Introduction
Hypopharyngeal carcinoma (HC) is a malignant tumor
of the head and neck, which has the highest mortality rate in all
patients with head and neck cancer. Hypopharyngeal squamous cell
carcinoma (HNSCC) is the most common pathological type of HC, which
is closely associated with smoking, drinking, air pollution,
long-term exposure to toxic chemicals, human papilloma virus, sex
hormones and other factors (1);
however, its etiology remains unclear. Patients with HC are usually
asymptomatic, and it is commonly diagnosed at the middle-late
stage. The 5-year survival rate for HC is ~40% and has not improved
over the last two decades (2);
therefore, the development of novel therapeutic strategies is
required to improve the survival rate.
Gold nanoparticles (AuNPs) have specific physical
and chemical properties; they are easy to make and exhibit good
stability and biocompatibility, as well as strong surface
plasticity. In addition, AuNPs can be conjugated to antibodies,
carbohydrates, DNA/RNA and other factors, which may specifically
target tumor cells. These features make AuNPs promising tools for
plasmonic photothermal therapy (PPTT) and tumor-targeting therapy
(3). PPTT involves the rapid
conversion of light into heat by plasmonic nanoparticles that are
targeted to a tumor, thus inducing hyperthermia-induced cell death.
These nanoparticles can be passively targeted using the enhanced
permeability and retention effect, or they can be actively targeted
using proteins, peptides and other small molecules. AuNPs have been
reported to induce plasmonic photothermal cell death, and are able
to monitor associated molecular alterations through time-dependent
surface-enhanced Raman spectroscopy within a single cell (4). Gold nanorods (AuNRs) are a specific
type of AuNP, which have been applied to treat head and neck
tumors. AuNRs have two characteristic surface plasmon resonance
(SPR) absorbance peaks. The transverse SPR is ~520 nm and is a
characteristic of all AuNPs, whereas the long SPR (LSPR) is a
variable of its aspect ratio and can therefore be manually tuned.
When the aspect ratio is 3.9, the LSPR is 800 nm, which is in the
middle of the near infrared region (i.e. 700-900 nm). Near infrared
spectroscopy (NIR) can penetrate deeply into living tissues with
few fluorescence effects on the biological content in the tissue;
therefore, it is considered beneficial for PPTT of deep tumors.
AuNR-mediated PPTT is able to selectively kill tumor cells labeled
with AuNRs, without damaging the surrounding normal cells.
Therefore, AuNR-induced PPTT may be an emerging strategy for cancer
therapy (4).
AuNRs have a strong affinity with antibodies,
proteins and DNA fragments, and the resulting conjugates can target
tumor cells more specifically. It has been reported that epidermal
growth factor receptor (EGFR) is highly expressed in various types
of human malignant cancer, including colorectal cancer, breast
cancer, pancreatic cancer, and head and neck squamous cell
carcinoma (5). Therefore, EGFR is
considered an important biotherapy target in numerous types of
solid tumor. EGFR monoclonal antibody-conjugated AuNRs
(EGFRmAb-AuNRs) have been generated, and the apoptotic potential of
EGFRmAb-AuNRs has been assessed in Hep-2 cells in vitro and
in vivo (6). However, few
studies have focused on the effects of EGFRmAb-AuNRs on HNSCC;
therefore, further investigation is required.
The phosphatidylinositol-3-kinase (PI3K)/AKT
serine//threonine kinase (Akt) pathway is one of the most important
pathways activated by EGFR (7,8).
During tumor development, it is critical for increasing tumor cell
proliferation, inhibiting apoptosis, enhancing angiogenesis, and
promoting invasion and metastasis (9,10).
Akt, which is also termed protein kinase B, is the key effector
downstream of PI3K (11). In
response to external stimulation, PI3K is activated, which in turn
transforms Akt into phosphorylated (p)-Akt (12).
Endogenous and exogenous factors, including abnormal
metabolites and ionizing radiation, induce DNA damage (13,14).
Usually, cells initiate the DNA damage response against such
damage, and when the damage is repairable, it can be fixed by the
internal repair system (15).
However, if the damage is too severe and cannot be recovered,
apoptosis is triggered (16).
Previously, we synthesized AuNRs using the
cetyltrimethylammonium bromide system and conjugated EGFRmAb to
AuNRs. Furthermore, it was revealed that 293T and FaDu human HC
cell lines overexpress EGFR on the surface of the cell membrane.
Subsequently, it was revealed that EGFRmAb-AuNRs selectively kill
FaDu cells under suitable NIR, thus suggesting that EGFRmAb may
have a synergistic role and may significantly improve the
efficiency of AuNR-induced PPTT (17). However, in vitro data may
not correctly reflect what occurs in vivo, since tumor
growth is a complex process that depends on the interaction between
tumor cells and their microenvironment. Therefore, the present
study aimed to investigate and verify the effects of AuNRs and
EGFRmAb-AuNRs PPTT on subcutaneous transplantable hypopharyngeal
tumors in nude mice. In addition, the roles of the PI3K/Akt and DNA
damage signaling pathways were explored in AuNR-mediated
apoptosis.
Materials and methods
Cell lines and cell culture
The FaDu human pharynx squamous cell carcinoma cell
line was purchased from the Shanghai Cell Bank of Chinese Academy
of Sciences (Shanghai, China). Cell lines were cultured in
RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS), 1%
penicillin-streptomycin and 1% L‑glutamine (all from Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) at 37°C in a humidified
atmosphere containing 5% CO2.
Preparation of AuNRs and conjugation with
EGFRmAb
AuNRs were obtained from Kunming Institute of
Precious Metals (Kunming, China). EGFRmAb was purchased from
Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). AuNRs and
EGFRmAb-AuNRs used for animal studies were prepared as previously
described (6). Briefly, EGFRmAb
was dissolved in HEPES buffer (20 nmol; pH 7.4) and mixed with
AuNRs solution to generate a solution with an optical density of
0.8 at 800 nm. Subsequently, the synthetic EGFRmAb-AuNRs (0.1
nmol/l) were examined under a transmission electron microscope (JEM
1010; JEOL Ltd., Tokyo, Japan) to determine the mean value of the
long and transverse diameter of the particles.
Generation of a HC model in nude
mice
A total of 70 healthy female BALB/C(nu/nu) nude mice
(age, 5-6 weeks; weight, 19-22 g) were purchased from Hunan Silaike
Jingda Laboratory Animal Co. Ltd. (Changsha, China). All mice were
housed in a standard pathogen-free facility under constant
temperature (25±2°C) and humidity (45‑50%) at the experimental
animal center of Kunming Medical University. All experimental
procedures were conducted in accordance with the institutional
guidelines for the care and use of laboratory animals, and the
present study was approved by the Institutional Animal Care and Use
Committee of Kunming Medical University.
FaDu cells in the logarithmic growth phase were
collected and resuspended in PBS (Gibco; Thermo Fisher Scientific,
Inc.) to make a single cell suspension. Subsequently, 200 µl
PBS solution containing 1×107 cells was injected into
the back of nude mice using 1 ml sterile syringes. Following
injection of FaDu cells, HC tumors began to develop after 7 days.
The mice were randomly divided into seven groups; each group
contained 10 mice. When the tumor size reached ~200 mm3,
a total of 3 weeks after injection, each group was treated
differently. In the control group [normal saline (NS).it], 0.9% NS
was injected directly into tumors. In the AuNRs.it group, AuNRs
were injected directly into tumors. In the AuNRs. iv + NIR group,
mice were treated with intravenous injection of AuNRs, and received
NIR laser irradiation 24 h post-injection. Mice in the NIR group
received local irradiation only, without any injection. In the
AuNRs.it + NIR group, AuNRs were injected directly into tumors,
followed by NIR treatment 24 h post-injection. In the EGFRmAb.it
group, EGFRmAb was injected directly into tumors. In the
EGFRmAb-AuNRs. it + NIR group, EGFRmAb-AuNRs was injected directly
into tumors, and NIR treatment was performed 24 h post-injection.
The total injection volume was 200 µl for all reagents (0.9%
N.S, EGFRmAb, AuNRs and EGFRmAb-AuNRs). In the present study, the
wavelength of NIR was 808 nm, the power was 5.0 W/cm2,
and the duration was 6 min. The laser-transmitting end was placed 2
cm away from the tumors to ensure the laser spot covered the entire
tumor.
Following treatment, the mice were observed every
other day and body weight, food intake, sleep, activity and
defecation were recorded. The long diameter (a), short diameter (b)
and height (c) of the tumors were also recorded every other day.
Tumor volume was calculated using the following equation: V = a × b
× c; tumor growth curves were generated accordingly. A total of 3
days after treatment, the mice were sacrificed using cervical
dislocation and tumors were dissected from the body. The inhibition
rate for tumor weight was calculated as follows: Average tumor
weight in the control group - average tumor weight in the
experimental group) × 100%. The inhibition rate for tumor volume
was calculated as follows: Average tumor volume in the control
group - average tumor volume in the experimental group) × 100%.
Subsequently, five tumors were taken randomly from each group and
stored in 4% polyformaldehyde solution at room temperature for
terminal-deoxynucleotidyl transferase dUTP nick end labeling
(TUNEL) staining. The other five tumors in each group were
maintained in PBS at room temperature for flow cytometry, reverse
transcription‑quantitative polymerase chain reaction (RT-qPCR) and
western blot analysis.
Apoptosis analysis using flow
cytometry
Tumor tissues (100 mg) were digested with 500
µl 0.25% pancreatin-EDTA solution at 37°C for 10 min, after
which, 500 µl RPMI‑1640 medium supplemented with 10% FBS was
added to terminate the reaction. The resulting solution was
centrifuged at 1,750 × g for 5 min, and the pellet was resuspended
in PBS to make a single cell suspension (5×105
cells/ml). Annexin V‑fluorescein isothiocyanate (FITC) and
propidium iodide (PI) were used to label apoptotic and necrotic
cells, according to the manufacturer’s protocol (cat. no. 556547;
BD Biosciences, Franklin Lakes, NJ, USA). The stained cells were
detected using flow cytometry and were analyzed with FlowJo 7.6.2
(FlowJo, LLC, Ashland, OR, USA). Surviving cells exhibited very low
fluorescence, whereas apoptotic cells were stained with Annexin
V‑FITC and exhibited strong green fluorescence, and necrotic cells
were stained with Annexin V-FITC and PI, thus exhibiting green and
red fluorescence.
Apoptosis detection using TUNEL
assay
Tumor samples were fixed and sectioned.
Subsequently, a TUNEL assay (cat. no. E607175; Sangon Biotech Co.,
Ltd., Shanghai, China) was performed to determine the presence of
apoptotic cells in situ. The TUNEL assay identifies
apoptotic cells by the terminal deoxynucleotidyl
transferase-mediated addition of DIG-labeled deoxyridine
triphosphate nucleotides (DIG-dUTPs) to the 3′-OH end of DNA strand
breaks. The TUNEL-labeled cells are subsequently visualized using
anti-DIG-biotin, streptavidin-biotin complex (SABC) and DAB.
Briefly, tumor tissues were fixed with 10% formalin
at room temperature for 12 h, and formalin‑fixed tissues were
embedded in paraffin. Subsequently, sections (5 µm) were
prepared and transferred onto glass slides; the formalin‑fixed,
paraffin‑embedded tumor sections were deparaffinized and rehydrated
in a coplin jar. Prepared slides were incubated with freshly made
3% H2O2 in the dark for 10 min at room
temperature, and were then washed three times with 0.01 M
Tris-buffered saline (TBS; 5 min/wash). Subsequently, the slides
were treated with 20 µg/ml proteinase K without DNase at
37°C for 5 min, and were then rinsed three times in 0.01 M TBS (2
min/wash). Each slide was treated with 20 µl working
solution containing 1 µl TdT, 1 µl DIG-dUTP and 18
µl labeling buffer at 37°C for 2 h, after which, 50
µl sealing solution was applied to each slide for 30 min at
room temperature. Subsequently, 50 µl anti-DIG-biotin
antibody (1:100) was added to each slide, and the slides were
incubated at 37°C for 30 min. SABC (1:100) and DAB were used
sequentially to visualize the signal. Apoptotic cells were stained
brown and were observed under a light microscope.
The sections were viewed under a light microscope
(400× magnification) to identify TUNEL staining. Nine fields were
randomly selected in each slide, and the number of TUNEL-positive
cells was determined as the average number of stained cells in the
observed fields. Image Pro Plus 6.0 software (Media Cybernetics,
Inc., Rockville, MD, USA) was used to determine the light intensity
of TUNEL staining. Similarly, nine fields were randomly selected
and observed in each slide. The light intensity of TUNEL-positive
cells in a slide was determined as the average number of the nine
fields.
mRNA expression detection using
RT‑qPCR
Tumor tissues (100 mg) were used for total RNA
extraction using 800 µl TRIzol® lysis buffer
(Invitrogen; Thermo Fisher Scientific, Inc.). Total mRNA was
initially extracted with chloroform, and then with isopropanol, and
was finally dissolved in 20 µl DEPC water. Subsequently,
first‑strand cDNA was synthesized using the RevertAid™ First Strand
cDNA Synthesis kit (Thermo Fisher Scientific, Inc.) according to
the manufacturer’s protocol. Gene‑specific primers used for RT‑qPCR
were designed with Beacon Designer 7.90 software (Premier Biosoft
International, Palo Alto, CA, USA) and synthesized by Invitrogen;
Thermo Fisher Scientific, Inc. Genes were amplified using a
SYBR‑Green master mix and the ABI 7300 Real-Time PCR system
(Applied Biosystems; Thermo Fisher Scientific, Inc.). β-actin was
used as an internal control. The RT-qPCR reaction protocol was as
follows: After denaturation at 95°C for 30 sec, the mixture was
amplified for 45 cycles. Each amplification cycle consisted of 5
min of denaturation at 95°C to activate the enzyme, 1 min of
denaturation at 95°C, 1 min of annealing at 58°C and 1 min at 72°C.
The final extension step was 15 min at 72°C. Each set of
experiments was repeated three times. The relative expression of
detected genes was calculated using the 2−ΔΔCq method
(18). The primer sequences were
as follows: β-actin, forward, 5′-TATGGAATCCTGTGGCATC-3′, reverse,
5′-GTGTTGGCA TAGAGGTCTT-3′; Akt, forward, 5′-TATTGTGAAGGA
GGGTTG-3′, reverse, 5′-ATTCTTGAGGAGGAAG TAG-3′; ATR
serine/threonine kinase (ATR), forward, 5′-AGAGACG GAATGAAGATT-3′,
reverse, 5′-GAGATTAGATTATTGA GGACTT-3′; checkpoint kinase 1 (Chk1),
forward, 5'-GTGTGA ATGACAACTACTG-3′, reverse, 5′-TGCTGTATGTTCGGT
ATT-3′; glycogen synthase kinase 3β (GSK3β), forward,
5′-TAATCTGGTGCTGGACTA-3′, reverse, 5′-CTGATACATA TACAACTTGACAT-3′;
p53, forward, 5′-AGTATTTGG ATGACAGAA-3′, reverse,
5′-ATGTAGTTGTAGTGGATG-3′; and phosphatase and tensin homolog
(PTEN), forward, 5′-ACGAACTGGTGTAATGATA-3′ and reverse, 5′-GTCTCT
GGTCCTTACTTC-3′.
Protein expression detection using
western blotting
Tumor tissues (100 mg) were used for total protein
extraction. Samples were lysed in 800 µl
radioimmunoprecipitation assay buffer containing protease
inhibitors (Beyotime Institute of Biotechnology, Haimen, China).
Protein concentrations were subsequently determined using a
bicinchoninic acid (Beyotime Institute of Biotechnology) assay at
562 nm, according to the manufacturer’s protocol. Equal amounts of
protein (70 µg) were denatured in 1X loading buffer at 100°C
for 10 min and were separated by 4% SDS-PAGE. Subsequently,
proteins were transferred to polyvinylidene fluoride membranes. The
resulting membranes were blocked with 5% bovine serum albumin
(Beijing Solarbio Science & Technology Co. Ltd., Beijing,
China) for 1 h at room temperature. The membranes were then
individually incubated overnight at 4°C with primary antibodies
against β-actin (cat. no. T40104), pan-Akt (cat. no. 4691), p-Akt
(cat. no. 4060), p-GSK3β (cat. no. 9322), GSK3β (cat. no. 5676),
PTEN (cat. no. 9552), p-ATR (cat. no. 2853), ATR (cat. no. 2790),
p-Chk1 (cat. no. 2348), Chk1 (cat. no. 2360), p-p53 (cat. no. 9286)
and p53 (cat. no. 2527). Subsequently, the membrane was incubated
with horseradish peroxidase-conjugated secondary antibodies
[1:1,000; M21001 (anti-mouse) and M21002 (anti-rabbit), Shanghai
Abmart Co., Ltd.] for 2 h at room temperature. Images of the
membranes were captured with a Bio-Rad GelDock system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) and the band intensity was
determined with ImageJ version 1.8.0 software (National Institutes
of Health, Bethesda, MD, USA). The majority of primary antibodies
were purchased from Cell Signaling Technology, Inc. (Danvers, MA,
USA) and were diluted to 1:1,000, whereas anti-β-actin antibodies
were obtained from Shanghai Abmart Co., Ltd. (Shanghai, China) and
were diluted to 1:2,000.
Statistical analysis
SPSS 18.0 software (SPSS, Inc., Chicago, IL, USA)
was used to analyze the data. Quantitative data from triplicate
experiments were presented as the means ± standard deviation.
One-way analysis of variance was used to compare multiple groups
and the least significant difference test was applied to make
comparisons between two groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Construction of a HC model in nude
mice
HC is one of the most severe malignant tumors in the
upper aerodigestive tract. To investigate the effects of AuNRs on
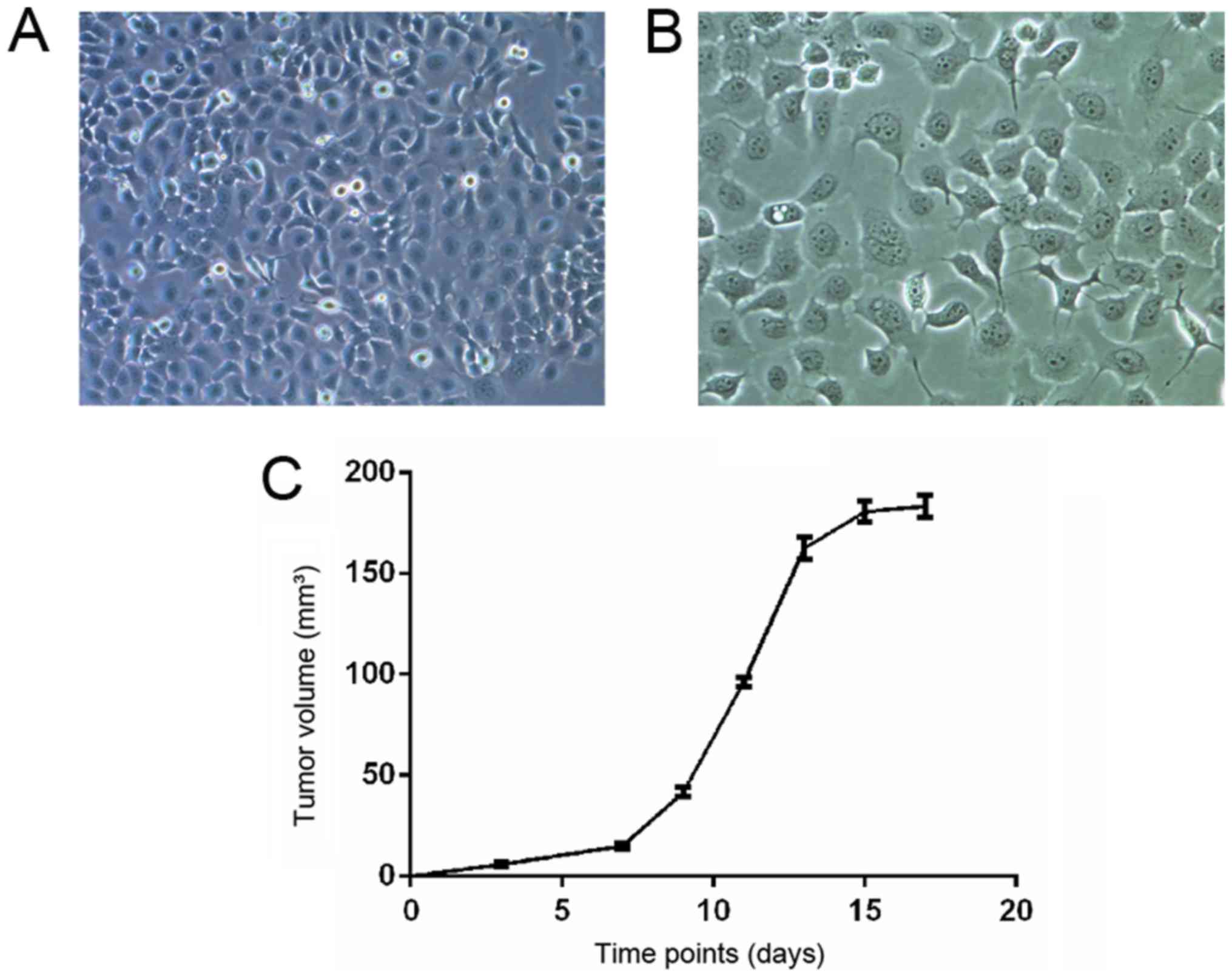
tumor growth in vivo, FaDu HC cells (Fig. 1A and B) were injected into the
backs of nude mice. There was no significant difference in body
weight prior to FaDu cell injection and no mice succumbed during
tumor development. Furthermore, there was no redness or swelling at
the site of injection. After 7 days of inoculation, subcutaneous
nodules with a diameter of ~7 mm developed, thus suggesting the
successful establishment of a HC model.
After FaDu cell injection, tumor growth was observed
and recorded every other day. As shown in Fig. 1C, the tumor gradually grew from the
first week, whereas tumor growth was more rapid in the second
week.
AuNR‑mediated photothermal therapy
effectively reduces tumor growth in vivo, and the inhibitory effect
is enhanced by EGFRmAb conjugation
Tumor size was measured before treatment, and no
significant difference was observed among the seven groups
(Table I). In the control group,
tumor volume was 179.00±3.30 mm3 prior to injection and
reached 268.57±24.98 mm3 72 h post-injection. Tumors
grew from 179.66±2.00 to 259.37±22.70 mm3 in the
AuNRs.it group, from 178.73±3.01 to 264.64±17.63 mm3 in
the NIR group, and from 180.39±1.46 to 252.76±10.85 mm3
in the AuNRs.iv + NIR group. There were no obvious differences in
tumor growth between the control group and the three aforementioned
experimental groups (P>0.05; Fig.
2 and Table I). Furthermore,
tumors were dissected from nude mice 72 h post-treatment and tumor
weight was measured. As shown in Fig.
2 and Table I, tumor weight
was similar among the control, AuNRs.it, NIR and AuNRs.iv + NIR
groups. These results suggested that AuNRs alone, NIR alone and
AuNRs.iv + NIR had no effect on tumor growth.
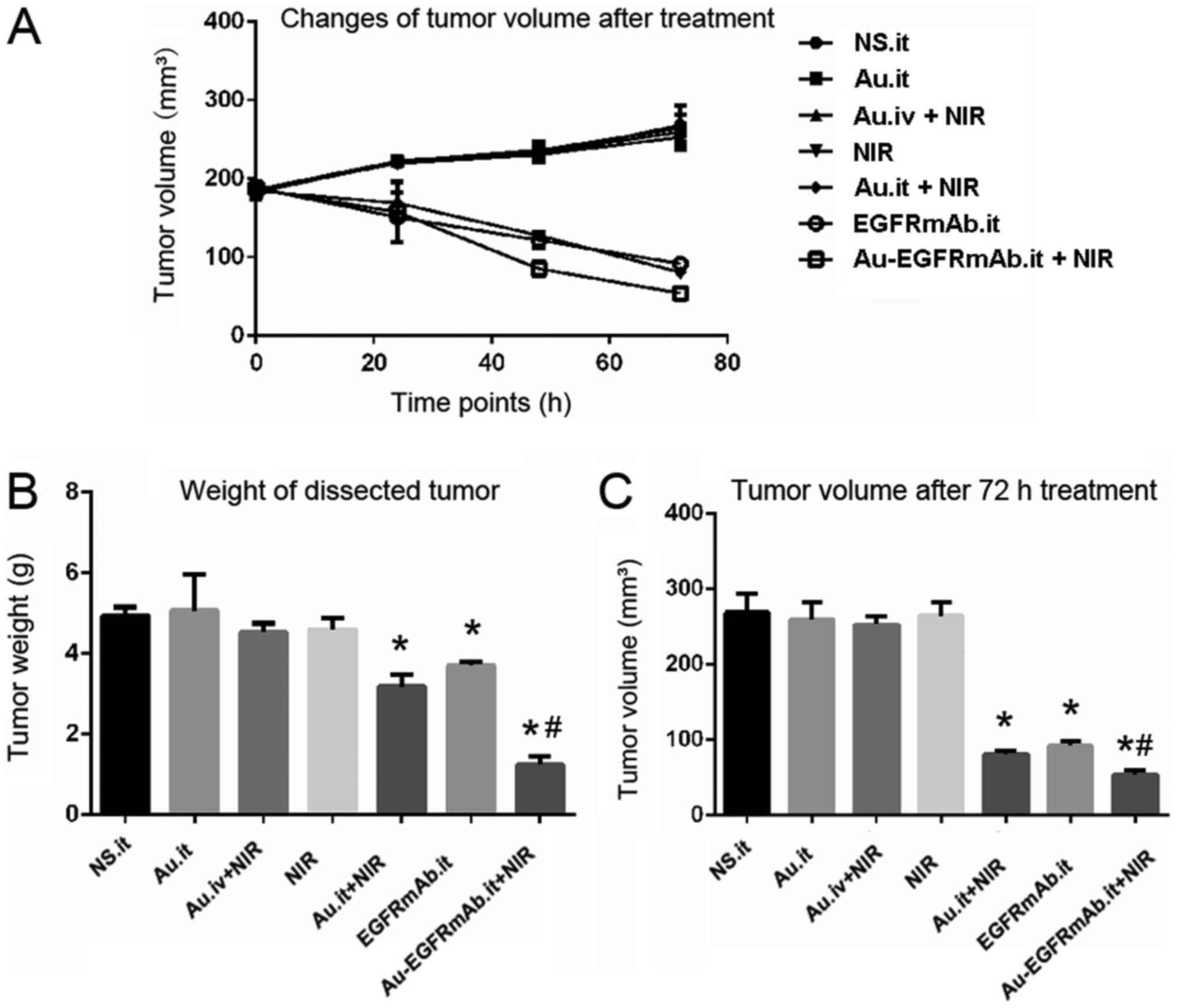 | Figure 2EGFRmAb increases the tumor
inhibitory effects of AuNRs on nude mice. (A) Tumor growth curves
following treatment. (B) Weight of the dissected tumor. After
treatment, mice in the control, Au.it, Au.iv + NIR and NIR groups
exhibited similar tumor growth. (C) Tumor volume after 72 h
treatment. The tumor volume in mice treated with Au.it + NIR,
EGFRmAb.it and Au‑EGFRmAb.it + NIR was significantly decreased.
*P<0.05 compared with the NS.it group;
#P<0.05 compared with the Au.it + NIR and EGFRmAb
groups. Au/AuNRs, gold nanorods; EGFRmAb, epidermal growth factor
monoclonal antibody; it, intratumoral; iv, intravenous; NS, normal
saline; NIR, near infrared spectroscopy. |
 | Table ITumor volume before and after
treatment (n=10). |
Table I
Tumor volume before and after
treatment (n=10).
| Group | Before treatment
| After treatment
|
|---|
| Tumor volume
(mm3) | Tumor volume
(mm3) | Tumor weight
(g) |
|---|
| NS.it | 179.00±3.30 |
268.57±24.98a | 4.93±0.21 |
| Au.it | 179.66±2.00 |
259.37±22.70a | 5.07±0.91 |
| Au.iv + NIR | 180.39±1.46 |
252.76±10.85a | 4.53±0.21 |
| NIR | 178.73±3.01 |
264.64±17.63a | 4.60±0.27 |
| Au.it + NIR | 179.53±2.04 | 80.99±4.18a-e | 3.18±0.31a-d |
| EGFRmAb.it | 180.18±0.83 | 91.95±5.93a,b | 3.70±0.10a |
| EGFRmAb-Au.it +
NIR | 179.24±2.48 | 54.12±5.33a,b,e-g | 1.23±0.21a,d-f |
The present study also determined whether local
application of AuNRs + NIR inhibited tumor growth. Notably, tumor
volume decreased by 54.89% (from 179.53±2.04 to 80.99±4.18
mm3) in the AuNRs.it + NIR group (P<0.05). In
addition, the tumor weight was 35.5% less compared with in the
control group (P<0.05). In the EGFRmAb.it group, tumor size was
reduced by 48.97% from 180.18±0.83 to 91.95±5.93 mm3
(P<0.05), and the tumor weight was 24.95% less than the weight
in the control group (P<0.05). EGFRmAb was conjugated to AuNRs
and injected into the tumor, followed by NIR. The results indicated
that EGFRmAb-ANRS.it + NIR presented significantly stronger tumor
inhibition efficiency than AuNRs.it + NIR or EGFRmAb (P<0.05).
The EGFRmAb-AuNRs.it + NIR mice exhibited the smallest tumor volume
(54.12±5.33 mm3) and the inhibition rate was highest
(69.8%). The tumor weight was 1.23±0.21 g, and the inhibition rate
was 75.1% (Fig. 2 and Table I).
EGFRmAb conjugation increases
AuNRs‑mediated apoptosis
The reduced tumor size and weight may be due to
increased cell apoptosis or decreased proliferation. Therefore,
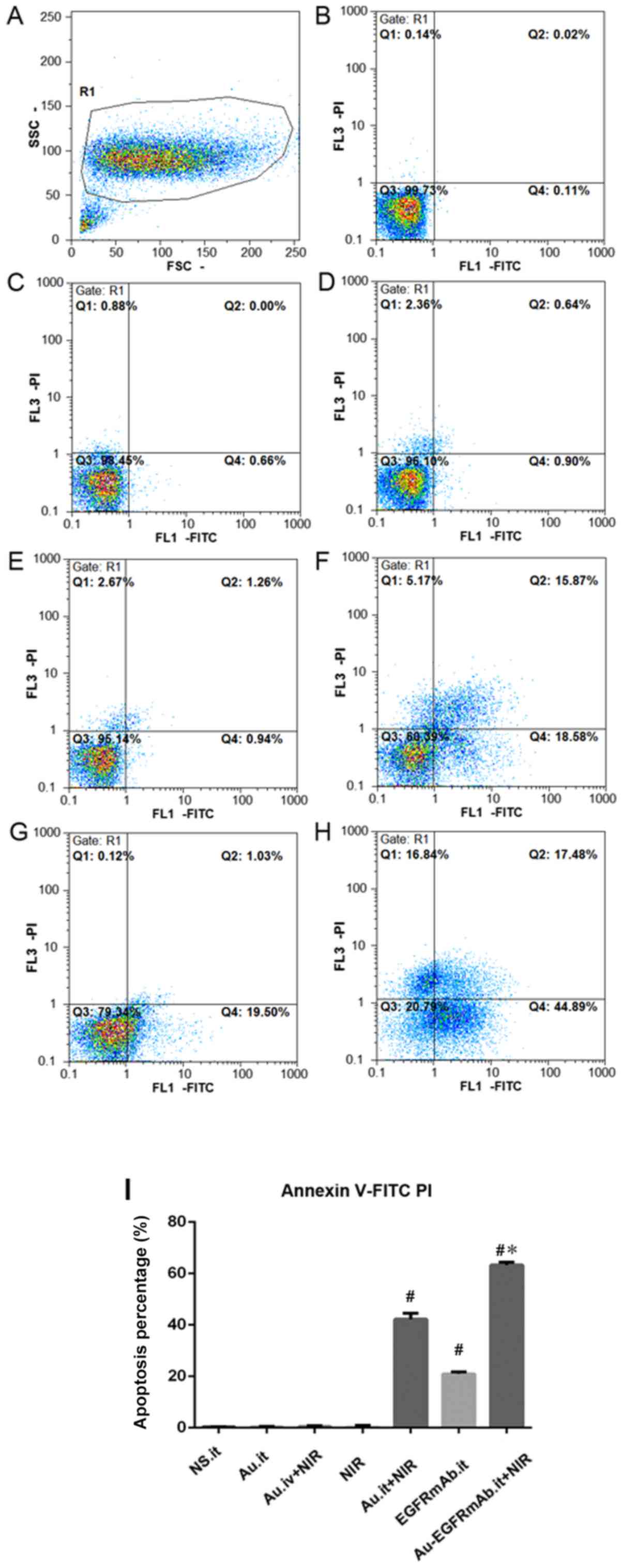
flow cytometry was performed to detect apoptosis of tumor cells in
each group. The results suggested that apoptotic rates in the
AuNRs.it, NIR and AuNRs.iv + NIR groups were not markedly different
compared with the control group. However, mice treated with
AuNRs.it + NIR or EGFRmAb alone exhibited markedly increased
apoptosis of tumor cells compared with in the control group
(P<0.05; Fig. 3 and Table II). There was no significant
difference between the AuNRs.it + NIR and EGFRmAb groups.
Furthermore, apoptosis was significantly increased in the
EGFRmAb-AuNRs.it + NIR group compared with in the AuNRs.it + NIR or
EGFRmAb groups (P<0.05; Fig. 3
and Table II). These results
suggested that EGFRmAb may act synergistically with AuNRs and may
trigger apoptosis following NIR treatment.
 | Table IIComparison of apoptotic rate in tumor
tissues from each group (n=5). |
Table II
Comparison of apoptotic rate in tumor
tissues from each group (n=5).
| Group | Apoptotic rate
(%) |
|---|
| NS.it | 0.29±0.14 |
| Au.it | 0.38±0.21 |
| Au.iv + NIR | 0.47±0.29 |
| NIR | 0.57±0.34 |
| Au.it + NIR | 42.23±2.33a-d |
| EGFRmAb.it | 20.97±0.72a |
| EGFRmAb-Au.it +
NIR | 63.30±1.11a,d-f |
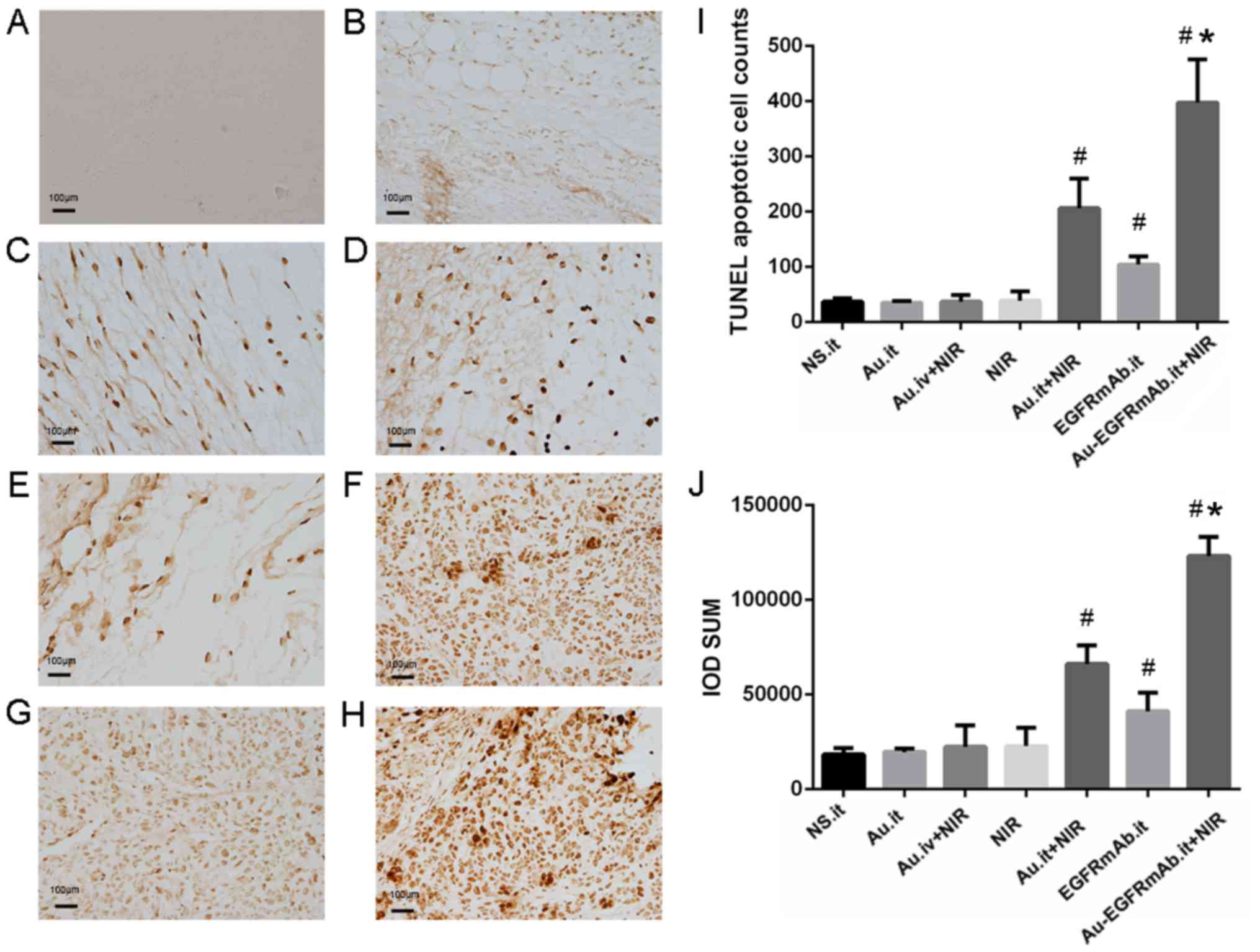
The number of apoptotic cells was counted and the
integrated optical density (IOD) of positively stained cells was
calculated by individual researchers that were blinded to the
groups. As shown in Fig. 4,
apoptotic cells were stained brown. In the control group, few
apoptotic cells were observed (37.25±6.24 cells), and the total IOD
was 18,413.80±3,268.79. Consistent with the flow cytometry data,
TUNEL assays indicated that the number of apoptotic cells and the
IOD value in the AuNRs.it, NIR and AuNRs.iv + NIR groups were
similar to the control group (P>0.05). However, the number of
apoptotic cells in the AuNRs.it + NIR, EGFRmAb.it and
EGFRmAb-AuNRs.it + NIR groups were ~5.5, 2.8 and 10.7 times higher
than in the control group; these differences were all statistically
significant (P<0.05; Fig. 4 and
Table III). Furthermore, the IOD
values in these three groups were significantly higher than in the
control group (P<0.05; Fig. 4
and Table III). In all test
groups, the most marked apoptotic effect was detected in mice
treated with EGFRmAb-AuNRs. it + NIR, further confirming the
synergistic effect of EGFRmAb on AuNR-induced PPTT.
 | Table IIIApoptotic cells and IOD values, as
detected using terminal-deoxynucleotidyl transferase dUTP nick end
labeling assays (n=5). |
Table III
Apoptotic cells and IOD values, as
detected using terminal-deoxynucleotidyl transferase dUTP nick end
labeling assays (n=5).
| Group | Number of apoptotic
cells | IOD sum |
|---|
| NS.it | 37.25±6.24 |
18,413.80±3,268.79 |
| Au.it | 35.00±3.37 |
19,634.14±1,860.90 |
| Au.iv + NIR | 37.25±12.09 |
22,441.31±11,318.44 |
| NIR | 39.00±16.85 |
22,774.24±9,644.58 |
| Au.it + NIR |
206.50±53.17a-e |
66,185.23±9,857.81a-e |
| EGFRmAb.it |
104.50±14.66a |
41,146.04±9,966.85a |
| EGFRmAb-Au.it +
NIR |
397.75±77.68a,d-f |
123,179.31±10,132.38a,d-f |
EGFRmAb‑AuNRs PPTT inhibits the PI3K/Akt
signaling pathway by increasing PTEN expression
Since AuNR-mediated PPTT inhibited tumor growth in
nude mice, and EGFRmAb enhanced the effect, the present study
further investigated the underlying molecular mechanism. The
PI3K/Akt pathway is activated in various types of cancer by EGFR,
and it has been reported that EGFR is overexpressed in HC (17). Akt and GSK3β are key factors of the
PI3K/Akt pathway; therefore, their mRNA expression levels were
detected using RT-qPCR. The results indicated that the mRNA
expression levels of Akt and GSK3β were similar in mice treated
with AuNRs.it, AuNRs. iv + NIR and NIR compared with in the control
group (P>0.05; Fig. 5A and B).
Compared with in the control group, the mRNA expression levels of
Akt and GSK3β were significantly reduced in mice treated with
AuNRs.it + NIR, EGFRmAb. it or EGFRmAb-AuNRs.it + NIR (P<0.01;
Fig. 5A and B). Since PTEN is a
negative regulator of the PI3K/Akt pathway, the present study aimed
to determine whether an increase in PTEN may contribute to reduced
activation of the PI3K/Akt pathway. The results of a RT-qPCR
analysis indicated that, compared with in the control group, PTEN
mRNA expression was not markedly altered in the AuNRs.it, AuNRs.iv
+ NIR and NIR groups (P>0.05), but was obviously increased in
the AuNRs.it + NIR, EGFRmAb.it and EGFRmAb-AuNRs. it + NIR groups
(P<0.05; Fig. 5C). In addition,
conjugation of EGFRmAb to AuNRs (EGFRmAb-AuNRs.it + NIR) markedly
reduced the mRNA expression levels of Akt and GSK3β, and increased
PTEN expression compared with in the AuNRs. it + NIR or EGFRmAb.it
groups (P<0.05; Fig. 5A–C).
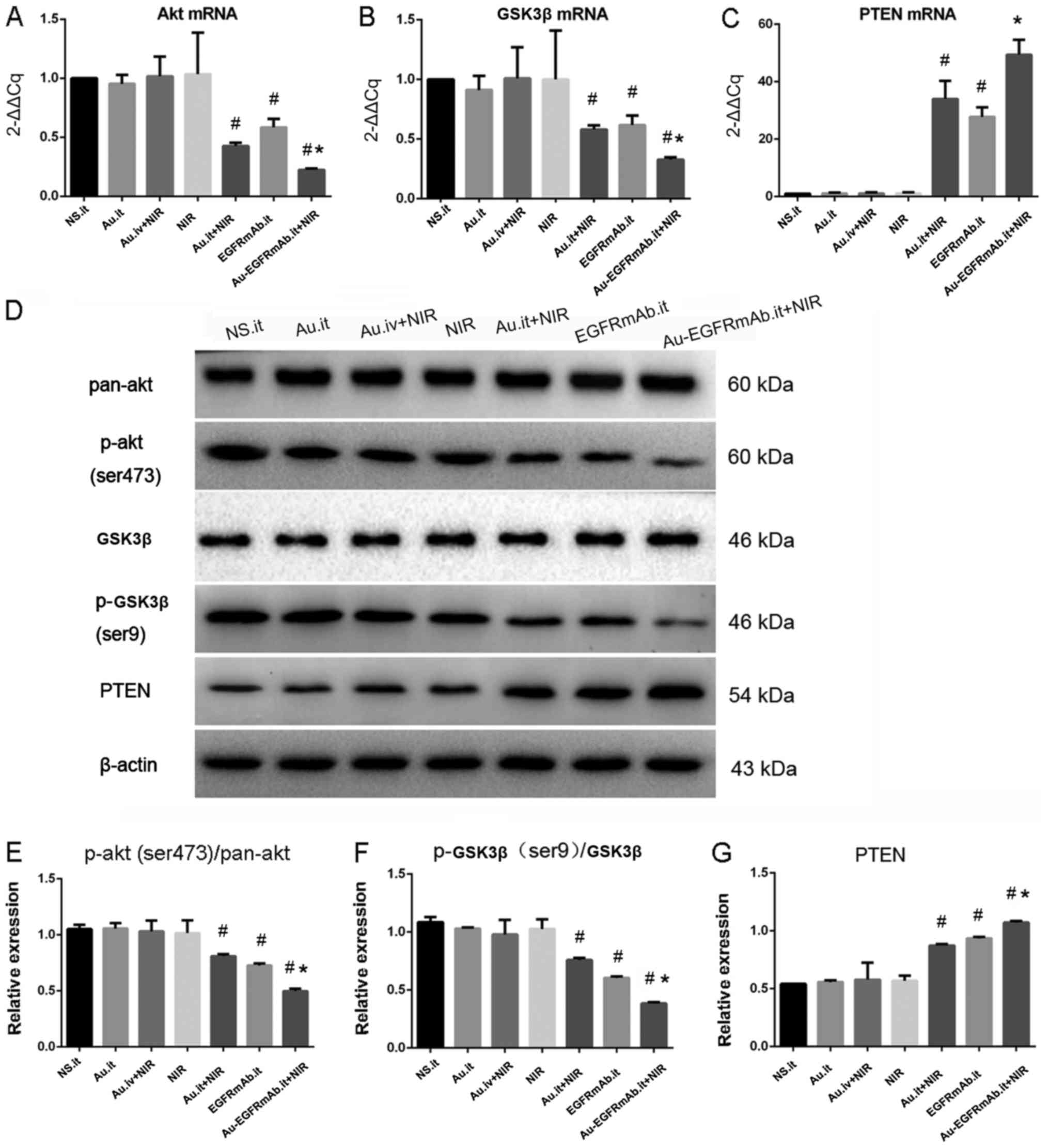 | Figure 5AuNRs plasmonic photothermal therapy
inhibits the phosphatidylinositol-3-kinase/Akt pathway, and EGFRmAb
conjugation enhances the effect. (A–C) mRNA expression levels of
Akt, GSK3 and PTEN. mRNA expression of each sample was detected by
reverse transcription-quantitative polymerase chain reaction. The
expression data of each group were normalized to the control group
(N.S.it). (D–G) Protein expression levels of p-Akt, p-GSK3β and
PTEN. Relative protein expression levels are presented in the bar
charts. β-actin was used as a control. #P<0.05
compared with the NS.it group; *P<0.05 compared with
the Au.it + NIR and EGFRmAb.it groups. All experiments were
repeated at least three times. Akt, AKT serine/threonine kinase;
Au, gold nanorods; EGFRmAb, epidermal growth factor monoclonal
antibody; GSK3β, glycogen synthase kinase 3β; it, intratumoral; iv,
intravenous; NS, normal saline; NIR, near infrared spectroscopy; p,
phosphorylated; PTEN, phosphatase and tensin homolog. |
To further verify the effects of AuNRs.it + NIR,
EGFRmAb. it and EGFRmAb-AuNRs.it + NIR on p-Akt, p-GSK3β and PTEN
expression, western blotting was conducted to detect their protein
levels. As shown in Fig. 5D–G, the
protein expression levels of p-Akt, p-GSK3β and PTEN were not
altered in the AuNRs.it, NIR and AuNRs.iv + NIR groups compared
with in the control group (P>0.05; Fig. 5D–G). However, treatment with
AuNRs.it + NIR, EGFRmAb. it and EGFRmAb-AuNRs.it + NIR
significantly reduced p-Akt and p-GSK3β protein expression, but
increased PTEN expression (P<0.01; Fig. 5D–G). Furthermore, p-Akt and p-GSK3β
were much lower, and PTEN was much higher, in the EGFRmAb-AuNRs.it
+ NIR group compared with in the AuNRs.it + NIR or EGFRmAb.it
groups (P<0.05; Fig. 5D–G).
These results suggested that EGFRmAb may synergistically interact
with AuNRs to inhibit the PI3K/Akt pathway, potentially through
increasing PTEN expression.
AuNR‑mediated PPTT activates the DNA
damage signaling pathway, and EGFRmAb conjugation enhances the
effect
The DNA damage pathway serves an important role in
cell apoptosis; therefore, the present study aimed to determine
whether it contributed to apoptosis triggered by AuNR-mediated PPTT
in vivo. RT-qPCR was performed to determine the relative
mRNA expression levels of key factors in this pathway, such as ATR,
Chk1 and p53. For each gene, no obvious changes were observed among
the control, AuNRs.it, AuNRs.iv + NIR and NIR groups (P>0.05;
Fig. 6A–C). Compared with in the
control group, the mRNA expression levels of all three genes were
significantly augmented in mice treated with AuNRs.it + NIR,
EGFRmAb. it or EGFRmAb-AuNRs.it + NIR (P<0.05; Fig. 6A–C). Furthermore, the
EGFRmAb-AuNRs.it + NIR group exhibited the highest mRNA expression
of all three genes, and the differences were statistically
significant compared with the AuNRs. it + NIR and EGFRmAb.it groups
(P<0.05; Fig. 6A–C).
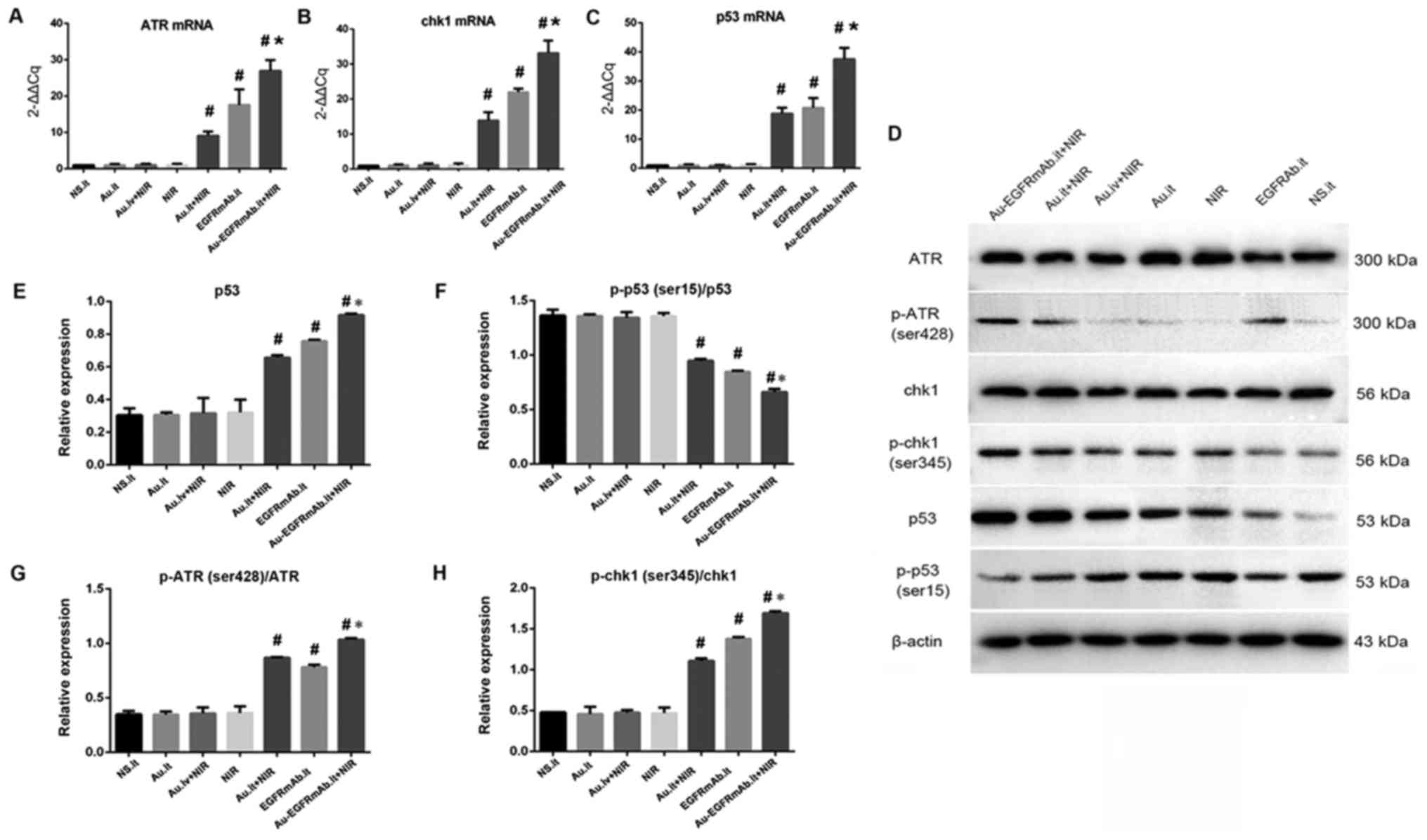 | Figure 6AuNRs plasmonic photothermal therapy
activates the DNA damage signaling pathway, and EGFRmAb conjugation
enhances the effect. (A–C) mRNA expression levels of ATR, Chk1 and
p53. mRNA expression of each sample was detected by reverse
transcription-quantitative polymerase chain reaction. The
expression data of each group were normalized to the control group
(N.S.it). (D-H) Protein expression levels of p-ATR, p-Chk1, p53 and
p-p53. Relative protein expression levels are presented in the bar
charts. β-actin was used as a control. #P<0.05
compared with the NS.it group; *P<0.05 compared with
the Au.it + NIR and EGFRmAb.it groups. All experiments were
repeated at least three times. ATR, ATR serine/threonine kinase;
Au, gold nanorods; Chk1, checkpoint kinase 1; EGFRmAb, epidermal
growth factor monoclonal antibody; it, intratumoral; iv,
intravenous; NS, normal saline; NIR, near infrared spectroscopy; p,
phosphorylated. |
To further verify the effects of AuNRs.it + NIR,
EGFRmAb.it and EGFRmAb-AuNRs.it + NIR on the DNA damage pathway,
western blotting was performed to detect relative protein levels.
As shown in Fig. 6D–H, the AuNRs.
it, NIR and AuNRs.iv + NIR groups exhibited similar expression
levels of all detected proteins compared with the control group
(P>0.05). However, treatment with AuNRs.it + NIR, EGFRmAb.it and
EGFRmAb‑AuNRs.it + NIR significantly increased p-ATR, p-Chk1 and
p53 protein expression, but decreased p-p53 expression (P<0.05).
The most marked alterations were detected in the EGFRmAb-AuNRs.it +
NIR group, and the differences were statistically significant
compared with the AuNRs.it + NIR and the EGFRmAb.it groups
(P<0.05; Fig. 6D–H). These
results suggested that EGFRmAb may synergistically interact with
AuNRs to activate the DNA damage signaling pathway under NIR.
Discussion
HC is one of the most common malignant tumors of the
head and neck, the survival rate of which is low due to
difficulties in its diagnosis and surgery; therefore, the
development of novel treatments for HC is essential. AuNRs absorb
NIR and efficiently convert light into thermal energy.
AuNR‑mediated PPTT has been effectively adopted for the treatment
of cancer in animal models; however, AuNRs alone have a poor
specificity to tumor cells and a high cytotoxicity to normal
tissues, as previously reported (6). It has been suggested that EGFR is
overexpressed on the cell surface of several tumors, and
EGFRmAb‑AuNRs have exhibited enhanced specificity to cancer cells.
For example, Durr et al (19) revealed that EGFRmAb‑AuNRs
specifically recognize epithelial cancer cells. In addition,
Dickerson et al (20)
constructed a mouse model of squamous carcinoma by injecting mice
with human HSC-3 cells; the results revealed that, compared with in
the control groups, EGFRmAb-AuNRs significantly inhibit tumor
growth in vivo. These studies indicated that anti-EGFR
antibodies may specifically target AuNRs to cancer cells and
greatly improve the specificity of AuNR‑induced PPTT; however, its
effect on HC remains unclear.
The present study investigated the effects of
EGFRmAb-AuNRs on inhibiting tumor growth in BALB/C(nu/nu) nude
mice, which are widely used for the study of malignant tumors. The
effects of intravenous injection of AuNRs followed by NIR were
tested; however, tumor growth was similar to that in the control
group. This finding may be due to the outward growth phenomenon of
tumor vessels and the first-pass elimination effect of the
reticuloendothelial system; these factors may prevent the effective
accumulation of AuNRs in tumor cells. Compared with intravenous
administration, local treatment was more selective and effective;
NIR triggered AuNR-mediated PPTT to prevent tumor growth in
vivo. In addition, EGFRmAb conjugation was revealed to
effectively target AuNRs to cancer cells and greatly enhance the
inhibitory effects of AuNRs-mediated PPTT.
It was suggested that the reduced tumor size and
weight caused by AuNR-mediated PPTT may be attributed to increased
apoptosis. To investigate this hypothesis, flow cytometry and TUNEL
experiments were performed separately, in order to detect apoptotic
cells in isolated tumors. The results were consistent, compared
with in the control group, the AuNRs.it + NIR, EGFRmAb. it and
EGFRmAb-AuNRs.it + NIR groups exhibited increased apoptosis. In
addition, the apoptotic rate in the EGFRmAb-AuNRs.it + NIR group
was markedly higher than in the AuNRs.it + NIR and EGFRmAb.it
groups. These observations indicated that EGFRmAb conjugation may
enhance apoptosis induced by AuNRs + NIR, which in turn may
contribute to reduced tumor growth in nude mice.
EGFR is a transmembrane tyrosine kinase receptor. It
is well known that EGFR overexpression is associated with the
occurrence and poor prognosis of numerous malignant tumors
(21). EGFRmAb can block the
binding of EGFR with epidermal growth factors, thus inhibiting
downstream pathways activated by EGFR. Cetuximab is an EGFRmAb that
targets the extracellular domain of EGFR (22); it has been used to treat head and
neck cancer by specifically blocking the activation of EGFR
downstream pathways. Bonner et al (23,24)
compared the effects of cetuximab + radiotherapy and radiotherapy
alone on patients with advanced oropharyngeal and laryngeal cancer,
and HC. The results revealed that the survival rate of patients
receiving cetuximab + radiotherapy is significantly higher than the
rate of patients receiving radiotherapy only. In the present study,
local injection of EGFRmAb into tumors induced apoptosis and
decreased tumor growth. It is possible that EGFRmAb conjugation not
only improves the specificity of AuNRs targeting to tumor cells,
but also blocks EGFR-activated pathways, thus leading to the
enhanced growth inhibitory effects of EGFRmAb-AuNRs.it + NIR.
The PI3K/Akt signaling pathway is activated by EGFR
overexpression in various types of cancer and serves an important
role in preventing apoptosis, promoting proliferation and
increasing metastasis, etc. (25).
PI3K/Akt pathway inhibitors, such as LY294002 and Wortmannin, have
been used to treat cancer (26,27).
When the pathway is activated,
phosphatidylinositol-3,4,5-trisphosphate is increased, which in
turn phosphorylates Akt, the key factor of the pathway (28,29).
Compared with in the control group, Akt levels were
reduced in the AuNRs.it + NIR, EGFRmAb.it and EGFRmAb-AuNRs. it +
NIR groups. Furthermore, the downstream factor GSK3β (30) was also decreased in these three
groups. Notably, the most marked alterations in Akt and GSK3β
expression occurred in the EGFRmAb-AuNRs.it + NIR group. To further
confirm the inhibition of the PI3K/Akt pathway, the present study
determined the effects of various treatments on PTEN, which is a
negative regulator of the PI3K/Akt pathway (31,32).
PTEN mRNA and protein levels were significantly augmented in the
AuNRs.it + NIR, EGFRmAb.it and EGFRmAb-AuNRs.it + NIR groups; the
increase was markedly increased in the EGFRmAb-AuNRs.it + NIR group
compared with in the other two groups. Taken together, AuNRs.it +
NIR may downregulate the PI3K/Akt signaling pathway by decreasing
Akt and GSK3β, as well as increasing PTEN. EGFRmAb conjugation
further increased the inhibition of this pathway.
When DNA damage cannot be repaired in a timely
manner, apoptosis occurs. Clinically, chemotherapy and radiotherapy
cause serious DNA damage to tumor cells, inducing cell apoptosis
and ultimately killing the tumor cells (33). Therefore, the present study
investigated the role of the DNA damage pathway in AuNRs-mediated
tumor inhibition. ATR is a key factor of this pathway. In response
to DNA damage, ATR is phosphorylated, consequently phosphorylating
Chk1 and activating p53 to induce cell death (34). In the present study, the mRNA and
protein expression levels of ATR, Chk1 and p53 were detected. The
results indicated that AuNRs augmented the mRNA expression of all
three genes, and EGFRmAb conjugation further enhanced mRNA
expression, as compared with single treatments using AuNRs or
EGFRmAb. Furthermore, all three local treatments (AuNRs.it + NIR,
EGFRmAb.it and EGFRmAb-AuNRs.it + NIR) significantly upregulated
p-ATR, p-Chk1 and p53. Similar with other results, the most marked
increase was observed in the EGFRmAb-AuNRs.it + NIR group. Overall,
AuNRs.it + NIR may trigger apoptosis partially by activating the
DNA damage signaling pathway, and EGFRmAb conjugation further
increased the effect. AuNR‑based PPTT has shown significant promise
for the selective ablation of cancer cells (35). It has been reported that AuNRs act
as efficient PPTT agents that induce cell death through
hyperthermia while scavenging the reactive oxygen species (ROS)
produced during treatment (35).
ROS can potentially interact with neighboring healthy tissues,
causing irreversible damage to DNA (36).
There were some limitations to the present study.
Firstly, tumor targeting was not investigated, since
administrations were intratumoral. Secondly, analysis of the
associated signaling pathway was insufficient, as other signaling
pathways, including oxidative stress pathways, were not
investigated. Therefore, further study on the underlying mechanism
is required.
In conclusion, the present study revealed that
EGFRmAb conjugation enhanced the inhibitory effects of
AuNRs-mediated PPTT on tumor growth in a mouse model of HC. Based
on our previous studies and the present study, EGFRmAb may target
AuNRs to tumor cells by binding to EGFR. The enhanced specificity
of AuNRs increased their inhibitory effects on tumor growth. In
addition, EGFRmAb conjugation may also increase tumor cell
apoptosis by down-regulating the PI3K/Akt pathway and upregulating
the DNA damage pathway. These results provided novel insights into
the effects of targeted PPTT on cancer treatment, particularly HC
treatment.
Funding
This study was supported by the National Natural
Science Foundation of China (grant no. 81160324).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors’ contributions
YZh analyzed the experimental data, perfomed in
vitro experiments and was a major contributor in writing the
manuscript. JH analyzed the experimental data. YW and JW performed
the in vivo experiments; YZo and ZY performed the in
vitro experiments. XH designed the study and provided the
research funds.
Ethics approval and consent to
participate
All experimental procedures were conducted in
accordance with the institutional guidelines for the care and use
of laboratory animals. The present study was approved by the
Institutional Animal Care and Use Committee of Kunming Medical
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank the Kunming
Institute of Precious Metals (Kunming, China) and the National
Center for Nanoscience and Technology (Kunming, China) for
technical support during the study.
References
|
1
|
Rivière D, Mancini J, Santini L, Giovanni
A, Dessi P and Fakhry N: Lymph-node metastasis following total
laryngectomy and total pharyngolaryngectomy for laryngeal and
hypopharyngeal squamous cell carcinoma: Frequency, distribution and
risk factors. Eur Ann Otorhinolaryngol Head Neck Dis. 135:163–166.
2018. View Article : Google Scholar
|
|
2
|
Krstevska V, Stojkovski I and Lukarski D:
Concurrent radiochemotherapy in advanced hypopharyngeal cancer.
Radiat Oncol. 5:392010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cabral RM and Baptista PV: Anti-cancer
precision theranostics: A focus on multifunctional gold
nanoparticles. Expert Rev Mol Diagn. 14:1041–1052. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aioub M and El-Sayed MA: A real-time
surface enhanced raman spectroscopy study of plasmonic photothermal
cell death using targeted gold nanoparticles. J Am Chem Soc.
138:1258–1264. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brand TM, Iida M, Li C and Wheeler DL: The
nuclear epidermal growth factor receptor signaling network and its
role in cancer. Discov Med. 12:419–432. 2011.PubMed/NCBI
|
|
6
|
Zhang S, Li Y, He X, Dong S, Huang Y, Li
X, Li Y, Jin C, Zhang Y and Wang Y: Photothermolysis mediated by
gold nanorods modified with EGFR monoclonal antibody induces Hep-2
cells apoptosis in vitro and in vivo. Int J Nanomedicine.
9:1931–1946. 2014.PubMed/NCBI
|
|
7
|
Normanno N, De Luca A, Bianco C, Strizzi
L, Mancino M, Maiello MR, Carotenuto A, De Feo G, Caponigro F and
Salomon DS: Epidermal growth factor receptor (EGFR) signaling in
cancer. Gene. 366:2–16. 2006. View Article : Google Scholar
|
|
8
|
Garrett TP, McKern NM, Lou M, Elleman TC,
Adams TE, Lovrecz GO, Zhu HJ, Walker F, Frenkel MJ, Hoyne PA, et
al: Crystal structure of a truncated epidermal growth factor
receptor extracellular domain bound to transforming growth factor
alpha. Cell. 110:763–773. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sourbier C, Lindner V, Lang H, Agouni A,
Schordan E, Danilin S, Rothhut S, Jacqmin D, Helwig JJ and
Massfelder T: The phosphoinositide 3-kinase/Akt pathway: A new
target in human renal cell carcinoma therapy. Cancer Res.
66:5130–5142. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hotfilder M, Sondermann P, Senss A, van
Valen F, Jürgens H and Vormoor J: PI3K/AKT is involved in mediating
survival signals that rescue Ewing tumour cells from fibroblast
growth factor 2-induced cell death. Br J Cancer. 92:705–710. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sandra CM, Eduardo CC, Simon HO, Teresa
RA, Antonio NC, Lijanova IV and Marcos MG: Anticancer activity and
anti-inflammatory studies of 5‑aryl‑1,4‑benzodiazepine derivatives.
Anticancer Agents Med Chem. 12:611–618. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Clemens MJ: Targets and mechanisms for the
regulation of translation in malignant transformation. Oncogene.
23:3180–3188. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vignard J, Mirey G and Salles B:
Ionizing-radiation induced DNA double-strand breaks: A direct and
indirect lighting up. Radiother Oncol. 108:362–369. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ciccia A and Elledge SJ: The DNA damage
response: Making it safe to play with knives. Mol Cell. 40:179–204.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Abraham RT: Cell cycle checkpoint
signaling through the ATM and ATR kinases. Genes Dev. 15:2177–2196.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Demoulin B, Hermant M, Castrogiovanni C,
Staudt C and Dumont P: Resveratrol induces DNA damage in colon
cancer cells by poisoning topoisomerase II and activates the ATM
kinase to trigger p53-dependent apoptosis. Toxicol In Vitro.
29:1156–1165. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang Y, Cong L, He J, Wang Y, Zou Y, Yang
Z, Hu Y, Zhang S and He X: Photothermal treatment with
EGFRmAb-AuNPs induces apoptosis in hypopharyngeal carcinoma cells
via PI3K/AKT/mTOR and DNA damage response pathways. Acta Biochim
Biophys Sin (Shanghai). 50:567–578. 2018. View Article : Google Scholar
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
19
|
Durr NJ, Larson T, Smith DK, Korgel BA,
Sokolov K and Ben-Yakar A: Two-photon luminescence imaging of
cancer cells using molecularly targeted gold nanorods. Nano Lett.
7:941–945. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dickerson EB, Dreaden EC, Huang X,
El-Sayed IH, Chu H, Pushpanketh S, McDonald JF and El-Sayed MA:
Gold nanorod assisted near-infrared plasmonic photothermal therapy
(PPTT) of squamous cell carcinoma in mice. Cancer Lett. 269:57–66.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Castellani R, Visscher DW, Wykes S, Sarkar
FH and Crissman JD: Interaction of transforming growth factor-alpha
and epidermal growth factor receptor in breast carcinoma. An
immunohistologic study. Cancer. 73:344–349. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Giampieri R, Scartozzi M, Del Prete M,
Maccaroni E, Bittoni A, Faloppi L, Bianconi M, Cecchini L and
Cascinu S: Molecular biomarkers of resistance to anti-EGFR
treatment in metastatic colorectal cancer, from classical to
innovation. Crit Rev Oncol Hematol. 88:272–283. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bonner JA, Harari PM, Giralt J, Azarnia N,
Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, et al:
Radiotherapy plus cetuximab for squamous-cell carcinoma of the head
and neck. N Engl J Med. 354:567–578. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bonner JA, Harari PM, Giralt J, Cohen RB,
Jones CU, Sur RK, Raben D, Baselga J, Spencer SA, Zhu J, et al:
Radiotherapy plus cetuximab for locoregionally advanced head and
neck cancer: 5-year survival data from a phase 3 randomised trial,
and relation between cetuximab-induced rash and survival. Lancet
Oncol. 11:21–28. 2010. View Article : Google Scholar
|
|
25
|
Ahlmann E, Patzakis M, Roidis N, Shepherd
L and Holtom P: Comparison of anterior and posterior iliac crest
bone grafts in terms of harvest-site morbidity and functional
outcomes. J Bone Joint Surg Am. 84-A:716–720. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
LoPiccolo J, Blumenthal GM, Bernstein WB
and Dennis PA: Targeting the PI3K/Akt/mTOR pathway: effective
combinations and clinical considerations. Drug Resist Updat.
11:32–50. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Catasus L, D’Angelo E, Pons C, Espinosa I
and Prat J: Expression profiling of 22 genes involved in the
PI3K‑AKT pathway identifies two subgroups of high‑grade endometrial
carcinomas with different molecular alterations. Mod Pathol.
23:694–702. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Priulla M, Calastretti A, Bruno P,
Azzariti A, Paradiso A, Canti G and Nicolin A: Preferential
chemosensitization of PTEN-mutated prostate cells by silencing the
Akt kinase. Prostate. 67:782–789. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang D and Fan D: Multidrug resistance in
gastric cancer: Recent research advances and ongoing therapeutic
challenges. Expert Rev Anticancer Ther. 7:1369–1378. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang L, Dan HC, Sun M, Liu Q, Sun XM,
Feldman RI, Hamilton AD, Polokoff M, Nicosia SV, Herlyn M, et al:
Akt/protein kinase B signaling inhibitor-2, a selective small
molecule inhibitor of Akt signaling with antitumor activity in
cancer cells overexpressing Akt. Cancer Res. 64:4394–4399. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu X, Dai X and Wu B: Study of 5-Aza-CdR
on transcription regulation of RASSF1A gene in the BIU87 cell line.
Urol Int. 82:108–112. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liao W, McNutt MA and Zhu WG: The comet
assay: A sensitive method for detecting DNA damage in individual
cells. Methods. 48:46–53. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lieberman HB: DNA damage repair and
response proteins as targets for cancer therapy. Curr Med Chem.
15:360–367. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Matsumoto K, Nagahara T, Okano J and
Murawaki Y: The growth inhibition of hepatocellular and
cholangiocellular carcinoma cells by gemcitabine and the roles of
extracellular signal-regulated and checkpoint kinases. Oncol Rep.
20:863–872. 2008.PubMed/NCBI
|
|
35
|
Aioub M, Panikkanvalappil SR and El-Sayed
MA: Platinum-coated gold nanorods: Efficient reactive oxygen
scavengers that prevent oxidative damage toward healthy, untreated
cells during plasmonic photothermal therapy. ACS Nano. 11:579–586.
2017. View Article : Google Scholar
|
|
36
|
Schieber M and Chandel NS: ROS function in
redox signaling and oxidative stress. Curr Biol. 24:R453–R462.
2014. View Article : Google Scholar : PubMed/NCBI
|