Introduction
Natural products isolated from plants have attracted
increased interest due to their potent biological and
pharmaceutical activities (1).
Baicalein (5,6,7-trihydroxyflavone) extracted from the dry root of
Scutellaria baicalensis Georgi, also known as Baikal
skullcap, has a wide range of pharmacological functions and has
been reported to possess potential antitumor activities against
human liver cancer (2), breast
cancer (3-6) and lung cancer cells (7). Multiple mechanisms are associated
with its antitumor activity, however, the detailed mechanisms of
baicalein-induced apoptosis in the HCT116 human colon cancer cell
line remain to be fully elucidated.
Decidual protein induced by progesterone (DEPP) was
originally identified from the human endometrial stromal cell cDNA
library as a progesterone-induced gene (8). It is expressed in multiple tissues,
including the placenta, ovary, kidney, white adipose and liver
(9,10). In our previous study, DEPP was
markedly upregulated in baicalein-treated HCT116 cells as assessed
by microarray. Additionally, previous reports have suggested an
association between DEPP and tumor cell death (8). However, there have been no reports on
the physiological functions of DEPP in human colon cancer cells.
Therefore, it was hypothesized that DEPP may be involved in the
baicalein-induced cell death of HCT116 cells.
Growth arrest and DNA damage-inducible 45α (Gadd45a)
is a member of the Gadd45 family, the constituents of which can
interact with pivotal cell effectors, including p21, p38,
proliferating cell nuclear antigen, Cdc2/Cyclin B1 and mitogen-
activated protein kinase kinase kinase 4 (11). In addition, Gadd45a is a vital
regulator of cell cycle arrest, apoptosis and differentiation, all
of which are strictly regulated by p53 (12). Gadd45a has been shown to be crucial
in suppressing tumor cell growth (13,14).
However, whether baicalein-induced apoptosis is associated with the
activity of Gadd45a remains to be elucidated. Therefore, in the
present study, experiments were performed to investigate whether
Gadd45a and DEPP are involved in the baicalein-stimulated apoptosis
of human colon cancer cells and to examine the mechanism involved
in this activity.
The results of the present study verified that
baicalein significantly stimulated the apoptosis of HCT116, A549
and Panc-1 cells, upregulated the expression of DEPP and Gadd45a,
and triggered the phosphorylation of MAPKs. Further experiments
revealed that, as DEPP increased the protein and mRNA levels of
Gadd45a, the expression of DEPP and Gadd45a contributed to
baicalein-induced apoptosis and MAPK activation. Inhibiting c-Jun
N-terminal kinase (JNK)/p38 signaling also reduced the expression
of Gadd45a, however, there was no such change in the inactivation
of extracellular signal-regulated kinase (ERK). Taken together,
these findings identified the essential role of DEPP and Gadd45a in
baicalein-induced apoptosis and indicated that baicalein may be a
promising antitumor agent for the treatment of colon cancer in
humans.
Materials and methods
Cell cultures and drug treatments
The HCT116, A549, Panc-1 and FHC (normal human
colorectal mucosal cells) cell lines were obtained from American
Type Culture Collection (Rockville, MD, USA) and were grown in RPMI
1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% fetal bovine serum (CellMax, Beijing,
USA) and penicillin- streptomycin (Beyotime Institute of
Biotechnology, Nanjing, China). The cells were cultured in a
humidified incubator at 37°C and 5% CO2 and were exposed
to the indicated concentrations (0, 10, 20 or 40 µM) of
baicalein (Jiangsu Institute for Food and Drug Control, Nanjing,
China), 1 µM gemcitabine (cat. no. G8970, Beijing Solarbio
Science & Technology Co., Ltd., Beijing, China), SP600125 (cat.
no. S1460, Selleck Chemicals, Houston, TX, USA), SB203580 (cat. no.
S1076, Selleck Chemicals) or SCH772984 (cat. no. S7101, Selleck
Chemicals).
Western blot analysis
The cell lysates were prepared with RIPA buffer
containing PMSF (Beyotime Institute of Biotechnology). Protein
concentration was determined by Enhanced Bicinchoninic Acid Protein
assay kit (cat. no. P0010, Beyotime Institute of Biotechnology).
Equal quantities (50 µg) of cellular protein were loaded
onto a 12% SDS-PAGE gel and then transferred onto a PVDF membrane
(cat. no. IPVH00010, EMD Millipore, Billerica, MA, USA). The
membranes were blocked by 5% non-fat milk in Tris-buffered saline
with 0.07% Tween-20 for 2 h and incubated with specific primary
antibodies (1:1,000) in 1% bovine serum albumin (Biosharp, Hefei,
China) in Tris-buffered saline with 0.07% Tween-20 at 4°C
overnight. The following primary antibodies were used: Rabbit
anti-DEPP (cat. no. 25833-1-AP, 1:1,000) from ProteinTech Group,
Inc. (Rosemont, IL, USA); mouse anti-β-actin (cat. no. 21800,
1:1,000) from SAB Signalway Antibody (Nanjing, China); and rabbit
anti-Gadd45a (cat. no. 4632, 1:1,000), rabbit anti-caspase-3 (cat.
no. 14220, 1:1,000), rabbit anti-caspase-9 (cat. no. 9502,
1:1,000), rabbit anti-cleaved-capase-9 (cat. no. 9505, 1:1,000),
mouse anti-JNK (cat. no. 3780, 1:1000), rabbit anti-phosphorylated
(p-)JNK (cat. no. 4668, 1:1,000), rabbit anti-ERK (cat. no. 4695,
1:1,000), rabbit anti-p-ERK (cat. no. 4370, 1:1,000), rabbit
anti-p38 (cat. no. 9212, 1:1,000) and rabbit anti-p-p38 (cat. no.
4511, 1:1,000) from Cell Signaling Technology, Inc. (Danvers, MA,
USA). The secondary antibodies used were goat anti-rabbit IgG (cat.
no. L3012-2, 1:5,000) or goat anti-mouse IgG (cat. no. L3032-2,
1:5,000) from SAB Signalway Antibody. The membranes were incubated
with diluted secondary antibodies (1:5,000) in 1% bovine serum
albumin at room temperature for 1.5 h. The signal was visualized
using the Immobilon Western Chemiluminescent HRP Substrate (cat.
no. WBKLS0500, EMD Millipore), using Image Lab 5.2.1 (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The quantitative
densitometric analysis was performed using ImageJ 1.48v with 32-bit
Java 1.6.0_20 (National Institutes of Health, Bethesda, MD,
USA).
Detection of apoptosis
Apoptosis induced by baicalein was determined using
an Annexin V-FITC/PI Apoptosis Detection kit (cat. no. A211-02,
Vazyme, Nanjing, China). The cells were seeded into a 12-well plate
at 5×104 cells/ml and treated with different
concentrations of baicalein for 48 h. The cells were detached with
0.25% trypsin without EDTA, washed twice with cold PBS and
resuspended in 100 µl binding buffer. The cells were then
incubated with 5 µl Annexin V-FITC and 5 µl PI for 10
min at room temperature in the dark, and analyzed with the Accuri
C6 flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA from the cultured cells was extracted
using TRIzol (cat. no. R401-01, Vazyme, Piscataway, NJ, USA) and
chloroform/isopropanol, as specified by the manufacturer, and then
reverse transcribed using a HiScript Q RT SuperMix for qPCR (+gDNA
wiper) kit (cat. no. R223-01, Vazyme). The qPCR was subsequently
performed using a ChamQ SYBR qPCR Master Mix kit (cat. no. Q311-02,
Vazyme) and each sample [5 µl 2X ChamQ SYBR qPCR Master Mix,
0.2 µl Primer 1 (10 µM), 0.2 µl Primer 2 (10
µM), 0.2 µl 50X ROX Reference Dye1, 2 µl
template cDNA and 2.4 µl ddH2O] was analyzed on
the StepOne™ Real-Time PCR system (Thermo Fisher Scientific, Inc.).
The thermocycling conditions were as follows: Stage 1, 95°C for 3
min; Stage 2, 95°C for 10 sec, 56°C for 30 sec, 72°C for 30 sec;
Stage 3, 95°C for 15 sec, 60°C for 60 sec, 95°C for 15 sec. The
following PCR primers were used: GAPDH forward, 5′-GGGAAACTG
TGGCGTGAT-3′ and reverse, 5′-GAGTGGGTGTCGCTGTTGA-3′; DEPP forward,
5′-ATACGTCCTGTGGTGGCATTG-3′ and reverse,
5′-CCTGATTCCCGTTCCCTGAT-3′; Gadd45a forward,
5′-TTGCAATATGACTTTGGAGGAA-3′ and reverse, 5′-CATC
CCCCACCTTATCCAT-3′; p21 forward, 5′-AGCAGAGGAAGA CCATGTGGA-3′ and
reverse, 5′-AATCTGTCATGCTGGTCTGCC-3′; and p53 forward,
5′-CTCTCCCCAGCCAAAGAAGAA-3′ and reverse,
5′-TCCAAGGCCTCATTCAGCTCT-3′ (Springen Biotechnology, Nanjing,
China). The relative mRNA expression was normalized to that of
GAPDH and was determined using the comparative Cq method
(2−ΔΔCq) (15).
Small interfering (si)RNA
transfection
siRNA targeting DEPP and Gadd45a, and non-specific
siRNA (NC) were synthesized by GenScript Co., Ltd. (Nanjing,
China): DEPP, 5′-GCAGUGUCCUCAGAACACU-3′; Gadd45a
5′-AAAGUCGCUACAUGGAUCAAU-3′; NC 5′-UUCUCCG AACGUGUCACGU-3′. The
cells were transfected with siDEPP, siGadd45a or non-specific siRNA
using Entranster™-R4000 (Engreen Biosystem, Ltd., Beijing, China)
according to the manufacturer's protocol.
Overexpression of DEPP
The HCT116 cells were transfected with 0.82
µg/µl of either empty pcDNA3.1 or pcDNA3.1 (GenScript
Co., Ltd.) containing DEPP cDNA using Lipofectamine 2000
transfection reagent (Thermo Fisher Scientific, Inc.). The cells
were harvested 72 h following transfection.
MTT proliferation assay
The cells were treated with the indicated
concentrations of Baicalein at 37°C for 48 h. Subsequently, MTT was
added to reach a final concentration of 5 mg/ml for 4 h. The
supernatant was removed, and the purple-colored formazan
precipitate was dissolved in 150 µl DMSO and measured at 490
nm with a microplate reader (iMark; Bio-Rad Laboratories,
Inc.).
Statistical analysis
All data are shown as the mean ± standard deviation
of at least three independent experiments. Statistical analyses
were performed using GraphPad Prism 5 (GraphPad Software, Inc., La
Jolla, CA, USA), and data were analyzed using one-way analysis of
variance, followed by the Dunnett's multiple comparison test. In
certain cases, Student's t-test was used for comparing two groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Baicalein induces the apoptosis of
HCT116, A549 and Panc-1 cells
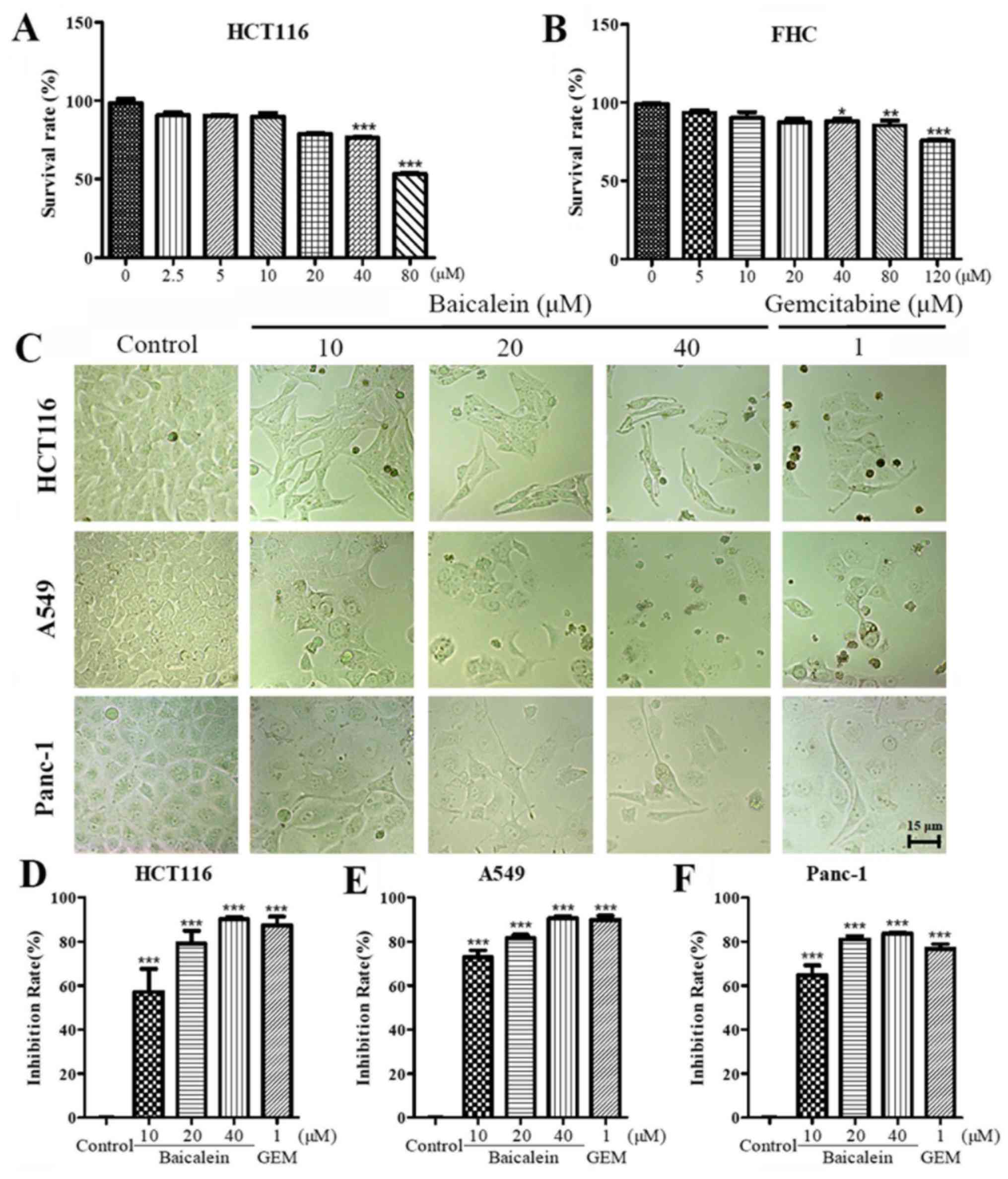
Firstly, an MTT assay was performed to confirm that
the appropriate concentration of baicalein was used. The data from
the HCT116 (Fig. 1A) and FHC
(Fig. 1B) cells demonstrated that
40 µM baicalein significantly inhibited the proliferation of
HCT116 (~24%) and cased less damage to FHC cells (~10%).
Subsequently, to detect the effect of baicalein on human cancer
cells, light microscopy was used to observe the morphological
changes of HCT116, A549 and Panc-1 cells following treatment with
0, 10, 20 or 40 µM baicalein with 1 µM gemcitabine
serving as a positive control. As shown in Fig. 1C, cells treated with baicalein (10,
20 or 40 µM) or gemcitabine (1 µM) were in flattened,
blebby and shrunken in appearance, consistent with cell death,
whereas the negative control cells presented with an intact and
polygonal morphology. Additionally, baicalein markedly inhibited
the proliferation of HCT116 (Fig.
1D), A549 (Fig. 1E), and
Panc-1 (Fig. 1F) cells in a
dose-dependent manner. For example, 40 µM baicalein
inhibited proliferation by up to 90%. Furthermore, the data
indicated that baicalein induced apoptosis of the HCT116 cells in a
dose-dependent manner as assessed by the Annexin V/PI double
staining assay (Fig. 2A), and
significantly increase the levels of cleaved-caspase-3 and
cleaved-caspase-9 (Fig. 2B)
following treatment for the indicated times. Similar results were
also observed in the A549 (Fig. 2C and
D) and Panc-1 (Fig. 2E and F)
cells, suggesting that baicalein induced the apoptosis of HCT116,
A549 and Panc-1 cells.
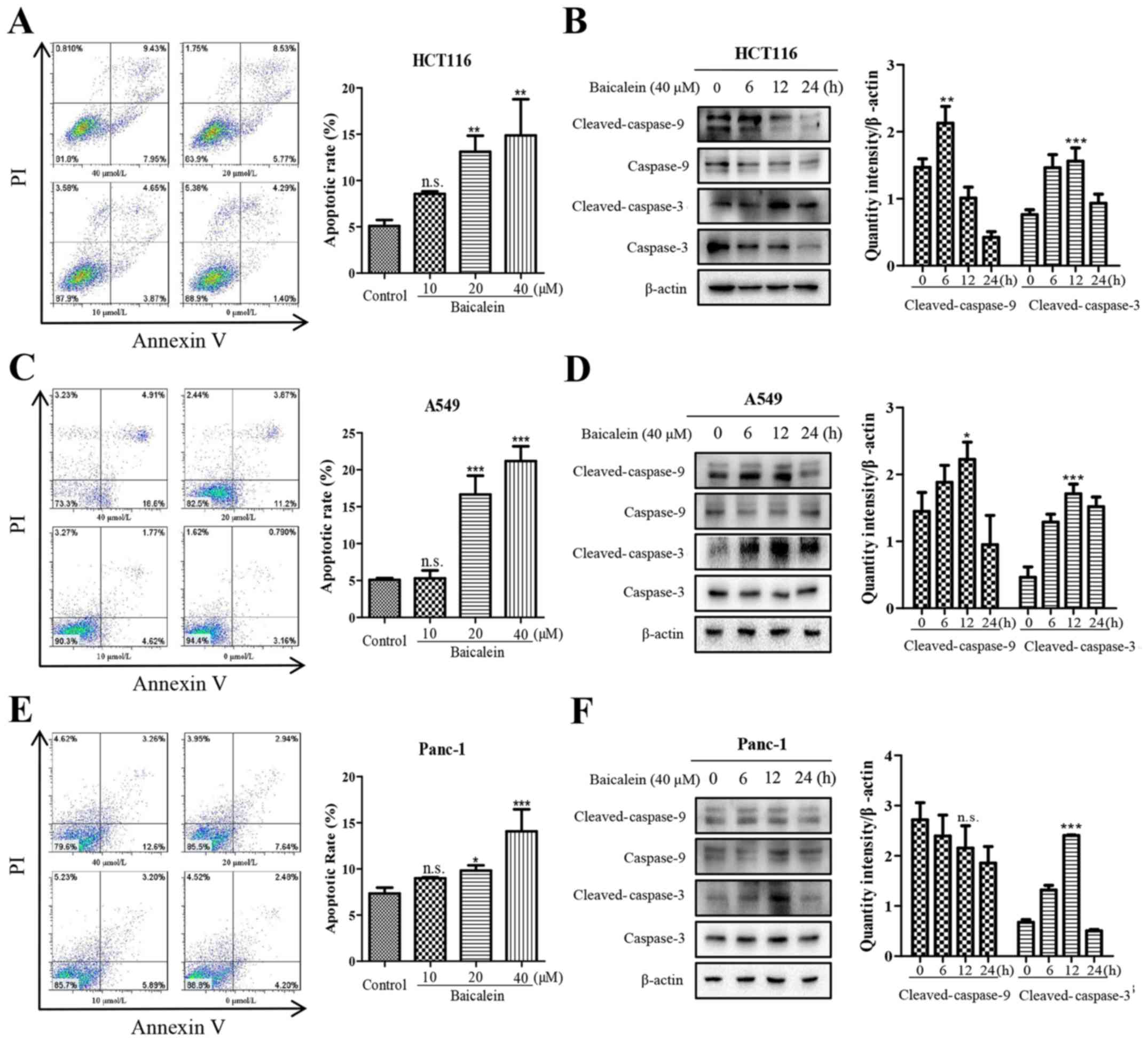 | Figure 2Baicalein induces the apoptosis of
HCT116, A549 and Panc-1 cells. (A) Flow cytometric analysis of
HCT116 cell apoptosis; (B) Immublot assay of cleaved-caspase-9 and
caspase-3 in HCT116 cells; (C) Flow cytometric analysis of A549
cell apoptosis; (D) Immublot assay of cleaved-caspase-9 and
caspase-3 in A549 cells; (E) Flow cytometric analysis of Panc-1
cell apoptosis; (F) Immublot assay of cleaved-caspase-9 and
caspase-3 in Panc-1 cells. Flow cytometric analysis was performed
following treatment with baicalein (0, 10, 20 or 40 µM) for
48 h. Representative results are shown and the histogram (right)
shows the mean apoptotic rate as detected by flow cytometry. For
immunoblotting, cells were treated with 40 µM baicalein for
0, 6, 12 or 24 h, and the induced cleavage of caspase-9 and
caspase-3 was detected. All data shown are presented as the mean ±
standard deviation from three independent experiments
(*P<0.05, **P<0.01 and
***P<0.001 compared with the control group). DEPP,
decidual protein induced by progesterone; Gadd45a, growth arrest
and DNA damage-inducible 45α; n.s., not significant. |
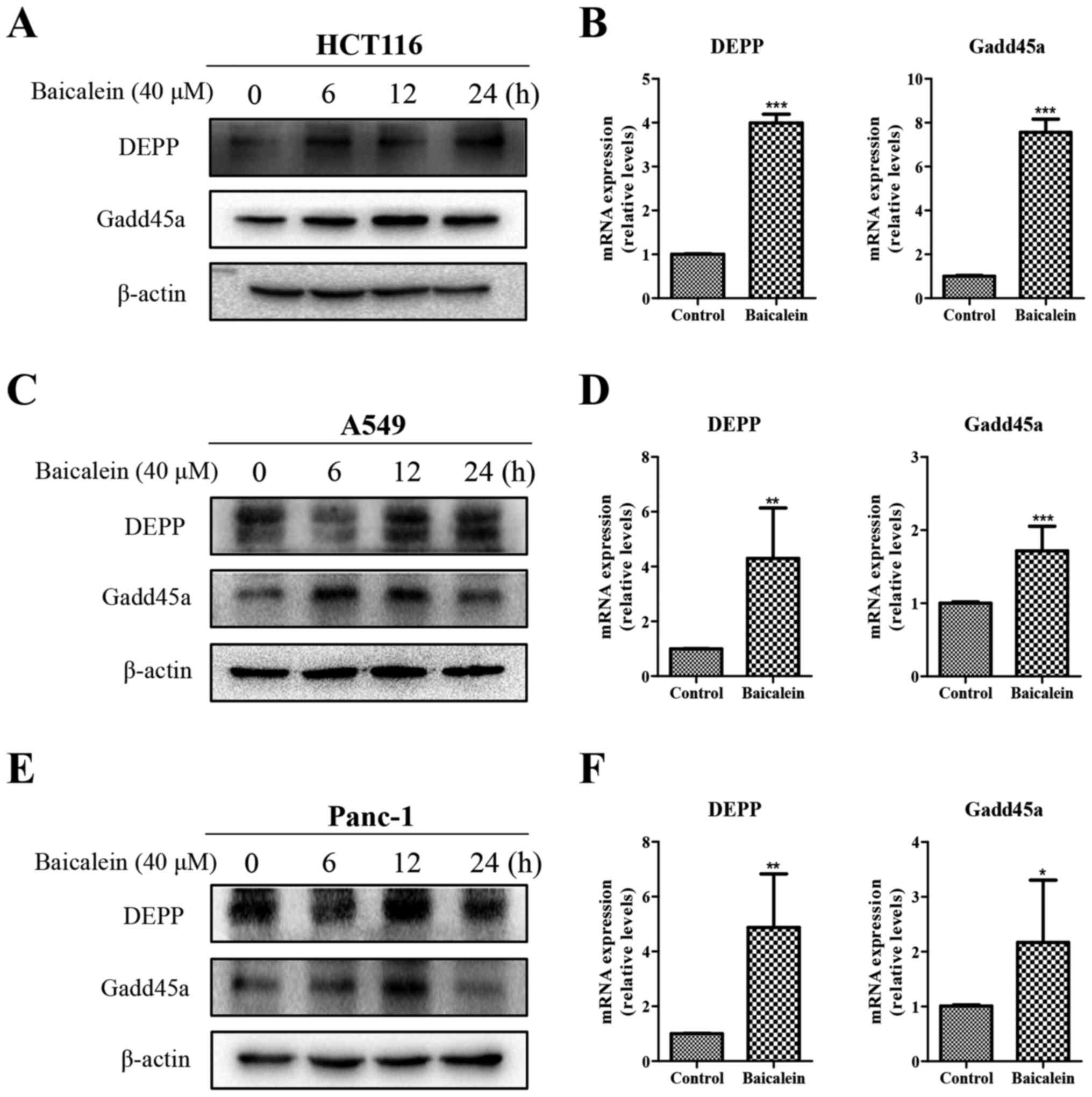
Expression levels of DEPP and Gadd45a are
elevated in cancer cells subjected to baicalein-induced
apoptosis
To verify the potential role of DEPP and Gadd45a in
baicalein- induced apoptosis, the present study investigated
whether the expression levels of DEPP and Gadd45a were altered in
baicalein-treated cells. Western blot analysis (Fig. 3A) and RT-qPCR analysis (Fig. 3B) showed that the protein and mRNA
expression levels of DEPP and Gadd45a were elevated more than
two-fold in the baicalein-treated HCT116 cells. The data for
protein levels in the A549 and Panc-1 cells were similar to those
for the HCT116 cells. However, the mRNA expression of DEPP and
Gadd45a in the A549 and Panc-1 cells had distinctly increased
following 6 h of baicalein treatment, vs. 24 h of treatment
(Fig. 3C–F), which may be
attributable to the delay between transcription and translation.
These results confirmed that baicalein upregulated DEPP and Gadd45a
in three distinct human cancer cell lines.
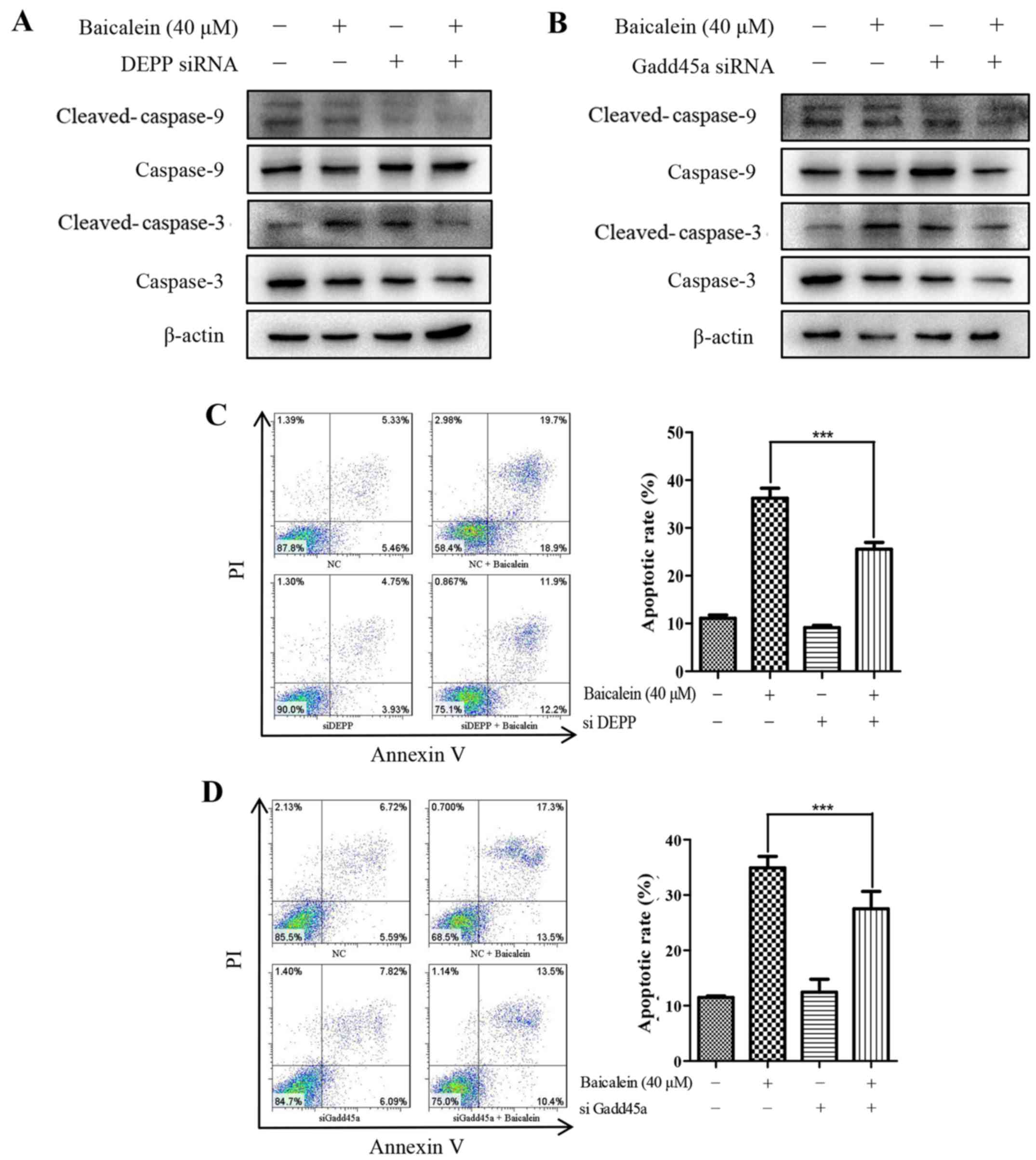
DEPP and Gadd45a deficiency inhibits
baicalein-induced apoptosis of HCT116 cells
To determine whether DEPP and Gadd45a were necessary
for baicalein-induced apoptosis, siRNA against either DEPP or
Gadd45a was transfected into HCT116 cells. Western blot analysis
showed that the accumulation of baicalein-induced cleaved-caspase-3
and cleaved-caspase-9 were markedly reduced when DEPP and Gadd45a
were silenced (Fig. 4A and B).
These results were further confirmed by the Annexin V/PI double
staining assay. As shown in Fig. 4C
and D, there was a decrease of ~10% in the apoptotic rate of
cells treated with 40 µM baicalein when transfected with
either DEPP siRNA or Gadd45a siRNA, compared with cells transfected
with non-specific siRNA. Taken together, these data showed that
baicalein upregulated the expression of DEPP and Gadd45a, which was
involved in the apoptotic response in HCT116 cells through the
activation of caspase-3 and caspase-9.
Baicalein-induced HCT116 cell apoptosis
via the upregulation of DEPP/Gadd45a is mediated by the
phosphorylation of MAPKs
To further elucidate the mechanism linking
baicalein-induced apoptosis and the expression of DEPP and Gadd45a,
the activation of MAPK was examined using an immunoblot assay in
HCT116 cells at 0, 6, 12 and 24 h post-baicalein treatment.
Notably, increases in the phosphorylation of JNK, ERK and p38 were
observed in the western blot analysis (Fig. 5A). The expression of DEPP was then
stably inhibited using siRNA to determine whether DEPP is required
for the baicalein-induced phosphorylation of MAPKs. Following
baicalein treatment, the absence of DEPP (Fig. 5B and C) had a negative effect on
the baicalein-mediated protein expression of p-JNK, p-ERK, p-p38
and Gadd45a, or on the mRNA expression of p21, p53 and Gadd45a. In
addition, a DEPP-overexpression plasmid was used to further
validate these findings (Fig. 5D).
Similarly, p-JNK, p-ERK, p-p38, p21, and p53 were induced only when
the overexpression of DEPP was present (Fig. 5D and E). Furthermore, the gene
expression of Gadd45a was increased by 1.60-fold. Together, these
results suggested that DEPP is required for the baicalein-induced
phosphorylation of MAPKs and upregulated expression of Gadd45a at
the protein and mRNA levels. The present study subsequently
examined whether Gadd45a also regulated the baicalein- induced
phosphorylation of MAPKs, and similar results were observed in the
levels of p-JNK, p-ERK and p-p38 in cells transfected with siRNA
against Gadd45a (Fig. 5F). In
summary, these data suggested that DEPP and Gadd45a are essential
in baicalein-induced apoptosis via MAPK signaling.
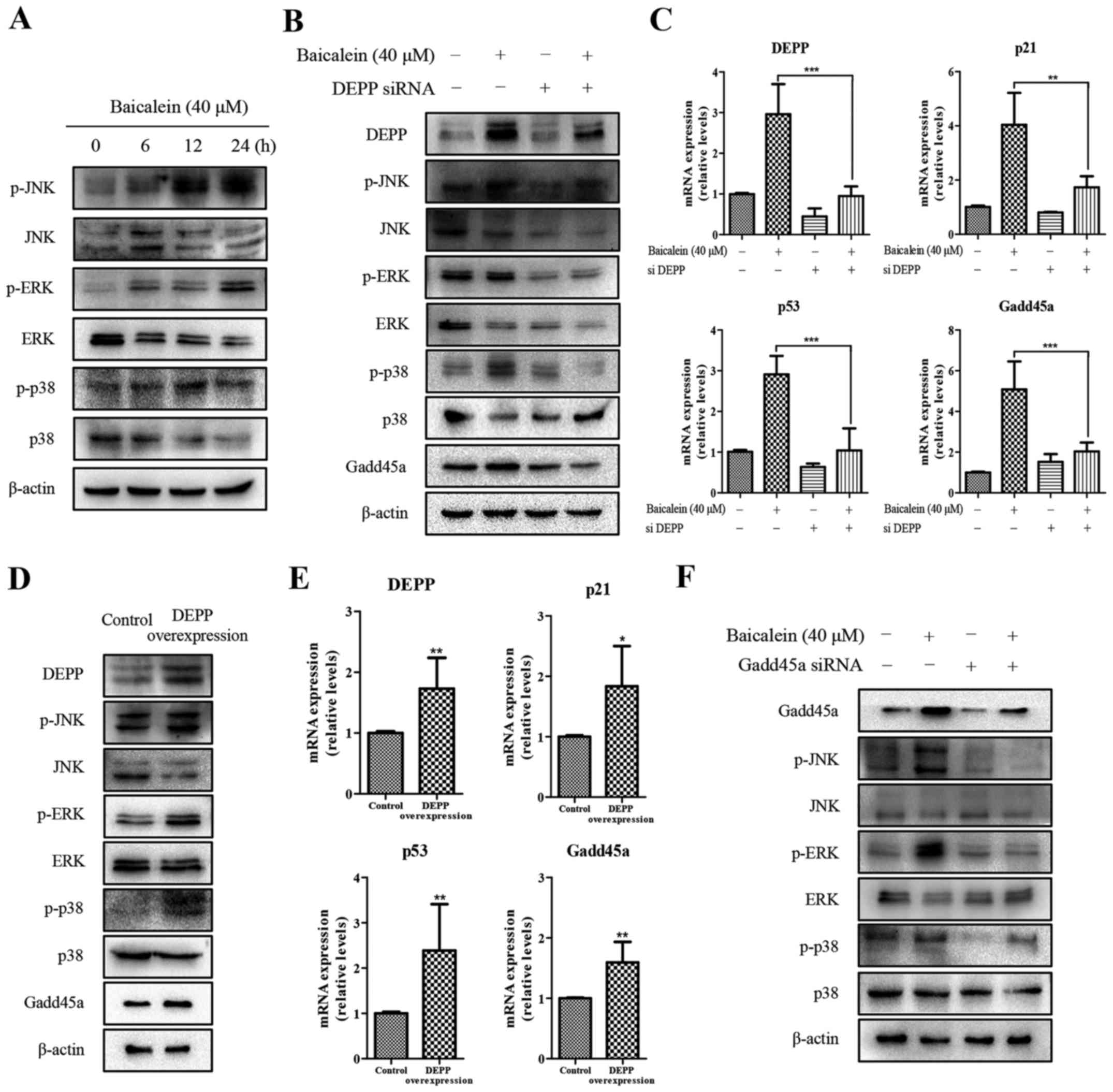 | Figure 5Expression of DEPP and Gadd45a
contribute to the baicalein-induced phosphorylation of MAPKs in
HCT116 cells. (A) Western blot analysis of the expression of p-JNK,
JNK, p-ERK, ERK, p-p38 and p38 in HCT116 cells treated with
baicalein (40 µM) for 0, 6, 12 or 24 h. (B) Western blot
analysis of the expression of DEPP, Gadd45a, p-JNK, JNK, p-ERK,
ERK, p-p38, p38 in DEPP siRNA-transfected HCT116 cells treated with
baicalein (40 µM) for 24 h. (C) RT-qPCR analysis of the mRNA
expression of DEPP, Gadd45a, p21 and p53 in DEPP siRNA-transfected
HCT116 cells treated with baicalein (40 µM) for 24 h. (D)
Western blot analysis of the expression of DEPP, Gadd45a, p-JNK,
JNK, p-ERK, ERK, p-p38 and p38 in HCT116 cells overexpressing DEPP
for 72 h. (E) RT-qPCR analysis of the mRNA expression of DEPP,
Gadd45a, p21 and p53 in HCT116 cells overexpressing DEPP for 72 h.
(F) Western blot analysis of the expression of Gadd45a, p-JNK, JNK,
p-ERK, ERK, p-p38 and p38 in Gadd45a siRNA-transfected HCT116 cells
treated with baicalein (40 µM) for 24 h. All data shown are
presented as the mean ± standard deviation from three independent
experiments (*P<0.05, **P<0.01 and
***P<0.001 compared with the control group, or as
indicated). DEPP, decidual protein induced by progesterone;
Gadd45a, growth arrest and DNA damage-inducible 45α; siRNA/si,
small interfering RNA; JNK, c-Jun N-terminal kinase; ERK,
extracellular signal-regulated kinase; p-, phosphorylated; RT-qPCR,
reverse transcription-quantitative polymerase chain reaction. |
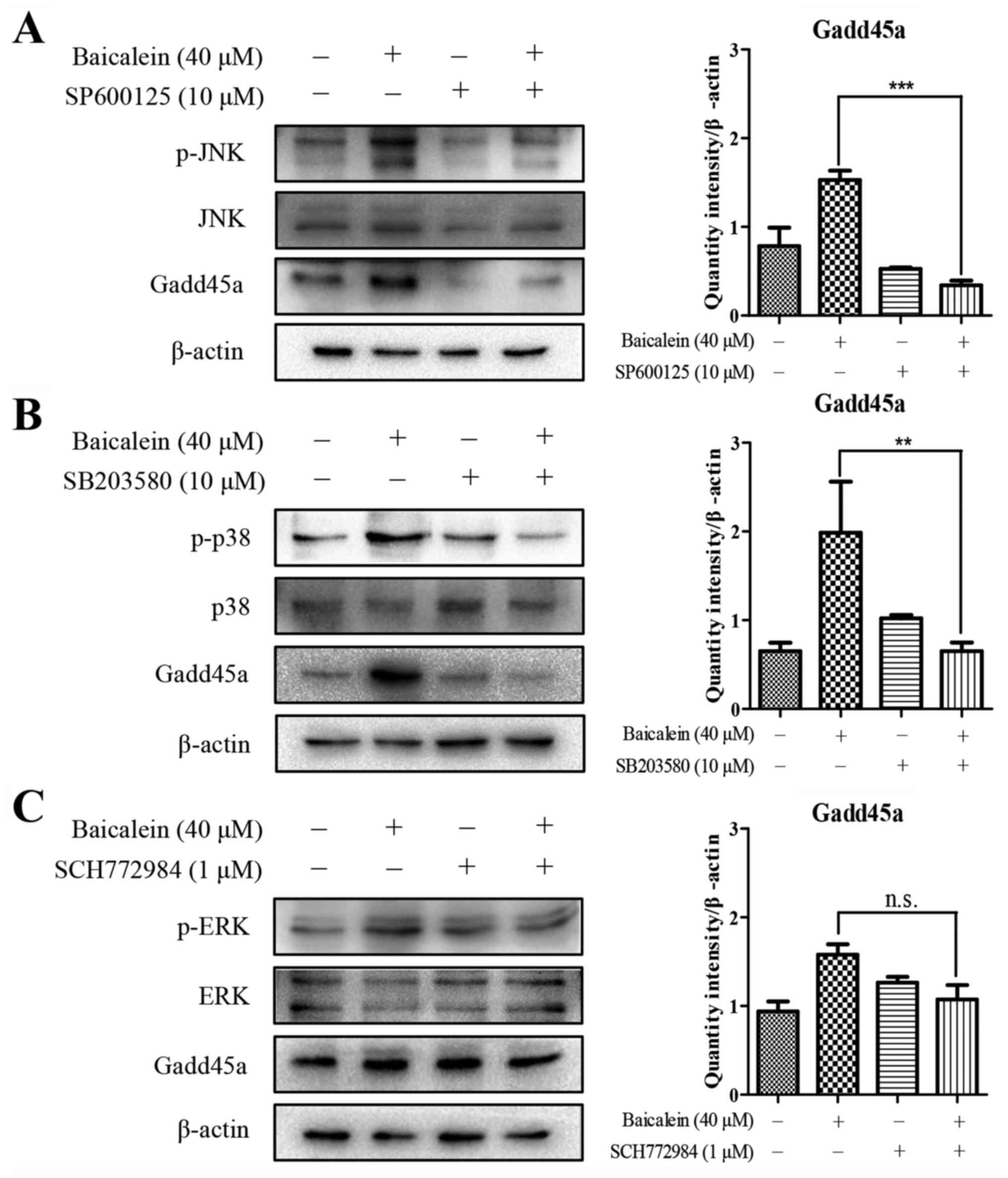
Baicalein-induced upregulation of Gadd45a
is attenuated by inhibiting the phosphorylation of MAPK
The abovementioned results showed that Gadd45a
upregulated the phosphorylation of JNK, ERK and p38 in the
baicalein-induced apoptosis of HCT116 cells. A previous report
demonstrated that MAPK signaling mediates the expression of Gadd45a
(14). Therefore, to determine
whether MAPK signaling was involved in the baicalein-mediated
upregulation of Gadd45a, the JNK-specific inhibitor SP600125, p38
inhibitor SB203580, and ERK inhibitor SCH772084 were used to
prevent the activation of JNK, p38 and ERK, respectively. The
inhibition of JNK and p38 was accompanied by a marked decrease in
the baicalein-induced expression of Gadd45a (Fig. 6A and B), however, no such change
was observed in the presence of the ERK inhibitor SCH772084
(Fig. 6C). These results suggested
that there was a positive feedback loop between Gadd45a and JNK/p38
when baicalein triggered the apoptotic response in human colon
cancer cells, whereas the suppression of ERK had no effect on the
expression of Gadd45a. Therefore, these data revealed the critical
role of the activation of MAPK in inducting of the expression of
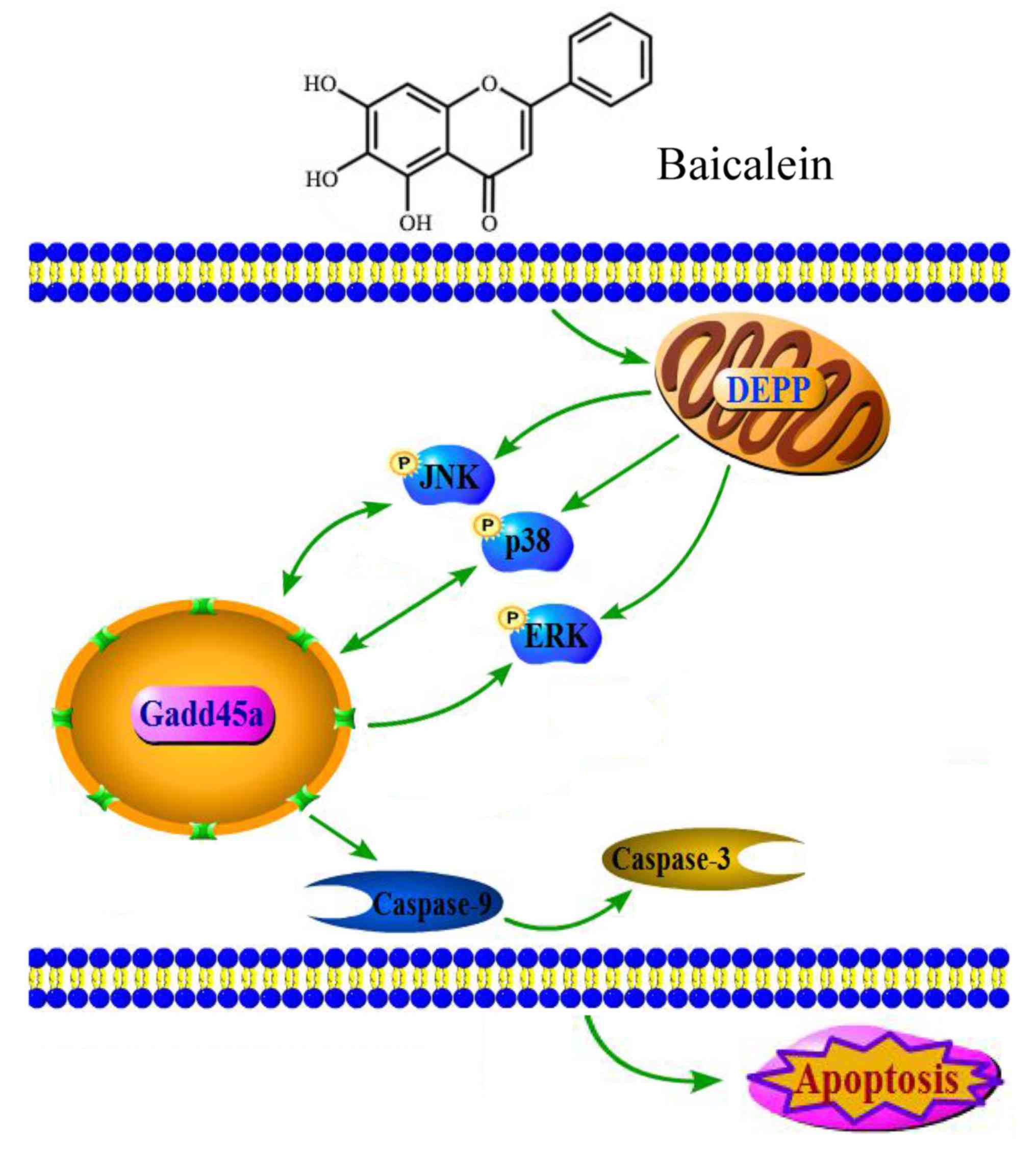
Gadd45a in baicalein-induced apoptosis (Fig. 7).
Discussion
In the present study, it was shown that baicalein
upregulated DEPP and Gadd45a, which activated caspase-3, caspaase-9
and the JNK/ERK/p38 MAPK pathways leading to apoptosis of HCT116
human colon cancer cells. As a natural compound extracted from
Scutellaria baicalensis Georgi, baicalein has been widely
investigated for its potent antitumor properties. The majority of
studies have revealed that baicalein exerts its antitumor ability
via the induction of apoptosis in human lung cancer (6), breast cancer (7) and osteosarcoma cells (16). In addition, it also has been
demonstrated that the MAPK signaling pathway results in the
baicalein-induced apoptosis of cancer cells (7). Therefore, the present study was
performed to validate these findings and observed not only marked
induction of apoptosis by baicalein in HCT116 colon cancer cells,
A549 lung carcinoma cells and Panc-1 pancreatic cancer cells, but
also the prominent activation of caspase-3, caspase-9 and
JNK/ERK/p38 in baicalein-treated HCT116 cells.
However, whether the MAPKs functioned upstream or
downstream to mediate baicalein-induced apoptosis remained to be
elucidated. Therefore, on the basis of our previous results that
DEPP and Gadd45a were markedly upregulated in baicalein-treated
HCT116 cells by microarray, it was hypothesized DEPP and Gadd45a
were important in baicalein-induced apoptosis and the activation of
caspase-3, caspase-9 and MAPKs in HCT116 cells. Consistent with
this hypothesis, the absence of DEPP notably decreased the
apoptotic rate and the levels of pro-apoptotic proteins
cleaved-caspase-3, cleaved-caspase-9, p-JNK/ERK/p38, p21, p53 and
Gadd45a, all of which were induced by baicalein. Similar results
were observed in HCT116 cells transfected with Gadd45a siRNA.
Furthermore, the overexpression of DEPP provoked the
phosphorylation of MAPKs and the expression of p21, p53 and
Gadd45a. Taken together, these results indicated that as DEPP
upregulated Gadd45a at the protein and mRNA levels, both these
genes contribute to the activation of JNK/ERK/p38 during
baicalein-induced apoptosis. However, further in vitro and
in vivo investigations are required to examine whether
baicalein has a direct impact on the expression of DEPP.
DEPP is a progesterone-induced gene that is
regulated by progesterone in endometrial stromal cells and by
insulin in adipose tissue. Additionally, it is induced in malignant
glioma cells under hypoxic conditions (9,10,17).
However, there are few reports on its physiological function.
Salcher et al found that the expression of DEPP contributes
to Forkhead Box O3 (FOXO3)-induced apoptosis, as DEPP knockdown
significantly reduced FOXO3-induced cell death (8). Another study demonstrated that the
upregulation of DEPP activated MAPK signaling pathways to stimulate
the transcription factor ELK1 (17). Therefore, the findings in the
present study that DEPP promoted baicalein-induced apoptosis by the
activation of Gadd45a, JNK/ERK/p38, caspase-3 and caspase-9, can
drive further investigations regarding the physiological function
of DEPP. However, there remain several questions in terms of how
baicalein increases the mRNA and protein levels of DEPP and how
DEPP induces the phosphorylation of MAPKs, two activities requiring
thorough investigation.
In contrast to DEPP, the induction of Gadd45a and
apoptosis via the MAPK signaling pathway has been examined
extensively (13,14). However, detailed conclusions
regarding the feedback loop between Gadd45a and MAPKs remain to be
fully elucidated as reports have conflicting data. Certain reports
have shown that Gadd45a is upstream of MAP signaling and activates
the JNK pathway (18), whereas
others have suggested that Gadd45a is downstream of MAPK pathways,
and is positively regulated by JNK and negatively regulated by
ERK/p38 (14). Accordingly, the
analyses in the present study of the mediation between the
expression of Gadd45a and baicalein-induced activation of
JNK/ERK/p38 in human colon cancer cells is useful as a supplement
to previously published data. The in vitro experiments
suggested the existence of a positive feedback loop between Gadd45a
and JNK/p38. However, further investigations on the detailed role
of Gadd45a and JNK/p38 on this loop, and the role of ERK outside
this loop, in baicalein-induced apoptosis are required.
In conclusion, the present study provided evidence
of a novel mechanism of baicalein-induced apoptosis in human colon
cancer cells and found for the first time, to the best of our
knowledge, that baicalein upregulated DEPP and Gadd45a, leading to
apoptosis via the MAPK signaling pathway and the activation of
caspase-3 and caspase-9 in HCT116 cells. In this context, the
existence of a positive feedback loop between Gadd45a and JNK/p38
was also confirmed. In general, the findings of the present study
may encourage the development of novel options to target DEPP and
the Gadd45a-JNK/p38 feedback loop in the treatment of colon
cancer.
Acknowledgments
Not applicable.
Funding
This study was supported by the National Natural
Science Foundation of China (grant no. 81472233), the Jiangsu
National Natural Science Foundation of China (grant no. BK20150700)
and the '111 Projec' from the Ministry of Education of China and
the State Administration of Foreign Expert Affairs of China (grant
no. 111-2-07).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MS, YZ and JD designed and performed the
experiments; XR, HY, XZ and XK contributed to the data analysis; BS
and CZ contributed to the conception of the study and helped with
data interpretation; GP drafted the manuscript and was responsible
for methodology; JD and CZ revised the manuscript critically; all
authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liu X and Liu S, Chen J, He L, Meng X and
Liu S: Baicalein suppresses the proliferation of acute
T-lymphoblastic leukemia Jurkat cells by inhibiting the
Wnt/β-catenin signaling. Ann Hematol. 95:1787–1793. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Han Z, Zhu S, Han X, Wang Z, Wu S and
Zheng R: Baicalein inhibits hepatocellular carcinoma cells through
suppressing the expression of CD24. Int Immunopharmacol.
29:416–422. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chung H, Choi HS, Seo EK, Kang DH and Oh
ES: Baicalin and baicalein inhibit transforming growth
factor-β1-mediated epithelial-mesenchymal transition in human
breast epithelial cells. Biochem Biophys Res Commun. 458:707–713.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang XF, Zhou QM, Du J, Zhang H, Lu YY and
Su SB: Baicalin suppresses migration, invasion and metastasis of
breast cancer via p38MAPK signaling pathway. Anticancer Agents Med
Chem. 13:923–931. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ma X, Yan W, Dai Z, Gao X, Ma Y, Xu Q,
Jiang J and Zhang S: Baicalein suppresses metastasis of breast
cancer cells by inhibiting EMT via downregulation of SATB1 and
Wnt/β-catenin pathway. Drug Des Devel Ther. 10:1419–1441. 2016.
View Article : Google Scholar :
|
|
6
|
Zhou QM, Wang S, Zhang H, Lu YY, Wang XF,
Motoo Y and Su SB: The combination of baicalin and baicalein
enhances apoptosis via the ERK/p38 MAPK pathway in human breast
cancer cells. Acta Pharmacol Sin. 30:1648–1658. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim HJ, Park C, Han MH, Hong SH, Kim GY,
Hong SH, Kim ND and Choi YH: Baicalein induces caspase-dependent
apoptosis associated with the generation of ROS and the activation
of AMPK in human lung carcinoma A549 Cells. Drug Dev Res. 77:73–86.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Salcher S, Hagenbuchner J, Geiger K,
Seiter MA, Rainer J, Kofler R, Hermann M, Kiechl-Kohlendorfer U,
Ausserlechner MJ and Obexer P: C10ORF10/DEPP, a transcriptional
target of FOXO3, regulates ROS-sensitivity in human neuroblastoma.
Mol Cancer. 13:2242014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kuroda Y, Kuriyama H, Kihara S, Kishida K,
Maeda N, Hibuse T, Nishizawa H, Matsuda M, Funahashi T and
Shimomura I: Insulin-mediated regulation of decidual protein
induced by progesterone (DEPP) in adipose tissue and liver. Horm
Metab Res. 42:173–177. 2010. View Article : Google Scholar
|
|
10
|
Chen S, Gai J, Wang Y and Li H: FoxO
regulates expression of decidual protein induced by progesterone
(DEPP) in human endothelial cells. FEBS Lett. 585:1796–1800. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sytnikova YA, Kubarenko AV, Schäfer A,
Weber AN and Niehrs C: Gadd45a is an RNA binding protein and is
localized in nuclear speckles. PLoS One. 6:e145002011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tong T, Ji J, Jin S, Li X, Fan W, Song Y,
Wang M, Liu Z, Wu M and Zhan Q: Gadd45a expression induces Bim
dissociation from the cytoskeleton and translocation to
mitochondria. Mol Cell Biol. 25:4488–4500. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tront JS, Hoffman B and Liebermann DA:
Gadd45a suppresses Ras-driven mammary tumorigenesis by activation
of c-Jun NH2-terminal kinase and p38 stress signaling resulting in
apoptosis and senescence. Cancer Res. 66:8448–8454. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yin F, Bruemmer D, Blaschke F, Hsueh WA,
Law RE and Herle AJ: Signaling pathways involved in induction of
GADD45 gene expression and apoptosis by troglitazone in human MCF-7
breast carcinoma cells. Oncogene. 23:4614–4623. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−∆ ∆ C(T)) Method. Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
16
|
Ye F, Wang H, Zhang L, Zou Y, Han H and
Huang J: Baicalein induces human osteosarcoma cell line MG-63
apoptosis via ROS-induced BNIP3 expression. Tumour Biol.
36:4731–4740. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Watanabe H, Nonoguchi K, Sakurai T, Masuda
T, Itoh K and Fujita J: A novel protein Depp, which is induced by
progesterone in human endometrial stromal cells activates Elk-1
transcription factor. Mol Hum Reprod. 11:471–476. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Song L, Li J, Zhang D, Liu ZG, Ye J, Zhan
Q, Shen HM, Whiteman M and Huang C: IKKbeta programs to turn on the
GADD45α-MKK4-JNK apoptotic cascade specifically via p50 NF-kappaB
in arsenite response. J Cell Biol. 175:607–617. 2006. View Article : Google Scholar : PubMed/NCBI
|