Introduction
Glioblastoma multiforme (GBM) is the most frequently
diagnosed and lethal type of primary brain tumor, and is
characterized by high invasive ability. Although surgery is the
primary treatment strategy for GBM, extensive diffuse parenchymal
invasion often results in failure of surgical resection (1-3).
Therefore, radiotherapy is a major adjuvant therapy for patients
with GBM (4). It has long been
recognized that GBM tumors are heterogeneous in their radiation
response, and the degree of radiosensitivity is thought to be
associated with intrinsic and extrinsic properties of the tumor
cell population (5-7). The effects and underlying molecular
mechanisms of GBM progression and radioresistance have yet to be
clarified.
Long non-coding RNAs (lncRNAs) are
non-protein-coding transcripts longer than ~200 nucleotides.
Accumulating evidence has indicated that certain lncRNAs serve
important functions in the regulation of various biological
processes, including proliferation, differentiation and cell death
(8-14). The lncRNA AHIF is the natural
antisense transcript of hypoxia-inducible factor-1α (HIF-1α), and
is exactly complementary to the 3'-untranslated region of HIF-1α
mRNA (15,16). A small number of studies have
addressed the function of AHIF in tumor progression (17-20).
The expression of AHIF was detected in invasive ductal carcinoma
samples, whereas adjacent non-cancer tissues did not exhibit AHIF
expression. AHIF is a poor prognostic marker in breast cancer
contributing in HIF-1α mRNA regulation (18).
Exosomes are nano-sized membrane vesicles with
diameters between 30 and 100 nm (21-23).
It has previously been reported that cancer-associated exosomes
serve important roles in regulating the cellular functions of
cancerous cells, fibroblasts, vascular smooth muscle cells and
endothelial cells through effectively delivering microRNAs, mRNAs
and proteins (24-29). However, the functions of exosomes
in GBM progression and radiotherapy remain unknown.
In the present study, the reverse
transcription-quantitative polymerase chain reaction (RT-qPCR), was
used to identify the expression of AHIF in GBM cancerous tissues
and radioresistant GBM cells. Functional experiments in vitro were
performed to address the hypothesis that AHIF could promote
glioblastoma progression and radioresistance via exosomes. Further
biochemical analysis identified that AHIF regulates factors
associated with migration and angiogenesis in exosomes. To the best
of our knowledge, the present study is the first to establish that
AHIF promotes glioblastoma progression and radioresistance via
exosomes, which may be a potential therapeutic target.
Materials and methods
Patients and tissue samples
The present study was approved by the Ethics
Committee of Renji Hospital, School of Medicine, Shanghai Jiao Tong
University (Shanghai, China). Written informed consent was obtained
from patients for participation in the study. A total of 31
patients (including 16 males and 15 females) with histologically
confirmed GBM were recruited at Renji Hospital between January 2016
and December 2017 for inclusion in the present study. The mean age
of patients was 49.38±15.87 years (range, 13-85 years). Adjacent
normal tissues were also collected from 7 of the patients with
GBM.
Cell culture
The human GBM cell lines U87-MG (glioblastoma of
unknown origin; the cell line was authenticated by short tandem
repeat profiling), U251-MG, A172 and T98G (purchased in 2014 from
the Cell Bank of the Chinese Academy of Sciences, Shanghai, China)
were cultured in Dulbecco's modified Eagle's medium (Hyclone; GE
Healthcare, Logan, UT, USA) supplemented with 10% fetal bovine
serum (FBS; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) and maintained in a humidified atmosphere at 37˚C with 5%
CO2.
Radiation treatment
Cells [U87-MG and U251-MG, as well as respective
AHIF-knockdown (KD) and AHIF-overexpression (OE) cells] in culture
were treated with an irradiator (GE3000) using a 137Cs
source at a dose rate of 4.0 Gy/min for 90 sec. During irradiation,
the cultures were maintained in the cell culture incubator (5%
CO2 at 37°C).
RT-qPCR
RNA extraction, cDNA synthesis and RT-qPCR were
performed as described previously (20). Total RNA was extracted from tissues
and/or cells using TRIzol® (Invitrogen; Thermo Fisher
Scientific, Inc.). A total of 1 μg RNA was used for first-strand
cDNA synthesis (99°C for 5 min and 42°C for 45 min) using an
oligo-dT primer and M-myeloblastosis virus reverse transcriptase XL
(Promega Corporation, Madison, WI, USA). The synthesized
first-strand cDNA was used for each qPCR. The qPCR primers were as
follows: Human AGIF forward, 5'-TCAACATACATTAAGGTGATGGCAC-3' and
reverse, 5'-ATTTGCTTCAACACCTCCAACTC-3'; human vascular endothelial
growth factor A (VEGF-A) forward, 5'-TTGCCTTGCTGCTCTACCTCCA-3' and
reverse, 5'-GAT GGCAGTAGCTGCGCTGATA-3'; human angiogenin forward,
5'-CAACAAGCGCAGCATCAAG-3' and reverse,
5'-CAAGTGGTGACCTGGAAAGAAG-3'. SYBR-Green PCR Master Mix (Applied
Biosystems; Thermo Fisher Scientific, Inc.) was used for the qPCR
experiments. β-actin was used as an internal control. The relative
expression of target genes was determined using the
2−∆∆Cq method (30).
The qPCR primers for β-actin were: Forward,
5'-CACCATTGGCAATGAGCGGTTC-3' and reverse,
5'-AGGTCTTTGCGGATGTCCACGT-3'. The thermocycling conditions for qPCR
were as follows: Initial denaturation for 3 min at 95°C, followed
by 45 cycles of 95°C for 10 sec and 58°C for 45 sec. Data were
acquired at the end of the annealing/extension phase. Melt curve
analysis was performed at the end of each run from 58 to 95°C.
Western blot analysis
Cells were lysed in radioimmunoprecipitation assay
buffer (Pierce; Thermo Fisher Scientific, Inc.), and protein was
quantified using Coomassie Blue protein standards (Pierce; Thermo
Fisher Scientific, Inc.). Protein samples (30 ’g) were subjected to
SDS-PAGE (10% gel) and transferred onto polyvinylidene difluoride
membranes (EMD Millipore, Billerica, MA, USA). The membranes were
incubated with blocking buffer [5% skimmed milk in Tris-buffered
saline with 0.1% Tween-20 (TBS-T)] at room temperature for 1 h and
then proteins were detected with the following antibodies at 1:500
dilution, incubated overnight at 4°C: Anti-cluster of
differentiation (CD)63 antibody (cat. no. 25682-1-AP; ProteinTech
Group, Inc., Chicago, IL, USA), anti-CD81 antibody (cat. no.
ab109201; Abcam, Cambridge, MA, USA), anti-cytochrome c oxidase IV
(Cox IV; cat. no. sc58348, Santa Cruz Biotechnology, Inc., Dallas,
TX, USA), anti-B-cell lymphoma 2 (Bcl-2; cat no. 2870; Cell
Signaling Technology, Inc., Danvers, MA, USA), anti-B-cell lymphoma
extra-large (Bcl-xl; cat no. 2764; Cell Signaling Technology,
Inc.), anti-myeloid cell leukemia-1 (Mcl-1; cat no. 94296; Cell
Signaling Technology, Inc.) and anti-β-actin antibody (cat no.
ab8227; Abcam). The membranes were washed with TBS-T, then
incubated with horseradish peroxidase-conjugated anti-rabbit or
anti-mouse secondary antibody (cat. nos. AP182P and AP308P,
respectively; 1:10,000 dilution; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) at room temperature for 2 h. Detection was
performed using western blot detection reagents (Odyssey; LI-COR
Biosciences, Lincoln, NE, USA).
Lentiviral vector-mediated gene KD or
OE
The AHIF-KD target sequence was:
5'-GATCCAAAGCTCTGAGTAA-3'. The AHIF-OE sequence (NCBI accession no.
NR_045406.1) was constructed by Hanyin Ltd., Co. (Shanghai, China).
The recombinant lentivirus and negative control (NC; PHY-008 for
AHIF-OE NC and PHY-310 for AHIF-KD NC) lentivirus were prepared and
titered to 109 transfection units/ml (Hanyin Ltd., Co.). After 48
h, the efficiency of AHIF-KD or AHIF-OE was confirmed using RT-qPCR
as aforementioned. To obtain stably transfected cells, GBM cells
(U87-MG, U251-MG, A172 and T98G) with AHIF-KD or AHIF-OE cells and
respective control cells were seeded in 6-well dishes at a density
of 1×105 cells/well. The cells were then infected with the same
virus titer on the following day with 8 µg/ml Polybrene
(Maokang Co., Shanghai, China). At ~72 h after viral infection, the
culture medium was replaced with Dulbecco's modified Eagle's medium
(DMEM; Hyclone; GE Healthcare) containing 4 µg/ml puromycin
supplemented with 10% FBS. The puromycin-resistant cells were
amplified in medium containing 2 µg/ml puromycin for 7 days
and then transferred to a medium without puromycin.
Cell Counting Kit-8 (CCK-8) assay
A CCK-8 assay (Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan) was performed according to the manufacturer's
protocol. In brief, exosome-treated GBM cells (U87-MG, U251-MG,
A172 and T98G), with AHIF-KD or AHIF-OE cells and respective
control cells, were cultured at equal cell density (2,500 cells/100
µl per well) in 96-well plates for continuous detection over
a 5-day period. The culture was terminated by adding 10 µl
CCK-8 (5 mg/ml) to the culture medium. After 2 h, the wells were
analyzed using a microplate reader (BioTek El×800; BioTek
Instruments, Inc., Winooski, VT, USA) at 490 nm.
Invasion assay
GBM cells (U87-MG, U251-MG, A172 and T98G), with
AHIF-KD or AHIF-OE cells and respective control cells, at
1×104 cells/100 µl were plated in the upper
chambers of Matrigel-coated Transwell assay inserts (EMD Millipore)
in 200 ml serum-free DMEM. The inserts were then placed into wells
of a 24-well plate containing DMEM with 10% FBS as a
chemoattractant. After 24 h at 37°C, the top layer of the insert
was wiped with a cotton swab to remove remaining cells. The
invading cells on the lower surface were stained with 0.1% crystal
violet at room temperature for 1 h and images were captured using
digital microscopy. The number of cells in five random fields of
each chamber was determined, and the mean number of cells was
calculated.
Cell apoptosis analysis
Apoptosis was analyzed by translocation of
phosphatidylserine to the cell surface using an Annexin and DAPI
Apoptosis Detection kit (BD Biosciences, Franklin Lakes, NJ, USA).
GBM cells (U87-MG, U251-MG, A172 and T98G), with AHIF-KD or AHIF-OE
cells and respective control cells, were treated with 6-Gy
radiation, then collected and washed in ice-cold PBS. Cells were
stained with AnnexinV-fluorescein isothiocyanate and DAPI for 30
min in the dark. Cell apoptosis was analyzed using BD CellQuest™
Pro software (BD Biosciences) on a FACSAria flow cytometer (BD
Biosciences). Fluorescence was captured with emission wavelength of
488 nm.
Exosome isolation and co-culture
In order to isolate exosomes, GBM cells (U87-MG,
U251-MG, A172 and T98G), with AHIF-KD or AHIF-OE cells and
respective control cells, were cultured for 48 h and the
supernatant was collected. The supernatants were then centrifuged
twice (1,000 × g for 10 min and 3,000 × g for 30 min at 4°C) to
deplete them of the cells and fragments. Then, Total Exosome
Isolation Reagent (Thermo Fisher Scientific, Inc.) was added
overnight, followed by centrifugation 10,000 × g for 1 h at 4°C.
Exosomes were resuspended in PBS and stored at −80°C. The
concentration of exosomes was determined using a Bicinchoninic Acid
Protein assay. Exosomes were then added to 105 GBM cells
at a concentration of 50 ng/ml serum-free DMEM for 24 h. AHIF-OE
cells were treated with exosomal inhibitor GW4869 (10 µM for
24 h at 37°C with 5% CO2) prior to collection of the
supernatant.
Electron microscopic observation of
exosomes
The exosome suspension was added to an equal volume
of 4% paraformaldehyde (Nacalai Tesque, Inc., Kyoto, Japan), and
the mixture was applied to a Formvar/carbon film-coated
transmission electron microscope (TEM) grid (Alliance Biosystems,
Inc., Osaka, Japan). Subsequently, the sample was fixed by
incubation with 1% glutaraldehyde for 5 min, washed with PBS, and
incubated with 1% uranyl acetate for 5 min. The sample was observed
under a TEM (Hitachi H-7650; Hitachi, Ltd., Tokyo, Japan).
Exosome labeling with PKH67
Exosomes derived from AHIF-KD or AHIF-OE cells were
labeled with PKH67, a Green Fluorescent Labeling kit
(Sigma-Aldrich; Merck KGaA). The concentration of PKH67 used for
exosome labeling was 2 µM per exosome from 5×105
cells. The labeled exosomes were assessed using an inverted
fluorescence microscopy (Olympus CKX41; Olympus Corporation, Tokyo,
Japan).
Statistical analysis
Results are presented as the mean ± standard error
of the mean. One-way analysis of variance with Tukey's test was
conducted to compare multiple groups. All statistical analyses were
performed using SPSS for Windows (version 17.0; SPSS, Inc.,
Chicago, IL, USA). Two-tailed P<0.05 was considered to indicate
a statistically significant difference.
Results
AHIF is highly expressed in GBM and in
response to radiotherapy
Fresh tissues were collected from patients with GBM
as well as adjacent non-cancerous tissues. The results of RT-qPCR
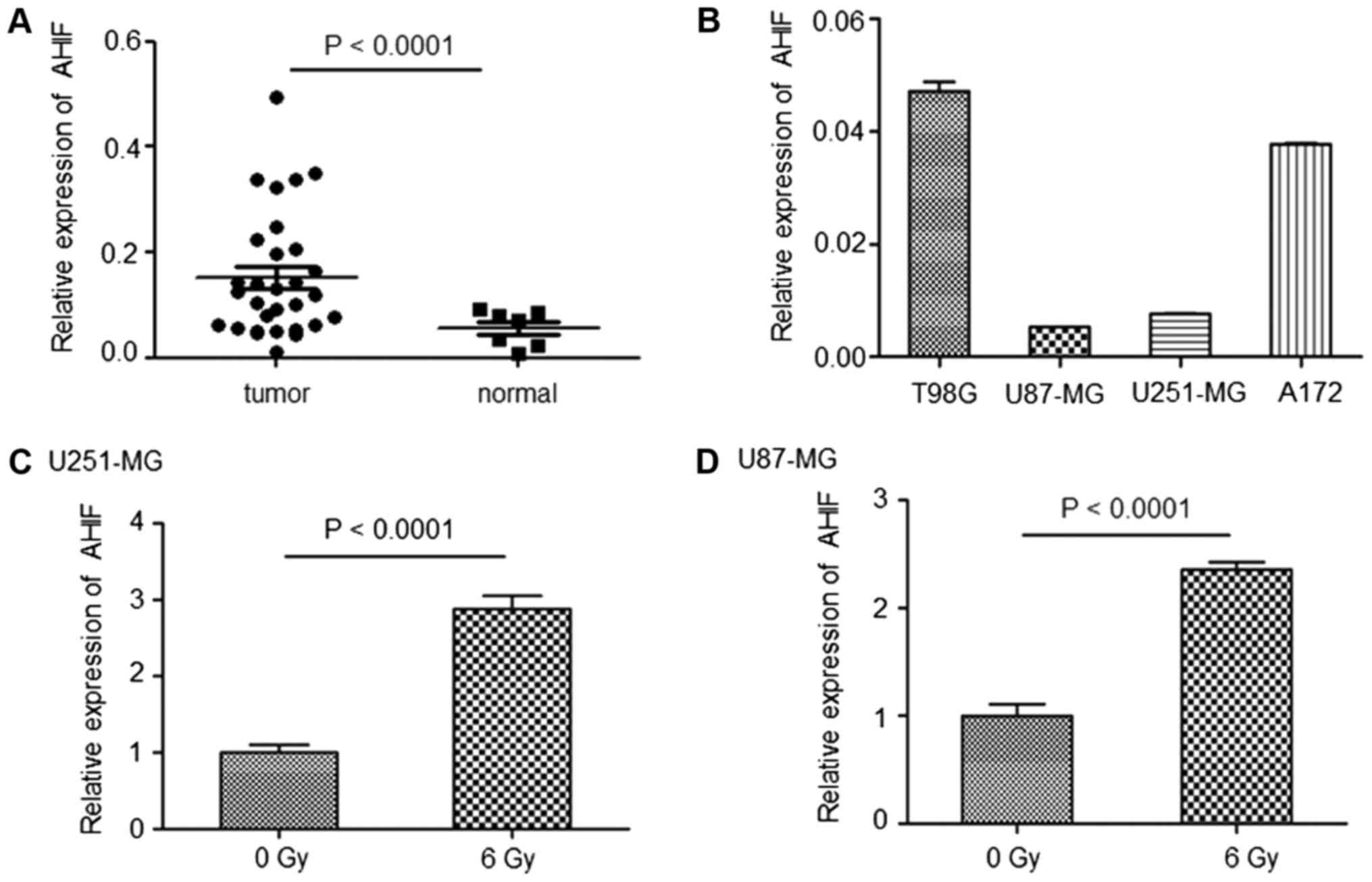
indicated that AHIF expression was significantly upregulated in GBM
tissues compared with in normal tissues (Fig. 1A). AHIF expression was then
investigated in an array of GBM cell lines (U87-MG, U251-MG, A172
and T98G). As indicated in Fig.
1B, T98G and A712 cells exhibited increased levels of AHIF
expression compared with in U87-MG and U251-MG cells. Furthermore,
AHIF expression was increased in U87-MG and U251-MG cells following
irradiation (Fig. 1C and D),
indicating that AHIF expression may be affected by
radiotherapy.
Suppression of AHIF in GBM cells
decreases cell viability, invasion and radioresistance
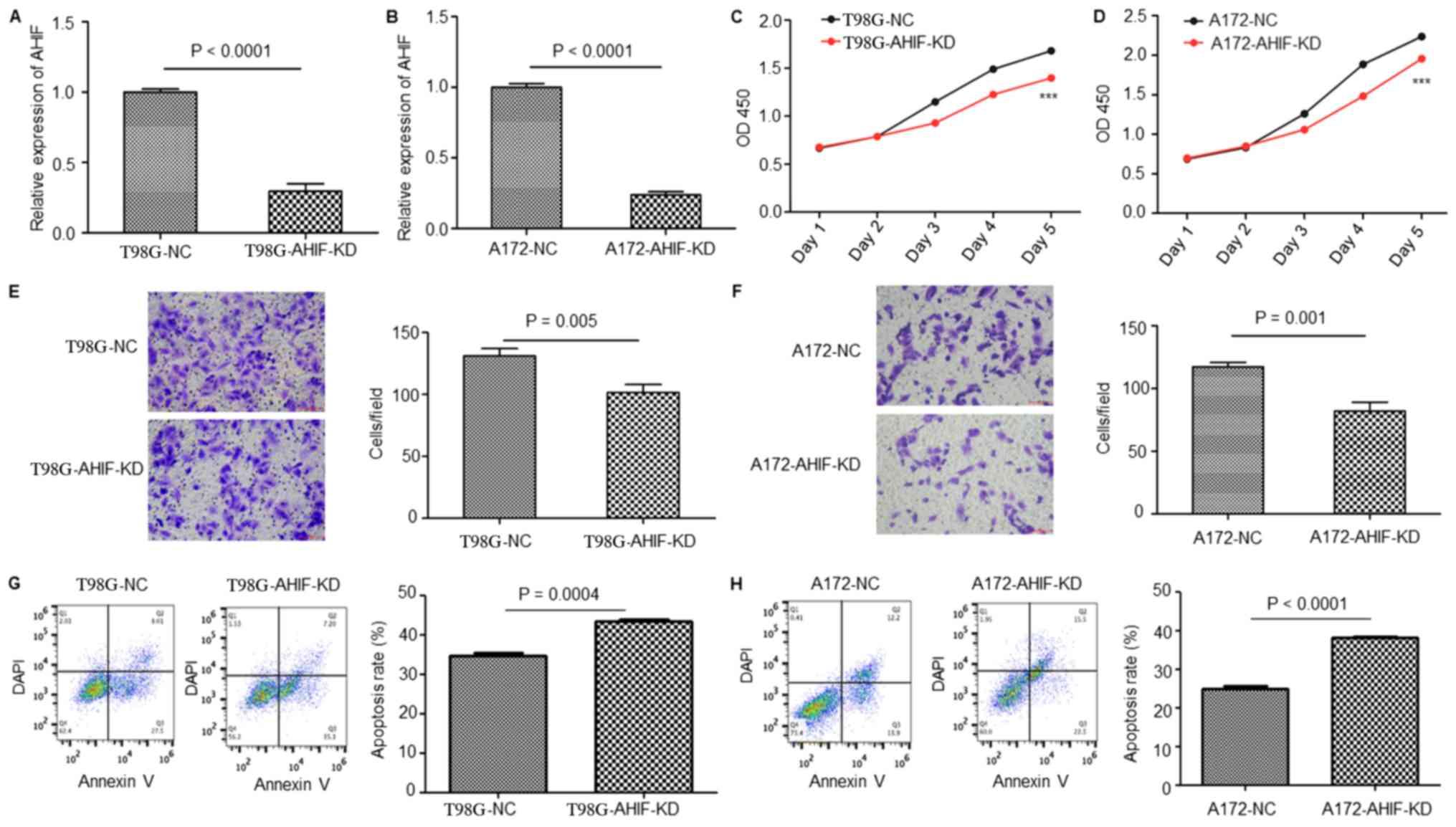
To investigate the function of AHIF in GBM
progression and radiotherapy, T98G and A172 cells with stable KD of
AHIF were constructed using a lentivirus. As presented in Fig. 2A and B, AHIF was effectively
inhibited in AHIF-KD cells compared with in the NC cells. CCK-8
analysis of these cells suggested that the viability of AHIF-KD
cells was significantly decreased compared with that of NC cells
(Fig. 2C and D). Invasion assay
results demonstrated that AHIF-KD cells had a significantly
decreased invasive capacity compared with that of NC cells
(Fig. 2E and F). Furthermore, an
apoptosis assay of AHIF-KD cells following 6-Gy treatment indicated
that the proportion of apoptotic cells was significantly increased
(Fig. 2G and H). In summary, these
results indicate that KD of AHIF in GBM cells decreased the
viability and invasive ability, and increased the proportion of
apoptotic cells following radiotherapy.
OE of AHIF in GBM cells increases cell
viability, invasion and radioresistance
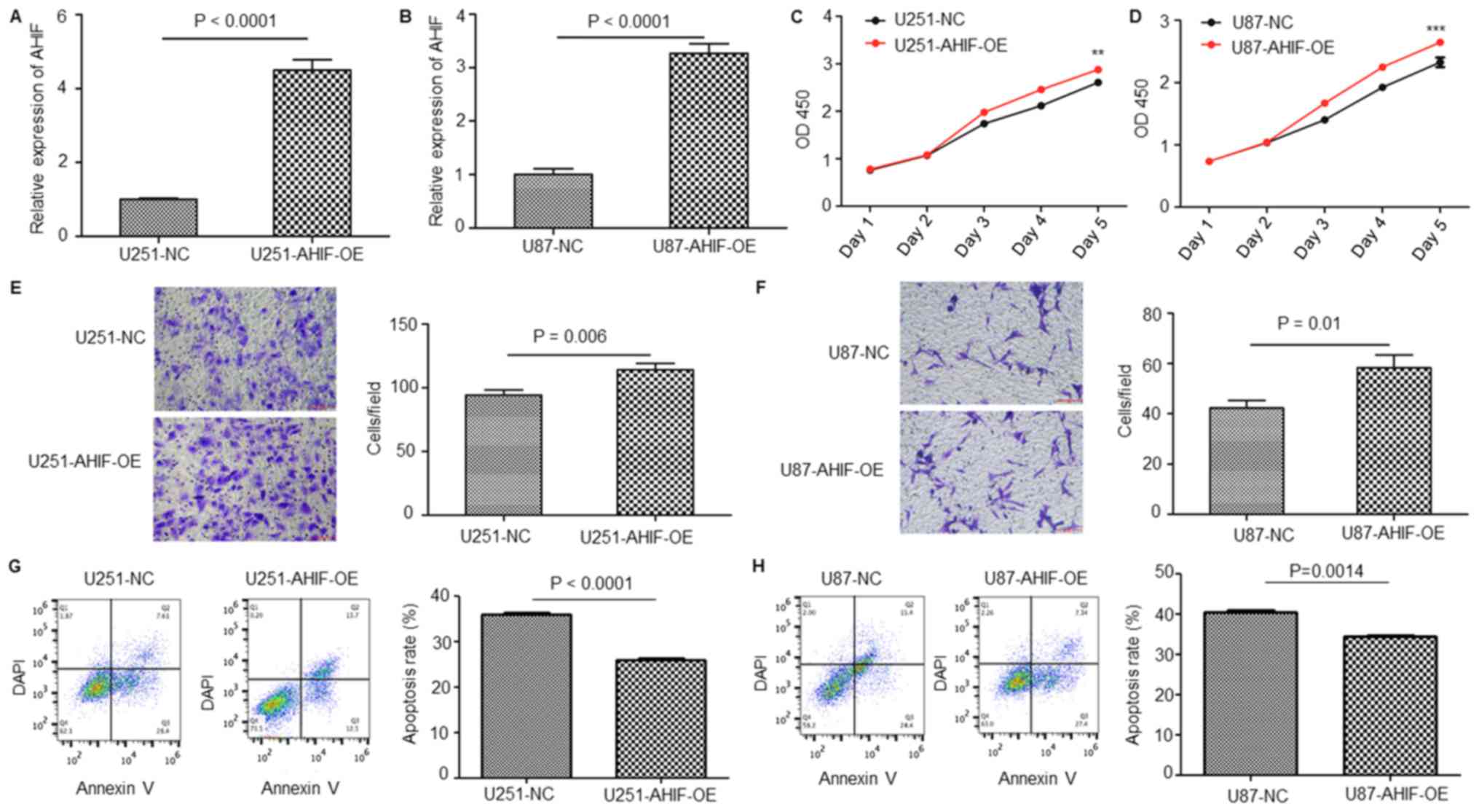
To further clarify the function of AHIF in GBM
progression and radiotherapy, GBM cells with stable OE of AHIF were
constructed using a lentivirus. As presented in Fig. 3A and B, AHIF was effectively
overexpressed in AHIF-OE U87-MG and U251-MG cells compared with in
the NC cells. CCK-8 analysis of these cells identified that the
viability of AHIF-OE cells was significantly increased compared
with that of NC cells (Fig. 3C and
D). Invasion assay results demonstrated that OE of AHIF
significantly increased the cell invasive ability (Fig. 3E and F). Furthermore, an apoptosis
assay of AHIF-OE cells following 6-Gy treatment indicated that the
proportion of apoptotic cells was significantly decreased (Fig. 3G and H). In summary, these results
indicate that OE of AHIF in GBM cells increased the viability and
invasive ability, and decreased the proportion of apoptotic cells
following radiotherapy.
Exosomes derived from AHIF-KD cells
inhibit GBM cell viability, invasion and radioresistance
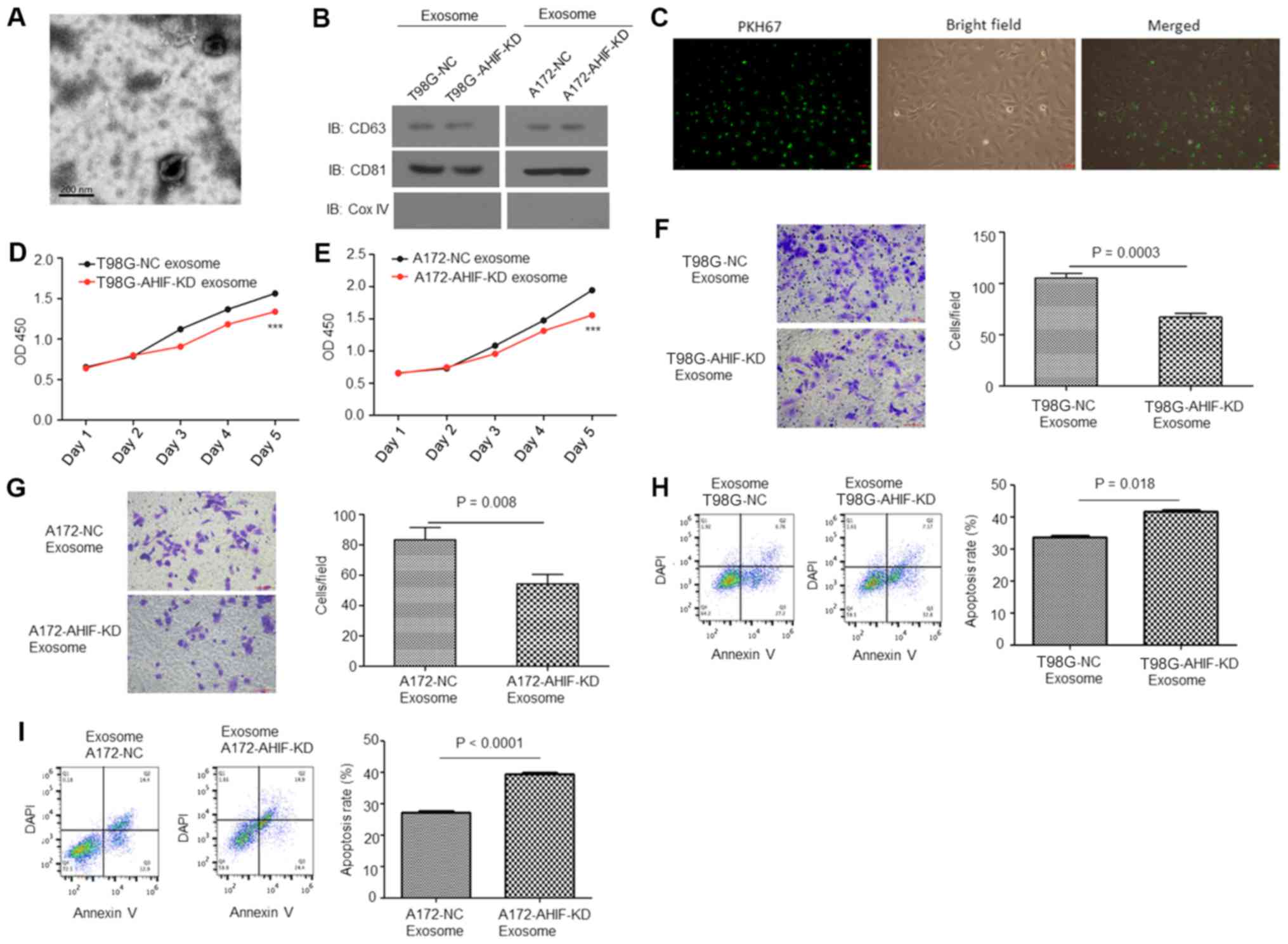
Exosomes have previously been identified to serve
important functions in hypoxia and tumor progression (26,27).
Therefore, in the present study, it was investigated whether AHIF
was able to regulate GBM progression and radiotherapy via exosomes.
Exosomes were isolated from GBM cell medium and their morphology
was observed under a TEM (Fig.
4A). Western blot analysis indicated that the exosomes were
enriched with the exosomal markers CD63 and CD81, but not the
mitochondrial marker Cox IV (Fig.
4B), indicating that exosomes had been successfully isolated.
Fig. 4C presents fluorescence
microscopy images of GBM cells co-cultured with exosomes [stained
with PKH67 (green)]. CCK-8 analysis indicated that the viability of
cells co-cultured with exosomes from AHIF-KD cells was
significantly decreased compared with cells treated with exosomes
derived from control cells (Fig. 4D
and E). Invasion assay results indicated that cells co-cultured
with exosomes derived from AHIF-KD cells exhibited significantly
decreased invasive ability compared with cells treated with
exosomes derived from control cells (Fig. 4F and G). Furthermore, an apoptosis
assay of these cells following 6-Gy treatment indicated that the
group co-cultured with exosomes derived from AHIF-KD cells
contained a significantly increased proportion of apoptotic cells
compared with the cell group treated with exosomes derived from
control cells (Fig. 4H and I). In
summary, these results indicate that AHIF-KD cells inhibited GBM
cell viability, invasion and radioresistance via exosomes.
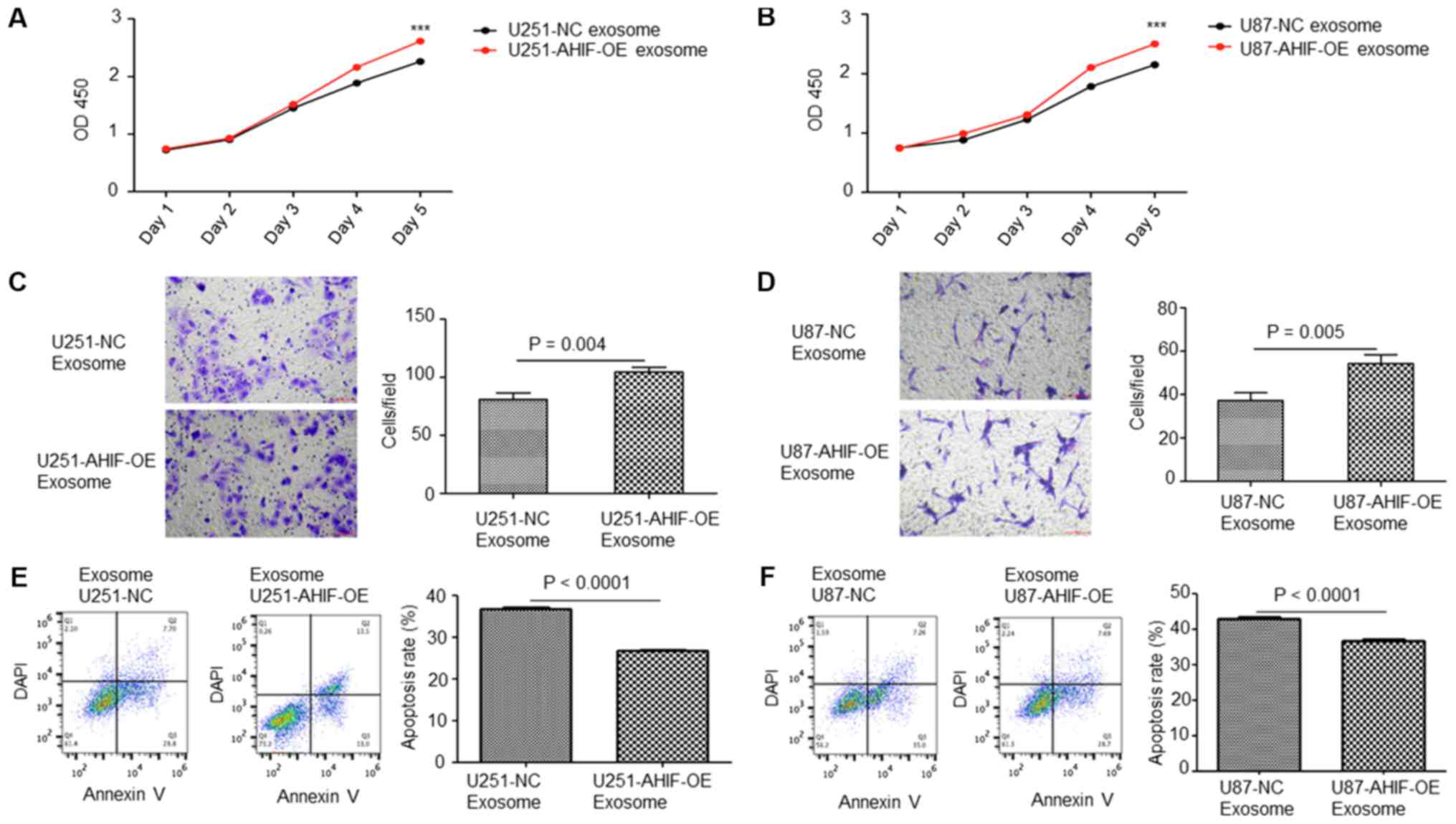
Exosomes derived from AHIF-OE cells
promote cell viability, invasion and radioresistance
Exosomes were collected from AHIF-OE cells. CCK-8
analysis indicated that the viability of cells co-cultured with
exosomes derived from AHIF-OE cells was significantly increased
compared with cells treated with exosomes derived from control
cells (Fig. 5A and B). Invasion
assay results suggested that cells co-cultured with exosomes
derived from AHIF-OE cells exhibited significantly increased
invasive ability compared with cells treated with exosomes derived
from control cells (Fig. 5C and
D). Furthermore, an apoptosis assay of these cells following
6-Gy treatment indicated that the cell group co-cultured with
exosomes derived from AHIF-OE cells contained a significantly
decreased percentage of apoptotic cells compared with the cell
group treated with exosomes derived from control cells (Fig. 5E and F). In summary, these results
indicate that AHIF-OE GBM cells promoted viability, invasion and
radio-resistance via exosomes.
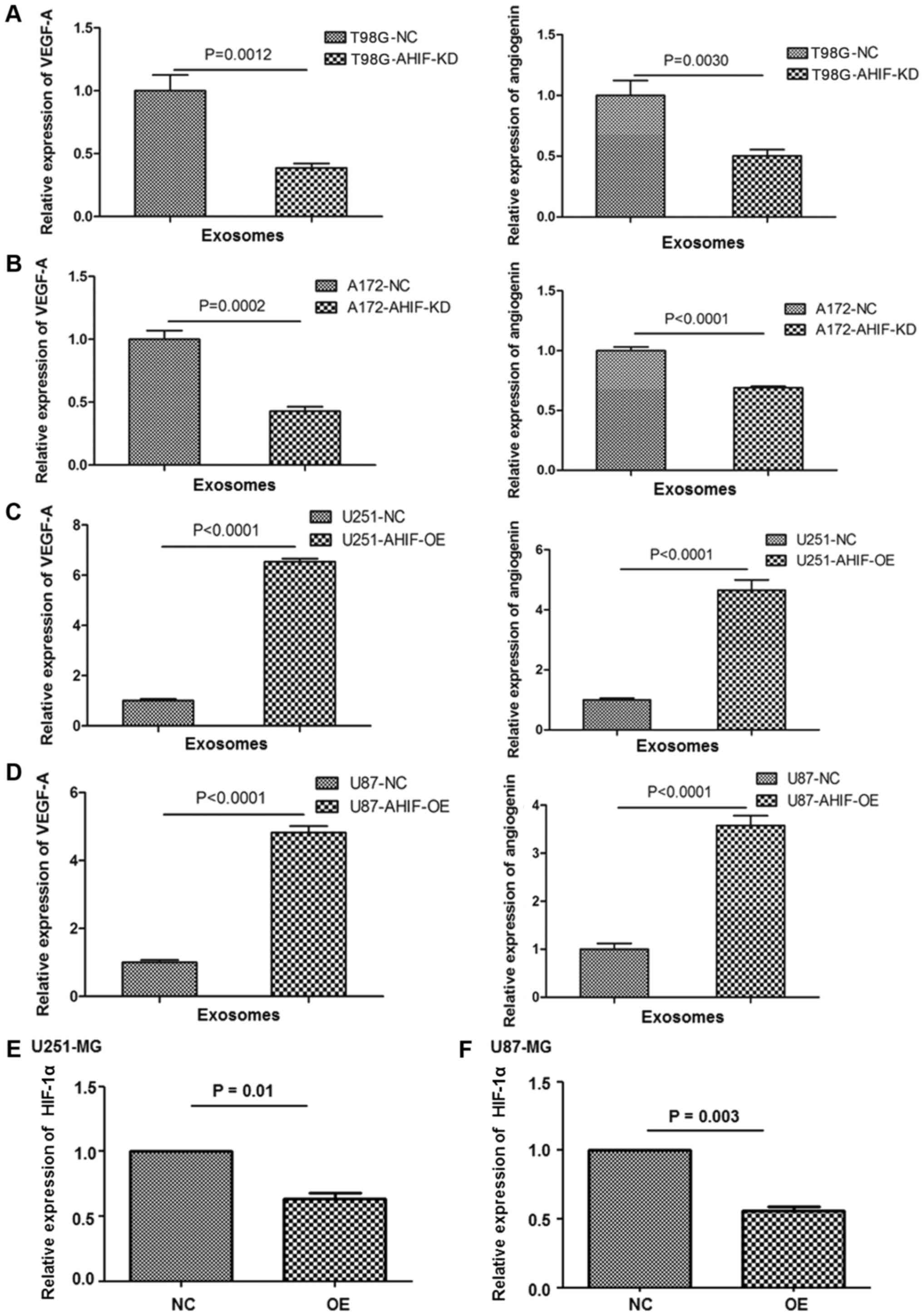
AHIF regulates factors associated with
invasion and apoptosis
In order to clarify the underlying molecular
mechanisms of AHIF in GBM progression and radiotherapy, invasion
and angiogenic genes were analyzed in AHIF-KD and AHIF-OE cells.
Expression levels of exosomal VEGF and angiogenin were
significantly decreased following KD of AHIF (Fig. 6A and B). By contrast, the
expression levels of exosomal VEGF and angiogenin were
significantly increased following OE of AHIF (Fig. 6C and D). Significantly
downregulated HIF-1α expression was observed in AHIF-OE cells
(Fig. 6E and F). Furthermore, the
expression levels of anti-apoptotic Bcl-2, Bcl-xl and Mcl-1 were
analyzed in AHIF-KD, AHIF-OE and control cells. The western blot
results indicated that the expression of anti-apoptotic Bcl-2,
Bcl-xl and Mcl-1 was decreased in AHIF-KD cells (Fig. 7A). By contrast, the levels of these
proteins were increased in AHIF-OE cells (Fig. 7B). These data indicated that AHIF
regulates factors associated with invasion and apoptosis.
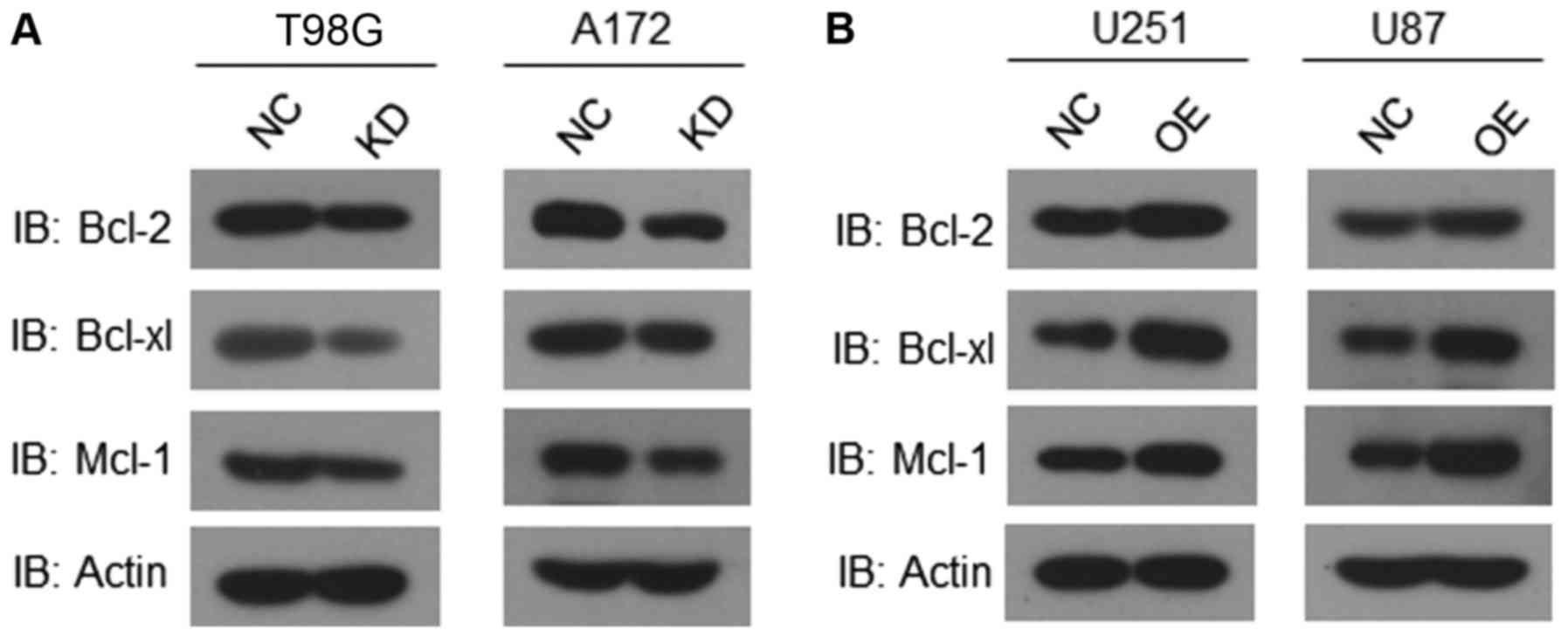 | Figure 7AHIF regulates factors associated
with apoptosis. (A) Western blot analysis of Bcl-2, Bcl-xl, Mcl-1
and actin in T98G cells and A172 cells with or without AHIF
knockdown. (B) Western blot analysis of Bcl-2, Bcl-xl, Mcl-1 and
actin in U251-MG cells and U87-MG cells with or without AHIF
overex-pression. All experiments were performed three times. AHIF,
antisense transcript of hypoxia-inducible factor 1α; Bcl-2, B-cell
lymphoma 2; Bcl-xl, B-cell lymphoma extra-large; Mcl-1, myeloid
cell leukemia-1; NC, negative control; KD, knockdown; OE
overexpression; IB, immunoblot. |
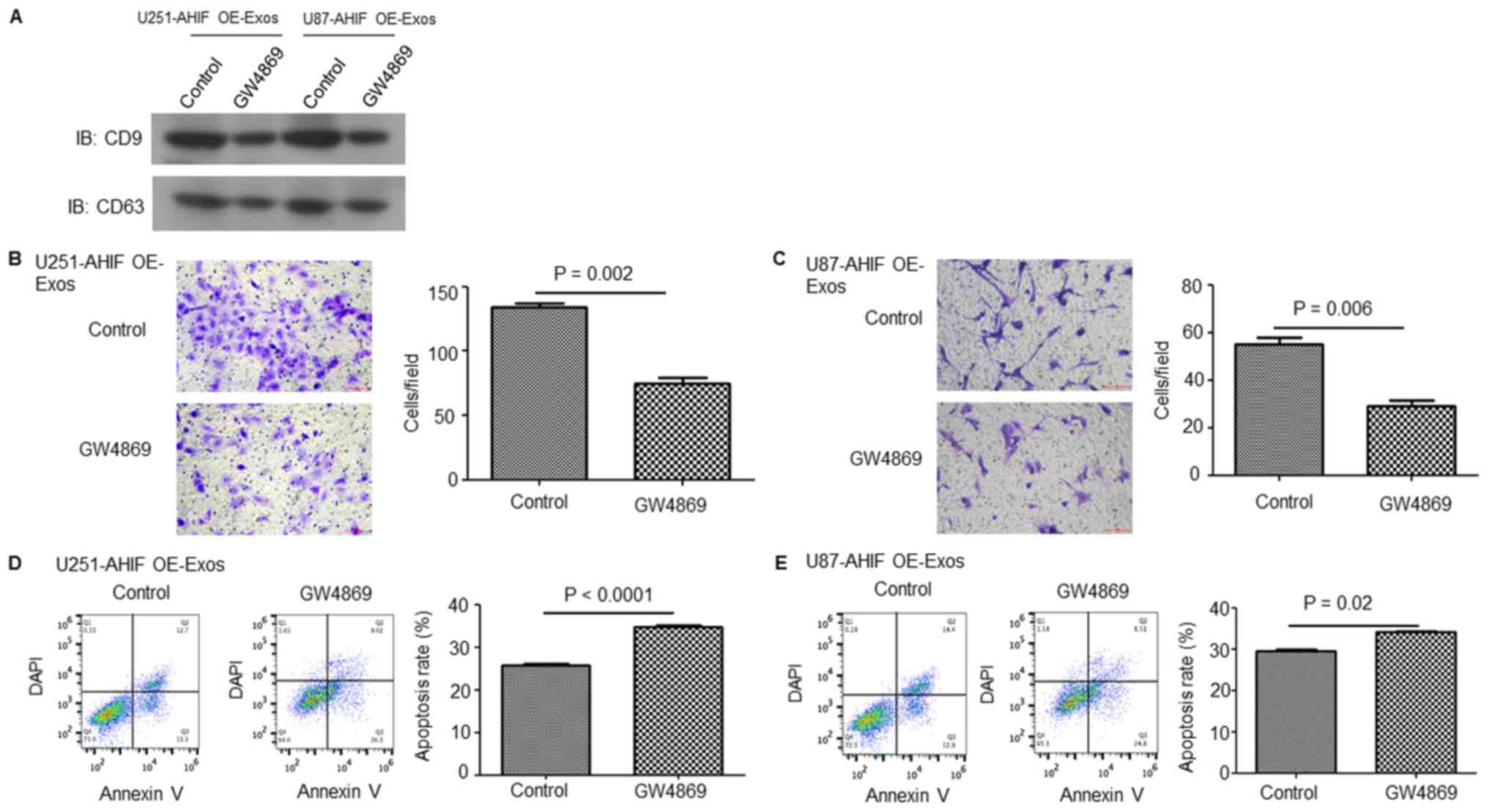
Suppression of exosome secretion in
AHIF-OE GBM cells decreases the invasive and anti-apoptosis
abilities of GBM cells
To further verify the function of exosomes in the
effect of AHIF expression on GBM cells, exosome generation was
blocked with the exosomal release inhibitor GW4869 (31,32).
As presented in Fig. 8A, exosome
release was effectively suppressed following GW4869 treatment.
Invasion assay results indicated that GW4869 significantly
decreased GBM cell invasion induced by exosomes derived from
AHIF-OE cells (Fig. 8B and C).
Furthermore, the apoptosis assay indicated that GW4869 treatment
significantly inhibited the cell survival ability induced by
exosomes derived from AHIF-OE cells (Fig. 8D and E). These results provided
further evidence that AHIF promotes the invasive and anti-apoptosis
abilities of GBM cells via exosomes.
Discussion
Despite the availability of aggressive therapeutic
regimens, the majority of patients with GBM suffer recurrence due
to its molecular heterogeneity (33-35).
Consequently, a number of genetic factors associated with GBM
progression and radiotherapy have been investigated, including
isocitrate dehydrogenase mutations, 1p19q deletion,
O6-methylguanine-DNA methyltransferase promoter
methylation and epidermal growth factor receptor variant III
amplification (36-39). In the present study, it was
identified that AHIF was significantly upregulated in cancerous GBM
tissues as well as radioresistant GBM cells, indicating that AHIF
may be a novel biomarker for GBM progression and radioresistance. A
non-cancerous glial cell line was not included in the present
study, which may need further clarification. These results were
consistent with a previous study, which identified that AHIF is
upregulated in breast cancer tissues (18). However, the function and underlying
molecular mechanisms of AHIF are largely unknown. In the present
study, the function of AHIF in GBM cells was revealed through KD or
OE of AHIF. The results indicated that AHIF regulates cell
viability, invasion and apoptosis in response to radiotherapy,
which may provide a therapeutic target. Although the differences
between groups were relatively small in the CCK-8 assay, these were
still observed to be significant. However, the difference between
groups was more obvious in the invasion and apoptosis experiments,
which may be due to regulatory effects of the tumor
microenvironment on tumor cells. LncRNAs such as HOTAIR have been
observed to be dysregulated in GBM and required for GBM cell
proliferation (40).
The results of the present study raise the question
of how AHIF promotes tumor invasiveness and radioresistance. The
expression of HIF1a is negatively regulated by AHIF, which forms a
double-stranded RNA molecule with the antisense transcript of
HIF-1α (15,16). Consistent with this, downregulated
HIF-1α expression was observed in AHIF-OE cells. HIF-1α stabilizes
the tumor suppressor gene p53 (41). Inhibition of HIF-1α by AHIF during
sustained hypoxia results in the loss of p53 and subsequent tumor
cell proliferation (41). The
results of the present study indicated that the expression of
anti-apoptotic Bcl-2, Bcl-xl and Mcl-1 decreased in AHIF-KD cells;
by contrast, these proteins were increased in AHIF-OE cells. Thus,
AHIF-mediated p53 downregulation and anti-apoptosis may be one of
the mechanisms by which AHIF conveys more aggressive tumor behavior
and radioresistance. Furthermore, AHIF-regulated exosomal secretion
of VEGF and angiogenin may a novel mechanism responsible for
invasion and radioresistance.
Exosomes are nanovesicles released by tumor cells to
modulate tumor progression. Accumulating evidence has revealed that
glioblastoma-derived exosomes contain multiple pro-angiogenic
factors that induce proliferation and progression (42-44).
VEGF-A has been identified to be overexpressed in hypoxic
GBM-derived exosomes (45).
Considering the classic function of hypoxia in angiogenesis and
invasion, angiogenic genes in exosomes derived from AHIF-KD and OE
cells were analyzed. In the present study, it was observed that
AHIF KD or OE in GBM cells altered the content of VEGF-A and
angiogenin in secreted exosomes, indicating that AHIF promotes
glioblastoma progression and radioresistance via exosomes. In
addition, exosomes collected from GBM cells with AHIF KD or OE
altered the viability, invasion and apoptosis in response to
radiotherapy of GBM cells. Although the interaction between AHIF
and HIF-1α has been suggested in a previous study (19), the molecular mechanisms underlying
the regulation of AHIF, VEGF and angiogenin require further
investigation. Increased AHIF expression has been observed to be in
parallel with that of VEGF (20).
Furthermore, hypoxic glioblastoma releases exosomal VEGF to induce
permeability of the blood-brain barrier (46).
To the best of our knowledge, the present study is
the first to establish that AHIF promotes glioblastoma progression
and radioresistance via exosomes. This could serve as a potential
therapeutic target in the treatment of GBM.
Funding
The present study was supported by the National
Science Foundation of Fujian Province (grant nos. 2017J0105 and
2018J01210) and the National Science Foundation of China (grant
nos. 81671203 and 81874215).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XD, KL, YQ and RL contributed to the experimental
design, performing experiments, acquiring data, analyzing data,
providing reagents and writing the manuscript. ZZhuang, BC, ZZhou,
SZ, GL, FZ, YL, YM, ZL and RH contributed to performing experiments
and acquiring data.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Renji Hospital, School of Medicine, Shanghai Jiao Tong
University (Shanghai, China). Written informed consent was obtained
from patients for participation in the study.
Patient consent for publication
Glioma tissues were collected from patients at
Shanghai Renji Hospital, School of Medicine, Shanghai Jiao Tong
University, following acquisition of written informed consent for
publication from the patients and with institutional review board
approval of the hospital. All patients obtained a confirmed
diagnosis of glioblastoma following resection.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Ostrom QT, Gittleman H, Farah P, Ondracek
A, Chen Y, Wolinsky Y, Stroup NE, Kruchko C and Barnholtz-Sloan JS:
CBTRUS statistical report: Primary brain and central nervous system
tumors diagnosed in the United States in 2006–2010. Neuro Oncol.
15(Suppl 2): ii1–ii56. 2013. View Article : Google Scholar :
|
|
2
|
Cuddapah VA, Robel S, Watkins S and
Sontheimer H: A neuro-centric perspective on glioma invasion. Nat
Rev Neurosci. 15:455–465. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Suvà ML, Rheinbay E, Gillespie SM, Patel
AP, Wakimoto H, Rabkin SD, Riggi N, Chi AS, Cahill DP, Nahed BV, et
al: Reconstructing and reprogramming the tumor-propagating
potential of glioblastoma stem-like cells. Cell. 157:580–594. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Caruso C, Carcaterra M and Donato V: Role
of radiotherapy for high grade gliomas management. J Neurosurg Sci.
57:163–169. 2013.PubMed/NCBI
|
|
5
|
Debus C, Waltenberger M, Floca R,
Afshar-Oromieh A, Bougatf N, Adeberg S, Heiland S, Bendszus M, Wick
W, Rieken S, et al: Impact of 18F-FET PET on target volume
definition and tumor progression of recurrent high grade glioma
treated with carbon-ion radiotherapy. Sci Rep. 8:72012018.
View Article : Google Scholar :
|
|
6
|
Yadav VN, Altshuler D, Kadiyala P, Zamler
D, Comba A, Appelman H, Dunn P, Koschmann C, Castro MG and
Löwenstein PR: Molecular ablation of tumor blood vessels inhibits
therapeutic effects of radiation and bevacizumab. Neuro Oncol.
20:1356–1367. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Song X, Shao Y, Jiang T, Ding Y, Xu B,
Zheng X, Wang Q, Chen X, Gu W, Wu C, et al: Radiotherapy
upregulates programmed death ligand-1 through the pathways
downstream of epidermal growth factor receptor in glioma.
EBioMedicine. 28:105–113. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ørom UA and Shiekhattar R: Long noncoding
RNAs usher in a new era in the biology of enhancers. Cell.
154:1190–1193. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wapinski O and Chang HY: Long noncoding
RNAs and human disease. Trends Cell Biol. 21:354–361. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang JX, Han L, Bao ZS, Wang YY, Chen LY,
Yan W, Yu SZ, Pu PY, Liu N, You YP, et al Chinese Glioma
Cooperative Group: HOTAIR, a cell cycle-associated long noncoding
RNA and a strong predictor of survival, is preferentially expressed
in classical and mesenchymal glioma. Neuro Oncol. 15:1595–1603.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li Z, Xu C, Ding B, Gao M, Wei X and Ji N:
Long non-coding RNA MALAT1 promotes proliferation and suppresses
apoptosis of glioma cells through derepressing Rap1B by sponging
miR-101. J Neurooncol. 134:19–28. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li H, Yuan X, Yan D, Li D, Guan F, Dong Y,
Wang H, Liu X and Yang B: Long non-coding RNA MALAT1 decreases the
sensitivity of resistant glioblastoma cell lines to temozolomide.
Cell Physiol Biochem. 42:1192–1201. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yu M, Ohira M, Li Y, Niizuma H, Oo ML, Zhu
Y, Ozaki T, Isogai E, Nakamura Y, Koda T, et al: High expression of
ncRAN, a novel non-coding RNA mapped to chromosome 17q25.1, is
associated with poor prognosis in neuroblastoma. Int J Oncol.
34:931–938. 2009.PubMed/NCBI
|
|
14
|
Zhu Y, Yu M and Li Z, Kong C, Bi J, Li J,
Gao Z and Li Z: ncRAN, a newly identified long noncoding RNA,
enhances human bladder tumor growth, invasion, and survival.
Urology. 77:510.e511-5152011. View Article : Google Scholar
|
|
15
|
Cayre A, Rossignol F, Clottes E and
Penault-Llorca F: aHIF but not HIF-1alpha transcript is a poor
prognostic marker in human breast cancer. Breast Cancer Res.
5:R223–R230. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Thrash-Bingham CA and Tartof KD: aHIF: A
natural antisense transcript overexpressed in human renal cancer
and during hypoxia. J Natl Cancer Inst. 91:143–151. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang Q, Matsuura K, Kleiner DE, Zamboni
F, Alter HJ and Farci P: Analysis of long noncoding RNA expression
in hepatocellular carcinoma of different viral etiology. J Transl
Med. 14:3282016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tasharrofi B, Soudyab M, Nikpayam E,
Iranpour M, Mirfakhraie R, Sarrafzadeh S, Geranpayeh L, Azargashb
E, Sayad A and Ghafouri-Fard S: Comparative expression analysis of
hypoxia-inducible factor-alpha and its natural occurring antisense
in breast cancer tissues and adjacent noncancerous tissues. Cell
Biochem Funct. 34:572–578. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rossignol F, de Laplanche E, Mounier R,
Bonnefont J, Cayre A, Godinot C, Simonnet H and Clottes E: Natural
antisense transcripts of HIF-1alpha are conserved in rodents. Gene.
339:121–130. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Span PN, Rao JU, Oude Ophuis SB, Lenders
JW, Sweep FC, Wesseling P, Kusters B, van Nederveen FH, de Krijger
RR, Hermus AR, et al: Overexpression of the natural antisense
hypoxia-inducible factor-1alpha transcript is associated with
malignant pheochromocytoma/paraganglioma. Endocr Relat Cancer.
18:323–331. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shen M and Ren X: New insights into the
biological impacts of immune cell-derived exosomes within the tumor
environment. Cancer Lett. 431:115–122. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Samanta S, Rajasingh S, Drosos N, Zhou Z,
Dawn B and Rajasingh J: Exosomes: New molecular targets of
diseases. Acta Pharmacol Sin. 39:501–513. 2018. View Article : Google Scholar
|
|
23
|
Bahrami A, Aledavood A, Anvari K,
Hassanian SM, Maftouh M, Yaghobzade A, Salarzaee O, ShahidSales S
and Avan A: The prognostic and therapeutic application of microRNAs
in breast cancer: Tissue and circulating microRNAs. J Cell Physiol.
233:774–786. 2018. View Article : Google Scholar
|
|
24
|
Lin XJ, Fang JH, Yang XJ, Zhang C, Yuan Y,
Zheng L and Zhuang SM: Hepatocellular carcinoma cell-secreted
exosomal microRNA-210 promotes angiogenesis in vitro and in vivo.
Mol Ther Nucleic Acids. 11:243–252. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kumata Y, Iinuma H, Suzuki Y, Tsukahara D,
Midorikawa H, Igarashi Y, Soeda N, Kiyokawa T, Horikawa M and
Fukushima R: Exosome-encapsulated microRNA-23b as a minimally
invasive liquid biomarker for the prediction of recurrence and
prognosis of gastric cancer patients in each tumor stage. Oncol
Rep. 40:319–330. 2018.PubMed/NCBI
|
|
26
|
Azmi AS, Bao B and Sarkar FH: Exosomes in
cancer development, metastasis, and drug resistance: A
comprehensive review. Cancer Metastasis Rev. 32:623–642. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lugea A and Waldron RT: Exosome-mediated
intercellular communication between stellate cells and cancer cells
in pancreatic ductal adenocarcinoma. Pancreas. 46:1–4. 2017.
View Article : Google Scholar
|
|
28
|
Umezu T, Ohyashiki K, Kuroda M and
Ohyashiki JH: Leukemia cell to endothelial cell communication via
exosomal miRNAs. Oncogene. 32:2747–2755. 2013. View Article : Google Scholar
|
|
29
|
Zhao X, Wu X, Qian M, Song Y, Wu D and
Zhang W: Knockdown of TGF-β1 expression in human umbilical cord
mesenchymal stem cells reverts their exosome-mediated EMT promoting
effect on lung cancer cells. Cancer Lett. 428:34–44. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
31
|
Richards KE, Zeleniak AE, Fishel ML, Wu J,
Littlepage LE and Hill R: Cancer-associated fibroblast exosomes
regulate survival and proliferation of pancreatic cancer cells.
Oncogene. 36:1770–1778. 2017. View Article : Google Scholar :
|
|
32
|
Ohsh i ma K, Ka nto K, Hat a keya ma K,
Ide T, Wakabayashi-Nakao K, Watanabe Y, Sakura N, Terashima M,
Yamaguchi K and Mochizuki T: Exosome-mediated extracellular release
of polyadenylate-binding protein 1 in human metastatic duodenal
cancer cells. Proteomics. 14:2297–2306. 2014. View Article : Google Scholar
|
|
33
|
Orzan F, De Bacco F, Crisafulli G,
Pellegatta S, Mussolin B, Siravegna G, D'Ambrosio A, Comoglio PM,
Finocchiaro G and Boccaccio C: Genetic Evolution of Glioblastoma
Stem-Like Cells From Primary to Recurrent Tumor. Stem Cells.
35:2218–2228. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Godlewski J, Ferrer-Luna R, Rooj AK, Mineo
M, Ricklefs F, Takeda YS, Nowicki MO, Salińska E, Nakano I, Lee H,
et al: MicroRNA signatures and molecular subtypes of glioblastoma:
the role of extracellular transfer. Stem Cell Reports. 8:1497–1505.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Abou-El-Ardat K, Seifert M, Becker K,
Eisenreich S, Lehmann M, Hackmann K, Rump A, Meijer G, Carvalho B,
Temme A, et al: Comprehensive molecular characterization of
multifocal glioblastoma proves its monoclonal origin and reveals
novel insights into clonal evolution and heterogeneity of
glioblastomas. Neuro Oncol. 19:546–557. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang L, He L, Lugano R, Roodakker K,
Bergqvist M, Smits A and Dimberg A: IDH mutation status is
associated with distinct vascular gene expression signatures in
lower-grade gliomas. Neuro Oncol. 20:1505–1516. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Darlix A, Deverdun J, Menjot de Champfleur
N, Castan F, Zouaoui S, Rigau V, Fabbro M, Yordanova Y, Le Bars E,
Bauchet L, et al: IDH mutation and 1p19q codeletion distinguish two
radiological patterns of diffuse low-grade gliomas. J Neurooncol.
133:37–45. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yamashita S, Yokogami K, Matsumoto F,
Saito K, Mizuguchi A, Ohta H and Takeshima H: MGMT promoter
methylation in patients with glioblastoma: is methylation-sensitive
high-resolution melting superior to methylation-sensitive
polymerase chain reaction assay. J Neurosurg:. May 4–2018.Epub
ahead of print. View Article : Google Scholar
|
|
39
|
Zhang X, Peng L, Liang Z, Kou Z, Chen Y,
Shi G, Li X, Liang Y, Wang F and Shi Y: Effects of aptamer to
U87-EGFRvIII cells on the proliferation, radiosensitivity, and
radiotherapy of glioblastoma cells. Mol Ther Nucleic Acids.
10:438–449. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tan SK, Pastori C, Penas C, Komotar RJ,
Ivan ME, Wahlestedt C and Ayad NG: Serum long noncoding RNA HOTAIR
as a novel diagnostic and prognostic biomarker in glioblastoma
multiforme. Mol Cancer. 17:742018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
An WG, Kanekal M, Simon MC, Maltepe E,
Blagosklonny MV and Neckers LM: Stabilization of wild-type p53 by
hypoxia-inducible factor 1alpha. Nature. 392:405–408. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Huang K, Fang C, Yi K, Liu X, Qi H, Tan Y,
Zhou J, Li Y, Liu M, Zhang Y, et al: The role of PTRF/Cavin1 as a
biomarker in both glioma and serum exosomes. Theranostics.
8:1540–1557. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Treps L, Perret R, Edmond S, Ricard D and
Gavard J: Glioblastoma stem-like cells secrete the pro-angiogenic
VEGF-A factor in extracellular vesicles. J Extracell Vesicles.
6:13594792017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zeng AL, Yan W, Liu YW, Wang Z, Hu Q, Nie
E, Zhou X, Li R, Wang XF, Jiang T, et al: Tumour exosomes from
cells harbouring PTPRZ1-MET fusion contribute to a malignant
phenotype and temozolomide chemoresistance in glioblastoma.
Oncogene. 36:5369–5381. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhao C, Wang H, Xiong C and Liu Y: Hypoxic
glioblastoma release exosomal VEGF-A induce the permeability of
blood-brain barrier. Biochem Biophys Res Commun. 502:324–331. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhao C, Wang H, Xiong C and Liu Y: Hypoxic
glioblastoma release exosomal VEGF-A induce the permeability of
blood-brain barrier. Biochem Biophys Res Commun. 502:324–331. 2018.
View Article : Google Scholar : PubMed/NCBI
|