Introduction
Gastric cancer (GC) is among the most lethal
malignancies worldwide, and has a particularly high mortality rate
in East Asian countries (1).
Despite numerous advances in its treatment, the overall survival of
patients with GC remains poor, as the majority are diagnosed at an
advanced stage for which there is a lack of effective therapies
(2). Therefore, there is an urgent
need to investigate the molecular mechanisms underlying gastric
tumourigenesis in order to facilitate the clinical management of GC
and identify novel therapeutic targets for patients with GC.
Cullin 4B (CUL4B) is a scaffold protein responsible
for assembling DDB1, RBX1 and substrate component to form the CRL4B
ubiquitin ligase complexes (3),
contributing to ubiquitin-mediated proteolysis and tumourigenesis
(4,5). Mounting evidence has highlighted the
upregulation and oncogenic potential of CUL4B in various types of
cancer, including hepatocellular carcinoma, lung cancer, colon
cancer and bladder cancer (6-10).
Mechanistically, CUL4B epigenetically represses a series of tumour
suppressors, including insulin-like growth factor-binding protein 3
(IGFBP3), phosphatase and tensin homolog (PTEN) and p16 through
physical interaction with the SIN3A/HDAC and SUV39H1/HP1/DNMT3A
complexes (11,12). Yuan et al demonstrated that
CUL4B positively regulated the Wnt/β-catenin signalling pathway by
repressing Wnt pathway antagonists in liver cancer (13). Moreover, CUL4B can control cell
cycle progression in osteosarcoma cells by targeting p21 for
ubiquitination and degradation (14). To date, the mechanisms underlying
CUL4B upregulation in GC have not yet been fully elucidated,
although CUL4B has recently been shown to be associated with a poor
prognosis and tumour progression in this malignancy (15).
Over the past few decades, a large body of evidence
generated regarding microRNAs (miRNAs or miRs) has unveiled a new
and promising strategy with which to control target gene expression
that may translate into clinical applications. miRNAs
post-transcriptionally regulate the expression of their target
genes by binding to 3′ untranslated regions (3′-UTRs) (16-18).
Of note, we as well as others have demonstrated that aberrantly
expressed miRNAs are closely linked to human malignancies,
including GC (19-21). miR-381 has been shown to inhibit
cell growth and invasion, while a low expression of miR-381 has
been shown to be associated with lymph node metastasis, an advanced
clinical tumour stage and a poor survival (22). miR-489 has been shown to be
downregulated in the GC tissues and cell lines, compared with their
normal counterparts (23).
However, the molecular basis for the regulatory effects of miR-381
and miR-489 targeting CUL4B in GC progression have not yet been
fully elucidated.
The Wnt/β-catenin pathway is dysregulated by
mutations or copy number alterations in multiple human cancers, and
regulates malignant progression, such as cell growth, apoptosis,
migration and invasion. The Wnt/β-catenin pathway is also highly
activated in gastric tumourigenesis. Nevertheless, whether miR-381
and miR-489 participate in the Wnt/β-catenin pathway remains
unclear.
In this study, we discovered miR-381 and miR-489 to
be novel, negative regulators of CUL4B in gastric carcinogenesis.
The expression of miR-381/miR-489 was found to be downregulated and
to inversely correlate with that of CUL4B in GC tissues and cell
lines. The overexpression of miR-381 and miR-489 suppressed cell
proliferation, invasion and epithelial-mesenchymal transition
(EMT). Moreover, we verified that the restoration of CUL4B
expression negated the miRNA-induced suppression of proliferation
and invasion, and this miR-381/489-CUL4B axis was shown to regulate
GC progression through the inactivation of the Wnt/β-catenin
pathway. On the whole, our data reveal the importance of the
miR-381/489-CUL4B axis in gastric carcinogenesis, which may provide
novel insight into the molecular mechanisms underlying GC
development, and may lead to the development of novel therapeutic
strategies in the future.
Materials and methods
Clinical tissue samples
All patients with GC in this study were
treatment-naïve prior to surgery. Primary GC tissues and
corresponding normal gastric tissues were obtained from 20 patients
undergoing surgical resection at the Department of Surgery of the
First Affiliated Hospital of Nanchang University (Nanchang, China)
from December, 2016 to November, 2017. Following resection, the
fresh tissue samples were frozen in liquid nitrogen and stored at
−80°C. The clinicopathological characteristics of the patients were
confirmed by two experienced pathologists and are summarized in
Table I. This study was approved
by the Independent Ethics Committee of the First Affiliated
Hospital of Nanchang University and complied with the Declaration
of Helsinki (Approval no. #021-2017). All patients agreed to
participate in this study and provided written informed
consent.
 | Table IThe clinicopathological
characteristics of the patients with gastric cancer. |
Table I
The clinicopathological
characteristics of the patients with gastric cancer.
| Patient no. | Age (years) | Sex | Tumor size
(cm) | Depth of
invasion | Differentiation
(well, moderate or poorly) | TNM stage | Lymph node
metastasis (N0 or NX) |
|---|
| 1 | 54 | Female | 3×3.1 | T2 | Poorly | III | N3 |
| 2 | 57 | Female | 4×2.5 | T3 | Moderate | II | N0 |
| 3 | 62 | Female | 2×1.5 | T3 | Moderate | I | N2 |
| 4 | 56 | Male | 5.5×4.1 | T3 | Well | II | N0 |
| 5 | 62 | Female | 2.4×2.8 | T2 | Poorly | I | N0 |
| 6 | 57 | Female | 5.8×2.7 | T4 | Poorly | III | N1 |
| 7 | 63 | Male | 2.6×3.2 | T2 | Moderate | III | N3 |
| 8 | 60 | Male | 3.5×2.1 | T3 | Moderate | II | N1 |
| 9 | 51 | Female | 5.9×3.0 | T1 | Well | I | N0 |
| 10 | 66 | Male | 2.1×1.8 | T4 | Poorly | III | N0 |
| 11 | 54 | Male | 4.2×2.0 | T1 | Moderate | I | N0 |
| 12 | 68 | Male | 5.4×2.9 | T1 | Moderate | I | N0 |
| 13 | 77 | Female | 4.6×2.6 | T4 | Moderate | I | N3 |
| 14 | 73 | Male | 2.8×2.2 | T2 | Poorly | II | N1 |
| 15 | 50 | Male | 5.5×3.3 | T1 | Well | I | N0 |
| 16 | 64 | Female | 3.2×2.2 | T1 | Well | I | N0 |
| 17 | 42 | Male | 2.3×2.1 | T4 | Poorly | III | N2 |
| 18 | 70 | Male | 5.1×3.2 | T1 | Moderate | I | N0 |
| 19 | 52 | Male | 2.9×.4 | T4 | Poorly | Ⅳ | N3 |
| 20 | 49 | Male | 5.4×2.7 | T1 | Well | I | N0 |
Cell lines and cell culture
Three GC cell lines (AGS, MGC803 and BGC823), the
normal gastric epithelial cell line, GES-1, and 293 cells were
obtained from Sun Yat-Sen University Cancer Center (Guangzhou,
China), and the GC cell line, SGC7901, was purchased from the
Shanghai Institute of Cell Biology, China Academy of Sciences
(Shanghai, China). The cells were cultured in RPMI-1640 medium
supplemented with 10% fetal bovine serum (FBS) (both from HyClone,
Logan, UT, USA) in a humidified chamber at 37°C in an atmosphere
containing 5% CO2.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
RNA was isolated from fresh human tissues or
harvested cells using TRIzol reagent (Invitrogen/Thermo Fisher
Scientific, Waltham, MA, USA) according to the manufacturer’s
instructions, as previously described (24). Complementary DNA was synthesised
using a PrimeScript RT Reagent kit (Takara, Ohtsu, Japan), and
quantitative PCR (qPCR) was performed using the ABI 7500 Real-Time
Fast PCR System (Applied Biosystems, Foster City, CA, USA) with
SYBR-Green qPCR SuperMix (Invitrogen/Thermo Fisher Scientific).
Primers targeting miR-381, miR-489 and CUL4B were purchased from
GenePharm (Shanghai, China). U6 or GAPDH were used as internal
controls, and all results were calculated using the
2−ΔΔCq method (25).
The primer sequences are presented in Table II.
 | Table IIPrimer sequences used in RT-qPCR. |
Table II
Primer sequences used in RT-qPCR.
| Gene | Primer
sequence |
|---|
| miR-381 | F:
TGGTACTTAAAGCGAGGTTGC |
| R
GGTCATGCACACACATACCAC |
| miR-489 | F:
ACACTCCAGCTGGGGTGACATCACATA |
| R:
TGGTGTCGTGGAGTCG |
| CUL4B | F:
TGGAAGTTCATTTACCACCAGAGATG |
| R:
TTCTGCTTTTAACACACAGTGTCCTA |
| U6 | F:
CTCGCTTCGGCAGCACA |
| R:
AACGCTTCACGAATTTGCGT |
| GAPDH | F:
GATTCCACCCATGGCAAATTC |
| R:
AGCATCGCCCCACTTGATT |
Western blot analysis
Briefly, the cells were lysed in lysis buffer
consisting 50 mM Tris/HCl (pH 7.5), 150 mM NaCl, 1 mM
dithiothreitol (DTT), 1 mM EDTA, 0.5% Nonidet P-40 (NP-40), 0.2 mM
phenylmethylsulfonyl fluoride (PMSF), 10 µM pepstatin A and
1 mM leupeptin. Equal amounts of clear cell lysates were separated
by 8 or 10% SDS-PAGE polyacrylamide gel electrophoresis on 8 or 10%
gels and electroblotted onto polyvinylidene difluoride membranes
(Bio-Rad Laboratories, Hercules, CA, USA). The membranes were
blocked with a 5% milk solution at room temperature, before being
incubated at 4°C with the following primary antibodies overnight:
CUL4B (diluted 1:1,000; 60151-1-Ig; Proteintech, Rosemont, IL,
USA), β-catenin (1:1,000; #9562), cyclin D1 (1:1,000; #2978), c-Myc
(1:1,000; #5605) (all from Cell Signaling Technology, Danvers, MA,
USA), E-cadherin (1:1,000; ab1416), N-cadherin (1:1,000; ab18203),
Vimentin (1:1,500; ab8978), β-actin (1:2,000; ab119716) (all from
Abcam, Cambridge, MA, USA) and GAPDH (1:2,000; 14C10; Santa Cruz
Biotechnology, Dallas, TX, USA). The following day, the membranes
were incubated with corresponding horseradish peroxidase-conjugated
secondary antibodies (anti-rabbit or anti-mouse; Cell Signaling
Technology; #7074, 1:3,000 and #7076, 1:4,000) for 1 h at room
temperature. The signal was detected with super sensitive regent
(Thermo Fisher Scientific), and β-actin or GAPDH was used as an
internal control. Quantification of the protein bands were analysed
using ImageJ software (Rawak Software, Stuttgart, Germany).
Cell transfection, lentiviral
transduction and stable cell lines
miR-381 and miR-489 mimics and the corresponding
negative control, CUL4B small interfering RNA (siRNA), control
siRNA, lentiviral vectors of hsa-miR-381, hsa-miR-489 and
hsa-miR-negative control were purchased from GenePharm. The siRNA
sequences were as follows: siRNA-NC, 5′-UUCUCC GAACGUGUCACGUTT-3′;
and siRNA-CUL4B, 5′-CCACCC AGAAGUCAUUAAUTT-3′. The transfection of
oligonucleotides into target cells was performed using TurboFect
reagent (Thermo Fisher Scientific) according to the manufacturer’s
instructions. Certain cells, as indicated, were transduced using
lentivirus-containing supernatant for 24 h in the presence of 2.5
µg/ml polybrene. Following infection, puromycin selection
was used to establish stable cell lines. An expression vector
carrying a CUL4B sequence lacking the 3′-UTR (CUL4B-no UTR) was
constructed by inserting the coding sequence into the psiCHECK-2
vector (Promega, Madison, WI, USA). RT-qPCR and western blot
analysis were performed to confirm successful transfection. The
data shown were derived from triplicate samples.
Dual-luciferase reporter assay
The identification of upstream regulatory miRNAs
targeting CUL4B was performed using two independent miRNA
databases: miRanda (http://www.microrna.org/) and TargetScan (http://www.targetscan.org). The full-length 3′-UTR of
the CUL4B gene and a variant sequence were amplified by PCR and
cloned into the pGL3 luciferase reporter vector (Promega). The
wild-type plasmid (CUL4B-Wt-3′-UTR) contained the full-length CUL4B
3′-UTR, including the sequences complementary to miR-381 and
miR-489. The mutant plasmids (CUL4B-Mut-3′-UTR) carried variant
3′-UTR sequences created by replacing ‘CUUGUAU’ with ‘GAACAUA’ in
the sequence complementary to miR-381 or ‘UAUGAUGU’ with ‘ACACUACA’
in that complementary to miR-489. The luciferase reporter assay was
performed as previously described. 293 cells were seeded at
2×105 cells per well in 12-well plates and transiently
co-transfected with 1 µg reporter plasmid and 100 nM miR-381
mimic, miR-489 mimic, or negative control using TurboFect reagent
for 48 h. Luciferase activity was measured using a Dual-Luciferase
Reporter Assay kit (E1910; Promega) according to the manufacturer’s
instructions. Renilla luciferase activity was used for
normalization, and all experiments included 3 biological
replicates.
Cell Counting kit-8 (CCK-8) assay
To assess long-term cell viability, a CCK-8 assay
(Dojindo Molecular Technologies, Rockville, MD, USA) was used
according to the manufacturer’s instructions as previously
described (26). Following
transfection for 24 h, the cells were seeded in 96-well plates.
Cell viability was measured by the addition of WST-8 at a final
concentration of 10% and measuring the absorbance of samples at 450
nm in a microplate reader (SpectraMax M5e; Molecular Devices,
Sunnyvale, CA, USA) every 24 h for 5 days. The data was derived
from triplicate samples and are presetned as means ± standard
errors of the mean (SEM).
Colony formation assay
The colony formation assay was carried out as
previously described (24). The
MGC803 and BGC823 cells (500 per well) were seeded in a 60-mm dish
24 h post-transfection and cultured in RPMI-1640 medium containing
10% FBS for 10 days. Colonies comprising >50 cells were then
fixed with 10% formaldehyde for 30 min and stained with 8.0%
crystal violet (#R40052; Thermo Fisher Scientific) for a further 30
min at room temperature. Each experiment was repeated 3 times.
Wound healing assay
To evaluate cell motility, the indicated cells, at a
density of 4.5×105 per well, were seeded in 6-well
plates and serum-starved for 24 h once fully confluent. A sterile
200-µl pipette tip was then used to scratch the culture to
form a wound. The cells were subsequently carefully washed with
phosphate-buffered saline and cultured in serum-free medium. Images
were captured at 0 and 48 h after scratching to evaluate wound
closure under a microscope (#CKX41; Olympus America, Inc., Center
Valley, MA, USA). Percentage wound closure was calculated by
comparing the wound area at a given time to that at 0 h. These
experiments were performed at least 3 times.
Transwell assay
The Transwell assay was performed as described
previously (26). For the
migration assay, the indicated cells were seeded in the upper
chambers of Transwell inserts with non-coated membranes
(8-µm pore size; BD Biosciences, Franklin Lakes, NJ, USA).
For the invasion assay, cells were suspended in 200 µl
serum-free medium and seeded in the upper chambers of Transwell
inserts pre-coated with Matrigel. Medium (500 µl) containing
15% FBS was added to the lower chambers as a chemoattractant.
Following 72 h of incubation at 37°C, cells that had invaded the
bottom surface of the membranes were fixed and stained with 10%
crystal violet at room temperature. These experiments were
performed using 3 biological replicates.
Mouse xenograft experiments
A total of 24 (6 weeks old; weighing, 18-21 g)
female NOD SCID mice were purchased from Jackson Laboratories (Bar
Harbor, ME, USA). Mice were supplied with free sterilized water and
food at 22±2°C with 40-70% humidity. The mice were randomly
allocated to 1 of 4 groups (6 mice in each) and inoculated with
5×106 MGC803 cells stably expressing miR-negative
control, hsa-miR-381, or hsa-miR-489 by subcutaneous injection into
both anterior flanks. Tumour growth in two dimensions was monitored
every 3 days using electronic digital callipers (Thermo Fisher
Scientific). Tumour volume was calculated with the following
formula: Tumour volume (mm3) = (length ×
width2)/2. The mice were euthanised, and tumours were
harvested and weighed. All animal experiments were approved by the
Animal Ethics Committee of Nanchang University. The maximum tumour
diameter allowed per tumour was 1.5 cm.
Statistical analysis
Statistical analysis was performed using SPSS 17.0
software (SPSS Inc., Chicago, IL, USA). All data are presented as
the means ± SEM, and each experiment was repeated at least 3 times.
Comparisons between 2 groups were made using a Student’s t-test,
while one-way ANOVA with a post hoc Dunnett or Bonferroni
correction test was used when analysing >2 groups. The
association between miRNA expression and CUL4B protein expression
was analysed by Pearson’s correlation coefficient. A P-value
<0.05 was considered to indicate a statistically significant
difference.
Results
Expression of CUL4B negatively correlates
with that of miR-381 and miR-489 in GC tissues and cell lines
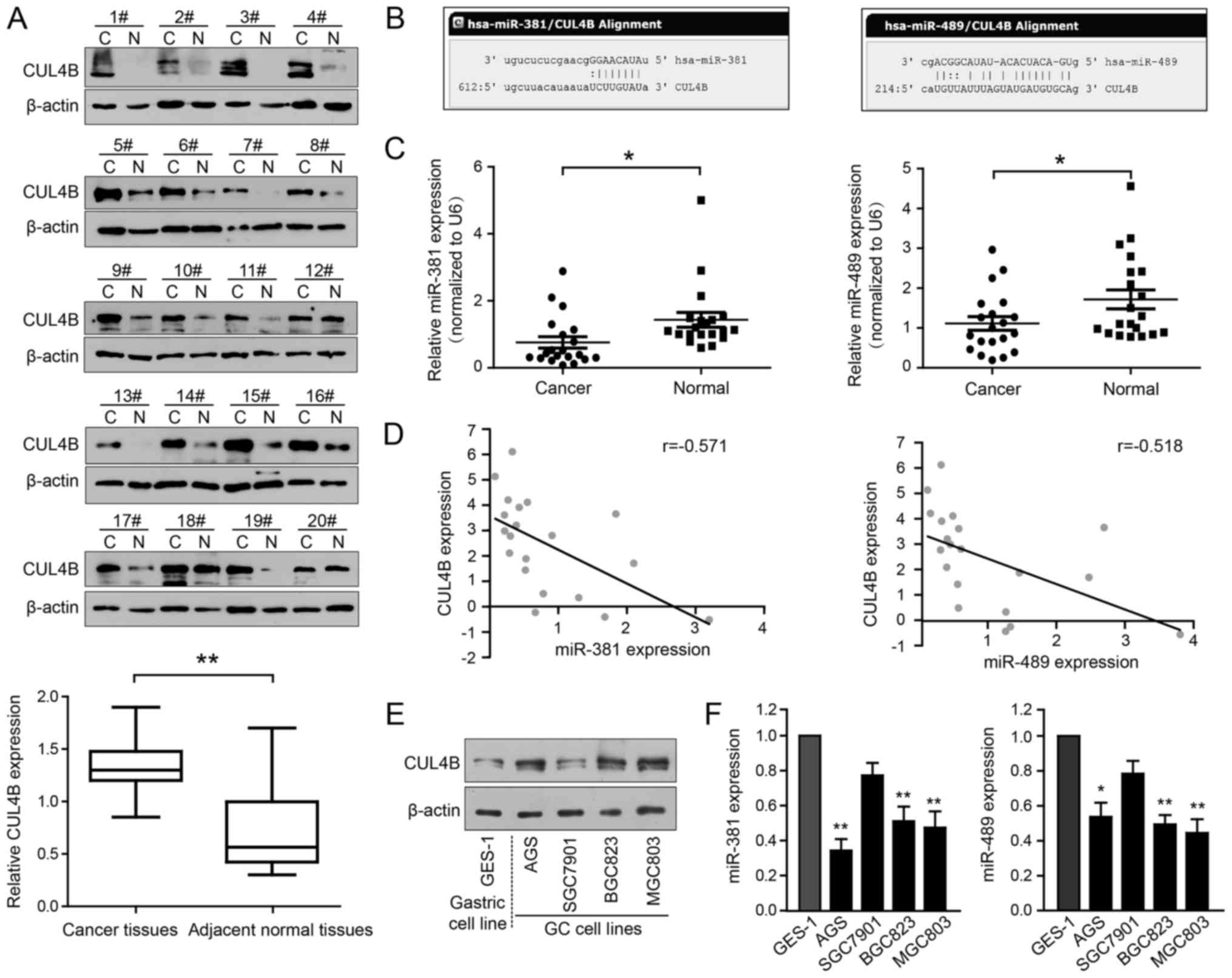
Our preliminary immunohistochemical data revealed
that CUL4B was upregulated in GC tissues compared with paired
adjacent normal gastric tissues (data not shown). In the present
study, we found that 15 of the 20 GC samples (75%) examined
exhibited a higher CUL4B protein expression than the matched
adjacent non-cancerous gastric tissues (Fig. 1A; P<0.01). To investigate the
underlying mechanisms of CUL4B upregulation, we searched for
potential miRNAs that target CUL4B using bioinformatics analysis
(http://www.microrna.org/microrna/home.do). We screened
out several miRNAs that may regulate CUL4B expression according to
the SVR score and conserved status. Moreover, with TargetScan
(www.Targetscan.org) for the second
screening, miR-381 and miR-489 attracted our attention. The reasons
for the selection of miR-381 and miR-489 for further investigation
in this study were as follows: i) Two algorithms both predicted
that miR-381 and miR-489 were potential regulatory miRNAs that
could target CUL4B; and ii) these two miRNAs have been reported to
be involved in cancer progression (27-29).
As shown in Fig. 1B, potential
miR-381 and miR-489 target sites are present in the CUL4B 3′-UTR.
As the expression patterns of miRNAs are often the opposite of
those of their target genes, we examined miR-381 and miR-489
expression in the above-mentioned 20 tissue sample pairs by
RT-qPCR. As clearly illustrated in Fig. 1C, the miR-381 and miR-489 levels
were consistently downregulated in the GC tissues compared with the
non-cancerous controls. Correlation analysis indicated that the
expression of miR-381 and miR-489 was negatively correlated with
CUL4B protein expression in the 20 clinical GC samples (Fig. 1D; r=−0.571 and r=−0.518,
respectively; P<0.01). The expression of these miRNAs and CUL4B
was also examined in the AGS, BGC823, MGC803 and SGC7901 human GC
cells and in GES-1 normal gastric epithelial cells. Of note, CUL4B
protein expression was much higher in the AGS, BGC823 and MGC803
cells, but lower in the SGC7901 cells, and was inversely associated
with miR-381 and miR-489 expression (Fig. 1E and F). Collectively, these data
suggest that the downregulation of miR-381 and miR-489 may play an
important role in the pathogenesis of GC by affecting CUL4B
expression.
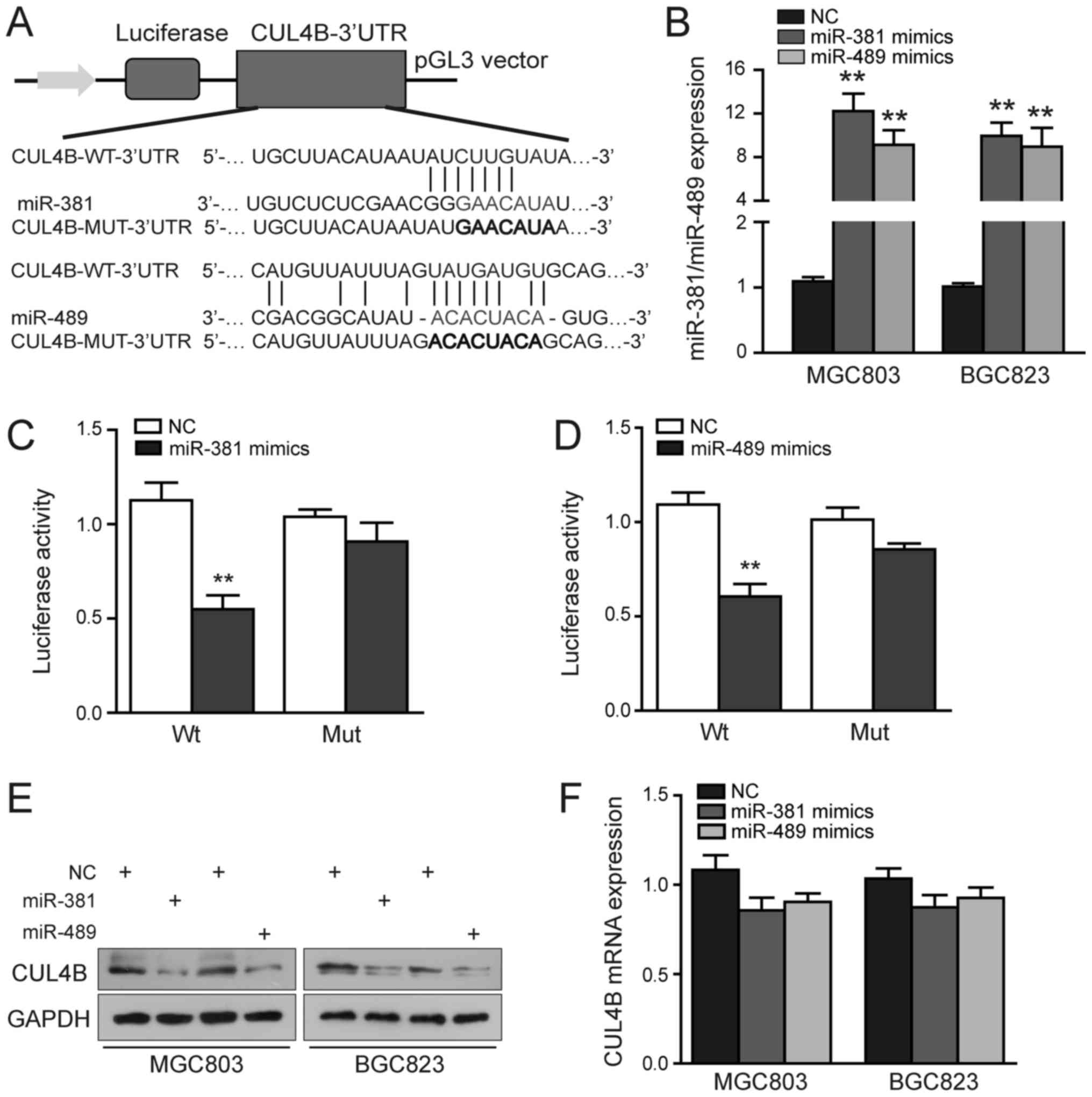
CUL4B is a direct target of miR-381 and
miR-489
To determine whether CUL4B is a direct target of
miR-381 and miR-489 in GC, fragments of its 3′-UTR containing the
predicted binding sites of these miRNAs or variants of these
sequences were separately subcloned into the pGL3 vector (Fig. 2A). Luciferase reporter assays were
then performed to verify the binding of the CUL4B 3′-UTR by these
two miRNAs. As depicted in Fig.
2B, the miR-381 and miR-489 levels were significantly increased
in the MGC803 and BGC823 cells upon transfection. We also found
that the ectopic expression of miR-381 or miR-489 exerted a
significant inhibitory effect on the luciferase activity of the
CUL4B-Wt-3′-UTR plasmid in the 293 cells, but had no marked effect
on that of the CUL4B-Mut-3′-UTR (P<0.01; Fig. 2C and D). As shown in Fig. 2E and F, miR-381 or miR-489
upregulation decreased CUL4B protein, but not mRNA expression in
the MGC803 and BGC823 cells, suggesting that these miRNAs regulate
CUL4B levels principally through translational repression.
Collectively, these results strongly support the hypothesis that
CUL4B is a direct target of miR-381/miR-489, and that they
specifically and directly suppress CUL4B expression by binding to
its 3′-UTR.
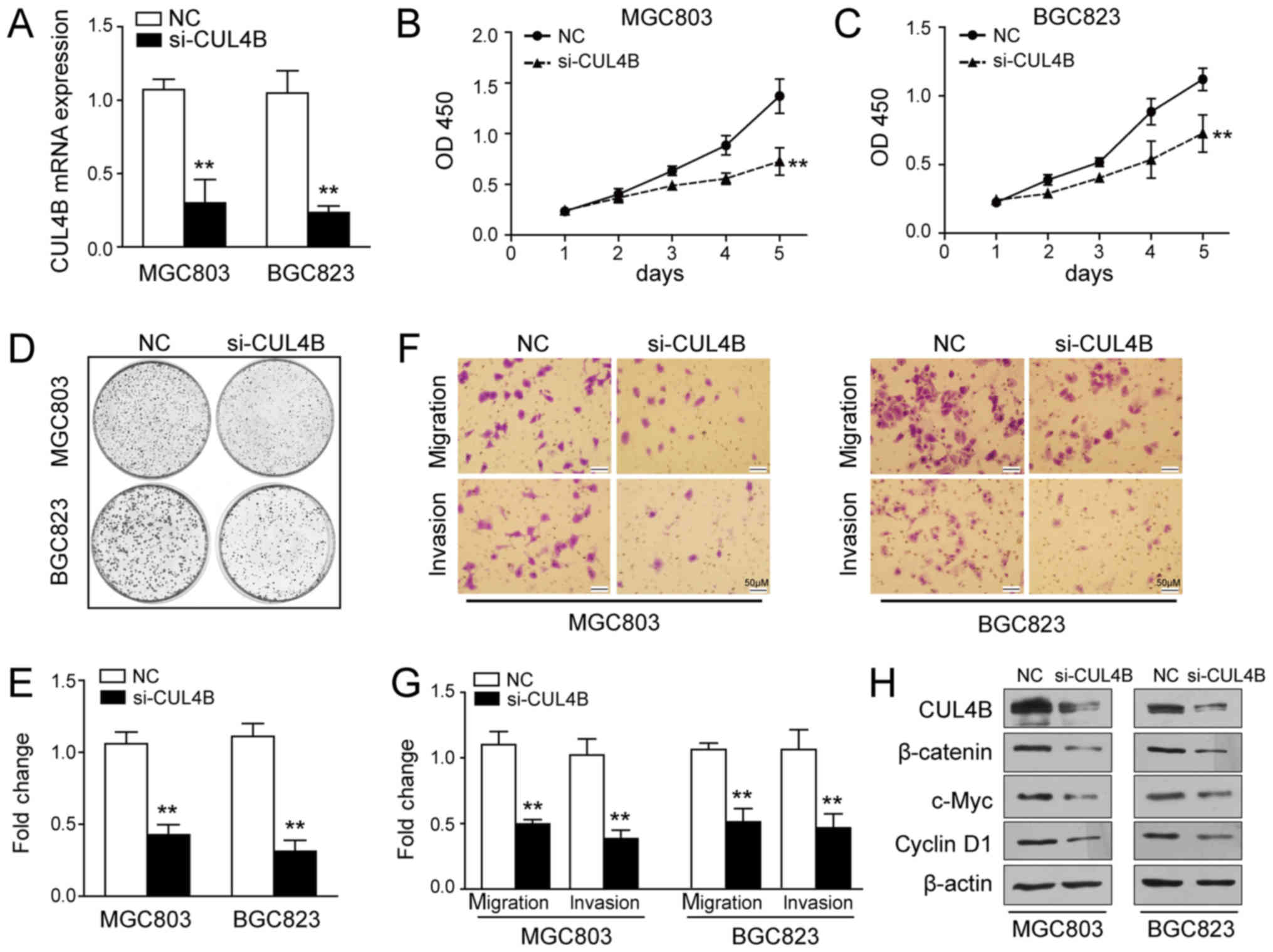
CUL4B silencing inhibits the
proliferation, migration and invasion of GC cells by inactivating
the Wnt/β-catenin pathway
To determine the functional effects of CUL4B on GC
cells, we examined cellular phenotypes following its knockdown
using siRNA. Successful knockdown was confirmed by RT-qPCR
(Fig. 3A) and western blot
analysis (Fig. 3H). As clearly
shown in Fig. 3B and C, the
silencing of CUL4B suppressed GC cell growth. Consistent with this
finding, a significant decrease in the colony formation ability was
detected in the cells in which CUL4B was knocked down when compared
with the negative control (P<0.01; Fig. 3D and E). Moreover, CUL4B silencing
markedly diminished the migratory and invasive abilities of the
MGC803 and BGC823 cells (P<0.01; Fig. 3F and G). In previous studies, CUL4B
was found to positively regulate the Wnt/β-catenin pathway in
pancreatic cancer and hepatocellular carcinoma, and the abnormal
activation of this pathway has been shown to be involved in
tumourigenesis (13,30). Therefore, in this study, we
examined whether Wnt/β-catenin signalling was affected by the
silencing of CUL4B. As shown in Fig.
3H, the expression of β-catenin, c-Myc and cyclin D1 was
downregulated in the MGC803 and BGC823 cells transfected with CUL4B
siRNA. These data suggest that the silencing of CUL4B exerts a
marked tumour suppressive effect on GC tumourigenesis via the
inactivation of the Wnt/β-catenin pathway.
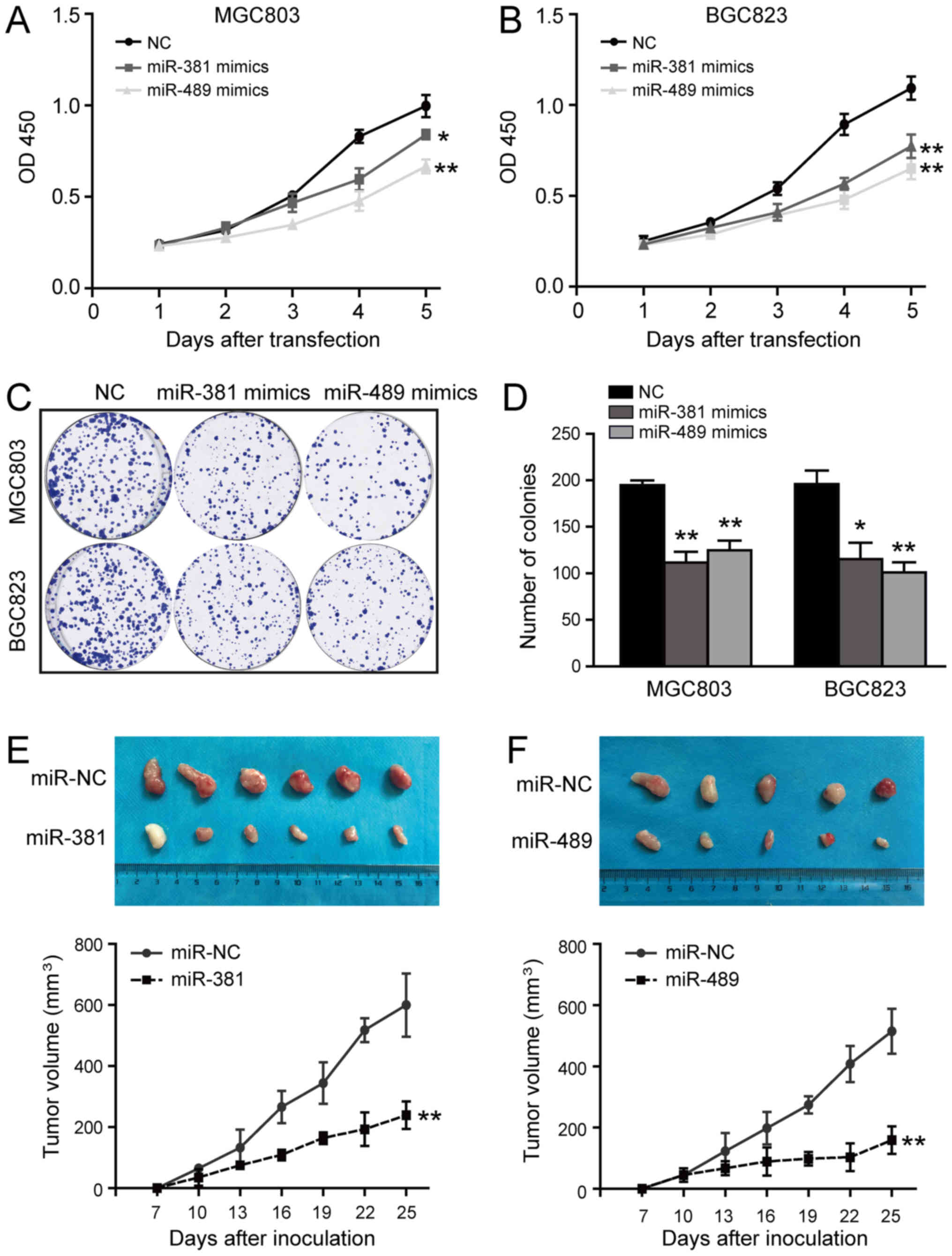
miR-381 and miR-489 retard GC growth in
vitro and in vivo
Given that miR-381 and miR-489 are frequently
downregulated in GC (22,23), we expressed these miRNAs
ectopically to explore their biological functions. As illustrated
in Fig. 4A and B, miR-381 and
miR-489 over-expression reduced cell viability. Consistent with
this, we observed a significant reduction in the number of colonies
formed by the miRNA transfectants compared with the negative
control (Fig. 4C and D).
Subsequently, to determine whether these miRNAs can inhibit tumour
growth in vivo, the MGC803 cells were infected with
lentivirus expressing miR-381, miR-489, or negative control miRNA,
and puro-mycin selection was used to establish lines stably
expressing these constructs. These cells (5×106 per
mouse) were then injected into the anterior flanks of nude mice.
Compared with the miR-381 group, the miR-NC group developed
significantly larger tumours (P<0.01; Fig. 4E). Similarly, miR-489
overexpression resulted in the formation of markedly smaller tumour
nodules and attenuated the growth of tumour xenografts in the nude
mice (P<0.01; Fig. 4F). Taken
together, these results clearly suggest that the ectopic expression
of miR-381/miR-489 strongly delays GC cell growth in vitro
and in vivo.
miR-381 and miR-489 negatively regulate
cell migration, invasion, and EMT of GC cells
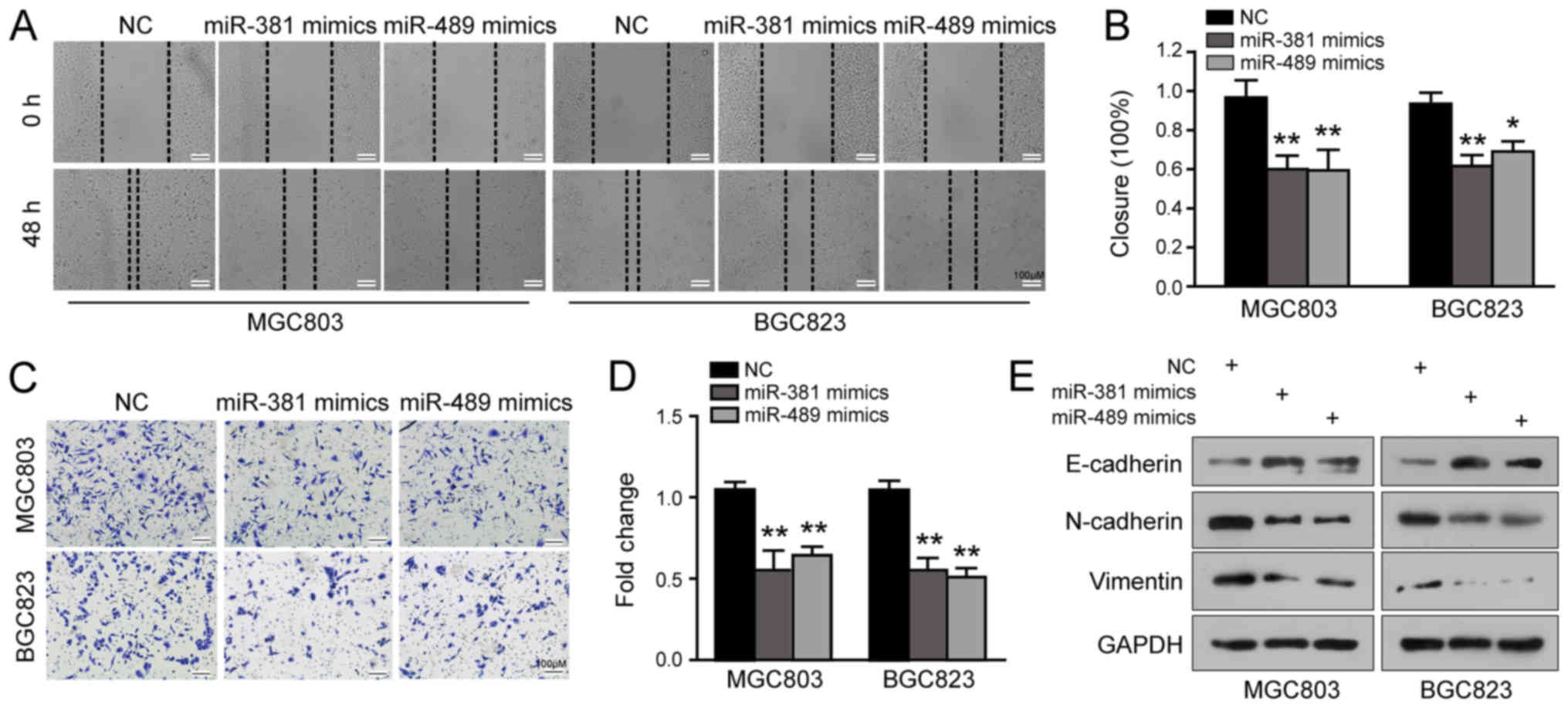
We then evaluated the effects of miR-381 and miR-489
on GC cell migration and invasion using wound healing and Transwell
invasion assays. The results of the wound healing assay revealed
that the cells overexpressing miR-381/miR-489 took a much longer
time to close the scratch wound than the controls (P<0.05;
Fig. 5A and B). Moreover, miR-381
or miR-489 overexpression significantly suppressed the invasive
abilities of the MGC803 and BGC823 cells (P<0.01; Fig. 5C and D). EMT is a critical process
contributing to the initiation of cancer cell invasion; therefore,
we also examined EMT markers in these two cell lines following
miR-381 or miR-489 upregulation. Surprisingly, the levels of
E-cadherin were increased and those of N-cadherin and Vimentin were
reduced due to the elevated expression of miR-381 or miR-489
(Fig. 5E). Furthermore, the
combination of miR-381 and miR-489 overexpression suppressed cell
growth, migration and invasion more markedly (data not shown). The
simultaneous overexpression of miR-381 and miR-489 exerted a more
potent tumour-suppressive effect on the GC cells (data not shown).
These data indicate a critical role of miR-381/miR-489 in cell
migration, invasion and EMT, apart from cell proliferation.
CUL4B overexpression counteracts the
phenotypic effects of miR-381 and miR-489
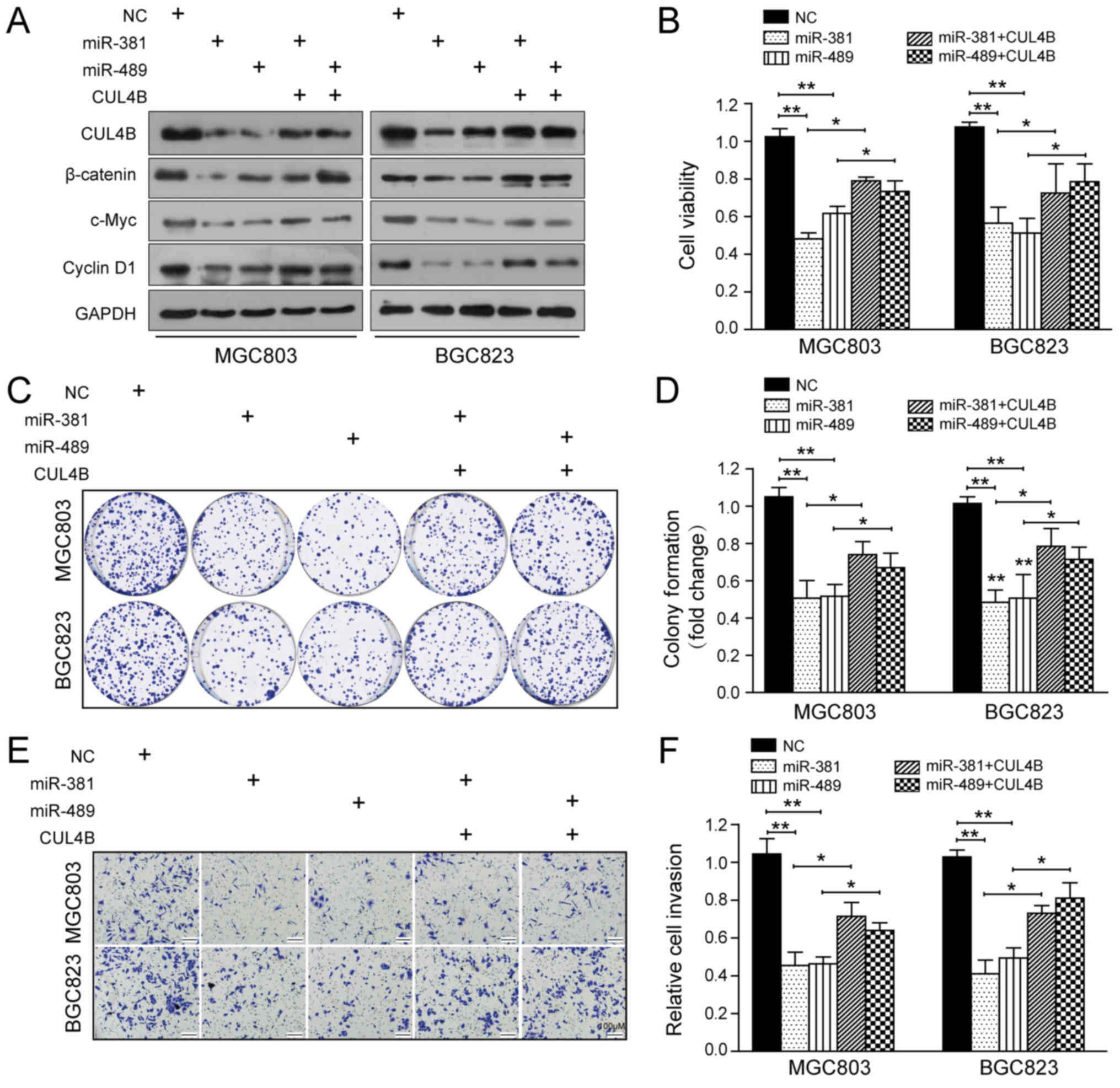
To further confirm that the functional effects of
miR-381 and miR-489 in GC are mediated by the inhibition of CUL4B,
we overexpressed a CUL4B sequence lacking the 3′-UTR in the
miRNA-transfected cells. As shown in Fig. 6A, the restoration of CUL4B
expression partially negated the effects of miR-381 and miR-489 on
the protein levels of CUL4B and components of the Wnt/β-catenin
pathway. Furthermore, CCK-8, colony formation and Transwell
invasion assays demonstrated that the restoration of CUL4B
expression also counteracted the inhibitory effects of miR-381 and
miR-489 on GC cell proliferation and invasion (Fig. 6B-F). Taken together, these results
further demonstrate that the altered CUL4B expression is a main
mediating factor in the functions of both miR-381 and miR-489.
Discussion
It has been well established that CUL4B is involved
in the initiation and development of human malignancies, as it
contributes to tumour progression by inducing cell proliferation,
invasion and metastasis (31,32).
In the current study, we demonstrated that CUL4B was upregulated in
GC tissues and cell lines, suggesting that it plays a role in GC
tumourigenesis. Moreover, we identified the downregulation of
miR-381 and miR-489 as a novel mechanism responsible, at least in
part, for CUL4B overexpression in GC and the consequent activation
of the Wnt/β-catenin pathway.
There is much evidence of a critical role for miRNAs
in gastric tumourigenesis via the modulation of cell proliferation,
invasion, EMT and chemoresistance (33-35).
Previous studies have indicated that some members of the Cullin
protein family may be targeted by miRNAs (26,36).
Moreover, CUL4B and miR-194 have been found to regulate each other
directly in a coordinated manner (10). In the present study, we identified
miR-381 and miR-489 as potential regulators of CUL4B using
computational prediction tools. We also noted that the expression
of these miRNAs was downregulated and inversely correlated with
that of CUL4B in GC tumour samples and cell lines. More credibly,
CUL4B was confirmed as a direct target gene of both miR-381 and
miR-489 by western blot analysis, RT-qPCR and luciferase reporter
assay. These data further uncovered a potentially critical role of
miR-381 and miR-489 in the suppression of GC tumourigenesis,
leading to CUL4B overexpression.
The altered expression of miR-381 and miR-489 has
been strongly implicated in human tumourigenesis and cancer
progression. However, the reported expression patterns and roles of
miR-381 in various types of cancer markedly differ. miR-381 has
been shown to be downregulated in malignancies and exerts tumour
suppressive effects on colon, breast and liver cancer and pituitary
tumours (27,37-39).
In lung cancer, this miRNA has been shown to inhibit cell migration
and invasion by targeting LRH-1, where it acts as a prognostic
marker (40). However, miR-381 may
even function as an oncogenic miRNA in certain other malignancies,
including glioma and osteosarcoma (41,42).
miR-489 has also been identified as a tumour suppressor miRNA in
multiple types of cancer, such as breast, ovarian, colorectal and
non-small cell lung cancer (43-46).
Recently, it has been reported in two different studies that
miR-381 and miR-489 inhibit GC growth and metastasis by targeting
TMEM16A and PROX1 (22,23), respectively. In the present study,
we further expanded the function of miR-381 and miR-489 in GC. We
revealed that these two miRNAs are critical for cell proliferation,
invasion, and the EMT process (Figs.
4 and 5), which recapitulates
the effects stimulated by CUL4B silencing (Fig. 3). Of note, the combined
overexpression of miR-381 and miR-489 suppressed GC cell growth,
migration and invasion more markedly due to a greater inhibition of
CUL4B protein expression (data not shown). For more conclusive
results, we performed rescue experiments by overexpressing CUL4B in
GC cells and evaluated the outcomes using CCK-8, colony formation,
and Transwell invasion assays. Surprisingly, re-expression of CUL4B
partially attenuated the effects of miR-381 and miR-489 on GC cell
proliferation and invasion (Fig.
6). These data provide direct evidence that miR-381/miR-489
inhibit GC progression by suppressing CUL4B expression. In
addition, it is worth noting that the ectopic expression of CUL4B
did not completely abrogate the observed miRNA-induced effects,
suggesting that other downstream targets may also contribute to the
functions of miR-381 and miR-489.
The involvement of Wnt/β-catenin signalling in a
wide variety of cellular processes, such as growth,
differentiation, invasion and tumourigenesis (47), has been well documented. Our data,
along with those of others, have revealed CUL4B to be a positive
regulator of the Wnt/β-catenin pathway (9,48).
In this study, based on our finding that the behaviour of GC cells
was affected by miR-381, miR-489 and CUL4B expression, we examined
whether the Wnt/β-catenin pathway was also involved in this
interplay. Our results revealed that miR-381 or miR-489
overexpression markedly decreased the expression of β-catenin,
c-Myc, and cyclin D1, suggesting that these miRNAs act as negative
regulators of Wnt/β-catenin signalling. Indeed, a previous study on
renal cancer indicated that the gene encoding β-catenin is a target
of miR-381 (49), although the
association between miR-489 and the Wnt/β-catenin pathway in
tumourigenesis has not yet been clarified. Furthermore, in this
study, we found that the re-establishment of CUL4B expression also
negated the suppressive effects of miR-381 and miR-489 on
Wnt/β-catenin signalling. Taken together, these data suggest that
miR-381/miR-489 inactivate the Wnt/β-catenin pathway by targeting
CUL4B. Thus, the inactivation of Wnt/β-catenin signalling is also
involved in the anti-tumour functions of miR-381 and miR-489 in
GC.
In conclusion, the data from the present study
demonstrate that the downregulation of miR-381 and miR-489 is
responsible for the aberrant CUL4B overexpression in GC
tumourigenesis. The miR-381/489-CUL4B axis greatly contributes to
the initiation and progression of GC via the Wnt/β-catenin pathway.
Our results not only expand the target pool of miR-381 and miR-489,
but also confirm a direct interaction between CUL4B and these
miRNAs, highlighting the miR-381/489-CUL4B axis as a promising
target for GC therapeutic strategies.
Funding
The study was supported by the National Natural
Science Foundation of China (no. 81660402), and grants from the
Science and Technology Department of Jiangxi Province (nos.
20161ACB21018 and 20171BBH80027), and the Graduate Student
Innovation Foundation of Jiangxi Province, China (YC2015-B010).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article or are available from the
corresponding author on reasonable request.
Authors’ contributions
ZF contributed to the analysis and interpretation of
the data and the drafting of the manuscript. MZ contributed to the
acquisition and analysis of the data. YW and XY contributed to data
analysis and technical support. HG and YY performed the western
blot analysis of the gastric cancer clinical samples. MF and JC
contributed to the acquisition of the data and revised the
manuscript for important intellectual content. JX and XX conceived
and designed, and supervised the study. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Independent Ethics
Committee of the First Affiliated Hospital of Nanchang University
and complied with the Declaration of Helsinki (Approval no.
#021-2017). All patients agreed to participate in this study and
gave written informed consent. All animal experiments were approved
by the Animal Ethics Committee of Nanchang University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wadhwa R, Song S, Lee JS, Yao Y, Wei Q and
Ajani JA: Gastric cancer-molecular and clinical dimensions. Nat Rev
Clin Oncol. 10:643–655. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Petroski MD and Deshaies RJ: Function and
regulation of cullin-RING ubiquitin ligases. Nat Rev Mol Cell Biol.
6:9–20. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yi J, Lu G, Li L, Wang X, Cao L, Lin M,
Zhang S and Shao G: DNA damage-induced activation of CUL4B targets
HUWE1 for proteasomal degradation. Nucleic Acids Res. 43:4579–4590.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Qian Y, Yuan J, Hu H, Yang Q, Li J, Zhang
S, Jiang B, Shao C and Gong Y: The CUL4B/AKT/β-catenin axis
restricts the accumulation of myeloid-derived suppressor cells to
prohibit the establishment of a tumor-permissive microenvironment.
Cancer Res. 75:5070–5083. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Qu Z, Li D, Xu H, Zhang R, Li B, Sun C,
Dong W and Zhang Y: CUL4B, NEDD4, and UGT1As involve in the TGF-β
signalling in hepatocellular carcinoma. Ann Hepatol. 15:568–576.
2016.PubMed/NCBI
|
|
7
|
Mok MT and Cheng AS: CUL4B: A novel
epigenetic driver in Wnt/β-catenin-dependent hepatocarcinogenesis.
J Pathol. 236:1–4. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jiang T, Tang HM, Wu ZH, Chen J, Lu S,
Zhou CZ, Yan DW and Peng ZH: Cullin 4B is a novel prognostic marker
that correlates with colon cancer progression and pathogenesis. Med
Oncol. 30:5342013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mao XW, Xiao JQ, Xu G, Li ZY, Wu HF, Li Y,
Zheng YC and Zhang N: CUL4B promotes bladder cancer metastasis and
induces epithelial-to-mesenchymal transition by activating the
Wnt/β-catenin signaling pathway. Oncotarget. 8:77241–77253. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mi J, Zou Y, Lin X, Lu J, Liu X, Zhao H,
Ye X, Hu H, Jiang B, Han B, et al: Dysregulation of the
miR-194-CUL4B negative feedback loop drives tumorigenesis in
non-small-cell lung carcinoma. Mol Oncol. 11:305–319. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hu H, Yang Y, Ji Q, Zhao W, Jiang B, Liu
R, Yuan J, Liu Q, Li X, Zou Y, et al: CRL4B catalyzes H2AK119
monoubiquitination and coordinates with PRC2 to promote
tumorigenesis. Cancer Cell. 22:781–795. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang Y, Liu R, Qiu R, Zheng Y, Huang W, Hu
H, Ji Q, He H, Shang Y, Gong Y, et al: CRL4B promotes tumorigenesis
by coordinating with SUV39H1/HP1/DNMT3A in DNA meth-ylation-based
epigenetic silencing. Oncogene. 34:104–118. 2015. View Article : Google Scholar
|
|
13
|
Yuan J, Han B, Hu H, Qian Y, Liu Z, Wei Z,
Liang X, Jiang B, Shao C and Gong Y: CUL4B activates Wnt/β-catenin
signalling in hepatocellular carcinoma by repressing Wnt
antagonists. J Pathol. 235:784–795. 2015. View Article : Google Scholar
|
|
14
|
Chen Z, Wang K, Hou C, Jiang K, Chen B,
Chen J, Lao L, Qian L, Zhong G, Liu Z, et al: CRL4BDCAF11 E3 ligase
targets p21 for degradation to control cell cycle progression in
human osteosarcoma cells. Sci Rep. 7:11752017. View Article : Google Scholar :
|
|
15
|
Qi M, Jiao M, Li X, Hu J, Wang L, Zou Y,
Zhao M, Zhang R, Liu H, Mi J, et al: CUL4B promotes gastric cancer
invasion and metastasis-involvement of upregulation of HER2.
Oncogene. 37:1075–1085. 2018. View Article : Google Scholar
|
|
16
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hammond SM: An overview of microRNAs. Adv
Drug Deliv Rev. 87:3–14. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lin S and Gregory RI: MicroRNA biogenesis
pathways in cancer. Nat Rev Cancer. 15:321–333. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xiang XJ, Deng J, Liu YW, Wan LY, Feng M,
Chen J and Xiong JP: MiR-1271 Inhibits Cell Proliferation, Invasion
and EMT in Gastric Cancer by Targeting FOXQ1. Cell Physiol Biochem.
36:1382–1394. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xia JT, Chen LZ, Jian WH, Wang KB, Yang
YZ, He WL, He YL, Chen D and Li W: MicroRNA-362 induces cell
proliferation and apoptosis resistance in gastric cancer by
activation of NF-κB signaling. J Transl Med. 12:332014. View Article : Google Scholar
|
|
21
|
Ma DH, Li BS, Liu JJ, Xiao YF, Yong X,
Wang SM, Wu YY, Zhu HB, Wang DX and Yang SM: miR-93-5p/IFNAR1 axis
promotes gastric cancer metastasis through activating the STAT3
signaling pathway. Cancer Lett. 408:23–32. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cao Q, Liu F, Ji K, Liu N, He Y, Zhang W
and Wang L: MicroRNA-381 inhibits the metastasis of gastric cancer
by targeting TMEM16A expression. J Exp Clin Cancer Res. 36:292017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang B, Ji S, Ma F, Ma Q, Lu X and Chen
X: miR-489 acts as a tumor suppressor in human gastric cancer by
targeting PROX1. Am J Cancer Res. 6:2021–2030. 2016.PubMed/NCBI
|
|
24
|
Fang Z, Zhang L, Liao Q, Wang Y, Yu F,
Feng M, Xiang X and Xiong X: Regulation of TRIM24 by miR-511
modulates cell proliferation in gastric cancer. J Exp Clin Cancer
Res. 36:172017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
26
|
Han X, Fang Z, Wang H, Jiao R, Zhou J and
Fang N: CUL4A functions as an oncogene in ovarian cancer and is
directly regulated by miR-494. Biochem Biophys Res Commun.
480:675–681. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
He X, Wei Y, Wang Y, Liu L, Wang W and Li
N: MiR-381 functions as a tumor suppressor in colorectal cancer by
targeting Twist1. Onco Targets Ther. 9:1231–1239. 2016.PubMed/NCBI
|
|
28
|
Yang X, Ruan H, Hu X, Cao A and Song L:
miR-381-3p suppresses the proliferation of oral squamous cell
carcinoma cells by directly targeting FGFR2. Am J Cancer Res.
7:913–922. 2017.PubMed/NCBI
|
|
29
|
Chen X, Wang YW, Xing AY, Xiang S, Shi DB,
Liu L, Li YX and Gao P: Suppression of SPIN1-mediated PI3K-Akt
pathway by miR-489 increases chemosensitivity in breast cancer. J
Pathol. 239:459–472. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
He YM, Xiao YS, Wei L, Zhang JQ and Peng
CH: CUL4B promotes metastasis and proliferation in pancreatic
cancer cells by inducing epithelial-mesenchymal transition via the
Wnt/β-catenin signaling pathway. J Cell Biochem. 119:5308–5323.
2018. View Article : Google Scholar
|
|
31
|
Dong J, Wang XQ, Yao JJ, Li G and Li XG:
Decreased CUL4B expression inhibits malignant proliferation of
glioma in vitro and in vivo. Eur Rev Med Pharmacol Sci.
19:1013–1021. 2015.PubMed/NCBI
|
|
32
|
Chen Z, Shen BL, Fu QG, Wang F, Tang YX,
Hou CL and Chen L: CUL4B promotes proliferation and inhibits
apoptosis of human osteosarcoma cells. Oncol Rep. 32:2047–2053.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li Q, Li Z, Wei S, Wang W, Chen Z, Zhang
L, Chen L, Li B, Sun G, Xu J, et al: Overexpression of miR-584-5p
inhibits proliferation and induces apoptosis by targeting WW
domain-containing E3 ubiquitin protein ligase 1 in gastric cancer.
J Exp Clin Cancer Res. 36:592017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zheng P, Chen L, Yuan X, Luo Q, Liu Y, Xie
G, Ma Y and Shen L: Exosomal transfer of tumor-associated
macrophage-derived miR-21 confers cisplatin resistance in gastric
cancer cells. J Exp Clin Cancer Res. 36:532017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Petersen B and Kingsley K: Differential
expression of miR-21, miR-133 and miR-155 from exosome fractions
isolated from oral squamous cell carcinomas in vitro. J Med Discov.
1:160102016.
|
|
36
|
Deng J, Lei W, Xiang X, Zhang L, Lei J,
Gong Y, Song M, Wang Y, Fang Z, Yu F, et al: Cullin 4A (CUL4A), a
direct target of miR-9 and miR-137, promotes gastric cancer
proliferation and invasion by regulating the Hippo signaling
pathway. Oncotarget. 7:10037–10050. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liang Y, Zhao Q, Fan L, Zhang Z, Tan B,
Liu Y and Li Y: Down-regulation of MicroRNA-381 promotes cell
proliferation and invasion in colon cancer through up-regulation of
LRH-1. Biomed Pharmacother. 75:137–141. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ming J, Zhou Y, Du J, Fan S, Pan B, Wang
Y, Fan L and Jiang J: miR-381 suppresses C/EBPα-dependent Cx43
expression in breast cancer cells. Biosci Rep. 35:352015.
View Article : Google Scholar
|
|
39
|
Zhang Q, Zhao S, Pang X and Chi B:
MicroRNA-381 suppresses cell growth and invasion by targeting the
liver receptor homolog-1 in hepatocellular carcinoma. Oncol Rep.
35:1831–1840. 2016. View Article : Google Scholar
|
|
40
|
Tian C, Li J, Ren L, Peng R, Chen B and
Lin Y: MicroRNA-381 serves as a prognostic factor and inhibits
migration and invasion in non-small cell lung cancer by targeting
LRH-1. Oncol Rep. 38:3071–3077. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang Z, Yang J, Xu G, Wang W, Liu C, Yang
H, Yu Z, Lei Q, Xiao L, Xiong J, et al: Targeting miR-381-NEFL axis
sensitizes glioblastoma cells to temozolomide by regulating
stemness factors and multidrug resistance factors. Oncotarget.
6:3147–3164. 2015.PubMed/NCBI
|
|
42
|
Li Y, Zhao C, Yu Z, Chen J, She X, Li P,
Liu C, Zhang Y, Feng J, Fu H, et al: Low expression of miR-381 is a
favorite prognosis factor and enhances the chemosensitivity of
osteosarcoma. Oncotarget. 7:68585–68596. 2016.PubMed/NCBI
|
|
43
|
Wang X, Wang X, Gu J, Zhou M, He Z, Wang X
and Ferrone S: Overexpression of miR-489 enhances efficacy of
5-fluorouracil-based treatment in breast cancer stem cells by
targeting XIAP. Oncotarget. 8:113837–113846. 2017. View Article : Google Scholar
|
|
44
|
Wu H, Xiao Z, Zhang H, Wang K, Liu W and
Hao Q: MiR-489 modulates cisplatin resistance in human ovarian
cancer cells by targeting Akt3. Anticancer Drugs. 25:799–809. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gao S, Liu H, Hou S, Wu L, Yang Z, Shen J,
Zhou L, Zheng SS and Jiang B: MiR-489 suppresses tumor growth and
invasion by targeting HDAC7 in colorectal cancer. Clin Transl
Oncol. 20:703–712. 2018. View Article : Google Scholar
|
|
46
|
Xie Z, Cai L, Li R, Zheng J, Wu H, Yang X,
Li H and Wang Z: Down-regulation of miR-489 contributes into NSCLC
cell invasion through targeting SUZ12. Tumour Biol. 36:6497–6505.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Clevers H and Nusse R: Wnt/β-catenin
signaling and disease. Cell. 149:1192–1205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhang JQ, Chen S, Gu JN, Zhu Y, Zhan Q,
Cheng DF, Chen H, Deng XX, Shen BY and Peng CH: MicroRNA-300
promotes apoptosis and inhibits proliferation, migration, invasion
and epithelial-mesenchymal transition via the Wnt/β-catenin
signaling pathway by targeting CUL4B in pancreatic cancer cells. J
Cell Biochem. 119:1027–1040. 2018. View Article : Google Scholar
|
|
49
|
Chen B and Liu B: MiRNA-381 inhibits the
invasion of renal carcinoma and the underlying mechanisms. Zhong
Nan Da Xue Xue Bao Yi Xue Ban. 40:1053–1059. 2015.In Chinese.
PubMed/NCBI
|
|
50
|
Washington K: 7th edition of the AJCC
cancer staging manual: Stomach. Ann Surg Oncol. 17:3077–3079. 2010.
View Article : Google Scholar : PubMed/NCBI
|