Introduction
Lung cancer remains the most common type of cancer
worldwide with an estimated 1.8 million new cases in 2012 (12.9% of
the total cancer cases) and accounting for 23.6% of total deaths
(1). While treatment for lung
cancer is available in the form of surgical resection, chemotherapy
and radiation, the 5-year survival rate remains very low at only
16.6% (2,3). It is therefore important to gain a
better understanding of the molecular mechanisms that are involved
in lung carcinogenesis with the aim to identify diagnostic and
prognostic markers for the early detection and a more targeted
treatment of lung cancer.
Apoptosis plays an important role during development
and in the maintenance of multicellular organisms through the
removal of damaged, aged or autoimmune cells (4). Studies conducted on non-small cell
lung cancer (NSCLC), which accounts for the majority of lung cancer
cases (5), have demonstrated that
the expression of B-cell lymphocyte xL (Bcl-xL), the other
major prototype of the anti-apoptotic Bcl-2 gene, is
elevated in NSCLC, leading to the inhibition of apoptosis and a
poor prognosis (6). A previous
study conducted by our group demonstrated that the silencing of
Bcl-xL induced alterations in microRNA (miRNA or miR)
expression in the A549 lung adenocarcinoma cell line, and the
rippling effects of the alteration in miRNA expression towards
target genes may contribute to the induction of apoptosis.
miR-361-5p was one of the miRNAs found to be significantly
downregulated following Bcl-xL silencing (7).
miRNAs are non-coding RNAs of approximately 19 to 23
nucleotides in length, which post-transcriptionally regulate gene
expression (8). These regulatory
elements play a role in a wide range of biological processes,
including cell proliferation (9),
differentiation (10), drug
sensitivity (11) and apoptosis
(12). A number of studies carried
out in recent years have aimed at elucidating the specific miRNAs
associated with apoptosis in cancer and their related target genes
(13-17).
In this study, we aimed to determine whether
miR-361-5p, which was downregulated in response to Bcl-xL
silencing, as shown in our previous study (7), is involved in the regulation of the
apoptosis of NSCLC cells and to investigate the molecular
mechanisms that mediate these effects.
Materials and methods
Cell lines and culture conditions
The human lung adenocarcinoma cell lines, A549
(Cancer Research Malaysia, Subang Jaya Medical Centre, Subang Jaya,
Selangor Malaysia) and SK-LU-1 (AseaCyte Pte. Ltd., Subang Jaya,
Selangor, Malaysia), were maintained in RPMI-1640 (HyClone/GE
Healthcare Life Sciences, Pittsburgh, PA, USA) and MEM-α
(Gibco/Thermo Fisher Scientific, Waltham, MA, USA), respectively,
containing 10% (v/v) fetal bovine serum (FBS) (HyClone/GE
Healthcare Life Sciences), and maintained in 5.0% CO2
levels at 37.0°C in a humidified atmosphere.
Transfection with miRNA mimic and
inhibitors
The cells were seeded 24 h prior to transfection
with 80.0 nM miRIDIAN microRNA human miR-361-5p mimics or hairpin
inhibitors (GE Healthcare Dharmacon, Lafayette, CO, USA) using the
DharmaFECT transfection reagent (GE Healthcare Dharmacon), as per
the manufacturer’s instructions. miRIDIAN microRNA Mimic Negative
Control #1 and miRIDIAN microRNA Hairpin Inhibitor Negative Control
#1 (GE Healthcare Dharmacon) were used as negative controls.
Combined transfection of siBcl-xL and
miR-361-5p
The cells were plated 24 h prior to transfection
with 100.0 nM Stealth RNAi (siBcl-xL) using Lipofectamine
2000 reagent (Invitrogen, Carlsbad, CA, USA), as per the
manufacturer’s instructions. At 24 h post-transfection, the spent
media were removed and the cells were transfected with miR-361-5p
mimics or mimic negative control (NC).
Annexin V-FITC apoptosis assay
Apoptosis was detected using the FITC Annexin V
apoptosis detection kit (BD Biosciences, Franklin Lakes, NJ, USA)
at 72 h post-transfection, as per the manufacturer’s instructions.
Apoptosis was detected in 1.0×104 cells using the BD
FACSCanto™ II flow cytometer (BD Biosciences) and analyzed using BD
FACSDiva™ software (BD Biosciences).
Caspase-3/7 activity assay
Caspase-3 and -7 activities were determined using
the Caspase-Glo 3/7 assay kit (Promega, Madison, WI, USA), at 48 h
post-transfection, as per the manufacturer’s instructions.
Luminescence was detected using the GloMax Multi Luminescence
Multimode Reader (Promega).
Zebrafish care and use
Experiments involving zebrafish were approved by the
University of Malaya, Faculty of Medicine, Institutional Care of
Use Committee (FOM IACUC) (Ethics reference no.
2015-181006/IBS/R/NO) and complied with all relevant animal welfare
laws, guidelines and policies. Wild-type Danio rerio
zebrafish embryos were cared for and maintained using standard
husbandry practices.
Zebrafish microinjection
Approximately 150 zebrafish embryos (Zebrafish
Laboratory, Department of Biomedical Science, Faculty of Medicine,
University of Malaya, Kuala Lumpur, Malaysia) were injected with
A549 cells transfected with 80.0 nm miR-361-5p inhibitors or their
corresponding negative controls. The A549 cells re-suspended in
serum-free RPMI were injected at the superficial location of the
yolk near to the perivitelline space of the embryos using a
FemtoJet Microinjector (Eppendorf, Hamburg, Germany) and InjectMan
NI 2 Micromanipulator (Eppendorf) with constant injection pressure
and injection time. The injection volume and cell suspension was
calibrated to be approximately 100-200 cells/injection in each
embryo. Following microinjection, the embryos were immediately
incubated at 37°C overnight.
Whole mount caspase-3
immunofluorescence
At 12 h post-injection, the embryos were fixed in 4%
paraformaldehyde overnight at 4°C, and then dehydrated in methanol
for 2 h at −20°C. Following rehydration, the embryos were washed
several times with 1% DMSO, 0.1% Triton in PBS (1X PDT) and blocked
for 1 h at 25°C with gentle shaking in 10% FBS, 2% BSA in PBST
(blocking buffer). The embryos were then stained with purified
rabbit anti-active caspase-3 antibody (Cat. no. 559565, BD
Biosciences) (1:500 dilution) for 2 h at 25°C followed by washes in
PDT. The embryos were again blocked with blocking buffer, and then
stained with anti-rabbit IgG Fab2 Alexa Fluor 647 Conjugate (Cat.
no. 4414S, Cell Signaling Technology, Danvers, MA, USA) (1:500
dilution) overnight at 4°C. The following day, the embryos were
washed with PDT prior to visualization and imaging using a Leica
confocal laser-scanning microscope SPII and Leica Application Suite
(LAS) software v5.0 (Leica, Wetzlar, Germany). Fluorescence was
quantified using ImageJ Analyst [National Institutes of Health
(NIH), Bethesda, MD, USA] software. The threshold was set to
eliminate background fluorescence and embryos were analyzed to
generate arbitrary fluorescence units.
Bioinformatics analyses of miRNA gene
targets
The putative miRNA targets were identified using
TargetScan Human v5.2, a database of conserved 3′UTR targets, found
at http://www.targetscan.org/ (Whitehead
Institute for Biomedical Research, Cambridge, MA, USA). TargetScan
allows for the ranking of predicted miRNA targets based upon a
total context+ score, which is the sum of the contribution of six
targeting factors which include site type, site number, site
location, local AU content, 3′-supplementary pairing, target site
abundance, and seed-pairing stability. The total context+ score
indicates the relative repression of mRNA, with low context scores
being more favorable (18).
Gene-annotation enrichment analyses of the predicted miRNA targets
with total context+ scores of <0 were then performed using the
web tool made up of an integrated biological knowledgebase and
analytic tools (19) called
Database for Annotation, Visualization and Integrated Discovery
(DAVID) v6.7 at http://david.abcc.ncifcrf.gov/summary.jsp (SAIC
Frederick, Inc. Frederick, MD, USA) using default parameters. Data
from TargetScan 5.2 and DAVID were combined to generate a
hypothetical pathway of the association between miR-361-5p and its
gene targets.
Dual luciferase reporter assay
system
The cells were plated 24 h prior to co-transfection
with 40.0 ng of 3′UTR reporter constructs containing wild-type or
mutated bindings sites and 80.0 nM of miR-361-5p mimic/hairpin
inhibitor or mimic NC/hairpin inhibitor NC using DharmaFECT
transfection reagent. Relative luciferase activity was assayed at
48 h post-transfection using the Dual Luciferase Reporter Assay
System (Promega) and detected on the GloMax Multi Luminescence
Multimode Reader (Promega). Luciferase activity was normalized to
the internal control Renilla activity in each well.
Protein extraction and western blot
analysis
Proteins were extracted at 48 h post-transfection
using the NE-PER® Nuclear and Cytoplasmic Extraction kit
(Thermo Fisher Scientific), as per the manufacturer’s instructions.
Size separation by electrophoresis in 12.0% (w/v) SDS-PAGE was
performed prior to transfer to nitrocellulose membranes. The
membranes were blocked with 5% (w/v) non-fat skimmed milk for 1 h
at 25°C and incubated overnight at 4°C with primary monoclonal
rabbit antibodies: Mothers against decapentaplegic homolog 2
(SMAD2) (Cat. no. 3122, Cell Signaling Technology) (1:1,000
dilution) and GAPDH (Cat. no. 2188, Cell Signaling Technology)
(1:10,000 dilution). The membranes were subsequently washed and
incubated with secondary goat anti-rabbit IgG HRP-linked antibody
(Cat. no. 7074, Cell Signaling Technology) (1:1,000 dilution) and
anti-biotin HRP-linked antibody (Cat. no. 7075, Cell Signaling
Technology) (1:1,000 dilution). Bands were visualized using Western
Bright Quantum (Advansta, Menlo Park, CA, USA) on the Fusion FX7
system (Vilber Lourmat, Eberhardzell, Germany) and quantified using
ImageJ Analyst software with band intensities normalized to
GAPDH.
Gene rescue experiments
The cells were plated 24 h prior to transfection
with 80.0 nM miR-361-5p mimics or mimic NC. At 6 h
post-transfection, the spent media were removed and the cells
transfected with 50.0 ng of plasmid expressing the SMAD2 gene
without its 3′UTR (pCMV6/SMAD2) (OriGene Technologies, Rockville,
MD, USA). Empty pCMV6 (OriGene Technologies) was used as a control.
The protein levels of SMAD2 were determined 48 h post-transfection
by western blot analysis, while apoptosis was detected using the
FITC Annexin V apoptosis detection kit and Caspase-Glo 3/7 assay
kit as described above.
Statistical analysis
All in vitro experiments were performed in
triplicate independent experiments and presented as the means ±
standard deviation (SD). In vivo experiments were performed
with sample size of 15 zebrafish embryos per treatment group. An
unpaired Student’s t-test was used to determine the statistical
significance of the differences between 2 groups of data, where a
P-value ≤0.05 was considered to indicate a statistically
significant difference. The analysis of statistical significance
between 3 or more groups of data was performed using one-way
analysis of variance (ANOVA), followed by Dunnett’s Multiple
Comparison post-hoc test where a P-value <0.05 was considered to
indicate a statistically significant difference.
Results
Suppression of miR-361-5p expression
promotes the apoptosis and induces cell cycle arrest of A549 and
SK-LU-1 lung adenocarcinoma cell lines
The silencing of Bcl-xL in the A549 cells has
been shown to downregulate miR-361-5p expression (7). In this study, to investigate the
biological effects of miR-361-5p, miR-361-5p hairpin inhibitor and
hairpin inhibitor negative control were transiently transfected
into the lung adenocarcinoma cells, A549 and SK-LU-1. The results
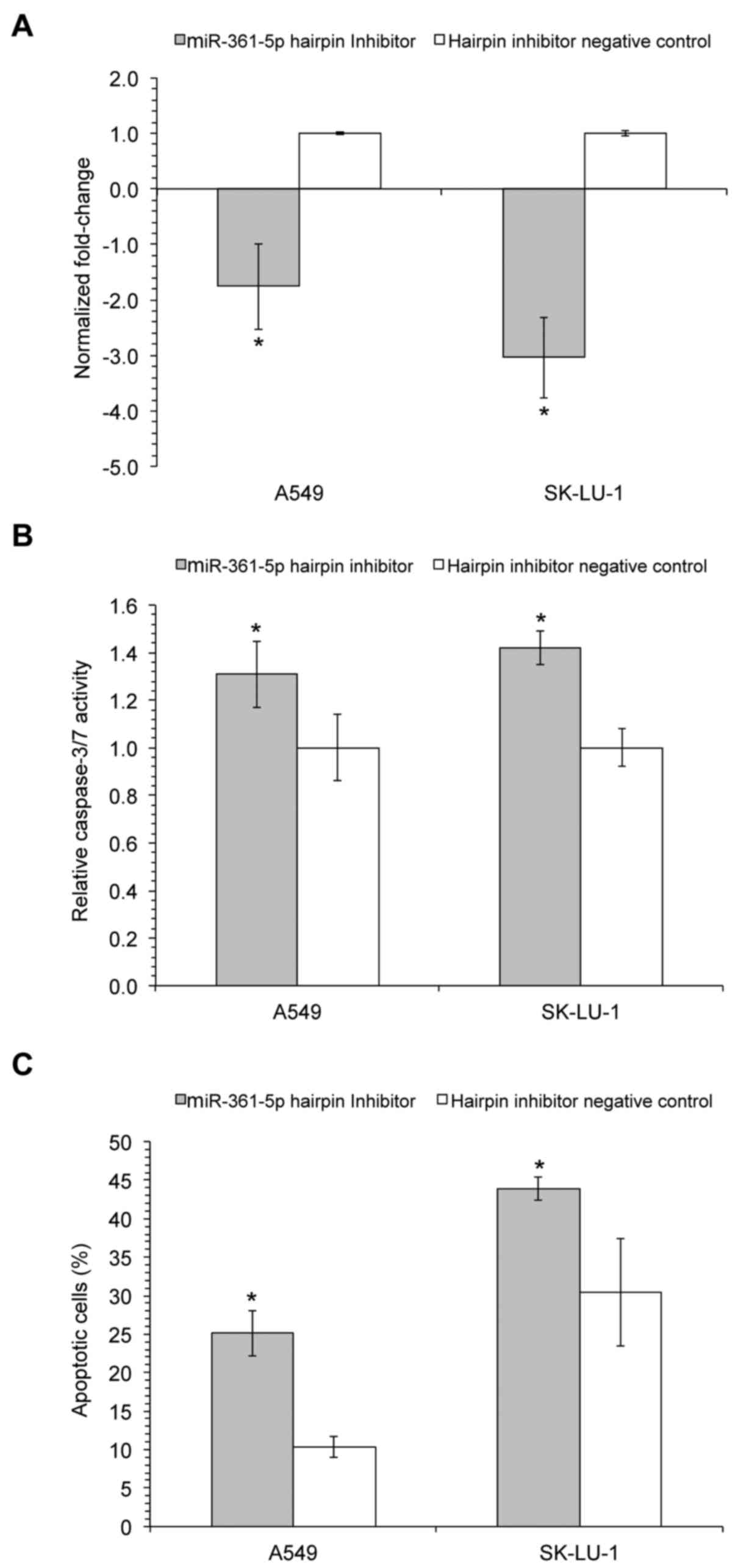
from RT-qPCR indicated that the transfection of miR-361-5p hairpin
inhibitor successfully suppressed miR-361-5p expression levels,
when compared to the hairpin inhibitor negative control (Fig. 1A), whereas transfection with
miR-361-5p mimics significantly increased miR-361-5p expression
(data not shown). Furthermore, the inhibition of miR-361-5p
expression resulted in a significant increase in caspase-3/7
activities in both the A549 and SK-LU-1 cells (1.31±0.14 and
1.42±0.07 relative activity, respectively; Fig. 1B). Correspondingly, the detection
of cell death at 72 h post-transfection, using flow cytometry after
Annexin V-FITC/PI staining, indicated that the inhibition of
miR-361-5p resulted in a significant increase in the percentage of
apoptotic A549 and SK-LU-1 cells (25.10±2.94 and 43.93±1.53%,
respectively) in comparison to the hairpin inhibitor negative
control (Fig. 1C). However, no
significant change in apoptosis and caspase activity was observed
when miR-361-5p was overexpressed using mimics (data not
shown).
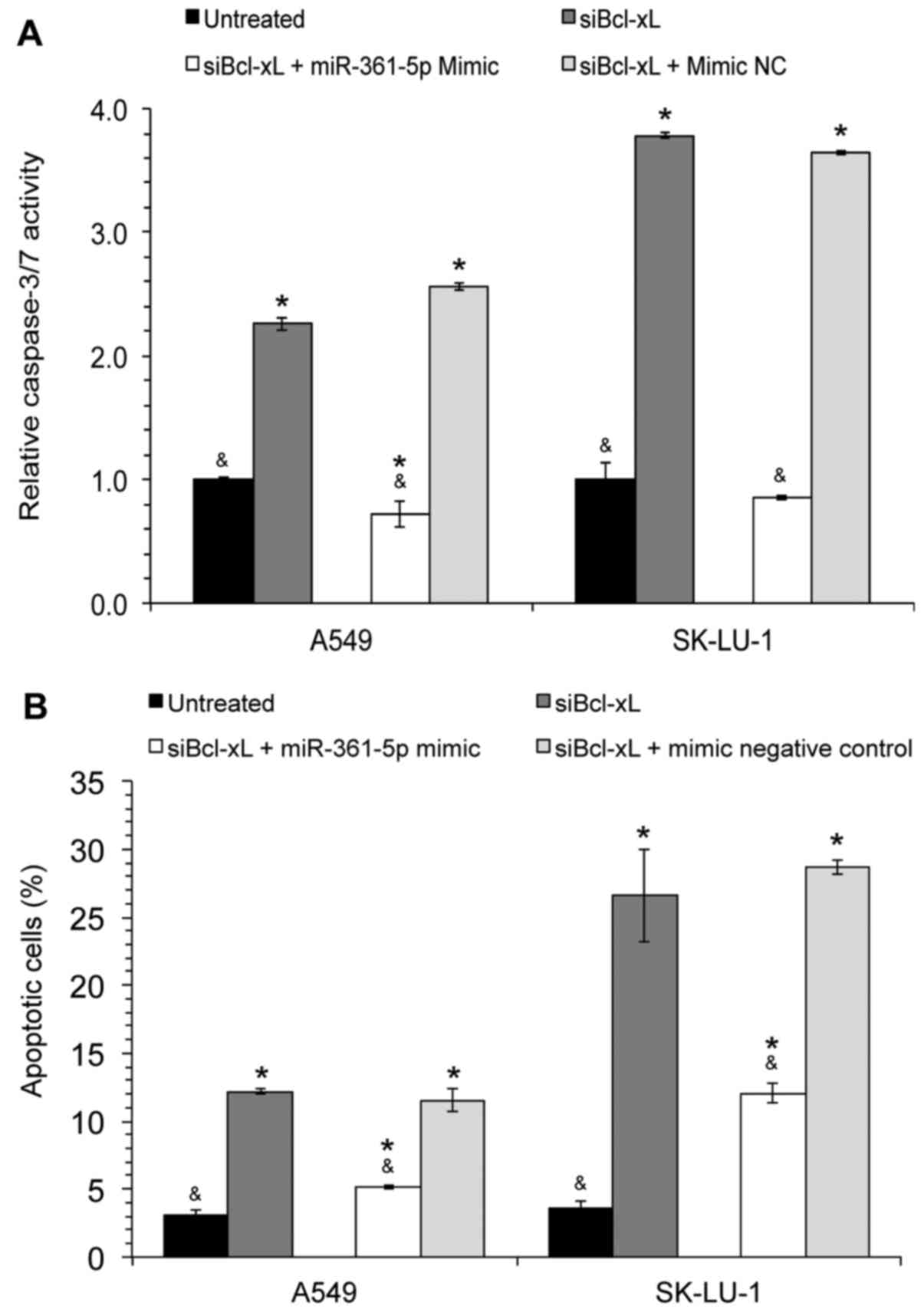
Transfection with miR-361-5p mimics
blocks siBcl-xL-induced cell death
To elucidate the association between Bcl-xL,
miR-361-5p and apoptosis, a combination study was performed whereby
the cells were first transfected with siBcl-xL followed by
transfection with miR-361-5p mimics. The data revealed that the
relative caspase-3/7 activity in the Bcl-xL-silenced cells
was significantly decreased following miR-361-5p mimic transfection
(Fig. 2A); a similar decrease in
the percentage of apoptotic A549 and SK-LU-1 cells was observed
(Fig. 2B), thus demonstrating that
the overexpression of miR-361-5p was able to block
siBcl-xL-induced cell death.
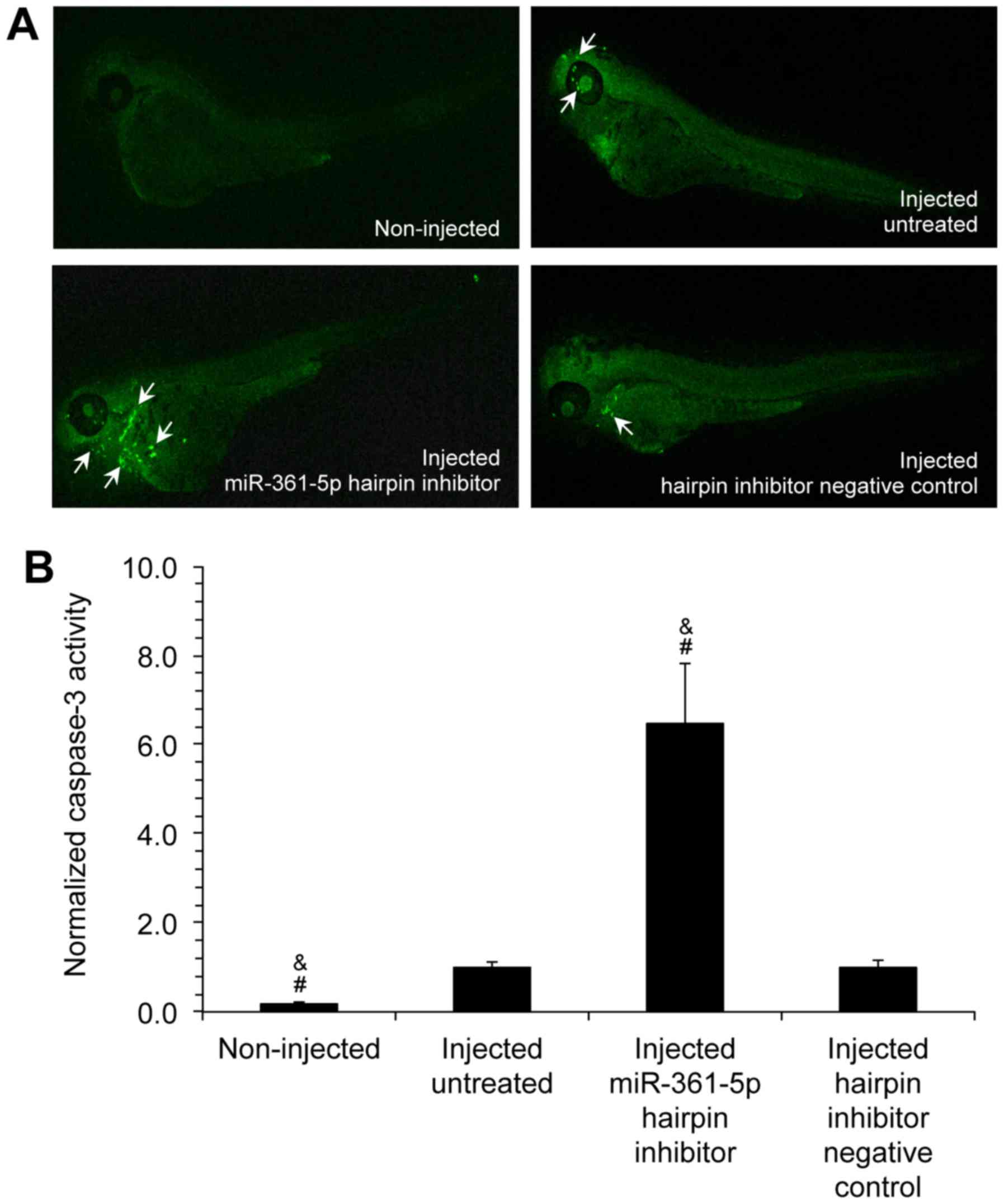
Transfection with miR-361-5p inhibitors
increases caspase-3 expression in zebrafish embryo animal
models
To analyze the in vivo effects of miR-361-5p
on apoptosis, zebrafish were used as an animal model. A549 cells
transfected with either miR-361-5p inhibitor or hairpin inhibitor
negative controls were microinjected into zebrafish embryos.
Following staining with anti-rabbit fluorophore-conjugated
antibodies, the embryos were visualized using a Leica confocal
microscope (Fig. 3A). The analysis
of the fluorescent images using ImageJ software indicated that th
caspase-3 levels were significantly increased by 6.41±1.04-fold in
the embryos injected with miR-361-5p hairpin inhibitor-transfected
cells in comparison to the embryos injected with hairpin inhibitor
negative control-transfected cells (Fig. 3B). These results suggested that the
downregulation of miR-361-5p expression was also able to induce
apoptosis in vivo.
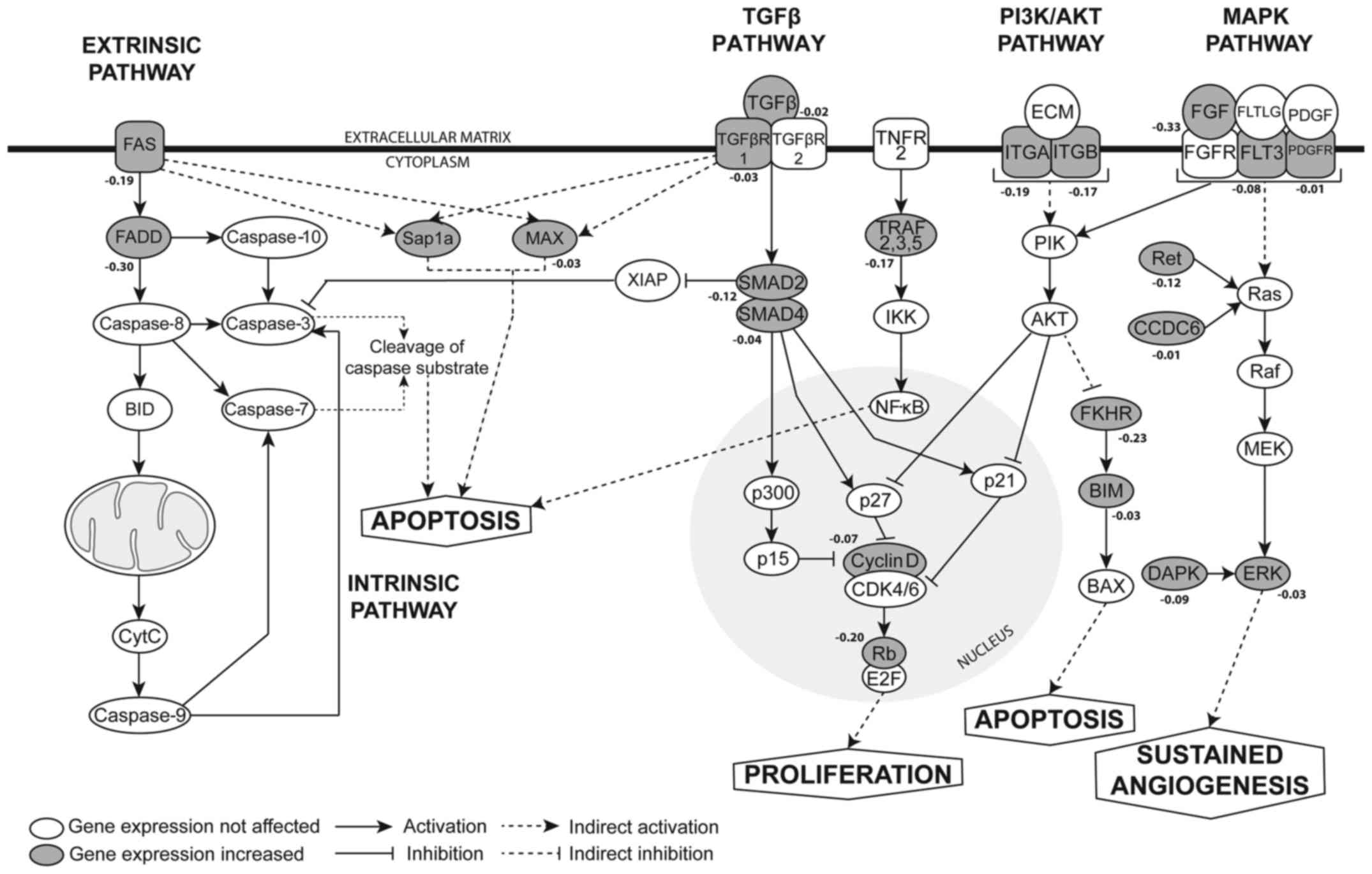
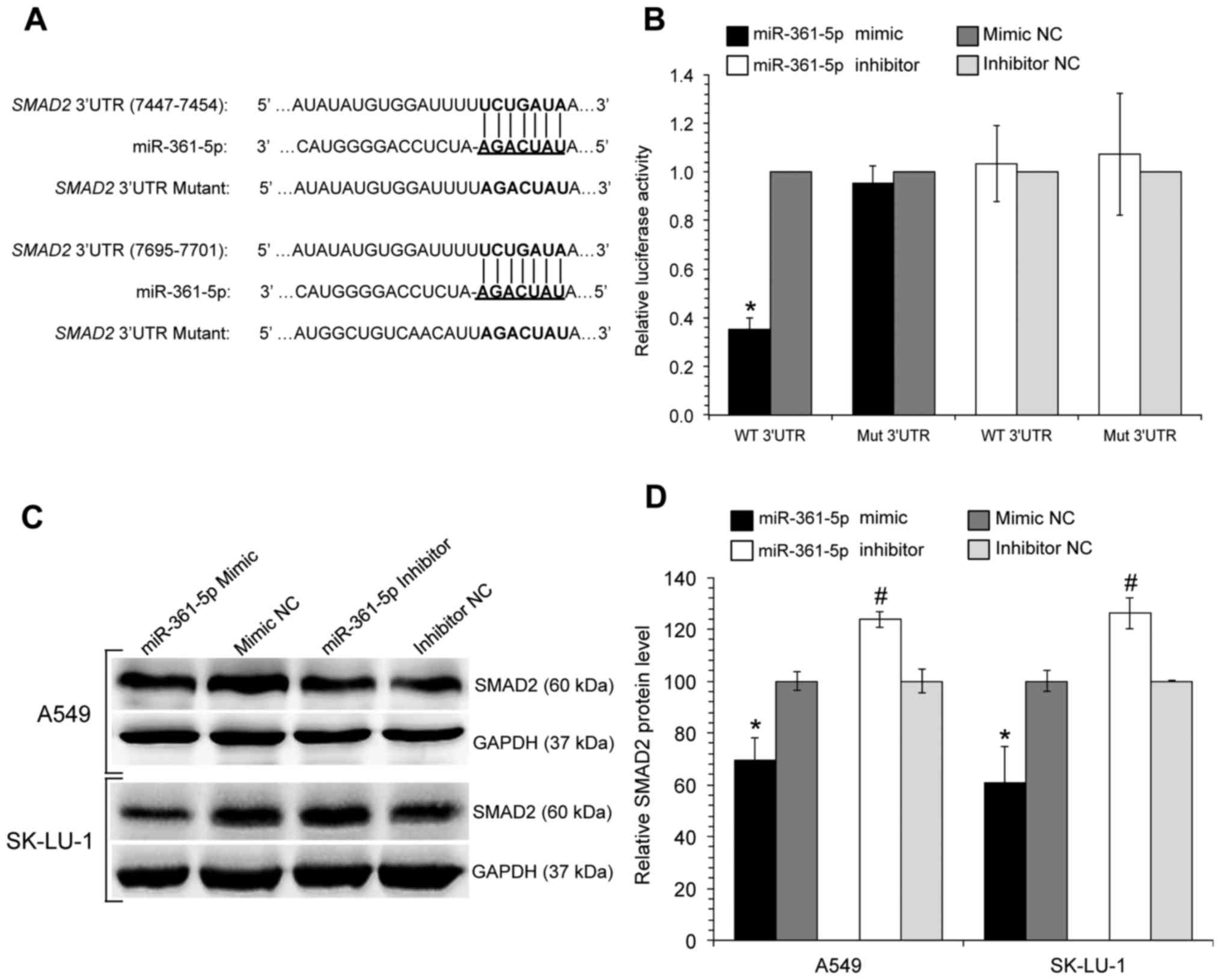
miR-361-5p is predicted to bind to SMAD2
3′UTR
To elucidate the underlying molecular mechanisms
responsible for miR-361-5p-mediated apoptosis, an in silico
approach was used to identify the putative target genes of
miR-361-5p using the TargetScan database, followed by
gene-annotation enrichment analyses using the web tool, DAVID,
which categorizes the predicted miR-361-5p targets into
apoptosis-related pathways. The enrichment of genes involved in
cancer pathways indicated the possible involvement of the
transforming growth factor β (TGFβ), phosphatidylinositol
3-kinase/protein kinase B (PI3K/AKT) pathway and mitogen-activated
protein kinase (MAPK) pathways, together with the intrinsic and
extrinsic apoptotic pathways as potential targets of miR-361-5p
(Fig. 4). SMAD2, a cytoplasmic
signaling protein, was identified as a predicted target of
miR-361-5p, with a total context+ score of −0.12.
miR-361-5p directly binds to the SMAD2
3′UTR, decreasing its protein expression
Among the various target genes, SMAD2 was
selected for further experimental validation as it contains two
seed-recognizing miR-361-5p binding sites and is known to be
involved in apoptosis and proliferation (20-22).
The SMAD2 3′UTR and its corresponding mutant counterpart,
were cloned into the 3′ end of the pmirGLO Dual-Luciferase miRNA
Target Expression Vector (Fig.
5A). Relative Firefly luciferase activity, measured in the
presence of miR-361-5p mimic/inhibitors or their respective
negative controls were measured. The results indicated that the
cells co-transfected with wild-type constructs (WT 3′UTR) and
miR-361-5p mimics exhibited a substantial 0.35-fold decrease in
relative luciferase activity in comparison to the cells in the
control group (Fig. 5B). In the
cells co-transfected with the mutant constructs (Mut 3′UTR), no
significant differences were observed between the relative
luciferase activities. This observation indicates that miR-361-5p
can directly bind to the binding sequence on the 3′UTR of
SMAD2, suppressing its gene expression; this was further
verified by a decrease in SMAD2 protein levels as determined by
western blot analysis (Fig. 5C and
D).
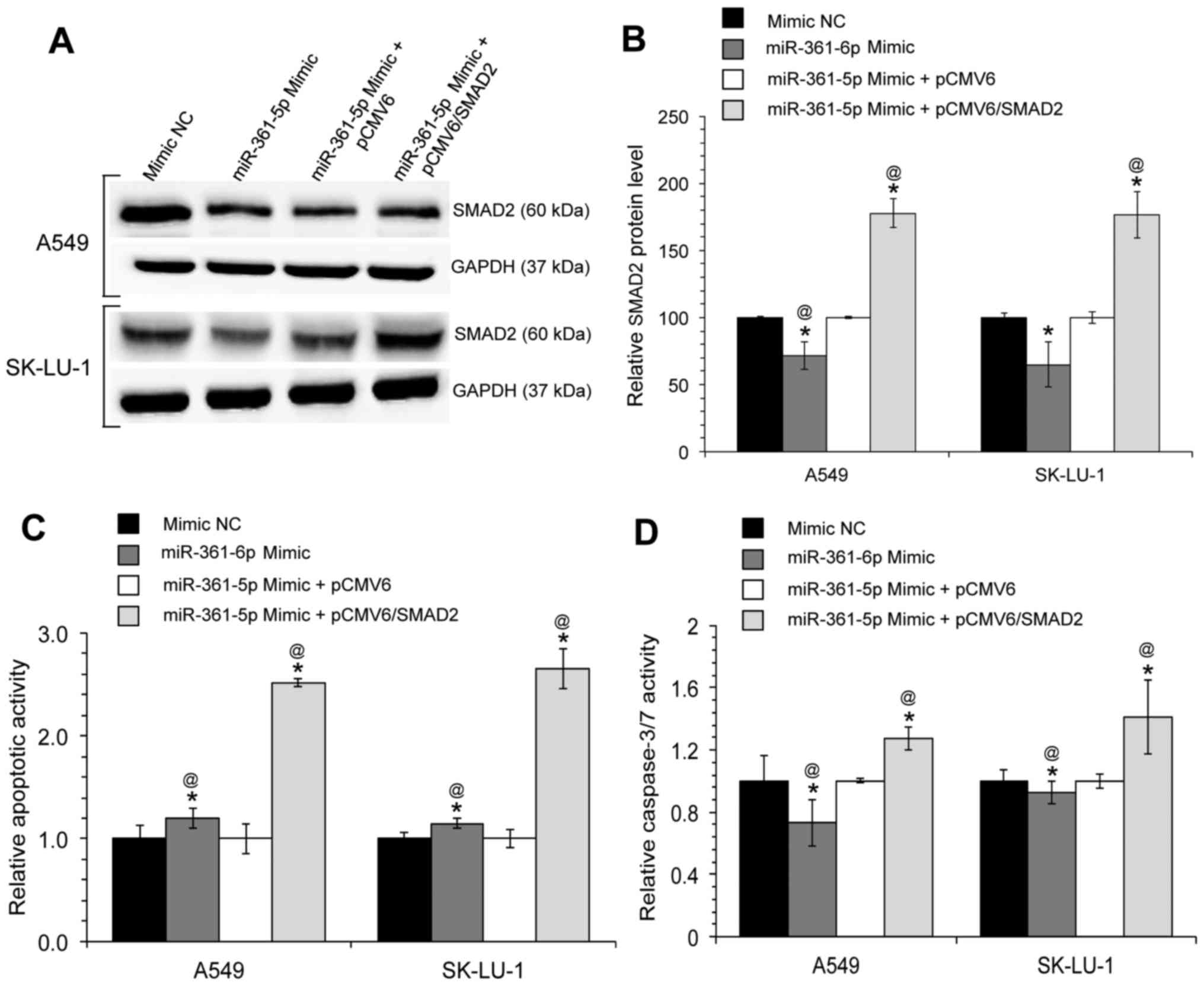
The ectopic overexpression of SMAD2,
without the 3′UTR, restores the effects of miR-361-5p on the A549
and SK-LU-1 lung adenocarcinoma cell lines
The results of this study indicated that miR-361-5p
suppresses the apoptosis of A549 and SK-LU-1 cells, and inhibits
SMAD2 expression. Therefore, it was predicted that the
miR-361-5p-mediated inhibition of apoptosis may in part be
attributed to the function of SMAD2. To confirm that the effects of
miR-361-5p on apoptosis are mediated by SMAD2, gene rescue
experiments were performed, whereby a SMAD2 expression
vector (pCMV6/SMAD2, lacking its 3′UTR) was co-transfected into the
A549 and SK-LU-1 cells along with the miR-361-5p mimic. The SMAD2
protein expression levels were measured at 48 h post transfection
and it was found that transfection with pCMV6/SMAD2 ‘rescued’ or
reversed the decrease in protein expression that was observed in
the cells transfected only with miR-361-5p mimics (Fig. 6A and B). Furthermore, transfection
with pCMV6/SMAD2 was able to partially reverse the inhibition of
apoptosis induced by miR-361-5p (Fig.
6C and D). Taken together, these observations suggest that the
oncogenic role of miR-361-5p in lung adenocarcinoma cells is at
least partially due to the inhibition of its target gene,
SMAD2.
Discussion
The anti-apoptotic Bcl-xL protein is
frequently found to be overexpressed in lung adenocarcinoma,
playing an important role in the inhibition of apoptosis, and is
therefore a critical contributor to tumor development and
progression (23). Our previous
study demonstrated that the silencing of Bcl-xL triggers
alterations in the miRNA expression profiles of A549 lung
adenocarcinoma cells and the rippling effects of these miRNA
expression alterations towards target genes may contribute to the
inducement of apoptosis (7).
miR-361-5p was one of the miRNAs found to be significantly
downregulated following Bcl-xL silencing, and the present
study aimed to determine the role of miR-361-5p in the apoptotic
properties of Bcl-xL-silenced human lung adenocarcinoma
cells.
Previous studies have demonstrated that in different
cancer types, miR-361-5p can function as either a tumor suppressive
or an oncogenic miRNA (oncomiR). For example, in colorectal and
gastric cancer, miR-361-5p has been shown to act as a tumor
suppressor, and to inhibit cancer cell proliferation, invasion and
metastasis by directly binding and decreasing the expression of
staphylococcal nuclease domain containing-1 (SND1) (24). miR-361-5p also functions as a tumor
suppressor in castration-resistant prostate cancer (25) and hepatocellular carcinoma
(26) by suppressing cell
proliferation and triggering apoptosis, through the direct
inhibition of signal transducer and activator of transcription 6
(STAT6) and C-X-C chemokine receptor type 6 (CXCR6), respectively.
Furthermore, an increased expression of miR-361-5p has reported to
be associated with a better prognosis in patients with breast
cancer (27). Conversely,
miR-361-5p functions as an oncomiR in cervical cancer, enhancing
the promotion of growth and increasing the migration/invasion
capacity of cells through mediation of epithelial-to-mesenchymal
(EMT) transition (28).
The results of this study indicated that the
inhibition of miR-361-5p expression led to an increase of the
apoptosis of A549 and SK-LU-1 cells in vitro and in
vivo. Furthermore, co-transfection experiments demonstrated
that miR-361-5p overexpression was able to block the apoptosis
induced by the silencing of Bcl-xL. These results suggest
that miR-361-5p plays an oncogenic role in the regulation of
apoptosis in NSCLC. The reasons that miR-361-5p plays differential
roles in various cancer types is not yet well understood; however,
it is suggested that the specific functions of miR-361-5p may be
tissue- or cell-specific and may strongly be dependent upon their
downstream targets. Further studies are warranted to elucidate the
role of miR-361-5p in different cell lines and types of cancer.
In this study, to identify the cellular target
through which miR-361-5p regulates apoptosis, bioinformatics
analysis revealed that two regions in the SMAD2 3′UTR mRNA
have perfect complementary binding sites to the seed sequence of
miR-361-5p. Luciferase activity assays indicated that miR-361-5p
binds to the 3′UTR sequence of SMAD2 mRNA, confirming that
SMAD2 is a direct target of miR-361-5p. Given that
miR-361-5p was downregulated in Bcl-xL-silenced A549 cells
(7) and that it negatively
regulates SMAD2 expression, we hypothesized that the
targeting of SMAD2 may be a mechanism through which
miR-361-5p acts as an oncomiR in NSCLC.
SMADS are a family of structurally related proteins
that function as signal transducers of TGFβ family member proteins
and play an important role in regulating cell growth inhibition,
cellular senescence, differentiation and apoptosis (29-33).
SMAD2, a receptor-regulated SMAD (R-Smads), has been proposed to be
a tumor suppressor and the decreased expression of SMAD2 has been
observed in human cancers (34-37).
Emerging evidence has demonstrated the critical role of SMAD2 in
apoptosis. For example, Yang et al (38) provided one of the first studies on
SMAD2 functioning as a tumor suppressor and identified SMAD2 as a
critical mediator of the TGFβ-induced apoptosis of prostate
epithelial cells. In another study, Yang et al also
demonstrated that SMAD2 together with Rb/E2F4, the cell
cycle-regulated repressor element (CDE) and the cell cycle gene
homology region (CHR), were able to suppress the expression of
survivin, an inhibitor of apoptosis, and to induce the
apoptosis of prostate epithelial cells (20). SMAD2 has also been shown to
activate the DAP-kinase promoter, thus associating SMAD2 with
mitochondrial-based pro-apoptotic events (32). Furthermore, various studies have
indicated that SMAD2 can downregulate the X-linked inhibitor of
apoptosis protein (XIAP), inducing caspase-3 activation and
TRAIL-induced apoptosis (39-41).
In conclusion, the findings of the present study
demonstrated that miR-361-5p acts as an oncogenic miRNA in NSCLC
and that the downregulation of this miRNA induces apoptosis in
vitro and in vivo. Its role as an oncogenic miRNA was
attributed towards the direct targeting of SMAD2, a novel
gene target. Notably, gene rescue experiments demonstrated that the
miR-361-5p-mediated inhibition of the apoptosis of A549 and SK-LU-1
cells was reversed by the re-introduction of the SMAD2 protein.
These results indicate that the downregulation of SMAD2 by
miR-361-5p is likely to be an authentic mechanism of
miR-361-5p-mediated oncogenesis in NSCLC, thus suggesting that the
inhibition of miR-361-5p may be a promising therapeutic strategy
for NSCLC.
Acknowledgments
The authors would like to thank Professor Ian
Charles Paterson (Department of Oral and Craniofacial Sciences,
Faculty of Dentistry, University of Malaya, Malaysia) for providing
technical editing, language editing and proofreading of this
manuscript.
Funding
This study was supported by the High Impact Research
Grant under Grant UM.C/625/1/HIR/MOE/CHAN/016 and the University of
Malaya Postgraduate Research Grant under Grant PG019-2016A. The
founding sponsors had no role in the design of the study; in the
collection, analyses, or interpretation of data; in the writing of
the manuscript, and in the decision to publish the results.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors’ contributions
NO and NHN conceived and designed the experiments;
NO performed the experiments; NO and NHN analyzed the data; NO and
NHN wrote the manuscript.
Ethics approval and consent to
participate
Approval was obtained from the University of Malaya,
Faculty of Medicine, Institutional Care of Use Committee (FOM
IACUC) (Ethics reference no. 2015-181006/IBS/R/NO) and complied
with all relevant animal welfare laws, guidelines and policies.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Soerjomataram I, Ervik M, Forman
D, Bray F, Dikshit R, Elser S, Mathers C, Rebelo M and Parkin DM:
GLOBOCAN 2012 v10, Lung Cancer Estimated Incidence, Mortality and
Prevalence Worldwide in 2012. International Agency for Research on
Cancer. World Health Organization; Lyon: 2013, http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx.
Accessed July 7, 2017.
|
|
2
|
Pfister DG, Johnson DH, Azzoli CG, Sause
W, Smith TJ, Baker S Jr, Olak J, Stover D, Strawn JR, Turrisi AT,
et al American Society of Clinical Oncology: American Society of
Clinical Oncology treatment of unresectable non-small-cell lung
cancer guideline: Update 2003. J Clin Oncol. 22:330–353. 2004.
View Article : Google Scholar
|
|
3
|
Howlader N, Noone A, Krapcho M, Miller D,
Bishop K, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, et
al: SEER Cancer Statistics Review, 1975–2013. National Cancer
Institute; Bethesda, MD: 2017, https://seer.cancer.gov/archive/csr/1975_2013/.
Accessed July 7, 2017.
|
|
4
|
Sorenson CM: Bcl-2 family members and
disease. Biochim Biophys Acta. 1644:169–177. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liam CK, Pang YK, Leow CH, Poosparajah S
and Menon A: Changes in the distribution of lung cancer cell types
and patient demography in a developing multiracial Asian country:
Experience of a university teaching hospital. Lung Cancer.
53:23–30. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Soini Y, Kinnula V, Kaarteenaho-Wiik R,
Kurttila E, Linnainmaa K and Pääkkö P: Apoptosis and expression of
apoptosis regulating proteins bcl-2, mcl-1, bcl-X, and bax in
malignant mesothelioma. Clin Cancer Res. 5:3508–3515.
1999.PubMed/NCBI
|
|
7
|
Othman N, In LL, Harikrishna JA and Hasima
N: Bcl-xL silencing induces alterations in hsa-miR-608 expression
and subsequent cell death in A549 and SK-LU1 human lung
adeno-carcinoma cells. PLoS One. 8:e817352013. View Article : Google Scholar
|
|
8
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hayashita Y, Osada H, Tatematsu Y, Yamada
H, Yanagisawa K, Tomida S, Yatabe Y, Kawahara K, Sekido Y and
Takahashi T: A polycistronic microRNA cluster, miR-17-92, is
overexpressed in human lung cancers and enhances cell
proliferation. Cancer Res. 65:9628–9632. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shivdasani RA: MicroRNAs: Regulators of
gene expression and cell differentiation. Blood. 108:3646–3653.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Phuah NH, Azmi MN, Awang K and Nagoor NH:
Downregulation of microRNA-210 confers sensitivity towards
1′S-1′-acetoxychavicol acetate (ACA) in cervical cancer cells by
targeting SMAD4. Mol Cells. 40:291–298. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mott JL, Kobayashi S, Bronk SF and Gores
GJ: mir-29 regulates Mcl-1 protein expression and apoptosis.
Oncogene. 26:6133–6140. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Othman N and Nagoor NH: The role of
microRNAs in the regulation of apoptosis in lung cancer and its
application in cancer treatment. BioMed Res Int. 2014:3180302014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Koo KH and Kwon H: MicroRNA miR-4779
suppresses tumor growth by inducing apoptosis and cell cycle arrest
through direct targeting of PAK2 and CCND3. Cell Death Dis.
9:77–91. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yuan L, Li S, Zhou Q, Wang D, Zou D, Shu J
and Huang Y: miR-124 inhibits invasion and induces apoptosis of
ovarian cancer cells by targeting programmed cell death 6. Oncol
Lett. 14:7311–7317. 2017.
|
|
16
|
Lv KT, Liu Z, Feng J, Zhao W, Hao T, Ding
WY, Chu JP and Gao LJ: miR-22-3p regulates cell proliferation and
inhibits cell apoptosis through targeting the eIF4EBP3 gene in
human cervical squamous carcinoma cells. Int J Med Sci. 15:142–152.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gang L, Qun L, Liu WD, Li YS, Xu YZ and
Yuan DT: MicroRNA-34a promotes cell cycle arrest and apoptosis and
suppresses cell adhesion by targeting DUSP1 in osteosarcoma. Am J
Transl Res. 9:5388–5399. 2017.
|
|
18
|
Grimson A, Farh KK, Johnston WK,
Garrett-Engele P, Lim LP and Bartel DP: MicroRNA targeting
specificity in mammals: Determinants beyond seed pairing. Mol Cell.
27:91–105. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang W, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2009. View Article : Google Scholar
|
|
20
|
Yang J, Song K, Krebs TL, Jackson MW and
Danielpour D: Rb/E2F4 and Smad2/3 link survivin to TGF-beta-induced
apoptosis and tumor progression. Oncogene. 27:5326–5338. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jang CW, Chen CH, Chen CC, Chen JY, Su YH
and Chen RH: TGF-beta induces apoptosis through Smad-mediated
expression of DAP-kinase. Nat Cell Biol. 4:51–58. 2002. View Article : Google Scholar
|
|
22
|
Van Themsche C, Chaudhry P, Leblanc V,
Parent S and Asselin E: XIAP gene expression and function is
regulated by autocrine and paracrine TGF-beta signaling. Mol
Cancer. 9:216–228. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Leech SH, Olie RA, Gautschi O, Simões-Wüst
AP, Tschopp S, Häner R, Hall J, Stahel RA and Zangemeister-Wittke
U: Induction of apoptosis in lung-cancer cells following bcl-xL
anti-sense treatment. Int J Cancer. 86:570–576. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ma F, Song H, Guo B, Zhang Y, Zheng Y, Lin
C, Wu Y, Guan G, Sha R, Zhou Q, et al: miR-361-5p inhibits
colorectal and gastric cancer growth and metastasis by targeting
staphylococcal nuclease domain containing-1. Oncotarget.
6:17404–17416. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu D, Tao T, Xu B, Chen S, Liu C, Zhang
L, Lu K, Huang Y, Jiang L, Zhang X, et al: miR-361-5p acts as a
tumor suppressor in prostate cancer by targeting signal transducer
and activator of transcription-6 (STAT6). Biochem Biophys Res
Commun. 445:151–156. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sun JJ, Chen GY and Xie ZT:
MicroRNA-361-5p inhibits cancer cell growth by targeting CXCR6 in
hepatocellular carcinoma. Cell Physiol Biochem. 38:777–785. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cao ZG, Huang YN, Yao L, Liu YR, Hu X, Hou
YF and Shao ZM: Positive expression of miR-361-5p indicates better
prognosis for breast cancer patients. J Thorac Dis. 8:1772–1779.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu X, Xi X, Yan Q, Zhang Z, Cai B, Lu W
and Wan X: MicroRNA-361-5p facilitates cervical cancer progression
through mediation of epithelial-to-mesenchymal transition. Med
Oncol. 30:751–762. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Derynck R, Zhang Y and Feng XH: Smads:
Transcriptional activators of TGF-beta responses. Cell. 95:737–740.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Itoh S, Itoh F, Goumans MJ and Ten Dijke
P: Signaling of transforming growth factor-beta family members
through Smad proteins. Eur J Biochem. 267:6954–6967. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Massagué J: How cells read TGF-beta
signals. Nat Rev Mol Cell Biol. 1:169–178. 2000. View Article : Google Scholar
|
|
32
|
Massagué J and Wotton D: Transcriptional
control by the TGF-beta/Smad signaling system. EMBO J.
19:1745–1754. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Miyazono K: TGF-beta signaling by Smad
proteins. Cytokine Growth Factor Rev. 11:15–22. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hoot KE, Lighthall J, Han G, Lu SL, Li A,
Ju W, Kulesz-Martin M, Bottinger E and Wang XJ:
Keratinocyte-specific Smad2 ablation results in increased
epithelial-mesenchymal transition during skin cancer formation and
progression. J Clin Invest. 118:2722–2732. 2008.PubMed/NCBI
|
|
35
|
Munker S, Weng HL, Li Q, Liu Y, Meyer C,
Dooley S and Li J: Differential Smad expression contributes to
severity of cholangiocarcinoma. Z Gastroenterol. 50:K1022012.
View Article : Google Scholar
|
|
36
|
Wu Y, Li Q, Zhou X, Yu J, Mu Y, Munker S,
Xu C, Shen Z, Müllenbach R, Liu Y, et al: Decreased levels of
active SMAD2 correlate with poor prognosis in gastric cancer. PLoS
One. 7:e356842012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Samanta D and Datta PK: Alterations in the
Smad pathway in human cancers. Front Biosci. 17:1281–1293. 2012.
View Article : Google Scholar
|
|
38
|
Yang J, Wahdan-Alaswad R and Danielpour D:
Critical role of Smad2 in tumor suppression and transforming growth
factor-beta-induced apoptosis of prostate epithelial cells. Cancer
Res. 69:2185–2190. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang H, Jiang JY, Zhu C, Peng C and Tsang
BK: Role and regulation of nodal/activin receptor-like kinase 7
signaling pathway in the control of ovarian follicular atresia. Mol
Endocrinol. 20:2469–2482. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xu G, Zhou H, Wang Q, Auersperg N and Peng
C: Activin receptor-like kinase 7 induces apoptosis through
up-regulation of Bax and down-regulation of Xiap in normal and
malignant ovarian epithelial cell lines. Mol Cancer Res. 4:235–246.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xu F, Zhou D, Meng X, Wang X, Liu C, Huang
C, Li J and Zhang L: Smad2 increases the apoptosis of activated
human hepatic stellate cells induced by TRAIL. Int Immunopharmacol.
32:76–86. 2016. View Article : Google Scholar : PubMed/NCBI
|