Introduction
Cancer metastasis, the spread of cells from a
primary site to distant organs, is an ominous feature of malignant
tumors. It is the primary obstacle for effective treatment, and the
primary cause of high mortality in patients with cancer (1,2).
Metastasis occurs in a series of discrete steps, including the
detachment of cells in the primary tumor mass from the
extracellular matrix (ECM), invasion to the surrounding tissues,
entrance into the lymphatic and blood vessels, survival and
migration in the circulation, extravasation into new distant
tissues, adaptation to the microenvironment, and final colonization
to form a secondary tumor (3,4).
This highly complicated process involves proteases including
urokinase-type plasminogen activator, cathepsin and matrix
metalloproteinases (MMPs), and angiogenic factors including
angiogenin, vascular endothelial growth factor (VEGF),
platelet-derived growth factor (PDGF) and interleukins, and
adhesion proteins including cadherins, integrins and selectins
(2,5). Among these components, MMPs have been
regarded as critical molecules assisting cancer cells in their
progressive proliferation, migration, invasion and angiogenesis. An
increase in the expression and proteolytic activity of MMPs in
tumors and/or plasma has been positively correlated with rapid
progression, metastasis, a high incidence of recurrence and a short
survival time (6). In addition,
tumors bearing extensive vasculature exhibit increased metastatic
potential and become more aggressive tumors (7,8).
Hypoxia is commonly observed in the microenvironment
of tumors. Under hypoxic conditions, proteasomal degradation of
hypoxia inducible factor (HIF)-1α is prevented by inactivation of
oxygen sensor enzymes including prolyl hydroxylase dehydrogenase
and factor inhibiting HIF. In addition, HIF-1α stabilization,
accumulation and translocation into the nucleus are increased,
leading to tumor vascularization, metastasis,
epithelial-mesenchymal transition (EMT), and resistance to
radiation and chemotherapy (9-11).
MMPs and the HIF pathway are therefore considered valuable markers
and potential therapeutic targets for the control of malignant
tumors.
Rheum undulatum
L. is a perennial herb that is widely distributed in
Asia, including China and Korea. Its pharmacological component is
mainly present in the roots. R. undulatum L. has been
traditionally used as a purgative, laxative, anti-inflammatory and
anti-blood stagnation agent in eastern Asia, and has been used to
treat dental disease in Korea. Chemical components isolated from
R. undulatum L., including rhein, emodine, chrysophanol,
physicon, resveratrol and rhapontigenin, have been reported to
possess anti-allergic, antioxidant, platelet aggregation,
antidiabetic and antitumor activities (12-16).
Rhaponticin (RA; 3′5-dihydroxy-4′-methoxystilbene
3-O-β-D-glucopyranoside; Fig.
1A) from R. undulatum L. has been identified to exhibit
beneficial effects, including anti-allergic, anti-diabetic and
anti-thrombotic activities (14,17).
Furthermore, RA effectively alleviated colitis, pulmonary fibrosis
and liver steatosis (18-20); however, to the best of our
knowledge, the effects of RA on metastasis and angiogenesis in
cancer cells have not been identified.
In the present study, the effects of RA on the
metastatic and angiogenic properties of cancer and endothelial
cells were investigated using an in vitro assay and an in
ovo chick chorioallantoic membrane (CAM) assay. In addition, the
underlying molecular mechanisms of its anti-metastatic and
anti-angiogenic activities were investigated.
Materials and methods
Cells and culture
Human breast adenocarcinoma MDA-MB231 cells [Korean
Cell Line Bank (KCLB) no. 30026] and human fibrosarcoma HT1080
cells (KCLB no. 10121) were purchased from the KCLB (Seoul, Korea)
and maintained in Dulbecco's modified Eagle's medium (DMEM) or
RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS;
Biotechnics Research, Lake Forest, CA, USA) containing 100 U/ml
penicillin and 100 µg/ml streptomycin (Cellgro; Corning
Incorporated, Corning, NY, USA) at 37°C in a 5% CO2
incubator. Human umbilical vein endothelial cells (HUVECs;
Innopharmascreen, Asan, Korea) were maintained in endothelial cell
growth medium-2 (EGM-2; PromoCell GmbH, Heidelberg, Germany) and
used at passages 3-8 in the experiments. RA (≥98% purity determined
using high-pressure liquid chromatography) was obtained from Faces
Biochemical (Wuhan, China).
Cell death and colony formation
analysis
Cells (5×103 cells/well/100 µl)
were seeded into 96-well culture plates and treated with or without
the specified concentrations of RA. After 48 h, the cell viability
was determined using the Cell Counting Kit-8 (CCK-8; Dojindo
Molecular Technologies, Inc., Kumamoto, Japan). To evaluate the
ability to form sizable colonies, cells (1×103
cells/well) seeded into 6-well plates were incubated for 10 days
with or without the indicated concentrations of RA. At the end of
the experiments, the cells were washed three times with PBS, and
the colonies were stained with 0.2% crystal violet/20% (w/v)
methanol solution for 30 min. The cells were washed with tap water
thoroughly and air-dried, and images were captured. To quantify
colony formation, the dye was extracted with dimethylsulfoxide
(DMSO) and the absorbance was measured at 590 nm using a
SpectraMaxi3 Multi-mode reader (Molecular Devices, LLC, Sunnyvale,
CA, USA).
Scratch and Transwell®
migration assays
For the scratch migration assay, cells
(2×104 cells/well/100 µl) were seeded into
96-well plates, allowed to adhere overnight and treated with 25
µg/ml mitomycin (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) for 30 min. Following the creation of wounds on the
confluent monolayers using a 96-pin Wound Maker (IncuCyte; Essen
Bioscience, Ann Arbor, MI, USA), the plates were placed into the
IncuCyte chamber (Essen Bioscience) and incubated with or without
RA in a 5% CO2 incubator at 37°C. The wound images were
captured every 3 h using an IncuCyte Zoom system (Essen
Bioscience). The wound closure proportion at each time point was
calculated based on the wound width at 0 h as 100%. For the
Transwell migration assay, a Transwell chamber (10 mm diameter, 8
µm pore size polycarbonate membrane; Corning Incorporated)
was used. In the lower chamber, 600 µl 10% FBS in DMEM or
EGM-2 was used for tumor cells or HUVECs, respectively. In the
upper chamber, cells (1×105 cells/well) suspended in 100
µl serum-free DMEM or endothelial cell basal medium-2
(EBM-2) were added. Following incubation at 37°C with 5%
CO2 for 12 h, the migrated cells were fixed, stained
with 0.2% crystal violet/20% (w/v) methanol solution, and counted
using a phase-contrast microscope (magnification, ×100).
Scratch, Transwell and three-dimensional
(3D) spheroid invasion assays
A scratch wound invasion assay using IncuCyte Zoom
and the Transwell invasion assay were used with
Matrigel® (BD Biosciences, Franklin Lakes, NJ, USA)
diluted 1:4 with serum-free medium as the intervening invasive
barrier. In addition, a 3D invasion assay was performed using the
Cultrex 96-well 3D Spheroid Cell Invasion assay (Trevigen;
Bio-Techne, Minneapolis, MN, USA), according to the manufacturer's
protocol. In brief, 3×105 cells were suspended in 50 µl
prechilled spheroid formation ECM, added to a Corning 96-well Clear
Round Bottom Ultra Low Attachment Microplate (Corning
Incorporated), centrifuged at 200 × g for 3 min at room
temperature, and then incubated for 3 days to assemble into compact
spheroids. Following the addition of 50 µl prechilled
invasion matrix, the plates were centrifuged at 300 × g for 5 min
at 4°C and incubated for 1 h at 37°C to promote gel formation.
Culture medium containing the indicated concentrations of RA was
then added into each well and the plates were incubated at 37°C in
a 5% CO2 incubator for between 3 and 5 days. Cells that
invaded the surrounding matrix were observed using a phase-contrast
inverted microscope (magnification, ×100) and images were captured
every 24 h.
Gelatin zymography
The MMP-9 activity in RA-treated or -untreated
HT1080 conditioned medium (CM) was measured by zymography using a
gelatin substrate as described previously (21). The activity of gelatinase was
detected as a clear band with a blue background at 92 kDa. Phorbol
12-myristate 13-acetate (PMA) was obtained from Sigma-Aldrich.
HUVEC tube formation assays
Capillary-like tube formation of endothelial cells
was measured using a Cultrex in vitro angiogenesis assay kit
(Trevigen; Bio-Techne), according to the manufacturer's protocol.
In brief, 50 µl basement membrane extract was coated on a
prechilled 96-well culture plate and polymerized for 30 min at
37°C. RA-treated or untreated HUVECs (5×104) suspended
in 100 µl EGM-2 were added to each well and incubated for
between 4 and 12 h at 37°C. The tube formation was visualized using
a phase-contrast inverted microscope (magnification, ×100).
Proteome profiler antibody arrays
The expression profile of angiogenesis-associated
proteins in the RA-treated or untreated CM was determined using a
Proteome Profiler™ Human Angiogenesis Array kit (R&D Systems,
Inc., Minneapolis, MN, USA). The blots were visualized using a
ChemiDoc™ Touch Imaging system using a Clarity™ western enhanced
chemiluminescence (ECL) substrate (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). For the preparation of CM under hypoxia, the
cells were incubated with or without 50 µM RA for 24 h in
complete medium, washed with 0.5% FBS medium three times, and then
incubated under hypoxic conditions (1% O2) for an
additional 24 h in 0.5% FBS medium. The culture medium was
harvested, centrifuged at 13,000 × g for 15 min at 4°C to remove
cell debris, and collected as CM.
Fluorescence immunocytochemistry
Cells were seeded into 35-mm-diameter glass-bottomed
dishes (SPL Life Sciences, Pocheon, Korea), treated with RA for 12
h, and then stimulated with 200 µM CoCl2 for 6 h.
Following washing with ice-cold PBS three times, the cells were
subjected to immunocytochemistry as described previously (22). Following counterstaining with DAPI,
the cells were analyzed for the nuclear expression of HIF-1α using
a fluorescence microscope (Eclipse Ti; Nikon Corporation, Tokyo,
Japan, magnification, ×100).
Western blotting
Whole cell lysates were prepared using Mammalian
Protein Extraction Reagent (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and the protein concentration was determined
using a Bicinchoninic Acid protein assay kit (Pierce; Thermo Fisher
Scientific, Inc.). Equal amounts of each samples (20 µg)
mixed with NuPAGE lithium dodecyl sample buffer (Invitrogen; Thermo
Fisher Scientific, Inc.) were denatured by heating for 10 min at
95°C and then separated by SDS-PAGE (8-15%). Following
electrotransfer onto a polyvinylidene difluoride membrane
(Immobilon-P; Merck Millipore, Darmstadt, Germany), membranes were
blocked in a 3% BSA/Tris-buffered saline solution containing 0.05%
Tween-20 (TBST) for 1 h at 25°C and washed three times with TBST.
Membranes were incubated with specific antibodies (1:1,000 dilution
in 3% BSA/TBST) overnight at 4°C, washed three times with TBST, and
then incubated with horseradish peroxidase-conjugated secondary
antibodies (1:4,000 dilution in 3% BSA/TBST) for 1 h at 25°C.
Following washing three times with TBST, immune-reactive protein
bands were detected using a ChemiDoc Touch Imaging system with a
Clarity western ECL substrate. The relative band intensity was
calculated using ImageJ software (version 1.50; National Institutes
of Health, Bethesda, MD, USA). Antibodies against protein kinase B
(Akt; cat. no. 4685), phospho (p)-Akt (cat. no. 9271), mammalian
target of rapamycin (mTOR; cat. no. 2983), p-mTOR (cat. no. 5536),
MMP-9 (cat. no. 2270), von Hippell-Lindau protein (VHL; cat. no.
68547) and tubulin (cat. no. 2125) were obtained from Cell
Signaling Technology, Inc. (Danvers, MA, USA), and an anti-HIF-1α
antibody (cat. no. 610958) was obtained from BD Biosciences. The
EMT Antibody Sampler kit (cat. no. 9782) including β-catenin,
neuronal (N-)cadherin, Slug, Snail, zinc finger E-box-binding
homeobox 1 (ZEB1), vimentin, claudin-1 and epithelial (E-) cadherin
was purchased from Cell Signaling Technology, Inc.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was extracted from RA-treated or
-untreated HT1080 under CoCl2 stimulation and then
reverse-transcribed into cDNA using an RNA extraction solution and
a First Strand cDNA Synthesis kit (BioAssay Co., Ltd., Daejeon,
Korea), respectively, according to the manufacturer's protocol.
Amplification of MMP-9, pentraxin-related protein 3 (PTX-3),
VEGF-α, placental growth factor (PlGF), HIF-1α and VHL was
performed using a Vertiti 96-well Thermal Cycler (Applied
Biosystems; Thermo Fisher Scientific, Inc.) using specific primers
(Table I). The PCR conditions were
as follows: 94°C for 3 min and 25-35 cycles of 94°C for 30 sec,
54°C for 30 sec, and 70°C for 30 sec, with a final extension of 10
min at 72°C. GAPDH was used as a reference gene. PCR products were
visualized on a 1% agarose gel by staining with GreenLight™
(BioAssay Co., Ltd.) and the relative band intensity was calculated
using ImageJ software.
 | Table IPrimers used for the polymerase chain
reaction. |
Table I
Primers used for the polymerase chain
reaction.
| Target gene | Sequence |
|---|
| MMP-9 | F:
5′-CAAGCTGGACTCGGTCTTTGA-3′ |
| R:
5′-TTCAACTCACTCCGGGAACTCA-3′ |
| PTX-3 | F:
5′-CATCCAGTGAGACCAATGAGG-3′ |
| R:
5′-GTAGCCGCCAGTTCACCATTT-3′ |
| VEGF-α | F:
5′-CCACTGAGGAGTCCAACATCA-3′ |
| R:
5′-CATTTACACGTCTGCGGATCTT-3′ |
| PlGF | F:
5′-ACTCAGCTCTTCTCCTCCTGTG-3′ |
| R:
5′-ACGGTAATAAATACACGAGCCG-3′ |
| HIF-1α | F:
5′-AACCACCTATGACCTGCTTGGT-3′ |
| R:
5′-TTGAGCGGCCTAAAAGTTCTTC-3′ |
| VHL | F:
5′-TACCGAGGTCACCTTTGGC-3′ |
| R:
5′-GGAGGCATCGCTCTTTCAG-3′ |
| GAPDH | F:
5′-TCATGACCACAGTCCATGCC-3′ |
| R:
5′-TCCACCACCCTGTTGCTGTA-3′ |
CAM assay
Fertilized chicken eggs purchased from Pulmuone Co.,
Ltd. (Seoul, Korea) were incubated in a digital egg incubator
(MX-190 CD, R-COM, Gimhae, Korea) at 37°C with 65% humidity. The
starting time point was designated as the chick embryonic
development (ED) day 0. On ED day 3, 3 ml albumin was removed using
a syringe and a round window was made in each egg. Following
resealing of the windows with adhesive tape, the eggs were returned
to the incubator. To investigate the effects of RA on angiogenesis,
5-mm-diameter discs loaded with RA and/or VEGF in 20 µl PBS
were placed on the CAM of individual embryos on ED day 6. Following
incubation for 3 days, the vasculature was observed and images were
captured.
Statistics
Results are expressed as the mean ± standard
deviation. Statistical significance of treatment effects were
analyzed using one-way analysis of variance (ANOVA) followed by
Dunnett's test with GraphPad Prism 5 software (GraphPad Software,
Inc., La Jolla, CA, USA). Analysis in two groups was performed
using Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
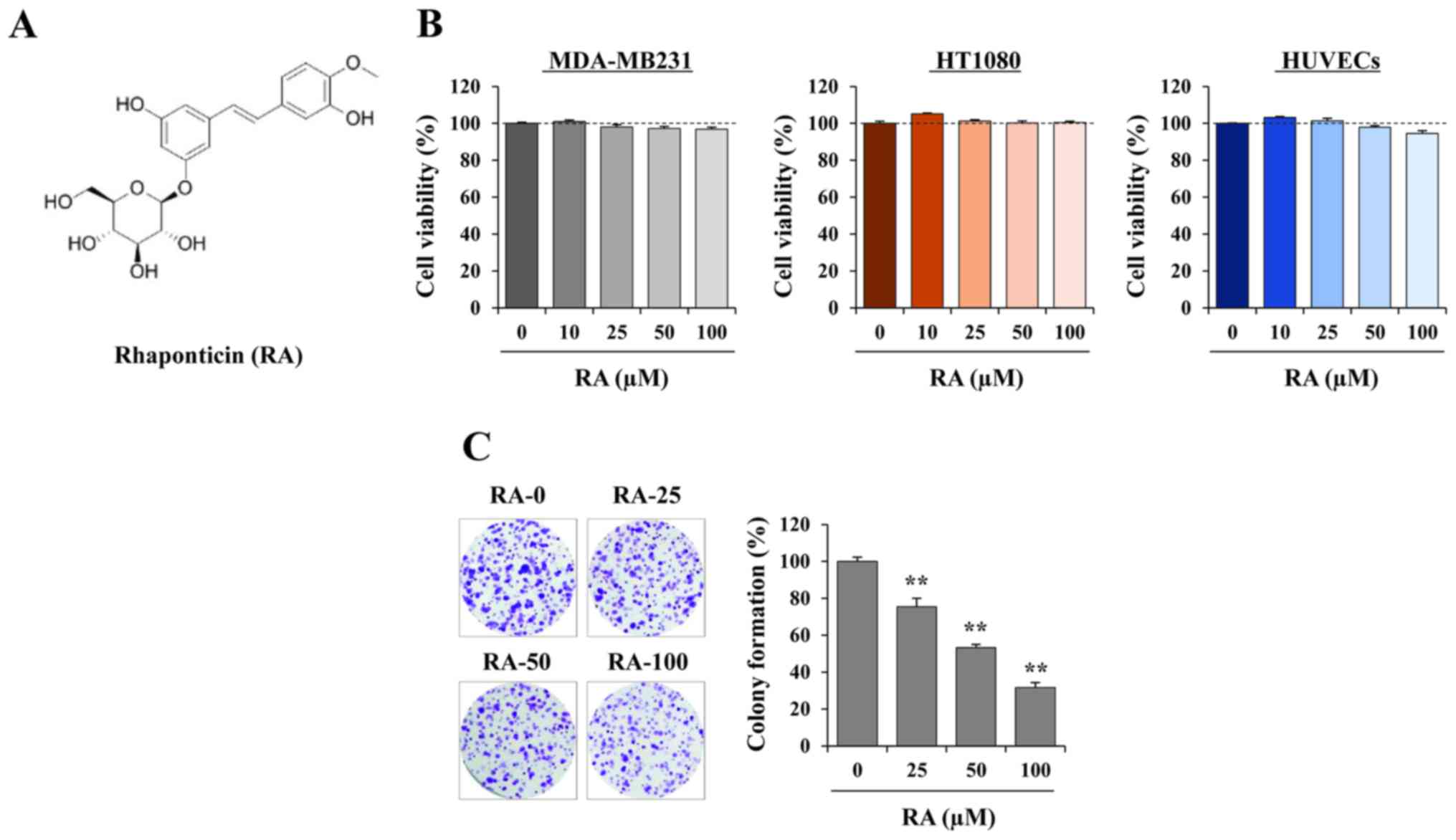
RA exhibits no cytotoxicity and
suppresses anchorage-dependent colony formation in MDA-MB231
cells
Prior to assessing the anti-metastatic activity of
RA, its cytotoxic effects on cancer and endothelial cells were
determined using a CCK-8 assay following incubation of the cells
with the indicated doses of RA for 48 h. Since RA was dissolved in
100% DMSO to a final concentration of 100 mM, 0.001% DMSO was used
as the vehicle control. Fig. 1B
indicates that, at concentrations up to 100 µM, RA did not
decrease the cell viability of MDA-MB231 cells, HT1080 cells or
HUVECs. In subsequent studies, cells were therefore treated with RA
at concentrations of 25, 50 and 100 µM. Since adhesion and
colony formation are essential steps in distant metastasis, the
ability of MDA-MB231 cells to form detectable colonies from a
single cell in the presence or absence of RA was investigated.
Fig. 1C indicates that RA
significantly inhibited anchorage-dependent colony formation, as
determined by decreases in the colony numbers and densities.
Quantification of colony formation by extracting the dye revealed a
signifi-cant decrease in cells treated with RA, resulting in a 70%
decrease using 100 µM RA (F=280.0, P=0.0096; one-way ANOVA).
Fig. 1B indicates that this
inhibitory effect was not due to cytotoxicity.
RA suppresses the migratory and invasive
abilities of MDA-MB231 cells
RA-untreated control MDA-MB231 cells migrated across
a scratch wound region, leading to 51.6 and 82.5% healing at 12 and
18 h, respectively. However, treatment with 100 µM RA
significantly inhibited wound migration by 35.91% at 12 h and
49.19% at 18 h, when compared with that of control cells (12 h,
F=7.018, P=0.0125; 18 h, F=30.37, P=0.0001; one-way ANOVA; Fig. 2A). Using a Transwell assay, RA
suppressed the serum-induced migration and invasion in a
dose-dependent manner, compared with that of the control cells,
exhibiting decreases of 76.6 and 89.9% at 100 µM RA,
respectively (migration, F=270.9, P<0.0001; invasion, F=605.2,
P<0.0001; one-way ANOVA; Fig.
2B). RA-untreated control cells invaded the Matrigel-coated
scratch wound at levels of 57.5 and 84.3% at 12 and 24 h,
respectively, whereas treatment with 100 µM RA decreased the
invasion by 49.9 and 46.0% at 12 h and 24 h, respectively (12 h,
F=9.970, P=0.0044; 24 h, F=17.27, P=0.0007; one-way ANOVA; Fig. 2C). In addition, invasion from 3D
spheroids was also suppressed by RA treatment in a dose-dependent
manner (3 days post-treatment, T3, F=247.0, P<0.0001; 5 days
post-treatment, T5, F=229.9, P<0.0001; one-way ANOVA; Fig. 2D). Together, these results
indicated that RA exhibits potent anti-metastatic activity with no
cytotoxicity in MDA-MB231 cells.
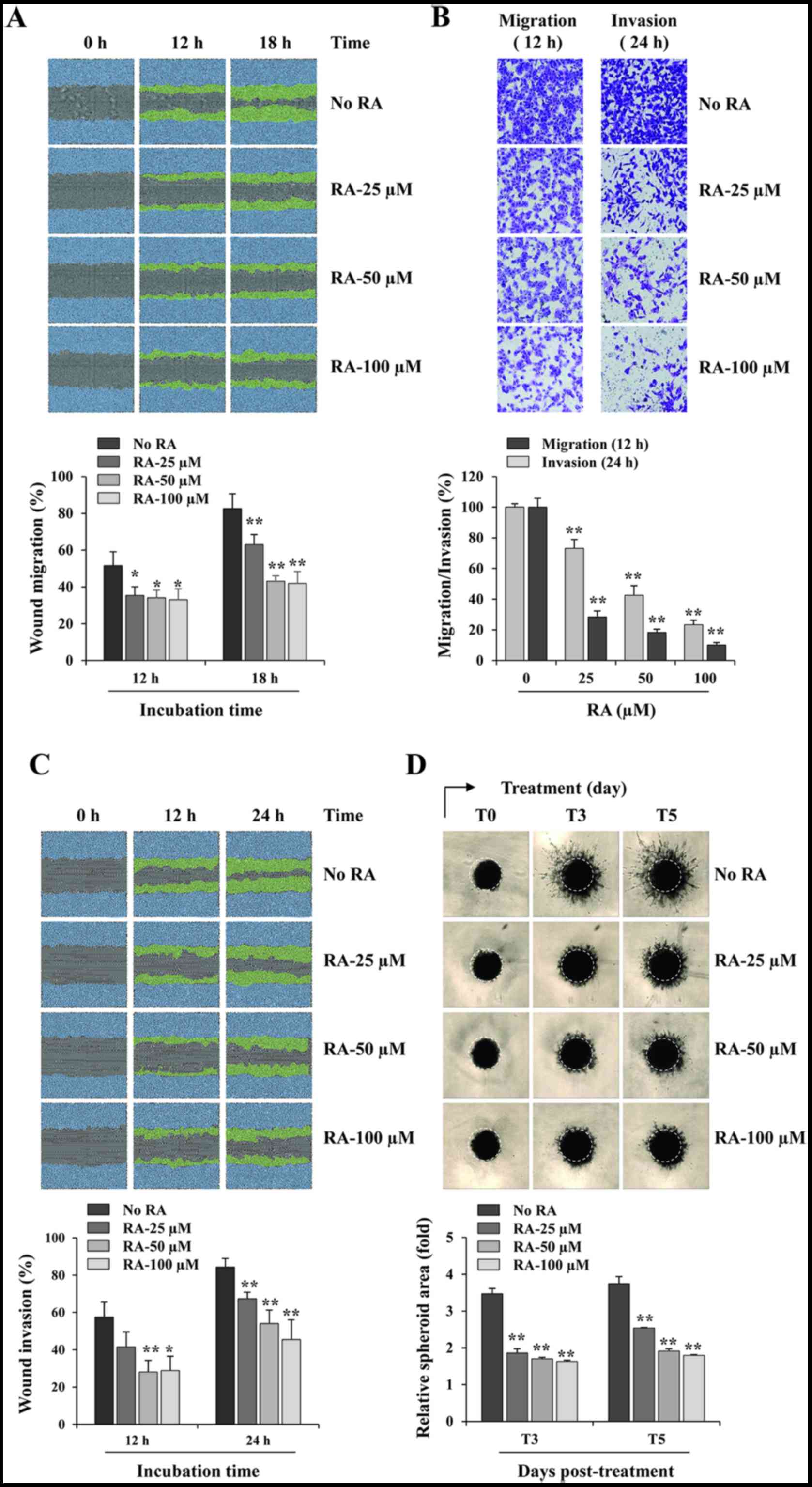 | Figure 2RA inhibits the migration and
invasion of MDA-MB231 cells. (A) In a scratch migration assay,
wound widths in RA-treated and -untreated cells were measured every
3 h following scratch wound formation using the IncuCyte Zoom. The
relative wound migrations at 12 and 18 h were calculated based on
the wound width at 0 h using ImageJ software, and are expressed as
the mean ± SD obtained from triplicate samples. (B) The migration
and invasion of RA-treated and -untreated cells across Transwell
membranes were measured following incubation for 12 and 24 h,
respectively. The number of cells in five random fields was
determined using phase-contrast microscopy, and the relative
migration and invasion were calculated and compared with
RA-untreated control cells. The data are representative of three
independent experiments and are expressed as the mean ± SD. (C) In
the scratch invasion assay, wound widths in RA-treated and
-untreated cells were measured every 3 h following scratch wound
formation using the IncuCyte Zoom. The relative wound invasions at
12 and 24 h were calculated based on the wound width at 0 h using
ImageJ software, and are expressed as the mean ± SD obtained from
triplicate samples. (D) Cells were cultured as three-dimensional
spheroids, treated with the indicated concentrations of RA and
incubated further for 5 days. The images of stellate structural
formations were captured at 3 and 5 days post-treatment, and the
relative spheroid areas were measured using ImageJ software.
Results are expressed as the mean ± SD from triplicate samples.
*P<0.05 and **P<0.01 vs. untreated
control. RA, rhaponticin; SD, standard deviation. |
RA inhibits the angiogenic activities of
HUVECs in vitro and in the CAM assay
To investigate the effects of RA on endothelial
cells, the ability of RA-treated and -untreated HUVECs to form
tube-like structures on Matrigel was assessed. To induce tube
formation, the cells were suspended in EGM-2 containing angiogenic
stimuli including endothelial growth factor (EGF), basic fibroblast
growth factor (FGF) and VEGF. Fig.
3A indicates that HUVECs in EBM-2 (panel a) did not induce
tube-like networks, whereas RA-untreated HUVECs in EGM-2 (panel b)
induced almost complete tube formation. RA-treated HUVECs in EGM-2
(panels c-e) exhibited weak tube formation in a dose-dependent
manner compared with RA-untreated HUVECs (F=488.9, P<0.0001;
one-way ANOVA). In addition, the ability of RA-treated and
-untreated HUVECs to migrate across a Transwell membrane was
investigated. Each group of HUVECs in EBM-2 was seeded in the upper
chamber and then incubated for 12 h to allow downward migration to
the lower chamber filled with EGM-2. Fig. 3B indicates that RA-untreated HUVECs
migrated efficiently, whereas RA-treated HUVECs exhibited decreased
migration in a dose-dependent manner to 23.6% of the control HUVECs
at 100 µM (F=255.8, P<0.0001; one-way ANOVA). Using the
CAM assay, spontaneous angiogenesis was observed on ED day 6, and
topical application of RA suppressed spontaneous angiogenesis by
29.16% compared with the vehicle control on ED day 9 (Fig. 3C, left). Furthermore, VEGF induced
a pronounced angiogenic response in terms of vessel length,
thickness and sprouting, whereas RA at 100 µg per embryo
exhibited a significant inhibition of VEGF-induced angiogenesis
(Fig. 3C, right), indicating that
RA directly suppressed the angiogenesis of endothelial cells.
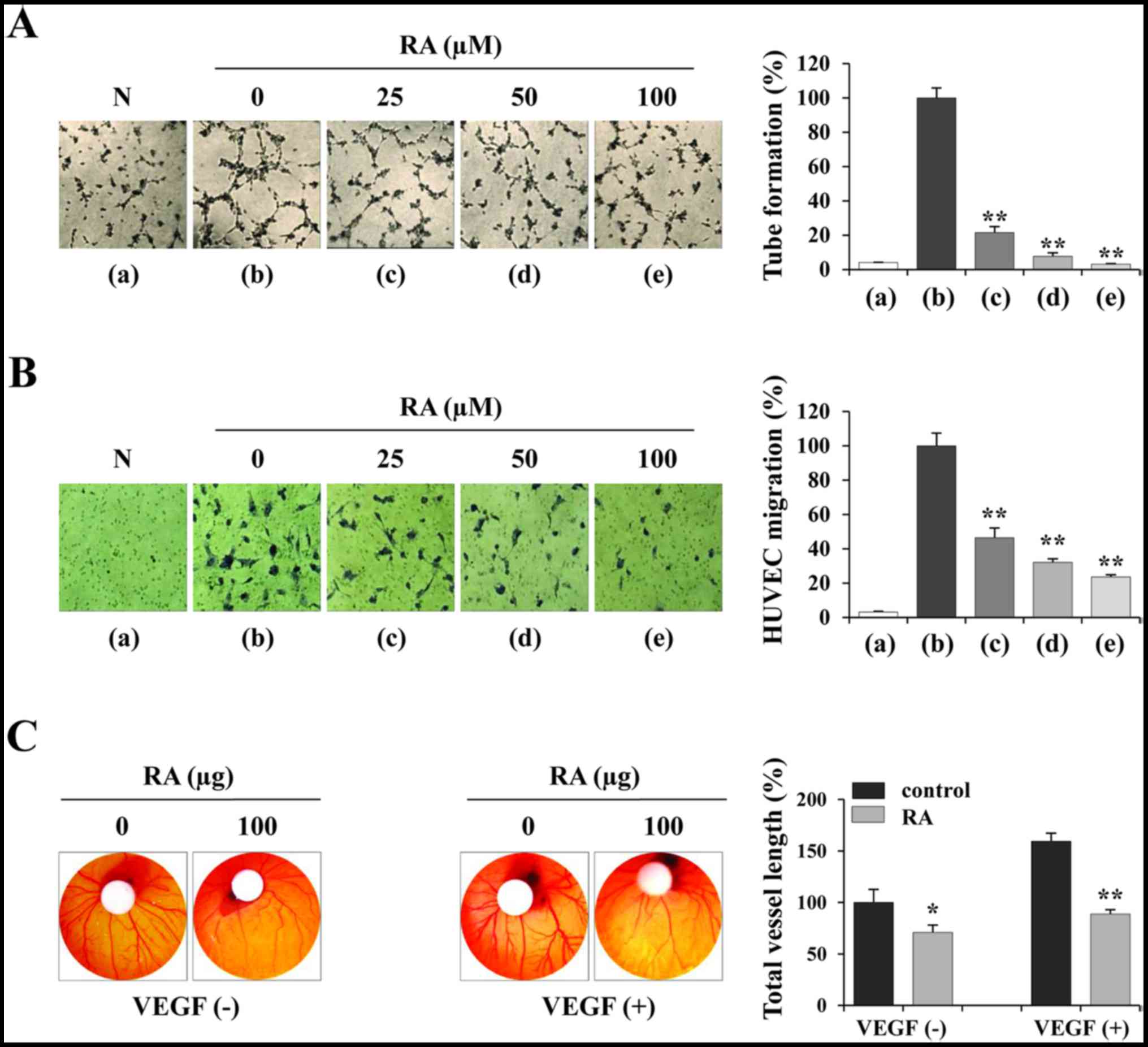 | Figure 3RA suppresses the angiogenic activity
of HUVECs. (A) HUVECs were treated with or without the indicated
concentrations of RA for 24 h, suspended in EGM-2, and seeded in
basement membrane extract-coated wells. Following incubation for 4
h, capillary-like tube formation was measured. RA-untreated HUVECs
in EBM-2 were used as a negative control. The number of tubes in
triplicate samples was counted. (B) RA-treated or untreated HUVECs
were suspended in EBM-2 and seeded into the upper chamber of the
Transwell. The EGM-2-induced migration of HUVECs for 12 h was
observed following staining with crystal violet solution (n=5 per
group). (C) On ED day 6, RA (100 µg) and VEGF (200 ng) in
PBS (20 µl) were loaded onto filter discs and then carefully
placed on the CAM. Following resealing the windows with adhesive
tape, the eggs were incubated for 3 days. On ED day 9, the
vasculature in the eggs was photographed and the total vessel
length (n=3 per group) was measured by ImageJ software. The data
are representative of three independent experiments and expressed
as the mean ± SD. *P<0.05 and **P<0.01
vs. RA-untreated control. RA, rhaponticin; HUVEC, human umbilical
vein endothelial cell; EGM-2, endothelial cell growth medium-2;
EBM-2, endothelial cell basal medium-2; N, negative control; ED,
embryonic development; VEGF, vascular endothelial growth
factor. |
RA decreases the production of
angiogenesis-associated proteins under normoxic and hypoxic
conditions in HT1080 cells
In the tumor microenvironment (TME), imbalances
favoring angiogenic activators (e.g. VEGF, FGF, PDGFB and EGF) over
inhibitors (e.g. thrombospondin-1, endostatin and angiostatin)
activate the angiogenic switch that induces tumor vascularization,
proliferation and metastasis (23,24).
Angiogenesis is therefore an important target for controlling solid
tumors. To assess the effects of RA on the secretion of pro-and
anti-angiogenic factors in HT1080 cells, the CM obtained from
RA-untreated control and RA-treated HT1080 cells under normoxic and
hypoxic conditions was analyzed using a Proteome Profiler Human
Angiogenesis Array kit. Fig. 4A
indicates that the levels of pro-angiogenic factors, including
MMP-9, PTX-3, interleukin 8 (IL-8), VEGF and PlGF were
significantly decreased in the RA-treated CM compared with the
control CM under normoxic conditions. Under hypoxic conditions, the
levels of these pro-angiogenic factors were increased between 15
and 99% compared with those under normoxic conditions, and RA
treatment also decreased the levels of the pro-angiogenic factors.
The transcriptional levels of MMP-9, PTX-3, VEGF-α and PlGF were
also increased by CoCl2 stimulation, mimicking hypoxic
conditions. However, the increase in the mRNA levels of these
pro-angiogenic factors were significantly inhibited by RA treatment
(MMP-9, F=99.31, P=0.0003; PTX-3, F=105.8, P=0.0003; VEGF-α,
F=92.63, P=0.0004; PlGF, F=77.26, P=0.0005; one-way ANOVA; Fig. 4B). Additionally, gelatin zymography
and western blotting indicated that RA markedly suppressed the
PMA-induced increase in the gelatinolytic activity of MMP-9 and
secretion of MMP-9 into the CM of HT1080 cells (gelatin zymography,
F=68.46; P=0.0007; western blotting, F=25.10, P=0.0047; one-way
ANOVA; Fig. 4C). These results
indicate that RA inhibits the angiogenic switch by suppression of
angiogenic factors in tumor cells.
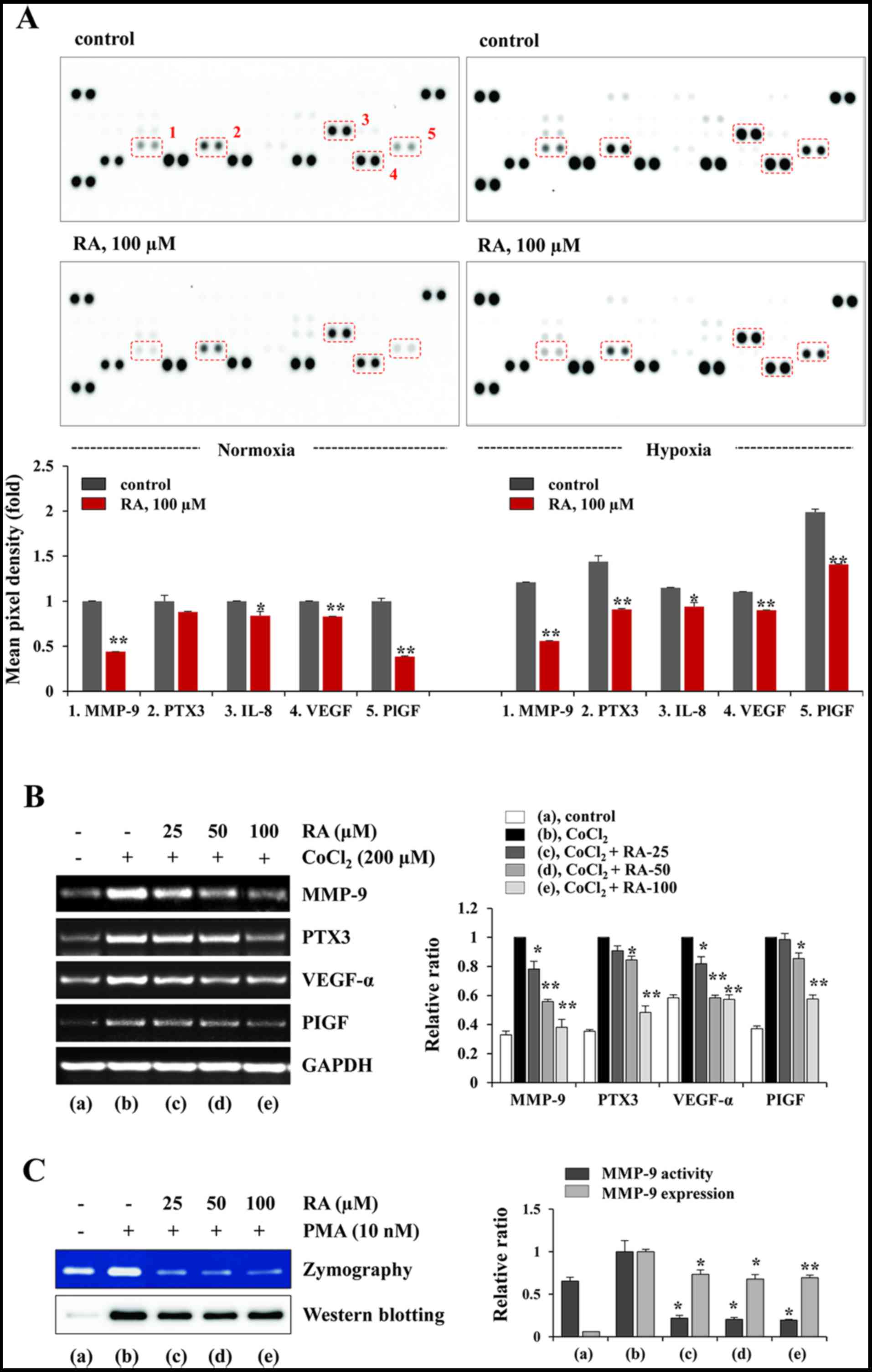 | Figure 4RA decreases the production of
pro-angiogenic proteins and MMP-9 activity of HT1080 cells. (A)
Using a Proteome Profiler Human Angiogenesis Array kit, the levels
of angiogenesis-associated proteins in CM were analyzed. The CM
were obtained from RA-treated and -untreated cells incubated under
normoxic (20% O2) and hypoxic (1% O2)
conditions. The pixel intensity was measured using ImageJ software
and the relative values are expressed as the mean ± SD calculated
from duplicate dots. The data are representative of two independent
experiments. (B) Cells pretreated with or without RA for 12 h were
stimulated with CoCl2 (200 µM) for 3 h, and the
mRNA levels of MMP-9, PTX-3, VEGF-α and PlGF were determined using
the reverse transcription-polymerase chain reaction. The relative
band intensities were analyzed using ImageJ software and calculated
following normalization to GAPDH. Results are expressed as the mean
± SD of two independent experiments. (C) The gelatinolytic activity
of MMP-9 and the levels of MMP-9 in RA-treated and -untreated CM
were measured using zymography and western blotting, respectively.
The band intensities were analyzed using ImageJ software and
expressed as the mean ± SD of two independent experiments.
*P<0.05 and **P<0.01 vs. RA-untreated
control. RA, rhaponticin; MMP, matrix metalloproteinase; CM,
conditioned media; SD, standard deviation; PTX-3, pentraxin-related
protein 3; VEGF, vascular endothelial growth factor; PlGF,
placental growth factor; PMA, phorbol 12-myristate 13-acetate. |
RA suppresses HIF-1α accumulation and
nuclear expression, and regulates EMT-associated proteins under
hypoxic conditions in HT1080 cells
Since pro-angiogenic factors are downstream targets
of HIF-1α, the effects of RA on the HIF-1α nuclear expression and
HIF-1α accumulation under CoCl2 stimulation conditions,
to mimic physiological hypoxia, were investigated. Fig. 5A indicates that cells with nuclear
HIF-1α induced by CoCl2 treatment were decreased by RA
treatment in a dose-dependent manner (F=213.4, P<0.0001; one-way
ANOVA). Western blotting indicated that RA efficiently suppressed
the CoCl2-induced accumulation of HIF-1α protein in a
dose-dependent manner (F=33.33, P=0.0027; one-way ANOVA). However,
the mRNA levels of HIF-1α were not influenced by RA treatment,
suggesting that RA did not affect the transcription of the HIF-1α
gene. In addition, RA significantly increased the VHL expression at
the mRNA and protein levels, leading to the rapid degradation of
HIF-1α (VHL mRNA, F=54.24, P=0.0011; VHL protein, F=33.58,
P=0.0027; one-way ANOVA; Fig. 5B).
These results indicated that RA decreased the production of
angiogenesis-associated proteins via suppression of HIF-1α
stability and induction of HIF-1α degradation. Hypoxia-induced
HIF-1α stabilization promotes EMT in a number of types of cancer,
and is associated with metastasis and resistance to chemo- and
radiotherapy (25,26). Several transcription factors,
including Slug, Snail and Twist, drive the EMT process and cause
the loss of cell-cell junctions, thereby allowing migration and
dissemination of tumor cells (27). Since RA efficiently decreased
hypoxia-induced HIF-1α accumulation, the effect of RA on the
expression of EMT-associated proteins under hypoxic conditions was
investigated. As presented in Fig.
5C, CoCl2 stimulation significantly increased
mesenchymal markers, including N-cadherin, Slug, Snail, ZEB1 and
vimentin, whereas it slightly decreased claudin-1 and E-cadherin.
In RA-treated cells, the levels of mesenchymal markers were
significantly decreased compared with those in RA-untreated control
cells, whereas the levels of claudin-1 and E-cadherin were
increased in a dose-dependent manner (claudin-1, F=40.46, P=0.0019;
E-cadherin, F=22.28, P=0.0059; N-cadherin, F=38.61, P=0.0021; Slug,
F=52.39, P=0.0011; Snail, F=55.40, P=0.0010; ZEB1, F=65.04,
P=0.0008; vimentin, F=30.27, P=0.0033; one-way ANOVA; Fig. 5C), suggesting that RA regulates
EMT-associated proteins via suppression of the HIF-1 signaling
pathway.
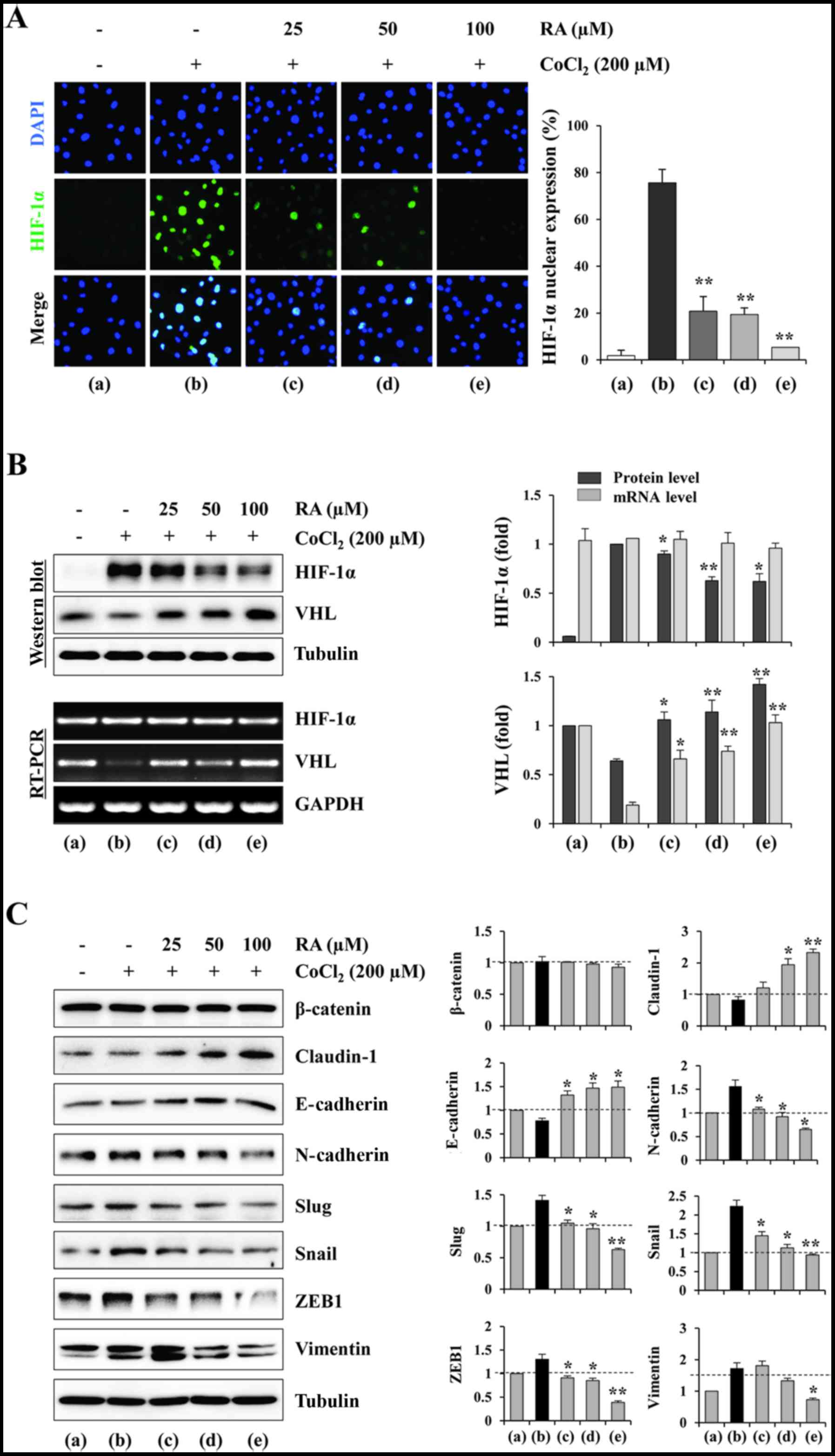 | Figure 5RA inhibits the nuclear expression
and accumulation of HIF-1α, and regulates EMT-associated proteins
in HT1080 cells under hypoxic conditions. (A) Cells grown in
glass-bottomed dishes were treated with RA for 12 h and then
stimulated with CoCl2 (200 µM) to mimic hypoxic
conditions. After 6 h, nuclear HIF-1α was visualized using
fluorescence immunocytochemistry. DAPI was used for counterstaining
nuclei. Results are presented as the mean ± SD of five selected
fields per sample, and are representative of three independent
experiments. (B) The cells were incubated with or without RA for 12
h and then treated with CoCl2 (200 µM) for 3 or 6
h. The mRNA and protein levels of HIF-1α and VHL were detected
using RT-PCR and western blotting, respectively. The relative band
intensities were quantitated using ImageJ software following
normalization to GAPDH and tubulin expression, respectively. (C)
The cells were treated with or without RA for 12 h and then
stimulated with CoCl2 (200 µM) for 24 h. Cell
lysates were subjected to western blotting against
endothelial-mesenchymal transition-associated proteins. The
relative band intensities were calculated using ImageJ software
following normalization to tubulin expression. Results are
expressed as the mean ± SD of two independent experiments.
*P<0.05 and **P<0.01 vs. RA-untreated
control. RA, rhaponticin; HIF, hypoxia-inducible factor; SD,
standard deviation; VHL, von Hippel-Lindau protein; RT-PCR, reverse
transcription-polymerase chain reaction; E-cadherin, epithelial
cadherin; N-cadherin, neuronal cadherin; ZEB1, zinc finger
E-box-binding homeobox 1. |
Discussion
Carcinogenesis, also called oncogenesis or
tumorigenesis, which involves the formation of a cancer, is a
complicated and multistep process consisting of the stages of
initiation, promotion and progression (28). The majority of human cancer types
exhibit various hallmarks, including sustained proliferation,
evasion of tumor suppressors, resistance to cell death,
angiogenesis, escape from the immune response, inflammation,
angiogenesis, invasion and metastasis (29). Previous studies have identified
that carcinogenesis occurs by close interaction of cancer cells
with the surrounding ECM and neighboring normal stromal cells.
Cancer cells secrete growth factors, chemokines and cytokines,
which efficiently recruit stromal cells, vascular cells and immune
cells in the TME. In turn, these recruited cells also affect cancer
cells by releasing growth-promoting signals and providing a
suitable environment for tumor progression and metastasis,
indicating that the TME is not simply a bystander, but an important
potential therapeutic target for cancer treatment (30-32).
Consistent with this possibility, several clinical and preclinical
studies that primarily target the TME for managing metastatic
cancer have been conducted. Therapeutic strategies include
targeting the tumor vasculature, cancer-associated inflammation,
the communication between tumor cells and the TME, and the hypoxia
in the TME (33,34). Angiogenesis during normal
physiological conditions, such as embryonic development, tissue
regeneration and wound healing, occurs by a regulated process to
form well-organized vascular networks. In contrast, in pathological
conditions such as ocular neovascularization in diabetes, arthritis
and cancer, the balance between the pro- and anti-angiogenic
factors shifts in favor of the pro-angiogenic factors, leading to
the formation of a disorganized vascular network (35). Blood vessels within tumors act as a
pathway to provide sufficient oxygen and nutrients, to eliminate
waste metabolites, and to facilitate cellular entry into the
circulation, thus promoting tumor growth and metastasis (36,37).
Hypoxia is common in a majority of malignant tumors
and is important for facilitating rapid formation of blood vessels,
by increasing the proliferation, migration and organization of
endothelial cells into new tubular structures. In addition, hypoxia
in the TME increases tumor cell survival, metastasis, the EMT and
resistance to cell death (31).
The transcription factor HIF-1α is primarily involved in these
processes, so targeting the HIF-1 pathway is considered to be a
promising strategy to control tumor growth, improve the efficacy of
treatment and prolong the survival of patients with cancer
(38,39). Consistent with these possibilities,
inhibition of HIF-1 activity using an antisense oligonucleotide was
an effective and safe treatment of metastatic cancer cells in Phase
I clinical trials (40,41). In addition, phytochemicals
including decursin, decursinol, curcumin, resveratrol and genistein
have been reported to exhibit anti-angiogenic effects in various
types of cancer (42-44).
RA isolated from a number of medicinal herbs has
been identified to possess various pharmacological effects,
including antioxidant, anti-thrombotic, anti-allergic and
vasorelaxant activities (12-19,45).
In addition, antitumor activities using PEGylated liposomal RA and
RA containing a stilbene moiety were identified to exhibit
growth-inhibiting and apoptosis-inducing activities (46). Notably, rhapontigenin (Rha), an
aglycone form of RA, has been identified to impede cancer
progression by disrupting EMT and angiogenesis via inhibition of
transforming growth factor-β-induced Snail expression (47). In addition, Rha suppressed the
migration and invasion of MDA-MB231 breast cancer cells by
inhibiting phosphoinositide 3-kinase-dependent Rac1 signaling, and
exhibited an anti-angiogenic effect in hypoxic PC-3 prostate cancer
cells by inhibiting HIF-1α accumulation (48,49).
Since it is known that the aglycone form did not always exhibit the
same pharmacological action as the glycone form, the present study
is therefore the first report to show the anti-metastatic and
anti-angiogenic activities of RA and its underlying molecular
mechanisms using both in vitro and in ovo systems.
In the present study, it was identified that RA at
non-cytotoxic concentrations efficiently suppressed the metastatic
ability of MDA-MB231 cells, including anchorage-dependent colony
formation, migration and invasion. In addition, it was also
observed that RA treatment decreased the ability of HUVECs to form
a vascular network and migrate through the Transwell. Furthermore,
RA treatment in the CAM assay significantly inhibited angiogenesis
in normal and VEGF-stimulation conditions, indicating that RA
directly regulates tumor cells and endothelial cells to limit tumor
progression. In HT1080 cells, RA treatment under normoxic and
hypoxic conditions inhibited the production of pro-angiogenic
factors, including MMP-9, PTX-3, IL-8, VEGF and PlGF, which
enhanced proliferation, migration, sprouting, permeability and
resistance to apoptosis of endothelial cells. In addition, RA
suppressed the PMA-induced increase of MMP-9 proteolytic activity,
reinforcing the inhibitory effects of RA on metastasis and
angiogenesis. Consistent with these results, RA inhibited
hypoxia-induced HIF-1α accumulation and nuclear expression via
enhancing HIF-1α degradation by increasing VHL expression,
indicating that RA acts as an important regulator of the HIF-1
pathway. Further investigation of the function of kinases which are
downstream targets of HIF-1, including Akt and mTOR, on the
RA-induced anti-metastatic and anti-angiogenic activities using
short interfering RNA.
EMT is a downstream target of the HIF-1 pathway, in
which epithelial cells acquire the phenotype of mesenchymal cells
via disruption of cell-cell interactions and loss of the tight
junction (TJ) barrier, thereby leading to the dissemination of
tumor cells from the primary tumor (50). Claudin-1, a transmembrane protein
of the TJ, has long been postulated to be a tumor suppressor in
breast cancer, because it is downregulated in breast cancers
compared with normal breast epithelia. In addition, the loss of
claudin-1 has been associated with increased recurrence, shorter
survival and enhanced invasiveness (51). In contrast, it was identified
previously that overexpression of claudin-1 in the colon,
colorectal area and in melanomas increased cell invasion,
indicating that claudin-1 was able to promote or suppress tumor
progression (52). In the present
study, RA significantly increased the levels of E-cadherin and
claudin-1 in HT1080 cells under hypoxic conditions, whereas it
decreased the levels of N-cadherin, Slug, Snail, ZEB-1 and
vimentin, which are positive regulators of EMT.
In summary, RA inhibited the metastatic potential of
malignant cancer cells, including MDA-MB231 and HT1080 cells, by
inhibiting the HIF-1 pathway and regulating the EMT-associated
proteins. In addition, RA efficiently decreased the angiogenic
activities of endothelial cells. Taken together, these results
indicate that RA may be a potent anticancer agent, which could be
used to treat highly metastatic malignant human cancers. Currently,
to verify the in vivo effectiveness of RA, we are
investigating whether repeated oral administration of RA is able to
suppress the metastasis of malignant cancer cells. In addition,
assessment of the safety of RA during experimental periods is also
underway.
Acknowledgments
Not applicable.
References
|
1
|
Valastyan S and Weinberg RA: Tumor
metastasis: Molecular insights and evolving paradigms. Cell.
147:275–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Leber MF and Efferth T: Molecular
principles of cancer invasion and metastasis (review). Int J Oncol.
34:881–895. 2009.PubMed/NCBI
|
|
3
|
Alizadeh AM, Shiri S and Farsinejad S:
Metastasis review: From bench to bedside. Tumour Biol.
35:8483–8523. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Patel LR, Camacho DF, Shiozawa Y, Pienta
KJ and Taichman RS: Mechanisms of cancer cell metastasis to the
bone: A multistep process. Future Oncol. 7:1285–1297. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guan X: Cancer metastases: Challenges and
opportunities. Acta Pharm Sin B. 5:402–418. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vasaturo F, Solai F, Malacrino C, Nardo T,
Vincenzi B, Modesti M and Scarpa S: Plasma levels of matrix
metalloproteinases 2 and 9 correlate with histological grade in
breast cancer patients. Oncol Lett. 5:316–320. 2013. View Article : Google Scholar
|
|
7
|
Deryugina EI and Quigley JP: Tumor
angiogenesis: MMP-mediated induction of intravasation- and
metastasis-sustaining neovasculature. Matrix Biol. 44–46:94–112.
2015. View Article : Google Scholar
|
|
8
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: Regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wilson WR and Hay MP: Targeting hypoxia in
cancer therapy. Nat Rev Cancer. 11:393–410. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wigerup C, Påhlman S and Bexell D:
Therapeutic targeting of hypoxia and hypoxia-inducible factors in
cancer. Pharmacol Ther. 164:152–169. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vaupel P and Mayer A: Hypoxia in cancer:
Significance and impact on clinical outcome. Cancer Metastasis Rev.
26:225–239. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yoo MY, Oh KS, Lee JW, Seo HW, Yon GH,
Kwon DY, Kim YS, Ryu SY and Lee BH: Vasorelaxant effect of
stilbenes from rhizome extract of rhubarb (Rheum undulatum) on the
contractility of rat aorta. Phytother Res. 21:186–189. 2007.
View Article : Google Scholar
|
|
13
|
Moon MK, Kang DG, Lee JK, Kim JS and Lee
HS: Vasodilatory and anti-inflammatory effects of the aqueous
extract of rhubarb via a NO-cGMP pathway. Life Sci. 78:1550–1557.
2006. View Article : Google Scholar
|
|
14
|
Matsuda H, Tewtrakul S, Morikawa T and
Yoshikawa M: Anti-allergic activity of stilbenes from Korean
rhubarb (Rheum undulatum L.): Structure requirements for inhibition
of antigen-induced degranulation and their effects on the release
of TNF-alpha and IL-4 in RBL-2H3 cells. Bioorg Med Chem.
12:4871–4876. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Song JH, Yang TC, Chang KW, Han SK, Yi HK
and Jeon JG: In vitro anti-cariogenic activity of dichloromethane
fraction from Rheum undulatum L. root. Arch Pharm Res. 29:490–496.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Matsuda H, Morikawa T, Toguchida I, Park
JY, Harima S and Yoshikawa M: Antioxidant constituents from
rhubarb: Structural requirements of stilbenes for the activity and
structures of two new anthraquinone glucosides. Bioorg Med Chem.
9:41–50. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ko SK, Lee SM and Whang WK: Anti-platelet
aggregation activity of stilbene derivatives from Rheum
undulatum. Arch Pharm Res. 22:401–403. 1999.
|
|
18
|
Tao L, Cao J, Wei W, Xie H, Zhang M and
Zhang C: Protective role of rhapontin in experimental pulmonary
fibrosis in vitro and in vivo. Int Immunopharmacol. 47:38–46. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wei W, Wang L, Zhou K, Xie H, Zhang M and
Zhang C: Rhapontin ameliorates colonic epithelial dysfunction in
experimental colitis through SIRT1 signaling. Int Immunopharmacol.
42:185–194. 2017. View Article : Google Scholar
|
|
20
|
Chen J, Ma M, Lu Y, Wang L, Wu C and Duan
H: Rhaponticin from rhubarb rhizomes alleviates liver steatosis and
improves blood glucose and lipid profiles in KK/Ay diabetic mice.
Planta Med. 75:472–477. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim A, Im M, Yim NH, Jung YP and Ma JY:
Aqueous extract of Bambusae Caulis in Taeniam inhibits PMA-induced
tumor cell invasion and pulmonary metastasis: Suppression of NF-κB
activation through ROS signaling. PLoS One. 8:e780612013.
View Article : Google Scholar
|
|
22
|
Kim A, Im M, Gu MJ and Ma JY: Ethanol
extract of Lophatheri Herba exhibits anti-cancer activity in human
cancer cells by suppression of metastatic and angiogenic potential.
Sci Rep. 6:362772016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gupta MK and Qin RY: Mechanism and its
regulation of tumor-induced angiogenesis. World J Gastroenterol.
9:1144–1155. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vaupel P: The role of hypoxia-induced
factors in tumor progression. Oncologist. 9(Suppl 5): 10–17. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shang Y, Cai X and Fan D: Roles of
epithelial-mesenchymal transition in cancer drug resistance. Curr
Cancer Drug Targets. 13:915–929. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Polyak K and Weinberg RA: Transitions
between epithelial and mesenchymal states: Acquisition of malignant
and stem cell traits. Nat Rev Cancer. 9:265–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Garg M: Epithelial-mesenchymal transition
- activating transcription factors - multifunctional regulators in
cancer. World J Stem Cells. 5:188–195. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Weinberg RA: Oncogenes, antioncogenes, and
the molecular bases of multistep carcinogenesis. Cancer Res.
49:3713–3721. 1989.PubMed/NCBI
|
|
29
|
Colotta F, Allavena P, Sica A, Garlanda C
and Mantovani A: Cancer-related inflammation, the seventh hallmark
of cancer: Links to genetic instability. Carcinogenesis.
30:1073–1081. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mbeunkui F and Johann DJ Jr: Cancer and
the tumor micro-environment: A review of an essential relationship.
Cancer Chemother Pharmacol. 63:571–582. 2009. View Article : Google Scholar
|
|
31
|
Finger EC and Giaccia AJ: Hypoxia,
inflammation, and the tumor microenvironment in metastatic disease.
Cancer Metastasis Rev. 29:285–293. 2010. View Article : Google Scholar
|
|
32
|
Li H, Fan X and Houghton J: Tumor
microenvironment: The role of the tumor stroma in cancer. J Cell
Biochem. 101:805–815. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cairns R, Papandreou I and Denko N:
Overcoming physiologic barriers to cancer treatment by molecularly
targeting the tumor microenvironment. Mol Cancer Res. 4:61–70.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Joyce JA: Therapeutic targeting of the
tumor microenvironment. Cancer Cell. 7:513–520. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Carmeliet P and Jain RK: Angiogenesis in
cancer and other diseases. Nature. 407:249–257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chang C and Werb Z: The many faces of
metalloproteases: Cell growth, invasion, angiogenesis and
metastasis. Trends Cell Biol. 11:S37–S43. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rak JW, St Croix BD and Kerbel RS:
Consequences of angiogenesis for tumor progression, metastasis and
cancer therapy. Anticancer Drugs. 6:3–18. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Semenza GL: HIF-1 and tumor progression:
Pathophysiology and therapeutics. Trends Mol Med. 8(Suppl):
S62–S67. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Semenza GL: Hypoxia-inducible factors:
Mediators of cancer progression and targets for cancer therapy.
Trends Pharmacol Sci. 33:207–214. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jeong W, Rapisarda A, Park SR, Kinders RJ,
Chen A, Melillo G, Turkbey B, Steinberg SM, Choyke P, Doroshow JH,
et al: Pilot trial of EZN-2968, an antisense oligonucleotide
inhibitor of hypoxia-inducible factor-1 alpha (HIF-1α), in patients
with refractory solid tumors. Cancer Chemother Pharmacol.
73:343–348. 2014. View Article : Google Scholar
|
|
41
|
Patnaik A, Chiorean EG, Tolcher A,
Papadopoulos K, Beeram M, Kee D, Waddell M, Gilles E and Buchbinder
A: EZN-2968, a novel hypoxia-inducible factor-1 alpha (HIF-1α)
messenger ribonucleic acid (mRNA) antagonist: Results of a phase I,
pharmacokinetic (PK), dose escalation study of daily administration
in patients (pts) with advanced malignancies. J Clin Oncol.
27:25642009.
|
|
42
|
Son SH, Kim MJ, Chung WY, Son JA, Kim YS,
Kim YC, Kang SS, Lee SK and Park KK: Decursin and decursinol
inhibit VEGF-induced angiogenesis by blocking the activation of
extracellular signal-regulated kinase and c-Jun N-terminal kinase.
Cancer Lett. 280:86–92. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yoysungnoen P, Wirachwong P, Bhattarakosol
P, Niimi H and Patumraj S: Effects of curcumin on tumor
angiogenesis and biomarkers, COX-2 and VEGF, in hepatocellular
carcinoma cell-implanted nude mice. Clin Hemorheol Microcirc.
34:109–115. 2006.PubMed/NCBI
|
|
44
|
Su SJ, Yeh TM, Chuang WJ, Ho CL, Chang KL,
Cheng HL, Liu HS, Cheng HL, Hsu PY and Chow NH: The novel targets
for anti-angiogenesis of genistein on human cancer cells. Biochem
Pharmacol. 69:307–318. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Jo SP, Kim JK and Lim YH:
Antihyperlipidemic effects of rhapontin and rhapontigenin from
rheum undulatum in rats fed a high-cholesterol diet. Planta Med.
80:1067–1071. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sun Y and Zhao Y: Enhanced
pharmacokinetics and anti-tumor efficacy of PEGylated liposomal
rhaponticin and plasma protein binding ability of rhaponticin. J
Nanosci Nanotechnol. 12:7677–7684. 2012. View Article : Google Scholar
|
|
47
|
Yeh YH, Wang SW, Yeh YC, Hsiao HF and Li
TK: Rhapontigenin inhibits TGF-β-mediated epithelial-mesenchymal
transition via the PI3K/AKT/mTOR pathway and is not associated with
HIF-1α degradation. Oncol Rep. 35:2887–2895. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kim JS, Kang CG, Kim SH and Lee EO:
Rhapontigenin suppresses cell migration and invasion by inhibiting
the PI3K-dependent Rac1 signaling pathway in MDA-MB-231 human
breast cancer cells. J Nat Prod. 77:1135–1139. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Jung DB, Lee HJ, Jeong SJ, Lee HJ, Lee EO,
Kim YC, Ahn KS, Chen CY and Kim SH: Rhapontigenin inhibited hypoxia
inducible factor 1 alpha accumulation and angiogenesis in hypoxic
PC-3 prostate cancer cells. Biol Pharm Bull. 34:850–855. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Martin TA and Jiang WG: Loss of tight
junction barrier function and its role in cancer metastasis.
Biochim Biophys Acta. 1788:872–891. 2009. View Article : Google Scholar
|
|
51
|
Morohashi S, Kusumi T, Sato F, Odagiri H,
Chiba H, Yoshihara S, Hakamada K, Sasaki M and Kijima H: Decreased
expression of claudin-1 correlates with recurrence status in breast
cancer. Int J Mol Med. 20:139–143. 2007.PubMed/NCBI
|
|
52
|
Dhawan P, Singh AB, Deane NG, No Y, Shiou
SR, Schmidt C, Neff J, Washington MK and Beauchamp RD: Claudin-1
regulates cellular transformation and metastatic behavior in colon
cancer. J Clin Invest. 115:1765–1776. 2005. View Article : Google Scholar : PubMed/NCBI
|