Introduction
During the past century, gastric cancer (GC) has
remained the fourth most prevalent type of malignant cancer and the
second leading cause of cancer-associated mortality worldwide
(1). Currently, the majority of
patients with GC are diagnosed at the advanced stage of the disease
due to the lack of effective diagnostic methods at the early
stages. The five-year survival rate of advanced patients with GC is
only 5-20%, and the median overall survival is <1 year (2). For advanced-stage patients,
chemotherapy is the first-line treatment. Unfortunately,
chemotherapy resistance is common, particularly multidrug
resistance (MDR), which affects treatment and prognosis (3,4).
Although the mechanisms underlying MDR have been extensively
explored, the key multiple-drug resistance features of this
clinical phenomenon remain unclear.
Micro (mi)RNAs are short (20-24 nt), stable,
non-coding RNA molecules that negatively regulate 60% of coding
genes by targeting the 3′ untranslated regions (3′UTRs) of specific
mRNAs to prevent translation and/or promote degradation (5). Accumulating evidence indicates that
miRNAs serve important roles in chemoresistance in various types of
cancer, including GC (6-14). However, the exact mechanisms
underlying the regulation of chemoresistance in GC by miRNAs remain
unclear. miR-874 has been reported in numerous types of cancer,
including GC, hepatocellular carcinoma, colorectal cancer, breast
cancer, non-small-cell lung cancer, and head and neck squamous
carcinoma (8,15-18).
In our previous studies, miR-874 was demonstrated to serve a
potential role in tumour growth, apoptosis and angiogenesis in GC.
However, to the best of our knowledge, the association between
miR-874 and chemotherapy drug resistance in GC has not been
reported. The present study demonstrated that miR-874 enhanced the
sensitivity of GC cells to chemotherapy. Furthermore,
autophagy-related 16-like 1 (ATG16L1) was identified as a direct
and functional target of miR-874. In addition, ATG16L1 expression
was revealed to be increased in GC MDR cells, and positively
associated with autophagy and chemotherapy resistance. Lastly, it
was demonstrated that miR-874 expression was downregulated in
chemoresistant patients, and was associated with ATG16L1 expression
and overall survival in patients with GC.
Materials and methods
Tissue samples
The acquisition of tissue specimens and the study
protocol were performed in strict accordance with the regulations
of the Institutional Review Board of Xuzhou Medical University
(Xuzhou, China). All patients signed informed consent forms. Human
GC specimens were collected between January 2010 and December 2012,
and detailed clinicopathological and follow-up information were
obtained from the Tissue Sample Centre of the Affiliated Huai’an
Hospital at Xuzhou Medical University in China. In all cases, the
diagnoses and grading were confirmed by two experienced
pathologists and were performed according to the criteria of the
American Joint Committee on Cancer (19,20).
Cells and cell culture
The human GC cell lines SGC7901, BGC823 (Type
Culture Collection of the Chinese Academy of Sciences, Shanghai,
China), AGS (American Type Culture Collection, Manassas, VA, USA),
and SGC7901/cisplatin (DDP) (established and maintained in our
laboratory) were cultured in RPMI-1640 supplemented with 10% foetal
bovine serum (both from Wisent Biotechnology, Nanjing, China) and
antibiotics (1% penicillin/streptomycin) (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). All cell lines were cultured
in a humidified chamber supplemented with 5% CO2 at
37°C. Cell resistance was induced by gradually increasing the
cisplatin concentration in the culture medium. SGC-7901 cells in
the logarithmic growth phase were seeded in the culture medium,
which contained DDP (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
at a low starting concentration of 0.05 μg/ml. After 48 h,
the culture medium was discarded, fresh medium was added and
culturing was continued. Once normal growth was observed, the cells
were continuously treated with 0.05 μg/ml DDP for 48 h after
digestion and passage. The cells were cultured and passaged in this
manner, and the DDP concentration was gradually increased (the
concentration of DDP was increased by 0.05 μg/ml each time;
range, 0.05-1 μg/ml) to continuously induce the cells.
Finally, a cell line that was tolerant of 1 μg/ml DDP was
established.
RNA extraction, cDNA synthesis and
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR)
Total RNA was extracted from frozen tissues or cells
using TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.) according
to the manufacturer’s protocol. RNA purity was assessed using a
Thermo NanoDrop 2000 (NanoDrop Technologies; Thermo Fisher
Scientific, Inc., Wilmington, DE, USA) using the standard
absorbance ratios of A260/A280 ≥1.8 and A260/A230 ≥1.5.
Complementary DNAs were synthesized from 1 μg of total RNA
using the TaqMan Reverse Transcription kit (Life Technologies;
Thermo Fisher Scientific, Inc.) as follows: 42°C for 15 min; 85°C
for 5 sec; followed by storage at 4°C. To determine the mRNA
expression of ATG16L1 and miR-874, RT-qPCR was performed on a 7500
Real-Time PCR system (Life Technologies; Thermo Fisher Scientific,
Inc.) using Fast Start Universal SYBR-Green Master mix (Roche,
Basel, Switzerland) according to the manufacturer’s protocol. The
following thermocycling conditions were maintained: 95°C for 10
min; followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min.
All mRNA and miRNA quantification data were normalized to GAPDH and
U6, respectively. All experiments were performed independently in
triplicate. The relative expression levels of target genes were
normalized to those of the internal control genes using the
2-ΔΔCq cycle quantification method (21). The primers were as follows: GAPDH
forward, 5′-ATCTCTGCCCCCTCTGCTGA-3′ and reverse,
5′-GATGACCTTGCCCACAGCCT-3′; miR-874 forward,
5′-GGCCCTGAGGAAGAACTGAG-3′ and reverse, 5′-TGAGAT
CCAACAGGCCTTGAC-3′; U6 forward, 5′-GCTTCGGCAGCA CATATACTAAAAT-3′
and reverse, 5′-CGCTTCACGAATTTG CGTGTCAT-3′; and ATG16L1 forward,
5′-AGGACAGGGAGAT GCAGATGA-3′ and reverse,
5′-GATTGGCTTCCTGGGCTTT-3′.
Vector constructs, lentivirus production
and cell transfection
The lentiviral vectors has-miR-874-pre-microRNA
(pre-miR-874) and has-miR-874-pre-inhibitor (miR-874 inhibitor)
were purchased from Shanghai GenePharma Co., Ltd. A scrambled
lentiviral construct (miR-NC) was used as a negative control (NC).
The sequence of pre-miR-874 was 5′-TTAGCCCTGCGG
CCCCACGCACCAGGGTAAGAGAGACTCTCGCTTCCTG
CCCTGGCCCGAGGGACCGACTGGCTGGGC-3′. The sequence of miR-874-inhibitor
was 5′-GCCCAGCCAGTCG GTCCCTCGGGCCAGGGCAGGAAGCGAGAGTCTCTCT
TACCCTGGTGCGTGGGGCCGCAGGGCTAA-3′. The sequence of miR-NC was
5′-TTGTATACAAAAGTACTGGC ATGAAAACACATCATGTTATGTACTACACATTATACTG
GTCAGAACACATATGTTTAA-3′ The lentiviral vectors [multiplicity of
infection (MOI) of 15] were used to transfect GC cells once they
had grown to 40-50% confluence with polybrene (hexadimethrine
bromide) (5 μg/ml) (Sigma-Aldrich; Merck KGaA). After
culturing for 8 h, the culture medium was replaced with fresh
medium. Puromycin culturing was used to select the aforementioned
stable cell lines. Subsequently, the expression of miR-874 was
detected to confirm the efficiency of transfection.
A lentiviral vector containing an shRNA targeting
human ATG16L1 was also purchased from Shanghai GenePharma Co., Ltd.
The target sequence of ATG16L1-shRNA was 5′-GAT
TACTGCCCTGGACTTAAA-3′. The sequence of 5′-CAGTAC TTTTGTGTAGTACAA-3′
was used as a negative control (sh-NC). The cells were infected and
selected as aforementioned. RT-qPCR and western blotting was then
performed to detect the expression of ATG16L1, and confirm the
efficiency of transfection.
In vitro and in vivo drug sensitivity
assays
Each well of a 96-well plate was seeded with
5×103 cells (SGC7901, SGC7901-NC, SGC7901-inhibitor,
SGC7901/DDP, SGC7901/DDP-NC, SGC7901/DDP-pre, SGC7901/DDP-sh-NC,
SGC7901/DDP-sh-ATG16L1 or SGC7901/DDP+CQ). After 24 h, culture
media containing different concentrations of the chemotherapeutic
drugs DDP (0-25 μg/ml), 5-fluorouracil (5-FU) (0-25
μg/ml) and vincristine (VCR) (0-50 μg/ml) (both from
Sigma-Aldrich; Merck KGaA) were added to each well. After the
plates were incubated for 48 h, a Cell Counting Kit 8 (CCK8;
Dojindo Molecular Technologies, Inc., Kumamoto, Japan) assay was
performed. The half maximal inhibitory concentration
(IC50) of each drug was calculated. For the in
vivo experiments, ~2.0×106 cells stably transfected
with pre-miR-874 or NC were inoculated subcutaneously into both
flanks of nude mice. After 2 weeks, the mice were intraperitoneally
injected with PBS containing 5-FU or DDP (10 mg/kg) once weekly.
The tumour size on their skin surfaces was measured once weekly.
The mice were humanely euthanized on day 28, and the tumours were
measured and images. A total of six female nude mice (BALB/c nude
mice, 5-weeks old, 15-18 g) were purchased from Shanghai
Experimental Animal Centre (Shanghai, China) and housed under
specific pathogen-free conditions. All animal experiments were
conducted according to the recommendations of the Xuzhou Medical
University Institutional Animal Care and Use Committee (approval
no. IACUC-201601012).
3′UTR luciferase constructs and
assay
The 3′UTR of ATG16L1 mRNA containing either the
putative or mutated miR-874 binding site was synthesized by Sangon
Biotech Co., Ltd. (Shanghai, China). The cells were co-transfected
with plasmids (Shanghai GenePharma Co., Ltd.) expressing wild-type
ATG16L1 or mutant ATG16L1 in addition to cells stably transfected
with pre-miR-874 or miR-NC. After 36 h of transfection, firefly and
Renilla luciferase activities were measured using the
Dual-Luciferase Reporter assay kit (Promega Corporation, Madison,
WI, USA).
Transmission electron microscopy
(TEM)
Cells were collected and centrifuged at 150 × g, 4°C
for 10 min and then fixed in 2.5% glutaraldehyde in 0.1 M phosphate
buffer (pH 7.2) overnight at 4°C. Next, the cells were fixed in 1%
OsO4 for 1 h at room temperature. Subsequently, the
samples were dehydrated using an increasing concentration gradient
of ethanol (50-100%) and the cells were then embedded in Epon. The
samples were cut into ultrathin (50 nm) sections and counterstained
with 0.3% lead citrate at room temperature for 20 min. Images were
generated using a JEM-1010 electron microscope at a magnification
of ×10,000 and ×40,000.
Western blotting
Protein extract was obtained using RIPA Lysis buffer
(Beyotime Institute of Biotechnology, Haimen, China) containing 1%
phenylmethylsulfonyl fluoride (Sigma-Aldrich; Merck KGaA). The
protein concentration of cell lysate was determined using an
Enhanced BCA Protein assay kit (Beyotime Institute of
Biotechnology). Equal amounts (30 μg protein/lane) of
samples were size-fractioned on a 10% SDS-PAGE gel and transferred
to polyvinylidene difluoride membranes (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The membranes were blocked with 5% non-fat milk
in Tris-buffered saline for 2 h at room temperature, and then
incubated with specific antibodies at 4°C overnight. Following
washing with TBS-Tween-20 for 15 min at room temperature, the
membranes were incubated with horseradish peroxidase
(HRP)-conjugated secondary antibodies for 2 h at room temperature.
The proteins were visualized using a SuperSignal West Femto Maximum
Sensitivity Substrate kit (Thermo Fisher Scientific, Inc.). The
software Image-Pro Plus (version 6.0; Media Cybernetics, Inc.,
Rockville, MD, USA) was used to quantify protein expression. Mouse
anti-human GAPDH primary antibodies (cat. no. SC-365062; dilution
1:1,000) were purchased from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). Goat anti-human ATG16L1 primary antibodies (cat.
no. ab223238; dilution 1:1,000) were purchased from Abcam
(Cambridge, UK) and rabbit anti-human LC3I/II primary antibodies
(cat. no. 4108; dilution 1:1,000) were purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA). The HRP-conjugated
second antibodies used were as follows: Donkey anti-goat IgG (cat.
no. A0181; dilution 1:1,000), goat anti-rabbit IgG (cat. no. A0208;
dilution 1:1,000) and goat anti-mouse IgG (cat. no. A0216; dilution
1:1,000) (all from Beyotime Institute of Biotechnology).
Immunohistochemistry (IHC)
All specimens were fixed in 4% formalin at
temperature for 24 h and embedded in paraffin prior to performing
the IHC analysis, as described in detail in our previous report
(18). The specimens were examined
in a blinded manner. Five fields were selected for examination, and
the percentage of positive tumour cells and the cell-staining
intensity were determined at the magnification of ×200 and ×400
with a Nikon Eclipse 90i digital microscope (Nikon Corporation,
Tokyo, Japan).
Bioinformatics
TargetScan (http://www.targetscan.org), miRanda (http://www.microrna.org/microrna/home.do), miRBase
(http://www.mirbase.org), miRDB (http://www.mirdb.org) and CLIPdb (http://clipdb.ncrnalab.org) were used to predict the
genes targeted by miR-874.
Chloroquine (CQ)
The autophagy inhibitor CQ (Sigma-Aldrich; Merck
KGaA) was used to examine whether autophagy affected
chemoresistance in GC cells. Cells were treated with or without CQ
(20 μmol/l) for 24 h prior to the other experiments.
Statistical analysis
SPSS software (version 18.0; SPSS, Inc., Chicago,
IL, USA) was used to perform statistical analyses. Student’s t-test
(two-tailed) or one-way analysis of variance followed by the
Student-Newman-Keuls post-hoc test was performed to analyse the
in vitro and in vivo data. The clinicopathological
factors were compared by performing unpaired t-tests or Pearson’s
χ2 tests. The quantitative data are presented as the
mean ± standard deviation. The Kaplan-Meier method was used to
estimate the survival curve. The differences in the survival
distributions were determined by performing log-rank tests.
P<0.05 was considered to indicate a statistically significant
difference.
Results
miR-874 regulates the sensitivity of GC
cells to chemotherapeutic drugs in vitro
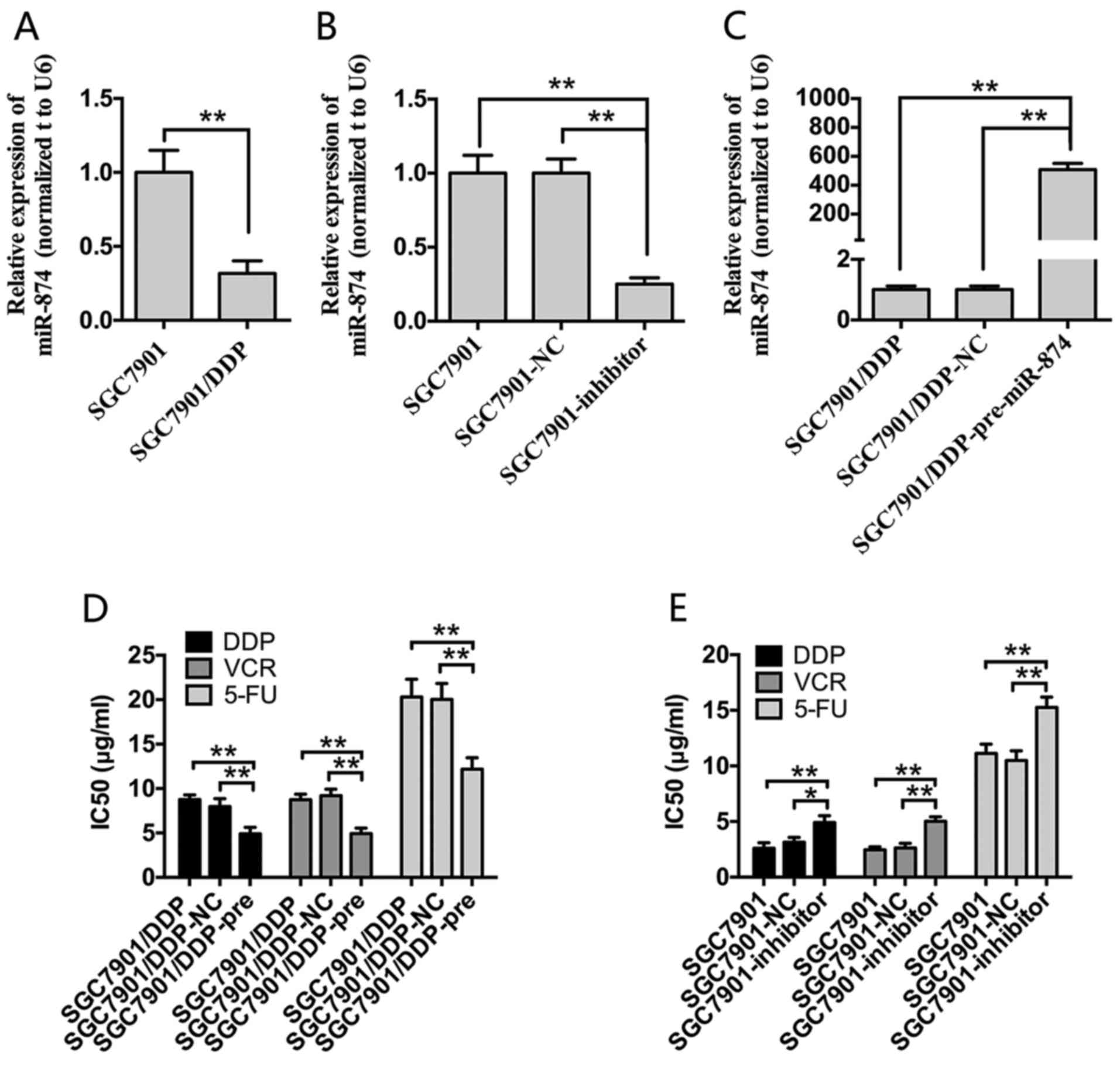
First, RT-qPCR analysis was performed to detect
miR-874 expression in SGC7901 and SGC7901/DDP cells. miR-874
expression was significantly downregulated in SGC7901/DDP cells
compared with SGC7901 cells (Fig.
1A). To investigate the effect of miR-874 on chemotherapeutic
resistance, miR-874 overexpression and knockdown cell lines were
established. SGC7901 and SGC7901/DDP cells were transfected with
miR-874 inhibitors or pre-miR-874. miR-874 inhibitor transfection
resulted in miR-874 being knocked down by ~80% in SGC7901 cells
compared with the NC control group (Fig. 1B). Furthermore, miR-874 was
significantly increased by ~30-fold in SGC7901/DDP cells following
transfection with pre-miR-974 compared with the NC control
(Fig. 1C). CCK8 assays were then
performed to generate cell growth curves and calculate the
IC50 values. Overexpression of miR-874 significantly
enhanced the sensitivity of SGC7901/DDP cells to DDP, VCR and 5-FU
compared with the blank control and NC groups. Conversely,
transfection of SGC7901 cells with inhibitors of miR-874
significantly increased the IC50 values of these three
chemotherapeutic agents compared with the two control groups
(Fig. 1D and E).
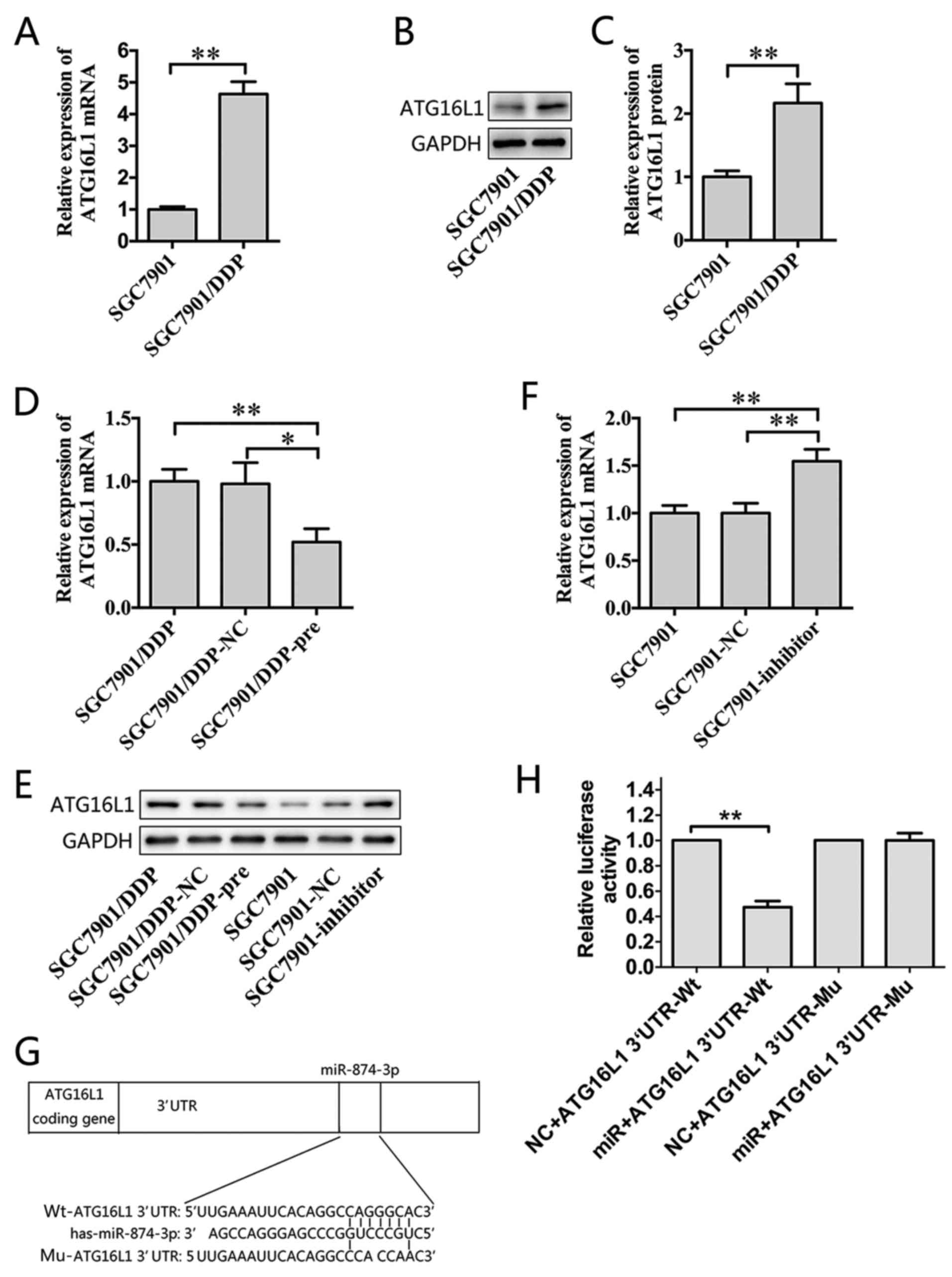
ATG16L1 is a direct target of
miR-874
ATG16L1 was identified as a potential target gene of
miR-874 through bioinformatic algorithms. RT-qPCR and western blot
assays were performed, which revealed that ATG16L1 was
significantly upregulated in SGC7901/DDP cells compared with
SGC7901 cells (Fig. 2A-C).
Furthermore, the overexpression of miR-874 reduced the expression
of ATG16L1 at the mRNA and protein levels in SGC7901/DDP cells
(Fig. 2D and E), whereas the
downregulation of miR-874 led to the opposite effect (Fig. 2E and F). A putative miR-874 binding
site was identified within the 3′UTR of ATG16L1 (Fig. 2G). A dual-luciferase reporter
system was used to validate whether ATG16L1 is a direct target of
miR-874. Wild-type (Wt) and mutant (Mu) versions of the ATG16L1
3′UTR were cloned into the reporter plasmids. Forced expression of
miR-874 significantly suppressed luciferase activity from the
wild-type reporter, but did not affect the mutant reporter
(Fig. 2H). Thus, ATG16L1 is likely
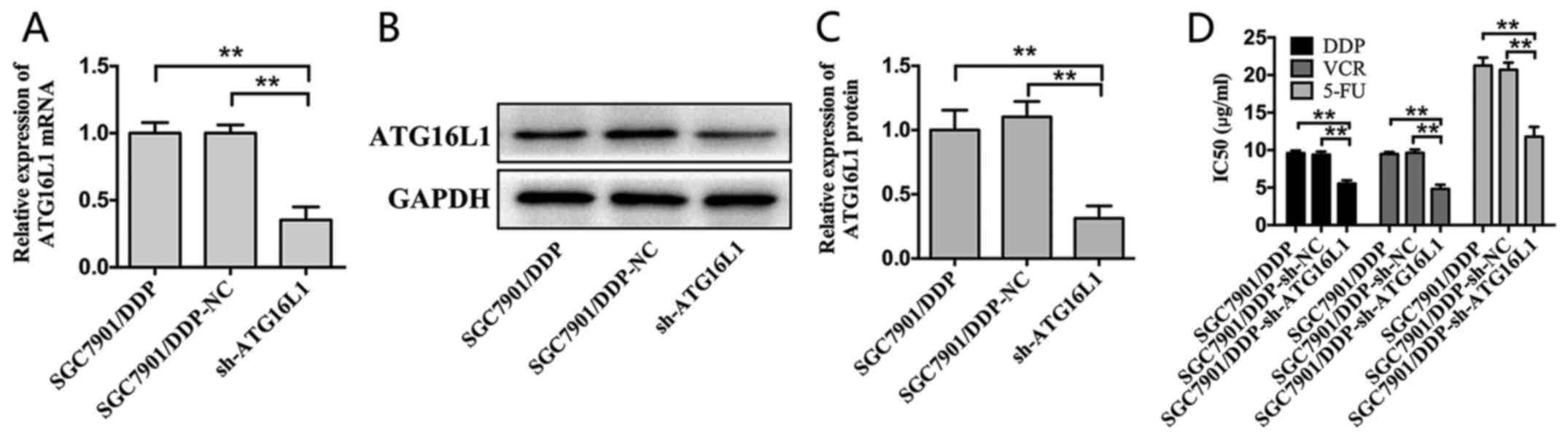
a direct target of miR-874. To further explore the function of
ATG16L1 in MDR in GC, a shRNA targeting ATG16L1 was constructed and
introduced using lentiviral gene transfer, and its inhibitory
effect was confirmed by RT-qPCR and western blot assays (Fig. 3A-C). Remarkably, the silencing of
ATG16L1 sensitized SGC7901/DDP cells to chemotherapeutic agents and
significantly decreased the IC50 values of these drugs
(Fig. 3D). Thus, ATG16L1 likely
serves an important role in GC as a direct functional target gene
of miR-874.
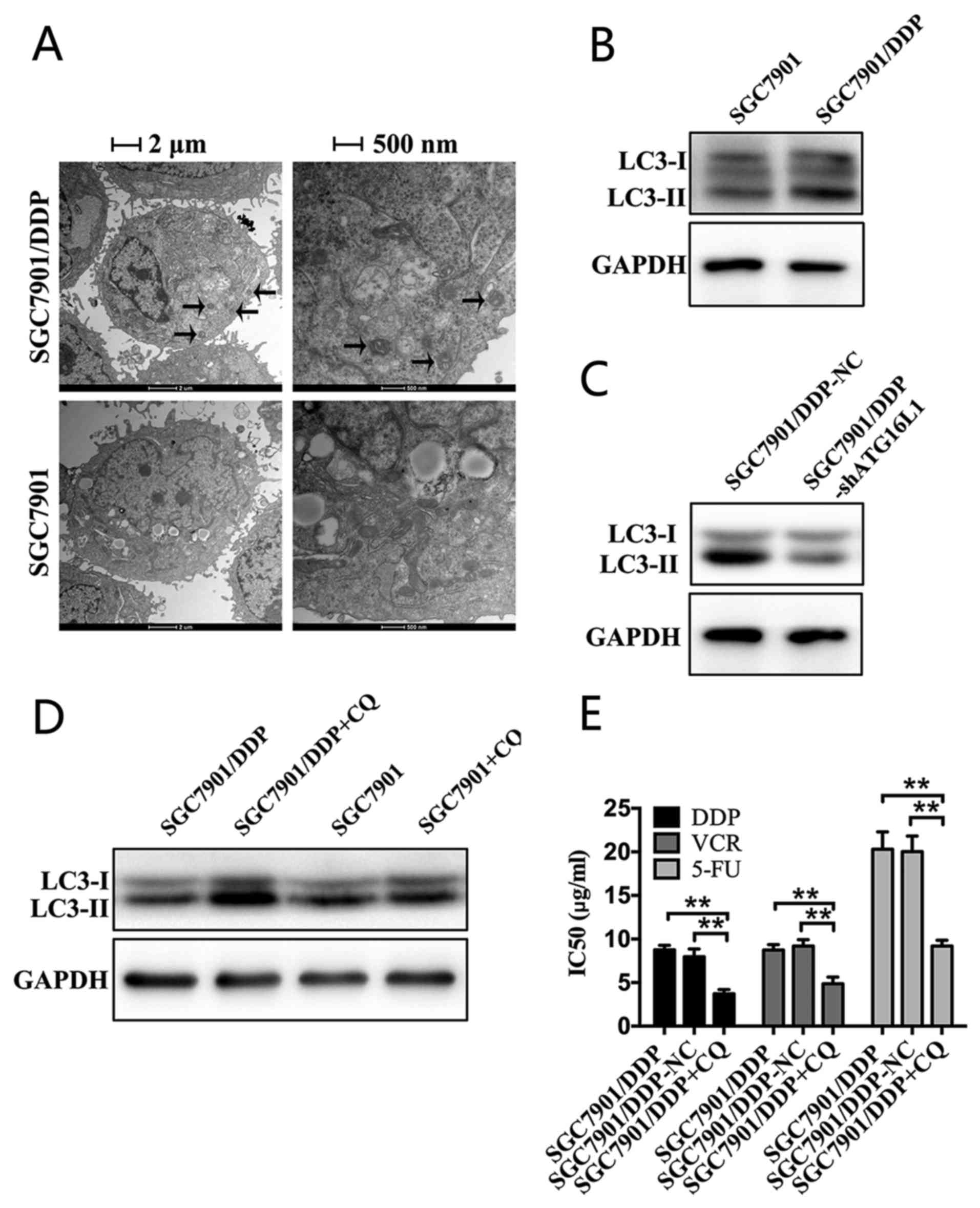
Chemoresistant GC cells exhibit increased
autophagy
ATG16L1 is a member of a large protein complex that
is necessary for autophagy (22).
As the expression of ATG16L1 was demonstrated to be increased at
the mRNA and protein levels, we hypothesized that autophagy may be
enhanced in SGC7901/DDP cells. To test this hypothesis, TEM was
performed to evaluate autophagosomes. Compared with SGC7901 cells,
autophagosomes markedly accumulated in the cytoplasm of SGC7901/DDP
cells (Fig. 4A). The processing of
the LC3-I protein to LC3-II, which is a hallmark of autophagy, was
also evaluated by performing western blot analysis. A marked
increased expression of LC3-II in SGC7901/DDP cells was observed
compared with that in SGC7901 cells (Fig. 4B). The results suggested that the
chemoresistant GC cells exhibited increased autophagy.
ATG16L1 inhibits chemosensitivity in
chemoresistant GC cells by promoting autophagy
The silencing of ATG16L1 increased the sensitivity
of SGC7901/DDP cells to chemotherapeutic agents. Next, whether
ATG16L1 regulated autophagy was investigated. According to western
blot analysis, autophagy was markedly decreased in SGC7901/DDP
cells in which ATG16L1 was silenced compared with the
NC-transfected group (Fig. 4C).
The expression of LC3-II was markedly increased in SGC7901/DDP
cells following CQ treatment for 24 h compared with the untreated
control group (Fig. 4D).
Additionally, according to the CCK8 assays, CQ treatment
significantly increased the sensitivity of SGC7901/DDP cells to
chemotherapeutic drugs (Fig. 4E).
In summary, ATG16L1 increased autophagic activity, weakening the
sensitivity of chemoresistant GC cells to chemotherapy.
miR-874 inhibits autophagy by regulating
ATG16L1
The aforementioned results suggested that ATG16L1
may be a target gene of miR-874 and that ATG16L1 regulated
autophagy. Next, we hypothesized that the suppression of miR-874
contributes to increased autophagy. To examine this hypothesis,
SGC7901/DDP or SGC7901 cells were transfected with pre-miR-874 or
inhibitors. Following transfection, autophagy was detected in the
aforementioned cells. Downregulation of miR-874 markedly increased
the expression of LC3-II in SGC7901 cells compared with the blank
and NC groups. In contrast, overexpression of miR-874 led to the
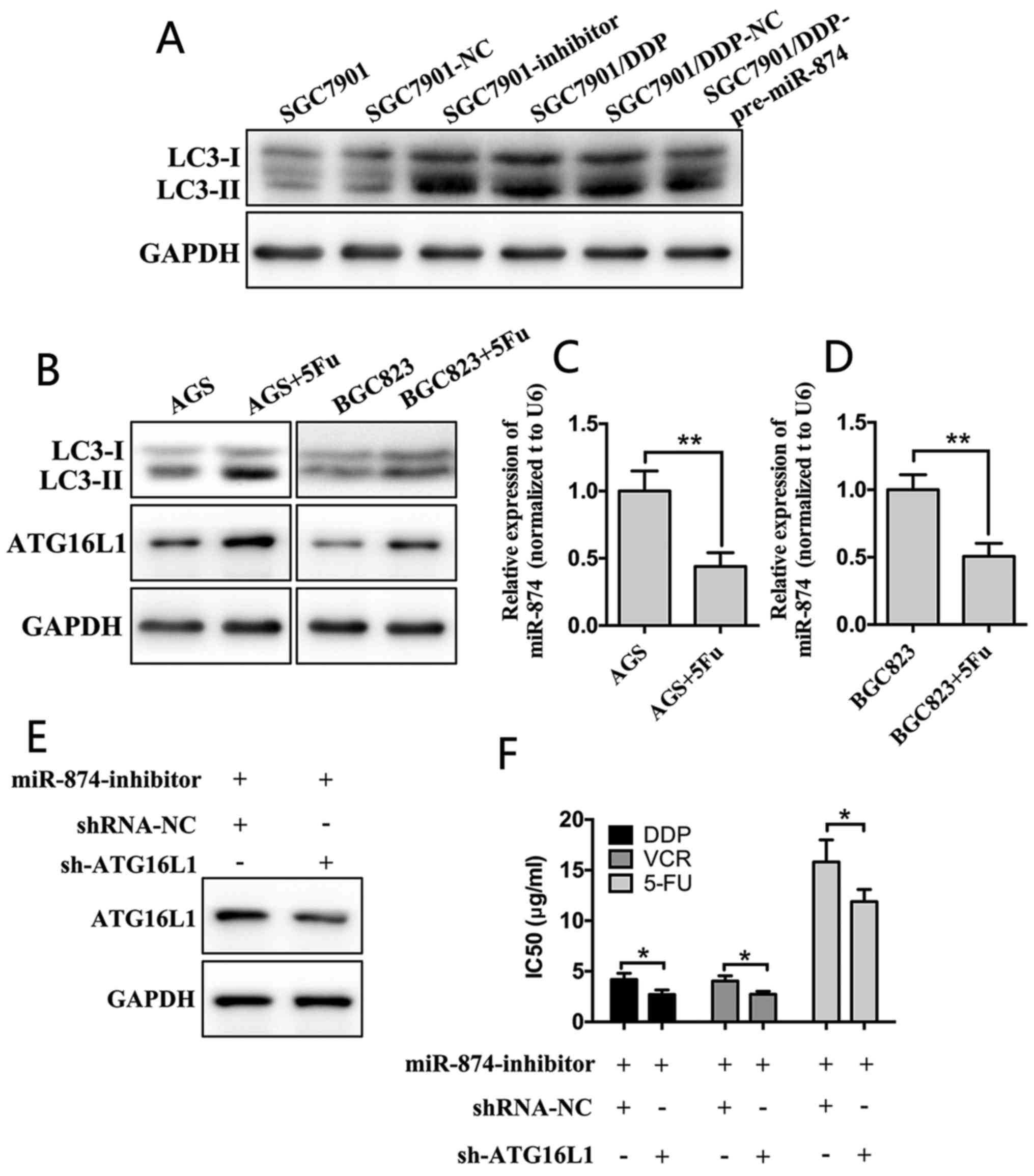
opposite effect in SGC7901/DDP cells (Fig. 5A). Furthermore, according to the
RT-qPCR and western blot analyses, following treatment of the cells
with a low concentration of 5-FU for 24 h, the expression of
miR-874 in AGS and BGC823 cells was significantly reduced, whereas
the expression levels of ATG16L1 and LC3-II were markedly
upregulated (Fig. 5B-D). SGC7901
cells were then transfected with miR-874 inhibitors and shRNAs
targeting ATG16L1. Downregulation of ATG16L1 attenuated the effects
of the miR-874 inhibitors compared with NC group (Fig. 5E and F). These results confirmed
that miR-874 inhibited autophagy by targeting ATG16L1.
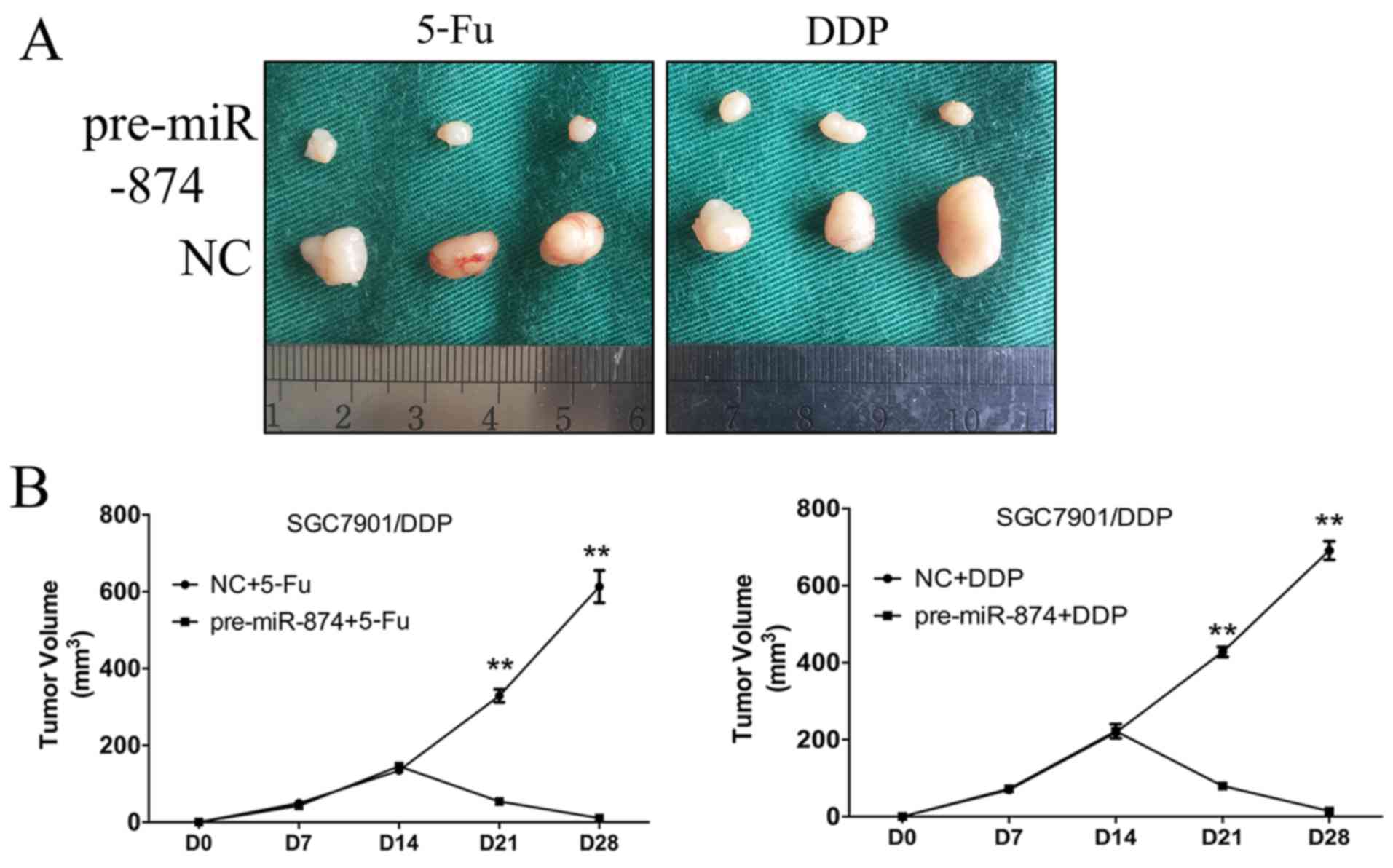
Restoration of miR-874 increases
chemosensitivity in GC cells in vivo
To determine whether miR-874 affected the
chemosensitivity of GC cells in vivo, we constructed
SGC7901/DDP cells that stably overexpressed miR-874. SGC7901/DDP-NC
or SGC7901/DDP-pre-miR-874 cells were then transplanted into nude
mice. The volumes of the pre-miR-874-transfected tumours were
significantly decreased following chemotherapy compared with the
SGC7901/DDP-NC group, indicating that ectopic miR-874 expression
reverted chemoresistance (Fig.
6).
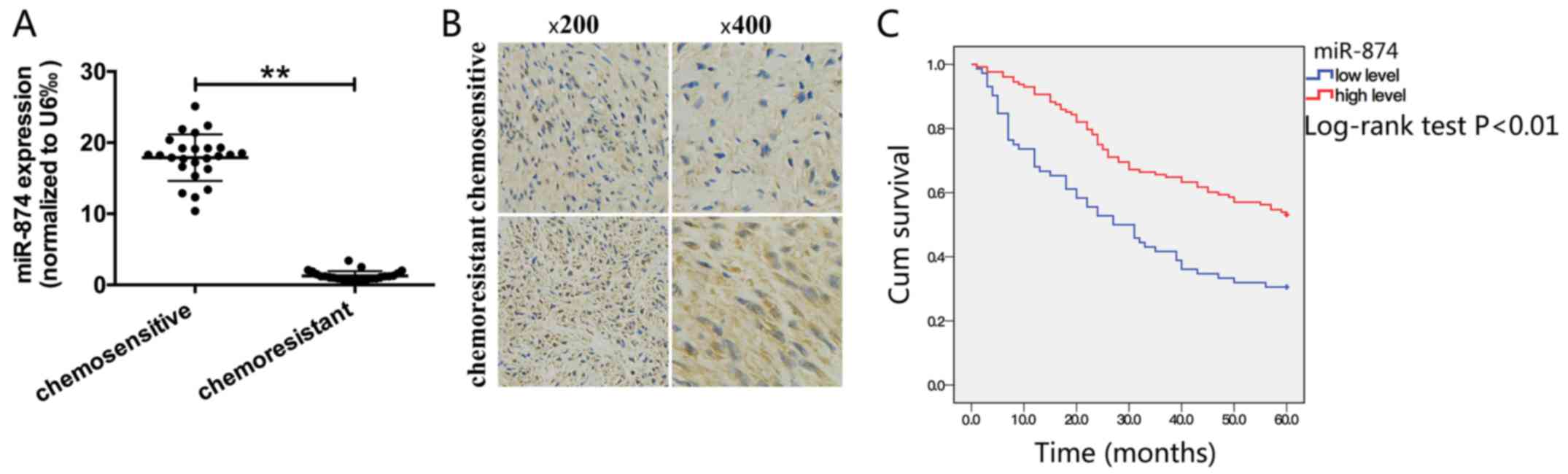
miR-874 expression is downregulated in
chemoresistant patients and is associated with overall survival in
patients with GC
RT-qPCR and immunohistochemistry were performed to
detect miR-874 and its targets, respectively, in clinical samples.
A total of 50 clinical samples (25 chemoresistant samples and 25
chemosensitive samples) were obtained from patients who received
neoadjuvant chemotherapy prior to surgery between 2014 and 2016 at
the Affiliated Huai’an Hospital, Xuzhou Medical University.
Chemosensitivity or resistance was the result of clinical
multidisciplinary discussions. In drug-resistant patients, the
expression of miR-874 was significantly decreased and the
expression of ATG16L1 was markedly upregulated compared with
drug-sensitive patients (Fig. 7A and
B). The association between miR-874 expression and
clinicopathological parameters from 200 cases of gastric malignant
tissues were analysed from Tissue Sample Center, which demonstrated
that miR-874 expression was significantly associated with distant
metastasis, whereas no significant association was observed between
miR-874 expression and patient age, sex, tumour differentiation,
tumour depth, nodal metastasis or tumour node metastasis stage
(Table I). In addition, patients
with low miR-874 expression had a significantly poorer prognosis
compared with those with high expression (Fig. 7C).
 | Table IAssociation between miR-874
expression and clinicopathological characteristics. |
Table I
Association between miR-874
expression and clinicopathological characteristics.
| Variables | No. of cases | Expression of
miR-874
| P-value |
|---|
| Low (%) | High (%) |
|---|
| Age, years | | | | 0.64 |
| ≥50 | 151 | 53 (35.1) | 98 (64.9) | |
| <50 | 49 | 20 (40.8) | 29 (59.2) | |
| Sex | | | | 0.22 |
| Male | 111 | 31 (27.9) | 80 (72.1) | |
| Female | 89 | 32 (36.0) | 57 (64.0) | |
| Degree of
differentiation | | | | 0.38 |
| Well and
moderately differentiated | 171 | 62 (36.3) | 109 (63.7) | |
| Poorly
differentiated | 29 | 13 (44.8) | 16 (55.2) | |
| T
classification | | | | 0.13 |
| T1 | 14 | 6 (42.9) | 8 (57.1) | |
| T2 | 58 | 15 (25.9) | 43 (74.1) | |
| T3 | 116 | 43 (37.1) | 73 (62.9) | |
| T4 | 12 | 7 (58.3) | 5 (41.7) | |
| N
classification | | | | 0.68 |
| N0 | 119 | 49 (41.2) | 70 (58.8) | |
| N≥1 | 81 | 31 (38.3) | 50 (61.7) | |
| M
classification | | | | <0.01 |
| M0 | 166 | 52 (31.3) | 114 (68.7) | |
| M1 | 34 | 21 (61.8) | 13 (38.2) | |
| TNM stage | | | | 0.12 |
| Stage I/II | 121 | 39 (32.2) | 82 (67.8) | |
| Stage III/IV | 79 | 34 (43.0) | 45 (57.0) | |
Discussion
GC is among the most common causes of
cancer-associated mortality. Currently, the rates of early
detection and diagnosis among patients with GC are low, and
chemotherapy remains a primary treatment method. However, due to
drug resistance, the effectiveness of chemotherapy is limited. The
5-year survival rate of patients with GC remains low (3,23).
Although numerous mechanisms contribute to chemoresistance,
including increased drug efflux, inactivation of detoxification
enzymes, alterations in drug metabolism, mutations of drug targets,
dysfunction of pro-apoptotic proteins and enhancement of DNA repair
activity, the mechanisms involved in cancer chemoresistance remain
poorly understood (24-28). Evidence suggests that miRNAs are
involved in chemoresistance in various types of cancer, including
GC (6,14). However, relatively few studies
investigating MDR in GC have been performed.
The dysregulation of miR-874 has been reported in
various cancer types, as aforementioned. In the present study, the
underlying mechanisms through which miR-874 regulates MDR in GC
were studied. miR-874 expression was demonstrated to be decreased
in MDR GC cells compared with parental GC cells, indicating that
miR-874 is involved in chemoresistance.
miRNAs usually have multiple target genes.
Therefore, a search for potential target genes of miR-874 in GC
using several computational algorithms was performed. Among the
potential targets, ATG16L1 was focused on as its function is
associated with chemoresistance in cancer (29). The results of the present study
revealed that the expression of ATG16L1 was increased in
drug-resistant GC cells. Furthermore, miR-874 reduced ATG16L1
expression at the mRNA and protein levels. Knockdown of ATG16L1
expression by shRNA increased the chemosensitivity of
chemoresistant GC cells, suggesting that ATG16L1 is associated with
chemosensitivity in GC.
Autophagy is increased in various cancer types and
contributes to drug resistance. ATG16L1 is a member of a large
protein complex that is necessary for autophagy, which is the major
process by which intracellular components are targeted to lysosomes
for degradation (30,31). The expression of ATG16L1 was
increased in MDR GC cells, suggesting that autophagy may be
involved in MDR. To examine this hypothesis, the level of autophagy
was evaluated by performing TEM and western blot analyses. The
results demonstrated that MDR cells exhibited increased autophagy,
which functioned as a mechanism of chemoresistance. The level of
autophagy decreased following the knockdown of ATG16L1 or treating
MDR cells with CQ, both of which resulted in increased sensitivity
to chemotherapy agents. These results suggest that autophagy in MDR
GC cells may be a mechanism that promotes chemotherapy resistance.
The inhibition of autophagy by interfering with ATG16L1 may be a
novel approach to improving chemotherapeutic efficacy.
miRNAs serve an important role in regulating
autophagy (32,33). The expression of miR-874 was
modified by transfecting GC cells with pre-miR-874 or inhibitors to
ascertain whether miR-874 regulated autophagy. The upregulation of
miR-874 significantly inhibited autophagy in MDR cells, while the
downregulation of miR-874 had the opposite effect. To verify
whether these effects were mediated by ATG16L1, SGC7901 cells were
co-transfected with miR-874 inhibitors and shRNAs targeting
ATG16L1. The downregulation of ATG16L1 by shRNAs reversed the
effect of miR-874 inhibition on autophagy. Taken together, these
findings indicated that miR-874 inhibits autophagy by targeting
ATG16L1 in MDR cells, highlighting the potential of miR-874 as a
target in human GC therapy.
To determine the effects of miR-874 in the
regulation of drug resistance in vivo, a xenograft tumour
model and clinical GC specimens were used. The results confirmed
that miR-874 also regulates drug resistance in vivo. In
addition, miR-874 was not associated with any clinicopathological
parameters except distant metastasis. Furthermore, according to a
Kaplan Meier analysis, the prognosis of patients with GC was
associated with the expression level of miR-874. Thus, miR-874 may
serve as an MDR marker in GC to guide chemotherapy and may be used
as a predictor of overall survival.
miR-874 was also demonstrated to be significantly
downregulated in chemoresistant cells and GC tissue samples. To
further explore whether chemotherapeutic agents influenced the
expression of miR-874, two typical gastric adenocarcinoma cell
lines, AGS and BGC823, were treated with 5-FU, which is widely used
in clinical settings as a chemotherapeutic agent. The results
revealed that 5-FU treatment significantly decreased the expression
of miR-874. Further studies are required to determine the exact
effects of chemotherapy on the expression of miR-874.
In summary, the results of the current study suggest
that miR-874 is a novel miRNA, which regulates MDR in GC. miR-874
inhibits autophagy by targeting ATG16L1 in MDR cells, leading to
increased chemotherapeutic sensitivity. These findings reveal a
novel miR-874/ ATG16L1/autophagy/chemosensitivity regulatory axis.
Furthermore, this study has clinical relevance as miR-874
overexpression and/or strategies that inhibit autophagy may have
potential therapeutic applications for the treatment of MDR in
GC.
Funding
This study was funded by a Huai’an International
Science and Technology Cooperation Research Project (grant no.
HAC201709), the 333 High-Level Talents Training Project of Jiangsu
Province (grant no. BRA2017247), and a Jiangsu Province Young
Medical Talent Project (grant no. QNRC 2016423).
Availability of data and materials
The datasets used and/or analysed in the current
study are available from the corresponding author on reasonable
request.
Authors’ contributions
HH, JT and LZ designed and performed the experiments
and contributed to the data analysis; YB enrolled the patients,
measured the RNA levels in the clinical samples and analysed the
relevant data; XZ initiated the work and wrote the manuscript; and
all authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The use of human tissues was approved by the Ethics
Committee of the Affiliated Huai’an Hospital of Xu Zhou Medical
University, and patient consent was obtained.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Van Cutsem E, Sagaert X, Topal B,
Haustermans K and Prenen H: Gastric cancer. Lancet. 388:2654–2664.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Charalampakis N, Economopoulou P,
Kotsantis I, Tolia M, Schizas D, Liakakos T, Elimova E, Ajani JA
and Psyrri A: Medical management of gastric cancer: A 2017 update.
Cancer Med. 7:123–133. 2018. View Article : Google Scholar :
|
|
4
|
Sitarz R, Skierucha M, Mielko J, Offerhaus
GJA, Maciejewski R and Polkowski WP: Gastric cancer: Epidemiology,
prevention, classification, and treatment. Cancer Manag Res.
10:239–248. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Araújo R, Santos JM, Fernandes M, Dias F,
Sousa H, Ribeiro J, Bastos MM, Oliveira PA, Carmo D, Casaca F, et
al: Expression profile of microRNA-146a along HPV-induced multistep
carcinogenesis: A study in HPV16 transgenic mice. J Cancer Res Clin
Oncol. 144:241–248. 2018. View Article : Google Scholar
|
|
6
|
Dehghanzadeh R, Jadidi-Niaragh F, Gharibi
T and Yousefi M: MicroRNA-induced drug resistance in gastric
cancer. Biomed Pharmacother. 74:191–199. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mohammadi A, Mansoori B and Baradaran B:
The role of microRNAs in colorectal cancer. Biomed Pharmacother.
84:705–713. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang R, Li P, Zhang G, Lu C, Wang H and
Zhao G: Long non-coding RNA XLOC_008466 functions as an oncogene in
human non-small cell lung cancer by targeting miR-874. Cell Physiol
Biochem. 42:126–136. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shea A, Harish V, Afzal Z, Chijioke J,
Kedir H, Dusmatova S, Roy A, Ramalinga M, Harris B, Blancato J, et
al: MicroRNAs in glioblastoma multiforme pathogenesis and
therapeutics. Cancer Med. 5:1917–1946. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jin F, Wang Y, Li M, Zhu Y, Liang H, Wang
C, Wang F, Zhang CY, Zen K and Li L: MiR-26 enhances
chemosensitivity and promotes apoptosis of hepatocellular carcinoma
cells through inhibiting autophagy. Cell Death Dis. 8:e25402017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang B, Ji S, Ma F, Ma Q, Lu X and Chen
X: miR-489 acts as a tumor suppressor in human gastric cancer by
targeting PROX1. Am J Cancer Res. 6:2021–2030. 2016.PubMed/NCBI
|
|
12
|
Wei T, Zhu W, Fang S, Zeng X, Huang J,
Yang J, Zhang J and Guo L: miR-495 promotes the chemoresistance of
SCLC through the epithelial-mesenchymal transition via Etk/BMX. Am
J Cancer Res. 7:628–646. 2017.PubMed/NCBI
|
|
13
|
Riquelme I, Letelier P, Riffo-Campos AL,
Brebi P and Roa JC: Emerging Role of miRNAs in the Drug Resistance
of Gastric Cancer. Int J Mol Sci. 17:4242016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Çalışkan M, Güler H and Bozok Çetintaş V:
Current updates on microRNAs as regulators of chemoresistance.
Biomed Pharmacother. 95:1000–1012. 2017. View Article : Google Scholar
|
|
15
|
Nohata N, Hanazawa T, Kinoshita T, Inamine
A, Kikkawa N, Itesako T, Yoshino H, Enokida H, Nakagawa M, Okamoto
Y, et al: Tumour-suppressive microRNA-874 contributes to cell
proliferation through targeting of histone deacetylase 1 in head
and neck squamous cell carcinoma. Br J Cancer. 108:1648–1658. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Que K, Tong Y, Que G, Li L, Lin H, Huang
S, Wang R and Tang L: Downregulation of miR-874-3p promotes
chemotherapeutic resistance in colorectal cancer via inactivation
of the Hippo signaling pathway. Oncol Rep. 38:3376–3386.
2017.PubMed/NCBI
|
|
17
|
Wang L, Gao W, Hu F, Xu Z and Wang F:
MicroRNA-874 inhibits cell proliferation and induces apoptosis in
human breast cancer by targeting CDK9. FEBS Lett. 588:4527–4535.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang X, Tang J, Zhi X, Xie K, Wang W, Li
Z, Zhu Y, Yang L, Xu H and Xu Z: miR-874 functions as a tumor
suppressor by inhibiting angiogenesis through STAT3/VEGF-A pathway
in gastric cancer. Oncotarget. 6:1605–1617. 2015.PubMed/NCBI
|
|
19
|
Brierley JD, Gospodarwicz MK and Wittekind
C: TNM classification of maligant tumours. 8th edition. Wiley
Blackwell; Oxford: 2017
|
|
20
|
Amin MB, Edge SB, Greene FL and Brierley
JD: AJCC cancer staging manual. 8th edition. Springer; New York:
2017, View Article : Google Scholar
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
22
|
Lu Y, Gao J, Zhang S, Gu J, Lu H, Xia Y,
Zhu Q, Qian X, Zhang F, Zhang C, et al: miR-142-3p regulates
autophagy by targeting ATG16L1 in thymic-derived regulatory T cell
(tTreg). Cell Death Dis. 9:2902018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee KW, Lee JH, Kim JW, Kim JW, Ahn S and
Kim JH: Population-based outcomes research on treatment patterns
and impact of chemotherapy in older patients with metastatic
gastric cancer. J Cancer Res Clin Oncol. 142:687–697. 2016.
View Article : Google Scholar
|
|
24
|
Annovazzi L, Mellai M and Schiffer D:
Chemotherapeutic drugs: DNA damage and repair in glioblastoma.
Cancers (Basel). 9:572017. View Article : Google Scholar
|
|
25
|
Adamska A, Elaskalani O, Emmanouilidi A,
Kim M, Abdol Razak NB, Metharom P and Falasca M: Molecular and
cellular mechanisms of chemoresistance in pancreatic cancer. Adv
Biol Regul. 68:77–87. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bourguignon LY, Earle C and Shiina M:
Activation of matrix hyaluronan-mediated CD44 signaling, epigenetic
regulation and chemoresistance in head and neck cancer stem cells.
Int J Mol Sci. 18:182017. View Article : Google Scholar
|
|
27
|
Butera G, Pacchiana R and Donadelli M:
Autocrine mechanisms of cancer chemoresistance. Semin Cell Dev
Biol. 78:3–12. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
de Oliveira Júnior RG, Christiane Adrielly
AF, da Silva Almeida JR, Grougnet R, Thiéry V and Picot L:
Sensitization of tumor cells to chemotherapy by natural products: A
systematic review of preclinical data and molecular mechanisms.
Fitoterapia. 129:383–400. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang K, Chen J, Zhou H, Chen Y, Zhi Y,
Zhang B, Chen L, Chu X, Wang R and Zhang C: PU1/microRNA-142-3p
targets ATG5/ATG16L1 to inactivate autophagy and sensitize
hepato-cellular carcinoma cells to sorafenib. Cell Death Dis.
9:3122018. View Article : Google Scholar
|
|
30
|
Bhat P, Kriel J, Shubha Priya B, Basappa,
Shivananju NS and Loos B: Modulating autophagy in cancer therapy:
Advancements and challenges for cancer cell death sensitization.
Biochem Pharmacol. 147:170–182. 2018. View Article : Google Scholar
|
|
31
|
Sannigrahi MK, Singh V, Sharma R, Panda NK
and Khullar M: Role of autophagy in head and neck cancer and
therapeutic resistance. Oral Dis. 21:283–291. 2015. View Article : Google Scholar
|
|
32
|
Gao Wu J, Xu F, Deng T, Wang X, Yang C, Hu
X, Long Z, He Y, Liang XG, et al: miR-503 suppresses the
proliferation and metastasis of esophageal squamous cell carcinoma
by triggering autophagy via PKA/mTOR signaling. Int J Oncol. Mar
16–2018.Epub ahead of print. View Article : Google Scholar
|
|
33
|
Wang S, Kobeissi A, Dong Y, Kaplan N, Yang
W, He C, Zeng K and Peng H: MicroRNAs-103/107 regulate autophagy in
the epidermis. J Invest Dermatol. 138:1481–1490. 2018. View Article : Google Scholar : PubMed/NCBI
|