Introduction
Malignant melanoma is an aggressive tumor of the
skin with a poor prognosis for patients with advanced disease.
Despite novel therapeutic treatments developed in recent years,
patients with advanced melanoma continue to succumb to the disease.
Over the past decade, many efforts have been made to enhance our
understanding of the molecular biological mechanisms associated
with the development of melanoma, and several of the key
alterations affecting the regulation of cellular proliferation- and
viability-related pathways have been identified (1,2).
Over 50% of melanoma cases harbor activating V600E mutations in
BRAF (BRAFV600E), which sustains the proliferation and
survival of melanoma cells by activating the mitogen-activated
protein kinase (MAPK) pathway (2–6).
Thus, there is an ongoing effort to develop small molecules acting
as inhibitors of the activated effectors into the MAPK pathway.
Among these, vemurafenib and dabrafenib, two inhibitors of the
mutated BRAF (mostly, BRAFV600E mutation), have been
documented to significantly improve the clinical outcome of
patients with metastatic melanoma (7,8).
Although both vemurafenib and dabrafenib positively affect
progression-free and overall survival, their prolonged use as
single agents is limited due to the consistent development of drug
resistance (9). Indeed, the
majority of patients who initially respond to treatment with BRAF
inhibitor (BRAFi), relapse within 6–8 months, suggesting that
chronic treatment with BRAFi may strongly induce the activation of
molecular mechanisms overcoming the anti-proliferative effects of
such drugs (10). The resistance
to therapy with BRAFi is mostly associated with the re-activation
of the MAPK pathway through different molecular mechanisms,
including NRAS upregulation (in some cases, through the functional
loss of the RAS-antagonist, NF1 protein), the acquisition of
activating mutations in the downstream MAP2K1 gene, BRAF
amplification and/or the expression of inhibition-escaping splicing
isoforms of BRAFV600E (11–17).
Specific inhibitors of MEK1 and MEK2 kinases, including
selumetinib, trametinib, cobimetinib and binimetinib, have been
introduced into clinical practice for the treatment of BRAF- and
NRAS-mutated metastatic melanomas (18–20).
In fact, the combination of BRAF and MEK inhibitors is recognized
as the standard of care for the targeted therapy of BRAF-mutated
metastatic melanomas (21,22). However, several molecular
alterations involved in the resistance to BRAFi may play a similar
role in the acquisition of resistance to MEK inhibitors (23,24).
Therefore, there is still an unmet need for additional therapeutic
strategies for the treatment of BRAFi-resistant melanomas.
The poly(ADP-ribose) polymerase (PARP) family
includes 17 different enzymes that regulate different cellular
functions, such as the regulation of the cell cycle, gene
transcription and the regulation of the repair mechanisms of DNA
damage (25). Since they seem to
be essential for the maintenance of genomic stability, PARP
proteins are indeed attractive therapeutic targets. PARP inhibitors
are being widely investigated as a class of useful drugs for use in
the treatment of tumors with underlying defects in DNA repair, or
when combined with DNA-damaging agents (26).
The most abundant isoform of the PARP enzyme family,
PARP1, plays a key role in the mechanisms of the repair of
single-strand DNA breaks through the base excision repair pathway
(27,28). In BRCA1- or BRCA2-deficient cells
which are defective of homologous recombination, the inhibition of
PARP1 leads to an increase in DNA double-strand breaks. This
results in chromosomal instability, cell cycle arrest and
subsequent apoptosis (26).
Accumulating evidence demonstrates that PARP1 interacts with
various oncogenic proteins and regulates several transcription
factors, thereby modulating a broad variety of cellular functions
(29). In an orthotopic murine
model of melanoma, PARP1 silencing has been shown to hamper
angiogenesis, reduce intra-tumor vascularization and tumor growth,
and to enhance the sensitivity to temozolomide (30). Among the PARP inhibitors, the
PARP1/2 inhibitor, ABT-888 (veliparib), is an orally bioavailable
small molecule with pre-clinical efficacy without signs of toxicity
in tumor models, including melanoma (31), and is currently being investigated
in a number of ongoing clinical trials for the treatment of solid
tumors (32,33) (NCT01690598, NCT02289690,
NCT02723864, NCT03123211). Emerging evidence indicates that the
efficacy of ABT-888 in delaying the PARP-mediated repair of DNA
damage is potentiated by the concomitant administration of chemo-
and/or radiotherapeutic agents which concur to accumulate DNA
strand breaks (31,34). In particular, Palma et al
demonstrated that ABT-888 enhanced the efficacy of temozolomide in
a variety of pre-clinical tumor models, including B-cell lymphoma,
pancreatic, breast, ovarian, non-small cell lung carcinoma and
small-cell lung carcinoma models (34).
In this study, using a number of human melanoma cell
lines harboring different mutations in the BRAF or NRAS genes, we
examined the effects of ABT-888 on the growth and invasiveness of
melanoma cells which are either sensitive or resistant to the
BRAFi, dabrafenib.
Materials and methods
Cell lines and treatments
The human melanoma cell line, A375, was purchased
from ATCC (Manassas, VA, USA); the SK-MEL-2, SK-MEL-5, 397-MEL,
LOX-IMVI and M14 cell lines were kindly provided by Dr F. M.
Marincola (Sidra Medical and Research Center, Doha, Qatar). The
human melanoma M-368 cells were provided by Dr A. Ribas (UCLA
Medical Center, Santa Monica, CA, USA). The LCP and COPA-159
melanoma cells were established in the laboratories of the Istituto
Nazionale Tumori 'Fondazione G. Pascale'-IRCCS and passaged for
<6 months. The LCP cells are derived from a primary lesion of a
patient with malignant melanoma, whereas the COPA-159 cells are
derived from an axillary lymph node metastasis removed from a
patient with a melanoma progressive disease (35,36).
The SK-MEL-2, SK-MEL-5, A375, COPA-159, LOX-IMVI, LCP, 397-MEL and
M-368 melanoma cell lines were grown in RPMI-1640 medium, and the
M14 cell line in DMEM medium, both supplemented with 10% fetal
bovine serum (FBS), 100 IU/ml penicillin and 50 µg/ml
streptomycin, and maintained in 25 cm2 tissue culture
flasks at 37°C in humidified air with 5% CO2. ABT-888
supplied by AbbVie Inc. (Chicago, IL, USA), was dissolved in DMSO
(Sigma-Aldrich, St. Louis, MO, USA) at a stock concentration of 50
mM, and stored at −20°C. The cells were treated with diluent, 5,
10, 25 or 50 µM ABT-888 for 24 or 72 h in complete growth
medium prior to being subjected to functional assays. The A375R
sub-line with acquired resistance to dabrafenib (purchased from
GlaxoSmithKline, Brentfort, UK) was generated by growing the
parental A375 cells in the presence of increasing concentrations of
dabrafenib (from 1 nM up to 25 nM) over a period of 4 months. BRAFi
IC50 values of 138 and 331 nM were determined for the
parental A375 and A375R cells, using the logistic function (or
sigmoidal) to fit dose-response curves, using GraphPad statistical
software. Following selection, the A375R cells were maintained in
growth medium supplemented with 25 nM dabrafenib for at least 8
weeks before performing the experiments.
Analysis of cell viability by
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
The viability of the melanoma cell lines was
assessed by using the MTT colorimetric assay. Viable cells with
active metabolism convert MTT into the corresponding,
purple-colored formazan, while dead cells lose their ability to
convert MTT into formazan. The cells (3×103 cells/well)
suspended in complete growth medium, were seeded in 96-well plates.
After 24 h, the medium was replaced with 100 µl fresh medium
containing the vehicle (0.001% DMSO), the indicated concentrations
of ABT-888 or dabrafenib. Following 72 h of incubation at 37°C in
humidified air with 5% CO2, the suspended cells were
removed and the adherent cells were stained with 0.5 mg/ml sterile
MTT dye solution (Invitrogen, Carlsbad, CA, USA) for 4 h at 37°C.
The resulting formazan was eluted with 100 µl DMSO and
measured at a wavelength of 540 nm using a microplate reader
(Bio-Rad, Hercules, CA, USA). For each cell line, data were
calculated as a percentage of the absorbance of untreated cells,
considered 100%.
Annexin V-FITC assay
The apoptotic response of the melanoma cells to
ABT-888 was evaluated using the Annexin V-FITC apoptosis detection
kit (eBioscience, Santa Clara, CA, USA), according to the
manufacturer's instructions. Briefly, the cells (1×105
cells/sample) were incubated with 5 µl Annexin V-FITC
diluted in 200 µl binding buffer for 10 min at room
temperature in the dark, washed with binding buffer, incubated with
10 µl propidium iodide (PI, 20 µg/ml) in 190
µl binding buffer and immediately analyzed using a BD
FACSAria II flow cytometer (BD Biosciences, Franklin Lakes, NJ,
USA).
Cell cycle analysis by flow
cytometry
The cells were harvested and washed once with
phosphate-buffered saline (PBS), fixed in pre-cold 70% ethanol and
incubated with 50 µg/ml PI (Sigma-Aldrich), 1 mg/ml RNase
solution, for 60 min at 4°C in the dark. The stained nuclei were
analyzed with a FACS Vantage cell sorter, and the data were
analyzed using a Mod-Fit 2.0 cell cycle analysis program (both from
BD Biosciences). Flow cytometric analysis was performed using a BD
FACSAria II flow cytometer (BD Biosciences), and the data were
analyzed using ModFit software (Verity Software House, Topsham, ME,
USA).
Western blot analysis
The cells were lysed in RIPA buffer (10 mM Tris pH
7.5, 140 mM NaCl, 0.1% SDS, 1% Triton X-100, 0.5% NP-40) containing
protease inhibitor mixture (Sigma-Aldrich) and the protein content
was measured by a colorimetric assay (Bio-Rad). Proteins (30
µg/sample) were separated on 10% SDS-PAGE and transferred
onto Immobilon PVDF membranes (Millipore, Darmstadt, Germany). The
membranes were blocked with 5% non-fat dry milk and probed with
anti-human PARP1 monoclonal antibody (mAb; 1:1,000; 551024; BD
Biosciences, Franklin Lakes, NJ, USA) recognizing both full length
enzyme (160 kDa) and the 85-kDa cleaved PARP fragment and then with
peroxidase AffiniPure goat anti-mouse IgG (H+L) (code: 115-035-003;
1:4,000; Jackson ImmunoResearch Europe Ltd., Cambridgeshire, UK).
Chemiluminescence was developed by ECL and detected using
ImageQuant LAS 500 software (both from GE Healthcare Life Sciences,
Little Chalfont, UK).
Fluorescence microscopy
To visualize the cytoskeleton, the cells
(~2×104/sample) were seeded on glass coverslips and
cultured for 24 h in growth medium. The slides were then washed
with PBS, fixed with 2.5% formaldehyde, permeabilized with 0.1%
Triton X-100 for 10 min at 4°C, and incubated with 0.1 µg/ml
rhodamine-conjugated phalloidin (Sigma-Aldrich) for 40 min. Nuclear
staining was performed with 4-6-diamidino-2-phenylindole (DAPI)
dye. Finally, coverslips were mounted using 20% (w/v) Mowiol, and
visualized with an Axiovert 200 M fluorescence inverted microscope
connected to a video camera (Carl Zeiss, Oberkochen, Germany).
Migration of cells monitored in
real-time
Cell migration was monitored in real-time using the
xCELLigence Real-Time Cell Analysis (RTCA) technology (Acea
Bioscience, San Diego, CA, USA) as previously described (37). For these experiments, we used
CIM-16-well plates which are provided with interdigitated gold
microelectrodes on bottom side of a filter membrane interposed
between a lower and an upper compartment. The lower chambers were
filled with serum-free medium (CTRL) or growth medium (10% FBS).
Viable cells assessed by exclusion trypan blue (2×104
cells/well) were seeded on filters in serum-free medium.
Microelectrodes detect impedance changes which are proportional to
the number of migrating cells and are expressed as cell index.
Migration was monitored in real-time for 15 h. Slopes represent the
alteration rate of the cell index generated in a 1–15 h time frame.
Each experiment was performed at least twice in quadruplicate.
Cell invasion assays
Cell invasion assays were performed in Boyden
chambers, as previously described (38). Briefly, 4×104
cells/chamber were allowed to invade in Matrigel for 18 h at 37°C,
5% CO2 using 8 µm pore size Nuclepore
Track-Etched Membranes (Whatman, Maidstone, UK) coated with 50
µg Matrigel (BD Biosciences) and 10% FBS in DMEM as a source
of chemoattractants. At the end of the assay, cells on the lower
filter surface were fixed with ethanol, stained with haematoxylin
and counted in 10 random fields/filter at ×200 magnification. The
extent of cell invasion was expressed as a percentage of the basal
cell invasion (CTRL), considered 100%.
Statistical analyses
Data are presented as the means ± SD and analyzed by
one-way analysis of variance and a post hoc Dunnett's t-test for
multiple comparisons. The software used was the SPSS statistical
software version 23.0 (SPSS Inc., Chicago, IL, USA). Values of
P<0.05 and P<0.01 were considered to indicate statistically
significant and highly statistically significant differences,
respectively.
Results
Effect of ABT-888 on melanoma cell
viability
First, we examined whether ABT-888 affects the
viability of human melanoma cell lines harboring different
mutations in the BRAF or NRAS genes. To this end,
dabrafenib-sensitive melanoma cell lines listed in Table I were exposed to increasing
concentrations of ABT-888 for 72 h and their viability was assayed
by measuring the mitochondrial activity of living cells by MTT
assay. Data were expressed as a percentage of the absorbance
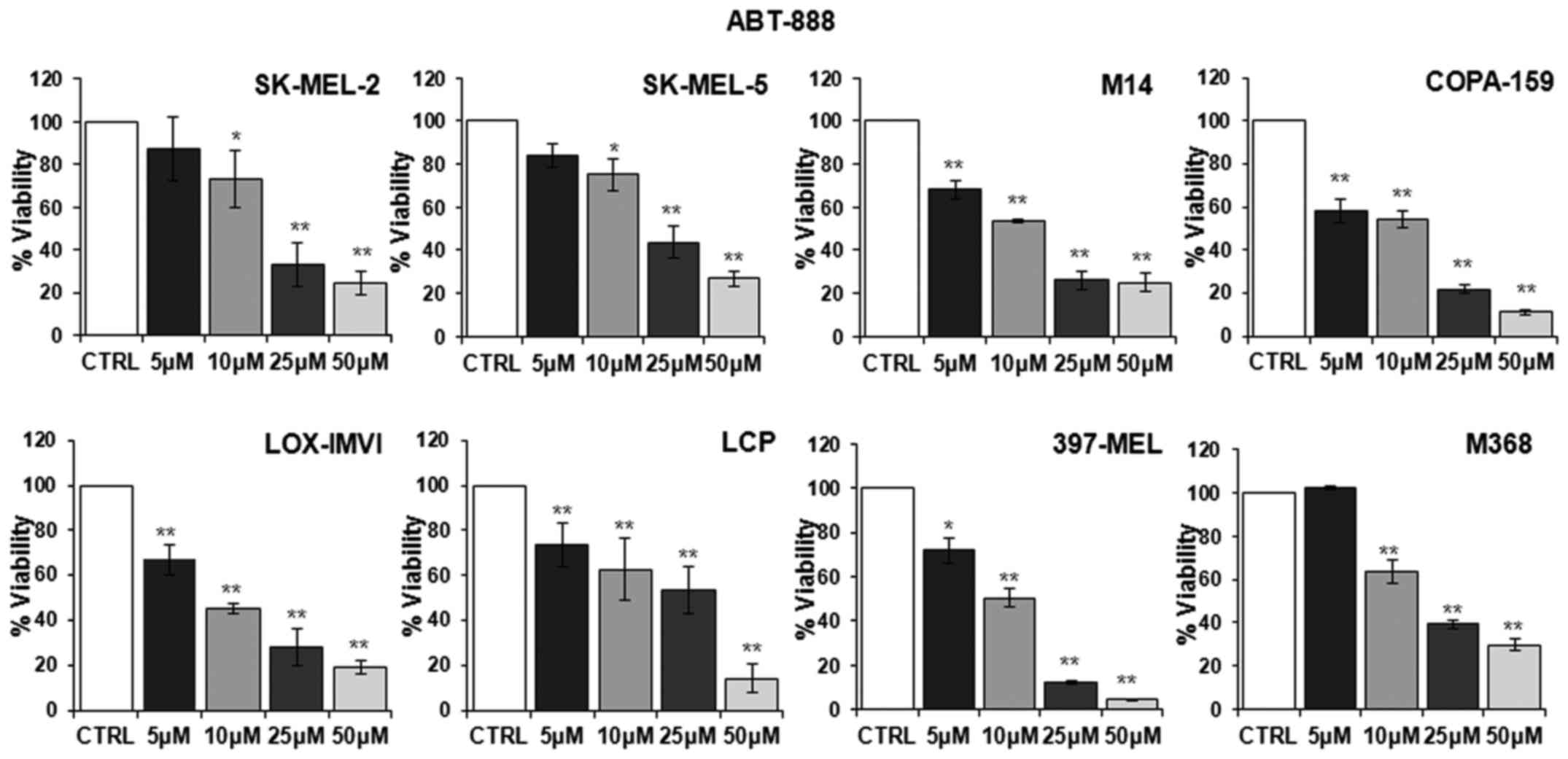
detected in untreated cells (CTRL). As shown in Fig. 1, a slight decrease in the number of
viable cells was observed from the concentration of 5 µM
ABT-888 in the majority of the cell lines, whereas an appreciable
and statistically significant decrease in cell viability was
observed in all the melanoma cell lines exposed to 10 µM
ABT-888. Following exposure of the cells to 25 µM ABT-888,
the number of total viable cells markedly decreased, apart from the
LCP cells, which lost only 46% of their viability compared to the
control. In any case, exposure of the cells to 50 µM ABT-888
led to a significant loss of cell proliferation, ranging from
95–70% of the cell population for each cell line compared to the
control (Fig. 1). These findings
indicated that, although to a varying extent, ABT-888 caused a
dose-dependent decrease in the viability of melanoma cells,
independently from their mutation status in the BRAF and/or NRAS
genes, with the maximal decrease being observed in the 25–50
µM concentration range.
 | Table IHuman melanoma cell lines harboring
different mutations in BRAF or NRAS genes. |
Table I
Human melanoma cell lines harboring
different mutations in BRAF or NRAS genes.
| Melanoma cell
lines | Mutation |
|---|
| SK-MEL-2 |
NRASQ61R |
| SK-MEL-5 |
BRAFV600E |
| M14 |
BRAFV600E |
| COPA-159 |
BRAFV600E |
| LOX-IMVI |
BRAFV600E |
| LCP |
BRAFV600R |
| 397-MEL |
BRAFV600D |
| M368 | BRAF wild-type |
| NRAS wild-type |
Effect of ABT-888 on the apoptosis of
melanoma cells
It has been reported that prolonged exposure to
ABT-888 induces the activation of the apoptotic program in the
majority of cancer cell lines (26,31).
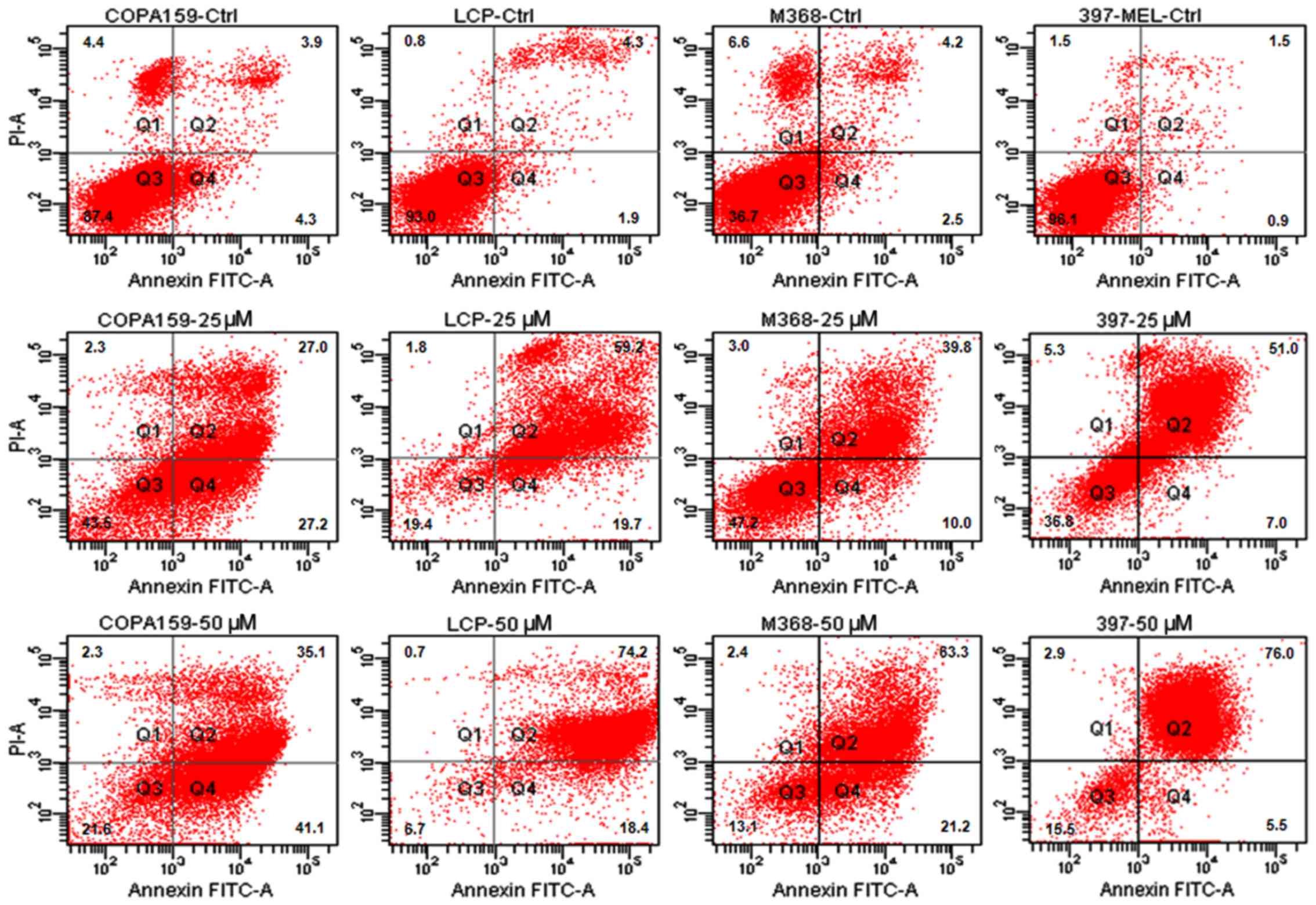
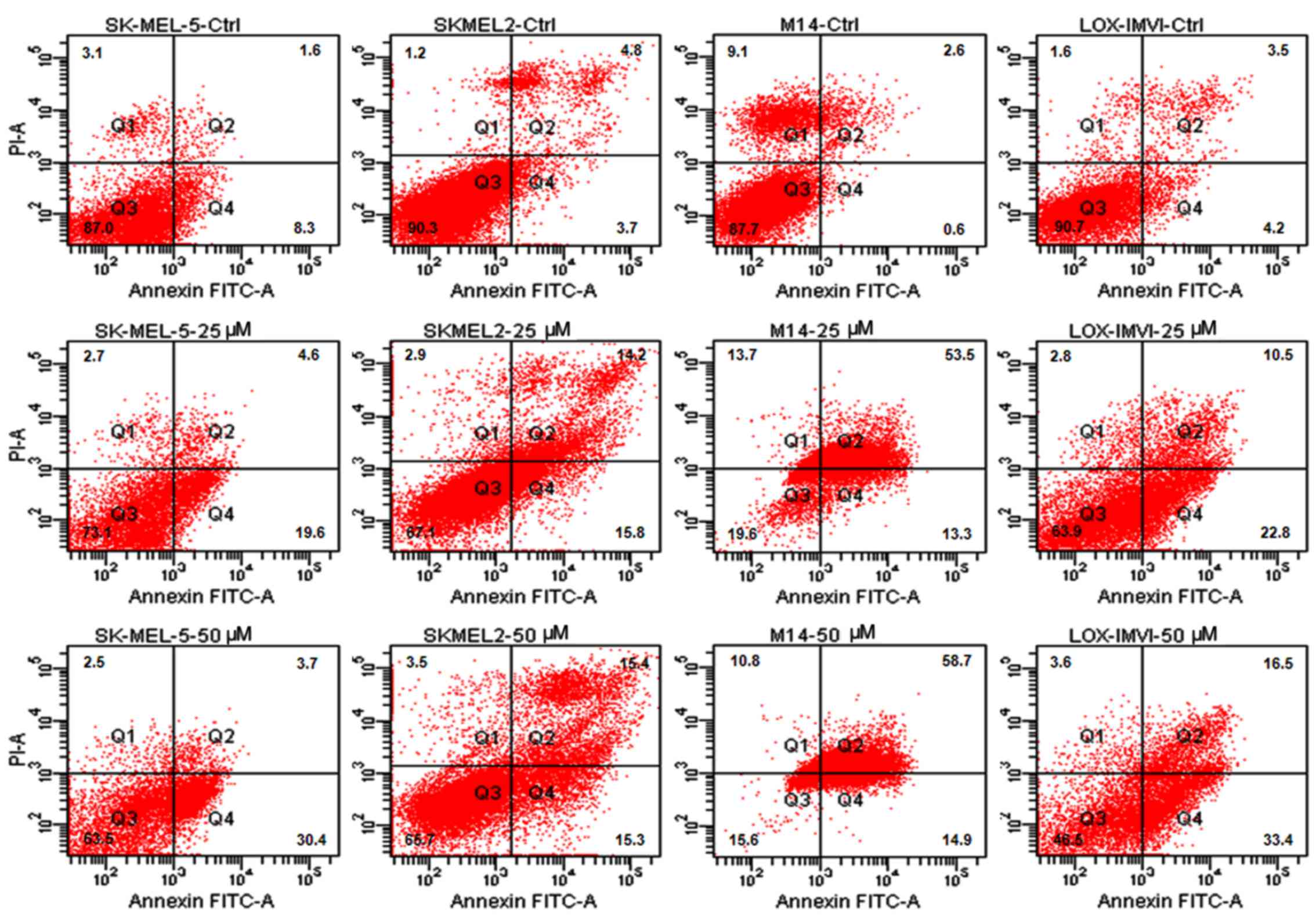
Thus, in this study, we investigated the apoptotic response of the
melanoma cells to increasing concentrations of ABT-888. The cells
were exposed to increasing concentrations of ABT-888 for 72 h and
apoptotic cells were identified by FACS analysis, counting Annexin
V-positive cells in the right upper (late apoptotic cells) and
right lower (early apop-totic cells) quadrants of each panel. We
observed that some of the untreated cells underwent overgrowth in
the absence of any treatment (necrotic cells in the left upper
quadrants). The majority of the melanoma cells underwent apoptosis
following exposure to 25 or 50 µM ABT-888 for 72 h (Figs. 2 and 3, and Table
II), whereas only a weak pro-apoptotic effect was elicited by 5
or 10 µM ABT-888 (Table
II). These findings indicated that, although to a varying
extent, ABT-888 induces the apoptosis of melanoma cells in a
dose-dependent manner and independently from their mutation status
in the BRAF and/or NRAS genes, with the maximal effect being
reached in the 25–50 µM concentration range.
 | Table IIDose-dependent apoptotic effect of
ABT-88 on melanoma cell lines. |
Table II
Dose-dependent apoptotic effect of
ABT-88 on melanoma cell lines.
| Apoptotic cells
(Q2+Q4)
|
|---|
| CTRL | 5 µM | 10 µM | 25 µM | 50 µM |
|---|
| COPA-159 | 8.2+0.01 | 19.4+0.08 |
31.3+0.02a |
54.2+0.14a |
76.2+0.16b |
| LCP | 6.2+0.07 |
39.6+0.11a |
58.1+0.16a |
78.9+0.11b |
92.6+0.08b |
| M638 | 6.7+0.02 | 17.5+0.13 | 10.9+0.05 |
49.9+0.02a |
84.5+0.12b |
| 397-MEL | 2.4+0.12 |
23.1+0.04a |
24.2+0.07a |
58+0.04b |
81.5+0.07b |
| SK-MEL-5 | 9.9+0.08 | 13.2+0.05 | 10.2+0.04 |
24.2+0.05a |
34.1+0.01a |
| SK-MEL-2 | 8.5+0.13 | 13.9+0.11 | 13.3+0.04 |
30+0.13a |
30.7+0.03a |
| M14 | 3.2+0.12 |
30.4+0.14a |
50.3+0.16a |
66.8+0.08b |
73.6+0.05b |
| LOX-IMVI | 7.7+0.01 |
12.1+0.06a | 7.8+0.08 |
33.3+0.12a |
49.9+0.12a |
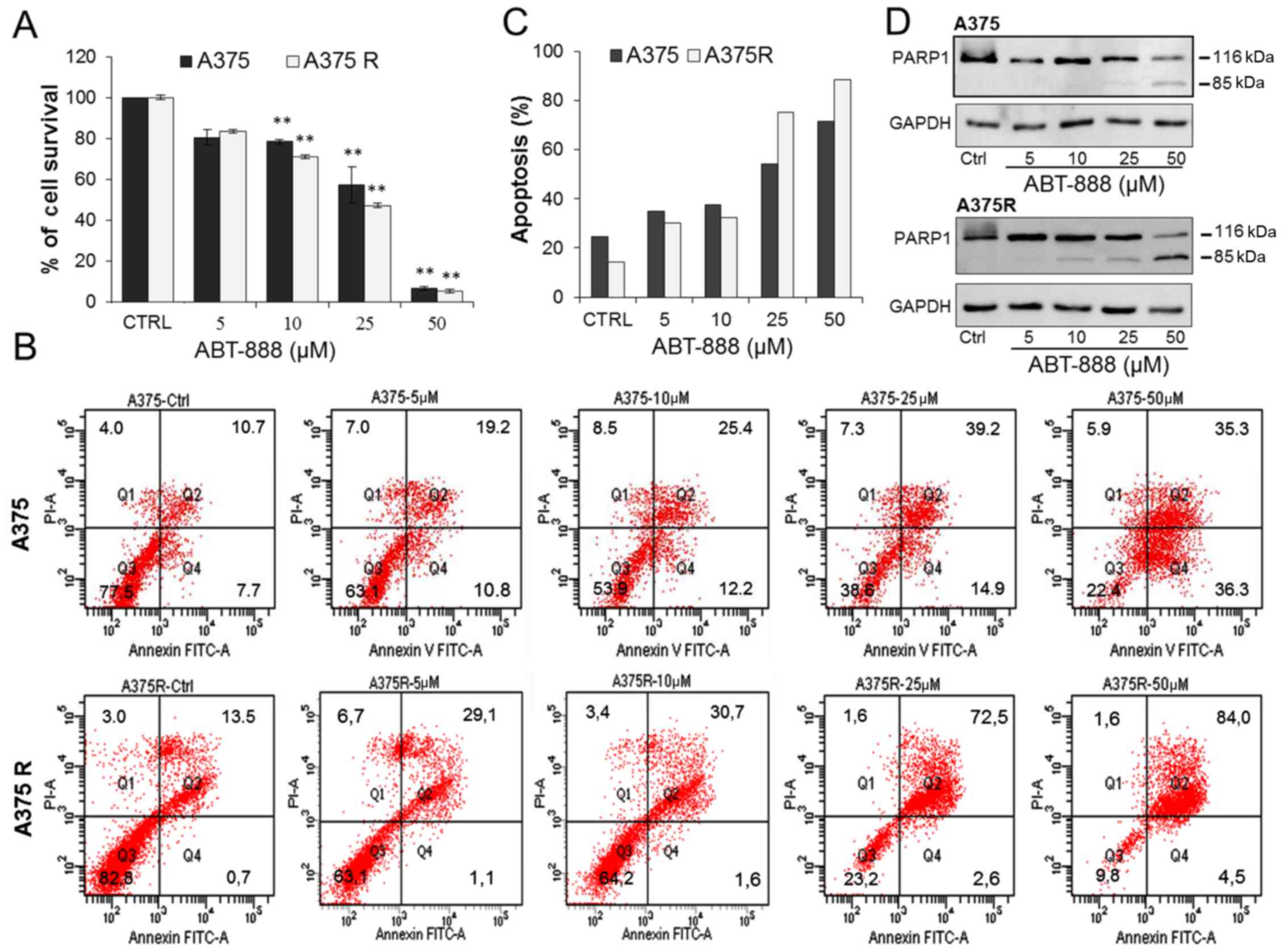
Effect of ABT-888 on the viability and
apoptosis of A375 and A375R melanoma cells
The chronic treatment of melanoma with BRAFi is
usually associated with the development of drug resistance
(10). Thus, in this study, we
investigated the possibility that ABT-888 exerts pro-apoptotic
effects on melanoma cells which are resistant to BRAFi. Since
approximately 50% of melanomas harbor a BRAF mutation and in 90% of
these cases, there is a substitution of a glutamic acid with a
valine residue at position 600 (V600E) (3,39),
we employed A375 cells harboring the V600E mutation to generate
dabrafenib-resistant A375 cells (A375R). The A375R cells were
obtained by growing the parental A375 cells in the presence of
increasing concentrations of dabrafenib (from 1–25 nM) for 4
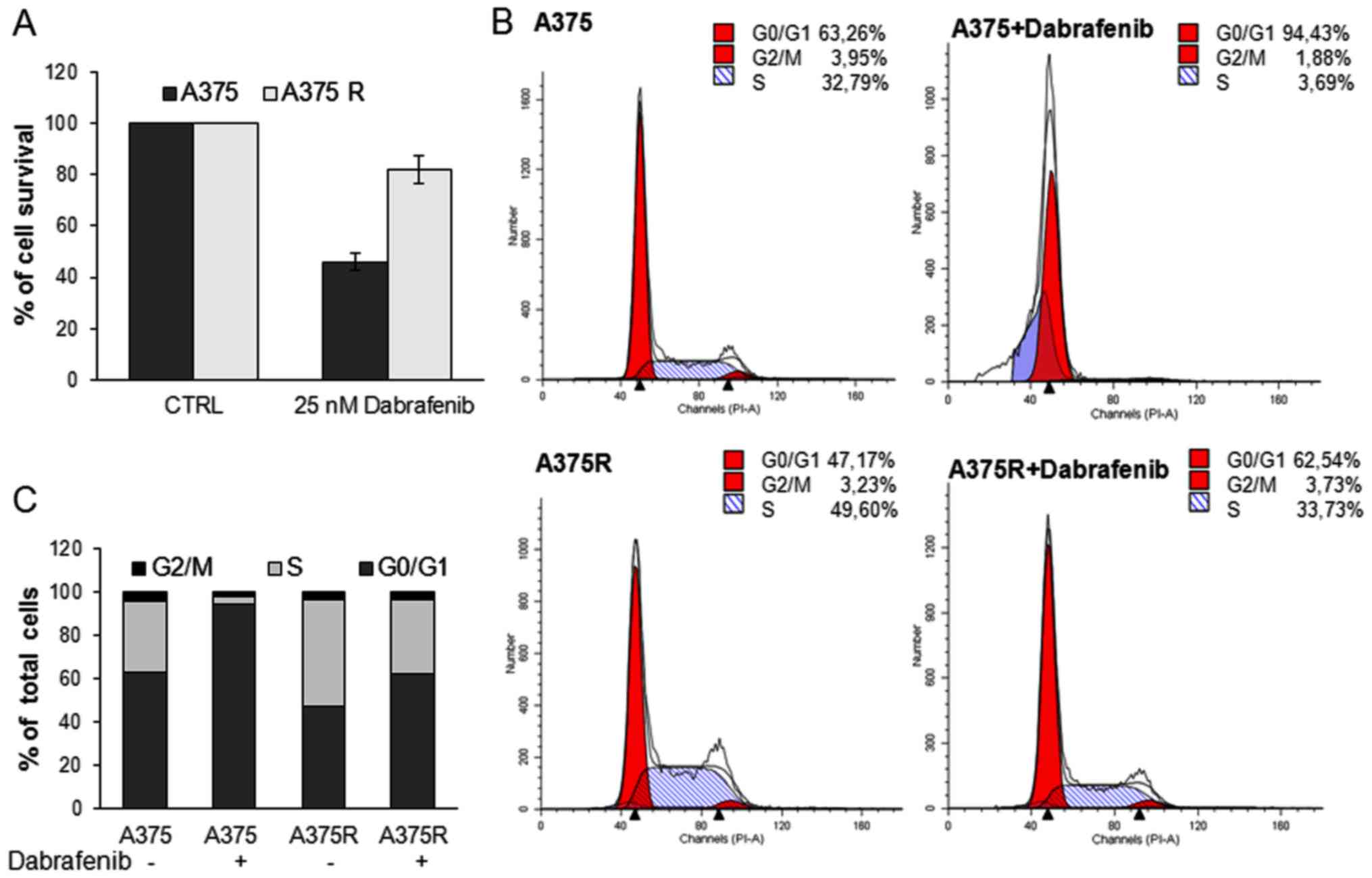
months. The resistant A375 cells were preliminary analyzed for
their ability to escape the dabrafenib-induced decrease in
viability and proliferation by MTT assay and FACS cell cycle
analysis, respectively. As shown in Fig. 4A, exposure of the cells to 25 nM
dabrafenib for 24 h elicited a marked cytotoxic effect on the A375
cells, but not on the A375R cells. FACS cell cycle analysis
revealed that 25 nM dabrafenib induced G0/G1 cell cycle arrest of
the A375 cells, but not the A375R cells. The A375R cells retained
the ability to progress into the S and G2/M phases, thus confirming
their acquisition of resistance to BRAFi (Fig. 4B–C). We then examined the effects
of ABT-888 on A375 and A375R cell viability. The cells were treated
with increasing concentrations of ABT-888 for 72 h and then
subjected to an MTT assay as described above. According to the data
shown in Fig. 5A, ABT-888 induced
a slight decrease in the number of viable cells from the
concentrations of 5–10 µM in both the A375 and A375R cells.
Upon exposure to 25 µM ABT-888, the viability of the A375
and A375R cells decreased to 43 and 53%, respectively, as compared
to that of the untreated cells, while only a few viable cells were
detected in both the sensitive and resistant cells treated with 50
µM ABT-888 (Fig. 5A). FACS
analysis of the Annexin V-positive A375 and A375R cells exposed to
25 or 50 µM ABT-888 revealed a significant increase in the
number of apoptotic cells in both cell populations, as compared to
the untreated cells (Fig. 5B–C).
At the 5 or 10 µM concentrations, ABT-888 exerted a slight
pro-apoptotic effect on both A375 and A375R cells. Upon the
exposure of the cells to 25 µM ABT-888, the number of
apoptotic A375 and A375R cells markedly increased, reaching 54,1
and 75,1% of the cell population, respectively. A further
enhancement of the apoptotic effect was exerted on both the
sensitive and resistant A375 cells exposed to 50 µM ABT-888
(Fig. 5B–C).
PARP-1 inactivation is accompanied by the production
of several specific proteolytic cleavage fragments with different
molecular weights, which are considered markers of apoptosis. To
confirm that ABT-888-dependent apoptosis occurs due to PARP-1
inhibition, the occurrence of a 85-kDa cleaved PARP1 fragment was
investigated in lysates from the A375 and A375R cells exposed to
ABT-888 by western blot analysis. For this subset of experiments,
the cells were exposed to increasing concentrations of ABT-888 for
24 h in order to detect early events occurring during the apoptotic
process. As shown in Fig. 5D, only
the full-length enzyme (116 kDa) was detected in the lysates from
the A375 or A375R cells treated with diluents or 5 µM
ABT-888 for 24 h. Apart from the full-length enzyme, in the lysates
from both the A375 and A375R cells exposed to 25 µM ABT-888,
we observed the appearance of the 85-kDa band corresponding to the
85-kDa cleaved PARP fragment that increased in the cells exposed to
50 µM ABT-888. Interestingly, a very faint 85-kDa band
appeared in lysates from A375R but not A375 cells treated with 10
µM ABT-888, suggesting that, unlike the sensitive cells, the
resistant A375 cells were more responsive to ABT-888-induced,
PARP1-mediated apoptosis (Fig.
5D). This finding is in agreement with the Annexin V assay
data, showing that a more robust increase in apoptosis was elicited
by ABT-888 in the resistant cells as compared to the
BRAFi-sensitive cell population (Fig.
5C). Taken together, these findings indicate that ABT-888
reduces the viability and induces the apoptosis of both A375 and
A375R melanoma cells in a dose-dependent manner. The findings also
suggest that BRAFi-resistant melanoma cells are more responsive to
the pro-apoptotic effects exerted by ABT-888 than their sensitive
counterparts.
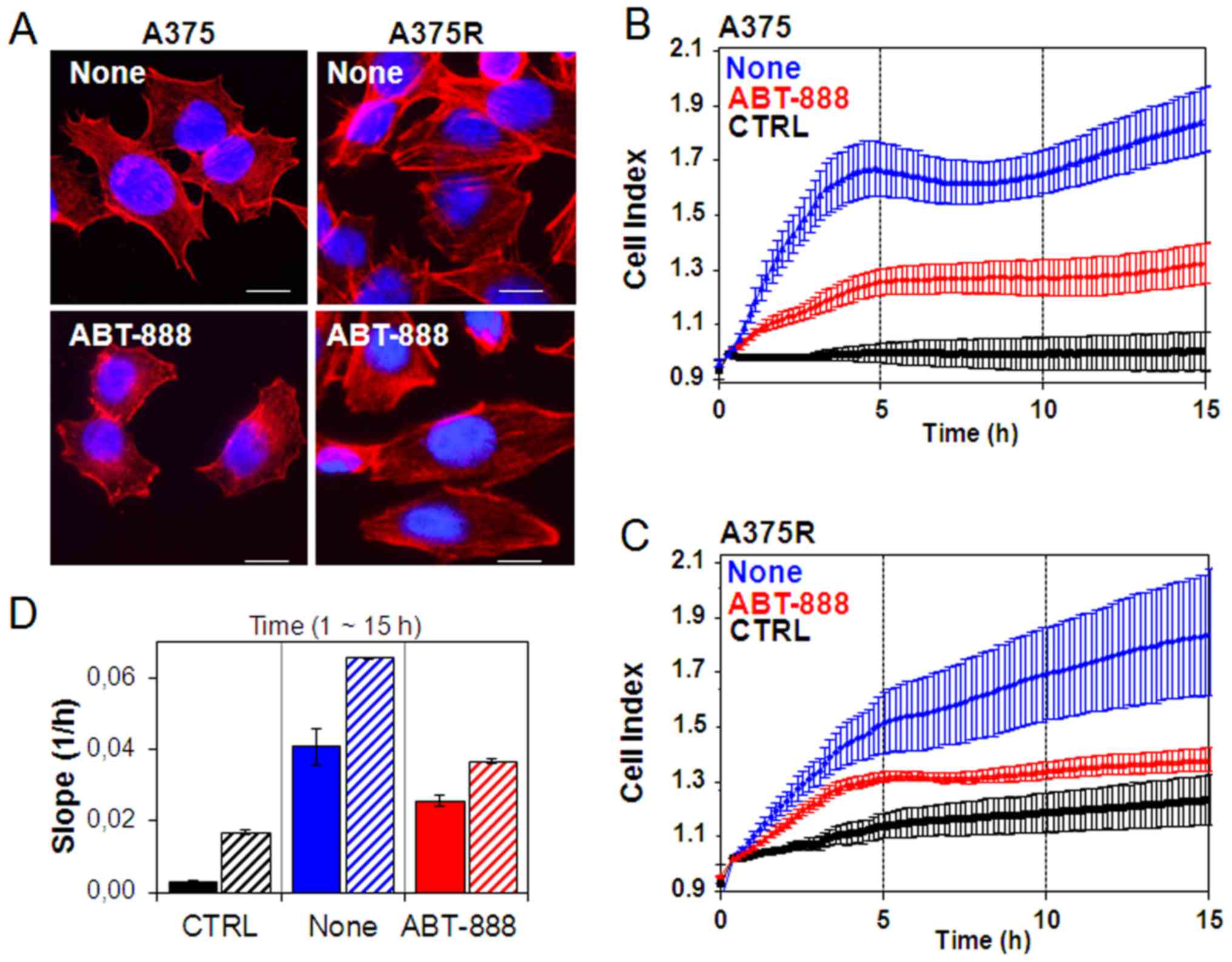
Effect of ABT-888 on cytoskeletal
organization, migration and the Matrigel invasion of melanoma
cells
Recently, it has been reported that PARP confers
increased melanoma cell motility and that PARP inactivation
represses actin cytoskeleton machinery, which is required for the
acquisition of cell migratory ability (40,41).
Therefore, in this study, we investigated whether ABT-888 treatment
exerts an effect on cytoskeletal organization and the motility of
sensitive and resistant A375 cells. In order to investigate the
early intracellular effects on cytoskeletal rearrangements
occurring during ABT-888 treatment and to avoid any effect of
ABT-888 on cell viability and apoptosis, cytoskeletal organization
and cell motility were analyzed in the A375 and A375R cells exposed
for 24 h to diluent (none) or to a low concentration of ABT-888 (5
µM). As the rhodamine-phalloidin staining of F-actin
polymerization revealed, the sensible A375 cells exhibited few
stress fibers parallel to the longitudinal axis of the cells and
actin aggregates near the membrane (Fig. 6A). Conversely, the acquisition of
resistance to BRAFi induced marked changes in cell morphology. The
A375R cells acquired a more elongated morphology which reflected a
marked reorganization of actin into stress fibres spanning the
length of the cells, often localized at one pole of the cell
(Fig. 6A). ABT-888 markedly
altered the cytoskeletal organization of both the A375 and A375R
cells with a general reduction in the amount of stress fibres. In
particular, treatment of the A375 cells with ABT-888 led to the
appearance of condensed aggregates, mostly local-ized below the
membrane (Fig. 6A). These effects
were not due to ABT-888-induced apoptosis as no form of cleaved
PARP was found in the cells exposed to 5 µM ABT-888 for 24 h
(Fig. 5D).
Alterations in cytoskeletal organization reflect the
capability of cells to migrate. In this study, the ability of the
sensitive and dabrafenib-resistant A375 cells to migrate toward
serum (employed as a source of chemoattractant), was assessed using
the xCELLigence RTCA technology. The A375 and A375R cells treated
with diluents or 5 µM ABT-888 for 24 h were seeded in the
upper compartment of CIM plates. The lower chambers were filled
with RPMI (CTRL) or RPMI containing 10% FBS (none). Cell migration
was monitored in real-time for 15 h as the cell index changes due
to the adhesion of migrating cells to microelectrodes (Fig. 6B–C). Of note, we found that BRAFi
resistance was associated with increased basal cell migration, as
compared to the sensitive A375 cells, probably due to the elongated
morphology that reflected the high concentration of the stress
fibers spanning the length of A375R cells. Accordingly, the A375R
cells were able to respond more efficiently to the chemotactic
gradient (approximately a 3-fold increase) than the A375 cells, as
shown in the plot which recapitulates the cell index changes
generated in the 1–15 h time frame (Fig. 6D). ABT-888 treatment did not affect
basal cell migration, as compared to the untreated cells (data not
shown). Despite different cell migratory abilities, both the A375
and A375R cells responded to ABT-888 treatment to a similar extent,
as ABT-888 reduced the directional migration of the A375 and A375R
cells by 37 and 45%, respectively (Fig. 6B–D). Since cell motility is a
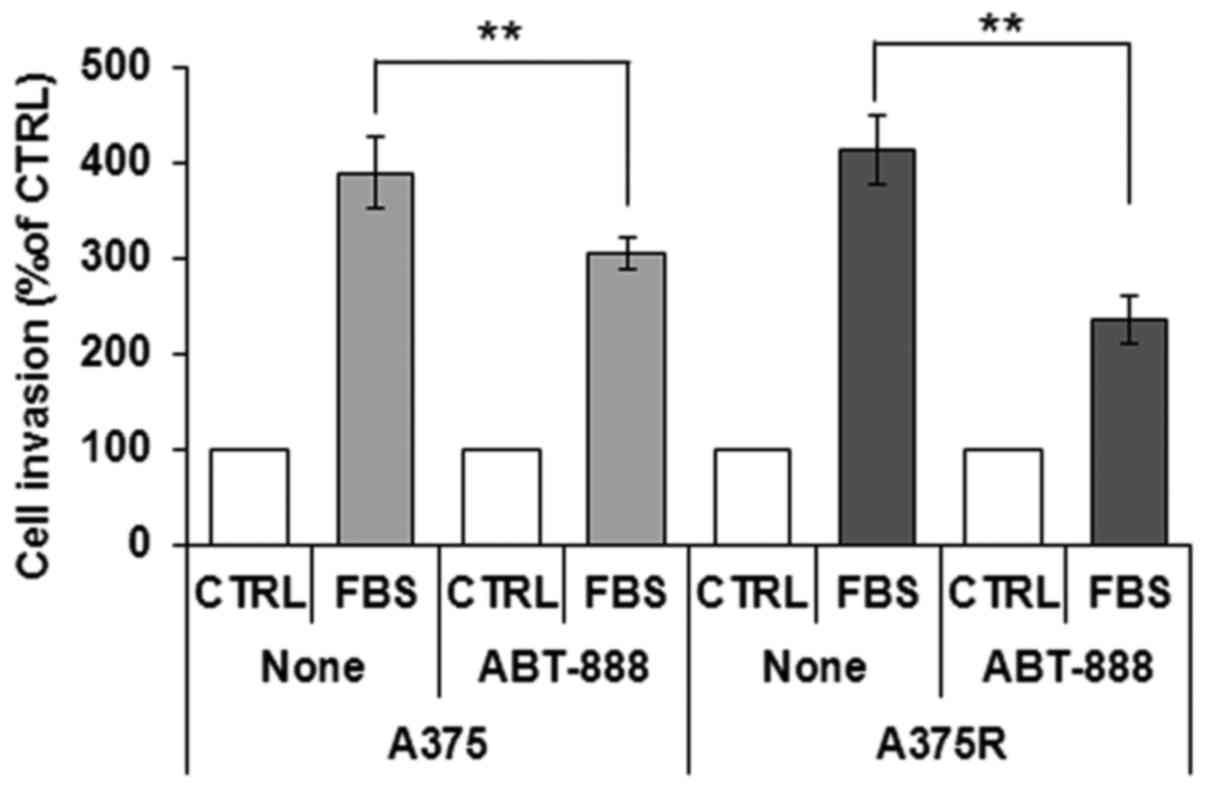
prerequisite for the acquisition of an invasive phenotype, we
examined the effects of ABT-888 treatment on the capability of A375
and A375R cells to cross the Matrigel. The cells were seeded in the
upper compartment of Boyden chambers. The lower chambers were
filled with RPMI (CTRL) or RPMI containing 10% FBS (FBS). We found
that the A375 and A375R cells were able to cross the Matrigel to a
similar extent (391 and 414% of the basal cell invasion,
respectively). According to the cell migration data, pre-exposure
of the cells to 5 µM ABT-888 for 24 h caused a 22 and 44%
inhibition of the A375 and A375R cell invasive ability,
respectively (Fig. 7). Taken
together, our data highlight the pivotal role of PARP1 in the
migratory and invasive ability of melanoma cells, raising the
possibility that ABT-888 may be considered, not only as a
pro-apoptotic drug for the treatment of BRAFi-resistant melanoma
cells, but also as a good candidate for preventing the migration
and invasion of melanoma cells.
Discussion
In recent years, the development of therapies
targeting BRAF and MEK to block the MAPK pathway has improved the
overall survival of patients with melanoma. Despite these landmark
changes in practice, the majority of patients are either
intrinsically resistant or rapidly acquire resistance to MAPK
pathway inhibitors, imposing more complex combinatorial therapeutic
strategies (42,43). Thus, the aim of this study was to
investigate the possibility that PARP1/2 inhibitors may be taken
into account for the design of novel combinatorial therapies. PARP1
expression is associated with the recurrence and/or progression of
the disease (44). Thus, the use
of PARP inhibitors in combination with other cytotoxic agents may
represent an efficacious strategy. Accumulating evidence indicates
that PARP1 inhibitors abrogate resistance to temozolomide,
increasing the survival of tumor-bearing mice with respect to
treatment with temozolomide as a single agent, and in preclinical
tumor models (including melanoma), as well as in clinical trials
(31,45–47).
Among the PARP inhibitors, ABT-888 (veliparib)
appears most effective when used to treat solid tumors with
underlying defects in DNA repair, or when combined with
DNA-damaging agents and is currently undergoing clinical trials
(48,49). In this study, we present evidence
that, in vitro, ABT-888 reduces the viability and induces
the apoptosis of melanoma cell lines, independently of their
BRAF/NRAS mutation status and in a dose-dependent manner, with the
maximal effect being reached in the 25–50 µM concentration
range. We found that ABT-888 promoted the apoptosis of both
dabrafenib-sensitive and -resistant A375 cells. Although we did not
explore the efficacy of ABT-888 alone in preventing melanoma growth
in mouse models, or its pharmacokinetic proprieties, our findings
strongly support the hypothesis that ABT-888 may prove to be useful
in the treatment of BRAFi-resistant melanoma cells, in combination
with other cytotoxic agents.
In addition to its direct role in DNA damage
recognition and repair, emerging evidence indicates that PARP1
regulates the expression of key factors, such as vimentin and
VE-cadherin and is involved in epithelial-mesenchymal transition
(41). Furthermore, PARP
inactivation has been documented to suppress actin cytoskeleton
machinery, which is required for the acquisition of the cell
migratory ability (40,50,51).
Consequently, targeting and inhibiting these important biological
functions may open up another avenue for melanoma therapy. In this
study, we provide evidence that ABT-888 prevents the directional
migration of dabrafenib-sensitive, as well as dabrafenib-resistant
A375 cells exposed to 5 µM ABT-888 for 24 h, arguing that
combinatorial approaches, including ABT-888, effectively improve
the prognosis of patients with metastatic melanoma. Notably, the
ABT-888 inhibitory effect on cell migration cannot be due to
apoptosis, since at the 5 µM concentration, ABT-888 failed
to elicit PARP fragmentation in both the dabrafenib-sensitive and
-resistant A375 cells (Fig. 5D).
Of note, we also found that the melanoma cells failed to cross the
Matrigel in Boyden chamber assays following treatment with 5
µM ABT-888 for 24 h. Zhu et al reported that the
PARP1 inhibitor, benzyl-isothiocyanate, prevented the invasion of
hepatocellular carcinoma cells by downregulating the expression of
matrix metalloproteinase (MMP)2 and MMP9 (52). In this study, although we did not
investigate the molecular mechanisms underlying the inhibition of
melanoma invasiveness by ABT-888, or whether, similar to
benzyl-isothiocyanate, ABT-888 decreases protease activity, our
findings encourage the inclusion of ABT-888 in combinatorial
therapies for the management of patients with metastatic disease.
Considering that, similar to other PARP1 inhibitors (53), ABT-888 has been proven to cross the
blood brain barrier (31), our
findings support the notion that ABT-888 may provide some
advantages for patients with melanoma with brain metastases.
In conclusion, our data highlight the pivotal role
of PARP1 in the migratory and invasive ability of melanoma cells,
raising the possibility that ABT-888 may be considered, not only as
a pro-apoptotic drug for the treatment of BRAFi-resistant melanoma
cells, but also a good candidate for preventing the migration and
invasion of melanoma cells, arguing that combinatorial approaches
including ABT-888 may effectively improve the prognosis of patients
with metastatic melanoma.
Acknowledgments
The authors would like to thank Dr F. M. Marincola
(Sidra Medical and Research Center, Doha, Qatar) and Dr A. Ribas
(UCLA Medical Center, Santa Monica, CA, USA) for kindly providing
the human melanoma cells. The authors would also like to thank
AbbVie Inc. (Chicago, IL, USA) for providing the ABT-888.
References
|
1
|
Tsao H, Chin L, Garraway LA and Fisher DE:
Melanoma: From mutations to medicine. Genes Dev. 26:1131–1155.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Akbani R, Akdemir KC, Aksoy BA, Albert M,
Ally A, Amin SB, Arachchi H, Arora A, Auman JT, Ayala B, et al:
Cancer Genome Atlas Network: Genomic classification of cutaneous
melanoma. Cell. 161:1681–1696. 2015. View Article : Google Scholar
|
|
3
|
Davies H, Bignell GR, Cox C, Stephens P,
Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W,
et al: Mutations of the BRAF gene in human cancer. Nature.
417:949–954. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dhomen N and Marais R: BRAF signaling and
targeted therapies in melanoma. Hematol Oncol Clin North Am.
23:529–545. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fecher LA, Amaravadi RK and Flaherty KT:
The MAPK pathway in melanoma. Curr Opin Oncol. 20:183–189. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Garnett MJ and Marais R: Guilty as
charged: B-RAF is a human oncogene. Cancer Cell. 6:313–319. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chapman PB, Hauschild A, Robert C, Haanen
JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M, et
al BRIM-3 Study Group; Improved survival with vemurafenib in
melanoma with BRAF V600E mutation. N Engl J Med. 364:2507–2516.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Flaherty KT, Puzanov I, Kim KB, Ribas A,
McArthur GA, Sosman JA, O'Dwyer PJ, Lee RJ, Grippo JF, Nolop K, et
al: Inhibition of mutated, activated BRAF in metastatic melanoma. N
Engl J Med. 363:809–819. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Eggermont AM, Spatz A and Robert C:
Cutaneous melanoma. Lancet. 383:816–827. 2014. View Article : Google Scholar
|
|
10
|
Palmieri G, Ombra M, Colombino M, Casula
M, Sini M, Manca A, Paliogiannis P, Ascierto PA and Cossu A:
Multiple molecular pathways in melanomagenesis: Characterization of
therapeutic targets. Front Oncol. 5:1832015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Johannessen CM, Boehm JS, Kim SY, Thomas
SR, Wardwell L, Johnson LA, Emery CM, Stransky N, Cogdill AP,
Barretina J, et al: COT drives resistance to RAF inhibition through
MAP kinase pathway reactivation. Nature. 468:968–972. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nazarian R, Shi H, Wang Q, Kong X, Koya
RC, Lee H, Chen Z, Lee MK, Attar N, Sazegar H, et al: Melanomas
acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS
upregulation. Nature. 468:973–977. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Poulikakos PI, Persaud Y, Janakiraman M,
Kong X, Ng C, Moriceau G, Shi H, Atefi M, Titz B, Gabay MT, et al:
RAF inhibitor resistance is mediated by dimerization of aberrantly
spliced BRAF(V600E). Nature. 480:387–390. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Whittaker SR, Theurillat JP, Van Allen E,
Wagle N, Hsiao J, Cowley GS, Schadendorf D, Root DE and Garraway
LA: A genome-scale RNA interference screen implicates NF1 loss in
resistance to RAF inhibition. Cancer Discov. 3:350–362. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Villanueva J, Infante JR, Krepler C,
Reyes-Uribe P, Samanta M, Chen HY, Li B, Swoboda RK, Wilson M,
Vultur A, et al: Concurrent MEK2 mutation and BRAF amplification
confer resistance to BRAF and MEK inhibitors in melanoma. Cell
Reports. 4:1090–1099. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wagle N, Van Allen EM, Treacy DJ,
Frederick DT, Cooper ZA, Taylor-Weiner A, Rosenberg M, Goetz EM,
Sullivan RJ, Farlow DN, et al: MAP kinase pathway alterations in
BRAF-mutant melanoma patients with acquired resistance to combined
RAF/MEK inhibition. Cancer Discov. 4:61–68. 2014. View Article : Google Scholar :
|
|
17
|
Shi H, Hugo W, Kong X, Hong A, Koya RC,
Moriceau G, Chodon T, Guo R, Johnson DB, Dahlman KB, et al:
Acquired resistance and clonal evolution in melanoma during BRAF
inhibitor therapy. Cancer Discov. 4:80–93. 2014. View Article : Google Scholar :
|
|
18
|
Ascierto PA, Schadendorf D, Berking C,
Agarwala SS, van Herpen CM, Queirolo P, Blank CU, Hauschild A, Beck
JT, St-Pierre A, et al: MEK162 for patients with advanced melanoma
harbouring NRAS or Val600 BRAF mutations: A non-randomised,
open-label phase 2 study. Lancet Oncol. 14:249–256. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim DW and Patel SP: Profile of
selumetinib and its potential in the treatment of melanoma. Onco
Targets Ther. 7:1631–1639. 2014.PubMed/NCBI
|
|
20
|
King JW and Nathan PD: Role of the MEK
inhibitor trametinib in the treatment of metastatic melanoma.
Future Oncol. 10:1559–1570. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Long GV, Weber JS, Infante JR, Kim KB,
Daud A, Gonzalez R, Sosman JA, Hamid O, Schuchter L, Cebon J, et
al: Overall survival and durable responses in patients with BRAF
V600-mutant metastatic melanoma receiving dabrafenib combined with
trametinib. J Clin Oncol. 34:871–878. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ascierto PA, McArthur GA, Dréno B,
Atkinson V, Liszkay G, Di Giacomo AM, Mandalà M, Demidov L,
Stroyakovskiy D, Thomas L, et al: Cobimetinib combined with
vemurafenib in advanced BRAF(V600)-mutant melanoma (coBRIM):
Updated efficacy results from a randomised, double-blind, phase 3
trial. Lancet Oncol. 17:1248–1260. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Van Allen EM, Wagle N, Sucker A, Treacy
DJ, Johannessen CM, Goetz EM, Place CS, Taylor-Weiner A, Whittaker
S, Kryukov GV, et al Dermatologic Cooperative Oncology Group of
Germany (DeCOG); The genetic landscape of clinical resistance to
RAF inhibition in metastatic melanoma. Cancer Discov. 4:94–109.
2014. View Article : Google Scholar
|
|
24
|
Welsh SJ, Rizos H, Scolyer RA and Long GV:
Resistance to combination BRAF and MEK inhibition in metastatic
melanoma: Where to next. Eur J Cancer. 62:76–85. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hassa PO and Hottiger MO: The diverse
biological roles of mammalian PARPS, a small but powerful family of
poly-ADP-ribose polymerases. Front Biosci. 13:3046–3082. 2008.
View Article : Google Scholar
|
|
26
|
Rouleau M, Patel A, Hendzel MJ, Kaufmann
SH and Poirier GG: PARP inhibition: PARP1 and beyond. Nat Rev
Cancer. 10:293–301. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schreiber V, Amé JC, Dollé P, Schultz I,
Rinaldi B, Fraulob V, Ménissier-de Murcia J and de Murcia G:
Poly(ADP-ribose) polymerase-2 (PARP-2) is required for efficient
base excision DNA repair in association with PARP-1 and XRCC1. J
Biol Chem. 277:23028–23036. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Memisoglu A and Samson L: Base excision
repair in yeast and mammals. Mutat Res. 451:39–51. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schiewer MJ and Knudsen KE:
Transcriptional roles of PARP1 in cancer. Mol Cancer Res.
12:1069–1080. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tentori L, Muzi A, Dorio AS, Bultrini S,
Mazzon E, Lacal PM, Shah GM, Zhang J, Navarra P, Nocentini G, et
al: Stable depletion of poly (ADP-ribose) polymerase-1 reduces in
vivo melanoma growth and increases chemosensitivity. Eur J Cancer.
44:1302–1314. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Donawho CK, Luo Y, Luo Y, Penning TD,
Bauch JL, Bouska JJ, Bontcheva-Diaz VD, Cox BF, DeWeese TL,
Dillehay LE, et al: ABT-888, an orally active poly(ADP-ribose)
polymerase inhibitor that potentiates DNA-damaging agents in
preclinical tumor models. Clin Cancer Res. 13:2728–2737. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nishikawa T, Matsumoto K, Tamura K,
Yoshida H, Imai Y, Miyasaka A, Onoe T, Yamaguchi S, Shimizu C,
Yonemori K, et al: Phase 1 dose-escalation study of single-agent
veliparib in Japanese patients with advanced solid tumors. Cancer
Sci. 108:1834–1842. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Middleton MR, Friedlander P, Hamid O, Daud
A, Plummer R, Falotico N, Chyla B, Jiang F, McKeegan E, Mostafa NM,
et al: Randomized phase II study evaluating veliparib (ABT-888)
with temozolomide in patients with metastatic melanoma. Ann Oncol.
26:2173–2179. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Palma JP, Wang YC, Rodriguez LE,
Montgomery D, Ellis PA, Bukofzer G, Niquette A, Liu X, Shi Y, Lasko
L, et al: ABT-888 confers broad in vivo activity in combination
with temozolomide in diverse tumors. Clin Cancer Res. 15:7277–7290.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Carta F, Demuro PP, Zanini C, Santona A,
Castiglia D, D'Atri S, Ascierto PA, Napolitano M, Cossu A, Tadolini
B, et al: Analysis of candidate genes through a proteomics-based
approach in primary cell lines from malignant melanomas and their
metastases. Melanoma Res. 15:235–244. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chang CC, Pirozzi G, Wen SH, Chung IH,
Chiu BL, Errico S, Luongo M, Lombardi ML and Ferrone S: Multiple
structural and epigenetic defects in the human leukocyte antigen
class I antigen presentation pathway in a recurrent metastatic
melanoma following immunotherapy. J Biol Chem. 290:26562–26575.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Carriero MV, Bifulco K, Ingangi V,
Costantini S, Botti G, Ragone C, Minopoli M, Motti ML, Rea D,
Scognamiglio G, et al: Retro-inverso urokinase receptor antagonists
for the treatment of metastatic sarcomas. Sci Rep. 7:13122017.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ragone C, Minopoli M, Ingangi V, Botti G,
Fratangelo F, Pessi A, Stoppelli MP, Ascierto PA, Ciliberto G,
Motti ML, et al: Targeting the cross-talk between Urokinase
receptor and Formyl peptide receptor type 1 to prevent invasion and
trans-endothelial migration of melanoma cells. J Exp Clin Cancer
Res. 36:1802017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ascierto PA, Kirkwood JM, Grob JJ, Simeone
E, Grimaldi AM, Maio M, Palmieri G, Testori A, Marincola FM and
Mozzillo N: The role of BRAF V600 mutation in melanoma. J Transl
Med. 10:852012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rom S, Zuluaga-Ramirez V, Reichenbach NL,
Dykstra H, Gajghate S, Pacher P and Persidsky Y: PARP inhibition in
leukocytes diminishes inflammation via effects on
integrins/cytoskeleton and protects the blood-brain barrier. J
Neuroinflammation. 13:2542016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rodríguez MI, Peralta-Leal A, O'Valle F,
Rodriguez-Vargas JM, Gonzalez-Flores A, Majuelos-Melguizo J, López
L, Serrano S, de Herreros AG, Rodríguez-Manzaneque JC, et al:
PARP-1 regulates metastatic melanoma through modulation of
vimentin-induced malignant transformation. PLoS Genet.
9:e10035312013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ascierto PA, Atkins M, Bifulco C, Botti G,
Cochran A, Davies M, Demaria S, Dummer R, Ferrone S, Formenti S, et
al: Future perspectives in melanoma research: meeting report from
the 'Melanoma Bridge': Napoli, December 3rd-6th 2014. J Transl Med.
13:3742015. View Article : Google Scholar
|
|
43
|
Leonardi GC, Falzone L, Salemi R, Zanghì
A, Spandidos DA, Mccubrey JA, Candido S and Libra M: Cutaneous
melanoma: From pathogenesis to therapy (Review). Int J Oncol.
52:1071–1080. 2018.PubMed/NCBI
|
|
44
|
Staibano S, Pepe S, Lo Muzio L, Somma P,
Mascolo M, Argenziano G, Scalvenzi M, Salvatore G, Fabbrocini G,
Molea G, et al: Poly(adenosine diphosphate-ribose) polymerase 1
expression in malignant melanomas from photoexposed areas of the
head and neck region. Hum Pathol. 36:724–731. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tentori L, Leonetti C, Scarsella M,
D'Amati G, Vergati M, Portarena I, Xu W, Kalish V, Zupi G, Zhang J,
et al: Systemic administration of GPI 15427, a novel
poly(ADP-ribose) polymerase-1 inhibitor, increases the antitumor
activity of temozolomide against intracranial melanoma, glioma,
lymphoma. Clin Cancer Res. 9:5370–5379. 2003.PubMed/NCBI
|
|
46
|
Lok BH, Gardner EE, Schneeberger VE, Ni A,
Desmeules P, Rekhtman N, de Stanchina E, Teicher BA, Riaz N, Powell
SN, et al: PARP inhibitor activity correlates with SLFN11
expression and demonstrates synergy with temozolomide in small cell
lung cancer. Clin Cancer Res. 23:523–535. 2017. View Article : Google Scholar :
|
|
47
|
Ohmoto A and Yachida S: Current status of
poly(ADP-ribose) polymerase inhibitors and future directions.
OncoTargets Ther. 10:5195–5208. 2017. View Article : Google Scholar
|
|
48
|
Wagner LM: Profile of veliparib and its
potential in the treatment of solid tumors. OncoTargets Ther.
8:1931–1939. 2015. View Article : Google Scholar
|
|
49
|
Sonnenblick A, de Azambuja E, Azim HA Jr
and Piccart M: An update on PARP inhibitors - moving to the
adjuvant setting. Nat Rev Clin Oncol. 12:27–41. 2015. View Article : Google Scholar
|
|
50
|
Barboro P, Ferrari N, Capaia M, Petretto
A, Salvi S, Boccardo S and Balbi C: Expression of nuclear matrix
proteins binding matrix attachment regions in prostate cancer.
PARP-1: New player in tumor progression. Int J Cancer.
137:1574–1586. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Inbar D, Cohen-Armon M and Neumann D:
Erythropoietin-driven signalling and cell migration mediated by
polyADP-ribosylation. Br J Cancer. 107:1317–1326. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhu M, Li W, Dong X, Chen Y, Lu Y, Lin B,
Guo J and Li M: Benzyl-isothiocyanate induces apoptosis and
inhibits migration and invasion of hepatocellular carcinoma cells
in vitro. J Cancer. 8:240–248. 2017. View Article : Google Scholar :
|
|
53
|
Tentori L, Leonetti C, Scarsella M,
Vergati M, Xu W, Calvin D, Morgan L, Tang Z, Woznizk K, Alemu C, et
al: Brain distribution and efficacy as chemosensitizer of an oral
formulation of PARP-1 inhibitor GPI 15427 in experimental models of
CNS tumors. Int J Oncol. 26:415–422. 2005.PubMed/NCBI
|