Introduction
Cervical carcinoma has a high incidence worldwide,
with ~500,000 women developing the disease each year. The majority
of cases occur in less developed countries where no effective
screening systems are available (1). Although surgery and chemoradiotherapy
are effective treatment for 80-95% of patients with early stage
cancer, recurrence and metastasis remain a major cause of
cancer-associated mortality (2).
The incidence of cervical cancer is increasing, thus resulting in a
need to develop novel effective drugs to treat this disease
(3).
Traditional Chinese medicine has been used to treat
a variety of illnesses for thousands of years. Rhubarb has been
widely used to treat intestinal dysmotility (4) and acute renal failure (5), and has been reported to inhibit
inflammatory and oxidative stresses (6) as well as tumor cell proliferation
(7). Rhein, a bioactive component
of rhubarb, has been demonstrated to have a number of antitumor
effects, including inhibiting ERK phosphorylation, inducing G0/G1
arrest and triggering apoptosis (8-11).
Rhein lysinate (RHL) is a novel compound obtained by modifying
Rhein. The carboxyl group of Rhein reacts with amino-group of
lysine to form RHL salt and its structure was presented in our
previously published study (12).
Compared with Rhein, RHL is easy to dissolve in water; however,
does not affect the function of Rhein. RHL has been extensively
studied for its anticancer properties (13-15).
Our group previously demonstrated that RHL inhibited HeLa cell
proliferation via a specific mechanism (14). In order to clarify the mechanism of
RHL in HeLa cells, its effects on vacuolar degeneration and
apoptosis were investigated.
Materials and methods
Materials
Rhein was purchased from Nanjing Qingze Medical
Technology Development Co., Ltd. (Nanjing, Jiangsu, China). Lysine
was purchased from Beijing Solarbio Science & Technology Co.,
Ltd. (Beijing, China). Rhein lysinate (RHL) was prepared in the MOH
Key Laboratory of Geriatrics, Beijing Hospital (Beijing, China)
with a purity of 98% detected by colorimetric method. Vitamin C,
2,7-dichlorfluorescein-diacetate (DCFH-DA),
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide
(MTT), N-acetyl cysteine (NAC), diphenylene iodonium (DPI),
Rhodamine 123 and Hoechst 33342 were purchased from Merck KGaA
(Sigma-Aldrich, Darmstadt, Germany). An Annexin V/propidium iodide
(PI) kit was obtained from Thermo Fisher Scientific, Inc. (Waltham,
MA, USA). Primary antibodies directed against B-cell lymphoma 2
(Bcl-2; cat. no. 15071; 1:1,000), Bcl-2-associated X protein (Bax;
cat. no. 2774; 1:1,000), caspase-3 (cat. no. 9662; 1:1,000) and
cleaved-caspase-3 (cat. no. 9661; 1:1,000) were purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA). The primary antibody
against β-actin (cat. no. sc-70319; 1:2,000) and rabbit (cat. no.
sc-2004; 1:5,000) or mouse (cat. no. sc-2005; 1:5,000) IgG
secondary antibodies were purchased from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA). Prestained Protein marker p7708V was
purchased from New England BioLabs, Inc. (Ipswich, MA, USA).
Western Blot Luminol reagent and polyvinylidene difluoride (PVDF)
membranes were purchased from EMD Millipore (Billerica, MA,
USA).
Determination of cell proliferation
Cell proliferation was examined using an MTT assay,
according to the manufacturer’s protocol. HeLa cells were obtained
from the American Type Culture Collection (Manassas, VA, USA) and
were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco,
Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine
serum (FBS), 100 µg/ml of streptomycin and 100 U/ml of
penicillin at 37°C with 5% CO2. HeLa cells were plated
onto 96-well plates (4×103 cells/well) for 24 h.
Subsequently, the cells were treated with RHL (0, 20, 40, 80, 160
or 320 µmol/l) for 48 h. All assays were performed in
triplicate.
Live cell imaging
HeLa cells treated with RHL 160 µmol/l were
used for live cell imaging for 24 h by placing the culture dishes
onto a sample stage within a heated chamber (37°C). Live imaging
was performed using an ImageXpress Micro XLS Widefield High-Content
Analysis system (Molecular Devices, LLC, Sunnyvale, CA, USA).
Images were captured at 40×` with 10 msec exposure times in 5 min
intervals using an MRm CCD camera (Carl Zeiss AG, Oberkochen,
Germany), and different Z sections were projected using the
softWoRx® Suite (version 2.0; Applied Precision, Inc.,
Mississauga, ON, Canada).
Transmission electron microscopy
To clarify whether the development of vesicles in
RHL-treated HeLa cells was due to autophagy, ultrastructural
analysis was performed as previously described (16). Briefly, HeLa cells were treated
with 160 µmol/l RHL for 24 h, harvested and washed twice
with PBS. After being fixed with ice-cold glutaraldehyde (3% in 0.1
M cacodylate buffer, pH 7.4) at 4°C for 30 min, the samples were
post-fixed with 1% OsO4 in the same buffer at 4°C for 1
h and subjected to electron microscopic analysis. Representative
areas were chosen for ultra thin sectioning and were observed at
500kX with a 200CX transmission electron microscope (JEOL Ltd.,
Tokyo, Japan).
Intracellular reactive oxygen species
(ROS) generation
The generation of intracellular ROS was assessed
using DCFH-DA staining. HeLa cells were cultured in 6-well plates
for determination of intracellular ROS generation. The cells were
treated with RHL for 24 h and then stained with 10 µmol/l
DCFH-DA at 37°C for 30 min according to the manufacturer’s
protocol. Following staining, cells were washed with PBS. DCF
fluorescence data were acquired at x20 or x10 using an inverted
fluorescence microscope (Olympus Corporation, Tokyo, Japan).
Fluorescence intensity was detected by ImageJ software [version
1.0; National Institutes of Health (NIH), Bethesda, MD, USA].
Detection of the mitochondrial membrane
potential (MMP) by Rhodamine 123 stain
HeLa cells were seeded in 6-well plates overnight
and subsequently treated with 80 or 160 µmol/l RHL at 24-h
intervals. Cells were then fixed with 4% paraformaldehyde at room
temperature for 20 min. PBS was used as a negative control.
Rhodamine 123 was added to cells at a final concentration of 5
µg/ml and incubated at 37°C for 30 min to stain the
mitochondria. Images were captured using an inverted fluorescence
microscope (magnification, x20). Fluorescence intensity was
detected by ImageJ software (version 1.0; NIH).
Mitochondrial isolation and proteomics
assay
Mitochondria were isolated using a Mitochondrial
Extraction kit (cat. no. SM0020) according to the manufacturer’s
protocol (Beijing Solarbio Science & Technology Co., Ltd.).
Protein was extracted from the mitochondria and a proteomics assay
was performed using label-free quantitative proteomics technology
(Fanxing Boao Ltd., Beijing, China). The procedure included protein
quantification, protein reduction alkylation, trypsin hydrolysis,
liquid chromatography-tandem mass spectrometry (LC-MS/MS) assay and
data analysis as previously described (17).
Hoechst staining fluorescence microscopy
and Annexin V/PI flow cytometry
HeLa cells were seeded in a 25 cm2 flask
over- night. Cells were treated with 160 µmol/l RHL for 24,
48 or 72 h and fixed with 4% paraformaldehyde at room temperature
for 20 min. PBS was used as a negative control. Hoechst was added
to cells at a final concentration of 5 µg/ml and incubated
at 37°C for 30 min for nuclear staining. Images were captured using
an inverted fluorescence microscope (magnification, x20). To assess
cell apoptosis, treated cells were collected and stained with
Annexin V and PI at room temperature for 30 min and subjected to
flow cytometry with FACSCalibur and Cell Quest software (version
5.1; BD Biosciences, Franklin Lakes, NJ, USA).
Western blotting
HeLa cells were grown to 90% confluence in 25
cm2 flasks in DMEM supplemented with 10% FBS, 100
µg/ml of streptomycin and 100 U/ml of penicillin. Cells were
subsequently incubated for 12 h in DMEM supplemented with 0.1% FBS.
The cells were treated with 160 µmol/l RHL for 0, 24, 48 and
72 h. Cells were scraped, and pellets were lysed using RIPA lysis
buffer containing 150 mmol/l NaCl, 1% NP-40, 0.5% deoxycholate,
0.1% SDS and 50 mmol/l Tris-HCl (pH 7.4) (Roche Diagnostics, Basel,
Switzerland) and lysed on ice for 15 min. Following centrifugation
at 22,000 × g for 4 min at 4°C, proteins in the supernatant were
quantified using a protein assay (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Samples were stored at −70°C until analysis.
Samples were mixed with an equal amount of Laemmli sample buffer
(Bio-Rad Laboratories, Inc.) and boiled at 100°C for 5 min.
Isolated proteins (40 µg) were resolved on 10% SDS-PAGE and
transferred to PVDF membranes using a semidry transfer apparatus
(both from Bio-Rad Laboratories, Inc.). The membranes were
incubated in blocking buffer [5% non-fat milk dissolved in TBS with
0.1% Tween-20 (TBST)] for 1 h at room temperature, followed by
overnight incubation with primary antibodies at 4°C. Subsequently,
the membranes were incubated with the secondary antibody at room
temperature for 2 h following washing three times with TBST.
β-actin was used as the loading control. Protein bands were
developed using Immobilon Western Chemiluminescent HRP substrate
(cat. no. WBKLS0100; EMD Millipore) and images were captured using
an enhanced ChemiImager 5500 chemiluminescence system
(ProteinSimple, San Jose, CA, USA). ImageJ software (version 1.0;
NIH) was used to quantify the optical density for treated samples,
which were normalized to the β-actin internal controls.
Statistical analysis
Data are expressed as the mean ± standard deviation.
The statistical analysis was conducted with one-way analysis of
variance with the least significant difference post hoc test using
IBM SPSS Statistics 19.0 software (IBM Corp., Armonk, NY, USA) and
P<0.05 was considered to indicate a statistically significant
difference.
Results
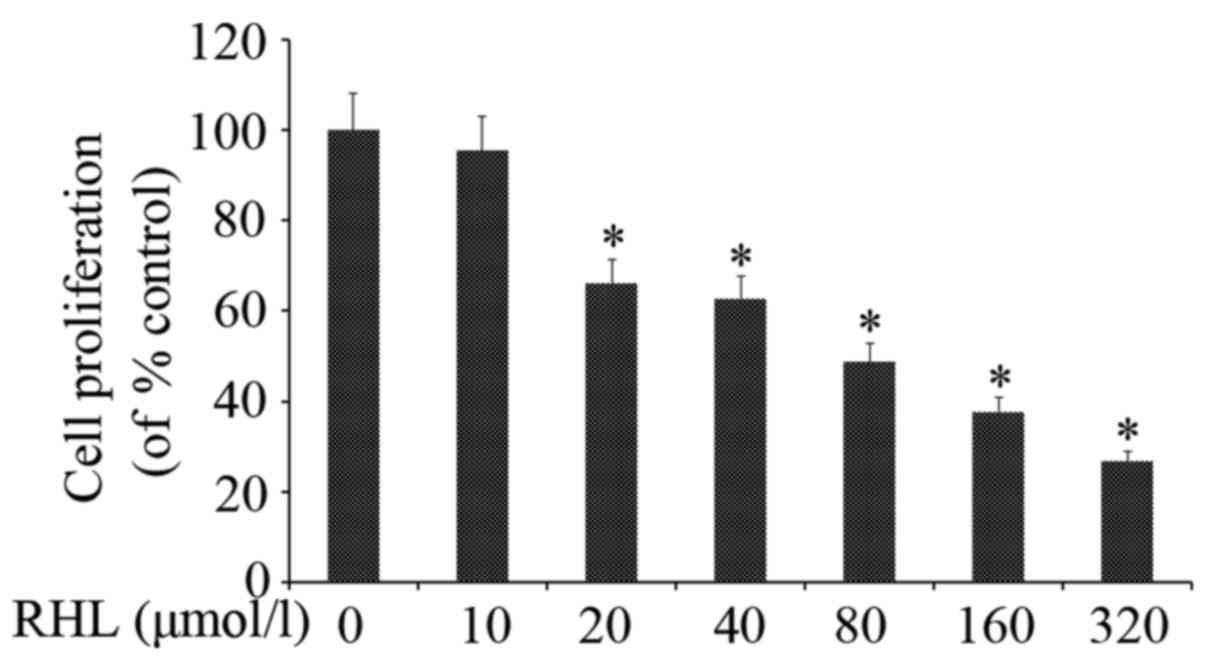
RHL inhibits HeLa cell proliferation
RHL inhibited HeLa cell proliferation in a
dose-dependent manner (Fig. 1). In
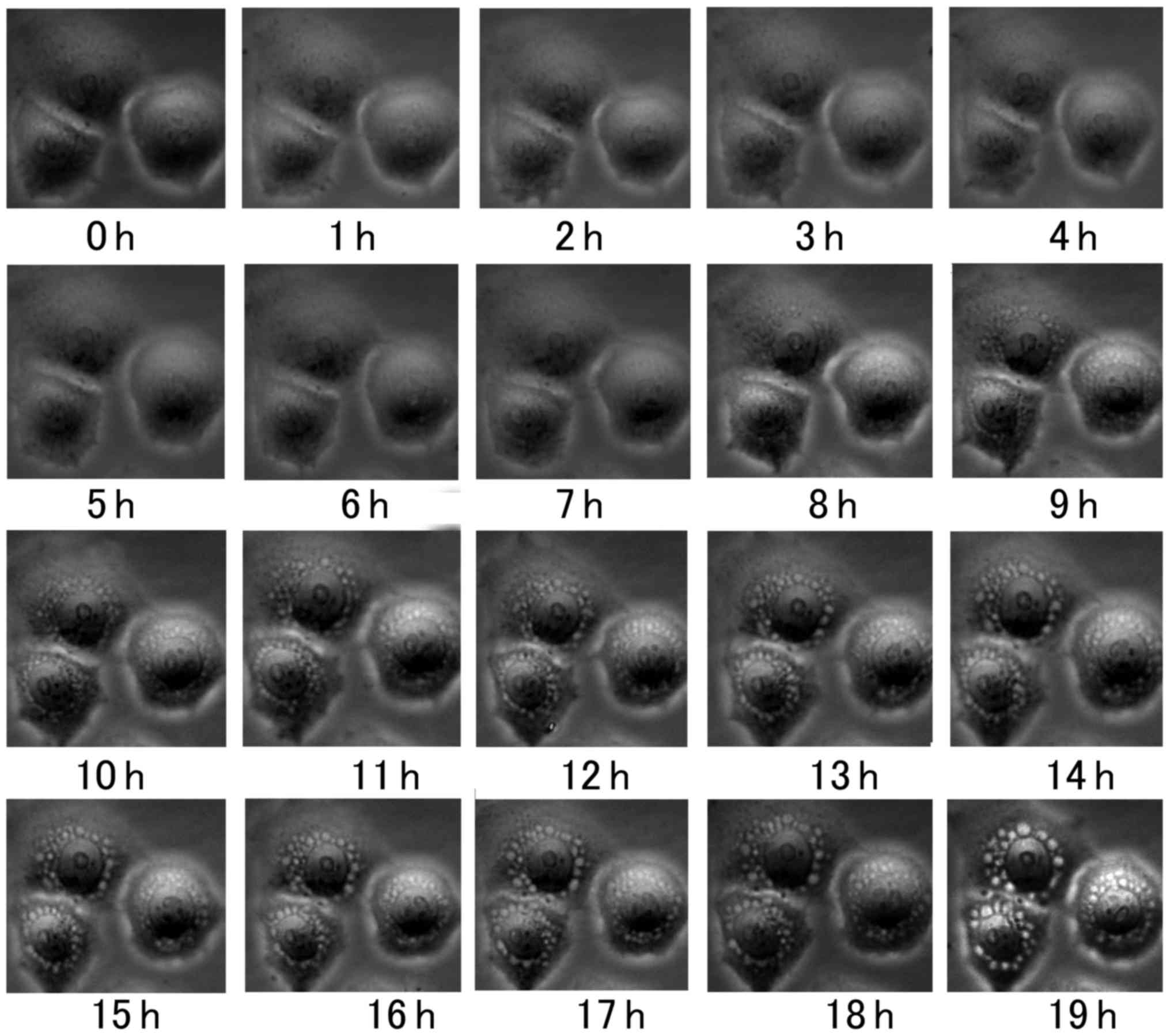
the present study, when HeLa cells were treated with 160
µmol/l RHL for 6 h, vacuoles were observed in the cytoplasm
near the nucleus. The formation of vacuoles was recorded using a
live cell-imaging system and increased in size over time (Fig. 2).
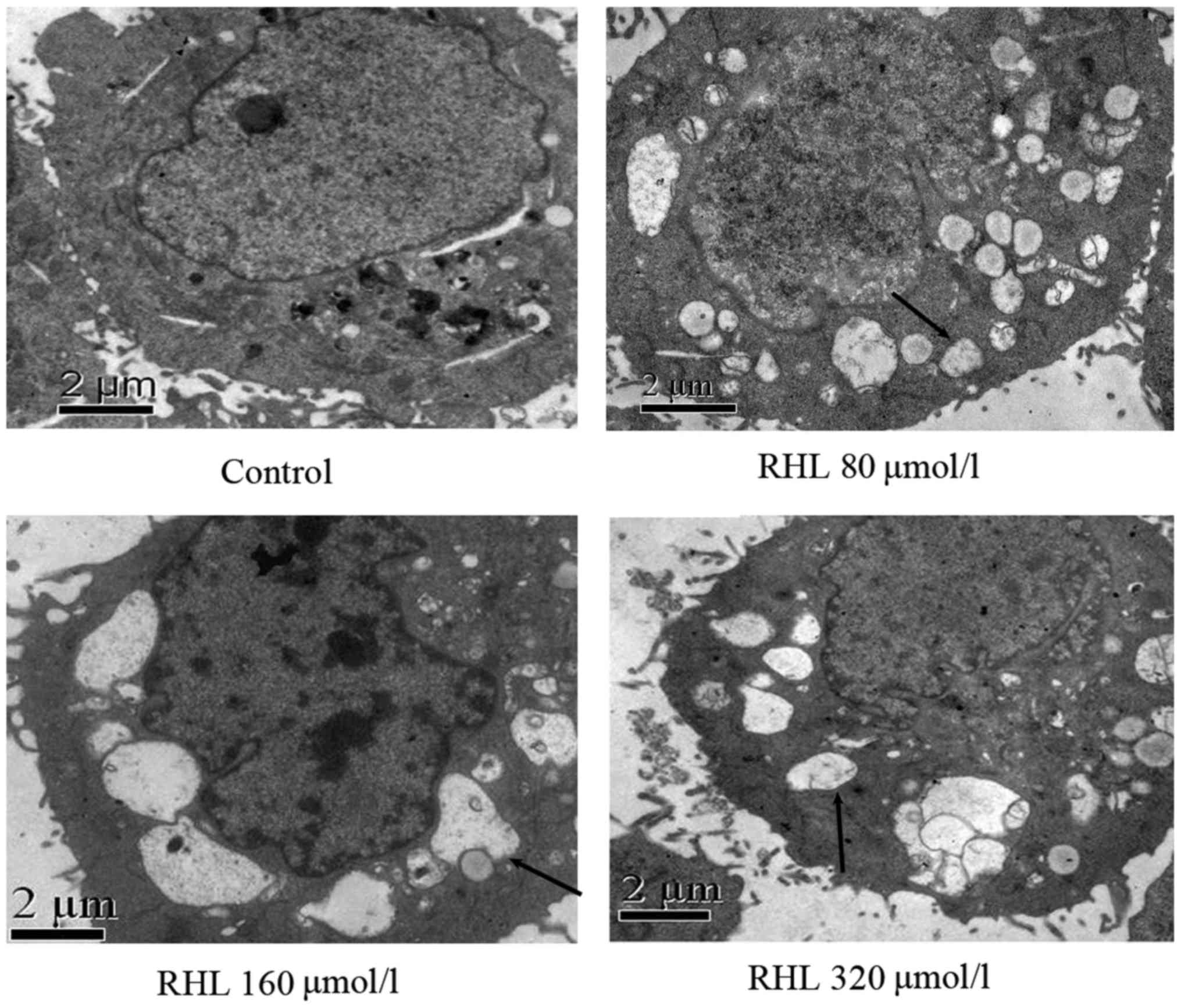
RHL-induced vacuoles in HeLa cells arise
from mitochondria or endoplasmic reticulum
Transmission electron microscopy was performed to
observe HeLa cells following treatment with 160 µmol/l for
24 h. Vacuoles had a complete membrane structure and some were also
observed to have mitochondrial cristae, suggesting that they formed
from endoplasmic reticulum or mitochondria damaged by RHL
treatment. Mitochondrial cristae were observed in small vacuoles;
however, they were not present in large vacuoles (Fig. 3).
Mitochondrial proteomic changes induced
by RHL
Following treatment with 0, 80 or 160 µmol/l
RHL for 24 h, mitochondria were extracted and a proteomic assay was
performed by LC-MS/MS (Fig. 4).
The results revealed that 138 genes were differentially expressed
between treatment groups (0 vs. 80 µmol/l, 0 vs. 160
µmol/l and 80 vs. 160 µmol/l). Approximately 23% of
these genes were associated with protein/nucleic acid binding,
while others were associated with structural molecular activity,
oxidoreductase activity, antioxidant activity and catalytic
activity (Fig. 4A and C). Further
analysis revealed that compared with control group, cytoskeletal
protein keratin and dermal papilla derived protein 12 (DERP12),
which is associated with the oxidation- reduction process, were
most significantly decreased in the two RHL-treated groups. Both of
which, are associated with mitochondrial structure and function.
The top 10 upregulated and downregulated proteins are presented in
Fig. 4B and D. In addition, the
expression of proteins associated with cell proliferation,
including Rac family small GTPase 1, signal transducer and
activator of transcription 3, mitogen-activated protein kinase
kinase 2, SMAD family member (SMAD)2 and SMAD4, were decreased
following RHL treatment.
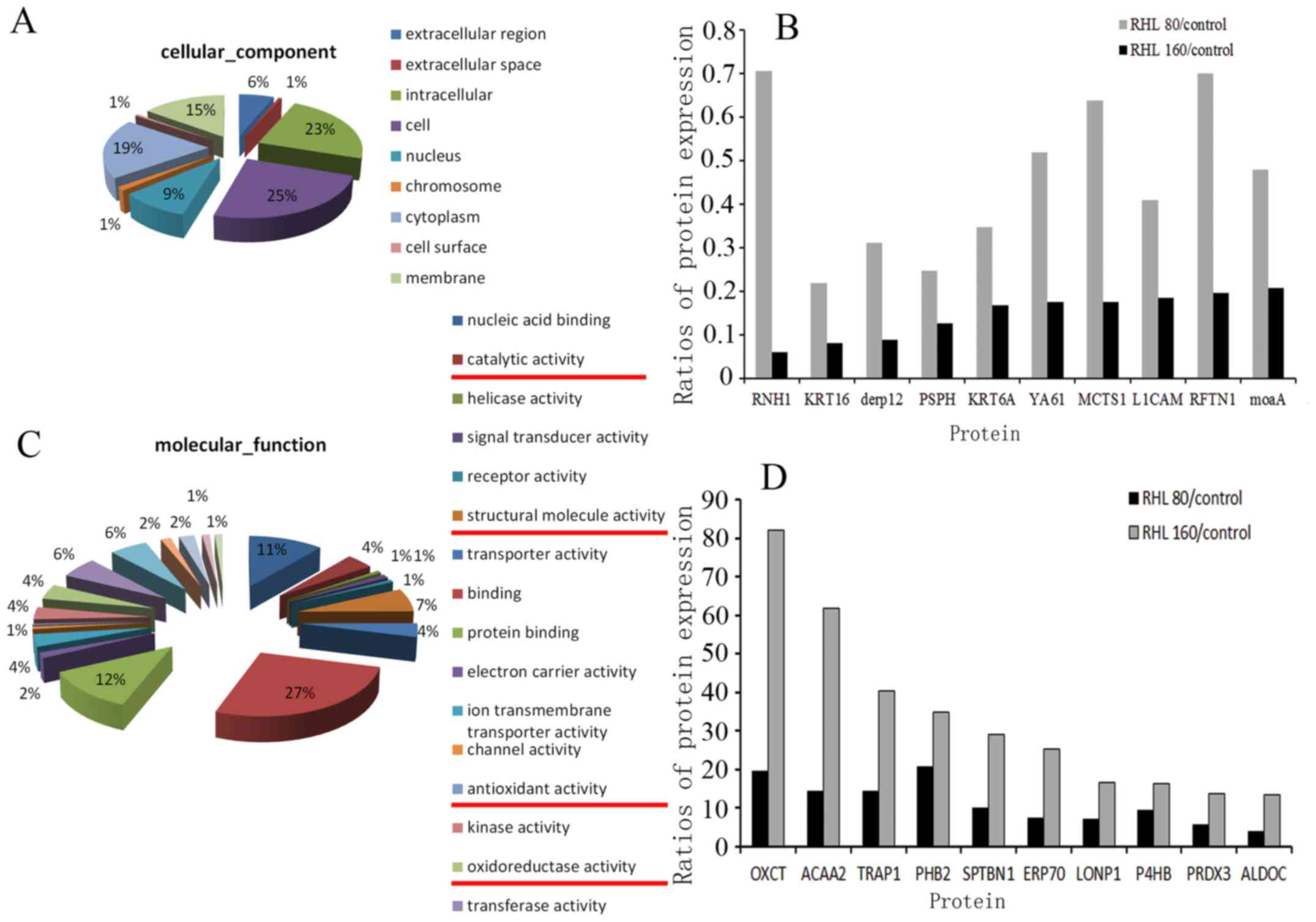 | Figure 4Vacuolar degeneration-associated
mitochondrial proteomics changes. Changes in (A) cellular
components following treatment with 160 µmol/l RHL. (B)
Compared with untreated control group, the expression levels of
RNH1, KRT16, derp12, PSPH, KRT6A, YA61, MCTS1, L1CAM, RFTN1 and
moaA were decreased in cells treated with 80 or 160 µmol/l
RHL. Changes in (C) molecular function following treatment with 160
µmol/l RHL. (D) Compared with control group, the expression
levels of OXCT, ACAA2, TRAP1, PHB2, SPTBN1, ERP70, LONP1, P4HB,
PRDX3 and ALDOC were increased in cells treated with 80 or 160
µmol/l RHL. RHL, Rhein lysinate. |
RHL increases ROS levels
ROS are primarily produced in the mitochondria by
the oxidative respiratory chain. If mitochondria are damaged, their
contents and function are affected. ROS levels were significantly
increased following RHL treatment in a dose-dependent manner
compared with the control group (Fig.
5). In addition, increased ROS levels following treatment with
160 µmol/l RHL were partly scavenged by vitamin C or DPI,
whereas NAC exhibited no significant effect (Fig. 5).
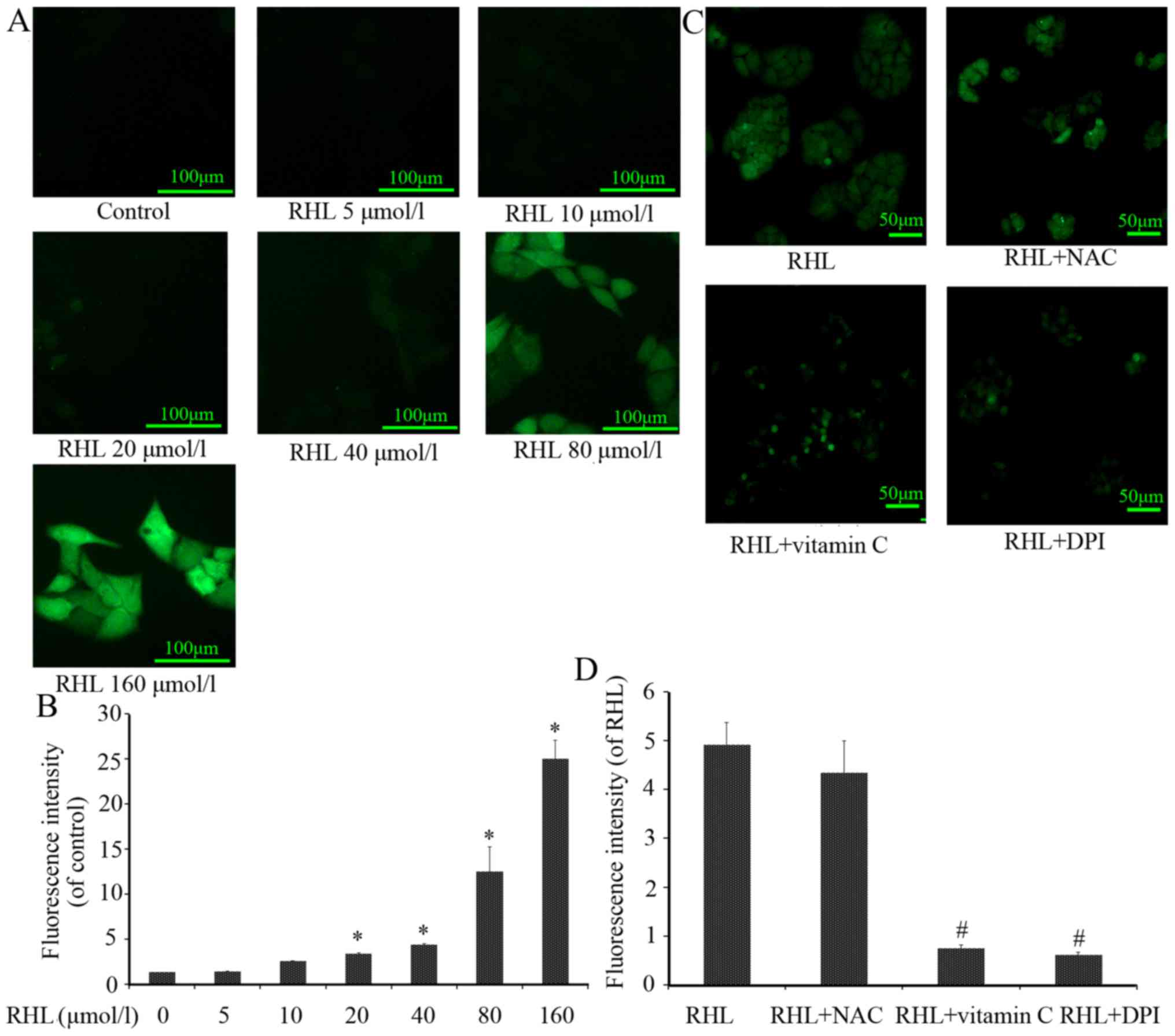 | Figure 5Effect of RHL on ROS. Intracellular
ROS generation is an early event of RHL-induced cell apoptosis. (A)
Representative images (magnification, x20) of the effects 0, 5, 10,
20, 40, 80 or 160 µmol/l RHL on intracellular ROS levels
following 24-h treatment. (B) Representative images (magnification,
x10) and quantification of HeLa cells treated with 160
µmol/l RHL, 160 µmol/l RHL+10 mmol/l NAC, 160
µmol/l RHL+10 mmol/l vitamin C, or 160 µmol/l RHL+5
µmol/l DPI for 24 h. Intracellular ROS levels were measured
using fluorescence microscopy. (C) Quantification of (A). (D)
Quantification of (B). *P<0.05, compared with the untreated
control group; #P<0.05, compared with RHL group. ROS,
reactive oxygen species; NAC, N-acetyl cysteine; DPI, diphenylene
iodonium; RHL, Rhein lysinate. |
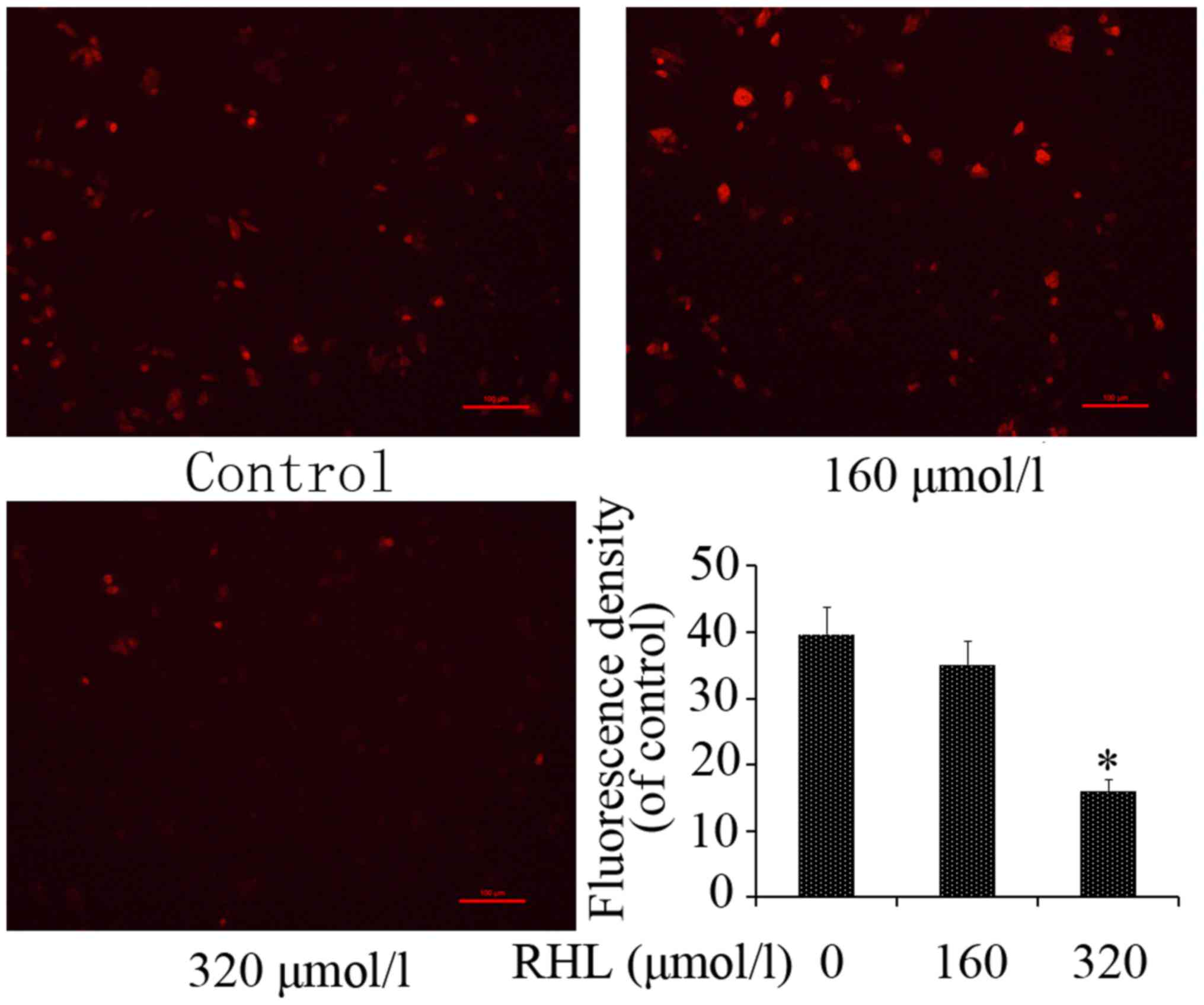
RHL destroys mitochondrial membrane
potential
MMP is a hallmark of mitochondrial function. To
identify mitochondrial dysfunction induced by RHL, MMP was assessed
following RHL treatment using Rhodamine 123 staining. Compared with
the control group, treatment with 320 µmol/l RHL
significantly inhibited Rhodamine 123 uptake in HeLa cells
(Fig. 6).
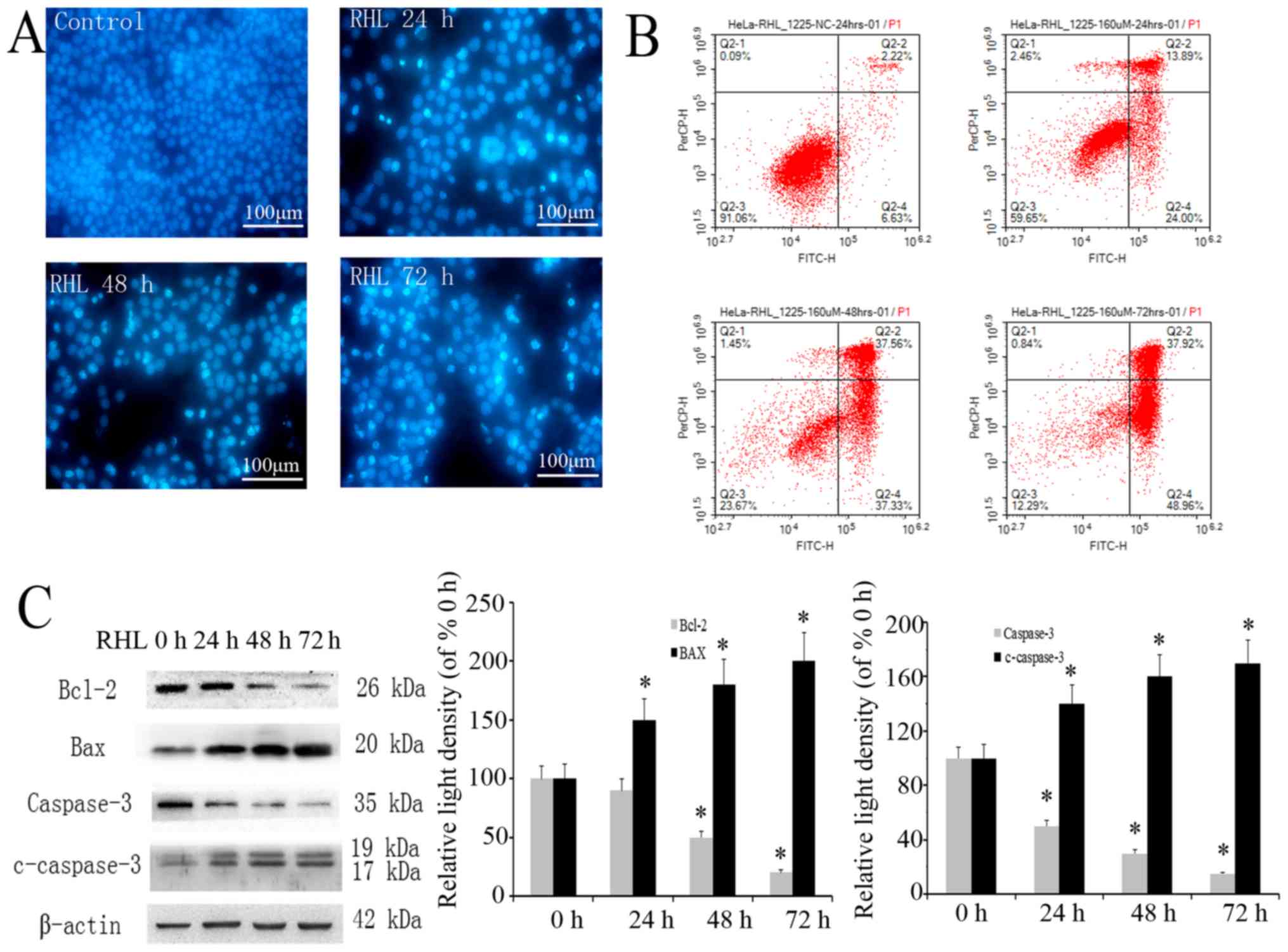
RHL induces mitochondrial
pathway-dependent apoptosis
In the present study, short-term RHL treatment
induced vacuolar degeneration in HeLa cells, while long-term RHL
treatment induced cell death. To assess whether this is due to
apoptosis, nuclear Hoechst and Annexin V/PI double-staining was
performed. Compared with control group, the nuclear concentration,
nucleus edge set and apoptotic body were observed in nucleus of
HeLa cells treated with 160 µmol/l RHL by fluorescence
microscope (Fig. 7A). Flow
cytometry analysis revealed that the number of Annexin V-positive
cells was increased by RHL treatment in a dose-dependent manner
compared with control group (Fig.
7B). To evaluate the apoptosis-associated pathway, caspase-3,
Bcl-2 and Bax expression was assessed using western blotting. As
expected, RHL increased the cleavage of caspase-3, while Bax
expression was significantly upregulated and Bcl-2 expression was
downregulated (Fig. 7C). These
results suggest that RHL induces apoptosis via regulating
apoptosis-associated proteins.
Discussion
Mitochondria serve important roles in the regulation
of a wide variety of intracellular processes, including providing a
source of ATP energy, generating reactive oxygen species (18,19),
and regulating intracellular Ca2+ homeostasis (20), iron-sulfur protein assembly
(21,22), apoptosis (23-25)
and mitophagy (26,27). During cell reprogramming and
cellular transformation, mitochondria undergo dynamic changes
(28). In the present study,
mitochondrial dynamic changes were first observed when HeLa cells
were treated with 160 µmol/l RHL. The earliest change in
mitochondrial morphology, which appeared 6 h following treatment,
was vacuolar degeneration. Rhein is a weakly acidic molecule.
Therefore, it exists in two forms: Non-dissociation and
dissociation. It is considered that the non-dissociation form of
Rhein penetrates the cell membrane by active diffusion (29). When levels of the non-dissociation
form of Rhein are reduced, the dissociation form of Rhein turns
into the non-dissociation form. In the current study, Rhein
lysinate was demonstrated to inhibit the expression of keratin 16
and keratin 6A in proteomics analysis. It was reported that keratin
16 was a type I cytokeratin and was paired with keratin 6 in a
number of epithelial tissues (30). Type I keratins (or Type I
cytokeratins) are cytokeratins that constitute the Type I
intermediate filaments of the intracytoplasmatic cytoskeleton
(31). Thus, it is considered that
vacuolar degeneration of mitochondrion is induced by the
destruction of the cytoskeleton.
Apoptosis is characterized by cell shrinkage,
blebbing of the plasma membrane, maintenance of organelle
integrity, condensation and fragmentation of DNA, followed by
ordered removal of phagocytes to minimize damage to surrounding
tissues (32). Apoptosis has been
classified into two types depending on the mechanistic pathway
responsible, namely the extrinsic pathway and the
mitochondria-mediated pathway (33,34).
Following treatment with 160 µmol/l RHL for 24, 48 or 72 h,
HeLa cell apoptosis was observed using flow cytometry. Bcl-2 is a
prototypical anti-apoptotic member of the Bcl-2 family (35), while Bax is pro-apoptotic (36). Bax is a functional antagonist of
Bcl-2, and the Bcl-2/Bax ratio regulates cell apoptosis (35,37,38).
In the present study, RHL treatment was demonstrated to decrease
Bcl-2 expression and increase Bax expression, thereby reducing the
Bcl-2/Bax ratio in HeLa cells.
A number of studies have reported that ROS serves an
important role in carcinogenesis. For example, at low physiological
concentrations ROS regulate cell growth; however, at high
concentrations, ROS serves a role in oxidative stress and the
induction of apoptosis (39-41).
Non-thermal plasma induces the mitochondria-mediated apoptotic
signaling pathway via ROS generation in HeLa cells (42). In the present study, it was
revealed that RHL induces ROS generation in HeLa cells in
dose-dependent manner. Antioxidants, including vitamin C and DPI,
are able to partially inhibit RHL-induced ROS generation. In
addition, the antioxidant activity of certain genes, such as
DERP12, may decrease and others, such as PRDX3, may increase, as
indicated by in proteomics analysis. As a whole, the ROS level of
mitochondrion was increased. It may be deduced that ROS
participates in the process by which RHL induces HeLa cell
apoptosis.
It should be noted that there are a number of
fluorescent mitochondrial dyes that may be used for MMP
measurements (43). Different
probes are recommended for each usage paradigm, depending on the
uptake kinetics, concentration and mitochondrial binding affinity
of the probe (44). Rhodamine 123
is recommended for applications that aim to measure rapid changes
in membrane potential (45,46).
In the present study, 320 µmol/l RHL was revealed to
decrease the MMP of HeLa cells. However, the mechanism of RHL
decreasing MMP is unclear and requires further study.
In conclusion, RHL exhibits a dynamic influence on
HeLa cells, causing vacuolar degeneration after 6 h and apoptosis
after 24 h of treatment (Fig. 8).
It was hypothesized that the vacuolar degeneration and
apoptosis-associated signal pathways may be an appealing target for
therapeutic interventions in cervical carcinoma. Future studies
should investigate the antitumor effect of RHL in patients with
cervical cancer.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 81671391)
and the Beijing Hospital Nova project (grant no. BJ-2016-033).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors’ contributions
YL and PT conceived and designed the experiments;
JL, YZ and JC performed the experiments; YL and RX analyzed the
data; JW assisted in flow cytometric analysis and manuscript
preparation; GH assisted in western blot analysis and manuscript
preparation; YL wrote the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Waggoner SE: Cervical cancer. Lancet.
361:2217–2225. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Peralta-Zaragoza O, Bermúdez-Morales VH,
Pérez-Plasencia C, Salazar-León J, Gómez-Cerón C and Madrid-Marina
V: Targeted treatments for cervical cancer: A review. OncoTargets
Ther. 5:315–328. 2012. View Article : Google Scholar
|
|
3
|
He AD, Wang SP, Xie W, Song W, Miao S,
Yang RP, Zhu Y, Xiang JZ and Ming ZY: Platelet derived TGF-β
promotes cervical carcinoma cell growth by suppressing KLF6
expression. Oncotarget. 8:87174–87181. 2017.PubMed/NCBI
|
|
4
|
Shimizu K, Kageyama M, Ogura H, Yamada T
and Shimazu T: Effects of Rhubarb on intestinal dysmotility in
critically ill patients. Intern Med. 57:507–510. 2018. View Article : Google Scholar :
|
|
5
|
Gao D, Zeng LN, Zhang P, Ma ZJ, Li RS,
Zhao YL, Zhang YM, Guo YM, Niu M, Bai ZF, et al: Rhubarb
anthraquinones protect Rats against Mercuric Chloride
(HgCl2)-induced acute renal failure. Molecules.
21:2982016. View Article : Google Scholar
|
|
6
|
Neyrinck AM, Etxeberria U, Taminiau B,
Daube G, Van Hul M, Everard A, Cani PD, Bindels LB and Delzenne NM:
Rhubarb extract prevents hepatic inflammation induced by acute
alcohol intake, an effect related to the modulation of the gut
microbiota. Mol Nutr Food Res. 61:612017. View Article : Google Scholar
|
|
7
|
Huang Q, Lu G, Shen HM, Chung MC and Ong
CN: Anti-cancer properties of anthraquinones from rhubarb. Med Res
Rev. 27:609–630. 2007. View Article : Google Scholar
|
|
8
|
Morrison DK: MAP kinase pathways. Cold
Spring Harb Perspect Biol. 4:42012. View Article : Google Scholar
|
|
9
|
Hsia TC, Yang JS, Chen GW, Chiu TH, Lu HF,
Yang MD, Yu FS, Liu KC, Lai KC, Lin CC, et al: The roles of
endoplasmic reticulum stress and Ca2+ on rhein-induced
apoptosis in A-549 human lung cancer cells. Anticancer Res.
29:309–318. 2009.PubMed/NCBI
|
|
10
|
Heo SK, Yun HJ, Park WH and Park SD: Rhein
inhibits TNF-alpha-induced human aortic smooth muscle cell
proliferation via mitochondrial-dependent apoptosis. J Vasc Res.
46:375–386. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun H, Luo G, Chen D and Xiang Z: A
comprehensive and system review for the pharmacologicalFront
Pharmacol mechanism of action of rhein, an active anthraquinone
ingredient. 7:2472016.
|
|
12
|
Lin YJ and Zhen YS: Rhein lysinate
suppresses the growth of breast cancer cells and potentiates the
inhibitory effect of Taxol in athymic mice. Anticancer Drugs.
20:65–72. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu J, Zhang K, Zhen YZ, Wei J, Hu G, Gao
JL, Tian YX and Lin YJ: Antitumor activity of rhein lysinate
against human glioma U87 cells in vitro and in vivo. Oncol Rep.
35:1711–1717. 2016. View Article : Google Scholar
|
|
14
|
Lin YJ, Zhen YZ, Zhao YF, Wei J and Hu G:
Rhein lysinate induced S-phase arrest and increased the anti-tumor
activity of 5-FU in HeLa cells. Am J Chin Med. 39:817–825. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhen YZ, Hu G, Zhao YF, Yan F, Li R, Gao
JL and Lin YJ: Synergy of Taxol and rhein lysinate associated with
the downregulation of ERK activation in lung carcinoma cells. Oncol
Lett. 6:525–528. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Paglin S, Hollister T, Delohery T, Hackett
N, McMahill M, Sphicas E, Domingo D and Yahalom J: A novel response
of cancer cells to radiation involves autophagy and formation of
acidic vesicles. Cancer Res. 61:439–444. 2001.PubMed/NCBI
|
|
17
|
Dzieciatkowska M, Qi G, You J, Bemis KG,
Sahm H, Lederman HM, Crawford TO, Gelbert LM, Rothblum-Oviatt C and
Wang M: Proteomic characterization of cerebrospinal fluid from
ataxia-telangiectasia (A-T) patients using a LC/MS-based label-free
protein quantification technology. Int J Proteomics.
2011:5789032011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Faulk A, Weissig V and Elbayoumi T:
Mitochondria-specific nano-emulsified therapy for myocardial
protection against doxorubicin-induced cardiotoxicity. Methods Mol
Biol. 991:99–112. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pohjoismäki JL and Goffart S: The role of
mitochondria in cardiac development and protection. Free Radic Biol
Med. 106:345–354. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Park SJ, Lee SB, Suh Y, Kim SJ, Lee N,
Hong JH, Park C, Woo Y, Ishizuka K, Kim JH, et al: DISC1 modulates
neuronal stress responses by gate-keeping ER-mitochondria
Ca2+transfer through the MAM. Cell Reports.
21:2748–2759. 2017. View Article : Google Scholar
|
|
21
|
Wachnowsky C, Fidai I and Cowan JA:
Iron-sulfur cluster biosynthesis and trafficking - impact on human
disease conditions. Metallomics. 10:9–29. 2018. View Article : Google Scholar
|
|
22
|
Stehling O, Wilbrecht C and Lill R:
Mitochondrial iron-sulfur protein biogenesis and human disease.
Biochimie. 100:61–77. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Burke PJ: Mitochondria, bioenergetics and
apoptosis in cancer. Trends Cancer. 3:857–870. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Duan H, Wang R, Yan X, Liu H, Zhang Y, Mu
D, Han J and Li X: Phloretin induces apoptosis of human esophageal
cancer via a mitochondria-dependent pathway. Oncol Lett.
14:6763–6768. 2017.PubMed/NCBI
|
|
25
|
Park S, Lim W, Bazer FW and Song G:
Naringenin induces mitochondria-mediated apoptosis and endoplasmic
reticulum stress by regulating MAPK and AKT signal transduction
pathways in endometriosis cells. Mol Hum Reprod. 23:842–854. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ułamek-Kozioł M, Kocki J, Bogucka-Kocka A,
Januszewski S, Bogucki J, Czuczwar SJ and Pluta R: Autophagy,
mitophagy and apoptotic gene changes in the hippocampal CA1 area in
a rat ischemic model of Alzheimer’s disease. Pharmacol Rep.
69:1289–1294. 2017. View Article : Google Scholar
|
|
27
|
Rodger CE, McWilliams TG and Ganley IG:
Mammalian mitophagy - from in vitro molecules to in vivo models.
FEBS J. 285:1185–1202. 2018. View Article : Google Scholar
|
|
28
|
Prieto J and Torres J: Mitochondrial
dynamics: In cell reprogramming as it is in cancer. Stem Cells Int.
2017:80737212017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Peng Y, Fan M, Peng C, Wang M and Li X:
Alleviating the intestinal absorption of rhein in Rhubarb through
herb compatibility in Tiaowei Chengqi Tang in Caco-2 cells. Evid
Based Complement Alternat Med. 2018:7835128eCollection. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Trost A, Desch P, Wally V, Haim M, Maier
RH, Reitsamer HA, Hintner H, Bauer JW and Onder K: Aberrant
heterodimerization of keratin 16 with keratin 6A in HaCaT
keratinocytes results in diminished cellular migration. Mech Ageing
Dev. 131:346–353. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Herrmann H, Bär H, Kreplak L, Strelkov SV
and Aebi U: Herrmann H1: Bär H, Kreplak L, Strelkov SV and Aebi U.
Intermediate filaments: From cell architecture to nanomechanics.
Nat Rev Mol Cell Biol. 8:562–573. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Brenner C and Kroemer G: Apoptosis.
Mitochondria - the death signal integrators. Science.
289:1150–1151. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Low IC, Kang J and Pervaiz S: Bcl-2: A
prime regulator of mitochondrial redox metabolism in cancer cells.
Antioxid Redox Signal. 15:2975–2987. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Indran IR, Tufo G, Pervaiz S and Brenner
C: Recent advances in apoptosis, mitochondria and drug resistance
in cancer cells. Biochim Biophys Acta. 1807.735–745. 2011.
|
|
35
|
Vucicevic K, Jakovljevic V, Colovic N,
Tosic N, Kostic T, Glumac I, Pavlovic S, Karan-Djurasevic T and
Colovic M: Association of Bax expression and Bcl2/Bax ratio with
clinical and molecular prognostic markers in chronic lymphocytic
leukemia. J Med Biochem. 35:150–157. 2016. View Article : Google Scholar
|
|
36
|
Pawlowski J and Kraft AS: Bax-induced
apoptotic cell death. Proc Natl Acad Sci USA. 97:529–531. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Siqueira EC, Souza FT, Diniz MG, Gomez RS
and Gomes CC: Hsp27 (HSPB1) differential expression in normal
salivary glands and pleomorphic adenomas and association with an
increased Bcl2/Bax ratio. Tumour Biol. 36:213–217. 2015. View Article : Google Scholar
|
|
38
|
Muhammad Nadzri N, Abdul AB, Sukari MA,
Abdelwahab SI, Eid EE, Mohan S, Kamalidehghan B, Anasamy T, Ng KB,
Syam S, et al: Inclusion complex of Zerumbone with hydroxypropyl- β
-cyclodextrin induces apoptosis in liver hepatocellular HepG2 cells
via caspase 8/BID cleavage switch and modulating Bcl2/Bax ratio.
Evid Based Complement Alternat Med. 2013:8106322013. View Article : Google Scholar
|
|
39
|
Fakhri A, Omranipour R, Fakhri S,
Mirshamsi M, Zangeneh F, Vatanpour H and Pourahmad J: Naja naja
oxiana venom fraction selectively induces ROS-mediated apoptosis in
human colorectal tumor cells by directly targeting mitochondria.
Asian Pac J Cancer Prev. 18:2201–2208. 2017.PubMed/NCBI
|
|
40
|
Pant K, Yadav AK, Gupta P, Islam R, Saraya
A and Venugopal SK: Butyrate induces ROS-mediated apoptosis by
modulating miR-22/SIRT-1 pathway in hepatic cancer cells. Redox
Biol. 12:340–349. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Alarifi S, Ali D, Alkahtani S and Almeer
RS: ROS-mediated apoptosis and genotoxicity induced by palladium
nanoparticles in human skin malignant melanoma cells. Oxid Med Cell
Longev. 2017.8439098:2017.
|
|
42
|
Li W, Yu KN, Ma J, Shen J, Cheng C, Zhou
F, Cai Z and Han W: Non-thermal plasma induces
mitochondria-mediated apoptotic signaling pathway via ROS
generation in HeLa cells. Arch Biochem Biophys. 633:68–77. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Perry SW, Norman JP, Barbieri J, Brown EB
and Gelbard HA: Mitochondrial membrane potential probes and the
proton gradient: A practical usage guide. Biotechniques. 50:98–115.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhu C, Martinez AF, Martin HL, Li M,
Crouch BT, Carlson DA, Haystead TAJ and Ramanujam N:
Near-simultaneous intravital microscopy of glucose uptake and
mitochondrial membrane potential, key endpoints that reflect major
metabolic axes in cancer. Sci Rep. 7:137722017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sun LL, Sun LR and Wang GY: Mitochondrial
membrane potential at HL-60 cell apoptosis induced by cytarabine.
Zhongguo Shi Yan Xue Ye Xue Za Zhi. 15:1196–1199. 2007.In Chinese.
PubMed/NCBI
|
|
46
|
Peatey CL, Chavchich M, Chen N, Gresty KJ,
Gray KA, Gatton ML, Waters NC and Cheng Q: Mitochondrial membrane
potential in a small subset of artemisinin-induced dormant
Plasmodium falciparum parasites in vitro. J Infect Dis.
212:426–434. 2015. View Article : Google Scholar : PubMed/NCBI
|