Introduction
Head and neck squamous cell carcinoma is considered
to be the sixth most common type of cancer worldwide, among which
the laryngeal squamous cell carcinoma (LSCC) is a common malignant
neoplasm (1). The incidence of
LSCC in China has been increasing gradually, particularly in the
northeast region (2). Current
treatments have a moderate effect in patients with early stage
disease, including radiation therapy, chemotherapy and surgical
intervention, however, these are less effective for more advanced
disease (3). Regional lymph node
metastasis and distant metastasis are considered to be the major
factors that negatively affect the survival rates of patients with
LSCC (4). It is necessary to
understand the molecular mechanisms underlying the progression of
LSCC in the diagnosis and therapy of LSCC.
MicroRNAs (miRNAs or miRs) are a class of
endogenous, non-coding, small, single-stranded RNAs consisting of
18-25 nucleotides, which mediate the post-transcriptional
regulation of target genes via inhibiting translation or inducing
RNA degradation (5,6). Increasing evidence has demonstrated
that miRNAs can function as oncogenes or tumor suppressor genes in
cancer, and can suppress cell signaling pathways to modulate
various cancer cell processes, including cell proliferation,
apoptosis, differentiation and migration (7,8). The
ectopic expression of miRNAs is key in the development of LSCC
(9), and certain miRNAs directly
modulate the apoptosis, proliferation and invasion of LSCC cells
(3,10). Tian et al revealed that
miRNA (miR)-203 is downregulated in LSCC tissues and cells, and
that the overexpression of miR-203 represses proliferation and
invasion, induces apoptosis and causes cell cycle arrest of Hep-2
cells in vitro (10).
miR-21 has been identified to be overexpressed in LSCC and
correlated with advanced stage, and the inhibition of miR-21
suppresses cellular proliferation and invasion (3). miR-370 functions as a tumor
suppressor via targeting Forkhead Box M1 in LSCC (11). miR-143-3p has also been extensively
reported to be downregulated in various types of cancer and serves
as a tumor suppressor by modulating cell proliferation, apoptosis,
invasion and metastasis (12,13).
However, the molecular mechanism underlying the function of
miR-143-3p in the progression and development of LSCC remains to be
elucidated.
k-Ras, a membrane-associated GTPase signaling
protein, modulates cell survival, proliferation and differentiation
(14). The k-Ras protein (21 kDa)
located at the inner plasma membrane, is tightly correlated with
the transduction of mitogenic signals (15). Accumulating evidence has
demonstrated that k-Ras is important in tumorigenesis, cellular
transformation and maintenance of a malignant phenotype (16-18).
The ectopic expression of k-Ras has been found in various human
tumors due to alterations in upstream or downstream signaling
components or the triggering of mutations in RAS genes (19,20).
The aberrant activation of downstream regulators in k-Ras pathways,
including the RAF/mitogen-activated protein kinase kinase
(MEK)/extracellular signal-regulated kinase (ERK) cascade, have
been found to result in k-Ras-driven tumorigenesis, which is
characterized by cellular transformation, metastasis and resistance
to apoptosis (21-24). A previous study demonstrated that
miR-21 increased the activation of oncogenic k-Ras and modulated
non-small-cell lung cancer tumorigenesis via suppressing the
negative regulators of the Ras/Raf/MEK/ERK pathway (25). In addition, Zhou et al
reported that miR-30a suppresses tumor progression in
hepatocellular carcinoma by inhibiting the Ras/Raf/MEK/ERK
signaling pathway (26).
Therefore, the miRNAs-mediated Ras/Raf/MEK/ERK pathway in LSCC
requires further clarification.
The present study aimed to investigate miRNA
expression profiles in the tumorigenesis of LSCC and examine the
molecular mechanism underlying the biological function of miRNAs in
the development of LSCC. The results revealed that miR-143-3p was
downregulated in LSCC tissues and its expression predicted poor
prognosis in LSCC. The findings also demonstrated that miR-143-3p
repressed cell growth, migration and invasion in LSCC via
modulating the k-Ras/Raf/MEK/ERK signaling pathway, and suggested
that miR-143-3p may act as a tumor suppressor in LSCC tumorigenesis
and represent a novel target for effective therapies.
Materials and methods
Patient tissue samples
Tumor tissues and matched normal tissues were
obtained from 52 patients with LSCC at the Ear Nose and Throat
Hospital, The First Affiliated Hospital of Zhengzhou University
(Zhengzhou, China) between November, 2015 and January, 2016. The
clinicopathological characteristics of the patients with LSCC are
summarized in Table I. The tissues
were collected from patients who had not received radiotherapy or
chemotherapy prior to surgical resection. Verification of the
specimens was performed by three pathologists according to the
World Health Organization classification system (27). All patients provided written
informed consent for the use of the tumor tissues for clinical
investigation. All the tissues were snap-frozen in liquid nitrogen
and stored at -80°C immediately following resection for subsequent
experiments. The Institute Research Medical Ethics Committee of
Zhengzhou University granted approval for the study.
 | Table IAssociation between miR-143-3p and
clinicopathological features of patients with laryngeal squamous
cell carcinoma. |
Table I
Association between miR-143-3p and
clinicopathological features of patients with laryngeal squamous
cell carcinoma.
| Clinicopathological
parameter | Total n=52 | miR-143-3p
| P-value |
|---|
| High (n) | Low (n) |
|---|
| Sex | | | | 0.8967 |
| Male | 35 | 13 | 22 | |
| Female | 17 | 6 | 11 | |
| Age (years) | | | | 0.8478 |
| ≤60 | 31 | 11 | 20 | |
| >60 | 21 | 8 | 13 | |
| T
classification | | | | 0.0025 |
| T1-2 | 24 | 14 | 10 | |
| T3-4 | 28 | 5 | 23 | |
|
Differentiation | | | | 0.0041 |
| G1 | 15 | 10 | 5 | |
| G2 | 37 | 9 | 28 | |
| Lymph node
metastasis | | | | 0.0111 |
| Negative | 21 | 12 | 9 | |
| Positive | 31 | 7 | 24 | |
| Primary
location | | | | 0.9382 |
| Supraglottic | 25 | 9 | 16 | |
| Glottic | 27 | 10 | 17 | |
| Clinical stage | | | | 0.0371 |
| I-II | 23 | 12 | 11 | |
| III-IV | 29 | 7 | 22 | |
Cell culture
The TU177, SNU899 and SNU46 human LSCC cell lines
were purchased from the Cell Biology Institute of Shanghai, Chinese
Academy of Science (Shanghai, China). Human oral keratinocyte (HOK)
cells (ScienCell Research Laboratories, Carlsbad, CA, USA) were
used as a normal control (28).
The LSCC cell lines were grown in RPMI-1640 (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA; cat. no. 11875) supplemented
with 10% fetal bovine serum (FBS; Sigma; EMD Millipore, Billerica,
MA, USA), 100 IU/ml penicillin and 100 mg/ml streptomycin
(Invitrogen/Thermo Fisher Scientific, Inc.; cat. no. 15140-122), at
37°C in a humidified atmosphere containing 5% CO2. The
HOK cells were maintained in oral keratinocyte medium (ScienCell
Research Laboratories) supplemented with oral keratinocyte growth
supplement (ScienCell Research Laboratories).
Cell transfection
The miR-143-3p mimics/inhibitor and mimics/inhibitor
negative control (NC) were designed and synthesized by Guangzhou
RiboBio Co., Ltd. (Guangzhou, China). The cells were seeded into
6-well plates and transfected with miR-143-3p mimics/inhibitor and
mimics/inhibitor NC (100 nM) using Lipofectamine 2000
(Invitrogen/Thermo Fisher Scientific, Inc.) according to the
manufacturer’s instructions. Following transfection for 48 h, the
cells were collected for further measurements. The sequences are as
follows: miR-143-3p mimics, 5′-UGAGAUGAAGCACUGUAGCUC-3′; miR-143-3p
inhibitor, 5′-GAGCUACAGUGCUUCAUCUCA-3′; mimics NC,
5′-UUUGUACUACACAAAAGUACUG-3′; inhibitor NC,
5′-CAGUACUUUUGUGUAGUACAAA-3′.
miRNA microarray analysis
miRNA microarray analysis was performed to identify
miRNA expression profiles in clinical samples. Total RNAs were
extracted by TRIzol reagent (Invitrogen/Thermo Fisher Scientific,
Inc.; cat. no. 15596-018), and the miRNA fraction was further
purified using a mirVana miRNA isolation kit (Ambion; Thermo Fisher
Scientific, Inc.; cat. no. AM1560) according to the manufacturer’s
protocol. The isolated miRNAs were labeled with Hy3 using the
miRCURY array labeling kit (Exiqon, Inc., Vedbaek, Denmark; cat.
no. 208032-A) and hybridized with miRCURY locked nucleic acid
microRNA arrays (v8.0; Exiqon, Inc.). Microarray images were
obtained using a Genepix 4000B scanner (Axon Instruments; Molecular
Devices LLC, Sunnyvale, CA, USA) and analyzed with Genepix Pro 6.0
software (Axon Instruments; Molecular Devices LLC).
Reverse transcription-quantitative
(RT-qPCR) analysis
Total RNA from the frozen tissues and cells were
isolated using TRIzol reagent (Molecular Research Center, Inc.,
Cincinnati, OH, USA; cat. no. RT118) according to the
manufacturer’s protocol. 5 µl of RNA was reverse transcribed
using a TaqMan Gene Expression Assays kit and TaqMan MicroRNA
Reverse Transcription for k-Ras and miRNA according to the
instructions of the manufacturer (Applied Biosystems/Thermo Fisher
Scientific, Inc.). For analysis on a Step One Plus PCR machine
(Applied Biosystems/Thermo Fisher Scientific, Inc.), 5 µl of
cDNA was added to TaqMan® Fast Universal PCR Master Mix
reagents (Applied Biosystems), to obtain final primer and probe
concentrations of 300 nM/primer and 250 nM/probe in a 20 µl
total volume. The sample was centrifuged briefly and run onthe PCR
machine using the default fast programme (40 cycles of 95°C for 1
sec, 60°C for 20 sec). GAPDH and U6 served as endogenous controls.
The primer sequences are as follows: miR-143-3p forward, 5′-UGAGAU
GAAGCACUGUAGCUC-3′, reverse, 5′-GTCGTATCCAGTG CGTGTCGTG-3′; k-Ras
forward, 5′-ACTGAATATAAACCT TGTGGTAG-3′, reverse,
5′-TCAAAGAATGGTCCTGGACC-3′; GAPDH forward,
5′-TGTGGGCATCAATGGATTTGG-3′, reverse, 5′-ACACCATGTATTCCGGGTCAAT-3′;
U6 forward, 5′-CTCGCTTCGGCAGCACA-3′, reverse,
5′-AACGCTTCACGAATTTGCGT-3′. The relative quantification
(2−∆∆Cq) method (29)
was used to calculate fold changes.
Cell viability analysis
Cell viability was measured using a Cell Counting
Kit-8 (CCK-8) assay according to the manufacturer’s protocol.
Briefly, the cells (5×104 cells/well) were seeded in
96-well plate with 100 µl RPMI-1640 medium supplemented with
10% FBS. Then, 10 µl of CCK-8 solution (Dojindo Molecular
Technologies, Inc., Gaithersburg, MD, USA) was added into each well
and the mixture was incubated for 1 h at 37°C with 5%
CO2. The absorbance rate at 450 nm was measured using a
microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Each experiment was repeated at least three times.
Analysis of apoptosis
Flow cytometric analysis was used to measure cell
apoptosis. The cells (1×106 cells) were harvested and
washed twice with cold PBS. The cells were then fixed with 70%
ice-cold methanol at 4°C for 30 min. Following two PBS washes, the
cells were resuspended in binding buffer and incubated with 5
µl of Annexin V-FITC (BD Biosciences, Franklin Lakes, NJ,
USA) and 1 µl of propidium iodide (PI; 50 µg/ml; BD
Biosciences). Flow cytometric analysis was performed within 5 min.
All samples were analyzed by flow cytometry (FACSCalibur; BD
Biosciences).
Western blot analysis
The cells were lysed as described previously
(30). The BCA protein assay kit
(Pierce; Thermo Fisher Scientific, Inc.) was used to measure
protein concentration. The protein samples (60 µg) were
separated in 10% SDS-polyacrylamide gels (Sigma-Aldrich; EMD
Millipore) and then transferred onto polyvinylidene difluoride
membranes (BD Pharmingen, San Diego, CA, USA). The membranes were
then blocked with 5% skim milk at room temperature for 1 h, and
were incubated primary antibodies at 4°C overnight against
cleaved-caspase-3 (1:1,000; cat. no. SC-5298; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), B cell lymphoma 2 (Bcl-2;
1:1,000; cat. no. SC-492; Santa Cruz Biotechnology, Inc.),
Bcl-2-associated X protein (Bax; 1:500; cat. no. SC-526; Santa Cruz
Biotechnology, Inc.), E-cadherin (1:1,000; cat. no. SC-7870; Santa
Cruz Biotechnology, Inc.), N-cadherin (1:1,000; cat. no. SC-7939;
Santa Cruz Biotechnology, Inc.), Vimentin (1:1,000; cat. no.
SC-5565; Santa Cruz Biotechnology, Inc.), matrix metalloproteinase
(MMP-9; 1:1,000; cat. no. SC-12759; Santa Cruz Biotechnology,
Inc.), k-Ras (1:1,000; cat. no. SC-30; Santa Cruz Biotechnology,
Inc.), phosphorylated (p)-Raf/1 (1:1,000; cat. no. ab130572; Abcam,
Cambridge, UK), Raf/1 (1:1,000; cat. no. ab137435; Abcam), p-MEK1/2
(1:1,000; cat. no. SC-436; Santa Cruz Biotechnology, Inc.), MEK1/2
(1:1,000; cat. no. 9154S; Cell Signaling Technology, Inc., Danvers,
MA, USA), p-ERK1/2 (1:1,000; cat. no. SC-81492; Santa Cruz
Biotechnology, Inc.) and ERK1/2 (1:1,000; cat. no. SC-514302; Santa
Cruz Biotechnology, Inc.). β-actin (1:750; cat. no. A5060;
Sigma-Aldrich; EMD Millipore) served as an internal control. The
membranes were then incubated for 2 h with horseradish
peroxidase-conjugated antibodies (1:1,000; cat. no. SC-2060; Santa
Cruz Biotechnology, Inc.) at room temperature. Subsequently, the
protein bands were scanned on the X-ray film using the enhanced
chemiluminescence detection system (PerkinElmer, Inc., Boston, MA,
USA). The Alpha Imager software 2000 (Alpha Innotech Corporation,
San Leandro, CA, USA) was performed to measure the relative
intensity of each band on the western blots.
Xenograft tumor model
All animal procedures were approved by the Animal
Care Committee of the Zhengzhou University. The female BALB/C
athymic nude mice (7-8 weeks old; n=20; weighing 26±4 g) were
obtained from Cancer Institute of the Chinese Academy of Medical
Science. The mice were housed in laminar flow cabinets under
specific pathogen-free conditions, and fed ad libitum. The
mice were housed on 12:12-h light–dark cycle in a temperature
controlled (24±2°C) and humidity-controlled room, with free access
to standard chow and tap water. Overall, 1×107 BGC-823
cells in 200 µl of sterile PBS were administered to the
BALB/c nude mice by subcutaneous injection. The female BALB/C
athymic nude mice (7-8 weeks; n=4) were inoculated with SNU899 and
SNU46 (1×107 cells in 200 µl of sterile PBS)
transfected with miR-143-3p mimic or mimic negative control (NC) in
the forelimb. The tumor cells were allowed to grow for 4 weeks. The
tumors were then excised and weighed from the sacrificed mice at 28
days.
Cell invasion assay
Cell invasion assays were performed using 24-well
Transwell chambers with an 8.0-µm pore size polycarbonate
membrane (Corning Incorporated, Corning, NY, USA). The TU177 and
SNU899 cells were seeded on the upper surface of the membrane
precoated with Matrigel (BD Biosciences). Subsequently, the
non-invading cells on the upper side of the membrane were removed
and invading cells on the lower surface of the membrane were fixed
with methanol and stained with crystal violet (cat. no. MAK083;
Sigma-Aldrich; EMD Millipore). The cell numbers were counted under
a microscope (Olympus IX81, Olympus Corp., Tokyo, Japan;
magnification, ×100).
Cell migration assay
The wound-healing assay was used to examine cell
migration. The TU177 and SNU899 cells were plated onto 6-well
plates. The cells were serum-starved overnight prior to wounding of
the monolayers by scratching with a 10-µl micropipette
pipette tip. Following 48 h of incubation, the migration status was
examined by measuring the movement of cells into the scraped area.
Images were captured at different points of time post-wounding. The
wound area was measured and the percentage of closure of the
denuded area was counted using Image J software (Version 1.42q,
National Institutes of Health, Bethesda, MD, USA).
Luciferase assay
The potential binding site between k-Ras and
miR-143-3p was searched using TargetScan (http://www.targetscan.org). The miR-143-3p
mimics/inhibitor and corresponding NC were synthesized by Guangzhou
RiboBio Co., Ltd. The wild-type k-Ras-3′-UTR (WT) and mutant
k-Ras-3′-UTR (mut) containing the putative binding site of
miR-143-3p were established (Fig.
4A) and cloned into the firefly luciferase expressing vector
pMIR-REPORT (Ambion; Thermo Fisher Scientific, Inc.). Site-directed
mutagenesis of the k-Ras 3′-UTR at the putative miR-143-3p binding
site was performed using a QuikChange kit (Qiagen, Inc., Valencia,
CA, USA). For the luciferase assay, TU177 cells (2×105
per well) were seeded into 24-well plates and co-transfected with
0.8 µg of pMIR-k-Ras-3′-UTR or pMIR-k-Ras-mut-3′-UTR, 50 nM
miR-143-3p mimic/inhibitor or mimic NC using Lipofectamine 2000
reagent (cat. no. 11668-027; Invitrogen/Thermo Fisher Scientific,
Inc.). The ratio of Firefly to Renilla was used to normalize
the relative Firefly luciferase activity 48 h following
transfection, and measured with the Dual-Light luminescent reporter
gene assay (Applied Biosystems; Thermo Fisher Scientific,
Inc.).
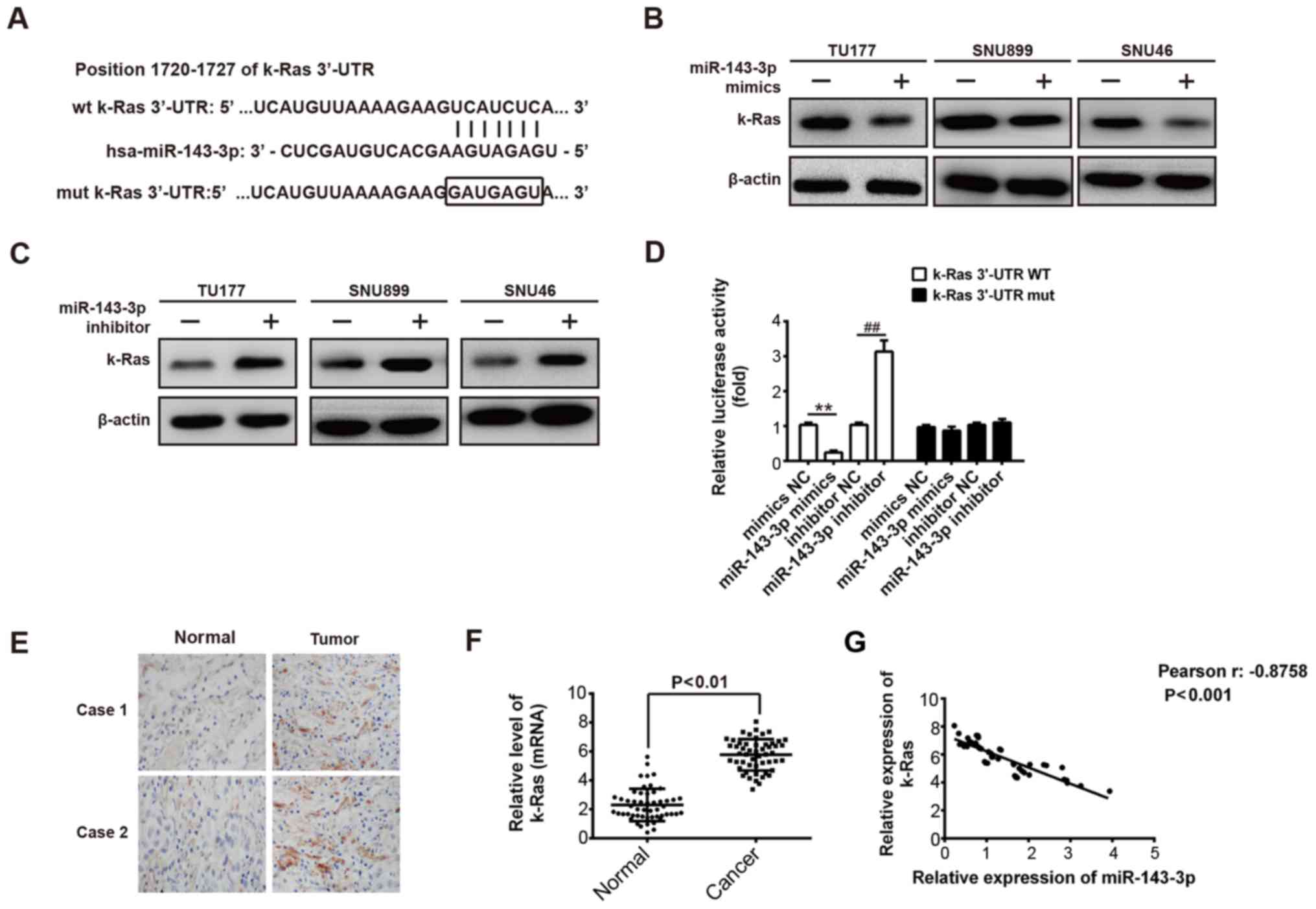 | Figure 4miR-143-3p inhibits expression of
k-Ras by directly targeting its 3′-UTR. (A) k-Ras 3′-UTR containing
the wt or mut binding site for miR-143-3p. (B) Western blot
analysis was performed to detect levels of k-Ras in three LSCC
cells (TU177, SNU899 and SNU46) following transfection with
miR-143-3p mimics or NC mimics. β-actin served as an internal
control. (C) Western blot analysis was used to measure the
expression of k-Ras in three LSCC cells (TU177, SNU899 and SNU46)
transfected with miR-143-3p inhibitor or NC inhibitor. β-actin
served as an internal control. (D) Relative luciferase activity of
k-Ras wt or mut 3′-UTR in TU177 cells following transfection with
the miR-143-3p mimic/inhibitor or corresponding NC.
**P<0.01, vs NC mimic. ##P<0.01, vs NC
inhibitor. (E) Representative images of immunohistochemical
staining of k-Ras in LSCC and adjacent normal tissues. (F) Levels
of miR-143-3p were measured using reverse
transcription-quantitative polymerase chain reaction analysis in
the LSCC and matched normal tissues (n=52). **P<0.01,
vs normal tissues. (G) Negative correlation between k-Ras and
miR-143-3p levels in patients with LSCC (r=-0.8758, P<0.001).
Data are presented as the means ± standard deviation of three
individual experiments. LSCC, laryngeal squamous cell carcinoma;
miR, microRNA; wt, wild-type; mut, mutant. |
Immunohistochemistry
Immunohistochemistry was performed on
paraformaldehyde-fixed paraffin sections (5 µm thickness).
The tissue slides were deparaffinized with xylene and rehydrated
with graded series of ethanol. Following antigen retrieval, the
sections were blocked with bovine serum (Gibco/Thermo Fisher
Scientific, Inc., 10%) in PBS for 30 min and incubated overnight at
4°C with the following primary antibodies: Anti-k-Ras (1:500; cat.
no. SC-30; Santa Cruz Biotechnology, Inc.), anti-p-Raf/1 (1:500;
cat. no. ab130572; Abcam) and anti-p-ERK1/2 (1:500; cat. no.
SC-81492; Santa Cruz Biotechnology, Inc.). The sections were then
incubated overnight at 4°C with secondary antibodies (IgG
antibodies; 1:1,000; cat. no. SC-2060; Santa Cruz Biotechnology,
Inc.). The immunostaining was visualized with diaminobenzidine
(cat. no. D5637; DAB; Sigma; EMD Millipore) for 3 min, covered with
a cover-slip, and analyzed under a light microscope.
Statistical analysis
All statistical analyses were performed using SPSS
software (version 19.0; IBM SPSS, Armonk, NY, USA). Data were
obtained from independent experiments repeated at least three
times. Numerical data are presented as the means ± standard
deviation. The association between the clinicopathological
characteristics of the patients with LSCC and miR-143-3p low and
high expression was determined by the Pearson’s Chi-square test.
Independent t-tests were used to compare differences between 2
groups. One-way ANOVA with Tukey’s post hoc tests were performed to
compare the differences between 3 or more groups. Kaplan-Meier
curve analysis with a log-rank test was used to evaluate the
survival rate between patients with high- and low-miR-143-3p
expressing tumors. The correlation between k-Ras and miR-143-3p
expression levels was determined by with Pearson’s correlation
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
miR-143-3p is downregulated in LSCC
tissues and is negatively associated with the patient survival
rate
To determine the expression of miRNAs involved in
LSCC tumorigenesis, microarray analysis was used to determine miRNA
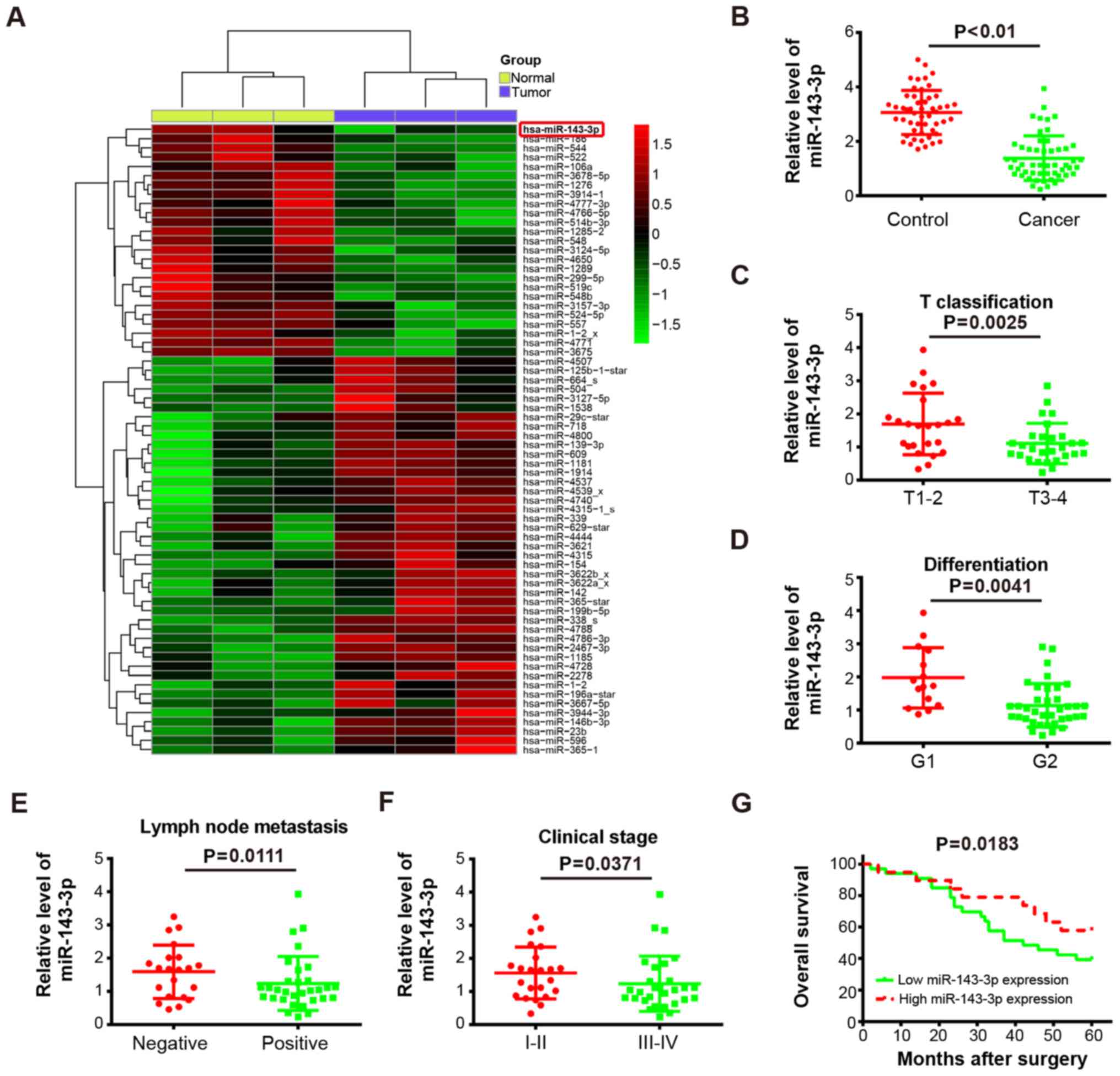
levels in LSCC tissues and matched normal tissues. As shown in
Fig. 1A, compared with the normal
tissues, several miRNAs were altered in tumor tissues, with the
most marked significant downregulation in miR-143-3p. Several
studies have demonstrated that miR-143-3p is downregulated in
cancer tissues and functions as a tumor suppressor in various types
of cancer, including esophageal cancer and breast cancer (12,13).
However, the role of miR-143-3p in LSCC remains to be elucidated.
Therefore, the present study investigate the role of miR-143-3p in
the development of LSCC. Consistent with the microarray analysis
results, the RT-qPCR analysis further confirmed that the levels of
miR-143-3p were significantly downregulated in cancer tissues
(n=52) compared with control tissues (P<0.01; Fig. 1B). Previous studies have reported
that the development of tumors is associated with the
clinicopathological features, including T classification,
differentiation and lymph node metastasis, in patients (31-33).
Therefore, the present study further analyzed the association
between miR-143-3p and the clinicopathological features of patients
with LSCC (Table I). It was
observed that a low expression of miR-143-3p was negatively
associated with T classification (P=0.0025; Fig. 1C), differentiation (P=0.0041;
Fig. 1D), lymph node metastasis
(P=0.0111; Fig. 1E) and clinical
stage (P=0.0371; Fig. 1F) in
patients with LSCC. However, there was no associated between the
expression of miR-143-3p and patient gender, age or primary
location (Table I). To assess the
correlation between the expression of miR-143-3p and the prognosis
of patients with LSCC, Kaplan-Meier survival analysis was used to
evaluate the LSCC survival curves. It was observed that patients
with low expression of miR-143-3p (n=33) had a lower overall
survival percentage, compared with those with high expression of
miR-143-3p (n=19; P=0.0183; Fig.
1G). Collectively, these results demonstrated that miR-143-3p
was downregulated in LSCC tissues and a low expression of
miR-143-3p predicts poor prognosis in LSCC, suggesting that
miR-143-3p may function as a tumor suppressor in LSCC
tumorigenesis.
Overexpression of miR-143-3p inhibits
LSCC cell proliferation, induces apoptosis in vitro and suppresses
tumor growth in vivo
To further examine the functions of miR-143-3p in
LSCC cells, the levels of miR-143-3p were measured in TU177, SNU899
and SNU46 LSCC cell lines, and HOK cells. As shown in Fig. 2A, the expression of miR-143-3p was
significantly downregulated in all LSCC cell lines compared with
the normal epithelial cell lines (P<0.01). Based on these data,
the two LSCC cells (TU177 and SNU899) with the lowest expression
level of miR-143-3p were selected for further experiments. The
TU177 and SNU899 cells were transfected with miR-143-3p
mimics/inhibitor or mimics/inhibitor NC, and the transfection
efficiency of miR-143-3p mimics/inhibitor were evaluated by RT-qPCR
analysis. As shown in Fig. 2B,
miR-143-3p was significantly downregulated or upregulated in these
TU177 and SNU899 cells, compared with the cells transfected with
mimics/inhibitor NC (P<0.01). Subsequently, the CCK-8 assay and
flow cytometric analysis were performed to measure cell viability
and apoptosis in TU177 and SNU899 cells following transfection with
miR-143-3p mimics or NC mimics, respectively. It was found that the
overexpression of miR-143-3p markedly reduced cell viability and
increased apoptotic cells compared with the control (P<0.01;
Fig. 2C and D). To further examine
the molecular mechanisms of miR-143-3p-induced apoptosis, western
blot analysis was used to determine the expression levels of
apoptosis-related proteins in TU177 and SNU899 cells transfected
with miR-143-3p mimics or NC mimics. It was found that the
overexpression of miR-143-3p significantly increased the protein
expression of pro-apoptotic cleaved-caspase-3 and Bax, and
decreased the protein expression of anti-apoptotic Bcl-2, compared
with the NC mimics (P<0.01; Fig.
2E). To investigate whether the overexpression of miR-143-3p
influences tumorigenesis in vivo, a xenograft mouse model
was established, which was subcutaneously injected with
miR-143-3p-overexpressing TU177 or SNU899 cells and their parallel
NC mimics-carrying cells. Following injection, the tumor growth was
determined by measuring the weight. It was found that the
miR-143-3p-overexpressing cells exhibited a significant decrease in
the tumor weight compared with the control cells (P<0.01;
Fig. 2F). These data indicated
that the overexpression of miR-143-3p possessed antitumor effects
via inhibiting cell proliferation and inducing apoptosis in
vitro, and repressing tumor growth in vivo.
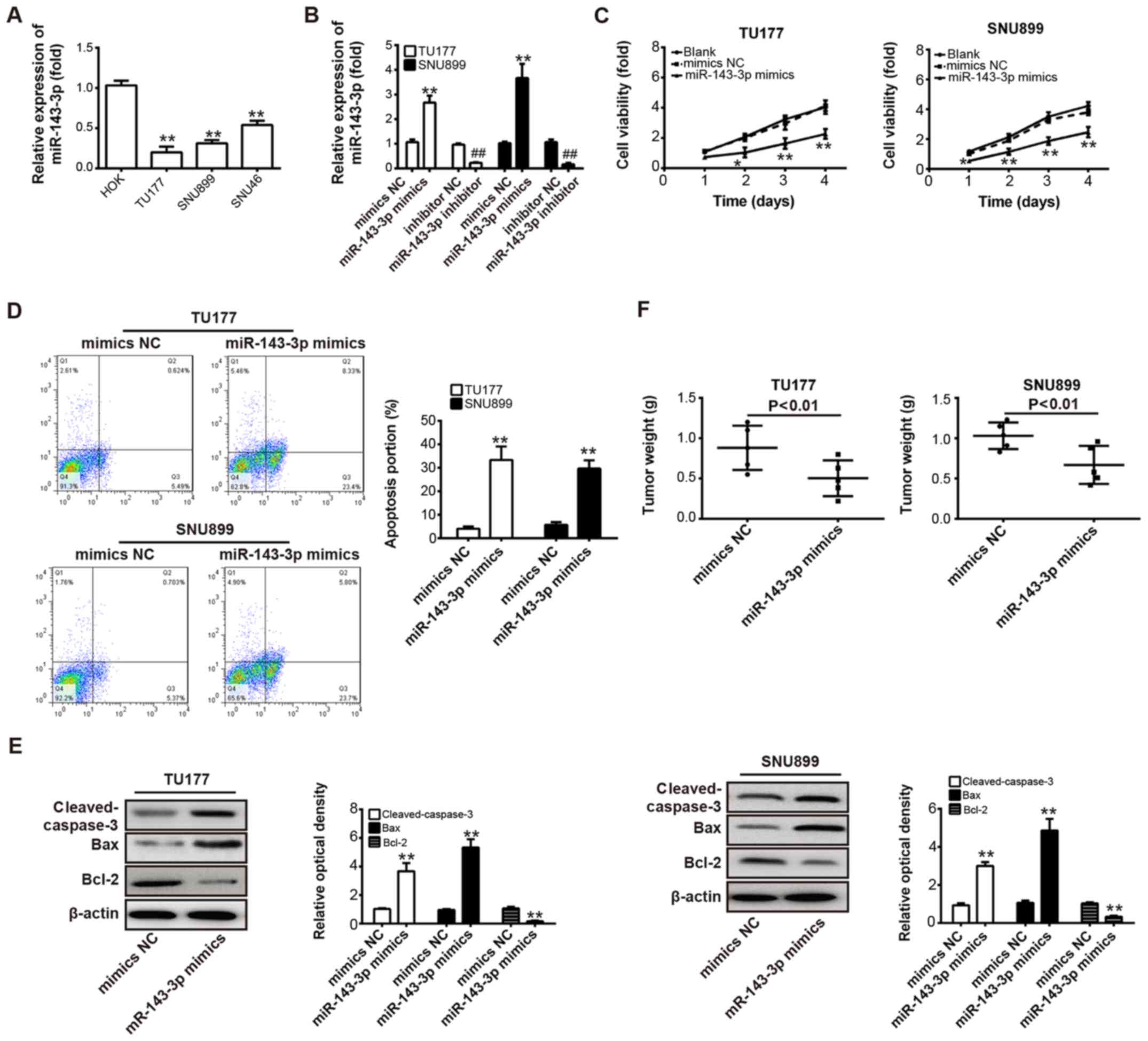 | Figure 2Overexpression of miR-143-3p
represses LSCC cell proliferation, induces apoptosis and suppresses
tumor growth. (A) Expression of miR-143-3p was measured using
RT-qPCR analysis in TU177, SNU899 and SNU46 LSCC cell lines, and
HOK normal epithelial cell lines. **P<0.01 vs. HOK.
(B) RT-qPCR analysis was used to determine the level of miR-143-3p
in TU177 and SNU899 cells transfected with miR-143-3p
mimics/inhibitor or NC mimics/inhibitor. **P<0.01 vs.
NC mimics; ##P<0.01 vs. NC inhibitor. (C) A Cell
Counting Kit-8 assay was performed to measure cell viability in
TU177 and SNU899 cells transfected with miR-143-3p mimics or NC
mimics. *P<0.05 and **P<0.01 vs. NC
mimics. (D) TU177 and SNU899 cells were transfected with miR-143-3p
mimics or NC mimics and cell apoptosis was determined by flow
cytometric analysis. **P<0.01 vs. NC mimics. (E)
Cleaved-caspase-3, Bax and Bcl-2 were detected in TU177 and SNU899
cells transfected with miR-143-3p mimics or NC mimics. β-actin was
used as an internal control for protein loading.
**P<0.01 vs. NC mimics. (F) Xenograft model was
injected with miR-143-3p-overexpressing TU177 or SNU899 cells and
tumor growth was determined by measuring tumor weight (n=5/group).
Data are presented as the means ± standard deviation of three
individual experiments. **P<0.01 vs. NC mimics. LSCC,
laryngeal squamous cell carcinoma; miR, microRNA; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; Bcl-2, B-cell
lymphoma 2; Bax, Bcl-2-associated X protein; NC, negative
control. |
Overexpression of miR-143-3p inhibits
cell migration and invasion in vitro
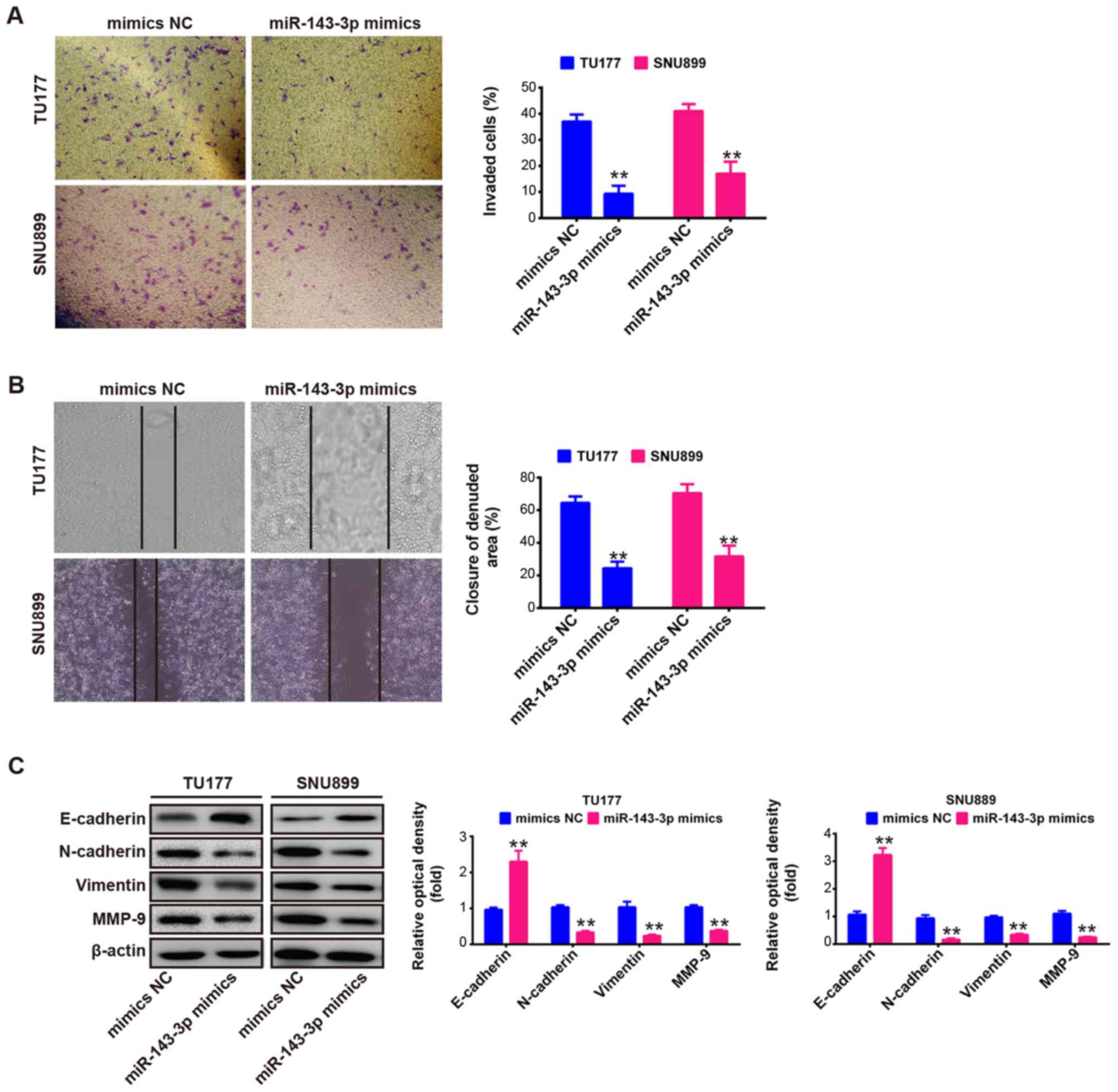
The present study further evaluated the effects of
the upregulation of miR-143-3p on cell invasion and migration,
which are key factors in malignant progression and metastasis. The
TU177 and SNU899 cells were transfected with miR-143-3p mimics or
NC mimics, and cell invasion was evaluated using a Transwell
invasion assay. As shown in Fig.
3A, the invaded cells were significantly inhibited by the
overexpression of miR-143-3p compared with the NC mimics
(P<0.01). Subsequently, a wound-healing assay was performed to
determine cell migration following transfection with miR-143-3p
mimics or NC mimics. It was observed that the overexpression of
miR-143-3p also markedly suppressed the migration of TU177 and
SNU899 cells (P<0.01; Fig. 3B).
These results suggested that the overexpression of miR-143-3p
suppressed cell migration and invasion in vitro, although
the potential molecular mechanism requires further investigation
for a deeper understanding.
It is well reported that tumor cell invasion and
metastasis are tightly correlated with several processes, including
epithelial-mesenchymal transition (EMT), matrix metalloproteinase
(MMP) upregulation and adhesion molecule downregulation in cancer
cells. Epithelial tumor progression to more aggressive metastatic
tumors, concurrent upregulation of mesenchymal protein markers
N-cadherin and vimentin, and loss of epithelial protein marker
E-cadherin are important cellular events during EMT (34-36).
E-cadherin, an EMT marker, is frequently downregulated in various
tumors, including LSCC (37,38),
and loss of its function or expression decreases cell-cell contacts
and results in tumor invasion and metastasis (39). MMP-9, a member of the MMP family,
is expressed at a high level in cancer tissues, and is correlated
with the processes of tumor metastasis and invasion in human
cancer, including LSCC (40,41).
Studies have demonstrated that EMT and MMP-9 in different types of
cancer are regulated by post-transcriptional mechanisms, including
miRNAs (40,42). Therefore, to further elucidate the
molecular mechanism by which the overexpression of miR-143-3p
suppresses cell migration and invasion in vitro, the TU177
and SNU899 cells were transfected with miR-143-3p mimics or NC
mimics, and the expression levels of E-cadherin, N-cadherin,
vimentin and MMP-9 were measured by western blot assays. As shown
in Fig. 3C, the overexpression of
miR-143-3p increased the protein level of E-cadherin, but decreased
the levels of N-cadherin, vimentin and MMP-9 in the TU177 and
SNU899 cells. Taken together, these data indicated that the
overexpression of miR-143-3p suppressed cell migration and invasion
through regulating EMT and MMP-9.
miR-143-3p inhibits the expression of
k-Ras by targeting its 3′-UTR
There is increasing evidence that k-Ras acts as an
oncogene via regulating tumorigenesis and cellular transformation
in various types of cancer (16,17,43).
A previous study reported that miR-143-3p serves as a tumor
suppressor via targeting oncogene k-Ras in renal cell carcinoma
(44). To determine whether k-Ras
is a target of miR-143-3p in LSCC cells, bioinformatics analysis
was performed to predicate the putative targets of miR-143-3p, and
it was found that k-Ras may be a target gene of miR-143-3p
(Fig. 4A). To investigate whether
the expression of k-Ras is controlled by miR-143-3p, the TU177,
SNU899 or SNU46 LSCC cell lines were transfected with
mimics/inhibitor or NC, and the protein level of k-Ras was
determined using western blot analysis. It was found that the
expression of k-Ras was decreased by the overexpression of
miR-143-3p (Fig. 4B), whereas the
knockdown of miR-143-3p increased the protein level of k-Ras in all
LSCC cells, compared with the level in the NC group (Fig. 4C). To validate whether the k-Ras is
the direct target of miR-143-3p, luciferase-reporter plasmids were
constructed containing the WT or mut 3′-UTR segments of k-Ras
(Fig. 4A). The WT or mut reporter
plasmid was co-transfected into TU177 cells with miR-143-3p
mimics/inhibitor or NC, and the luciferase activity was measured.
The results showed that the miR-143-3p mimic markedly repressed the
luciferase activity compared with the NC mimic, whereas the
miR-143-3p inhibitor increased the luciferase activity compared
with the NC inhibitor in the presence of the WT 3′-UTR (P<0.01;
Fig. 4D). miR-143-3p did not
suppress the luciferase activity of the reporter vector containing
the 3′-UTR of k-Ras with mutations in the miR-143-3p-binding site
(Fig. 4D). These data indicated
that k-Ras is a direct target of miR-143-3p in LSCC cells.
To further clarify the association between
miR-143-3p and k-Ras in LSCC, immunohistochemistry and RT-qPCR
assays were performed to detect the expression of k-Ras in the 52
paired LSCC and matched normal tissues. It was observed that the 52
cases of tumors showed enhanced expression of k-Ras when compared
with adjacent normal tissues (P<0.01; Fig. 4E and F). Correlation analysis
showed that the expression levels of k-Ras in tumor tissues were
inversely correlated with the levels of miR-143-3p (r=-0.8758,
P<0.001; Fig. 4G). These
results suggested that miR-143-3p negatively modulated the
expression of k-Ras and their inverse correlation was determined in
clinical samples.
Overexpression of k-Ras rescues the
suppressive effects of the upregulation of miR-143-3p on LSCC
cells
To further investigate whether the ectopic
expression of k-Ras rescued the suppressive effect of miR-143-3p,
the TU177 and SNU899 cells were transfected with miR-143-3p mimics
or were co-transfected with miR-143-3p mimics and the pcDNA-k-Ras
plasmid. Cells without transfection are used as a control (blank
group). The results showed that the overexpression of miR-143-3p
significantly decreased the expression of k-Ras compared with the
blank group, however, the pcDNA-k-Ras plasmid led to marked k-Ras
upregulation compared with the miR-143-3p mimics group (P<0.01;
Fig. 5A). Subsequently, cell
viability, apoptosis, migration and invasion in each group were
measured using a CCK-8 assay, flow cytometry, a wound-healing assay
and Transwell invasion assay, respectively. It was found that the
overexpression of miR-143-3p inhibited cell proliferation,
migration and invasion, and promoted apoptosis, however, the
upregulation of k-Ras led to a marked increase in cell
proliferation, migration and invasion, and decrease in apoptosis in
the TU177 and SNU899 cells following co-transfection with
miR-143-3p mimics and the pcDNA-k-Ras plasmid (P<0.01; Fig. 5B-F). These results indicated that
the suppressive effects of miR-143-3p on cell growth, migration and
invasion were rescued by the overexpression of k-Ras in LSCC
cells.
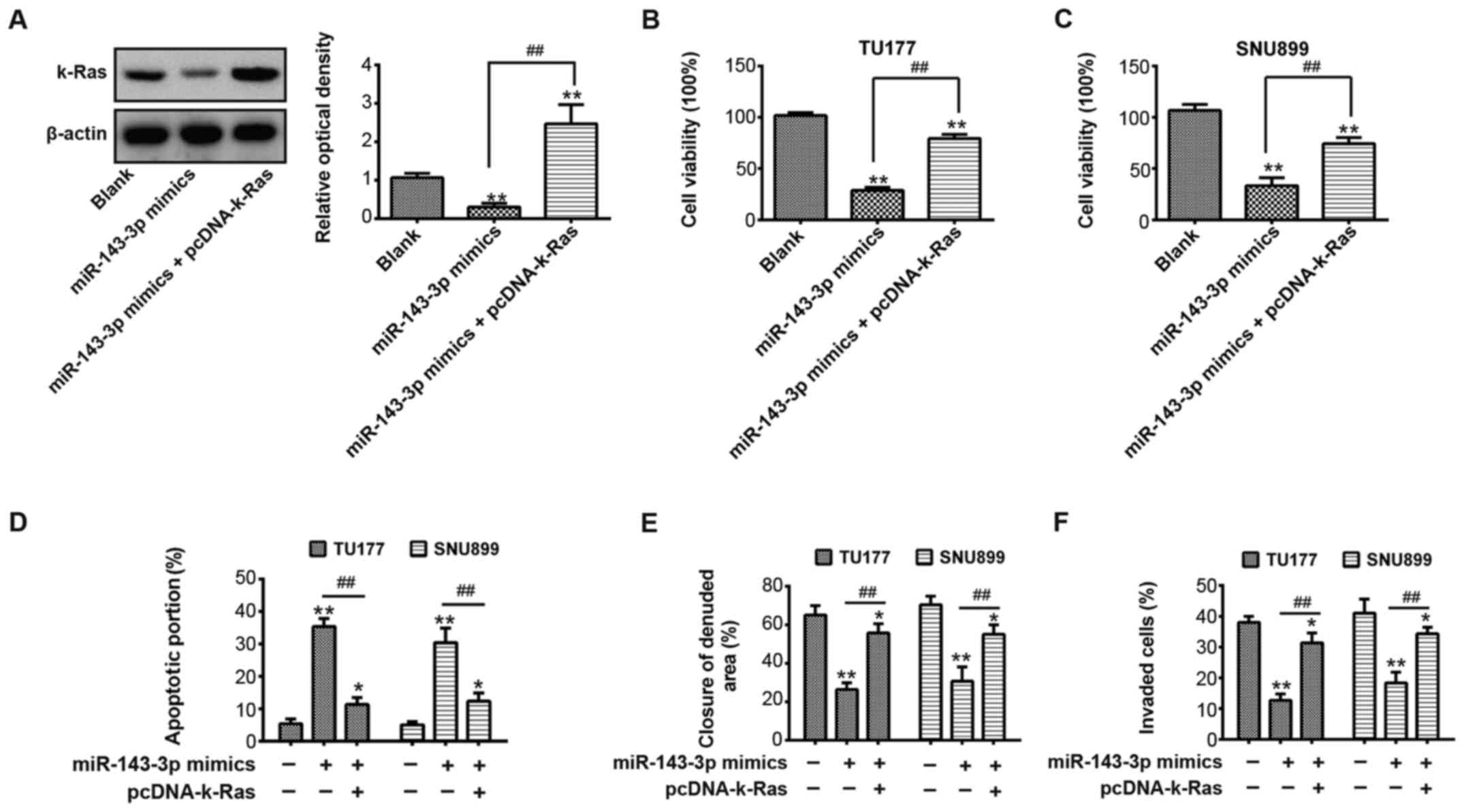 | Figure 5Suppressive effects of miR-143-3p on
laryngeal squamous cell carcinoma cells are rescued by
overexpression of k-Ras. TU177 or SNU899 cells were transfected
with miR-143-3p mimics or were co-transfected with miR-143-3p
mimics and the pcDNA-k-Ras plasmid. (A) Expression of k-Ras was
measured in TU177 cells using western blot analysis. β-actin served
as an internal control. **P<0.01, vs blank group.
##P<0.01, vs. miR-143-3p mimics group. A Cell
Counting-Kit-8 assay was used to determine the cell viability of
(B) TU177 and (C) SNU899 cells. **P<0.01, vs. blank
group. ##P<0.01, vs. miR-143-3p mimics group. (D)
Cell apoptosis was measured by flow cytometry in TU177 or SNU899
cells. *P<0.05 and **P<0.01, vs. blank
group. ##P<0.01, vs. miR-143-3p mimics group. (E)
Wound-healing assay was performed to evaluate the cell migration of
TU177 or SNU899 cells. *P<0.05 and
**P<0.01, vs. blank group. ##P<0.01,
vs. miR-143-3p mimics group. (F) Transwell invasion assay was used
to measure the cell invasion of TU177 or SNU899 cells.
*P<0.05 and **P<0.01, vs. blank group.
##P<0.01, vs. miR-143-3p mimics group. Data are
presented as the means ± standard deviation of three individual
experiments. miR, microRNA. |
miR-143-3p suppresses the
k-Ras/Raf/MEK/ERK signaling pathway
The k-Ras/Raf/MEK/ERK intracellular signaling
pathway is involved in the regulation of diverse cellular
functions, including survival, proliferation, apoptosis, motility
and metabolism (45,46). A previous study reported that
miR-221 inhibits cell proliferation in endothelial progenitor cells
via modulating the Raf/MEK/ERK pathway (47). The present study examined whether
the aberrant expression of miR-143-3p regulates the Raf/MEK/ERK
signaling pathway via targeting k-Ras in LSCC cells. To confirm
this hypothesis, the TU177 and SNU899 cells were transfected with
miR-143-3p mimic or NC mimic, and western blot analysis was
performed to detect the levels of k-Ras, Raf/1, MEK1/2 and ERK1/2.
As shown in Fig. 6A-C, the
overexpression of miR-143-3p significantly inhibited the expression
of p-k-Ras, p-Raf/1, p-MEK1/2 and p-ERK1/2 in the TU177 and SNU899
cells compared with the NC mimic (P<0.01). The previous results
demonstrated that miR-143-3p was downregulated in LSCC tissues. To
confirm whether the lower expression of miR-143-3p is associated
with augmented expression of Raf/1 and ERK1/2 in LSCC, clinical
tumor samples were collected for the immunohistochemistry assay. As
shown in Fig. 6D and E, a lower
expression of miR-143-3p in LSCC tissues was associated with
increased expression of p-Raf/1 and p-ERK1/2. These data suggested
that miR-143-3p may suppress cell growth, migration and invasion in
LSCC via repressing the k-Ras/Raf/MEK/ERK signaling pathway.
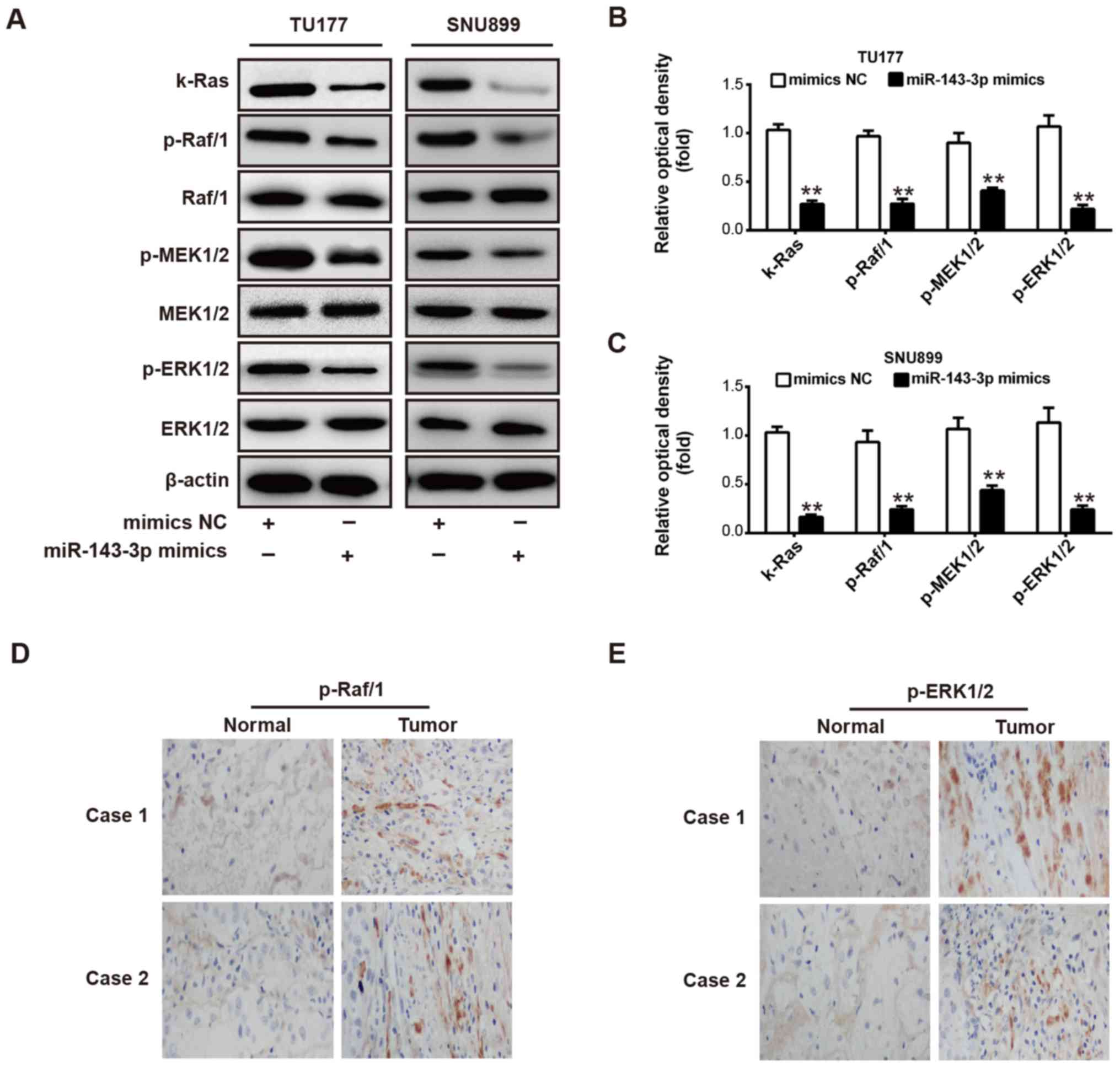 | Figure 6miR-143-3p modulates the
k-Ras/Raf/MEK/ERK signaling pathway. (A) TU177 and SNU899 cells
were transfected with miR-143-3p mimic or NC mimic, and western
blot analysis was used to detect the expression of k-Ras, Raf/1,
MEK1/2 and ERK1/2. Levels of k-Ras, Raf/1, MEK1/2 and ERK1/2 are
shown in bar graphs for (B) TU177 and (C) SNU899 cells,
respectively. Immunohistochemistry assays were used to detect the
expression of (D) p-Raf/1 and (E) p-ERK1/2 in laryngeal squamous
cell carcinoma and adjacent normal tissues, respectively
(magnification, ×100). Data are presented as the mean ± standard
deviation of three individual experiments. **P<0.01,
vs. NC. miR, microRNA; NC, negative control; MEK, mitogen-activated
protein kinase; ERK, extracellular signal-regulated kinase; p-,
phosphorylated. |
Downregulation of miR-143-3p suppresses
the mitochondrial apoptotic pathway and induces the EMT
cascade
Our data provided direct evidence that miR-143-3p
was downregulated in LSCC tissues and that a low expression of
miR-143-3p predicts the poor prognosis of patients with LSCC,
suggesting that miR-143-3p may serve as a potential prognostic
biomarker and therapeutic target. The downregulation of miR-143-3p
in LSCC resulted in k-Ras, p-Raf/1, p-MEK1/2 and p-ERK1/2
upregulation, inhibited the mitochondrial apoptotic pathway and
activated the EMT cascade, which is associated diverse cellular
responses, including cell growth and metastasis (Fig. 7).
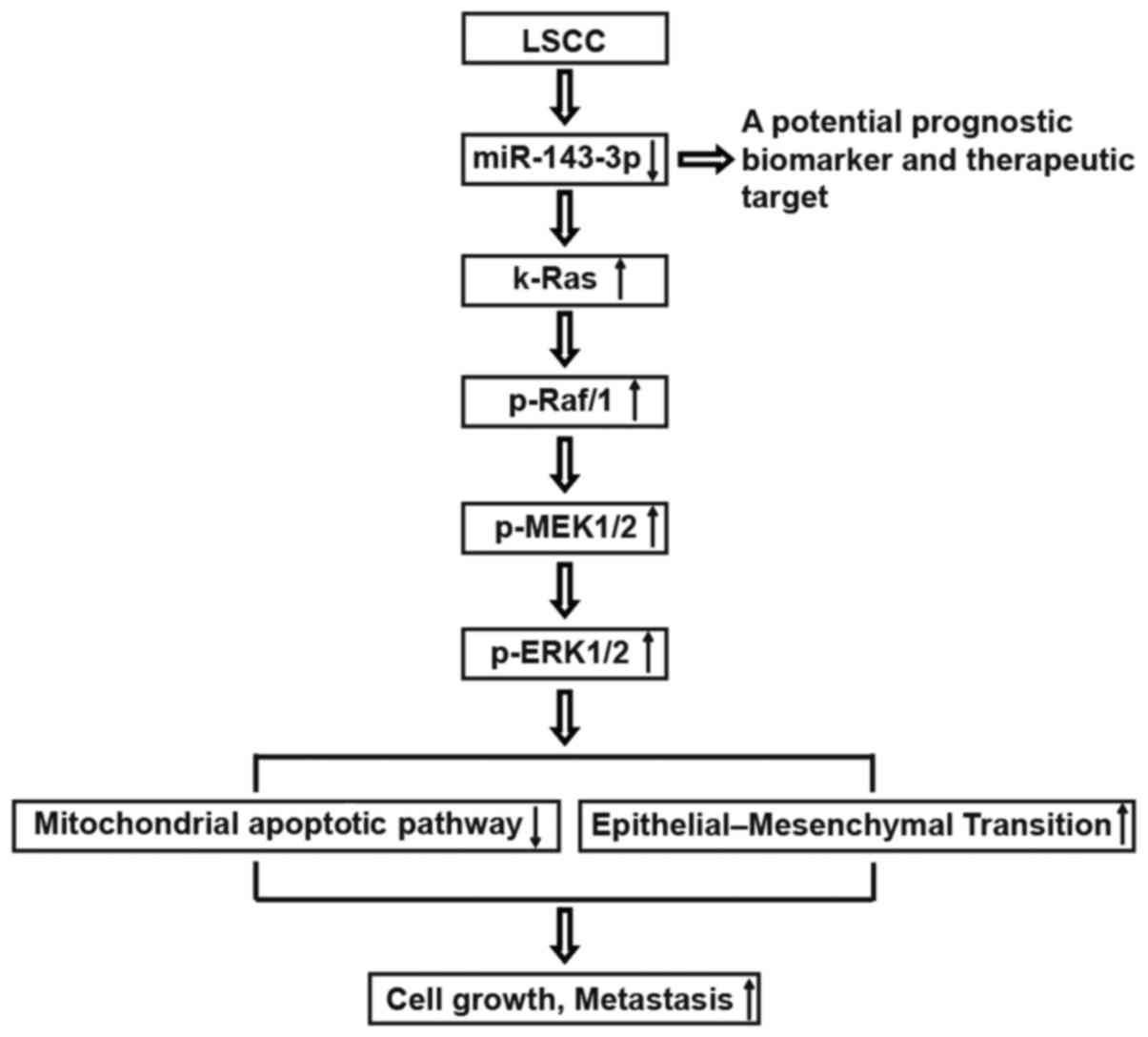 | Figure 7Schematic diagram of the regulatory
and signaling network of miR-143-3p in LSCC. The schematic diagram
illustrates the inducing effect of miR-143-3p on the
k-Ras/Raf/MEK/ERK signaling pathway in LSCC. Downregulation of
miR-143-3p in LSCC induces the upregulation of k-Ras, p-Raf/1,
p-MEK1/2 and p-ERK1/2, inhibits the mitochondrial apoptotic pathway
and activates the epithelial-mesenchymal transition cascade, which
is involved in diverse cellular responses, including cell growth
and metastasis. LSCC, laryngeal squamous cell carcinoma; miR,
microRNA; MEK, mitogen-activated protein kinase; ERK, extracellular
signal-regulated kinase; p-, phosphorylated. |
Discussion
miRNAs are reported to act as key regulator in the
development of cancer through inhibiting specific genes involved in
cellular mechanisms, including drug resistance, proliferation,
apoptosis, invasion and metastasis (8,48).
Previous studies have also demonstrated that several miRNAs can
function as a tumor suppressor in LSCC by suppressing cellular
proliferation, invasion, and promoting apoptosis, including miR-203
and miR-370 (3,11). However, the precise molecular
mechanisms for aberrant miRNA expression in LSCC require further
investigation for a more detailed understanding. In the present
study, the miRNA expression profiles in LSCC were investigated and
the molecular mechanism underlying the biological function of
miRNAs in the development of LSCC were examined. It was found that
miR-143-3p was downregulated in LSCC tissues and that the ectopic
expression of miR-143-3p predicted poor prognosis in LSCC. The
overexpression of miR-143-3p inhibited cellular proliferation and
promoted apoptosis in vitro, and suppressed tumor growth
in vivo. In addition, the upregulation of miR-143-3p
repressed cell migration and invasion via suppressing EMT and
MMP-9. The results demonstrated that miR-143-3p may exert
anticancer effects on LSCC via targeting the k-Ras/Raf/MEK/ERK
signaling pathway.
Previous studies have reported that miR-143-3p is
down-regulated in different types of cancer and functions as a
tumor suppressor to control various biological processes, including
cell proliferation, apoptosis and migration (12,13).
However, the role of miR-143-3p in LSCC remained unclear. In the
present study, microarray analysis showed that the expression
levels of several miRNAs were altered in LSCC tissues, with
miR-143-3p being downregulated the most compared with normal
tissues. Therefore, miR-143-3p was selected in the present study
and its function in the development of LSCC was investigated. The
results demonstrated that a low expression of miR-143-3p was
positively associated with T classification, differentiation, lymph
node metastasis and clinical stage in patients with LSCC.
Kaplan-Meier survival analysis illustrated that patients with a low
expression of miR-143-3p had a lower overall survival rates. These
data suggested that the downregulation of miR-143-3p may be key in
the development and progression of LSCC. Subsequently, it was
demonstrated that the upregulation of miR-143-3p markedly inhibited
cell proliferation and induced apoptosis in vitro.
Furthermore, miR-143-3p-induced apoptosis in LSCC cells modulated
the levels of apoptosis-related proteins (cleaved-caspase-3, Bax
and Bcl-2). It is reported that Bcl-2 is involved in mitochondria
and that a mitochondrial apoptotic pathway may be
caspase-3-independent (49,50).
Therefore, it was hypothesized that miR-143-3p may promote
apoptosis in LSCC cells through regulation of the mitochondrial
apoptotic pathway. Additionally, the xenograft model demonstrated
that the overexpression of miR-143-3p suppressed tumor growth in
vivo. Taken together, these results suggested that a low
expression of miR-143-3p was significantly associated with poor
prognosis in patients with LSCC, and acts as a tumor suppressor in
LSCC tumorigenesis.
Cell invasion and migration are known to be key
factors in malignant progression and metastasis. A previous study
also indicated that recurrence and metastasis are major factors
limiting the successful treatment of LSCC (4). To further evaluate the effects of
miR-143-3p on tumor cell metastasis, Transwell invasion and
wound-healing assays were performed to investigate LSCC cell
invasion and migration following the upregulation of miR-143-3p. It
was found that the overexpression of miR-143-3p significantly
repressed cell migration and invasion in vitro. EMT is one
of the key molecular steps in the process of distant metastasis,
which results in invasion and emigration in different types of
cancer (51,52). The EMT cascade can result in loss
of apicobasolateral polarity and dissolution of cell-cell
junctions, causing the formation of migratory mesenchymal cells
with invasive properties (42). A
previous study reported that miR-143-3p serves as a tumor
suppressor via regulating the EMT pathway in esophageal squamous
cell carcinoma (12).
Additionally, MMP-9 was shown to be involved in the development of
EMT phenotype in LSCC (41), and
patients with LSCC with a low expression of MMP-9 had a higher
5-year survival rate (53).
Therefore, a western blot assay was performed in the present study
to detect the protein expression of EMT markers (E-cadherin,
N-cadherin and vimentin) and MMP-9 in LSCC cells following the
overexpression of miR-143-3p. The results demonstrated that the
upregulation of miR-143-3p increased the protein level of
E-cadherin and reduced the levels of N-cadherin, vimentin and
MMP-9. Collectively, these data indicated that miR-143-3p repressed
cell migration and invasion via inhibiting the EMT cascade and
expression of MMP-9 in LSCC cells.
The oncogene k-Ras modulates a multilayered
signaling network in various cancer cells, including proliferation,
apoptosis, survival, transformation and EMT (21-24,54).
k-Ras is considered to be one of the most prominent oncogenes owing
to its ability to transform human cells into malignant tumor cells,
with missense mutations at codons 12 or 13 (55). In addition, miR-143-3p has been
shown to act as a tumor suppressor via targeting oncogene k-Ras in
renal cell carcinoma (44). In the
present study, it was confirmed that k-Ras is a direct target of
miR-143-3p in LSCC cells. In addition, the expression levels of
k-Ras in LSCC tumor tissues were inversely correlated with levels
of miR-143-3p. The overexpression of k-Ras abrogated the
suppressive effects of miR-143-3p on cell growth, migration and
invasion in LSCC cells. These results suggested that miR-143-3p
possessed suppressive effects on LSCC cells through targeting
k-Ras.
Raf/1 is one of the RAF kinase family members, also
termed c-Raf (56). Evidence
indicates that Raf/1 is involved in the regulation of endothelial
apoptosis and angiogenesis, which is essential in the development
and metastasis of tumors (57,58).
Raf/1 protein phosphorylates MEK1/2, which can phosphorylate ERK1/2
(56). A previous study documented
that k-Ras activated the Raf/MEK/ERK pathway, occurring in ~30% of
cancer cases (16). Therefore, to
evaluate whether miR-143-3p can regulate the Raf/MEK/ERK pathway in
LSCC cells via targeting k-Ras, the present study measured the
levels of k-Ras, Raf/1, MEK1/2 and ERK1/2 in LSCC cells transfected
with miR-143-3p mimic or NC mimic. The western blot analysis showed
that the overexpression of miR-143-3p resulted in the
downregulation of p-k-Ras, p-Raf/1, p-MEK1/2 and p-ERK1/2.
Furthermore, the results demonstrated that a low expression of
miR-143-3p in LSCC tissues was associated with increased expression
of p-Raf/1 and p-ERK1/2. Taken together, these data suggested that
miR-143-3p may inhibit cell growth, migration and invasion in LSCC
through suppressing the k-Ras/Raf/MEK/ERK signaling pathway.
In conclusion, the results of the present study
demonstrated that miR-143-3p was downregulated in LSCC tumor
tissues, and that the low expression of miR-143-3p predicted poor
prognosis in LSCC. The overexpression of miR-143-3p suppressed
tumor cell growth and metastasis in vitro and in
vivo. Furthermore, it was found that miR-143-3p possesses
suppressive effects on LSCC via inhibiting the k-Ras/Raf/MEK/ERK
signaling pathway. The effects of miR-143-3p may be closely
associated with the mitochondrial apoptotic pathway and EMT cascade
(Fig. 7). Collectively, these
findings provide evidence supporting the role of miR-143-3p as a
tumor suppressor in LSCC by targeting k-Ras.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during the present
study are included in the published article.
Authors’ contributions
FZ performed the experiments, contributed to data
analysis and wrote the manuscript. HC analyzed the data. FZ and HC
conceptualized the study design, contributed to data analysis and
experimental materials. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All patients provided written informed consent for
the use of the tumor tissues for clinical investigation. The
Institute Research Medical Ethics Committee of Zhengzhou University
granted approval for the study. All animal procedures were approved
by the Animal Care Committee of the Zhengzhou University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Chu EA and Kim YJ: Laryngeal cancer:
diagnosis and preoperative work-up. Otolaryngol Clin North Am.
41:673–695. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tian Y, Fu S, Qiu GB, Xu ZM, Liu N, Zhang
XW, Chen S, Wang Y, Sun KL and Fu WN: MicroRNA-27a promotes
proliferation and suppresses apoptosis by targeting PLK2 in
laryngeal carcinoma. BMC Cancer. 14:6782014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ren J, Zhu D, Liu M, Sun Y and Tian L:
Downregulation of miR-21 modulates Ras expression to promote
apoptosis and suppress invasion of Laryngeal squamous cell
carcinoma. Eur J Cancer. 46:3409–3416. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rudolph E, Dyckhoff G, Becher H, Dietz A
and Ramroth H: Effects of tumour stage, comorbidity and therapy on
survival of laryngeal cancer patients: A systematic review and a
meta-analysis. Eur Arch Otorhinolaryngol. 268:165–179. 2011.
View Article : Google Scholar
|
|
5
|
Croce CM: Causes and consequences of
microRNA dysregulation in cancer. Nat Rev Genet. 10:704–714. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhuang LK, Xu GP, Pan XR, Lou YJ, Zou QP,
Xia D, Yan WW, Zhang YT, Jia PM and Tong JH: MicroRNA-181a-mediated
downregulation of AC9 protein decreases intracellular cAMP level
and inhibits ATRA-induced APL cell differentiation. Cell Death Dis.
5:e11612014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang B, Pan X, Cobb GP and Anderson TA:
microRNAs as oncogenes and tumor suppressors. Dev Biol. 302:1–12.
2007. View Article : Google Scholar
|
|
9
|
Shen Z, Zhan G, Ye D, Ren Y, Cheng L, Wu Z
and Guo J: MicroRNA-34a affects the occurrence of laryngeal
squamous cell carcinoma by targeting the antiapoptotic gene
survivin. Med Oncol. 29:2473–2480. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tian L, Li M, Ge J, Guo Y, Sun Y, Liu M
and Xiao H: miR-203 is downregulated in laryngeal squamous cell
carcinoma and can suppress proliferation and induce apoptosis of
tumours. Tumour Biol. 35:5953–5963. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yungang W, Xiaoyu L, Pang T, Wenming L and
Pan X: miR-370 targeted FoxM1 functions as a tumor suppressor in
laryngeal squamous cell carcinoma (LSCC). Biomed Pharmacother.
68:149–154. 2014. View Article : Google Scholar
|
|
12
|
He Z, Yi J, Liu X, Chen J, Han S, Jin L,
Chen L and Song H: miR-143-3p functions as a tumor suppressor by
regulating cell proliferation, invasion and epithelial-mesenchymal
transition by targeting QKI-5 in esophageal squamous cell
carcinoma. Mol Cancer. 15:512016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li D, Hu J, Song H, Xu H, Wu C, Zhao B,
Xie D, Wu T, Zhao J and Fang L: miR-143-3p targeting LIM domain
kinase 1 suppresses the progression of triple-negative breast
cancer cells. Am J Transl Res. 9:2276–2285. 2017.PubMed/NCBI
|
|
14
|
Campbell SL, Khosravi-Far R, Rossman KL,
Clark GJ and Der CJ: Increasing complexity of Ras signaling.
Oncogene. 17(11 Reviews): 1395–1413. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vojtek AB and Der CJ: Increasing
complexity of the Ras signaling pathway. J Biol Chem.
273:19925–19928. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Campbell PM, Groehler AL, Lee KM,
Ouellette MM, Khazak V and Der CJ: K-Ras promotes growth
transformation and invasion of immortalized human pancreatic cells
by Raf and phosphatidylinositol 3-kinase signaling. Cancer Res.
67:2098–2106. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim MJ, Woo SJ, Yoon CH, Lee JS, An S,
Choi YH, Hwang SG, Yoon G and Lee SJ: Involvement of autophagy in
oncogenic K-Ras-induced malignant cell transformation. J Biol Chem.
286:12924–12932. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Voice JK, Klemke RL, Le A and Jackson JH:
Four human ras homologs differ in their abilities to activate
Raf-1, induce transformation, and stimulate cell motility. J Biol
Chem. 274:17164–17170. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Maehara Y, Saeki H and Morita M: Molecular
mechanisms of esophageal squamous cell carcinogenesis: Clues to
improve treatment outcomes. Ann Thorac Cardiovasc Surg. 16:387–388.
2010.
|
|
20
|
Keohavong P, Mady HH, Gao WM, Siegfried
JM, Luketich JD and Melhem MF: Topographic analysis of K-ras
mutations in histologically normal lung tissues and tumours of lung
cancer patients. Br J Cancer. 85:235–241. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Karnoub AE and Weinberg RA: Ras oncogenes:
Split personalities. Nat Rev Mol Cell Biol. 9:517–531. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pylayeva-Gupta Y, Grabocka E and Bar-Sagi
D: RAS oncogenes: Weaving a tumorigenic web. Nat Rev Cancer.
11:761–774. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Samatar AA and Poulikakos PI: Targeting
RAS-ERK signalling in cancer: Promises and challenges. Nat Rev Drug
Discov. 13:928–942. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cox AD, Fesik SW, Kimmelman AC, Luo J and
Der CJ: Drugging the undruggable RAS: Mission possible? Nat Rev
Drug Discov. 13:828–851. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hatley ME, Patrick DM, Garcia MR,
Richardson JA, Bassel-Duby R, van Rooij E and Olson EN: Modulation
of K-Ras-dependent lung tumorigenesis by MicroRNA-21. Cancer Cell.
18:282–293. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou K, Luo X, Wang Y, Cao D and Sun G:
MicroRNA-30a suppresses tumor progression by blocking
Ras/Raf/MEK/ERK signaling pathway in hepatocellular carcinoma.
Biomed Pharmacother. 93:1025–1032. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou L, Rui JA, Ye DX, Wang SB, Chen SG
and Qu Q: Edmondson-Steiner grading increases the predictive
efficiency of TNM staging for long-term survival of patients with
hepatocellular carcinoma after curative resection. World J Surg.
32:1748–1756. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ma J, Ren Y, Zhang L, Kong X, Wang T, Shi
Y and Bu R: Knocking-down of CREPT prohibits the progression of
oral squamous cell carcinoma and suppresses cyclin D1 and c-Myc
expression. PLoS One. 12:e01743092017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Δ ΔC(T)) Method. Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
30
|
Lai YJ, Lin CI, Wang CL and Chao JI:
Expression of survivin and p53 modulates honokiol-induced apoptosis
in colorectal cancer cells. J Cell Biochem. 115:1888–1899.
2014.PubMed/NCBI
|
|
31
|
Schipper JH, Frixen UH, Behrens J, Unger
A, Jahnke K and Birchmeier W: E-cadherin expression in squamous
cell carcinomas of head and neck: Inverse correlation with tumor
dedifferentiation and lymph node metastasis. Cancer Res.
51:6328–6337. 1991.PubMed/NCBI
|
|
32
|
Gotoda T, Yanagisawa A, Sasako M, Ono H,
Nakanishi Y, Shimoda T and Kato Y: Incidence of lymph node
metastasis from early gastric cancer: Estimation with a large
number of cases at two large centers. Gastric Cancer. 3:219–225.
2000. View Article : Google Scholar
|
|
33
|
Hartgrink HH, van de Velde CJ, Putter H,
Bonenkamp JJ, Klein Kranenbarg E, Songun I, Welvaart K, van Krieken
JH, Meijer S, Plukker JT, et al: Extended lymph node dissection for
gastric cancer: Who may benefit? Final results of the randomized
Dutch gastric cancer group trial. J Clin Oncol. 22:2069–2077. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bergers G, Brekken R, McMahon G, Vu TH,
Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, et al:
Matrix metallopro-teinase-9 triggers the angiogenic switch during
carcinogenesis. Nat Cell Biol. 2:737–744. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kang Y and Massagué J:
Epithelial-mesenchymal transitions: Twist in development and
metastasis. Cell. 118:277–279. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Natalwala A, Spychal R and Tselepis C:
Epithelial-mesenchymal transition mediated tumourigenesis in the
gastrointestinal tract World. J Gastroenterol. 14:3792–3797.
2008.
|
|
37
|
Eriksen JG, Steiniche T, Sogaard H and
Overgaard J: Expression of integrins and E-cadherin in squamous
cell carcinomas of the head and neck. APMIS. 112:560–568. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang SY, Lu ZM, Lin YF, Chen LS, Luo XN,
Song XH, Chen SH and Wu YL: miR-144-3p, a tumor suppressive
microRNA targeting ETS-1 in laryngeal squamous cell carcinoma.
Oncotarget. 7:11637–11650. 2016.PubMed/NCBI
|
|
39
|
Schmalhofer O, Brabletz S and Brabletz T:
E-cadherin, beta-catenin, and ZEB1 in malignant progression of
cancer. Cancer Metastasis Rev. 28:151–166. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li JZ, Gao W, Lei WB, Zhao J, Chan JY, Wei
WI, Ho WK and Wong TS: MicroRNA 744-3p promotes MMP-9-mediated
metastasis by simultaneously suppressing PDCD4 and PTEN in
laryngeal squamous cell carcinoma. Oncotarget. 7:58218–58233.
2016.PubMed/NCBI
|
|
41
|
Zhang W, Liu Y and Wang CW: S100A4
promotes squamous cell laryngeal cancer Hep-2 cell invasion via
NF-κB/MMP-9 signal. Eur Rev Med Pharmacol Sci. 18:1361–1367.
2014.
|
|
42
|
Hur K, Toiyama Y, Takahashi M, Balaguer F,
Nagasaka T, Koike J, Hemmi H, Koi M, Boland CR and Goel A:
MicroRNA-200c modulates epithelial-to-mesenchymal transition (EMT)
in human colorectal cancer metastasis. Gut. 62:1315–1326. 2013.
View Article : Google Scholar :
|
|
43
|
Han Z, Yang Q, Liu B, Wu J, Li Y, Yang C
and Jiang Y: MicroRNA-622 functions as a tumor suppressor by
targeting K-Ras and enhancing the anticarcinogenic effect of
resveratrol. Carcinogenesis. 33:131–139. 2012. View Article : Google Scholar
|
|
44
|
Zhai W, Sun Y, Guo C, Hu G, Wang M, Zheng
J, Lin W, Huang Q, Li G, Zheng J, et al: lncRNA-SARCC suppresses
renal cell carcinoma (RCC) progression via altering the androgen
receptor(AR)/miRNA-143-3p signals. Cell Death Differ. 24:1502–1517.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ramos JW: The regulation of extracellular
signal-regulated kinase (ERK) in mammalian cells. Int J Biochem
Cell Biol. 40:2707–2719. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
McCubrey JA, Steelman LS, Chappell WH,
Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M,
Tafuri A, et al: Roles of the Raf/MEK/ERK pathway in cell growth,
malignant transformation and drug resistance. Biochim Biophys Acta.
1773:1263–1284. 2007. View Article : Google Scholar
|
|
47
|
Zhang X, Mao H, Chen JY, Wen S, Li D, Ye M
and Lv Z: Increased expression of microRNA-221 inhibits PAK1 in
endothelial progenitor cells and impairs its function via
c-Raf/MEK/ERK pathway. Biochem Biophys Res Commun. 431:404–408.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Coburn GA and Cullen BR: siRNAs: A new
wave of RNA-based therapeutics. J Antimicrob Chemother. 51:753–756.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Cheng EH, Wei MC, Weiler S, Flavell RA,
Mak TW, Lindsten T and Korsmeyer SJ: BCL-2, BCL-X(L) sequester BH3
domain-only molecules preventing BAX- and BAK-mediated
mitochondrial apoptosis. Mol Cell. 8:705–711. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ochs K and Kaina B: Apoptosis induced by
DNA damage O6-methylguanine is Bcl-2 and caspase-9/3 regulated and
Fas/caspase-8 independent. Cancer Res. 60:5815–5824.
2000.PubMed/NCBI
|
|
51
|
Yang J and Weinberg RA:
Epithelial-mesenchymal transition: At the crossroads of development
and tumor metastasis. Dev Cell. 14:818–829. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Jou J and Diehl AM: Epithelial-mesenchymal
transitions and hepatocarcinogenesis. J Clin Invest. 120:1031–1034.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Gou X, Chen H, Jin F, Wu W, Li Y, Long J,
Gong X, Luo M, Bi T, Li Z, et al: Expressions of CD147, MMP-2 and
MMP-9 in laryngeal carcinoma and its correlation with poor
prognosis. Pathol Oncol Res. 20:475–481. 2014. View Article : Google Scholar
|
|
54
|
Malumbres M and Barbacid M: RAS oncogenes:
The first 30 years. Nat Rev Cancer. 3:459–465. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Shimizu K, Goldfarb M, Suard Y, Perucho M,
Li Y, Kamata T, Feramisco J, Stavnezer E, Fogh J and Wigler MH:
Three human transforming genes are related to the viral ras
oncogenes. Proc Natl Acad Sci USA. 80:2112–2116. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Davies H, Bignell GR, Cox C, Stephens P,
Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W,
et al: Mutations of the BRAF gene in human cancer. Nature.
417:949–954. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Alavi A, Hood JD, Frausto R, Stupack DG
and Cheresh DA: Role of Raf in vascular protection from distinct
apoptotic stimuli. Science. 301:94–96. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Hood JD, Bednarski M, Frausto R, Guccione
S, Reisfeld RA, Xiang R and Cheresh DA: Tumor regression by
targeted gene delivery to the neovasculature. Science.
296:2404–2407. 2002. View Article : Google Scholar : PubMed/NCBI
|