Introduction
Dentin sialophosphoprotein (DSPP) is a member of the
small integrin-binding ligand N-linked glycoprotein family, which
activates kinases and transcription factors, and controls signal
transduction pathways that regulate cell adhesion, migration and
survival (1). DSPP, the expression
of which was previously thought to be limited to mineralizing
tissues such as dentin and bone, is now known to be expressed in
metabolically active ductal epithelial tissues, including normal
salivary glands, nephrons and eccrine sweat glands (2–4). The
DSPP gene encodes two main noncollagenous dentin proteins: Dentin
sialoprotein (DSP) and dentin phosphoprotein (DPP), as well as the
much smaller dentin glycoprotein (DGP) (5). DSP occupies the N-terminal domain and
presents a Cys205 (the only cysteine in DSPP) domain (6,7).
C-terminal DPP possesses 165 Asp-Ser-Ser sequence motifs, has a low
isoelectric point of 2.84 and exhibits high calcium affinity
(7,8). The short DGP fragment is sandwiched
between the N-terminal DSP and the C-terminal DPP. It is difficult
to isolate the full-length DSPP protein because soon after its
translation, matrix metal-loproteinase (MMP)20 and MMP2 mediate the
proteolytic fragmentation of DSPP into DSP, DPP and DGP (5). The role of DGP remains unclear,
whereas the functional roles of DSP include initiation of dentin
mineralization and DPP promotes dentin maturation (6).
Within the past decade, the expression of DSPP has
been detected in numerous human epithelial cancers, including
breast, lung, colon, prostate and oral cancer (9–12).
In oral squamous cell carcinoma (OSCC), DSPP is significantly
upregulated in poorly differentiated lesions (12). Recently, we reported the
coexpression and potential interactions of MMP20 and DSPP in human
OSCC tissues and cell lines (13).
Furthermore, the report established the specific cognate partnering
of MMP20 and DSPP, which also is present in metabolically active
ductal epithelial systems, such as the salivary gland and nephron
(13). In particular, the DSP
portion of DSPP strongly interacts with MMP20 promoter proximal
elements (13).
Endoplasmic reticulum (ER) is a major store of
intracellular calcium, which is central to regulating cellular
calcium homeostasis and various calcium signaling pathways
(14). ER serves a key role in
proper protein folding and targeting during protein synthesis;
unfolded proteins entering the ER are chemically modified in an
oxidizing and calcium-rich environment (15). ER-regulated calcium homeostasis is
mediated by various calcium proteins, the functions of which are
modulated by several factors, including redox mechanisms (16,17).
These calcium-related proteins function as influx pumps, which
transfer calcium from the cytosol to the lumen, as luminal storing
buffers, and as controlled-release calcium channels to the cytosol
(16). ER homeostasis is a dynamic
process that often exhibits sensitivity to minor environmental
alterations (18); therefore,
changes in redox state, ischemic state, nutrient status and
Ca2+ levels, high protein synthesis rate and
inflammation may disrupt proper ER function, thus resulting in the
accumulation of unfolded or misfolded proteins (18,19).
This outcome is referred to as ER stress, which in turn triggers
numerous molecular signaling pathways aimed at preventing
intraluminal accumulation and/or secretion of unfolded proteins,
also known as the unfolded protein response (UPR), by promoting the
degradation of misfolded proteins through the ER-associated protein
degradation pathway (20,21).
Disturbances in ER homeostasis are detected by three
ER transmembrane proteins-sensors: Protein kinase R-like
endoplasmic reticulum kinase (PERK), activating transcription
factor 6 (ATF6a and b), and serine/threonine-protein
kinase/endoribonuclease IRE1 (IRE1). Initiation of the UPR and
activation of sensor proteins and related downstream molecules
reduce the duration of stress, re-establishe normal ER function and
promote survival. However, depending on stress severity, the UPR
may trigger apoptosis and cell death (16,18).
In addition, 78 kDa glucose-regulated protein (GRP78), also known
as binding immunoglobulin protein, had been proposed as the master
UPR chaperone that negatively regulates PERK, ATF6 and IRE1
activity (15,22).
The accumulation of unfolded proteins releases PERK,
ATF6 and IRE1 from the strong GRP78 engagement (15,22).
The tumor microenvironment is characterized by a lack of nutrients,
low pH, hypoxia and oxidative stress (23,24).
Consequently, changes in ER stress and UPR serve a critical role in
the ability of cancer cells to survive in this demanding
environment and prevent ER stress-induced apoptosis (25,26).
Previous studies have reported that GRP78 cysteine oxidation
enhances cell survival during stress (27), and that GRP78, PERK, IRE1 and ATF6
overexpression in cancer is correlated with cell proliferation and
survival signaling (28–31). Similarly, sarcoplasmic/endoplasmic
reticulum calcium ATPase (SERCA) has been proposed as a potential
target for cancer treatment (15).
The present study aimed to investigate the potential
association of DSPP with the ER homeostatic mechanism, the possible
inter-regulatory effects in OSCC biology, and the molecular
pathways implicated.
Materials and methods
Stable DSPP-silenced OSC2 cell lines
Our laboratory recently established stable
lentiviral-mediated DSPP-, MMP20- and combined DSPP +
MMP20-silenced OSC2 cells; OSC2 cells infected with scrambled short
hairpin (sh)RNA (SCR) were used as controls (13). shRNA Plasmid A (cat. no.
sc-108060), which was used as a negative control, was obtained from
Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). The stably
silenced (75% silencing) phenotype of these cell lines was
routinely verified by western blotting (data not shown).
Subsequently, cells were cultured as a monolayer in Dulbecco's
modified Eagle's medium (DMEM)/F12 (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.), 1%
penicillin/streptomycin and 500 ng/ml hydrocortisone (Sigma
Aldrich; Merck KGaA, Darmstadt, Germany) at 37°C in the presence of
5% CO2 humidified air. Untreated DSPP-silenced stable
cells were incubated in media devoid of puromycin for 4 days prior
to experimentation, whereas treated DSPP-silenced stable cells were
continuously treated with 3 µg/ml puromycin (cat. no.
sc-108071; Santa Cruz Biotechnology, Inc.). Omission of puromycin
in the untreated DSPP-silenced group did not affect cell
stability.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
To evaluate the effects of DSPP silencing on the
mRNA expression levels of proteins associated with ER stress and
the UPR, GRP78, SERCA2b, inositol 1,4,5-trisphosphate receptor
(IP3r), PERK, IRE1 and ATF6 expression was detected by RT-qPCR
analysis. RT-qPCR was conducted on total RNA extracted from
DSPP-silenced and SCR OSC2 cells according to established
protocols. Briefly, total RNA was extracted from cells using
TRIzol® reagent (cat. no. 15596-026; Invitrogen; Thermo
Fisher Scientific, Inc.), according to a standardized protocol, and
the concentration of each sample was measured. qSTAR (Origene
Technologies, Inc., Rockville, MD, USA) qPCR primer pairs against
human genes were used in the present study; the primer sequences
were as follows (5′-3′): DSPP forward, CAACCATAGAGAAAGCAAACGCG and
reverse, TTTCTGTTGCCACTGCTGGGAC; MMP20 forward,
GACCAGACCACAATGAACGT and reverse, GTCCACTTCTCAGGATTGTC; PERK
forward, ATCCCCCATG GAACGACCTG and reverse, ACCCGCCAGGGACAAAAATG;
ATF6 forward, TTGGCATTTATAATACTGAACTATGGA and reverse,
TTTGATTTGCAGGGCTCAC; SERCA2b forward, TCATCTTCCAGATCACACCGC and
reverse, GTCAAGACCAGAACATATC; IP3r forward, GGTTTCATTTG
CAAGTTAATAAAG and reverse, AATGCTTTCATGGAA CACTCGGTC; IRE1 forward,
CGGGAATTCGGCCGAGTC CTCGCCATG and reverse, CAAGCGGCCGCCTTTCCCA
ACTATCACCACGCT; GRP78 forward, TGTTCAACCAATTATCAGCAAACTC and
reverse, TTCTGCTGTATCCTCTTCACCAGT; and β-actin forward,
GTCTCCTCTGACTTCAACAGCG and reverse, ACCACCCTGTTGCTGTAGCCAA.
Briefly, total RNA (1 µg) was reverse transcribed using
iScript™ RT Supermix (cat. no. 1708841; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA), according to the manufacturer's protocol.
RT-qPCR was conducted using synthesized cDNA on a qPCR machine
using iTaq™ Universal SYBR® Green Supermix (cat, no.
1725124; Bio-Rad Laboratories, Inc.). PCR thermocycling was as
follows: 94°C for 5 min, followed by 40 cycles at 94°C for 30 sec,
60°C for 20 sec and 72°C for 40 sec, followed by a final extension
step at 72°C for 5 min. A standard curve was generated from three
serial dilutions of cDNA. Samples, including negative controls,
were analyzed in triplicate, and PCR products were verified using
dissociation curve analysis. mRNA expression levels were normalized
to β-actin and were analyzed using Bio-Rad CFX Manager™ software
(Version Number 3.0; Bio-Rad Laboratories, Inc.). The log2-fold
change between the SCR and experimental treated samples was
calculated using the 2−ΔΔCq method (32).
Western blot analysis
Cells were washed twice with ice-cold PBS, and were
then lysed with radioimmunoprecipation assay buffer [cat. no.
156034; Abcam, Cambridge, MA, USA; 50 mM Tris (pH 7.4), 150 mM
NaCl, 1% Triton X-100, 1% deoxycholic acid sodium salt, 0.1% sodium
dodecyl sulfate, 100 mg/ml phenylmethylsulfonyl fluoride, 1 mg/ml
aprotinin, 1 mM dichlorodiphenyltrichlo-roethane and 1 mM sodium
orthovanadate] for 10 min at 4°C. The wells were scraped, and the
recovered cell products were centrifuged (Sorvall™ CC40; Thermo
Fisher Scientific, Inc.) at 40,000 × g for 15 min at 4°C. The
concentration of the recovered proteins was measured using the
Bio-Rad Protein Assay kit (Bio-Rad Laboratories, Inc.), according
to the manufacturer's protocol. Equal amount of proteins (30–50
µg, depending on the particular protein) were separated by
SDS-PAGE (10% separation gel and 5% spacer gel) and were
electrotransferred to polyvinylidene difluoride membranes (Bio-Rad
Laboratories, Inc.). Membranes were then placed in blocking
solution for 1 h at room temperature, after which they were probed
with the following primary antibodies overnight at 4°C: Mouse
monoclonal B-cell lymphoma 2 (Bcl2; cat. no. sc-7382; 1:250), mouse
monoclonal Bcl2-associated X protein (Bax; cat. no. sc-7480;
1:200), rabbit polyclonal cytochrome c (cat. no. sc-7159;
1:200) and rabbit polyclonal proliferating cell nuclear antigen
(PCNA; cat. no. sc-7907; 1:200) (all from Santa Cruz Biotechnology,
Inc.). The film was then washed thoroughly and incubated with goat
polyclonal anti-rabbit immunoglobulin G (IgG) horseradish
peroxidase-conjugated secondary antibody (cat. no. sc-2030;
1:3,000; Santa Cruz Biotechnology, Inc.) or anti-mouse IgG antibody
(cat. no. sc-2031; 1:3,000; Santa Cruz Biotechnology, Inc.) with
agitation at room temperature (25°C) for 1 h. β-actin (1:2,000) was
used as a loading control (cat. no. sc-47778; Santa Cruz
Biotechnology, Inc.). Proteins were visualized using an enhanced
chemiluminescence (ECL) system (Pierce™ ECL; Thermo Fisher
Scientific, Inc) and band intensity was semi-quantified using
ImageJ software1.48 (https://imagej.nih.gov/ij/; National Institutes of
Health, Bethesda, MD, USA).
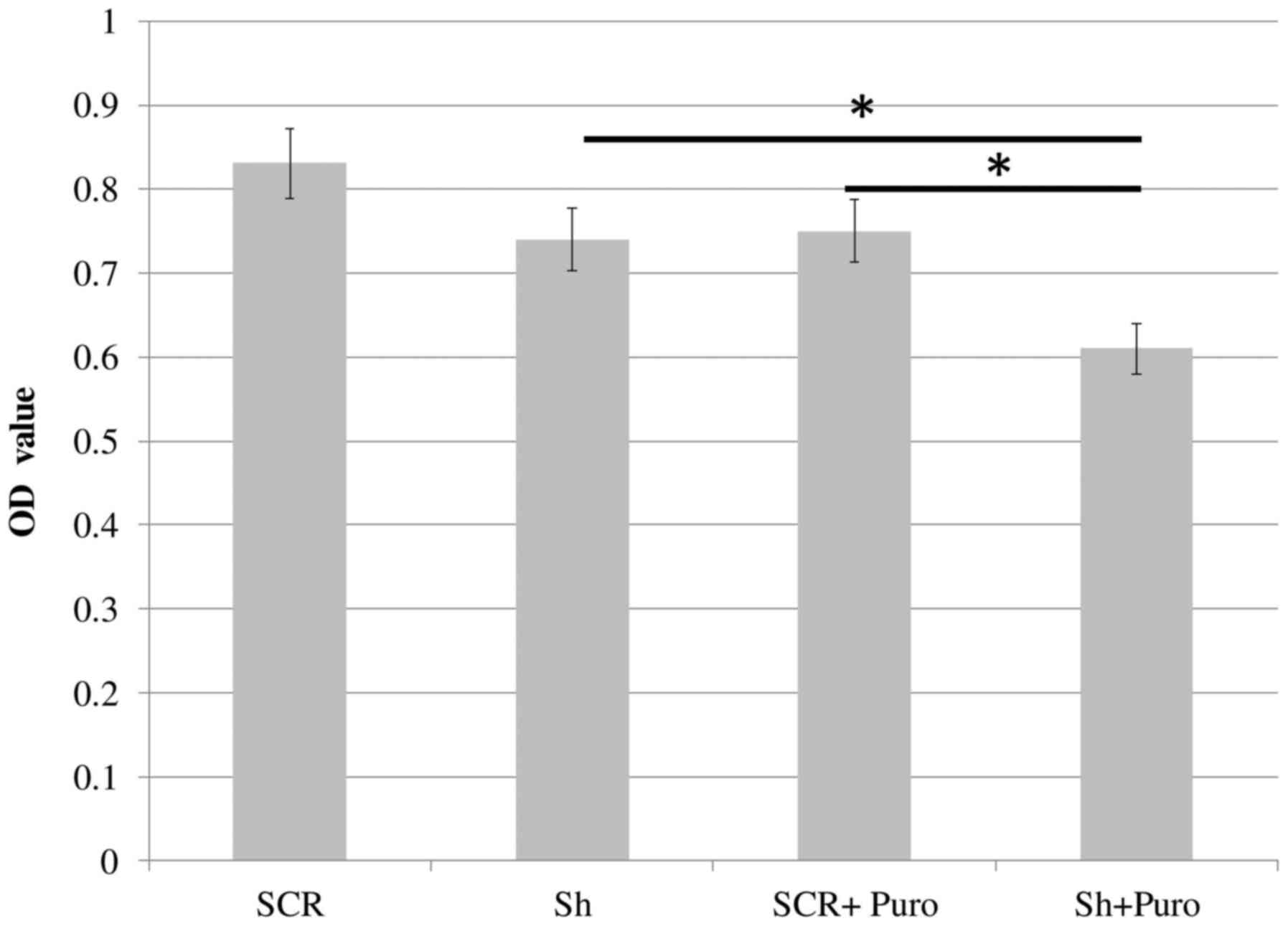
MTT assay
Cell viability was assessed by detecting the
conversion of MTT to formazan, which is induced by mitochondrial
oxidation. Cell cultures (5×103 cells/well), including
SCR or DSPP-silenced (puromycin-treated and puromycin-free) cells,
were incubated in the presence of 0.5 mg/ml MTT for 3 h at 37°C in
96-well plates. Water insoluble, purple formazan crystals were
formed, indicating the presence of viable cells. Crystals were then
dissolved in dimethyl sulfoxide and optical density (OD) values of
the solutions were measured using a spectrophotometer at a
wavelength of 570 nm. Assays were performed in triplicate and data
are presented as the means of OD values ± standard error of the
mean.
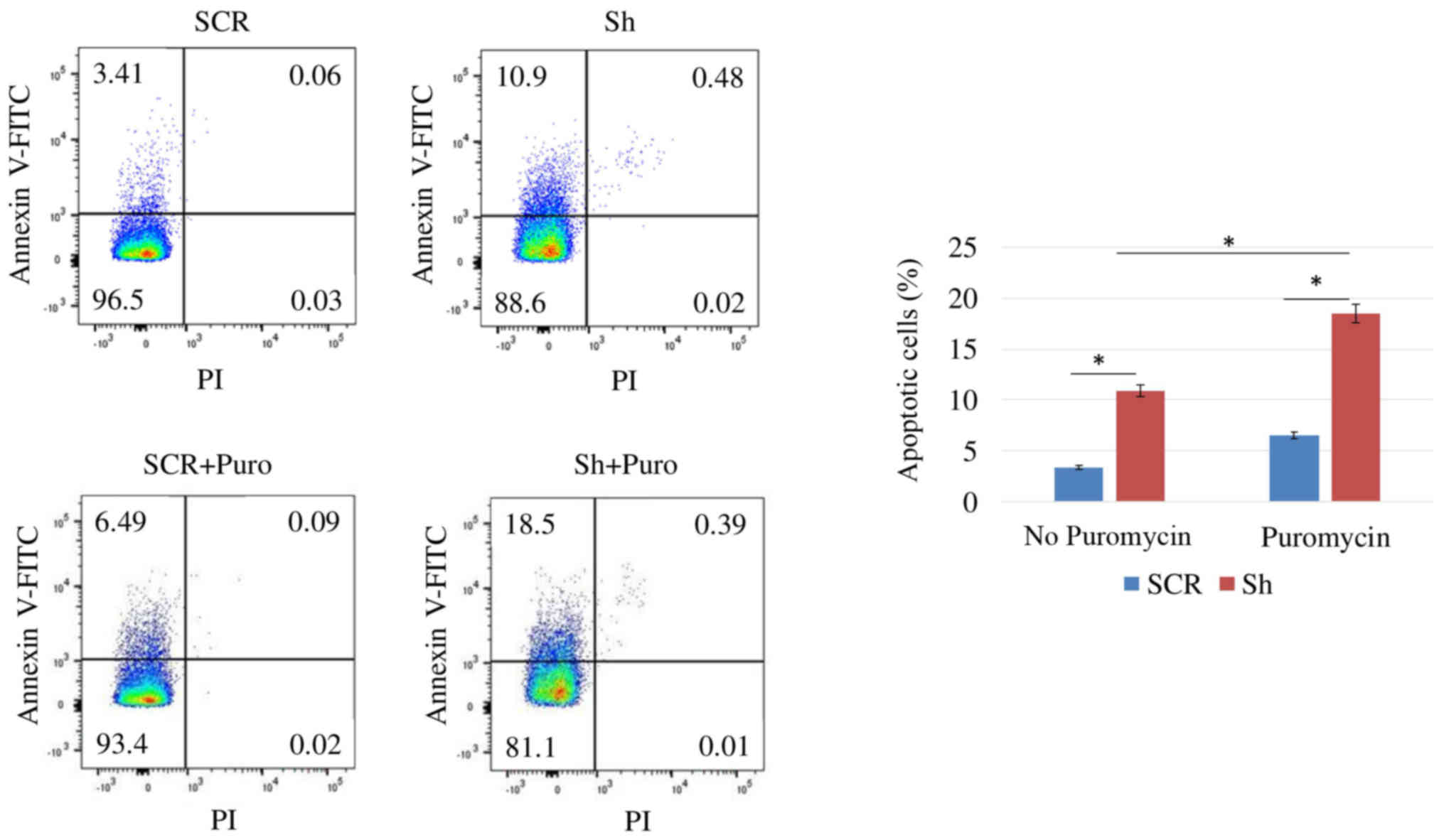
Apoptosis analyses by flow cytometry
For apoptosis analyses, Annexin V/propidium iodide
(PI) staining of SCR and DSPP-silenced (puromycin-treated and
puromycin-free) cells was conducted. Briefly, cells were washed
with 1X PBS and resuspended at 106 cells/ml in Annexin
V-binding buffer prior to aliquoting the suspension into 100
µl/tube fractions. Subsequently, 5 µl Annexin
V-fluorescein isothiocyanate (FITC) and 10 µl PI buffer were
added to each tube and incubated in the dark for 15 min at room
temperature. Finally, 400 µl 1X Annexin V-binding buffer was
added to each tube and flow cytometric analysis was performed
within 1 h. Samples were analyzed on a FACSCalibur flow cytometer
(BD Biosciences, Franklin Lakes, NJ, USA). Gates in the right angle
scatter versus forward scatter diagrams were used to exclude
debris. At least 100,000 events were recorded prior to analysis.
All flow cytometric data were analyzed using BD Cell Quest Pro
software (Version 5.0; BD Biosciences).
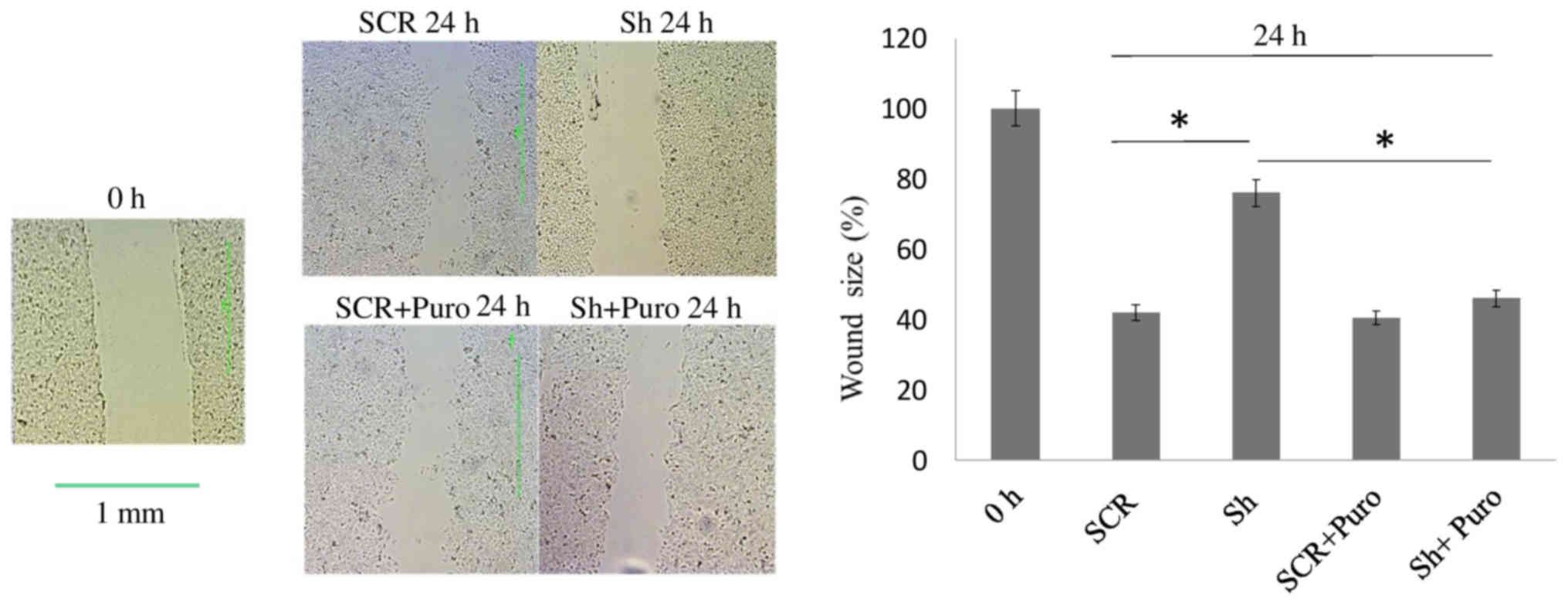
Scratch wound-healing assay
Cells were cultured to 90% confluence in 35-mm
dishes and scratched with a sterile 200-µl pipette tip. The
border of the denuded area was immediately marked with a fine line,
and cells were incubated with DMEM/F12 containing 10% FBS with or
without puromycin at 37°C. Cultures were photographed after 24 h
using an inverted phase contrast microscope (Olympus Corporation,
Tokyo, Japan). The assay was performed in triplicate.
Statistical analyses
Statistical analysis was performed using SPSS
version 21 (IBM Corp., Armonk, NY, USA). Paired groups were
compared using Student's t-test, whereas one-way analysis of
variance was applied to compare multiple groups, followed by
post-hoc pairwise comparisons with the application of Dunn's test.
All experiments were performed in triplicate. P<0.05 was
considered to indicate a statistically significant difference.
Results
DSPP silencing downregulates critical ER
stress and UPR-related protein expression in OSC2 cells
As shown in Fig.
1A, DSPP silencing in OSC2 cells resulted in a significant
reduction in the mRNA expression levels of GRP78, SERCA2b, PERK and
ATF6 (P<0.05), whereas IRE1 and IP3r mRNA expression was not
significantly altered compared with the SCR group. Conversely,
DSPP-silenced OSC2 cells treated with puromycin exhibited reduced
GRP78, SERCA2b, IP3r, PERK, IRE1 and ATF6 mRNA levels compared with
in the puromycin-treated SCR group. However, decreases in mRNA
expression between puromycin-treated DSPP-silenced and SCR cells
(Fig. 1B) were less pronounced
compared with between the puromycin-free DSPP-silenced and SCR
cells (Fig. 1A). In addition, IP3r
was only downregulated in puromycin-treated cells, and PERK
exhibited comparable downregulation in puromycin-free and
puromycin-treated cells. Statistically significant differences were
detected between GRP78 and PERK mRNA expression in DSPP-silenced
cells compared with SCR cells following puromycin treatment.
Notably, puromycin treatment resulted in a 38% decrease in DSPP as
a result of silencing (Fig. 1B)
compared with a 55% decrease in puromycin-free silenced cells
(Fig. 1A). However, there was no
difference on the effects of DSPP silencing on UPR activity between
puromycin-treated and puromycin-free DSPP-silenced OSC2 cells.
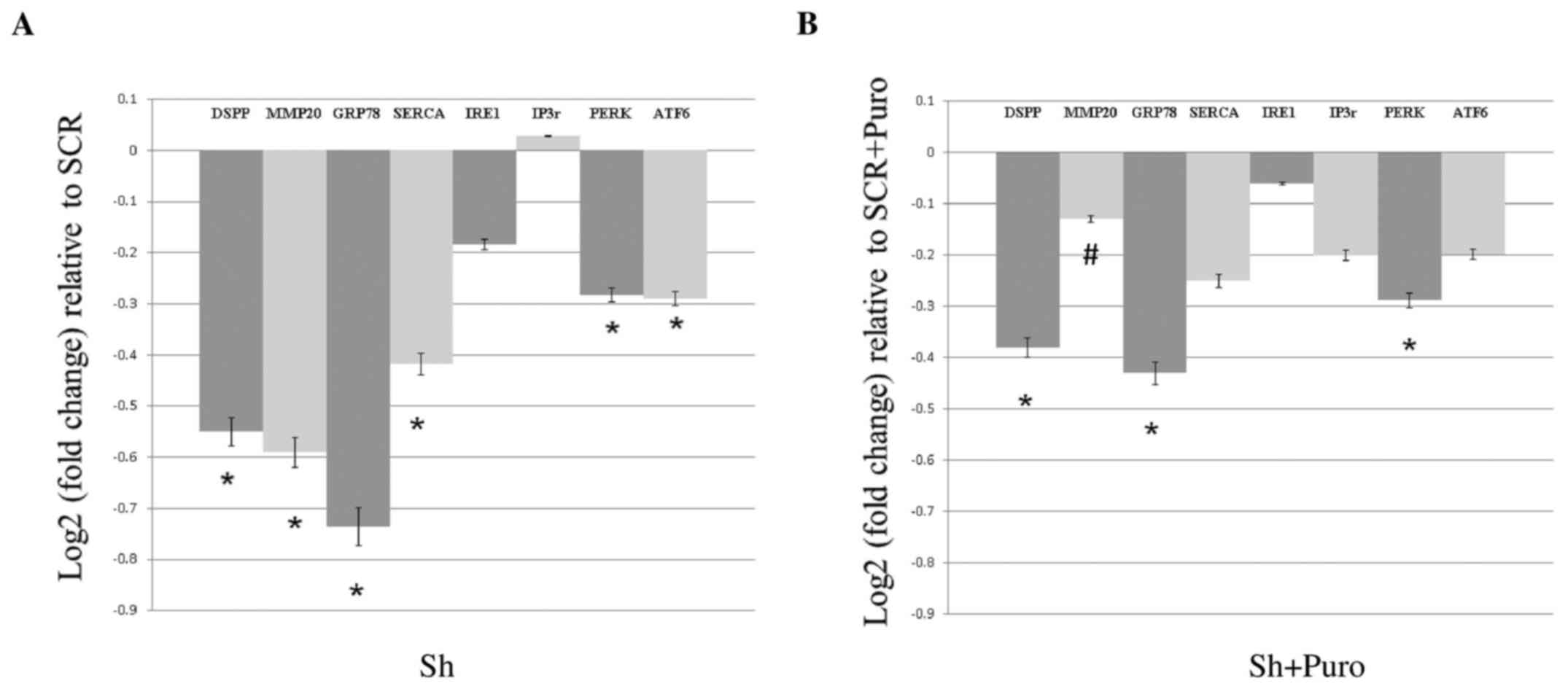 | Figure 1Effects of DSPP silencing on the mRNA
expression levels of proteins associated with endoplasmic reticulum
stress and the unfolded protein response in oral squamous cell
carcinoma cells, as assessed by reverse transcription-quantitative
polymerase chain reaction analyses. (A) DSPP mRNA expression
exhibited a 0.55 log2-fold change decrease in Sh compared with in
SCR cells. mRNA expression levels of GRP78, SERCA2b, PERK and ATF6,
as well as those of MMP20, were significantly reduced, whereas IRE1
and IP3r expression was not significantly altered compared with in
SCR cells. (B) Puromycin treatment decreased DSPP silencing; the
reduction in DSPP mRNA expression (38%) was less pronounced
compared with in DSPP-silenced puromycin-free cells (55%). MMP20
mRNA expression was only minimally decreased in DSPP-silenced
puromycin-treated cells, and puromycin-treated DSPP-silenced cells
exhibited significantly (#P<0.05) increased MMP20
mRNA expression compared with in puromycin-free DSPP-silenced
cells. Results are expressed as log2-fold changes relative to the
expression levels in SCR cells. Data are presented as the means ±
standard error of the mean; each experiment was performed in
triplicate. *P<0.05 vs. SCR cells. ATF6, activating
transcription factor 6; DSPP, dentin sialophosphoprotein; GRP78, 78
kDa glucose-regulated protein; IP3r, inositol 1,4,5-trisphosphate
receptor; IRE1, serine/threonine-protein kinase/endoribonuclease
IRE1; MMP20, matrix metalloproteinase 20; P, puromycin; PERK,
protein kinase R-like endoplasmic reticulum kinase; SCR, scrambled
shRNA; SERCA2b, sarcoplasmic/endoplasmic reticulum calcium ATPase
2b; Sh, DSSP shRNA; shRNA, short hairpin RNA. |
DSPP silencing downregulates MMP20
expression and decreases the migratory potential of OSC2 cells
To determine the effects of DSPP silencing on the
migratory capacity of OSC2 cells, closure of a scratch wound in
cell culture plates was measured. As shown in Fig. 2, there was a significant delay in
wound closure (P<0.05) in DSPP-silenced cells compared with in
SCR cells after 24 h. This finding may be associated with the
significant reduction in MMP20 mRNA expression (P<0.05), which
was observed in DSPP-silenced cells compared with in SCR cells
(Fig. 1A), thus suggesting that
DSPP, along with its cognate partner MMP20, may regulate the
migratory capacity of OSC2 cells.
With respect to puromycin-treated OSC2 cells, wound
closure rate was similar between DSPP-silenced and SCR cells after
24 h (Fig. 2). However, wound
healing exhibited a significantly increased closure rate
(P<0.05) in puromycin-treated DSPP-silenced cells compared with
in puromycin-free DSPP-silenced cells. Conversely, MMP20 mRNA
expression levels were minimally decreased following DSPP silencing
in puromycin-treated cells (Fig.
1B), and puromycin-treated DSPP-silenced cells exhibited
significantly (P<0.05) increased MMP20 mRNA expression compared
with puromycin-free DSPP-silenced cells (Fig. 1A and B). These results suggested
that puromycin may antagonize the effects of DSPP silencing on
MMP20 expression and may increase migration of OSC2 cells.
DSPP silencing inhibits cell
proliferation and increases apoptosis of OSC2 cells
DSPP-silenced and SCR OSC2 cells were cultured in
96-well plates for 24 h, and mitochondrial activity was determined
using the MTT colorimetric assay. As shown in Fig. 3, DSPP-silenced cells exhibited a
slight, but insignificant, reduction in OD values compared with in
SCR cells. The rate of apoptosis of DSPP-silenced OSC2 cells was
assessed by Annexin V-FITC flow cytometry; the apoptotic cell
fraction was increased from 3.41% in SCR cells to 10.9% in
DSPP-silenced cells (Fig. 4).
Taken together, DSSP silencing reduced cell proliferation and
enhanced apoptosis, thus suggesting that DSPP may exert a
tumorigenic influence on OSC2 cells. With the addition of
puromycin, DSPP-silenced cells exhibited significantly lower OD
values (P<0.05) compared with in SCR cells and puromycin-free
DSPP-silenced cells (Fig. 3).
Annexin V-FITC flow cytometry detected an increase in the apoptotic
cell fraction from 3.41 to 6.49%, and from 10.9 to 18.5%, in
response to 48-h puromycin treatment of SCR and DSPP-silenced
cells, respectively (Fig. 4).
Notably, puromycin-treated DSPP-silenced cells exhibited a higher
apoptotic rate than puromycin-free DSPP-silenced cells.
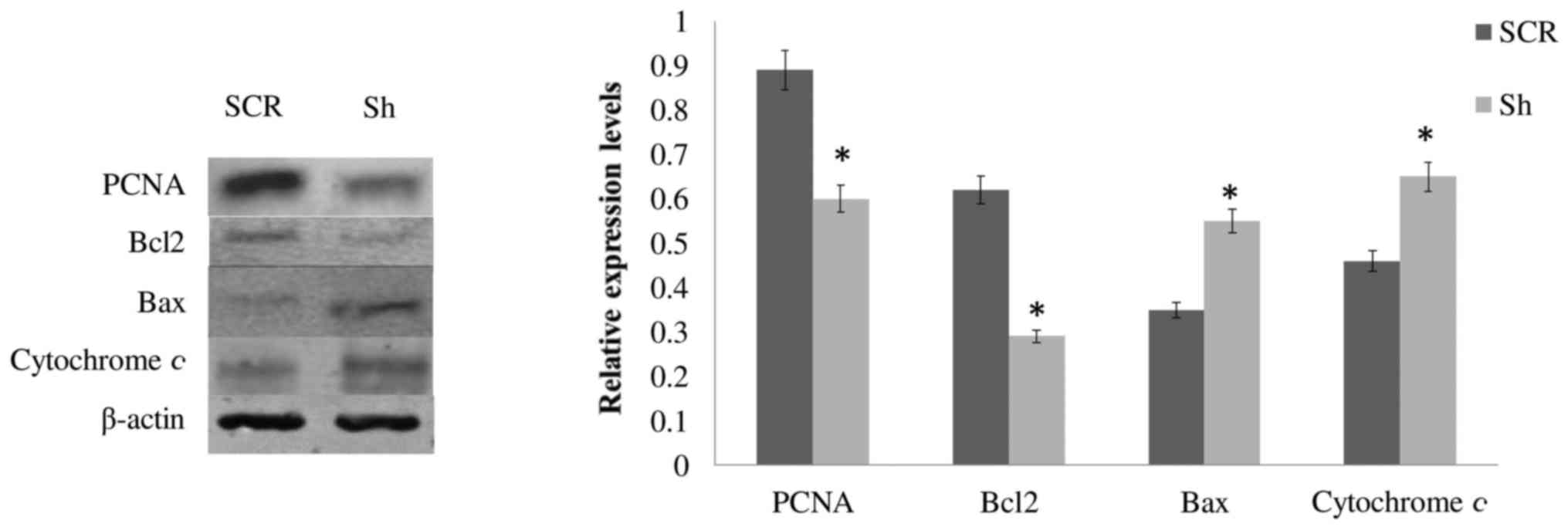
Effects of DSPP silencing on the
expression of cell proliferation- and apoptosis-associated proteins
in OSC2 cells
Since the aforementioned results indicated that
puromycin-treated and puromycin-free DSPP-silenced OSC2 cells
presented comparable effects with regards to the mRNA expression
levels of UPR-related proteins, puromycin seems to only partially
counteract, without significantly reversing, the effects of DSPP
silencing on OSC2 cells. Therefore, although there were significant
differences between puromycin-free and puromycin-treated cells with
respect to apoptosis and proliferation, puromycin treatment, per
se, did not alter the original overall effect of DSPP silencing on
apoptosis and cell proliferation. Therefore, to evaluate the
effects of DSPP silencing on cell proliferation and
apoptosis-related proteins, western blot analysis was conducted
only on whole cell lysates from puromycin-free DSPP-silenced and
SCR OSC2 cells. As shown in Fig.
5, the protein expression levels of the proliferation marker
PCNA and the anti-apoptotic molecule Bcl2 were significantly
decreased (P<0.05), whereas levels of the proapoptotic molecule,
Bax, and cytochrome c were increased, compared with SCR
cells. These results, which are concordant with the results of cell
proliferation (Fig. 3) and
apoptosis (Fig. 4) analyses,
indicated that alterations in DSPP expression may affect the
expression and activity of cell proliferation- and
apoptosis-associated molecules, thus modulating the survival of
OSC2 cells.
Discussion
The present study demonstrated that DSPP silencing
may result in perturbation of ER calcium homeostasis in OSC2 cells,
as evidenced by reductions in the mRNA expression levels of GRP78,
SERCA2b, IRE1, PERK and ATF6. Conversely, IP3r levels remained
unchanged. Furthermore, the results indicated that DSPP silencing
induced a reduction in Bcl2 and PCNA protein expression, and an
increase in Bax and cytochrome c levels. The present data
also verified the findings of a previously published study, which
reported that DSPP downregulation may decrease cell proliferation
and increase apoptosis of OSC2 cancer cells (33). Bcl2 serves a cytoprotective role,
exerting its prosurvival effect by binding, inhibiting and
sequestrating proapoptotic proteins [Bax/Bcl2 homologous
antagonist/killer (Bak)], and by lowering ER Ca2+ stores
(34,35). Bcl2, expressed in ER, has also been
reported to exert anti-apoptotic effects and to antagonize
Bax/Bak-independent paraptotic cell death in transformed mouse
kidney epithelial cells (35).
Furthermore, ER stress enhances Bax translocation and insertion
into the ER membrane in MEF cells (36), and Bax or Bak upregulation induces
ER Ca2+ efflux and cytochrome c release from the
mitochondria (37,38).
In general, cancer cells demonstrate increased
levels of misfolded proteins and ER stress as a result of genetic
mutations, stressful microenvironment, or in response to anticancer
therapy (39,40). Furthermore, changes in calcium
homeostasis help tumor cells rearrange calcium signaling according
to the activity of various cancer-associated proteins and
acclimatize to a new hostile environment (41). Several components of the UPR have
been revealed to be overexpressed in numerous cancer types;
therefore, targeting UPR proteins has been proposed as a promising
therapeutic strategy (15,39,42).
The present data indicated that DSPP silencing may
decrease GRP78 and SERCA2b mRNA expression, and induce
oncosuppressive effects. GRP78 is a major chaperone and modulator
of the UPR that demonstrates high protein-binding capacity and
directly manages UPR signaling proteins IRE1, PERK and ATF6
(43). Previous studies have
suggested that GRP78 promotes tumor progression (44,45),
and increased GRP78 expression has been correlated with reduced
patient survival and shorter recurrence time in esophageal squamous
cell carcinoma, prostate and breast cancer (41,46,47).
Other studies have indicated that GRP78 silencing reduces tumor
cell invasion, cell growth and metastasis in xenograft models of
gastric cancer (48). Furthermore,
decreased GRP78 expression reduces multidrug resistance (49).
SERCA regulates cellular Ca2+ homeostasis
via its pump activity, which channels Ca2+ into the ER,
thereby maintaining the requisite ionic balance between ER and
cytosolic calcium (50). In
addition to this role, SERCA is involved in survival pathways,
including the Notch1 signaling pathway (51). SERCA inhibition has been reported
to cause ER Ca2+ depletion, while increasing cytosolic
and mitochondrial Ca2+ concentration. SERCA pump
inhibitors, such as thapsigargin, which are capable of interfering
with calcium homeostasis, have been revealed to exert cytotoxic
effects on cancer cells in vitro (15,52).
Specifically, persistent ER Ca2+ depletion may activate
the ER stress response and intrinsic apoptotic pathways involving
caspase 12, whereas high cytosolic calcium levels may trigger
extrinsic proapoptotic and anti-apoptotic signaling of Bcl2 family
members with subsequent release of mitochondrial cytochrome
c (15). It has also been
suggested that Bcl2 directly interacts with SERCA resulting in
alterations in calcium pumps and calcium levels required for
initiating apoptosis (53).
The present results also revealed that DSPP
silencing induced downregulation of the three major UPR-sensors
(PERK, ATF6 and IRE1), whose activities have been associated with
tumorigenic effects. Cancer cells appear more resistant to
environmental stress due to alterations in the UPR protein activity
(18). PERK and its target
molecule eukaryotic initiation factor 2α have been associated with
increased tumor growth and survival under hypoxic conditions
(28). Conversely, PERK inhibition
decreases tumor growth in vitro and in vivo (54), impedes cell cycle progression and
reduces metastatic propensity in mammary cancer in mice xenografts
(55). Similarly, whereas IRE1
contributes to resistance against ER stress-mediated cell death
through its effects on Bax/Bak activity, its inhibition results in
oligomerization of Bax and Bak and an increase in ER membrane
permeabilization (56). High
levels of ATF6 protein have also been detected in several human
solid tumors and it has been suggested to induce proliferation and
survival under nutrient-deficient conditions (30). Previous reports suggested that ATF6
enhances X-box binding protein 1 expression, and both proteins
increase GRP78 activity in liver cancer (57). Furthermore, ATF6 activation results
in radiation-induced upregulation of GRP78 and Notch1, whereas ATF6
knockdown promotes radiation-induced cell death in glioblastoma
(58).
The results of a scratch wound-healing assay
indicated that DSPP silencing in OSC2 cells reduced migration
compared with the control cells. This effect may be attributable to
a decrease in MMP20 levels upon DSPP silencing. Recently, MMP20 was
identified as the cognate MMP partner of DSPP (13). Furthermore, it has been reported
that the DSPP cleaved product, DSP, interacts with MMP20 through
its N-terminal domain. It has been suggested that this interaction
may bridge MMP20 to cell surface receptors, thus triggering
signaling pathways associated with proliferation, migration,
invasion and metastasis (13).
Other investigators have reported that GRP78 downregulation, which
results in decreased MMP2 and MMP9 expression, significantly
reduces the metastatic potential of esophageal SCC cells (59). Furthermore, GRP78 knockdown
downregulates TIMP metallopeptidase inhibitor (TIMP)1, TIMP2,
MMP14, MMP2 and MMP9 expression in human pancreatic adenocarcinoma
cells (60). It therefore may be
hypothesized that the decreased migratory ability of DSPP-silenced
cells is due to the consequent reduction in MMP20 and GRP78 mRNA
expression. As has been revealed for other MMPs, this finding
indicated that GRP78 may be a potential regulator of MMP20.
The present study analyzed the effects of puromycin
treatment on DSPP-silenced oral cancer cells, and demonstrated that
treatment of OSC2 cells with puromycin partially counteracted the
effects of DSSP silencing on GRP78, SERCA, IRE1 and ATF6
expression, without reversing the overall effects of DSPP silencing
on OSC2 cells. Puromycin-treated DSPP-silenced OSC2 cells exhibited
less pronounced reductions in GRP78, SERCA, IRE1 and ATF6
expression compared with DSPP-silenced cells without puromycin
treatment. Furthermore, puromycin-treated DSPP-silenced cells
exhibited further reductions in cell proliferation and increased
apoptosis compared with DSPP-silenced cells without puromycin
treatment. A plausible explanation for this result is that
puromycin may trigger the UPR, disrupting protein synthesis and the
metabolic activity of OSC2 cells. Both puromycin-treated and
untreated DSPP-silenced cells exhibited low SERCA2b mRNA
expression.
As a translocon opener, puromycin treatment of cells
may result in increased ER calcium release and higher rates of
apoptosis. For example, Oguma et al reported that puromycin
treatment induces GRP78 expression, resulting in caspase
12-mediated cell death in cluster of differentiation
(CD)4+CD8+ thymic lymphoma cells and in
normal thymocytes (61). Johnson
et al also revealed that malignant glioma cells treated with
a low dose of puromycin exhibit increased GRP78 protein and mRNA
levels, and enhanced activation of caspase 4. Caspase 4 activation
has been suggested to result from the direct effect of calcium
leakage through open translocons, leading to ~93% reduction of cell
viability (62). Similarly, it has
been reported that puromycin treatment of human prostate
adenocarcinoma cells increases GRP78 protein expression, enhances
ER calcium release via translocons, and induces apoptosis (63).
In the context of the present study, the
aforementioned effects of puromycin on DSPP-silenced cells may also
account for the less pronounced reduction in MMP20 mRNA.
Alternatively, the less pronounced downregulation of MMP20 caused
by puromycin treatment in DSPP-silenced cells may be due to less
pronounced reductions in GRP78 levels in puromycin-treated cells.
The composite effect may be increased cell migration, as indicated
by the scratch wound-healing assay. Previous studies reported that
puromycin treatment induces GRP78 expression and leads to caspase
activation in several malignancies (62,63);
this has been suggested to be a direct effect of enhanced ER
calcium release via translocon (62,63).
Therefore, in the present study, puromycin was used as a vehicle to
cause alterations in the expression of GRP78 and UPR-related
proteins, as well as in ER calcium homeostasis. Since the present
study focused on the tumorigenic role of DSPP and its potential
correlation with alterations in the UPR and ER stress, puromycin
treatment revealed how the observed changes may affect DSPP
tumorigenic activity in OSC2 cells. To the best of our knowledge,
the present study is the first to provide an insight into the
potential associations between DSPP and GRP78 functional roles, and
the modulation of ER stress in OSCC.
DSPP is an evolutionary younger member of the acidic
secretory calcium-binding phosphoprotein gene family (7). The two major DSPP cleaved functional
units, DSP and DPP, are highly acidic, with human DPP presenting a
very low isoelectric point of 2.84 and high calcium affinity
(7,8). It is therefore conceivable that DSPP
serves as a buffer protein in ER calcium homeostasis with a similar
functional role to other calcium-dependent proteins: Calnexin,
calreticulin and GRP78 (64).
Furthermore, the DSPP cysteine residue may function similarly to
its GRP78 counterpart, which has been suggested to promote cell
survival under ER stress (27),
and by doing so may contribute to cell stability during ER stress.
Therefore, DSPP silencing may lead to a sudden alteration in the ER
calcium balance, provoking collapse of the UPR as a result of the
calcium-demanding tumor microenvironment.
In conclusion, the present study provided evidence
to suggest that DSPP is involved in ER stress mechanisms.
Downregulation of DSPP in OSCC cells resulted in changes in major
ER stress-associated proteins (GRP78, SERCA2b and UPR sensor
proteins), thus leading to collapse of the UPR system and function.
These results also validated previously published reports,
indicating that DSPP silencing resulted in decreased cell
proliferation, migration, invasion and increased apoptosis. Further
in-depth studies are required to determine the nature of the
interaction/relationship between DSPP and major UPR regulators,
such as GRP78, in OSCC. Increased understanding may advance the
understanding of the ability of cancer cells to survive under
stressful conditions, and to elucidate potential therapeutic
strategies.
Acknowledgments
Not applicable.
Funding
The present study was supported by faculty startup
research funding (to KUEO) from the University of Texas Health
Science Center at Houston (Houston, TX, USA).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
NGN and KUEO made substantial contributions to the
conception and design of the study, reviewed data, reviewed/edited
draft manuscripts, and reviewed/edited the final draft of the
manuscript. IG and JA carried out experiments related to the study,
acquired, analyzed and interptreted data, and provided the initial
draft of the manuscript. All authors gave their approval of the
final draft of the manuscript, and agree to be accountable for all
aspects of the study related to accuracy or integrity of all parts
of the study.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bellahcène A, Castronovo V, Ogbureke KU,
Fisher LW and Fedarko NS: Small integrin-binding ligand N-linked
glycoproteins (SIBLINGs): Multifunctional proteins in cancer. Nat
Rev Cancer. 8:212–226. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ogbureke KU and Fisher LW: Expression of
SIBLINGs and their partner MMPs in salivary glands. J Dent Res.
83:664–670. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ogbureke KU and Fisher LW: SIBLING
expression patterns in duct epithelia reflect the degree of
metabolic activity. J Histochem Cytochem. 55:403–409. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Koli K, Saxena G and Ogbureke KU:
Expression of matrix metal-loproteinase (MMP)-20 and potential
interaction with dentin sialophosphoprotein (DSPP) in human major
salivary glands. J Histochem Cytochem. 63:524–533. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yamakoshi Y, Hu JC, Iwata T, Kobayashi K,
Fukae M and Simmer JP: Dentin sialophosphoprotein is processed by
MMP-2 and MMP-20 in vitro and in vivo. J Biol Chem.
281:38235–38243. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Suzuki S, Sreenath T, Haruyama N,
Honeycutt C, Terse A, Cho A, Kohler T, Müller R, Goldberg M and
Kulkarni AB: Dentin sialoprotein and dentin phosphoprotein have
distinct roles in dentin mineralization. Matrix Biol. 28:221–229.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang J, Kawasaki K, Lee M, Reid BM, Nunez
SM, Choi M, Seymen F, Koruyucu M, Kasimoglu Y, Estrella-Yuson N, et
al: The dentin phosphoprotein repeat region and inherited defects
of dentin. Mol Genet Genomic Med. 4:28–38. 2015. View Article : Google Scholar
|
|
8
|
Alvares K, Stern PH and Veis A: Dentin
phosphoprotein binds annexin 2 and is involved in calcium transport
in rat kidney ureteric bud cells. J Biol Chem. 288:13036–13045.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fisher LW, Jain A, Tayback M and Fedarko
NS: Small integrin binding ligand N-linked glycoprotein gene family
expression in different cancers. Clin Cancer Res. 10:8501–8511.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jain A, McKnight DA, Fisher LW, Humphreys
EB, Mangold LA, Partin AW and Fedarko NS: Small integrin-binding
proteins as serum markers for prostate cancer detection. Clin
Cancer Res. 15:5199–5207. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Anunobi CC, Koli K, Saxena G, Banjo AA and
Ogbureke KU: Expression of the SIBLINGs and their MMP partners in
human benign and malignant prostate neoplasms. Oncotarget.
7:48038–48049. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ogbureke KU, Nikitakis NG, Warburton G,
Ord RA, Sauk JJ, Waller JL and Fisher LW: Up-regulation of SIBLING
proteins and correlation with cognate MMP expression in oral
cancer. Oral Oncol. 43:920–932. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Saxena G, Koli K, de la Garza J and
Ogbureke KU: Matrix metal-loproteinase 20-dentin
sialophosphoprotein interaction in oral cancer. J Dent Res.
94:584–593. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rao RV, Hermel E, Castro-Obregon S, del
Rio G, Ellerby LM, Ellerby HM and Bredesen DE: Coupling endoplasmic
reticulum stress to the cell death program. Mechanism of caspase
activation. J Biol Chem. 276:33869–33874. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Denmeade SR and Isaacs JT: The SERCA pump
as a therapeutic target: Making a 'smart bomb' for prostate cancer.
Cancer Biol Ther. 4:14–22. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mekahli D, Bultynck G, Parys JB, De Smedt
H and Missiaen L: Endoplasmic-reticulum calcium depletion and
disease. Cold Spring Harb Perspect Biol. 3:32011. View Article : Google Scholar
|
|
17
|
Raturi A, Ortiz-Sandoval C and Simmen T:
Redox dependence of endoplasmic reticulum (ER) Ca2+
signaling. Histol Histopathol. 29:543–552. 2014.
|
|
18
|
Giampietri C, Petrungaro S, Conti S,
Facchiano A, Filippini A and Ziparo E: Cancer microenvironment and
endoplasmic reticulum stress response. Mediators Inflamm.
2015:4172812015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang M and Kaufman RJ: The impact of the
endoplasmic reticulum protein-folding environment on cancer
development. Nat Rev Cancer. 14:581–597. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lemus L and Goder V: Regulation of
endoplasmic reticulum-associated protein degradation (ERAD) by
ubiquitin. Cells. 3:824–847. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yadav RK, Chae SW, Kim HR and Chae HJ:
Endoplasmic reticulum stress and cancer. J Cancer Prev. 19:75–88.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bertolotti A, Zhang Y, Hendershot LM,
Harding HP and Ron D: Dynamic interaction of BiP and ER stress
transducers in the unfolded-protein response. Nat Cell Biol.
2:326–332. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Brown JM and Giaccia AJ: The unique
physiology of solid tumors: Opportunities (and problems) for cancer
therapy. Cancer Res. 58:1408–1416. 1998.PubMed/NCBI
|
|
24
|
He B: Viruses, endoplasmic reticulum
stress, and interferon responses. Cell Death Differ. 13:393–403.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Martinon F: Targeting endoplasmic
reticulum signaling pathways in cancer. Acta Oncol. 51:822–830.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Moenner M, Pluquet O, Bouchecareilh M and
Chevet E: Integrated endoplasmic reticulum stress responses in
cancer. Cancer Res. 67:10631–10634. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang J and Sevier CS: Formation and
reversibility of BiP protein cysteine oxidation facilitate cell
survival during and post oxidative stress. J Biol Chem.
291:7541–7557. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Koumenis C: ER stress, hypoxia tolerance
and tumor progression. Curr Mol Med. 6:55–69. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fu Y, Li J and Lee AS: GRP78/BiP inhibits
endoplasmic reticulum BIK and protects human breast cancer cells
against estrogen starvation-induced apoptosis. Cancer Res.
67:3734–3740. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ye J, Kumanova M, Hart LS, Sloane K, Zhang
H, De Panis DN, Bobrovnikova-Marjon E, Diehl JA, Ron D and Koumenis
C: The GCN2-ATF4 pathway is critical for tumour cell survival and
proliferation in response to nutrient deprivation. EMBO J.
29:2082–2096. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Thorpe JA and Schwarze SR: IRE1alpha
controls cyclin A1 expression and promotes cell proliferation
through XBP-1. Cell Stress Chaperones. 15:497–508. 2010. View Article : Google Scholar
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
33
|
Joshi R, Tawfik A, Edeh N, McCloud V,
Looney S, Lewis J, Hsu S and Ogbureke KU: Dentin
sialophosphoprotein (DSPP) gene-silencing inhibits key tumorigenic
activities in human oral cancer cell line, OSC2. PLoS One.
5:e139742010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Brunelle JK and Letai A: Control of
mitochondrial apoptosis by the Bcl-2 family. J Cell Sci.
122:437–441. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Heath-Engel HM, Wang B and Shore GC: Bcl2
at the endoplasmic reticulum protects against a Bax/Bak-independent
paraptosis-like cell death pathway initiated via p20Bap31. Biochim
Biophys Acta. 1823:335–347. 2012. View Article : Google Scholar
|
|
36
|
Wang X, Olberding KE, White C and Li C:
Bcl-2 proteins regulate ER membrane permeability to luminal
proteins during ER stress-induced apoptosis. Cell Death Differ.
18:38–47. 2011. View Article : Google Scholar
|
|
37
|
Nutt LK, Pataer A, Pahler J, Fang B, Roth
J, McConkey DJ and Swisher SG: Bax and Bak promote apoptosis by
modulating endoplasmic reticular and mitochondrial Ca2+
stores. J Biol Chem. 277:9219–9225. 2002. View Article : Google Scholar
|
|
38
|
Scorrano L, Oakes SA, Opferman JT, Cheng
EH, Sorcinelli MD, Pozzan T and Korsmeyer SJ: BAX and BAK
regulation of endoplasmic reticulum Ca2+: A control
point for apoptosis. Science. 300:135–139. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nagelkerke A, Bussink J, Sweep FC and Span
PN: The unfolded protein response as a target for cancer therapy.
Biochim Biophys Acta. 1846:277–284. 2014.PubMed/NCBI
|
|
40
|
Corazzari M, Gagliardi M, Fimia GM and
Piacentini M: Endoplasmic reticulum stress, unfolded protein
response, and cancer cell fate. Front Oncol. 7:782017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Marchi S and Pinton P: Alterations of
calcium homeostasis in cancer cells. Curr Opin Pharmacol. 29:1–6.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lee E, Nichols P, Spicer D, Groshen S, Yu
MC and Lee AS: GRP78 as a novel predictor of responsiveness to
chemotherapy in breast cancer. Cancer Res. 66:7849–7853. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
de Ridder G, Ray R, Misra UK and Pizzo SV:
Modulation of the unfolded protein response by GRP78 in prostate
cancer. Methods Enzymol. 489:245–257. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Dong D, Ni M, Li J, Xiong S, Ye W, Virrey
JJ, Mao C, Ye R, Wang M, Pen L, et al: Critical role of the stress
chaperone GRP78/BiP in tumor proliferation, survival, and tumor
angiogenesis in transgene-induced mammary tumor development. Cancer
Res. 68:498–505. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Fu Y, Wey S, Wang M, Ye R, Liao CP,
Roy-Burman P and Lee AS: Pten null prostate tumorigenesis and AKT
activation are blocked by targeted knockout of ER chaperone
GRP78/BiP in prostate epithelium. Proc Natl Acad Sci USA.
105:19444–19449. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Daneshmand S, Quek ML, Lin E, Lee C, Cote
RJ, Hawes D, Cai J, Groshen S, Lieskovsky G, Skinner DG, et al:
Glucose-regulated protein GRP78 is up-regulated in prostate cancer
and correlates with recurrence and survival. Hum Pathol.
38:1547–1552. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ren P, Chen C, Yue J, Zhang J and Yu Z:
High expression of glucose-regulated protein 78 (GRP78) is
associated with metastasis and poor prognosis in patients with
esophageal squamous cell carcinoma. OncoTargets Ther. 10:617–625.
2017. View Article : Google Scholar
|
|
48
|
Zhang J, Jiang Y, Jia Z, Li Q, Gong W,
Wang L, Wei D, Yao J, Fang S and Xie K: Association of elevated
GRP78 expression with increased lymph node metastasis and poor
prognosis in patients with gastric cancer. Clin Exp Metastasis.
23:401–410. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kang J, Zhao G, Lin T, Tang S, Xu G, Hu S,
Bi Q, Guo C, Sun L, Han S, et al: A peptide derived from phage
display library exhibits anti-tumor activity by targeting GRP78 in
gastric cancer multidrug resistance cells. Cancer Lett.
339:247–259. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Toyoshima C, Nomura H and Sugita Y:
Crystal structures of Ca2+-ATPase in various
physiological states. Ann N Y Acad Sci. 986:1–8. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Roti G, Carlton A, Ross KN, Markstein M,
Pajcini K, Su AH, Perrimon N, Pear WS, Kung AL, Blacklow SC, et al:
Complementary genomic screens identify SERCA as a therapeutic
target in NOTCH1 mutated cancer. Cancer Cell. 23:390–405. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Casemore D and Xing C: SERCA as a target
for cancer therapies. Integr Cancer Sci Ther. 2:100–103. 2015.
|
|
53
|
Dremina ES, Sharov VS, Kumar K, Zaidi A,
Michaelis EK and Schöneich C: Anti-apoptotic protein Bcl-2
interacts with and destabilizes the sarcoplasmic/endoplasmic
reticulum Ca2+-ATPase (SERCA). Biochem J. 383:361–370.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Axten JM, Medina JR, Feng Y, Shu A,
Romeril SP, Grant SW, Li WH, Heerding DA, Minthorn E, Mencken T, et
al: Discovery of
7-methyl-5-(1-{[3-(trifluoromethyl)phenyl]acetyl}-2,3-dihydro-1H-indol-5-yl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine
(GSK2606414), a potent and selective first-in-class inhibitor of
protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK). J
Med Chem. 55:7193–7207. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Bobrovnikova-Marjon E, Grigoriadou C,
Pytel D, Zhang F, Ye J, Koumenis C, Cavener D and Diehl JA: PERK
promotes cancer cell proliferation and tumor growth by limiting
oxidative DNA damage. Oncogene. 29:3881–3895. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kanekura K, Ma X, Murphy JT, Zhu LJ, Diwan
A and Urano F: IRE1 prevents endoplasmic reticulum membrane
permeabili-zation and cell death under pathological conditions. Sci
Signal. 8:ra622015. View Article : Google Scholar
|
|
57
|
Shuda M, Kondoh N, Imazeki N, Tanaka K,
Okada T, Mori K, Hada A, Arai M, Wakatsuki T, Matsubara O, et al:
Activation of the ATF6, XBP1 and grp78 genes in human
hepatocellular carcinoma: A possible involvement of the ER stress
pathway in hepatocarcinogenesis. J Hepatol. 38:605–614. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Dadey DY, Kapoor V, Khudanyan A, Urano F,
Kim AH, Thotala D and Hallahan DE: The ATF6 pathway of the ER
stress response contributes to enhanced viability in glioblastoma.
Oncotarget. 7:2080–2092. 2016. View Article : Google Scholar :
|
|
59
|
Zhao G, Kang J, Jiao K, Xu G, Yang L, Tang
S, Zhang H, Wang Y, Nie Y, Wu K, et al: High expression of GRP78
promotes invasion and metastases in patients with esophageal
squamous cell carcinoma. Dig Dis Sci. 60:2690–2699. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Yuan XP, Dong M, Li X and Zhou JP: GRP78
promotes the invasion of pancreatic cancer cells by FAK and JNK.
Mol Cell Biochem. 398:55–62. 2015. View Article : Google Scholar
|
|
61
|
Oguma T, Ono T, Kajiwara T, Sato M,
Miyahira Y, Arino H, Yoshihara Y and Tadakuma T: CD4(+)CD8(+)
thymocytes are induced to cell death by a small dose of puromycin
via ER stress. Cell Immunol. 260:21–27. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Johnson GG, White MC, Wu JH, Vallejo M and
Grimaldi M: The deadly connection between endoplasmic reticulum,
Ca2+, protein synthesis, and the endoplasmic reticulum
stress response in malignant glioma cells. Neuro-oncol.
16:1086–1099. 2014. View Article : Google Scholar
|
|
63
|
Hammadi M, Oulidi A, Gackière F,
Katsogiannou M, Slomianny C, Roudbaraki M, Dewailly E, Delcourt P,
Lepage G, Lotteau S, et al: Modulation of ER stress and apoptosis
by endoplasmic reticulum calcium leak via translocon during
unfolded protein response: Involvement of GRP78. FASEB J.
27:1600–1609. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Michalak M, Milner RE, Burns K and Opas M:
Calreticulin. Biochem J. 285:681–692. 1992. View Article : Google Scholar : PubMed/NCBI
|