Introduction
Breast cancer is the most commonly diagnosed cancer
and the second leading cause of cancer associated-mortality in
women in the United States (1).
The majority of breast cancer types are adenocarcinomas (invasive
ductal carcinoma) originating from epithelial cells (2). Terminal end buds, which contain
proliferating mammary epithelial stem cells, appear to be the
target of mammary neoplastic transformation (3,4). The
development of chemotherapeutic agents without side effects and
drug resistance has been an active area of cancer research.
Sphingolipids are present in all eukaryotic cell
membranes, specific prokaryotes, and in certain foods (5,6).
Anti-tumorigenic in vivo effects of complex sphingolipids
could be the result of the conversion of complex sphingolipids to
sphingolipid metabolites including sphingosine, sphinganine and
ceramide. Ceramides are composed of sphingosine and a fatty acid
with a variable chain length. These sphingolipid metabolites
function as second messengers in signal transduction pathways to
regulate cell proliferation, differentiation, apoptosis, and cell
migration (6,7). Sphingosine is a metabolic precursor
to sphingosine-1-phosphate (S1P), however sphingosine and S1P
exhibit opposing effects (e.g., sphingosine is anti-proliferative
and pro-apoptotic; S1P is growth-stimulatory and anti-apoptotic
effects) (6-8). It is suggested that the balance
between sphingosine and S1P can form a rheostat model: When this
balance moves toward sphingosine it triggers death of the cancer
cell, whereas increased S1P levels lead to cancer cell survival
(8). Most studies have examined
effects of ceramides and S1P, while fewer have been performed on
elucidating the action of sphingosine, its stereoisomers, and
sphinganine.
Previously the authors have developed a stem
cell-derived breast carcinogenesis model that encompasses Type I
and Type II normal human breast epithelial cells (HBECs) and
transformed cells, which represent multiple stages of breast
carcinogenesis. Type I HBECs display stem cell characteristics and
have been characterized by: The expression of a stem cell marker
octamer-binding transcription factor 4 (9), estrogen receptor α (10), and luminal epithelial markers
(11,12); a deficiency in gap-junction
associated intercellular communication (11,13);
the ability to display anchorage-independent growth (13); the ability to differentiate into
Type II (normal differentiated) HBECs (11,13);
reduced expression of maspin (14); and the ability to form
budding/ductal organoids on Matrigel in conjunction with Type II
HBECs (13). Furthermore, Type I
HBECs have been sequentially transformed into
immortal/non-tumorigenic cells (M13SV1), weakly tumorigenic cells
(M13SV1R2) and highly tumorigenic cells (M13SV1R2N1) by oncogenic
treatments, the SV40 large T-antigen (SV40-T), X-rays, and the
receptor tyrosine-protein kinase erbB-2/neu oncogene (11,15).
Type I HBECs are more susceptible to the oncogenic treatments than
Type II HBECs. In contrast, Type II HBECs rarely become immortal
following transfection with SV40-T (10,11,13,16).
Type II HBECs demonstrate basal epithelial phenotypes and do not
express the estrogen receptor α. This suggests that Type I HBECs
appear to be the major target cells for breast carcinogenesis.
The unique HBEC model system described above has
enabled the authors to evaluate chemotherapeutic and
cancer-protective properties of sphingolipid metabolites. The
effects of sphingosine, its stereoisomers, sphinganine, and
C2-ceramide (N-acetyl-D-erythro-sphingosine) have
been evaluated on Type I (normal stem) HBECs, Type II (normal
differentiated) HBECs, and highly tumorigenic cells transformed
from the parental normal stem cells. In addition, the effects of
sphingosine and C2-ceramide were tested on MCF7 breast
cancer cells. The following criteria were applied to define
chemotherapeutic and cancer-protective agents: i) A
chemotherapeutic agent is expected to preferentially inhibit the
proliferation and induce apoptosis in tumorigenic cells and normal
stem cells (Type I), while it has no or little effect on normal
differentiated cells (Type II); ii) A potential cancer-protective
agent is expected to induce the differentiation of normal stem
cells (Type I) to normal differentiated cells (Type II), thereby
decreasing the number of stem cells that are more susceptible to
neoplastic transformation.
Materials and methods
Sphingolipids
D-erythro-sphingosine (hereinafter referred
to as sphingosine) was purchased from Sigma-Aldrich; Merck KGaA
(Darmstadt, Germany). Sphingosine stereoisomers (D-threo,
L-threo, and L-erythro), sphinganine
(D-erythro-dihydro-sphingosine), and C2-ceramide
were obtained from Matreya LLC (State College, PA, USA). These
sphingolipid metabolites were dissolved and prepared at working
concentrations as described previously (14,17).
During experimental periods, sphingosine and sphingosine
stereoisomers were delivered to cells in 0.1% bovine serum albumin
(BSA; Sigma-Aldrich; Merck KGaA).
Development of normal HBECs from
reduction mammoplasty tissues and derivation of in vitro
neoplastically transformed cells
Normal HBECs were developed as described previously
(11,14). In vitro neoplastically
transformed HBEC lines (M13SV1, M13SV1R2, and M13SV1R2N1) were
sequentially derived from Type I HBECs (11,13,15,18).
In the present study, Type I and Type II HBECs and the human breast
transformed highly tumorigenic cells (M13SV1R2N1) were tested. The
transformed highly tumorigenic cells (M13SV1R2N1, hereinafter
referred to as tumorigenic cells) examined in the present study
were authenticated by short tandem repeat (STR) DNA profiling
(Genetica DNA Laboratories, Cincinnati, OH, USA). The STR
DNA-profile of the tumorigenic cells is unique among the known
3,274 cell lines reposited in American Type Culture Collection
(ATCC, Manassas, VA, USA), Deutsche Sammlung von Mikroorganismen
und Zellkulturen GmbH, Japanese Collection of Research
Bioresources, or RIKEN and does not match any of these known
repository cell lines, indicating no cross-contamination or
misidentification of the cells. The STR DNA-profiling results for
tumorigenic cells are presented in brackets for each STR locus:
D3S1358 [14], TH01 [5], D21S11 [28], D18S51
[12,21], Penta E [11], D5S818 [11,12], D13S317
[9], D7S820 [8,9], D16S539 [12,15], CSF1PO
[12,13], Penta D [9,13], vWA [14,17], D8S1179
[12,15], TPOX [11], FGA [21],
Amelogenin/Gender marker [X].
Cell culture and assessment of cell
proliferation
Type I and Type II HBECs and tumorigenic cells
(M13SV1R2N1) were cultured in the MSU-1 medium with ‘supplements
(S)’ (called ‘MSU-1+S medium’ hereafter) as described previously
(14). The composition of MSU-1
media (11) is equivalent to the
1:1 (vol:vol) mixture of DMEM with low glucose and
keratinocyte-serum free media (both from Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). The ‘supplements’ (11) are 0.5 µg/ml hydrocortisone,
5 µg/ml insulin, 4 µg/ml transferrin (all from
Sigma-Aldrich; Merck KGaA), 0.5 ng/ml epidermal growth factor (EGF;
Invitrogen; Thermo Fisher Scientific, Inc.), 10−8 M
17-β-estradiol (Sigma-Aldrich, Merck KGaA), and 50 µg/ml
gentamicin (Invitrogen; Thermo Fisher Scientific, Inc.). MCF7
breast cancer cells, obtained from the ATCC, were cultured using
the same procedures as for the breast tumorigenic cells
(M13SV1R2N1). Total nucleic acid concentrations representing cell
numbers were determined as described previously (14).
Analysis of DNA fragmentation by agarose gel
electrophoresis
Cells were trypsinized and centrifuged at 379 × g at
4°C for 10 min. Pellets were resuspended in 200 µl PBS and
DNA was extracted with 400 µl phenol/chloroform, and then
was centrifuged at max speed (13,000 × g) at 4°C for 5 min.
Following incubation with 0.2 mg/ml RNase A at 37°C for 1 h, the
extraction was repeated with 400 µl phenol/chloroform to
inactivate RNase A. A total of 20 µl of 3 M NaOAc and 500
µl of 100% ethanol were added to the solution prior to
storing at −20°C overnight. Following centrifugation at max speed
(13,000 × g) at 4°C for 30 min, the DNA pellets were washed with
70% ethanol and dried en vacuo. Finally, the pellets were
dissolved in Tris-EDTA (pH 8) and the 5 µg DNA was separated
on a 2% agarose gel at 100 V for 1 h and the gel was stained with
ethidium bromide.
Flow cytometry analysis of apoptotic
cells
Cells were collected and resuspended in DNA staining
reagent [0.1 mM EDTA (pH 7.4), 0.1% of Triton X-100, 0.05 mg/ml
RNase A (50 U/mg) and 50 µg/ml propidium iodide (Thermo
Fisher Scientific, Inc.) in PBS at pH 7.4], and then were processed
as described previously (14).
Cells were analyzed via Fluorescence-activated cell sorting (FACS)
Vantage cytometer (BD Biosciences, San Jose, CA, USA). The
percentage of cells in each phase of the cell cycle and apoptotic
cells was calculated using the FCS Express 2.0 and Win cycle
(MultiCycle AV) programs (Phoenix Flow Systems, San Diego, CA,
USA). Apoptotic cells (A0 peaks) with reduced DNA
content were determined as the percentage of cells in the
hypo-diploid sub G0/G1 area to the left of
G0/G1 diploid peak.
Assessment of Type I HBEC
differentiation
Differentiation of Type I to Type II HBEC
experiments were conducted as described previously (14). At the end of each differentiation
culture period, Type I and Type II colonies were visually
identified using a light microscope by placing the cell culture 60
mm dish into the dish holder of the microscope and sliding the
stage in a controlled manner. Then, the Type I and Type II colonies
of each differentiation culture dish were counted, respectively.
The identity of the sphingolipid and cholera toxin (Sigma-Aldrich;
Merck KGaA) treatments was unknown (blind) during counting to
ensure objectivity.
Polymerase chain reaction (PCR)-based
telomerase assay
Cells were harvested by trypsinization. Following
cell counting, the cells were centrifuged at 78 × g at 4°C for 6
min to remove trypsin solution. Cell pellets were washed with 10 ml
PBS and then centrifuged at 113 × g at 4°C for 8 min to remove the
PBS. Cells were then suspended at 1×106 cells/ml in PBS
and aliquoted into Eppendorf tubes. After cells were centrifuged at
176 × g at 4°C for 8 min and the PBS was carefully removed, the
cell pellets were stored at −80°C. Telomerase activities were
measured using the TRAPeze™ Telomerase Detection kit (cat. no.
S7700; EMD Millipore, Billerica, MA, USA) and the procedures were
performed according to the company's protocol. The cell pellets
were thawed and resuspended in 200 µl of 1X CHAPS lysis
buffer (EMD Millipore)/106 cells and left on ice for 30
min. Samples were centrifuged at 12,000 × g for 20 min at 4°C. The
TRAPeze kit includes a 36 base pair primer to serve as an internal
standard for amplification, therefore providing a positive control
for accurate quantitation of telomerase activity. Each analysis
included a negative control (CHAPS-lysis buffer without sample),
heat-inactivated control (sample incubated at 85°C for 10 min prior
to the assay), and a positive cell line control (MCF7 breast cancer
cells: See the section of Methods-Cell culture). The products (25
µl) of the telomerase repeat amplification protocol (TRAP)
assay were resolved via a non-denaturing 12% PAGE in a buffer
containing 54 mM Tris-HCL (pH 8.0), 54 mM boric acid, and 1.2 mM
EDTA. The gel was stained with SYBR-Green for ~30 min at room
temperature (Molecular Probes, Inc; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and visualized using a 302 nm UV
transilluminator. The density of DNA bands was analyzed/quantified
by AlphaImager™ 3300 (Alpha Innotech Corporation, San Leandro, CA,
USA) and Adobe Photoshop CS6 (Adobe Systems Inc., San Jose, CA,
USA).
Statistical analysis
Statistical analyses were carried out using Sigma
plot version 12.0 (Systat Software, Inc., San Jose, CA, USA) or R
(program version 3.0.2 for Windows www.r-project.org). Data of cell growth at different
concentrations of sphingolipids and multiple culture periods were
analyzed by two-way factorial analysis of variance (ANOVA). A
one-way ANOVA was applied for the cell growth data at different
concentrations of sphingolipids for a single culture period.
Following the application of two-way ANOVA or one-way ANOVA, the
Holm-Sidak method was applied for multiple comparisons. Differences
between the control and sphingosine groups at a single
concentration for: Apoptotic cells at
pre-G0/G0 regions and telomerase activity
results were analyzed by Mann-Whitney rank sum test and t-test,
respectively. P<0.05 was considered to indicate a statistically
significant difference, unless otherwise specified. Differentiation
of Type I to Type II HBEC results was analyzed by Chi-Square test.
For the differentiation data with multiple (>two) groups and
with P<0.05, post-hoc tests were done by conducting multiple
pairwise Chi-Square tests with Bonferroni corrections. P-value
cutoff used to identify statistical significance was adjusted for
each set of pairwise comparisons as part of the Bonferroni
correction (Adjusted P-value =0.05/number of pairwise comparisons).
Data were considered statistically significant if the P-value of
each pairwise comparison was below the Bonferroni correction
adjusted P-value.
Results
Sphingosine selectively inhibits
proliferation and causes death of normal stem cells (Type I) and
tumorigenic/cancer cells, but not normal differentiated cells (Type
II)
In normal stem cells (Type I HBECs: Fig. 1A), control cultures grew slowly
over the culture period with the total concentration of nucleic
acid increasing by ~70% in 5 days. Sphingosine at 2 and 5 µM
did not affect cell proliferation; however, sphingosine at 8
µM significantly reduced the nucleic acid concentration to
~60% of the corresponding control cultures at day 1 (P<0.05) and
floating dead cells were visible in the medium. Thereafter, for
cells treated with 8 µM sphingosine, the total nucleic acid
concentration remained the same through day 5 suggesting that
sphingosine blocked cell proliferation.
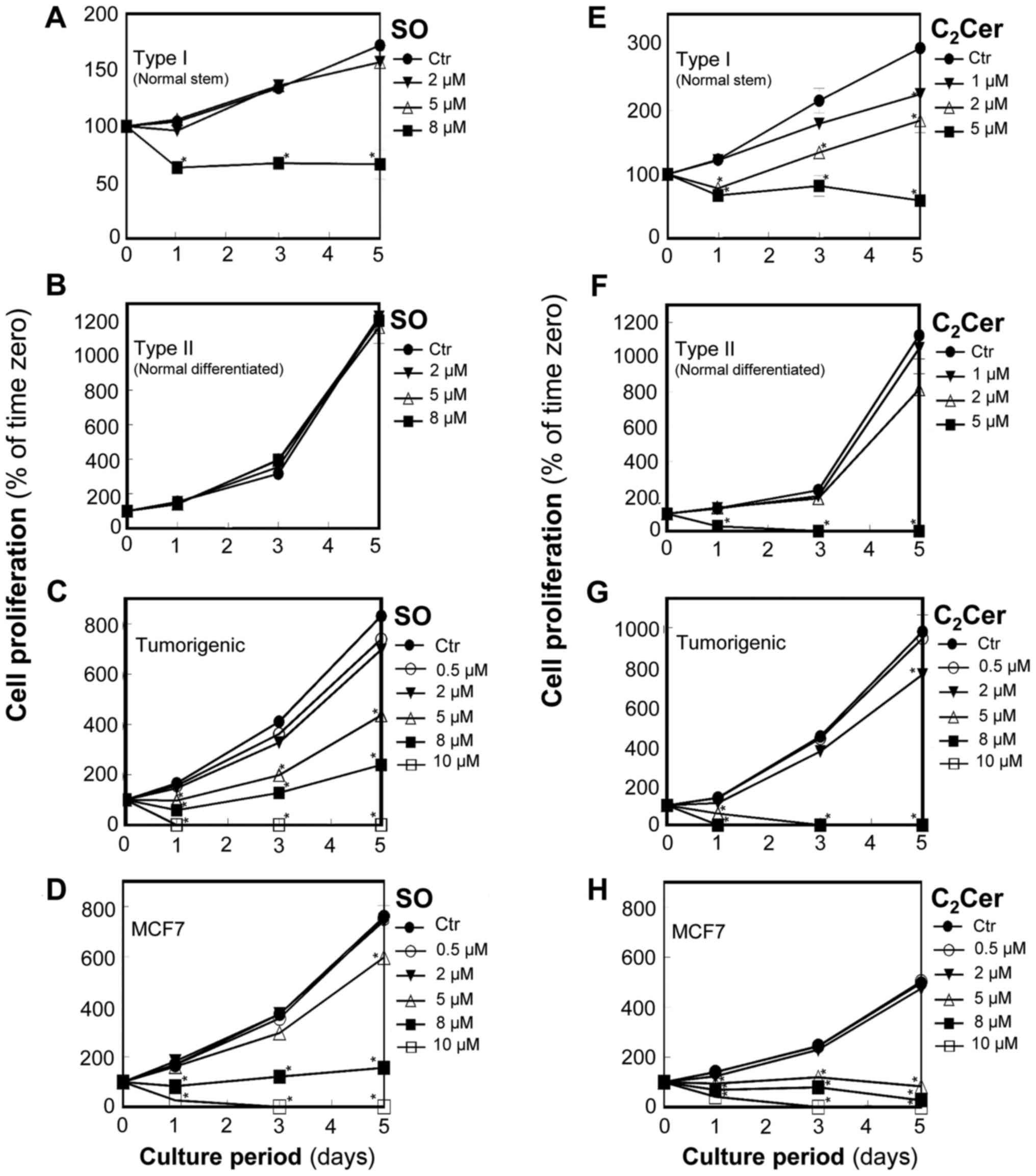 | Figure 1SO selectively inhibits the
proliferation of normal stem cells (Type I), tumorigenic cells, and
MCF7 cancer cells, but not normal differentiated cells (Type II).
In contrast, C2Cer inhibits the proliferation of all
breast cells tested in this study (Type I HBECs, Type II HBECs,
tumorigenic cells, and MCF7 cancer cells). Subconfluent cells
(6×104) were cultured in 6-well plates in triplicate and
treated with SO (A) Type I HBECs, (B) Type II HBECs, (C)
tumorigenic cells, and (D) MCF7 cancer cells and C2Cer
(E) Type I HBECs, (F) Type II HBECs, (G) tumorigenic cells, and (H)
MCF7 cancer cells at various concentrations (0.5-10 µM) on
day 0 and day 3. Total nucleic acid concentrations were measured as
an index of cell number for Type I (normal stem: A and E), Type II
(normal differentiated: B and F), tumorigenic (C and G), and MCF7
(D and H) cells. Results are expressed as a percentage of the
quantity at zero hours (mean ± standard deviation, n=3).
*P<0.05 vs. the Ctr. Where an error bar is not seen,
it lies within the dimensions of the symbol. HBECs, human breast
epithelial cells; Ctr, control; SO, sphingosine; Cer, ceramide. |
In normal differentiated cells (Type II HBECs:
Fig. 1B), the control culture cell
number tripled in 2 days and remained in log-phase at 5 days of
culture. Cell proliferation was not affected by sphingosine at
concentrations as high as 8 µM, which was the highest
concentration tested.
In breast tumorigenic cells (M13SV1R2N1) and MCF7
breast cancer cells, sphingosine caused concentration and
time-dependent decreases in total nucleic acid concentration
(Fig. 1C and D). Sphingosine at 5
to 10 µM significantly inhibited the proliferation and
caused death of breast tumorigenic cells and MCF7 cells
(P<0.05). Sphingosine at 5 µM reduced total nucleic acid
content to ~50% compared with the control and to ~80% of control at
day 5 in breast tumorigenic cells (Fig. 1C) and MCF7 cells (Fig. 1D), respectively.
C2-ceramide inhibits
proliferation and causes cell death in normal stem cells (Type I),
normal differentiated cells (Type II), and tumorigenic/cancer
cells
For Type I HBECs (Fig.
1E), C2-ceramide at 2 and 5 µM significantly
inhibited cell proliferation (P<0.05) and caused death within 1
day. C2-ceramide at 5 µM reduced total nucleic
acid content to ~60% of the corresponding control at day 1 and to
~25% of the control by day 5 (Fig.
1E). C2-ceramide at 8 µM killed all of the
cells by day 3. For Type II HBECs (Fig. 1F), C2-ceramide at 5
µM significantly inhibited cell proliferation (P<0.05)
and caused cell death within 1 day.
In breast tumorigenic cells (Fig. 1G) and MCF7 breast cancer cells
(Fig. 1H), C2-ceramide
significantly inhibited cell proliferation at 5 µM and
above, and caused death. C2-ceramide was more potent
than sphingosine as effects of 5 µM C2-ceramide
were similar to those of 8 to 10 µM sphingosine.
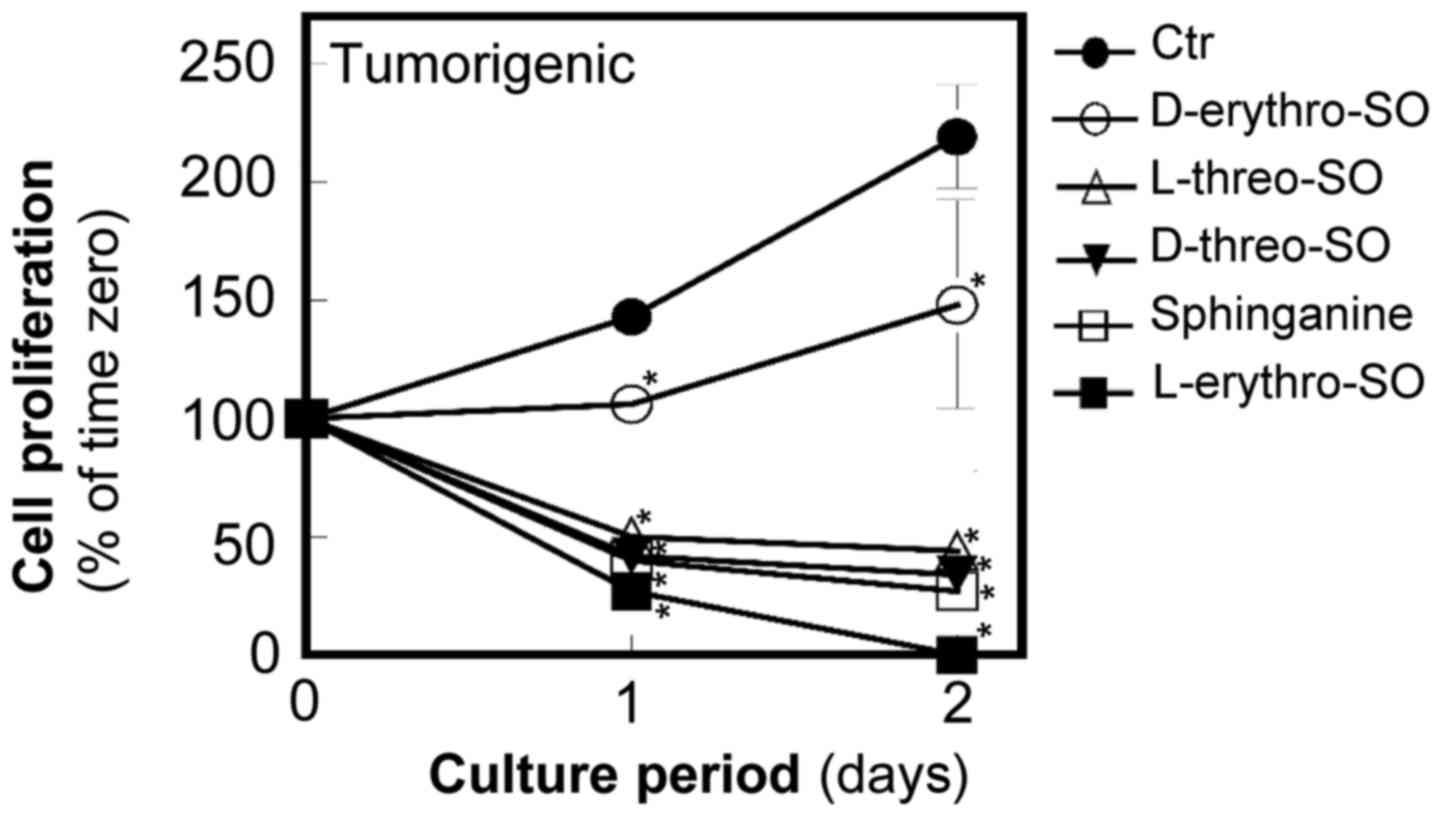
The unnatural stereoisomers of
sphingosine more potently inhibit proliferation and cause death of
breast tumorigenic cells compared with the natural form of
sphingosine in tumorigenic cells
To study the structural requirements for sphingosine
to inhibit proliferation, three unnatural stereoisomers,
D-threo, L-threo, and L-erythro-sphingosine at
5 µM were examined along with the natural form
D-erythro-sphingosine at 5 µM (Fig. 2). Since the authors of the present
study previously demonstrated that sphinganine is more potent than
D-erythro-sphingosine in inhibiting proliferation and
inducing apoptosis (14), the
effects of sphingosine isomers with sphinganine at the same
concentration were also compared. All three unnatural stereoisomers
of sphingosine were more potent compared with
D-erythro-sphingosine in inhibiting proliferation and
causing death of tumorigenic cells. L-erythro-sphingosine
was the most potent among the tested stereoisomers and sphinganine.
All isomers significantly inhibited cell growth compared with the
control (P<0.05).
Sphingosine and C2-ceramide
induce apoptosis in normal stem cells (Type I) and tumorigenic
cells
To examine whether sphingosine and
C2-ceramide caused cell death by inducing apoptosis, the
presence of apoptotic cells in Type I HBECs and tumorigenic cells
was detected using DNA fragmentation gel electrophoresis (Fig. 3A and B) and flow cytometry
(Fig. 3C-G) assays. In Type I
HBECs, control cultures exhibited intact, high molecular weight DNA
that remained at the top of the agarose gel (Fig. 3A; lane 2), while DNA extracted from
cells cultured with 10 µM C2-ceramide for 1, 2
and 4 days was fragmented and demonstrated a characteristic DNA
ladder pattern, indicative of apoptosis (Fig. 3A; lanes 3-5).
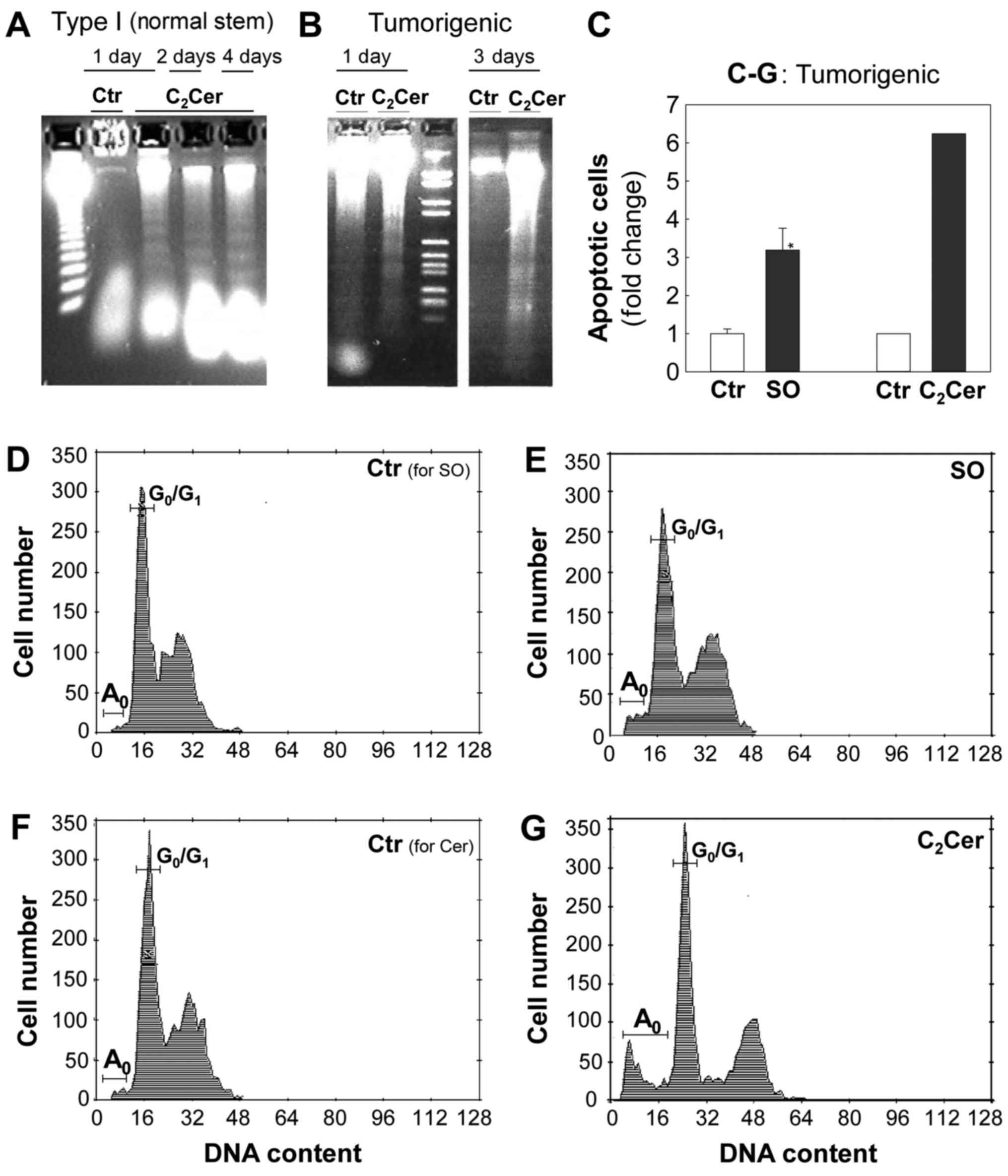 | Figure 3SO and C2Cer induce
apoptosis in tumorigenic cells. Apoptotic cells were detected by
using DNA fragmentation gel electrophoresis (A and B) and flow
cytometry (C-G) assays. (A and B) C2Cer causes
internucleosomal DNA fragmentation, indicative of apoptosis, in
Type I HBECs (normal stem) and tumorigenic cells. DNA was extracted
on indicated culture dates and analyzed via electrophoresis. (A)
Type I HBECs were cultured with 10 µM C2Cer for 1
day (lane 3), 2 days (lane 4), or 4 days (lane 5). Lane 1 presents
DNA size markers and lane 2 is the Ctr at day 1. (B) Tumorigenic
cells were cultured without C2Cer (lanes 1 and 4), with
10 µM C2Cer (lane 2) for 1 day, or with 5
µM C2Cer (lane 5) for 3 days. Lane 3 presents DNA
size markers. (C-G) SO and C2Cer increase the number of
apoptotic cells (A0) at pre-G0/G1
regions in tumorigenic cells. Cells were stained with propidium
iodide to determine DNA content and cell cycle via flow cytometry.
(C) The effects of SO and C2Cer on apoptosis of
tumorigenic cells are presented by a fold-change in the number of
apoptotic cells. Data are presented as the mean ± standard error of
the mean (n=5) for SO from three independent culture experiments.
*P<0.05 vs. the Ctr. Subconfluent tumorigenic cells
were cultured with or without treatments for 1 day: (D) Ctr for SO;
(E) 10 µM SO; (F) Ctr for C2-Cer; (G) 10
µM C2-Cer. Ctr, control; Cer, ceramide; SO,
sphingosine; HBECs, human breast epithelial cells. |
In tumorigenic cells, C2-ceramide at 5
µM (Fig. 3B; lane 5) and 10
µM (Fig. 3B; lane 2) also
caused the formation of a DNA ladder, an indicator of apoptosis.
Flow cytometry analysis indicated that sphingosine (Fig. 3C-E) and C2-ceramide
(Fig. 3C, F and G) increased by
~5-7-fold the apoptotic cell populations (as demonstrated by
A0 peaks in the hypo-diploid
pre-G0/G1 region).
Sphingosine, but not
C2-ceramide, induces differentiation of normal stem
cells (Type I HBECs)
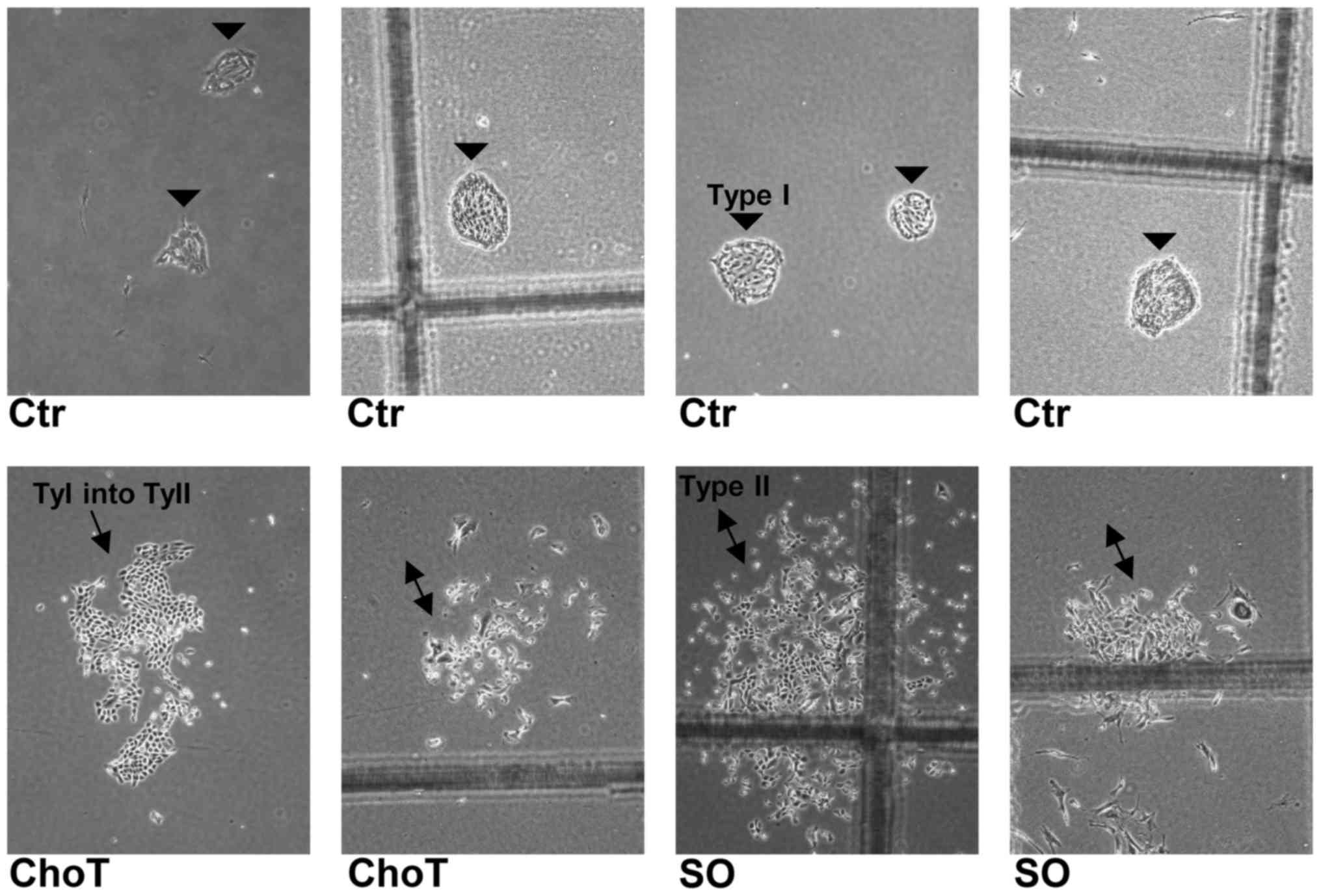
Type I HBECs were cultured with sphingosine and
C2-ceramide at non-cytotoxic concentrations.
Differentiation was measured by counting the numbers of Type II and
Type I colonies that were morphologically distinguishable (Fig. 4).
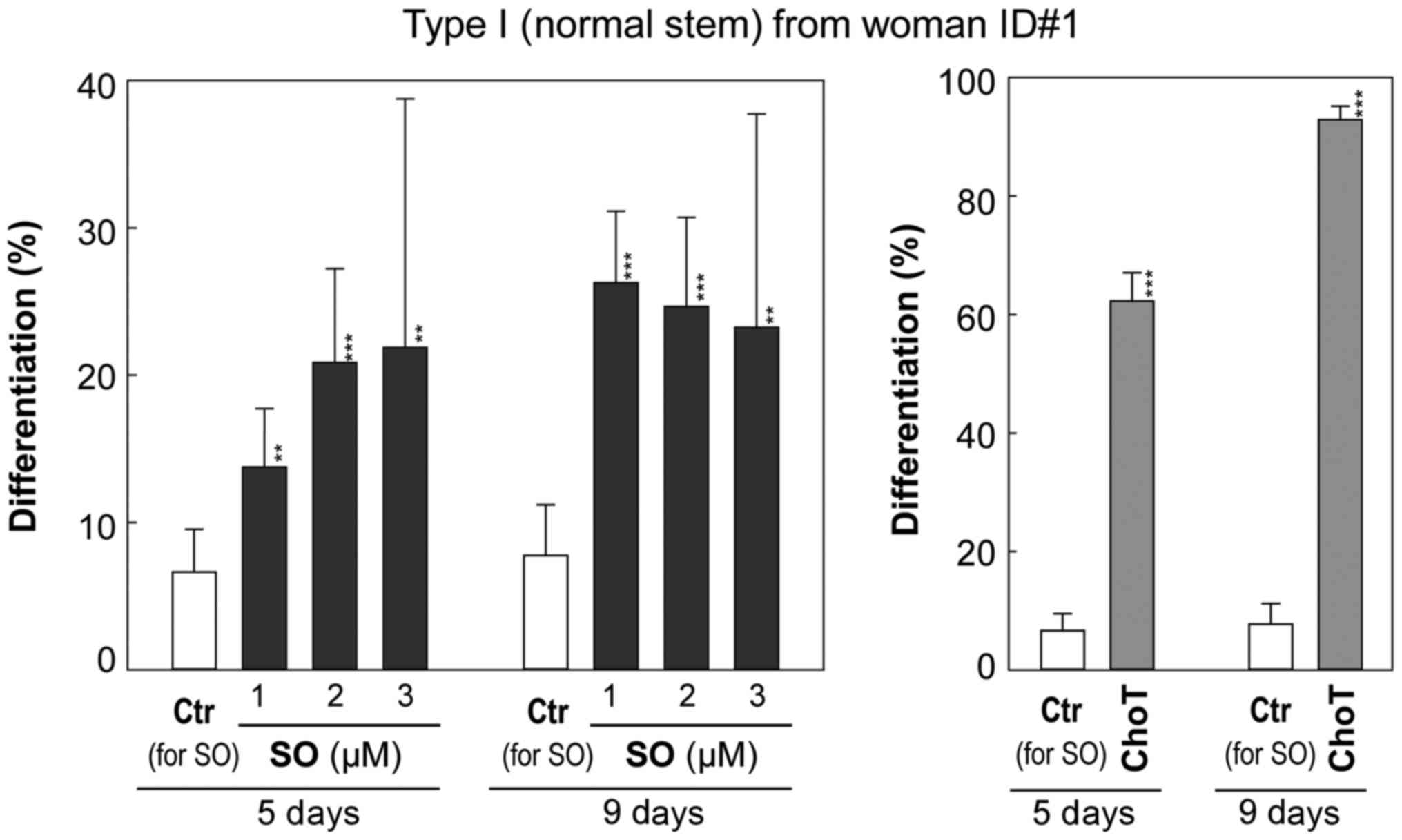
As presented in Fig.
5 and Table I, control cells
exhibited a low rate of differentiation of ~6.6% by day 5 and ~7.8%
by day 9. Cholera toxin (1 ng/ml), a positive control known to
induce differentiation (11,14),
significantly increased the number of Type II containing colonies
to 62% by day 5 and 93% by day 9 (P<0.001) without significant
alteration in the total number of colonies. Sphingosine at
non-cytotoxic concentrations (1 to 3 µM) significantly
increased the frequency of Type II HBEC-containing colonies
starting at day 5 (P<0.01; Fig.
5) compared with the vehicle control (0.1% BSA) culture. This
trend became more obvious at day 9 (P<0.01; Fig. 5). The induction of differentiation
of Type I to Type II HBECs by 2 and 3 µM sphingosine was
accompanied by inhibition of colony forming efficiency, as
presented by the decrease in the total number of colonies (Fig. 5 and Table I).
 | Table ISO induces differentiation of Type I
HBECs to Type II HBECs. |
Table I
SO induces differentiation of Type I
HBECs to Type II HBECs.
| Culture (days) | Treatments | Total colonies | Type II containing
colonies | % of Type II
containing colonies (95% CI) |
|---|
| Type I (normal
stem) HBEC from woman ID#1 | | | |
| 5 | Ctr for SO (0.1%
BSA) | 392 | 26 | 6.6% (−2.1%) |
| ChoT 1 ng/ml | 371 | 231 | 62.3%
(−5.0%)b |
| SO 1 µM | 356 | 49 | 13.8%
(−3.2%)a |
| SO 2 µM | 187 | 39 | 20.9%
(−5.2%)b |
| SO 3 µM | 32 | 7 | 21.9%
(−10.9%)a |
| 9 | Ctr for SO (0.1%
BSA) | 322 | 25 | 7.8% (−2.5%) |
| ChoT 1 ng/ml | 335 | 311 | 92.8%
(−3.3%)b |
| SO 1 µM | 350 | 92 | 26.3%
(−4.3%)b |
| SO 2 µM | 223 | 55 | 24.7%
(−5.2%)b |
| SO 3 µM | 43 | 10 | 23.3%
(−10.1%)a |
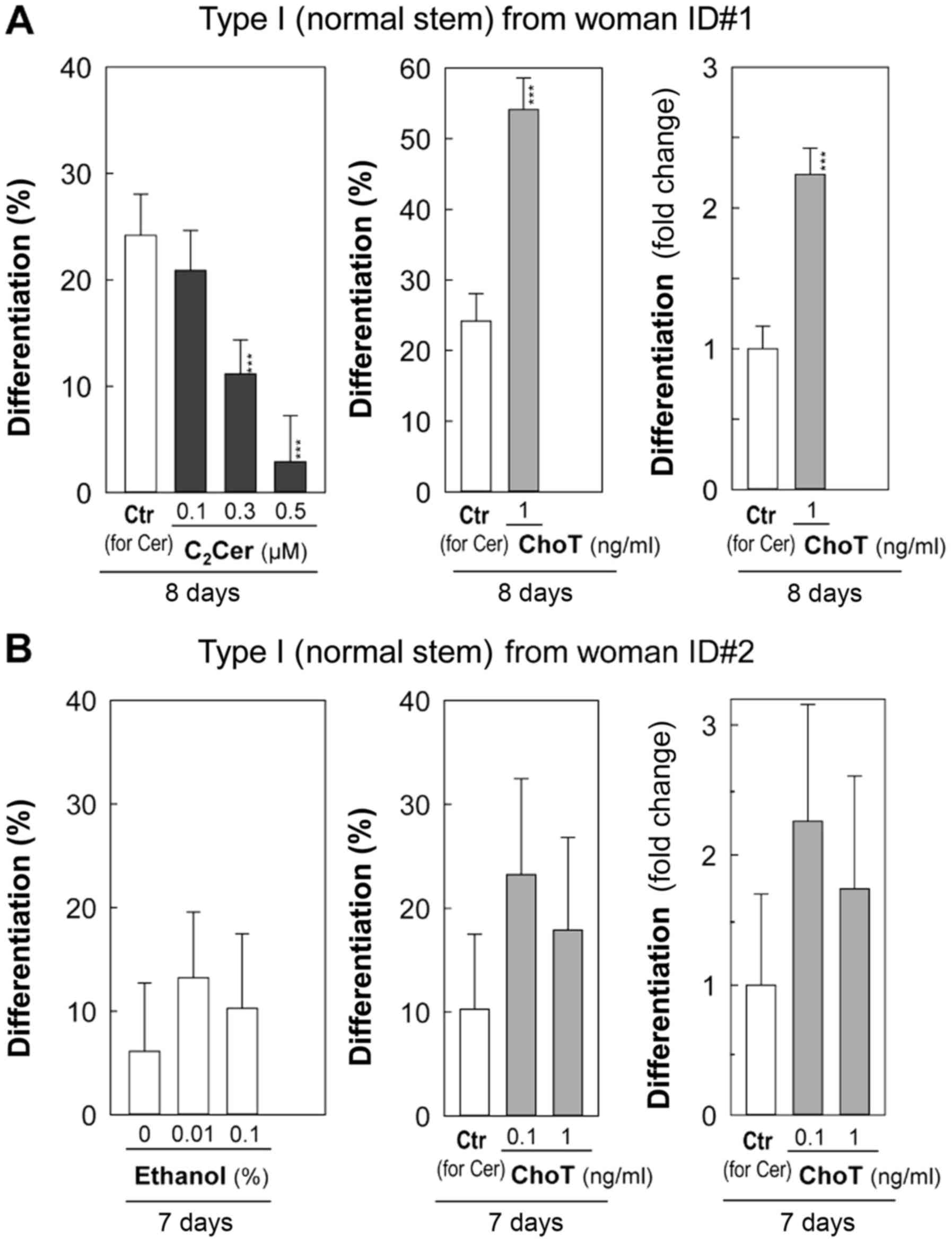
C2-ceramide at 0.1 µM exhibited
little effect on differentiation, while 0.3 and 0.5 µM
inhibited differentiation compared with the vehicle control (0.1%
ethanol) by day 8 (Fig. 6A).
C2-ceramide at 0.5 µM inhibited colony-forming
efficiency, as indicated by a significant decrease in the number of
total colonies (P<5×10−5; Fig. 6A and Table II).
 | Table IIEffects of C2Cer and
ethanol on differentiation of Type I HBECs. |
Table II
Effects of C2Cer and
ethanol on differentiation of Type I HBECs.
| Culture (days) | Treatments | Total Total
colonies | Type II- containing
colonies | % of Type
II-containing colonies (95% CI) | Fold- change
(ChoT/Ctr for Cer) |
|---|
| Type I HBECs from
woman ID#1 | | | | |
| 8 | Ethanol 0.1% (Ctr
for Cer) | 517 | 125 | 24.2% (−3.5%) | |
| ChoT 1 ng/ml | 462 | 250 | 54.1%
(−4.6%)a | ↑ 2.24 |
| C2Cer
0.1 µM | 503 | 105 | 20.9% (−3.3%) | |
| C2Cer
0.3 µM | 466 | 52 | 11.2%
(−2.6%)a | |
| C2Cer
0.5 µM | 138 | 4 | 2.9%
(−1.8%)a | |
| Type I HBECs from
woman ID#2 | | | | |
| 7 | Control (nothing
added) | 98 | 6 | 6.1% (−3.3%) | |
| Ethanol 0.01% | 151 | 20 | 13.3% (−4.5%) | |
| Ethanol 0.1% (Ctr
for Cer) | 107 | 11 | 10.3% (−4.4%) | |
| ChoT 0.1 ng/ml | 99 | 23 | 23.2% (−7.2%) | ↑ 2.26 |
| ChoT 1 ng/ml | 95 | 17 | 17.9% (−6.4%) | ↑ 1.74 |
Since the vehicle control (0.1% ethanol) for
C2-ceramide treatments demonstrated a higher level of
differentiation (Fig. 6A: Woman
ID#1) compared with the vehicle control (0.1% BSA) for sphingosine
treatments (Fig. 5), an additional
independent experiment was conducted (Fig. 6B: Woman ID#2) to determine the
effects of ethanol on the differentiation of Type I HBECs developed
from reduction mammoplasty tissues of a different healthy
(cancer-free) woman. Cholera toxin at 0.1 and 1 ng/ml was tested as
a positive differentiation inducer of Type I HBECs (Fig. 6B). Although variations exist in %
differentiation between different women and independent cell
culture experiments, the fold-increases of differentiation induced
by cholera toxin compared with those of controls for ceramide are
comparable between woman ID#1 for day 8 (Fig 6A; Table II) and woman ID#2 for day 7
experiments (Fig. 6B; Table II). This suggests cholera toxin
induced relatively similar levels of differentiation in two
different normal Type I HBECs derived from two different women
(Fig. 6). In this independent
experiment (woman ID#2), percentages of differentiation of Type I
HBECs were slightly increased in the 0.01 and 0.1% ethanol cultures
compared with the corresponding control (nothing added) culture by
day 7, however, these differences were not statistically
significant (Fig. 6B and Table II).
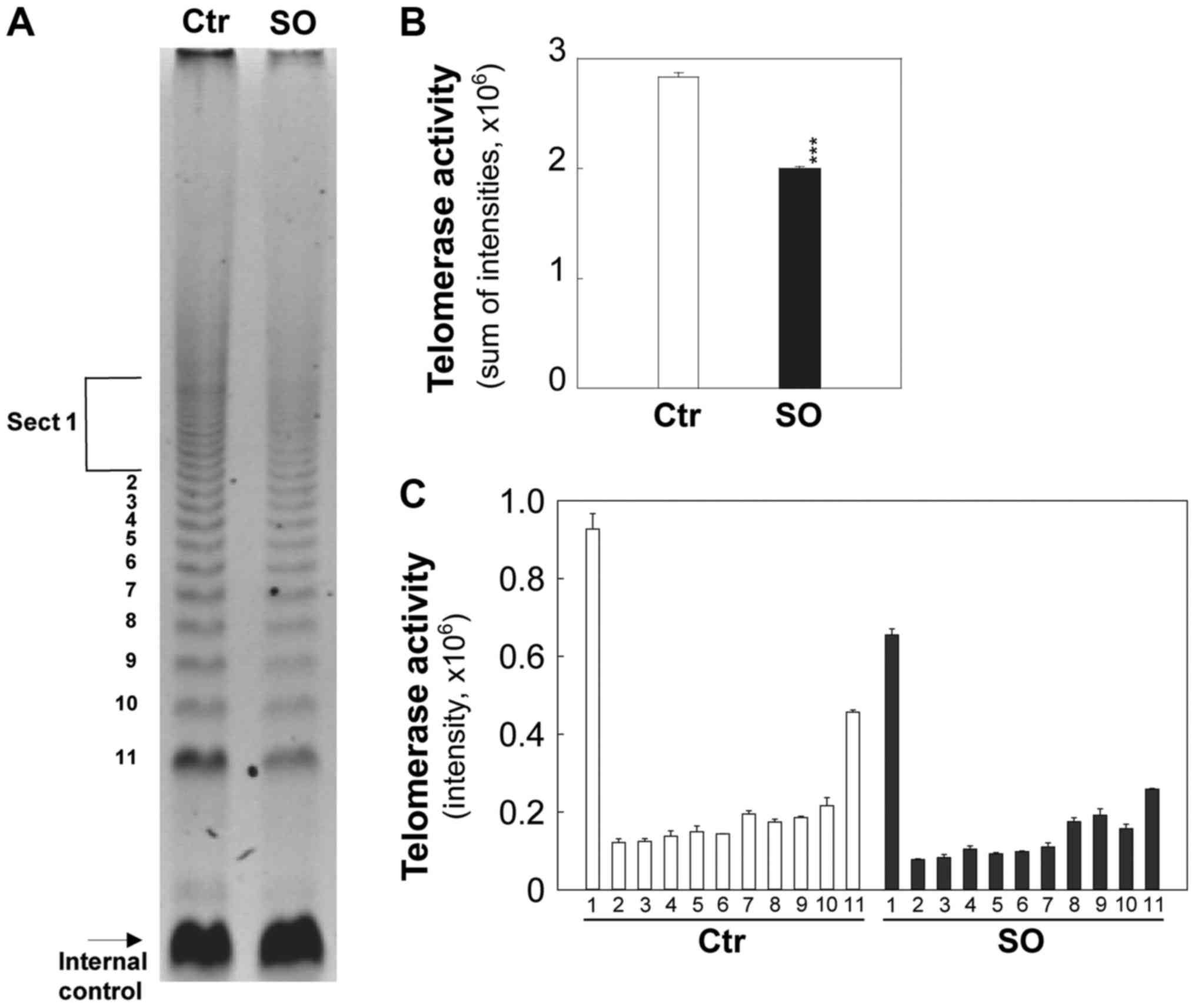
Sphingosine decreases telomerase activity
in tumorigenic cells
The effects of sphingosine on telomerase activity
were examined (Fig. 7A) using a
TRAP assay in tumorigenic cells. In this assay,
telomerase-synthesized extension products were amplified by PCR and
a ladder of products with 6 base increments, starting at 50
nucleotides, indicated positive telomerase activity. An internal
control was included to quantify telomerase activity and identify
false negative results.
Intensities of telomerase-synthesized/PCR-amplified
DNA extension ladders were quantified and presented in Fig. 7B and C. The comparable internal
standard indicates similar amplification efficiency in both control
and sphingosine-treated groups (Fig.
7A). Sphingosine at 5 µM significantly reduced
telomerase activity by ~1.5-fold within 2 days (P<0.001;
Fig. 7) in comparison to the
control.
Discussion
A unique aspect of the current study is that it
evaluated the plausibility of using sphingolipid metabolites as
chemotherapeutic drugs by comparing their effects on the
proliferation and death of tumorigenic breast cells to normal
breast differentiated (Type II) cells. Furthermore, since Type I
HBECs have stem cell characteristics and are more susceptible to
neoplastic transformation (16),
the present study also evaluated the cancer-protective potential of
sphingosine and C2-ceramide by examining their effects
on proliferation, death, and differentiation of Type I HBECs.
A major result of the present study is that
sphingosine preferentially inhibits proliferation and causes death
of normal breast stem cells (Type I HBECs) and tumorigenic cells,
while having little or no effect on the proliferation of normal
differentiated breast cells (Type II HBECs). Type II HBECs were
used as normal cell counterparts of breast cancer cells. The fact
that sphingosine selectively inhibits tumorigenic cells but has
little effect on Type II HBECs implies that sphingosine may be an
ideal therapeutic agent with low potential for side effects.
Furthermore, since apoptosis induced by sphingosine is a
well-regulated process, sphingosine is likely to have the advantage
of targeting cancer cells without eliciting an inflammatory
response in the surrounding normal tissue.
Tumorigenic breast cells, MCF7 breast cancer cells,
and Type I HBECs (normal stem) demonstrated similar sensitivity to
anti-proliferative and pro-apoptotic effects of sphingosine. This
may be because the phenotypes of breast tumorigenic cells
(M13SV1R2N1) and breast cancer cells (e.g. MCF7 and MDA-MB-231)
have been demonstrated to be more similar to those of Type I HBECs
rather than Type II HBECs (10-12).
These similar sensitivities of Type I HBECs, tumorigenic cells, and
MCF7 cancer cells to the effects of sphingosine could be evidence
for the cancer stem cell hypothesis (19).
Ceramides with an N-acyl chain shorter than 8 to 10
carbon atoms are generally called ‘short-chain ceramides’. Since
most naturally occurring ceramides cannot be dispersed in water,
due to their hydrophobicity, short-chain ceramides and, in
particular, C2-ceramide, have been extensively used as
agonists when ceramide effects need to be produced, both in intact
cells and in cell-free systems (20). In the present study, effects of
C2-ceramide were compared with those of sphingosine.
Unlike sphingosine, C2-ceramide does not demonstrate the
selective anti-proliferative effects. C2-ceramide
inhibits proliferation of normal differentiated cells, tumorigenic
cells, and MCF7 cancer cells with similar potency. Although
C2-ceramide seems to be more potent compared with
sphingosine in that 5 µM C2-ceramide causes
similar cytotoxic effects as 8 to 10 µM sphingosine,
C2-ceramide does not appear to be an ideal
chemotherapeutic agent due to its anti-proliferative and cytotoxic
effects on normal differentiated cells (Type II HBECs). Mechanisms
are unclear for nonselective anti-proliferative effects of a
short-chain cell-permeable ceramide, including
C2-ceramide, in normal differentiated cells. Goñi et
al (21) demonstrated that
biophysical properties of short-chain ceramides are affected by
their different N-acyl chain lengths. N-acyl chain length also
affects flip-flop lipid motion (22). Taken together, the presence of
N-acyl chain in C2-ceramide may affect cellular uptake,
membrane permeabilities, or binding specific sites in the target
proteins, which may account for different effects on proliferation
of Type II HBECs in comparison to sphingosine.
The results of the present study indicate that
unnatural D-threo-, L-threo-, and
L-erythro-stereoisomers of sphingosine are more potent
compared with natural D-erythro-sphingosine in inhibiting
proliferation and causing death in breast tumorigenic cells. In
contrast, L-erythro and DL-threo-sphingosine isomers did not
induce apoptosis in RD embryonal rhabdomyosarcoma cell line, while
D-erythro-sphingosine induced apoptosis (23). This suggests that the
stereo-specific efficacy of sphingosine isomers on proliferation or
apoptosis may be influenced by different cellular and genetic
backgrounds of tumor origins, which could affect the uptake and
metabolism of sphingosine and unnatural sphingosine isomers.
Another major result of the present study is that
non-cytotoxic (sub-lethal) concentrations of sphingosine induce
differentiation of Type I (normal stem) to Type II HBECs, thereby
reducing the number of stem cells, which are the target cells of
carcinogenesis. C2-ceramide, however, did not induce
differentiation of Type I to Type II HBECs at non-cytotoxic
concentrations. This suggests that sphingosine can be a potential
cancer-protective agent, whereas C2-ceramide does not
appear to be a cancer-protective agent due to its inability of
inducing the differentiation of Type I to Type II HBECs and its
anti-proliferative effects on Type II HBECs.
The roles of ethanol in proliferation and
differentiation are not clearly understood. The effects of ethanol
may vary depending on concentration, origins of cell types, culture
conditions, or stages of cellular immortalization and neoplastic
transformation. Ethanol enhanced neural differentiation of PC12 rat
pheochromocytoma cells with the involvement of protein kinase C
(24). Ethanol inhibited skeletal
muscle cell proliferation and delays its differentiation in cell
culture (25). Ethanol at 0.3%
stimulated proliferation of MCF7 human breast cancer cells, while
ethanol at higher than 0.3% inhibited the proliferation (26). This stimulation of proliferation by
ethanol was mediated by elevated activity of extracellular
signal-regulated kinase-1/2 in MCF7 cells (26). Ethanol at 10−3 M
increased proliferation and reduced differentiation of head and
neck squamous carcinoma cells (27). In the present study, although it is
not statistically significant, ethanol at 0.01 and 0.1%, which are
lower concentrations than most previous ethanol studies examined,
demonstrated a tendency of inducing differentiation of normal Type
I HBECs. A future study which focuses on ethanol can examine the
effects of ethanol at a broader range of concentrations on normal
stem cells derived from breast tissues of additional subjects to
more firmly establish the roles of ethanol on differentiation.
The mechanism by which sphingosine induces
differentiation of Type I (normal stem) HBECs is not clear. Most
differentiation studies have examined the effects of
sphingosine-1-phosphate (S1P) or various chain length analogs of
ceramide. Less is known about effects of sphingosine on
differentiation of cancer cells or normal cells, particularly on
normal stem cells. Hui et al (28) demonstrated that sphinganine
facilitated the retinoic acid induced differentiation of HL60
promyelocytic leukemia cells. In a study by Ohta et al
(29), differentiation induced by
4 β-phorbol 12-myristate 13-acetate increased cellular sphingosine
levels in HL60 cells. Sphingosine levels in the cells increased
concurrently with the increasing proportion of apoptotic cells
during cell differentiation (29).
Sphingosine treatment induced apoptosis and downregulated
c-myc mRNA expression in HL60 cells (29). This suggests that sphingosine may
induce differentiation by regulating c-myc expression.
Cholera toxin, a well-known inducer of cAMP, induced the
differentiation of Type I to Type II HBECs in the authors' previous
(11,14) and current studies. S1P, which
displays the opposite effects to sphingosine, inhibited
differentiation of placental trophoblasts (30) and adipogenic differentiation of
C3H10T1/2 murine mesenchymal stem cells (31) by reducing intracellular cAMP
accumulation. This suggests that sphingosine
induced-differentiation of Type I HBECs may involve a mechanism
that increases intracellular cAMP.
Telomerase, the ribonucleoprotein reverse
transcriptase that maintains the ends of human chromosomes
(telomeres), is activated in the majority of breast cancer types
but not in normal adjacent tissues (32) and its activity is associated with
poor prognosis and more aggressive phenotypes (33). Therefore, telomerase is an
attractive therapeutic target and can be a useful biomarker for
breast cancer diagnosis and prognosis. In the present study, it was
demonstrated that sphingosine at growth inhibitory concentrations
decreased telomerase activity in breast tumorigenic cells. Several
mechanisms may explain how sphingosine reduces telomerase activity.
First, c-myc has been demonstrated to activate the
transcription of telomerase reverse transcriptase in Epstein Barr
virus-immortalized B cells engineered to express c-MYC-activated or
c-MYC-repressed genes (34).
Second, sphingosine induces apoptosis and down-regulates
c-myc expression in HL60 cells (29). Therefore, sphingosine may reduce
telomerase activity by downregulating c-myc expression
(29). Among protein kinase C
inhibitors tested in human nasopharyngeal cancer cells,
bisindolylma-leimide I and H-7 demonstrated a big inhibition,
staurosporine demonstrated a moderate inhibition, and sphingosine
produced a small inhibition of telomerase activity (35). Sphingosine inhibits protein kinase
C activity in vitro (36)
and in glioma C6 cells (37).
Therefore, sphingosine may suppress telomerase activity by
inhibiting protein kinase C in breast tumorigenic cells. Third, a
close correlation exists between differentiation and the suppressed
activity of telomerase. For example, the inhibition of telomerase
activity was a common response to the induction of differentiation
in HL60, K562, F9, and CCE24 cells by dimethyl sulfoxide, retinoic
acid, vitamin D3, or 12-0-tetradecanoyl-1-phorbol-13-acetate
(38) and during the monocytic or
granulocytic differentiation of HL60 cells by vitamin D3, all-trans
retinoic acid, and Am80 (39).
This suggests that sphingosine may induce the differentiation of
breast tumorigenic cells by inhibiting telomerase activity.
In conclusion, the chemotherapeutic and
cancer-protective properties of sphingosine and
C2-ceramide for breast cancer were evaluated by
examining their effects on proliferation, apoptosis, and
differentiation of normal stem, normal differentiated, and
tumorigenic breast cells. The results indicate that sphingosine: i)
preferentially inhibits proliferation and causes death of Type I
(normal stem) HBECs and tumorigenic breast cells but has little
effect on Type II (normal differentiated) HBECs; ii) induces
apoptosis in breast tumorigenic cells; iii) induces differentiation
of Type I to Type II HBECs at non-cytotoxic concentrations; and iv)
inhibits telomerase activity in breast tumorigenic/cancer cells.
These results suggest that sphingosine has the potential to be used
as a chemotherapeutic and cancer-protective agent for human breast
cancer. C2-ceramide demonstrates comparable
growth-inhibitory effects on proliferation of Type I HBECs and
tumorigenic breast cells by inducing apoptosis. However,
C2-ceramide is cytotoxic to Type II HBECs and fails to
induce differentiation of Type I to Type II HBECs. Therefore,
C2-ceramide does not appear to be an ideal
chemotherapeutic or cancer-protective agent for human breast
cancer.
Funding
The present study was supported by U.S. Army
Medical Research and Material Command under DAMD17-96-1-6099 to
CCC., Michigan State University Agricultural Experiment Station to
JJS., National Institute of Environmental Health Sciences (NIEHS)
sponsored University of Washington Center for Ecogenetics and
Environmental Health and ITHS grant no. NIH/NIEHS P30ES007033 to
EHA and University of Washington Office of Research Royalty
Research Fund grant to EHA.
Availability of data and materials
All data generated or analyzed during this study
are included in this published article.
Authors' contributions
Conceived and designed the experiments: HY, EHA,
CCC, JJS. Performed the experiments: HY, EHA, CYH, WS, CCC.
Analyzed the data: EHA, HY. Contributed reagents/materials/analysis
tools: EHA, HY, CCC, JJS. Wrote the paper: EHA, HY, CCC, JJS.
Ethics approval and consent to
participate
Patients' written consent was obtained in
accordance with institutional guidelines for obtaining breast
tissues during reduction mammoplasty. Procedures were approved by
Sparrow Hospital (Lansing, MI, USA) Institutional Review Board and
Michigan State University Human Research Projection Program.
Patient consent for publication
Patients' written consent was obtained in
accordance with institutional guidelines for obtaining breast
tissues during reduction mammoplasty.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors thank Ms. Susan Kim for graphical
assistance, Mr. Howard Nebeck for proofreading the manuscript, and
Dr Normal Hord for comments on this manuscript.
References
|
1
|
American Cancer Society Cancer: Facts and
Figures 2017. American Cancer Society Inc.; Atlanta, GA: 2017
|
|
2
|
Eheman CR, Shaw KM, Ryerson AB, Miller JW,
Ajani UA and White MC: The changing incidence of in situ and
invasive ductal and lobular breast carcinomas: United States,
1999–2004. Cancer Epidemiol Biomarkers Prev. 18:1763–1769. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Russo J and Russo IH: Biological and
molecular bases of mammary carcinogenesis. Lab Invest. 57:112–137.
1987.PubMed/NCBI
|
|
4
|
Oakes SR, Gallego-Ortega D and Ormandy CJ:
The mammary cellular hierarchy and breast cancer. Cell Mol Life
Sci. 71:4301–4324. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ahn EH and Schroeder JJ: Bioactive
sphingolipids are significant constituents of soy and dairy
products. J Food Sci. 67:522–524. 2002. View Article : Google Scholar
|
|
6
|
Hannun YA and Obeid LM: Principles of
bioactive lipid signalling: Lessons from sphingolipids. Nat Rev Mol
Cell Biol. 9:139–150. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ogretmen B: Sphingolipid metabolism in
cancer signalling and therapy. Nat Rev Cancer. 18:33–50. 2018.
View Article : Google Scholar :
|
|
8
|
Huang YL, Huang WP and Lee H: Roles of
sphingosine 1-phosphate on tumorigenesis. World J Biol Chem.
2:25–34. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tai MH, Chang CC, Kiupel M, Webster JD,
Olson LK and Trosko JE: Oct4 expression in adult human stem cells:
Evidence in support of the stem cell theory of carcinogenesis.
Carcinogenesis. 26:495–502. 2005. View Article : Google Scholar
|
|
10
|
Kang KS, Morita I, Cruz A, Jeon YJ, Trosko
JE and Chang CC: Expression of estrogen receptors in a normal human
breast epithelial cell type with luminal and stem cell
characteristics and its neoplastically transformed cell lines.
Carcinogenesis. 18:251–257. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kao CY, Nomata K, Oakley CS, Welsch CW and
Chang CC: Two types of normal human breast epithelial cells derived
from reduction mammoplasty: Phenotypic characterization and
response to SV40 transfection. Carcinogenesis. 16:531–538. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kao CY, Oakley CS, Welsch CW and Chang CC:
Growth requirements and neoplastic transformation of two types of
normal human breast epithelial cells derived from reduction
mammoplasty. In Vitro Cell Dev Biol Anim. 33:282–288. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chang CC, Sun W, Cruz A, Saitoh M, Tai MH
and Trosko JE: A human breast epithelial cell type with stem cell
characteristics as target cells for carcinogenesis. Radiat Res.
155:201–207. 2001. View Article : Google Scholar
|
|
14
|
Ahn EH, Chang CC and Schroeder JJ:
Evaluation of sphinganine and sphingosine as human breast cancer
chemotherapeutic and chemopreventive agents. Exp Biol Med
(Maywood). 231:1664–1672. 2006. View Article : Google Scholar
|
|
15
|
Kang KS, Sun W, Nomata K, Morita I, Cruz
A, Liu CJ, Trosko JE and Chang CC: Involvement of tyrosine
phosphorylation of p185(c-erbB2/neu) in tumorigenicity induced by
X-rays and the neu oncogene in human breast epithelial cells. Mol
Carcinog. 21:225–233. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun W, Kang KS, Morita I, Trosko JE and
Chang CC: High susceptibility of a human breast epithelial cell
type with stem cell characteristics to telomerase activation and
immortalization. Cancer Res. 59:6118–6123. 1999.
|
|
17
|
Ahn EH and Schroeder JJ: Sphingoid bases
and ceramide induce apoptosis in HT-29 and HCT-116 human colon
cancer cells. Exp Biol Med (Maywood). 227:345–353. 2002b.
View Article : Google Scholar
|
|
18
|
Chang CC: Recent translational research:
Stem cells as the roots of breast cancer. Breast Cancer Res.
8:1032006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kakarala M and Wicha MS: Implications of
the cancer stem-cell hypothesis for breast cancer prevention and
therapy. J Clin Oncol. 26:2813–2820. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Goñi FM and Alonso A: Biophysics of
sphingolipids I. Membrane properties of sphingosine, ceramides and
other simple sphingolipids. Biochim Biophys Acta. 1758:1902–1921.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Goñi FM, Contreras FX, Montes LR, Sot J
and Alonso A: Biophysics (and sociology) of ceramides. Biochem Soc
Symp. 72:177–188. 2005. View Article : Google Scholar
|
|
22
|
Contreras FX, Basañez G, Alonso A,
Herrmann A and Goñi FM: Asymmetric addition of ceramides but not
dihydroceramides promotes transbilayer (flip-flop) lipid motion in
membranes. Biophys J. 88:348–359. 2005. View Article : Google Scholar
|
|
23
|
Phillips DC, Martin S, Doyle BT and
Houghton JA: Sphingosine-induced apoptosis in rhabdomyosarcoma cell
lines is dependent on pre-mitochondrial Bax activation and
post-mitochondrial caspases. Cancer Res. 67:756–764. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Messing RO: Ethanol as an enhancer of
neural differentiation. Alcohol Alcohol Suppl. 2:289–293.
1993.PubMed/NCBI
|
|
25
|
Garriga J, Adanero E, Fernández-Solá J,
Urbano-Márquez A and Cussó R: Ethanol inhibits skeletal muscle cell
proliferation and delays its differentiation in cell culture.
Alcohol Alcohol. 35:236–241. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Izevbigie EB, Ekunwe SI, Jordan J and
Howard CB: Ethanol modulates the growth of human breast cancer
cells in vitro. Exp Biol Med (Maywood). 227:260–265. 2002.
View Article : Google Scholar
|
|
27
|
Kornfehl J, Temmel A, Formanek M and
Knerer B: Effects of ethanol treatment of proliferation and
differentiation in a head and neck squamous cell carcinoma cell
line. Alcohol Clin Exp Res. 23:1102–1107. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hui EK, Yang YH and Yung BY:
Schedule-dependent sphinganine potentiation of retinoic
acid-induced differentiation, cell growth inhibition, and
nucleophosmin translocation in a human leukemia cell line (HL-60).
Exp Hematol. 20:454–461. 1992.PubMed/NCBI
|
|
29
|
Ohta H, Sweeney EA, Masamune A, Yatomi Y,
Hakomori S and Igarashi Y: Induction of apoptosis by sphingosine in
human leukemic HL-60 cells: A possible endogenous modulator of
apoptotic DNA fragmentation occurring during phorbol ester-induced
differentiation. Cancer Res. 55:691–697. 1995.PubMed/NCBI
|
|
30
|
Johnstone ED, Chan G, Sibley CP, Davidge
ST, Lowen B and Guilbert LJ: Sphingosine-1-phosphate inhibition of
placental trophoblast differentiation through a G(i)-coupled
receptor response. J Lipid Res. 46:1833–1839. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hashimoto Y, Matsuzaki E, Higashi K,
Takahashi-Yanaga F, Takano A, Hirata M and Nishimura F:
Sphingosine-1-phosphate inhibits differentiation of C3H10T1/2 cells
into adipocyte. Mol Cell Biochem. 401:39–47. 2015. View Article : Google Scholar
|
|
32
|
Herbert BS, Wright WE and Shay JW:
Telomerase and breast cancer. Breast Cancer Res. 3:146–149. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kulić A, Plavetić ND, Gamulin S,
Jakić-Razumović J, Vrbanec D and Sirotković-Skerlev M: Telomerase
activity in breast cancer patients: Association with poor prognosis
and more aggressive phenotype. Med Oncol. 33:232016. View Article : Google Scholar
|
|
34
|
Wu KJ, Grandori C, Amacker M, Simon-Vermot
N, Polack A, Lingner J and Dalla-Favera R: Direct activation of
TERT transcription by c-MYC. Nat Genet. 21:220–224. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ku WC, Cheng AJ and Wang TC: Inhibition of
telomerase activity by PKC inhibitors in human nasopharyngeal
cancer cells in culture. Biochem Biophys Res Commun. 241:730–736.
1997. View Article : Google Scholar
|
|
36
|
Hannun YA, Loomis CR, Merrill AH Jr and
Bell RM: Sphingosine inhibition of protein kinase C activity and of
phorbol dibutyrate binding in vitro and in human platelets. J Biol
Chem. 261:12604–12609. 1986.PubMed/NCBI
|
|
37
|
Czajkowski R and Barańska J: Sphingosine
and phorbol ester modulate protein kinase C activity and modify
ATP-evoked calcium mobilization in glioma C6 cells. Biochem Biophys
Res Commun. 260:614–618. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sharma HW, Sokoloski JA, Perez JR, Maltese
JY, Sartorelli AC, Stein CA, Nichols G, Khaled Z, Telang NT and
Narayanan R: Differentiation of immortal cells inhibits telomerase
activity. Proc Natl Acad Sci USA. 92:12343–12346. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yamada O, Ozaki K, Nakatake M, Akiyama M,
Kawauchi K and Matsuoka R: Multistep regulation of telomerase
during differentiation of HL60 cells. J Leukoc Biol. 83:1240–1248.
2008. View Article : Google Scholar : PubMed/NCBI
|