Introduction
Colorectal cancer (CRC) is the second most commonly
diagnosed cancer in females and the third in males, with an
estimated 1.4 million new cases and 693,900 associated mortalities
occurring globally in 2012 (1). In
China, CRC is the fifth leading cause of cancer-associated
mortality (2). In developing
countries, it is predicted that between the years 2015 and 2030,
the incidence of CRC will increase by as much as 60% (3). Patients diagnosed at early stages
have a better prognosis, with a survival rate of 5 years. However,
numerous patients are often diagnosed at an advanced stage,
therefore have low response and high recurrence rates (4). Chemotherapy is a common therapeutic
option for advanced CRC, but has limited effectiveness, with a poor
prognosis and cancer relapse (5).
Therefore, there is a requirement to identify novel prognostic
biomarkers and key target molecules for CRC treatment.
Cancerous inhibitor of protein phosphatase 2A
(CIP2A), originally known as KIAA1524 or P90, has been cloned from
patients with hepatocarcinoma (6).
CIP2A functions as an oncoprotein that promotes the growth,
transformation, drug resistance and maintenance of a malignant
cellular phenotype of various cancer types, including CRC, head and
neck cancer, oral squamous cell carcinoma, esophageal squamous cell
carcinoma, breast cancer, gastric cancer, tongue cancer, prostate
cancer lung cancer, cervical cancer and leukemia (6–10).
CIP2A is a cellular inhibitor of protein phosphatase 2A (PP2A)
(6). PP2A serves as a key tumor
suppressor that regulates signaling pathways and has a high
relevance in human cancer (11).
As a tumor suppressor, PP2A serves a critical role in the
regulation of survival, differentiation and cell-cycle progression
by negatively regulating the phosphoinositide 3-kinase/protein
kinase B (Akt) pathway and inactivating extracellular
signal-regulated kinase/dual specificity mitogen-activated protein
kinase kinase 1 family kinases (10). Therefore, CIP2A is considered as a
prognostic indicator, and targeting CIP2A/PP2A/Akt may be
attractive for cancer therapy.
Ethoxysanguinarine (Eth) is a benzophenanthridine
alkaloid natural product that is mainly found in Macleaya
cordata (Willd) R. Br. (also known as Bo Luo Hui) of the
Papaveraceae family (12). In
China, M. cordata is used as an herbal medicine and has been
widely applied for a long time due to its wide bioactivities, e.g.,
antimicrobial, antifungal, antiinflammatory, antioxidant and
antitumor activities (10,13–15).
M. cordata has also been used for the treatment of insect
stings or bites and tinea infection in China, Europe and North
America (10). Eth is converted by
sanguinarine upon crystallization with ammoniated ethanol during
the isolation process (16). Eth
is reported to exhibit antiviral activity by downregulation of the
porcine reproductive and respiratory syndrome virus-induced
cytopathic effect. However, the effect of Eth on human tumor cells
remains unclear. To the best of our knowledge, only one study has
investigated the effect of Eth on human tumor cells. In 2013, Liu
et al (12) revealed that
Eth can induce inhibitory effects and downregulate CIP2A in human
lung cancer cells. The effect and mechanism of Eth in other cancer
types requires investigation. The present study aimed to
investigate the antitumor activities and the possible mechanisms of
Eth against CRC.
Materials and methods
Patients
In total, 6 pairs of tumor and adjacent normal colon
tissues were collected from patients (mean age, 63 years; age
range, 48–76 years; sex ratio, 3:3) who were treated between July
2016 and July 2017 at the Department of Clinical Oncology, Taihe
Hospital, Hubei University of Medicine (Shiyan, Hubei, China) and
stored in liquid nitrogen until further use. Samples were ground in
liquid nitrogen and suspended in lysis buffer [50 mM Tris-HCl (pH
7.4), 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1%
SDS, 1 mM PMSF and complete protease inhibitor cocktail) and
cleared by centrifugation (14,000 × g at 4°C for 15 min). Proteins
extracted from the tissues were used for western blotting. The
tissue specimens were collected and used with the approval of the
Institutional Review Board of Hubei University of Medicine (Shiyan,
Hubei, China) and Taihe Hospital affiliated to Hubei University of
Medicine (Shiyan, Hubei, China). Written informed consent was
obtained from all patients.
Quantitative polymerase chain reaction
(qPCR)
The expression level of the CIP2A gene was
examined by qPCR. GAPDH was used as an endogenous control for each
sample. Total RNA from SW620 or HT29 cells or patients' tissues
were extracted using TRIzol reagent (Invitrogen; Thermo Fisher
Scientifc, Inc., Waltham, MA, USA) according to the manufacturer's
protocols. Total RNA (2 μg) and the ReverTra Ace qPCR RT kit
(Toyobo Life Science, Osaka, Japan) was used for qPCR analysis of
CIP2A. Reverse transcription occurred at 37°C for 15 min and
98°C for 5 min, with storage at −20°C. RNA (2 μg), 4
μl 5X RT Buffer, 1 μl RT Enzyme mix, 1 μl
Primer mix and Nuclease-free Water were mixed to a 20-μl
total volume. The primers used in this study were as follows:
CIP2A forward, 5′-TGCGGCACTTGGAGGTAATTTC-3′ and reverse,
5′-AGCTCTACAAGGCAACTCAAGC-3′; and GAPDH forward,
5′-TGTTGCCATCAATGACCCCTT-3′ and reverse, 5′-CTCCACGACGTACTCAGCG-3′.
qPCR was performed using an ABI StepOnePlus™ Real-Time PCR system
(Applied Biosystems; Thermo Fisher Scientific, Inc.) with the Power
SYBR®-Green PCR Master mix (Toyobo Life Science).
SYBR-Green PCR Master Mix (10 μl), forward and reverse
primers (200 nM), cDNA template (100 ng) and ddH2O were
mixed to a 20-μl total volume. PCR conditions consisted of
the following: 95°C for 3 min, 95°C for 15 sec and 60°C for 1 min,
for 40 cycles. The threshold cycle for each sample was selected
from the linear range and converted to a starting quantity by
interpolation from a standard curve generated on the same plate for
each set of primers. The CIP2A mRNA levels were evaluated
using the 2−ΔΔCq method, standardized to levels of GAPDH
amplification (17). Each test was
performed in triplicate.
Western blot analysis
SW620 or HT29 cells were harvested and lysed with
RIPA buffer containing 50 mM Tris, 150 mM NaCl, 0.1% SDS, 0.5%
deoxycholate, 1% NP-40, 1 mM DTT, 1 mM NaF, 1 mM sodium vanadate, 1
mM PMSF (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and a 1%
protease inhibitor cocktail (Merck KGaA). Lysates from cells or the
patients' tissues were normalized for total protein (25 μg)
and loaded on 8–12% SDS-PAGE gels. Subsequently, the gels were
electrophoretically transferred to polyvinylidene difluoride
membranes (EMD Millipore, Billerica, MA, USA). Subsequent to being
blocked with 5% skimmed milk at room temperature for 1 h, the
membranes were incubated at 4°C with primary antibodies overnight
and washed three times with Tris-buffered saline containing 0.5%
Tween-20. The primary antibodies used were anti-CIP2A (1:500
dilution; cat. no. sc-80662), anti-phospho-Akt (S473) (1:500
dilution; catalog no. sc-7985), anti-Akt (1:500 dilution; cat. no.
sc-8312) (all from Santa Cruz Biotechnology, Inc., Dallas, TX,
USA), anti-caspase-3 (1:1,000 dilution; cat. no. 9662), anti- poly
ADP ribose polymerase (PARP; 1:1,000 dilution; cat. no. 9542),
anti-PP2A (1:1,000 dilution; cat. no. 2038) (all from Cell
Signaling Technology, Inc., Danvers, MA, USA) and anti-GAPDH
(1:5,000 dilution; cat. no. M20006; Abmart Co., Ltd., Shanghai,
China). Subsequently the membranes were incubated with horseradish
peroxidase-conjugated secondary antibody [1:10,000 dilution;
catalog nos. E030120-01 (rabbit) and E030110-01 (mouse); EarthOx,
LLC, San Francisco, CA, USA] at room temperature for 1.5 h.
Detection was performed using a SuperSignal® West Pico
Trial kit (cat. no. QA210131; Pierce; Thermo Fisher Scientific,
Inc.) (18). The defined sections
of the film were scanned for image capture and quantification using
Adobe Photoshop CS4 software (Adobe Systems Inc., San Jose, CA,
USA) and ImageJ software (National Institutes of Health, Bethesda,
MD, USA).
Reagents
Eth with a purity of up to 98%, obtained from
Shanghai Yuanye Bio-Technology Co., Ltd. (Shanghai, China), was
dissolved in dimethyl sulfoxide (Sigma-Aldrich; Thermo Fisher
Scientific, Inc.) at a stock solution of 50 mM and stored at
−20°C.
Cell culture
A total of 4 CRC cell lines, SW620, SW480, HT29 and
HCT116, were procured from the American Type Culture Collection
(Manassas, VA, USA). SW620 and SW480 cells were grown in
Leibovitz's-15 (L-15) medium (Gibco; Thermo Fisher Scientific,
Inc.) containing 10% fetal bovine serum (FBS; HyClone; GE
Healthcare Life Sciences, Logan, UT, USA) and antibiotics. All
cells were then incubated in a humidified atmosphere without
CO2 at 37°C. HT29 and HCT116 cells were grown in
Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc.) containing with 10% FBS and antibiotics, and
incubated at 37°C in an incubator containing 5% CO2.
Cytotoxicity assay and cell
viability
An MTT assay (MTT dissolved in phosphate-buffered
saline) was used to calculate cell cytotoxicity. A total of
5×105 cells were seeded into a 96-well plate and
pre-cultured with L-15 or DMEM for 24 h, then treated with Eth
(0.5–12 μM) for 24 h. The absorbance was measured at 490 nm
(A490) with an enzyme immunoassay analyzer (BioTek
Instruments, Inc., Winooski, VT, USA), and the inhibition rate was
calculated as followed: Inhibition rate (%) = (average
A490 of the control group-average A490 of the
experimental group)/(average A490 of the control
group-average A490 of the blank group) × 100. Cell
viability was estimated by trypan blue dye exclusion as described
previously (19,20).
Soft-agar colony formation assay
This assay was performed in 6-well plates containing
0.6% agarose and 10% FBS. A total of 1×103 cells were
seeded in 1 ml L-15 or DMEM containing 10% FBS with 0.3%
low-melting-point agarose (Amresco, LLC, Solon, OH, USA), and
layered onto the base. After 2 weeks, the plates were stained with
0.2% gentian violet at room temperature for 20 min and the number
of colonies was counted under a light microscope (IX70; Olympus
Corporation, Tokyo, Japan) (18).
Apoptosis determination by DAPI
staining
Approximately 5×105 cells/well in a
12-well plate were treated with Eth (0–3 μM for SW620 cells;
0–5 μM for HT29 cells) for 24 h. The cells in each treatment
and control were then stained with DAPI at room temperature for 8
min. Representative images were captured using fluorescence
microscopy, as previously described (21).
Cycloheximide (CHX) treatment
SW620 (or HT29) cells were treated with 50
μg/ml CHX in the absence or presence of 2 μM (or 4.5
μM) Eth for 0, 6, 8 and 12 h, and cell lysates were
harvested for western blot assays.
Flow cytometric assays for Annexin V
(AV)
Cell apoptosis was evaluated using an AV-FITC kit
(BD Biosciences, San Jose, CA, USA). According to the
manufacturer's instructions, the samples were subjected to flow
cytometry to assess the apoptotic cells, as described previously
(22).
Transfection of small interfering RNA
(siRNA)
Two sequences of CIP2A siRNA were designed and then
synthesized by Shanghai GenePharma Co., Ltd., (Shanghai, China),
referred to as siRNA1 and siRNA2. The sequences of the CIP2A siRNA
were as follows: CIP2A siRNA1, 5′-CUGUGGUUGUGUUUGCACUTT-3′;
CIP2AsiRNA2, 5′-ACCAUUGAUAUC CUUAGAATT-3′; and negative control
siRNA, 5′-UUCUCCGAACGUGUCACGUTT-3′. CRC cells were transfected with
100 nM siRNA using Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocols. At 48 h post-transfection, the cells were used for
western blotting and cell viability analysis.
Transfection of DNA
The pOTENT-1-CIP2A expression plasmid was purchased
from Youbio Co. (Changcha, China). The pOTENT-1-CIP2A plasmid (1
μg/μl) was transfected into CRC cells using
Lipofectamine® 3000 transfection reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) following the manufacturer's
protocols.
PP2A activity assay
PP2A phosphatase activity was tested using a PP2A
immunoprecipitation phosphatase assay kit (Upstate Biotechnology,
Inc., Lake Placid, NY, USA). According to the manufacturer's
instructions, 100 μg protein isolated from the cells and 4
μg anti-PP2A monoclonal antibody (1:100 dilution; cat. no.
2038; Cell Signaling Technology, Inc.) were incubated together at
4°C overnight. Protein A agarose beads (40 μl) were added to
the mixture and incubated at 4°C for 2 h, and then the beads were
collected and washed three times with 700 μl ice-cold TBS
and once with 500 μl Ser/Thr Assay Buffer (Upstate
Biotechnology, Inc.). The beads were further incubated with 750 mM
phosphopeptide in assay buffer at 30°C for 10 min with continuous
agitation. Malachite Green Phosphate Detection Solution (100
μl) was added and the absorbance at 650 nm was measured, as
described previously (23).
Human CRC xenograft experiments
Equal numbers of female and male (n=20), 5-week-old,
nude immunodeficient mice (nu/nu) (weighing ~16 g) were purchased
from Hunan SJA Laboratory Animal Co., Ltd. (Changsha, China), and
maintained and monitored in a specific pathogen-free environment
(temperature, 22–24°C; barrier environment; 12 h dark/12 h light
cycle; sterile water and full nutritive feed). All animal studies
were conducted according to protocols approved by the Hubei
University of Medicine Animal Care and Use Committee, complying
with the rules of Regulations for the Administration of Affairs
Concerning Experimental Animals (approved by the State Council of
China). The mice were injected subcutaneously with SW620 cells
(5×106) suspended in 100 μl L-15 medium into the
right flank of each mouse. Treatments were started when the tumors
reached a palpable size. Mice were randomly divided into two groups
and treated with Eth (0.5 mg/kg; n=8), or vehicle control (n=8) for
4 weeks. Caliper measurements of the longest perpendicular tumor
diameters were performed twice a week to estimate the tumor volume,
using the following formula: 4π/3 × (width/2)2 ×
(length/2), representing the 3-dimensional volume of an ellipse.
Animals were sacrificed when tumors reached 1.5 cm in diameter. At
the time of death, tumors were excised for western blotting.
Statistical analysis
All statistical analyses were conducted using
GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA) and
SPSS 22.0 software for Windows (IBM Corporation Armonk, NY, USA).
Results from three independent experiments were presented as the
mean ± standard deviation unless otherwise noted. Statistically
significant values were compared using Student's t-test of unpaired
data or one-way analysis of variance and Bonferroni's post hoc
test. P<0.05 was used to indicate a statistically significant
difference.
Results
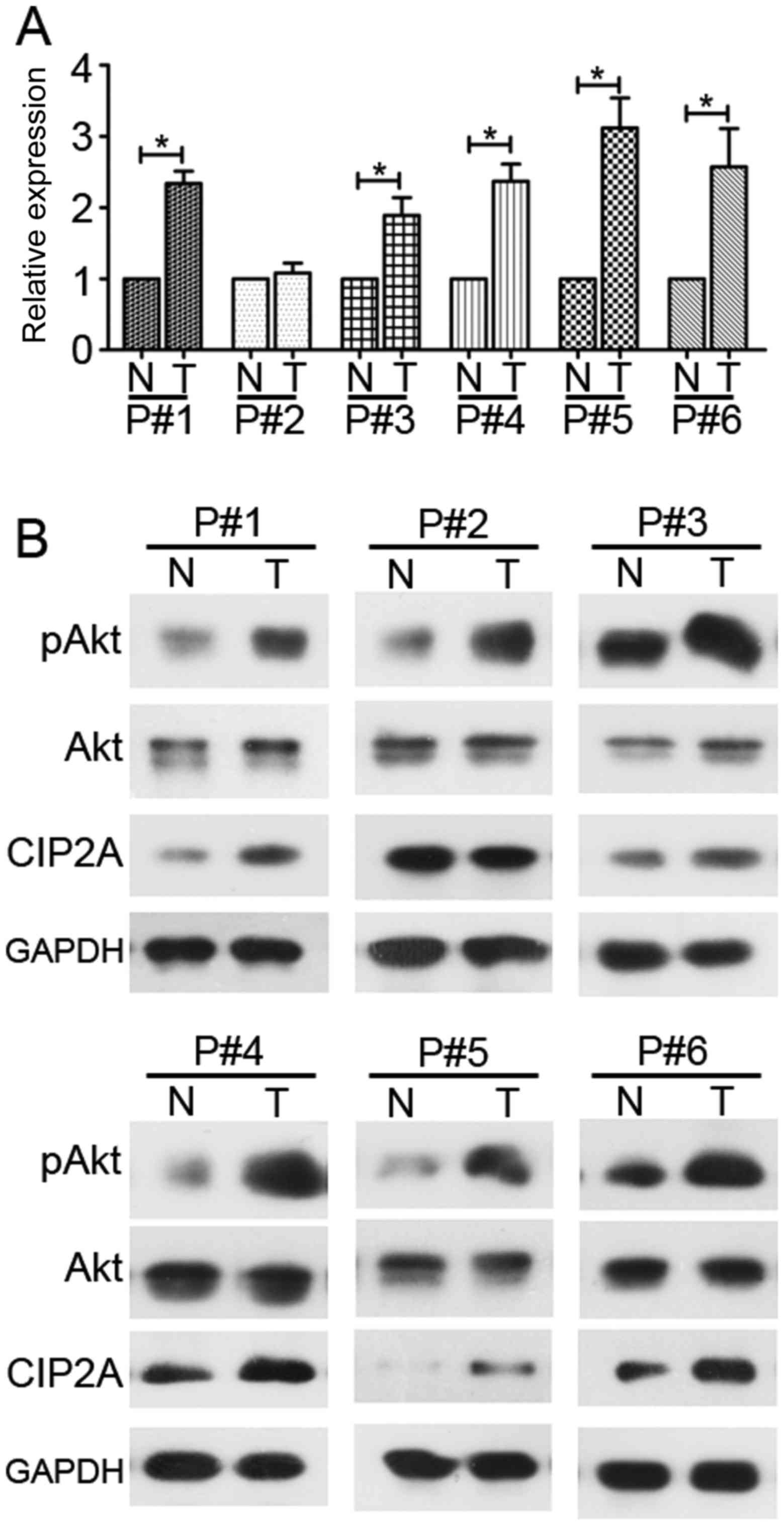
High expression of CIP2A and high
phosphorylation of Akt in human CRC tissues
qPCR and western blot analyses were used to detect
the mRNA and protein levels of CIP2A and its downstream molecule
Akt in the human CRC tissues of 6 patients. CIP2A exhibited higher
expression in all tumor tissues, with the exception of case no. 2,
compared with the patient-matched adjacent normal colonic tissues
at the mRNA and protein levels (Fig.
1). Similarly, Akt phosphorylation was elevated in 6 cases
(Fig. 1B).
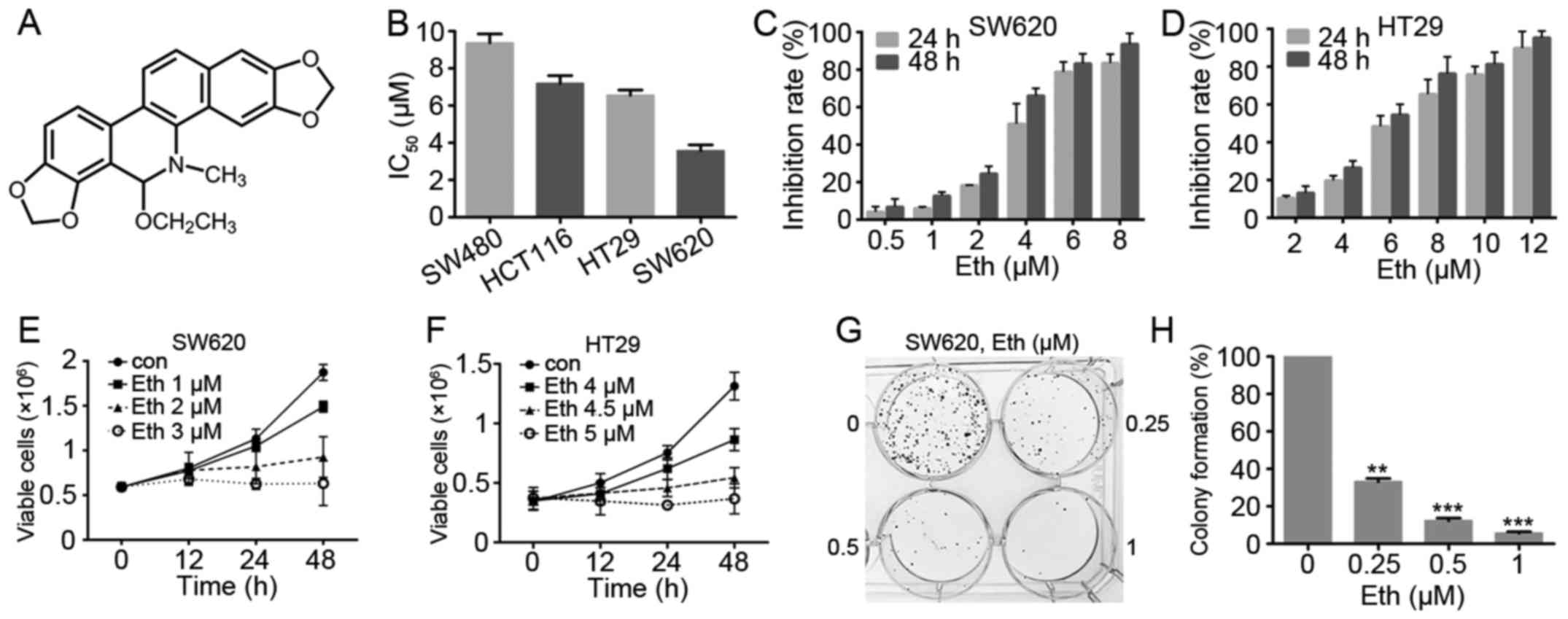
Eth inhibits the viability of CRC
cells
The effect of Eth (Fig.
2A) on cell viability was investigated with the four CRC cell
lines, SW620, SW480, HT29 and HCT116. An MTT assay showed that Eth
exhibited moderate cytotoxicity to these cell lines, with an half
maximal inhibitory concentration of 3.57–9.37 μM (Fig. 2B; Table I). As shown in Fig. 2C and D, Eth was effective in
inhibiting the growth of the SW620 and HT29 CRC cells. Trypan blue
exclusion assay showed that Eth rapidly reduced the number of
viable SW620 (Fig. 2E) and HT29
(Fig. 2F) cells in a dose- and
time-dependent manner. Colony formation activity showed that Eth
significantly inhibited the clonogenic ability of SW620 cells
(Fig. 2G and H).
 | Table IIC50 values of Eth in
colorectal cancer cell lines. |
Table I
IC50 values of Eth in
colorectal cancer cell lines.
| Cell line | IC50,
μM |
|---|
| SW480 | 9.37±0.83 |
| HCT116 | 7.19±0.73 |
| HT29 | 6.55±0.50 |
| SW620 | 3.57±0.54 |
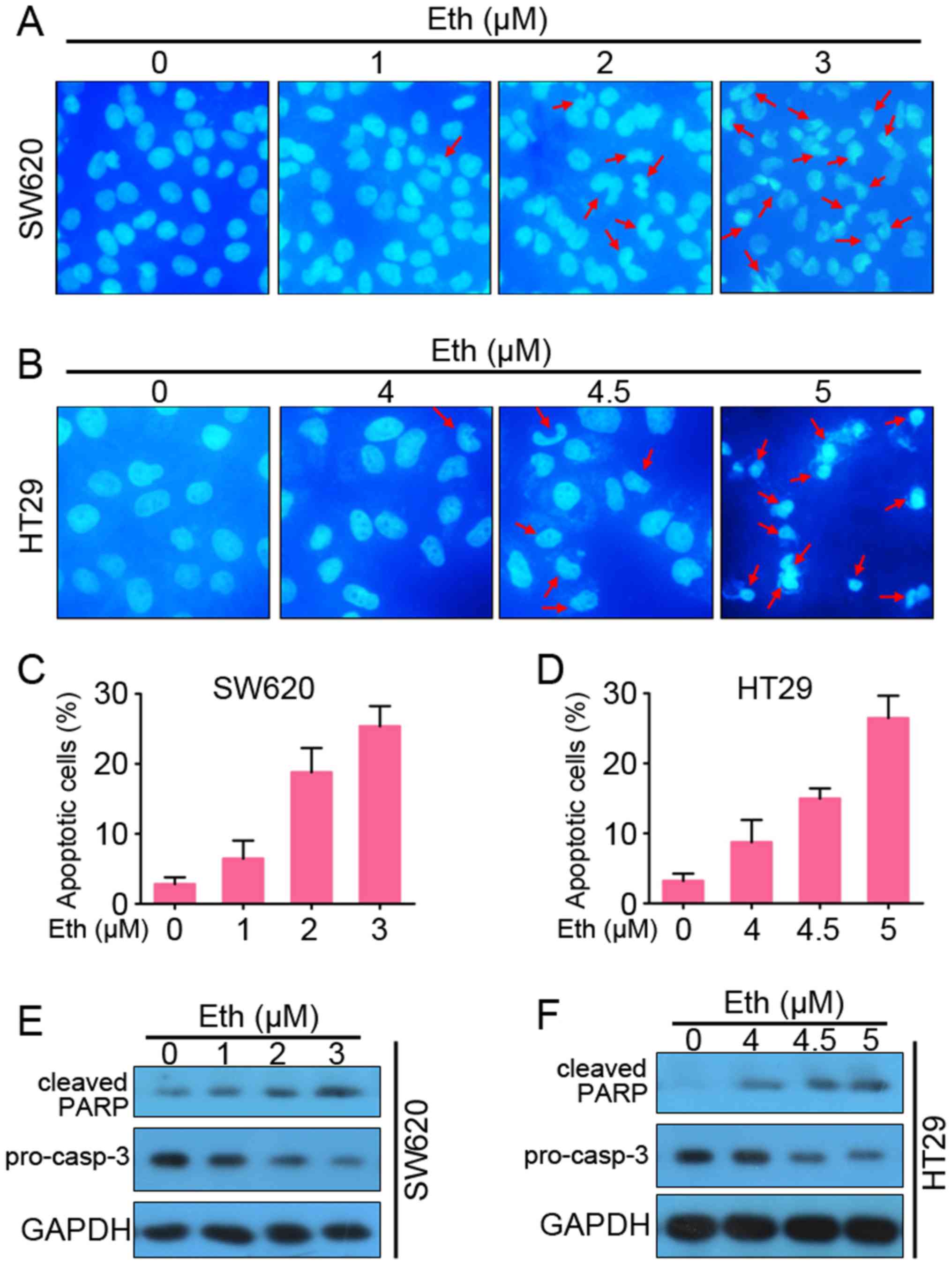
Eth induces apoptosis of CRC cells in a
caspase-dependent manner
The study next tested whether Eth is able to induce
apoptosis. DAPI staining showed that Eth induced chromatin
condensation and fragmentation in SW620 and HT29 cells, which are
typical apoptotic nuclear morphological changes (Fig. 3A and B). AV-FITC/propidium iodide
staining and flow cytometry assays were then used to confirm that
Eth induced apoptosis in the SW620 and HT29 cells (Fig. 3C and D). Furthermore, western blot
analysis detected that Eth induced a decrease in the prosomal form
of caspase-3 (pro-casp-3) and cleavage of PARP (cleaved-PARP) in a
dose-dependent manner in the SW620 (Fig. 3E) and HT29 (Fig. 3F) cells. These results suggested
that Eth induced caspase-dependent apoptosis in CRC cells.
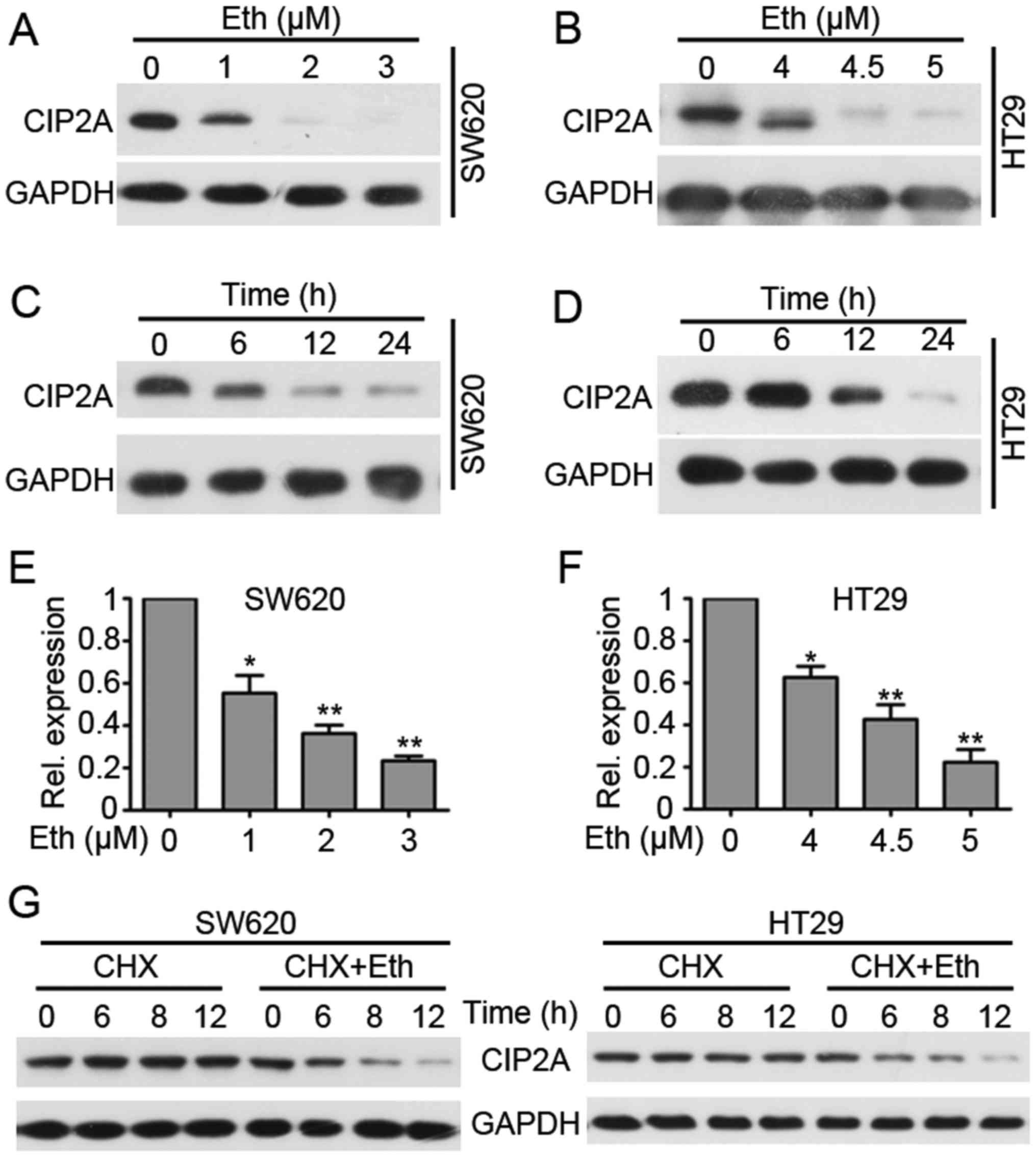
Eth treatment inhibits the expression of
CIP2A in CRC cells
The effect of Eth on the expression of CIP2A was
investigated by western blotting. It was found that treatment with
Eth at 1–3 μM could downregulate CIP2A expression in SW620
cells (4.5–5 μM in HT29 cells) (Fig. 4A and 4B). Fig.
4A shows that the protein level of CIP2A was decreased in SW620
cells treated with Eth at 1 μM and then much less detectable
in cells treated with Eth at 2 μM. In addition, treatment of
HT29 cells with Eth at 4.5 μM induced an apparent
downregulation of CIP2A (Fig. 4B).
Furthermore, it was found that Eth induced downregulation of CIP2A
in a time-dependent manner (Fig. 4C
and D). Next, qPCR was used to investigate whether Eth affected
CIP2A gene transcription. The result showed that Eth
treatment significantly downregulated CIP2A mRNA expression
(Fig. 4E and F). These results
indicate that Eth downregulates CIP2A transcription. Since
Eth induced a relatively rapid degradation of CIP2A, we speculated
that Eth may also influence CIP2A stability. Thus, a protein
synthesis inhibitor, cycloheximide (CHX), was next used to block
protein synthesis, and the results showed that CIP2A was stable
under CHX treatment for at least 12 h. However, when cells were
co-incubated with CHX and Eth, CIP2A expression was downregulated
in 6 h (Fig. 4G). Conclusively,
these results indicate that Eth decreases CIP2A
transcription and induces CIP2A proteolysis.
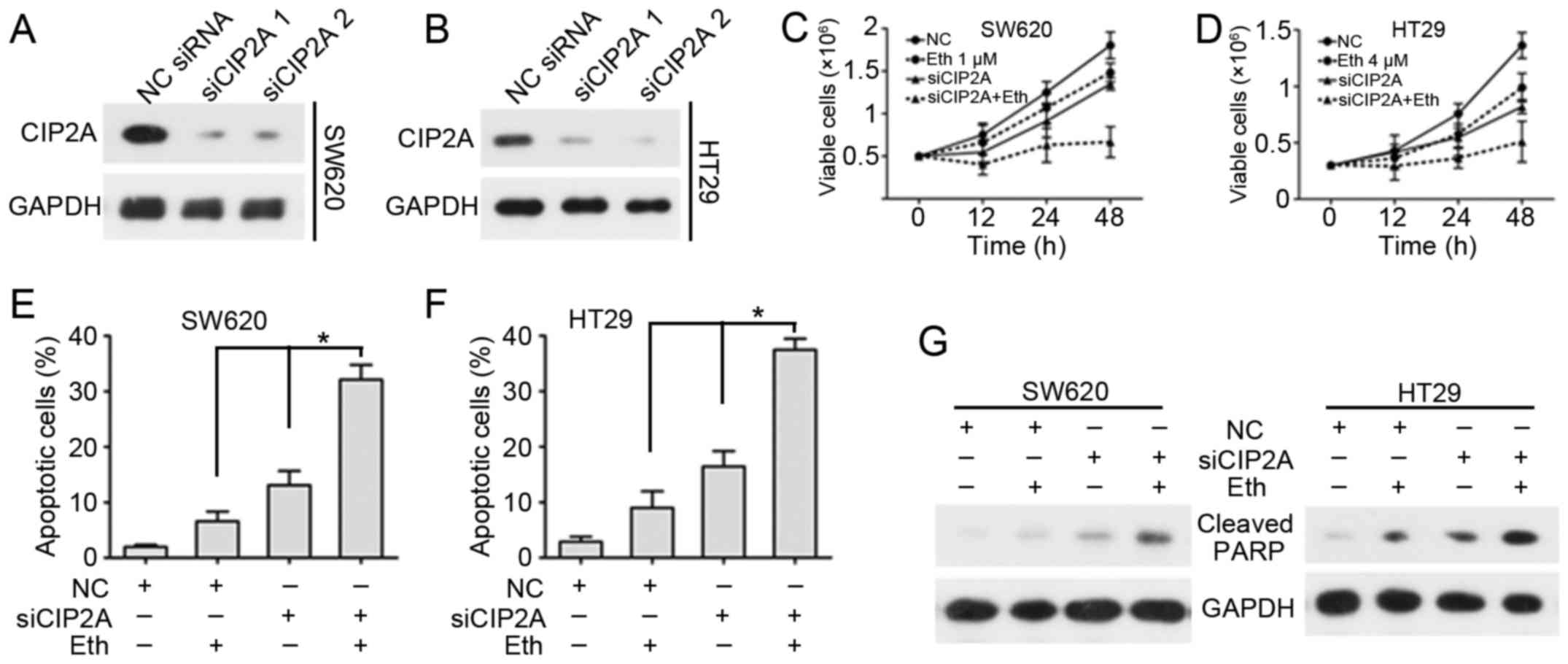
CIP2A-knockdown enhances the inhibition
of viability and apoptosis induction effects of Eth
Next, the effect of CIP2A depletion on the
inhibition of viability and the induction of apoptosis by Eth were
determined. Two siRNAs targeting CIP2A were synthesized and applied
to SW620 and HT29 cells. siRNA1 and siRNA2 knockdown considerably
decreased CIP2A protein expression in each CRC cell line (Fig. 5A and B). This finding validated the
specificity and effectiveness of the CIP2A siRNAs. To examine the
effect of CIP2A in Eth-induced inhibition of viability, SW620 and
HT29 cells were transfected with CIP2A siRNA and then subjected to
Eth treatment. Cell viability and flow cytometric assays were used
to detect variations in cell viability and apoptosis. Notably,
CIP2A depletion promoted the Eth-induced inhibition of viability
(Fig. 5C and D) and enhanced
Eth-induced apoptotic effects (Fig. 5E
and F). Detection of PARP cleavage suggested that Eth caused a
marked rise in PARP cleavage in SW620/siCIP2A and HT29/siCIP2A
cells (Fig. 5G).
CIP2A overexpression antagonizes the
inhibition of viability and the apoptosis induced by Eth
To certify the functional role of CIP2A in
Eth-induced viability inhibition and apoptosis, SW620 and HT29
cells were also transfected with CIP2A plasmid (SW620/CIP2A or
HT29/CIP2A), and CIP2A expression was confirmed using western blot
analysis (Fig. 6A and B). Cell
viability and flow cytometric assays were used to investigate
variations in cell viability and apoptosis. Notably, CIP2A
overexpression abolished Eth-induced viability inhibition (Fig. 6C and D) and inhibited Eth-induced
apoptosis effects (Fig. 6E–G).
These data certify the role of CIP2A in Eth-induced viability
inhibition and apoptosis.
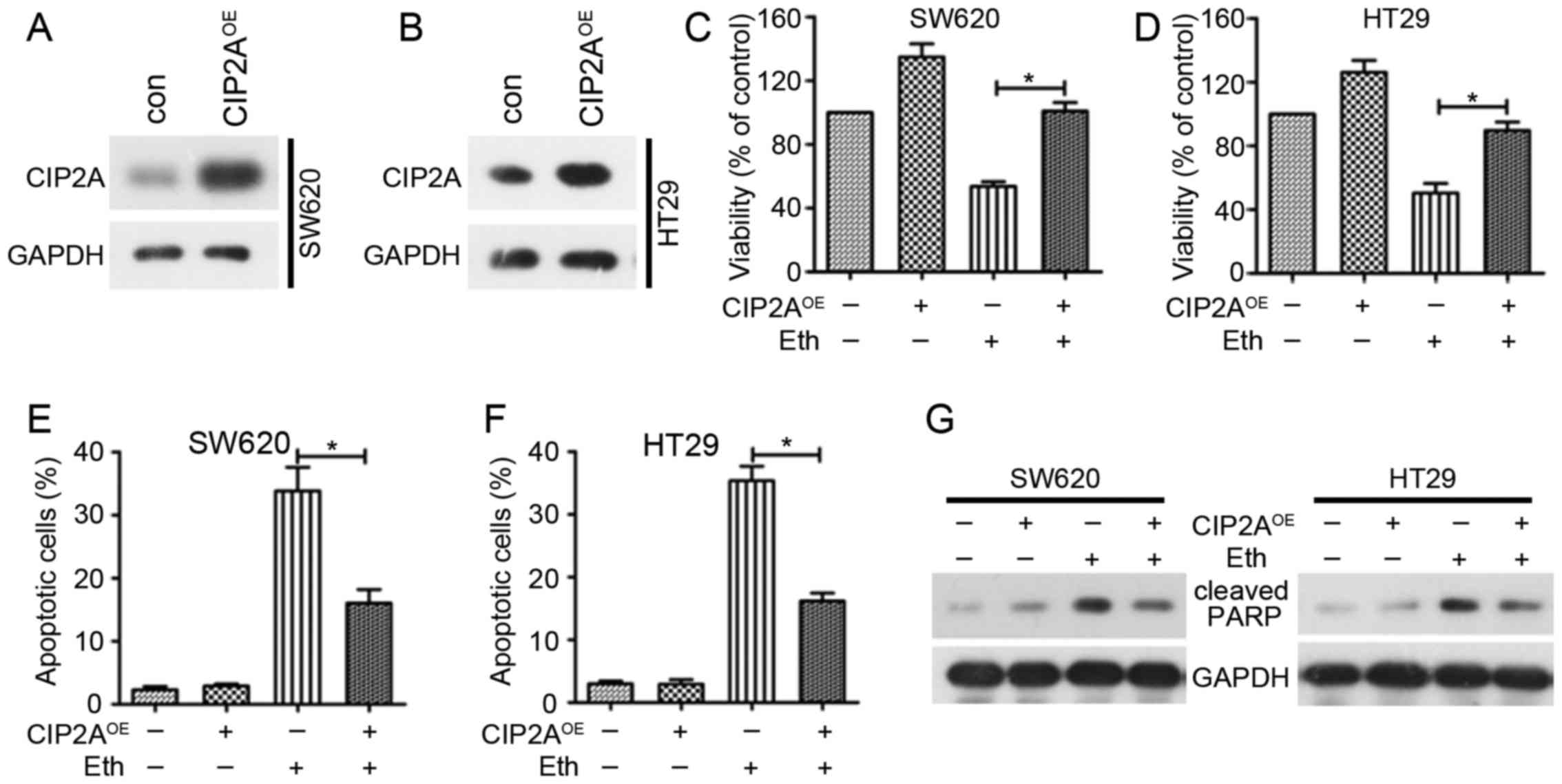 | Figure 6Overexpression of CIP2A reduces
Eth-induced viability inhibition and apoptosis. (A and B) SW620 and
HT29 cells were transfected with the pOTENT-1-CIP2A expression
plasmid (CIP2AOE), and then western blotting was used to
detect CIP2A expression at 48 h post-transfection. (C and D) SW620
(or HT29) cells were transfected with CIP2AOE, and after
6 h of transfection, the cells were treated with 4 μM (or 6
μM) Eth for 24 h. MTT assay were used to detect the
viability of the cells. (E and F) SW620 (or HT29) cells were
transfected with CIP2AOE, and after 6 h of transfection,
the cells were treated with 4 μM (or 6 μM) Eth for 24
h. Cells were stained with Annexin V/propidium iodide for apoptosis
analyses. *P<0.05. (G) SW620 (or HT29) cells were
transfected with CIP2AOE, and after 6 h of transfection,
the cells were treated with 3 μM (or 5 μM) Eth for 24
h. Cells were then lysed for western blotting using the indicated
antibodies. Eth, ethoxysanguinarine; CIP2A, cancerous inhibitor of
protein phosphatase 2A; CIP2AOE, CIP2A overexpression
plasmid. |
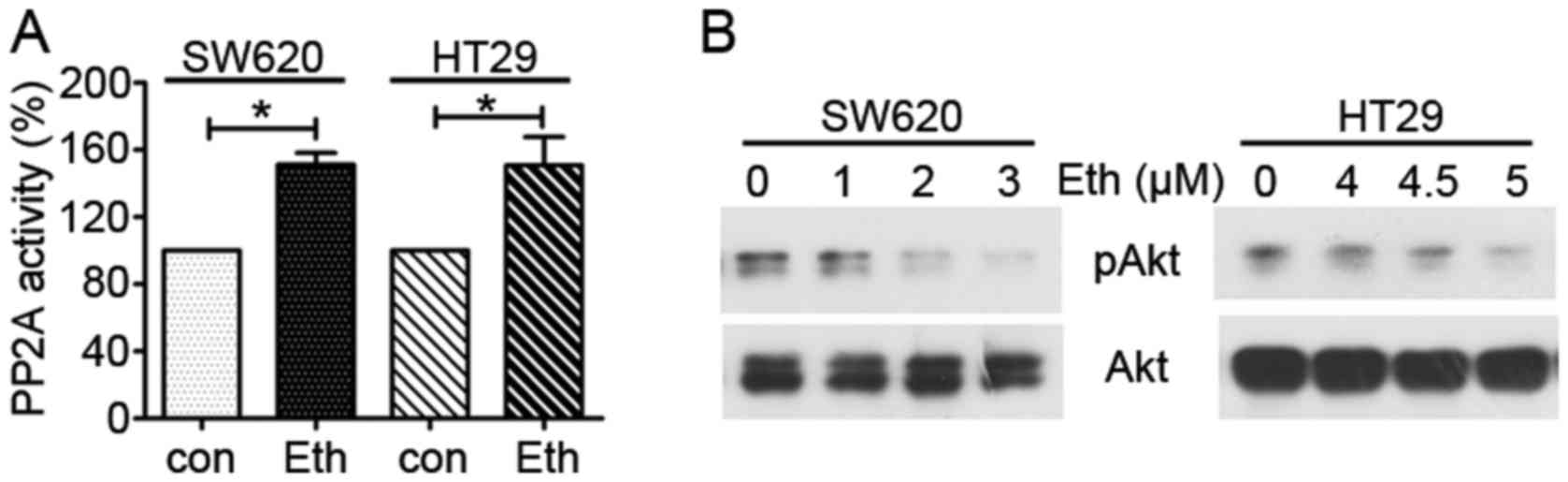
Eth downregulates the CIP2A/PP2A/Akt
pathway
Overexpression of CIP2A causes the activation of
PP2A downstream molecule Akt (24). In the present study, the activity
of PP2A was next tested, and it was found that PP2A activity was
increased in the Eth-treated SW620 and HT29 cells compared with
that in the control group (Fig.
7A). Furthermore, the expression of PP2A downstream molecule
Akt was examined and it was found that Eth downregulated
phospho-Akt in the SW620 and HT29 cells (Fig. 7B). The total Akt level did not
markedly change in the Eth-treated SW620 and HT29 cells compared
with that in the control group. These results suggest that Eth
decreases CIP2A/PP2A/Akt pathway expression in CRC cells.
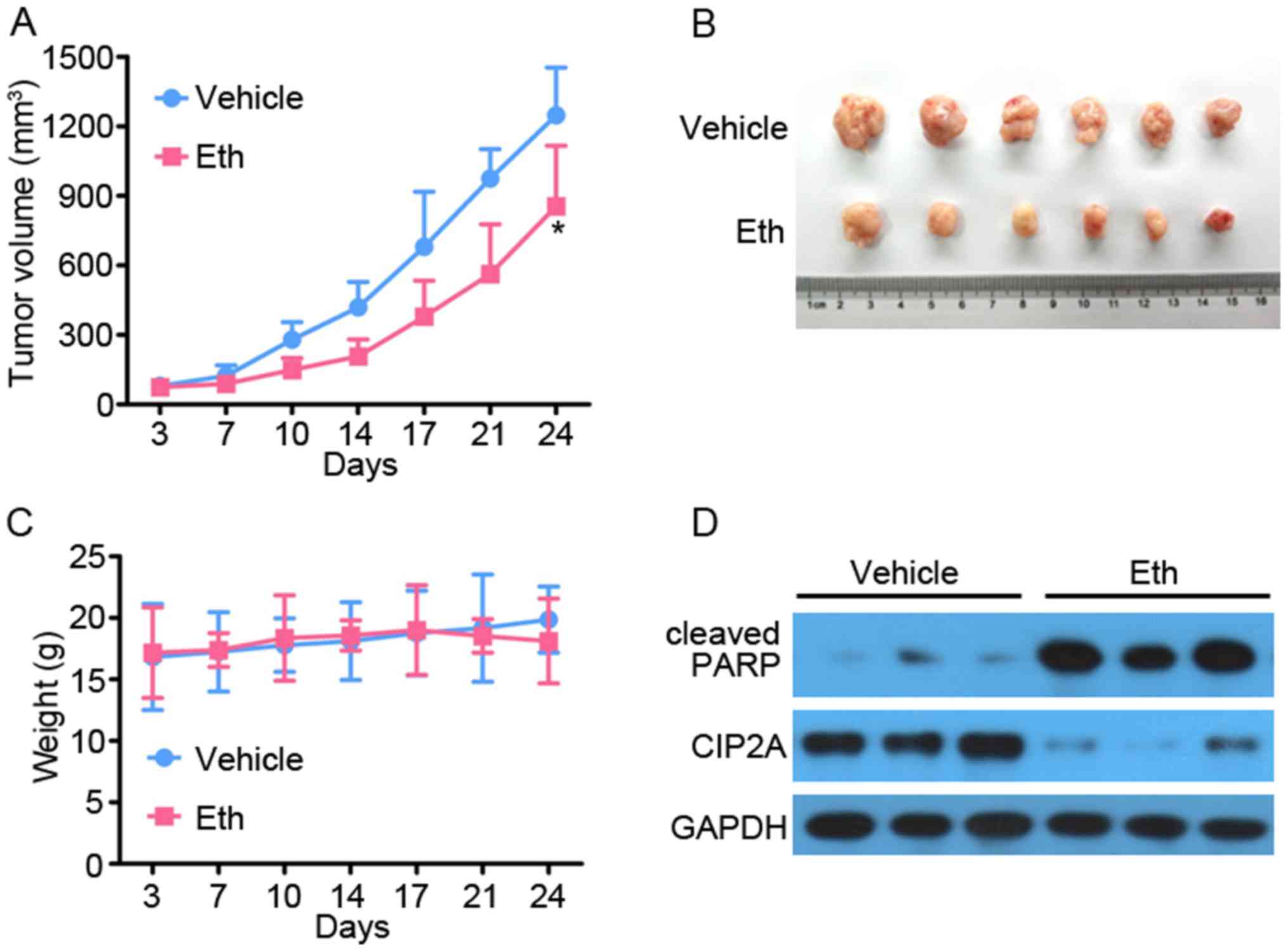
Eth exhibits antitumor effects on CRC
xenograft tumors in vivo
To access the antitumor effect on CRC in
vivo, 5×106 SW620 cells in 100 μl serum-free
L-15 medium were subcutaneously inoculated into the right flank of
nude mice to generate xenografted murine models. When the tumors
grew to a measurable size, each group (10 mice for each random
allocation) was administrated with vehicle control (0.5% DMSO/10%
Cremophor/5% ethanol in normal saline) or Eth (0.5 mg/kg) 5 times
per week for 4 weeks. Tumor-bearing mice were humanely sacrificed
when their tumors reached 1.5 cm in diameter or when paralysis or a
major compromise in their quality of life occurred. As expected, it
was found that Eth efficiently repressed tumor growth compared with
the vehicle control (P<0.05) (Fig.
8A and B). Additionally, Eth treatment did not reduce the body
weight of the mice, which suggested that Eth had no apparent side
effect (Fig. 8C). All the mice
were euthanized, the tumors were isolated and imaged, and the tumor
cell samples were harvested to extract protein for determination of
whether Eth inhibited CIP2A and induced apoptosis, as determined
via western blotting. As shown in Fig.
8D, the level of CIP2A in the Eth treatment group was decreased
compared with that in the vehicle control. Meanwhile, consistent
with the aforementioned results, the level of cleaved-PARP in the
Eth treatment group was increased compared with that in the vehicle
control. These results suggested that Eth has immense potential for
CRC therapy.
Discussion
The present study found that Eth treatment caused a
decrease in CIP2A expression in SW620 and HT29 CRC cells, which was
associated with the inhibition of cell viability and the induction
of apoptosis. Furthermore, it was demonstrated that CIP2A-knockdown
sensitized Eth-induced cellular effects, and conversely, that CIP2A
overexpression antagonized Eth-induced viability inhibition and
apoptosis. These data suggested that Eth specifically affects CIP2A
in CRC cells and that the decrease in CIP2A expression may serve a
key role in its antitumor effects. This study identifies Eth as a
novel CIP2A inhibitor and provides data to improve our
understanding of the inhibition of viability and induction of
apoptosis caused by Eth.
Previous research has indicated that CIP2A is a
potential diagnostic and prognostic marker of several cancer types,
including CRC (25,26). In the present study, the expression
of CIP2A was examined in 6 human CRC cases by western blotting and
qPCR (Fig. 1). The results showed
that the mRNA and protein expression of CIP2A in CRC tissues was
increased in comparison with that in adjacent normal tissue,
indicating that CIP2A serves a role in the tumorigenesis of
CRC.
Natural products continue to be targeted as a novel
dependable source for the treatments of numerous fatal diseases,
such as cancer, due to their preferential properties, including
reduced toxicity, the ability to elicit fewer adverse side effects
compared with synthetic compounds, their high bioavailability and
their reliability (10). M.
cordata is a perennial herb that belongs to the Papaveraceae
family, which is distributed in hills or low mountains, at an
elevation of 150–830 meters above sea level in China (27). The Shennongjia National Nature
Reserve has abundant distribution of this species. M.
cordata has been approved by the European Food Safety Authority
(EFSA) as a safe plant for the manufacture of feed additives,
suggesting its low toxicity. M. cordata is a perfect
substitute for bloodroot as a commercial source for the industrial
production of sanguinarine (27).
In the present study, it was demonstrated that Eth, a derivative of
sanguinarine, significantly inhibited CRC cell viability and colony
formation at low micromolar concentrations, suggesting its
potential for use in CRC control (Fig.
2).
One of the most efficient strategies in cancer
therapy is the induction of cell apoptosis. In the present study,
Eth was found to induce nuclear shrinkage and chromatin
condensation (Fig. 3A and B),
indicating that Eth induces apoptosis in CRC cells. Furthermore,
using flow cytometry, 3 and 5 μM Eth was found to cause
apoptosis at a ratio of 25.39 and 26.49% in SW620 and HT29 cells,
respectively (Fig. 3C and D).
These results further indicated that Eth induces apoptosis in CRC
cells. The extrinsic and intrinsic apoptotic pathways finally
result in the activation of effector caspases (casp)-3, -2 and -7.
The latter is associated with PARP degradation; a hallmark of
apoptosis due it being a substrate of activated effector caspases
(28,29). The potential association between
Eth-induced apoptosis and caspase activation was also examined
(Fig. 3E and F). Thus, Eth may
induce apoptosis by activating the apoptosis pathway, which leads
to the activation of effector casp-3.
Significant inhibition by Eth of CIP2A expression at
the transcriptional and translational levels has been reported in
lung cancer cells (12). However,
the effects of Eth on CIP2A expression in other cancer types have
not been determined and require further investigation. The present
study found that Eth was also able to induce a marked dose- and
time-dependent reduction of CIP2A at the mRNA and protein levels in
CRC (Fig. 4). Moreover, it was
shown that the depletion of CIP2A enhanced the growth inhibition
and apoptotic effects of Eth in CRC (Fig. 5). Additionally, overexpression of
CIP2A antagonized the inhibition of viability and the apoptosis
induced by Eth (Fig. 6). The
activity of CIP2A downstream molecules PP2A and Akt was detected,
and it was demonstrated that Eth upregulated PP2A activity and
downregulated phospho-Akt, but not total Akt, in SW620 and HT29
(Fig. 7) cells. The
dephosphorylation of Akt is frequently mediated by PP2A. In a
xenograft murine model of SW620, Eth significantly inhibited tumor
growth, but the body weight of the mice remained unaffected
(Fig. 8A–C). The results also
showed that Eth inhibited CIP2A and induced apoptosis in
vivo (Fig. 8D).
In conclusion, the present study demonstrated the
mechanism by which Eth induced cancer cell apoptosis in CRC, which
was at least in part by inhibiting the CIP2A/PP2A/Akt signaling
cascades.
Acknowledgments
Not applicable.
Notes
[1]
Funding
This study was supported by grants from the National
Natural Science Foundation of China (no. 81702930), the Open Ended
Design Project of the Hubei Province Key Laboratory of Conservation
Biology for Shennongjia Golden Monkey (no. 2016SNJ001), the Natural
Science Foundation of Hubei Provincial Department of Education (no.
B2014048), the Key Discipline Project of Hubei Province; the
Foundation of Health and Family planning Commission of Hubei
Province (nos. WJ2017F065 and WJ2017F067) and the Foundation of
Hubei University of Medicine (no. FDFR201605).
[2] Availability
of data and materials
Materials described in the manuscript will be freely
available to any scientist wishing to use them for non-commercial
purposes, without breaching participant confidentiality.
[3] Authors'
contributions
The project was conceived and designed by YG and YL.
Patient specimens were harvested/provided by ZL and MX. The
experiments were conducted by LJ, YS, XH, PL, BZ, HY, XZ and SQ.
Data were analyzed by YG and LJ. The manuscript was written by YG
and YL.
[4] Ethics
approval and consent to participate
All animal studies were conducted according to
protocols approved by the Hubei University of Medicine Animal Care
and Use Committee (approval no. 2017-56), complying with the rules
of Regulations for the Administration of Affairs Concerning
Experimental Animals (Approved by the State Council of China).
Written informed consent was obtained from all patients.
[5] Consent for
publication
Each patient provided written informed consent.
[6] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Center MM, DeSantis C and Ward
EM: Global patterns of cancer incidence and mortality rates and
trends. Cancer Epidemiol Biomarkers Prev. 19:1893–1907. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jonas S, Thelen A, Benckert C, Spinelli A,
Sammain S, Neumann U, Rudolph B and Neuhaus P: Extended resections
of liver metastases from colorectal cancer. World J Surg.
31:511–521. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pericàs JM, Corredoira J and Miró JM;
Hospital Clínic-Lucus Augusti Working Group for endocarditis:
Colorectal adenomas. N Engl J Med. 375:387–388. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Junttila MR, Puustinen P, Niemelä M, Ahola
R, Arnold H, Böttzauw T, Ala-aho R, Nielsen C, Ivaska J, Taya Y, et
al: CIP2A inhibits PP2A in human malignancies. Cell. 130:51–62.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li W, Ge Z, Liu C, Liu Z, Björkholm M, Jia
J and Xu D: CIP2A is overexpressed in gastric cancer and its
depletion leads to impaired clonogenicity, senescence, or
differentiation of tumor cells. Clin Cancer Res. 14:3722–3728.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ren J, Li W, Yan L, Jiao W, Tian S, Li D,
Tang Y, Gu G, Liu H and Xu Z: Expression of CIP2A in renal cell
carcinomas correlates with tumour invasion, metastasis and
patients' survival. Br J Cancer. 105:1905–1911. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu CY, Shiau CW, Kuo HY, Huang HP, Chen
MH, Tzeng CH and Chen KF: Cancerous inhibitor of protein
phosphatase 2A determines bortezomib-induced apoptosis in leukemia
cells. Haematologica. 98:729–738. 2013. View Article : Google Scholar :
|
|
10
|
Cai F, Zhang L, Xiao X, Duan C, Huang Q,
Fan C, Li J, Liu X, Li S and Liu Y: Cucurbitacin B reverses
multidrug resistance by targeting CIP2A to reactivate protein
phosphatase 2A in MCF-7/adriamycin cells. Oncol Rep. 36:1180–1186.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Perrotti D and Neviani P: Protein
phosphatase 2A: A target for anticancer therapy. Lancet Oncol.
14:e229–e238. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu Z, Ma L, Wen ZS, Cheng YX and Zhou GB:
Ethoxysanguinarine induces inhibitory effects and downregulates
CIP2A in lung cancer cells. ACS Med Chem Lett. 5:113–118. 2013.
View Article : Google Scholar
|
|
13
|
Kosina P, Gregorova J, Gruz J, Vacek J,
Kolar M, Vogel M, Roos W, Naumann K, Simanek V and Ulrichova J:
Phytochemical and antimicrobial characterization of Macleaya
cordata herb. Fitoterapia. 81:1006–1012. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ouyang L, Su X, He D, Chen Y, Ma M, Xie Q
and Yao S: A study on separation and extraction of four main
alkaloids in Macleaya cordata (Willd) R. Br. with strip dispersion
hybrid liquid membrane. J Sep Sci. 33:2026–2034. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yao JY, Shen JY, Li XL, Xu Y, Hao GJ, Pan
XY, Wang GX and Yin WL: Effect of sanguinarine from the leaves of
Macleaya cordata against Ichthyophthirius multifiliis in grass carp
(Ctenopharyngodon idella). Parasitol Res. 107:1035–1042. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Konda Y, Urano M, Harigaya Y and Onda M:
Studies on the constituents of bocconia-cordata 0.4. Transformation
of sanguinarine into bocconine. J Heterocycl Chem. 28:1841–1843.
1991. View Article : Google Scholar
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
18
|
Cao W, Liu Y, Zhang R, Zhang B, Wang T,
Zhu X, Mei L, Chen H, Zhang H, Ming P, et al: Homoharringtonine
induces apoptosis and inhibits STAT3 via IL-6/JAK1/STAT3 signal
pathway in Gefitinib-resistant lung cancer cells. Sci Rep.
5:84772015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu X, Duan C, Ji J, Zhang T, Yuan X,
Zhang Y, Ma W, Yang J, Yang L, Jiang Z, et al: Cucurbitacin B
induces autophagy and apoptosis by suppressing CIP2A/PP2A/mTORC1
signaling axis in human cisplatin resistant gastric cancer cells.
Oncol Rep. 38:271–278. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Feng T, Cao W, Shen W, Zhang L, Gu X, Guo
Y, Tsai HI, Liu X, Li J, Zhang J, et al: Arctigenin inhibits STAT3
and exhibits anticancer potential in human triple-negative breast
cancer therapy. Oncotarget. 8:329–344. 2017.
|
|
21
|
Chou CC, Yang JS, Lu HF, Ip SW, Lo C, Wu
CC, Lin JP, Tang NY, Chung JG, Chou MJ, et al: Quercetin-mediated
cell cycle arrest and apoptosis involving activation of a caspase
cascade through the mitochondrial pathway in human breast cancer
MCF-7 cells. Arch Pharm Res. 33:1181–1191. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu Y, Dong Y, Zhang B and Cheng YX: Small
compound 6-O-angeloylplenolin induces caspase-dependent apoptosis
in human multiple myeloma cells. Oncol Lett. 6:556–558. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu H, Gu Y, Wang H, Yin J, Zheng G, Zhang
Z, Lu M, Wang C and He Z: Overexpression of PP2A inhibitor SET
oncoprotein is associated with tumor progression and poor prognosis
in human non-small cell lung cancer. Oncotarget. 6:14913–14925.
2015.PubMed/NCBI
|
|
24
|
Rincón R, Cristóbal I, Zazo S, Arpí O,
Menéndez S, Manso R, Lluch A, Eroles P, Rovira A, Albanell J, et
al: PP2A inhibition determines poor outcome and doxorubicin
resistance in early breast cancer and its activation shows
promising therapeutic effects. Oncotarget. 6:4299–4314. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cristóbal I, Zazo S, Torrejón B, Pedregal
M, Madoz-Gúrpide J, Lluch A, Eroles P, Rovira A, Albanell J,
García-Foncillas J, et al: CIP2A confirms its prognostic value in
triple-negative breast cancer. Oncogene. 36:3357–3358. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Balliu M, Cellai C, Lulli M, Laurenzana A,
Torre E, Vannucchi AM and Paoletti F: HDAC1 controls CIP2A
transcription in human colorectal cancer cells. Oncotarget.
7:25862–25871. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang Q, Qin S, Yuan X, Zhang L, Ji J, Liu
X, Ma W, Zhang Y, Liu P, Sun Z, et al: Arctigenin inhibits
triple-negative breast cancers by targeting CIP2A to reactivate
protein phosphatase 2A. Oncol Rep. 38:598–606. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nicholson DW: Caspase structure,
proteolytic substrates, and function during apoptotic cell death.
Cell Death Differ. 6:1028–1042. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Johnstone RW, Ruefli AA and Lowe SW:
Apoptosis: A link between cancer genetics and chemotherapy. Cell.
108:153–164. 2002. View Article : Google Scholar : PubMed/NCBI
|