Introduction
Two- or three-dimensional actin assembly is a
prerequisite for formation of actin-rich protrusions such as
lamellipodia and filopodia, which are required for migration,
invasion, and metastasis of cancer cells (1). Actin filaments in lamellipodia
exhibit complex branching and combine with each other to form a
mesh-like structure, while those in filopodia are bundled by
various actin-crosslinking and -binding proteins (2).
The F-actin-binding protein cortactin contributes to
cell migration and invasion (3)
and has three functional domains: an N-terminal acidic region that
binds to the Arp2/3 complex, an F-actin-binding cortactin repeat
region that contains 6.5 tandem repeats, and a C-terminal Src
homology 3 (SH3) region that binds to proline-rich domain
(PRD)-containing proteins, such as dynamin (4-6),
Wiskott-Aldrich syndrome protein (WASP) (7) and WASP-interacting protein (8).
Dynamin, a well-known endocytic protein required for
vesicle scission, copolymerizes with cortactin to form a ring-like
complex via an interaction between the PRD of dynamin and the SH3
domain of cortactin, and this complex bundles and stabilizes actin
filaments (5,6). Actin bundling by the
dynamin-cortactin complex regulates formation of filopodia in human
neuroblastoma cells (5) and
non-small lung carcinoma cells (6)
and is thus implicated in cell motility. However, the mechanism by
which this complex regulates actin bundling is largely unknown.
Cyclin-dependent kinase 5 (CDK5) is a
proline-directed serine/threonine kinase belonging to the
cyclin-dependent kinase family (9). The role of CDK5 has been mostly
studied in terminally differentiated cells, including neurons and
neuroendocrine cells. CDK5 contributes to neurite outgrowth,
neuronal migration, and neuronal and β cell differentiation
(10,11). In addition, it has recently been
demonstrated that CDK5 is highly expressed in several cancer cell
lines and tissues, suggesting this kinase is functionally
associated with tumorigenesis and/or malignancy (12). Furthermore, CDK5 participates in
actin-based cancer cell motility, including migration, invasion
(13) and metastasis of pancreatic
cancer cells (14). However, it
remains unknown whether CDK5-mediated phosphorylation regulates
actin-based motility.
The present study investigated phosphorylation of
cortactin by CDK5 in vitro and its effect on actin bundling
and complex formation with dynamin in vitro and in
glioma-derived cells. The potential roles of CDK5 and cortactin in
anticancer therapy are also discussed.
Materials and methods
Antibodies and reagents
A rabbit polyclonal antibody against c-myc (C3956)
and a mouse monoclonal antibody against β-actin (clone AC-15) were
purchased from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). A
mouse monoclonal antibody against c-myc (sc-40) was purchased from
Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Mouse monoclonal
antibodies against cortactin (clone 4F-11; cat. no. 05-180) and
CDK5 (clone DC17; cat. no. 05-364) were purchased from EMD
Millipore (Billerica, MA, USA). Rabbit polyclonal anti-dynamin 1
antibodies (PA1-660), Alexa Fluor 488-conjugated anti-rabbit
immunoglobulin G (IgG; cat. no. A21206), rhodamine-conjugated
anti-mouse IgG (cat. no. R6393), rhodamine- (cat. no. R415) or
Alexa Fluor 488-labeled phalloidin (cat. no. A12379), Horseradish
peroxidase-coniugated goat anti-rabbit IgG (H+L; cat. no. 31460)
and rabbit anti-mouse IgG (H+L; cat. no. 31450) were purchased from
Thermo Fisher Scientific, Inc. (Waltham, MA, USA).
Cell culture
NG108-15 (HB-12317) cells were obtained from the
American Type Culture Collection (Manassas, VA, USA) and cultured
in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal
bovine serum (FBS; cat. no. 26140079) (both from Thermo Fisher
Scientific, Inc.) at 37°C in 5% CO2. To induce
differentiation of NG108-15 cells, cells were treated with 1 mM
dibutyryl-cyclic-AMP (cat. no. D0627; Sigma-Aldrich; Merck KGaA) at
37°C for 48 h.
Expression and purification of cortactin
and its mutants
cDNA encoding full-length rat cortactin was produced
as previously described (5).
Glutathione-S-transferase (GST)-tagged W525K, T145D, T219D,
T145DT219D and T145AT219A mutants were generated by mutating
cortactin in pGEX-6P using a QuickChange Site-Directed Mutagenesis
kit (Agilent Technologies, Inc., Santa Clara, CA, USA). GST-tagged
proteins were expressed in Escherichia coli (cat. no.
200131; Agilent Technologies, Inc.) and purified as described
previously (5). Histidine-tagged
human dynamin 1 was expressed using the Bac-to-Bac baculoviral
expression system (Thermo Fisher Scientific, Inc.) and purified as
described previously (5). Purified
dynamin solutions were concentrated using Centriplus YM50 (EMD
Millipore). Protein solutions (1-2 mg/ml protein) were stored at
−80°C and thawed at 37°C prior to use.
For protein expression in cells, wild-type (WT)
cortactin and the T145D, T219D and T145DT219D mutants were
separately subcloned into the pEF1 myc-His vector (Thermo Fisher
Scientific, Inc.) as EcoRI/XbaI fragments. The
nucleotide sequences of the constructs were verified using a BigDye
Terminator v3.1 Cycle Sequencing kit (Thermo Fisher Scientific,
Inc.). These vectors were transfected using Lipofectamine LTX
(Thermo Fisher Scientific, Inc.) according to the manufacturer’s
manual. Following 48 h of transfection, transfected cells were used
for subsequent experiments.
Fluorescence microscopy
NG108-15 cells were fixed for 15 min in 4%
paraformaldehyde (PFA) and processed for immunofluorescence
analysis as described previously (6). To measure filopodial length,
non-transfected cells and those transfected with WT or mutant
cortactin were fixed as described above and stained with Alexa
Fluor 488-conjugated phalloidin (1:40) for 1 h. All steps were
performed at room temperature. Digital images of actin-rich
structures, including filopodia, lamellipodia, and cellular
protrusions, were acquired at ×100 magnification. Up to three
filopodia per cellular protrusion were randomly selected, and their
lengths were measured using ImageJ software, version 1.40g
(National Institutes of Health, Bethesda, MD, USA).
Migration assay
NG108-15 cells (1.6×105/well) in a 6-well
plate were transfected with 2.5 µg of myc-tagged WT or
T145DT219D cortactin using Lipofectamine LTX. Following 6 h
transfection, cells were re-seeded in 35 mm glass base dishes (with
grid; AGC Techno Glass Co., Ltd., Shizuoka, Japan) precoated with
poly-L-lysin, and further cultured with 10% FBS/DMEM for 48 h.
Time-lapsed imaging of the cells was performed using differential
interference contrast microscopy (DIC) at ×100 magnification for 4
h at 30-sec intervals. During the experiments, the cells were
maintained at 37°C under 5% CO2. To determine the WT or
T145DT219D cortactin expressing cells, cells were stained with
anti-myc antibodies (1:300) at room temperature for 1 h following
the time lapse-imaging. Images were acquired by MetaMorph software
version 7.8.13.0. (Universal Imaging, Inc., Bedford Hills, NY,
USA). The migration path was processed using ImageJ, Adobe
Photoshop CS3 version 10 or Illustrator CS3 software version 13
(Adobe Systems, Inc., San Jose, CA, USA).
Phosphorylation assay
In vitro phosphorylation reactions were
performed in cytosolic buffer (25 mM HEPES-KOH, 25 mM KCl, 2.5 mM
magnesium acetate and 100 mM potassium glutamate, pH 7.2)
containing 0.5 mM ATP and 300 dpm/pmol [γ32P]-ATP.
Purified WT, T145AT219A cortactin or dynamin 1 was incubated with 1
µg/ml recombinant CDK5/p35 (Merck KGaA) at 30°C for 1 h.
Reactions were terminated by addition of SDS sample buffer and
subsequent boiling. The reaction products were analyzed by SDS-PAGE
in 10% polyacrylamide gel followed by SYPRO Orange staining at room
temperature for 1 h and autoradiography. Autoradiography images
were scanned using a FLA7000 Imager (Fuji Photo Film Co., Ltd.,
Tokyo, Japan). The results were obtained from independent two
experiments.
Actin-crosslinking assay
Non-muscle actin (Cytoskeleton, Inc., Denver, CO,
USA) was polymerized in F-buffer (5 mM Tris-HCl, 0.5 mM DTT, 0.2 mM
CaCl2, 2 mM MgCl2, 50 mM KCl and 1 mM ATP, pH
7.5) for 1 h. Thereafter, 3.3 µM F-actin was incubated with
5 µM WT cortactin or its phosphomimetic mutants alone or
together with dynamin 1 for 1 h, and then with 3 µM Alexa
Fluor 488-conjugated phalloidin for an additional 30 min. Samples
were dispersed on glass slides and mounted. All steps were
performed at room temperature. Cross-linked or bundled F-actin was
observed under epifluorescence microscopy at ×400 magnification.
When necessary, images were processed using Adobe Photoshop CS3 or
Illustrator CS3 software.
In vitro actin assembly assay
Actin assembly was quantified using pyrene-actin as
described previously (5). Briefly,
11.4 µM pyrene-actin (AP05; Cytoskeleton, Inc.) prepared in
assay buffer (5 mM Tris-HCl, 50 mM KCl, 2 mM MgCl2, 0.2
mM CaCl2 and 1 mM ATP, pH 7.5) was incubated for 60-90
min in a microtiter plate (Sumitomo Bakelite Co., Ltd., Tokyo,
Japan). Pyrene fluorescence at 407 nm (10-nm slit width) was then
measured using a fluorescence microplate reader (MTP-600F; Corona
Electric Co., Ltd., Ibaraki, Japan) with an excitation wavelength
of 365 nm. All steps were performed at room temperature.
Pulldown assay
The GST pulldown assay was performed as described
previously (5). GST-fusion
proteins (10 µg) bound to glutathione-Sepharose beads (GE
Healthcare Life Sciences, Little Chalfont, UK) were incubated with
5 µg recombinant dynamin 1 prepared in 0.1% Tween-20, 100 mM
KCl, 1 mM MgCl2 and 20 mM Tris-HCl (pH 7.8), and a
protease inhibitor cocktail tablet (Roche Diagnostics, Basel,
Switzerland) at 4°C for 1 h. Bead-bound dynamin 1 was separated by
centrifugation at 1,300 × g at 4°C for 3 min, and SDS sample buffer
was added to the sample. After boiling at 95°C for 5 min, the beads
were analyzed by SDS-PAGE in 10% polyacrylamide gel followed by
western blotting.
Preparation of cells and whole brain
homogenate
NG108-15 cells (1×106 cells) or whole
brains of 10 male mice (C57BL/6J; age, 6 weeks; 22-27 g; Japan SLC,
Inc., Hamamatsu, Japan) were homogenized in PBS containing a
protease inhibitor cocktail tablet (Roche Diagnostics) with a
Potter-type glass-Teflon homogenizer. The homogenate was
centrifuged at 20,000 × g for 30 min at 4°C. The supernatant was
sampled in SDS sample buffer. Samples were boiled for 5 min and
subjected to western blotting.
Western blotting
Samples were subjected to SDS-PAGE in 10%
polyacrylamide gel and transferred electrophoretically to a
nitrocellulose membranes (cat. no. 10600003; GE Healthcare Life
Sciences). The membrane was blocked with 140 mM NaCl, 1 mM EDTA, 20
mM Tris-HCl (pH 7.4), containing 0.1% Tween-20 and 5% skimmed milk
for 4 h at room temperature, and incubated with primary antibodies
(1:1,000) for 2 h followed by incubation with horseradish
peroxidase-conjugated secondary antibodies (1:10,000) for 1 h.
Bands were visualized using the ECL western blotting detection
system (cat. no. RPN2106; GE Healthcare Life Sciences).
Protein assay
Protein concentration was determined using a
bicinchoninic acid assay kit (cat. no. 23235; Thermo Fisher
Scientific, Inc.) with bovine serum albumin as the standard.
Morphometric analysis
To measure filopodial length, NG108-15 cells
transfected with WT or mutant cortactin were fixed with 4%
paraformaldehyde for 15 min and stained with Alexa Fluor
488-conjugated phalloidin (1:40) for 1 h. All steps were performed
at room temperature. Actin-rich cellular protrusions with
lamellipodia and filopodia at their tips were imaged. Digital
images were acquired at ×600 magnification. Up to three filopodia
per growth cone were randomly selected, and their lengths were
measured using ImageJ software, version 1.40g.
Electron microscopy
Dynamin 1-cortactin complexes were negatively
stained as described previously (5). Briefly, complexes were formed by
incubating 1 µM dynamin 1 and 1 µM cortactin in
cytosolic buffer at 37°C for 15 min. Samples were absorbed onto a
Formvar- and carbon-coated copper grid and stained with 1.5% uranyl
acetate prepared in ddH2O at room temperature for 2 min.
For morphometric analysis of ring complex formation,
negatively-stained samples with a similar density were imaged at
×20,000 magnification using a transmission electron microscope
(H7650; Hitachi, Ltd., Tokyo, Japan). A total of 6 regions of
interest, corresponding to 0.84 µm2, were randomly selected
and the number of ring complexes was counted. The dynamin
1-cortactin complexes observed were divided into the following four
categories according to the completeness of their circular shape:
Closed rings, incomplete rings that were ≥75% closed, incomplete
rings that were >50% and <75% closed, and rod-like or
irregularly shaped structures that were >60 nm in length.
Mass spectrometry analysis
Phosphorylation sites in cortactin were identified
by mass spectrometry, as described previously (15). Briefly, cortactin was
phosphorylated in vitro by CDK5 and separated by SDS-PAGE in
10% polyacrylamide gel. Polypeptide bands corresponding to
cortactin were excised from the gel and digested with trypsin
(Promega Corporation, Madison, WI, USA). Digested peptides were
extracted with acetonitrile and subjected to matrix-assisted laser
desorption ionization time-of-flight mass spectrometry (MALDI-MS;
Thermo Fisher Scientific, Inc.).
Ethics and Animal Use Statement
The present study was conducted in strict accordance
to the recommendations in the Guide for the Care and Use of
Laboratory Animals in Japan. Animals were housed at 23±2°C with a
12-h light/dark cycle and free access to water and standard rodent
chow in the Department of Animal Resources of Okayama University.
All surgery was performed under general anesthesia with
sevoflurane, and all efforts were made to minimize animal
suffering. After sacrificing mice, whole brains were removed.
Statistical analysis
Statistical analyses were performed using
KaleidaGraph software for Macintosh, version 4.1 (Synergy Software,
Inc., Essex Junction, VT, USA). One-way analysis of variance and
Tukey’s least significant difference post hoc test were used to
compare multiple groups. Student’s t-test was used to compare two
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
CDK5 directly phosphorylates recombinant
cortactin
Dynamin 1 is reported to be an endogenous substrate
of CDK5, and this phosphorylation is implicated in the regulation
of synaptic vesicle endocytosis (16,17).
Consistent with previous results, CDK5/p35 clearly phosphorylated
recombinant dynamin 1 (Fig. 1A).
Under the same conditions, CDK5/p35 also markedly phosphorylated
cortactin in the presence of [γ32P]-ATP (Fig. 1A). Next, the phosphorylation sites,
T145 and T219, were determined by MALDI-MS (Fig. 1B). Cortactin substitution mutant
with alanine residues replacing T145 and T219 (Cort T145AT219A)
exhibited only trace CDK5 radiolabeling (Fig. 1C). The two phosphorylation sites,
T145 and T219, are located in the F-actin-binding cortactin repeat
region of cortactin (RRRRRR 1/2; Fig.
1B).
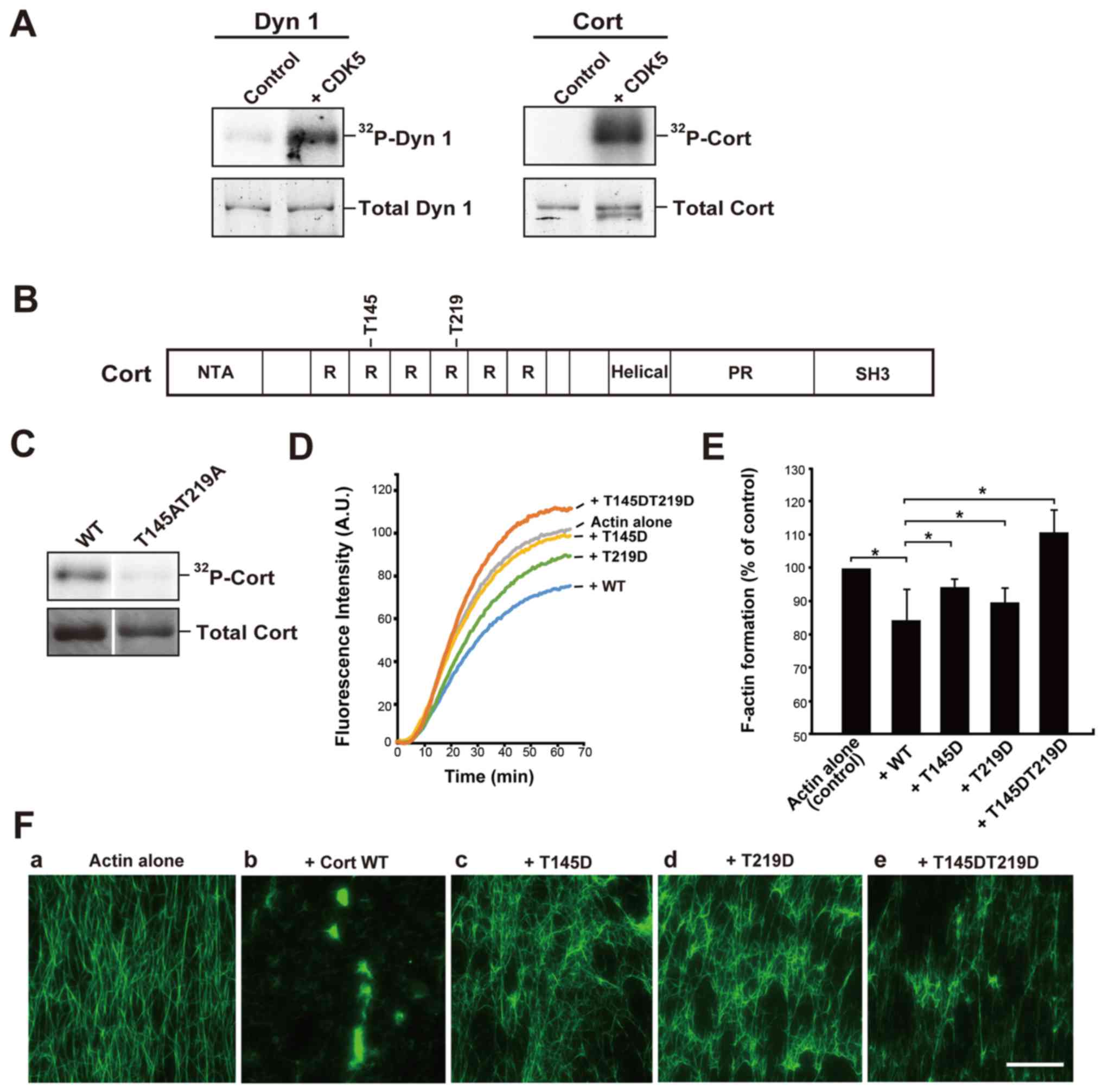 | Figure 1CDK5 directly phosphorylates Cort
in vitro and this phosphorylation alters the interaction
between Cort and F-actin. (A) Dyn 1 or Cort was incubated with or
without 1 µg/ml CDK5 in the presence of
[γ32P]-ATP. 32P-Dyn1 and 32P-Cort
was then analyzed by SDS-PAGE and visualized by autoradiography
(upper panels). Total protein was stained with SYPRO Orange (bottom
panels). Proteins were incubated without CDK5 as a control. (B)
Sites phosphorylated by CDK5 in Cort (T145 and T219) identified by
matrix-assisted laser desorption ionization time-of-flight mass
spectrometry analysis. These residues are located in the second and
fourth Cort R units. (C) Verification of the identified
phosphorylation sites in Cort. WT Cort (left) and a Cort mutant in
which the T145/T219 phosphorylation sites were substituted with
alanine (right) at 3.2 µg were incubated with CDK5/p35 and
[γ32P]-ATP for 1 h. Total Cort stained with coomassie
brilliant blue is presented (bottom). 32P-Cort was
detected by autoradiography. (D) In vitro actin
polymerization in the presence of WT Cort or the T145D, T219D, or
T145DT219D mutant. Actin polymerization was initiated by adding
K+ and Mg2+. Actin filament formation was
measured by determining the change in the fluorescence intensity of
pyrene-actin. (E) F-actin formation at 1 h following initiation of
actin polymerization with or without WT Cort or the T145D, T219D or
T145DT219D mutant was measured by analyzing pyrene-actin
fluorescence. Actin alone was used as a control. The mean ±
standard error of the mean of 3-6 independent experiments is
plotted. *P<0.05. (F) Phosphorylation of Cort by CDK5
decreases its actin-crosslinking capability. (a) Actin alone
appeared as individual filaments. (b) Tightly cross-linked F-actin
in the presence of WT Cort (+Cort WT). Partially assembled
filaments were observed in the presence of the (c) T145D, (d) T219D
or (e) T145DT219D mutants. Preformed F-actin (3.3 µM) was
incubated with or without the indicated proteins (5 µM each;
b-e). F-actin was stained with Alexa Fluor 488-conjugated
phalloidin. Scale bar, 30 µm. CDK5, cyclin-dependent kinase
5; Dyn 1, dynamin 1; Cort, cortactin; 32P,
phosphorylated; WT, wild-type; NTA, N-terminal acidic region; R,
repeat; PR, a proline-rich region; SH3, Src homology 3. |
Phosphorylation of cortactin by CDK5
alters the interaction between cortactin and F-actin
The finding that the phosphorylation sites were
located in the F-actin-binding region of cortactin suggested that
this phosphorylation affects the binding affinity of cortactin for
F-actin (Fig. 1B). To investigate
this, the kinetics of in vitro F-actin formation in the
presence or absence of WT cortactin or the phosphomimetic mutants
were examined. To imitate phosphorylation, residues T145 and/or
T219 were substituted by aspartate. Pyrene-labeled monomeric actin
was polymerized in the presence of high concentrations of
K+ and Mg2+. In the absence of cortactin,
actin polymerization plateaued at 1 h. Addition of WT cortactin
reduced the K+/Mg2+-dependent actin
polymerization rate and decreased F-actin formation by ~15% at 1 h
(Fig. 1D and E). Addition of the
T145D or T219D mutant decreased F-actin formation by 5-10%.
However, the T145DT219D mutant had no inhibitory effect (Fig. 1D and E). These results suggest that
phosphorylation of the T145 and/or T219 residues of cortactin by
CDK5 disrupts binding between cortactin and F-actin.
Next, it was investigated whether phosphorylation of
cortactin by CDK5 affects its actin-crosslinking activity in
vitro. Preformed F-actin was incubated with or without WT
cortactin or the T145D, T219D or T145DT219D mutant and then
stabilized and visualized using fluorescent phalloidin. F-actin
alone appeared as individual filaments in fluorescence microscopy
(Fig. 1Fa). Addition of WT
cortactin led to marked crosslinking of these filaments (Fig. 1Fb), as previously reported
(5,6). However, each of the phosphomimetic
mutants had a reduced actin-crosslinking capacity (Fig. 1Fc-e).
Phosphorylation of cortactin by CDK5
decreases actin bundling by the dynamin 1-cortactin complex, but
does not affect binding of cortactin to dynamin 1
Next, the effect of cortactin phosphorylation by
CDK5 on binding of cortactin to dynamin 1 was examined. Pulldown
assays demonstrated that WT cortactin and the T145D, T219D and
T145DT219D mutants bound to dynamin 1 comparably (Fig. 2A and B). By contrast, W525K, a
mutant incapable of binding to dynamin 1 (18), exhibited little binding. Thus,
phosphorylation of cortactin by CDK5 has little effect on its
binding to dynamin 1.
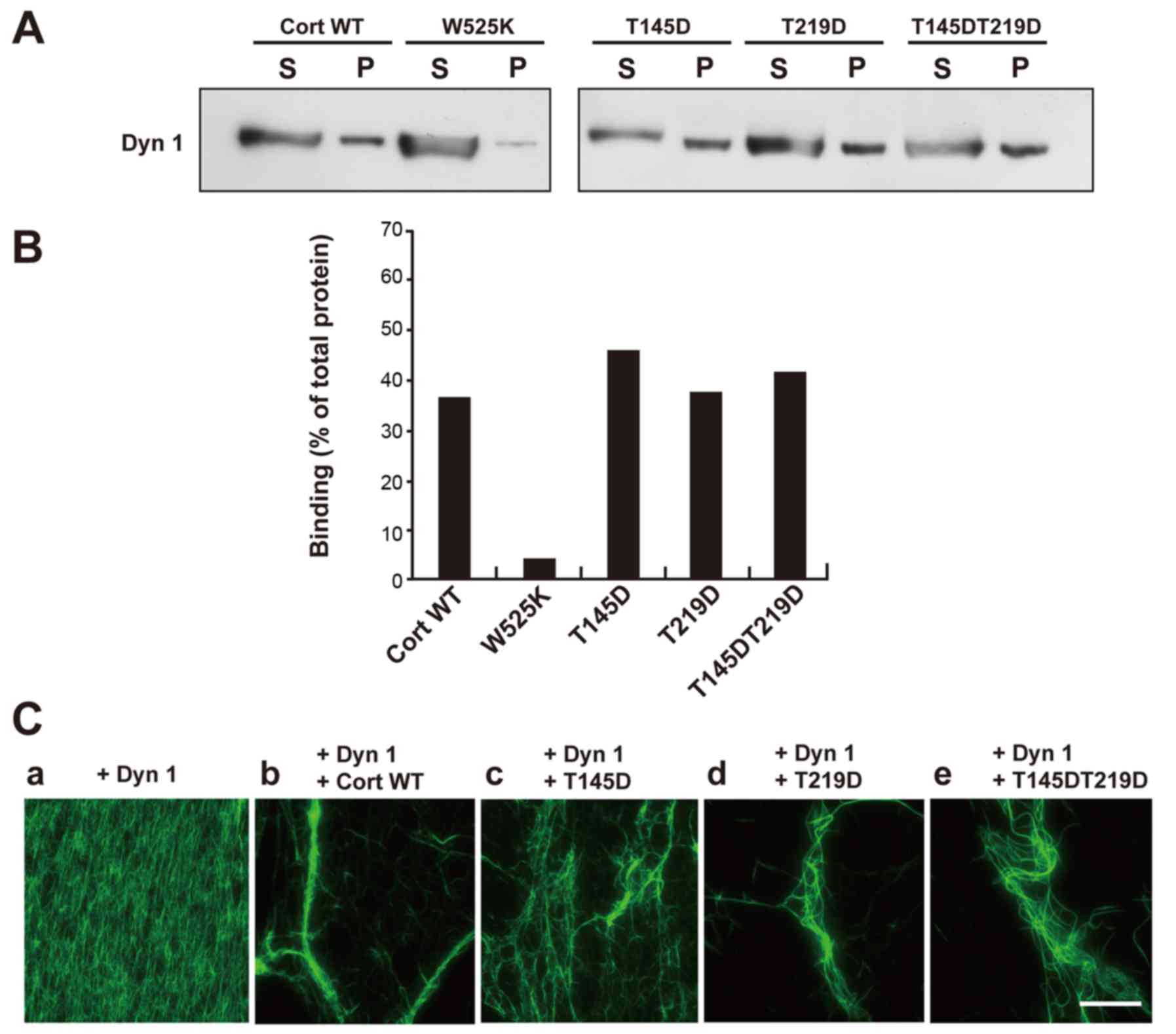 | Figure 2Phosphorylation of Cort by
cyclin-dependent kinase 5 decreases actin bundling by the Dyn
1-Cort complex, but does not affect binding between Dyn 1 and Cort.
(A) GST pulldown assay demonstrating that GST-tagged WT Cort and
the T145D, T219D, and T145DT219D mutant bound to Dyn 1 comparably.
The Cort Src homology 3 point mutant W525K hardly bound to Dyn 1.
(B) The ratio of Dyn 1 co-precipitated with WT or mutant Cort
relative to the total amount of Dyn 1. Levels were estimated by
densitometry. The results were obtained in two independent
experiments. (C) Dyn 1-mutant Cort complexes caused defective actin
bundling. (a) The presence of Dyn 1 did not lead to any visible
change of F-actin. (b) Long and thick actin bundles formed in the
presence of Dyn 1 and WT Cort. Partially and loosely bundled short
actin filaments were observed in the presence of Dyn 1 and the (c)
T145D, (d) T219D or (e) T145DT219D mutant. Preformed F-actin (3.3
µM) was incubated with or without the indicated proteins (5
µM each; a-e). F-actin labeled with Alexa Fluor
488-conjugated phalloidin was visualized under fluorescence
microscopy. Scale bar, 30 µm. Cort, cortactin; Dyn 1,
dynamin 1; GST, glutathione-S-transferase; WT, wild-type; S,
supernatant; P, pellet. |
Formation of F-actin bundles by dynamin 1 and WT
cortactin or the phosphomimetic mutants was examined visually.
Addition of dynamin 1 alone to F-actin did not lead to any visible
changes (Fig. 2Ca). Incubation of
F-actin with dynamin 1 and WT cortactin led to formation of long
and thick F-actin bundles (Fig.
2Cb). Partially bundled and short F-actin filaments were
observed upon incubation of the phosphomimetic mutants and dynamin
1 with F-actin (Fig. 2Cc-e). These
findings suggest that phosphorylation of the T145 and T219 residues
of cortactin reduces actin bundling by the dynamin 1-cortactin
complex.
Cortactin phosphorylated by CDK5 fails to
form a ring-like complex with dynamin 1
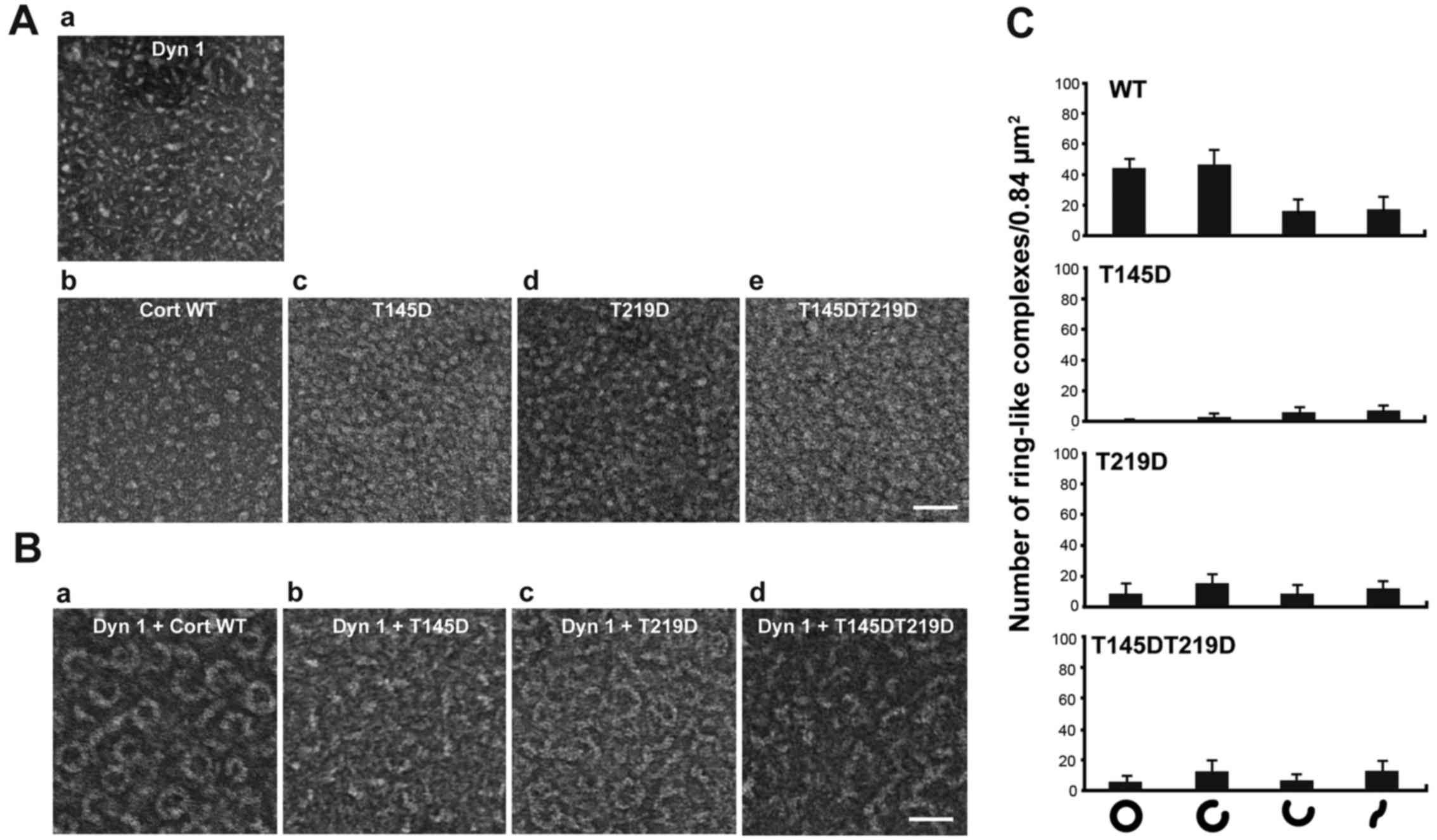
Dynamin 1 co-assembles with cortactin into a
ring-like complex, the shape of which is ‘open’ or ‘closed’
depending on the guanine nucleotide conditions (5). Structural changes are required for
actin bundling (5). Formation of
this ring-like complex was examined by negative-staining electron
microscopy. WT cortactin and the T145D, T219D, T145DT219D mutants
alone had a granular shape with a diameter of 3-7 nm (Fig. 3Ab-e). Dynamin 1 alone was short and
had a rod-like shape (Fig. 3Aa).
Consistent with the previous report (5), WT cortactin and dynamin 1
copolymerized into ring-like complexes with an outer diameter of
34.8±3.1 nm and an inner diameter of 23.5±3.1 nm (n=30; Fig. 3Ba). Formation of ring-like
complexes was markedly decreased upon incubation of dynamin 1 with
each of the phosphomimetic mutants (Fig. 3B and C). These results suggest that
phosphorylation of cortactin by CDK5 altered the higher order
structure of the dynamin 1-cortactin complex.
Cells expressing cortactin phosphomimetic
mutants exhibit aberrant lamellipodia and short filopodia
Finally, the effect of cortactin phosphorylation by
CDK5 on formation of pseudopodia in NG108-15 glioma-derived cells
was examined. Many actin-rich protrusions with lamellipodia and
filopodia at their tips formed in NG108-15 cells (19). Dynamin 1, cortactin, and CDK5 were
detected in the cell lysate by western blotting (Fig. 4A). Endogenous dynamin 1, cortactin,
and CDK5 colocalized in foci along filopodia in immunofluorescence
analysis (Fig. 4B). Next, NG108-15
cells were transfected with c-myc-tagged WT cortactin, T145D, T219D
or T145DT219D and formation of filo-podia was examined. Filopodia
were clearly observed in cells expressing c-myc-tagged WT
cortactin, similar to non-transfected cells (Fig. 4Ca and b). Filopodial length was
8.9±2.9 nm in control cells and 6.5±2.8 nm in WT
cortactin-expressing cells. By contrast, T145D, T219D and
T145DT219D were diffusely distributed in filopodia (Fig. 4Cc-e). Expression of each
phosphomimetic mutant led to deformation of lamellipodia (Fig. 4Cb-e), and filopodial length was
~50% shorter in cells expressing a phosphomimetic mutant than in
cells expressing WT cortactin (Fig.
4D). The effect of phosphorylation mimetic mutant, T145DT219D
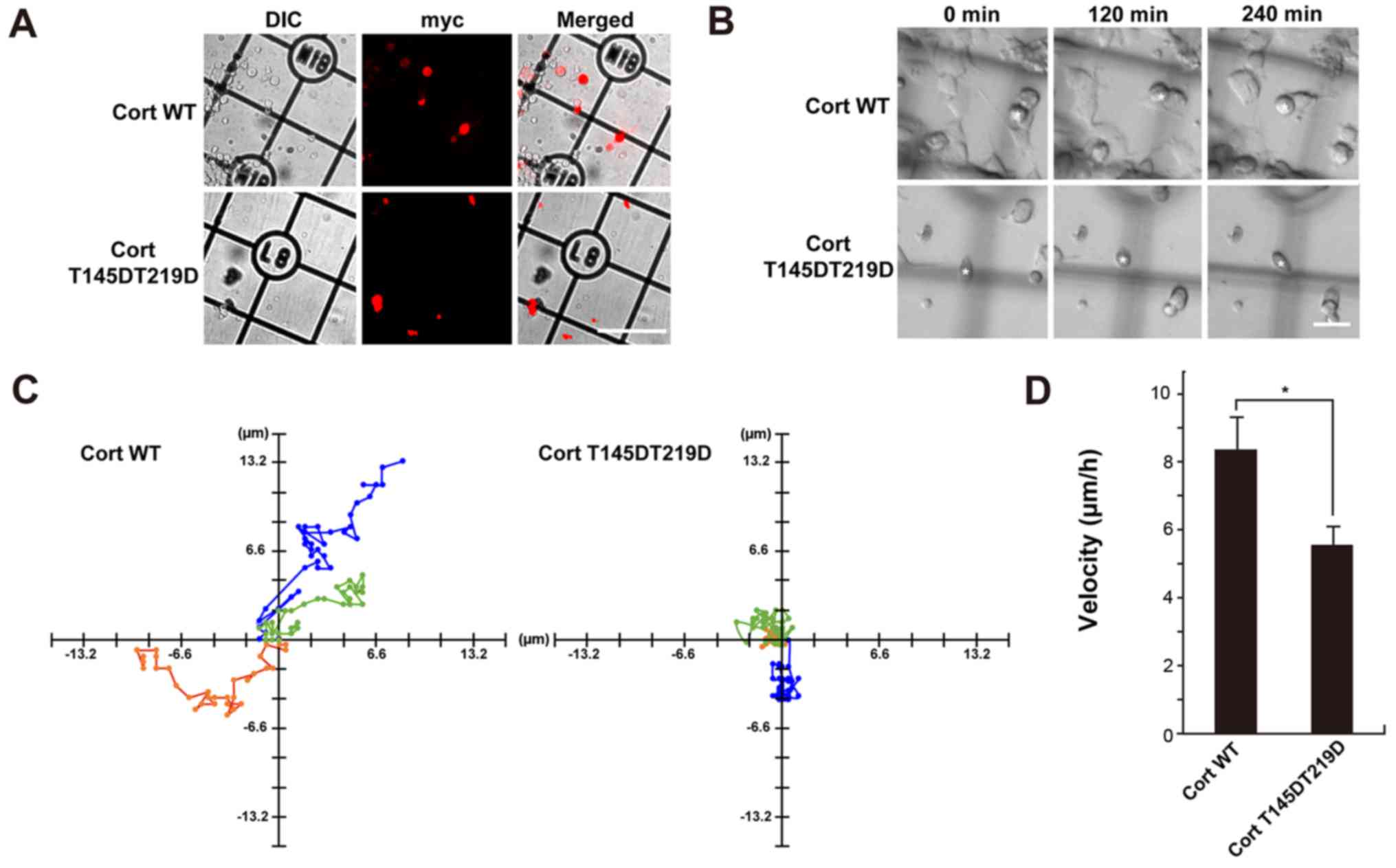
on cell migration of time-lapse DIC imaging was further determined.
Cells with cortactin mutant were identified by immunofluorescence
microscopy following the live-imaging (Fig. 5A). Cells with T145DT219D exhibited
defective migration capability compared with that of
WT-cortactin-expressing cells (Fig. 5B
and C). Furthermore, migration velocity of T145DT219D
expressing cells was slower than that of WT-cortactin expressing
cells (Fig. 5D). These results
suggest that phosphorylation of cortactin by CDK5 modulates not
only formation of lamellipodia and filopodia but also cell
migration.
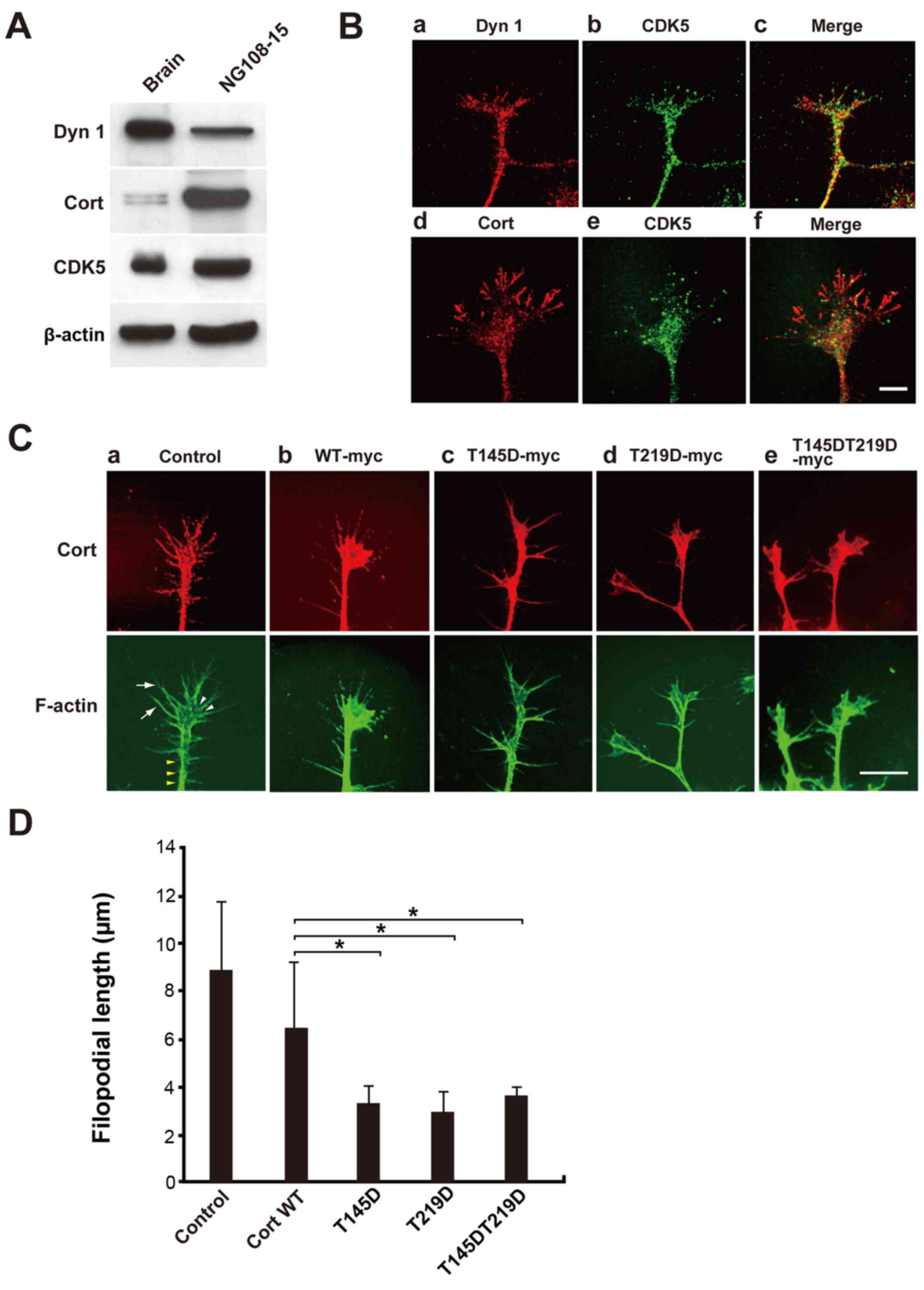 | Figure 4NG108-15 cells expressing
phosphomimetic Cort mutants exhibit aberrant lamellipodia and short
filopodia. (A) Expression of Dyn 1, Cort and CDK5 in NG108-15 cell
lysates was confirmed by western blotting. In total, 10 µg
of mouse brain homogenate or 20 µg of NG108-15 cell lysate
was loaded per lane. (B) Immunofluorescence analysis showing that
endogenous Dyn 1, Cort and CDK5 partially colocalized in
lamellipodia and filopodia of NG108-15 cells. Scale bar, 10
µm. (C) NG108-15 cells were not transfected (a; control) or
transfected with c-myc-tagged (b) WT Cort or the (c) T145D, (d)
T219D or (e) T145DT219D mutant, fixed, and stained with a
monoclonal anti-c-myc antibody (red). F-actin was stained with
Alexa Fluor 488-conjugated phalloidin (green). (Ca) Typical
cellular protrusion (yellow arrow heads), lamellipodia (white arrow
heads) and filopodia (white arrows) are presented. Filipodia were
markedly shorter in T145D-, T219D- and T145DT219D-expressing cells
than in WT Cort-expressing cells. Scale bar, 10 µm. (D)
Morphometric analysis of filopodial length in (C). Data are
presented as the mean ± standard error of the mean of 33-60
filopodia per experimental condition (n=3 independent experiments;
*P<0.05). Cort, cortactin; Dyn 1, dynamin 1; CDK5,
cyclin-dependent kinase 5; WT, wild-type. |
Discussion
Cortactin and dynamin have been independently
implicated in migration, invasion and metastasis of cancer cells
(3,20). It was recently reported that
cortactin and dynamin form a ring-like complex that bundles and
stabilizes actin filaments (5,6). The
actin bundling is essential for formation of actin-rich protrusions
in SH-SY5Y human neuroblastoma cells (5) and migration of H1299 human non-small
cell lung carcinoma cells (6).
However, the regulatory mechanism of actin bundling is largely
unknown. Dynamin 1 is a substrate of CDK5 (16,17).
Furthermore, recent reports implicated CDK5 in cancer progression
and aggressiveness (12).
Therefore, the potential regulation of cortactin function by CDK5
was investigated in vitro and in vivo.
It was demonstrated that CDK5 directly
phosphorylated cortactin in vitro. In addition, MALDI-MS
analysis identified T145 and T219 as the phosphorylation sites of
cortactin. To investigate the effects of this phosphorylation,
three phosphomimetic mutants of cortactin (T145D, T219D and
T145DT219D) were prepared by substituting T145 and/or T219 with
aspartate. These mutants exhibited reduced binding to F-actin.
F-actin bundles formed by the dynamin 1-mutant cortactin complex
dissociated more or were looser than those formed by the dynamin
1-WT cortactin complex. Although the T145D, T219D, and T145DT219D
mutants bound to dynamin 1, they could not form a ring-like
complex. Filopodia were shorter and lamellipodia were smaller in
rat glioma-derived NG108-15 cells expressing each phosphomimetic
mutant than in those expressing WT cortactin (Fig. 4). Furthermore, T145DT219D mutant
expressing cells exhibited significant decreased cell migration
compared with that of WT expressing cells (Fig. 5). These results strongly suggest
that phosphorylation of cortactin by CDK5 regulates formation of
pseudopodia by modulating actin bundle formation in
vivo.
CDK5 has been investigated in terminally
differentiated cells, such as neurons and neuroendocrine cells
(10,11). In synapses, phosphorylation of
dynamin 1 by CDK5 is crucial for regulation of endocytosis, and
dynamin 1 is phosphorylated at S774 and S778 in rats and sheep
(16) as well as T780 in bovine
(17). CDK5 also phosphorylates
endophilin 1 (21) and amphiphysin
1 (17,22), a physiological binding partner of
dynamin 1. Co-phosphorylation of dynamin 1 and amphiphysin 1 by
CDK5 leads to defective formation of the dynamin 1-amphiphysin 1
complex and strongly inhibits endocytosis (17). In the present study,
phosphorylation of cortactin by CDK5 not only decreased the
interaction between cortactin and F-actin, but also disrupted
formation of the ring-like dynamin 1-cortactin complex. These two
effects may regulate actin bundling. Co-phosphorylation of dynamin
1 and its binding partner likely controls endocytic activity in
vivo. Actin regulation by co-phosphorylation of dynamin 1 and
cortactin would be possible. The effect of co-phosphorylation of
dynamin 1 and cortactin on complex formation and actin bundling
should be investigated in detail in the future.
Brain-derived cancer cells, including glioma-derived
cells, and tissues highly express CDK5, and this kinase is
implicated in proliferation (23)
and invasion (24) of cancer
cells, and development and progression of tumors (25). NG108-15 glioma-derived cells
expressed dynamin 1, cortactin, and CDK5, and these proteins
colocalized in filopodia and lamellipodia.
The present study demonstrates that CDK5
phosphorylates two threonine residues (T145 and T219) of cortactin.
Martin et al (26)
previously exhaustively examined cortactin phosphorylation sites in
human embryonic kidney 293 cells by mass spectrometry. However,
they did not identify T145 and T219 as phosphorylation sites.
Phosphorylation of cortactin by CDK5 might differ between
brain-derived and non-neuronal cells as expression of this kinase
is lower in the latter cells than in the former cells.
In conclusion, cortactin was identified as a novel
substrate of CDK5. Phosphorylation of cortactin by CDK5 decreased
the actin-bundling activity of the dynamin 1-cortactin complex and
consequently reduced pseudopodal formation, which is required for
migration, invasion and metastasis of cancer cells. Most molecular
targets of current anticancer drugs are involved in cell division
or DNA replication. Therefore, targeting of dynamin-dependent actin
dynamics via modulation of CDK5 activity may be a more effective
anticancer therapy, especially for brain-derived tumors. Further
studies are required to determine the precise mechanism(s) by which
intracellular phosphorylation modulates actin bundling by the
dynamin 1-cortactin complex.
Funding
The present study was supported in part by grants
from the Ministry of Education, Science, Sports, and Culture of
Japan (grant nos. 16K10756 to TA and 17K08808 to HY).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors’ contributions
HY and KTa designed research and wrote the paper.
HY, TA, YM, EO and TT performed mutant protein construction,
protein purification and actin bundling experiments. TA and YM
performed electron microscopy. EO, TML, KS and KF performed
immunofluorescent microscopy, cell migration assay and analyzed
data. FYW and KTo identified phosphorylation sites by MALDI-MS. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
All procedures and animal protocols were approved by
the Committee on the Ethics of Animal Experimentation at Okayama
University (Okayama, Japan; approval no. OKU-2015052 and
OKU-2018109).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank Dai Shimizu (Okayama
University, Okayama, Japan) and Natsuki Wakita (Okayama University,
Okayama, Japan) for technical assistance.
References
|
1
|
Arjonen A, Kaukonen R and Ivaska J:
Filopodia and adhesion in cancer cell motility. Cell Adhes Migr.
5:421–430. 2011. View Article : Google Scholar
|
|
2
|
Ridley AJ: Life at the leading edge. Cell.
145:1012–1022. 2011. View Article : Google Scholar
|
|
3
|
MacGrath SM and Koleske AJ: Cortactin in
cell migration and cancer at a glance. J Cell Sci. 125:1621–1626.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
McNiven MA, Kim L, Krueger EW, Orth JD,
Cao H and Wong TW: Regulated interactions between dynamin and the
actin-binding protein cortactin modulate cell shape. J Cell Biol.
151:187–198. 2000. View Article : Google Scholar
|
|
5
|
Yamada H, Abe T, Satoh A, Okazaki N, Tago
S, Kobayashi K, Yoshida Y, Oda Y, Watanabe M, Tomizawa K, et al:
Stabilization of actin bundles by a dynamin 1/cortactin ring
complex is necessary for growth cone filopodia. J Neurosci.
33:4514–4526. 2013. View Article : Google Scholar
|
|
6
|
Yamada H, Takeda T, Michiue H, Abe T and
Takei K: Actin bundling by dynamin 2 and cortactin is implicated in
cell migration by stabilizing filopodia in human non-small cell
lung carcinoma cells. Int J Oncol. 49:877–886. 2016. View Article : Google Scholar
|
|
7
|
Weaver AM, Heuser JE, Karginov AV, Lee WL,
Parsons JT and Cooper JA: Interaction of cortactin and N-WASp with
Arp2/3 complex. Curr Biol. 12:1270–1278. 2002. View Article : Google Scholar
|
|
8
|
Kinley AW, Weed SA, Weaver AM, Karginov
AV, Bissonette E, Cooper JA and Parsons JT: Cortactin interacts
with WIP in regulating Arp2/3 activation and membrane protrusion.
Curr Biol. 13:384–393. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Contreras-Vallejos E, Utreras E and
Gonzalez-Billault C: Going out of the brain: Non-nervous system
physiological and pathological functions of Cdk5. Cell Signal.
24:44–52. 2012. View Article : Google Scholar
|
|
10
|
Nikolic M, Dudek H, Kwon YT, Ramos YF and
Tsai LH: The cdk5/p35 kinase is essential for neurite outgrowth
during neuronal differentiation. Genes Dev. 10:816–825. 1996.
View Article : Google Scholar
|
|
11
|
Liu KC, Leuckx G, Sakano D, Seymour PA,
Mattsson CL, Rautio L, Staels W, Verdonck Y, Serup P, Kume S, et
al: Inhibition of Cdk5 Promotes β-Cell Differentiation From Ductal
Progenitors. Diabetes. 67:58–70. 2018. View Article : Google Scholar
|
|
12
|
Pozo K and Bibb JA: The emerging role of
Cdk5 in cancer. Trends Cancer. 2:606–618. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Quintavalle M, Elia L, Price JH,
Heynen-Genel S and Courtneidge SA: A cell-based high-content
screening assay reveals activators and inhibitors of cancer cell
invasion. Sci Signal. 4:ra492011. View Article : Google Scholar
|
|
14
|
Feldmann G, Mishra A, Hong SM, Bisht S,
Strock CJ, Ball DW, Goggins M, Maitra A and Nelkin BD: Inhibiting
the cyclin-dependent kinase CDK5 blocks pancreatic cancer formation
and progression through the suppression of Ras-Ral signaling.
Cancer Res. 70:4460–4469. 2010. View Article : Google Scholar
|
|
15
|
Yamada H, Kikuchi T, Masumoto T, Wei FY,
Abe T, Takeda T, Nishiki T, Tomizawa K, Watanabe M, Matsui H, et
al: Possible role of cortactin phosphorylation by protein kinase Cα
in actin-bundle formation at growth cone. Biol Cell. 107:319–330.
2015. View Article : Google Scholar
|
|
16
|
Tan TC, Valova VA, Malladi CS, Graham ME,
Berven LA, Jupp OJ, Hansra G, McClure SJ, Sarcevic B, Boadle RA, et
al: Cdk5 is essential for synaptic vesicle endocytosis. Nat Cell
Biol. 5:701–710. 2003. View
Article : Google Scholar
|
|
17
|
Tomizawa K, Sunada S, Lu YF, Oda Y, Kinuta
M, Ohshima T, Saito T, Wei FY, Matsushita M, Li ST, et al:
Cophosphorylation of amphiphysin I and dynamin I by Cdk5 regulates
clathrin-mediated endocytosis of synaptic vesicles. J Cell Biol.
163:813–824. 2003. View Article : Google Scholar
|
|
18
|
Schafer DA, Weed SA, Binns D, Karginov AV,
Parsons JT and Cooper JA: Dynamin2 and cortactin regulate actin
assembly and filament organization. Curr Biol. 12:1852–1857. 2002.
View Article : Google Scholar
|
|
19
|
Nozumi M, Nakagawa H, Miki H, Takenawa T
and Miyamoto S: Differential localization of WAVE isoforms in
filopodia and lamellipodia of the neuronal growth cone. J Cell Sci.
116:239–246. 2003. View Article : Google Scholar
|
|
20
|
Jeannot P and Besson A: Cortactin function
in invadopodia. Small GTPases. Dec 31–2017.Epub ahead of print.
View Article : Google Scholar
|
|
21
|
Wong AS, Lee RH, Cheung AY, Yeung PK,
Chung SK, Cheung ZH and Ip NY: Cdk5-mediated phosphorylation of
endophilin B1 is required for induced autophagy in models of
Parkinson’s disease. Nat Cell Biol. 13:568–579. 2011. View Article : Google Scholar
|
|
22
|
Liang S, Wei FY, Wu YM, Tanabe K, Abe T,
Oda Y, Yoshida Y, Yamada H, Matsui H, Tomizawa K, et al: Major
Cdk5-dependent phosphorylation sites of amphiphysin 1 are
implicated in the regulation of the membrane binding and
endocytosis. J Neurochem. 102:1466–1476. 2007. View Article : Google Scholar
|
|
23
|
Moutal A, Villa LS, Yeon SK, Householder
KT, Park KD, Sirianni RW and Khanna R: CRMP2 Phosphorylation Drives
Glioblastoma Cell Proliferation. Mol Neurobiol. 55:4403–4416.
2018.
|
|
24
|
Liu R, Tian B, Gearing M, Hunter S, Ye K
and Mao Z: Cdk5-mediated regulation of the PIKE-A-Akt pathway and
glioblastoma cell invasion. Proc Natl Acad Sci USA. 105:7570–7575.
2008. View Article : Google Scholar
|
|
25
|
Yushan R, Wenjie C, Suning H, Yiwu D,
Tengfei Z, Madushi WM, Feifei L, Changwen Z, Xin W, Roodrajeetsing
G, et al: Insights into the clinical value of cyclin-dependent
kinase 5 in glioma: A retrospective study. World J Surg Oncol.
13:2232015. View Article : Google Scholar
|
|
26
|
Martin KH, Jeffery ED, Grigera PR,
Shabanowitz J, Hunt DF and Parsons JT: Cortactin phosphorylation
sites mapped by mass spectrometry. J Cell Sci. 119:2851–2853. 2006.
View Article : Google Scholar
|