, et al:
Involvement of chemokine receptors in breast cancer metastasis.
Nature. 410:50–56. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dubový P, Klusáková I, Svízenská I and
Brázda V: Spatio-temporal changes of SDF1 and its CXCR4 receptor in
the dorsal root ganglia following unilateral sciatic nerve injury
as a model of neuropathic pain. Histochem Cell Biol. 133:323–337.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hart CA, Brown M, Bagley S, Sharrard M and
Clarke NW: Invasive characteristics of human prostatic epithelial
cells: Understanding the metastatic process. Br J Cancer.
92:503–512. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Schimanski CC, Bahre R, Gockel I, Müller
A, Frerichs K, Hörner V, Teufel A, Simiantonaki N, Biesterfeld S,
Wehler T, et al: Dissemination of hepatocellular carcinoma is
mediated via chemokine receptor CXCR4. Br J Cancer. 95:210–217.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kollmar O, Rupertus K, Scheuer C, Junker
B, Tilton B, Schilling MK and Menger MD: Stromal cell-derived
factor-1 promotes cell migration and tumor growth of colorectal
metastasis. Neoplasia. 9:862–870. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Xu Q, Wang Z, Chen X, Duan W, Lei J, Zong
L, Li X, Sheng L, Ma J, Han L, et al: Stromal-derived
factor-1α/CXCL12-CXCR4 chemotactic pathway promotes perineural
invasion in pancreatic cancer. Oncotarget. 6:4717–4732.
2015.PubMed/NCBI
|
|
44
|
Kang H, Mansel RE and Jiang WG: Genetic
manipulation of stromal cell-derived factor-1 attests the pivotal
role of the autocrine SDF-1-CXCR4 pathway in the aggressiveness of
breast cancer cells. Int J Oncol. 26:1429–1434. 2005.PubMed/NCBI
|
|
45
|
Matteucci E, Locati M and Desiderio MA:
Hepatocyte growth factor enhances CXCR4 expression favoring breast
cancer cell invasiveness. Exp Cell Res. 310:176–185. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Vaday GG, Hua SB, Peehl DM, Pauling MH,
Lin YH, Zhu L, Lawrence DM, Foda HD and Zucker S: CXCR4 and CXCL12
(SDF-1) in prostate cancer: inhibitory effects of human single
chain Fv antibodies. Clin Cancer Res. 10:5630–5639. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Libura J, Drukala J, Majka M, Tomescu O,
Navenot JM, Kucia M, Marquez L, Peiper SC, Barr FG,
Janowska-Wieczorek A, et al: CXCR4-SDF-1 signaling is active in
rhabdomyosarcoma cells and regulates locomotion, chemotaxis, and
adhesion. Blood. 100:2597–2606. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Esencay M, Newcomb EW and Zagzag D: HGF
upregulates CXCR4 expression in gliomas via NF-kappaB: Implications
for glioma cell migration. J Neurooncol. 99:33–40. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wu M, Chen Q, Li D, Li X, Li X, Huang C,
Tang Y, Zhou Y, Wang D, Tang K, et al: LRRC4 inhibits human
glioblastoma cells proliferation, invasion, and proMMP-2 activation
by reducing SDF-1 alpha/CXCR4-mediated ERK1/2 and Akt signaling
pathways. J Cell Biochem. 103:245–255. 2008. View Article : Google Scholar
|
|
50
|
Roh J, Muelleman T, Tawfik O and Thomas
SM: Perineural growth in head and neck squamous cell carcinoma: A
review. Oral Oncol. 51:16–23. 2015. View Article : Google Scholar
|
|
51
|
Zhang J, Sarkar S and Yong VW: The
chemokine stromal cell derived factor-1 (CXCL12) promotes glioma
invasiveness through MT2-matrix metalloproteinase. Carcinogenesis.
26:2069–2077. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhu Y, Yang P, Zhang X, Zhang L, Cui G,
Wang Q, Lv L, Zhang Y, Xin X, Yan T, et al: The effect and
mechanism of CXCR4 silencing on metastasis suppression of human
glioma U87 cell line. Anat Rec (Hoboken). 296:1857–1864. 2013.
View Article : Google Scholar
|
|
53
|
Marchesi F, Locatelli M, Solinas G, Erreni
M, Allavena P and Mantovani A: Role of CX3CR1/CX3CL1 axis in
primary and secondary involvement of the nervous system by cancer.
J Neuroimmunol. 224:39–44. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Bazan JF, Bacon KB, Hardiman G, Wang W,
Soo K, Rossi D, Greaves DR, Zlotnik A and Schall TJ: A new class of
membrane-bound chemokine with a CX3C motif. Nature. 385:640–644.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Pan Y, Lloyd C, Zhou H, Dolich S, Deeds J,
Gonzalo JA, Vath J, Gosselin M, Ma J, Dussault B, et al:
Neurotactin, a membrane-anchored chemokine upregulated in brain
inflammation. Nature. 387:611–617. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Verge GM, Milligan ED, Maier SF, Watkins
LR, Naeve GS and Foster AC: Fractalkine (CX3CL1) and fractalkine
receptor (CX3CR1) distribution in spinal cord and dorsal root
ganglia under basal and neuropathic pain conditions. Eur J
Neurosci. 20:1150–1160. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Balkwill FR: Tumour necrosis factor and
cancer. Prog Growth Factor Res. 4:121–137. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zeng Y, Jiang J, Huebener N, Wenkel J,
Gaedicke G, Xiang R and Lode HN: Fractalkine gene therapy for
neuroblastoma is more effective in combination with targeted IL-2.
Cancer Lett. 228:187–193. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Locatelli M, Boiocchi L, Ferrero S,
Martinelli Boneschi F, Zavanone M, Pesce S, Allavena P, Maria Gaini
S, Bello L and Mantovani A: Human glioma tumors express high levels
of the chemokine receptor CX3CR1. Eur Cytokine Netw. 21:27–33.
2010.PubMed/NCBI
|
|
60
|
Marchesi F, Piemonti L, Fedele G, Destro
A, Roncalli M, Albarello L, Doglioni C, Anselmo A, Doni A, Bianchi
P, et al: The chemokine receptor CX3CR1 is involved in the neural
tropism and malignant behavior of pancreatic ductal adenocarcinoma.
Cancer Res. 68:9060–9069. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Andre F, Cabioglu N, Assi H, Sabourin JC,
Delaloge S, Sahin A, Broglio K, Spano JP, Combadiere C, Bucana C,
et al: Expression of chemokine receptors predicts the site of
metastatic relapse in patients with axillary node positive primary
breast cancer. Ann Oncol. 17:945–951. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Shulby SA, Dolloff NG, Stearns ME, Meucci
O and Fatatis A: CX3CR1-fractalkine expression regulates cellular
mechanisms involved in adhesion, migration, and survival of human
prostate cancer cells. Cancer Res. 64:4693–4698. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Muller A, Sonkoly E, Eulert C, Gerber PA,
Kubitza R, Schirlau K, Franken-Kunkel P, Poremba C, Snyderman C,
Klotz LO, et al: Chemokine receptors in head and neck cancer:
Association with metastatic spread and regulation during
chemotherapy. Int J Cancer. 118:2147–2157. 2006. View Article : Google Scholar
|
|
64
|
Fang WB, Jokar I, Zou A, Lambert D,
Dendukuri P and Cheng N: CCL2/CCR2 chemokine signaling coordinates
survival and motility of breast cancer cells through Smad3 protein-
and p42/44 mitogen-activated protein kinase (MAPK)-dependent
mechanisms. J Biol Chem. 287:36593–36608. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Dagouassat M, Suffee N, Hlawaty H, Haddad
O, Charni F, Laguillier C, Vassy R, Martin L, Schischmanoff PO,
Gattegno L, et al: Monocyte chemoattractant protein-1 (MCP-1)/CCL2
secreted by hepatic myofibroblasts promotes migration and invasion
of human hepatoma cells. Int J Cancer. 126:1095–1108. 2010.
|
|
66
|
Vaday GG, Peehl DM, Kadam PA and Lawrence
DM: Expression of CCL5 (RANTES) and CCR5 in prostate cancer.
Prostate. 66:124–134. 2006. View Article : Google Scholar
|
|
67
|
Borczuk AC, Papanikolaou N, Toonkel RL,
Sole M, Gorenstein LA, Ginsburg ME, Sonett JR, Friedman RA and
Powell CA: Lung adenocarcinoma invasion in TGFbetaRII-deficient
cells is mediated by CCL5/RANTES. Oncogene. 27:557–564. 2008.
View Article : Google Scholar
|
|
68
|
Singh S, Singh R, Singh UP, Rai SN,
Novakovic KR, Chung LW, Didier PJ, Grizzle WE and Lillard JW Jr:
Clinical and biological significance of CXCR5 expressed by prostate
cancer specimens and cell lines. Int J Cancer. 125:2288–2295. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Qi XW, Xia SH, Yin Y, Jin LF, Pu Y, Hua D
and Wu HR: Expression features of CXCR5 and its ligand, CXCL13
associated with poor prognosis of advanced colorectal cancer. Eur
Rev Med Pharmacol Sci. 18:1916–1924. 2014.PubMed/NCBI
|
|
70
|
El-Haibi CP, Singh R, Sharma PK, Singh S
and Lillard JW Jr: CXCL13 mediates prostate cancer cell
proliferation through JNK signalling and invasion through ERK
activation. Cell Prolif. 44:311–319. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Zhu Z, Zhang X, Guo H, Fu L, Pan G and Sun
Y: CXCL13-CXCR5 axis promotes the growth and invasion of colon
cancer cells via PI3K/AKT pathway. Mol Cell Biochem. 400:287–295.
2015. View Article : Google Scholar
|
|
72
|
Kim HJ, Kim JS, Kang CD, Lee SJ, Kim JY,
Yeon JE, Park JJ, Shim JJ, Byun KS, Bak YT, et al: Expression of
epidermal growth factor receptor, ErbB2 and matrix
metalloproteinase-9 in hepatolithiasis and cholangiocarcinoma.
Korean J Gastroenterol. 45:52–59. 2005.In Korean. PubMed/NCBI
|
|
73
|
Duan L, Hu XQ, Feng DY, Lei SY and Hu GH:
GPC-1 may serve as a predictor of perineural invasion and a
prognosticator of survival in pancreatic cancer. Asian J Surg.
36:7–12. 2013. View Article : Google Scholar
|
|
74
|
Itatsu K, Sasaki M, Yamaguchi J, Ohira S,
Ishikawa A, Ikeda H, Sato Y, Harada K, Zen Y, Sato H, et al:
Cyclooxygenase-2 is involved in the up-regulation of matrix
metalloproteinase-9 in cholangiocarcinoma induced by tumor necrosis
factor-alpha. Am J Pathol. 174:829–841. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Yang X, Dai J, Li T, Zhang P, Ma Q, Li Y,
Zhou J and Lei D: Expression of EMMPRIN in adenoid cystic carcinoma
of salivary glands: Correlation with tumor progression and
patients' prognosis. Oral Oncol. 46:755–760. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Yang X, Zhang P, Ma Q, Kong L, Li Y, Liu B
and Lei D: EMMPRIN contributes to the in vitro invasion of human
salivary adenoid cystic carcinoma cells. Oncol Rep. 27:1123–1127.
2012. View Article : Google Scholar
|
|
77
|
Yang X, Zhang P, Ma Q, Kong L, Li Y, Liu B
and Lei D: EMMPRIN silencing inhibits proliferation and perineural
invasion of human salivary adenoid cystic carcinoma cells in vitro
and in vivo. Cancer Biol Ther. 13:85–91. 2012. View Article : Google Scholar
|
|
78
|
Anton ES, Weskamp G, Reichardt LF and
Matthew WD: Nerve growth factor and its low-affinity receptor
promote Schwann cell migration. Proc Natl Acad Sci USA.
91:2795–2799. 1994. View Article : Google Scholar
|
|
79
|
Zhu Z, Kleeff J, Kayed H, Wang L, Korc M,
Büchler MW and Friess H: Nerve growth factor and enhancement of
proliferation, invasion, and tumorigenicity of pancreatic cancer
cells. Mol Carcinog. 35:138–147. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Zhu Z, Friess H, diMola FF, Zimmermann A,
Graber HU, Korc M and Büchler MW: Nerve growth factor expression
correlates with perineural invasion and pain in human pancreatic
cancer. J Clin Oncol. 17:2419–2428. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
DeSchryver-Kecskemeti K, Balogh K and Neet
KE: Nerve growth factor and the concept of neural-epithelial
interactions. Immunohistochemical observations in two cases of
vasitis nodosa and six cases of prostatic adenocarcinoma. Arch
Pathol Lab Med. 111:833–835. 1987.PubMed/NCBI
|
|
82
|
Okada Y, Eibl G, Duffy JP, Reber HA and
Hines OJ: Glial cell-derived neurotrophic factor upregulates the
expression and activation of matrix metalloproteinase-9 in human
pancreatic cancer. Surgery. 134:293–299. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Okada Y, Eibl G, Guha S, Duffy JP, Reber
HA and Hines OJ: Nerve growth factor stimulates MMP-2 expression
and activity and increases invasion by human pancreatic cancer
cells. Clin Exp Metastasis. 21:285–292. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Moscatelli I, Pierantozzi E, Camaioni A,
Siracusa G and Campagnolo L: p75 neurotrophin receptor is involved
in proliferation of undifferentiated mouse embryonic stem cells.
Exp Cell Res. 315:3220–3232. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Wang L, Sun M, Jiang Y, Yang L, Lei D, Lu
C, Zhao Y, Zhang P, Yang Y and Li J: Nerve growth factor and
tyrosine kinase A in human salivary adenoid cystic carcinoma:
expression patterns and effects on in vitro invasive behavior. J
Oral Maxillofac Surg. 64:636–641. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Taylor S, Herrington S, Prime W, Rudland
PS and Barraclough R: S100A4 (p9Ka) protein in colon carcinoma and
liver metastases: Association with carcinoma cells and
T-lymphocytes. Br J Cancer. 86:409–416. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Jiang WG: E-cadherin and its associated
protein catenins, cancer invasion and metastasis. Br J Surg.
83:437–446. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Schmidt KN, Amstad P, Cerutti P and
Baeuerle PA: Identification of hydrogen peroxide as the relevant
messenger in the activation pathway of transcription factor
NF-kappaB. Adv Exp Med Biol. 387:63–68. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Wang CY, Mayo MW and Baldwin AS Jr: TNF-
and cancer therapy-induced apoptosis: Potentiation by inhibition of
NF-kappaB. Science. 274:784–787. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Huang S, Pettaway CA, Uehara H, Bucana CD
and Fidler IJ: Blockade of NF-kappaB activity in human prostate
cancer cells is associated with suppression of angiogenesis,
invasion, and metastasis. Oncogene. 20:4188–4197. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Huang S, DeGuzman A, Bucana CD and Fidler
IJ: Nuclear factor-kappaB activity correlates with growth,
angiogenesis, and metastasis of human melanoma cells in nude mice.
Clin Cancer Res. 6:2573–2581. 2000.PubMed/NCBI
|
|
92
|
Sun X, Cheng G, Hao M, Zheng J, Zhou X,
Zhang J, Taichman RS, Pienta KJ and Wang J: CXCL12 / CXCR4 / CXCR7
chemokine axis and cancer progression. Cancer Metastasis Rev.
29:709–722. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Zheng Y, Miu Y, Yang X, Yang X and Zhu M:
CCR7 Mediates TGF-β1-induced human malignant glioma invasion,
migration, and epithelial-mesenchymal transition by activating
MMP2/9 through the nuclear factor kappaB signaling pathway. DNA
Cell Biol. 36:853–861. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Zhong W, Tong Y, Li Y, Yuan J, Hu S, Hu T
and Song G: Mesenchymal stem cells in inflammatory microenvironment
potently promote metastatic growth of cholangiocarcinoma via
activating Akt/NF-κB signaling by paracrine CCL5. Oncotarget.
8:73693–73704. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Wang H, Cai J, Du S, Guo Z, Xin B, Wang J,
Wei W and Shen X: Fractalkine/CX3CR1 induces apoptosis resistance
and proliferation through the activation of the AKT/NF-κB cascade
in pancreatic cancer cells. Cell Biochem Funct. 35:315–326. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Anwar TE and Kleer CG: Tissue-based
identification of stem cells and epithelial-to-mesenchymal
transition in breast cancer. Hum Pathol. 44:1457–1464. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Olmeda D, Montes A, Moreno-Bueno G, Flores
JM, Portillo F and Cano A: Snai1 and Snai2 collaborate on tumor
growth and metastasis properties of mouse skin carcinoma cell
lines. Oncogene. 27:4690–4701. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Carpenter RL, Paw I, Dewhirst MW and Lo
HW: Akt phosphorylates and activates HSF-1 independent of heat
shock, leading to Slug overexpression and epithelial-mesenchymal
transition (EMT) of HER2-overexpressing breast cancer cells.
Oncogene. 34:546–557. 2015. View Article : Google Scholar
|
|
99
|
Hotz B, Arndt M, Dullat S, Bhargava S,
Buhr HJ and Hotz HG: Epithelial to mesenchymal transition:
Expression of the regulators snail, slug, and twist in pancreatic
cancer. Clin Cancer Res. 13:4769–4776. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
He Q, Zhou X, Li S, Jin Y, Chen Z, Chen D,
Cai Y, Liu Z, Zhao T and Wang A: MicroRNA-181a suppresses salivary
adenoid cystic carcinoma metastasis by targeting MAPK-Snai2
pathway. Biochim Biophys Acta. 1830:5258–5266. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Chang B, Yang H, Jiao Y, Wang K, Liu Z, Wu
P, Li S and Wang A: SOD2 deregulation enhances migration, invasion
and has poor prognosis in salivary adenoid cystic carcinoma. Sci
Rep. 6:259182016. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Wang H, Liang X, Li M, Tao X, Tai S, Fan
Z, Wang Z, Cheng B and Xia J: Chemokine (CC motif) ligand 18
upregulates Slug expression to promote stem-cell like features by
activating the mammalian target of rapamycin pathway in oral
squamous cell carcinoma. Cancer Sci. 108:1584–1593. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Zhong G, Chen L, Yin R, Qu Y, Bao Y, Xiao
Q, Zhang Z, Shen Y, Li C, Xu Y, et al: Chemokine (C-C motif) ligand
21/C-C chemokine receptor type 7 triggers migration and invasion of
human lung cancer cells by epithelial-mesenchymal transition via
the extracellular signal-regulated kinase signaling pathway. Mol
Med Rep. 15:4100–4108. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Li G, Yang Y, Xu S, Ma L, He M and Zhang
Z: Slug signaling is up-regulated by CCL21/CCR7 [corrected] to
induce EMT in human chondrosarcoma. Med Oncol. 32:4782015.
|
|
107
|
Hou X, Zhang Y and Qiao H: CCL18 promotes
the invasion and migration of gastric cancer cells via ERK1/2/NF-κB
signaling pathway. Tumour Biol. 37:641–651. 2016. View Article : Google Scholar
|
|
108
|
Zhao S, Wang J and Qin C: Blockade of
CXCL12/CXCR4 signaling inhibits intrahepatic cholangiocarcinoma
progression and metastasis via inactivation of canonical Wnt
pathway. J Exp Clin Cancer Res. 33:1032014. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Murre C, McCaw PS, Vaessin H, Caudy M, Jan
LY, Jan YN, Cabrera CV, Buskin JN, Hauschka SD, Lassar AB, et al:
Interactions between heterologous helix-loop-helix proteins
generate complexes that bind specifically to a common DNA sequence.
Cell. 58:537–544. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Ip YT, Park RE, Kosman D, Yazdanbakhsh K
and Levine M: dorsal-twist interactions establish snail expression
in the presumptive mesoderm of the Drosophila embryo. Genes Dev.
6:1518–1530. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Yang J, Mani SA, Donaher JL, Ramaswamy S,
Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A and
Weinberg RA: Twist, a master regulator of morphogenesis, plays an
essential role in tumor metastasis. Cell. 117:927–939. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Kwok WK, Ling MT, Lee TW, Lau TC, Zhou C,
Zhang X, Chua CW, Chan KW, Chan FL, Glackin C, et al: Up-regulation
of TWIST in prostate cancer and its implication as a therapeutic
target. Cancer Res. 65:5153–5162. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Rosivatz E, Becker I, Specht K, Fricke E,
Luber B, Busch R, Höfler H and Becker KF: Differential expression
of the epithelial-mesenchymal transition regulators snail, SIP1,
and twist in gastric cancer. Am J Pathol. 161:1881–1891. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Wang D, Rai B, Qi F, Liu T, Wang J, Wang X
and Ma B: Influence of the Twist gene on the invasion and
metastasis of colon cancer. Oncol Rep. 39:31–44. 2018.
|
|
115
|
Chen W, Gao Q, Han S, Pan F and Fan W: The
CCL2/CCR2 axis enhances IL-6-induced epithelial-mesenchymal
transition by cooperatively activating STAT3-Twist signaling.
Tumour Biol. 36:973–981. 2015. View Article : Google Scholar
|
|
116
|
Low-Marchelli JM, Ardi VC, Vizcarra EA,
van Rooijen N, Quigley JP and Yang J: Twist1 induces CCL2 and
recruits macrophages to promote angiogenesis. Cancer Res.
73:662–671. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Xu C, Liu Y, Xiao L, Guo C, Deng S, Zheng
S and Zeng E: The involvement of anterior gradient 2 in the stromal
cell-derived factor 1-induced epithelial-mesenchymal transition of
glioblastoma. Tumour Biol. 37:6091–6097. 2016. View Article : Google Scholar
|
|
118
|
Koo YJ, Kim TJ, Min KJ, So KA, Jung US and
Hong JH: CXCL11 mediates TWIST1-induced angiogenesis in epithelial
ovarian cancer. Tumour Biol. 39:10104283177062262017. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Li K, Xu B, Xu G and Liu R: CCR7 regulates
Twist to induce the epithelial-mesenchymal transition in pancreatic
ductal adenocarcinoma. Tumour Biol. 37:419–424. 2016. View Article : Google Scholar
|
|
120
|
Franciszkiewicz K, Boissonnas A, Boutet M,
Combadière C and Mami-Chouaib F: Role of chemokines and chemokine
receptors in shaping the effector phase of the antitumor immune
response. Cancer Res. 72:6325–6332. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Wang D, Dubois RN and Richmond A: The role
of chemokines in intestinal inflammation and cancer. Curr Opin
Pharmacol. 9:688–696. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Celesti G, Di Caro G, Bianchi P, Grizzi F,
Marchesi F, Basso G, Rahal D, Delconte G, Catalano M, Cappello P,
et al: Early expression of the fractalkine receptor CX3CR1 in
pancreatic carcinogenesis. Br J Cancer. 109:2424–2433. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Schioppa T, Uranchimeg B, Saccani A,
Biswas SK, Doni A, Rapisarda A, Bernasconi S, Saccani S, Nebuloni
M, Vago L, et al: Regulation of the chemokine receptor CXCR4 by
hypoxia. J Exp Med. 198:1391–1402. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Ceradini DJ, Kulkarni AR, Callaghan MJ,
Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP
and Gurtner GC: Progenitor cell trafficking is regulated by hypoxic
gradients through HIF-1 induction of SDF-1. Nat Med. 10:858–864.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
125
|
Rong Y, Durden DL, Van Meir EG and Brat
DJ: 'Pseudopalisading' necrosis in glioblastoma: A familiar
morphologic feature that links vascular pathology, hypoxia, and
angiogenesis. J Neuropathol Exp Neurol. 65:529–539. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Lin S, Wan S, Sun L, Hu J, Fang D, Zhao R,
Yuan S and Zhang L: Chemokine C-C motif receptor 5 and C-C motif
ligand 5 promote cancer cell migration under hypoxia. Cancer Sci.
103:904–912. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Li Y, Qiu X, Zhang S, Zhang Q and Wang E:
Hypoxia induced CCR7 expression via HIF-1alpha and HIF-2alpha
correlates with migration and invasion in lung cancer cells. Cancer
Biol Ther. 8:322–330. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Zhao T, Gao S, Wang X, Liu J, Duan Y, Yuan
Z, Sheng J, Li S, Wang F, Yu M, et al: Hypoxia-inducible factor-1α
regulates chemotactic migration of pancreatic ductal adenocarcinoma
cells through directly transactivating the CX3CR1 gene. PLoS One.
7:e433992012. View Article : Google Scholar
|
|
129
|
Xiao LJ, Chen YY, Lin P, Zou HF, Lin F,
Zhao LN, Li D, Guo L, Tang JB, Zheng XL, et al: Hypoxia increases
CX3CR1 expression via HIF-1 and NF-κB in androgen-independent
prostate cancer cells. Int J Oncol. 41:1827–1836. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Trusolino L, Cavassa S, Angelini P, Andó
M, Bertotti A, Comoglio PM and Boccaccio C: HGF/scatter factor
selectively promotes cell invasion by increasing integrin avidity.
FASEB J. 14:1629–1640. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Matteucci E, Modora S, Simone M and
Desiderio MA: Hepatocyte growth factor induces apoptosis through
the extrinsic pathway in hepatoma cells: Favouring role of
hypoxia-inducible factor-1 deficiency. Oncogene. 22:4062–4073.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Zhang YW, Su Y, Volpert OV and Vande Woude
GF: Hepatocyte growth factor/scatter factor mediates angiogenesis
through positive VEGF and negative thrombospondin 1 regulation.
Proc Natl Acad Sci USA. 100:12718–12723. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Tacchini L, De Ponti C, Matteucci E,
Follis R and Desiderio MA: Hepatocyte growth factor-activated
NF-kappaB regulates HIF-1 activity and ODC expression, implicated
in survival, differently in different carcinoma cell lines.
Carcinogenesis. 25:2089–2100. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Semenza GL: Targeting HIF-1 for cancer
therapy. Nat Rev Cancer. 3:721–732. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Niu G and Chen X: Vascular endothelial
growth factor as an anti-angiogenic target for cancer therapy. Curr
Drug Targets. 11:1000–1017. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Owusu BY, Galemmo R, Janetka J and
Klampfer L: Hepatocyte growth factor, a key tumor-promoting factor
in the tumor microenvironment. Cancers (Basel). 9:92017. View Article : Google Scholar
|