Introduction
Nasopharyngeal carcinoma (NPC) is a tumor that
arises from the epithelial lining of the nasopharynx, and it is
considered the most common cancer of the head and neck (1). NPC has a distinct racial, sex and
geographical distribution with a multifactorial etiology. China,
Southeast Asia and North Africa have the highest prevalence
worldwide. According to World Health Organization (WHO) estimates,
the Western Pacific and Southeast Asia regions accounted for ~77%
of all cases internationally. Furthermore, NPC is particularly more
frequent in males (sex ratio 2.3:1) and certain ethnicities, such
as Chinese (2,3). The occurrence of NPC in childhood is
rare. It has been estimated that 5% of childhood primary tumors
arise in the head and neck area, while NPC only constitutes 2% of
head and neck tumors in children (4,5).
Multiple etiological factors have been reported to
be as associated with the risk of developing of NPC, including
genetics [certain human leukocyte antigen (HLA) types],
environmental factors (nitrosamine and tobacco) and viral infection
[Epstein Barr virus (EBV)] (6,7). The
WHO has classified the NPC into three types, based on
histopathological criteria: Keratinizing squamous cell carcinoma
(type 1), non-keratinizing carcinoma (type 2) and undifferentiated
carcinoma (type 3). It has been reported that these types are
geographically diversified. In Southern China, where NPC is highly
prevalent, nearly all cases are of the undifferentiated type,
whereas in the USA, where NPC is rare, ~20% cases are the
keratinizing type (8).
Although EBV has long been associated with
nasopharyngeal carcinogenesis, several studies have reported an
association of oncogenic human papillomaviruses (HPV) with a
sub-group of NPCs (9-12). Furthermore, HPV is considered as a
leading cause of cervical carcinoma (13). Whilst HPV has been reported as one
of the causative agents of NPC in adults, little is known about the
implication of HPV in the pathogenesis of childhood NPC.
In the present study, a rare case of a patient with
a low-risk NPC type IIb with T2N0M0, which was expected to have a
good prognosis, is reported. Surprisingly, the tumor was refractory
to therapy and even allogenic stem cell transplantation failed,
which ultimately led to mortality. The poor prognosis was not
predictable by standard clinicopathological parameters. Thus, the
expression of a panel of biomarkers was analyzed by
immunohistochemistry in formalin-fixed, paraffin-embedded (FFPE)
tumor material from this patient and compared with biopsies from 8
other childhood NPC cases. All 9 patients were enrolled in the
prospective multicenter study [Nasopharyngeal Carcinoma (NPC) 2003
German Society of Pediatric Oncology and Hematology/German
Children's Oncology Group (NPC-2003-GPOH/DCOG)] (14,15).
The biomarkers included human papilloma virus markers (E6, E7),
immunological markers (CD4, CD8 and CD56), proliferation markers
[Ki-67, 14-3-3τ, eukaryotic translation initiation factor 3 subunit
E (eIF3e)], tumor suppressors [p53, Wilms tumor 1 (WT1)], apoptosis
markers [terminal deoxynucleo-tidyl transferase dUTP nick-end
labeling (TUNEL) assay, p53, ATP-ADP translocase (AAC)] and other
markers [glutathione S-transferase π 1 (GSTP1), CD31, inducible
nitric oxide synthetase (iNOS)].
Materials and methods
Patients
A 17 year-old female patient was diagnosed with a
low-risk nasopharyngeal tumor at the Department for Pediatrics
(Medical Center of the University of Tübingen, Tübingen, Germany)
on March 16th, 2007. The histology was determined as
non-keratinizing squamous epithelial carcinoma with lymphoid
infiltration of type IIb with T2N0M0 stage. Therapy was performed
according to NPC-2003-GPOH (14)
and consisted of: i) Tumor irradiation including lymph vessels with
59.4 and 45 Gy, respectively; ii) two chemotherapy cycles with
cisplatin concomitant to irradiation during the first cycle and
carboplatin during the second cycle (because of tinnitus as side
effect); and iii) β-interferon (β-IFN).
A systemically refractory tumor was diagnosed on
February 7th, 2008. The patient suffered from severe pain symptoms
and atelectasis pneumonia of the left lower lobe. Chemotherapy was
started according to NPC-2003-GPOH with cisplatin and
5-fluorouracil in the first cycle and cisplatin, 5-fluorouracil and
docetaxel in the second cycle.
A second refractory tumor was diagnosed on October
17th, 2008. The patient was treated with chemotherapy again
(cisplatin, 5-fluorouracil and docetaxel). Recurrence was diagnosed
on February 7th, 2008. Irradiation could not be performed, because
of the large expansion of the tumor (intra-abdominal, retro-crural,
intra-thoracic, along the Truncus coeliacus and
para-aortic). AS chemotherapy alone was not curative, allogenic
stem cell transplantation was suggested. In parallel, there were
signs of immunodeficiency, indicated by infection with Herpes
simplex virus (HSV), human-HSV-6 (HHV-6), Varicella zoster
virus (VZV) and Candida ssp. in addition to EBV. Thus,
allogenic stem cell transplantation from the HLA-identical 9
year-old brother of the patient was suggested as a potential
curative treatment approach. As the brother was EBV-IgG-positive,
it was expected that a T-cell-mediated antiviral response against
EBV may be transferred by transplantation. The patient's tumor
cells expressed EBV antigens. Therefore, an antiviral T-cell
response may act as allo-versus-tumor response and, thus, as
prophylaxis against renewed recurrence of the tumor.
Following agreement of the patient, the stem cell
transplantation was performed. Conditioning took place at June 4th,
2008 with treosulfan and cyclophosphamide and was well tolerated
except for a transient increase of liver transaminases and nausea.
The allogenic bone marrow stem cells transferred on June 10th, 2008
contained 1.25×108 mononuclear cells/kg body weight
(BW), 2.45×106 CD34-positive progenitor cells/kg BW,
0.70×106 AC133-positive progenitor cells/kg BW and
1,661.43×104 CD3-positive cells/kg BW. Antibiotic
prophylaxis was performed with metronidazole (800 mg/day for 29
days) and cotrimoxazol (160 mg/day for 28 days), fungal prophylaxis
with amphotericin B (150 mg/day for 29 days) and viral prophylaxis
with acyclovir (2,400 mg/day for 35 days). Graft-versus-host
disease was treated with cyclosporine (90 mg/day for 16 days),
methotrexate (15 mg/day for 1 day) and prednisolone (100 mg/day for
5 days). Granulocyte colony stimulating factor growth factor was
applied 11 times between day 4 and 14 after transplantation. The
course of the transplantation was free of complications.
Toxicity (WHO grade IV) occurred in the aplastic
phase (mucositis, nausea, transient increase of transaminases).
Graft-versus-host disease of the skin (grade I) appeared on day 13
after transplantation. The engraftment took place on June 24th,
2008 (day 14 after transplantation). Arterial hypertension was
observed as side effect of drug therapy. The atelectasis of the
left lower lobe was persisting. Recurrence following stem cell
transplantation was diagnosed on October 17th, 2009 and the patient
died on January 25th, 2009.
In addition to this problematic patient, 8 pediatric
NPC patients (age range, 11-17 years at diagnosis; 5:3 male:female
ratio) that achieved complete remission following treatment
according to the NPC-2003-GPOH regimen were investigated. FFPE
tumor biopsies from all patients were provided by Dr Rolf Mertens
(University Hospital Aachen, Aachen, Germany) and analyzed for
comparison with the aforementioned clinical case. The tumor tissues
were fixed in 10% neutral-buffered formalin at room temperature for
24 h. These FFPE samples have been investigated within the frame of
the NPC-2003-GPOH/DCOG study (14).
Ethical approval (reference no. EK034/03) for the
use of tumor material for experimental purposes was obtained from
the Ethics Committee of the University of Aachen (Aachen, Germany),
and local ethical approval was obtained from the participating
centers in Germany (14). Written
informed consent was obtained from all patients.
Immunohistochemistry staining and
evaluation
Commercially available antibodies were applied on
paraffin-embedded tissue slides using a polymeric labeling
technique. Briefly, all slides were washed twice with xylene (98.5%
xylene for 5 min each wash at room temperature) to remove paraffin.
Primary antibodies used in immunohistochemistry staining were: iNOS
(1:150; clone K13-A; cat. no. DB 003-0.05; Acris Antibodies;
OriGene Technologies, Inc., Rockville, MD, USA), p53 (1:100; clone
Y5; cat. no. MA5-14467; Thermo Fisher Scientific, Inc., Waltham,
MA, USA), Ki-67 (1:200; clone SP6; cat. no. ab16667; Abcam,
Cambridge, UK), c-Myc (1:50; clone 9E10.3; cat. no. AHO0062), CD31
(1:150; clone JC/70A; cat. no. MA5-13188), CD56 (1:100; clone
56C04; cat. no. MA5-11563), CD8 (1:50; clone SP16; cat. no.
MA5-14548), CD4 (1:10; clone 4B12; cat. no. MA5-12259) (all from
Thermo Fisher Scientific, Inc.), AAC (1:50 polyclonal; cat. no.
51031-1-AP; ProteinTech Group, Inc., Chicago, IL, USA), GSTP1
(1:1,500; clone 3F2C2; cat. no. LS-B1576-50; Acris Antibodies;
OriGene Technologies, Inc.), WT1 (1:10; clone 6F-H2; cat. no.
MA1-46028; Thermo Fisher Scientific, Inc.), 14-3-3-τ (1:100; clone
AT1A1; cat. no. AM09060PU-N; Acris Antibodies; OriGene
Technologies, Inc.), E6 (1:50; clone C1P5; cat. no. MA1-46057;
Thermo Fisher Scientific, Inc.) and E7 (1:100; clone TVG701Y; cat.
no. MA5-14132; Thermo Fisher Scientific, Inc.). The TUNEL assay
used was Apoptag® Peroxidase Detection Kit (EMD
Millipore, Billerica, MA, USA). Then, sample tissues were
rehydrated through graded washes with isopropanol in water
(starting from 99% to 70% isopropanol for 1 min in each wash at
room temperature). Heat-induced epitope retrieval was performed
using a pressure cooker (100°C for 20 min) as a heating device.
Retrieval solutions were used (1% citrate buffer pH 6 in PBS), then
cooling was performed on ice for 25 min. H2O2
(3%) and Ultra-vision protein block (cat. no. TA-060-UB; Thermo
Fisher Scientific, Inc.) were added for 10 min in each solution at
room temperature to block endogenous peroxidase and avoid
non-specific background staining. Slides were incubated overnight
at 4°C following addition of primary antibodies, and then
horseradish peroxidase-labeled polymers conjugated with secondary
antibodies (cat. no. TL-060-QPH; Thermo Fisher Scientific, Inc.)
were added. The final staining reaction was performed with
diaminobenzidine and slides were counterstained with hematoxylin
(cat. nos. TA-060-QHSX and TA-002-QHCX; Thermo Fisher Scientific,
Inc.). All solutions used for blocking and detection were provided
from Thermo Fisher Scientific, Inc. (UltraVision Quanto Detection
System HRP DAB; cat. no. TL-060-QHD) and the protocol was followed
according to the manufacturer's instructions. The immunostained
slides were scanned by Panoramic Desk and quantified by Panoramic
Viewer software version 1.15.4 (NuclearQuant and MembraneQuant)
(both from 3DHISTECH Ltd., Budapest, Hungary). Quantification
(percentage of positivity) was calculated by dividing the number of
positively stained cells by an overall number of cells found each
six independently annotated tumor areas. Mean values revealed the
average protein expression, while standard deviation values
indicated the heterogeneity of expression in the tissue. Higher
standard deviation indicated higher heterogeneity of expression of
the corresponding protein.
Assessment of tumor-infiltrating
lymphocytes (TILs)
Histological hematoxylin and eosin (H&E) routine
staining (16) was performed on
tumor sections, in order to assess TILs. The cells were counted
with panoramic viewer software (NuclearQuant; 3DHISTECH Ltd.)
according to the recommendations of the International TILs Working
Group 2015 (17): i) The
percentage of TILs in the stromal tumor tissue within the borders
of the invasive tumor has been determined. TILs in normal areas
outside the tumor were not considered; ii) tumor areas with crush
artifacts, necrosis, or regressive hyalinization were not
evaluated; iii) only mono-nuclear, but not polymorphonuclear
leukocytes were counted; iv) the average values of the full tumor
area were assesses, rather than focusing on hotspots.
Hierarchical cluster analysis
In the present study, hierarchical cluster analysis
was performed to group heterogeneous objects into clusters of
homogeneous objects. All objects were assembled into a cluster tree
(dendrogram). Thus, objects with tightly related features appear
together, whereas the separation in the cluster tree increases with
progressive dissimilarity. The merging of objects with similar
features leads to formation of a cluster, the shortest the distance
of the branch the closest degree of relatedness (18). Hierarchical clustering was
conducted using WinSTAT software (R. Fitch Software, Cambridge, MA,
USA) and the Ward method (19) was
performed. Previously, cluster methods have been validated for gene
expression profiling, identification of candidate genes for drug
resistance and sensitivity and for understanding molecular biology
of cancer (20,21).
Results
Immunohistochemistry
An expression of a panel of biomarkers for tumor
aggressiveness and progression was analyzed in a female patient
with NPC type IIb (case 9). The expression patterns were compared
with those of eight other patients with NPC (cases 1 to 8). Using
immunohistochemistry, 15 different markers were detected, which may
indicate tumor aggressiveness and may also predict tumor
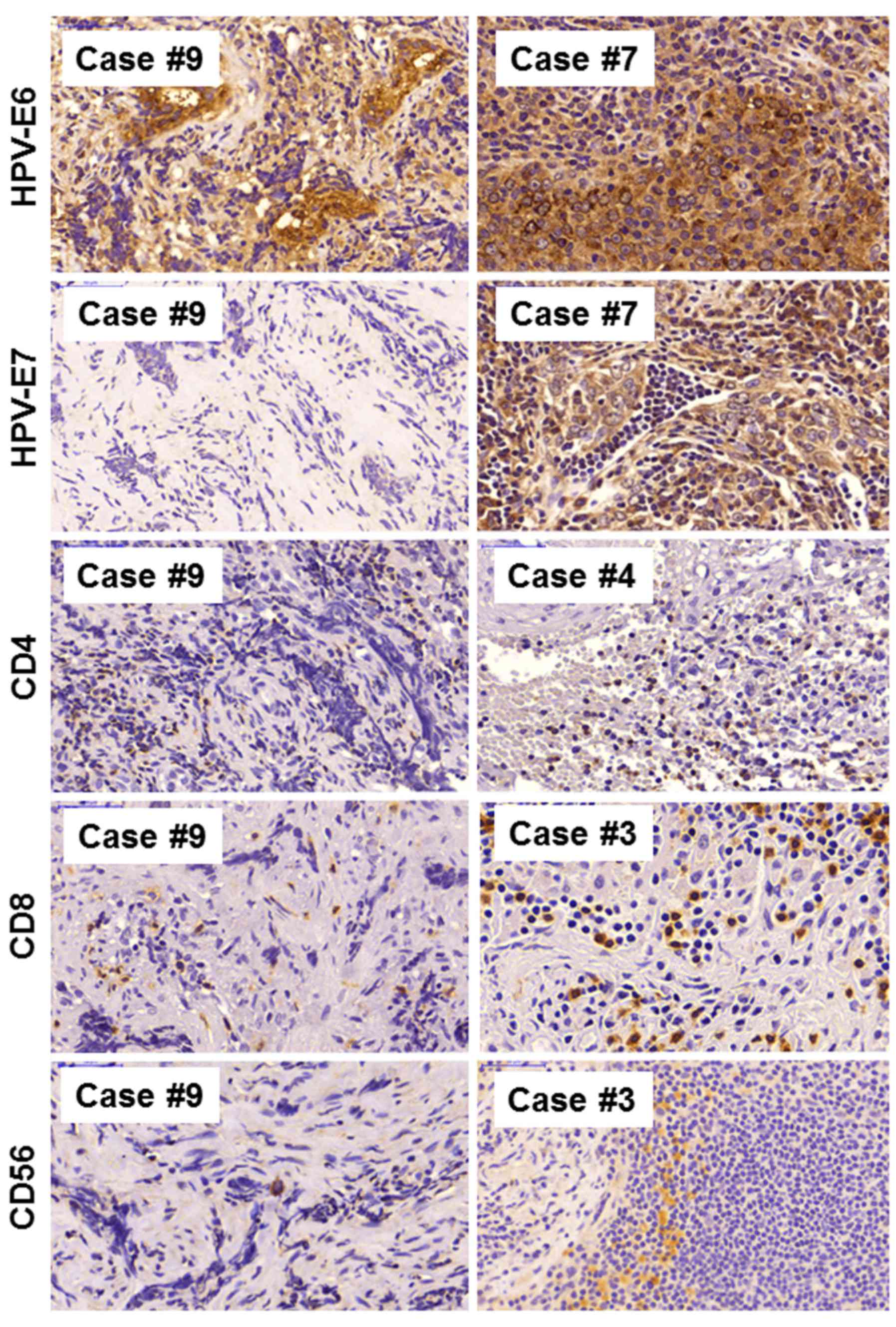
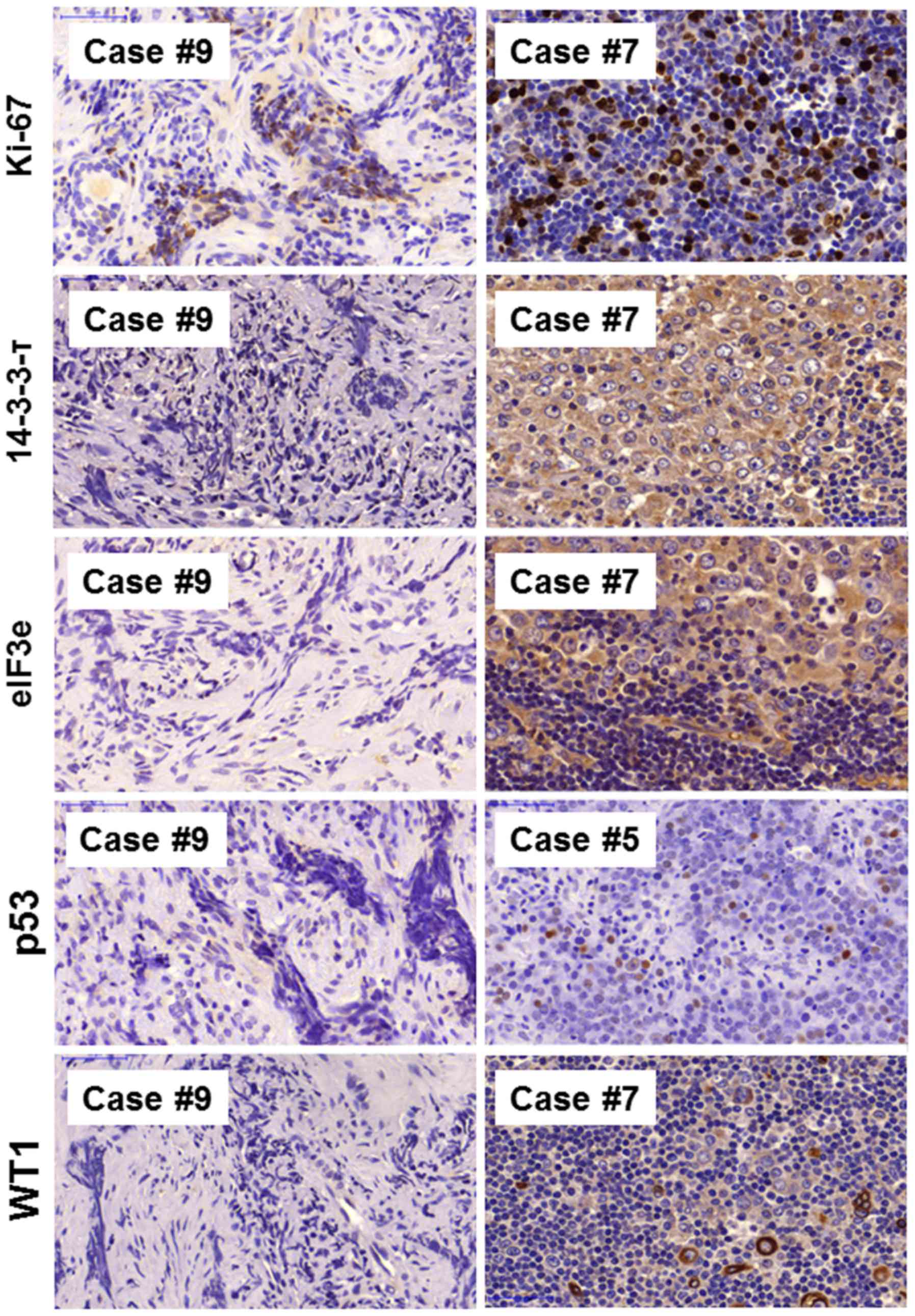
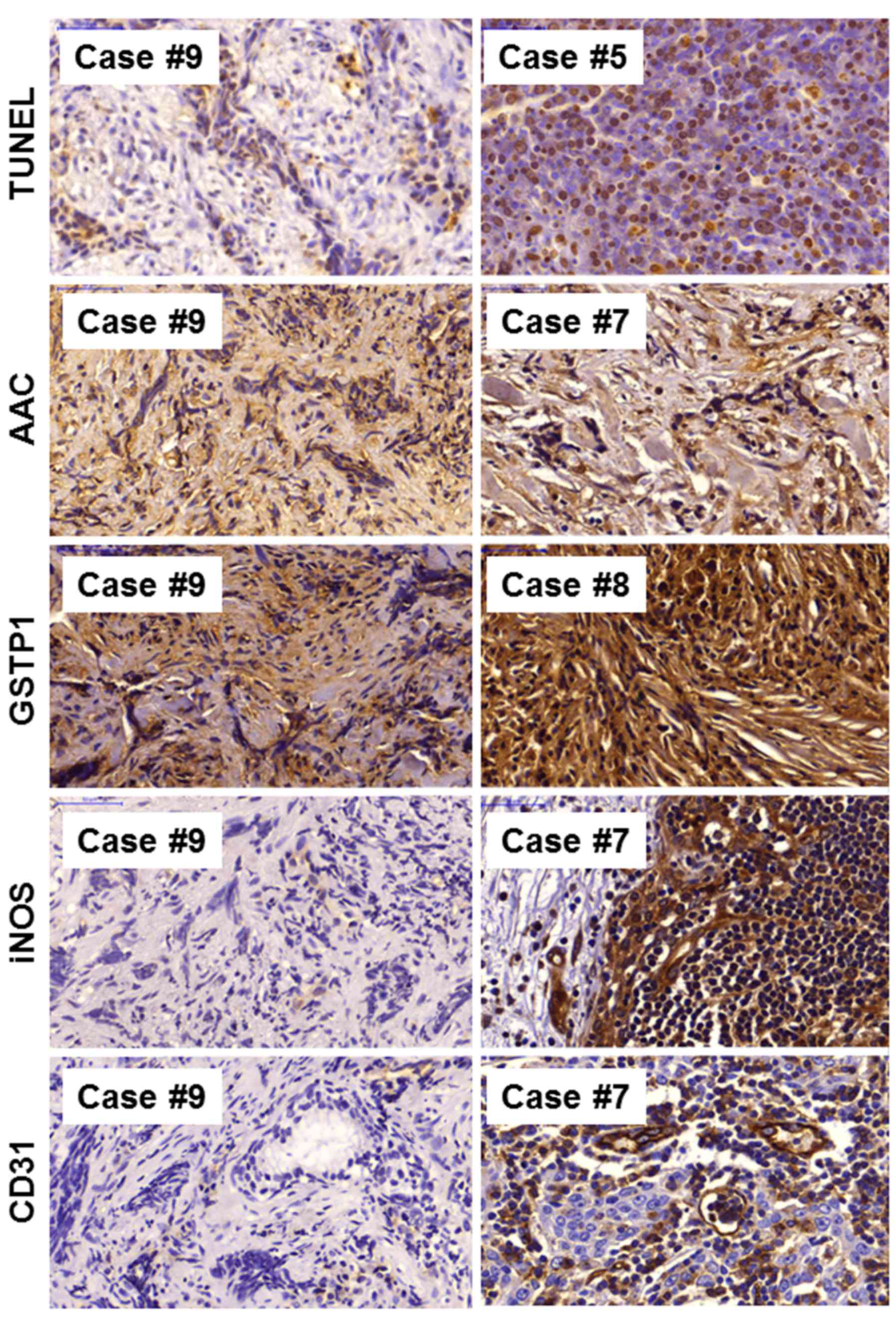
responsiveness to therapy. Representative immunostaining images of
NPC case 9 and selected tumors of the other patients with NPC are
depicted in Figs. 1Figure 2–3; staining for human papilloma virus
markers (E6, E7; Fig. 1),
immunological markers (CD4, CD8 and CD56; Fig. 1), proliferation markers (Ki-67;
14-3-3τ, eIF3e; Fig. 2), tumor
suppressors (p53, WT1; Fig. 2),
apoptosis markers (TUNEL assay, p53, AAC; Fig. 3) and other markers (GSTP1, CD31,
iNOS; Fig. 3) is shown.
A general observation in most tumor tissues was that
the expression pattern exhibited considerable heterogeneity; the
number of stained cells and the staining intensities varied between
different areas of the same tumor. For this reason, the
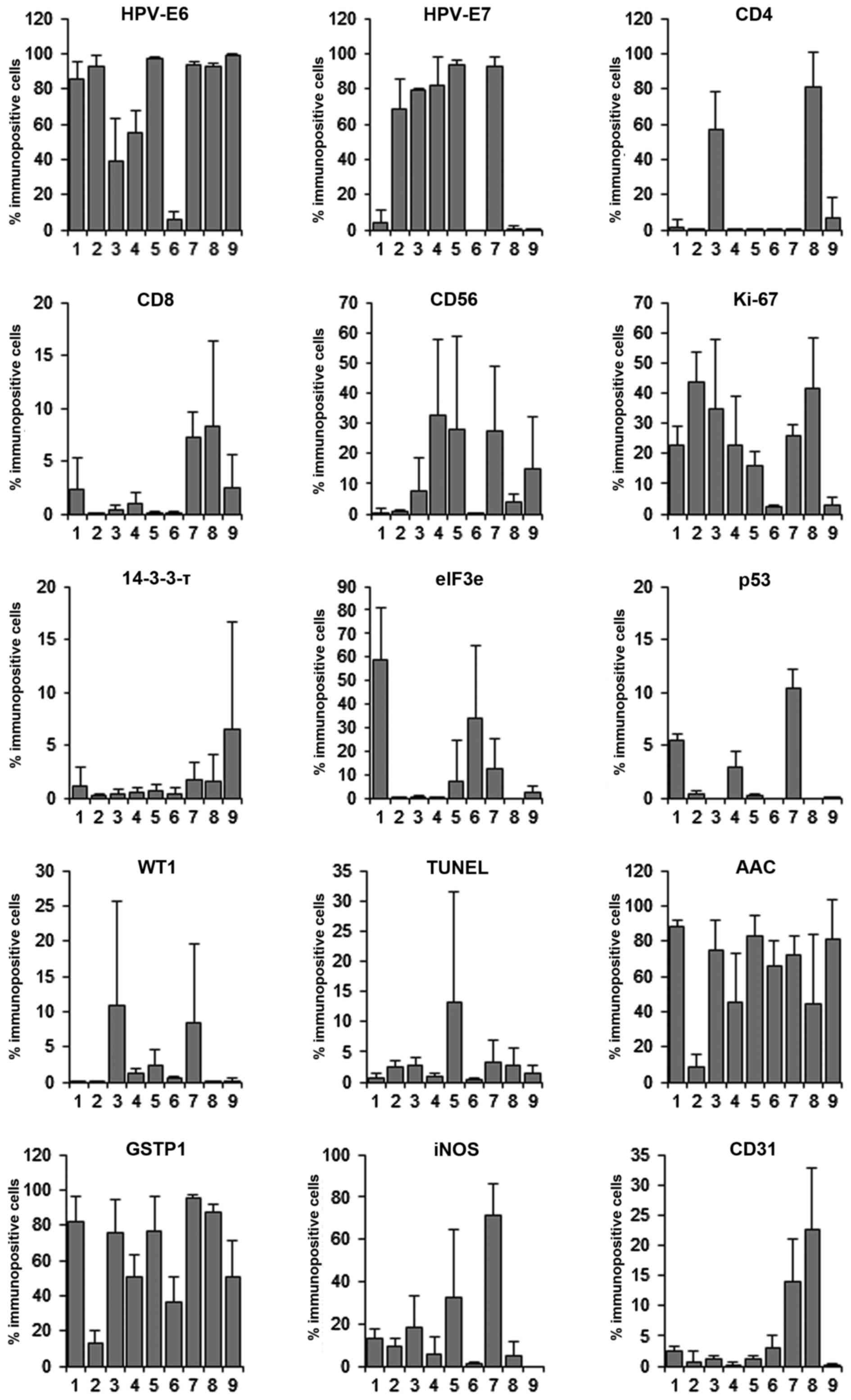
immunostaining for all antibody and all tumors was quantified. Six
representative areas were chosen from each tumor and the number of
positively stained cells was counted using a digital slide scanning
(Fig. 4).
Hierarchical cluster analysis
This technique is a widely used methodology to
extract relevant results from transcriptomic data sets. It uses a
distance/proximity-based approach to identify structures in large
data sets. In systems biology, cluster analyses are valuable to
define profiles of genes ('gene signatures'), whose expression is
linked to biological phenomena, i.e. histological subtypes of
tumors, resistance or sensitivity of tumors towards anticancer
treatments, survival chances of cancer patients, etc. Among the
numerous clustering methods (e.g. topological interaction models,
influence maps, physical regulatory maps, self-organizing maps,
principal component analysis, etc.), supervised, hierarchical and
aggregative techniques provide advantages for pharmacological
questions in cancer biology and pharmacology, because of their
flexibility, possibility to include biological knowledge with
different weighting, and detection of higher-order relationships
between clusters of profiles (22). Aggregative hierarchical clustering
is a frequently used method to investigate gene expression
signatures (23-25).
The quantified expression values obtained from
immunohistochemistry were subjected to hierarchical cluster
analysis in order to investigate, whether the protein expression
profile of patient 9, who died following standard therapy and
allogenic stem cell transplantation, could be differentiated from
the profiles of the eight other NPC samples from patients that
responded well to treatment.
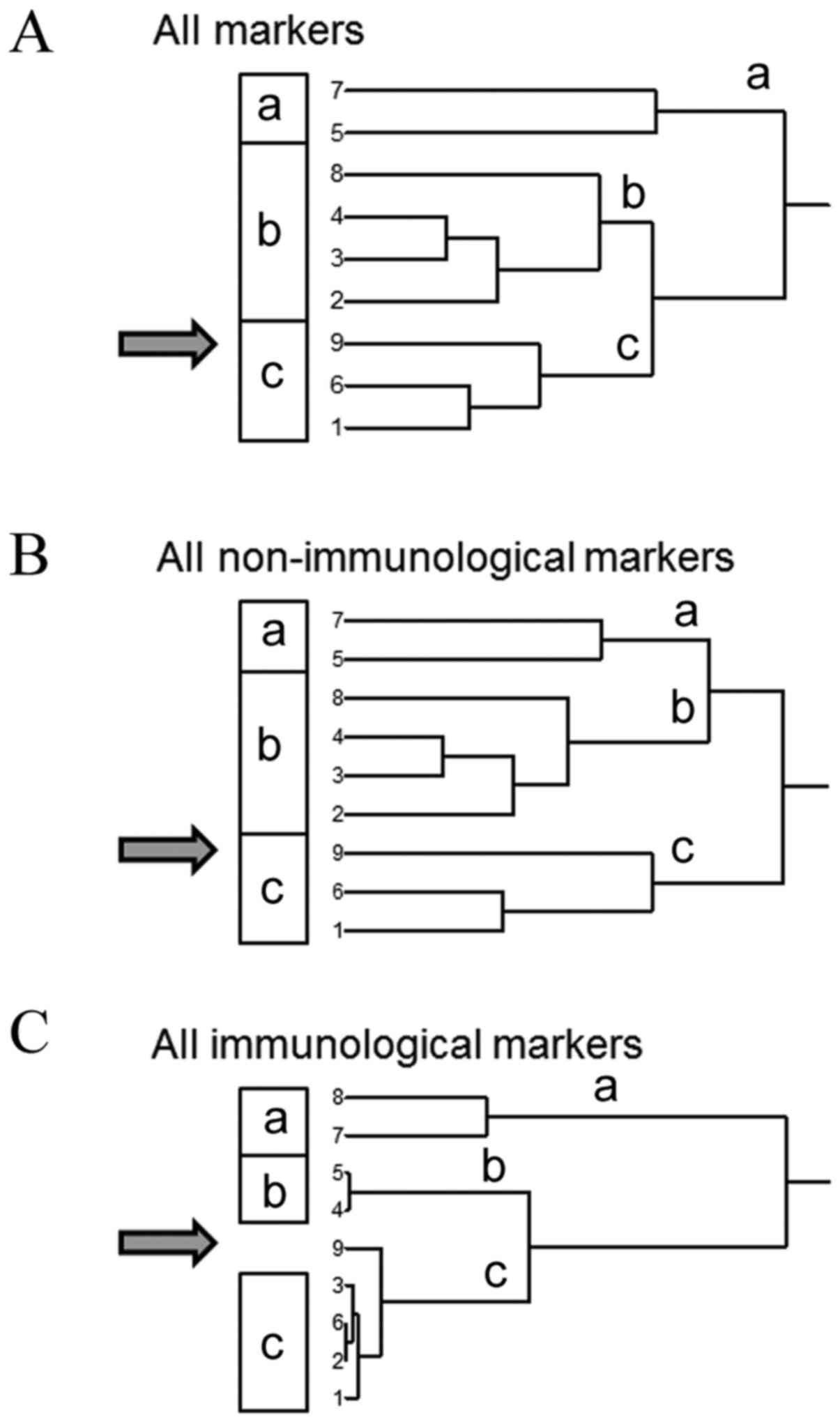
If the results of all immunostainings were subjected
to cluster analysis, a dendrogram with two clearly distinguishable
branches appeared (Fig. 5A).
However, case 9 did not appear as isolated branch separated from
all other tumors, indicating that the panel of immunohistochemical
parameters in its entirety was not able to identify this tumor.
Additionally, immunohistochemical staining markers
were divided into groups consisting of immunological parameters
[CD4 and CD8 as T-cell markers, and CD56 expression as marker of
natural killer (NK) cells] and the remaining biomarkers were
non-immunological markers. As shown in Fig. 5B and C, case 9 appeared isolated as
single tumor in the dendrogram of the three immunological markers.
The CD counts in case 9 were not the lowest of all samples, but the
intermediate to low expression was different from the expression
profile of these markers in the other NPC cases. Thus, cluster
analysis separated case 9 as being distinct from the others when
stained with immunological markers. The immunodeficient state of
the patient was also validated by H&E-staining of TILs, where
the lack of TILs in this patient became apparent. This was not
observed in the dendrogram of all other non-immunological markers,
indicating a potential specific role of the immune response in
patient 9, which may differ from the other patients with NPC.
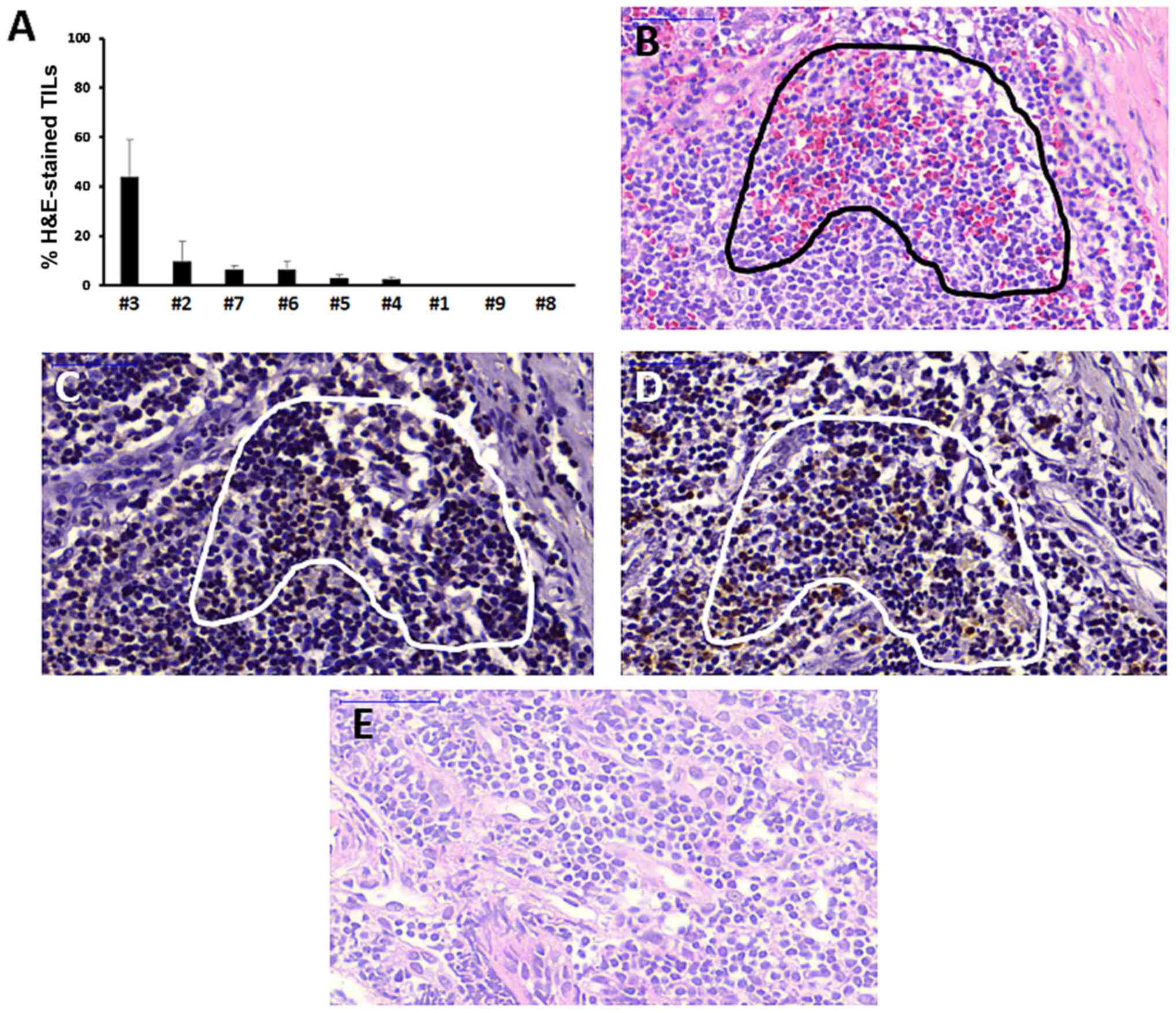
Assessment of TILs
To further validate that a deficient immune response
may have contributed to the treatment outcome of NPC case 9, the
percentage of TILs in tumor samples was evaluated using
H&E-staining. TILs were red-stained and were counted with the
same microscopic slide scanning procedure as used for
immunohistochemistry, with the exception that NuclearQuant software
was used for counting red-stained cells, rather than brown-stained
cells. The bar chart in Fig. 6A
shows that NPC case 9 and another tumor (case 8) did not contain
TILs at all, whereas the other NPC cases contained variable
fractions of TILs ranging from 0.06±0.05 to 43.7±15.3%. To further
validate the specificity of TIL-staining by H&E, serial NPC
sections stained with CD4 or CD8 antibodies were compared. As
revealed by staining of serial sections of case 3 tumor, the TIL
population identified by H&E-staining was also CD4- and
CD8-positive in immunohistochemistry (Fig. 6B–D).
Discussion
NPC is a complex disease associated with EBV
infection, environmental factors, and genetic aberrations (26). Although childhood NPC generally has
a good prognosis, certain patients do not benefit from chemo- and
radiotherapy (27). Although
β-IFN-containing therapy regimens improve outcomes (13,14),
cure rates of 100% are still achieved. The current study presents a
case of a 17 year-old patient that could not be cured and finally
died from the disease. This was surprising, as the patient was
diagnosed with a low-risk type IIb tumor (T2N0M0), which was
expected to have a good prognosis. Cases like these are still
enigmatic, as treatment outcome or prognosis cannot be predicted by
established clinical or pathoclinical parameters. Therefore, such
cases deserve further attention and investigation.
To identify biomarkers that can predict rare cases
of treatment non-responders, we immunohistochemical analyses were
performed using a broad panel of antibodies against proteins
involved in diverse cancer- and NPC-associated functions, including
HPV, immune response, proliferation, tumor suppression, apoptosis
and others. The quantified results were subjected to hierarchical
cluster analysis, which is valuable for microarray-based
mRNA-expression profiling, and also for protein expression data
(28-30). The combination of three markers of
immunological responses (CD4, CD8 and CD56) provided better
separation of case 9 than when all markers were included.
CD4 and CD8 are key for the adoptive T-cell immune
response. CD56 is a marker of NK cells, which are important for the
innate immune system. Both categories of immune responses are
involved in NPC. CD4+ and CD8+ T-cells (TILs)
infiltrate NPC (28,29), and CD56+ NK cells act
against EBV-infected cells (31).
Animal experiments demonstrated that CD8-positive T-cells enhance
immunity to EBV-associated malignancies; CD4+ and
CD8+ T-cells act together synergistically in the
antitumor response (32). In adult
patients with NPC, it has been reported they have reduced numbers
and function of cytotoxic T lymphocytes (CTLs) compared with normal
subjects or long-term NPC survivors (33). In those patients, the cytolytic
effects of NK cells were higher than that of healthy donors and NPC
survivors (33). CTLs and NK cells
compensate each other and are involved in immunity against NPC
(33).
Innate and adaptive immunity may exert antitumor
responses in some, but not all patients with tumors. The reasons
are not fully understood. CD4+ T-regulatory cells
(Tregs) of the Th1-type and follicular helper cells were reported
to be associated with favorable prognosis, whereas Th2-type Tregs
were demonstrated to inhibit the antitumor immune responses
(33,34). The presence of CD8+ TILs
is significantly correlated with response to therapy and survival
of patients (34). While TILs have
been investigated by H&E-staining in lung cancer, breast
cancer, laryngeal squamous cell carcinoma and melanoma (15,36-38),
to the best of our knowledge, there is no such report on NPC. The
present investigation demonstrated, for the first time, that TILs
can also be detected by H&E-staining in NPC. As NPC case 9
contained no TILs in the stromal parts of the tumor, we hypothesize
that the patient exhibited immunodeficiency.
Several immunotherapeutic strategies against NPC
have been suggested. EBV-latent membrane proteins (LMP1 and LMP2)
on the surface of NPC cells may act as targets for CD4+
and CD8+ T-cells (35).
Treatment of NPC using dendritic cells loaded with virus-associated
antigens (such as LMP2) elicited antitumor immune responses
(40,41). Following stimulation of EBV
antigenicity with gemcitabine and valproic acid, treatment of NPC
with valganciclovir increased weak antitumor immune responses
(36). Bispecific antibodies
simultaneously target tumor and T-cell antigens to bring T-cells in
close contact with NPC cells (37). From a previous study, a patient
with relapsed NPC received salvage adoptive immunotherapy with
EBV-specific CTLs, which were reactivated ex vivo from a
HLA-identical sibling; a marked increase of endogenous
tumor-infiltrating CD8+ T-cells was observed, indicating
the induction of antitumor effects in the patient (38).
The patient presented here was also refractory to
chemo-, radio- and β-INF therapy, and to allogenic stem cell
transplantation. It was hoped that IgG autoantibodies against
EBV-associated antigens of the EBV-infected, but otherwise healthy
brother could elicit a T-cell-mediated antiviral response
(graft-versus-tumor response) against the tumor of the sister, and
that this expected immune response may be transferred with the
transplantation. Unfortunately, a sustainable tumor remission was
not reached. The patient was severely immunodeficient. The addition
of β-INF to the NPC-2003-GPOH treatment protocol was ineffective,
although the beneficial effect of β-INF for the outcome of patients
with NPC has been convincingly demonstrated by several clinical
trials (13,14). Another indicator of the
immunodeficient state of the patient was the infection with several
other viruses and fungi in addition to EBV, including HSV, HHV-6,
VZV and Candida. Although immunotherapeutic approaches may
be attractive alternatives for non-responders to conventional
treatments, immunodeficiency represents a resistance mechanism to
immunostimulatory strategies, as indicated by the patient presented
in this study.
The rationale to investigate the other biomarkers
was their prognostic value for NPC reported in the literature.
Although these markers were not able to discriminate case 9 from
the other NPC cases, they are valuable markers to describe the
aggressiveness of larger cohorts of NPC and other tumor types.
AAC staining was strong in all tumors, whereas, p53
detection was minimal. AAC is a mitochondrial protein that
facilitates the exchange of ADP and ATP across the inner
mitochondrial membrane and has an essential role in cellular energy
metabolism. AAC is involved in metabolic adaptation during tumor
development (Warburg effect) (39)
causing apop-tosis resistance in cancer cells. This supports the
observation of low rates of apoptosis in the current study (TUNEL
assay). It is not surprising that wild-type p53 was not highly
detected, if the high AAC expression and low apoptosis rates are
taken into consideration. Wild-type p53 is a nuclear phosphoprotein
that triggers apoptosis or cell cycle arrest under cellular stress
conditions (40-42). TP53 gene mutations are the
most common genetic feature of tumors, identified in >50% of
tumors (49,50). Loss of p53 function is associated
with poor prognosis and drug resistance (51,52).
The HPV-E6 protein expression was high in almost all
NPC biopsies investigated. HPV-E6 expression results from the
integration HPV genomes into host chromosomes. HPV-E6 binds to p53
and promotes p53 degradation through the ubiquitin-proteasome
pathway (43) and the p53 levels
decrease (44). This observation
coincides with the findings in NPC biopsies in the current study.
The HPV-E7 protein was expressed in certain patients in the present
study. HPV-E7 targets retinoblastoma-associated protein, pRb, which
negatively regulates G1/S and G2/M cell cycle transitions (45).
Ki-67 is expressed during all cell cycle phases (G1,
S, G2 and M) (46). It is a marker
of cell proliferation and poor prognosis (47,48).
Ki-67 was detected in almost all of our NPC specimens. The clinical
significance of Ki-67 in patients with NPC has been reported
previously (49).
Eukaryotic translation initiation factors (eIFs) are
involved in the initiation of translation. The eIF3 complex binds
to 40S ribosomal subunits and promotes the binding of
methionyl-tRNA and mRNA to form the 40S initiation complex
(50). The eIF3a is involved in
cell cycle progression (51) and
has a role in tumorigenesis, re-sensitization to chemotherapeutics
and improved prognosis (52). In
the current study, the expression of eIF3e was detected in only 20%
of NPC cases.
Nitric oxide (NO) is an inflammatory mediator and
contributes to the inhibition of tumor suppressor functions and DNA
repair, activation of oncogenes, angiogenesis, and metastasis
(53-55). iNOS generates high amounts of NO
over prolonged periods (63,64). The overexpression of iNOS is
associated with high apoptosis rates, whereas low iNOS expression
is associated with increased incidences of local tumor recurrence
and metastasis following radiotherapy in patients with NPC
(56). Tumor-derived iNOS is a
pro-angiogenic factor and has been strongly implicated in
angiogenesis via upregulation of vascular endothelial growth factor
(57). In the present study, iNOS
and CD31 (blood vessel marker) were observed in ~1/3 of
patients.
In conclusion, childhood NPC has an excellent
prognosis (13,14). However, as not all children are
cured, research efforts have to be undertaken to improve current
therapy options. The current study presented a patient with
non-keratinizing NPC type IIb (T2N0M0), that was resistant to
chemo-, radio- and β-IFN therapy, and to allogenic stem cell
transplantation. Immunohistochemistry was performed to identify
factors distinguishing this patient from a panel of other patients
with NPC, who experienced complete remission following conventional
therapy. By hierarchical cluster analysis, detection of
immunological factors (CD4, CD8 and CD56) separated this patient
from the others. The tumor in this patient recurred following β-IFN
therapy, and EBV-directed autoantibodies of the HLA-identical
brother did not provoke a graft-versus-tumor response upon
allogenic stem cell transplantation. This and several concomitant
infections indicated severe immunodeficiency as factor contributing
to the fatal outcome. The analysis of more rare cases like this one
may help to further improve treatment success of refractory
childhood NPC in the future.
Acknowledgments
We thank Mrs. Doris Rohr (Department of
Pharmaceutical Biology, Institute of Pharmacy and Biochemistry,
Johannes Gutenberg University, Mainz, Germany) for technical
assistance with immunohistochemistry staining.
Abbreviations:
|
AAC
|
ATP-ADP translocase
|
|
CTL
|
cytotoxic T-lymphocyte
|
|
EBV
|
Epstein-Barr virus
|
|
eIF3
|
eukaryotic translation initiation
factor 3
|
|
GSTP1
|
glutathione S-transferase pi 1
|
|
H&E
|
hematoxylin and eosin
|
|
HHV-6
|
human herpes virus 6
|
|
HPV
|
human papilloma virus
|
|
HSV
|
herpes simplex virus
|
|
iNOS
|
inducible nitric oxide synthetase
|
|
INF
|
interferon
|
|
NK
|
natural killer
|
|
NPC
|
nasopharyngeal carcinoma
|
|
TIL
|
tumor-infiltrating lymphocyte
|
|
Tregs
|
T-regulatory cells
|
|
WHO
|
world health organization
|
|
VZV
|
Varicella zoster virus
|
|
WT1
|
Wilms tumor 1
|
Funding
The authors are grateful for the financial support
of the Förderkreis Hilfe für krebskranke Kinder Aachen. e.V.
Availability of data and materials
The data that support the findings of this study are
available from Department of Pediatric and Adolescent Medicine,
University Hospital Aachen (Aachen, Germany) and Department of
Paediatric Haematology/Oncology, Children's University Hospital
(Tübingen, Germany), but restrictions apply to the availability of
these data, which were used under patients' consent for the current
study, and so are not publicly available. Data are however
available from the Department of Pediatric and Adolescent Medicine,
University Hospital Aachen, and Department of Paediatric
Haematology/Oncology, Children's University Hospital.
Authors' contributions
TE, RM and RH designed the study; MEMS performed the
immunostaining and wrote the manuscript; RM and RH provide the
material and treated the patients; TE performed the statistical
analysis, wrote the paper, supervised the work and provided the
facilities for the study. All authors read the manuscript and
approved the final version.
Ethics approval and consent to
participate
Ethical approval (reference number: EK034/03) for
the use of tumor material for experimental purposes was obtained
from the Ethics Committee of the University of Aachen (Aachen,
Germany) on May 19th, 2003, and local ethical approval was obtained
from the participating centers in Germany (14). Written informed consent for
experimental work was obtained from all patients.
Consent for publication
Written informed consent for publication was
obtained from all patients.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Parkin DM and Muir CS: Cancer Incidence in
Five Continents. Comparability and quality of data. IARC Sci Publ.
120:45–173. 1992.
|
|
2
|
Chong VH, Telisinghe PU, Lim E, Abdullah
MS, Idris F and Chong CF: Declining incidence of nasopharyngeal
carcinoma in brunei darussalam: A three decade study (1986-2014).
Asian Pac J Cancer Prev. 16:7097–7101. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar
|
|
4
|
Healy GB: Malignant tumors of the head and
neck in children: Diagnosis and treatment. Otolaryngol Clin North
Am. 13:483–488. 1980.PubMed/NCBI
|
|
5
|
Cunningham MJ, Myers EN and Bluestone CD:
Malignant tumors of the head and neck in children: A twenty-year
review. Int J Pediatr Otorhinolaryngol. 13:279–292. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang Y, Zhang Y and Ma S: Racial
differences in nasopharyngeal carcinoma in the United States.
Cancer Epidemiol. 37:793–802. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chan AT, Teo PM and Johnson PJ:
Nasopharyngeal carcinoma. Ann Oncol. 13:1007–1015. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Society AC: Cancer Facts & Figures
2015 Nasopharyngeal Cancer. American Cancer Society; Atlanta, GA:
pp. 1–44. 2015
|
|
9
|
Singhi AD, Califano J and Westra WH:
High-risk human papillomavirus in nasopharyngeal carcinoma. Head
Neck. 34:213–218. 2012. View Article : Google Scholar
|
|
10
|
Robinson M, Suh YE, Paleri V, Devlin D,
Ayaz B, Pertl L and Thavaraj S: Oncogenic human
papillomavirus-associated nasopharyngeal carcinoma: An
observational study of correlation with ethnicity, histological
subtype and outcome in a UK population. Infect Agent Cancer.
8:302013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lo EJ, Bell D, Woo J, Li G, Hanna EY,
El-Naggar AK and Sturgis EM: Human papillomavirus & WHO type I
nasopharyngeal carcinoma. Laryngoscope. 120(Suppl 4): S1852010.
View Article : Google Scholar
|
|
12
|
Laantri N, Attaleb M, Kandil M, Naji F,
Mouttaki T, Dardari R, Belghmi K, Benchakroun N, El Mzibri M and
Khyatti M: Human papillomavirus detection in moroccan patients with
nasopharyngeal carcinoma. Infect Agent Cancer. 6:32011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Giannoudis A, Ergazaki M, Segas J,
Giotakis J, Adamopoulos G, Gorgoulis V and Spandidos DA: Detection
of Epstein-Barr virus and human papillomavirus in nasopharyngeal
carcinoma by the polymerase chain reaction technique. Cancer Lett.
89:177–181. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Buehrlen M, Zwaan CM, Granzen B, Lassay L,
Deutz P, Vorwerk P, Staatz G, Gademann G, Christiansen H,
Oldenburger F, et al: Multimodal treatment, including interferon
beta, of nasopharyngeal carcinoma in children and young adults:
Preliminary results from the prospective, multicenter study
NPC-2003-GPOH/DCOG. Cancer. 118:4892–4900. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kontny U, Franzen S, Behrends U, Bührlen
M, Christiansen H, Delecluse H, Eble M, Feuchtinger T, Gademann G,
Granzen B, et al: Diagnosis and treatment of nasopharyngeal
carcinoma in children and adolescents - Recommendations of the
GPOH-NPC Study Group. Klin Padiatr. 228:105–112. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fischer AH, Jacobson KA, Rose J and Zeller
R: Hematoxylin and eosin staining of tissue and cell sections. CSH
Protoc. 2008:pdb.prot4986. 2008.
|
|
17
|
Salgado R, Denkert C, Demaria S, Sirtaine
N, Klauschen F, Pruneri G, Wienert S, Van den Eynden G, Baehner FL,
Penault-Llorca F, et al International TILs Working Group 2014: The
evaluation of tumor-infiltrating lymphocytes (TILs) in breast
cancer: Recommendations by an International TILs Working Group
2014. Ann Oncol. 26:259–271. 2015. View Article : Google Scholar
|
|
18
|
Kadioglu O and Efferth T: Pharmacogenomic
characterization of cytotoxic compounds from Salvia officinalis in
cancer cells. J Nat Prod. 78:762–775. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schielke HJ, Fishman JL, Osatuke K and
Stiles WB: Creative consensus on interpretations of qualitative
data: The Ward method. Psychother Res. 19:558–565. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Efferth T, Fabry U and Osieka R: Apoptosis
and resistance to daunorubicin in human leukemic cells. Leukemia.
11:1180–1186. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Scherf U, Ross DT, Waltham M, Smith LH,
Lee JK, Tanabe L, Kohn KW, Reinhold WC, Myers TG, Andrews DT, et
al: A gene expression database for the molecular pharmacology of
cancer. Nat Genet. 24:236–244. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mocellin S, Provenzano M, Rossi CR, Pilati
P, Nitti D and Lise M: DNA array-based gene profiling: From
surgical specimen to the molecular portrait of cancer. Ann Surg.
241:16–26. 2005.
|
|
23
|
Eisen MB, Spellman PT, Brown PO and
Botstein D: Cluster analysis and display of genome-wide expression
patterns. Proc Natl Acad Sci USA. 95:14863–14868. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Perou CM, Sørlie T, Eisen MB, van de Rijn
M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA,
et al: Molecular portraits of human breast tumours. Nature.
406:747–752. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ross DT, Scherf U, Eisen MB, Perou CM,
Rees C, Spellman P, Iyer V, Jeffrey SS, Van de Rijn M, Waltham M,
et al: Systematic variation in gene expression patterns in human
cancer cell lines. Nat Genet. 24:227–235. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang J, Zhou M, Zhao R, Peng S, Luo Z, Li
X, Cao L, Tang K, Ma J, Xiong W, et al: Identification of candidate
biomarkers for the early detection of nasopharyngeal carcinoma by
quantitative proteomic analysis. J Proteomics. 109:162–175. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cheuk DK, Billups CA, Martin MG, Roland
CR, Ribeiro RC, Krasin MJ and Rodriguez-Galindo C: Prognostic
factors and long-term outcomes of childhood nasopharyngeal
carcinoma. Cancer. 117:197–206. 2011. View Article : Google Scholar
|
|
28
|
Volm M, Koomägi R, Mattern J and Efferth
T: Expression profile of genes in non-small cell lung carcinomas
from long-term surviving patients. Clin Cancer Res. 8:1843–1848.
2002.PubMed/NCBI
|
|
29
|
Volm M, Koomägi R, Mattern J and Efferth
T: Protein expression profiles indicative for drug resistance of
non-small cell lung cancer. Br J Cancer. 87:251–257. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Volm M, Koomägi R, Mattern J and Efferth
T: Protein expression profile of primary human squamous cell lung
carcinomas indicative of the incidence of metastases. Clin Exp
Metastasis. 19:385–390. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Trempat P, Tabiasco J, Andre P, Faumont N,
Meggetto F, Delsol G, Gascoyne RD, Fournie JJ, Vivier E and
Brousset P: Evidence for early infection of nonneoplastic natural
killer cells by Epstein-Barr virus. J Virol. 76:11139–11142. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yuling H, Ruijing X, Li L, Xiang J, Rui Z,
Yujuan W, Lijun Z, Chunxian D, Xinti T, Wei X, et al: EBV-induced
human CD8+ NKT cells suppress tumorigenesis by
EBV-associated malignancies. Cancer Res. 69:7935–7944. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zheng Y, Cao KY, Ng SP, Chua DT, Sham JS,
Kwong DL, Ng MH, Lu L and Zheng BJ: Complementary activation of
peripheral natural killer cell immunity in nasopharyngeal
carcinoma. Cancer Sci. 97:912–919. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mahmoud SM, Paish EC, Powe DG, Macmillan
RD, Grainge MJ, Lee AH, Ellis IO and Green AR: Tumor-infiltrating
CD8+ lymphocytes predict clinical outcome in breast
cancer. J Clin Oncol. 29:1949–1955. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Haigh TA, Lin X, Jia H, Hui EP, Chan AT,
Rickinson AB and Taylor GS: EBV latent membrane proteins (LMPs) 1
and 2 as immunotherapeutic targets: LMP-specific CD4+
cytotoxic T cell recognition of EBV-transformed B cell lines. J
Immunol. 180:1643–1654. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Stoker SD, Novalić Z, Wildeman MA, Huitema
AD, Verkuijlen SA, Juwana H, Greijer AE, Tan IB, Middeldorp JM and
de Boer JP: Epstein-Barr virus-targeted therapy in nasopharyngeal
carcinoma. J Cancer Res Clin Oncol. 141:1845–1857. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Taylor GS, Haigh TA, Gudgeon NH, Phelps
RJ, Lee SP, Steven NM and Rickinson AB: Dual stimulation of
Epstein-Barr Virus (EBV)-specific CD4+- and
CD8+-T-cell responses by a chimeric antigen construct:
Potential therapeutic vaccine for EBV-positive nasopharyngeal
carcinoma. J Virol. 78:768–778. 2004. View Article : Google Scholar :
|
|
38
|
Comoli P, De Palma R, Siena S, Nocera A,
Basso S, Del Galdo F, Schiavo R, Carminati O, Tagliamacco A, Abbate
GF, et al: Adoptive transfer of allogeneic Epstein-Barr virus
(EBV)-specific cytotoxic T cells with in vitro antitumor activity
boosts LMP2-specific immune response in a patient with EBV-related
nasopharyngeal carcinoma. Ann Oncol. 15:113–117. 2004. View Article : Google Scholar
|
|
39
|
Chevrollier A, Loiseau D, Reynier P and
Stepien G: Adenine nucleotide translocase 2 is a key mitochondrial
protein in cancer metabolism. Biochim Biophys Acta. 1807:562–567.
2011. View Article : Google Scholar
|
|
40
|
Wang Z and Sun Y: Targeting p53 for novel
anticancer therapy. Transl Oncol. 3:1–12. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Vogelstein B, Lane D and Levine AJ:
Surfing the p53 network. Nature. 408:307–310. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Giaccia AJ and Kastan MB: The complexity
of p53 modulation: Emerging patterns from divergent signals. Genes
Dev. 12:2973–2983. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Scheffner M, Werness BA, Huibregtse JM,
Levine AJ and Howley PM: The E6 oncoprotein encoded by human
papillomavirus types 16 and 18 promotes the degradation of p53.
Cell. 63:1129–1136. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Matlashewski G, Banks L, Wu-Liao J, Spence
P, Pim D and Crawford L: The expression of human papillomavirus
type 18 E6 protein in bacteria and the production of anti-E6
antibodies. J Gen Virol. 67:1909–1916. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yim EK and Park JS: The role of HPV E6 and
E7 oncoproteins in HPV-associated cervical carcinogenesis. Cancer
Res Treat. 37:319–324. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kim KH and Sederstrom JM: Assaying cell
cycle status using flow cytometry. Curr Protoc Mol Biol.
111:28.6.1–11. 2015. View Article : Google Scholar
|
|
47
|
Urruticoechea A, Smith IE and Dowsett M:
Proliferation marker Ki-67 in early breast cancer. J Clin Oncol.
23:7212–7220. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Liu P, Sun YL, Du J, Hou XS and Meng H:
CD105/Ki67 coexpression correlates with tumor progression and poor
prognosis in epithelial ovarian cancer. Int J Gynecol Cancer.
22:586–592. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Masuda M, Shinokuma A, Hirakawa N,
Nakashima T and Komiyama S: Expression of bcl-2-, p53, and Ki-67
and outcome of patients with primary nasopharyngeal carcinomas
following DNA-damaging treatment. Head Neck. 20:640–644. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hershey JW: The role of eIF3 and its
individual subunits in cancer. Biochim Biophys Acta. 1849:792–800.
2015. View Article : Google Scholar
|
|
51
|
Dong Z, Liu Z, Cui P, Pincheira R, Yang Y,
Liu J and Zhang JT: Role of eIF3a in regulating cell cycle
progression. Exp Cell Res. 315:1889–1894. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yin JY, Shen J, Dong ZZ, Huang Q, Zhong
MZ, Feng DY, Zhou HH, Zhang JT and Liu ZQ: Effect of eIF3a on
response of lung cancer patients to platinum-based chemotherapy by
regulating DNA repair. Clin Cancer Res. 17:4600–4609. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lala PK and Orucevic A: Role of nitric
oxide in tumor progression: Lessons from experimental tumors.
Cancer Metastasis Rev. 17:91–106. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wink DA, Ridnour LA, Hussain SP and Harris
CC: The reemergence of nitric oxide and cancer. Nitric Oxide.
19:65–67. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Beckman JS, Beckman TW, Chen J, Marshall
PA and Freeman BA: Apparent hydroxyl radical production by
peroxynitrite: Implications for endothelial injury from nitric
oxide and superoxide. Proc Natl Acad Sci USA. 87:1620–1624. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Jayasurya A, Dheen ST, Yap WM, Tan NG, Ng
YK and Bay BH: Inducible nitric oxide synthase and bcl-2 expression
in naso-pharyngeal cancer: Correlation with outcome of patients
after radiotherapy. Int J Radiat Oncol Biol Phys. 56:837–845. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kane AJ, Barker JE, Mitchell GM, Theile
DR, Romero R, Messina A, Wagh M, Fraulin FO, Morrison WA and
Stewart AG: Inducible nitric oxide synthase (iNOS) activity
promotes ischaemic skin flap survival. Br J Pharmacol.
132:1631–1638. 2001. View Article : Google Scholar : PubMed/NCBI
|