Introduction
Colorectal cancer (CRC) is associated with a high
mortality rate worldwide (1). It
is the second and third most common cause of cancer-related
mortality for males and females, respectively (2). Despite advances in the diagnosis of
CRC and treatment with surgery, chemotherapy and radiotherapy,
numerous patients still succumb to the disease (3). Therefore, additional studies focusing
on novel methods for the diagnosis and treatment of the disease are
required.
Transmembrane 4 superfamily member 5 protein
(TM4SF5) is eminently expressed in colon, liver, esophageal and
pancreatic cancers (4–10). TM4SF5 is composed of four
transmembrane regions, two cytoplasmic tails (N- and C-terminal),
and two extracellular loops [short extracellular loop 1 (EC1) and
long extracellular loop 2 (EC2)] (11). TM4SF5 forms an enormous
tetraspanin-enriched domain by interacting with other TM4SFs and
integrins to accomplish various functional roles, including roles
in cell growth, migration, tumor progression, and metastasis
(12,13). TM4SF5 induces
epithelial-mesenchymal transition by increasing p27kip1
expression and stabilizing the cytosolic form of
p27kip1, resulting in abnormal cell growth, the loss of
contact inhibition and tumor progression (5). TM4SF5 activates focal adhesion kinase
(FAK) and c-Src, which in turn increase cell adhesion, migration
and lung metastasis (14).
Furthermore, epidermal growth factor receptor and integrin α5
complexes with TM4SF5 mediate cell migration and invasion (15). EC2 of TM4SF5 contains
N-glycosylation sites that mediate the cross-talk with integrins,
thereby affecting cellular migration and invasion (16). Blocking the EC2 domain with a
synthetic anti-TM4SF5 compound, 4′-(p-toluenesulfonylam
ido)-4-hydroxychalcone (TSAHC), has been shown to inhibit
TM4SF5-mediated tumorigenesis (6).
Therefore, TM4SF5 is considered a major molecular target for
anticancer therapy and EC2 is regarded as a key region for the
inhibition of TM4SF5 activity.
We previously developed a prophylactic and
therapeutic vaccine for colon and hepatocellular carcinoma (HCC)
using an EC2 domain TM4SF5 peptide epitope (hTM4SF5R2-3,
138NRTLWDRCEAPPRV151) in combination with our
own adjuvant formulation complex [Lipoplex(O)] (7–9). We
then generated a mouse monoclonal antibody against the hTM4SF5R2-3
epitope, which displayed therapeutic effects in colon and HCC mouse
tumor models (17,18). Moreover, using a novel type of
cyclic peptide epitope targeting the EC2 domain (hTM4SF5EC2-C,
131TACAYLLNRTLWDRSEAPPRVVPWNCT157),
we also produced a humanized anti-TM4SF5 antibody that decreased
the generation and growth of lung metastasis in a mouse colon
cancer model (19).
In this study, we successfully produced a monoclonal
antibody targeting hTM4SF5 using another cyclic peptide epitope
mimicking the natural structure of the EC2 domain (hTM4SF5EC2-CF,
116PRCLMNGEWGYHFEDTAGAYLLNRTLWDRCEAPPRVVP153).
We also examined the utility of this monoclonal antibody for TM4SF5
recognition in cancer tissues and then employed this antibody in
evaluation of TM4SF5 as a prognostic marker for patients with
CRC.
Materials and methods
Cell culture
The 293F cell line was purchased from the Thermo
Fisher Scientific (Waltham, MA, USA) and maintained in Freestyle
293 Expression Medium (Thermo Fisher Scientific). The human colon
cancer cell lines, HT-29, LoVo and HCT-116, were purchased from the
Korean Cell Line Bank (Seoul, Korea) and maintained in RPMI-1640
medium (Thermo Fisher Scientific). All media were supplemented with
10% fetal bovine serum (FBS; Thermo Fisher Scientific), 25 mM
HEPES, 100 U/ml penicillin and 100 µg/ml streptomycin. All
cell lines were incubated at 37°C in an atmosphere of 95% air and
5% CO2.
Synthesis of TM4SF5 B-cell-epitope
peptides
To select B-cell-epitope peptides for
hTM4SF5-specific antibody production, we analyzed the epitope
probability, surface accessibility and antigenicity index
(http://tools.iedb.org/bcell) of the
hTM4SF5 protein. We selected several epitope sequences from the EC2
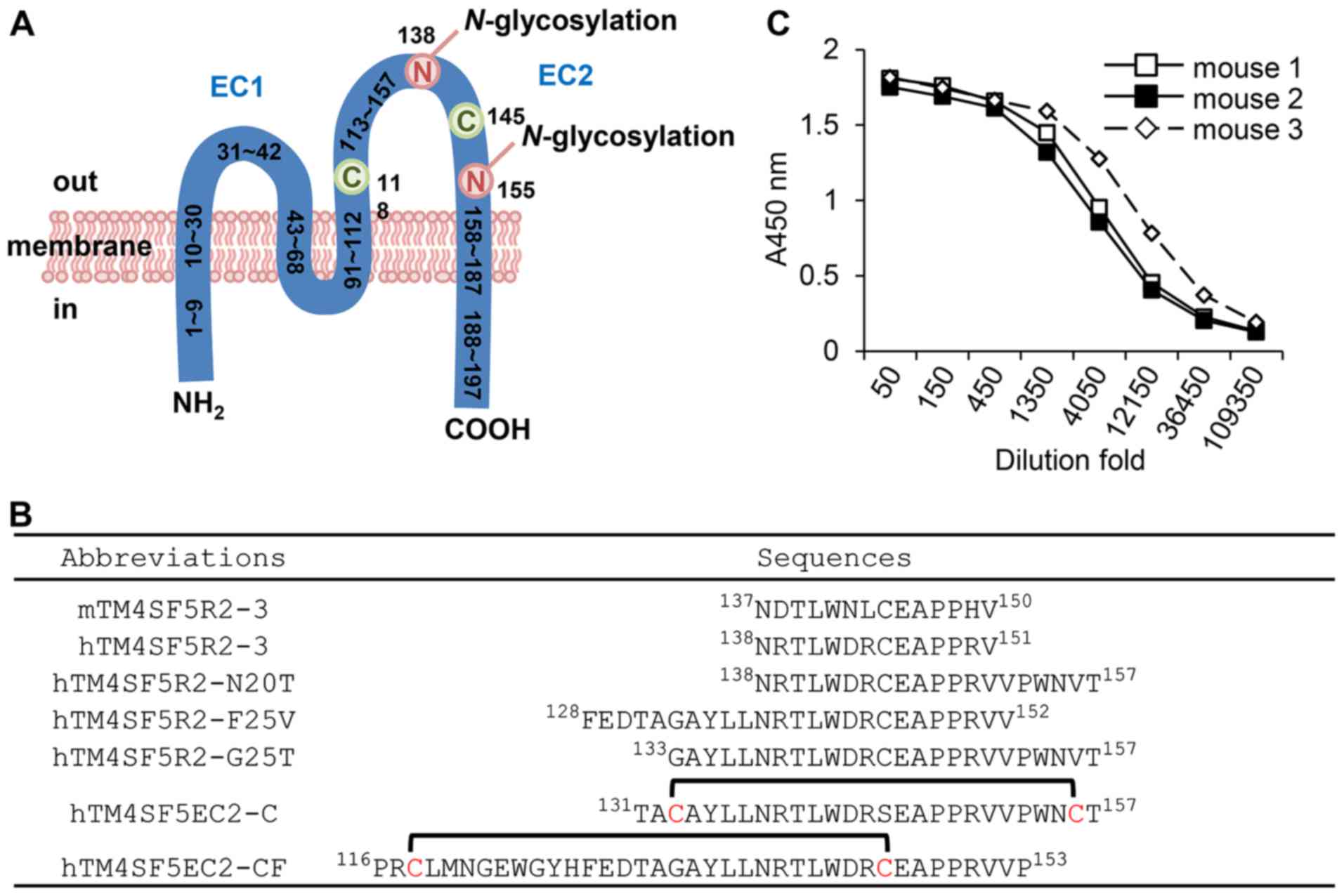
domain of TM4SF5 (Fig. 1A and B).
We synthesized each peptide, including cyclic peptides, with an
automated peptide synthesizer (Peptron III-R24; Peptron, Daejeon,
Korea). The peptides were purified by reversed-phase HPLC
(Prominence HPLC; Shimadzu Corp., Tokyo, Japan) to a >90%
purity.
Mice
Four-week-old female BALB/c (H-2b) mice
(n=11; weight, 19–20 g) were purchased from Nara-Biotec (Seoul,
Korea). Our animal experiments were approved by the Institutional
Animal Care and Use Committee of Hallym University (Permit no.
Hallym 2015-55, Hallym 2015-56). The mice were maintained under
specific pathogen-free conditions in a controlled environment
(20–25°C, 40–45% humidity, 12-h light/dark cycle; ad libitum
access to food and water). The mice were euthanized by
CO2 inhalation (CO2 inhalation was performed
with 100% CO2 at a fill rate of 10–30% of the chamber
volume per min) when the mice lost and gained 20% of adult body
weight, exhibited evidence of debilitation, pain or distress, such
as a hunched posture, rough haircoat, decreased food consumption,
emaciation, inactivity, difficulty ambulating, respiratory problems
and solid tumor growth. After the experiments were completed, the
mice were sacrificed by CO2 inhalation (as mentioned
above) and all efforts were made to minimize suffering.
Immunization
Epitope peptide-CpG-DNA-liposome complex
[Lipoplex(O)] was formulated as previously described (20,21).
Briefly, the liposome complexes consisting of hTM4SF5EC2-CF cyclic
peptide and CpG-DNA [MB-ODN 4531(O)] co-encapsulated with
phosphatidyl-β-oleoyl-γ-palmitoyl ethanolamine (DOPE):cholesterol
hemisuccinate (CHEMS) were prepared. The MB-ODN 4531(O)
encapsulated in DOPE:CHEMS (at a 1:1 ratio) complex as was named
Lipoplex(O). The mixture of DOPE and CHEMS was prepared in ethanol
at a molar ratio of 1:1, and evaporated with nitrogen gas, and then
resuspended in water-soluble CpG-DNA and hTM4SF5EC2-CF cyclic
peptide. Following vigorous stirring at room temperature for 30
min, the mixture solution was adjusted the pH to 7.0. The mixture
solution was slightly sonicated for 30 sec with a sonicator,
filtered with a 0.22 µm filter, and then freeze-thawed 3
times with liquid nitrogen. The mice (n=6; now weighing 20–22 g)
were injected intraperitoneally with 50 µg of the
hTM4SF5EC2-CF cyclic peptide epitope mixed with 50 µg of
CpG-DNA encapsulated in liposomes as previously described (20). All mice were injected on 3
occasions at 10-day intervals.
Antigen-specific Ig enzyme-linked
immunosorbent assay (ELISA)
Ten days after final injection of a complex of
hTM4SF5EC2-CF cyclic peptide and Lipoplex(O) into the mice (n=3;
weight, 20–25 g), sera (100 µl/mouse) were collected by
retro-orbital bleeding under isoflurane (2–3%) inhalation
anesthesia with RC2-Rodent Circuit Controller (Lab Etc Inc. Store,
Clayton, MO, USA) to detect peptide epitope-specific antibody
production. The hTM4SF5EC2-CF peptide was coated onto 96-well
immunoplates (5 µg/well; Thermo Fisher Scientific) and the
plates were then blocked with phosphate-buffered saline (PBS) with
0.2% Tween-20 (PBST) containing 1% bovine serum albumin (BSA).
Antibody production levels in sera were analyzed as previously
described (21). To identify the
specificity of the monoclonal antibody, various TM4SF5 peptides
(Fig. 1B) were coated onto 96-well
immunoplates and the plates were incubated at 4°C overnight. The
plates were blocked with PBS containing 1% BSA for 1 h and
incubated with the mEC2-CF monoclonal antibody (serial 1:4
dilutions in PBST, beginning at 10 µg/ml) for 2 h. After the
plates were washed, they were treated with horseradish peroxidase
(HRP)-conjugated goat anti-mouse IgG (1:5,000, cat. no.
115-035-003; Jackson ImmunoResearch Laboratories, West Grove, PA,
USA). The immunoreactivity was measured with a kit purchased from
KPL (Gaithersburg, MD, USA) and quantified as previously described
(22). The IgG isotypes were
identified with HRP-conjugated goat anti-mouse IgG (each isotype)
antibodies (1:500, cat. no. 5300-05; Southern Biotech, Birmingham,
AL, USA). The binding affinity of the mEC2-CF monoclonal antibody
was measured as previously described (14). To measure the binding affinity
(EC50 value) of the mEC2-CF monoclonal antibody, 96-well
immunoplates were coated with hTM4SF5EC2-CF peptide (5
µg/well) and then treated with PBST containing 1% BSA. The
mEC2-CF monoclonal antibody was applied to the top row of each well
with serial 1:5 dilutions in PBST, starting at 1 µM. The
mixture was incubated for 2 h at room temperature, and then washed
with PBST. The mEC2-CF monoclonal antibody binding to hTM4SF5EC2-CF
cyclic peptide was detected by incubation with the HRP-conjugated
goat anti-mouse IgG (1:5,000; Jackson ImmunoResearch Laboratories)
for 1 h. The amounts of bound antibodies were analyzed with
tetramethylbenzidine (TMB) peroxidase substrate from KPL. The
absorbance was detected with the Spectra Max 250 microplate reader
at 405 nm, and the results were then analyzed with the SigmaPlot
program to calculate the EC50 value.
Production of the mEC2-CF monoclonal
antibody
The hybridoma clone (2D7-1F7) producing the
anti-hTM4SF5EC2-CF peptide-specific monoclonal antibody was
obtained with the standard hybridoma technique (23,24).
Five days after the final injection of a complex of hTM4SF5EC2-CF
cyclic peptide and Lipoplex(O), the mice (n=3) were anesthetized
with isoflurane. Splenocytes producing the anti-hTM4SF5EC2-CF
antibodies were extracted and hybridized with the SP2/0 mouse
myeloma cell line (cat. no. CRL-1581; ATCC, Manassas, VA, USA). The
2D7-1F7 clone was selected by media containing
hypoxanthine-aminopterin-thymidine (HAT) and hypoxanthine-thymidine
(HT). The remaining 5 of the 11 BALB/c (H-2b) mice
(weight, 20–22 g) were primed with pristine (Sigma-Aldrich, St.
Louis, MO, USA; 0.5 ml/mouse) and injected into the peritoneal
cavity with the hybridoma cells (2D7-1H7 clone, 1×105
cells/mouse) for the production of ascitic fluid. Following the
collection of ascitic fluid, the anti-hTM4SF5EC2-CF
peptide-specific monoclonal antibody (mEC2-CF) was purified by
Protein A column chromatography. The purified antibody was resolved
by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) using a 12.5%
gel and confirmed by Coomassie Blue (cat. no. B0149; Sigma-Aldrich;
0.5 g/l) staining at room temperature for 1 h.
Western blot analysis
To assess the ability of the monoclonal antibody to
detect hTM4SF5, the 293F cells were transfected with hTM4SF5/pcDNA
and 293F cells overexpressing hTM4SF5 were selected (19). Cell lysates were prepared using a
lysis buffer [10 mM HEPES (pH 8.4), 150 mM NaCl, 5 mM EDTA, 100 mM
NaF, 2 mM Na3VO4, 1% Nonidet P40 (NP-40) and
protease inhibitor cocktail tablet (cat. no. 11697498001;
Sigma-Aldrich)]. The protein concentration was measured using
Copper(II) surface solution (cat. no. C2284) and bicinchoninic acid
solution (cat. no. B9643) (both from Sigma-Aldrich). Equal amounts
of proteins in the cell lysates were resolved by SDS-PAGE using
12.5% gel and electro-transferred onto nitro-cellulose membranes.
The membranes were blocked with 5% low-fat dry milk (Difco™ Skim
Milk, cat. no. 232100; BD Biosciences, San Jose, CA, USA) in
phosphate-buffered saline-Tween-20 (PBS-T; 140 mM NaCl, 2.7 mM KCl,
10 mM Na2HPO4, 2 mM
KH2PO4 and 0.1% Tween-20) for 1 h at room
temperature, and treated with mEC2-CF (10 µg/ml), a mouse
anti-c-Myc tag antibody (1:1,000, cat. no. 2276; Cell Signaling
Technology, Danvers, MA, USA), and monoclonal mouse anti-β-actin
antibody (1:5,000, cat. no. A5316; Sigma-Aldrich) for 2 h at room
temperature. Immunoreactive proteins were visualized by
HRP-conjugated donkey anti-mouse IgG (1:5,000, cat. no.
715-035-150; Jackson-ImmunoResearch Laboratories) secondary
antibody and an ECL solution (1:1 ratio, cat. nos. 1856135 and
1856136; Thermo Fisher Scientific) was performed for
immunoprecipitation analyses.
Immunoprecipitation
Cell lysates were incubated with mEC2-CF (10
µg/ml) or mouse anti-c-Myc antibodies (1:500; Cell Signaling
Technology) for 4 h at 4°C and then immunoprecipitated using
Protein A beads. The samples were resolved using 12.5% SDS-PAGE and
relevant proteins were detected using mEC2-CF and a mouse anti-Myc
tag antibody (1:1,000; Cell Signaling Technology). To assess
whether mEC2-CF can detect the glycosylated form of hTM4SF5,
lysates from hTM4SF5-overexpressing 293F cells were incubated with
N-glycosidase F (PNGase F; Elpis-Biotech, Daejeon, Korea; cat. no.
EBG-1005) in 1% β-mercaptoethanol and 0.05% SDS for 1 h at 37°C and
immunoprecipitated with mEC2-CF (10 µg/ml). The obtained
immunocomplex was examined by 12.5% SDS-PAGE and western blot
analysis.
Confocal images
To monitor antibody internalization, the HT-29, LoVo
and HCT-116 cells were plated in 12-well plates and incubated with
mouse normal IgG (1 µg/ml, cat. no. 10400C; Thermo Fisher
Scientific) and mEC2-CF (1 µg/ml) in RPMI-1640 medium
containing 1% FBS. After 3 h, the cells were fixed with 4%
formaldehyde for 10 min, and blocked with PBS containing 3% BSA and
0.1% Triton X-100 for 10 min. The cells were then incubated with
Alexa 488-conjugated goat anti-mouse IgG (ratio: 1:500, cat. no.
A11001; Thermo Fisher Scientific) for 1 h at room temperature. To
stain the nuclei, 10 µg/ml of Hoechst 33258 dye (Thermo
Fisher Scientific) was added to the cells for 10 min. After
mounting with Fluoromount G (SouthernBiotech, Birmingham, AL, USA),
the cells were scanned with a Carl Zeiss LSM 710 microscope (Carl
Zeiss, Jena, Germany), in the Cooperative Center for Research
Facilities, Hallym University (Chuncheon, Korea). To identify the
co-localization of mEC2-CF and hTM4SF5, the HT-29 cells which
express hTM4SF5 were incubated with Alexa 488-conjugated humanized
anti-hTM4SF5 antibody (hEC2-C-2) recognizing different region of
TM4SF5 (19) in RPMI-1640 medium
containing 1% FBS. After 3 h, the cells were fixed with 4%
formaldehyde for 10 min, and blocked with PBS containing 3% BSA and
0.1% Triton X-100 for 10 min. The cells were incubated with mEC2-CF
(1 µg/ml) and Alexa 594-conjugated goat anti-mouse IgG
(1:500, cat. no. 53044; Thermo Fisher Scientific) for 1 h at room
temperature.
MTT assay
The HT-29, LoVo and HCT-116 cells were plated in
24-well plates and the viability of the cells was examined with
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;
Sigma-Aldrich). The cells were treated with mEC2-CF (0, 11, 33 and
100 nM) for 5 days. MTT solution was added to each well followed by
incubation for 4 h at 37°C, and the formazan crystals were
dissolved in DMSO. The absorbance was read at 595 nm with a
spectrophotometer (SpectraMAX 250; Molecular Devices, San Jose, CA,
USA).
Patients and tissue samples
In this study, we included 204 patients with primary
CRC who underwent radical surgical resection from November, 2006 to
December, 2012 with no pre-operative chemotherapy or radiotherapy
administered. All tissue samples were retrieved from the archive of
the Department of Pathology, Hallym University Chuncheon Sacred
Heart Hospital. Two independent pathologists (J.-Y.P. and K.C.C.)
reviewed all the hematoxylin and eosin-stained tissue sample
slides. Clinicopathological characteristics including tumor size,
depth of invasion, histological grade, lymph node metastases,
lymphatic invasion, vascular invasion and perineural invasion were
assessed. Lymph node metastases were assessed in only 196 patients
as the remaining 8 patients were diagnosed at the early stage of
cancer and their lymph nodes were not dissected. All other
clinicopathological characteristics were evaluated in the 204
patients with CRC. Patient age ranged from 19 to 87 years (mean and
median, 67 years). During a mean follow-up period of 51 months
(range, 2–113 months; median, 54 months), 15% (27/186) of the
patients with CRC succumbed to the disease. The survival of 18
patients was not evaluated as we could not contact the patients.
Research protocols for the use of human tissues were approved by,
and conducted in accordance with the policies of the Institutional
Review Board at Hallym University Chuncheon Sacred Heart Hospital
(Permit no. 2014-108). Informed consent was obtained from all study
subjects.
Tissue microarray and
immunohistochemistry
A tissue microarray was constructed with
formalin-fixed, and paraffin-embedded tissue blocks from the 204
patients with CRC mentioned above. Representative sections from
cases were selected through microscopic examination. Sample cores
(2 mm in diameter) from each tissue block of CRC were inserted into
recipient paraffin blocks in a grid pattern using a tissue arrayer
(TMA-1; Unitma, Seoul, Korea). The slides were deparaffinized with
xylene and rehydrated in ethanol, and endogenous peroxidase
activity was blocked with 3% hydrogen peroxide for 15 min. For
antigen retrieval, all sections were boiled in a citrate buffer (pH
6.0; cat. no. CBB-500; ScyTek Laboratories, West Logan, UT, USA)
for 15 min. The slides were incubated overnight in PBST containing
the mEC2-CF antibody (10 µg/ml) at 4°C, followed by
incubation with biotinylated anti-mouse IgG antibody (Histostain
Plus kit; cat. no. 85-6643; Invitrogen, Carlsbad, CA, USA). They
were sequentially treated with streptavidin-conjugated peroxidase,
3′,3′-diaminobenzinidine (0.5 mg/ml), and 0.3% of hydrogen
peroxide, and then counterstained with hematoxylin. After rinsing,
the sections were mounted, dehydrated and covered with
coverslips.
Evaluation of immunohistochemical
staining
TM4SF5 expression was scored based on a
semi-quantitative scoring method. Briefly, an intensity score was
assigned as follows: 0, negative staining; 1, weak staining; 2,
moderate staining; or 3, strong staining; as was a proportion score
as follows: 0, none; 1, <25%; 2, 25–50%; 3, 50–75%; and 4,
>75%. The products of the intensity and proportion scores were
calculated to obtain the total score. We defined a high expression
of TM4SF5 as a total score of ≥4. All slides were examined and
scored by two independent pathologists (J.-Y.P. and K.C.C.) who
were blinded to the clinicopathological data and patient identity.
Any discrepancy between assigned scores was resolved through
re-examination by both pathologists to achieve a consensus.
Statistical analysis
The MTT assay results are shown as the means ±
standard deviation. Statistical significance of differences between
2 samples was evaluated using the Student's t-test; A value of
P<0.05 was considered to indicate a statistically significant
difference. The association between TM4SF5 expression and
clinicopathological characteristics was evaluated by Pearson's
Chi-square test. The Kaplan-Meier test was used to analyze the
survival rates and the significance of the difference in survival
was determined using the log-rank test. Statistical significance
was defined as a P-value <0.05. The SPSS statistical package
version 15.0 (SPSS, Inc., Chicago, IL, USA) was used for
statistical analysis.
Results
Selection of a candidate peptide and
production of the mEC2-CF monoclonal antibody
Considering the structure of the hTM4SF5 EC2 domain
and its functional implications (Fig.
1A), we designed a cyclic peptide, hTM4SF5EC2-CF (Fig. 1B). In particular, we designed the
epitope focusing early part of the EC2 region by using several
additionally extended amino acids in the N-terminal to obtain a new
type of antibody.
We immunized BALB/c mice with the hTM4SF5EC2-CF
peptide and Lipoplex(O) complex. When the sera were analyzed by
ELISA, we found that the anti-hTM4SF5EC2-CF antibody had been
successfully produced (Fig. 1C).
Subsequently, 2D7-1F7 hybridoma clone cells producing the
anti-hTM4SF5EC2-CF monoclonal antibody were selected and the
production of the antibodies in the ascitic fluid of 5 mice was
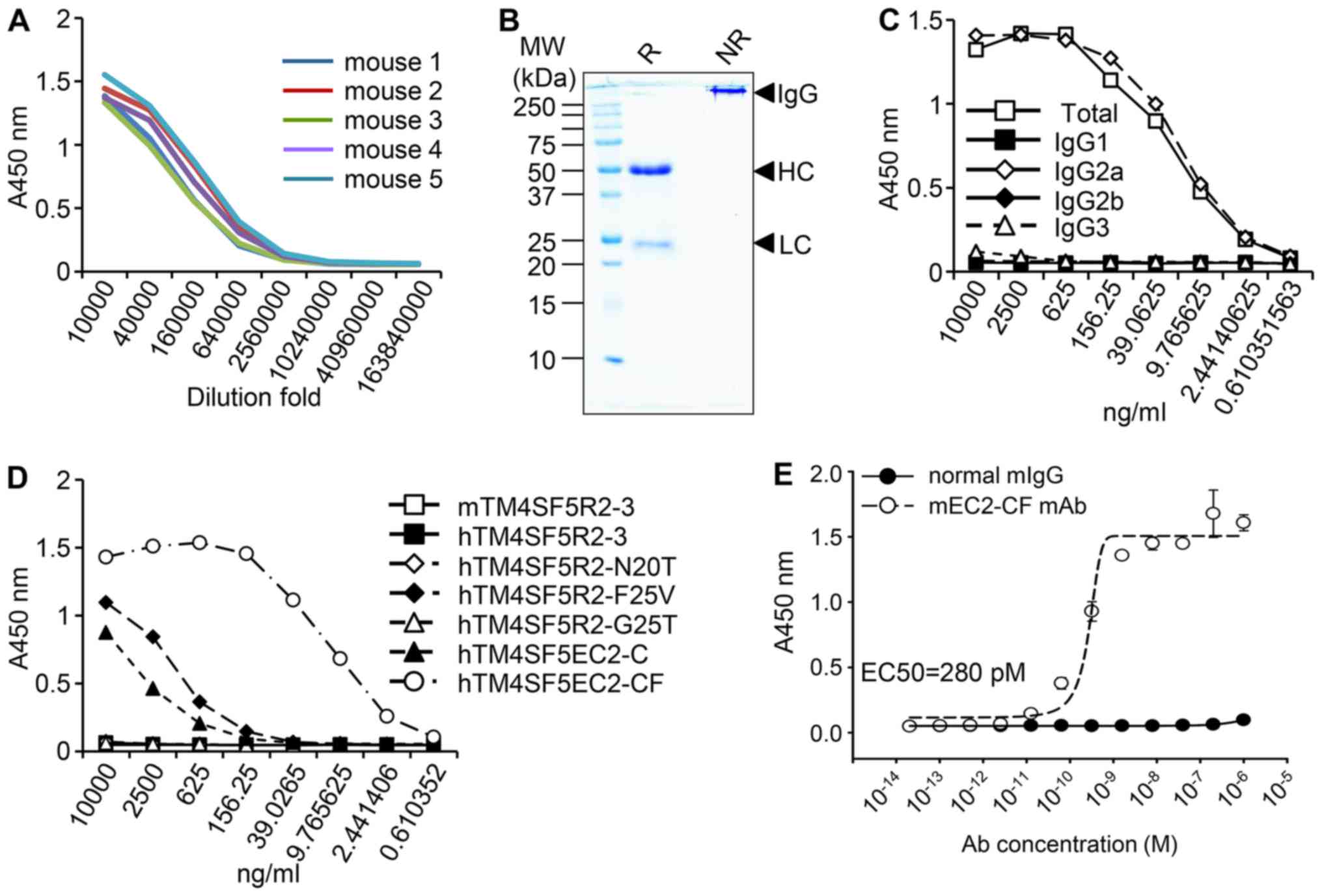
confirmed by ELISA (Fig. 2A). The
antibody was separated and purified from the ascitic fluid by
Protein A chromatography (Fig.
2B). ELISA identified the monoclonal antibody to be of the
IgG2a isotype (Fig. 2C). To
determine the specificity of the antibody, ELISA was performed with
various TM4SF peptides (shown in Fig.
1B). The antibody exhibited strong binding to the hTM4SF5EC2-CF
peptide, very weak binding to two other peptides (hTM4SF5R2-F25V
and hTM4SF5EC2-C) at high concentrations (Fig. 2D), and no binding to the other
peptides tested. These results suggest that the monoclonal antibody
is highly specific to hTM4SF5EC2-CF, which possibly mimics the
structure of the natural EC2 region of TM4SF5. The antibody binding
affinity was evaluated and the EC50 was 280 pM (Fig. 2E). These results demonstrated that
the hTM4SF5EC2-CF peptide epitope induced production of an
IgG2a-type antibody with strong and specific affinity towards the
hTM4SF5EC2-CF peptide. The anti-hTM4SF5EC2-CF monoclonal antibody
was named mEC2-CF.
Characterization of mEC2-CF
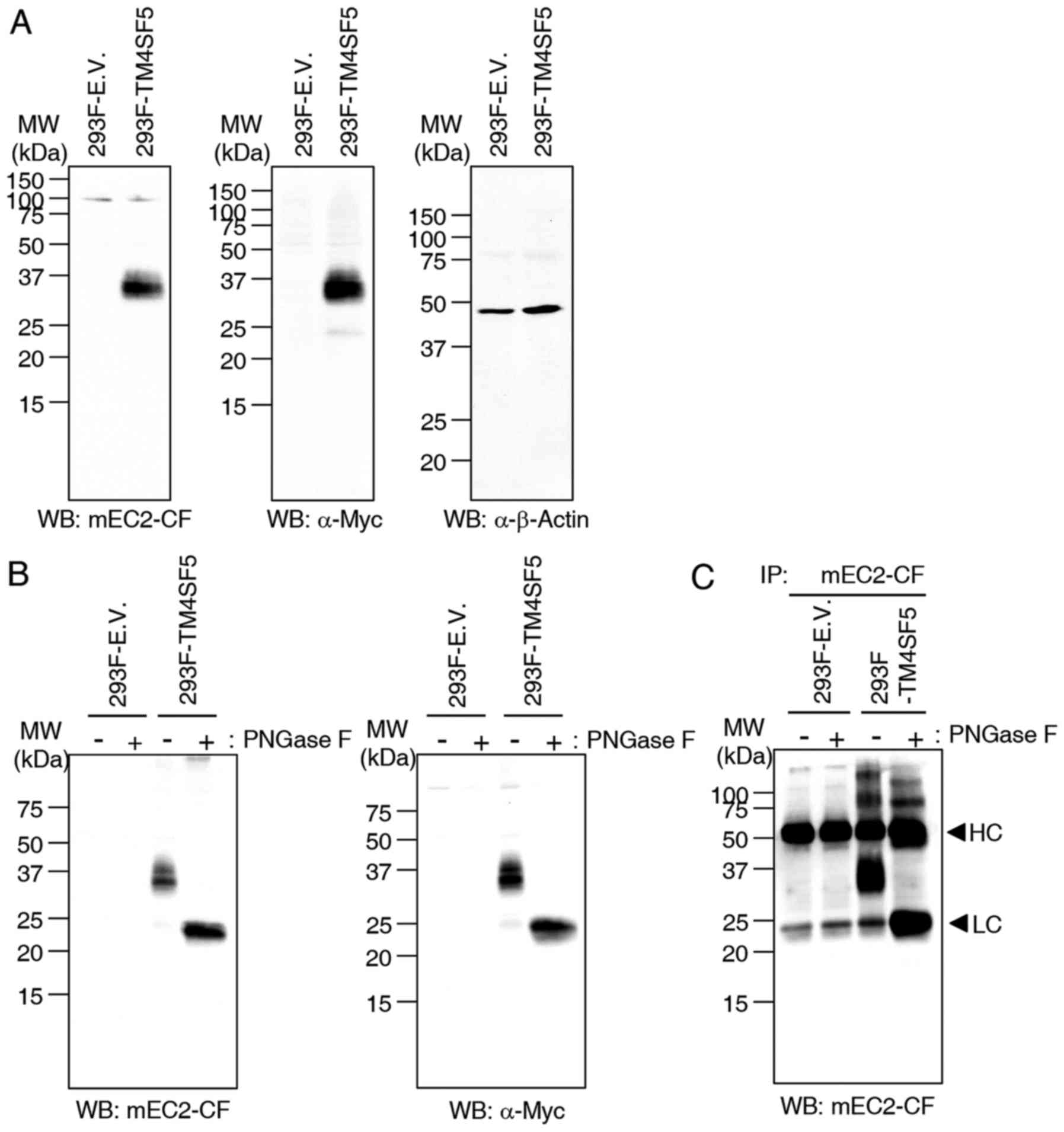
To investigate the function of mEC2-CF, 293F cells
were transfected with empty vector or Myc-tagged hTM4SF5
(hTM4SF5-myc) expression vector and the cell lysates were examined
by western blot analysis and immunoprecipitation using the mEC2-CF
antibody. The results of western blot analysis demonstrated that
mEC2-CF effectively recognizes hTM4SF5-myc protein in cell lysates
and the specificity was confirmed using an anti-Myc antibody
(Fig. 3A). We then de-glycosylated
hTM4SF5-myc protein by treating the cell lysates with N-glycosidase
F (PNGase F) and examined the reactivity of mEC2-CF by western blot
analysis and immunoprecipitation. When the cell lysates were
treated with PNGase F, the mobility of the detected protein band
was enhanced compared to the untreated control. Considering that
TM4SF5 has two N-glycosylation sites, the more rapidly moving
protein band seemed to be unglycosylated hTM4SF5-myc. It was also
confirmed by western blot analysis with an anti-Myc antibody
(Fig. 3B). Therefore, these
results suggested that the mEC2-CF antibody could detect both
glycosylated and unglycosylated hTM4SF5-myc protein. mEC2-CF was
also able to immunoprecipitate both forms of hTM4SF5-myc protein,
which was confirmed by western blot analysis with mEC2-CF (Fig. 3C). When the cell lysates were
treated with PNGase F, the protein band of unglycosylated
hTM4SF5-myc protein was detected to be overlapped with the light
chain of antibody due to their similar mobility. Therefore, we
concluded that mEC2-CF is reactive to the hTM4SF5 protein and that
the glycosylation of Asn at 138 of hTM4SF5 is likely not required
for the recognition of hTM4SF5 by the mEC2-CF antibody.
Internalization of TM4SF5 bound by
mEC2-CF
Considering the antibody-drug conjugate strategy,
the ability of an antibody to internalize into cells is an
important parameter for the therapeutic efficacy. In this study, to
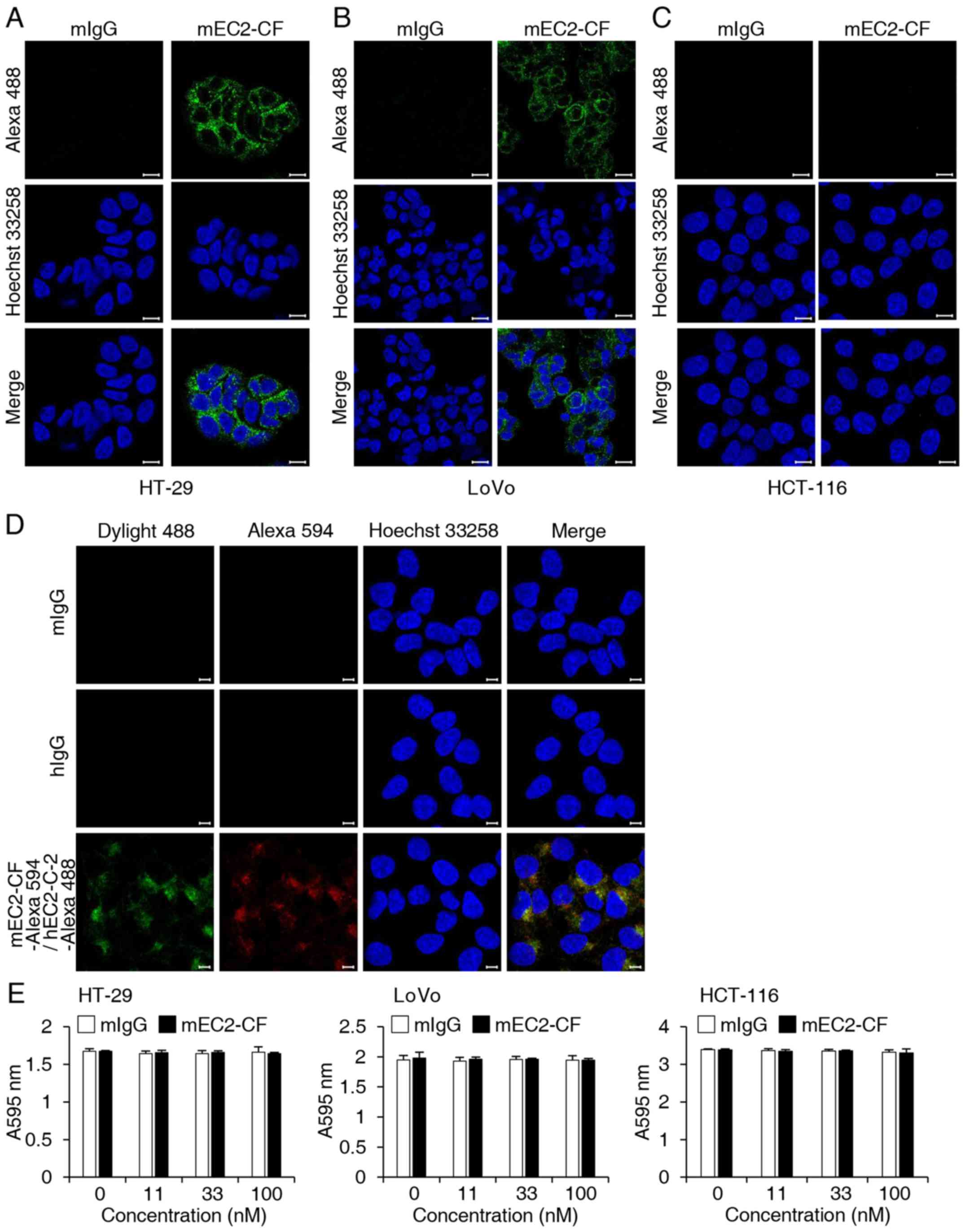
determine whether mEC2-CF could be internalized into cells, HT-29,
HCT-116 and LoVo cells were treated with mEC2-CF and then incubated
with Alexa 488-conjugated goat anti-mouse IgG. Immunofluorescence
analysis using confocal microscopy revealed that mEC2-CF was
successfully internalized into the HT-29 and LoVo cells, which
express hTM4SF5 (18,19) (Fig. 4A
and B). No such internalization was observed in the HCT-116
cells, which do not express hTM4SF5 (19), suggesting that the internalization
of mEC2-CF is mediated by its binding to hTM4SF5 (Fig. 4C). To further confirm that the
internalization is mediated by targeting hTM4SF5, the HT-29 cells
were incubated with Alexa 488-conjugated humanized anti-TM4SF5
antibody (hEC2-C-2) recognizing another epitope of EC2 (19). After 3 h, the cells were fixed,
permeabilized and then stained with mEC2-CF and Alexa
594-conjugated goat anti-mouse IgG. As shown in Fig. 4D, both the antibodies were
co-localized in the confocal images. Based on these observations,
we concluded that mEC2-CF effectively recognizes hTM4SF5 and then
internalizes into hTM4SF5 protein-expressing target cells. To
further identify the biological effect of mEC2-CF on cell growth,
the viability of the CRC cells in the presence of mEC2-CF was
examined but there was no significant effect (Fig. 4E).
Detection of TM4SF5 protein expression in
CRC tissues with mEC2-CF
To determine the effect of hTM4SF5 expression on the
clinicopathological characteristics of patients with CRC, we
examined the protein expression of hTM4SF5 in CRC tissues using
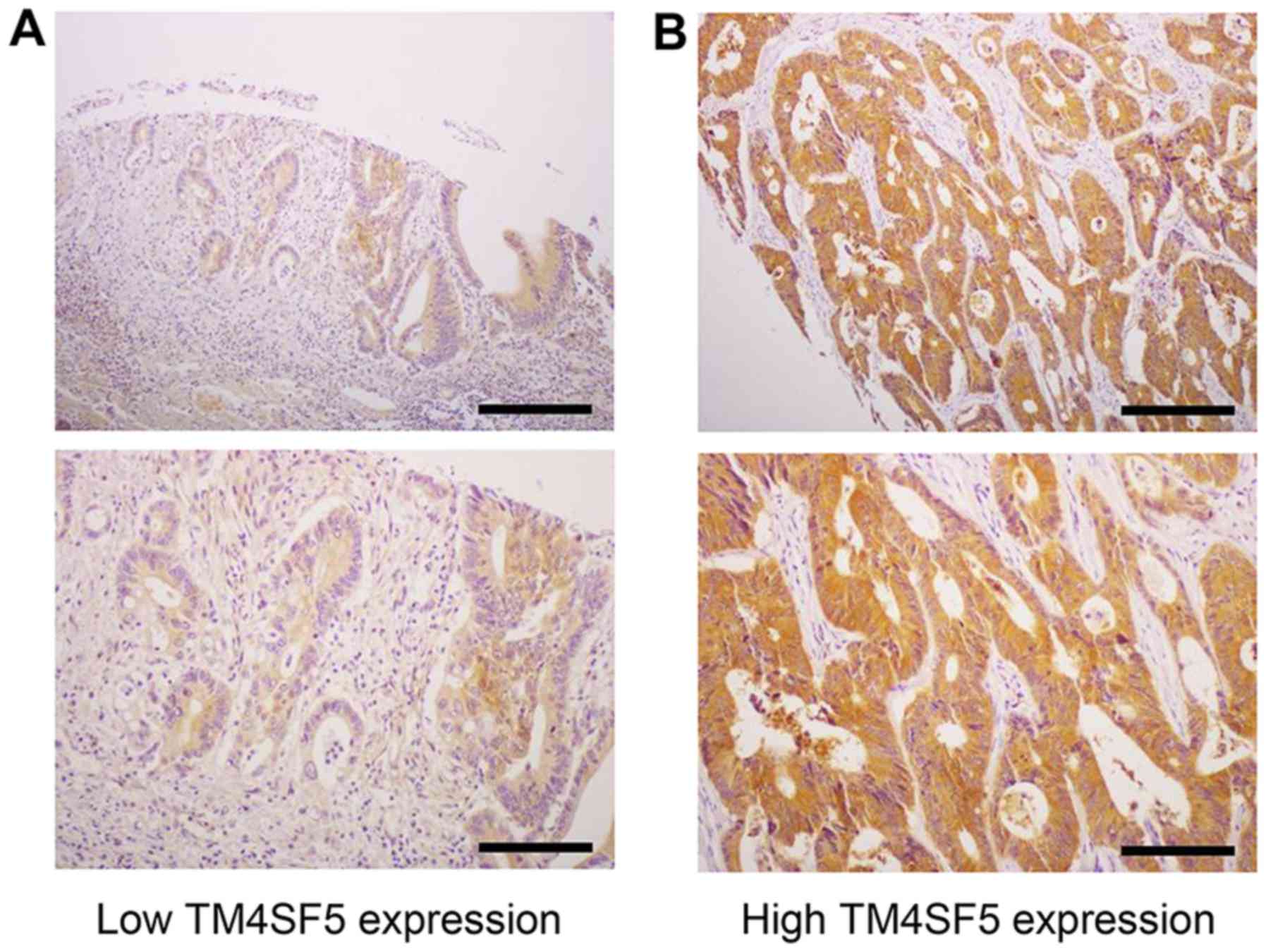
immunohistochemistry with mEC2-CF (Fig. 5). A high expression of hTM4SF5 was
detected in 55 of the 204 CRC cases (27%). We then examined whether
TM4SF5 expression is associated with tumor size, depth of invasion,
histological grade, lymph node metastases, lymphatic invasion,
vascular invasion and perineural invasion. The patients with a high
TM4SF5 expression exhibited significantly increased tumor invasion
compared with those with a low TM4SF5 expression (P=0.027). Other
clinicopathological characteristics evaluated were not associated
with TM4SF5 expression (Table
I).
 | Table IThe association between TM4SF5 and
the clinico-pathological characteristics of patients with
colorectal cancer. |
Table I
The association between TM4SF5 and
the clinico-pathological characteristics of patients with
colorectal cancer.
|
Clinicopathologicalcharacteristics | TM4SF5 expression
| P-value |
|---|
| Low (n=149) | High (n=55) |
|---|
| Tumor size | | | |
| ≤5 cm | 86 (74.1%) | 30 (25.9%) | 0.685 |
| >5 cm | 63 (71.6%) | 25 (28.4%) | |
| Depth of
invasion | | | |
| T1 and T2 | 41 (85.4%) | 7 (14.6%) | 0.027a |
| T3 and T4 | 108 (69.2%) | 48 (30.8%) | |
| Histological
grade | | | |
| WD | 30 (78.9%) | 8 (21.1%) | 0.343 |
| MD and PD | 119 (71.7%) | 47 (28.3%) | |
| Lymphatic
invasion | | | |
| Present | 58 (72.5%) | 22 (27.5%) | 0.889 |
| Absent | 91 (73.4%) | 33 (26.6%) | |
| Vascular
invasion | | | |
| Present | 141 (73.8%) | 50 (26.2%) | 0.343 |
| Absent | 8 (61.5%) | 5 (38.5%) | |
| Perineural
invasion | | | |
| Present | 106 (71.1%) | 43 (28.9%) | 0.315 |
| Absent | 43 (78.2%) | 12 (21.8%) | |
| Lymph node
metastasisb | | | |
| Present | 87 (72.5%) | 33 (27.5%) | 0.984 |
| Absent | 55 (72.4%) | 21 (27.6%) | |
Survival analysis
To investigate the possible involvement of hTM4SF5
expression in the prognosis of patients with CRC, Kaplan-Meier
tests for overall survival and disease-free survival were
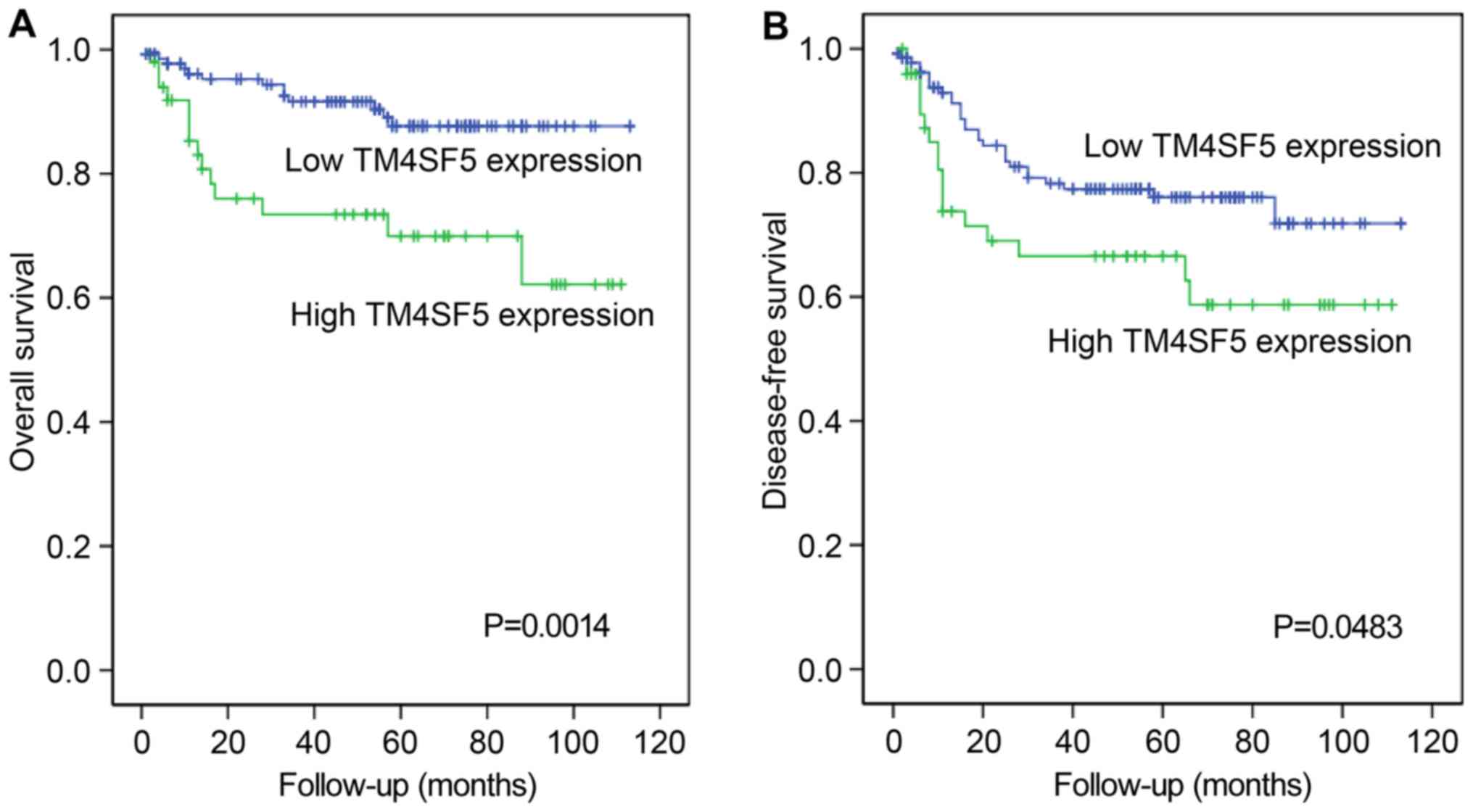
performed. As shown in Fig. 6, a
high expression of hTM4SF5 was significantly associated with a
worse overall survival (P=0.0014) and disease-free survival
(P=0.0483) of patients with CRC.
In addition, our analysis revealed that the
decreased patient survival was significantly associated with the
depth of invasion (P=0.0227), lymphatic invasion (P=0.0047), lymph
node metastasis (P=0.002) and perineural invasion (P=0.0005). Worse
disease-free survival was also significantly associated with the
depth of invasion (P=0.0221), lymphatic invasion (P=0.0081),
vascular invasion (P<0.0001), lymph node metastasis
(P<0.0001) and perineural invasion (P=0.0009) (Table II). These data are in accordance
with current knowledge regarding the prognosis of patients with
CRC.
 | Table IIUnivariate analyses for the overall
survival and disease-free survival of patients with colorectal
cancer. |
Table II
Univariate analyses for the overall
survival and disease-free survival of patients with colorectal
cancer.
| Clinicopathological
characteristics | Overall survival
P-value | Disease-free
survival P-value |
|---|
| Size | 0.4708 | 0.4817 |
| Depth of
invasion | 0.0227a | 0.0021a |
| Lymphatic
invasion | 0.0047a | 0.0081a |
| Vascular
invasion | 0.1555 | <0.0001a |
| Perineural
invasion | 0.0005a | 0.0009a |
| Histological
grade | 0.0769 | 0.0716 |
| Lymph node
metastasis | 0.0002a | <0.0001a |
Discussion
Previously, we screened an efficacious B-cell
peptide epitope (hTM4SF5R2-3) by immunizing BALB/c mice with
various TM4SF5 peptides and characterized the prophylactic and the
therapeutic effects of the peptide vaccine on hepatocellular
carcinoma and colon cancer cell lines and tumor mouse models
(8,9,25).
We also produced TM4SF5-specific monoclonal antibodies and
evaluated their therapeutic effects (18,19,26).
Based on these data, we hypothesized that the hTM4SF5 protein is a
reasonable target for anticancer therapy and that anti-hTM4SF5
antibodies warrant further development as therapeutic agents for
the inhibition of hTM4SF5 function in cancer. To isolate more
effective monoclonal antibodies targeting hTM4SF5, we recently
isolated an anti-hTM4SF5 monoclonal antibody using a cyclic peptide
that mimics the native EC2 region (hTM4SF5EC2-C) and characterized
the anti-metastatic effects of its humanized antibody in a colon
cancer model (19). In this study,
we produced and verified a monoclonal antibody (mEC2-CF) against
another cyclic peptide epitope targeting the EC2 domain
(hTM4SF5EC2-CF) of hTM4SF5.
The monoclonal antibody, mEC2-CF, was highly
specific to the cyclic peptide hTM4SF5EC2-CF, with low
cross-reactivity to two other TM4SF5 peptides, possibly due to the
partial overlap of the epitopes. It is hard to determine the exact
epitope of the antibody due to the complexity involving structure
and sequence. Considering that two glycosylation sites (N138 and
N155) are known to be essential for the function of TM4SF5
(16) and that mEC2-CF cannot
inhibit the growth of TM4SF5-expressing cells, it is likely that
the antibody recognizes the early part of EC2 region rather than
the late part of EC2, including the glycosylation sites as we
originally planned.
The antibody recognized recombinant hTM4SF5 protein
produced by ectopic overexpression in 293F cells, as well as
endogenous hTM4SF5 protein overexpressed in colon cancer cell
lines. Therefore, we postulated that this antibody may be useful
for detection of hTM4SF5 in clinical samples of patients with CRC.
The results of immunohistochemistry revealed that mEC2-CF
successfully recognized hTM4SF5 in the colon tissues from patients
with CRC. When we performed immunohistochemistry on the clinical
samples with the previously developed anti-hTM4SF5 antibody
targeting hTM4SF5EC2-C, we did not obtain the expected results. One
important thing to be considered is that the isotype of the
antibody is IgG3 and IgG3 antibodies are generally unstable. In the
case of its humanized antibody, it is not suitable for the staining
of human tissues. Accounting that the isotype of mEC2-CF is IgG2a
and the target epitope seems different from the original one, we
tried to use this antibody in immunohistochemistry to analyze
TM4SF5 expression in clinical samples and found that the new
antibody has a unique advantage for the purposes of
immunohistochemistry.
Our analysis also revealed that mEC2-CF can be
internalized after binding to hTM4SF5, further supporting the
utility of this antibody in development of cancer therapy. However,
in contrast to previously-isolated antibodies, mEC2-CF did not
reduce the growth of hTM4SF5-expressing cells, suggesting that the
antibody binds to hTM4SF5, but may not inhibit the function of
hTM4SF5 in cells. Therefore, this antibody may have limited utility
when applied as a single agent in the treatment of cancer. However,
given the specificity of its targeting and its internalization into
cells, the development of antibody-drug conjugates of mEC2-CF may
be an alternative strategy for its application in anticancer
therapeutics.
Previously, we demonstrated a higher expression of
hTM4SF5 in colon cancer tissues compared to normal tissues by
analyzing commercially-available tissue microarrays using
immunohistochemistry (18). The
vendor provided information regarding sex, age, tumor grade, along
with the tumor, node and metastasis (TNM) stage of the tissues
included in the microarray. However, there was no information
regarding the prognosis of the patients. In this study, we analyzed
the association between hTM4SF5 expression and the prognosis of
patients with CRC after surgery using the newly-isolated mEC2-CF
antibody and tumor tissues obtained from CRC patients. The results
clearly revealed that a high expression of hTM4SF5 in the cancer
tissues was associated with a poor prognosis, represented by the
low overall and disease-free survival rates. Therefore, we suggest
that hTM4SF5 expression is suitable as a marker for a poor
prognosis in CRC.
To date, we have isolated several monoclonal
antibodies against hTM4SF5 and produced one humanized antibody. In
this study, we isolated and characterized another candidate
antibody and verified its utility as a tool to investigate hTM4SF5
expression. Different antibodies have different characteristics as
we expected. For example, the anti-hTM4SF5 antibody targeting
hTM4SF5EC2-C was effective in the inhibition of tumor growth in
vitro and in vivo (19). By contrast, mEC2-CF did not inhibit
the growth of cancer cells in vitro, although it proved to
be useful in detection using immunohistochemistry. Therefore, we
consider that obtaining more diverse candidate antibodies may
provide us with an array of choices when considering antibodies for
diagnostic, prognostic and therapeutic applications in cancer
treatment. For future application, the in vivo stability and
efficacy of the candidate antibodies will have to be determined in
detail and strategies to strengthen the efficacy of antibodies in
the inhibition of tumor growth will have to be evaluated and
verified.
Abbreviations:
|
CRC
|
colorectal cancer
|
|
EC1
|
short extracellular loop 1
|
|
EC2
|
long extracellular loop 2
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
HCC
|
hepatocellular carcinoma
|
|
mEC2-CF
|
anti-hTM4SF5EC2-CF monoclonal
antibody
|
|
PNGase
|
N-glycosidase
|
|
TM4SF5
|
transmembrane 4 superfamily member 5
protein
|
Acknowledgments
Not applicable.
Funding
This study was supported by grants from the National
Research Foundation (2013M3A9A9050126, 2015R1A2A2A01007209,
2016M3A9B6916708 and 2009-0093812) funded by the Ministry of
Science and ICT in the Republic of Korea. BKP was supported by the
Hallym University Postdoctoral Fellowship Program of 2017
(HLM-PF-2017-0001).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YL, HJK and KCC conceived the study and its design
and wrote the manuscript. BKP, JYP, THK, DK, GW, AG, SM and SIL
performed the experiments. JYP and KCC performed the
clinicopathological analysis. BKP, THK, DK, GW and SIL were
involved in the animal experiments. BKP, JYP, YL, HJK and KCC
performed the revision of the manuscript and edited the manuscript.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Research protocols for the use of human tissues were
approved by, and conducted in accordance with the policies of the
Institutional Review Board at Hallym University Chuncheon Sacred
Heart Hospital (Permit no. 2014-108). Informed consent was obtained
from all study subjects. the animal experiments were approved by
the Institutional Animal Care and Use Committee of Hallym
University (Permit no. Hallym 2015-55, Hallym 2015-56).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
World Health Organization: GLOBOCAN 2012:
Estimated Cancer Incidence, Mortality and Prevalence Worldwide in
2012. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx.
Retrieved April, 2015.
|
|
2
|
Wilkinson N and Scott-Conner CE: Surgical
therapy for colorectal adenocarcinoma. Gastroenterol Clin North Am.
37:253–267. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Goodwin RA and Asmis TR: Overview of
systemic therapy for colorectal cancer. Clin Colon Rectal Surg.
22:251–256. 2009. View Article : Google Scholar :
|
|
4
|
Müller-Pillasch F, Wallrapp C, Lacher U,
Friess H, Büchler M, Adler G and Gress TM: Identification of a new
tumour-associated antigen TM4SF5 and its expression in human
cancer. Gene. 208:25–30. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee SA, Lee SY, Cho IH, Oh MA, Kang ES,
Kim YB, Seo WD, Choi S, Nam JO, Tamamori-Adachi M, et al:
Tetraspanin TM4SF5 mediates loss of contact inhibition through
epithelial-mesenchymal transition in human hepatocarcinoma. J Clin
Invest. 118:1354–1366. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee SA, Ryu HW, Kim YM, Choi S, Lee MJ,
Kwak TK, Kim HJ, Cho M, Park KH and Lee JW: Blockade of
four-transmembrane L6 family member 5 (TM4SF5)-mediated
tumorigenicity in hepatocytes by a synthetic chalcone derivative.
Hepatology. 49:1316–1325. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kwon S, Kim D, Park BK, Cho S, Kim KD, Kim
YE, Park CS, Ahn HJ, Seo JN, Choi KC, et al: Prevention and therapy
of hepatocellular carcinoma by vaccination with TM4SF5
epitope-CpG-DNA-liposome complex without carriers. PLoS One.
7:e331212012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kwon S, Kim D, Park BK, Wu G, Park MC, Ha
YW, Kwon HJ and Lee Y: Induction of immunological memory response
by vaccination with TM4SF5 epitope-CpG-DNA-liposome complex in a
mouse hepatocellular carcinoma model. Oncol Rep. 29:735–740. 2013.
View Article : Google Scholar
|
|
9
|
Kwon S, Kim YE, Kim D, Park BK, Wu G, Kim
TH, Choi SH, Kim DS, Kwon HJ and Lee Y: Prophylactic effect of a
peptide vaccine targeting TM4SF5 against colon cancer in a mouse
model. Biochem Biophys Res Commun. 435:134–139. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu YB, Huang YS, Xu YP, Sun YF, Yu DL,
Zhang XQ, Long X, Zhu SQ, Zhou JL and Xu JJ: A high level of TM4SF5
is associated with human esophageal cancer progression and poor
patient survival. Dig Dis Sci. 58:2623–2633. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wright MD, Ni J and Rudy GB: The L6
membrane proteins - a new four-transmembrane superfamily. Protein
Sci. 9:1594–1600. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Detchokul S, Williams ED, Parker MW and
Frauman AG: Tetraspanins as regulators of the tumour
microenvironment: Implications for metastasis and therapeutic
strategies. Br J Pharmacol. 171:5462–5490. 2014. View Article : Google Scholar
|
|
13
|
Yáñez-Mó M, Barreiro O, Gordon-Alonso M,
Sala-Valdés M and Sánchez-Madrid F: Tetraspanin-enriched
microdomains: A functional unit in cell plasma membranes. Trends
Cell Biol. 19:434–446. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jung O, Choi S, Jang SB, Lee SA, Lim ST,
Choi YJ, Kim HJ, Kim DH, Kwak TK, Kim H, et al: Tetraspan
TM4SF5-dependent direct activation of FAK and metastatic potential
of hepatocarcinoma cells. J Cell Sci. 125:5960–5973. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim HJ, Kwon S, Nam SH, Jung JW, Kang M,
Ryu J, Kim JE, Cheong JG, Cho CY, Kim S, et al: Dynamic and
coordinated single-molecular interactions at TM4SF5-enriched
microdomains guide invasive behaviors in 2- and 3-dimensional
environments. FASEB J. 31:1461–1481. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee SA, Kim YM, Kwak TK, Kim HJ, Kim S, Ko
W, Kim SH, Park KH, Kim HJ, Cho M, et al: The extracellular loop 2
of TM4SF5 inhibits integrin alpha2 on hepatocytes under collagen
type I environment. Carcinogenesis. 30:1872–1879. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kwon S, Choi KC, Kim YE, Ha YW, Kim D,
Park BK, Wu G, Kim DS, Lee Y and Kwon HJ: Monoclonal antibody
targeting of the cell surface molecule TM4SF5 inhibits the growth
of hepatocellular carcinoma. Cancer Res. 74:3844–3856. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim YE, Kwon S, Wu G, Kim D, Park BK, Park
JA, Choi KC, Kim DS, Kwon HJ and Lee Y: Therapeutic effect of a
TM4SF5-specific monoclonal antibody against colon cancer in a mouse
model. Oncotarget. 5:8402–8415. 2014. View Article : Google Scholar :
|
|
19
|
Wu G, Kim D, Park BK, Park S, Ha JH, Kim
TH, Gautam A, Kim JN, Lee SI, Park HB, et al: Anti-metastatic
effect of the TM4SF5-specific peptide vaccine and humanized
monoclonal antibody on colon cancer in a mouse lung metastasis
model. Oncotarget. 7:79170–79186. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim D, Kwon S, Ahn CS, Lee Y, Choi SY,
Park J, Kwon HY and Kwon HJ: Adjuvant effect of
liposome-encapsulated natural phosphodiester CpG-DNA. BMB Rep.
44:758–763. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Park BK, Choi SH, Kim YE, Park S, Lee Y,
Lee KW and Kwon HJ: Monoclonal antibodies against the human
respiratory syncytial virus obtained by immunization with epitope
peptides and CpG-DNA-liposome complex. Monoclon Antib Immunodiagn
Immunother. 34:101–109. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Park BK, Lee SI, Lee Y and Kwon HJ: Effect
of a monoclonal antibody against human relaxin-2 on cancer cell
growth inhibition. Appl Biol Chem. 59:739–746. 2016. View Article : Google Scholar
|
|
23
|
Yokoyama WM: Production of monoclonal
antibodies. Curr Protoc Cytom 2006. Appendix 3:Appendix 3J. 2006.
View Article : Google Scholar
|
|
24
|
Yokoyama WM, Christensen M, Santos GD and
Miller D: Production of monoclonal antibodies. Curr Protoc Immunol.
74:1–25. 2006.
|
|
25
|
Kim D, Kwon S, Rhee JW, Kim KD, Kim YE,
Park CS, Choi MJ, Suh JG, Kim DS, Lee Y, et al: Production of
antibodies with peptide-CpG-DNA-liposome complex without carriers.
BMC Immunol. 12:292011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kwon S, Kim YE, Park JA, Kim DS, Kwon HJ
and Lee Y: Therapeutic effect of a TM4SF5-specific peptide vaccine
against colon cancer in a mouse model. BMB Rep. 47:215–220. 2014.
View Article : Google Scholar :
|