Introduction
Breast cancer (BC) is a common cancer and leading
cause of cancer-associated mortality in women and is the second
most lethal cancer worldwide, following lung adenocarcinoma
(1,2). In the last decades, approaches used
for the treatment of BC have advanced, including surgery,
radiotherapy, chemotherapy and endocrine therapy, leading to a
significant reduction of the rates of premature mortality (3). However, the molecular mechanisms
responsible for BC development and progression remain unclear.
There are diverse BC subtypes, and clear and definitive targets are
limited, resulting in ambiguity of the pathophysiology of BC
(4). It was demonstrated that, in
response to therapy, distinct signaling pathways may be activated
in different subtypes of BC (5,6). The
features of tumorigenesis include uncontrolled cell proliferation,
and high rates of metastasis and stemness (7,8).
Therefore, determining the mechanisms of dysregulation of cell
proliferation, metastasis and stemness is urgently required to
identify key regulators in the development of BC and improve BC
therapy.
Long non-coding RNAs (lncRNAs) are a family of
transcripts, with lengths >200 nucleotides, which contain no
open reading frames, and these RNAs are less abundant and more
variable among tissues compared with mRNA expression (9). lncRNAs have been identified to serve
key roles in the regulation of a broad array of cancer processes,
including proliferation (10),
apoptosis (11), metastasis
(12) and drug resistance
(13). lncRNAs may function
through a wide range of mechanisms (14), including serving as signals,
decoys, guides and scaffolds to modulate the transcriptional or
post-transcriptional regulation of gene expression in multiple
cancer types. Serving as guides or molecular scaffolds, lncRNAs may
recruit co-regulators of transcription to a specific DNA region or
raise enzymes with chromatin-modifying activity to form
ribonucleoprotein complexes, leading to the bridging of regulatory
proteins and regulation of transcription (15). In addition, functioning as decoys,
lncRNAs may bind directly with microRNAs (miRNAs) or proteins and
thus modulate the functions of key regulators (16). Therefore, lncRNAs may serve an
oncogenic or a tumor-suppressive role, making them good prognostic
biomarkers and therapeutic targets.
lncRNA FOXD2 adjacent opposite strand RNA 1
(FOXD2-AS1; accession no. NR_026878), is located on chromosome 1p33
and consists of 2,527 nucleotides (17). It was firstly identified that
FOXD2-AS1 expression is increased in gastric cancer (18,19).
Previous studies demonstrated that FOXD2-AS1 is critical for cell
proliferation, apoptotic cell death, invasion, migration and
drug-resistance in non-small cell lung cancer (20), esophageal squamous cell carcinoma
(21), nasopharyngeal carcinoma
carcinogenesis (17), colorectal
cancer (22) and bladder cancer
(23). However, whether FOXD2-AS1
is dysregulated in BC and its underlying mechanisms remain
unclear.
The present study aimed to identify the possible
role of FOXD2-AS1 in the proliferation, migration, invasion and
stemness in BC, and to clarify the clinical features of FOXD2-AS1
in BC. In the present study, an increased expression of FOXD2-AS1
was identified in BC tissues and cells. Critical roles of FOXD2-AS1
in the regulation of proliferation, epithelial-mesenchymal
transition and stemness were identified. The results suggested that
FOXD2-AS1 promoted BC malignancy and tumorigenesis by targeting the
miRNA (miR)-150-5p/profilin 2 (PFN2) axis.
Materials and methods
Patients and tissue specimens
In total, 34 pairs of BC tissues and paired adjacent
normal tissues were collected from the Department of Breast
Surgery, Affiliated Cancer Hospital and Institute of Guangzhou
Medical University (Guangzhou, China) between Jan 2012 and May
2012. The present study was approved by the Ethics Committee of the
Affiliated Cancer Hospital and Institute of Guangzhou Medical
University (approval no. ACHIGMU-2012-1-3-05). The specimens were
immediately frozen in liquid nitrogen and subsequently stored at
−80°C for further determination. The female patients did not
receive any radiation or chemotherapy prior to operation, and
patients with hypertension and diabetes mellitus were excluded in
the present study. All tissue sections were reviewed by at least
two experienced pathologists. The tumor, node, metastasis (TNM)
stage was evaluated according to the American Joint Committee on
Cancer (24). Written informed
consent was obtained from all the enrolled patients. A 5-year
follow-up was performed and overall survival was defined as the
length of time between surgery and mortality or the last follow-up
(if mortality did not occur).
Cell lines, culture and transfection
A human normal breast epithelial cell line (MCF-10A)
and human breast cancer cell lines (MCF-7, MDA-MB-231, MDA-MB-453
and MDA-MB-468) were purchased from The American Type Culture
Collection (Manassas, VA, USA). The cell lines were incubated in
RPMI-1640 medium (Corning, Inc., Corning, NY, USA) supplemented
with 10% fetal bovine serum (FBS; Corning, Inc.) and cultured at
37°C with 5% CO2. Cells in the exponential phase were
used in the experiments. Small interfering RNA (siRNA) targeting
FOXD2-AS1 (cat. no. 4390771; 100 nM) and its scrambled control
siRNA (cat. no. AM4636; 100 nM) (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) were employed to downregulate FOXD2-AS1. Short
hairpin RNA (shRNA) targeting FOXD2-AS1 (100 nM) was prepared based
on those siRNAs using pSilencer 3.1-H1 puro plasmids (Shanghai
GenePharma Co. Ltd., Shanghai, China). Lentivirus carrying shRNA
targeting FOXD2-AS1 (1×107 IFU/ml) was constructed using
pre-packaged lentivirus (Shanghai GenePharma Co. Ltd.). The
miR-150-5p mimics (cat. no. 4464084; 100 nM), inhibitors (cat. no.
4464066; 100 nM) and scrambled control oligonucleotides (cat. nos.
4464059 and 4464076; 100 nM) were purchased from Thermo Fisher
Scientific, Inc. Oligonucleotide and lentivirus transfection into
cells were conducted using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. After 48 h of transfection, subsequent
experiments were performed.
Cell proliferation
Cell proliferation was determined by Cell Counting
Kit-8 (CCK-8; Beyotime Institute of Biotechnology, Haimen, China),
according to the manufacturer's protocol. Following the treatment,
cells were incubated in 10 µl CCK-8 solution for 1 h at
37°C. Finally, the absorbance at 450 nm was measured following the
plate incubation at 37°C for 2 h (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Triplicate experiments were performed.
RNA extraction and reverse
transcription-quantitative poly- merase chain reaction
(RT-qPCR)
Tissues and cells were lysed and total RNA was
extracted using TRIzol® (Thermo Fisher Scientific, Inc.)
reagent, according to the manufacturer's protocol. RNA quality was
measured using Nanodrop equipment (Thermo Fisher Scientific, Inc.,
Wilmington, DE, USA). For the detection of mRNA and lncRNA, 500 ng
RNA was reverse transcribed into cDNA using a cDNA synthesis kit
(Thermo Fisher Scientific, Inc.) according to the following
conditions: 65°C for 5 min, 25°C for 10 min, 50°C for 15 min and
85°C for 5 min. The sequences of specific primers used for RT-qPCR
were as follows: GAPDH, forward, 5′-GCGAGATC GCACTCATCATCT-3′ and
reverse, 5′-TCAGTGGTGGACCT GACC-3′; FOXD2-AS1, forward,
5′-TGGACCTAGCTGCAGC TCCA-3′ and reverse,
5′-AGTTGAAGGTGCACACACTG-3′; E-cadherin, forward,
5′-CTGCTGCAGGTCTCCTCTTG-3′ and reverse, 5′-TGTCGACCGGTGCAATCTTC-3;
Vimentin, forward, 5′-AAGGCGAGGAGAGCAGGATT-3′ and reverse,
5′-GGTCATCGTGATGCTGAGAAG-3′; N-cadherin, forward,
5′-GTGCCATTAGCCAAGGGAATTCAGC-3′ and reverse,
5′-GCGTTCCTGTTCCACTCATAGGAG-3′; Nanog, forward,
5′-GGTCCCAGTCAAGAAACAGA-3′ and reverse, 5′-GAG
GTTCAGGATGTTGGAGA-3′; Oct4, forward, 5′-CCCGAAAGAGAAAGCGAACC-3′ and
reverse, 5′-GCAGCCTCAAAATCCTCTCG-3′; and Sox2, forward,
5′-CATGTCCCAGCACTA CCAGA-3′ and reverse,
5′-TTTGAGCGTACCGGGTTTTC-3′. Reactions of RT-qPCR were conducted on
an ABI 7500 real-time PCR System (Applied Biosystems; Thermo Fisher
Scientific, Inc.). GAPDH was used as an internal control for
normalization.
For the detection of miRNA, the qScript miRNA cDNA
Synthesis kit (Quantabio, Beverly, MA, USA) was employed to
synthesize cDNA, according to the following conditions: 50°C for 60
min and 85°C for 5 sec. miRNA RT-qPCR was performed using a
miScript SYBR Green PCR kit (Qiagen GmbH, Hilden, Germany)
according to the manufacturer's protocol. The sequences of specific
primers used for RT-qPCR were as follows: U6, forward, 5′-CTCGCTT
CGGCAGCACA-3′; reverse, 5′-AACGCTTCACGAATTTGCGT-3′; miR-150-5p,
forward, 5′-TCTCCCAACCCTTGTACCAGTG-3′; reverse,
5′-CTCAACTGGTGTCGTGGTA-3′. U6 was used as an internal control for
normalization. The level of target gene was calculated relative to
internal control using the 2−∆∆Cq method (25). The PCR conditions were as follows:
95°C for 5 min, 40 cycles of 95°C for 20 sec and 62°C for 30 sec,
followed by 72°C for 3 min.
Cytoplasmic and nuclear isolation of
RNA
The location of lncRNA FOXD2-AS1 was measured using
the Cytoplasmic and Nuclear RNA Purification kit (Norgen Biotek
Corp., Thorold, ON, Canada) according to the manufacturer's
protocol.
Luciferase reporter assay
The fragments of wild-type (WT) or mutated (MUT)
3′untranslated region (UTR) of FOXD2-AS1 and PFN2 were synthesized,
inserted into the vector pGL3 (Shanghai GeneChem Co. Ltd.,
Shanghai, China), and subsequently miR-150-5p mimics and their
respective control were transfected into 293T cells (American Type
Culture Collection) using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.). The Renilla
luciferase plasmid was used for normalization. The luciferase
activity was measured using the Dual-Luciferase Reporter Assay
System (Promega Corporation, Madison, WI, USA) according to the
manufacturer's protocol. After 48 h of transfection relative
luciferase activity was calculated by comparison with
Renilla luciferase activity.
Sphere-formation assay
The formation of spheres was evaluated as previously
described (26). BC cells were
suspended and 5×104 cells/well were seeded into 6-well
plates (Corning, Inc.). In total, 2 ml serum-free Dulbecco's
modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific,
Inc.) was added to each well to re-suspend cells. The medium
contained 4 U/l insulin, 20 mg/l epidermal growth factor and 20
mg/l human fibroblast growth factor. Sphere-formation was observed
using a light microscope (magnification, ×400) and the number of
spheroids was counted (Olympus Corporation, Tokyo, Japan).
Flow cytometry analysis
CD44 antigen (CD44)+/signal transducer
CD24 (CD24)− cells percentage was determined using a
Breast Cancer Stem Cell Isolation kit (R&D Systems Inc.,
Minneapolis, MN, USA) using flow cytometer analysis. Cells were
trypsinized and resuspended to a density of 2×105
cells/well. Cells were incubated in PBS containing 2% FBS and human
CD24 biotinylated antibody, human CD44 biotinylated antibody, human
CD24 detection antibody and human CD44 detection antibody provided
in the kit for 15 min at 2-8°C. A flow cytometer was used (BD
Biosciences, Franklin Lakes, NJ, USA) and
CD44+/CD24− cells percentage was measured
(Kaluza 1.2 software; Beckman Coulter, Inc., Brea, CA, USA).
Bioinformatics analysis
The lncRNA-miRNA interaction prediction was
performed using StarBase v 2.0 (http://starbase.sysu.edu.cn/starbase2). The miRNA-mRNA
interaction prediction was conducted using TargetScanHuman 7.1
(http://www.targetscan.org/vert_71/).
Migration and invasion assays
A wound healing assay was conducted to evaluate the
migration of the cells. 5×105 cells were plated in
6-well plates and when cells were grown to 90% confluence, a
scratch was made through the monolayer using a sterile pipette tip.
The floating cells were removed with PBS and subsequently, cells
were cultured in serum-free medium. Images were captured at 0 and
24 h. The width of the scratches at 0 and 24 h was measured. The
migration distance was calculated following the formula: Migration
rate = migration distance/original distance. Relative migration
distance was expressed as fold-change of the control.
A Matrigel assay was conducted to evaluate the
invasion of the cells. 1×105 cells in 200 µl
serum-free medium were added to the upper chamber of Transwell
chambers (Corning, Inc.) were coated with DMEM-diluted Matrigel (BD
Biosciences). In total, 800 µl medium containing 10% FBS was
added to the lower chamber. Following incubation at 37°C for 48 h,
the upper membrane surface was scraped using a cotton tip to remove
non-invaded cells. Finally, the membrane was fixed with 4%
paraformaldehyde for 30 min at room temperature and stained with 1%
crystal violet for 15 min at room temperature. Staining was
observed using a light microscope (magnification, ×400; Olympus
Corporation) and the number of invaded cells was counted and
expressed as the fold-change of the control.
Nude mice experiments
Xenograft mice were established as per the
experimental protocols and the study was approved by the Affiliated
Cancer Hospital and Institute of Guangzhou Medical University. The
mice were housed in a specific pathogen-free animal laboratory
under temperature (23±2°C) and humidity (55±5%) condition with a
standard light cycle (12 h light/dark) and free access to food and
water. In total, 12 female BALB/c nu/nu mice (4-weeks; weighing
16±2 g; purchased from The Animal Centre of Guangzhou Medical
University) were randomly divided into two groups with six mice in
each group. In total, 1×107 MCF-7 cells were suspended
in physiological saline. An equal volume of Matrigel (Corning,
Inc.) was added to the cell suspension. Each mouse was
subcutaneously injected with 150 µl mixture to generate a
tumor. 17b-Estradiol pellets (0.72 mg; 60 day release; Innovative
Research of America, Sarasota, FL, USA) were implanted
subcutaneously using a precision trochar (10 gages) at the time of
cell injection. The tumor size was measured using a caliper every 3
days and the volume of the tumor was calculated using the following
formula: Volume = (length/2) × width2. The experimental
period was 21 days. Subsequently, the mice were sacrificed, and
tumors were resected and weighed. Tumor tissues were stored at
−80°C for further analysis.
Statistical analysis
The data are presented as the mean ± standard
deviation. A total of three independent experiments were performed.
Differences between two groups were analyzed with Student's t-test.
Differences among more than two groups were analyzed with analysis
of variance followed by Tukey's test. Overall survival was analyzed
using the Kaplan-Meier method and log-rank test. P<0.05 was
considered to indicate a statistically significant difference.
GraphPad Prism 6.01 software (GraphPad Software, Inc., La Jolla,
CA, USA) was used to perform statistical analyses.
Results
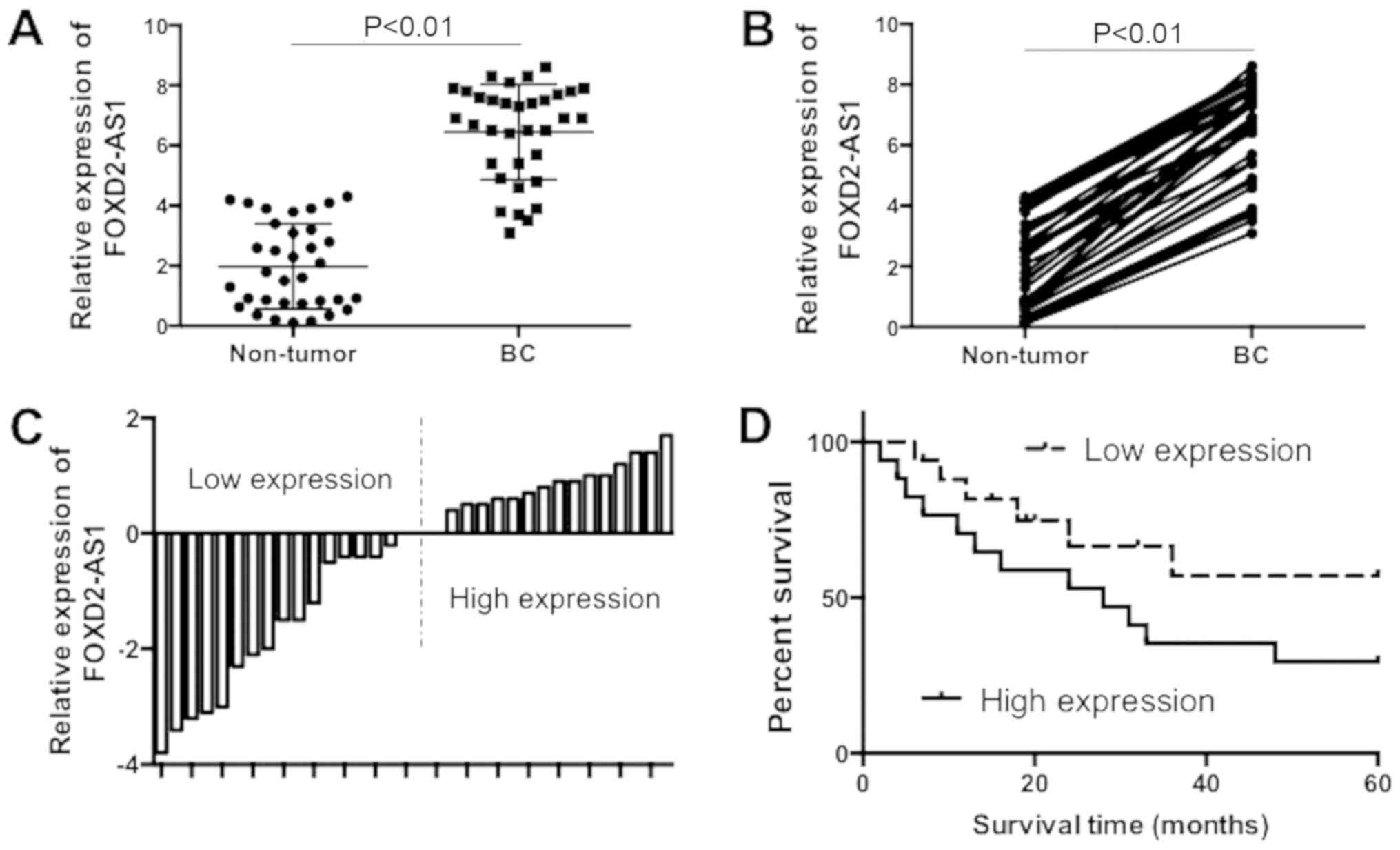
lncRNA FOXD2-AS1 expression is increased
in BC tissues and is associated with poor prognosis
To examine the pattern of lncRNA FOXD2-AS1
expression in BC tissues and non-tumor tissues, the mRNA expression
of FOXD2-AS1 in 34 paired BC tissues and adjacent normal tissues
was determined. The results demonstrated that mRNA expression of
FOXD2-AS1 was significantly increased in BC tissue samples,
compared with adjacent normal tissues (Fig. 1A and B; P<0.01). According to
the median expression (6.9) of FOXD2-AS1, the BC tissues specimens
were divided into two groups: A higher FOXD2-AS1 expression group
and the other group with lower expression of FOXD2-AS1 (Fig. 1C). The differences of overall
survival between groups with either higher or lower expression of
FOXD2-AS1 were compared, using Kaplan-Meier curves and a log-rank
test. The results demonstrated that the overall survival was poor
in patients with BC with high expression levels of FOXD2-AS1,
compared with patients with low expression levels of FOXD2-AS1
(Fig. 1D). The clinicopathologic
features of the patients are presented in Table I. It was demonstrated that higher
FOXD2-AS1 expression was associated the positive expression of
estrogen receptor, human epidermal growth factor receptor 2,
distant metastases, lymphatic metastasis and tumor, node,
metastasis stage. Overall, the present results suggested that
FOXD2-AS1 expression was increased in BC tissues and upregulation
of FOXD2-AS1 was associated with a poor prognosis BC. The results
demonstrated that FOXD2-AS1 may serve an oncogenic role in the
tumorigenesis of BC.
 | Table IAssociation between FOXD2-AS1
expression and clinicopathological characteristics of patients with
breast cancer. |
Table I
Association between FOXD2-AS1
expression and clinicopathological characteristics of patients with
breast cancer.
| Clinicopathological
characteristics | Number of
patients | FOXD2-AS1
expression
| P-value |
|---|
| Low, n=15 | High, n=19 |
|---|
| Age | | | | |
| <50 | 18 | 8 | 9 | 0.327 |
| ≥50 | 16 | 7 | 10 | |
| ER | | | | |
| Positive | 23 | 8 | 14 | 0.006a |
| Negative | 11 | 7 | 5 | |
| PR | | | | |
| Positive | 19 | 9 | 9 | 0.623 |
| Negative | 15 | 6 | 10 | |
| HER2 | | | | |
| Positive | 20 | 9 | 13 | 0.025a |
| Negative | 14 | 6 | 6 | |
| EGFR | | | | |
| Positive | 18 | 8 | 9 | 0.712 |
| Negative | 16 | 7 | 10 | |
| Distant
metastases | | | | |
| Yes | 21 | 7 | 14 | 0.017a |
| No | 13 | 8 | 5 | |
| Lymphatic
metastasis | | | | |
| Positive | 22 | 8 | 15 | 0.008a |
| Negative | 12 | 7 | 4 | |
| TNM stage | | | | |
| I-II | 20 | 9 | 12 | 0.013a |
| III | 14 | 6 | 7 | |
FOXD2-AS1 knockdown reduces the breast
cancer stem cell (BCSC) properties
It was investigated whether FOXD2-AS1 served a role
in the maintenance of BCSC properties in BC. The FOXD2-AS1
expression level was significantly increased in BC cell lines,
MCF-7, MDA-MB-231, MDA-MB-453 and MDA-MB-468, compared with normal
human breast epithelial cells (MCF-10A; Fig. 2A; P<0.01). Furthermore, the
FOXD2-AS1 expression level was significantly increased in MCF-7 and
MDA-MB-468 sphere cells compared with parental cells (Fig. 2B; P<0.01). BCSC cells [MCF-7
cancer stem cells (CSCs) and MDA-MB-468 CSCs] were trans-fected
with lentivirus-mediated interfering oligonucleotides targeting
FOXD2-AS1 to knockdown the expression of FOXD2-AS1 (Fig. 2C and D). Knockdown of FOXD2-AS1
significantly decreased the percentage of
CD44+/CD24− in the BCSC cell (MCF-7 CSC and
MDA-MB-468 CSC) subpopulation (Fig.
2E; P<0.01). The results additionally demonstrated that
downregulation of FOXD2-AS1 resulted in a significant decrease of
stem factor expression, including Nanog, octamer-binding
transcription factor 4 (Oct4) and sex determining region Y-box 2
(SOX2; Fig. 2F and G; P<0.01).
Therefore, the data suggested that knockdown of FOXD2-AS1 decreased
the properties of BCSC.
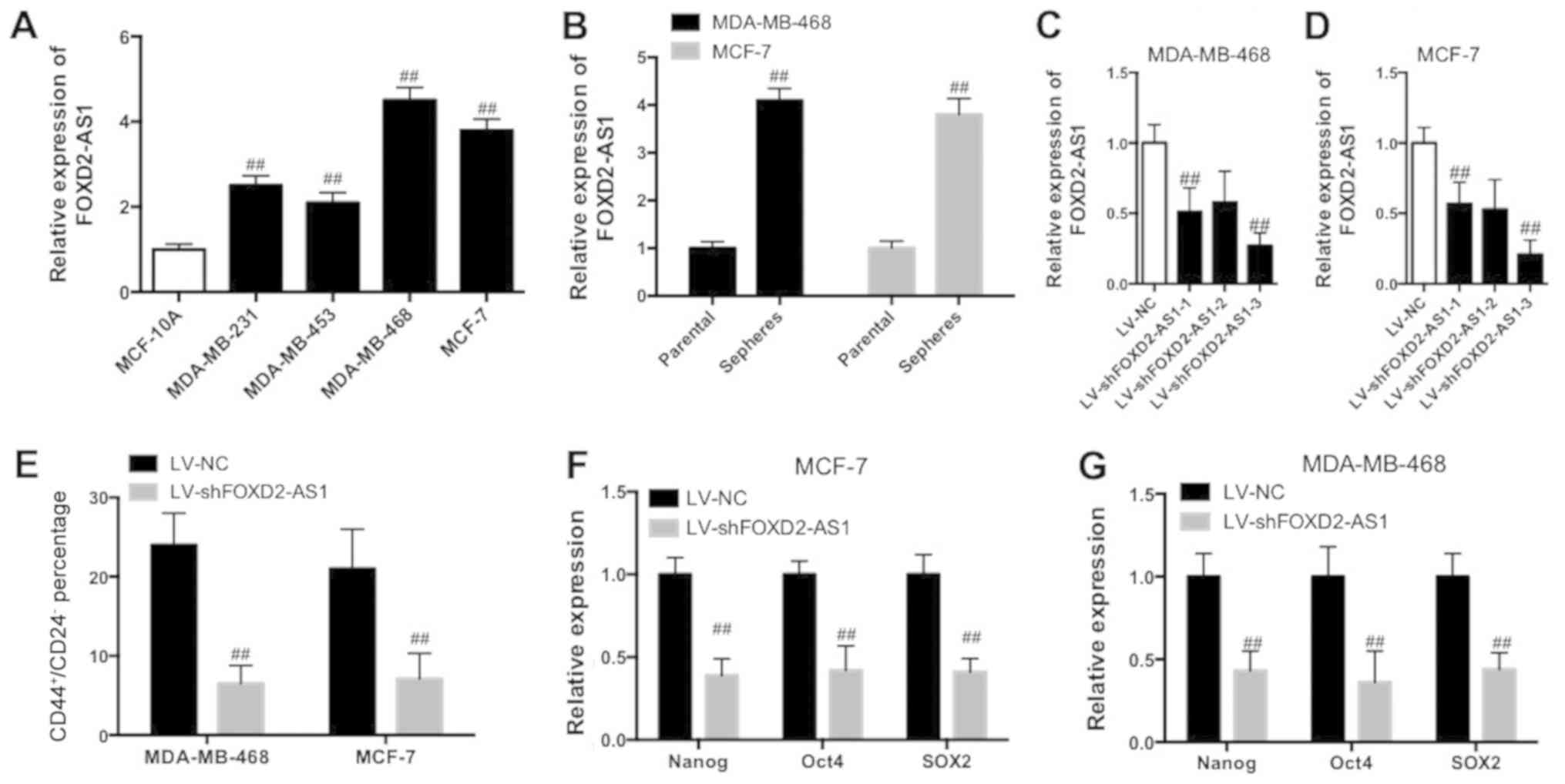 | Figure 2FOXD2-AS1 knockdown reduces BCSC
properties in BC cells. (A) RT-qPCR determination of FOXD2-AS1 in
BC cell lines, MCF-7, MDA-MB-231, MDA-MB-453 and MDA-MB-468, and
human normal breast epithelial cells (MCF-10A).
##P<0.01 vs. MCF-10A. (B) Expression of FOXD2-AS1 in
MCF-7 and MDA-MB-468 spheres and parental cells was determined
using RT-qPCR. ##P<0.01 vs. respective parental.
Knockdown efficiency of FOXD2-AS1 in (C) MDA-MB-468 and (D) MCF-7
cells. (E) Percentage of CD44+/CD24− cells in
BCSC cells (MCF-7 and MDA-MB-468) was analyzed by flow cytometry.
Expression of stem factors (Nanog, Oct4, SOX2) in (F) MCF-7 and (G)
MDA-MB-468 cells with downregulation of FOXD2-AS1 was determined
using RT-qPCR. ##P<0.05 vs. respective LV-NC.
FOXD2-AS1, FOXD2 adjacent opposite strand RNA 1; BCSC, breast
cancer stem cell; BC, breast cancer; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; CD44, CD44
antigen; CD24, signal transducer CD24; Oct4, octamer-binding
transcription factor 4; SOX2, sex determining region Y-box 2; LV,
lentivirus; NC, negative control; sh, small hairpin. |
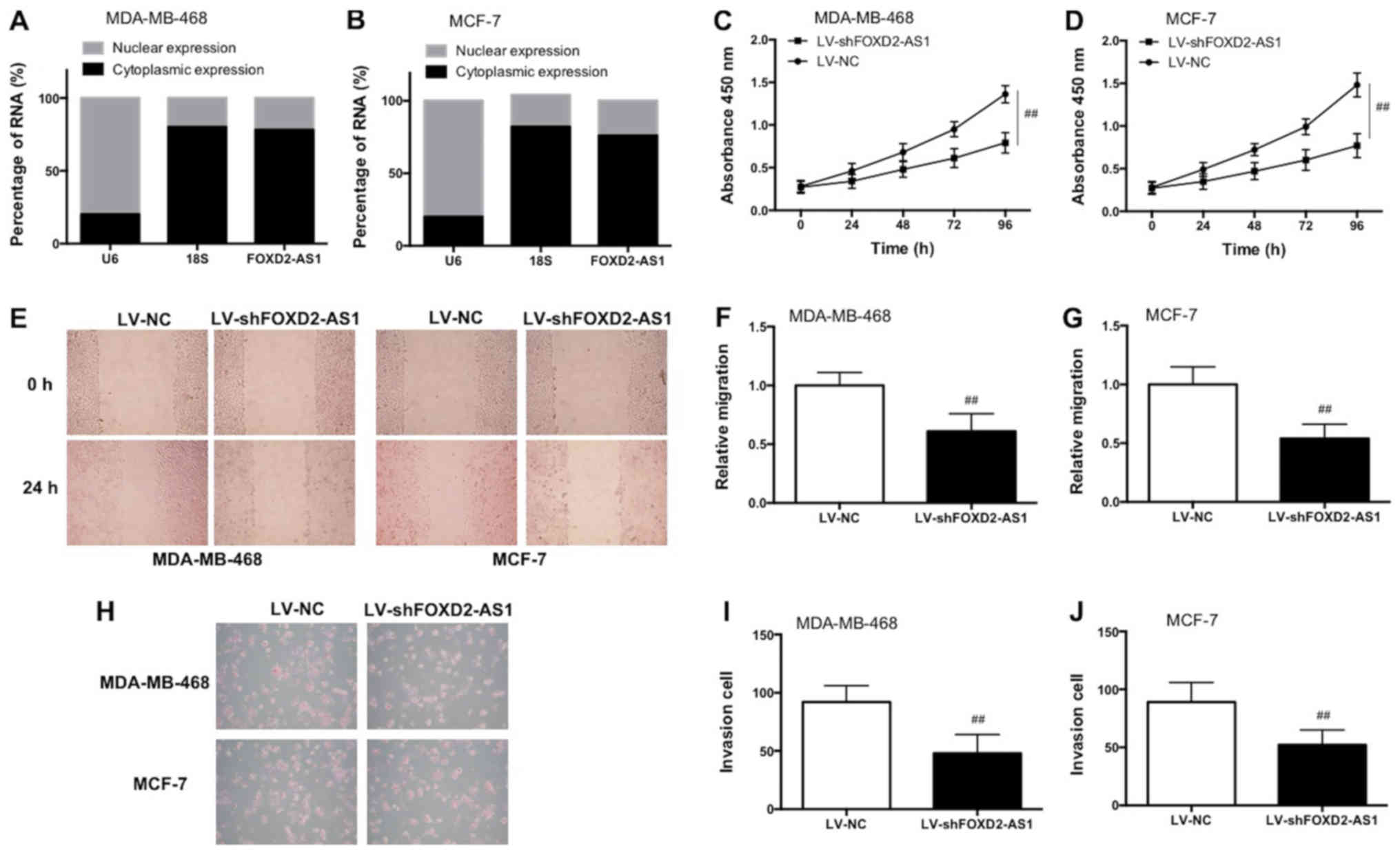
Knockdown of FOXD2-AS1 inhibits the
proliferation, migration and invasion of BC cells in vitro
The nuclear and cytoplasmic expression of FOXD2-AS1
was measured and it was identified that FOXD2-AS1 was primarily
located in the cytoplasm in MCF-7 and MDA-MB-468 cells (Fig. 3A and B). Knockdown of FOXD2-AS1
inhibited cell proliferation in MCF-7 and MDA-MB-468 cells, as
demonstrated by the CCK-8 results (Fig. 3C and D). As demonstrated in the
wound-healing assay, FOXD2-AS1 knockdown significantly decreased
the relative distance of migration (Fig. 3E–G; P<0.01). As demonstrated in
the Matrigel assay, knockdown of FOXD2-AS1 decreased the number of
invaded cells (Fig. 3H–J). Taken
together, the data demonstrated that knockdown of FOXD2-AS1
suppressed the proliferation, invasion and migration of BC cells
in vitro.
Knockdown of FOXD2-AS1 suppresses BC
tumor growth in vivo
To investigate the role of FOXD2-AS1 downregulation
on BC growth, a xenograft mice in vivo assay was conducted.
The knockdown of FOXD2-AS1 significantly decreased the tumor volume
in xenograft mice (Fig. 4A;
P<0.01). Additionally, knockdown of FOXD2-AS1 significantly
decreased the weight of the tumor (Fig. 4B; P<0.01). The expression levels
of Nanog, Oct4 and SOX2 were significantly decreased by FOXD2-AS1
downregulation (Fig. 4C;
P<0.01). Furthermore, it was demonstrated that FOXD2-AS1
knockdown significantly decreased N-cadherin expression, and
increased E-cadherin and vimentin expression (Fig. 4D; P<0.01). Therefore, the data
suggested that FOXD2-AS1 knockdown suppressed the tumor growth of
BC in vivo.
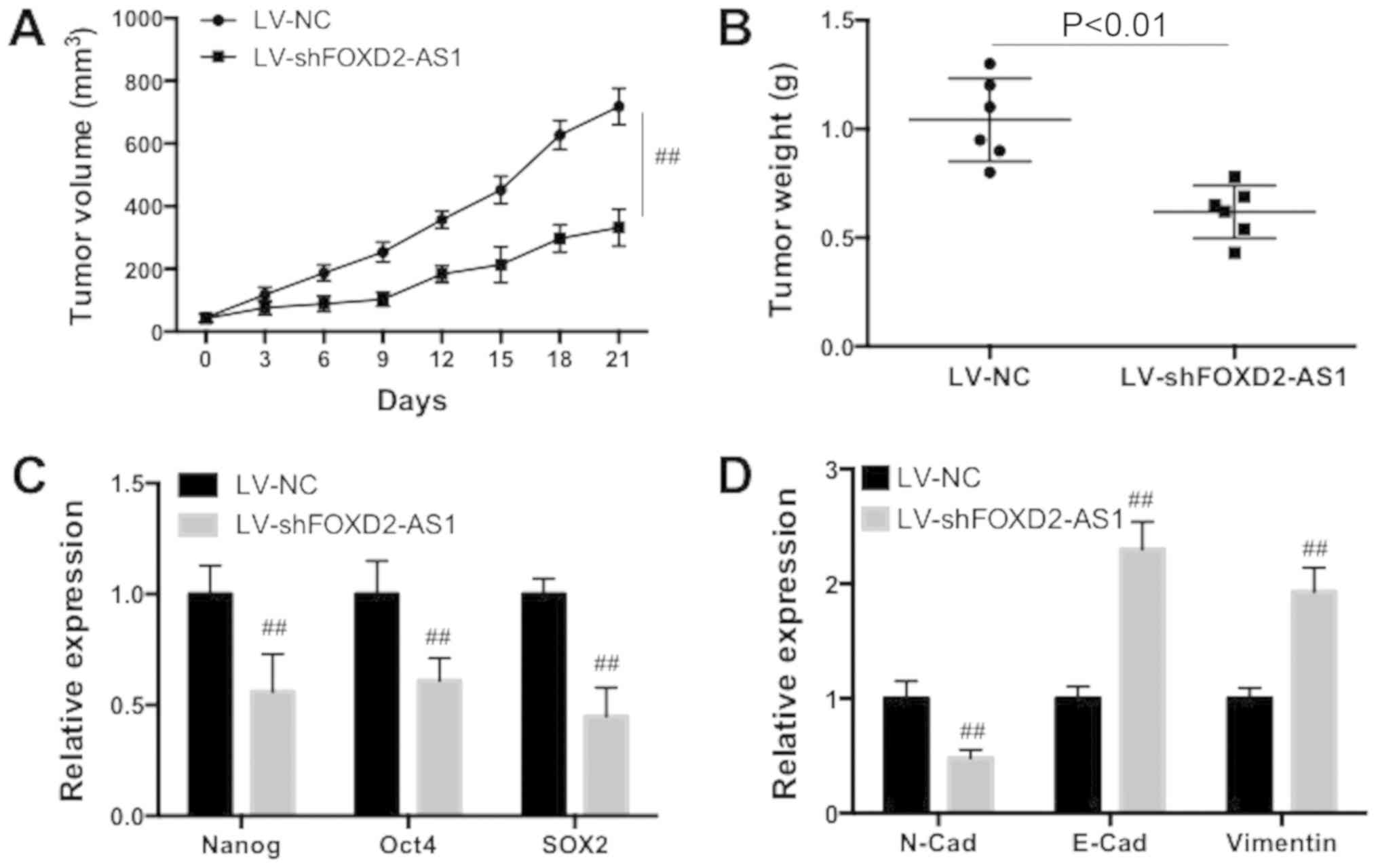 | Figure 4FOXD2-AS1 downregulation suppresses
the tumor growth of breast cancer in vivo. (A) Tumor volume
was measured in transplanted mice. (B) Tumor weight was determined.
(C) Expression of Nanog, Oct4 and SOX2 was determined using
RT-qPCR. (D) Expression of N-Cad, E-Cad and vimentin was determined
using RT-qPCR. Data are presented as the mean ± standard deviation.
##P<0.05 vs. respective LV-NC. FOXD2-AS1, FOXD2
adjacent opposite strand RNA 1; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; Oct4,
octamer-binding transcription factor 4; SOX2, sex determining
region Y-box 2; Cad, cadherin; LV, lentivirus; NC, negative
control; sh, small hairpin. |
FOXD2-AS1 promotes BC through modulation
of PFN2 by sponging miR-150-5p
To examine the molecular mechanism underlying
FOXD2-AS1-exhibited regulation of proliferation, invasion,
migration and BCSC properties of BC, the possible targets of
FOXD2-AS1 were investigated. Using bioinformatics analysis and
validation assays, it was identified that there were complementary
binding sites between miR-150-5p and the 3′-UTR of FOXD2-AS1
(Fig. 5A). The results of the
reporter gene assay provided evidence that miR-150-5p was able to
bind with the FOXD2-AS1 3′-UTR (Fig.
5B). The expression of miR-150-5p was lower in MDA-MB-468 and
MCF-7 sphere cells compared with respective parental cells
(Fig. 5C). Transfection of
miR-150-5p mimics (Fig. 5D)
significantly decreased mRNA expression levels of Nanog, Oct4 and
SOX2 in MDA-MB-468 and MCF-7 cells (Fig. 5E and F; P<0.01). As observed in
the wound-healing assay, miR-150-5p mimics significantly decreased
the relative migration distance, demonstrating a decrease in
migratory ability (Fig. 5G-I;
P<0.01). As demonstrated in the Matrigel assay, miR-150-5p
mimics decreased the number of invaded cells, suggesting a
reduction of invasive ability (Fig.
5J–L). Taken together, these data demonstrated that miR-150-5p
inhibited the proliferation, invasion, migration and BCSC
properties of BC in vitro.
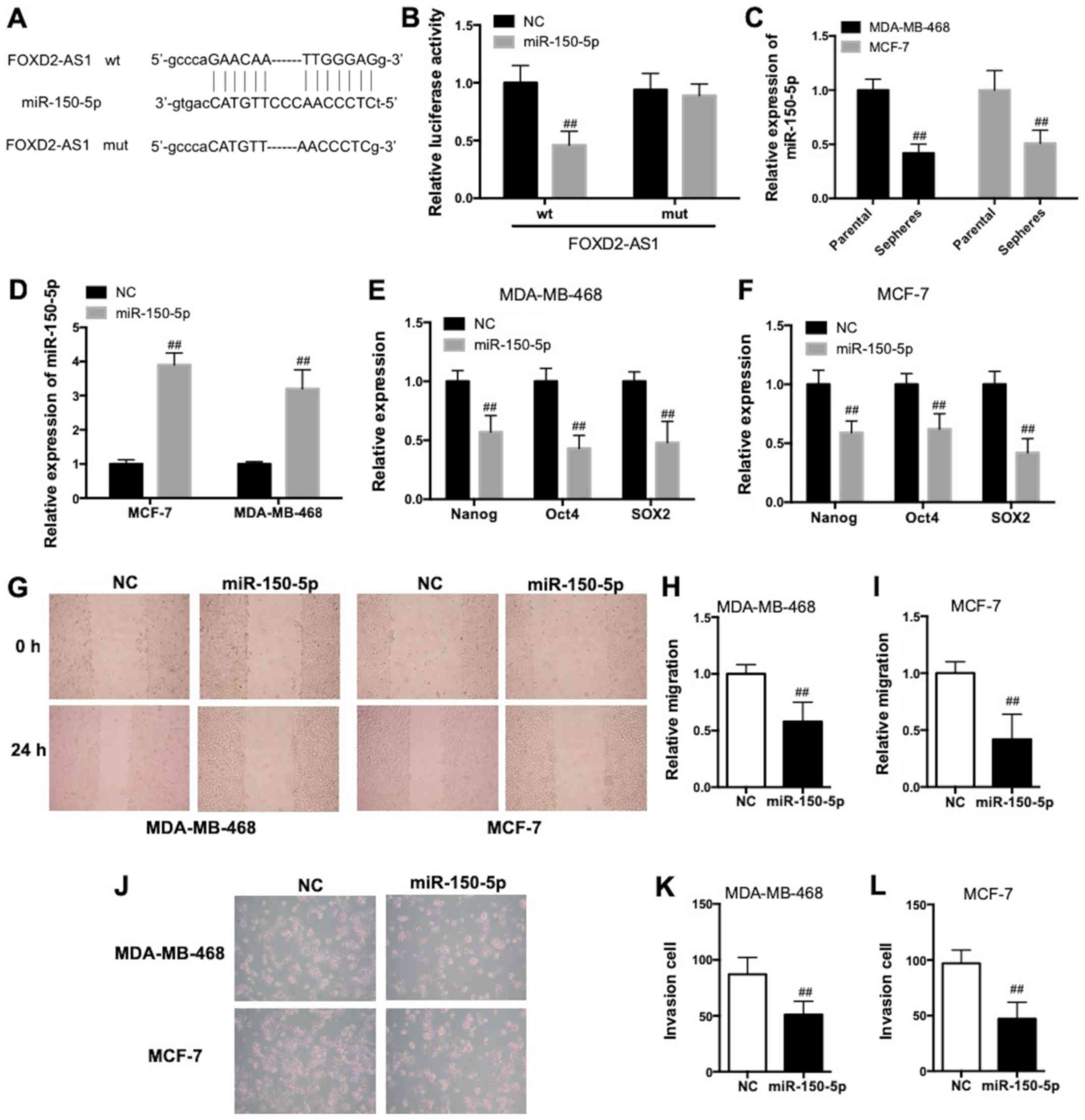 | Figure 5FOXD2-AS1 regulates BC malignancy
through interaction with miR-150-5p. (A) Bioinformatics analysis of
the binding between miR-150-5p and 3′UTR of wt FOXD2-AS1 and the
mutation of putative binding sites. (B) Direct binding of
miR-150-5p with FOXD2-AS1 3′-UTR was detected by a lucif-erase
reporter assay. (C) RT-qPCR determination of miR-150-5p in
MDA-MB-468 and MCF-7 spheres and parental cells.
##P<0.01 vs. respective parental. (D) MDA-MB-468 and
MCF-7 cells were transfected with miR-150-5p mimics and
transfection efficiency was evaluated. MDA-MB-468 and MCF-7 cells
were transfected with miR-150-5p mimics. RT-qPCR determination of
Nanog, Oct4 and SOX2 in (E) MDA-MB-468 and (F) MCF-7 cells. (G)
Representative images of the wound-healing assay. Magnification,
×400. Migration in (H) MDA-MB-468 and (I) MCF-7 cells was
determined by a wound-healing assay. (J) Representative images of
the Matrigel assay. Invasion of (K) MDA-MB-468 and (L) MCF-7 cells
was determined by a Matrigel assay. ##P<0.05 vs.
respective NC. FOXD2-AS1, FOXD2 adjacent opposite strand RNA 1; BC,
breast cancer; miR, microRNA; UTR, untranslated region; wt,
wild-type; RT-qPCR, reverse transcription-quantitative polymerase
chain reaction; Oct4, octamer-binding transcription factor 4; SOX2,
sex determining region Y-box 2; NC, negative control; mut,
mutant. |
Using bioinformatics analysis, it was identified
that there were complementary binding sites between miR-150-5p and
the PFN2 3′-UTR (Fig. 6A).
Furthermore, the direct interaction between miR-150-5p and PFN2
3′-UTR was confirmed by the results of the luciferase reporter
assay (Fig. 6B). Transfection of
miR-150-5p mimics decreased FOXD2-AS1 and PFN2 expression (Fig. 6C), whereas, miR-150-5p inhibitors
(Fig. 6D) increased FOXD2-AS1 and
PFN2 expression (Fig. 6E).
FOXD2-AS1 knockdown significantly inhibited miR-150-5p
inhibitor-induced increase of Nanog, Oct4 and SOX2 expression
(Fig. 6F; P<0.01).
Additionally, the miR-150-5p inhibitor-induced increase of
N-cadherin, and decrease of E-cadherin and vimentin was inhibited
by FOXD2-AS1 knockdown (Fig.
6G).
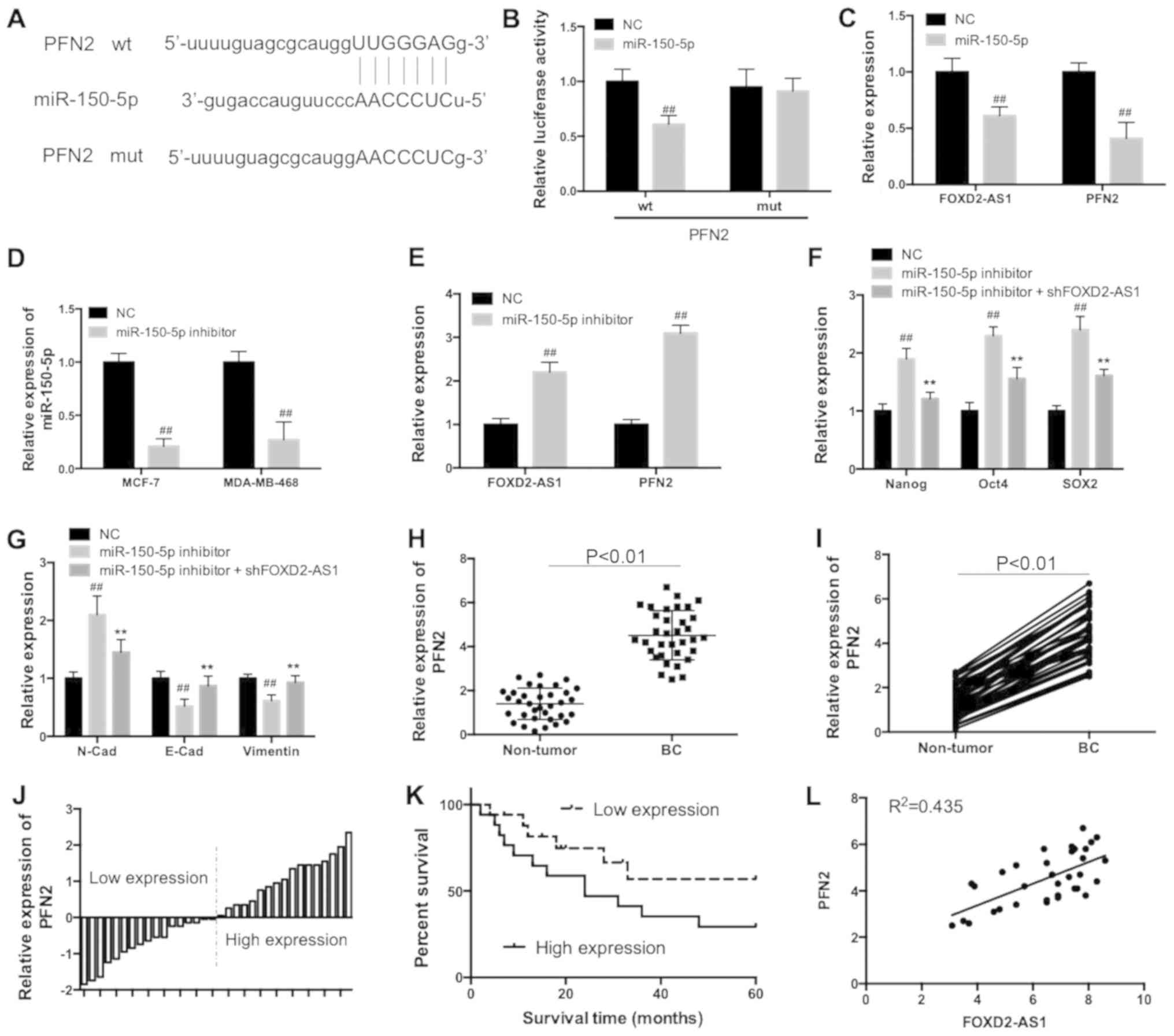 | Figure 6FOXD2-AS1 modulated the expression of
PFN2 by sponging miR-150-5p. (A) Bioinformatics analysis of the
binding between miR-150-5p and 3′UTR of wt PFN2 and the mutation of
putative binding sites. (B) Direct binding of miR-150-5p with PFN2
3′-UTR was detected by a luciferase reporter assay. (C) RT-qPCR
determination of FOXD2-AS1 and PFN2 expression in MCF-7 cells
following transfection of miR-150-5p mimics. (D) MCF-7 cells were
transfected with miR-150-5p inhibitors and the transfection
efficiency was examined. (E) RT-qPCR determination of FOXD2-AS1 and
PFN2 expression in MCF-7 cells following transfection of miR-150-5p
inhibitors. MCF-7 cells were transfected with miR-150-5p inhibitors
in the presence or absence of lentivirus-shFOXD2-AS1. (F) RT-qPCR
determination of Nanog, Oct4 and SOX2. (G) RT-qPCR determination of
N-Cad, E-Cad and vimentin. (H) RT-qPCR determination of PFN2 in 34
pairs of BC tissues and paired adjacent normal tissues. (I)
Respective association of PFN2 expression between each BC tissue
specimen and paired adjacent normal tissue. (J) A total of 34
tissues samples were divided into two groups: Higher PFN2
expression group and lower PFN2 expression group, according to the
median expression of PFN2. (K) Overall survival in patients with BC
with high/low PFN2 expression levels. (L) Correlation between
FOXD2-AS1 and PFN2 expression in BC tissues. ##P<0.05
vs. respective NC; **P<0.05 vs. respective miR-150-5p
inhibitor. FOXD2-AS1, FOXD2 adjacent opposite strand RNA 1; PFN2,
profilin 2; miR, microRNA; UTR, untranslated region; wt, wild-type;
RT-qPCR, reverse transcription-quantitative polymerase chain
reaction; sh, small hairpin; Oct4, octamer-binding transcription
factor 4; SOX2, sex determining region Y-box 2; BC, breast cancer;
NC, negative control; mut, mutant; Cad, cadherin. |
The expression of PFN2 was significantly increased
in BC tissues specimens compared with adjacent normal tissues
(Fig. 6H and I; P<0.01).
According to the median expression of PFN2 (4.35), the BC tissues
specimens were divided into two groups: A higher PFN2 expression
group and the other group with lower expression of PFN2 (Fig. 6J). The differences of overall
survival between groups with either higher or lower expression of
PFN2 were compared using Kaplan-Meier curves and a log-rank test.
The results demonstrated that overall survivals were poor in
patients with BC with high expression levels of PFN2, compared with
patients with low expression levels of PFN2 (Fig. 6K). Furthermore, the expression of
FOXD2-AS1 was positively correlated with the PFN2 expression level
in tumor tissues of patients with BC (Fig. 6L). The present results concluded
that FOXD2-AS1 promoted BC through modulation of PFN2 by sponging
miR-150-5p (Fig. 7).
Discussion
BC is one of the most prevalent malignant tumors in
women worldwide and one of the leading causes of cancer mortality
(27). The high rate of metastasis
and recurrence of BC typically contribute to accelerated
progression (28). lncRNAs are
emerging as important regulators in the process of cancer stemness
and tumorigenesis (12,29). In the present study, it was
demonstrated that FOXD2-AS1 expression was significantly increased
in BC tissue, cells and sphere subpopulation. Additionally, the
upregulation of FOXD2-AS1was closely associated with poor prognosis
of patients with BC. Furthermore, downregulation of FOXD2-AS1
decreased cell proliferation, migration and invasion in BC cells,
and inhibited tumor growth in the transplanted tumor in
vivo. Knockdown of FOXD2-AS1 decreased the percentage of
CD44+/CD24− cells and the ability to form
spheres in the BCSC cell (MCF-7 CSC and MDA-MB-468 CSC)
subpopulation, suggesting the inhibitory role of FOXD2-AS1
knockdown in BC stemness. Furthermore, knockdown of FOXD2-AS1
decreased the expression of stem factors, including Nanog, Oct4 and
SOX2. It was identified that CSCs limit the efficiency of surgical
resection or post-chemoradiotherapy as these cells may endow the
growth or proliferation potential (30,31).
The results suggested that FOXD2-AS1 serves an oncogenic role in BC
and high FOXD2-AS1 expression was associated with poor prognosis of
patients with BC.
FOXD2-AS1 was primarily located in the cytoplasm,
suggesting that the primary reason for FOXD2-AS1-exhibited
regulation of BC malignancy was due to post-transcriptional
regulation. At present, serving as an miRNA 'sponge' is believed to
be the most prevalent mechanism of lncRNA-mediated biological
regulation (32). In the present
study, it was demonstrated that the expression of FOXD2-AS1 was
increased in the sphere subpopulation of BCSC, and FOXD2-AS1 and
PFN2 expression was positively correlated. Furthermore, it was
identified that miR-150-5p targeted the 3′-UTR of FOXD2-AS1 and
PFN2 mRNA, and miR-150-5p expression was negatively associated with
FOXD2-AS1 and PFN2 expression. miR-150-5p mimics decreased cell
proliferation, migration and invasion in BC cells. Additionally,
PFN2 expression was significantly upregulated in BC tissues.
Furthermore, the upregulation of PFN2 indicated poor prognosis of
patients with BC. FOXD2-AS1 and PFN2 expression was positively
correlated. Previous studies demonstrated that miR-150-5p and PFN2
were able to regulate the development of certain cancer types
(33-35). miR-150-5p inhibits cancer cell
aggressiveness by targeting SPARC (osteonectin), cwcv and kazal
like domain proteoglycan 1 in head and neck squamous cell carcinoma
(33). PFN2 was correlated with
poor prognosis of esophageal squamous cell carcinoma, which is
proposed to be a therapeutic target (34). It was additionally demonstrated
that PFN2 promoted migration, invasion and stemness of HT29 human
colorectal cancer stem cells (35). However, at present, to the best of
the authors' knowledge, there is no evidence for the direct
interaction between FOXD2-AS1, miR-150-5p and PFN2. In summary, all
the data suggested that FOXD2-AS1 regulated the expression of PFN2
and BCSC by serving as a sponge of miR-150-5p.
In conclusion, the findings demonstrated that
FOXD2-AS1 was crucial for BC proliferation, invasion, migration and
stemness, and tumor growth. FOXD2-AS1 regulates BC malignancy
through modulation of PFN2 by sponging miR-150-5p. The present
results suggested that the FOXD2-AS1/miR-150-5p/PFN2 axis is
involved in the development of BC, and provides novel targets for
the treatment of BC, and potential biomarkers for the diagnosis and
prognosis of BC.
Funding
The present study was supported by General Project
of Western Medicine of Guangzhou Health and Family Planning
Commission (grant no. 20181A011099), Natural Science Foundation of
Guangdong Province (grant no. 22017A030313551) and Guangzhou Key
Medical Discipline Construction Project Fund.
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
XA, MJ and NQ designed the study and wrote the
paper. MJ, NQ, HX, HLia and HLi performed the experiments and
analyzed the data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present patient and animal studies were approved
by the Ethics Committee of the Affiliated Cancer Hospital and
Institute of Guangzhou Medical University (approval no.
ACHIGMU-2012-1-3-05; Guangzhou, China). Written informed consent
was obtained from all the enrolled patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar
|
|
2
|
Landero J, Touloei K and Glick BP:
Invasive ductal breast carcinoma underneath a lipoma in a male
patient. J Clin Aesthet Dermatol. 5:33–37. 2012.PubMed/NCBI
|
|
3
|
Hurvitz SA, Hu Y, O'Brien N and Finn RS:
Current approaches and future directions in the treatment of
HER2-positive breast cancer. Cancer Treat Rev. 39:219–229. 2013.
View Article : Google Scholar
|
|
4
|
Jia T, Zhang L, Duan Y, Zhang M, Wang G,
Zhang J and Zhao Z: The differential susceptibilities of MCF-7 and
MDA-MB-231 cells to the cytotoxic effects of curcumin are
associated with the PI3K/Akt-SKP2-Cip/Kips pathway. Cancer Cell
Int. 14:1262014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jovanovic J, Rønneberg JA, Tost J and
Kristensen V: The epigenetics of breast cancer. Mol Oncol.
4:242–254. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Parton M, Dowsett M and Smith I: Studies
of apoptosis in breast cancer. BMJ. 322:1528–1532. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim K, Chie EK, Han W, Noh DY, Oh DY, Im
SA, Kim TY, Bang YJ and Ha SW: Prognostic factors affecting the
outcome of salvage radiotherapy for isolated locoregional
recurrence after mastectomy. Am J Clin Oncol. 33:23–27. 2010.
View Article : Google Scholar
|
|
9
|
Mercer TR, Dinger ME and Mattick JS: Long
non-coding RNAs: Insights into functions. Nat Rev Genet.
10:155–159. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Prensner JR, Iyer MK, Sahu A, Asangani IA,
Cao Q, Patel L, Vergara IA, Davicioni E, Erho N, Ghadessi M, et al:
The long noncoding RNA SChLAP1 promotes aggressive prostate cancer
and antagonizes the SWI/SNF complex. Nat Genet. 45:1392–1398. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huarte M, Guttman M, Feldser D, Garber M,
Koziol MJ, Kenzelmann-Broz D, Khalil AM, Zuk O, Amit I, Rabani M,
et al: A large intergenic noncoding RNA induced by p53 mediates
global gene repression in the p53 response. Cell. 142:409–419.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gupta RA, Shah N, Wang KC, Kim J, Horlings
HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, et al: Long
non-coding RNA HOTAIR reprograms chromatin state to promote cancer
metastasis. Nature. 464:1071–1076. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fan Y, Shen B, Tan M, Mu X, Qin Y, Zhang F
and Liu Y: Long non-coding RNA UCA1 increases chemoresistance of
bladder cancer cells by regulating Wnt signaling. FEBS J.
281:1750–1758. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang KC and Chang HY: Molecular mechanisms
of long noncoding RNAs. Mol Cell. 43:904–914. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tsai MC, Manor O, Wan Y, Mosammaparast N,
Wang JK, Lan F, Shi Y, Segal E and Chang HY: Long noncoding RNA as
modular scaffold of histone modification complexes. Science.
329:689–693. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Qu L, Ding J, Chen C, Wu ZJ, Liu B, Gao Y,
Chen W, Liu F, Sun W, Li XF, et al: Exosome-transmitted lncARSR
promotes sunitinib resistance in renal cancer by acting as a
competing endogenous RNA. Cancer Cell. 29:653–668. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen G, Sun W, Hua X, Zeng W and Yang L:
Long non-coding RNA FOXD2-AS1 aggravates nasopharyngeal carcinoma
carcinogenesis by modulating miR-363-5p/S100A1 pathway. Gene.
645:76–84. 2018. View Article : Google Scholar
|
|
18
|
Li J, Zhao Y, Lu Y, Ritchie W, Grau G,
Vadas MA and Gamble JR: The poly-cistronic miR-23-27-24 complexes
target endothelial cell junctions: Differential functional and
molecular effects of miR-23a and miR-23b. Mol Ther Nucleic Acids.
5:e3542016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li CY, Liang GY, Yao WZ, Sui J, Shen X,
Zhang YQ, Peng H, Hong WW, Ye YC, Zhang ZY, et al: Integrated
analysis of long non-coding RNA competing interactions reveals the
potential role in progression of human gastric cancer. Int J Oncol.
48:1965–1976. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rong L, Zhao R and Lu J: Highly expressed
long non-coding RNA FOXD2-AS1 promotes non-small cell lung cancer
progression via Wnt/β-catenin signaling. Biochem Biophys Res
Commun. 484:586–591. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bao J, Zhou C, Zhang J, Mo J, Ye Q, He J
and Diao J: Upregulation of the long noncoding RNA FOXD2-AS1
predicts poor prognosis in esophageal squamous cell carcinoma.
Cancer Biomark. 21:527–533. 2018. View Article : Google Scholar
|
|
22
|
Shang W, Tang Z, Gao Y, Qi H, Su X, Zhang
Y and Yang R: lncRNA RNCR3 promotes Chop expression by sponging
miR-185-5p during MDSC differentiation. Oncotarget.
8:111754–111769. 2017. View Article : Google Scholar
|
|
23
|
An Q, Zhou L and Xu N: Long noncoding RNA
FOXD2-AS1 accelerates the gemcitabine-resistance of bladder cancer
by sponging miR-143. Biomed Pharmacother. 103:415–420. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kalli S, Semine A, Cohen S, Naber SP,
Makim SS and Bahl M: American Joint Committee on Cancer's Staging
System for Breast Cancer, Eighth Edition: What the radiologist
needs to know. Radiographics. 38:1921–1933. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−∆∆C(T)) Method. Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
26
|
Peng F, Li TT, Wang KL, Xiao GQ, Wang JH,
Zhao HD, Kang ZJ, Fan WJ, Zhu LL, Li M, et al: H19/let-7/LIN28
reciprocal negative regulatory circuit promotes breast cancer stem
cell maintenance. Cell Death Dis. 8:e25692017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cai Z and Liu Q: Cell Cycle regulation in
treatment of breast cancer. Adv Exp Med Biol. 1026:251–270. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang T: Association between HIF1α 1772 C/T
polymorphism and breast cancer susceptibility: A systematic review.
Crit Rev Eukaryot Gene Expr. 27:297–304. 2017. View Article : Google Scholar
|
|
29
|
Wang J and Sun G: FOXO1-MALAT1-miR-26a-5p
feedback loop mediates proliferation and migration in osteosarcoma
cells. Oncol Res. 25:1517–1527. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu Y, Choi DS, Sheng J, Ensor JE, Liang
DH, Rodriguez-Aguayo C, Polley A, Benz S, Elemento O, Verma A, et
al: HN1L promotes triple-negative breast cancer stem cells through
LEPR-STAT3 pathway. Stem Cell Reports. 10:212–227. 2018. View Article : Google Scholar :
|
|
31
|
Safa AR: Resistance to cell death and its
modulation in cancer stem cells. Crit Rev Oncog. 21:203–219. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gao YL, Zhao ZS, Zhang MY, Han LJ, Dong YJ
and Xu B: Long noncoding RNA PVT1 facilitates cervical cancer
progression via negative regulating of miR-424. Oncol Res.
25:1391–1398. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Koshizuka K, Hanazawa T, Kikkawa N, Katada
K, Okato A, Arai T, Idichi T, Osako Y, Okamoto Y and Seki N:
Antitumor miR-150-5p and miR-150-3p inhibit cancer cell
aggressiveness by targeting SPOCK1 in head and neck squamous cell
carcinoma. Auris Nasus Larynx. 45:854–865. 2018. View Article : Google Scholar
|
|
34
|
Cui XB, Zhang SM, Xu YX, Dang HW, Liu CX,
Wang LH, Yang L, Hu JM, Liang WH, Jiang JF, et al: PFN2, a novel
marker of unfavorable prognosis, is a potential therapeutic target
involved in esophageal squamous cell carcinoma. J Transl Med.
14:1372016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim MJ, Lee YS, Han GY, Lee HN, Ahn C and
Kim CW: Profilin 2 promotes migration, invasion, and stemness of
HT29 human colorectal cancer stem cells. Biosci Biotechnol Biochem.
79:1438–1446. 2015. View Article : Google Scholar : PubMed/NCBI
|