Introduction
Renal cell carcinoma (RCC) is a common malignancy of
the genitourinary system that accounts for 5% of new cancer cases
in males and 3% in females worldwide; RCC was responsible for
14,240 cases of mortality in the United States of America in 2016
(1). The majority of patients with
localised RCC are effectively treated with radical nephrectomy, and
25-30% of these patients ultimately present with disseminated
disease. Furthermore, nearly a third of patients who undergo
surgery will experience recurrence or progression (2). The prognosis of metastatic RCC is
poor, with a median overall survival of 12 months and a 5-year
survival rate of <10% (3).
Over the past decade, steps have been made towards
developing targeted therapies for patients with advanced RCC
(4,5), which have increased the therapeutic
response rate and improved survival outcomes. Tyrosine kinase
inhibitors targeting vascular endothelial growth factor (VEGF)
receptors are considered first-line therapies for RCC (6-8). In
addition, RCC appears to be particularly sensitive to strategies
that target tumour associated angiogenesis; however, complete and
long term responses to the current targeted therapies are rare, and
the cancer usually progresses (9).
Notably, RCC is not a single disease but is a process associated
with complex and heterogeneous tumourigenesis, which may confer
resistance to targeted therapies (10,11).
Therefore, it is essential that novel therapeutic strategies or
targets be explored in future research.
Vascular endothelial growth inhibitor (VEGI) is a
member of the tumour necrosis factor (TNF) superfamily, which has
been identified as an anti-angiogenic cytokine (12,13).
It is located on human chromosome 9q32 (Gene ID: 9966). The full
length VEGI gene is ~17 kb, and consists of four exons and three
introns. Three alternatively spliced isoforms, VEGI174, VEGI192 and
VEGI251, have been documented and share 151 common C-terminal amino
acids (AAs) with different N terminal regions. The initially
reported VEGI protein consists of 174 AAs that can be divided into
two parts: AA residues 1-25 at the N-terminus constitute an
intracel lular transmembrane domain, and AA residues 26-174 at the
C-terminus form an extracellular domain (14,15).
VEGI is highly expressed in kidney, bladder, prostate, lung, breast
and colon tissues (16-21). Our previous studies revealed that
overexpression of VEGI174 significantly inhibited RCC cell
proliferation, motility, adhesion and epithelial mesenchymal
transition (EMT) in vitro and in vivo (21-24).
Taken together, these previous data suggested that VEGI174 exerts
inhibitory effects on RCC; therefore, it may be valuable to be
further study VEGI174 with regards to RCC treatment.
As an endothelial cell-secreted cytokine, the role
of VEGI174 in RCC is unclear, and whether its functional domain
peptides exert the same effects on RCC has yet to be reported. The
present study designed different peptide fragments based on the
functional domain of the VEGI174 protein. Furthermore, the
biological functions of VEGI174 and its functional domain peptides
were assessed on RCC in vitro and in vivo, and how
well the compounds were tolerated was investigated.
Materials and methods
Selection of effective functional domains
of VEGI174
The present study was approved by the ethics
committee of Beijing Institute for Cancer Research (Beijing,
China). Based on the human VEGI sequence (GenBank: NC_000009.12;
https://www.ncbi.nlm.nih.gov/genbank/), eight
different segments (V1-V8) were designed that encode the VEGI174
functional extracellular domains (23). In our prestudies, the full length
human VEGI174 and functional domain (V1-V8) genes were respectively
cloned into mammalian expression plasmid vectors (pEF/His TOPO TA;
Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and
were then transfected into human umbilical vein endothelial cells
(HUVECs; American Type Culture Collection, Manassas, VA, USA).
Briefly, HUVECs (confluence, 50-60%) were transfected with the
vectors using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) at 37˚C for 10 h; overexpression was
confirmed by western blotting and reverse
transcription-quantitative polymerase chain reaction. An electric
cell-substrate impedance sensing measuring system (ibidi GmbH,
Planegg, Germany) revealed that overexpression of VEGI174 or V1 V8
was able to inhibit the motility and adhesion of HUVEC cells
(25,26). Specifically, the inhibitory effects
of overexpressing the V7 or V8 gene fragments were more significant
(Fig. 1). Therefore, the
full-length VEGI174 protein and V7 and V8 peptides were selected as
target agents in the present study.
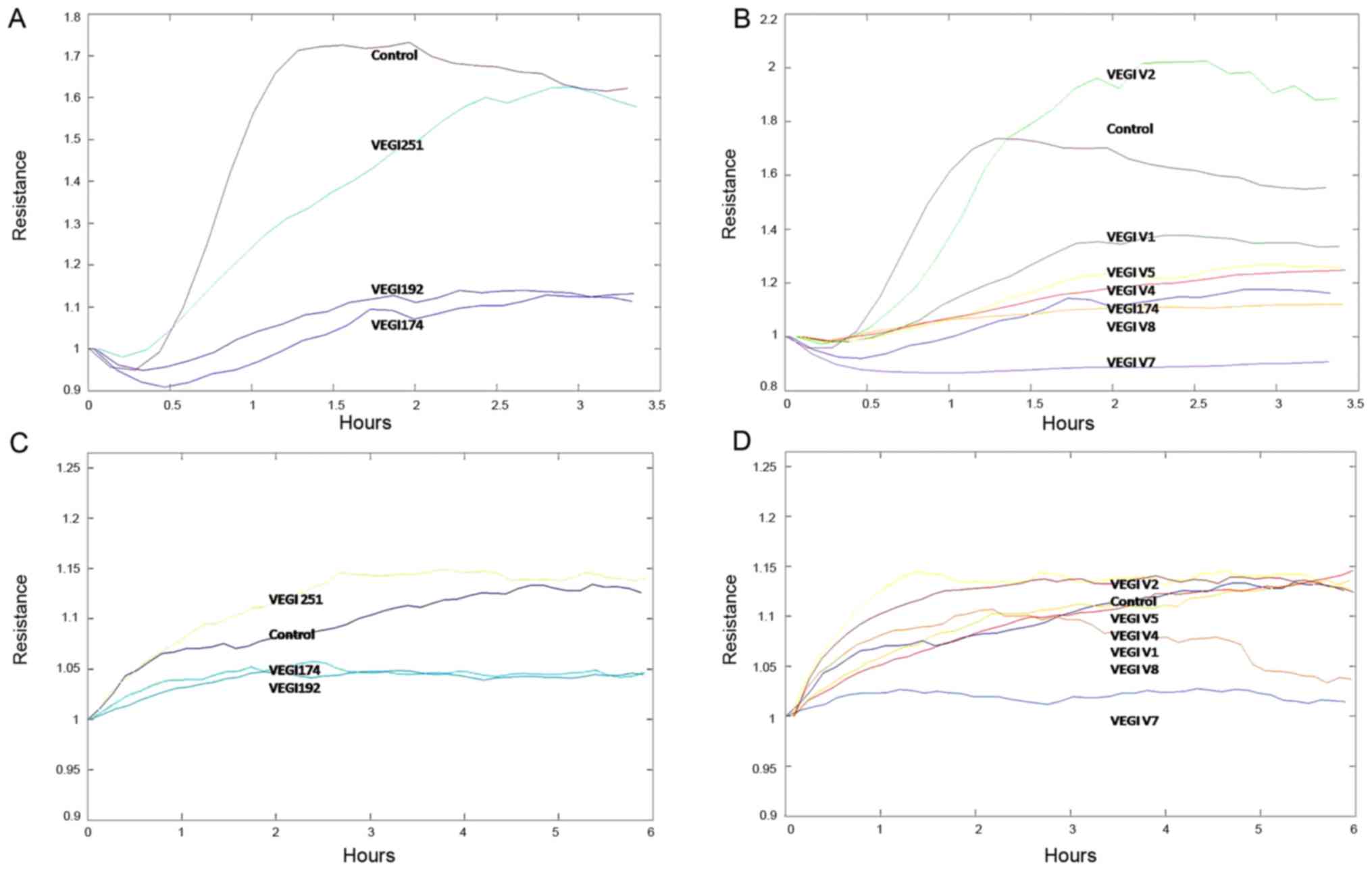 | Figure 1Cell adhesion and motility of HUVECs
overexpressing full length VEGI174, VEGI192 and VEGI251, and the
VEGI174 functional fragments V1 V8, were detected using an electric
cell-substrate impedance sensing cell function detector. (A and B)
Overexpression of VEGI174, VEGI192, V4, V5, V7 and V8 inhibited
HUVEC adhesion; V7 and V8 exhibited more potent inhibition than the
other functional domain peptides. (C and D) Overexpression of
VEGI174, VEGI192, V1, V4, V7 and V8 inhibited HUECV motility; V7
and V8 exhibited more potent inhibition than the other functional
domain peptides. HUVECs, human umbilical vein endothelial cells;
VEGI, vascular endothelial growth inhibitor. |
Biosynthesis of the VEGI174 protein and
its domain peptides
VEGI174 protein and its domain peptides (V7 and V8)
were biosynthesised and qualified by the School of Pharmaceutical
Sciences, Sun Yat-Sen University (Guangzhou, China). The AA
sequence of the VEGI174 protein and its domain peptides are listed
in Table I.
 | Table IAmino acid sequence of VEGI174
protein (V7 peptide contains AA 81-145, V8 peptide contains AA
118-145). |
Table I
Amino acid sequence of VEGI174
protein (V7 peptide contains AA 81-145, V8 peptide contains AA
118-145).
| Name | AA sequence |
|---|
| VEGI174a | MRRFLSKVYS
FPMRKLILFL VFPVVRQTPT QHFKNQFPAL HWEHELGLAF/50 AA |
| TKNRMNYTNK
FLLIPESGDY FIYSQVTFRG MTSECSEIRQ AGRPNKPDSI/100 AA |
| TVVITKVTDS
YPEPTQLLMG TKSVCEVGSN WFQPIYLGAM FSLQEGDKLM/150 AA |
| VNVSDISLVD
YTKEDKTFFG AFLL/174 AA |
Cell culture
The human RCC cell lines, A498 and 786-O, were
provided by Sun Yat-Sen University Laboratory (Guangzhou, China).
In vitro cell culture was conducted in Dulbecco's modified
Eagle's medium (HyClone; GE Healthcare, Logan, UT, USA) or RPMI
1640 medium (HyClone; GE Healthcare), supplemented with 10% foetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) and 100
U/ml penicillin and streptomycin (Gibco; Thermo Fisher Scientific,
Inc.). Cells were grown at 37°C in a humidified atmosphere
containing 5% CO2 air.
Animals
The animal studies were approved by the ethics
committee of Beijing Institute for Cancer Research, and the
Institutional Animal Care and Use Committee (IACUC) of Crown
Bioscience (Beijing, China). Female BALB/c nude and NOD/SCID mice
(n=5/group; age, 4-6 weeks; weight, 16-20 g; specific pathogen free
degree) were supplied by Beijing FuKang Bioscience (Beijing, China;
Animal Certificate no. 11401300025891). Mice were maintained under
the following conditions: Temperature, 18-22°C; humidity, 50-60%;
12 h light/dark cycle; ad libitum food/water access Procedures
related to animal handling, care and treatment in this study were
performed according to the guidelines approved by the IACUC of
Crown Bioscience and following the guidance of the Association for
Assessment and Accreditation of Laboratory Animal Care.
Cell proliferation assay
Cancer cells were harvested during the logarithmic
growth phase and were counted using a Countstar automated cell
counter (ALIT Life Science Co., Ltd., Shanghai, China). Cell
concentration was adjusted to 5.0×104/ml in culture
medium. Cell suspensions, ~100 \µl/well, were seeded in 96
well plates (Corning Incorporated, Corning, NY, USA) and were
cultured in a humidified incubator at 37°C under 5% CO2
for 24 h. Subsequently, VEGI174 and its domain peptides (V7 and
V8), at various concentrations, were separately added to each well
at final concentrations of 1 and 100 pM, 10 nM and 1 \µM.
Cytoactivity was detected using a Cell Counting Kit 8 assay
(Dojindo Molecular Technologies, Inc., Kumamoto, Japan), according
to the manufacturer's protocol, at 24, 48, 72, 96 and 120 h.
Real-time measurement of cell migration
and invasion
Cell migration and invasion were monitored using the
xCELLigence system (Roche Applied Science, Penzberg, Germany)
(27-29). To examine cell migration, the RCC
cells were incubated in a cell invasion/migration (CIM) plate 16 (8
\µm pore size; Roche Applied Science) coated with
fibronectin. The lower chambers were filled with fresh medium
containing 10% FBS. The upper chambers were filled with serum-free
medium, and the plate was incubated at 37°C in an atmosphere
containing 5% CO2. Subsequently, VEGI174 and its domain
peptides (V7 and V8), at various concentrations, were separately
added to each well, at final concentrations of 1 and 100 pM, 10 nM
and 1 \µM. The CIM plate was assembled onto the Real Time
Cell Analyzer (RTCA) with dual plate format instrument, and cell
migration was assessed at 15 min intervals for 24-48 h. Data
acquisition and analysis were performed using RTCA software
(version 1.2; Roche Diagnostics, Basel, Switzerland).
Invasion was measured with the RTCA in the same
manner as migration; however, a layer of Matrigel (BD Biosciences,
Franklin Lakes, NJ, USA) was added to the upper side of the
membranes.
In vivo xenograft studies
A498 and 786-O cell lines were implanted into
NOD/SCID mice and BALB/c nude mice, respectively. Briefly, the mice
were subcutaneously injected into the right flank with RCC cells
(3×106) mixed with Matrigel (100 \µl). Tumour
dimensions were measured using a Vernier caliper. Tumour sizes were
calculated using the following formula: Tumour volume
(mm3) = (length × width2)/2. When the tumours
grew to 130-190 mm3 in size, the animals were randomised
into an untreated control group and treatment groups (n=5
mice/group). VEGI174, V7 and V8 were admin istered as single
agents, and PBS was used as a blank control. Mice were treated via
intratumoural injections every other day and received a total of
four doses (Q2Dx4; Table II).
Tumour growth and behavioural data, including mobility, visual
estimation of food and water consumption, and body weight
alterations, were assessed twice weekly.
 | Table IIDose regimen. |
Table II
Dose regimen.
| Group | n | Dosage (mg/kg) | Route | Administration
time |
|---|
| Blank
controla | 5 | - | Intratumoural
injection | Q2Dx4 |
| VEGI174 | 5 | 5 | Intratumoural
injection | Q2Dx4 |
| V7 | 5 | 5 | Intratumoural
injection | Q2Dx4 |
| V8 | 5 | 5 | Intratumoural
injection | Q2Dx4 |
Effectiveness and safety assessment
Tumour growth inhi bition (TGI) and tumour growth
delay time (T-C) were calculated, in order to assess the response
of the tumours to the agents. TGI was calculated using the
following formula: TGI (%) = [(DVc DVe)/DVc] × 100%, in which DVc
is the difference between the final and initial tumour volumes of
the blank control group, and DVe represents the change in tumour
volume in each agent treated group. T-C refers to the difference in
time (days) required for a tumour to reach a certain volume between
the implanted tumour of a treatment group and that of the control
group; T is the number of days required for the treatment group to
reach a specific tumour volume, and C represents the number of days
required for the control group to reach the same value.
Mouse behaviour, including temperament, appetite,
activity, body weight changes and cases of mortality were recorded,
in order to conduct a safety evaluation.
Statistical analysis
All statistical analyses were performed using SPSS
software (version 20.0; IBM Corp., Armonk, NY, USA). The measured
data (three replicates) are presented as the means ± standard
deviation. One way analysis of variance and a least-significant
difference multiple comparisons test were used to analyse the
equality of the means among three or more groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
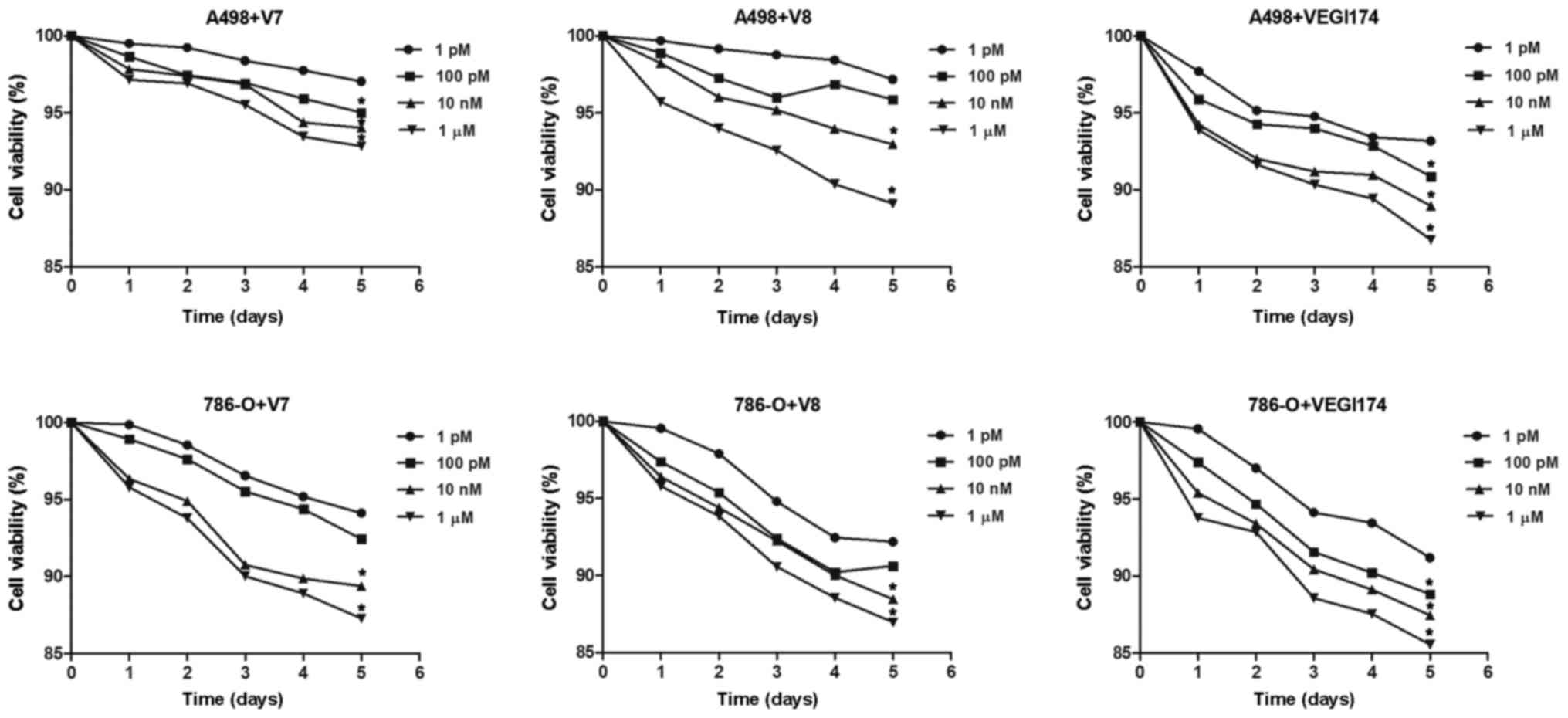
VEGI174, V7 and V8 inhibit the
proliferation of RCC cells in vitro
Viability of A498 and 786-O cells was continuously
monitored for 5 days following treatment with the VEGI174 protein
and the V7 and V8 peptides. Cell activity of the blank control
group was set as the baseline. Compared with the blank control
group, proliferation was inhibited following treatment with
VEGI174, V7 and V8 at concentrations of 1 and 100 pM, 10 nM and 1
\µM in both cell lines (Fig.
2). The inhibitory effects of treatment on RCC cells exhibited
a dose dependent effect in vitro; however, the inhibitory
effects among the VEGI174-, V7- and V8-treated groups showed no
significant differences (P>0.05).
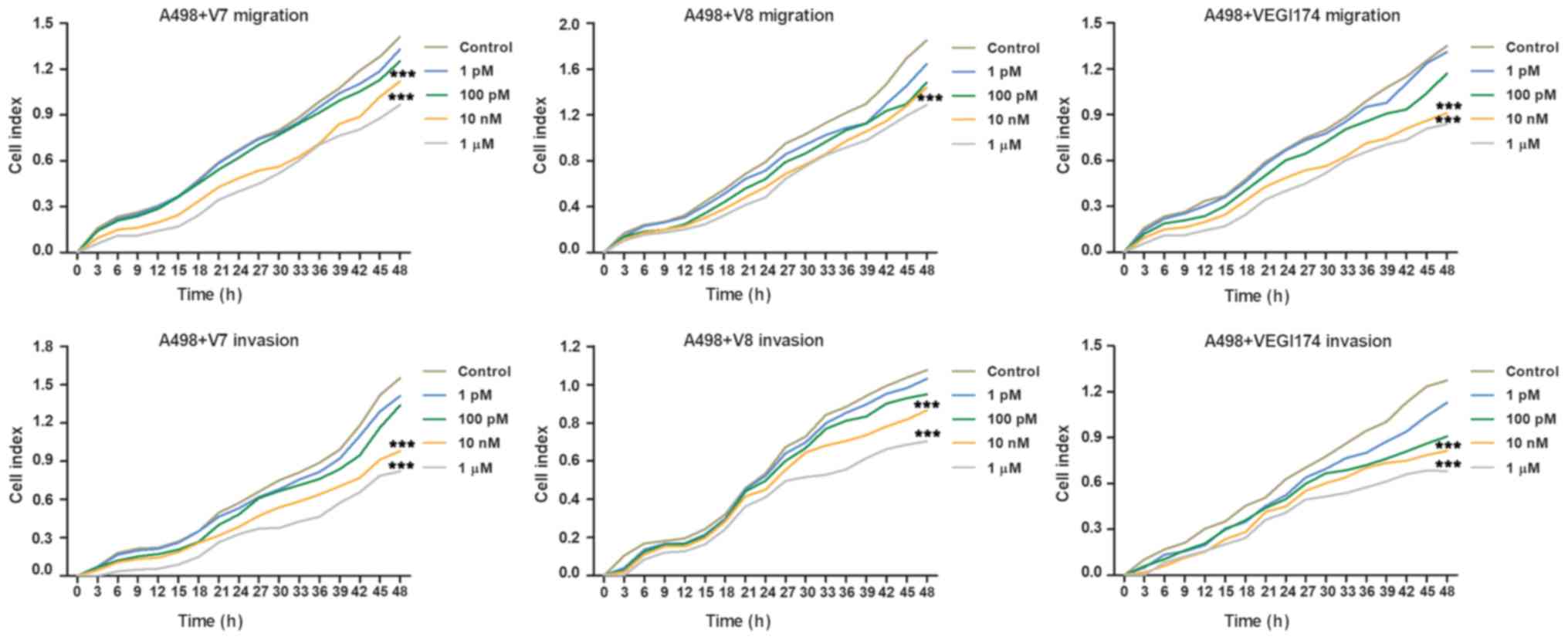
VEGI174, V7 and V8 inhibit RCC cell
migration and invasion in vitro
The present study used the xCELLigence system to
investigate whether the VEGI174 protein and V7 and V8 peptides were
involved in the regulation of RCC cell migration and invasion.
These assays revealed that VEGI174, V7 and V8 significantly
inhibited the migration and invasion of A498 cells compared with
the control group at concentrations of 10 nM and 1 \µM
(P<0.001; Fig. 3). Inhibition
of cell migration and invasion exhibited dose and time dependent
effects. As the treatment concentration increased, the inhibitory
effect became more obvious. In addition, cell migration and inva
sion were more notably affected after ≥24 h of treatment.
Conversely, no significant differences were observed in the
inhibitory effects among the VEGI174 , V7 and V8 treated
groups.
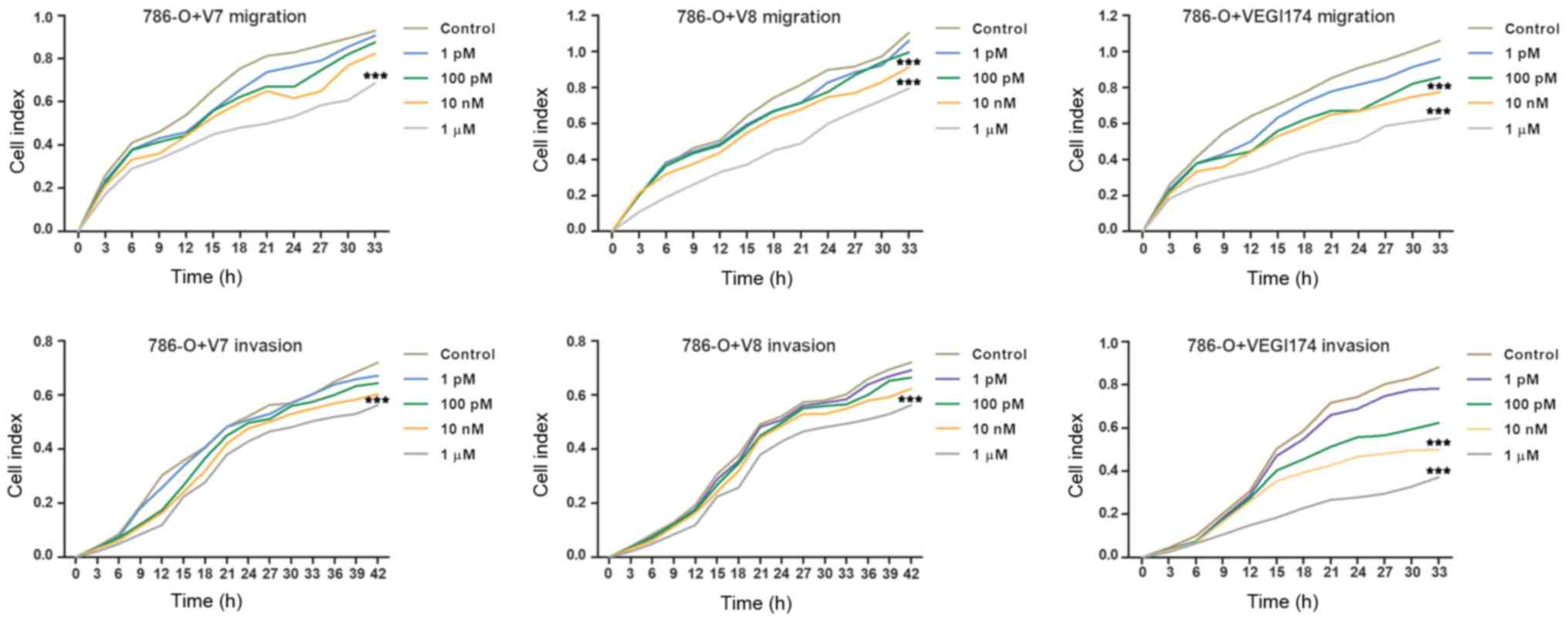
Consistently, treatment with the VEGI174 protein and
V7 and V8 peptides significantly inhibited the migration and
invasion of 786-O cells compared with in the control group at
concentrations of 10 nM and 1 \µM (P<0.001; Fig. 4). Inhibition of cell migration and
invasion exhibited dose and time depen dent effects. Notably, cell
migration and invasion were more obviously affected after ≥24 h of
treatment. These findings suggested that the V7 and V8 peptides may
inhibit RCC cell migration and invasion, as well as VEGI174
protein, in vitro.
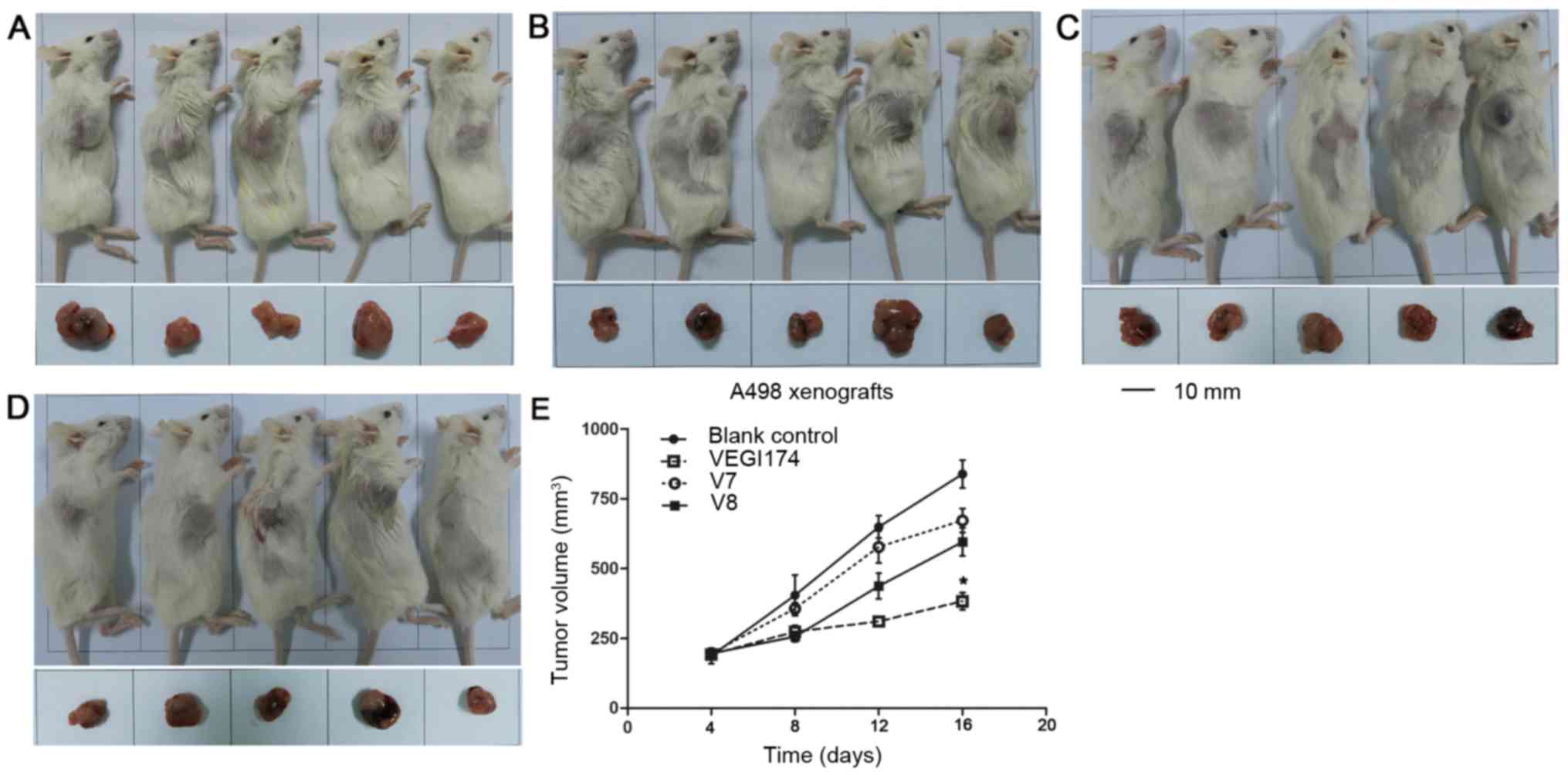
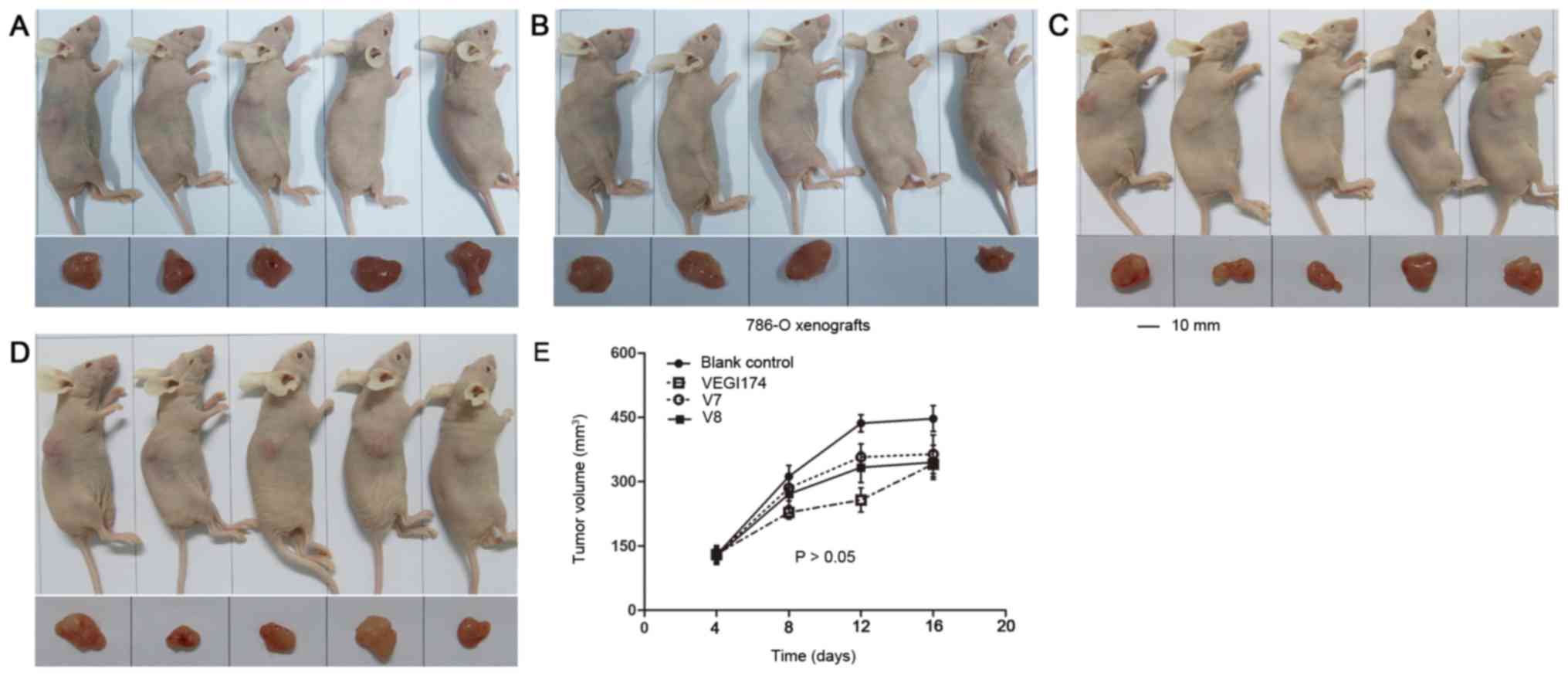
VEGI174, V7 and V8 suppress A498
xenograft growth in vivo
NOD/SCID mice were used to establish A498 xeno
grafts. The effects of VEGI174, V7 and V8 were determined on A498
xenograft tumour growth over an 8 day (Q2Dx4) period (Fig. 5). Growth inhibition was observed in
the VEGI174 , V7 and V8 treated groups compared with in the blank
control group (Table III). The
final tumour volume in the VEGI174-treated group was significantly
smaller than that in the control group (P<0.05). The TGI of the
VEGI174 treated group was 71%, which was higher than in the V7
(20%) and V8 treated (31%) groups. In A498 xenografts, for T-C
analysis, the average tumour volume was set at 350 mm3.
The T-C values of the VEGI174 , V7 and V8 treated groups were 5, 1
and 3 days, respectively (Table
III). There was no significant loss in body weight and no cases
of mortality in all mice groups (Fig.
6A). The mice had a good temperament and appe tite, and
exhibited normal activity levels. Overall, VEGI174, V7 and V8
treatments were well tolerated and safe.
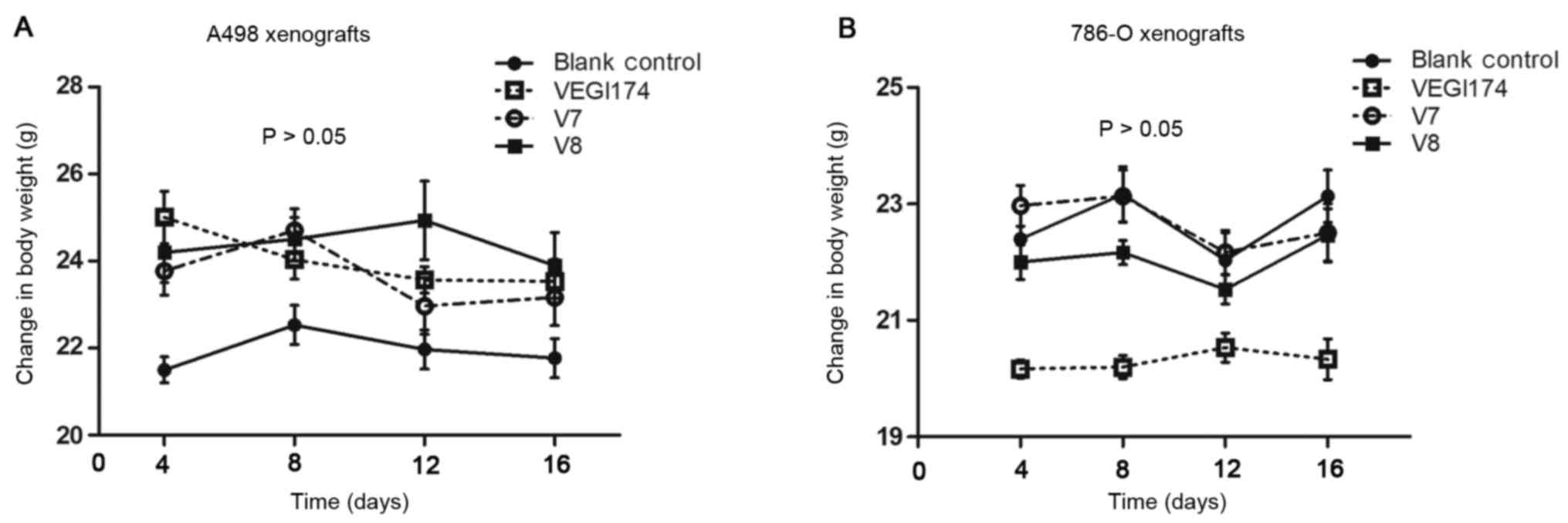 | Figure 6(A) Body weight alterations in the
A498 xenograft mice. At the end of the study, the average weight of
the blank control group was 21.7±2.1 g, and the average weights for
the VEGI174-, V7- and V8-treated groups were 23.9±0.9, 23.3±0.6 and
23.9±0.7 g, respectively (P>0.05). (B) Body weight alterations
in the 786-O xenograft mice. At the end of the study, the average
weight of the blank control group was 23.2±0.7 g, and the average
weights for the VEGI174-, V7- and V8-treated groups were 20.3±0.4,
22.6±0.6 and 22.2±0.7 g, respectively (P>0.05). VEGI174,
vascular endothelial growth inhibitor 174. |
 | Table IIITV, TGI and T-C following treatment
with VEGI174, V7 and V8 in A498 xenografts. |
Table III
TV, TGI and T-C following treatment
with VEGI174, V7 and V8 in A498 xenografts.
| Group | T-C (days) | TV before treatment
(mm3) | TV after final
treatment (mm3) | TGI (%) | P-valuea |
|---|
| Blank control | - | 187±28 | 838±50 | - | - |
| VEGI174 | 5 | 192±9 | 383±30 | 71 | 0.006 |
| V7 | 1 | 196±12 | 672±43 | 20 | 0.685 |
| V8 | 3 | 196±14 | 595±50 | 31 | 0.501 |
VEGI174, V7 and V8 suppress 786-O
xenograft growth in vivo
A second xenograft study was performed using 786-O
cells to determine the effects of VEGI174, V7 and V8 on tumour
growth over an 8 day (Q2Dx4) period (Fig. 7). TGI was also observed in the
VEGI174 , V7 and V8 treated groups; however, the rates were not
significant compared with in the blank control treated group
(Table IV). The TGI rates of the
VEGI174 , V7 and V8 treated groups were 34, 26 and 31%,
respectively. In 786-O xenografts, for T-C anal ysis, the average
tumour volume was set at 300 mm3. The T-C values of the
VEGI174 , V7 and V8 treated groups were 3.5, 1 and 1.5 days,
respectively (Table IV). The body
weights of all 786-O xenograft implanted mice were stable, and
there were no agent related cases of mortality (Fig. 6B). The mice had a good temperament
and appetite, and exhibited normal activity levels during the
experimental period. These findings indicated that VEGI174, V7 and
V8 were well tolerated and safe in the 786-O xenograft mouse
models.
 | Table IVTV, TGI and T-C following treatment
with VEGI174, V7 and V8 in 786-O xenografts. |
Table IV
TV, TGI and T-C following treatment
with VEGI174, V7 and V8 in 786-O xenografts.
| Group | T-C (days) | TV before treatment
(mm3) | TV after final
treatment (mm3) | TGI (%) | P-valuea |
|---|
| Blank control | - | 129±22 | 447±30 | - | - |
| VEGI174 | 3.5 | 130±13 | 341±35 | 34 | 0.171 |
| V7 | 1 | 129±17 | 364±45 | 26 | 0.547 |
| V8 | 1.5 | 127±17 | 345±40 | 31 | 0.427 |
Discussion
VEGI174 is a cytokine that belongs to the TNF ligand
family. It is abundantly expressed in endothelial cells and is a
ligand for the TNF receptor superfamily member (TNFRSF)25 receptor
and the TNFRSF21/death receptor 6 decoy receptor (30,31).
Numerous studies have investigated its expression and biological
functions in cancer, and have demonstrated that VEGI expression is
absent in the tumour vasculature in various types of cancer
(32,33). Parr et al (18) reported that VEGI is aberrantly
expressed in breast cancer and has prognostic relevance. Patients
with breast cancer with low expression levels of VEGI have a poorer
prognosis than those with high VEGI expression. Our previous study
also observed a decrease in the expression of VEGI in RCC tissues
(22). In addition, Chew et
al (14) demonstrated that
overexpression of VEGI abrogates xenograft tumour progression by
reducing tumour growth rate and microvessel density. It has also
been demonstrated that overexpression of VEGI inhibits RCC cell
motility, EMT, vascular endothelial tube formation and tumour
growth (21-24). Furthermore, VEGI participates in
regulating the biological functions of several tumour cell lines,
including breast carcinoma (MCF 7), cervical carcinoma (HeLa) and
myeloid tumour (U 937 and ML 1a) cell lines (34,35).
Notably, Hou et al (36) reported that systemic administra
tion of recombinant human VEGI192 protein gave rise to a marked
inhibition of tumour growth and an increased survival time in a
Lewis lung carcinoma murine tumour model. VEGI174 is one of three
alternatively spliced isoforms of VEGI. It is important to further
demonstrate how VEGI174 protein and its peptide fragments function
in RCC. Among the VEGI174 functional domains, our prestudies
verified that the inhibitory effects produced by the V7 and V8
domains are more significant than those produced by the other
functional domains. In the present study, VEGI174 and its
functional domain peptides (V7 and V8) were biosynthesized, and
their effects on RCC proliferation, migration and invasion were
detected.
The cell viability of A498 and 786-O cell lines was
inhibited following treatment with various concentrations of
VEGI174, V7 and V8. In addition, the xCELLigence system clearly
monitored A498 and 786-O cell migration and invasion. The data
confirmed that VEGI174, V7 and V8 significantly regulated the cell
migration and invasion of A498 and 786-O cells in vitro.
Furthermore, inhibition of cell proliferation, migration and
invasion exhibited dose and time dependent effects, which require
further exploration.
The in vivo study provided support for the
ability of VEGI174, V7 and V8 to inhibit RCC growth. TGI was
observed in the A498 and 786-O xenograft models. In the A498
xenograft mice, the TGI rates of the VEGI174 , V7 and V8 treated
groups were 71, 20 and 31%, respectively. The T-C values of the
VEGI174 , V7 and V8 treated groups were 5, 1 and 3 days,
respectively. In the 786-O xenografts, the TGI rates of the VEGI174
, V7 and V8 treated groups were 34, 26 and 31%, respectively. The
T-C values of the VEGI174 , V7 and V8 treated groups were 3.5, 1
and 1.5 days, respectively. These findings indicated that the V7
and V8 peptides were slightly inferior to the VEGI174 protein in
terms of antitumour activity. The V7 and V8 peptides are only parts
of the VEGI174 protein, and their spatial structures and biological
functions are different; therefore, these features require more
intensive study. Any drug resulting in a TGI of ≥58% (the National
Cancer Institute standard criterion for antitumour activity) is
considered a valid anticancer agent (37). VEGI174, V7 and V8 have exerted
potential antitumour effects in vivo. Notably, the V7 and V8
peptides inhibited RCC cell proliferation, migration and invasion
as well as the VEGI174 protein did in vitro. These data
strongly support the fact that V7 and V8 are important functional
domains of VEGI174.
The safety and tolerance of the three agents were
also evaluated in vivo. Mouse behaviour, including
temperament, appetite, activity, body weight gain/loss and
mortality, were recorded. The mice in all groups had no abnormal
appearance or behaviour, all of the mice survived, and their body
weights were stable. These results preliminarily demonstrated that
VEGI174, V7 and V8 were safe and well tolerated.
The VEGI174 protein and its domain peptides (V7 and
V8) exhibited inhibitory effects on RCC proliferation, migra tion
and invasion; however, the exact mechanism remains unclear. Zhang
et al (38) reported that
VEGF expression could be suppressed by VEGI stimulated TNF
superfamily member 15 activation of the Jun N terminal kinase GATA
binding protein 3 signalling pathway. Qi et al (39) reported that VEGI inhibits
vasculogenesis by regulating the relative levels of membrane bound
and soluble isoforms of VEGF receptor 1. Overall, these data
suggested that the VEGI174 protein and its domain peptides may
negatively regulate the VEGF pathway, in order to inhibit tumour
growth. Our previous study also demonstrated that there was an
inverse correlation between VEGI174 and microvessel density
(22). Notably, in the present
study, it was demonstrated that the VEGI174 protein and V7 and V8
peptides inhibited the proliferation, migration and invasion of
A498 and 786-O cells in vitro. These data indicated that
VEGI174, and V7 and V8 peptides may directly interact with tumour
cells and regulate cellular functions. In addition, the mechanism
underlying the inhibitory effects of VEGI174 on tumour growth may
not be limited to the VEGF pathway but may involve other signalling
pathways; this should be analysed in future research.
In conclusion, based on the overall antitumour
activity of VEGI174, V7 and V8 in RCC, V7 and V8 may be considered
important functional domains of VEGI174. The differences in the
secondary and tertiary structures between the V7 and V8 peptides,
and the VEGI174 protein, should be analysed in future studies, in
order to improve antitumour effects and provide information for
novel drug development. In addition, the regulatory mechanisms of
VEGI174 and the V7 and V8 peptides on RCC cell functions should be
carefully elucidated.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81372738).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
NZ and YY were involved in study conception and
design. YY, LY and KG developed the methodology. QZ, BAH, TZL, XXT
and YPJ were involved in data acquisition and analysis. NZ, QZ, BAH
and LY were involved in writing, reviewing and/or revising the
manuscript. XXT and LY provided technical and material support. KG
and YY provided study supervision.
Ethics approval and consent to
participate
The present study was approved by the ethics
committee of Beijing Institute for Cancer Research. The animal
studies were approved by the ethics committee of Beijing Institute
for Cancer Research, and IACUC of Crown Bioscience. Procedures
related to animal handling, care, and treatment in this study were
performed according to the guidelines approved by the IACUC of
Crown Bioscience and following the guid ance of the Association for
Assessment and Accreditation of Laboratory Animal Care.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Russo P: Renal cell carcinoma:
Presentation, staging, and surgical treatment. Semin Oncol.
27:160–176. 2000.PubMed/NCBI
|
|
3
|
Liu L, Zhang W, Qi X, Li H, Yu J, Wei S,
Hao X and Ren X: Randomized study of autologous cytokine induced
killer cell immunotherapy in metastatic renal carcinoma. Clin
Cancer Res. 18:1751–1759. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jonasch E and Motzer RJ: Ten years of
progress in renal cell carcinoma. J Natl Compr Canc Netw.
10:690–693. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Barata PC and Rini BI: Treatment of renal
cell carcinoma: Current status and future directions. CA Cancer J
Clin. 67:507–524. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bedke J, Gauler T, Grünwald V, Hegele A,
Herrmann E, Hinz S, Janssen J, Schmitz S, Schostak M, Tesch H, et
al: Systemic therapy in metastatic renal cell carcinoma. World J
Urol. 35:179–188. 2017. View Article : Google Scholar :
|
|
7
|
Vachhani P and George S: VEGF inhibitors
in renal cell carcinoma. Clin Adv Hematol Oncol. 14:1016–1028.
2016.
|
|
8
|
Posadas EM, Limvorasak S and Figlin RA:
Targeted therapies for renal cell carcinoma. Nat Rev Nephrol.
13:496–511. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rini BI and Atkins MB: Resistance to
targeted therapy in renal cell carcinoma. Lancet Oncol.
10:992–1000. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Haddad AQ and Margulis V: Tumour and
patient factors in renal cell carcinoma towards personalized
therapy. Nat Rev Urol. 12:253–262. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hsieh JJ, Manley BJ, Khan N, Gao J, Carlo
MI and Cheng EH: Overcome tumor heterogeneity imposed therapeutic
barriers through convergent genomic biomarker discovery: A braided
cancer river model of kidney cancer. Semin Cell Dev Biol.
64:98–106. 2017. View Article : Google Scholar
|
|
12
|
Tan KB, Harrop J, Reddy M, Young P,
Terrett J, Emery J, Moore G and Truneh A: Characterization of a
novel TNF like ligand and recently described TNF ligand and TNF
receptor superfamily genes and their constitutive and inducible
expression in hemato poietic and non hematopoietic cells. Gene.
204:35–46. 1997. View Article : Google Scholar
|
|
13
|
Zhai Y, Yu J, Iruela-Arispe L, Huang WQ,
Wang Z, Hayes AJ, Lu J, Jiang G, Rojas L, Lippman ME, et al:
Inhibition of angio genesis and breast cancer xenograft tumor
growth by VEGI, a novel cytokine of the TNF superfamily. Int J
Cancer. 82:131–136. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chew LJ, Pan H, Yu J, Tian S, Huang WQ,
Zhang JY, Pang S and Li LY: A novel secreted splice variant of
vascular endothelial cell growth inhibitor. FASEB J. 16:742–744.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang N, Sanders AJ, Ye L and Jiang WG:
Vascular endothelial growth inhibitor in human cancer (Review). Int
J Mol Med. 24:3–8. 2009.PubMed/NCBI
|
|
16
|
Zhai Y, Ni J, Jiang GW, Lu J, Xing L,
Lincoln C, Carter KC, Janat F, Kozak D, Xu S, et al: VEGI, a novel
cytokine of the tumor necrosis factor family, is an angiogenesis
inhibitor that suppresses the growth of colon carcinomas in vivo.
FASEB J. 13:181–189. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liang PH, Tian F, Lu Y, Duan B, Stolz DB
and Li LY: Vascular endothelial growth inhibitor (VEGI; TNFSF15)
inhibits bone marrow derived endothelial progenitor cell
incorporation into Lewis lung carcinoma tumors. Angiogenesis.
14:61–68. 2011. View Article : Google Scholar
|
|
18
|
Parr C, Gan CH, Watkins G and Jiang WG:
Reduced vascular endothelial growth inhibitor (VEGI) expression is
associated with poor prognosis in breast cancer patients.
Angiogenesis. 9:73–81. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang N, Sanders AJ, Ye L, Kynaston HG and
Jiang WG: Vascular endothelial growth inhibitor, expression in
human prostate cancer tissue and the impact on adhesion and
migration of prostate cancer cell in vitro. Int J Oncol.
35:1473–1480. 2009.PubMed/NCBI
|
|
20
|
Zhang N, Sanders AJ, Ye L, Kynaston HG and
Jiang WG: Expression of vascular endothelial growth inhibitor
(VEGI) in human urothelial cancer of the bladder and its effects on
the adhesion and migration of bladder cancer cells in vitro.
Anticancer Res. 30:87–95. 2010.PubMed/NCBI
|
|
21
|
Zhang N, Wu P, Shayiremu D, Wu L, Shan H,
Ye L, Zhao X, Cai J, Jiang WG, Gong K, et al: Suppression of renal
cell carcinoma growth in vivo by forced expression of vascular
endothelial growth inhibitor. Int J Oncol. 42:1664–1673. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu L, Li X, Ye L, Shayiremu D, Deng X,
Zhang X, Jiang W, Yang Y, Gong K and Zhang N: Vascular endothelial
growth inhibitor 174 is a negative regulator of aggressiveness and
microvascular density in human clear cell renal cell carcinoma.
Anticancer Res. 34:715–722. 2014.PubMed/NCBI
|
|
23
|
Zhang N, Hong B, Lian W, Zhou C, Chen S,
Du X, Deng X, Duoerkun S, Li Q, Yang Y, et al: Vascular endothelial
growth inhibitor 174 and its functional domains inhibit epithelial
mesen chymal transition in renal cell carcinoma cell in vitro. Int
J Mol Med. 40:569–575. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao Q, Liu T, Hong B, Wang F, Zhou C, Du
X, Chen S, Deng X, Duoerkun S, Li Q, et al: Vascular Endothelial
Growth Inhibitor, a Cytokine of the Tumor Necrosis Factor Family,
is Associated With Epithelial-Mesenchymal Transition in Renal Cell
Carcinoma. Appl Immunohistochem Mol Morphol. 2017. View Article : Google Scholar
|
|
25
|
Szulcek R, Bogaard HJ and van Nieuw
Amerongen GP: Electric cell-substrate impedance sensing for the
quantification of endo thelial proliferation, barrier function, and
motility. J Vis Exp. 85:e513002014.
|
|
26
|
Chen SW, Yang JM, Yang JH, Yang SJ and
Wang JS: A compu tational modeling and analysis in cell biological
dynamics using electric cell-substrate impedance sensing (ECIS).
Biosens Bioelectron. 33:196–203. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dowling CM, Herranz Ors C and Kiely PA:
Using real time impedance-based assays to monitor the effects of
fibroblast-derived media on the adhesion, proliferation, migration
and invasion of colon cancer cells. Biosci Rep. 34:342014.
View Article : Google Scholar
|
|
28
|
Stefanowicz Hajduk J, Adamska A,
Bartoszewski R and Ochocka JR: Reuse of E plate cell sensor arrays
in the xCEL Ligence Real Time Cell Analyzer. Biotechniques.
61:117–122. 2016. View Article : Google Scholar
|
|
29
|
Kho D, MacDonald C, Johnson R, Unsworth
CP, O'Carroll SJ, du Mez E, Angel CE and Graham ES: Application of
xCEL Ligence RTCA Biosensor Technology for Revealing the Profile
and Window of Drug Responsiveness in Real Time. Biosensors (Basel).
5:199–222. 2015. View Article : Google Scholar
|
|
30
|
Migone TS, Zhang J, Luo X, Zhuang L, Chen
C, Hu B, Hong JS, Perry JW, Chen SF, Zhou JX, et al: TL1A is a TNF
like ligand for DR3 and TR6/DcR3 and functions as a T cell
costimulator. Immunity. 16:479–492. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tian F, Grimaldo S, Fujita M, Cutts J,
Vujanovic NL and Li LY: The endothelial cell produced
antiangiogenic cytokine vascular endothelial growth inhibitor
induces dendritic cell maturation. J Immunol. 179:3742–3751. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhou J, Yang Z, Tsuji T, Gong J, Xie J,
Chen C, Li W, Amar S and Luo Z: LITAF and TNFSF15, two downstream
targets of AMPK, exert inhibitory effects on tumor growth.
Oncogene. 30:1892–1900. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Deng W, Gu X, Lu Y, Gu C, Zheng Y, Zhang
Z, Chen L, Yao Z and Li LY: Down modulation of TNFSF15 in ovarian
cancer by VEGF and MCP 1 is a pre requisite for tumor
neovascularization. Angiogenesis. 15:71–85. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xiao Q, Hsu CY, Chen H, Ma X, Xu J and Lee
JM: Characterization of cis regulatory elements of the vascular
endothelial growth inhibitor gene promoter. Biochem J. 388:913–920.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Haridas V, Shrivastava A, Su J, Yu GL, Ni
J, Liu D, Chen SF, Ni Y, Ruben SM, Gentz R, et al: VEGI, a new
member of the TNF family activates nuclear factor kappa B and c Jun
N terminal kinase and modulates cell growth. Oncogene.
18:6496–6504. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hou W, Medynski D, Wu S, Lin X and Li LY:
VEGI-192, a new isoform of TNFSF15, specifically eliminates tumor
vascular endothelial cells and suppresses tumor growth. Clin Cancer
Res. 11:5595–5602. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Meyer CJ, Krauth M, Wick MJ, Shay JW,
Gellert G, De Brabander JK, Northcote PT, Miller JH and Peloruside
A: Peloruside A Inhibits Growth of Human Lung and Breast Tumor
Xenografts in an Athymic nu/nu Mouse Model. Mol Cancer Ther.
14:1816–1823. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang K, Cai HX, Gao S, Yang GL, Deng HT,
Xu GC, Han J, Zhang QZ and Li LY: TNFSF15 suppresses VEGF
production in endothelial cells by stimulating miR 29b expression
via activation of JNK GATA3 signals. Oncotarget. 7:69436–69449.
2016.PubMed/NCBI
|
|
39
|
Qi JW, Qin TT, Xu LX, Zhang K, Yang GL, Li
J, Xiao HY, Zhang ZS and Li LY: TNFSF15 inhibits vasculogenesis by
regu lating relative levels of membrane bound and soluble isoforms
of VEGF receptor 1. Proc Natl Acad Sci USA. 110:13863–13868. 2013.
View Article : Google Scholar
|