Introduction
Pediatric sarcomas represent ~10% of all childhood
malignancies (1). Treatment
generally consists of a combination of surgery, chemotherapy and/or
radiotherapy. Due to the continuously improving diagnostics and
treatment techniques, the 5-year survival rate is currently 60-70%
(2,3). However, the survival rate of
pediatric sarcoma patients with recurrent disease is <40%
(4-6). The treatment options for this patient
group are limited, since therapies are associated with (late)
adverse events (7,8), and applying a second curative
treatment scheme may increase the risk of adverse events to
unacceptable levels. This is particularly relevant for patients
with local recurrence following previous radiotherapy.
Hyperthermia, i.e., heating of tumors to 39-43°C for
1 h, is a treatment modality that may be combined with radiotherapy
and/or chemotherapy to significantly enhance their effectiveness
(9,10). Several randomized trials in adults
have demonstrated the effectiveness of hyperthermia in the control
of soft tissue sarcomas and recurrent breast, cervix and head-neck
carcinomas, among others, with no significant hyperthermia-related
increase in side effects (11-17).
For example, low-dose re-irradiation combined with hyperthermia
applied for recurrent breast cancer can increase the complete
response rate from 38 to 78%, compared with re-irradiation alone
(13).
Although hyperthermia is a clinically proven
treatment for several tumors in adults (11-15),
it is not commonly applied for pediatric tumors. The effectiveness
of chemotherapy and hyperthermia has been investigated for children
with sarcomas and germ cell tumors that respond poorly to or recur
after chemotherapy (18). A phase
II trial demonstrated that chemotherapy plus hyperthermia are
successful as local therapy, with a 5-year survival of 52%
(19). A prospective study for
recurrent or refractory malignant non-testicular germ cell tumors
further demonstrated the effectiveness of locoregional hyperthermia
and chemotherapy, with a 5-year survival of 72% (20); the long-term prognosis for patients
with poor response to therapy or after the first relapse was almost
similar to that for patients receiving first-line treatment.
A possible role of radiotherapy plus hyperthermia
for pediatric tumors has not been investigated thus far, despite
the positive results of chemotherapy plus hyperthermia in pediatric
patients and all successful clinical applications of hyperthermia
combined with chemotherapy or radiotherapy in adults. This
treatment combination may prove to be very effective for previously
irradiated recurrent sarcomas, since a second curative dose would
increase the risk of toxicity to unacceptable levels and the
clinical feasibility of re-irradiation plus hyperthermia for adults
with radiation-associated sarcoma has been demonstrated (21). Given the poor prognosis of
pediatric patients with recurrent sarcoma, this treatment option is
worth investigating further.
Before initiating a clinical feasibility study, the
theoretical feasibility should be explored. Treatment planning
combined with biological modelling is helpful for evaluating this
theoretical feasibility. The radiosensitizing effect of
hyperthermia may be considered as a local increase in tumor dose,
which may be quantified using biological modelling, with a
temperature-dependent change in the radiosensitivity parameters of
the linear-quadratic model (22,23).
According to this concept, the possible effectiveness of
re-irradiation and hyperthermia can be estimated by calculating the
equivalent 3D dose distribution (24-26),
i.e., the radiation dose that exerts a biological effect equivalent
to that of the combined re-irradiation plus hyperthermia
treatment.
The aim of the present study was to investigate the
theoretical feasibility of re-irradiation plus hyperthermia for
infield recurrent pediatric sarcomas. Re-irradiation and
hyperthermia treatment plans were simulated and equivalent 3D dose
distributions were calculated and evaluated based on dose-volume
histograms (DVH).
Materials and methods
Patient selection
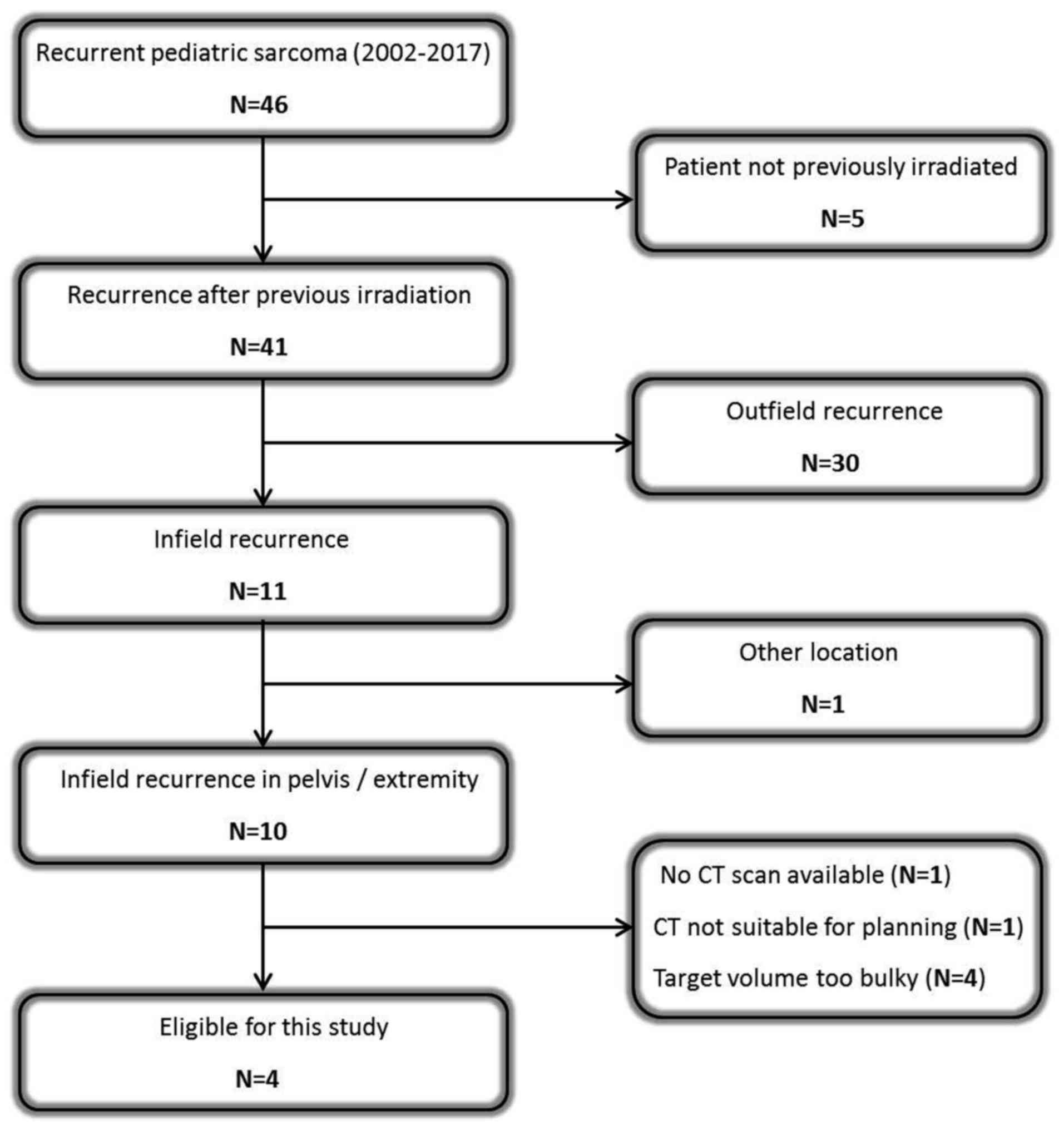
In the Emma Children's Hospital - Academic Medical
Center (Amsterdam, The Netherlands), 46 pediatric patients (aged
<18 years) were diagnosed with recurrent sarcoma (29 Ewing's
sarcomas, 10 rhabdomyosarcomas and 7 non-rhabdomyosarcoma soft
tissue sarcomas) between 2002 and 2017, 41 of which developed after
previous irradiation and 11 were infield recurrences. Only pelvic
and extremity tumors were included, due to our ample experience
with clinical hyperthermia for these locations in adults. Bulky
pelvic target volumes with a diameter >15 cm were excluded,
since locoregional hyperthermia at 70 MHz (see subsection
‘Hyperthermia treatment planning’) yields a heating focus of
~10-15 cm and, thus, effective heating of bulkier volumes is
difficult. This selection process resulted in 2 patients with a
recurrent pelvic tumor and 2 patients with a recurrence in an
extremity (Fig. 1).
Recurrent pelvic sarcomas
Patient 1
A 3-year-old male patient presented with recurrent
embryonal rhabdomyosarcoma, located perivesically in the left side
of the pelvis. The tumor was first diagnosed at the age of 1 year.
The primary tumor was treated with chemotherapy, followed by
resection and proton radiotherapy (50.4 Gy in 28 fractions)
combined with pulmonary radiotherapy due to lung metastases.
Patient 2
A 17-year-old female patient presented with a second
recurrence of a pararectal alveolar rhabdomyosarcoma. The primary
tumor was diagnosed at the age of 15 years and treated with
radiotherapy (54 Gy; 30×1.8 Gy) and chemotherapy. The first
recurrence at 16 years of age was located in both breasts and the
left inguinal area, and was treated with radiotherapy [41.4 Gy;
23×1.8 Gy, followed by a boost to 50.4 Gy for positron emission
tomography (PET)-positive breast lesions].
Recurrent extremity sarcomas
Patient 3
A 14-year-old male patient presented with recurrent
Ewing's sarcoma in the second metatarsal bone of the right foot.
The first diagnosis was at 12 years of age and treatment consisted
of chemotherapy, followed by resection and postoperative
radiotherapy (54 Gy; 30×1.8 Gy). Lung metastases were resected,
followed by pulmonary radiotherapy (15 Gy; 10×1.5 Gy).
Patient 4
A 13-year-old male patient presented with recurrent
alveolar rhabdomyosarcoma in the left lower leg. The tumor was
first diagnosed at 11 years of age at an advanced stage, with lymph
node (inguinal, iliac and para-aortal), bone and lung metastases.
Initial treatment consisted of chemotherapy followed by
radiotherapy to all primary locations. The location of the
recurrence initially received 54 Gy (30×1.8 Gy).
Treatment simulations
Radiotherapy treatment planning
External beam re-irradiation treatment plans for an
Elekta Agillity 10MV machine were created using the diagnostic
computed tomography scans. The gross tumor volume (GTV), planning
target volume (PTV) and organs at risk (OARs; femur, rectum and
bladder), if relevant, were delineated. Treatment plans were
created for a re-irradiation schedule of 23×2 Gy and weekly
hyperthermia, as applied clinically for recurrent breast cancer.
Treatment planning was performed at 2×2×2 mm3 resolution
using Raystation (version 6.0; RaySearch Labs, Stockholm, Sweden).
Volumetric modulated arc therapy (VMAT) plan optimizations were
started with objective values that were individually optimized to
minimize OAR dose, while maintaining ICRU-based PTV coverage (D98%
>95%, D2% <107%) (27).
Hyperthermia treatment planning
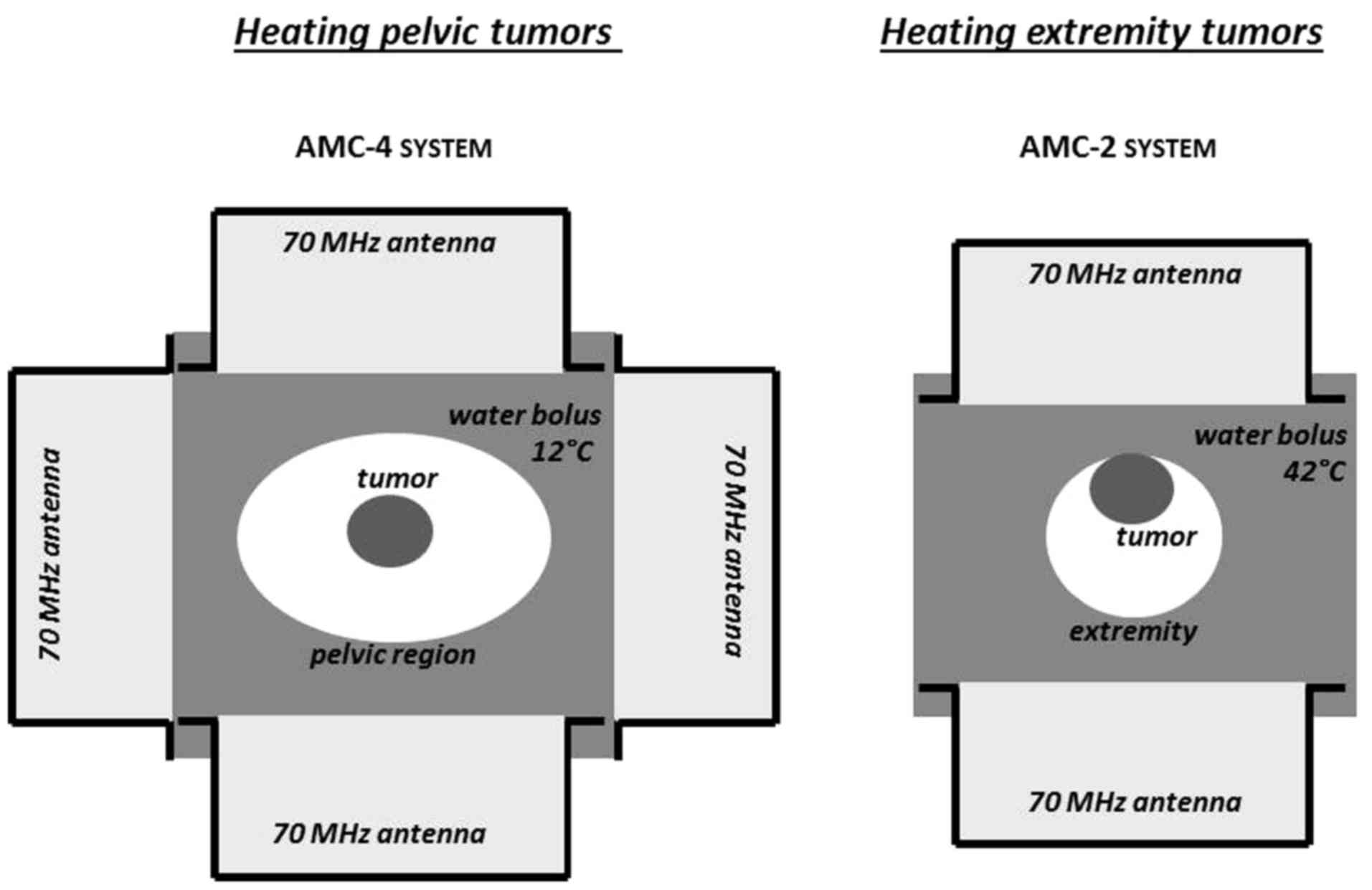
Hyperthermia treatment of pelvic tumors was
simulated for the 70 MHz AMC-4 system, which consists of a ring of
four waveguides (fixed aperture size 20×34 cm2)
positioned around the patient (28). For the extremities, the 70 MHz
AMC-2 system was simulated, consisting of two waveguides positioned
at the top and bottom of the target (29). Waveguides with different aperture
sizes may be selected for this system, and an aperture size of
20×34 cm2 was selected. For both systems, water boluses
provide skin cooling and coupling of the electromagnetic energy
into the tissue. Following regular clinical practice, aggressive
cooling (bolus temperature 12°C) was simulated for deep heating of
the pelvis to avoid overheating of the skin, and a higher bolus
temperature (42°C) was modelled for heating the extremities, since
the skin is part of the target volume. The set-up for pelvic and
extremity tumors is shown in Fig.
2. Hyperthermia treatment planning was performed using
Plan2Heat (30). Hounsfield
unit-based tissue segmentation into muscle, fat, bone and air was
combined with delineations to create a patient model, which was
inserted in the applicator model for the AMC-4 or AMC-2 system.
Literature-based dielectric and thermal properties were assigned,
and electric field and thermal computations were performed using
finite difference methods (30,31)
at 2.5×2.5×2.5 mm3 (pelvis) or 1×1×2.5 mm3
(extremities). Temperature-based optimization was performed to
maximize the T90, i.e., the temperature achieved in at
least 90% of the PTV, with hard constraints of 45°C to all normal
tissues (30,32), since a pain sensation is
experienced when tissue temperatures exceed 45°C (33).
Biological modeling
Biological evaluation of the combined radiotherapy
and hyperthermia treatment was performed using the in-house
developed X-Term software package, which calculates the 3D
equivalent radiotherapy dose distribution (EQDRT) for a
user-specified treatment schedule (26). Calculations are based on the
linear-quadratic (LQ) model, expressing the survival fraction (SF)
of cells after delivering n fractions with a fraction dose
d (Gy) according to the following equation:
SF(n,d,α,β)=e−n(αd+βd2),
where α (Gy−1) and β (Gy−2) are the
radiosensitivity parameters. X-Term uses an extension of the
LQ-model, in which α and β depend on the temperature and the time
interval between radiotherapy and hyperthermia. Additionally,
X-Term accounts for the direct hyperthermic cytotoxicity in tumor
tissue (
34). Mathematical
functions were parametrized such that the EQDRT depends
mainly on the α/β ratio and on the hyperthermia-enhancing factors,
rather than on the individual parameters (
34). More details on the software
package, mathematics and the derivation of the temperature
dependency of α and β may be found in earlier publications
(
26,
34).
Weekly application of hyperthermia directly after
the first radiotherapy fraction was modelled. Ratios α/β=10 Gy and
α/β=3 Gy were assumed for tumor and OARs, respectively, under
non-hyperthermic conditions (37°C). Since curative radiation
schedules for primary sarcomas typically use a fraction dose of 1.8
Gy, the reference fraction dose for all EQDRT
calculations was 1.8 Gy.
The reliability of biological modelling is affected
by the statistical accuracy of the model parameters. Therefore, a
95% confidence interval for the parameters was translated into a
confidence interval for EQDRT, as described by van
Leeuwen et al (34).
Statistical analysis
The planned temperature distributions were evaluated
by indexed temperatures T10, T50 and
T90, i.e., the temperature achieved in at least 10, 50
and 90% of the PTV, following the quality assurance guidelines
(35). Ideally, the
EQDRT should approach the standard curative dose (54
Gy). The EQDRT distributions were analyzed using
standard DVH parameters. The increase in D98% and D95% by adding
hyperthermia was evaluated for the PTV (36). Additionally, the increase in D2%
for the OARs was evaluated (36).
Results
Recurrent pelvic sarcomas
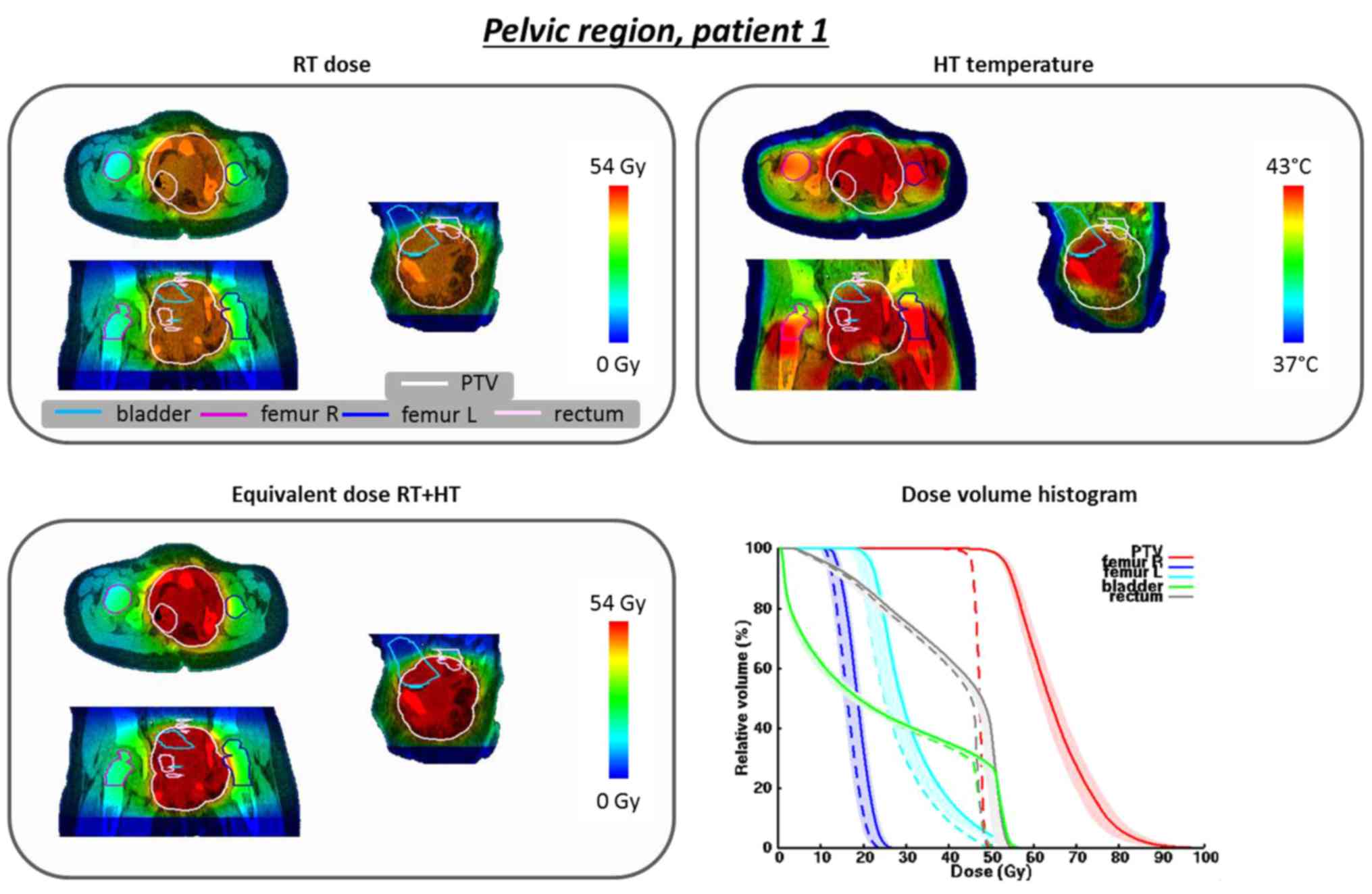
For patient 1 (3-year-old male) the planned PTV
temperatures T10, T50 and T90 were
43.9, 42.7 and 41.1°C, respectively, with therapeutic temperatures
between 39 and 43°C. The total absorbed power in the patient was
168 W. Fig. 3 shows the radiation
dose distribution, converted to a reference fraction dose of 1.8
Gy, the temperature distribution, the predicted EQDRT of
the combined treatment and the DVH for the original and equivalent
radiation dose distributions. A substantial increase in equivalent
dose for the PTV is predicted, with a considerably lower increase
in equivalent OAR dose. The D98% in the PTV is predicted to
increase from 44.4 to 51.6 Gy; the D95% increases from 45.4 to 53.4
Gy, which approaches the curative dose of 54 Gy. Although the
rectum and bladder are located within the PTV, the effect is rather
tumor-selective: the D2% increases from 49.2 to 54.3 Gy and from
49.0 to 53.8 Gy for the bladder and rectum, respectively; i.e.,
approximately half of the increase in D95%.
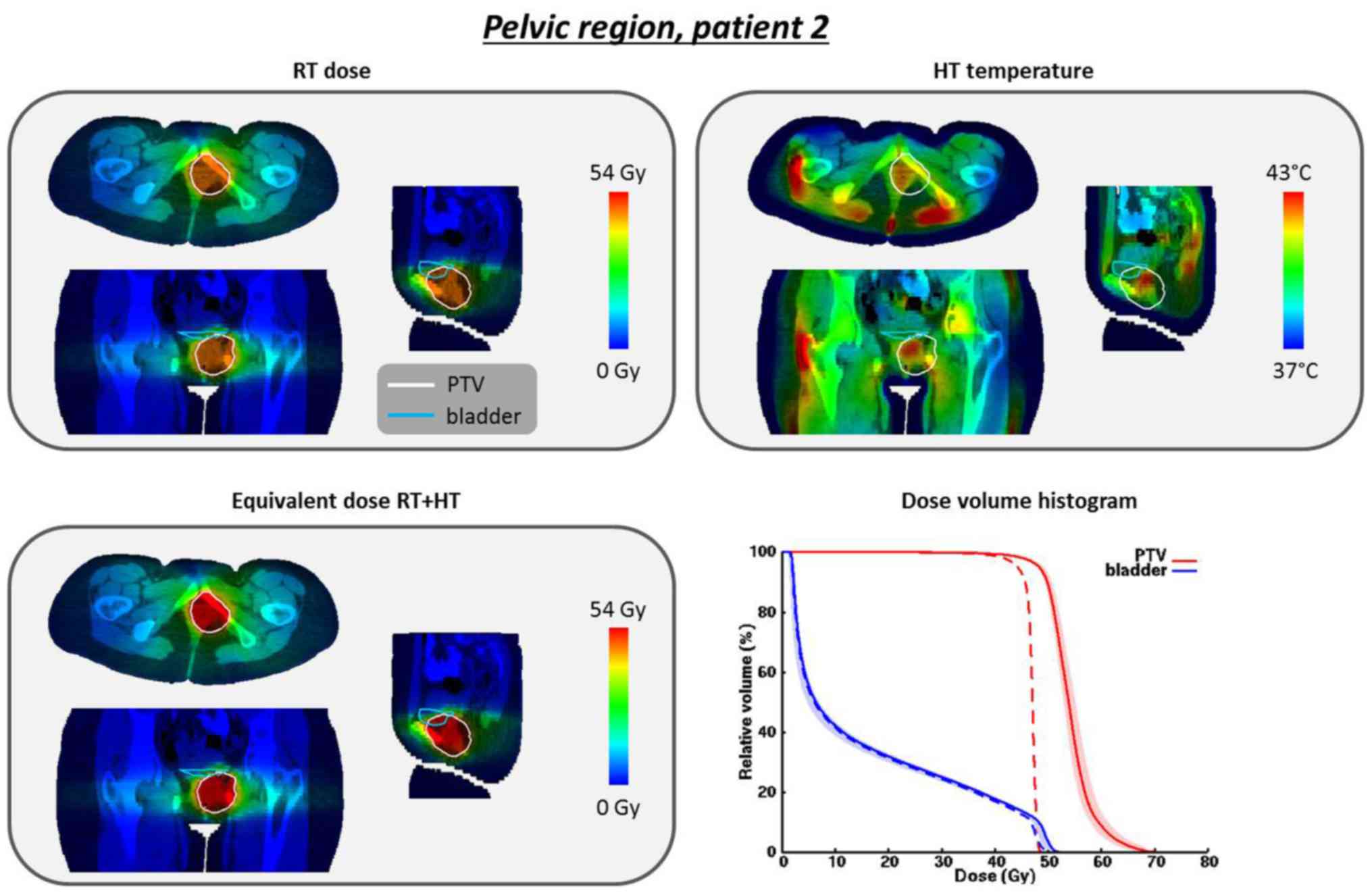
For patient 2 (17-year-old female) the planned PTV
temperatures T10, T50 and T90 were
41.8, 40.8 and 39.5°C, respectively. The total absorbed power in
the patient was 471 W. Fig. 4
shows the dose and temperature distributions. Although therapeutic
heating is possible, achievable temperatures are lower compared
with those for patient 1, which is also reflected by a lower
enhancement in EQDRT. The D98% increases from 41.7 to
45.3 Gy and the D95% increases from 44.2 to 48.3 Gy. The overlap of
the OAR (bladder) with the PTV is very small; thus, the predicted
EQDRT in the bladder is practically the same as the
original dose.
Recurrent extremity sarcomas
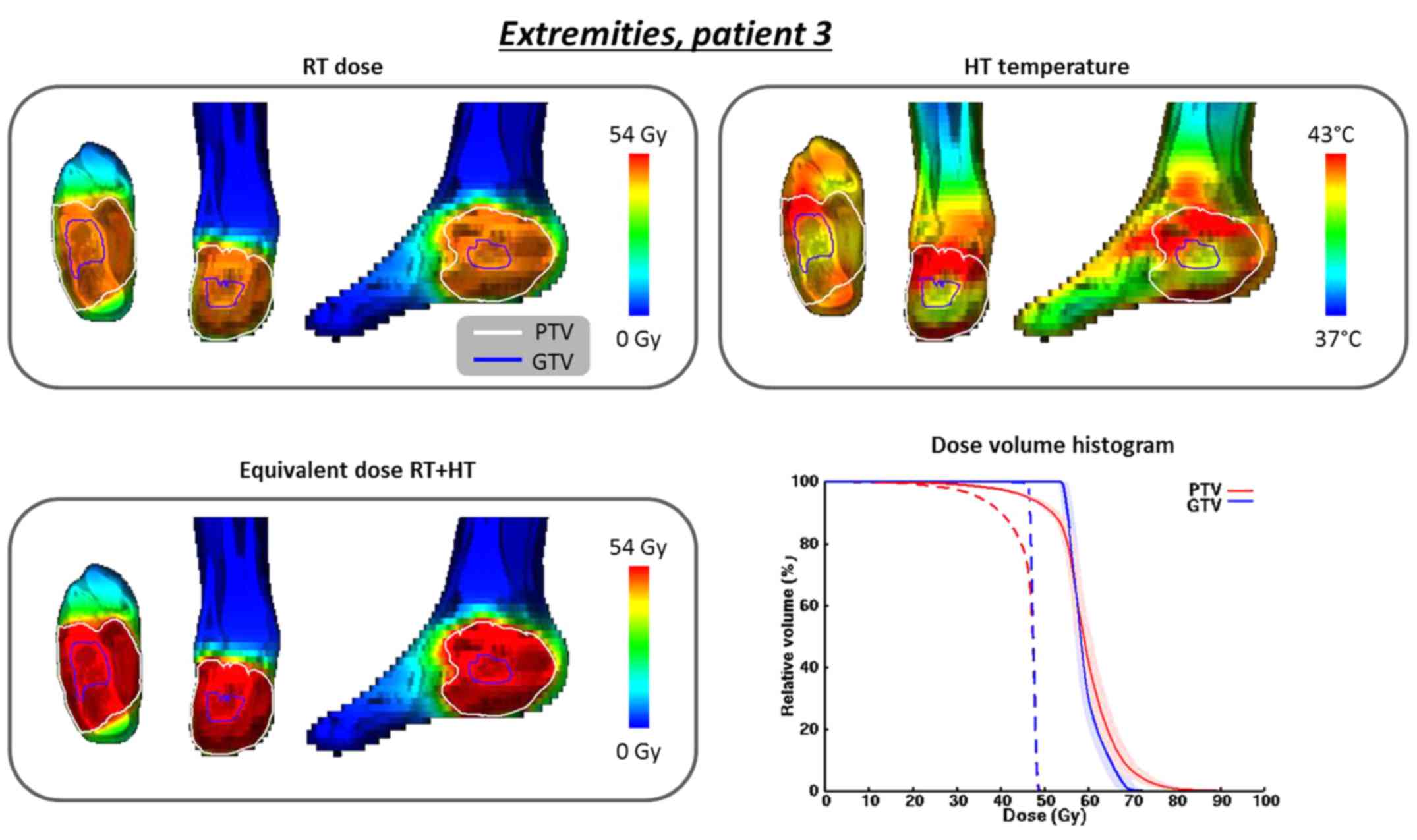
For patient 3 (foot; 14-year-old male) the planned
PTV temperatures T10, T50 and T90
were 43.3, 42.2 and 41.4°C, respectively. The total absorbed power
in the patient was 19 W. Fig. 5
shows the radiation dose distribution, converted to a reference
fraction dose of 1.8 Gy, the temperature distribution, the
EQDRT of the combined treatment and the DVHs for the
original and equivalent radiation dose distributions. A substantial
increase in equivalent PTV dose is predicted: the D98% increases
from 28.0 to 38.2 Gy and the D95% increases from 35.0 to 46.1 Gy.
As a result of skin sparing in the extremities, the D98% and D95%
of the original PTV dose are quite low, reflecting an underdosage
of the skin. Adding hyperthermia yields a substantial increase of
the equivalent dose in this region, while no substantial additional
toxicity in the healthy skin is expected due to the tumor
selectivity of hyperthermia. For the GTV, the D98% and D95%
increase from 46.4 to 54.4 Gy and from 46.5 to 54.7 Gy,
respectively, thereby realizing a curative dose of 54 Gy.
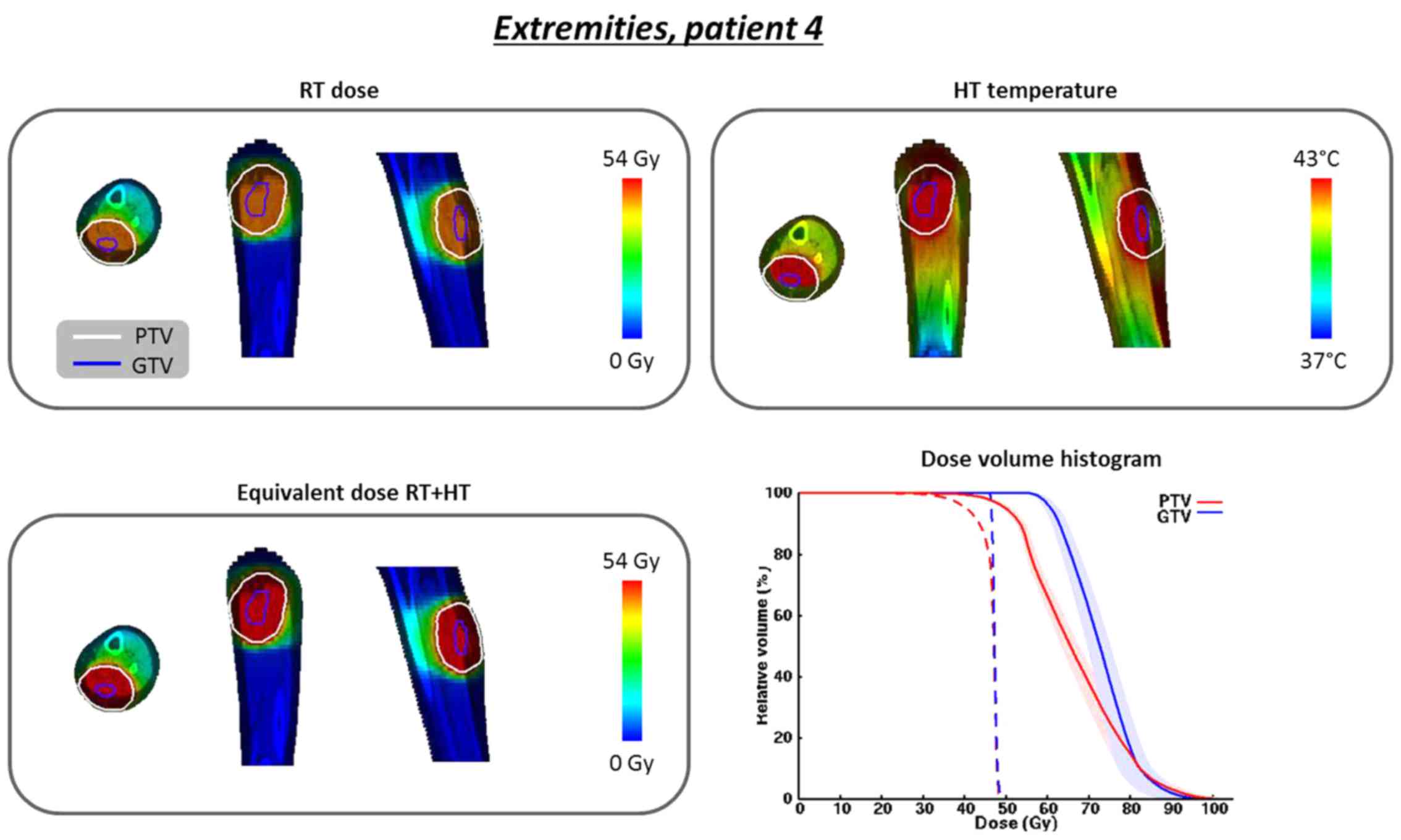
For patient 4 (lower leg; 13-year-old male) the
planned temperatures T10, T50 and
T90 were 44.2, 43.0 and 41.3°C. The total absorbed power
in the patient was 61 W. Fig. 6
shows the dose and temperature distributions. The D98% in the PTV
increases from 36.5 to 45.8 Gy and the D95% increases from 40.6 to
50.0 Gy. For the GTV, the D98% and D95% increase from 46.3 to 58.9
Gy and from 46.5 to 60.9 Gy, respectively, again realizing a
curative dose.
Discussion
This simulation study demonstrates that
re-irradiation plus hyperthermia may be a feasible treatment
combination for infield recurrent pediatric sarcomas in the pelvic
region or the extremities. 3D equivalent dose calculations
combining clinically representative dose and temperature
distributions predicted that radiosensitization by hyperthermia
yields an increase in equivalent D95% of typically 10 Gy, thereby
delivering a possible curative dose without a substantial
additional risk of normal tissue toxicity. This treatment
combination may thus improve the currently disappointing survival
rate of sarcoma patients with infield recurrence and warrants
further clinical evaluation. The long-term effectiveness and
possible late complications of this treatment combination in
pediatric recurrent sarcoma patients must be assessed in a clinical
study.
An important aspect of biological modelling is the
choice of the parameter values used. The value of α/β for tumor
tissue may vary between different pathologies (37). The present study assumed the
commonly applied value of α/β=10 Gy for tumor tissue under
normothermic conditions. The temperature-dependent behavior of the
enhancement factor of α/β was estimated based on detailed
experiments for cervical cancer cells, as described in an earlier
study (34). The accuracy of the
prediction of the equivalent dose would be expected to improve if
more exact parameter values were available, but derivation of such
parameter values is very challenging; thus, a commonly used value
was adopted in this study. Although the exact increase in
equivalent dose may be somewhat different when the precise
parameters are known, the overall conclusion that hyperthermia can
strongly increase the equivalent tumor dose is not likely to change
substantially. A clinically relevant enhancement in
radiosensitivity has also been demonstrated in clinical studies
when adding hyperthermia for cervical cancer (α/β >10 Gy) and
(recurrent) breast cancer (α/β <10 Gy) (12,13).
Biological modelling studies evaluating radiotherapy plus
hyperthermia for cervical and prostate cancer also predicted the
same order of magnitude increase in equivalent tumor dose (24,25).
The radiosensitization effect by hyperthermia is thus more
determinant of the increase in equivalent radiation dose than the
exact initial reference value of α/β. Realistic uncertainties in
hyperthermic radiosensitization were included in the modelling, as
reflected by the confidence intervals.
Regarding normal tissue and OARs, the conventional
value of α/β=3 Gy for late effects was applied, as commonly used
for biological modelling in both adults and children (38,39).
Clinical studies combining radiotherapy and hyperthermia in adults
for several tumor sites have demonstrated that the toxicity in
normal tissue is generally not significantly increased compared
with radiation or re-irradiation alone (12,13).
Although there is no reason to hypothesize that normal tissue
toxicity will increase in children, it is currently unknown whether
this value of α/β is fully appropriate for pediatric patients, as
the OARs at a young age may behave radiobiologically differently
compared with the same OARs in adults. Nevertheless, most relevant
and determining for the effect of re-irradiation plus hyperthermia
is the increase in equivalent radiation dose by adding
hyperthermia, which is determined more by the radiosensitization
due to hyperthermia rather than by the initial normothermic
reference value of α/β.
There are usually some challenges regarding the
maximum cumulative dose in normal tissue when applying
re-irradiation (40). Although the
hyperthermic enhancement of the radiation dose is already predicted
to be very low in normal tissue in the examples discussed in this
study, a time interval (e.g., 1 h) between radiotherapy and
hyperthermia may be applied to further minimize the enhancement in
normal tissue, if desired (41,42).
This may be required when there is a risk of exceeding the
tolerance dose to a specific OAR, in case of slowly proliferating
tissues (e.g., bone and central nervous system) receiving a
relatively high cumulative dose, or in case the time interval
between the initial radiation treatment and re-irradiation is
relatively short. Predictably, applying a time interval will also
somewhat reduce the equivalent tumor dose, and biological modelling
may be helpful in determining a good balance, such that a
sufficiently high tumor dose can still be expected.
The results exhibited some variation regarding
increased effectiveness among the four patients, which is due to
differences in heatability. With locoregional hyperthermia,
excessive temperatures (hot spots) in normal tissue may develop at
tissue interfaces (e.g., fat, muscle and bone) due to the
differences in tissue properties (43,44).
These hot spots should be avoided and, therefore, all tissue
temperatures were constrained to 45°C, which can limit the maximum
achievable target temperature. Extremity tumors are easier to heat
compared with pelvic tumors, due to their smaller anatomical size
and the lower number of tissue interfaces at which hot spots may
occur. Due to the differences in heatability between patients, it
is advisable to perform pre-planning to determine whether effective
heating is possible (i.e., T90 >39°C) (45,46),
prior to deciding clinical treatment with re-irradiation and
hyperthermia. Particularly for relatively large tumor volumes, and
in case of multiple treatment options, it should be determined
first whether sufficient target coverage can be achieved.
When pre-planning indicates that the target region
cannot be effectively heated and the treatment intent is still
curative, re-irradiation plus whole-body hyperthermia may be
considered. There is some, albeit limited, clinical experience with
whole-body heating for children in combination with chemotherapy
(47-49). Whole-body heating requires a
dedicated heating device, which heats the patient to a maximum of
41.8°C. This ensures a homogeneous target temperature and, thus,
effective enhancement (~10 Gy) of the radiotherapy. Whole-body
hyperthermia is not the first choice of treatment, however, since
deep analgesia and sedation or general anesthesia are required, and
greater efforts are needed (including intensive medical care)
compared with locoregional hyperthermia.
The AMC-4 system, as it is extensively used for
adults (12,50,51),
was modelled for heating pelvic tumors. The 17-year-old patient had
the body sizes of a small adult, and the 3-year-old patient had a
tumor located relatively low in the pelvis; therefore, heating with
the AMC-4 system was considered feasible. However, the axial length
of the patient covered by the waveguides and boluses is 40 cm,
which may not be feasible for very small children or tumors located
relatively high in the pelvis. In those cases, a system with
smaller antennas would be desired, e.g., the BSD Sigma-30 (Pyrexar
Medical, Salt Lake City, UT, USA) (20,52).
However, this system operates at a higher frequency, yielding a
smaller heating focus. Additionally, a higher frequency (typically
90-130 MHz) also improves the steering capabilities, which would
possibly allow further reduction of normal tissue heating, for
example of the femoral heads for patient 1. Pre-planning should
evaluate whether and to which extent this affects target
coverage.
Sarcomas often contain hypoxic regions with reduced
radiosensitivity. Hypoxic tumors are more sensitive to hyperthermia
(53) and the mechanism
responsible for part of the apparent hyperthermic
radiosensitization of hypoxic tumors is direct cell kill. The rate
of direct cell kill depends on the oxygenation status. A strong
dose-effect relationship exists, both for direct cytotoxicity and
for radiosensitization, due to inhibition of DNA repair by
hyperthermia (54-56). Enhanced direct cytotoxicity was not
accounted for in the present study, since no information on the
oxygenation status was available. Therefore, the predicted increase
in equivalent dose is likely an underestimation of the real
enhancement. The accuracy of biological treatment planning may be
improved by including hypoxia in the models (57). This is subject of further research
and requires determination of the oxygenation status and tumor
reoxygenation in individual patients by hypoxia PET/MRI techniques
(58,59).
Additionally, the tissue properties (fibrosis,
necrosis) and tumor perfusion may also be altered in previously
irradiated tissues, which may affect the temperature distribution
and, thereby, the equivalent dose calculations. Incorporating these
influences on pre-irradiated tissue in the X-Term calculation
models requires further research as well as dedicated imaging
techniques.
To avoid complications due to excessive temperatures
developing in normal tissue, temperature feedback during treatment
is crucial. For adults, standard (minimally) invasive thermometry
probes are placed in or close to the tumor and normal tissue
heating is monitored by patient feedback. When tissue temperatures
exceed 45°C, a pain sensation is experienced (33) and the operator changes the system
settings to resolve the hot spot (60). In general, this procedure should
also be feasible for children; however, anesthesia might be
required for very young children and exact hot spot locations
cannot be communicated. Sedation does not increase the risk of
burns at elevated temperatures (20). For those cases, 3D non-invasive
thermometry feedback by MR is desired, particularly in the pelvis.
Additionally, some studies suggest that heating pelvic malignancies
in pediatric patients may increase the risk of osteonecrosis or
avascular necrosis (AVN) (61,62).
Young age is also considered a possible risk factor for AVN
(61,62). Therefore, high temperatures in the
femoral heads should be avoided. This may be achieved by setting
more strict temperature constraints to the femoral heads when
optimizing antenna settings and/or using a higher operating
frequency, as mentioned above.
MR-thermometry is not generally available and, based
on the previous considerations, a first clinical feasibility study
evaluating re-irradiation plus hyperthermia for infield recurrent
pediatric sarcomas will be initiated for patients with pelvic
malignancies not requiring anesthesia, and extremity sarcomas. For
the latter category, age will be no limitation, since OARs from
hyperthermia are absent and the incidence of treatment-limiting hot
spots should be low. Standard thermometry probes will then be
sufficient to monitor treatment quality.
In the future, once the clinical effectiveness of
re-irradiation plus hyperthermia for recurrent pediatric sarcoma
has been established, the effectiveness of radiotherapy plus
hyperthermia for primary tumors may be explored. Radiotherapy may
also be necessary in the first-line treatment of childhood
sarcomas, particularly when surgery alone (R1/R2 resection) is not
sufficient for local tumor control or results in mutilating
sequelae. However, radiotherapy is associated with a significant
risk of late toxicity, such as atrophy, fibrosis and bone growth
abnormalities in the extremities (63). To improve normal tissue sparing and
reduce the risk of (late) toxicities, proton beam therapy is
emerging, which yields more precisely focused dose distributions,
thereby reducing the dose in the low-to-medium dose regions, but
not for the OARs in close proximity to the tumor (64). Adding hyperthermia may allow
reduction of the delivered dose, thereby significantly reducing the
incidence of late side effects.
In conclusion, re-irradiation with 23×2 Gy plus
hyperthermia is a theoretically feasible and possibly effective
treatment for recurrent pediatric sarcoma in the pelvic region or
the extremities. Hyperthermic radiosensitization is predicted to
yield a target-selective additional D95% of typically 10 Gy,
thereby delivering a curative equivalent dose of 54 Gy.
Funding
The present study was financially supported by Kika
(grant no. 253).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
HPK: Software, investigation, methodology, formal
analysis, writing original draft; IWEMvD: Data curation, review and
editing; KFC: Investigation, review and editing; NAP:
Conceptualization, review and editing; CRN: Conceptualization,
funding acquisition, review and editing; JHMM: Conceptualization,
funding acquisition, data curation, review and editing. JC:
Conceptualization, funding acquisition, review and editing; BVB:
Conceptualization, data curation, review and editing; AB: Funding
acquisition, conceptualization, project administration, review and
editing of the manuscript.
Ethics approval and consent to
participate
This retrospective study was not subject to the
Medical Research Involving Human Subjects Act and has been
conducted in accordance with the ethical standards and according to
the principles outlined in the Declaration of Helsinki.
Patient consent for publication
Consent for the publication of the clinical data was
obtained from all patients who were involved in this study.
Competing interests
J. Crezee, C.R.N. Rasch and A. Bel report a research
collaboration with Medlogix, Rome, Italy, outside this study. A.
Bel reports grants and non-financial support from Elekta AB,
Stockholm, Sweden, outside this work.
Abbreviations:
|
PTV
|
planning target volume
|
|
GTV
|
gross tumor volume
|
|
OAR
|
organ at risk
|
|
VMAT
|
volumetric modulated arc therapy
|
Acknowledgments
Not applicable.
References
|
1
|
Kaatsch P: Epidemiology of childhood
cancer. Cancer Treat Rev. 36:277–285. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gatta G, Zigon G, Capocaccia R, Coebergh
JW, Desandes E, Kaatsch P, Pastore G, Peris-Bonet R, Stiller CA and
Group EW; EUROCARE Working Group: Survival of European children and
young adults with cancer diagnosed 1995-2002. Eur J Cancer.
45:992–1005. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Magnani C, Pastore G, Coebergh JW, Viscomi
S, Spix C and Steliarova-Foucher E: Trends in survival after
childhood cancer in Europe, 1978-1997: Report from the Automated
Childhood Cancer Information System project (ACCIS). Eur J Cancer.
42:1981–2005. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hawkins DS, Spunt SL and Skapek SX:
Children's Oncology Group's 2013 blueprint for research: Soft
tissue sarcomas. Pediatr Blood Cancer. 60:1001–1008. 2013.
View Article : Google Scholar
|
|
5
|
Gorlick R, Janeway K, Lessnick S, Randall
RL and Marina N: Children's Oncology Group's 2013 blueprint for
research: Bone tumors. Pediatr Blood Cancer. 60:1009–1015. 2013.
View Article : Google Scholar
|
|
6
|
Chisholm JC, Marandet J, Rey A, Scopinaro
M, de Toledo JS, Merks JH, O'Meara A, Stevens MC and Oberlin O:
Prognostic factors after relapse in nonmetastatic rhabdomyosarcoma:
A nomogram to better define patients who can be salvaged with
further therapy. J Clin Oncol. 29:1319–1325. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Oeffinger KC, Mertens AC, Sklar CA,
Kawashima T, Hudson MM, Meadows AT, Friedman DL, Marina N, Hobbie
W, Kadan-Lottick NS, et al Childhood Cancer Survivor Study: Chronic
health conditions in adult survivors of childhood cancer. N Engl J
Med. 355:1572–1582. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Geenen MM, Cardous-Ubbink MC, Kremer LC,
van den Bos C, van der Pal HJ, Heinen RC, Jaspers MW, Koning CC,
Oldenburger F, Langeveld NE, et al: Medical assessment of adverse
health outcomes in long-term survivors of childhood cancer. JAMA.
297:2705–2715. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wust P, Hildebrandt B, Sreenivasa G, Rau
B, Gellermann J, Riess H, Felix R and Schlag PM: Hyperthermia in
combined treatment of cancer. Lancet Oncol. 3:487–497. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Peeken JC, Vaupel P and Combs SE:
Integrating hyperthermia into modern radiation oncology: What
Evidence Is Necessary? Front Oncol. 7:1322017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cihoric N, Tsikkinis A, van Rhoon G,
Crezee H, Aebersold DM, Bodis S, Beck M, Nadobny J, Budach V, Wust
P, et al: Hyperthermia-related clinical trials on cancer treatment
within the http://ClinicalTrials.govurisimpleClinicalTrials.gov
registry. Int J Hyperthermia. 31:609–614. 2015. View Article : Google Scholar
|
|
12
|
van der Zee J, González González D, van
Rhoon GC, van Dijk JD, van Putten WL and Hart AA; Dutch Deep
Hyperthermia Group: Comparison of radiotherapy alone with
radiotherapy plus hyperthermia in locally advanced pelvic tumours:
A prospective, randomised, multicentre trial. Lancet.
355:1119–1125. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vernon CC, Hand JW, Field SB, Machin D,
Whaley JB, van der Zee J, van Putten WL, van Rhoon GC, van Dijk JD,
González González D, et al International Collaborative Hyperthermia
Group: Radiotherapy with or without hyperthermia in the treatment
of superficial localized breast cancer: Results from five
randomized controlled trials. Int J Radiat Oncol Biol Phys.
35:731–744. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huilgol NG, Gupta S and Sridhar CR:
Hyperthermia with radiation in the treatment of locally advanced
head and neck cancer: A report of randomized trial. J Cancer Res
Ther. 6:492–496. 2010. View Article : Google Scholar
|
|
15
|
Issels RD, Lindner LH, Verweij J,
Wessalowski R, Reichardt P, Wust P, Ghadjar P, Hohenberger P,
Angele M, Salat C, et al European Organization for the Research and
Treatment of Cancer-Soft Tissue and Bone Sarcoma Group and the
European Society for Hyperthermic Oncology: Effect of Neoadjuvant
Chemotherapy Plus Regional Hyperthermia on Long-term Outcomes Among
Patients With Localized High-Risk Soft Tissue Sarcoma: The EORTC
62961-ESHO 95 Randomized Clinical Trial. JAMA Oncol. 4:483–492.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Datta NR, Puric E, Klingbiel D, Gomez S
and Bodis S: Hyperthermia and radiation therapy in locoregional
recurrent breast cancers: A systematic review and meta-analysis.
Int J Radiat Oncol Biol Phys. 94:1073–1087. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Datta NR, Rogers S, Ordóñez SG, Puric E
and Bodis S: Hyperthermia and radiotherapy in the management of
head and neck cancers: A systematic review and meta-analysis. Int J
Hyperthermia. 32:31–40. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Seifert G, Budach V, Keilholz U, Wust P,
Eggert A and Ghadjar P: Regional hyperthermia combined with
chemotherapy in paediatric, adolescent and young adult patients:
Current and future perspectives. Radiat Oncol. 11:652016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wessalowski R, Schneider DT, Mils O,
Hannen M, Calaminus G, Engelbrecht V, Pape H, Willers R, Engert J,
Harms D, et al: An approach for cure: PEI-chemotherapy and regional
deep hyperthermia in children and adolescents with unresectable
malignant tumors. Klin Padiatr. 215:303–309. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wessalowski R, Schneider DT, Mils O,
Friemann V, Kyrillopoulou O, Schaper J, Matuschek C, Rothe K,
Leuschner I, Willers R, et al MAKEI study group: Regional deep
hyperthermia for salvage treatment of children and adolescents with
refractory or recurrent non-testicular malignant germ-cell tumours:
An open-label, non-randomised, single-institution, phase 2 study.
Lancet Oncol. 14:843–852. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
de Jong MA, Oldenborg S, Bing Oei S,
Griesdoorn V, Kolff MW, Koning CC and van Tienhoven G:
Reirradiation and hyperthermia for radiation-associated sarcoma.
Cancer. 118:180–187. 2012. View Article : Google Scholar
|
|
22
|
Myerson RJ, Roti Roti JL, Moros EG,
Straube WL and Xu M: Modelling heat-induced radiosensitization:
Clinical implications. Int J Hyperthermia. 20:201–212. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Franken NA, Oei AL, Kok HP, Rodermond HM,
Sminia P, Crezee J, Stalpers LJ and Barendsen GW: Cell survival and
radiosensitisation: Modulation of the linear and quadratic
parameters of the LQ model (Review). Int J Oncol. 42:1501–1515.
2013.Review. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kok HP, Crezee J, Franken NAP, Stalpers
LJA, Barendsen GW and Bel A: Quantifying the combined effect of
radiation therapy and hyperthermia in terms of equivalent dose
distributions. Int J Radiat Oncol Biol Phys. 88:739–745. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Crezee J, van Leeuwen CM, Oei AL, van
Heerden LE, Bel A, Stalpers LJ, Ghadjar P, Franken NA and Kok HP:
Biological modelling of the radiation dose escalation effect of
regional hyperthermia in cervical cancer. Radiat Oncol. 11:142016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
van Leeuwen CM, Crezee J, Oei AL, Franken
NA, Stalpers LJ, Bel A and Kok HP: 3D radiobiological evaluation of
combined radiotherapy and hyperthermia treatments. Int J
Hyperthermia. 33:160–169. 2017. View Article : Google Scholar
|
|
27
|
International Commission on Radiation
Units and Measurements: ICRU Report 50 Prescribing, recording, and
reporting photon beam therapy. ICRU; Bethesda, MD: 1993
|
|
28
|
van Dijk JDP, Schneider C, van Os R, Blank
LE and Gonzalez DG: Results of deep body hyperthermia with large
waveguide radiators. Adv Exp Med Biol. 267:315–319. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
van Stam G, Kok HP, Hulshof MCCM, Kolff
MW, van Tienhoven G, Sijbrands J, Bakker A, Zum Vörde Sive Vörding
PJ, Oldenborg S, de Greef M, et al: A flexible 70 MHz
phase-controlled double waveguide system for hyperthermia treatment
of superficial tumours with deep infiltration. Int J Hyperthermia.
33:796–809. 2017.PubMed/NCBI
|
|
30
|
Kok HP, Kotte ANTJ and Crezee J: Planning,
optimisation and evaluation of hyperthermia treatments. Int J
Hyperthermia. 33:593–607. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Taflove A and Hagness SC: Computational
Electrodynamics. 2nd edition. Artech House; Boston, London:
2000
|
|
32
|
Das SK, Clegg ST and Samulski TV:
Computational techniques for fast hyperthermia temperature
optimization. Med Phys. 26:319–328. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Stoll AM and Greene LC: Relationship
between pain and tissue damage due to thermal radiation. J Appl
Physiol. 14:373–382. 1959. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
van Leeuwen CM, Oei AL, Ten Cate R,
Franken NA, Bel A, Stalpers LJ, Crezee J and Kok HP: Measurement
and analysis of the impact of time-interval, temperature and
radiation dose on tumour cell survival and its application in
thermoradiotherapy plan evaluation. Int J Hyperthermia. 34:30–38.
2018. View Article : Google Scholar
|
|
35
|
Bruggmoser G, Bauchowitz S, Canters R,
Crezee H, Ehmann M, Gellermann J, Lamprecht U, Lomax N, Messmer MB,
Ott O, et al Atzelsberg Research Group; European Society for
Hyperthermic Oncology: Guideline for the clinical application,
documentation and analysis of clinical studies for regional deep
hyperthermia: Quality management in regional deep hyperthermia.
Strahlenther Onkol. 188(Suppl 2): 198–211. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
International Commission on Radiation
Units Measurements: ICRU report 83 Prescribing, Recording, and
Reporting Intensity-Modulated Photon-Beam Therapy (IMRT). ICRU;
Bethesda, MD: 2010
|
|
37
|
van Leeuwen CM, Oei AL, Crezee J, Bel A,
Franken NA, Stalpers LJ and Kok HP: The alfa and beta of tumours: A
review of parameters of the linear-quadratic model, derived from
clinical radiotherapy studies. Radiat Oncol. 13:962018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
van Dijk IW, van Os RM, van de Kamer JB,
Franken NA, van der Pal HJ, Koning CC, Caron HN, Ronckers CM and
Kremer LC: The use of equivalent radiation dose in the evaluation
of late effects after childhood cancer treatment. J Cancer Surviv.
8:638–646. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Brahme A: Individualizing cancer
treatment: Biological optimization models in treatment planning and
delivery. Int J Radiat Oncol Biol Phys. 49:327–337. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kaidar-Person O, Oldenborg S and Poortmans
P: Re-irradiation and Hyperthermia in Breast Cancer. Clin Oncol (R
Coll Radiol). 30:73–84. 2018. View Article : Google Scholar
|
|
41
|
Overgaard J: Simultaneous and sequential
hyperthermia and radiation treatment of an experimental tumor and
its surrounding normal tissue in vivo. Int J Radiat Oncol Biol
Phys. 6:1507–1517. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
van Leeuwen CM, Crezee J, Oei AL, Franken
NA, Stalpers LJ, Bel A and Kok HP: The effect of time interval
between radiotherapy and hyperthermia on planned equivalent
radiation dose. Int J Hyperthermia. 34:901–909. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
ESHO Taskgroup Committee: ‘Treatment
Planning and Modelling in Hyperthermia, a Task Group Report of the
European Society for Hyperthermic Oncology. Tor Vergata, Rome.
1992.
|
|
44
|
de Greef M, Kok HP, Correia D, Borsboom
PP, Bel A and Crezee J: Uncertainty in hyperthermia treatment
planning: The need for robust system design. Phys Med Biol.
56:3233–3250. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Dewhirst MW, Vujaskovic Z, Jones E and
Thrall D: Re-setting the biologic rationale for thermal therapy.
Int J Hyperthermia. 21:779–790. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sreenivasa G, Gellermann J, Rau B, Nadobny
J, Schlag P, Deuflhard P, Felix R and Wust P: Clinical use of the
hyperthermia treatment planning system HyperPlan to predict
effectiveness and toxicity. Int J Radiat Oncol Biol Phys.
55:407–419. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Willnow U, Lindner H, Brock D, Wild L,
Diestelhorst C, Greiner C and Eichstädt H: Treatment of otherwise
incurable tumor diseases in childhood using whole-body hyperthermia
and chemotherapy. Dtsch Med Wochenschr. 114:208–213. 1989.In
German. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lindner H and Tillig B: Treatment of
recurrent neuroblastoma in childhood with whole body
thermochemotherapy. Padiatr Grenzgeb. 31:187–194. 1993.In
German.
|
|
49
|
Ismail-Zade RS, Zhavrid EA and Potapnev
MP: Whole body hyperthermia in adjuvant therapy of children with
renal cell carcinoma. Pediatr Blood Cancer. 44:679–681. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Geijsen ED, de Reijke TM, Koning CCE, Zum
Vörde Sive Vörding PJ, de la Rosette JJ, Rasch CRN, van Os RM and
Crezee J: Combining Mitomycin C and Regional 70 MHz Hyperthermia in
Patients with Nonmuscle Invasive Bladder Cancer: A Pilot Study. J
Urol. 194:1202–1208. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hulshof MC, Van Haaren PM, Van Lanschot
JJ, Richel DJ, Fockens P, Oldenborg S, Geijsen ED, Van Berge
Henegouwen MI and Crezee J: Preoperative chemoradiation combined
with regional hyperthermia for patients with resectable esophageal
cancer. Int J Hyperthermia. 25:79–85. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Turner PF, Tumeh A and Schaefermeyer T:
BSD-2000 approach for deep local and regional hyperthermia: Physics
and technology. Strahlenther Onkol. 165:738–741. 1989.PubMed/NCBI
|
|
53
|
Horsman MR and Overgaard J: Hyperthermia:
A potent enhancer of radiotherapy. Clin Oncol (R Coll Radiol).
19:418–426. 2007. View Article : Google Scholar
|
|
54
|
Sapareto SA and Dewey WC: Thermal dose
determination in cancer therapy. Int J Radiat Oncol Biol Phys.
10:787–800. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Dewey WC, Hopwood LE, Sapareto SA and
Gerweck LE: Cellular responses to combinations of hyperthermia and
radiation. Radiology. 123:463–474. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Sapareto SA, Hopwood LE and Dewey WC:
Combined effects of X irradiation and hyperthermia on CHO cells for
various temperatures and orders of application. Radiat Res.
73:221–233. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Crezee H, van Leeuwen CM, Oei AL, Stalpers
LJ, Bel A, Franken NA and Kok HP: Thermoradiotherapy planning:
Integration in routine clinical practice. Int J Hyperthermia.
32:41–49. 2016. View Article : Google Scholar
|
|
58
|
Wijsman R, Kaanders JH, Oyen WJ and
Bussink J: Hypoxia and tumor metabolism in radiation oncology:
Targets visualized by positron emission tomography. Q J Nucl Med
Mol Imaging. 57:244–256. 2013.PubMed/NCBI
|
|
59
|
Niendorf T, Pohlmann A, Arakelyan K,
Flemming B, Cantow K, Hentschel J, Grosenick D, Ladwig M, Reimann
H, Klix S, et al: How bold is blood oxygenation level-dependent
(BOLD) magnetic resonance imaging of the kidney? Opportunities,
challenges and future directions Acta Physiol (Oxf). 213:19–38.
2015.
|
|
60
|
Kok HP, Korshuize-van Straten L, Bakker A,
De Kroon-Oldenhof R, Geijsen ED, Stalpers LJA and Crezee J: On-line
adaptive hyperthermia treatment planning during locoregional
heating to suppress treatment limiting hot spots. Int J Radiat
Oncol Biol Phys. 99:1039–1047. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Balzer S, Schneider DT, Bernbeck MB, Jäger
M, Mils O, Schaper J, Willers R, Krauspe R, Göbel U and Wessalowski
R: Avascular osteonecrosis after hyperthermia in children and
adolescents with pelvic malignancies: A retrospective analysis of
potential risk factors. Int J Hyperthermia. 22:451–461. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Jäger M, Balzer S, Wessalowski R, Schaper
J, Göbel U, Li X and Krauspe R: Hyperthermia associated
osteonecrosis in young patients with pelvic malignancies.
Anticancer Agents Med Chem. 8:571–575. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Paulino AC: Late effects of radiotherapy
for pediatric extremity sarcomas. Int J Radiat Oncol Biol Phys.
60:265–274. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
DeLaney TF and Haas RL: Innovative
radiotherapy of sarcoma: Proton beam radiation. Eur J Cancer.
62:112–123. 2016. View Article : Google Scholar : PubMed/NCBI
|